Abstract
In 1999, Engelen and coworkers investigated colonization in Amsterdam among 259 children attending 16 day-care centers (DCCs) and among 276 children who did not attend day-care centers (NDCCs). A 1.6- to 3.4-fold increased risk for nasopharyngeal colonization was observed in children attending DCCs compared with NDCC children, while no difference in antibiotic resistance was found between groups. The serotype and genotype distributions of 305 nasopharyngeal Streptococcus pneumoniae isolates of the latter study were investigated. The predominant serotypes in both the DCC and the NDCC groups included 19F (19 and 18%, respectively), 6B (14 and 16%, respectively), 6A (13 and 7%, respectively), 23F (9 and 7%, respectively), and 9V (7 and 7%, respectively). The theoretical vaccine coverage of the 7-valent conjugate vaccine was 59% for the DCC children and 56% for the NDCC group. Genetic analysis of the pneumococcal isolates revealed 75% clustering among pneumococci isolated from DCC attendees versus 50% among the NDCC children. The average pneumococcal cluster size in the DCC group was 3.8 and 4.6 isolates for two respective sample dates (range, 2 to 13 isolates per cluster), while the average cluster size for the NDCC group was 3.0 (range, 2 to 6 isolates per cluster). Similar to observations made in other countries, these results indicate a higher risk for horizontal spread of pneumococci in Dutch DCCs than in the general population. This study emphasizes the importance of molecular epidemiological monitoring before, during, and after implementation of pneumococcal conjugate vaccination in national vaccination programs for children.
Streptococcus pneumoniae is worldwide one of the major causes of severe infections such as meningitis, septicemia, and respiratory tract infections. In addition, S. pneumoniae is, together with Moraxella catarrhalis and Haemophilus influenzae, a dominant pathogen in middle ear infections and sinusitis. Risk groups for pneumococcal infections are young children under the age of 2 years, elderly people, and immunocompromised patients (1).
Pneumococci are often part of the nasopharyngeal flora; probably all humans are colonized with this organism at least once early in life. The risk of pneumococcal colonization is high, especially under conditions with crowding, such as day-care centers (DCCs), nursing homes, hospitals, and jails (16, 22, 23). A strong relation between carriage and middle ear infections has been found, but the association between colonization and invasive disease has not been confirmed (11, 31).
The emergence of penicillin- and multidrug-resistant pneumococci has been observed in various countries over the last decade. In some countries and populations, up to 60% of the pneumococcal isolates are resistant to one or more antibiotics (3, 12, 17). A significant proportion of pneumococcal resistance is the result of the worldwide spread of a limited number of multidrug-resistant clones (4, 14, 30, 35). Carriage of organisms with decreased antibiotic susceptibility is associated with young age, female sex, winter season, and exposure to antimicrobial drugs during the previous month (37).
Children attending DCCs have several risk factors for carriage, i.e., young age, crowding, and frequent usage of antimicrobial agents. Furthermore, it is believed that DCCs may be a global reservoir for multidrug-resistant pneumococci (27). Therefore, prevention of carriage and infection with S. pneumoniae in these risk groups will become an important tool in the battle against (antibiotic-resistant) S. pneumoniae.
Prevention of infections caused by S. pneumoniae and of spread of this pathogen is an important goal of an effective vaccine. Therefore, new vaccines have been developed that are also immunogenic in risk groups, such as young children, the elderly, and immunocompromised patients. Results with these conjugate vaccines, containing polysaccharides from up to 11 different serotypes conjugated to a protein carrier (tetanus-diphtheria toxoid-Hib protein or meningococcal outer membrane protein), are promising (6, 9, 21, 28, 29). Recently, one of these vaccines, the 7-valent pneumococcal conjugate vaccine from Wyett Lederle, has been approved by the Department of Health and Human Services in the United States and the European Agency for the Evaluation of Medicine for Europe.
The introduction of the conjugate vaccines underscores the need for detailed and long-term epidemiological surveillance of S. pneumoniae in the target groups in order to calculate the theoretical vaccine coverage and to evaluate the (long-term) effects of large-scale introduction of this vaccine in the general population by using serological and molecular techniques. So far, no data are available on the molecular epidemiology of S. pneumoniae carriage in young children in The Netherlands.
