Abstract
Background
The risk of coccidioidomycosis (CM) as a life-threatening respiratory illness or disseminated CM (DCM) increases as much as 150-fold in immunosuppressed patients. The safety of biologic response modifiers (BRMs) as treatment for patients with autoimmune disease (AI) in CM-endemic regions is not well defined. We sought to determine that risk in the Tucson and Phoenix areas.
Methods
We conducted a retrospective study reviewing demographics, Arizona residency length, clinical presentations, specific AI diagnoses, CM test results, and BRM treatments in electronic medical records of patients ≥18 years old with International Classification of Diseases (ICD-10) codes for CM and AI from 1 October 2017 to 31 December 2019.
Results
We reviewed 944 charts with overlapping ICD-10 codes for CM and AI, of which 138 were confirmed to have both diagnoses. Male sex was associated with more CM (P = .003), and patients with African ancestry were 3 times more likely than those with European ancestry to develop DCM (P < .001). Comparing CM+/AI+ (n = 138) with CM+/AI– (n = 449) patients, there were no significant differences in CM clinical presentations. Patients receiving BRMs had 2.4 times more DCM compared to pulmonary CM (PCM).
Conclusions
AI does not increase the risk of any specific CM clinical presentation, and BRM treatment of most AI patients does not lead to severe CM. However, BRMs significantly increase the risk of DCM, and prospective studies are needed to identify the immunogenetic subset that permits BRM-associated DCM.
Keywords: autoimmune disease, biologic response modifiers, coccidioidomycosis, tumor necrosis factor inhibitors, Valley fever
Coccidioidomycosis (CM) is a fungal infection endemic to the southwestern United States [1] and elsewhere in the Western Hemisphere [2]. Although a small percentage of infections in otherwise healthy persons result in life-threatening respiratory failure [3] or disseminated CM (DCM) [4], the risk increases as much as 150-fold in immunosuppressed patients [5].
Those receiving biological response modifiers (BRMs) are an emerging at-risk group for some infections [6–8]. BRMs are a diverse and expanding number of medications used for autoimmune diseases (AIs). Earliest and still dominant among BRMs are tumor necrosis factor–α inhibitors (TNFIs). Immunosuppression, including BRM treatment, might either worsen the consequences of newly acquired coccidioidal infection or allow a prior, latent coccidioidal infection to reactivate [9]. Since most coccidioidal infections occur within endemic regions, both are possible, and it is not known whether new or reactivated infections are the larger problem for immunosuppressed patients. Previous studies and clinical observations suggest that some, perhaps many, but not all patients with CM can be safely treated with BRMs [6]. Currently, there are no guidelines to manage this risk. As a result, the choices for clinicians are either to never use BRMs if CM is a risk, thereby forgoing very effective therapies for AI, or to routinely use BRMs and incur potentially life-threatening consequences for some patients.
Recommendations for managing BRM risk are incomplete. The most recent (2016) revised Infectious Diseases Society of America guidelines on CM are silent regarding how to avoid serious complications while using TNFIs and other BRMs [10]. A more recent article offers helpful proposed guidelines for managing CM in patients receiving TNFIs, while acknowledging that the risk for CM with the use of other BRMs besides TNFIs is not known [11]. The authors raise questions about CM incidence, timing concerning TNFI use, subsequent risk, and utility of CM screening. To further explore CM risk in AI patients on BRMs, we conducted a retrospective cohort study in the Tucson and Phoenix areas to determine the risk posed by both AI itself and these medications in patients with AI.
METHODS
Study Population
We conducted a retrospective chart review from 1 October 2017 through 31 December 2019 for patient records in Banner University Medical Group, Banner Medical Group, and Banner Urgent Care System in the Tucson and Phoenix areas. During the study period, these portions of Banner Health used in common a Cerner electronic medical record (EMR) system [12]. During this period there were 839 362 encounters recorded in the EMR. Using International Classification of Diseases (ICD-10) codes for CM and AI (Supplementary Table 1), 944 records were found to have at least 1 CM and 1 AI ICD-10 code and formed the basis of our cohort. These were distributed approximately equally for review among our research team, comprised of 2 physicians and 5 medical students.
