Abstract
Herpes infections are among the most common sexually transmitted diseases and are the most common cause of genital ulcer disease in the United States. This study addresses the changing distribution of herpes simplex virus type 1 (HSV-1) and HSV-2 in patients presenting for evaluation of herpetic infections. Viral culture results from the University of Kentucky Clinical Microbiology Laboratory were reviewed for a 6-year period (1994 through 1999). Data were collected on patient sex, site of culture, and culture result. These data were analyzed statistically to identify yearly trends. Of the 4,498 cultures analyzed, nearly equal proportions of HSV-1 (13.3%) and HSV-2 (12.0%) were detected for an overall culture positivity rate of 25.3%. Approximately two-thirds of all positive cultures were from women. Although HSV-2 remained the predominant type of genital herpes, over the 6-year span of this study, there was a trend toward increasing proportions of HSV-1 genitalis, with 31.8% of male patients and 44.8% of female patients demonstrating HSV-1 genitalis by 1999. The majority of patients with HSV in nongenital sites grew HSV-1. Although there was significant yearly variation, HSV-2 was isolated from only 9.4% of patients with nongenital HSV for the entire 6-year period. This study therefore concludes that HSV-2 remains primarily a genital pathogen, while HSV-1 is taking on an increasingly important role in causing genital ulcer disease in addition to being the primary nongenital HSV.
The herpes simplex viruses (HSVs), HSV type 1 (HSV-1) and HSV-2, infect millions of people worldwide. HSV-1 is ubiquitous, with a seroprevalence of about 60 to 85% in the adult population (16, 28). Acute HSV-1 infection most commonly occurs in children and young adults as a gingivostomatitis, pharyngitis, or tonsillitis and is readily transmitted through oral secretions (13). Reactivation of latent infection generally presents as oral-facial disease (cold sores) (3).
By comparison, HSV-2 is much less common. The National Health and Nutrition Examination Survey (NHANES II) from 1970 to 1984 indicated that HSV-2 seropositivity was rare in children and that HSV-2 infected only about 15% of adults in the United States (16). According to the 1988 to 1994 NHANES III survey, HSV-2 seroprevalence in the cross-section of the U.S. population sampled had risen to over 20%, reflecting an increase of over 30% in a single decade (10). The seroprevalence is higher for patients being seen in sexually transmitted disease (STD) clinics (25 to 65%) (21, 28, 31, 34), for prostitutes (78%), and for homosexual men in the United States (83%) (28), populations generally considered high risk for having STDs. Regional, racial, and gender-related differences in seroprevalence have similarly been noted (10, 16). Contributing to the rapid spread of genital HSV is the fact that most individuals infected with HSV-2 are unaware of their infection, but are still capable of transmitting virus to sexual contacts (10, 11, 23, 35). Onset of illness with HSV-2 peaks in early adulthood, corresponding to the initiation of sexual activity, underscoring the role of HSV-2 as primarily an STD.
Using HSV-2 seroprevalence alone as an indicator of sexually transmitted genital herpes infection significantly underestimates the true occurrence of genital herpes, however, since HSV-1 also may produce genital infections. It is estimated that approximately 10 to 15% of all cases of primary genital HSV are caused by HSV-1 (8, 21, 25, 27, 29), with increased proportions of HSV-1 being described focally in the United States, the United Kingdom, and Japan (2, 5, 17).
Distinguishing HSV-1 from HSV-2 in genital lesions is important in predicting subsequent patient symptomatology and response to treatment. Patients presenting with symptomatic genital HSV-2 can be anticipated to have significantly more morbidity than patients with genital HSV-1 infections (4, 22, 29). Patients with genital HSV-2 demonstrate symptomatic reactivation disease in 60 to 90% of cases, whereas only 25% of patients with HSV-1 genitalis have reactivations (22, 29). Genital HSV-2 also has a significantly higher monthly recurrence rate than HSV-1 (4). Koelle et al. (20) have demonstrated that subclinical shedding of virus is also more common for genital HSV-2 than for HSV-1. Although symptomatic reactivation of HSV-1 and HSV-2 may diminish in numbers in subsequent years (18), patients may still continue shedding infective viral particles even in the asymptomatic state (20, 36).