In 1999, a study was performed in Amsterdam, The Netherlands, among 259 children attending 16 DCCs and 276 children who did not attend day-care centers (NDCC). We investigated nasopharyngeal carriage rates and susceptibility to antibiotics of the nasopharyngeal flora. Carriage rates for S. pneumoniae, M. catarrhalis, and H. influenzae of 37, 48, and 11%, respectively, were observed in the NDCC group. Increased risks of 1.6, 1.7, and 3.4 for carriage of S. pneumoniae, M. catarrhalis, and H. influenzae, respectively, were observed among DCC attendees. Finally, DCC attendees were ill more frequently and used more antibiotics than the controls. Similar to earlier surveillance data from The Netherlands (20), only 2% of the pneumococcal isolates showed reduced susceptibility to erythromycin and no penicillin resistance was found (P. Peerbooms, M. Engelen, A. van Belkum, and R. Coutinho, 11th Eur. Cong. Clin. Microbiol. Infect. Dis., abstr. 8, 2001). These results are in contrast to data from previous DCC studies in other countries, where antibiotic resistance among pneumococcal isolates was high, associated with previous antibiotic consumption, and correlated to increased spread of drug-resistant pneumococci among DCC attendees (2, 5, 24, 33, 37). Because drug resistance among pneumococci is negligible in our study group, we hypothesized that crowding, which is also a risk factor for nasopharyngeal carriage, is playing an important role in facilitating the transmission of bacteria among children in DCCs. To obtain insight in the transmission of pneumococci in children, the molecular epidemiology of the pneumococcal isolates collected from both the general population and DCCs was investigated by serotyping and genotyping.
MATERIALS AND METHODS
Bacterial sampling.
S. pneumoniae strains were isolated from the nasopharynges of 259 children, aged 3 to 36 months, attending 16 DCCs in Amsterdam, The Netherlands, from January to March 1999. All children were sampled twice, with a time interval of 4 weeks. In the same period, an additional 276 children from three well-baby clinics in Amsterdam, aged 3 to 36 months, who did not attend DCCs were evaluated for S. pneumoniae carriage (P. Peerbooms et al., 11th Eur. Cong. Clin. Microbiol. Infect. Dis., 2001). Nasopharyngeal samples were obtained with a dacron pernasal swab (Medical Wire & Equipment Co., Wiltshire, England). The swabs were transported in Amies transport medium to the Microbiology Laboratory of the Municipal Health Service (Amsterdam, The Netherlands), immediately plated on 5% sheep blood agar plates, and grown overnight at 36°C in a CO2-enriched atmosphere. S. pneumoniae isolates were identified according to standard microbiological procedures (18). Molecular analyses were performed on all the pneumococcal isolates that were available for use, i.e., 115 and 129 strains collected from the 16 DCCs on the two occasions, respectively, and 61 strains collected from the NDCC children.
Serotyping.
Pneumococci were serotyped by the capsular quellung method (Quellung reaction) and observed microscopically using commercially available antisera (Statens Seruminstitut, Copenhagen, Denmark).
RFEL.
Typing of the 305 pneumococcal strains by restriction fragment end labeling (RFEL) analysis was performed as described by Van Steenbergen et al. (36) and as adapted by Hermans et al. (15). Briefly, purified pneumococcal DNA was digested by the restriction enzyme EcoRI. The DNA restriction fragments were end labeled at 72°C with [α-32P]dATP using DNA polymerase (Goldstar; Eurogentec, Seraing, Belgium). The radiolabeled fragments were denatured and separated electrophoretically on a 6% polyacrylamide sequencing gel containing 8 M urea. Subsequently, the gel was transferred onto filter paper, vacuum dried (HBI, Saddlebrook, N.Y.), and exposed for variable lengths of time at room temperature to ECL Hyperfilms (Amersham, Little Chalfont, United Kingdom).
Computer-assisted analysis of RFEL banding patterns.
