Abstract
Simple Summary
MicroRNAs (miRNAs) are short RNA molecules that can interfere with messenger RNA and thus influence protein translation. In recent years, it has been revealed that miRNAs are also involved in carcinogenesis. However, the effect of each miRNA can differ significantly, and they may exhibit pro-tumorigenic or anti-tumorigenic properties. Breast cancer is one of the most common cancer entity in women and distant metastases are frequently observed in the skeleton. The progression of breast cancer bone metastasis largely depends on the interaction of tumor cells and cells of the bone microenvironment. In this review, we summarize the current findings related to miRNAs in metastatic bone disease with a focus on breast cancer. This review emphasizes the impact of miRNAs on both cancer cells and key cells of the bone microenvironment. Additionally, we discuss the potential use of miRNAs as a therapeutic target and elaborate advantages and hurdles of miRNA treatment.
Abstract
Bone metastasis is a frequent complication in patients with advanced breast cancer. Once in the bone, cancer cells disrupt the tightly regulated cellular balance within the bone microenvironment, leading to excessive bone destruction and further tumor growth. Physiological and pathological interactions in the bone marrow are mediated by cell–cell contacts and secreted molecules that include soluble proteins as well as RNA molecules. MicroRNAs (miRNAs) are short non-coding RNAs that post-transcriptionally interfere with their target messenger RNA (mRNA) and subsequently reduce protein abundance. Since their discovery, miRNAs have been identified as critical regulators of physiological and pathological processes, including breast cancer and associated metastatic bone disease. Depending on their targets, miRNAs can exhibit pro-tumorigenic or anti-tumorigenic functions and serve as diagnostic and prognostic biomarkers. These properties have encouraged pre-clinical and clinical development programs to investigate miRNAs as biomarkers and therapeutic targets in various diseases, including metastatic cancers. In this review, we discuss the role of miRNAs in metastatic bone disease with a focus on breast cancer and the bone microenvironment and elaborate on their potential use for diagnostic and therapeutic purposes in metastatic bone disease and beyond.
Keywords: microRNA, bone metastasis, breast cancer, bone microenvironment, targeted therapy
1. Introduction
Breast cancer is the most commonly diagnosed cancer in women worldwide [1]. Increased awareness, improved screening methods, and novel treatment strategies have had a significant impact on disease management, and survival rates for patients with primary breast cancer are now above 90% [2]. Nevertheless, breast cancer remains the leading cause of cancer-related deaths in female patients [1], with the majority of cancer deaths being a consequence of metastatic disease [3]. About 70% of patients with advanced breast cancer will develop metastases in the skeleton, making bone the most frequent site of breast cancer metastasis [4]. Patients suffering from breast cancer bone metastasis are confronted with a tremendous reduction in quality of life, predominantly due to the accelerated cancer-induced bone loss. They often suffer from skeletal-related events (SREs) such as bone pain, spinal cord compression, fractures, and consequently increased morbidity [5].
The establishment of metastatic disease requires several sequential steps, including the detachment of cancer cells from the primary tumor, intravasation and circulation in the blood stream, followed by extravasation at secondary organs, adaptation to the new environment, and, ultimately, proliferation and the formation of overt metastasis [6]. Disseminated breast cancer cells that home to bone arrive in a heterogenous microenvironment that comprises several cell types originating from either hematopoietic or mesenchymal stem cells (HSCs or MSCs, respectively). Briefly, bone-resorbing osteoclasts, which are derived from HSCs, and bone-forming osteoblasts, derived from MSCs, maintain skeletal integrity through a tightly balanced remodeling cycle [7]. Key signaling pathways that regulate osteoclast and osteoblast activity involve the receptor activator of nuclear factor-κB (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) axis and canonical Wnt signaling [8]. Additionally, the bone marrow comprises a dense vascular system that couples osteogenesis and angiogenesis [9]. Tight interactions between bone cells and disseminated tumor cells (DTCs) are critical for successful establishment of bone metastasis. Tumor-supportive environments, so-called “niches”, are thought to regulate tumor cell homing, dormancy, and colonization at secondary sites [10,11,12,13,14]. Over the last years, significant progress has been made in identifying and characterizing the cellular and molecular composition of the bone metastasis niche [14,15,16,17,18], which includes the HSC, endosteal (osteoblasts, osteoclasts, fibroblasts), and vascular (endothelial cells) niches [14,16]. However, several other cell types that are present in the bone, such as adipocytes, megakaryocytes, and immune cells, have been reported to regulate metastatic breast cancer growth in bone [17,19,20]. The precise location of these niches remains to be defined; similarly, the extent to which these niches overlap and/or interact remains challenging to elaborate.
2. The Bone Microenvironment as a Therapeutic Target in Breast Cancer Bone Metastasis
Once disseminated breast cancer cells proliferate in bone, the tumor–bone cell interactions result in accelerated osteoclast-mediated bone resorption, a key characteristic of the disease. Briefly, tumor cell-derived factors (e.g., parathyroid hormone-related protein (PTHrP) and interleukin 11 (IL-11)) alter the RANKL/OPG ratio in favor of osteoclast activity [21,22]. During the increased osteolysis, tumor growth-promoting factors are released from the bone matrix (e.g., bone morphogenetic proteins (BMPs), insulin-like growth factor 1 (IGF-1), and transforming growth factor-beta1 (TGF-β1)), resulting in a feedforward loop referred to as the vicious cycle of bone metastasis [21,22]. Osteoclasts are key drivers of the breast-cancer-induced osteolysis; therefore, standard-of-care treatment includes, besides conventional radiation and chemotherapy, agents that inhibit excessive bone resorption [23]. In clinical practice, bisphosphonates (e.g., Zoledronic acid) and Denosumab, an antibody against RANKL, are approved for treatment, while several other osteoclast-modifying agents are still under investigation (e.g., mTOR-inhibitors, Src-inhibitors, cathepsin K-inhibitors) [23,24,25,26]. However, these treatments are only palliative, and the disease remains incurable. In order to counteract the cancer-induced osteolysis, bone-anabolic agents (e.g., Romosozumab, an antibody against Sclerostin [27]), which are used in clinical practice to treat osteoporosis, are currently emerging as promising treatment approaches [28]. Indeed, sclerostin-antibody treatment altered the number and activity of osteoblasts and osteoclasts in vivo, protected against cancer-induced bone destruction, and reduced the bone-metastatic burden in mouse models of breast cancer bone metastasis [29].
Despite these recent advances, disrupting the tight interaction between DTCs and cells of the bone microenvironment remains a challenge that has yet to be overcome. Besides direct cell–cell contact, pathological crosstalk between tumor cells and bone cells is mediated via secreted factors. In this context, microRNAs, small non-coding RNAs that can be transferred between cell types, e.g., via extracellular vesicles (EVs) and exosomes [30,31,32], have been suggested as potential therapeutic targets to intervene with the vicious cycle of bone metastasis.