During the 6 months for data collection, the research team met monthly to maintain data extraction quality and standardization. Data extracted from the patients’ EMRs included (1) demographics (age, sex, race, ethnicity, and length of endemic residence); (2) types of AI and therapeutic measures to treat the AI such as BRMs, steroids, or disease-modifying antirheumatic drugs (DMARDs); and (3) CM presentations including various CM clinical signs, symptoms, imaging, and laboratory studies. The extracted data from Cerner was stored using Research Electronic Data Capture (REDCap) tools hosted at the University of Arizona [13, 14].
Records were excluded from analysis if (1) patients were <18 years old at the time of CM diagnosis, (2) the CM ICD-10 codes were entered in error, (3) the CM diagnoses were not laboratory confirmed [10], or (4) the CM tests were negative. Serologic confirmation was a positive coccidioidal enzyme-linked immunoassay (EIA) immunoglobulin M (IgM) or immunoglobulin (IgG), a positive immunodiffusion IgM or IgG, or complement fixing (CF) antibody titer ≥1:2 [15]. Indeterminant EIA results were interpreted as negative. Cultures of clinical specimens yielding Coccidioides species or tissue demonstrating spherules were also CM diagnostic. AI ICD-10 codes were also determined to be accurate if the confirmatory data (eg, AI serology results or pathology results were identified in the chart review), as discussed in the results. CM+ patients without a confirmed AI served as a comparison group to those CM+ patients with AI.
CM clinical presentations were categorized by type: (1) pulmonary CM (PCM); (2) chronic cavitary CM (CAV); (3) asymptomatic patients with positive CM serology (tested as a screen or evaluation of a pulmonary nodule) (ASYM); and (4) DCM. Incomplete or unknown results were further investigated and clarified by the lead investigator (F. M. D.) via additional EMR review.
The University of Arizona institutional review board approved this study.
Statistical Analysis
Categorical variables are summarized using counts and percentages. Groups were compared using Fisher exact test [16]. Continuous measures were assessed using the nonparametric 2-sided Mann-Whitney U test for independent samples [17]. Statistical analyses were conducted using R version 3.6.0 [18]. P values < .05 were considered statistically significant (hereafter “significant”) with no correction for multiple comparisons.
RESULTS
Demographic and Clinical Characteristics
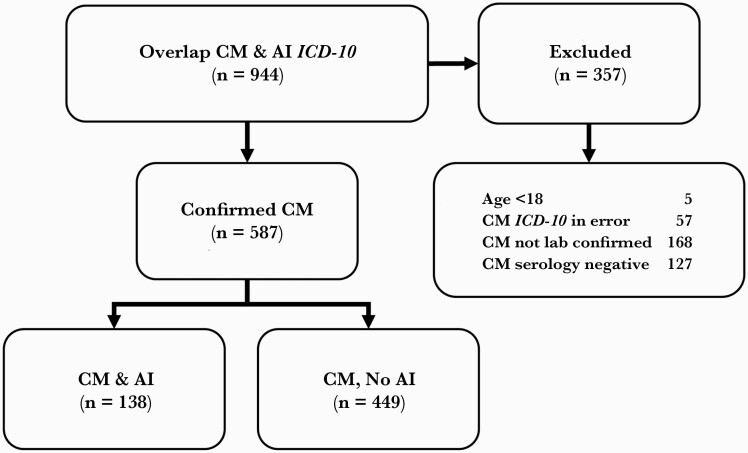
In our retrospective study, a total of 9960 EMRs with ICD-10 codes for AI and 1363 with codes for CM were identified (Supplementary Table 1). Of these, 944 had ICD-10 codes for both CM and AI (Figure 1). Three hundred fifty-seven EMRs were excluded because either the patients were <18 years of age at the time of CM diagnosis, CM ICD-10 codes were entered in error, the CM diagnoses had no confirmatory laboratory tests done, or CM tests that were done were negative. As a result, we identified 587 CM-positive (CM+) patients, of which 138 had AI (AI+) and 449 patients had no AI (AI–). Because of our methods for selecting EMRs, those patients with CM but without AI constitute a comparable group to assess the influence AI has on the CM manifestations.
Figure 1.
Data extraction algorithm. Abbreviations: CM, coccidioidomycosis; AI, autoimmune disease; ICD-10, International Classification of Diseases, Tenth Revision.
Table 1 demonstrates patient demographics in the various CM clinical presentations. CM was diagnosed more frequently in male patients (56.6%) than in females (P = .003). There is a significant difference between CM presentations in all races, but the primary difference is noted for African ancestry with DCM (32.4%) compared to the PCM group (9.8%) (P < .001). Median length of Arizona residence prior to being diagnosed with CM was 16 years (data not shown) and was similar in all CM clinical presentations (Table 1).