The RFEL types were analyzed using the Windows version of the Gelcompar software (version 4; Applied Maths, Kortrijk, Belgium) after imaging the RFEL autoradiograms using the Image Master DTS (Pharmacia Biotech, Uppsala, Sweden). For this purpose, DNA fragments in the molecular weight range of 160 to 400 bp were explored. The DNA banding patterns were normalized using pneumococcus-specific bands present in the RFEL banding patterns of all strains. Comparison of the banding patterns was performed by unweighted pair group method using arithmetic averages (25) and the Jaccard similarity coefficient applied to peaks (32). Computer-assisted analysis and methods and algorithms used in this study were carried out according to the instructions of the manufacturer of Gelcompar. A tolerance of 1.2% in band position was applied during comparison of the DNA patterns. For evaluation of the genetic relatedness of the isolates, we used the following definitions: score of 1, strains of the particular RFEL type are considered 100% identical by RFEL analysis; score of 2, the RFEL cluster represents a group of RFEL types that differs in only one band (>95% genetic relatedness); score of 3, the RFEL lineage represents a group of RFEL types that differs in less than four bands (>85% genetic relatedness).
The genotypes of the pneumococcal isolates were also compared with an international collection of pneumococcal strains representing about 320 distinct RFEL types originating from 17 different countries in America, Europe, Africa, and Asia (M. Sluijter, unpublished observations), including the 16 international clones described by the Pneumococcal Epidemiology Network (http://www.wits.ac.za/pmen/pmen.htm).
Statistical analysis.
For statistical analysis of the results, the Fisher exact test was used.
RESULTS
We investigated the serotypes of 244 DCC isolates and 61 NDCC isolates (Table 1). The predominant serotypes in both the DCC and NDCC groups were the serotypes 19F (19 and 18%, respectively), 6B (14 and 16%, respectively), 6A (13 and 7%, respectively), 23F (9 and 7%, respectively), 9V (7 and 7%, respectively), and 14 (7 and 5%, respectively). The serotypes 19F, 6B, 23F, 9V, and 14 are covered by the 7-valent pneumococcal conjugate vaccine. The other two serotypes covered by the 7-valent conjugate vaccine are serotypes 4 and 18C. No children were colonized with vaccine type 4, and only 1 to 3% of the children were colonized with vaccine serotype 18. The theoretical vaccine coverages of the 7-valent conjugate vaccine are 59% for the DCC group and 57% for the NDCC group. The theoretical vaccine coverage of the 11-valent conjugate vaccine, in which the additional capsular types 1, 3, 5, and 7F are included, is 62% for both the DCC and NDCC groups.
TABLE 1.
Contribution of vaccine serotypes to carriage of S. pneumoniae in Dutch infants
| Serotype | % of children with the serotype
|
|
|---|---|---|
| DCCa | NDCC | |
| 19F | 19 | 18 |
| 6B | 14 | 16 |
| 9V | 7 | 7 |
| 23F | 9 | 7 |
| 14 | 7 | 5 |
| 18 | 1 | 3 |
| 4 | 0 | 0 |
| 6A | 13 | 7 |
| 19A | 4 | 2 |
| 23A+23B | 4 | 5 |
| 7-valent conjugate vaccine (4, 6B, 9V, 14, 18C, 19F, and 23F) | 59 | 57 |
| 7-valent conjugate vaccine (including 6A) | 72 | 64 |
| 11-valent conjugate vaccine (7-valent + 1, 3, 5, and 7F) | 62 | 62 |
| 11-valent conjugate vaccine (including 6A) | 75 | 70 |
Average percentages were calculated from the two sampling dates.