3. MicroRNAs (miRNAs)
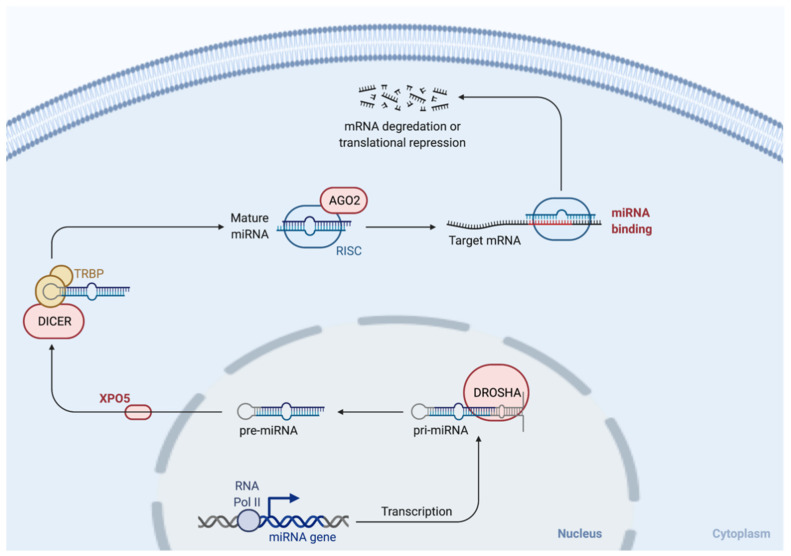
miRNAs are small (~20 nucleotides in length), non-coding RNA molecules that post-transcriptionally control gene expression [33]. Mechanistically, miRNAs bind to their complementary sequences on the 3′ untranslated region (UTR) of their target mRNA and consequently repress the production of the target protein [33,34]. miRNA biogenesis involves several sequential steps, including the generation of primary miRNAs (pri-miRNA) and precursor miRNAs (pre-miRNA, around 60–70 nucleotides), with the subsequent release of mature miRNAs [35]. These processes involve cleaving steps that are performed by Drosha in the nucleus and Dicer in the cytoplasm, two ribonuclease III endonucleases [35,36,37] (Figure 1). Since their original discovery in C. elegans in 1993 [33], a great number of miRNAs have been reported and characterized. Functionally, miRNAs are crucial in regulating physiological cell processes, including differentiation, proliferation, migration, and apoptosis [38,39]. miRNAs are dysregulated in several cancers [36], including breast cancer [40]. Studies have now also reported unique miRNA expression patterns depending on the breast cancer subtype [41,42]. Importantly, miRNAs can act as both tumor suppressors and oncogenes, depending on their target gene, and the expression of miRNA clusters with both pro- and anti-tumorigenic roles has been reported in breast cancer [43].
Figure 1.
miRNA biogenesis. Following the creation of a pri-miRNA transcript, a complex that contains an RNA-binding protein (DiGeorge Syndrome Critical Region 8 (DGCR8)) and the ribonuclease III enzyme, Drosha, is formed [44]. Pre-miRNAs are formed upon the cleavage of pri-miRNA by Drosha [44]. This process takes place in the nucleus. Following exportation to the cytoplasm by exportin 5 (XPO5), Dicer, another ribonuclease III enzyme, processes the pre-miRNA into its mature form [45]. miRNAs are further processed by the Argonaute (AGO) family of proteins to form an RNA-induced silencing complex (RISC) [45]. Finally, unwinding of the miRNA duplex takes place, enabling the binding of the miRNA to its target mRNA with consequent degradation or transcriptional repression [44,45].
4. miRNAs in Breast Cancer Bone Metastasis
Tightly regulated crosstalk of bone and tumor cells drives the progression of breast cancer growth in bone. miRNAs have been shown to affect both bone and tumor cells [32,46,47,48], highlighting their capability to interfere with the vicious cycle of bone metastasis (Figure 2). Indeed, altered expression of miRNAs has been associated with disease progression and clinical outcome in breast cancer patients, and they are emerging as attractive non-invasive clinical biomarkers [49,50]. Although several established biomarkers for breast cancer diagnosis (e.g., CA 15-3 and CEA) exist, the combination of two or more tumor markers with miRNAs has been shown to increase their diagnostic value [51,52]. A study by Zaleski et al. demonstrated an improvement in both the sensitivity and specificity of breast cancer diagnosis when using miR-34a in addition to the well-established breast cancer tumor marker CA15-3 [52]. Furthermore, lower levels of miR-34a were observed in patients suffering from breast cancer in comparison to benign breast disease and healthy controls. These data underline the potential use of miR-34a in breast cancer diagnosis, as well as differential diagnosis of malignant and benign breast disease. In the same study, Zaleski et al. found a correlation between miR-34a and the Union for International Cancer Control (UICC) stage. The authors observed lower levels of miR-34a in breast cancer patients of UICC stage II or higher, underlining the potential role of miRNAs as prognostic biomarkers in breast cancer [52]. Thus, the significance of miRNAs is not limited to diagnosis; they may also contribute as prognostic biomarkers [53], as miR-10b was one of the first identified miRNAs highly expressed in metastatic breast cancer [54]. High expression levels of miR-10b were observed in breast cancer patients with lymphatic node metastasis [55], as well as in patients with distant metastases in the bone [56] and brain [57]. Furthermore, studies have shown that lower levels of miR-124 in primary breast cancer tissues correlate with shorter bone-metastasis-free survival in breast cancer patients [46]. As another example, miR-218 serum levels have been shown to be elevated in patients with breast cancer bone metastasis when compared to those in patients without metastasis [32]. Similarly, altered miR-124 and miR-218 expression in breast cancer cells has been associated with increased aggressiveness in vitro and in vivo [46,58,59].
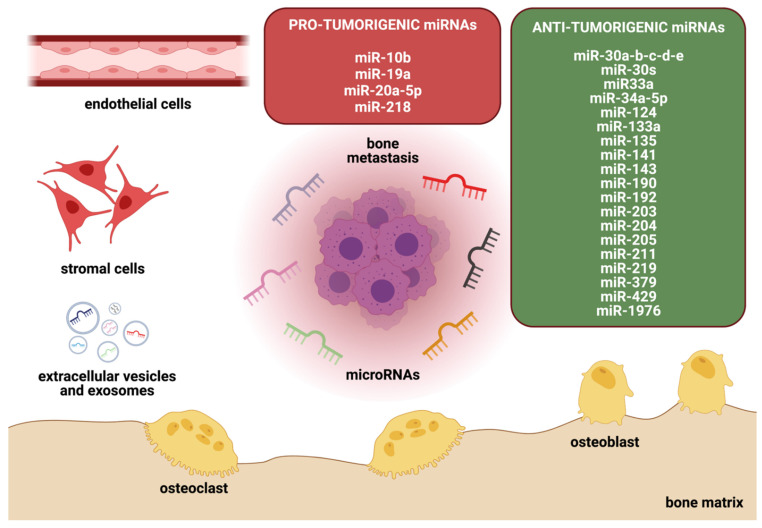
Figure 2.
miRNAs in the bone metastatic environment. The bone microenvironment consists of several cell types, including osteoblasts, osteoclasts, stromal cells, and endothelial cells, that orchestrate the tightly regulated bone metabolism. Cancer- and bone-cell-derived miRNAs can either promote or suppress metastatic outgrowth of tumor cells within the bone. The figure depicts the role of miRNAs in breast cancer bone metastasis and groups them according to their influence on tumor growth.