This study addresses the incidence of positive cultures processed at the University of Kentucky Clinical Microbiology Laboratory from 1994 to 1999. These data were analyzed to determine the distribution of HSV-1 and HSV-2 in both genital and nongenital sources from male and female patients.
MATERIALS AND METHODS
Study population.
Following Institutional Review Board protocol approval, specimen log books and computer records from the University of Kentucky Clinical Microbiology Laboratory were reviewed for all patients cultured for HSV between 1 January, 1994 and 31 December 1999. Data on patient sex, culture results, and culture site were tabulated for statistical analysis, dissociating all individual identifiers to assure patient confidentiality. For the purposes of statistical analysis, the culture sites were designated as genital (external and internal genitalia, urine, urethra, perineum, thighs, anus, perianal lesions, rectum, and buttocks) or nongenital (lips, mouth, face, eyes, nasal sources, gastrointestinal sources [not ano-rectal], respiratory specimens, and cerebrospinal fluid). Culture results were collected on a yearly basis and statistically analyzed by HSV type, sex, and culture site distribution. For the evaluation of yearly trends, repeatedly sampled patients were represented only once for genital and nongenital sites.
Virologic studies.
HSV cytopathic effect was detected in standard tube culture on MRC-5 cells (Biowhittacker, Walkersville, Md, or Viromed, Minneapolis, Minn.) All positive cultures were speciated with immunofluorescent HSV-1 and HSV-2 monoclonal antibodies (Trinity Biotech, Wicklow, Ireland).
RESULTS
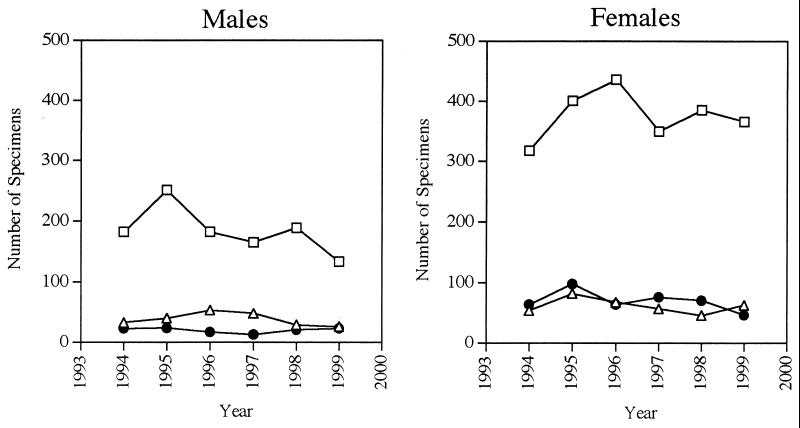
A total of 4,594 HSV cultures were performed during 1994 through 1999. Of these, 4,498 contained data extensive enough to allow at least partial analysis. Five hundred ninety-nine cultures were positive for HSV-1, 541 were positive for HSV-2, and 3,358 were negative. The 6-year culture positivity rate was 25.3% for both HSVs combined, 13.3% for HSV-1, and 12.0% for HSV-2. The distribution of HSV-1- and HSV-2-positive cultures between males and females over the study period is summarized in Table 1. Of the 1,140 positive cultures, 69.3% were found in women, and 30.7% were from men. Women accounted for the majority of both HSV subtype isolates cultured, with 61.8% of the HSV-1-positive cultures and 77.6% of the HSV-2-positive cultures being derived from this population. HSV-1 and HSV-2 were cultured in nearly equivalent proportions from women patients, with 46.2% of all female isolates being HSV-1 and 53.8% being HSV-2. In comparison, 65.4% of the male-derived isolates were HSV-1, and only 34.5% were HSV-2. The number of positive cultures derived from males and females fluctuated minimally throughout the study period (Fig. 1). Likewise, the rate of negative cultures over the 6-year study varied only slightly on a yearly basis (between 74.2 and 77.5%) and was essentially the same for men (averaging 75.9%, with a range of 72.2 to 79.7%) and women (average 74.1%, ranging from 69.9 to 76.9%).
TABLE 1.
Distribution of specimens by sex and culture result over the 6-year studya
| Demographic parameter (specimen source) | No. of specimens
|
|||
|---|---|---|---|---|
| HSV-1 positive | HSV-2 positive | Negative | Total | |
| All | 599 | 541 | 3,358 | 4,498 |
| From females | 370 | 420 | 2,256 | 3,046 |
| From males | 229 | 121 | 1,102 | 1,452 |
Includes data on repeatedly sampled patients.