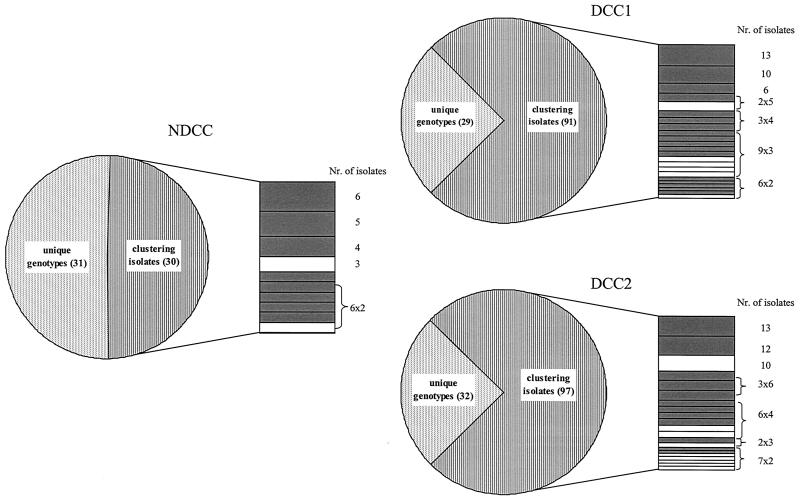
Genotyping of the 305 pneumococcal isolates from this study was performed by RFEL analysis. The 61 isolates from the NDCC children showed 50 different genotypes; 50% of the strains represented 10 distinct genetic clusters. Cluster sizes in the NDCC group ranged from two to six strains per cluster with an average cluster size of 3.0. The DCC group displayed 66 and 75 different genotypes at the first and second sampling dates, respectively. Seventy-five percent of the strains from both the first and the second sampling dates represented 24 and 20 clusters, respectively. This percentage differed significantly from the 50% genetic clustering observed in the NDCC group (P < 0.01). Cluster sizes within the individual sampling data ranged from 2 to 13 strains, with an average cluster size of 3.8 and 4.6 for the two sampling dates, respectively (Fig. 1). The majority of the clusters in both the DCC group (29 of 44 clusters) and the NDCC group (8 of 10 clusters) displayed a serotype covered by the 7-valent conjugate vaccine, including the cross-protective serotype 6A (Fig. 1). We also investigated carriage turnover in the DCC group, defined as the percentage of children with positive pneumococcal samples at both sampling dates that changed genotypes within the 4-week interval between sampling dates. A total of 209 of 259 children in the DCC group were carrying a pneumococcus on the nasopharynx at least once. Of the 259 children, 107 were carrying pneumococci on the nasopharynx at both sampling dates. The isolates from 69 children who were identified as being colonized by S. pneumoniae at both sampling dates were available for molecular analysis. Forty-four of these 69 children had changed genotypes between the two sampling dates, i.e., a carriage turnover of 64%.
FIG. 1.
Number and distribution of pneumococcal isolates with unique genotypes or genetically clustered isolates from both the NDCC children and the DCC attendees. The clustered isolates are further grouped into separate clusters. Cluster sizes are depicted on the right; clusters representing conjugate-vaccine serotypes are depicted in gray, and nonvaccine serotypes are depicted in white. DCC1 and DCC2 refer to the two different sampling dates.
Comparison of the RFEL data with the 16 international clones described by the Pneumococcal Epidemiology Network demonstrated that six isolates (10%) of the NDCC group were identical to the clones Taiwan19F-14 (1 isolate), France9V-3 (3 isolates), Slovakia14-10 (1 isolate), and Tennessee23F-4 (1 isolate), whereas 25 of the isolates from the DCC group (10%) belonged to the clones France9V-3 (15 isolates), Slovakia14-10 (6 isolates), and Tennessee23F-4 (4 isolates). All but one of the isolates were susceptible to the antibiotics penicillin, tetracycline, erythromycin, and cotrimoxazole, in contrast to the antibiotic resistance patterns of their genetically homologous clones. Only the isolate identical to clone Taiwan19F-14 was resistant to erythromycin (Peerbooms et al., 11th Eur. Cong. Clin. Microbiol. Infect. Dis., 2001).