Table 1.
Patient Demographics by Coccidioidomycosis Clinical Presentation
| Characteristic | PCM (n = 305) | CAV (n = 117) | ASYM (n = 94) | DCM (n = 71) | Totala (N = 587) | P Value∗ |
|---|---|---|---|---|---|---|
| Age, y, median (range) | 54 (18–87) | 52 (18–88) | 58 (18–85) | 42 (18–88) | 53 (18–88) | .324 |
| Sex | .003∗∗ | |||||
| Male | 165 (54.1) | 66 (56.4) | 47 (50) | 54 (76.1) | 332 (56.6) | |
| Female | 140 (45.9) | 51 (43.6) | 47 (50) | 17 (23.9) | 255 (43.4) | |
| Race | <.001∗∗ | |||||
| African American | 30 (9.8) | 10 (8.5) | 10 (10.6) | 23 (32.4) | 73 (12.4) | |
| AI/AN | 21 (6.9) | 13 (11.1) | 6 (6.4) | 4 (5.6) | 44 (7.5) | |
| Asian | 11 (3.6) | 5 (4.3) | 4 (4.3) | 4 (5.6) | 24 (4.1) | |
| White | 216 (70.8) | 86 (73.5) | 66 (70.2) | 36 (50.7) | 404 (68.8) | |
| Unspecifiedb | 27 (8.9) | 3 (2.6) | 8 (8.5) | 4 (5.6) | 42 (7.2) | |
| Ethnicity | .411 | |||||
| Hispanic | 65 (21.7) | 25 (21.7) | 26 (28.9) | 13 (18.3) | 129 (22.4) | |
| Non-Hispanic | 234 (78.3) | 90 (78.3) | 64 (71.1) | 58 (81.7) | 446 (77.6) | |
| Length of residencec | .318 | |||||
| <1 y | 28 (10.5) | 5 (4.9) | 7 (10.3) | 6 (7.8) | 46 (8.9) | |
| 1–10 y | 100 (37.5) | 31 (30.1) | 26 (38.2) | 26 (33.8) | 183 (35.5) | |
| 11–20 y | 29 (10.9) | 14 (13.6) | 5 (7.4) | 14 (18.2) | 62 (12) | |
| >20 y | 110 (41.2) | 53 (51.5) | 30 (44.1) | 31 (40.3) | 224 (43.5) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AI/AN, American Indian/Alaska Native; ASYM, asymptomatic; CAV, chronic cavitary coccidioidomycosis; DCM, disseminated coccidioidomycosis; PCM, pulmonary coccidioidomycosis.
Total of 944 patient electronic medical records (EMRs) reviewed; variables may not sum to total or presentations due to unavailable demographic data.
Race was not identifiable or available in EMR review.
Length of residence was evaluated as a continuous measure.
Fisher exact test was used to evaluate categorical groups; Wilcoxon rank-sum test was used to evaluate continuous measures.
Statistically significant difference between coccidioidomycosis clinical presentations.
Table 2 compares demographics and clinical presentations of the 587 CM+ patients with or without AI. Patients with CM and AI were older (56.5 years, P < .001) and more frequently female (56.5%, P < .001). Ethnicity or race was equally distributed among both groups. Overall, duration of Arizona residence was shorter (median, 7 years) in patients with both CM and AI compared to CM patients without AI (20 years) (P < .005). CM clinical presentations were similarly distributed among both groups. Of the 19 patients with DCM, 5 had central nervous system disease, 4 had cutaneous lesions, 4 had musculoskeletal disease, and 6 had soft tissue involvement. Supplementary Table 2 shows details on DCM patients including age, sex, race, and CM dissemination site, as well as immunomodulator use.
Table 2.
Patient Demographics and Coccidioidomycosis Presentation by Autoimmune Status
| Characteristic | CM+/ AI+ (n = 138) | CM+/AI– (n = 449) | Totala (N = 587) | P Value∗ |
|---|---|---|---|---|
| Age, y, median (range) | 56.5 (19–88) | 52 (18–88) | 53 (18–88) | <.001∗∗ |
| Sex | <.001∗∗ | |||
| Male | 60 (43.5) | 272 (60.6) | 332 (56.6) | |
| Female | 78 (56.5) | 177 (39.4) | 255 (43.4) | |
| Race | .9379 | |||
| African American | 15 (10.9) | 58 (12.9) | 73 (12.4) | |
| AI/AN | 10 (7.2) | 34 (7.6) | 44 (7.5) | |
| Asian | 4 (2.9) | 20 (4.5) | 24 (4.1) | |
| White | 94 (68.1) | 310 (69) | 404 (68.8) | |
| Unspecifiedb | 15 (10.9) | 27 (6) | 42 (7.2) | |
| Ethnicity | .6906 | |||
| Hispanic | 27 (20.8) | 102 (22.9) | 129 (22.4) | |
| Non-Hispanic | 103 (79.2) | 343 (77.1) | 446 (77.6) | |
| Length of residencec | .0047 | |||
| <1 y | 9 (6.4) | 37 (9.9) | 46 (8.9) | |
| <1–10 y | 67 (47.5) | 116 (31) | 183 (35.5) | |
| 11–20 y | 17 (12.1) | 45 (12) | 62 (12) | |
| >20 y | 48 (34) | 176 (47.1) | 224 (43.5) | |
| Clinical presentation | .5607 | |||
| PCM | 75 (54.3) | 230 (51.2) | 305 (52) | |
| CAV | 22 (15.9) | 95 (21.2) | 117 (19.9) | |
| ASYM | 22 (15.9) | 72 (16) | 94 (16) | |
| DCM | 19 (13.8) | 52 (11.6) | 71 (12.1) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AI–, without autoimmune disease; AI+, with autoimmune disease; AI/AN, American Indian/Alaska Native; ASYM, asymptomatic; CAV, chronic cavitary coccidioidomycosis; CM+, coccidioidomycosis-positive; DCM, disseminated coccidioidomycosis; PCM, pulmonary coccidioidomycosis.
Total of 944 patient electronic medical records (EMRs) reviewed; variables may not sum to total or presentations due to unavailable demographic data.
Race was not identifiable or available in EMR review.
Length of residence was evaluated as a continuous measure.
Fisher exact test was used to evaluate categorical groups; Wilcoxon rank-sum test was used to evaluate continuous measures.
Statistically significant difference between CM+/AI+ and CM+/AI– groups.
Comparison of CM clinical presentations with any prescribed immunomodulator in AI patients are shown in Table 3. The most common rheumatological diseases observed were rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and psoriatic arthritis. Among the gastrointestinal disorders, Crohn disease and ulcerative colitis were the most common presentations. Psoriasis was the most commonly observed condition identified in dermatological disease (data not shown). Among patients with PCM, 22.7% were on BRMs, whereas 47.4% of DCM patients had received BRMs (P = .06) at the time of CM diagnosis. Only 7 of 138 (5.1%) with CM were on TNFIs, and of these, all but 1 had PCM. Although not significant, a substantial number of DCM patients were on BRMs, steroids, or both (12 of 19 [63.2%]). The comparison of steroid and DMARD use among different CM clinical presentations showed no significant differences. Of the 19 patients with DCM, 9 (47%) were on BRMs and 5 (26%) were on DMARDs (P = .53).
Table 3.
Coccidioidomycosis Presentations With Any Prescribed Immunomodulator in Autoimmune Patients
| Immunomodulator | PCM (n = 75) | CAV (n = 22) | ASYM (n = 22) | DCM (n = 19) | Total (n = 138) | P Value∗ | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | ||
| BRM | .06 | ||||||||||
| Yes | 17 | (22.7) | 3 | (13.6) | 3 | (13.6) | 9 | (47.4) | 32 | (23.2) | |
| No | 58 | (77.3) | 19 | (81.8) | 19 | (81.8) | 10 | (52.6) | 106 | (75.4) | |
| TNFI | .48 | ||||||||||
| Yes | 6 | (8.0) | 0 | (0.0) | 1 | (4.5) | 0 | (0.0) | 7 | (5.1) | |
| No | 69 | (92.0) | 22 | (100.0) | 21 | (95.5) | 19 | (100.0) | 131 | (94.9) | |
| Steroid | .27 | ||||||||||
| Yes | 24 | (32.0) | 4 | (18.2) | 9 | (40.9) | 8 | (42.1) | 45 | (32.6) | |
| No | 51 | (68.0) | 18 | (81.8) | 13 | (54.5) | 11 | (57.9) | 93 | (66.7) | |
| Steroid + BRMa | .15 | ||||||||||
| Yes | 34 | (45.3) | 6 | (27.3) | 11 | (50.0) | 12 | (63.2) | 63 | (45.7) | |
| No | 41 | (54.7) | 16 | (72.7) | 11 | (50.0) | 7 | (36.8) | 75 | (54.3) | |
| DMARD | .96 | ||||||||||
| Yes | 16 | (21.3) | 5 | (22.7) | 4 | (18.2) | 5 | (26.3) | 30 | (21.7) | |
| No | 59 | (77.3) | 17 | (77.3) | 18 | (72.7) | 14 | (73.7) | 108 | (76.1) | |
Abbreviations: ASYM, asymptomatic; BRM, biologic response modifier; CAV, chronic cavitary coccidioidomycosis; DCM, disseminated coccidioidomycosis; DMARD, disease-modifying antirheumatic drug; PCM, pulmonary coccidioidomycosis; TNFI, tumor necrosis factor–α inhibitor.
Either steroid or biologic response modifier identified in the electronic medical record, while those not counted had neither therapy.
Fisher exact test was used to evaluate categorical groups.
The number of immunomodulators prescribed before or after a CM diagnosis is illustrated in Supplementary Figure 1. Notably, 24 of 25 AI patients received BRMs prior to their CM diagnosis and only 1 patient had a BRM started post–CM diagnosis. In contrast, 14 patients were started on steroids and 7 were started on DMARDs post–CM diagnosis (Supplementary Figure 1). Overall, these results may reflect practitioners’ comfort starting steroids or DMARDs after a CM diagnosis compared to starting BRMs. The steroid dosing and treatment duration were not included in this analysis.
Comparison of CM+/AI+ patients with or without any immunomodulators (n = 69 in each group) did not show any significant differences in all clinical presentations (data not shown). When CM+/AI+ patients on BRMs were compared to those without BRM treatment, our data indicated no significant differences in all clinical presentations (P = .063). Nonetheless, when we compared the clinically symptomatic subgroups of PCM and DCM, we found that 34.6% of patients with AI on BRM treatment developed DCM compared to only 14.7% without BRM treatment (P = .045; Table 4).
Table 4.
Comparison of Biologic Response Modifier Used in Pulmonary and Disseminated Coccidioidomycosis Among Patients With Autoimmune Disease
| Clinical Presentation | BRM | No BRM | P Value∗ | ||
|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||
| PCM | 17 | (65.4) | 58 | (85.3) | .045∗∗ |
| DCM | 9 | (34.6) | 10 | (14.7) | |
| Totala | 26 | (100) | 68 | (100) | |
Abbreviations: BRM, biologic response modifier; DCM, disseminated coccidioidomycosis; PCM, pulmonary coccidioidomycosis..
Total of 94 patients identified with both PCM/DCM presentation and autoimmune syndrome.
Fisher exact test was used to evaluate categorical groups.
Statistically significant difference between coccidioidomycosis-positive groups.
Supplementary Table 3 demonstrates that for all immunomodulators (BRMs, TNFIs, steroids, steroids plus BRMs, and DMARDs) a greater percentage of patients with a CF titer <1:2 either remained on or were started an immunomodulator compared to patients with a CF titer >1:2. It should be noted that the difference was only significant for the steroid and the steroid plus BRM categories (P = .01). We found that steroids and methotrexate were the most commonly prescribed immunomodulators for patients who eventually developed PCM. TNFIs (adalimumab, infliximab, and etanercept) were the most commonly prescribed BRMs. Their use led to more PCM than CAV, ASYM, or DCM. Besides steroids, infliximab, rituximab, and hydroxychloroquine were the most common immunomodulators prescribed for AI patients prior to their DCM diagnosis (Supplementary Table 4).
DISCUSSION
Our study has uncovered several important findings. First, it should be noted that, despite including a broader range of AI patients than many previous studies, our demographic data are concordant with earlier research. The median age at the time of CM diagnosis, 53 years (Table 1), matches Arizona Department of Health statistics [19]. As noted in Table 2, CM was diagnosed at a younger age in patients without AI than those with AI. This could be secondary to patients without AI being more engaged in outdoor activities and occupations, leading to increased CM risk at a younger age. Also, it may be due to AI patients being older and more CM susceptible. Another explanation is the overlap of CM and AI presentations resulting in CM symptoms being attributed to AI aggravation and thus a delayed CM diagnosis. Our results support previous studies that male patients are more frequently diagnosed with CM, both in general and in some immunocompromised hosts [5, 20, 21]. Male sex preponderance in CM+ patients has been reported and thought to be partially explained by the greater participation of men in high-exposure activities, or certain sex hormones that could stimulate the growth of Coccidioides in vitro [20, 22]. Although males are more frequently diagnosed with CM overall, women comprise the majority of CM patients with AI (Table 2). This difference may reflect that 80% of AI patients are female [23]. The preponderance of women with AI is proposed to be related to variation within the sex chromosomes and hormonal changes [22].
We found a higher proportion of African Americans (12.4%) with CM+ status (Table 1) than that in the 2020 Arizona census (5.3%) (P < .001) [24]. Similarly, comparing CM clinical presentations in African American patients indicates higher risk of DCM (32.4%) than PCM (9.8%) in this population (P < .001) (Table 1). These results support previous studies and indicate a continued increased CM/DCM risk among people of African descent [25–27]. Our findings reinforce ongoing research to uncover genetic variants among these patients to explain the increased CM risk [4].
Since contracting CM is clearly a risk of residing within a Coccidioides-endemic region, we included duration of Arizona residency as a parameter in our evaluation. A prior Arizona-wide report found the median length of residence to be 12 years [19]; this is comparable to our finding that showed median length of residence of 16 years. In our study, CM+ patients with AI showed a shorter duration of endemic residence as compared to CM+ patients without AI (P < .005; Table 2). Multiple explanations could account for this difference including testing frequency, comorbidities, and AI treatments in this population. If much of the diagnosed CM in AI+ patients was due to reactivation, one might expect these patients would have longer, not shorter, endemic exposure. If, in future studies, CM in AI+ patients is confirmed to be shorter, this may be evidence that much of active CM is the result of newly acquired rather than reactivated infection. This question could be addressed by further investigations utilizing available skin testing and/or cytokine profiles [28, 29]. Spherusol, the only currently available and approved skin test for CM immunity, is limited by availability (many clinicians do not offer this test to their patients), subject inconvenience/discomfort, injection technique, time of reading, difficulty in measuring induration size, and Food and Drug Administration approval only for patients known to have had CM by other means [30, 31].
A second important finding is the result of our focus on prescribing trends in this study. By including multiple types of AI and a variety of BRM medications we found some interesting results. As noted in Supplementary Figure 1, just 1 of 25 AI patients was started on a BRM post–CM diagnosis. These results, coupled with acknowledgment that an elevated CF titer is generally considered a marker of increased CM severity [32], suggest that clinicians are more comfortable maintaining or starting immunomodulator treatments if the coccidioidal CF titers are low. This is especially true for patients treated with steroids or steroids plus BRMs (Supplementary Table 3). The patterns likely reflect their reluctance to start or continue BRMs on CM+ patients without clear, evidence-based clinical guidelines on how to do so safely.
A third and the key aspect of our study involves the evaluation of CM risk in AI patients. It might be anticipated having an AI would predispose an individual to symptomatic CM. To the contrary, our results indicate that AI presence does not increase the risk of any specific CM presentation (Table 2). As demonstrated in Table 3, for 23 of 32 (72%) of AI patients receiving a BRM who developed CM, their infection did not progress to DCM. Most importantly, 9 of the 32 developed DCM, which represents a 2.4-fold greater risk of DCM than for AI patients not on a BRM (Table 4). These results are consistent with the findings of Blair et al [9]. In addition, a 2021 retrospective study reviewed the CM clinical course for 49 patients on TNFIs from 2010 through 2017. The authors concluded that CM was a rare consequence of TNFI treatment but noted that 14% of patients developed extrapulmonary infections (DCM) [33]. Our results indicate that BRM therapies pose a significant risk for some but not all AI patients. It raises the important question about why there are differences in susceptibilities among patients. This question has been initially addressed by Odio et al, who have reported several genetic variants in a young and diverse group of patients with DCM. Our study supports their suggestion that “younger patients with severe DC [DCM] or patients whose illness relapses should be considered for genetic screening for discrete primary immune defects” [4]. Hsu et al in a recent study demonstrated the involvement of gene variants in impaired CM fungal recognition and host cellular responses to be predisposing factors for disease susceptibility [34]. Our results reinforce the need for prospective studies to clarify the DCM risk for AI patients starting BRMs. These results reinforce the need for methods to improve CM awareness and education for AI patients and their providers who consider BRM treatment in endemic regions. Careful CM screening and close clinical monitoring of this population would be an important aspect in caring for AI patients at risk to develop CM.
Our study has strengths and limitations. The overall number of charts reviewed represent a broad geographic and demographic range of a CM-endemic region. Data collection involved a large number of parameters. The AIs were not limited to 1 discipline and included a wide range of diseases. The final results demonstrate the ability to obtain an adequate number of subjects to power a prospective study of DCM risk with BRM use. A study limitation includes its retrospective nature. Nonetheless, it has provided important findings that should prompt a prospective study to identify DCM risk in AI patients on BRMs. Another limitation is the use of EMR for clinical research data extraction. We speculate that many of the initial 9960 patients with AI-type presentations should have been tested for CM in our endemic region [35]. This would be particularly true when a clinician utilized an AI diagnostic code for a clinical presentation that mimicked CM without ordering CM serologies.
In conclusion, while expanding both the number of AIs examined and considering an ever-increasing number of BRMs, our findings generally support more recent studies’ conclusions that BRM use increases DCM risk [9, 36]. It does not, however, completely answer the clinical question of the extent to which BRM use increases DCM risk in endemic areas. Conceding that the subset of AI patients on BRMs who develop DCM would be small, for these individuals the consequences of infection can be serious or even life- threatening. Our findings support the need for a prospective study and demonstrate the potential to obtain the likely-needed subject sample size in a CM-endemic region such as the Tucson and Phoenix areas.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1. Number of immunomodulators prescribed before or after CM diagnosis. Abbreviation: CM, coccidioidomycosis.
Notes
Acknowledgments. We express our appreciation to Donald Saner and Mario Arteaga of the Banner Electronic Data Warehouse for their support in obtaining the electronic medical record data required for this study.
Patient consent. This study did not include factors necessitating patient consent.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors submitted the ICMJE form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol 2019; 57(Suppl 1):S30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laniado-Laborin R, Arathoon EG, Canteros C, Muniz-Salazar R, Rendon A.. coccidioidomycosis in Latin America. Med Mycol 2019; 57(Suppl 1):S46–55. [DOI] [PubMed] [Google Scholar]
- 3. Arsura EL, Bellinghausen PL, Kilgore WB, Abraham JJ, Johnson RH.. Septic shock in coccidioidomycosis. Crit Care Med 1998; 26:62–5. [DOI] [PubMed] [Google Scholar]
- 4. Odio CD, Marciano BE, Galgiani JN, Holland SM.. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis 2017; 23:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen IM, Galgiani JN, Potter D, Ogden DA.. Coccidioidomycosis in renal replacement therapy. Arch Intern Med 1982; 142:489–94. [PubMed] [Google Scholar]
- 6. Taroumian S, Knowles SL, Lisse JR, et al. Management of coccidioidomycosis in patients receiving biologic response modifiers or disease-modifying antirheumatic drugs. Arthritis Care Res (Hoboken) 2012; 64:1903–9. [DOI] [PubMed] [Google Scholar]
- 7. Dobler CC. Biologic agents and tuberculosis. Microbiol Spectr 2016; 4. doi: 10.1128/microbiolspec.TNMI7-0026-2016. [DOI] [PubMed] [Google Scholar]
- 8. Ogawa E, Wei MT, Nguyen MH.. Hepatitis B virus reactivation potentiated by biologics. Infect Dis Clin North Am 2020; 34:341–58. [DOI] [PubMed] [Google Scholar]
- 9. Blair JE, Ampel NM, Hoover SE.. Coccidioidomycosis in selected immunosuppressed hosts. Med Mycol 2019; 57(Suppl 1):S56–63. [DOI] [PubMed] [Google Scholar]
- 10. Galgiani JN, Ampel NM, Blair JE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis 2016; 63:e112–46.. [DOI] [PubMed] [Google Scholar]
- 11. Blair J, Wack E, Mertz L, Galgiani J.. Approach to management of coccidioidomycosis in patients receiving inhibitors of tumor necrosis factor-α. Infect Dis Clin Pract 2016; 25:1. [Google Scholar]
- 12. Cerner. Cerner Corporation: International Directory of Company Histories, vol 16. USA: St. James Press; 1997. www.cerner.com. [Google Scholar]
- 13. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomedical Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pappagianis D, Zimmer BL.. Serology of coccidioidomycosis. Clin Microbiol Rev 1990; 3:247–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim H-Y. Statistical notes for clinical researchers: chi-squared test and Fisher’s exact test. Restor Dent Endod 2017; 42:152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mann HB, Whitney DR.. On a test of whether one of 2 random variables is stochastically larger than the other. Ann Math Stat 1947; 18:50–60. [Google Scholar]
- 18. Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 19. Tsang CA, Anderson SM, Imholte SB, et al. Enhanced surveillance of coccidioidomycosis, Arizona, USA, 2007-2008. Emerg Infect Dis 2010; 16:1738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown J, Benedict K, Park BJ, Thompson GR 3rd. Coccidioidomycosis: epidemiology. Clin Epidemiol 2013; 5:185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. Increase in coccidioidomycosis—California, 2000-2007. MMWR Morb Mortal Wkly Rep 2009; 58:105–9. [PubMed] [Google Scholar]
- 22. Drutz DJ, Huppert M, Sun SH, McGuire WL.. Human sex hormones stimulate the growth and maturation of Coccidioides immitis. Infect Immun 1981; 32:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Angum F, Khan T, Kaler J, Siddiqui L, Hussain A.. The prevalence of autoimmune disorders in women: a narrative review. Cureus 2020; 12:e8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. United States Census Bureau. QuickFacts Arizona. Suitland, MD: US Census Bureau;2020. [Google Scholar]
- 25. Rosenstein NE, Emery KW, Werner SB, et al. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995–1996. Clin Infect Dis 2001; 32:708–15. [DOI] [PubMed] [Google Scholar]
- 26. Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc 2011; 86:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foley CGTCA, Christ C, Anderson SM.. Impact of disseminated coccidioidomycosis in Arizona, 2007-2008. In: Proceedings of the 55th Annual Coccidioidomycosis Study Group, University of California, Davis, 2 April 2011. [Google Scholar]
- 28. Ampel NM, Bejarano GC, Salas SD, Galgiani JN.. In vitro assessment of cellular immunity in human coccidioidomycosis: relationship between dermal hypersensitivity, lymphocyte transformation, and lymphokine production by peripheral blood mononuclear cells from healthy adults. J Infect Dis 1992; 165:710–5. [DOI] [PubMed] [Google Scholar]
- 29. Wack EE, Ampel NM, Sunenshine RH, Galgiani JN.. The return of delayed-type hypersensitivity skin testing for coccidioidomycosis. Clin Infect Dis 2015; 61:787–91. [DOI] [PubMed] [Google Scholar]
- 30. Ampel NM. Measurement of cellular immunity in human coccidioidomycosis. Mycopathologia 2003; 156:247–62. [DOI] [PubMed] [Google Scholar]
- 31. Johnson R, Kernerman SM, Sawtelle BG, Rastogi SC, Nielsen HS, Ampel NM.. A reformulated spherule-derived coccidioidin (Spherusol) to detect delayed-type hypersensitivity in coccidioidomycosis. Mycopathologia 2012; 174:353–8. [DOI] [PubMed] [Google Scholar]
- 32. Smith CE, Saito MT, Beard RR, Kepp RM, Clark RW, Eddie BU.. Serological tests in the diagnosis and prognosis of coccidioidomycosis. Am J Epidemiol 1950; 52:1–21. [DOI] [PubMed] [Google Scholar]
- 33. Delafield NL, Mesbah Z, Lacy CR, et al. Coccidioidomycosis in patients with various inflammatory disorders treated with tumor necrosis factor alpha inhibitors. Med Mycol 2021; 59:720–7. [DOI] [PubMed] [Google Scholar]
- 34. Hsu AP, Davis J, Chaput AL, et al. Common population variants cause susceptibility to disseminated coccidioidomycosis. Open Forum Infect Dis 2020; 7(Suppl 1):S22–3. [Google Scholar]
- 35. Pu J, Donovan FM, Ellingson K, et al. Clinician practice patterns that result in the diagnosis of coccidioidomycosis before or during hospitalization. Clin Infect Dis 2021; 73:e1587–93. [DOI] [PubMed] [Google Scholar]
- 36. Sudano D, Kwoh CK, Zhou LL, Ashbeck EL, Lo-Ciganic WH.. Use of disease-modifying antirheumatic drugs, biologic response modifiers and corticosteroids, and subsequent risk of coccidioidomycosis infection among Medicare beneficiaries. Arthritis Rheumatol 2017; 69(Suppl 10). American College of Rheumatology, Conference proceedings, Abstract number 2906, November 3–8, 2017. San Diego, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.