FIG. 1.
Number of HSV cultures each year derived from males and females. □, negative cultures; ▵, HSV-1-positive cultures; ●, HSV-2-positive cultures.
For the data to be completely analyzed, information on patient sex, culture site, and culture results had to be available. There were 4,014 complete sets of data available for analysis (Table 2). Three independent variables were identified as sex, culture result (negative, HSV-1, or HSV-2), and site of culture (genital or nongenital location). Genital sources comprised the majority of cultured sites: 53.7% from external and internal genitalia and 5.1% from anorectal sources. Cutaneous sites and oral-respiratory and gastrointestinal sources made up nearly equal proportions of the remaining 41.2% of cultures analyzed and together represented “nongenital” sites. Nongenital culture sites produced positive culture results in 22.9% of specimens over the 6-year period, while genital sources produced positive cultures in 26.6% of specimens.
TABLE 2.
Distribution of specimens by site of culture, sex, and culture results over the 6-year studya
| Culture source | No. of specimens
|
|||
|---|---|---|---|---|
| HSV-1 positive | HSV-2 positive | HSV negative | Total | |
| Genital | 191 | 437 | 1,732 | 2,360 |
| Females | 167 | 356 | 1,418 | 1,941 |
| Males | 24 | 81 | 314 | 419 |
| Nongenital | 339 | 39 | 1,276 | 1,654 |
| Females | 153 | 21 | 628 | 802 |
| Males | 186 | 18 | 648 | 852 |
Includes data on repeatedly sampled patients.
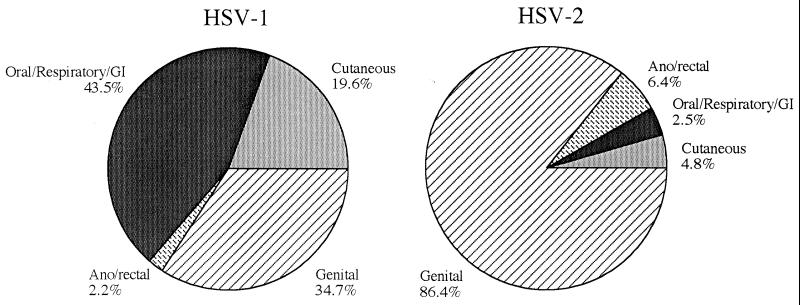
Data were then analyzed for unique patients by eliminating the repeat results on multiply cultured patients. These resamplings represented 10% of the original cultures analyzed. Data were pooled for the entire 6-year period to determine the overall distribution of HSV-1 and HSV-2 in unique patients between the genital and nongenital sites (Fig. 2). Five hundred eighty-one patients had culture-positive genital herpes, while 330 patients had nongenital herpetic infections. These data reflected 473 infections with HSV-1 (51.9%) and 438 infections with HSV-2 (48.1%). HSV-2 was isolated predominantly from genital and anorectal sources, with only 7.3% of patients with HSV-2 having isolates recovered from nongenital sources. In comparison, HSV-1 was found most often in nongenital sites. Of the cases of HSV-1, 43.5% were from oral, respiratory, or gastrointestinal sources, while 19.6% were cutaneous. Over the study period, 63.1% of all cases of HSV-1 represented nongenital infections, while the remaining 36.9% reflected HSV-1 genitalis.
FIG. 2.
Distribution of HSV-1 and HSV-2 in unique patients by site of culture over the cumulative 6-year period. GI, gastrointestinal.