DISCUSSION
In The Netherlands, the prevalence of pneumococcal colonization in the pediatric population was found to be 58% in DCC attendees versus 37% in the NDCC group, i.e., a 1.6-fold higher risk of pneumococcal colonization in DCC attendees compared to age-matched NDCC children (Peerbooms et al., 11th Eur. Cong. Clin. Microbiol. Infect. Dis., 2001). Known risk factors for carriage of S. pneumoniae are young age, crowding, and antibiotic usage. Since only 2% of the pneumococcal isolates were resistant to erythromycin and no resistance to the antibiotics penicillin, cotrimoxazole, or tetracycline was found, crowding is presumed to be the most important contributor to the difference in carriage seen in this study. We hypothesize that crowding facilitates horizontal transfer of bacteria from one child to another. Therefore, we investigated the molecular epidemiology of 305 pneumococcal isolates from this study. Both the DCC group and the NDCC group showed a serotype distribution comparable to what is found in many other countries, such as Israel, Finland, Canada, and South Africa (6, 10, 16, 19, 34). The most predominant serotypes were 19F (19 and 18% in DCC and NDCC attendees, respectively), 6B (14 and 16%, respectively), 6A (13 and 7%, respectively), 23F (9 and 7%, respectively), and 9V (7 and 7%, respectively). This serotype distribution implicates a theoretical coverage of 57 to 59% by the 7-valent conjugate vaccine. The additive value of an 11-valent conjugate vaccine is only 3 to 5% (62% total coverage for both study groups). An additive cross-protective effect is expected at least for serotype 6A (26), which increases the theoretical coverage of the 7-valent conjugate vaccine to 64% for the NDCC group and to 72% for the DCC group. It is unknown whether a cross-protective effect can be expected for the serotypes 19A, 23A, and 23B (13, 26, 38). With respect to the theoretical vaccine coverage, the long-term effect of large-scale implementation of the conjugate vaccine for the Dutch pediatric population remains unknown. Eskola et al. have found a similar serotype distribution before vaccination with the 7-valent pneumococcal conjugate vaccine and a shift towards nonvaccine serotypes causing middle ear infection after vaccination (10). Such a shift in distribution after conjugate vaccination was also observed among nasopharyngeal carriage isolates (7, 8, 19; S. K. Obaro, R. A. Adegbola, W. A. Banya, and B. M. Greenwood, Letter, Lancet 348:271–272, 1996). Therefore, it is concluded that a shift in distribution towards nonvaccine serotypes will reduce the efficacy of conjugate vaccination with respect to carriage and disease.
In the DCC group, 75% of the pneumococci represented genetic clusters, in contrast to 50% in the NDCC group. These molecular epidemiological data suggest augmented spread of pneumococci among DCC attendees compared to the NDCC group. In addition, the average cluster size for the first and second sampling dates in the DCC group were 3.8 and 4.6, respectively, with a range of 2 to 13 isolates per cluster, compared to 3.0 for the control group with a range of 2 to 6 isolates per cluster (Fig. 1). These data show larger clusters in the DCC group, which supports the hypothesis that pneumococci are spread more frequently by horizontal transfer between DCC attendees than among NDCC attendees. A carriage turnover of 64% in the DCC children with two positive pneumococcal isolates at both sampling dates was observed. At present, no carriage turnover data are available for the NDCC population. Whether the genotype shift in the DCC population is due to recolonization of the nasopharyngeal niche by new genotypes or whether it is due to unmasking of genotypes which were already present but not detected as a result of the abundant presence of other genotypes needs to be further investigated.
The RFEL patterns of both the DCC group and the NDCC group were compared with the 16 international (multidrug-resistant) clones described by the Pneumococcal Epidemiology Network. Twenty-five isolates (10%) in the DCC group were homologous to 3 of the reference clones (100% identical), whereas 6 isolates (10%) in the NDCC group matched with 4 of these clones. In contrast to the multidrug-resistant reference clones, all isolates but one were fully susceptible to penicillin, erythromycin, and cotrimoxazole. The resistant isolate, identical to the clone Taiwan19F-14, had reduced susceptibility to erythromycin only, whereas the reference clone was resistant to erythromycin, penicillin, and tetracycline. These results suggest that these Dutch isolates represent members of the ancestor lineages of the resistant reference clones. The overall absence of resistant pneumococcal strains in The Netherlands may be explained by the restricted use of antibiotics in general compared to that in many other countries.
In conclusion, an increased frequency of horizontal spread of S. pneumoniae strains was shown in DCCs. At least 56% of the nasopharyngeal pneumococcal isolates would be theoretically covered by a 7-valent conjugate vaccine. Furthermore, the majority of the horizontal spreading genotypes (70 to 80%) express capsular types that are covered by the conjugate vaccine. These data indicate that implementation of the pneumococcal conjugate vaccine in the near future in Dutch infants, and especially in risk groups like DCC attendees, should be considered. Importantly, to investigate the long-term efficacy of the vaccine against pneumococcal infections, detailed molecular epidemiological monitoring of pneumococcal colonization and infection is required.
ACKNOWLEDGMENTS
We thank M. Sluijter for technical support.
This study was supported by the Sophia Foundation for Medical Research (grant 268), Rotterdam, The Netherlands, and NWO (grant SGO-Inf.005), The Hague, The Netherlands.
REFERENCES
- 1.Anonymous. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 1997;46(RR-8):1–24. [PubMed] [Google Scholar]
- 2.Arnold K E, Leggiadro R J, Breiman R F, Lipman H B, Schwartz B, Appleton M A, Cleveland K O, Szeto H C, Hill B C, Tenover F C, Elliott J A, Facklam R R. Risk factors for carriage of drug-resistant Streptococcus pneumoniae among children in Memphis, Tennessee. J Pediatr. 1996;128:757–764. doi: 10.1016/s0022-3476(96)70326-8. [DOI] [PubMed] [Google Scholar]
- 3.Baquero F, Garcia-Rodriguez J A, Garcia de Lomas J, Aguilar L. Antimicrobial resistance of 1,113 Streptococcus pneumoniae isolates from patients with respiratory tract infections in Spain: results of a 1-year (1996–1997) multicenter surveillance study. The Spanish Surveillance Group for Respiratory Pathogens. Antimicrob Agents Chemother. 1999;43:357–359. doi: 10.1128/aac.43.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corso A, Severina E P, Petruk V F, Mauriz Y R, Tomasz A. Molecular characterization of penicillin-resistant Streptococcus pneumoniae isolates causing respiratory disease in the United States. Microb Drug Resist. 1998;4:325–337. doi: 10.1089/mdr.1998.4.325. [DOI] [PubMed] [Google Scholar]
- 5.Dagan R, Leibovitz E, Greenberg D, Yagupsky P, Fliss D M, Leiberman A. Dynamics of pneumococcal nasopharyngeal colonization during the first days of antibiotic treatment in pediatric patients. Pediatr Infect Dis J. 1998;17:880–885. doi: 10.1097/00006454-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Dagan R, Melamed R, Muallem M, Piglansky L, Greenberg D, Abramson O, Mendelman P M, Bohidar N, Yagupsky P. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174:1271–1278. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]
- 7.Dagan R, Melamed R, Zamir O, Leroy O. Safety and immunogenicity of tetravalent pneumococcal vaccines containing 6B, 14, 19F and 23F polysaccharides conjugated to either tetanus toxoid or diphtheria toxoid in young infants and their boosterability by native polysaccharide antigens. Pediatr Infect Dis J. 1997;16:1053–1059. doi: 10.1097/00006454-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Dagan R, Muallem M, Melamed R, Leroy O, Yagupsky P. Reduction of pneumococcal nasopharyngeal carriage in early infancy after immunization with tetravalent pneumococcal vaccines conjugated to either tetanus toxoid or diphtheria toxoid. Pediatr Infect Dis J. 1997;16:1060–1064. doi: 10.1097/00006454-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Eskola J. Immunogenicity of pneumococcal conjugate vaccines. Pediatr Infect Dis J. 2000;19:388–393. doi: 10.1097/00006454-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 10.Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, Takala A, Kayhty H, Karma P, Kohberger R, Siber G, Makela P H, Lockhart S, Eerola M. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 11.Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics J Infect Dis. 1997;175:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 12.Fairchok M P, Ashton W S, Fischer G W. Carriage of penicillin-resistant pneumococci in a military population in Washington, D.C.: risk factors and correlation with clinical isolates. Clin Infect Dis. 1996;22:966–972. doi: 10.1093/clinids/22.6.966. [DOI] [PubMed] [Google Scholar]