4.1. Direct Effects of miRNAs on Breast Cancer Cells and Metastasis
Extensive evidence indicates that miRNAs directly affect breast cancer cell behavior and bone metastasis progression. For example, knockdown of miR-1976 in breast cancer cell lines stimulated migration, invasion, and adhesion in vitro when compared to a control [60]. Similarly, lack of miR-1976 promoted epithelial-to-mesenchymal transition (EMT), a key step in metastasis establishment [61], and enhanced cancer stem cell (CSC) properties [60]. Opposing results were observed when cells were transfected with miR-1976 mimics, and the authors attributed a reduced presence of lung metastasis in vivo to miR-1976-induced alterations in EMT and the CSC pool [60]. Similarly, miR-429 has been shown to reduce proliferation, migration, and invasion, as well as EMT, in breast cancer cells in vitro and to inhibit bone metastasis in vivo [62]. A reduced invasion capacity was also observed in the MDA-B02 bone metastatic breast cancer cell line upon overexpression of miR-30b-d, miR-30b-c, or miR-30a-b-c-d-e [63]. Others have shown that transglutaminase 2 (TG2) downregulates miR-205 in breast cancer cells and thereby promotes bone metastasis [64]. Overexpression of miR-143 in breast cancer cells reduced cell viability, migration, and invasion in vitro through targeting mitogen-activated protein kinase 3 [65]. In contrast, others have shown that overexpression of miR-20a-5p stimulates the migration and invasion of breast cancer cells [66]. Studies have also suggested that miR-34a-5p regulates Met expression in breast carcinomas and, thus, progression to metastasis in bone [67]. Met receptor and its ligand hepatocyte growth factor (HGF) are involved in several cellular signaling pathways that regulate proliferation, migration, and invasion, and aberrant Met signaling has been reported in several types of cancer [68]. Indeed, an inverse correlation of miR-34a-5p and the tyrosine kinase receptor Met in breast cancer bone metastasis has been reported [67]. When associated with Met/HGF, miR-34a-5p has thus been suggested as a diagnostic marker predicting poor prognosis [67]. Furthermore, overexpression of miR-203 and miR-135 reduced the migration, proliferation, and viability of breast cancer cells in vitro, with reduced tumor growth in bone observed in vivo [69].
Osteomimicry-Related Genes in Breast Cancer Cells Are Altered by miRNAs
In order to increase their chance of survival in bone, disseminated breast cancer cells are capable of acquiring a bone-cell-like phenotype, a process known as osteomimicry [70]. Osteomimicry factors expressed by breast cancer cells that home to bone include, for example, Runt-related transcription factor 2 (Runx2), Bone Morphogenetic Proteins (BMPs), Alkaline Phosphatase (ALP), PTHrP, RANKL, or OPG—reviewed in great detail in [71]. Interestingly, miRNAs can affect the expression of several osteomimicry-related genes in breast cancer cells, which could account for their metastasis regulatory function [58,63]. For example, overexpression of miR-30s in MDA-B02 cells reduced osteomimetic genes, including CX43 and CDH11, as well as Dickkopf-related protein 1 (DKK1) [63]. On the other hand, miR-218 increased the expression of bone sialoprotein (BSP), osteopontin (OPN), and a chemokine receptor, CXCR4, in MDA-MB-231 breast cancer cells [58], suggesting that miR-218 supports osteomimicry and, thus, breast cancer cells homing to bone. Furthermore, miR-218 expression in breast cancer cells was associated with elevated Wnt-signaling. Compared to MCF10A breast epithelial cells, metastatic MDA-MB-231 breast cancer cells expressed higher levels of miR-218 and Wnt target genes LEF1 and TCF-4 [58]. In osteoblasts, miR-218 induced and stimulated differentiation, and it induced Wnt signaling by targeting DKK2, Sost, and Sfrp2 [58]. These studies suggest a miR-218/Wnt signaling loop between breast cancer cells and osteoblasts that supports breast cancer bone metastasis [58]. Indeed, overexpression of miR-218 in breast cancer cells promoted osteolytic disease in vivo, while antagonizing miR-218 attenuated tumor growth and bone destruction [59].
Runx2 has been shown to be a direct target of several miRNAs. miR-30 family members reduced the expression of Runx2 in MDA-MB-231 breast cancer cells [63]. Others have shown that ectopic expression of miR-135 and miR-203 in combination with systemic administration of miR-135 and miR-203 reduces orthotopic tumor growth and spontaneous metastasis of MDA-MB231 cells to bone in vivo [69]. Consistently, reduced tumor growth in bone was observed when cells expressing miR-135 and miR-203 were directly injected into the tibiae [69]. This was accompanied by reduced cancer-induced osteolysis and fewer TRAP+ osteoclasts. The authors suggest reduced expression of Runx2 and related target genes, including IL-11, MMP13, and PThrP, in breast cancer cells in the presence of miR-135 and miR-203 as a working mechanism [69].
4.2. miRNAs Disrupting the Tumor Cell–Bone Cell Crosstalk
The studies described in the previous section report direct effects of miRNAs on tumor cell behavior (e.g., migration, invasion, EMT). However, miRNAs can also indirectly affect tumor growth via effects on cells of the tumor/bone microenvironment (Figure 3). The bone microenvironment consists of various cell types, including bone-specific cells such as osteoclasts, osteoblasts, stromal cells, and endothelial cells [16]. A complex signaling network mediates the crosstalk of the plethora of cells within the bone. Especially over the past years, the bone microenvironment has gained tremendous attention, as research suggests an extensive interplay between tumor cells and cells of the tumor microenvironment [14,16]. In the following sections we review the effects of miRNAs on key players within the bone microenvironment.
Figure 3.
Direct and indirect roles of miRNAs in bone metastasis. miRNAs can affect cancer cells directly, either promoting (green arrows) or inhibiting (red arrows) tumor growth as depicted in the top panel. In addition, miRNAs affect other cells in the bone microenvironment, including osteoblasts, osteoclasts, and endothelial cells.
4.2.1. Osteoclasts
Given the osteolytic nature of breast cancer bone metastases, the role of osteoclasts in metastatic bone disease has been extensively studied. Various miRNAs have been discovered as important players in this process. For instance, lentivirus-mediated restoration of miR-124 in breast cancer cells reduced metastatic burden and osteolysis in hind limbs of mice when compared to a control [46]. Similar results were observed when mice received treatment with ago-miR-124 after tumor cell injection [46]. Reduced osteoclast number and activity are suggested to be at least partially responsible for the reduced metastatic burden in vivo. Indeed, complementary mechanistic in vitro studies support this hypothesis, as conditioned medium from miR-124-transfected breast cancer cells reduced the viability and differentiation of osteoclasts in vitro [46]. Guo and colleagues showed that breast-cancer-cell-derived exosomes containing miR-20a-5p stimulated the proliferation of bone marrow macrophages and differentiation into osteoclasts in vitro [66]. Recently, Wu et al. reported that exosomal miR-19a not only is a factor secreted by ER+ bone metastatic breast cancer cells, but also mediates osteolysis by creating an osteoclast-enriched environment within the bone in the presence of integrin-binding sialoprotein [72].
miRNAs can also directly affect osteoclast differentiation. For instance, ectopic expression of miR-141, miR-190, miR-219, miR-33a, and miR-133a reduced osteoclast differentiation and/or activity in vitro [73]. Combined ectopic expression of miR-141/190/219 was even more effective in reducing osteoclast differentiation and activity than single agents; these effects were further enhanced upon the addition of zoledronic acid, a standard-of-care agent used to ease cancer-induced bone disease [73]. Similarly, systemic miRNA treatment resulted in increased trabecular bone volume in BALB/c mice in vivo [73]. Additionally, miR-141 and miR-219 reduced bone metastasis of SCP28 cells in vivo [73].