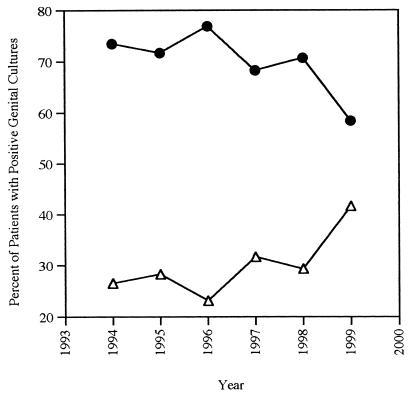
Although HSV-2 remained the predominant HSV type isolated from genital specimens, a significant proportion of genital cultures (29.9%) contained HSV-1. This proportion changed with time, demonstrating an overall increase in proportion of HSV-1 in genital specimens (Fig. 3). Although the proportion of HSV-1 in genital cultures from males was lower than that seen in females, the same trend was revealed in both populations. In men, 23.3% of culture-positive genital herpes was caused by HSV-1 over the 6-year study period (yearly range, 11.1 to 31.8%). In women, 31.7% of genital herpes was caused by HSV-1, with a yearly range of 24.3 to 44.8%. Furthermore, the proportion of genital HSV-1 showed an overall increase in percentage compared to HSV-2. Although there was some yearly variation, there was an overall upward trend, with 1999 data indicating that 41.6% of all cases of herpes genitalis were caused by HSV-1: 31.8% of males and 44.8% of females evaluated.
FIG. 3.
Percentage of genital cultures positive for HSV-1 and HSV-2 each year from 1994 through 1999. Δ, HSV-1; ●, HSV-2.
A similar site-specific analysis was performed for the nongenital specimens. The vast majority of nongenital isolates were HSV-1. Relatively few HSV-2 cultures were seen each year from nongenital sites, ranging from 2 to 16 isolates. Since the overall number of patients with nongenital herpes was small (330 males and females for the entire study), even insignificant changes in the total number of HSV-2 cases potentially inflated the proportion of nongenital disease caused by HSV-2. Overall, only 9.4% of all nongenital isolates in this study were HSV-2 (11.9% of women and 7.0% of men). Only one year, 1998, had a rate of nongenital herpes above 10%, with 11 of 42 cases (26.2%) having been caused by HSV-2.
DISCUSSION
Since HSV infections are not reportable diseases, good statistics on the occurrence of infections in the United States are not available. The NHANES II study conducted from 1970 to 1984 (16) and the NHANES III study conducted between 1985 and 1994 (10) demonstrated that a significant number of adults in the United States are infected with HSV-2, the STD of herpesviruses. A 30% increase in HSV-2 seroprevalence has been seen in the last decade alone (10). This study confirms the earlier observation that HSV-2, in general, is more prevalent in women than in men (10, 16). This conclusion is supported by the observation that over two-thirds of all positive cultures in this institution were obtained from women, and over three-fourths of all HSV-2 cultured from clinical specimens originated from women. HSV-2 was seen primarily in genital cultures, with only a small proportion seen in nongenital sites. The year 1998 stands out as an exception to this rule, with 26.2% of patients having HSV-2 in nongenital locations. Although only 11 patients with nongenital HSV-2 were identified during this year, this represented over one-third of the cases identified over the 6-year period. The relative decrease in the total number of positive cultures made this increase in nongenital HSV-2 cases seem even more inflated when percentages were determined. Overall, patients presenting with HSV-2 in nongenital sites represented less than 10% of cases for the remaining years, a proportion similar to that seen in other studies (8).
While HSV-2 has traditionally been viewed as the marker for sexually transmitted HSV, this ignores the role that HSV-1 also serves in causing genital herpes. It has generally been accepted that approximately 10 to 15% of newly acquired genital herpes is actually caused by HSV-1 (1, 8, 25, 27, 29). Regional variation in the percent of genital herpes caused by HSV-1 has been noted, however. Several locations in Britain have reported that up to 50% of cases of newly acquired herpes genitalis are caused by HSV-1 (5). The current data suggest that the proportion of HSV-1 in genital specimens also has increased dramatically in Central Kentucky, with the 1999 data demonstrating that over 43% of female genital isolates and over 30% of male genital isolates are HSV-1. Since HSV-2 is known to produce more genital reactivations than HSV-1 (22), it would be expected that HSV-2 would have more opportunities to be cultured. This would potentially skew detection of genital herpes in favor of HSV-2 isolates. Despite this fact, HSV-1 genital herpes has shown a significant increase in genital sites in this study.
The explanation for this increased proportion of genital HSV-1 is not clear, but might be explained by several potential mechanisms. First, this trend may be due to the decreasing rate of HSV-1 immunity in young adults. Several studies support this theory. A cross-sectional study of 1,057 college students found that the 18- to 19-year-old population had only 32.7% seropositivity compared to those over 20 who demonstrated 43.5% HSV-1 seropositivity (12). Similarly, a cohort of 14- to 15-year-olds who were monitored for 15 years initially had a 25% seropositivity for HSV-1, but had a 59% seropositivity 15 years later (6). A 1975 university-based study of HSV respiratory disease indicated that only 30% of incoming students initially had HSV-1 antibodies, but there was an approximate 10% annual rate of seroconversion noted in this population (13). Of note, in a recent study looking at 124 healthy 17- to 24-year-olds in Central Kentucky, only 35.1% of the 17- to 19-year-olds had antibodies to HSV-1. In the 20- to 24-year-old group, the seroprevalence had risen to 46.8% indicating that there was a gradual seroconversion seen in this population (unpublished data).
Linked to this apparent expanded population at risk for delayed infection also is probably a change in the sexual practices leading to an increase in oral-genital contact. Demographic analysis of individuals with primary genital HSV-1 has indicated that 50 to 100% of these patients had experienced oral-genital contact within weeks of their outbreak (8, 21, 22). These studies suggest that the practice of oral-genital sex is ubiquitous among those individuals acquiring herpes genitalis. A survey of sexual practices administered to individuals presenting to a Denver clinic for HIV testing likewise indicated that oral sex was quite commonly practiced in this patient population, with 88.7% acknowledging having had oral sex as a risk factor for acquiring an STD (26). While one might not be able to generalize these observations to the general population, it seems likely that individuals presenting for evaluation of genital ulcer disease will have engaged in oral sex a significant proportion of the time.
Several issues may be influencing this apparent increase in the practice of oral-genital sex. In a recent survey of college students, 59% indicated that they felt that oral-genital contact did not constitute actually “having had sex” for the sake of preserving virginity (30). A potential benefit of limiting sexual activity to oral-genital intercourse could therefore include maintaining ones “virginal state.” In a 1983 study of female university students, 35% of those who considered themselves “virgins” had engaged in oral-genital acts. This compared to 97% of “non-virgins.” suggesting that other factors also motivate oral-genital behaviors (15). Oral-genital intercourse appears to be a socially acceptable current practice, with over 80% of men and women surveyed in 1999 acknowledging having had some “oral-genital experience.”
Other influences on having oral-genital sex might include the perceived notion that oral sex is “safe sex.” Although oral sex does preclude conception, unprotected receptive oral genital intercourse is not safe from an infectious disease standpoint. Transmissions of Neisseria gonorrhoeae (19), human immunodeficiency virus (32), human papilloma virus, hepatitis C, and molluscum contagiosum (9) through unprotected oral-genital contact have all been documented. Transmission of HSV from an individual with active herpes pharyngitis or herpes labialis may likewise occur during oral-genital contact (9). The inoculation of HSV in high titer onto uninfected genital mucosa has been demonstrated to cause lesions and lead to reactivation disease in these new locations (24). Since individuals with herpes infections may shed infective virus without any evidence of active lesions (20, 27, 35), there would be no disease-free periods during which oral contact could be absolutely risk-free with regard to viral transmission.
Nonsexual transmission of HSV-1 also may occur by autotransmission. This could include transmission of viral particles from oral lesions through the gut to the perineum or by the direct inoculation of virus to the genitals on fingers or fomites. The parasthesias associated with herpes labialis often lead patients to repeatedly touch the infected lesion. Virus carried on the fingers could then be transferred directly to the perineum during the placement of tampons or indirectly transferred on toilet paper if adequate hand washing prior to toileting is not performed. In vivo studies of autoinoculation from one site to another with the patient's own strain of HSV has been demonstrated to cause new foci of disease and reactivation (24). The spread of genital HSV onto the patient's fingers or eyes or onto mucocutaneous sites adjacent to primary genital regions late in the disease course suggests that autoinoculation is a common occurrence (7, 14, 33). It is unclear why this rate of transmission would be showing such a dramatic increase with time, unless it was concluded that personal hygiene and hand washing practices have changed significantly.
ACKNOWLEDGMENTS
D. J. Baker and J. A. Ribes received funding for this project from the Women's Health Initiative through the Chandler Medical Center, Medical Center Research Fund, University of Kentucky.
REFERENCES
- 1.Arvin A M, Prober G C. Herpes simplex viruses. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. pp. 878–887. [Google Scholar]
- 2.Ashley R L, Wald A. Genital herpes: review of the epidemic and potential use of type-specific serology. Clin Microbiol Rev. 1999;12:1–8. doi: 10.1128/cmr.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bader C, Crumpacker C S, Schnipper L E, Ransil B, Clark J E, Arndt K, Freedberg I M. The natural history of recurrent facial-oral infection with herpes simplex virus. J Infect Dis. 1978;138:897–905. doi: 10.1093/infdis/138.6.897. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121:847–854. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Brugha R, Keersmaekers K, Renton A, Meheus A. Genital herpes infection: a review. Int J Epidemiol. 1997;26:698–709. doi: 10.1093/ije/26.4.698. [DOI] [PubMed] [Google Scholar]
- 6.Christenson B, Bottiger M, Svensson A, Jeansson S. A 15 year surveillance study of antibodies to herpes simplex virus types 1 and 2 in a cohort of young girls. J Infect. 1992;25:147–154. doi: 10.1016/0163-4453(92)93943-k. [DOI] [PubMed] [Google Scholar]
- 7.Crane L R, Lerner A M. Herpetic whitlow: a manifestation of primary infection with herpes simplex virus type 1 or type 2. J Infect Dis. 1978;137:155–156. doi: 10.1093/infdis/137.6.855. [DOI] [PubMed] [Google Scholar]
- 8.Corey L, Adams H G, Brown Z A, Holmes K K. Genital herpes simplex virus infections: clinical manifestations, course and complications. Ann Intern Med. 1983;98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 9.Edwards S, Carne C. Oral sex and the transmission of viral sexually transmitted infections. Sex Transm Infect. 1998;74:6–10. doi: 10.1136/sti.74.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming D T, McQuillan G M, Johnson R E, Nahmias A J, Aral S O, Lee F K, St. Louis M E. Herpes simplex virus type 2 in the United States 1976 to 1994. N Engl J Med. 1997;337:1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 11.Frenkel L M, Garratty E M, Shen J P, Wheeler N, Clark O, Bryson Y J. Clinical reactivation of herpes simplex type 2 infection in seropositive pregnant women with no history of genital herpes. Ann Intern Med. 1993;118:414–418. doi: 10.7326/0003-4819-118-6-199303150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Gibson J J, Hornung C A, Alexander G R, Lee F K, Potts W A, Nahmias A J. A cross-sectional study of herpes simplex virus types 1 and 2 in college students: occurrence and determinants of infection. J Infect Dis. 1990;162:306–312. doi: 10.1093/infdis/162.2.306. [DOI] [PubMed] [Google Scholar]
- 13.Glezen W P, Fernald G W, Lohr J A. Acute respiratory disease of university students with special reference to the etiologic role of Herpesvirus hominis. Am J Epidemiol. 1975;101:111–121. doi: 10.1093/oxfordjournals.aje.a112077. [DOI] [PubMed] [Google Scholar]
- 14.Glogau R, Hanna L, Jawetz E. Herpetic whitlow as part of genital virus infection. J Infect Dis. 1977;136:689–692. doi: 10.1093/infdis/136.5.689. [DOI] [PubMed] [Google Scholar]
- 15.Herold E S, Way L. Oral-genital sexual behavior in a sample of university females. J Sex Res. 1983;19:327–338. [Google Scholar]
- 16.Johnson R E, Nahmias A J, Magder L S, Lee F K, Brooks C A, Snowden C B. A seroepidemiologic survey of the prevalence of herpes simplex virus type 2 infection in the United States. N Engl J Med. 1989;321:1–12. doi: 10.1056/NEJM198907063210102. [DOI] [PubMed] [Google Scholar]
- 17.Kawana T, Kawaguchi T, Sakamoto S. Clinical and virological studies on genital herpes. Lancet. 1976;ii:964. doi: 10.1016/s0140-6736(76)90928-4. [DOI] [PubMed] [Google Scholar]
- 18.Kinghorn G R. Genital herpes: natural history and treatment of acute episodes. J Med Virol Suppl. 1993;1:33–38. doi: 10.1002/jmv.1890410508. [DOI] [PubMed] [Google Scholar]
- 19.Knapp J S, Rice R J. Neisseria and Branhamela. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D. C.: ASM Press; 1995. pp. 324–340. [Google Scholar]
- 20.Koelle D M, Benedetti J, Langenberg A, Corey L. Asymptomatic reactivation of herpes simplex virus in women after the first episode of genital herpes. Ann Intern Med. 1992;116:433–437. doi: 10.7326/0003-4819-116-6-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koutsky L A, Stevens C E, Holme K K, Ashley R L, Kiviat N B, Critchlow C W, Corey L. Underdiagnosis of genital herpes by current clinical and viral-isolation procedures. N Engl J Med. 1992;326:1533–1539. doi: 10.1056/NEJM199206043262305. [DOI] [PubMed] [Google Scholar]
- 22.Lafferty W E, Coombs R W, Benedetti J, Critchlo C, Corey L. Recurrences after oral and genital herpes simplex virus infection: influence of site of infection and viral type. N Engl J Med. 1987;31:1444–1449. doi: 10.1056/NEJM198706043162304. [DOI] [PubMed] [Google Scholar]
- 23.Langenberger A, Benedetti J, Jenkins J, Ashley R, Winter C, Corey C. Development of clinically recognizable genital lesions among women previously identified as having “asymptomatic” herpes simplex virus type 2 infection. Ann Intern Med. 1989;110:882–887. doi: 10.7326/0003-4819-110-11-882. [DOI] [PubMed] [Google Scholar]
- 24.Lazar M P. Vaccination for recurrent herpes simplex infection: initiation of a new disease site following use of unmodified material containing the live virus. Arch Dermatol. 1956;73:70–71. doi: 10.1001/archderm.1956.01550010072010. [DOI] [PubMed] [Google Scholar]
- 25.Lowhagen G B, Jansen E, Nordenfelt E, Lycke E. Epidemiology of genital herpes infections in Sweden. Acta Dermato Venereol. 1990;70:330–334. [PubMed] [Google Scholar]
- 26.McFarlane M, Bull S S, Rietmeijer C A. The internet as a newly emerging risk environment for sexually transmitted diseases. JAMA. 2000;284:443–446. doi: 10.1001/jama.284.4.443. [DOI] [PubMed] [Google Scholar]
- 27.Mertz G J, Schmidt O, Jourden J L, Guinan M E, Remington M L, Fahnlander A, Winter C, Holmes K K, Corey L. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contact. Sex Transm Dis. 1985;12:33–39. doi: 10.1097/00007435-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Nahmais A J, Lee F K, Beckman-Nahmias S. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand J Infect Dis Suppl. 1990;69:19–36. [PubMed] [Google Scholar]
- 29.Reeves W C, Corey L, Adams H G, Vontver L A, Holmes K K. Risk of recurrence after first episodes of genital herpes: relation to HSV type and antibody response. N Engl J Med. 1981;305:315–319. doi: 10.1056/NEJM198108063050604. [DOI] [PubMed] [Google Scholar]
- 30.Sanders S A, Reinisch J M. Would you say you “had sex” if…? JAMA. 1999;281:275–277. doi: 10.1001/jama.281.3.275. [DOI] [PubMed] [Google Scholar]
- 31.Slokma M J. Seroepidemiology and control of genital herpes: the value of type specific antibodies to herpes simplex virus. Communicable Dis Rep CDC Rev. 1996;6:41–45. [PubMed] [Google Scholar]
- 32.Stephenson J. HIV risk from oral sex higher than many realize. JAMA. 2000;283:1279. [PubMed] [Google Scholar]
- 33.Sumers K D, Sugar J, Levine R. Endogenous dissemination of genital Herpesvirus hominis type 2 to the eye. Br J Ophthalmol. 1980;64:770–772. doi: 10.1136/bjo.64.10.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Laar M J W, Termorshuizen F, Slomka M J, Ossewaarde J M, Brown D W G, Coutinho R A, van den Hoek J A R. Prevalence and correlates of herpes simplex virus type 2 infection: evaluation of behavioral risk factors. Int J Epidemiol. 1998;27:127–134. doi: 10.1093/ije/27.1.127. [DOI] [PubMed] [Google Scholar]
- 35.Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women: effect of acyclovir treatment. J Clin Investig. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wald A, Zeh J, Selke S, Warren T, Ryncarz A J, Ashley R, Krieger J N, Corey L. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]