- 13.Giebink G S, Meier J D, Quartey M K, Liebeler C L, Le C T. Immunogenicity and efficacy of Streptococcus pneumoniae polysaccharide-protein conjugate vaccines against homologous and heterologous serotypes in the chinchilla otitis media model. J Infect Dis. 1996;173:119–127. doi: 10.1093/infdis/173.1.119. [DOI] [PubMed] [Google Scholar]
- 14.Hermans P W, Sluijter M, Dejsirilert S, Lemmens N, Elzenaar K, van Veen A, Goessens W H, de Groot R. Molecular epidemiology of drug-resistant pneumococci: toward an international approach. Microb Drug Resist. 1997;3:243–251. doi: 10.1089/mdr.1997.3.243. [DOI] [PubMed] [Google Scholar]
- 15.Hermans P W, Sluijter M, Hoogenboezem T, Heersma H, van Belkum A, de Groot R. Comparative study of five different DNA fingerprint techniques for molecular typing of Streptococcus pneumoniae strains. J Clin Microbiol. 1995;33:1606–1612. doi: 10.1128/jcm.33.6.1606-1612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellner J D, Ford-Jones E L. Streptococcus pneumoniae carriage in children attending 59 Canadian child care centers. Toronto Child Care Centre Study Group. Arch Pediatr Adolesc Med. 1999;153:495–502. doi: 10.1001/archpedi.153.5.495. [DOI] [PubMed] [Google Scholar]
- 17.Lee H J, Park J Y, Jang S H, Kim J H, Kim E C, Choi K W. High incidence of resistance to multiple antimicrobials in clinical isolates of Streptococcus pneumoniae from a university hospital in Korea. Clin Infect Dis. 1995;20:826–835. doi: 10.1093/clinids/20.4.826. [DOI] [PubMed] [Google Scholar]
- 18.Lenette E H, Balows A, Hausler W J, Jr, Shadomy H J. Manual of clinical microbiology. 4th ed. Washington, D.C.: American Society for Microbiology; 1985. [Google Scholar]
- 19.Mbelle N, Huebner R E, Wasas A D, Kimura A, Chang I, Klugman K P. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180:1171–1176. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 20.Neelingde A J D. Resistentie tegen antibiotica bij pneumokokken in Nederland en Europa. Bilthoven, The Netherlands: National Institute for Public Health and the Environment; 2001. [Google Scholar]
- 21.Nieminen T, Kayhty H, Leroy O, Eskola J. Pneumococcal conjugate vaccination in toddlers: mucosal antibody response measured as circulating antibody-secreting cells and as salivary antibodies. Pediatr Infect Dis J. 1999;18:764–772. doi: 10.1097/00006454-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Nuorti J P, Butler J C, Crutcher J M, Guevara R, Welch D, Holder P, Elliott J A. An outbreak of multidrug-resistant pneumococcal pneumonia and bacteremia among unvaccinated nursing home residents. N Engl J Med. 1998;338:1861–1868. doi: 10.1056/NEJM199806253382601. [DOI] [PubMed] [Google Scholar]
- 23.Principi N, Marchisio P, Schito G C, Mannelli S. Risk factors for carriage of respiratory pathogens in the nasopharynx of healthy children. Ascanius Project Collaborative Group. Pediatr Infect Dis J. 1999;18:517–523. doi: 10.1097/00006454-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Reichler M R, Allphin A A, Breiman R F, Schreiber J R, Arnold J E, McDougal L K, Facklam R R, Boxerbaum B, May D, Walton R O, et al. The spread of multiply resistant Streptococcus pneumoniae at a day care center in Ohio. J Infect Dis. 1992;166:1346–1353. doi: 10.1093/infdis/166.6.1346. [DOI] [PubMed] [Google Scholar]
- 25.Romesburg H. Cluster analysis for researchers. Malabar, Fla: Krieger; 1990. pp. 9–28. [Google Scholar]
- 26.Saeland E, Jakobsen H, Ingolfsdottir G, Sigurdardottir S T, Jonsdottir I. Serum samples from infants vaccinated with a pneumococcal conjugate vaccine, PncT, protect mice against invasive infection caused by Streptococcus pneumoniae serotypes 6A and 6B. J Infect Dis. 2001;183:253–260. doi: 10.1086/317934. [DOI] [PubMed] [Google Scholar]
- 27.Sa-Leao R, Tomasz A, Sanches I S, Brito-Avo A, Vilhelmsson S E, Kristinsson K G, de Lencastre H. Carriage of internationally spread clones of Streptococcus pneumoniae with unusual drug resistance patterns in children attending day care centers in Lisbon, Portugal. J Infect Dis. 2000;182:1153–1160. doi: 10.1086/315813. [DOI] [PubMed] [Google Scholar]
- 28.Shinefield H R, Black S. Efficacy of pneumococcal conjugate vaccines in large scale field trials. Pediatr Infect Dis J. 2000;19:394–397. doi: 10.1097/00006454-200004000-00036. [DOI] [PubMed] [Google Scholar]
- 29.Shinefield H R, Black S, Ray P, Chang I, Lewis N, Fireman B, Hackell J, Paradiso P R, Siber G, Kohberger R, Madore D V, Malinowski F J, Kimura A, Le C, Landaw I, Aguilar J, Hansen J. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 1999;18:757–763. doi: 10.1097/00006454-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Sibold C, Wang J, Henrichsen J, Hakenbeck R. Genetic relationships of penicillin-susceptible and -resistant Streptococcus pneumoniae strains isolated on different continents. Infect Immun. 1992;60:4119–4126. doi: 10.1128/iai.60.10.4119-4126.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sluijter M, Faden H, de Groot R, Lemmens N, Goessens W H, van Belkum A, Hermans P W. Molecular characterization of pneumococcal nasopharynx isolates collected from children during their first 2 years of life. J Clin Microbiol. 1998;36:2248–2253. doi: 10.1128/jcm.36.8.2248-2253.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sneath P. Numerical taxonomy. San Francisco, Calif: Freeman; 1973. [Google Scholar]
- 33.Syrogiannopoulos G A, Grivea I N, Beratis N G, Spiliopoulou A E, Fasola E L, Bajaksouzian S, Appelbaum P C, Jacobs M R. Resistance patterns of Streptococcus pneumoniae from carriers attending day-care centers in southwestern Greece. Clin Infect Dis. 1997;25:188–194. doi: 10.1086/514526. [DOI] [PubMed] [Google Scholar]
- 34.Takala A K, Vuopio-Varkila J, Tarkka E, Leinonen M, Musser J M. Subtyping of common pediatric pneumococcal serotypes from invasive disease and pharyngeal carriage in Finland. J Infect Dis. 1996;173:128–135. doi: 10.1093/infdis/173.1.128. [DOI] [PubMed] [Google Scholar]
- 35.Tomasz A, Corso A, Severina E P, Echaniz-Aviles G, Brandileone M C, Camou T, Castaneda E, Figueroa O, Rossi A, Di Fabio J L. Molecular epidemiologic characterization of penicillin-resistant Streptococcus pneumoniae invasive pediatric isolates recovered in six Latin-American countries: an overview. PAHO/Rockefeller University Workshop. Pan American Health Organization. Microb Drug Resist. 1998;4:195–207. doi: 10.1089/mdr.1998.4.195. [DOI] [PubMed] [Google Scholar]
- 36.van Steenbergen T J, Colloms S D, Hermans P W, de Graaff J, Plasterk R H. Genomic DNA fingerprinting by restriction fragment end labeling. Proc Natl Acad Sci USA. 1995;92:5572–5576. doi: 10.1073/pnas.92.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yagupsky P, Porat N, Fraser D, Prajgrod F, Merires M, McGee L, Klugman K P, Dagan R. Acquisition, carriage, and transmission of pneumococci with decreased antibiotic susceptibility in young children attending a day care facility in southern Israel. J Infect Dis. 1998;177:1003–1012. doi: 10.1086/515239. [DOI] [PubMed] [Google Scholar]
- 38.Yu X, Gray B, Chang S, Ward J I, Edwards K M, Nahm M H. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J Infect Dis. 1999;180:1569–1576. doi: 10.1086/315096. [DOI] [PubMed] [Google Scholar]