Increased levels of matrix metalloproteinase (MMP)-13 have been shown to stimulate osteoclast activity in the metastatic bone environment [74], suggesting that MMPs mediate tumor cell–bone cell communication in the metastatic environment. Conditioned medium from breast cancer cells transfected with miR-124 inhibited the expression of MMP-13 in MC3T3 osteoblasts when compared to a control [46], which could, further on, indirectly reduce osteoclast activity and have consequences on the establishment of breast cancer bone metastasis. In agreement, miR-203 and miR-135 reduced the expression of MMP-13 in breast cancer cells [69], which could partially account for the reduced bone metastases in mouse models in these studies. Others have shown that breast-cancer-cell-derived miR-429 reduces osteoclast differentiation in vitro via MMP-9 and V-crk sarcoma virus CT10 oncogene homolog-like (CrkL) [48]. In vivo, mice injected with miR-429-transfected breast cancer cells had reduced cancer-induced osteolysis when compared to a control, and histological analysis of the bone metastases showed a reduction in both CrKL and MMP-9 expression in the miR-429 group [48]. Others have shown that overexpression of miR-20a-5p in MDA-MB-231 breast cancer cells increases the expression of MMP-2 and MMP-9, which is associated with increased migration and invasion in vitro [66]. In addition, breast-cancer-cell-derived miR-20a-5p was able to stimulate osteoclastogenesis in vitro [66].
Interleukins (ILs) have been identified as key regulatory soluble factors that mediate the tumor–bone cell interaction in breast cancer bone metastasis [75]. Several studies have shown that ILs affect the function and maturation of both osteoblasts and osteoclasts [76,77,78]. The majority of the published literature on the matter reports osteoclast stimulatory effects of ILs [79,80,81,82], which highlights them as a therapeutic target in breast-cancer-mediated osteolysis. Studies by Cai and colleagues have attributed the anti-metastatic effects of miR-124 to downregulation of IL-11 and identified IL-11 as a direct downstream target of miR-124 [46]. Briefly, in these studies, mice injected with control cells had significantly increased metastatic burden and osteolysis in hind limbs when compared to mice injected with MDA-MB-231-miR-124 cells, with similar results observed when using ago-miR-124 treatment [46]. The reduced metastatic burden was accompanied by reduced osteoclast number and activity in vivo. Complementary in vitro studies, in which conditioned medium from miR-124-transfected breast cancer cells reduced the viability and differentiation of osteoclasts, support this hypothesis [46]. Importantly, breast cancer cells expressing miR-124 had significantly lower mRNA and protein levels of IL-11 when compared to control [46]. This observation, in addition to complementary in vitro and in vivo studies using IL-11 neutralizing antibodies and recombinant human IL-11, demonstrated that miR-124 induced downregulation of IL-11 is partially responsible for reduced breast cancer bone metastasis [46].
TGF-ß is a key regulator of the vicious cycle of bone metastasis; it is released from the bone matrix during osteolysis and consequently stimulates tumor progression [83]. Studies have shown that TGF-ß increases the secretion of osteoclast-stimulating ILs (IL-11 and IL-8) in breast cancer cells [84]. In this context, miR-204, miR-211, and miR-379 have been identified as key regulators of the TGF-ß-induced production of IL-11 in bone metastatic MDA-MB-231 breast cancer cells [85]. By binding to the IL-11 3′ UTR, these miRNAs reduce IL-11 mRNA and protein secretion [85].
Additionally, conditioned medium from bone metastatic MDA-B02-cells that overexpressed miR-30 family members reduced osteoclast formation and differentiation in vitro, potentially through reduced expression of the osteoclast-promoting cytokines IL-11 and IL-8 [63]. Furthermore, conditioned medium from MDA-B02 breast cancer cells stably transfected with miR-30s decreased the formation of TRAP-positive multinucleated osteoclasts in vitro [63]. In vivo, mice injected with MDA-B02-pmiR30a-b-c-d-e tumor cells had reduced osteolytic bone disease, reduced bioluminescence signal, and reduced TRAP+ osteoclasts when compared to a control [63].
4.2.2. Osteoblasts
Studies by Liu et al. reported that overexpression of miR-218 in breast cancer cells supports bone metastasis via direct and indirect effects on osteoblasts [32]. The authors reported two independent mechanisms by which miR-218 could affect breast cancer bone metastasis. First, bone metastatic breast cancer cells secrete EVs that contain elevated levels of miR-218 when compared to the parental cell line [32]. The addition of EVs from MDA-231-miR-218 cells to osteoblast cultures reduced type I collagen mRNA expression and the bone formation marker P1NP—a measure of osteoblast activity—in the medium. Similar results, namely, reduced P1NP serum levels, were observed in vivo upon injection of EVs from MDA-231-miR-218 cells when compared to a control [32]. Interestingly though, no effect on osteoblast differentiation was observed in these experiments [32]. As a second mechanism, the authors suggested that overexpression of miR-218 in breast cancer cells alters the expression and secretion of inhibin beta subunits, which consequently affects procollagen processing in osteoblasts [32].
Others have shown that, via reducing DKK1—a key inhibitor of osteoblast differentiation [86]—in breast cancer cells, miR-30s stimulates osteoblast differentiation as compared to a control [63]. Additional key regulatory pathways in bone remodeling as well as cancer-induced bone disease include the RANK/RANKL/OPG axis [87] and Wnt signaling [88]. Several miRNAs have been shown to alter the RANKL/OPG ratio with potential consequences on the progression of breast cancer bone metastasis [46,48]. Both miR-124 and miR-429, in independent studies, decreased RANKL and increased OPG in osteoblasts, leading to an altered RANKL/OPG ratio [46,48].
4.2.3. Further Components of the (Bone) Tumor Microenvironment
Besides the heterogenous cell populations and soluble factors, the tumor microenvironment also comprises the extracellular matrix (ECM). MMPs are proteolytic enzymes that are involved in remodeling and/or degrading the ECM, a requirement for metastasis establishment. Additionally, MMPs mediate several steps of metastasis, including tumor angiogenesis and tumor cell proliferation, migration, and invasion [89,90]. Indeed, studies have shown that miRNAs can affect metastatic breast cancer growth in bone via altering the availability of MMPs [46].
The bone microenvironment is highly vascularized [9], and evidence supports the detrimental role of the bone marrow vascular niche in the initiation and progression of breast cancer bone metastasis [11,14,16,91]. miRNAs are also proposed to be involved in regulating tumor angiogenesis. In mouse models of lung cancer bone metastasis, miR-192 has demonstrated anti-metastatic potential. Mice injected with cancer cells overexpressing miR-192 showed reduced osteolytic bone lesions and decreased metastatic burden in the bones [31]. Interestingly, tumors in the miR-192 group were less vascularized, and in vitro studies showed that miR-192 reduces the migration of HUVEC cells, suggesting that miR-192 exerts anti-metastatic effects via vascular endothelial cells [31]. In these experiments, miRNAs were transferred between cell types via exosome-like vesicles [31].
5. Therapeutic Implications of miRNAs in Metastatic Bone Disease
As stated before and summarized in Table 1, miRNAs may serve as a potential novel therapeutic targets in cancer as they modify both bone and tumor cells. However, to date, no clinical trials targeting breast cancer or metastatic bone disease have been registered in the clinical trials database (www.clinicaltrials.gov, accessed on 22 November 2021). Nevertheless, a handful of Phase I trials and one Phase II clinical trial have been registered in the clinical trials database, targeting miRNAs or using miRNA mimics as a drug in cancer treatment of leukemia, lymphoma, and other solid cancer entities [92].
Table 1.
miRNAs involved in breast cancer bone metastasis.
| MicroRNA | Target | Effect on Bone Metastasis | Reference |
|---|---|---|---|
| miR-10b | Promoting | [56,93,94] | |
| miR-1976 | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma (PIK3CG) | Inhibiting | [60] |
| miR-429 | V-crk sarcoma virus CT10 oncogene homolog-like (CrkL) and Matrix metalloprotease 9 (MMP-9) | Inhibiting | [62] [32,46,47,48] |
| miR-30 family | Osteomimicry genes e.g., Cadherin 11 (CDH11) and Integrin Alpha 5 (ITGA5), Interleukins | Inhibiting | [63] |
| miR-205 | Inhibiting | [64] | |
| miR-143 | Mitogen-activated protein kinase 3 (MAPK3) | Inhibiting | [65] |
| miR-20a-5p | SRC Kinase Signaling Inhibitor 1 (SRCIN1) | Promoting | [66] |
| miR-34a-5p | Met | Inhibiting | [67] |
| miR-135 | Runt-related transcription factor 2 (Runx2) | Inhibiting | [69] |
| miR-203 | Runx2 | Inhibiting | [69,93] |
| miR-124 | Interleukin-11 (IL-11) | Inhibiting | [46] |
| miR-19a | Phosphatase and Tensin homolog (PTEN) | Promoting | [72] |
| miR-141 | Microphthalmia-associated transcription factor (Mitf) | Inhibiting | [73] |
| miR-219 | Mitf, TNF receptor associated factor (Traf-6) | Inhibiting | [73] |
| miR-204, miR-211, and miR-379 | IL-11 | Inhibiting | [85] |
| miR-218 | Dickkopf-related protein 2 (DKK2), Secreted frizzled-related protein 2 (sFRP2), Sost | Promoting | [32,58] |
| miR-192 | IL-8, Intercellular Adhesion Molecule (ICAM) and C-X-C Motif Chemokine Ligand 1 (CXCL1) | Inhibiting | [31] |
Regarding breast cancer and metastatic bone disease, several in vivo studies demonstrated miRNA-mediated effects on the bone microenvironment leading to reduced tumor growth and attenuated osteolytic disease. For example, high levels of miR-30 [63], miR-124 [46], miR-192 [31], and miR-429 [62] have been shown to have a beneficial effect with reduced osteolysis in vivo. In addition to osteolytic lesions, the frequency of bone metastasis or tumor burden in general can be altered by differential miRNA expression. For instance, reduced tumor burden, especially in the bone, mediated by miR-135 and miR-203 has been observed in mice [69]. The identification of disease-specific miRNAs brings us a step forward towards more personalized medicine, utilizing an endogenous molecule.
With respect to breast cancer, Ell and colleagues demonstrated that after systemic application of pre-miR-141 and pre-miR-219, the number of osteoclasts was significantly decreased. Although the analysis revealed that osteoblast differentiation was not affected, the authors could not rule out the possibility that other cells are affected by systemic pre-miRNA treatment [73].
6. Future Perspectives
In general, RNA-based medicine has received tremendous attention within the last decade [95]. Beyond mRNA-based drugs, non-coding RNAs, including miRNAs, are amenable for therapeutic development. Although miRNA-based drugs have not reached clinical approval yet, several compounds are in pre-clinical and clinical development. These drugs target various diseases, including cancer, where great progress has been made in recent years. In particular, the fact that miRNAs and other oligonucleotide drugs are adaptable molecules holds great promise in personalized medicine with individual and agile drug design.
However, given their biological properties, there are still potential pitfalls related to miRNA-related medicine. As mentioned before, miRNAs are usually short non-coding RNA molecules with an approximate length of 20 nucleotides. Their small size may be an advantage for drug delivery but may lack target specificity. One potential solution to overcome the low specificity could be a mixture of different miRNAs with the same target to ensure reliable target inhibition. Nonetheless, this approach might also increase the rate of off-target effects and, therefore, potentially also the risk of adverse events. In addition to off-target effects, unwanted and unpredictable side-effects within the complex signaling networks should be considered and extensively investigated.
Once the obstacles—including off-target effects, tissue specificity, and delivery systems—have been overcome, it can be assumed that treatments using miRNAs as targets will further progress, and novel drug candidates will be developed for diseases with high unmet medical needs, such as metastatic bone disease. Given that miRNAs were first discovered only a few decades ago, miRNA-based drug development is still in its infancy, but the conditions are promising for the development of next-generation miRNA-based drugs. More fundamental and translational studies are needed to better understand the mechanisms of action, as well as potential adverse effects related to miRNAs in various disease conditions.
7. Conclusions
The currently available results of preclinical studies suggest an important role of miRNAs in metastatic bone disease. Several miRNAs have already been characterized, and the data indicate a potential novel druggable target in cancer therapy that should be further evaluated in pre-clinical development and clinical trials.
Acknowledgments
The figures were created using Biorender.com, accessed on 25 December 2021.
Author Contributions
M.-T.H., D.J.S. and H.T. reviewed the literature and wrote and critically revised the manuscript. D.J.S. prepared the figures for this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Deutsche Forschungsgemeinschaft (DFG), grant numbers TA 1154/1-2 and TA 1154/2-1 to H.T.
