Abstract
In light of current knowledge on the role of reactive oxygen species and other oxidants in skin diseases, it is clear that oxidative stress facilitates inflammation and is an important factor involved in skin diseases, i.e., acne. Taking into consideration the fact that some Cotoneaster plants are valuable curatives in skin diseases in traditional Asian medicine, we assumed that thus far untested species C. hsingshangensis and C. hissaricus may be a source of substances used in skin diseases. The aim of this study was to evaluate the antioxidant, anti-inflammatory, antimicrobial, and cytotoxic activities of their various extracts. LC-MS analysis revealed the presence of 47 compounds (flavonoids, phenolic acids, coumarins, sphingolipids, carbohydrates), while GC-MS procedure allowed for the identification of 42 constituents (sugar derivatives, phytosterols, fatty acids, and their esters). The diethyl ether fraction of C. hsingshangensis (CHs-2) exhibited great ability to scavenge free radicals and good capacity to inhibit cyclooxygenase-1, cyclooxygenase-2, lipoxygenase, and hyaluronidase. Moreover, it had the most promising power against microaerobic Gram-positive strains, and importantly, it was non-toxic toward normal skin fibroblasts. Taking into account the value of the calculated therapeutic index (>10), it is worth noting that CHs-2 can be subjected to in vivo study and constitutes a promising anti-acne agent.
Keywords: Cotoneaster, Rosaceae, antioxidant, anti-inflammatory, anti-acne, skin diseases, antimicrobial
1. Introduction
The results obtained by Sarici et al. [1] concerning the evaluation of parameters associated with oxidative stress, such as nitric oxide (NO), xanthine oxidase (XO), malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT), in the venous blood of patients using spectrophotometrical methods indicate that oxidative damage of tissues is a significant etiological factor of acne. In view of the above, the role of oxidative stress in the etiology of acne seems to be unquestionable.
The cutaneous propionibacteria (P. acnes, P. avidum, P. granulosum, P. propionicum, and P. lymphophilum) are involved in the maintenance of healthy skin; however, they can also reveal adverse activity as opportunistic pathogens [2]. The three predominant genera, including Propionibacteria, Staphylococci, and Corynebacteria, form the microbial community established on the skin [3].
According to [4], the classification of Propionibacterium is extremely complicated. The major types of Propionibacterium (I, II, III clades) should be classified as P. acnes subsp. acnes, P. acnes subsp. defendens, and P. acnes subsp. elongatum, respectively. The latest research justifies the necessity to divide the genus Propionibacterium into four genera. Additionally, the cutaneous Propionibacterium, participating in seborrheic skin disorders, should be reclassified into the new genus Cutibacterium. Nevertheless, the previous classification is still valid and practiced among the medical microbiology community [4]. In our article, the new nomenclature (C. acnes) was used.
Although the association of acne lesion aggravation with the presence of C. acnes is well established, the mechanism of involvement of C. acnes in the formation of acne is not fully understood [5]. Nevertheless, it is well known that C. acnes participates in the production of pro-inflammatory cytokines, such as interleukin (IL)-8, IL-1β, IL-12, and tumor necrosis factor (TNF)-α, by stimulating keratinocytes, as well as phagocytic cells, which consequently leads to severe inflammation [5]. Furthermore, according to [6], enhancement of the mRNA expression levels of certain cytokines, such as (IFN)-γ, TNF-β, IL-8, IL-4, IL-10, IL-1α, IL-1β, and IL-17A, within acne lesions can be observed.
The presence of C. acnes on the skin leads to the formation of superoxide anions, which form peroxidants after combination with nitric oxide. As a consequence, this phenomenon causes keratinocyte damage [7].
Although synthetic antioxidant compounds are considered very effective, they also cause substantial side effects. Herbal sources possess the ability to participate in various phases of the oxidation mechanism. According to Soleymani and co-authors [8], phenolic compounds show significant anti-acne activity due to their ability to decrease oxidative stress. Many mechanisms of action are arranged in this biological activity and involve activation of TLR-2/4, p38/JNK/MAPK pathways, adjustment of MAPK signaling pathways, and regulation of Nrf2/Keapl-mediated antioxidant pathways. Additionally, upregulation of SOD, GP x, GSH, CAT, and heme oxygenase HO-1 was noted. Furthermore, polyphenolic compositions demonstrate the capability to downregulate endothelial ROS, NOX-4, and MDA (malondialdehyde) levels, which consequently leads to reduced amounts of H2O2 and MDA and to the inhibition of the expression of MAPKs, such as P38, ERK, and JNK. Pathways of particular significance, such as the ROS/MAPK/NF-ĸB pathway and PI3K/Akt/NF-ĸB pathway, are being suppressed. The decrease in lipid peroxidation linked with the downregulation of cytochrome P450 (CYP) expression and upregulation of STAT-1 2E1 expression seem to be notably relevant.
Medicinal plants have been traditionally used in the treatment of several human diseases, and their therapeutic properties have been assigned to different chemical compounds isolated from them. Of considerable importance, compounds with antioxidant properties may be found at great concentrations in plants and can be responsible for their preventive effects in various diseases caused by oxidative stress. Therefore, the antioxidant activities of plant extracts have prospective applications in healthcare [9].
A diversity of occurrence among the Cotoneaster genus is worth underlining and encompasses river valleys and banks, woods, thickets, rocky, and calcareous sites, as well as mountain areas approximately 800–4100 m above sea level. The individual species are indigenous, particularly to the Palearctic region (temperate Asia, Europe, and North Africa). Nevertheless, they are cultivated not only throughout Europe but also in various parts of the world due to their significant aesthetic qualities: white, red, or pink flowers and red, brownish red, orange, or black fruits. The pivotal area of occurrence, with 60% of species, can be observed in mountain regions of China [10]. Cotoneaster hsingshangensis is indigenous to China, and C. hissaricus is vernacular throughout Asia. These two species, similar to many others of Cotoneaster, are cultivated in Poland as ornamental plants in parks, gardens, and other urban places.
It has been reported that many Cotoneaster species have valuable biological properties and have been used in traditional medicine in many countries [11]. Swati and co-authors [12] reported that fruits of Cotoneaster microphyllus are applied on skin against irritation. Furthermore, leaves of C. microphyllus are used for dermatitis. The medicinal uses of Cotoneaster species in skin disorders have been reported in Pakistan, Turkey, India, Lebanon, and Iran as therapeutic agents in the treatment of cuts and wounds [13]. In addition, an anti-itching effect of Cotoneaster has been observed [14,15]. According to Akbar [16], Cotoneaster removes hyperpigmentation of the skin.
Taking into consideration the fact that some representatives of Cotoneaster are valuable curatives applied in various skin diseases in traditional Asian medicine, we assumed that thus far untested species—C. hsingshangensis and C. hissaricus—may be a source of active substances used in skin diseases. In view of the above, the present study reports primarily on a thorough examination of the composition (especially phenolic components) and biological activities with an emphasis on dermatological diseases, such as acne. An exploration of Cotoneaster species has been undertaken to provide an explanation for their capability to ameliorate skin conditions.
2. Materials and Methods
2.1. Chemicals and Reagents
Ascorbic acid, 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), 2,2′-azino-bis-(3-ethyl-benzothiazole-6-sulfonic acid) (ABTS•+), (−)-epigallocatechin gallate (EGCG), nordihydroguaiaretic acid (NDGA), indomethacin, hyaluronidase from bovine tests, hyaluronic acid sodium salt from rooster comb, Folin–Ciocalteu reagent, ethylene-diaminetetraacetic acid, disodium dihydrate (Na2EDTA*2H2O), Tricine (≥99%; titration) were obtained from Sigma-Aldrich (Steinheim, Germany). Phosphate-buffered saline (PBS) was purchased from Gibco (Carlsbad, CA, USA). Reference substances were supplied by ChromaDex (Irvine, CA, USA), while acetonitrile, formic acid, and water were supplied for LC analysis by Merck (Darmstadt, Germany). All other chemicals were of analytical grade and were obtained from the Polish Chemical Reagent Company (POCH, Gliwice, Poland).
2.2. Plant Material
Leaves of Cotoneaster hsingshangensis Fryer et B.Hylmö (Figure 1), as well as C. hissaricus Pojark. (Figure 2) were collected in the Maria Curie-Skłodowska University (UMCS) Botanical Garden in Lublin (Poland), at an altitude of 181.2 m a.s.l. (coordinates 52°14′34.4″ N 17°05′32.4″ E and 46°57′51.7″ N 142°45′22.1″ E, respectively) in September 2020. Taxonomical identification was confirmed by Dr. A. Cwener, an employee of the Botanical Garden who specializes in Cotoneaster. C. hsingshangensis was introduced to cultivation in the Botanical Garden in Lublin in 1966. This species was derived from the Institute of Dendrology of the Polish Academy of Sciences, Kórnik Arboretum. C. hissaricus was introduced to the Botanical Garden in Lublin in 2003 from Sakhalin Botanical Garden. Voucher specimens were deposited in the Department of Pharmaceutical Botany (CHs-0921 and CHi-0921).
Figure 1.
Photos of C. hsingshangensis under study collected in: (A) May, (B,C) September, and (D) October.
Figure 2.
Photos of C. hissaricus under study were collected in (A) April, (B) May, (C) September, and (D) October.
2.3. Preparation of the Extracts
The plant materials were dried in the shade at 24 °C (±0.5 °C) to achieve a constant weight [17]. Extracts were prepared using a mixture of methanol–acetone–water (3:1:1, v/v/v; 3 × 100 mL), and then sonicated at a controlled temperature (40 ± 2 °C) for 30 min [13]. The combined extracts were filtered, concentrated under reduced pressure, and after freezing, lyophilized in a vacuum concentrator (Free Zone 1 apparatus; Labconco, Kansas City, KS, USA) to obtain dried residues. The obtained extracts were dissolved in hot water, filtered after 24 h, and subjected to liquid-liquid extraction with diethyl ether, ethyl acetate, and n-butanol, successively. The obtained fractions of diethyl ether, ethyl acetate, and n-butanol, as well as the water residue, were evaporated in vacuo and lyophilized using a vacuum concentrator.
2.4. Total Flavonoid, Phenolic, and Phenolic Acids Content
Total flavonoid (TFC) and total phenolic content (TPC) were established using colorimetric assays as described previously [18]. The absorbance was measured at 430 and 680 nm, respectively, using a Pro 200F Elisa Reader (Tecan Group Ltd., Männedorf, Switzerland). TPC was estimated from the calibration curve (R2 = 0.9845), using gallic acid as a standard (concentration ranged 0.002–0.1 mg/mL). The results were expressed as mg of gallic acid equivalent (GAE) per 1 g of dry extract (DE). TFC was estimated from the calibrated curve (R2 = 0.995), using quercetin (0.004–0.11 mg/mL) as a standard. The results were expressed as mg of quercetin equivalent (QE) per 1 g of DE. Total phenolic acid (TPAC) content was assessed using Arnov’s reagent as described in the Polish Pharmacopoeia IX (an official translation of PhEur 7.0) [17]. The absorbance was measured at 490 nm. TPAC was estimated from the calibration curve (R2 = 0.9999), using caffeic acid as a standard in a concentration of 3.36–23.52 μg/mL. The results were ex-pressed as mg of caffeic acid equivalent (CAE) per 1 g of DE.
2.5. LC-MS Analysis
The chromatographic measurements were performed using the LC/MS system from Thermo Scientific (Q-EXATCTIVE and ULTIMATE 3000, San Jose, CA, USA) equipped with an ESI source. The ESI was operated in negative polarity modes under the following conditions: spray voltage—3.5 kV; sheath gas—40 arb. units; auxiliary gas—10 arb. units; sweep gas—10 arb. units; and capillary temperature—320 °C. Nitrogen (>99.98%) was employed as sheath, auxiliary, and sweep gas. The scan cycle used a full-scan event at a resolution of 60,000. A Gemini C18 column (4.6 × 100 mm, 3 μm) (Phenomenex, Torrance, CA, USA) was employed for chromatographic separation, which was performed using gradient elution. Mobile phase A was 25 mM formic acid in water; mobile phase B was 25 mM formic acid in acetonitrile. The gradient program started at 5% B, increasing to 95% for 60 min, followed by isocratic elution (95% B) for 10 min. The total run time was 70 min at the mobile phase flow rate 0.4 mL/min. The column temperature was 25 °C. In the course of each run, MS spectra in the range of 100–700 m/z were collected continuously. Additionally, the MS2 functions were used to carry out a detailed qualitative analysis. The collision energy for each examined compound was 25%.
The amounts of the identified compounds were carried out based on the calibration curves obtained for the standard. In the case of quantitative analysis of compounds that do not have standards, calibration curves for substances of similar structure were used. All the results are presented as the mean of three independent measurements (n = 3).
2.6. GC-MS Analysis
The qualification of the sample extract was performed using a GC-MS/MS system (GCMS-TQ8040; Shimadzu, Kyoto, Japan) equipped with a ZB5-MSi fused-silica capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness; Phenomenex, Torrance, CA, USA). Grade 5.0 helium was used as the carrier gas. Column flow was 1 mL/min. The injection of a 1 μL sample was performed using an AOC-20i + s type autosampler (Shimadzu, Kyoto, Japan). The injector was working at a temperature of 310 °C. The following temperature program was applied: the oven temperature was held at 60 °C for 2 min and was subsequently increased linearly at a rate of 6 °C/min to 310 °C, where it was held for 15 min. The mass spectrometer was operated in EI mode at 70 eV; the ion source temperature was 225 °C. The mass spectra were measured in the range 40–450 amu. The amounts of the individual analytes were estimated by the peak normalization method.
2.7. Antioxidant Activity
All antioxidant and enzyme inhibitory assays were done in 96-well plates (Nun-clon, Nunc, Roskilde, Denmark) using Infinite Pro 200F Elisa Reader (Tecan Group Ltd., Männedorf, Switzerland). The experiments were performed in triplicate.
2.7.1. DPPH• Assay
The 2,2-diphenyl-1-picryl-hydrazyl (DPPH•) free radical scavenging activity of Cotoneaster extracts and the positive control—ascorbic acid (AA)—was studied using the method described previously [18], but with some modifications. After 30 min of incubation at 28 °C, the decrease in DPPH• absorbance, caused by the tested extracts, was measured at 517 nm. The results were expressed as values of IC50.
2.7.2. ABTS•+ Assay
The ABTS•+ decolorization assay was the second method applied for the assessment of antioxidant activity [18]. The absorbance was measured at 734 nm. Trolox was used as a positive control. The results were expressed as values of IC50.
2.7.3. Metal Chelating Activity (CHEL)
The metal chelating activity was established using the method described by Guo et al. [19], modified in our previous study [18,20]. The absorbance was measured at 562 nm. As a positive control, Na2EDTA*2H2O was used. Results were expressed as the IC50 values of the Cotoneaster extracts based on concentration-inhibition curves.
2.8. Enzyme Inhibitory Activity
2.8.1. Cyclooxygenase-1 (COX-1) and Cyclooxygenase-2 (COX-2) Inhibitory Activity
The extracts of Cotoneaster species were examined for cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) inhibitory activity using a COX (ovine/human) Inhibitor Screening Assay Kit (Cayman Chemical, MI, USA) according to the protocol of the manufacturer. The extracts were tested at different concentrations. Indomethacin was used as a positive control.
2.8.2. Lipoxygenase Inhibitory Activity
Anti-lipoxygenase activity of Cotoneaster extracts was determined using the Lipoxygenase Inhibitor Screening Assay Kit (Cayman Chemical, MI, USA) according to the protocol of the manufacturer. The extracts were tested at different concentrations. The effective concentration (μg/mL) in which lipoxygenase activity is inhibited by 50% (IC50) was estimated graphically. Nordihydroguaiaretic acid (NDGA) was used as a positive control.
2.8.3. Hyaluronidase Inhibitory Activity
Anti-hyaluronidase activity was established using the method described by Liyanaarachchi et al. [21]. After 20 min incubation at 37 °C, the absorbance was measured at 585 nm. The extracts were tested at different concentrations. Epigallocatechin gallate was used as a positive control.
2.9. Bacterial Strains
The antibacterial power of Cotoneaster extracts was determined using bacterial strains causing skin diseases (including seborrhea and acne). We used microaerobic Gram-positive bacteria: Cutibacterium granulosum PCM 2462, C. acnes PCM 2334, C. acnes PCM 2400, (possessed from the Polish Collection of Microorganisms PCM Institute of Immunology and Experimental Therapy Polish Academy of Sciences, Poland, as Propionibacterium, now—Cutibacterium), C. acnes ATCC 11827; aerobic Gram-positive: Staphylococcus epidermidis ATCC 12228 and S. aureus ATCC 25923; and aerobic Gram-negative strains Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25992. Each bacterial strain was pre-incubated overnight at 37 °C on agar plates. Mueller–Hinton (BioMaxima S.A., Lublin, Poland) agar or broth (MH-agar, MH-broth) for aerobic strains and Brain–Heart Infusion (Oxoid Ltd, Altrincham, England) agar or broth (BHI-agar, BHI-broth) for microaerobic bacteria were used. The bacteria were suspended in 5 mL of sterile saline water, and the absorbance of this inoculum was adjusted to 108 CFU/mL (0.5 Mc’Farland scale).
2.10. Disc Diffusion Assay
This disc diffusion assay can evaluate the antibacterial potency of tested extracts and was made according to proven methods [22,23]. Briefly, solid medium in Petri dishes was smeared with inoculum of 0.5 Mc’Farland. The plant extracts were dissolved in DMSO (10 mg/mL) then loaded over sterile filter paper discs (8 mm in diameter) to obtain a final concentration of 0.1 mg per disc. Inoculated plates with samples were incubated at 37 °C for 24 h (aerobic stains) or 48 h (microaerobic bacteria).
The zones of growth inhibition around plant samples were measured [mm] and recognized as antibacterial potency. The larger the zone of growth inhibition, the greater the antibiotic activity.
2.11. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Determination
The MIC is the lowest concentration of a test compound that inhibits microbial proliferation. The test examined the MIC of the Cotoneaster extracts against Staphylococcus aureus ATCC 25923, S. epidermidis ATCC 12228, C. acnes ATCC 11827, C. acnes PCM 2334, C. acnes PCM 2400, and C. granulosum PCM 2462, whose optical density was 0.5 Mc’Farland scale. Double microdilution in the 96-well plate assay was used according to the CLSI method with some modification, as described in our paper [23,24]. Such an amount of the extract solution to the wells was added, that using the principle of double microdilution to obtain final concentrations in the range of 3.9–1000 µg/mL. Then, each well was inoculated (2 µL of inoculum density of 0.5 Mc’Farland). A background control (broth with extract), a negative control (the broth alone), and a positive control (broth and inoculum) were also prepared. The plates were incubated under conditions proper to bacterial growth (aerobic bacteria at 37 °C, for 24 h; anaerobic bacteria at 37 °C, for 48 h). Finally, microbial growth was determined using a BioTek Synergy H4 (BioTek, Winooski, VT, USA) plate reader at a wavelength of 600 nm.
MBC is the lowest concentration of the agent with bactericidal properties. To determine this, 96-well plates obtained in the previous experiment were used to determine the MIC of the tested extracts. Ten µL of solutions taken from the wells in which no bacterial growth was observed were applied to the new Petri plates. Then, the plates were incubated under optimal conditions for the growth of the given bacteria. After the incubation was completed, a visual assessment of the agar surface of the plates was made. The MBC was considered to be the one in which there was no visible bacterial growth on the solid medium.
To visualize the obtained results, it is advantageous to present the MBC/MIC ratio. Thus, MBC/MIC values ≤4 demonstrate that the agent is bactericidal. MBC/MIC values >4 indicate the bacteriostatic nature of the tested agents [25].
Microbiological tests were performed in three separate experiments (n = 3).
2.12. Cytotoxic Activity
This experiment was carried out according to the protocol described by us previously [26]. The cytotoxic activity of selected substances was evaluated toward the BJ cell line (normal human fibroblasts, ATCC CRL-2522TM). Briefly, the BJ cells were seeded in 96-well plates, and then after 24 h incubation at 37 °C in suitable conditions (5% CO2, 95% air), the culture medium was replaced with two-fold serial dilutions of investigated substances (1.95–1000 μg/mL). After 24 h incubation, the cell viability was assessed using the MTT assay. The obtained data were presented as mean values ± standard deviations (SD). These results were subjected to four-parameter nonlinear regression analyses (GraphPad Prism 5, version 5.04, GraphPad Software, San Diego, CA, USA) in order to determine values of half-maximum cytotoxic concentration (CC50).
2.13. Statistical Analysis
The results were expressed as mean values ± standard deviation (SD) of three independent experiments. The data from cell culture experiments were subjected to statistical analysis using unpaired Student’s t-test or One-Way ANOVA test, followed by a Tukey’s multiple comparison test, and differences were considered significant when p < 0.05 (GraphPad Prism 5, version 5.04, GraphPad Software, San Diego, CA, USA), whereas Principal Component Analysis was carried out in R version 3.6.3 (64-bit, Windows 10), using built-in “prcomp” function.
3. Results
3.1. Phytochemical Analysis
Total phenolic content (TPC) for Cotoneaster extracts and fractions was determined using Folin-Ciocalteu reagent, and the results were estimated as gallic acid equivalents (GAE) per g of dry extract (DE) (Table 1). Our results showed that the leaves of C. hissaricus (CHi) have the highest phenolic content (296.13 ± 1.52 mg GAE/g DE) than C. hsingshangensis (CHs) (193.84 ± 1.14 mg GAE/g DE). The results obtained in our study were better compared to data presented for extracts derived from the leaves of other Cotoneaster species. For instance, Kicel et al. [27] demonstrated that TPC for the 70% aqueous methanolic extracts of leaves of different Cotoneaster species cultivated in Poland varied from 51.7 (C. tomentosus) to 154.3 mg GAE/g (C. bullatus) of dry weight of the plant material. The same authors [28] also found lower amounts of phenolic compounds in 70% aqueous methanolic extracts of the fruits (from 26 to 43.5 mg GAE/g PM) of different Cotoneaster species.
Table 1.
The total content of phenolic (TPC), flavonoid (TFC), and phenolic acids (TPAC) in the C. hissaricus and C. hsingshangensis leaf extracts.
| Sample | Extraction Yield (% DE) |
Total Phenolic Content [mg GAE/g DE] |
Total Phenolic Acids [mg CAE/g DE] |
Total Flavonoid Content [mg QE/g DE] |
|---|---|---|---|---|
| CHi | 15.30 | 193.84 ± 1.14 e,f,g,h,i | 33.80 ± 1.03 e,f,g,h,i | 25.38 ± 0.35 e,f,g,h,i |
| CHi-1 | 3.00 | 83.87 ± 0.23 a,e,f,g,h,i | 10.31 ± 0.11 a,e,f,g,h,i | 0.12 ± 0.05 a,e,g,h,i |
| CHi-2 | 0.89 | 348.05 ± 2.81 a,b,e,f,g,h,i | 40.57 ± 0.19 a,b,e,f,g,h,i | 72.12 ± 0.35 a,b,e,f,g,h,i |
| CHi-3 | 17.29 | 219.00 ± 0.49 a,b,c,e,f,g,h,i | 37.83 ± 0.15 a,b,c,e,f,g,h,i | 58.71 ± 0.49 a,b,c,e,f,g,h,i |
| CHi-4 | 6.86 | 463.16 ± 3.94 a,b,c,d,e,f,g,h,i | 56.12 ± 0.27 a,b,c,d,e,f,g,h | 134.89 ± 0.51 a,b,c,d,i |
| CHs | 27.22 | 296.13 ± 1.52 | 61.27 ± 0.93 | 47.72 ± 0.37 |
| CHs-1 | 6.73 | 143.30 ± 1.09 e | 19.65 ± 0.30 e | 0.23 ± 0.02 e |
| CHs-2 | 2.07 | 486.04 ± 3.17 e,f | 83.94 ± 0.25 e,f | 97.66 ± 0.18 e,f |
| CHs-3 | 14.04 | 381.83 ± 2.53 e,f,g | 70.17 ± 0.13 e,f,g | 146.58 ± 1.10 e,f,g |
| CHs-4 | 5.76 | 559.77 ± 3.76 e,f,g,h | 91.95 ± 0.48 e,f,g,h | 252.27 ± 0.24 e,f,g,h |
DE—dry extract; GAE—Gallic Acid Equivalent; CAE—Caffeic Acid Equivalent; QE—Quercetin Equivalent; CHi—methanol–acetone–water (3:1:1, v/v) extract of C. hissaricus; CHs—methanol–acetone–water (3:1:1, v/v) extract of C. hsingshangensis; CHi-1—water fraction of C. hissaricus, CHi-2—diethyl ether fraction of C. hissaricus, CHi-3—butanol fraction of C. hissaricus, CHi-4—ethyl acetate fraction of C. hissaricus; CHs-1—water fraction of C. hsingshangensis, CHs-2—diethyl ether fraction of C. hsingshangensis, CHs-3—butanol fraction of C. hsingshangensis, CHs-4—ethyl acetate fraction of C. hsingshangensis. Values were presented as mean ± standard deviation (n = 9). Statistical analysis: a—significantly different results compared to CHi; b—significantly different results compared to CHi-1; c—significantly different results compared to CHi-2; d—significantly different results compared to CHi-3; e—significantly different results compared to CHs; f—significantly different results compared to CHs-1; g—significantly different results compared to CHs-2; h—significantly different results compared to CHs-3; i—significantly different results compared to CHs-4; One-Way ANOVA test, followed by a Tukey’s multiple comparison test, p < 0.05.
In our study, we also examined the content of phenolics in fractions obtained after fractionating crude extracts between solvents of different polarity. The results showed that the TPC level was in the range of 83.87 ± 0.23 (water fraction of C. hissaricus) to 559.77 ± 3.76 mg GAE/g DE (ethyl acetate fraction of C. hsingshangensis). Kicel and co-authors [29] obtained higher values with the highest TPC level for ethyl acetate fractions (470.9–650.8 mg GAE/g dw) and diethyl-ether fractions (453.1–546.9 mg GAE/g dw) of defatted methanol extracts.
Thus, the results obtained in our study indicated that the leaves of C. hissaricus and C. hsingshangensis are a rich source of phenolic compounds.
The total flavonoid content of the leaves of C. hissaricus and C. hsingshangensis was estimated by a previously described colorimetric method [27]. The data were expressed as quercetin equivalents (QE) per g of dry extract (DE). The results presented in Table 1 show that the content of total flavonoids in both species is at an average level. The comparable content was noted for the leaves of C. hissaricus (CHi) and C. hsingshangensis (CHs) (25.38 ± 2.35 and 47.72 ± 0.37 mg QE/g DE, respectively). The results obtained in our study were higher than those found by Mahmutović-Dizdarević et al. [11]. In their study, quantitative estimation revealed that methanolic extracts from leaves of C. tomentosus possessed 18.17 ± 0.30 mg QE/g dw. flavonoid content, followed by C. integerrimus—16.42 ± 0.35 mg QE/g dw. and C. horizontalis—10.55 ± 0.51 mg QE/g dw. Moreover, they found that fruits of these three species contain a lower amount of flavonoids—2.76–9.38 mg QE/g dw. A higher amount of flavonoids was found in the methanolic extract of leaves of C. wilsonii Nakai (36.46 ± 1.89 mg QE/g dw), while in the stems and fruits, the content of flavonoids was lower (6.09 ± 0.71 and 0.23 ± 0.20 mg QE/g dw, respectively) [30]. Kicel et al. [29] also obtained lower results for defatted methanol extracts of the three Cotoneaster species cultivated in Poland, and the values were from 14.70 ± 0.05 to 67.71 ± 0.80 mg/g dw. These authors obtained slightly higher results for the fractions obtained from crude extracts compared to the results in our study. The highest level of flavonoids was determined for the ethyl acetate fraction of C. integerrimus leaves (403.56 ± 7.48 mg/g dw). In our study, the lowest amount of total flavonoids was found in water fractions of C. hissaricus and C. hsingshangensis (0.12 ± 0.05 and 0.23 ± 0.02 mg QE/g DE, respectively) and the highest was in the ethyl acetate fraction of C. hsingshangensis (252.27 ± 0.24 mg QE/g DE).
The total phenolic acid content (TPAC) in Cotoneaster extracts is presented in Table 1. As for the content of polyphenols and flavonoids, a higher content of phenolic acids was noted for crude extract (61.27 ± 0.93 mg CAE/g DE) and fractions (19.65 ± 0.30–91.95 ± 0.48 mg CAE/g DE) of C. hsingshangensis.
The use of plants in the cure of different diseases depends on their phytochemical composition. Our research of the presence of active compounds in the crude methanol–acetone–water (3:1:1, v/v/v) extracts and different fractions (diethyl ether, ethyl acetate, butanol, and aqueous residual) indicated that methanol–acetone–water extracted the greatest range of constituents from the leaves of both Cotoneaster species. Therefore, in our study, we used only the crude extracts for the LC-MS and GC–MS analyses.
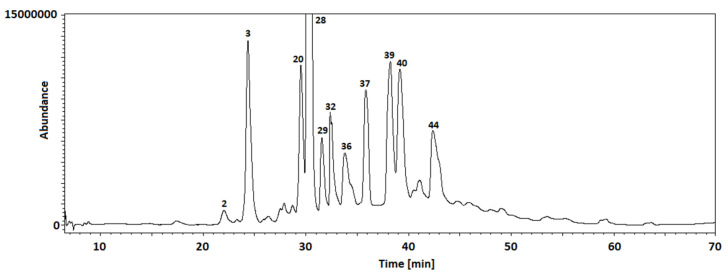
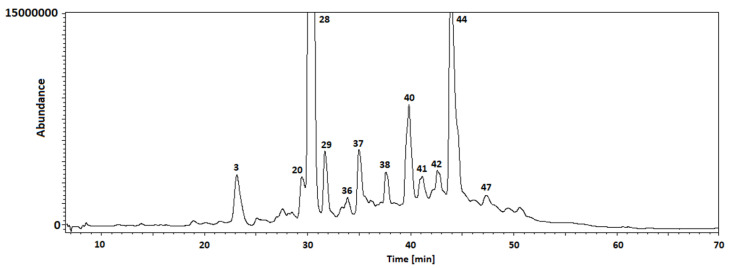
Thus, in the next step of our study, the chemical composition of the extracts obtained from Cotoneaster extracts was investigated using the LC-MS method. Table 2 shows 47 identified compounds, including their molecular formula, theoretical and experimental molecular mass, both errors in ppm and mDa, and the fragments. In our study, flavonoids, phenolic acids, coumarins, cyanogenic glycosides, sphingolipids, and carbohydrates were identified using LC-MS analysis. The chromatograms with marked main peaks are displayed in Figure 3 and Figure 4. The results of the quantitative analysis are presented in Table 3. The amounts of the identified compounds were carried out based on the calibration curves obtained for the standard. In the case of quantitative analysis of compounds that do not have standards, calibration curves for substances of similar structure were used.
Table 2.
High-resolution mass spectrometry (HR-MS) of [M − H]− ion and MS2 data.
| Peak No. | Name of Compound |
[M − H]− | MS2 | Theoretical Mass [M − H]− (Da) | Experimental Mass [M − H]− (Da) | Δ ppm | Δ mDa | Elemental Composition |
|---|---|---|---|---|---|---|---|---|
| 1 | Mannitol | 181 | 59,71,73,85,89,101,113,119,163 | 181.07122 | 181.07118 | 0.22 | −0.04 | C6H13O6 |
| 4 | Quercetin-3-O-(2″-O-xylosyl)galactoside | 595 | 271,301b,435 | 595.12992 | 595.12999 | 0.12 | 0.07 | C26H27O16 |
| 5 | Quercetin-3-O-gentiobioside | 625 | 271,301b,463 | 625.14048 | 625.14038 | 0.16 | −0.10 | C27H29O17 |
| 6 | Vitexin-2″-O-arabinoside | 563 | 283,432b | 563.14009 | 563.14001 | 0.14 | −0.08 | C26H27O14 |
| 7 | Apigenin-6,8-C-dicelobioside | 593 | 325,386b,387 | 593.15065 | 593.15077 | 0.20 | 0.12 | C27H29O15 |
| 8 | Vitexin-2″-O-rhamnoside | 577 | 283,432b | 577.15574 | 577.15571 | 0.05 | −0.03 | C27H29O14 |
| 9 | Quercetin-3-O-glucoside (Isoquercitrin) | 463 | 271,300b,301 | 463.08766 | 463.08775 | 0.19 | 0.09 | C21H19O12 |
| 10 | Quercetin-3-O-galactoside (Hyperoside) | 463 | 271,300b,301 | 463.08766 | 463.08763 | 0.06 | −0.03 | C21H19O12 |
| 11 | Kaempferol-3-O-glucoside (Astragalin) | 447 | 284b,300 | 447.09274 | 447.09289 | 0.34 | 0.15 | C21H19O11 |
| 12 | Quercetin-3-O-rhamnoside (Quercitrin) | 447 | 284b,300 | 447.09274 | 447.09271 | 0.07 | −0.03 | C21H19O11 |
| 13 | 7-Methylkaempferol-4′-O-glucoside | 461 | 298b,315 | 461.10839 | 461.10849 | 0.22 | 0.10 | C22H21O11 |
| 14 | 3′,4′-dihydroxy-6-methoxyflavone-7-O-rhamnoside | 429 | 255,283b,400,401 | 429.11856 | 429.11853 | 0.07 | −0.03 | C22H21O9 |
| 15 | Apigenin-8-C-glucoside (Vitexin) | 431 | 283,311b,312,341 | 429.11856 | 429.11842 | 0.33 | −0.14 | C21H19O10 |
| 16 | Apigenin-7-O-glucoside | 447 | 269b,270,431 | 431.09783 | 431.09797 | 0.32 | 0.14 | C21H19O10 |
| 17 | Biochanin A-7-O-glucoside (Sissotrin) | 445 | 132,211,223,224,239b,240,267 | 445.11348 | 445.11355 | 0.16 | 0.07 | C22H21O10 |
| 18 | 5,7,2′,5′-tetrahydroxyflavanone-7-O-glucoside | 449 | 286b,302 | 449.10839 | 449.10825 | 0.31 | −0.14 | C21H21O11 |
| 19 | 5-Methylgenistein-4′-O-glucoside | 445 | 255,283b,417,430 | 445.11348 | 445.11339 | 0.20 | −0.09 | C22H21O10 |
| 20 | Gentisic acid 2-O-glucoside (Orbicularin) | 315 | 153b,271 | 315.07161 | 315.07149 | 0.38 | −0.12 | C13H15O9 |
| 27 | Caffeoylmalic acid | 295 | 133,135,179b | 295.0454 | 295.04533 | 0.24 | −0.07 | C13H11O8 |
| 28 | Prunasin | 294 | 161b | 294.09777 | 294.09771 | 0.20 | −0.06 | C14H16NO6 |
| 29 | Amygdalin | 456 | 89,119,143,179,221,263,323b | 456.15059 | 456.15047 | 0.26 | −0.12 | C20H26NO11 |
| 33 | 7,8-dimethoxy-6-hydroxycoumarin | 221 | 191,206b,207 | 221.045 | 221.04506 | 0.27 | 0.06 | C11H9O5 |
| 35 | Cotonoate A | 291 | 121,165,247b | 291.15964 | 291.15975 | 0.38 | 0.11 | C17H23O4 |
| 36 | Horizontoate A | 263 | 148,164,219b | 263.12834 | 263.12852 | 0.68 | 0.18 | C15H19O4 |
| 37 | 3,3′,4′-tri-O-methylellagic acid | 343 | 255,284,299b,329 | 343.0454 | 343.04554 | 0.41 | 0.14 | C17H11O8 |
| 40 | Scopoletin | 191 | 104,105,120,148b,176 | 191.03444 | 191.03439 | 0.26 | −0.05 | C10H7O4 |
| 41 | Arbutin | 271 | 71,101,108b,109,113,161 | 271.08178 | 271.08175 | 0.11 | −0.03 | C12H15O7 |
| 42 | 5-Methylgenistein | 283 | 239b,255,269 | 283.06065 | 283.06053 | 0.42 | −0.12 | C16H11O5 |
| 44 | Eriodictyol | 287 | 151,162b | 287.05557 | 287.05551 | 0.21 | −0.06 | C15H11O6 |
| 45 | 5,7,2′,5′-tetrahydroxyflavanone | 287 | 151,162b,259 | 287.05557 | 287.05564 | 0.24 | 0.07 | C15H11O6 |
| 46 | Naringenin | 271 | 119,151b,187 | 271.06065 | 271.06061 | 0.15 | −0.04 | C15H11O5 |
| 47 | Horizontoate C | 482 | 210,272b,288 | 482.42093 | 482.42097 | 0.08 | 0.04 | C29H56NO4 |
b—base peak.
Figure 3.
TIC chromatogram of C. hissaricus extract.
Figure 4.
TIC chromatogram of C. hsingshangensis extract.
Table 3.
Content of active compounds in the leaves of C. hissaricus (CHi) and C. hsingshangensis (CHs).
| No | Compound | Calibration Standard | Amounts [μg/g DE] | |
|---|---|---|---|---|
| CHi | CHs | |||
| 1 | mannitol | glucose | 6834 ± 249 | 3104 ± 123 * |
| 2 | ascorbic acid | ascorbic acid | 298 ± 10 | 1726 ± 66 * |
| 3 | quercetin 3-O-rutinoside (rutin) | rutin | 18,028 ± 650 | 3823 ± 131* |
| 4 | quercetin 3-O-(2″-O-xylosyl)galactoside | rutin | 7318 ± 289 | 2667 ± 99 * |
| 5 | quercetin 3-O-gentiobioside | rutin | 2759 ± 109 | 2310 ± 87 |
| 6 | vitexin 2″-O-arabinoside | rutin | 5625 ± 233 | 3249 ± 121 * |
| 7 | apigenin 6,8-C-dicelobioside | rutin | 5926 ± 225 | 1273 ± 50 * |
| 8 | vitexin 2″-O-rhamnoside | rutin | 3923 ± 154 | 1268 ± 49 * |
| 9 | quercetin 3-O-glucoside (isoquercitrin) | rutin | 10,079 ± 353 | 8926 ± 327 * |
| 10 | quercetin 3-O-galactoside (hyperoside) | rutin | 9119 ± 331 | 6184 ± 237 * |
| 11 | kaempferol 3-O-glucoside (astragalin) | rutin | 2480 ± 92 | 4430 ± 168 * |
| 12 | quercetin 3-O-rhamnoside (quercitrin) | rutin | 2158 ± 80 | 6726 ± 251 * |
| 13 | 7-methylkaempferol 4′-O-glucoside | rutin | 3939 ± 137.9 | 2079 ± 79 * |
| 14 | 3′,4′-dihydroxy-6-methoxyflavone 7-O-rhamnoside | rutin | 1538 ± 50 | 443 ± 16 * |
| 15 | apigenin 8-C-glucoside (vitexin) | rutin | 2454 ± 94 | 1930 ± 77 * |
| 16 | apigenin 7-O-glucoside | rutin | 1486 ± 57 | 3734 ± 149 * |
| 17 | biochanin A 7-O-glucoside (sissotrin) | rutin | 724 ± 26 | 709 ± 23 |
| 18 | 5,7,2′,5′-tetrahydroxyflavanone 7-O-glucoside | rutin | 8067 ± 290 | 2469 ± 93 * |
| 19 | 5-methylgenistein 4′-O-glucoside | rutin | 1205 ± 43 | 943 ± 34 * |
| 20 | orbicularin | quercetin | 3838 ± 154 | 1818 ± 79 * |
| 21 | p-hydroxybenzoic acid | p-hydroxybenzoic acid | 897 ± 30 | 823 ± 27 * |
| 22 | benzoic acid | benzoic acid | 339 ± 12 | 441 ± 16 |
| 23 | gentisic acid | gentisic acid | 258 ± 10 | <LOQ |
| 24 | protocatechuic acid | protocatechuic acid | 509 ± 20 | 69 ± 3 * |
| 25 | syringic acid | syringic acid | 753 ± 31 | 1130 ± 39 * |
| 26 | vanillic acid | vanillic acid | <LOQ | <LOQ |
| 27 | caffeoylmalic acid | caffeic acid | 2828 ± 109 | 1459 ± 51 * |
| 28 | chlorogenic acid | chlorogenic acid | 37,932 ± 1330 | 60,043 ± 2231 * |
| 29 | prunasin | glucose | 3360 ± 127 | 1226 ± 61 |
| 30 | p-coumaric acid | p-coumaric acid | 329 ± 13 | 539 ± 19 * |
| 31 | amygdalin | glucose | 1803 ± 63 | 555 ± 19 * |
| 32 | caffeic acid | caffeic acid | 6118 ± 223 | 2220 ± 81 * |
| 33 | cinnamic acid | cinnamic acid | 924 ± 31 | 2429 ± 89 * |
| 34 | ferulic acid | ferulic acid | 644 ± 24 | 1567 ± 65 * |
| 35 | salicylic acid | salicylic acid | 657 ± 27 | 823 ± 30 * |
| 36 | 7,8-dimethoxy-6-hydroxycoumarin | umbelliferone | 1209 ± 43 | <LOQ |
| 37 | cotonoate A | benzoic acid | 1564 ± 55 | <LOQ |
| 38 | horizontoate A | benzoic acid | <LOQ | <LOQ |
| 39 | 3,3′,4′-tri-O-methylellagic acid | quercetin | 2053 ± 81 | 1169 ± 45 * |
| 40 | scopoletin | umbelliferone | 12,219 ± 440 | 10,481 ± 371 * |
| 41 | arbutin | glucose | 1126 ± 41 | 1084 ± 46 * |
| 42 | 5-methylgenistein | quercetin | 1109 ± 39 | 1423 ± 59 * |
| 43 | quercetin | quercetin | 229 ± 7 | 115 ± 4 * |
| 44 | horizontoate C | oleic acid | 5516 ± 219 | 2939 ± 119 * |
| 45 | eriodictyol | quercetin | 59 ± 3 | <LOQ |
| 46 | 5,7,2′,5′-tetrahydroxyflavanone | quercetin | 89 ± 5 | <LOQ |
| 47 | naringenin | quercetin | 2627 ± 97 | 2121 ± 88 * |
LOQ—limit of quantification; DE—dry extract. Asterisk (*) denotes significantly different data (p < 0.05, unpaired t-test) compared to CHi.
Among flavonoids, the most abundant in both species were quercetin derivatives. In the leaves of C. hissaricus rutin (18,028 ± 650 μg/g DE), isoquercitrin (10,079 ± 353 μg/g DE), hyperoside (9119 ± 331 μg/g DE), 5,7,2′,5′-tetrahydroxyflavanone 7-O-glucoside (8067 ± 290 μg/g DE), and quercetin 3-O-(2″-O-xylosyl)galactoside (7318 ± 289 μg/g DE) were found in the largest amount. In the leaves of C. hsingshangensis, isoquercitrin (8926 ± 327 μg/g DE), quercitrin (6726 ± 251 μg/g DE), and hyperoside (6184 ± 237 μg/g DE) were observed in the greatest amount. Isoquercitrin, rutin, hyperoside, and quercitrin were previously identified as dominant in the other Cotoneaster species [27,28,31,32,33,34,35,36,37]. Vitexin 2″-O-arabinoside, vitexin-2″-O-rhamnoside, and 5-methylgenistein, which were found in large quantities in both studied species, were previously noticed only in leaves of C. thymaefolia [32], aqueous alcoholic extracts of leafy twigs of C. obricularis [38], and chloroform extracts of C. simonsii leafy twigs [39], respectively. Subsequent compounds of rare occurrence in the Cotoneaster genus are 5,7,2′,5′-tetrahydroxyflavanone and its 7-O-glucoside, which were identified in C. thymaefolia leaves [32]. It is worth noting that rare in nature flavonoids, such as biochanin A 7-O-glucoside (sissotrin) and 5-methylgenistein-4′-O-glucoside were observed in both studied species. Previously, sissotrin was found only in the n-butanol fraction of the methanolic extract of leaves of C. mongolica [37], methanol extract of C. serotina flowers [40], and methanol extract of flowers and fruits of C. pannosa [40]. 5-methylgenistein-4′-O-glucoside was previously observed only in the chloroform extract of leafy twigs of C. simonsii [39]. Quercetin 3-O-gentiobioside (quercetin 3-O-β-d-glucopyranosyl(l-6)glucopyranoside) is noteworthy regarding the fact that it was one of the first compounds isolated from the Cotoneaster genus and is infrequent, being only reported in C. oligantha stems [41]. Furthermore, kaempferol 3-O-glucoside (astragalin) is of rare occurrence in the Cotoneaster genus and reported only in the methanol extract of C. mongolica leaves by Odontuya and co-authors [37].
The second largest group of active compounds were phenolic acids, and among them, chlorogenic acid, gentisic acid 2-O-glucoside, and caffeoylmalic acid were the most abundant in both species. Apart from these typical phenolic acids, cotonoate A and horizontoate A were observed in the leaves of C. hissaricus and C. hsingshangensis, but only in C. hissaricus was cotonoate A identified in quantifiable amounts (1564 ± 55 μg/g DE). In previous research, cotonoate A and horizontoate A were isolated only from the leafy twigs of the C. racemiflora chloroform soluble fraction of the methanolic extract [14] and methanolic extract of C. horizontalis [15], respectively.
Chlorogenic acid seems to be a ubiquitous ingredient among the Cotoneaster genus. What most attracts attention is that both studied extracts of leaves of C. hissaricus and C. hsingshangensis constitute a significant percentage of this acid (37,932 ± 1330 and 60,043 ± 2231 μg/g DE, respectively). According to [42], this compound participates in diminishing P. acnes-induced matrix metalloproteinase-9 levels, obstructing nuclear factor-κB (NF-κB) activation, inactivating mitogen-activated protein kinases (MAPK), and decreasing the migration of neutrophils and interleukin (IL)-1β+ populations in vivo. Therefore, it is increasingly being recognized as possibly efficacious in the management of dermatological conditions, such as acne.
Among the other polyphenolic compounds, some coumarins were identified in the leaves of C. hissaricus and C. hsingshangensis, and the most abundant in both species was scopoletin (12,219 ± 440 and 10,481 ± 371 μg/g DE, respectively). This compound was isolated earlier from the leafy twigs of C. racemiflora from methanolic extract [43] and the ethyl acetate-soluble fraction of methanolic extract [44,45].
In both studied species, great amounts of mannitol were also observed (C. hissaricus—6834 ± 249 μg/g DE and 3104 ± 123 μg/g DE—C. hsingshangensis). This compound is the predominant polysaccharide of manna, which is produced by the young shoots of C. discolor, C. nummularius, C. tricolor, and C. nummularioides [46]. From cyanogenic glycosides, prunasin, and amygdalin were found. These glycosides were also previously identified in the fruits and leaves of C. congesta, C. praecox, and C. integerrimus [47] and in the ethanol extract of C. horizontalis leafy twigs [48].
Between phenolic compounds occurring in C. hsingshangensis and C. hissaricus, caffeic acid [44,45], ferulic acid [49,50], chlorogenic acid [51], cinnamic acid [52], quercetin [53], and vitexin [54] are substances with proven anti-acne properties on skin.
The appropriate GC-MS procedure allowed for the identification of 42 and 41 compounds in the leaves of C. hissaricus (Table 4, Figure 5) and C. hsingshangensis (Table 5, Figure 6), respectively. Among the identified compounds, four main groups of analytes can be distinguished—sugar derivatives, phytosterols, fatty acids, and their esters.
Table 4.
Composition of the extracts of leaves of C. hissaricus (% of total fraction; mass%, GC).
| No. | tr | Area | %Area | Compound |
|---|---|---|---|---|
| 1 | 9.776 | 39557931 | 2.52 | Benzofuran, 2,3-dihydro- |
| 2 | 10.908 | 12341078 | 0.79 | Isosorbide |
| 3 | 12.913 | 3452024 | 0.22 | Furan, 3-(4-methyl-5-trans-phenyl-1,3-oxazolidin-2-yl)- |
| 4 | 13.952 | 192528086 | 12.29 | 1,3:2,5-Dimethylene-L-rhamnitol |
| 5 | 14.242 | 135536063 | 8.65 | d-Glycero-d-galacto-heptose |
| 6 | 15.48 | 7430877 | 0.47 | Megastigmatrienone |
| 7 | 15.988 | 32418863 | 2.07 | 3-Hydroxy-.beta.-damascone |
| 8 | 16.329 | 229108049 | 14.62 | 2-Deoxy-d-galactose |
| 9 | 17.009 | 21401978 | 1.37 | 2-Hydroxyhexadecyl butanoate |
| 10 | 17.229 | 21671810 | 1.38 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 11 | 17.448 | 5862283 | 0.37 | Myristic acid methyl ester |
| 12 | 17.709 | 6588889 | 0.42 | 9-(3,3-Dimethyloxiran-2-yl)-2,7-dimethylnona-2,6-dien-1-ol |
| 13 | 18.137 | 7027919 | 0.45 | 6-Hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one |
| 14 | 18.951 | 19840196 | 1.27 | Neophytadiene |
| 15 | 19.034 | 6060805 | 0.39 | 2-Pentadecanone, 6,10,14-trimethyl- |
| 16 | 19.222 | 10207664 | 0.65 | [1,1′-Bicyclopropyl]-2-octanoic acid, 2′-hexyl-, methyl ester |
| 17 | 19.496 | 10733207 | 0.69 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol |
| 18 | 20.028 | 157760294 | 10.07 | Hexadecanoic acid, methyl ester |
| 19 | 20.525 | 95409334 | 6.09 | Palmitic acid |
| 20 | 21.372 | 11475712 | 0.73 | Cyclohexanebutanoic acid |
| 21 | 21.913 | 3242083 | 0.21 | Methyl trans-4-(2-nonylcyclopentyl)butanoate |
| 22 | 22.029 | 10523560 | 0.67 | cis-11,14-Eicosadienoic acid, methyl ester |
| 23 | 22.104 | 33503994 | 2.14 | Linolenic acid methyl ester |
| 24 | 22.17 | 5141879 | 0.33 | 9-Octadecenoic acid (Z)-, methyl ester |
| 25 | 22.251 | 247573718 | 15.79 | Phytol, acetate |
| 26 | 22.387 | 53091621 | 3.39 | Methyl stearate |
| 27 | 23.854 | 32049624 | 2.05 | Benzyl.beta.-d-glucoside |
| 28 | 24.561 | 3681746 | 0.24 | Methyl 18-methylnonadecanoate |
| 29 | 24.852 | 4060228 | 0.26 | 4,8,12,16-Tetramethylheptadecan-4-olide |
| 30 | 25.205 | 2015357 | 0.13 | 3,7,11,15-Tetramethylhexadec-2-en-1-yl acetate |
| 31 | 26.197 | 5666720 | 0.36 | 1-Heptacosanol |
| 32 | 26.403 | 17982618 | 1.15 | 1-Docosanol, methyl ether |
| 33 | 27.053 | 3938497 | 0.25 | Phytyl palmitate |
| 34 | 28.102 | 4304975 | 0.27 | 9-Hexacosene |
| 35 | 28.421 | 2176024 | 0.14 | Methyl 18-methylnonadecanoate |
| 36 | 31.436 | 1813814 | 0.12 | Cholesta-4,6-dien-3-ol, (3.beta.)- |
| 37 | 31.551 | 8162207 | 0.52 | Octatriacontyl trifluoroacetate |
| 38 | 31.851 | 40546560 | 2.59 | dl-.alpha.-Tocopherol |
| 39 | 33.331 | 54433136 | 3.47 | beta.-Sitosterol |
| 40 | 33.63 | 1502659 | 0.10 | Acetyl betulinaldehyde |
| 41 | 33.87 | 1185800 | 0.08 | Urs-12-en-28-al |
| 42 | 34.019 | 3680922 | 0.23 | Acetyl betulinaldehyde |
Figure 5.
GC/MS chromatogram (in TIC mode) of C. hissaricus extract.
Table 5.
Composition of the extracts of leaves of C. hsingshangensis (% of total fraction; mass%, GC).
| No. | tr | Area | %Area | Compound |
|---|---|---|---|---|
| 1 | 9.655 | 1862168 | 0.19 | Benzofuran, 2,3-dihydro- |
| 2 | 10.990 | 2840045 | 0.28 | Isosorbide |
| 3 | 12.933 | 560231 | 0.06 | Furan, 3-(4-methyl-5-trans-phenyl-1,3-oxazolidin-2-yl)- |
| 4 | 13.854 | 146558815 | 14.62 | 1,3:2,5-Dimethylene-L-rhamnitol |
| 5 | 14.235 | 38707499 | 3.86 | d-Glycero-d-galacto-heptose |
| 6 | 14.608 | 21753970 | 2.17 | Lauric acid methyl ester |
| 7 | 15.280 | 11348450 | 1.13 | Tridecanoic acid |
| 8 | 15.501 | 4033264 | 0.40 | Megastigmatrienone |
| 9 | 16.125 | 55318375 | 5.52 | 2-Deoxy-d-galactose |
| 10 | 16.648 | 30284734 | 3.02 | d-Glucitol, 2,5-anhydro-1-O-octyl- |
| 11 | 17.070 | 6971059 | 0.70 | 2-Hydroxyhexadecyl butanoate |
| 12 | 17.456 | 8567378 | 0.85 | Myristic acid methyl ester |
| 13 | 17.725 | 2962331 | 0.30 | 9-(3,3-Dimethyloxiran-2-yl)-2,7-dimethylnona-2,6-dien-1-ol |
| 14 | 18.153 | 4592151 | 0.46 | 6-Hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one |
| 15 | 18.416 | 10513893 | 1.05 | Benzenesulfonamide, N-butyl- |
| 16 | 18.952 | 13881898 | 1.38 | Neophytadiene |
| 17 | 19.037 | 7043508 | 0.70 | 2-Pentadecanone, 6,10,14-trimethyl- |
| 18 | 19.269 | 4920685 | 0.49 | [1,1′-Bicyclopropyl]-2-octanoic acid, 2′-hexyl-, methyl ester |
| 19 | 19.500 | 7077957 | 0.71 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol |
| 20 | 20.027 | 170606966 | 17.02 | Hexadecanoic acid, methyl ester |
| 21 | 20.515 | 51495640 | 5.14 | Palmitic acid |
| 22 | 21.239 | 2788195 | 0.28 | Cyclohexanebutanoic acid |
| 23 | 21.920 | 3248248 | 0.32 | Methyl trans-4-(2-nonylcyclopentyl)butanoate |
| 24 | 22.033 | 9779382 | 0.98 | cis-11,14-Eicosadienoic acid, methyl ester |
| 25 | 22.105 | 34030060 | 3.39 | Linolenic acid methyl ester |
| 26 | 22.172 | 3962623 | 0.40 | 9-Octadecenoic acid (Z)-, methyl ester |
| 27 | 22.247 | 216221222 | 21.55 | Phytol, acetate |
| 28 | 22.386 | 56126780 | 5.60 | Methyl stearate |
| 29 | 23.876 | 3905372 | 0.39 | Benzyl.beta.-d-glucoside |
| 30 | 24.565 | 3540084 | 0.35 | Methyl 18-methylnonadecanoate |
| 31 | 24.857 | 1679444 | 0.17 | 4,8,12,16-Tetramethylheptadecan-4-olide |
| 32 | 25.213 | 1068195 | 0.11 | 3,7,11,15-Tetramethylhexadec-2-en-1-yl acetate |
| 33 | 26.211 | 1769239 | 0.18 | 1-Heptacosanol |
| 34 | 27.057 | 2638778 | 0.26 | Phytyl palmitate |
| 35 | 28.117 | 1110457 | 0.11 | 9-Hexacosene |
| 36 | 28.430 | 1074607 | 0.11 | Methyl 18-methylnonadecanoate |
| 37 | 28.792 | 1507426 | 0.15 | Neophytadiene |
| 38 | 29.305 | 1886233 | 0.19 | 2,6,10,14,18-Pentamethyl-2,6,10,14,18-eicosapentaene |
| 39 | 31.856 | 19787504 | 1.97 | dl-α-Tocopherol |
| 40 | 33.332 | 33729826 | 3.36 | β-Sitosterol |
| 41 | 34.009 | 802361 | 0.08 | Acetyl betulinaldehyde |
Figure 6.
GC/MS chromatogram (in TIC mode) of C. hsingshangensis extract.
The dominant group of compounds included in the extract from C. hissaricus are sugar derivatives; their content exceeds 37%. 1,3:2,5-dimethylene-l-rhamnitol, the content of which in the extract is 12.29%, and 2-deoxy-d-galactose, the content of which is 14.62%, are noteworthy. In the case of the extract of C. hsingshangensis, the content of sugar derivatives does not exceed 30%. The estimated amount of 1,3:2,5-dimethylene-l-rhamnitol (14.62%) is at a similar level of concentrations, while the content of 2-deoxy-d-galactose is nearly three times lower. The second major group of identified compounds is phytosterols. The content of those compounds in the extract of C. hissaricus exceeds 21%, while the amount of the main phytosterol, phytol acetate, is 15.79%. The extract of C. hsingshangensis, in turn, contains an approximately 25% higher concentration of phytosterols (more than 26%). The third major group of compounds in both extracts was fatty acids and their esters. Among the most important are hexa-decanoic acid, methyl ester (which constitutes 10.07%—CHi; 17.02%—CHs), followed by palmitic acid (6.09%—CHi; 5.14%—CHs), and linolenic acid (2.14%—CHi; 3.39%—CHs). Linolenic and palmitic acids were previously recorded as the major fatty acids in the fruits of C. zabelii, C. splendens, C. hjelmqvistii, and C. horizontalis [28] and the aerial parts of C. horizontalis [55].
Based on the GC/MS analysis, it was found that most of the identified compounds were present in both C. hissaricus and C. hsingshangensis. However, ((E)-2,6-dimethoxy-4-(prop-1-en-1-yl)phenol, 1-docosanol, methyl ether, 3-hydroxy-β-damascone, cholesta-4,6-dien-3-ol, (3β)-, octatriacontyl trifluoroacetate, and urs-12-en-28-al were only detected in C. hissaricus leaves, and 2,6,10,14,18-pentamethyl-2,6,10,14,18-eicosapentaene, benzenesulfonamide, N-butyl-, d-glucitol, 2,5-anhydro-1-O-octyl-, lauric acid methyl ester, and tridecanoic acid were only found in C. hsingshangensis.
It Is worth mentioning that the pronounced bioactivity of phenols toward skin disorders is enhanced by the simultaneous presence of such components as 6-hydroxy-4,4,7a-trimethyl-5,6,7,7a tetrahydrobenzofuran-2(4H)-one, which was reported as a strong anti-inflammatory agent [56], and which was identified in both studied Cotoneaster species.
A broad variety of active compounds in Cotoneaster species lead to overlapping activities of miscellaneous agents, hence acting on several levels of different diseases caused by oxidative stress is possible.
3.2. Biological Activity
Taking into account that both oxidation and inflammation play significant roles in disease pathogenesis, recent research has focused on the evaluation of the antioxidant and anti-inflammatory activity of plants. In our study, we examined in vitro antioxidant, anti-cyclooxygenase, anti-lipoxygenase, anti-hyaluronidase, antibacterial, and cytotoxic activities of leaves of C. hissaricus and C. hsingshangensis.
3.2.1. Antioxidant Activity
The antioxidant activity was studied on the microplate scale in cell-free systems. The Cotoneaster extracts were evaluated in a concentration ranging from 10 to 150 μg/mL. It was demonstrated that all investigated extracts exhibited moderate scavenging capacity in a concentration-dependent manner (Table 6). For comparison, the radical scavenging activity of ascorbic acid (AA; IC50 = 4.75 ± 0.16 μg/mL), quercetin (IC50 = 2.05 ± 0.10 μg/mL) and Trolox (IC50 = 3.68 ± 0.09 μg/mL) were tested in the same conditions. The highest DPPH scavenging activity was shown for the ethyl acetate fraction (CHs-4) (IC50 = 2.08 ± 0.03 μg/mL), followed by the butanol fraction (CHs-3), and the diethyl ether fraction (CHs-2) of C. hsingshangensis (IC50 = 3.43 ± 0.02 and 4.15 ± 0.05 μg/mL, respectively). The weakest activity was noted for the water fraction (CHi-1; IC50 = 32.37 ± 0.19 μg/mL) and crude extract of C. hissaricus (CHi; IC50 = 21.73 ± 0.13 μg/mL).
Table 6.
The IC50 values determined in antioxidant tests.
| Sample | IC50 | ||
|---|---|---|---|
| DPPH [μg/mL] | ABTS [μg/mL] | CHEL [μg/mL] | |
| CHi | 21.73 ± 0.13 e,f,g,h,i,k,l | 4.14 ± 0.10 e,f,g,h,i,j,k,l | 3.54 ± 0.15 f |
| CHi-1 | 32.37 ± 0.19 a,e,f,g,h,i,k,l | 5.48 ± 0.12 a,e,f,g,h,i,j,k,l | 182.67 ± 4.19 a,e,f,g,h,i,m |
| CHi-2 | 8.83 ± 0.10 a,b,e,f,g,h,i,j,k,l | 1.68 ± 0.03 a,b,e,f,g,h,i,k,l | 2.94 ± 0.03 b,f |
| CHi-3 | 13.69 ± 0.14 a,b,c,e,f,g,h,i,j,k,l | 2.74 ± 0.11 a,b,c,e,f,g,h,i,j,k,l | 4.04 ± 0.16 b,f |
| CHi-4 | 5.40 ± 0.10 a,b,c,d,e,f,g,h,i,j,k,l | 0.90 ± 0.02 a,b,c,d,e,f,i,j,k,l | 1.19 ± 0.05 b,f,i,m |
| CHs | 10.16 ± 0.02 j,k,l | 1.92 ± 0.13 j,l | 1.73 ± 0.10 j,m |
| CHs-1 | 18.15 ± 0.13 e,j,k,l | 1.53 ± 0.09 e,j | 76.50 ± 1.45 e,j,m |
| CHs-2 | 4.15 ± 0.05 e,f,j,k,l | 0.80 ± 0.02 e,f,j,k,l | 1.10 ± 0.03 f,j,m |
| CHs-3 | 3.43 ± 0.02 e,f,g,j,k | 0.68 ± 0.05 e,f,j,k,l | 1.01 ± 0.01 f,j,m |
| CHs-4 | 2.08 ± 0.03 e,f,g,h,k,l | 0.37 ± 0.01 e,f,g,h,j,k,l | 0.50 ± 0.01 f,j,m |
| quercetin | 2.05 ± 0.10 | 3.27 ± 0.23 | 6.24 ± 0.19 |
| AA | 4.75 ± 0.16 | 1.73 ± 0.09 | nt |
| Trolox | 3.68 ± 0.09 | 1.31 ± 0.05 | nt |
| Na2EDTA*2H2O | nt | nt | 4.15 ± 0.10 |
Data were expressed as mean ± SD, n = 3. AA—ascorbic acid; Na2EDTA*2H2O—ethylenediaminetetraacetic acid, disodium dihydrate; nt—not tested; CHi—crude extract of C. hissaricus, CHs—crude extract of C. hsingshangensis, CHi-1/CHs-1—water fraction, CHi-2/CHs-2—diethyl ether fraction, CHi-3/CHs-3—butanol fraction, CHi-4/CHs-4—ethyl acetate fraction; ABTS—2,2′-azino-bis-(3-ethyl-benzothiazole-6-sulfonic acid); CHEL—metal chelating activity. Statistical analysis: a—significantly different results compared to CHi; b—significantly different results compared to CHi-1; c—significantly different results compared to CHi-2; d—significantly different results compared to CHi-3; e—significantly different results compared to CHs; f—significantly different results compared to CHs-1; g—significantly different results compared to CHs-2; h—significantly different results compared to CHs-3; i—significantly different results compared to CHs-4; j—significantly different results compared to quercetin; k—significantly different results compared to AA; l—significantly different results compared to Trolox; m—significantly different results compared to Na2EDTA*2H2O; One-Way ANOVA test, followed by a Tukey’s multiple comparison test, p < 0.05.
The results of other published reports seem to be difficult to compare due to the other conditions used during experiments. Nevertheless, antioxidant activity using the DDPH assay was studied for different fractions of 70% methanol extract of the leaves of three Cotoneaster species cultivated in Poland [29]. The authors found that the studied extracts and fractions possessed significant scavenging effects with IC50 values ranging from 3.19 μg/mL for the ethyl acetate fraction of C. bullatus to 27.88 μg/mL for the water residue of C. integerrimus (the IC50 values for positive controls—quercetin, (−)-epicatechin, chlorogenic acid, butylated hydroxyanisole, and Trolox were 1.70, 2.35, 4.60, 2.90, and 4.05 μg/mL, respectively). Holzer et al. [26] also studied antioxidant capacity with DPPH tests of different extracts of sterile shoots, old stems, and leaves of C. melanocarpus. They found that the water extract had IC50 values from 53.53 to 86.72 μg/mL, methanolic extracts—30.91–106.41 μg/mL, ethyl acetate extracts—74.88–200 μg/mL, dichloromethane extracts—134.60–200 μg/mL. Ten polyphenol compounds isolated from the leaves of C. bullatus and C. zabelii and crude hydroalcoholic extracts were also evaluated by the DPPH radical scavenging assay. All these compounds showed significant inhibitory activity. Importantly, (−)-epicatchin (IC50 = 2.35 μg/mL), procyanidin B2 (IC50 = 2.46 μg/mL), and procyanidin C1 (IC50 = 2.55 μg/mL) showed stronger activity compared to Trolox (IC50 = 4.06 ± 0.11 μg/mL), which was used as a positive control [36]. Significant antioxidant activities were also observed for the compounds isolated from the leafy twigs of C. racemiflora, including racemiside (IC50 = 11.1 μM), scopoletin (IC50 = 3.8 μM), 7,8-dimethoxy-6-hydroxycoumarin (IC50 = 1.8 μM), 3,3′,4′-tri-O-methylellagic acid (IC50 = 15.1 μM), and cereotagloperoxide (IC50 = 5.9 μM) (IC50 for BHA used as positive control was 44.2 μM) [44].
As shown in Table 6, similar to the DPPH test, the ABTS•+ assay revealed that the ethyl acetate fraction (CHs-4) of the leaves of C. hsingshangensis possessed the strongest ability to scavenge free radicals (IC50 = 0.37 ± 0.01 μg/mL), followed by the butanol fraction (CHs-3; IC50 = 0.68 ± 0.05 μg/mL) and the diethyl ether fraction (CHs-2; IC50 = 0.80 ± 0.02 μg/mL) of C. hsingshangensis. The ABTS•+ assay was also used to test different extracts of C. nummularia. The authors found that the greatest ABTS inhibition was caused by the methanol (IC50 = 0.020 mg/mL) and water extract (IC50 = 0.023 mg/mL) (IC50 for BHA used as a positive control was 0.015 mg/mL) [57]. Mahmutović-Dizdarević and co-authors [11] reported that IC50 values of methanolic extracts of leaves and barks of C. horizontalis, C. integerrimus, and C. tomentosum ranged from 0.12 (leaves of C. tomentosum) to 0.87 mg/mL (barks of C. tomentosum).
Phenolic compounds can reduce oxidative stress by several mechanisms that depend on their chemical structure. One of them is the chelation of metal ions, such as iron, which plays a key role in the production of damaging oxygen species [58].
The chelating ability was determined based on measurement of the percentage of inhibition of formation of the ferrozine-Fe2+ complex. As reported in Table 6, the extracts and fractions from leaves of both studied Cotoneaster species possessed the capacity to interfere with the formation of iron and ferrozine complexes, which suggests their high chelating capacity and ability to capture iron ions before ferrozine. The IC50 values of most of these extracts showed higher chelating activity than the positive control–Na2EDTA*2H2O (IC50 = 4.15 ± 0.10 µg/mL). Among the investigated extracts, the best activity was noticed in the ethyl acetate fraction of C. hsingshangensis (IC50 = 0.50 µg/mL). Zengin and co-authors [57] also evaluated the metal chelating capacity of different extracts of aerial parts of C. nummularia. They found that the highest chelating activity was expressed by the ethyl acetate extract (IC50 = 0.25 mg EDTA/g of extract). Among the ethanol extracts of 34 species belonging to Rosaceae, Cotoneaster meyeri, C. morulus, and C. numullaria caused moderate metal-chelating effects (5.91, 21.48, and 26.19 chelation%, respectively) [34].
3.2.2. Enzyme Inhibitory Activity
Lipoxygenases (LOXs) are present in the human body and play a crucial role in the stimulation of inflammatory reactions. Exaggerated quantities of reactive oxygen species can induce inflammation that stimulates the release of cytokines and then the activation of lipoxygenases. They are connected with the spread of many diseases, and their inhibition is viewed as a relevant step in their prevention [59]. The lipoxygenase inhibitor screening assay kit is a popular method for lipoxygenase detection, which estimates the presence of hydroperoxides (4-hydroperoxy cis-trans 1,3-conjugated pentadienyl moiety within the unsaturated fatty acid) at various positions (5, 12-, 15-), which are produced in the lipoxygenation reaction using a purified lipoxygenase.
Sinha et al. [60] noted that perioxisome proliferator activated receptor (PRAR) ligands induce lipogenesis in cultured human sebocytes; hence, 5-lipoxygenase inhibitors demonstrate the ability to decline acne lesions, due to the fact that they possess the capability to reduce lipogenesis. The presence of PPARα receptors within sebocytes has been observed in peroxisomes, mitochondria, and microsomes.
The results of the inhibition of lipoxygenase are shown in Table 7. The diethyl ether fraction (CHs-2) and ethyl acetate fraction (CHs-4) of C. hsingshangensis showed a considerable ability to inhibit lipoxygenase activity (IC50 = 4.15, and 5.72 μg/mL, respectively), while the water fraction of C. hissaricus showed the lowest activity (IC50 = 129.46 μg/mL). Both CHs-2 and CHs-4 exhibited significantly higher inhibitory activity than that of nordihydroguaiaretic acid (NDGA) used as a positive standard (IC50 = 5.89 μg/mL). In a recent study of Cotoneaster species in regard to their capacity to inhibit lipoxygenase, it was found that different extracts of the leaves of C. bullatus, C. integerrimus, and C. zabelii have moderate activity with IC50 values in the range of 95.19 to 684.84 μg/mL. The best LOX inhibition was achieved by the butanol fraction of C. bullatus (IC50 = 95.19 μg/mL) [29]. The hydromethanolic extracts of fruits of nine Cotoneaster species cultivated in Poland showed the strongest inhibition of LOX, with IC50 values in the range of 62.54 (C. zabelii) to 165.76 (C. nanshan) μg/mL [28].
Table 7.
Anti-lipoxygenase, anti-hyaluronidase, and anti-cyclooxygenase activities of the leaves of C. hissaricus and C. hsingshangensis.
| Sample | IC50 [µg/mL] | |||
|---|---|---|---|---|
| Lipoxygenase Inhibition | Hyaluronidase Inhibition | COX-1 Inhibition | COX-2 Inhibition | |
| CHi | 28.73 ± 2.01 e,f,g,h,i,l | 15.09 ± 0.61 e,g,h,i,k | 24.77 ± 0.35 e,f,g,h,i,j | 19.95 ± 0.08 e,f,g,h,i,j |
| CHi-1 | 129.46 ± 6.35 a,i,l | 24.95 ± 0.52 a,e,f,g,h,i,k | 62.54 ± 1.02 a,e,f,g,h,i,j | 100.36 ± 1.27 a,e,f,g,h,i,j |
| CHi-2 | 19.77 ± 1.56 b,i,l | 13.19 ± 0.08 a,b,e,f,g,h,i,k | 11.15 ± 0.42 a,b,e,f,g,h,i,j | 16.68 ± 0.19 a,b,e,f,g,h,i j |
| CHi-3 | 45.69 ± 4.21 a,b,c,i,l | 10.28 ± 0.23 a,b,c,e,f,g,h,i,k | 46.71 ± 0.69 a,b,c,e,f,g,h,i,j | 38.50 ± 0.32 a,b,c,e,f,g,h,i,j |
| CHi-4 | 97.12 ± 3.86 a,b,c,d,i,l | 19.80 ± 0.51 a,b,c,d,e,f,g,h,i,k | 45.35 ± 0.47 a,b,c,e,f,g,h,i,j | 81.69 ± 0.78 a,b,c,d,e,f,g,h,i,j |
| CHs | 11.06 ± 1.14 l | 6.82 ± 0.15 k | 13.02 ± 0.17 j | 9.21 ± 0.09 j |
| CHs-1 | 72.15 ± 1.57 e,l | 14.76 ± 0.19 e,k | 34.12 ± 0.28 e,j | 57.59 ± 0.65 e,j |
| CHs-2 | 4.15 ± 0.31 e,f | 1.17 ± 0.02 e,f,k | 6.39 ± 0.04 e,f,j | 5.09 ± 0.06 e,f |
| CHs-3 | 11.56 ± 0.98 f,g,l | 7.41 ± 0.05 e,f,g,k | 15.56 ± 0.13 e,f,g,j | 10.32 ± 0.25 e,f,g,j |
| CHs-4 | 5.72 ± 0.26 e,f,h | 1.89 ± 0.05 e,f,g,h,k | 9.54 ± 0.04 e,f,g,j | 15.03 ± 0.19 e,f,g,j |
| IND | nt | nt | 4.34 ± 0.05 | 3.82 ± 0.09 |
| EGCG | nt | 6.25 ± 0.02 | nt | nt |
| NDGA | 5.89 ± 0.15 | nt | nt | nt |
CHi—crude extract of C. hissaricus, CHs—crude extract of C. hsingshangensis, CHi-1/CHs-1—water fraction, CHi-2/CHs-2—diethyl ether fraction, CHi-3/CHs-3—butanol fraction, CHi-4/CHs-4—ethyl acetate fraction; EGCG—epigallocatechin gallate, NDGA—nordihydroguaiaretic acid; IND—Indomethacin. Statistical analysis: a—significantly different results compared to CHi; b—significantly different results compared to CHi-1; c—significantly different results compared to CHi-2; d—significantly different results compared to CHi-3; e—significantly different results compared to CHs; f—significantly different results compared to CHs-1; g—significantly different results compared to CHs-2; h—significantly different results com-pared to CHs-3; i—significantly different results compared to CHs-4; j—significantly different results compared to IND; k—significantly different results compared to EGCG; l—significantly different results compared to NDGA; One-Way ANOVA test, followed by a Tukey’s multiple comparison test, p < 0.05.
Skin is especially sensitive to reactive oxygen species because it is exposed to oxidative stress from both endogenous and exogenous sources. Although oxidative stress is a key factor in this process, hyaluronic acid also plays a notable role. Its integrity inside the dermal matrix is substantial for cell integrity and proliferation. Under oxidative stress, hyaluronidase, which is responsible for hyaluronic acid depolymerization, is overactivated and breaks down this anionic glycosaminoglycan, carrying on to the destruction of the proteoglycan system. This results in the deregulation of skin homeostasis and increases inflammatory and allergic conditions [61,62].
In our study, the ethyl acetate fraction of C. hsingshangensis exhibited the best hyaluronidase inhibition activity (IC50 = 1.89 μg/mL) compared to the other extracts (Table 7). Most importantly, such activity was even better than the positive standard—EGCG (IC50 = 6.25 μg/mL). In turn, the IC50 values for the crude extracts of C. hissaricus and C. hsingshangensis were 15.09 ± 0.61, and 6.82 ± 0.15 μg/mL, respectively. Previous studies have shown that methanol extracts of the leaves of C. bullatus, C. integerrimus, and C. zabelii reduced the activity of hyaluronidase in a concentration-dependent manner. The most active were the butanol fraction of C. bullatus and C. zabelii (IC50 = 2.81, and 6.08 μg/mL, respectively) and they had inhibition activity better than indomethacin used as a positive control (IC50 = 8.61 μg/mL) [29]. Moreover, methanol extracts of fruits of C. bullatus, C. dielsianus, C. divaricatus, C. hjelmqvistii, C. horizontalis, C. lucidus, C. nanshan, C. splendens, and C. zabelii showed moderate inhibition of LOX with IC50 values in the range of 25.65 (C. lucidus) to 45.64 μg/mL (C. nanshan) [28].
Cyclooxygenases catalyze two reactions, the first being a cyclooxygenase function consisting of the addition of molecular oxygen to arachidonic acid to form prostaglandin G2 (PGG2). The second is the conversion of PGG2 to PGH2 by a peroxidase function. Therefore, this enzyme performs the initial reaction in the arachidonic acid metabolic cascade, carrying on to the formation of pro-inflammatory, i.e., prostaglandins, which regulate smooth muscle contractility, platelet aggregation, and mediate pain. Cyclooxygenase constitutive (COX-1) is responsible for the maintenance of physiological prostanoid biosynthesis, and COX-2 (an inducible isoform) is connected to inflammatory cell types and tissues [63].
To determine the potential anti-inflammatory properties of Cotoneaster leaves, we also estimated the ability of the extracts and fractions to inhibit the conversion of arachidonic acid to PGH2 by ovine COX-1 and human recombinant COX-2 using a COX inhibitor screening assay kit (Cayman Chemical, MI, USA). As shown in Table 7, most of the studied extracts and fractions showed good activity against COX-1 and COX-2. The most active against COX-1 were CHs-2 (IC50 = 6.39 µg/mL) and CHs-4 (IC50 = 9.54 µg/mL), followed by CHi-2 (IC50 = 11.15 µg/mL) and CHs (IC50 = 13.02 µg/mL), while the weakest was fraction CHi-1 (IC50 = 62.54 µg/mL). Except for the water residual of C. hissaricus—CHi-1 (IC50 = 100.36 µg/mL) and ethyl acetate fraction of the C. hissaricus—CHi-4 (IC50 = 81.69 µg/mL), all extracts and fractions of both Cotoneaster species showed good activity against COX-2 (IC50 = 5.09–57.59 µg/mL).
3.2.3. Antibacterial Activity
Diffusion Test in Solid Medium
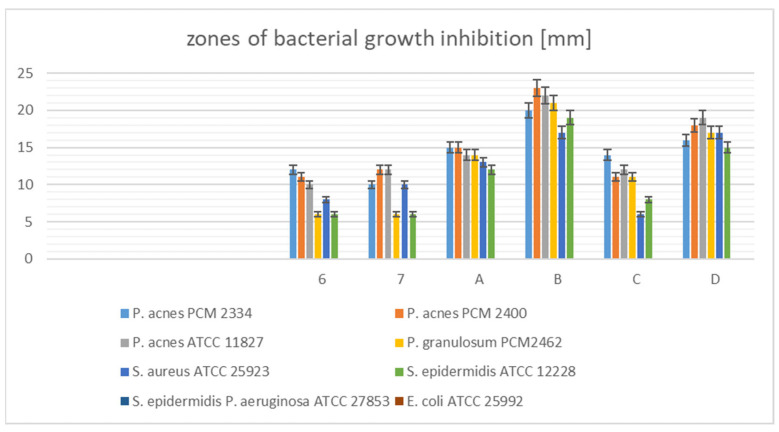
The antibacterial activity of the Cotoneaster extracts and fractions was assessed by diffusion test in a solid medium and measuring the zones of bacterial growth inhibition. The larger the zone around the applied samples, the more active the extract/fraction is.
The data show (Figure 7, Table S1) that the Cotoneaster fractions, not the starting crude extracts, possessed the strongest antimicrobial properties. The largest zones of growth inhibition of Gram-positive microaerobic bacteria were produced by CHs-2 fraction (23 mm–21 mm), then CHs-4 (19 mm–16 mm), CHs-1 (15 mm–14 mm), and CHs-3 fractions (14 mm–11 mm). These fractions against Gram-positive aerobic strains showed weaker activity (in the range 19 mm–6 mm) than against microaerobic bacteria. Crude Cotoneaster extracts CHi and CHs showed moderate activity against all tested Gram-positive strains (12 mm–6 mm). None of the samples were active against Gram-negative bacteria. Previous studies have reported the resistance of both Gram-positive and Gram-negative bacteria to the leaf and bark of C. integerrimus, C. tomentosus, and C. horizontalis methanolic extracts. Mahmutović-Dizdarević and co-authors [11] found that these extracts have significant antimicrobial activity against Salmonella enterica, Pseudomonas aeruginosa, Escherichia coli, Extended Spectrum Beta-Lactamase producing E. coli or ESBL E. coli, Enterococcus faecalis, Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus or MRSA, and Bacillus subtilis. In a further study, the antibacterial activity of the ethanolic extract of roots of C. acuminatus was also investigated against Bacillus pumilus, B. subtilis, E. coli, Microccocus glutamicus, S. aureus, Proteus vulgaris, and P. aeruginosa. The greatest effect was found for 100 μg/mL extract, with growth inhibition zones 10–18 mm [64]. Using the broth microdilution method, the antibacterial activity of the water, methanol, and ethyl acetate extracts of C. nummularia was evaluated against E. coli, Bacillus cereus, P. aeruginosa, methicillin sensitive S. aureus (MSSA), Klebsiella pneumoniae, Salmonella enteritidis, S. pneumoniae, Sarcina lutea, Enterococcus faecalis, methicillin resistant S. aureus (MRSA), and strains of methicillin resistant S. aureus isolated from clinical samples. The authors found that E. faecalis was the most sensitive bacteria, and B. cereus, K. pneumoniae, and S. enteritidis were the most resistant bacteria against all extracts except for the ethyl acetate extract [57].
Figure 7.
Zones of bacterial growth inhibition of the Cotoneaster extracts.
To the best of our knowledge, this study represents the first report of the antibacterial activity against C. acnes, S. epidermidis, and C. granulosum strains, which can be responsible for skin diseases of Cotoneaster species.
Results of MIC and MBC Determination
In order to determine the MIC values of the selected active fractions of crude C. hsingshangensis extract (CHi, CHs, CHs-1-CHs-4), an assay was performed using the double dilution method in a 96-well plate. After the plates were incubated under the appropriate conditions for the given strain, they were analyzed in an automatic plate reader (at 600 nm) against the control. The concentration at which no bacterial growth was observed was taken as the MIC. Next, the MBC value was determined by dispensing 10 µL of the solutions taken from the wells of 96-well plates in which no bacterial growth was observed.
The data contained in Table 8 confirm the results obtained in the earlier diffusion assay; namely, the tested fractions are the most active against the acne strains of Cutibacterium spp.
Table 8.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration of the fractions of C. hsingshangensis crude extract.
| Sample |
S. aureus ATCC 25923 |
S. epidermidis ATCC 12228 |
C. acnes PCM 2400 |
C. acnes PCM 2334 |
C. acnes ATCC 11827 |
C. granulosu PCM 2462 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC [µg/mL] | MIC [µg/mL] | MIC [µg/mL] | MIC [µg/mL] | MIC [µg/mL] | MIC [µg/mL] | |||||||
| CHi | >1000 | >4 | >1000 | >4 | >1000 | >4 | >1000 | >4 | >1000 | >4 | >1000 | >4 |
| CHs | >1000 | >4 | >1000 | >4 | >1000 | >4 | >1000 | >4 | >1000 | >4 | >1000 | >4 |
| CHs-1 | >1000 | >4 | 1000 | >4 | 500 | >4 | 1000 | >4 | 1000 | >4 | 500 | >4 |
| CHs-2 | 500 | >4 | 1000 | >4 | 31.25 | 8 | 62.5 | 8 | 125 | 8 | 62.5 | 8 |
| CHs-3 | >1000 | >4 | >1000 | >4 | 1000 | >4 | 500 | >4 | 1000 | >4 | 1000 | >4 |
| CHs-4 | 1000 | >4 | >1000 | >4 | 250 | >4 | 250 | >4 | 125 | >8 | 250 | >4 |
CHs-1—water fraction of C. hsingshangensis, CHs-2—diethyl ether fraction of C. hsingshangensis, CHs-3—butanol fraction of C. hsingshangensis, CHs-4—ethyl acetate fraction of C. hsingshangensis.
The most favorable, i.e., the lowest MIC values against these bacteria, were obtained by fractions CHs-2 (MIC 31.25–125 µg/mL), CHs-4 (125–250 µg/mL), CHs-1, and CHs-3 (both 500–1000 µg/mL).
Next, the MBC—as the lowest concentration of an antibacterial sample required to kill the bacterium—can be assayed based on an MIC test by subculturing samples on agar plates. Thus, the clear medium from MIC plates was spread on fresh agar to check its sterility. The concentration of the sample that did not produce colonies was considered the MBC power. According to the ratio MBC/MIC, we visualized and appreciated antibacterial activity. If the ratio MBC/MIC ≤ 4, the effect was considered bactericidal, but if the ratio MBC/MIC > 4, the effect was defined as bacteriostatic. Data of MBC/MIC ratio displayed in Table 8 show that none of C. hsingshangensis fractions showed any bactericidal activity in relation to the acne bacteria. The most promising power against microaerobic Gram-positive strains was displayed for CHs-2 (diethyl ether fraction of C. hsingshangensis). The remaining extract power (CHs-1, CHs-3, CHs-4) was not measurable.
3.2.4. Cytotoxic Activity
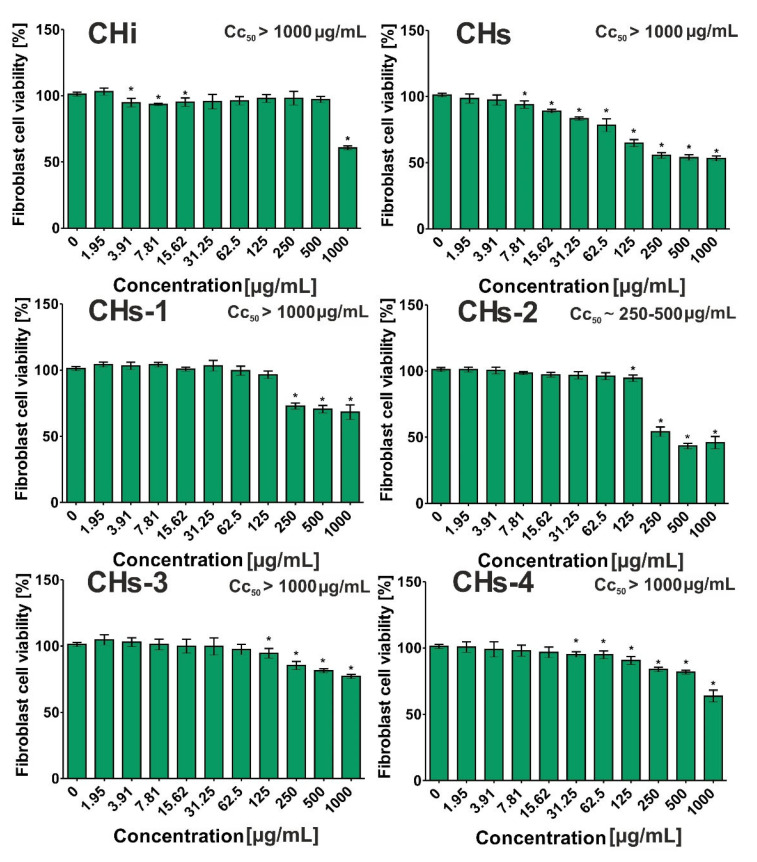
The obtained data revealed that all Cotoneaster extracts possessed low cytotoxicity toward normal human fibroblasts (Figure 8), as it was not possible to determine their CC50 values at tested concentrations (1.95–1000 μg/mL). The CC50 value denotes the extract concentration that inhibits BJ viability to 50%. Among the tested Cotoneaster extracts, CHs-3 exhibited the lowest ability to inhibit the viability of BJ cells because at the highest tested concentration (1000 μg/mL), the cell viability was 77.06 ± 1.57%. For comparison, fibroblast viability treated with CHi, CHs, CHs-1, and CHs-4 at the same concentration was 60.60 ± 1.47%, 53.18 ± 1.84%, 68.28 ± 5.55%, and 63.80 ± 4.39%, respectively. In the case of CHs-2, cell viability after incubation at concentrations of 250 μg/mL, 500 μg/mL, and 1000 μg/mL was 54.40 ± 3.56%, 43.35 ± 2.02%, and 45.89 ± 4.57%, respectively. Thus, these results may suggest that the CC50 value for this substance should be detected in the range 250–500 μg/mL. As a consequence, it was demonstrated that CHs-2 possessed the highest ability to inhibit BJ cell viability compared to other substances.
Figure 8.
Cytotoxic effect of Cotoneaster extracts on human normal fibroblasts. (BJ cell line, ATCC CRL-2522TM). The cell viability was assessed after 24-h incubation using the MTT assay. Asterisk (*) denotes significantly different data (p < 0.05, unpaired t-test) compared to the control, namely culture medium without substances—0 μg/mL.
3.3. Multivariate Analysis of the Results
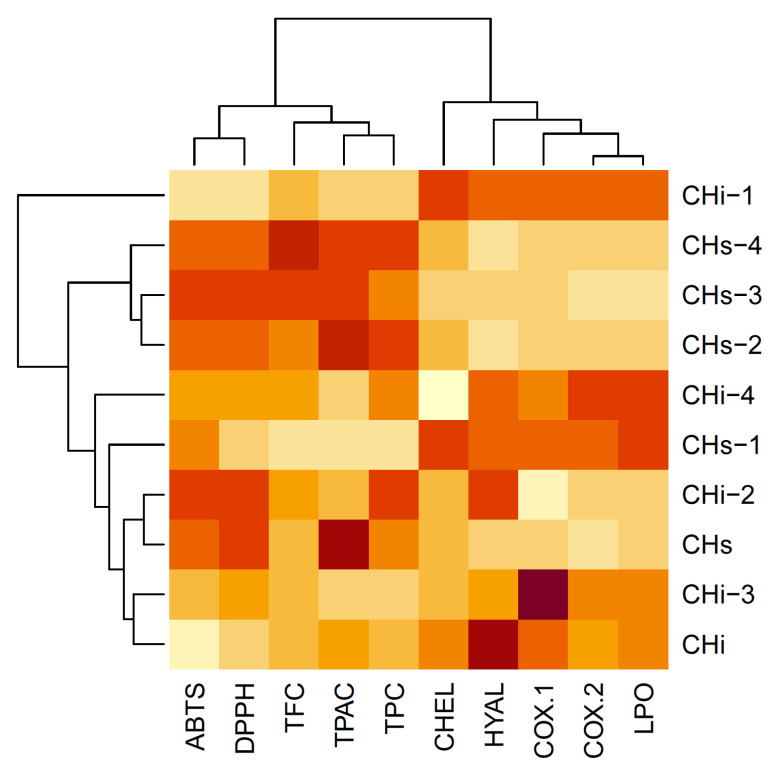
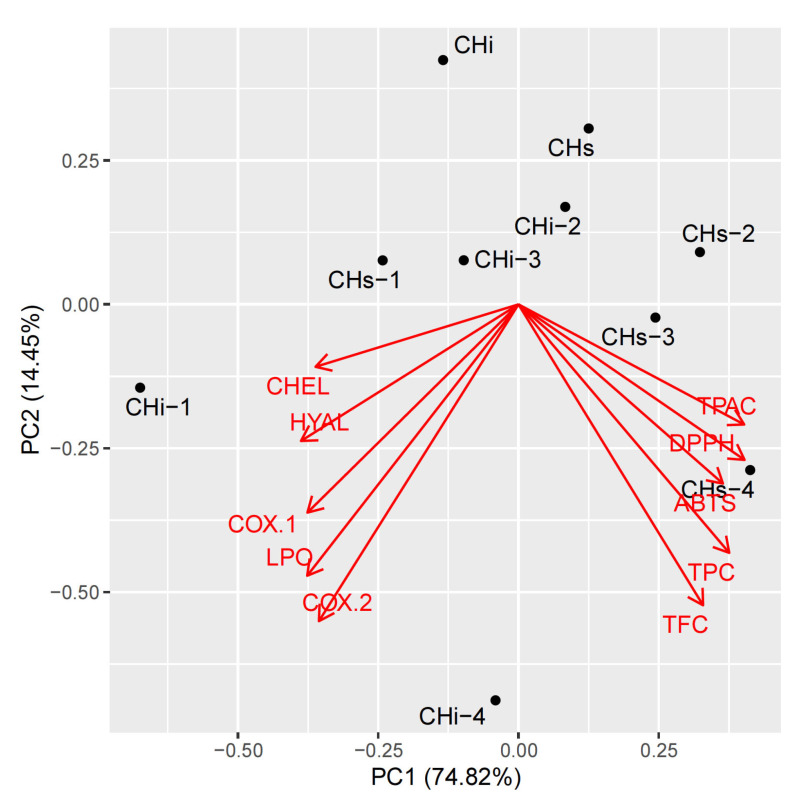
To perform a holistic view of the results, we used a chemometric multivariate approach. It allows us to examine some independent trends in changes among the investigated properties. They were investigated using hierarchical cluster analysis (HCA, Figure 9) and principal component analysis (PCA, Figure 10).
Figure 9.
The heatmap analysis of the scaled matrix, with dendrograms based on Euclidean distance. TPC—Total phenolic content; TPCA—Total phenolic acids content; TFC—Total flavonoid content; CHEL—Metal chelating activity; LPO—Lipoxygenase inhibition; HYAL—Hyaluronidase inhibition.
Figure 10.
Principal component analysis loadings of the investigated dataset: PC1 vs. PC2 plot. TPC—Total phenolic content; TPCA—Total phenolic acids content; TFC—Total flavonoid content; CHEL—Metal chelating activity; LPO—Lipoxygenase inhibition; HYAL—Hyaluronidase inhibition.
The analysis was done on a column-wise scaled matrix, as each parameter is ex-pressed with different units. Additionally, two of the parameters, DPPH and ABTS, were expressed as negative values in this matrix. This was done to convert the strong correlation between them and TPC, TPAC, and TFC from negative to positive. This places the loading vectors in the same direction and allows us to see everything visually in more detail.
It can be clearly seen that the parameters form two distinct groups, expressed as two separate groups of loading arrows and two main branches of the dendrogram. The first group contains antioxidant parameters TPC, TPAC, and TFC, together with DPPH and ABTS. The other parameters form the second group.
The increase or decrease of all investigated parameters in opposite directions is responsible for 75% of total variance and is modeled as PC1. PC2 represents an intercorrelated increase or decrease of all investigated parameters and is responsible for 14% of the variability. Further PCs do not contain any interpretable information (results not shown).
4. Conclusions
The management of acne vulgaris should be “multidirectional”. Therapeutics should reduce sebum production, as well as follicular hyperkeratinization of the epidermal cells. For this reason, they should primarily exhibit antioxidant, anti-inflammatory, and antimicrobial activities, without cytotoxic effects.
Our analysis has been designed due to missing data in the available literature, namely considerable gaps in the knowledge considering C. hissaricus and C. hsingshangensis are observed. The unique chemical composition of the representatives of the Rosaceae family makes it possible to obtain a wide range of pharmaceutical, medicinal, and cosmetic products. Thus, Cotoneaster species seem to be promising candidates for further research. Chemical drugs have limitations because of their high toxic activity, as well as their ability to induce adverse side effects. Thus, a growing interest in the use of herbal medicines for the management of acne and other diseases is increasingly recognized.
Among approximately 2500 species belonging to the family Rosaceae, in vitro anti-acne research has been carried out only with a small number of them, namely Rosa damascene [65], Rosa multiflora [66], Prunus jamasakura [67], and apple polyphenols [54]. Interestingly, there is a lack of research on other plants from the Rosaceae family for anti-acne activity and the effect of phytochemicals on the skin. Simultaneously, indisputable evidence that phenolic compounds exhibit the desired effects and contribute to alleviate skin disorders can be found in the available literature [51,68]. Furthermore, clindamycin and kaempferol in combination with quercetin possess more beneficial effects than other formulations [69].
In our in vitro research, we characterized composition and evaluated the biological properties of extracts from C. hissaricus and C. hsingshangensis. Thus, we identified the main compounds present in extracts, as well as determined the antioxidant, anti-inflammatory, antimicrobial, and cytotoxic properties of such extracts.
To sum up our results, it seems to be clear that comprehensive and well-designed future research on phenolic compounds from Cotoneaster in greater detail will constitute significant importance in pharmacy and medicine. Among the studied extracts, the diethyl ether fraction of C. hsingshangensis (CHs-2) exhibited great ability to scavenge free radicals and good capacity to inhibit cyclooxygenase-1, cyclooxygenase-2, lipoxygenase, and hyaluronidase. Moreover, it had the most promising power against microaerobic Gram-positive strains, and importantly, it was non-toxic toward normal skin fibroblasts. Taking into account the value of the calculated therapeutic index (>10), it is worth noting that CHs-2 can be subjected to in vivo study and constitutes a promising anti-acne agent.
To the best of our knowledge, there is no reported study on the chemical composition and skin-related properties of the examined species.
Acknowledgments
We would like to thank Łukasz Komsta from the Chair and Department of Medicinal Chemistry, Faculty of Pharmacy, Medical University of Lublin for chemometric analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11030367/s1, Table S1: Zones of bacterial growth inhibition of the Cotoneaster extracts and fractions.
Author Contributions
Conceptualization, K.D.S.S. and B.K.; methodology, K.K., M.P.D., R.T., M.M.-K. and K.K.; formal analysis, B.K., K.D.S.S., M.P.D., M.M.-K. and K.K.; resources, K.D.S.S. and K.K.; data curation, K.D.S.S., M.P.D., M.M.-K. and K.K.; writing—original draft preparation, B.K. and K.D.S.S.; writing—review and editing, K.D.S.S., M.M.-K. and K.K.; supervision, K.D.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education in Poland DS45 and PBmb1 projects of the Medical University of Lublin. This paper was developed using the equipment purchased within agreement No. POPW.01.03.00-06-010/09-00 Operational Program Development of Eastern Poland 2007–2013, Priority Axis I, Modern Economy, Operations 1.3. Innovations Promotion.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sarici G., Cinar S., Armutcu F., