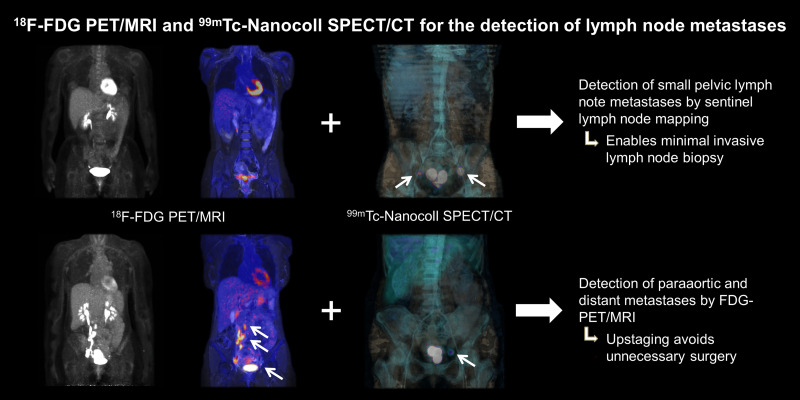
Visual Abstract
Keywords: 18F-FDG PET/MRI, 99mTc-nanocolloid SPECT/CT, sentinel node, cervical carcinoma, combined protocol
Abstract
Lymph node metastasis (LNM) is present in a minority of patients with early stages of cervical carcinomas. As conventional imaging including PET/CT has shown limited sensitivity, systematic lymphadenectomies are often conducted for staging purposes. Therefore, the aim of this prospective study was to analyze the impact of 18F-FDG PET/MRI in addition to sentinel lymph node (SLN) biopsy on lymph node (LN) status. Methods: Forty-two women with an initial diagnosis of Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) IA–IIB cervical carcinoma were included between March 2016 and April 2019. Each patient underwent preoperative whole-body 18F-FDG PET/MRI and SLN imaging with SPECT/CT after intracervical injection of 99mTc-labeled nanocolloid. Systematic lymphadenectomy and SLN biopsy served as the reference standard. Staging using PET/MRI was performed by nuclear medicine and radiology experts working in consensus. Results: One patient was excluded from surgical staging because of liver metastases newly diagnosed on PET/MRI. The overall prevalence of LNM in the remaining 41 patients was 29.3% (12/41). Five of 12 patients with LNM had solely small metastases with a maximum diameter of 5 mm. The consensus interpretation showed PET/MRI to have a specificity of 100% (29/29; 95% CI, 88.3%–100%) for LNM staging but a low sensitivity, 33.3% (4/12; 95% CI, 12.8%–60.9%). LN size was the most important factor for the detectability of metastases, since only LNMs larger than 5 mm could be identified by PET/MRI (sensitivity, 57.1% for >5 mm and 0% for ≤5 mm). Paraaortic LNM was evaluated accurately in 3 of the 4 patients with paraaortic LN metastasis. SLNs were detectable by SPECT/CT in 82.9% of the patients or 69.0% of the hemipelves. In cases with an undetectable SLN on SPECT/CT, the malignancy rate was considerably higher (31.2% vs. 19.3%). The combination of PET/MRI and SLN SPECT/CT improved the detection of pelvic LNM from 33.3% to 75%. Conclusion: 18F-FDG PET/MRI is a highly specific N-staging method and improves LNM detection. Because of the limited sensitivity in frequently occurring small LNMs, PET/MRI should be combined with SLN mapping. The proposed combined protocol helps to decide whether extensive surgical staging is necessary in patients with FIGO I/II cervical cancer.
Staging of cervical carcinoma is still based on clinical criteria and surgical specimens according to the Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) classification (1). However clinical staging becomes less accurate with increasing tumor stage, with a reported under- or overstaging in up to 65% of patients (2–5).
As the presence of lymph node metastasis (LNM) is the most important prognostic factor in early stages (4,6–11), accurate staging is essential. Therefore, imaging methods are increasingly used in addition to clinical staging and are gradually being mentioned in current guidelines (1,4,6,10).
Unfortunately, LNM can occur in any stage of cervical carcinoma, with an increasing prevalence from 15% in early-stage tumors to about 35% in carcinomas larger than 4 cm (10,12,13). Therefore, pelvic systematic lymphadenectomy—sometimes extended to paraaortic lymph nodes (LNs)—remains the gold standard for N-staging (1,4,6,10). Regrettably, this procedure is often associated with high morbidity and seems to be very invasive for the low prevalence of LNM in early tumor stages (1,4,6,10,14).
To minimize overtreatment, sentinel LN (SLN) biopsy is increasingly performed in several centers and has been established by guidelines for cervical cancer staging (4,6). The minimally invasive SLN biopsy is usually performed after an intracervical injection of fluorescent dye or 99mTc labeled nanocolloid, whereby the combination of both is superior (10,15).
Furthermore, a targeted removal of only a few LNs enables a more intensive histopathologic processing, by ultrastaging protocol. By means of ultrastaging, the LN is lamellated more finely and, if necessary, processed immunohistochemically so that even single tumor cells can be detected (16). This procedure results in a higher detection rate (upstaging in 5%–15% of patients) of micrometastases (16), which were shown to have an impact on recurrence probability and overall survival (17)
As a noninvasive imaging method that was first introduced for local tumor staging (5), MRI revealed its advantages quickly for pelvic LNs, with a high pooled specificity of about 95% but low sensitivity of about 55% (3,6,10,18).
Moreover, 18F-FDG PET enables whole-body staging, with excellent results in local tumor staging and in the detection of metastases (19–21). However, the use of PET/CT is still controversial (4,10,18).
According to the National Comprehensive Cancer Network guidelines, PET/CT can be considered for primary staging starting from FIGO IB (4), whereas the European Society of Medical Oncologists guidelines recommend PET/CT in locally advanced disease (10).
On the basis of the current data, whole-body 18F-FDG PET/MRI has the potential to combine the strength of MRI for locoregional tumors and LN staging with the capability of whole-body PET to exclude distant metastases, including lung metastases, in a 1-stop shop (19).
We hypothesize that a combined protocol of 18F-FDG PET/MRI and SPECT/CT for SLN detection could be an accurate, minimally invasive whole-body staging procedure. In particular, ultrastaging in the SLN enables the detection of micrometastases and isolated tumor cells that can hardly be visualized by any imaging method (16). A more precise preoperative staging could save patients with nonresectable N- or M-stage from a possibly unnecessary surgery.
MATERIALS AND METHODS
Forty-two patients with histopathologically confirmed cervical carcinoma and clinically determined stage FIGO IA to IIB were consecutively enrolled into this prospective study. The study was approved by the institutional review board (registry no. 173/2015BO01) and is listed in the German Clinical Trial Register (DRKS identifier DRKS00014346) (22). All patients gave written informed consent.
Each patient underwent whole-body 18F-FDG PET/MRI, preoperative SLN mapping with SPECT/CT, intraoperative SLN detection with a γ-probe, and surgical staging between March 2016 and April 2019.
There was 1 dropout due to newly diagnosed liver metastases on PET/MRI (clinical stage: pT1b1,L1,V0,R1,G1; final stage after PET/MRI: T2b,N1,M1). Two patients (3 hemipelves) were excluded from the evaluations with histologic correlation (1 patient without pelvic LN extraction, 1 hemipelvis due to unclear anatomic allocation). Detailed patient characteristics are presented in Table 1.
TABLE 1.
Patient Characteristics (n = 41)
| Characteristic | Average ± SD | Range |
|---|---|---|
| Age at PET/MRI (y) | 48.1 ± 12.2 | 28.1–80.9 |
| Patient size (cm) | 166 ± 6.7 | 152–187 |
| Patient weight (kg) | 70.8 ± 17.0 | 44.0–117.0 |
| Body mass index (kg/m2) | 25.8 ± 6.0 | 15.2–40.0 |
| Time between PET/MRI and LN histology (d) | 22.4 ± 15.7 | 1–71 |
PET/MRI Protocol
A Biograph mMR (Siemens Healthineers) was used for the PET/MRI acquisition, which started 65.3 ± 13.9 min after injection of 238.3 ± 14.8 MBq of 18F-FDG. To minimize intestinal movement, 20 mg of intravenous butylscopolamine bromide were administered concomitantly with the radiotracer injection unless contraindicated. All patients fasted for at least 8 h, and the blood sugar level at injection was below 140 mg/dL. The minimum acquisition time per bed position was 4 min. Detailed MRI parameters are listed in detail in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org). PET/MR images were evaluated in consensus by board-certified radiology and nuclear medicine specialists with at least 8 y of experience in PET and MRI.
SLN Injection Technique
Approximately 200 MBq of 99mTc-nanocolloid (Nanocoll; GE Healthcare) diluted with 0.9% NaCl to a volume of 0.8 mL were injected intracervically, evenly distributed at the 3-, 6-, 9-, and 12-o’clock positions.
SLN SPECT/CT
LN mapping was performed 3–5 h after 99mTc-nanocolloid injection. All patients were scanned on a hybrid SPECT/CT device (Discovery 670 Pro; GE Healthcare) including an area from pelvis to caudal liver with the 2 camera heads in H-Mode. The SPECT acquisition parameters were an energy window of 140.5 keV ± 10%, a 128 × 128 matrix, 30 angular steps with a 6° interval, an acquisition time of 15 min/step, and a pixel size of 4.42 × 4.42 mm. SPECT data were reconstructed using an ordered-subsets expectation maximization iterative protocol (2 iterations, 10 subsets).
The technical parameters of the 16-slice CT scanner were a gantry rotation speed of 0.8 s and a table feed of 20 mm/gantry rotation. CT scans were obtained with a dose modulation system (120 kV, 10–80 mAs [OptiDose; GE Healthcare]). A contrast agent (90 mL of Ultravist 370; Bayer Vital GmbH) was injected unless contraindicated.
The SLN SPECT/CT was analyzed by 2 nuclear medicine physicians with more than 10 y of experience in pelvic SLN imaging. An SLN was defined as focal activity enrichment on SPECT in a plausible anatomic region. SLN mapping was defined as successful if at least 1 SLN per hemipelvis was clearly detectable.
SLN Biopsy
Surgical staging was performed laparoscopically the day after 99mTc-nanocolloid injection. Blue dye or indocyanine green was injected intracervically at the 3- and 9-o’clock positions (0.2 mL per injection) before surgery.
SLNs were localized and identified intraoperatively either by direct visualization or using a laparoscopic γ-probe (Neoprobe, models 1017 and 1100; Devicor Medical Products, Inc.), resected separately, assigned meticulously, and sent for rapid tissue sectioning.
All patients underwent bilateral systematic lymphadenectomy with resection of SLNs and of macroscopically suggestive LNs. Additional paraaortic LNs were removed in cases of higher tumor stages or after histopathologic proof of LNM by frozen sectioning. Ultrastaging was performed for the SLN, with complete preparation of the entire LN with 200-μm slices.
Statistical Analysis
Statistical analysis was performed with SPSS Statistics software, version 25.0 (IBM Inc.), and MedCalc software, version 19.3. Test performance was calculated with 4-fold tables. The Fisher exact test (2-tailed) was used to verify significant differences in the prevalence of LNM. A P value of less than 0.05 was considered statistically significant.
RESULTS
Tumor Histology and Prevalence of LNM
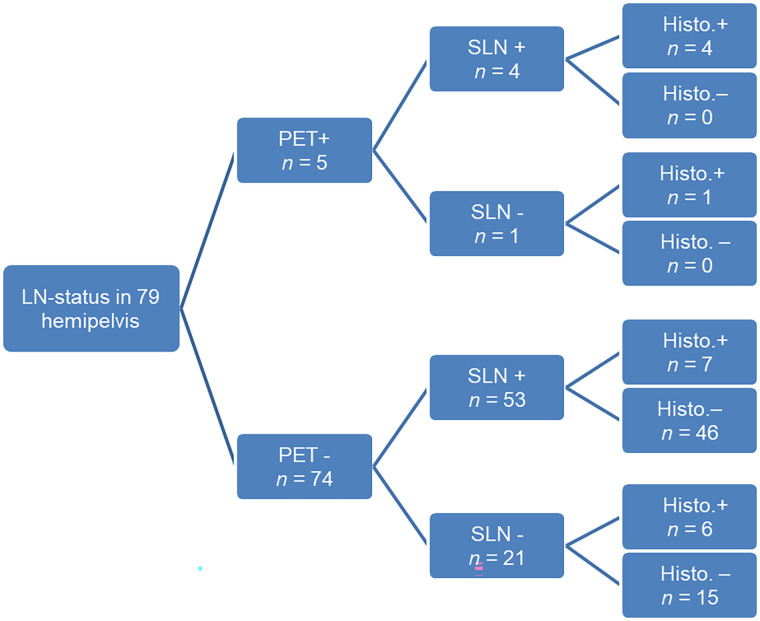
Almost every tumor stage from pT1a to pT2b was represented in this cohort (Table 2. Tumor grade was balanced between G1/G2 and G3 (G1: n = 4, G2: n = 17, G3: n = 20). In 1 case, because a peritoneal involvement could not be ruled out histologically (pTx), the clinical assessment of the tumor border (cT2b, M1 [peritoneum]) was applied. The prevalence of LNM was 29.3% per patient (12/41) and 22.8% per hemipelvis (18/79). Five of 12 patients presented with solely small LNMs at a maximum diameter of 5 mm.
TABLE 2.
Distribution of Patients by Tumor Stage
| Number of patients | ||||
|---|---|---|---|---|
| Tumor stage | G1 | G2 | G3 | |
| pT1 | pT1a | 1 (0) | 3 (0) | 1 (0) |
| pT1b | 2 (0) | 8 (2) | 14 (2) | |
| pT1c | — | — | — | |
| pT2 | pT2a | — | 1 (0) | — |
| pT2b | 1 (0) | 3 (3) | 6 (4) | |
| pTx (cT2b) | — | — | 1 (1) | |
Data in parentheses are patients with pelvic LNM. Subgroups of tumor stages are summarized.
The presence of LNM was significantly higher in pT2 (63.6%) than pT1 stages (13.8%, P < 0.05). No LNM was found with G1 tumors or T1a tumor stages.
Paraaortic LNM was strictly associated with higher tumor stage and grade (3/4 pT2b [1xG1, 2xG2], 1/4 pTx [G3]). All patients with paraaortic LNM presented with LNM in both hemipelves.
18F-FDG PET/MRI
The prospective reading of PET/MRI as an expert consensus demonstrated a very high specificity for pelvic LN staging in both patient-based (29/29 [100%]; 95% CI, 88.1%–100%) and hemipelvis-based (61/61 [100%]; 95% CI, 94.1%–100%) analyses.
However, sensitivity for pelvic LNM detection was considerably limited (patient-based analysis: 4/12 [33.3%]; 95% CI, 9.9%–65.1%; hemipelvis-based analysis: 5/18 [27.8%]; 95% CI, 9.7%–53.5%). Here, the size of the LN played a decisive role for LNM detection by PET/MRI, as it enabled detection of only those LNMs larger than 5 mm (4/7; 57%); no LNMs 5 mm or smaller (0/5) were found.
Thus, the accuracy, PPV, and NPV of PET/MRI for LNM detection were 80.5% (33/41; 95% CI, 65.1%–91.2%), 100% (474), and 78.4% (29/37), respectively, on a patient level and 83.5% (66/79; 95% CI, 73.5–90.9), 100% (5/5), and 82% (61/74), respectively, on a hemipelvis level.
Tumor grade had no significant impact on detectability on PET/MRI, as the distribution of G3 and G2 status was comparable in detectable LNMs (G3: n = 2, G2: n = 2) and nondetectable LNMs (G3: n = 4, G2: n = 4).
Pelvic lymphadenectomy was extended to a paraaortic LN sampling in 16 of 41 patients. Four of these 16 presented with paraaortic LNMs at histology. These paraaortic LNMs were rated correctly by PET/MRI in 3 of 4 patients, resulting in a sensitivity of 75% (95% CI, 19.4.%–99.4%), a specificity of 100% (12/12; 95% CI, 73.5%–100%), and an accuracy of 94% (15/16; 95% CI, 66.8%–99.8%). All detected paraaortic LNMs had diameters between 8 and 45 mm and were related to pT2b, G3 tumors. The single missed LNM was from a G2 tumor and had a maximal diameter of 7 mm.
SLN Detection
SPECT/CT detected at least 1 SLN in 82.9% of all patients (34/41) and in 70.7% of hemipelves (58/82). SLNs in both hemipelves were detectable in 61.0% of patients (25/41). The rate of detecting paraaortic SLNs was substantially lower (31.7%, 13/41). In 1 patient, SLNs were visualized solely by dye, increasing the overall detection rate to 85.4% (35/41) on a patient-based level.
The presence of LNM was higher in patients or hemipelves without a detectable SLN on SPECT/CT (50% [3/6] or 31.2% [7/22], respectively) than when an SLN was detectable (23.5% [8/34] or 19.3% [11/57], respectively). A representative case is shown in Figure 1. However, the difference was not significant on a patient or hemipelvis level (P = 0.32 and 0.25, respectively). Only 1 of 4 patients with paraaortic LNM showed corresponding SLNs on SPECT/CT.
FIGURE 1.
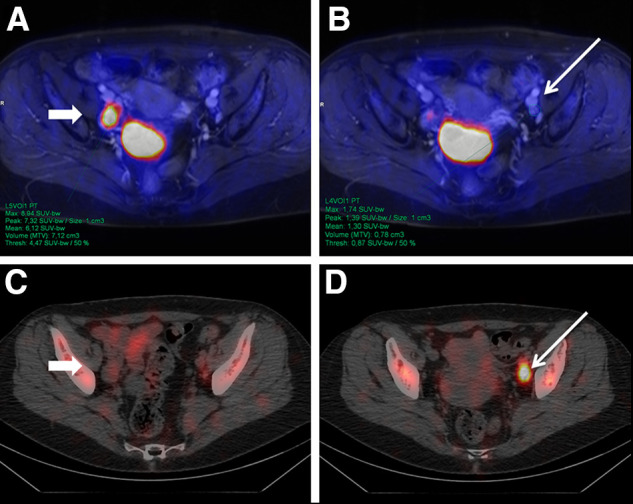
A 58-y-old woman with newly diagnosed T2b G3 cervical carcinoma. PET/MRI (top row) detected enlarged LN in right hemipelvis (A, arrow) with intense 18F-FDG uptake. In contrast, 99mTc-nanocolloid SPECT/CT showed clearly definable SLN in left hemipelvis (D, arrow) with benign characteristics on PET/MRI (B). After surgery, 1 LN in right hemipelvis (A and C) was confirmed as LNM. SLN on left (B and D) had benign histology.
To analyze the representativeness of SLN biopsy for assessment of N-status on a hemipelvis level, we performed an additional subanalysis on SLNs that were clearly assignable on SPECT/CT and on γ-probe visualization (44 SLNs assignable in 35 hemipelves of 24 patients). The prevalence of malignancy was 22.9% (8/35), and sensitivity was 87.5% (7/8; 95% CI, 47.4%–99.7%); specificity, 100% (27/27; 95% CI, 87.2%–100%); accuracy, 97% (34/35; 95% CI, 85.1–99.9); PPV, 100% (7/7); and NPV, 96.4% (27/28).
Combined PET/MRI and SLN Protocol
Combining 18F-FDG PET/MRI and SLN imaging improved the detection rate of LNM substantially from 33.3% to 75.0% on a patient-based level and from 27.8% to 66.7% on a hemipelvis level. In return, PET/MRI was superior to SLN imaging for the detection of paraaortic LNM. Further details are shown in Table 3.
TABLE 3.
Detection Rate of LNM in 18F-FDG PET/MRI, SLN SPECT/CT, and Combined Protocol
| Parameter | PET/MRI | SLN | PET/MRI + SLN |
|---|---|---|---|
| Per patient | 33.3% (4/12) | 66.7% (8/12) | 75.0% (9/12) |
| ≤pT2a1 and N0 in MRI | 0% (0/3) | 100% (3/3) | 100% (3/3) |
| ≥pT2a2 | 14.3% (1/7) | 42.9% (3/7) | 42.9% (3/7) |
| Hemipelvis | 27.8% (5/18) | 61.1% (11/18) | 66.7% (12/18) |
| ≤pT2a1 and N0 in MRI | 0% (0/4) | 75.0% (3/4) | 75.0% (3/4) |
| ≥pT2a2 | 18.2% (2/11) | 45.5% (5/11) | 54.5% (6/11) |
| Paraaortic LNM | 75% (3/4) | 25% (1/4) | 75% (3/4) |
In a subanalysis of 28 patients with tumors 4 cm or smaller, no parametrial invasion, and no enlarged LNs, SLN imaging detected LNM in 3 of 3 patients or 3 of 4 hemipelves. Implementing additional PET data could not improve LNM detection (0/3) in this small subgroup with metastases of 0.5–4 mm.
A representative example of the synergies between the 2 modalities is presented in Figure 2. However, our data imply that PET/MRI cannot predict LN status precisely in cases of failed SLN detection (Fig. 3).
FIGURE 2.
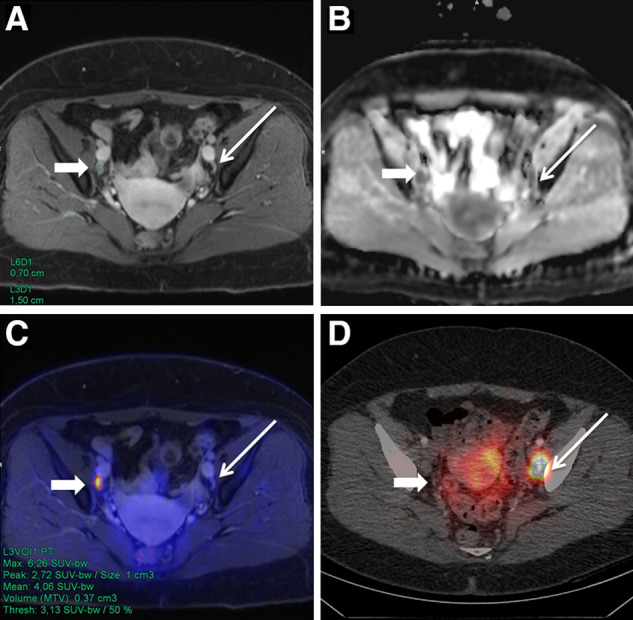
A 55-y-old woman with initial diagnosis of T1b G2 cervical carcinoma. PET/MRI showed slightly enlarged contrast-enhanced LN in right hemipelvis (A, thick arrow) with diffusion restriction in apparent-diffusion-coefficient map (B) and focal 18F-FDG uptake in PET-fused image (C) but no 99mTc-nanocolloid uptake on SPECT/CT (D). In contrast, SLN in left hemipelvis (C, thin arrow) was not suggestive on PET/MR images (A–C). Both LNs were removed and histologically confirmed as LNMs.
FIGURE 3.
Overview of distribution of LNM depending on PET/MRI findings and detectability of SLNs.
DISCUSSION
To our knowledge, this was the first prospective study on a combined protocol of 18F-FDG PET/MRI and SLN SPECT/CT for the purpose of LN staging in patients with cervical carcinoma. The data suggest that PET/MRI has a high specificity in LN staging but only a limited sensitivity because of the presence of LNMs smaller than 5 mm, which seem to occur frequently in early-stage tumors.
In contrast to intravenously injected tracers and contrast media, the SLN technique provides decisive information about lymphatic drainage of the tumor and identifies the LN with the highest risk for metastasis independent of its size. Thus, the combination of metabolic and morphologic information from PET/MRI with functional information from lymph drainage resulted in a significant improvement in sensitivity and detection rate.
Diagnostic Power of PET/MRI
Detection of small metastases is known to be a major challenge for PET. However, the sensitivity in our study was lower than in earlier PET/CT (44%) and PET/MRI studies (33% vs. 44%–88%) (20,21,23,24). This discrepancy might be explained by the high rate of small LNMs compared with previous studies (20,21,23,24), which might be due to the defined cohort of solely FIGO I–II stage, exhibiting a reported prevalence of micrometastases of more than 40% (25,26).
In addition, this was the first PET study on this topic implementing ultrastaging as gold standard, which increases the detection of micrometastases by up to 28% (16). In our study, the implementation of ultrastaging enabled the detection of micrometastases down to 0.5 mm. As the partial-volume effect increases with decreasing lesion size, PET has a poor sensitivity in the lower millimeter range, thus missing smaller metastases (27,28). Consequently, it can be hypothesized that studies without ultrastaging as the gold standard might overrate the sensitivity of PET.
Unfortunately, MRI was only able to compensate for the difficulties of PET to a limited extent, especially in small metastases. Because of the limited spatial resolution of diffusion-weighted images, only LNs larger than 5 mm were evaluated sufficiently on apparent-diffusion-coefficient images (29,30). The described improvement in LNM detection by calculating the ratio of apparent-diffusion-coefficient LN to primary tumor (29) was not applicable to our cohort, as most of the patients underwent diagnostic conization before PET/MRI.
Contrary to the limited sensitivity, the specificity of PET/MRI was very high in the assessment of LNM. This high specificity refers not only to pelvic LNM but also to paraaortic LNM and is comparable to previous PET/CT and PET/MRI studies with reported specificities of 84%–94% (20,21,23,24).
As the presence of pelvic or paraaortic LNM results in an upstaging to FIGO IIIB or FIGO IVB, PET/MRI can have a major impact on clinical management. For example, the histologic confirmation of a PET-positive paraaortic LNM via minimally invasive LN biopsy could shift the patient’s treatment from an unnecessary and stressful hysterectomy to radiochemotherapy.
Furthermore, MRI has the potential to detect infiltration of the pelvic wall, which is important for the differentiation between T3a and T3b or FIGO IIIA and FIGO IIIB stage and has accuracy equal to or better than that of clinical staging (5,24,31).
SLN Detection Rate
Our study confirms previous work that stated that the removal of single SLNs is representative of pelvic LN status (15,32). The presence of pelvic LNM was correctly demonstrated by SLN removal in all our patients with successful SPECT/CT-confirmed radioactive SLN labeling.
Our SLN detection rate on SPECT/CT was slightly lower than described in the current literature (33,34). As higher tumor stages and LNM prevalence are associated with a significantly lower SLN detection rate (33), the composition of this cohort—with higher tumor stages than in other SLN studies—might be a potential explanation. Current data indicate an up to 50% higher risk for LNM in cases of failed SLN detection on SPECT/CT (33,34). Although the significance level was not reached because of the limited number of LNs, it might be hypothesized that missed SLN detection on imaging is not necessarily indicative of an insufficient injection. Rather, metastatic tissue might inhibit lymph drainage or disrupt the filtering function of involved LNs.
Combination of PET/MRI and SLN SPECT/CT
The combination of PET/MRI and SLN SPECT/CT resulted in the highest detection rate for LNM, and the additional value of PET/MRI increases with advanced tumor stage and parametrial invasion. However, PET/MRI could not improve N-staging in cases of small tumors restricted to the cervix; in such cases, the false-negative rate has been described as being below 0.1% if there is successful SLN mapping and no suggestive LNs on MRI (32). Thus PET/MRI seems to contribute little to this specific patient group. In clinical practice, however, successful SLN marking and the final tumor stage often become apparent only during surgery. If whole-body imaging is omitted when there is a clinically assumed low T-stage, paraaortic LNM or distant metastases may be missed, as demonstrated here by a patient with pT1 tumor but liver metastases.
The fact that 18F-FDG PET/MRI contributes to the detection of LNMs might be explained by loss of colloid avidity. In addition, the SLN technique is independent of LN size and thus enables the biopsy-based detection of small LNMs beyond the resolution limit of PET and MRI, improving sensitivity considerably.
The detection rate of paraaortic LNs using SPECT/CT was higher in the current study than in previous studies (35,36) but was not sufficient as a stand-alone method for reliable assessment of the paraaortic status.
Our results demonstrate that PET/MRI has the potential to identify paraaortic LNMs; however, the statistical power is still limited (23,24). As imaging with 18F-FDG allows assessment of all organ systems, a full-body PET/MRI staging can be performed in 30–40 min.
Limitations
The limitation of this study is that the data represent an interim evaluation of an ongoing prospective study. Although the study covers the largest collective to date, the available data tend to indicate trends in some issues. For confirmation of these trends, more data are needed.
So far, the evaluation has been conducted at only a patient level and a hemipelvis level. However, because of the limited sensitivity of PET/MRI, further evaluation of the multiparametric data at an LN level is necessary. Thus, a larger study including a higher number of LNMs is desirable to further evaluate PET/MRI in this entity.
CONCLUSION
18F-FDG PET/MRI is a highly specific method in LN staging that improves LNM detection. Because of the limited sensitivity in frequently occurring small LNMs, PET/MRI should be combined with SLN SPECT/CT. The combined protocol helps to decide whether extensive surgical staging is needed in patients with FIGO I/II cervical cancer.
DISCLOSURE
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy (EXC 2180-390900677). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does the combination of 18F-FDG PET/MRT and SLN SPECT/CT improve LN staging in FIGO I/II cervical carcinoma?
PERTINENT FINDINGS: In this prospective study, PET/MRI exhibited a specificity of 100% but limited sensitivity of 33%. The combination of PET/MRI and SPECT/CT-guided SLN biopsy increased the detection rate of LNM to 75%.
IMPLICATIONS FOR PATIENT CARE: 18F-FDG PET/MRI can save metastasized patients from unnecessary surgery but should be combined with SLN biopsy because of its limited sensitivity in frequently occurring small metastases.
REFERENCES
- 1. Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145:129–135. [DOI] [PubMed] [Google Scholar]
- 2. Lagasse LD, Creasman WT, Shingleton HM, Ford JH, Blessing JA. Results and complications of operative staging in cervical cancer: experience of the Gynecologic Oncology Group. Gynecol Oncol. 1980;9:90–98. [DOI] [PubMed] [Google Scholar]
- 3. Selman TJ, Mann C, Zamora J, Appleyard TL, Khan K. Diagnostic accuracy of tests for lymph node status in primary cervical cancer: a systematic review and meta-analysis. CMAJ. 2008;178:855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koh WJ, Abu-Rustum NR, Bean S, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:64–84. [DOI] [PubMed] [Google Scholar]
- 5. Thomeer MG, Gerestein C, Spronk S, van Doorn HC, van der Ham E, Hunink MG. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: systematic review and meta-analysis. Eur Radiol. 2013;23:2005–2018. [DOI] [PubMed] [Google Scholar]
- 6. S3 Guideline for Diagnostics, Therapy and Aftercare of Patients with Cervical Cancer: Long Version, 1.0—AWMF Registration Number 032/033OL [in German]. AWMF Online; 2014. https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Zervixkarzinom/LL_Zervixkarzinom_Langversion_1.0.pdf. Accessed May 27, 2021.
- 7. Tanaka Y, Sawada S, Murata T. Relationship between lymph node metastases and prognosis in patients irradiated postoperatively for carcinoma of the uterine cervix. Acta Radiol Oncol. 1984;23:455–459. [DOI] [PubMed] [Google Scholar]
- 8. Fuller AF, Jr, Elliott N, Kosloff C, Hoskins WJ, Lewis JL, Jr. Determinants of increased risk for recurrence in patients undergoing radical hysterectomy for stage IB and IIA carcinoma of the cervix. Gynecol Oncol. 1989;33:34–39. [DOI] [PubMed] [Google Scholar]
- 9. Kim SM, Choi HS, Byun JS. Overall 5-year survival rate and prognostic factors in patients with stage IB and IIA cervical cancer treated by radical hysterectomy and pelvic lymph node dissection. Int J Gynecol Cancer. 2000;10:305–312. [DOI] [PubMed] [Google Scholar]
- 10. Marth C, Landoni F, Mahner S, et al.; ESMO Guidelines Committee. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv72–iv83. [DOI] [PubMed] [Google Scholar]
- 11. Pieterse QD, Kenter GG, Gaarenstroom KN, et al. The number of pelvic lymph nodes in the quality control and prognosis of radical hysterectomy for the treatment of cervical cancer. Eur J Surg Oncol. 2007;33:216–221. [DOI] [PubMed] [Google Scholar]
- 12. Kadkhodayan S, Hasanzadeh M, Treglia G, et al. Sentinel node biopsy for lymph nodal staging of uterine cervix cancer: a systematic review and meta-analysis of the pertinent literature. Eur J Surg Oncol. 2015;41:1–20. [DOI] [PubMed] [Google Scholar]
- 13. SiSaia P., Creasman W, Mannel R, Scott D. Clinical Gynecologic Oncology. Vol 9: Elsevier; 2017:38–101. [Google Scholar]
- 14. Holman LL, Levenback CF, Frumovitz M. Sentinel lymph node evaluation in women with cervical cancer. J Minim Invasive Gynecol. 2014;21:540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van de Lande J, Torrenga B, Raijmakers PG, et al. Sentinel lymph node detection in early stage uterine cervix carcinoma: a systematic review. Gynecol Oncol. 2007;106:604–613. [DOI] [PubMed] [Google Scholar]
- 16. Bats AS, Mathevet P, Buenerd A, et al. The sentinel node technique detects unexpected drainage pathways and allows nodal ultrastaging in early cervical cancer: insights from the multicenter prospective SENTICOL study. Ann Surg Oncol. 2013;20:413–422. [DOI] [PubMed] [Google Scholar]
- 17. Marchiolé P, Buenerd A, Benchaib M, Nezhat K, Dargent D, Mathevet P. Clinical significance of lympho vascular space involvement and lymph node micrometastases in early-stage cervical cancer: a retrospective case-control surgico-pathological study. Gynecol Oncol. 2005;97:727–732. [DOI] [PubMed] [Google Scholar]
- 18. Choi HJ, Ju W, Myung SK, Kim Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: meta-analysis. Cancer Sci. 2010;101:1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li K, Sun H, Guo Q. Combinative evaluation of primary tumor and lymph nodes in predicting pelvic lymphatic metastasis in early-stage cervical cancer: a multiparametric PET-CT study. Eur J Radiol. 2019;113:153–157. [DOI] [PubMed] [Google Scholar]
- 20. Stecco A, Buemi F, Cassara A, et al. Comparison of retrospective PET and MRI-DWI (PET/MRI-DWI) image fusion with PET/CT and MRI-DWI in detection of cervical and endometrial cancer lymph node metastases. Radiol Med (Torino). 2016;121:537–545. [DOI] [PubMed] [Google Scholar]
- 21. Kim SK, Choi HJ, Park SY, et al. Additional value of MR/PET fusion compared with PET/CT in the detection of lymph node metastases in cervical cancer patients. Eur J Cancer. 2009;45:2103–2109. [DOI] [PubMed] [Google Scholar]
- 22. DRKS-ID: DRKS00014346. German Clinical Trials Register website. https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00014346. Accessed April 7, 2021.
- 23. Sarabhai T, Schaarschmidt BM, Wetter A, et al. Comparison of 18F-FDG PET/MRI and MRI for pre-therapeutic tumor staging of patients with primary cancer of the uterine cervix. Eur J Nucl Med Mol Imaging. 2018;45:67–76. [DOI] [PubMed] [Google Scholar]
- 24. Grueneisen J, Schaarschmidt BM, Heubner M, et al. Integrated PET/MRI for whole-body staging of patients with primary cervical cancer: preliminary results. Eur J Nucl Med Mol Imaging. 2015;42:1814–1824. [DOI] [PubMed] [Google Scholar]
- 25. Lentz SE, Muderspach LI, Felix JC, Ye W, Groshen S, Amezcua CA. Identification of micrometastases in histologically negative lymph nodes of early-stage cervical cancer patients. Obstet Gynecol. 2004;103:1204–1210. [DOI] [PubMed] [Google Scholar]
- 26. Juretzka MM, Jensen KC, Longacre TA, Teng NN, Husain A. Detection of pelvic lymph node micrometastasis in stage IA2-IB2 cervical cancer by immunohistochemical analysis. Gynecol Oncol. 2004;93:107–111. [DOI] [PubMed] [Google Scholar]
- 27. Bellevre D, Blanc Fournier C, Switsers O, et al. Staging the axilla in breast cancer patients with 18F-FDG PET: how small are the metastases that we can detect with new generation clinical PET systems? Eur J Nucl Med Mol Imaging. 2014;41:1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cysouw MCF, Kramer GM, Hoekstra OS, et al. Accuracy and precision of partial-volume correction in oncological PET/CT studies. J Nucl Med. 2016;57:1642–1649. [DOI] [PubMed] [Google Scholar]
- 29. Lin G, Ho KC, Wang JJ, et al. Detection of lymph node metastasis in cervical and uterine cancers by diffusion-weighted magnetic resonance imaging at 3T. J Magn Reson Imaging. 2008;28:128–135. [DOI] [PubMed] [Google Scholar]
- 30. Roy C, Bierry G, Matau A, Bazille G, Pasquali R. Value of diffusion-weighted imaging to detect small malignant pelvic lymph nodes at 3 T. Eur Radiol. 2010;20:1803–1811. [DOI] [PubMed] [Google Scholar]
- 31. Ozsarlak O, Tjalma W, Schepens E, et al. The correlation of preoperative CT, MR imaging, and clinical staging (FIGO) with histopathology findings in primary cervical carcinoma. Eur Radiol. 2003;13:2338–2345. [DOI] [PubMed] [Google Scholar]
- 32. Tax C, Rovers MM, de Graaf C, Zusterzeel PL, Bekkers RL. The sentinel node procedure in early stage cervical cancer, taking the next step; a diagnostic review. Gynecol Oncol. 2015;139:559–567. [DOI] [PubMed] [Google Scholar]
- 33. Balaya V, Bresset A, Guani B, et al. Risk factors for failure of bilateral sentinel lymph node mapping in early-stage cervical cancer. Gynecol Oncol. 2020;156:93–99. [DOI] [PubMed] [Google Scholar]
- 34. Cibula D, Kuzel D, Slama J, et al. Sentinel node (SLN) biopsy in the management of locally advanced cervical cancer. Gynecol Oncol. 2009;115:46–50. [DOI] [PubMed] [Google Scholar]
- 35. Díaz-Feijoo B, Perez-Benavente MA, Cabrera-Diaz S, et al. Change in clinical management of sentinel lymph node location in early stage cervical cancer: the role of SPECT/CT. Gynecol Oncol. 2011;120:353–357. [DOI] [PubMed] [Google Scholar]
- 36. Ogawa S, Kobayashi H, Amada S, et al. Sentinel node detection with 99mTc phytate alone is satisfactory for cervical cancer patients undergoing radical hysterectomy and pelvic lymphadenectomy. Int J Clin Oncol. 2010;15:52–58. [DOI] [PubMed] [Google Scholar]