Conflicts of Interest
H.T. is a co-founder and shareholder of Sirana Pharma GmbH. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Lim B., Hortobagyi G.N. Current challenges of metastatic breast cancer. Cancer Metastasis Rev. 2016;35:495–514. doi: 10.1007/s10555-016-9636-y. [DOI] [PubMed] [Google Scholar]
- 4.Manders K., van de Poll-Franse L.V., Creemers G.J., Vreugdenhil G., van der Sangen M.J.C., Nieuwenhuijzen G.A.P., Roumen R.M.H., Voogd A.C. Clinical management of women with metastatic breast cancer: A descriptive study according to age group. BMC Cancer. 2006;6:179. doi: 10.1186/1471-2407-6-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sathiakumar N., Delzell E., Morrisey M.A., Falkson C., Yong M., Chia V., Blackburn J., Arora T., Brill I., Kilgore M.L. Mortality following bone metastasis and skeletal-related events among women with breast cancer: A population-based analysis of U.S. Medicare beneficiaries, 1999–2006. Breast Cancer Res. Treat. 2012;131:231–238. doi: 10.1007/s10549-011-1721-x. [DOI] [PubMed] [Google Scholar]
- 6.Pantel K., Brakenhoff R.H. Dissecting the metastatic cascade. Nat. Rev. Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 7.Parfitt A.M. The bone remodeling compartment: A circulatory function for bone lining cells. J. Bone Miner. Res. 2001;16:1583–1585. doi: 10.1359/jbmr.2001.16.9.1583. [DOI] [PubMed] [Google Scholar]
- 8.Kenkre J.S., Bassett J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018;55:308–327. doi: 10.1177/0004563218759371. [DOI] [PubMed] [Google Scholar]
- 9.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghajar C.M., Peinado H., Mori H., Matei I.R., Evason K.J., Brazier H., Almeida D., Koller A., Hajjar K.A., Stainier D.Y.R., et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price T.T., Burness M.L., Sivan A., Warner M.J., Cheng R., Lee C.H., Olivere L., Comatas K., Magnani J., Lyerly H.K., et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 2016;8:340ra73. doi: 10.1126/scitranslmed.aad4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malanchi I., Santamaria-Martínez A., Susanto E., Peng H., Lehr H.A., Delaloye J.F., Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–91. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 13.Haider M.T., Holen I., Dear T.N., Hunter K., Brown H.K. Modifying the osteoblastic niche with zoledronic acid in vivo-Potential implications for breast cancer bone metastasis. Bone. 2014;66:240–250. doi: 10.1016/j.bone.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarrer J., Haider M.-T., Smit D.J., Taipaleenmäki H. Pathological Crosstalk between Metastatic Breast Cancer Cells and the Bone Microenvironment. Biomolecules. 2020;10:337. doi: 10.3390/biom10020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Psaila B., Lyden D. The metastatic niche: Adapting the foreign soil. Nat. Rev. Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haider M.T., Smit D.J., Taipaleenmäki H. The Endosteal Niche in Breast Cancer Bone Metastasis. Front. Oncol. 2020;10:335. doi: 10.3389/fonc.2020.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Templeton Z.S., Lie W.R., Wang W., Rosenberg-Hasson Y., Alluri R.V., Tamaresis J.S., Bachmann M.H., Lee K., Maloney W.J., Contag C.H., et al. Breast Cancer Cell Colonization of the Human Bone Marrow Adipose Tissue Niche. Neoplasia. 2015;17:849–861. doi: 10.1016/j.neo.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H., Yu C., Gao X., Welte T., Muscarella A.M., Tian L., Zhao H., Zhao Z., Du S., Tao J., et al. The Osteogenic Niche Promotes Early-Stage Bone Colonization of Disseminated Breast Cancer Cells. Cancer Cell. 2015;27:193–210. doi: 10.1016/j.ccell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maroni P. Megakaryocytes in Bone Metastasis: Protection or Progression? Cells. 2019;8:134. doi: 10.3390/cells8020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y.H.V., Xu L., Mei X., Middleton K., You L. Mechanically stimulated osteocytes reduce the bone-metastatic potential of breast cancer cells in vitro by signaling through endothelial cells. J. Cell. Biochem. 2019;120:7590–7601. doi: 10.1002/jcb.28034. [DOI] [PubMed] [Google Scholar]
- 21.Clezardin P., Teti A. Bone metastasis: Pathogenesis and therapeutic implications. Clin. Exp. Metastasis. 2007;24:599–608. doi: 10.1007/s10585-007-9112-8. [DOI] [PubMed] [Google Scholar]
- 22.Clines G.A., Guise T.A. Molecular mechanisms and treatment of bone metastasis. Expert Rev. Mol. Med. 2008;10:e7. doi: 10.1017/S1462399408000616. [DOI] [PubMed] [Google Scholar]
- 23.D’Oronzo S., Coleman R., Brown J., Silvestris F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J. Bone Oncol. 2019;15:100205. doi: 10.1016/j.jbo.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dougall W., Chaisson M. Monoclonal antibody targeting RANKL as a therapy for cancer-induced bone diseases. Clin. Calcium. 2006;16:627–635. [PubMed] [Google Scholar]
- 25.Rodan G.A., Fleisch H.A. Bisphosphonates: Mechanisms of action. J. Clin. Investig. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleisch H. Development of bisphosphonates. Breast Cancer Res. 2002;4:30–34. doi: 10.1186/bcr414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S., Hofbauer L.C., Lau E., Lewiecki E.M., Miyauchi A., et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 28.Suvannasankha A., Chirgwin J.M. Role of bone-anabolic agents in the treatment of breast cancer bone metastases. Breast Cancer Res. 2014;16:484. doi: 10.1186/s13058-014-0484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hesse E., Schröder S., Brandt D., Pamperin J., Saito H., Taipaleenmäki H. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight. 2019;4:e125543. doi: 10.1172/jci.insight.125543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 31.Valencia K., Luis-Ravelo D., Bovy N., Antón I., Martínez-Canarias S., Zandueta C., Ormazábal C., Struman I., Tabruyn S., Rebmann V., et al. MiRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol. Oncol. 2014;8:689–703. doi: 10.1016/j.molonc.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X., Cao M., Palomares M., Wu X., Li A., Yan W., Fong M.Y., Chan W.C., Wang S.E. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type i collagen deposition by osteoblasts. Breast Cancer Res. 2018;20:127. doi: 10.1186/s13058-018-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., Zhou W., Liu Y., Liu T., Li C., Wang L. Oncogenic role of microRNA-532-5p in human colorectal cancer via targeting of the 5′UTR of RUNX3. Oncol. Lett. 2018;15:7215–7220. doi: 10.3892/ol.2018.8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y., Jeon K., Lee J.T., Kim S., Kim V.N. MicroRNA maturation: Stepwise processing and subcellular localization. EMBO J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S., Song M.L., Min H., Hwang I., Baek S.K., Kwon T.K., Park J.W. MiRNA biogenesis-associated RNase III nucleases drosha and dicer are upregulated in colorectal adenocarcinoma. Oncol. Lett. 2017;14:4379–4383. doi: 10.3892/ol.2017.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michlewski G., Cáceres J.F. Post-transcriptional control of miRNA biogenesis. RNA. 2019;25:1–16. doi: 10.1261/rna.068692.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang H.W., Mendell J.T. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer. 2006;94:776–780. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osada H., Takahashi T. MicroRNAs in biological processes and carcinogenesis. Carcinogenesis. 2007;28:2–12. doi: 10.1093/carcin/bgl185. [DOI] [PubMed] [Google Scholar]
- 40.Heneghan H.M., Miller N., Lowery A.J., Sweeney K.J., Newell J., Kerin M.J. Circulating micrornas as novel minimally invasive biomarkers for breast cancer. Ann. Surg. 2010;251:499–505. doi: 10.1097/SLA.0b013e3181cc939f. [DOI] [PubMed] [Google Scholar]
- 41.Bockmeyer C.L., Christgen M., Müller M., Fischer S., Ahrens P., Länger F., Kreipe H., Lehmann U. MicroRNA profiles of healthy basal and luminal mammary epithelial cells are distinct and reflected in different breast cancer subtypes. Breast Cancer Res. Treat. 2011;130:735–745. doi: 10.1007/s10549-010-1303-3. [DOI] [PubMed] [Google Scholar]
- 42.Lowery A.J., Miller N., Devaney A., McNeill R.E., Davoren P.A., Lemetre C., Benes V., Schmidt S., Blake J., Ball G., et al. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res. 2009;11:R27. doi: 10.1186/bcr2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kandettu A., Radhakrishnan R., Chakrabarty S., Sriharikrishnaa S., Kabekkodu S.P. The emerging role of miRNA clusters in breast cancer progression. Biochim. Biophys. Acta Rev. Cancer. 2020;1874:188413. doi: 10.1016/j.bbcan.2020.188413. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wahid F., Shehzad A., Khan T., Kim Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta Mol. Cell Res. 2010;1803:1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Cai W.L., Huang W.D., Li B., Chen T.R., Li Z.X., Zhao C.L., Li H.Y., Wu Y.M., Yan W.J., Xiao J.R. microRNA-124 inhibits bone metastasis of breast cancer by repressing Interleukin-11. Mol. Cancer. 2018;17:9. doi: 10.1186/s12943-017-0746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Surapaneni S.K., Bhat Z.R., Tikoo K. MicroRNA-941 regulates the proliferation of breast cancer cells by altering histone H3 Ser 10 phosphorylation. Sci. Rep. 2020;10:17954. doi: 10.1038/s41598-020-74847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X., Yu X., Zhao Z., Yuan Z., Ma P., Ye Z., Guo L., Xu S., Xu L., Liu T., et al. MicroRNA-429 inhibits bone metastasis in breast cancer by regulating CrkL and MMP-9. Bone. 2020;130:115139. doi: 10.1016/j.bone.2019.115139. [DOI] [PubMed] [Google Scholar]
- 49.Kawaguchi T., Yan L., Qi Q., Peng X., Edge S.B., Young J., Yao S., Liu S., Otsuji E., Takabe K. Novel MicroRNA-Based Risk Score Identified by Integrated Analyses to Predict Metastasis and Poor Prognosis in Breast Cancer. Ann. Surg. Oncol. 2018;25:4037–4046. doi: 10.1245/s10434-018-6859-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hackl M., Heilmeier U., Weilner S., Grillari J. Circulating microRNAs as novel biomarkers for bone diseases—Complex signatures for multifactorial diseases? Mol. Cell. Endocrinol. 2016;432:83–95. doi: 10.1016/j.mce.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 51.Duffy M.J. Serum tumor markers in breast cancer: Are they of clinical value? Clin. Chem. 2006;52:345–351. doi: 10.1373/clinchem.2005.059832. [DOI] [PubMed] [Google Scholar]
- 52.Zaleski M., Kobilay M., Schroeder L., Debald M., Semaan A., Hettwer K., Uhlig S., Kuhn W., Hartmann G., Holdenrieder S. Improved sensitivity for detection of breast cancer by combination of miR-34a and tumor markers CA 15-3 or CEA. Oncotarget. 2018;9:22523–22536. doi: 10.18632/oncotarget.25077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGuire A., Brown J.A.L., Kerin M.J. Metastatic breast cancer: The potential of miRNA for diagnosis and treatment monitoring. Cancer Metastasis Rev. 2015;34:145–155. doi: 10.1007/s10555-015-9551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma L., Teruya-Feldstein J., Weinberg R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 55.Chen W., Cai F., Zhang B., Barekati Z., Zhong X.Y. The level of circulating miRNA-10b and miRNA-373 in detecting lymph node metastasis of breast cancer: Potential biomarkers. Tumour Biol. 2013;34:455–462. doi: 10.1007/s13277-012-0570-5. [DOI] [PubMed] [Google Scholar]
- 56.Zhao F.-L., Hu G.-D., Wang X.-F., Zhang X.-H., Zhang Y.-K., Yu Z.-S. Serum overexpression of microRNA-10b in patients with bone metastatic primary breast cancer. J. Int. Med. Res. 2012;40:859–866. doi: 10.1177/147323001204000304. [DOI] [PubMed] [Google Scholar]
- 57.Ahmad A., Sethi S., Chen W., Ali-Fehmi R., Mittal S., Sarkar F.H. Up-regulation of microRNA-10b is associated with the development of breast cancer brain metastasis. Am. J. Transl. Res. 2014;6:384–390. [PMC free article] [PubMed] [Google Scholar]
- 58.Hassan M.Q., Maeda Y., Taipaleenmaki H., Zhang W., Jafferji M., Gordon J.A.R., Li Z., Croce C.M., Van Wijnen A.J., Stein J.L., et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J. Biol. Chem. 2012;287:42084–42092. doi: 10.1074/jbc.M112.377515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taipaleenmäki H., Farina N.H., van Wijnen A.J., Stein J.L., Hesse E., Stein G.S., Lian J.B. Antagonizing miR-218-5p attenuates Wnt signaling and reduces metastatic bone disease of triple negative breast cancer cells. Oncotarget. 2016;7:79032–79046. doi: 10.18632/oncotarget.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J., Li M., Han X., Wang H., Wang X., Ma G., Xia T., Wang S. MiR-1976 knockdown promotes epithelial–mesenchymal transition and cancer stem cell properties inducing triple-negative breast cancer metastasis. Cell Death Dis. 2020;11:500. doi: 10.1038/s41419-020-2711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Felipe Lima J., Nofech-Mozes S., Bayani J., Bartlett J. EMT in Breast Carcinoma—A Review. J. Clin. Med. 2016;5:65. doi: 10.3390/jcm5070065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L., Liu Q., Mu Q., Zhou D., Li H., Zhang B., Yin C. MiR-429 suppresses proliferation and invasion of breast cancer via inhibiting the Wnt/β-catenin signaling pathway. Thorac. Cancer. 2020;11:3126–3138. doi: 10.1111/1759-7714.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Croset M., Pantano F., Kan C.W.S., Bonnelye E., Descotes F., Alix-Panabieres C., Lecellier C.H., Bachelier R., Allioli N., Hong S.S., et al. miRNA-30 family members inhibit breast cancer invasion, osteomimicry, and bone destruction by directly targeting multiple bone metastasis–associated genes. Cancer Res. 2018;78:5259–5273. doi: 10.1158/0008-5472.CAN-17-3058. [DOI] [PubMed] [Google Scholar]
- 64.Seo S., Moon Y., Choi J., Yoon S., Jung K.H., Cheon J., Kim W., Kim D., Lee C.H., Kim S.-W., et al. The GTP binding activity of transglutaminase 2 promotes bone metastasis of breast cancer cells by downregulating microRNA-205. Am. J. Cancer Res. 2019;9:597–607. [PMC free article] [PubMed] [Google Scholar]
- 65.Du Y., Zhang J., Meng Y., Huang M., Yan W., Wu Z. MicroRNA-143 targets MAPK3 to regulate the proliferation and bone metastasis of human breast cancer cells. AMB Express. 2020;10:134. doi: 10.1186/s13568-020-01072-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo L., Zhu Y., Li L., Zhou S., Yin G., Yu G., Cui H. Breast cancer cell-derived exosomal miR-20a-5p promotes the proliferation and differentiation of osteoclasts by targeting SRCIN1. Cancer Med. 2019;8:5687–5701. doi: 10.1002/cam4.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maroni P., Puglisi R., Mattia G., Carè A., Matteucci E., Bendinelli P., Desiderio M.A. In bone metastasis miR-34a-5p absence inversely correlates with Met expression, while Met oncogene is unaffected by miR-34a-5p in non-metastatic and metastatic breast carcinomas. Carcinogenesis. 2017;38:492–503. doi: 10.1093/carcin/bgx027. [DOI] [PubMed] [Google Scholar]
- 68.Organ S.L., Tsao M.S. An overview of the c-MET signaling pathway. Ther. Adv. Med. Oncol. 2011;3:S7–S19. doi: 10.1177/1758834011422556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taipaleenmäki H., Browne G., Akech J., Zustin J., Van Wijnen A.J., Stein J.L., Hesse E., Stein G.S., Lian J.B. Targeting of Runx2 by miR-135 and miR-203 impairs progression of breast cancer and metastatic bone disease. Cancer Res. 2015;75:1433–1444. doi: 10.1158/0008-5472.CAN-14-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Awolaran O., Brooks S.A., Lavender V. Breast cancer osteomimicry and its role in bone specific metastasis; an integrative, systematic review of preclinical evidence. Breast. 2016;30:156–171. doi: 10.1016/j.breast.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 71.Rucci N., Teti A. Osteomimicry: How tumor cells try to deceive the bone. Front. Biosci. Sch. 2010;2:907–915. doi: 10.2741/s110. [DOI] [PubMed] [Google Scholar]
- 72.Wu K., Feng J., Lyu F., Xing F., Sharma S., Liu Y., Wu S.-Y., Zhao D., Tyagi A., Deshpande R.P., et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat. Commun. 2021;12:5196. doi: 10.1038/s41467-021-25473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ell B., Mercatali L., Ibrahim T., Campbell N., Schwarzenbach H., Pantel K., Amadori D., Kang Y. Tumor-Induced Osteoclast miRNA Changes as Regulators and Biomarkers of Osteolytic Bone Metastasis. Cancer Cell. 2013;24:542–556. doi: 10.1016/j.ccr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pivetta E., Scapolan M., Pecolo M., Wassermann B., Abu-Rumeileh I., Balestreri L., Borsatti E., Tripodo C., Colombatti A., Spessotto P. MMP-13 stimulates osteoclast differentiation and activation in tumour breast bone metastases. Breast Cancer Res. 2011;13:R105. doi: 10.1186/bcr3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haider M.-T., Ridlmaier N., Smit D.J., Taipaleenmäki H. Interleukins as Mediators of the Tumor Cell-Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis. Int. J. Mol. Sci. 2021;22:2898. doi: 10.3390/ijms22062898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kurihara N., Civin C., Roodman G.D. Osteotropic factor responsiveness of highly purified populations of early and late precursors for human multinucleated cells expressing the osteoclast phenotype. J. Bone Miner. Res. 1991;6:257–261. doi: 10.1002/jbmr.5650060307. [DOI] [PubMed] [Google Scholar]
- 77.Girasole G., Passeri G., Jilka R.L., Manolagas S.C. Interleukin-11: A new cytokine critical for osteoclast development. J. Clin. Investig. 1994;93:1516–1524. doi: 10.1172/JCI117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bendre M.S., Montague D.C., Peery T., Akel N.S., Gaddy D., Suva L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/S8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 79.Amarasekara D.S., Yun H., Kim S., Lee N., Kim H., Rho J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018;18:e8. doi: 10.4110/in.2018.18.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuura T., Ichinose S., Akiyama M., Kasahara Y., Tachikawa N., Nakahama K.I. Involvement of CX3CL1 in the Migration of Osteoclast Precursors Across Osteoblast Layer Stimulated by Interleukin-1ß. J. Cell. Physiol. 2017;232:1739–1745. doi: 10.1002/jcp.25577. [DOI] [PubMed] [Google Scholar]
- 81.Liu X.H., Kirschenbaum A., Yao S., Levine A.C. Cross-talk between the interleukin-6 and prostaglandin E2 signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-κB (RANK) ligand/RANK system. Endocrinology. 2005;146:1991–1998. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- 82.Liang M., Ma Q., Ding N., Luo F., Bai Y., Kang F., Gong X., Dong R., Dai J., Dai Q., et al. IL-11 is essential in promoting osteolysis in breast cancer bone metastasis via RANKL-independent activation of osteoclastogenesis. Cell Death Dis. 2019;10:353. doi: 10.1038/s41419-019-1594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang Y., Siegel P.M., Shu W., Drobnjak M., Kakonen S.M., Cordón-Cardo C., Guise T.A., Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/S1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 84.Gupta J., Robbins J., Jilling T., Seth P. TGFβ-dependent induction of interleukin-11 and interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol. Ther. 2011;11:311–316. doi: 10.4161/cbt.11.3.14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pollari S., Leivonen S.K., Perälä M., Fey V., Käkönen S.M., Kallioniemi O. Identification of microRNAs inhibiting TGF-β-induced IL-11 production in bone metastatic breast cancer cells. PLoS ONE. 2012;7:e37361. doi: 10.1371/journal.pone.0037361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qiang Y.-W., Barlogie B., Rudikoff S., Shaughnessy J.D.J. Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone. 2008;42:669–680. doi: 10.1016/j.bone.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Ando K., Mori K., Redini F., Heymann D. RANKL/RANK/OPG: Key Therapeutic Target in Bone Oncology. Curr. Drug Discov. Technol. 2008;5:263–268. doi: 10.2174/157016308785739857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valkenburg K.C., Steensma M.R., Williams B.O., Zhong Z. Skeletal metastasis: Treatments, mouse models, and the Wnt signaling. Chin. J. Cancer. 2013;32:380–396. doi: 10.5732/cjc.012.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tauro M., Lynch C.C. Cutting to the chase: How matrix metalloproteinase-2 activity controls breast-cancer-to-bone metastasis. Cancers. 2018;10:185. doi: 10.3390/cancers10060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deryugina E.I., Quigley J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 91.Kaplan R.N., Riba R.D., Zacharoulis S., Bramley A.H., Vincent L., Costa C., MacDonald D.D., Jin D.K., Shido K., Kerns S.A., et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forterre A., Komuro H., Aminova S., Harada M. A Comprehensive Review of Cancer MicroRNA Therapeutic Delivery Strategies. Cancers. 2020;12:1852. doi: 10.3390/cancers12071852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu S.-B., Fan R.-H., Qin X., Han R.-M. microRNA Prognostic Signature for Postoperative Success of Metastatic Orthopedic Cancers: Implications for Precision Microsurgery. Front. Cell Dev. Biol. 2021;9:704505. doi: 10.3389/fcell.2021.704505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Croset M., Goehrig D., Frackowiak A., Bonnelye E., Ansieau S., Puisieux A., Clézardin P. TWIST1 expression in breast cancer cells facilitates bone metastasis formation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014;29:1886–1899. doi: 10.1002/jbmr.2215. [DOI] [PubMed] [Google Scholar]
- 95.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]