Abstract
This study investigated whether radiomic features derived from preoperative PET images could predict both tumor biology and prognosis in women with invasive squamous cell carcinoma of the vulva. Methods: Patients were retrospectively included if they had a unifocal primary cancer at least 2.6 cm in diameter, received a preoperative 18F-FDG PET/CT scan followed by surgery, and had at least 6 mo of follow-up data. 18F-FDG PET images were analyzed by semiautomatically drawing a volume of interest on the primary tumor in each PET image, followed by extraction of 83 radiomic features. Unique radiomic features were identified by principal-component analysis (PCA), after which they were compared with histopathology using nonpairwise group comparison and linear regression. Univariate and multivariate Cox regression analyses were used to correlate the identified features with progression-free survival (PFS) and overall survival (OS). Survival curves were estimated using the Kaplan–Meier method. Results: Forty women were included. PCA revealed 4 unique radiomic features, which were not associated with histopathologic characteristics such as grade, depth of invasion, lymph-vascular space invasion, and metastatic lymph nodes. No statistically significant correlation was found between the identified features and PFS. However, Moran’s I, a feature that identifies global spatial autocorrelation, correlated with OS (P = 0.03). Multivariate Cox regression analysis showed that extracapsular invasion of the metastatic lymph nodes and Moran’s I were independent prognostic factors for PFS and OS. Conclusion: Our data show that PCA is usable to identify specific radiomic features. Although the identified features did not correlate strongly with tumor biology, Moran’s I was found to predict patient prognosis. Larger studies are required to establish the clinical relevance of the observed findings.
Keywords: vulvar cancer, radiomics, 18F-FDG PET/CT, principal component analysis
Vulvar cancer is a rare gynecologic malignancy, with an incidence rate of 1.5–2.4 per 100,000 women per year (1,2).Vulvar squamous cell carcinoma (VSCC) accounts for 90% of all vulvar cancers (3), which occur mainly in elderly women (4). The pattern of dissemination of vulvar cancer is predominantly lymphogenic to the inguinofemoral lymph nodes, and distant metastases are rare (5). Thus, the most important prognostic factor is the presence of metastatic lymph nodes (6). Indeed, the 5-y overall survival (OS) varies from 94.7% in women without metastatic lymph nodes to 61% in women with lymph node metastases (7). Additional pathologic prognostic factors include the depth of stromal invasion and lymph-vascular space invasion (8).
Currently, the standard treatment for VSCC includes radical resection of the primary tumor followed by unilateral or bilateral surgical staging of the inguinofemoral lymph nodes. In particular, sentinel lymph node biopsy is the standard of care in cases of a naïve unifocal tumor with a diameter of 4 cm or less and clinically negative lymph nodes (cN0). Radical inguinofemoral lymphadenectomy is thus indicated in the remaining cases of negative lymph nodes and in all cases of metastatic lymph nodes (8–10), even if sentinel lymph node biopsy is now under investigation, being potentially safe also in the many cN0 cases considered unfit for minimal surgical procedures (11). Adjuvant radiotherapy is indicated on the basis of primary risk factors, such as resection margin status, depth of stromal invasion, and nodal involvement (8,12).
Recently, PET/CT imaging using the glucose analog 18F-FDG has been included in the preoperative workup of vulvar cancer, mainly for staging (8). However, there is growing interest in radiomics, that is, high-throughput extraction of large amounts of imaging features (13), where several of these features, including descriptors that quantify tracer uptake—morphology and tracer uptake heterogeneity—are predictive of tumor biologic behavior, response to therapy, and prognosis (14). In the area of precision medicine, an improved characterization of the primary tumor could help to plan a more personalized treatment strategy. In this perspective, the identification of imaging features associated with tumor-specific biologic properties and metastatic potential could be clinically relevant in women with VSCC. Some 18F-FDG PET–related radiomics approaches have already been recently explored in other gynecologic malignancies such as cervical cancer (15).
The aim of this study was to investigate whether quantitative imaging features derived from 18F-FDG PET/CT scans can be used to predict both biologic behavior of the vulvar malignancy and patient prognosis. An innovative method for identifying imaging features in the dataset was achieved by principal-component analysis (PCA) (16), an unsupervised technique to perform dimensionality reduction.
MATERIALS AND METHODS
Patients and Study Design
This retrospective study was approved by the Ethical Committee of the Fondazione Policlinico Universitario A. Gemelli-IRCCS (study code 1633). The medical records of all patients with histologically proven VSCC, referred to the Division of Gynecologic Oncology between June 2013 and December 2016, were reviewed. With their written informed consent, women were included if they were at least 18 y old, had a unifocal VSCC with stromal invasion more than 1 mm deep and diameter of at least 26 mm (17), underwent a preoperative 18F-FDG PET/CT scan, received vulvar cancer surgery, and had at least 6 mo of follow-up data available. Women were excluded if they had multifocal vulvar cancer, a contraindication for surgery due to age or comorbidities, prior chemotherapy or locoregional radiation therapy within the last 5 y, prior locoregional surgery, or a plasma glucose level higher than 200 mg/dL before 18F-FDG PET/CT acquisition. Surgical procedures and adjuvant treatments (radiotherapy with or without concomitant chemotherapy) were provided according to international guidelines after multidisciplinary decision making. Follow-up was performed every 3 mo for the first 2 y and every 6 mo up to the fifth year.
18F-FDG PET/CT Image Acquisition
PET/CT studies were performed as previously described (18). Briefly, each patient fasted for at least 6 h. Following intravenous administration of 201 ± 58 MBq of 18F-FDG, PET images were acquired after 66 ± 16 min using either a Gemini GXL (Philips Healthcare) or a Biograph mCT (Siemens Heathineers) PET/CT scanner. A low-dose CT scan (110–120 kVp, 20–40 mAs) was acquired for anatomic reference and attenuation correction purposes, followed by a PET scan using 2.5 min (Biograph) or 3 min (Gemini) per bed position. All PET images were reconstructed according to the European Association of Nuclear Medicine guidelines for tumor PET imaging (19) using either a line-of-response row-action maximum-likelihood algorithm (3 iterations and 33 subsets, voxel size of 4 × 4 × 4 mm, no additional gaussian smoothing) or a 3-dimensional (3D) ordered-subsets expectation-maximization algorithm with resolution modeling (2 iterations and 21 subsets, voxel size of 3.2 × 3.2 × 5 mm, additional gaussian smoothing of 8.2 mm in full width at half maximum) for the Gemini or Biograph, respectively.
Image Analysis
Radiomic analysis was performed on all 18F-FDG PET/CT images using software built in-house (20), extracting 83 features based on intensity (n = 23), local intensity (n = 1), intensity-volume histograms (n = 1), morphology (n = 14), fractal (n = 3), or texture (n = 41) (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). These features were derived from gray-level cooccurrence matrices or from gray-level run-length matrices that were calculated by merging the obtained matrices over all 13 directions while applying discretization with a fixed bin size of 0.25 g/mL (20). Only uniformity, entropy, and local entropy were also calculated, with discretization with 64 gray-level bins (17,21). All features were implemented according to the definitions set by the image biomarker standardization initiative (22).
All 18F-FDG PET/CT images were reviewed by consensus between 2 experienced nuclear medicine physicians, masked to clinical and histopathologic information. Volumes of interest of the primary tumor were drawn semiautomatically on all PET images using an isocontour method that applied a 50% threshold of the SUVpeak corrected for local background activity (23). SUVpeak was defined as the highest SUVmean of a 1-mL sphere within the volume of interest, not necessarily centered around the SUVmax within the volume of interest. In cases of urinary contamination or the presence of high-uptake areas near the tumor (e.g., bladder), a freehand box was drawn that excluded the urinary contamination or other high-uptake areas before semiautomatic delineation.
Statistical Analysis
Dimensionality Reduction and Feature Selection
Radiomic features that correlated strongly (r > 0.9) were grouped, and only one feature from each group was selected as input for PCA. The dimensionality of radiomic features was then reduced by PCA (SPSS, version 23; IBM Statistics). During this analysis, an orthogonal rotation (varimax with Kaiser normalization) was applied to convert the set of linearly uncorrelated variables, also known as principal components, in such a way that the first principal component explained the largest possible variance in the dataset and each succeeding component had the highest variance possible under the constraint that it was orthogonal to the preceding components. To minimize false discoveries, no more than 4 principal components were allowed in the final analysis, since the rule of thumb is that at least 10 patients are required to test one radiomic feature (24). The sampling adequacy for each component in the model and for the complete model was determined by the Kaiser-Meyer-Olkin measure, which had to be 0.5 or higher.
Correlation of Radiomic Features with Histopathology
Radiomic features identified with PCA were correlated with histopathologic features such as histologic grade, depth of stromal invasion, presence of lymph-vascular space invasion, and metastatic lymph nodes. These features were tested for normality using the Shapiro–Wilk test. The significance of differences between histologic groups (grade, presence of lymph-vascular space invasion, and metastatic lymph nodes) was analyzed using the Mann–Whitney U test. Furthermore, linear regression was used to correlate the depth of stromal invasion with the radiomic features.
Correlation of Clinical Characteristics, Histopathologic Parameters, and Radiomic Features with Clinical Outcome
The correlation of clinical characteristics, histopathologic parameters, and radiomic features with prognosis was determined using univariate Cox regression analysis for progression-free survival (PFS) and OS. PFS was defined as the interval between surgery and the first clinical detection of recurrence. OS was defined as the interval between surgery and death. From the univariate Cox regression analysis, candidate covariates were identified for multivariate Cox regression models on the basis of significance level (P ≤ 0.2). Multivariate Cox regression analyses were performed by an iterative forward and backward selection of the candidate covariates based on the likelihood ratio. Furthermore, survival curves were estimated using the Kaplan–Meier analysis. Variables were stratified in a low group and a high group at their median. The Kaplan–Meier curves were compared using the Mantel–Cox statistics. P values of less than 0.05 were considered statistically significant.
RESULTS
Patients
The records of 120 women with VSCC were reviewed. The inclusion criteria were fulfilled by 40 women, whose characteristics are summarized in Tables 1–3.
TABLE 1.
Characteristics of the 40 Patients
| Characteristic | Data |
| Mean age ± SD (y) | 70 ± 10 |
| Mean body mass index (kg/m2) | 27 (range, 16–42) |
| Mean time ± SD between PET/CT and surgery (d) | 20 ± 17 (range, 1–74) |
| Primary vulvar tumor site | |
| Anterior | 20 (50) |
| Lateral | 17 (42.5) |
| Posterior | 3 (7.5) |
| Tumor growth pattern | |
| Exophytic | 29 (72.5) |
| Ulcerative | 7 (17.5) |
| Infiltrative | 4 (10) |
| Neighboring tissues involved | |
| Extravulvar skin | 4 (10) |
| Urologic tract | 4 (10) |
| None | 32 (80) |
| Vulvar surgery | |
| Partial vulvectomy | 6 (15) |
| Radical vulvectomy | 34 (85) |
| Inguinofemoral lymph node surgery | |
| Unilateral | 0 |
| Bilateral | 39 (97.5) |
| None | 1 (2.5) |
| SLNB | 3 (8) |
| SLNB followed by IFL | 16 (41) |
| ILF | 20 (51) |
| Pelvic lymph node surgery | |
| Unilateral | 8 (20) |
| Bilateral | 1 (2.5) |
| None | 31 (77.5) |
SLNB = sentinel lymph node biopsy; IFL 5 inguinofemoral lymphadenectomy.
Data are n followed by percentage in parentheses, unless otherwise specified.
TABLE 3.
Follow-Up Closeout Data (February 2018)
| Parameter | Data |
| Follow-up for entire study (mo) | |
| Median | 15 (range, 2–50) |
| Mean ± SD | 19 ± 13 |
| Follow-up for surviving patients (mo) | |
| Median | 26 (range, 6–50) |
| Mean ± SD | 25 ± 13 |
| PFS | |
| Median | 10 (range, 2–50) |
| Mean ± SD | 17 ± 14 |
| OS | |
| Median | 16 (range, 3–50) |
| Mean ± SD | 20 ± 13 |
| Local or distant recurrence (n) | 18 (45%)* |
| Death (n) | 18 (45%)† |
15 of these 18 patients died due to cancer progression.
During median follow-up of 11 mo (mean, 12 ± 8; range, 2–34).
TABLE 2.
Histopatologic Characteristics, Staging, and Postoperative Therapy of the 40 Patients
| Characteristic | Data |
| Tumor diameter (mm) | |
| Median | 50 (range, 30–90) |
| 26–40 | 17 (42.5) |
| >40 | 23 (57.5) |
| Histologic grade | |
| G1 | 2 (5) |
| G2 | 32 (80) |
| G3 | 6 (15) |
| Depth of stromal invasion (mm) | |
| Median | 9 (range, 4–20) |
| 1–4 | 3 |
| >5 | 34 |
| Unknown | 3 |
| Resection margin status | |
| R0 | 32 (80) |
| R1 | 4 (10) |
| VIN | 4 (10) |
| Lymph-vascular space invasion | |
| Yes | 19 (47.5) |
| No | 21 (52.5) |
| Metastatic lymph nodes | |
| Yes | 23 (57.5) |
| No | 17 (42.5) |
| Extracapsular invasion of metastatic lymph nodes | |
| Yes | 12 (52) |
| No | 11 (48) |
| Median no. of removed lymph nodes | 17 (range, 0–28) |
| Median no. of metastatic lymph nodes | 1 (range, 0–7) |
| Median size of lymph node metastases (mm) | 13 (range, 1–27) |
| FIGO stage | |
| IB | 5 (12.5) |
| II | 12 (30) |
| III | 18 (45) |
| IVA | 5 (12.5) |
| Adjuvant therapy | |
| Yes | 27 (67.5) |
| No | 13 (32.5) |
G1 = well differentiated; G2 = moderately differentiated; G3 = poorly differentiated; R0 = no tumor; R1 = microscopic tumor; VIN = vulvar intraepithelial neoplasia; FIGO = International Federation of Gynecology and Obstetrics.
Data are n followed by percentage in parentheses, unless otherwise specified.
Data Dimensionality Reduction and Radiomic Feature Selection
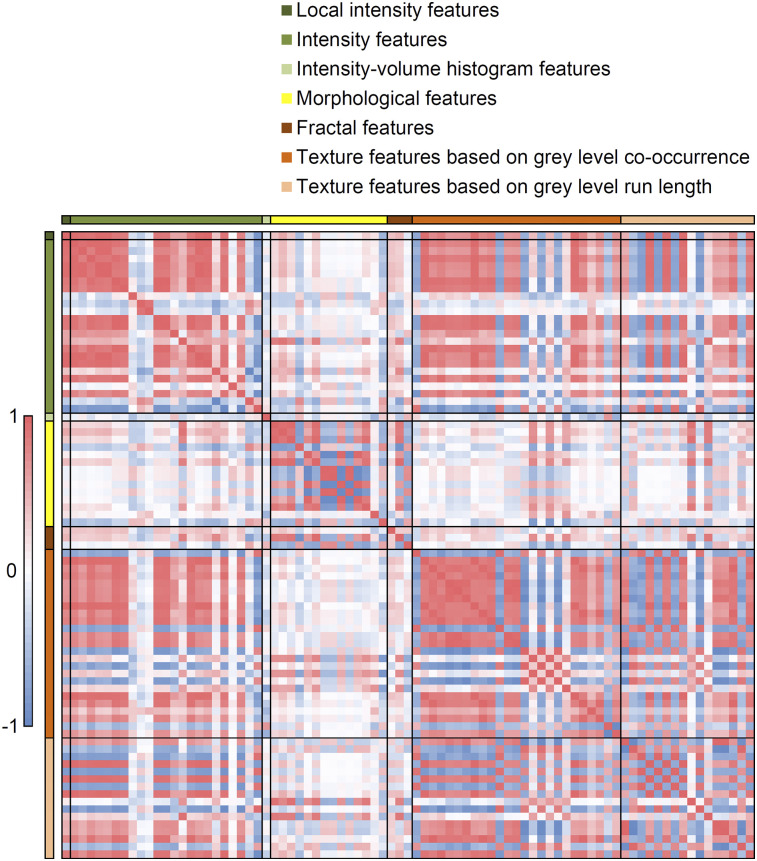
Analysis of the 83 radiomic features revealed 19 prominent groups of strongly correlating features (Fig. 1; Supplemental Table 2), of which one feature per group was selected for PCA. Three of the 19 features (coefficient of variation, mean Laplacian, and kurtosis) did not meet the minimum Kaiser-Meyer-Olkin value of 0.5 and were excluded from the final model. The Kaiser-Meyer-Olkin value of the final model was middling (0.7). Four principal components were found that explained 84% of the variance, corresponding best to 4 radiomic features: SUVmax, local entropy (64 gray-level bins), maximum 3D diameter, and Moran’s I (Supplemental Table 3). Moran’s I did not show the best correlation with the component but showed the least correspondence to other components and was therefore selected instead of the area under the cumulative SUV–volume histogram curve (25).
FIGURE 1.
Correlation map for all radiomic features. Red = high positive correlation; blue = high negative correlation; white = no correlation.
Correlation of Radiomic Features with Histopathology
In 5 of 40 women, urinary contamination or high-uptake areas near the tumors were found and the delineation was adapted as mentioned earlier. No significant association was found between radiomic features and histologic grade (Table 4). Moreover, there was no strong correlation between depth of stromal invasion and radiomic features, as revealed by linear regression. Furthermore, radiomic features of the primary tumor were not predictive for presence of lymph-vascular space invasion or metastatic lymph nodes.
TABLE 4.
Association Between Histopathologic Characteristics and Imaging Features Derived from 18F-FDG PET Images
| Histologic grade |
Lymph-vascular space invasion |
Metastatic lymph nodes |
||||||||
| Radiomic feature | 1–2 | 3 | P | Depth of invasion (mm) |
No | Yes | P | No | Yes | P |
| SUVmax | 12.1 ± 4.2 | 13.9 ± 3.0 | 0.3 | R2 = 0.07 | 11.2 ± 3.2 | 13.7 ± 4.48 | 0.05 | 11.5 ± 4.2 | 13.0 ± 3.9 | 0.2 |
| Local entropy | 3.9 ± 0.11 | 3.9 ± 0.09 | 1 | R2 = 0.10 | 3.9 ± 0.12 | 3.9 ± 0.10 | 0.6 | 3.9 ± 0.10 | 3.9 ± 0.11 | 0.4 |
| Maximum 3D diameter | 48.5 ± 19.4 | 44.6 ± 16.1 | 0.8 | R2 = 0.08 | 47.6 ± 22.0 | 48.3 ± 15.0 | 0.7 | 46.9 ± 20.2 | 48.6 ± 18.0 | 0.9 |
| Moran’s I | 0.062 ± 0.018 | 0.057 ± 0.021 | 0.8 | R2 = 0.013 | 0.059 ± 0.018 | 0.064 ± 0.020 | 0.6 | 0.062 ± 0.017 | 0.061 ± 0.020 | 0.9 |
R2 indicates the coefficient of determination.
Correlation of Clinical and Histopathologic Characteristics with Clinical Outcome
As shown in Table 5, univariate Cox regression analysis revealed that, in this patient cohort, age was not predictive for PFS (P = 0.06) but was significantly associated with OS (P = 0.006). Regarding the histopathologic features from the primary tumor, lymph-vascular space invasion was not significantly associated with PFS (P = 0.08) but was significantly associated with OS (P = 0.03). Furthermore, number of metastatic lymph nodes, bilateral inguinofemoral lymph node metastases, and extracapsular invasion of metastatic lymph nodes were significantly associated with both PFS and OS.
TABLE 5.
Univariate and Multivariate Cox Regression Analysis for PFS and OS
| PFS |
OS |
|||
| Parameter | Hazard ratio | P | Hazard ratio | P |
| Univariate Cox regression analysis | ||||
| Age | 1.1 (1.0–1.1) | 0.06 | 1.1 (1.0–1.1) | 0.006 |
| Maximum diameter of primary tumor | 1.0 (0.8–1.4) | 0.9 | 1.1 (1.0–1.1) | 0.6 |
| Histologic grade | 0.8 | 0.3 | ||
| I–II | 1 | 1 | ||
| III | 0.9 (0.3–3.0) | 0.6 (0.2–1.7) | ||
| Lymph-vascular space invasion | 2.3 (0.9–6.1) | 0.08 | 3.0 (1.1–7.9) | 0.03 |
| Depth of stromal invasion | 1.0 (0.9–1.2) | 0.4 | 1.1 (0.9–1.2) | 0.4 |
| Resection margin status | 0.6 | 0.4 | ||
| R0 | 1 | 1 | ||
| R1 | 2.5 (0.3–18.7) | 3.3 (0.4–25.3) | ||
| VIN | 1.9 (0.2–21.4) | 2.5 (0.2–27.4) | ||
| Metastatic lymph nodes* | 2.1 (0.7–5.9) | 0.2 | 3.0 (1.0–9.2) | 0.05 |
| Bilateral metastatic lymph nodes | 3.0 (1.2–7.6) | 0.02 | 2.7 (1.0–6.8) | 0.04 |
| Number of metastatic lymph nodes* | 1.3 (1.0–1.5) | 0.02 | 1.3 (1.0–1.5) | 0.03 |
| Extracapsular invasion of metastatic lymph nodes | 5.3 (2.1–13.7) | <0.0001 | 4.2 (1.6–10.6) | 0.003 |
| FIGO stage | 0.4 | 0.1 | ||
| IB | 1 | 1 | ||
| II | 0.6 (0.1–3.9) | 0.7 (0.01–5.0) | ||
| III | 0.3 (0.06–1.5) | 0.4 (0.05–2.6) | ||
| IVA | 0.8 (0.2–3.0) | 1.7 (0.4–7.6) | ||
| Radiomic feature | ||||
| SUVmax | 1.0 (0.9–1.2) | 0.6 | 1.0 (0.9–1.1) | 0.6 |
| Local entropy | 1.7 (0.03–99.1) | 0.8 | 0.6 (0–33.2) | 0.8 |
| 3D maximum diameter | 1.0 (1.0–1.0) | 0.5 | 1.0 (1.0–1.0) | 0.8 |
| Moran’s I | 1.3 (1.0–1.6) | 0.06 | 1.3 (1.0–1.7) | 0.03 |
| Multivariate Cox regression analysis | ||||
| Iterative forward selection | ||||
| Extracapsular invasion | 9.5 (3.1–28.6) | <0.0001 | 6.5 (2.3–18.4) | <0.0001 |
| Moran’s I | 1.6 (1.2–2.3) | 0.03 | 1.5 (1.1–2.1) | 0.009 |
| Iterative backward selection | ||||
| Extracapsular invasion | 9.5 (3.1–28.6) | 0.0001 | 4.3 (1.4–12.6) | 0.009 |
| Moran’s I | 1.6 (1.2–2.3) | 0.03 | 1.4 (1.1–2.0) | 0.02 |
Metastatic lymph nodes indicates patients with metastatic lymph nodes; number of metastatic lymph nodes is the number of metastatic lymph nodes per patient.
R0 = no tumor; R1 = microscopic tumor; VIN = vulvar intraepithelial neoplasia; FIGO = International Federation of Gynecology and Obstetrics.
Data in parentheses are 95% confidence intervals.
Correlation of Radiomic Features with Clinical Outcome
None of the 4 radiomic features were statistically significantly associated with PFS. Only Moran’s I correlated with OS (P = 0.03; Table 5). During multivariate Cox regression analysis, extracapsular invasion of the metastatic lymph node and Moran’s I were found to be independent prognostic factors for PFS and OS (Table 5).
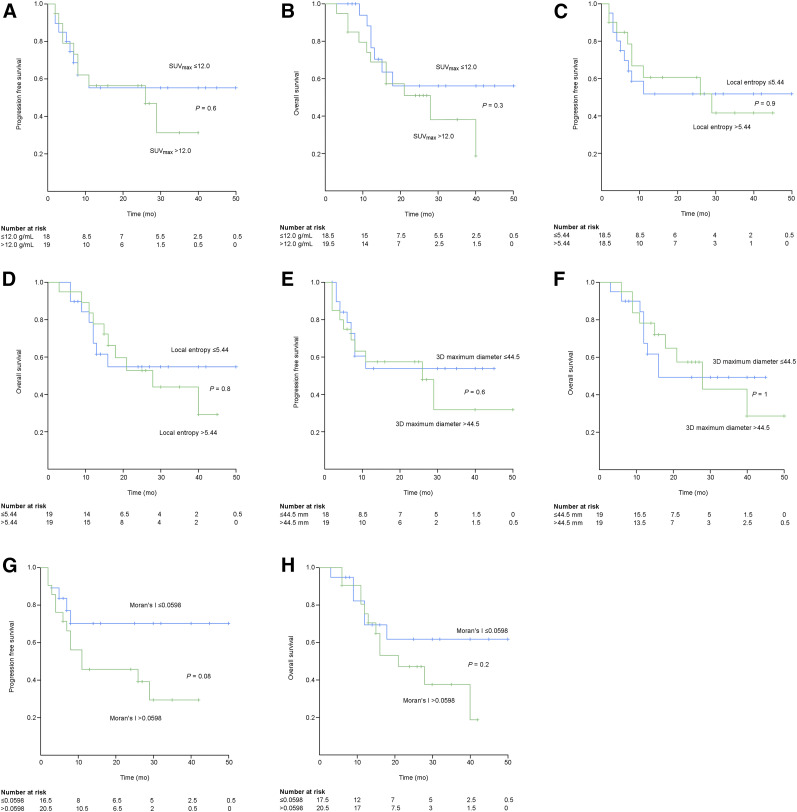
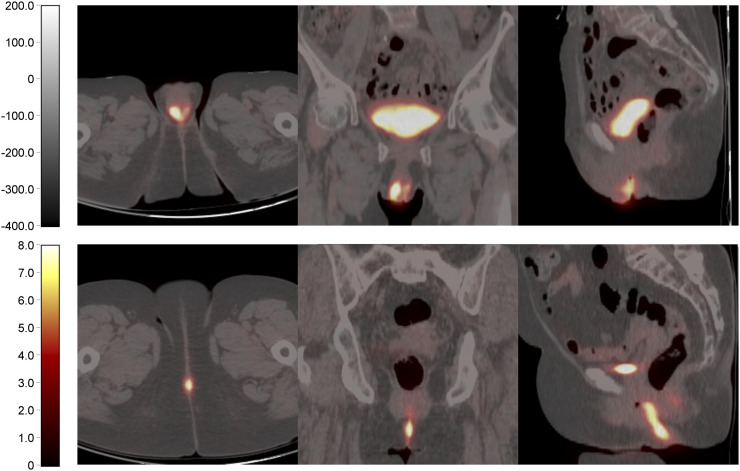
Estimated Kaplan–Meier survival curves revealed that the PFS and OS curves of the 4 radiomic features did not significantly differ (Fig. 2). The PFS and OS curves of the low and high group of SUVmax, local entropy, and maximum 3D diameter showed high similarity and no clear separation (Fig. 2). For Moran’s I, the survival curves showed a clear separation, albeit nonsignificant, between the low and high groups, particularly at later times (Fig. 2). A tendency associating a low value of Moran’s I with a longer PFS and OS was observed. Figure 3 shows 18F-FDG PET/CT images of 2 women with VSCC with either a high or a low Moran’s I value.
FIGURE 2.
Estimated Kaplan–Meier curves for PFS of SUVmax (A), OS of SUVmax (B), PFS of local entropy (C), OS of local entropy (D), PFS of 3D maximum diameter (E), OS of 3D maximum diameter (F), PFS of Moran’s I (G), and OS Moran’s I (H).
FIGURE 3.
From left to right, transverse, coronal, and sagittal 18F-FDG PET/CT images of 2 women with vulvar carcinoma. (Top) An 81-y-old woman who had anterior midline 4-cm-diamter tumor with heterogeneous 18F-FDG uptake and relatively high Moran’s I (0.0924). Inguinal lymph node recurrence and death occurred 4 and 16 mo after surgery, respectively. (Bottom) A 74-y-old woman who had posterior midline 3-cm-diamter tumor with homogeneous 18F-FDG uptake and low Moan I (0.0213). At time of last follow-up, patient was alive and without recurrence.
DISCUSSION
This retrospective study evaluated the clinically added value of quantitative imaging features, also known as radiomic features, derived from preoperative 18F-FDG PET/CT images to predict both tumor biology and patient prognosis in VSCC. Especially, the identification of radiomic features able to predict aggressiveness and poor prognosis before treatment may be of great value for planning a more personalized treatment strategy (e.g., surgery) in women with VSCC.
To the best of our knowledge, no prior studies have evaluated radiomics in vulvar cancer or used PCA to select unique radiomic features from a dataset with a limited number of patients. In radiomics studies, the problem of multiple testing, or the look-elsewhere effect, yields the problem of finding falsely statistically significant results (14,16,24). We addressed this problem by performing dimensionality reduction, leaving only 4 imaging features that explained most of the variance of our dataset.
On the basis of our findings, the identified image features correlated poorly with histopathologic features. A possible explanation may be that we included only women whose primary tumor was at least 26 mm in diameter. In accordance with previous publications, this minimum size was chosen to reliably quantify tracer uptake heterogeneity features (17), but hereby, the considered patient cohort may lack variation. Furthermore, only 6 women were diagnosed with a histologic grade 3 tumor, and the depth of stromal invasion was principally more than 4 mm, limiting the possibility of investigating the correlation between radiomic and these histopathologic features.
In our study, parameters such as age, lymph-vascular space invasion, and lymph node features were prognostic factors for PFS or OS. This finding is concordant with the literature and emphasizes that these parameters are risk factors for the prognosis of patients with vulvar cancer (8). In contrast, none of the selected radiomic features were found to be a prognostic factor for PFS at univariate analysis, although Moran’s I almost reached significance. Nevertheless, Moran’s I found a prognostic factor for OS at univariate analysis and an independent prognostic factor for PFS and OS at multivariate analysis. In detail, Moran’s I is a radiomic feature that reflects the global spatial autocorrelation of the voxels, and its value ranges from −1 to 1, where −1 reflects a perfect dispersion of the voxels, 0 reflects a completely random placement, and 1 reflects a perfect clustering (22). Our results showed that a higher Moran’s I value, reflecting a higher degree of clustering voxels with similar uptake (i.e., an indication of tracer uptake heterogeneity), predicts a high risk of recurrence and poor survival. On the basis of our results, Moran’s I appears to be usable in predicting the prognosis before treatment. Thus, if confirmed by further, larger, studies, Moran’s I could be included in the parameters considered in treatment planning.
Although both the PFS and the OS Kaplan–Meier curves of the low and high group of Moran’s I showed no significant difference, a tendency associating Moran’s I low values with longer PFS and OS was observed. One likely explanation for nonsignificance may be the relatively short median follow-up for some patients. However, only 4 living patients had a follow-up of less than 12 mo.
The relatively small sample size, which reflects the low incidence of vulvar cancer (1,2), may be another reason for the borderline association. Another possible underlying cause could be the middling PCA results, with a Kaiser-Meyer-Olkin value of 0.7. Although this value is higher than the minimal requirement (Kaiser-Meyer-Olkin > 0.5), the value should ideally be higher to make definitive conclusions. A larger number of patients are needed to have a more reliable PCA. Therefore, we recommend that a large multicenter study be designed to validate the results obtained from this study. To facilitate multicenter studies, future studies should focus on further standardization of image acquisition methods, reconstruction settings, segmentation methods (14,19), and radiomic analysis (22).
In this study, the clinically added value of 18F-FDG PET as a single imaging modality was investigated. However, combination of information derived from different imaging modalities, including MRI and CT, may complement the information concerning tumor biology. This possibility was recently emphasized in a study involving patients with locally advanced cervical cancer (26). In that study, combined radiomic analysis of both 18F-FDG PET and diffusion-weighted MRI was able to predict patient outcome after chemoradiotherapy.
There were several limitations to our study. First, it was a retrospective, single-center study. Second, it concerned a relatively small patient cohort with a low incidence of gynecologic malignancy (1,2). Third, a bias concerning population selection was introduced; however, this bias is required for radiomics (17). Finally, our results should be interpreted with caution because of the relatively short median follow-up.
CONCLUSION
The present study showed that PCA may be used to perform dimensionality reduction for radiomics, going beyond commonly used radiomic features in the current clinical workup, such as SUVmax or maximum diameter. Although the identified features poorly predicted the biologic characteristics of the primary tumor, Moran’s I was identified as an independent prognostic predictor for PFS and OS. However, the explorative character of this study demands further validation in larger, prospective multicenter studies.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Emiliano Barbante for his help in the collection of PET data and Marianne Valdés Olmos for language editing.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2013. National Cancer Institute website. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed September 27, 2018.
- 2.Bayne L, Butler J, Colombo N, et al. Gynaecological cancers in Europe: facts and figures 2015. ASACO website. http://www.asociacionasaco.es/wp-content/uploads/2015/10/Facts-datos-y-figures-estadisticas-2015-imprimible.pdf. Published September 2015. Accessed September 27, 2018.
- 3.Eifel PJ, Berek JS, Markman MA. Cancer of the cervix, vagina, and vulva. In: DeVita VT, Jr, Lawrence TS, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2011:1311–1344. [Google Scholar]
- 4.Del Pino M, Rodriguez-Carunchio L, Ordi J. Pathways of vulvar intraepithelial neoplasia and squamous cell carcinoma. Histopathology. 2013;62(suppl 1):161–175. [DOI] [PubMed] [Google Scholar]
- 5.Hacker NF. Vulvar cancer. In: Berek JS, Hacker NF, eds. Practical Gynecologic Oncology. 4th ed. Philadelphia, PA: Williams and Wilkins; 2005:585–602. [Google Scholar]
- 6.Homesley HD, Bundy BN, Sedlis A, et al. Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study). Am J Obstet Gynecol. 1991;164(suppl 4):997–1003. [DOI] [PubMed] [Google Scholar]
- 7.Burger MP, Hollema H, Emanuels AG, Krans M, Pras E, Bouma J. The importance of the groin node status for the survival of T1 and T2 vulval carcinoma patients. Gynecol Oncol. 1995;57(suppl 3):327–334. [DOI] [PubMed] [Google Scholar]
- 8.Koh WJ, Abu-Rustum NR, Bean S, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines), Vulvar cancer (squamous cell carcinoma): version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf. Updated August 30, 2018. Accessed September 27, 2018.
- 9.Levenback CF, Ali S, Coleman RL, et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a gynecologic oncology group study. J Clin Oncol. 2012;30(suppl 31):3786–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van der Zee AG, Oonk MH, De Hullu JA, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26(suppl 6):884–889. [DOI] [PubMed] [Google Scholar]
- 11.Garganese G, Collarino A, Fragomeni SM, et al. Groin sentinel node biopsy and 18F-FDG PET/CT-supported preoperative lymph node assessment in cN0 patients with vulvar cancer currently unfit for minimally invasive inguinal surgery: the GroSNaPET study. Eur J Surg Oncol. 2017;43(suppl 9):1776–1783. [DOI] [PubMed] [Google Scholar]
- 12.Oonk MHM, Planchamp F, Baldwin P, et al. European Society of Gynaecological Oncology guidelines for the management of patients with vulvar cancer. Int J Gynecol Cancer. 2017;27(suppl 4):832–837. [DOI] [PubMed] [Google Scholar]
- 13.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(suppl 4):441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatt M, Tixier F, Pierce L, Kinahan PE, Le Rest CC, Visvikis D. Characterization of PET/CT images using texture analysis: the past, the present… any future? Eur J Nucl Med Mol Imaging. 2017;44(suppl 1):151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsujikawa T, Rahman T, Yamamoto M, et al. 18F-FDG PET radiomics approaches: comparing and clustering features in cervical cancer. Ann Nucl Med. 2017;31(suppl 9):678–685. [DOI] [PubMed] [Google Scholar]
- 16.Limkin EJ, Sun R, Dercle L, et al. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol. 2017;28(suppl 6):1191–1206. [DOI] [PubMed] [Google Scholar]
- 17.Hatt M, Majdoub M, Vallières M, et al. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med. 2015;56(suppl 1):38–44. [DOI] [PubMed] [Google Scholar]
- 18.Collarino A, Garganese G, Valdés Olmos RA, et al. Evaluation of dual-timepoint 18F-FDG PET/CT imaging for lymph node staging in vulvar cancer. J Nucl Med. 2017;58(suppl 12):1913–1918. [DOI] [PubMed] [Google Scholar]
- 19.Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(suppl 2):328–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Velden FH, Kramer GM, Frings V, et al. Repeatability of radiomic features in non-small-cell lung cancer [18F]FDG-PET/CT studies: impact of reconstruction and delineation. Mol Imaging Biol. 2016;18(suppl 5):788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tixier F, Hatt M, Le Rest CC, Le Pogam A, Corcos L, Visvikis D. Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18F-FDG PET. J Nucl Med. 2012;53(suppl 5):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwanenburg A, Leger S, Vallières M, Löck S. Image biomarker standardisation initiative. https://arxiv.org/abs/1612.07003. arXiv.org website. Updated September 17, 2018. Accessed September 27, 2018.
- 23.Frings V, van Velden FH, Velasquez LM, et al. Repeatability of metabolically active tumor volume measurements with FDG PET/CT in advanced gastrointestinal malignancies: a multicenter study. Radiology. 2014;273(suppl 2):539–548. [DOI] [PubMed] [Google Scholar]
- 24.Chalkidou A, O’Doherty MJ, Marsden PK. False discovery rates in PET and CT studies with texture features: a systematic review. PLoS One. 2015;10(suppl 5):e0124165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Velden FH, Cheebsumon P, Yaqub M, et al. Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoural FDG uptake in non-small cell lung cancer PET studies. Eur J Nucl Med Mol Imaging. 2011;38(suppl 9):1636–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucia F, Visvikis D, Desseroit MC, et al. Prediction of outcome using pretreatment 18F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2018;45(suppl 5):768–786. [DOI] [PubMed] [Google Scholar]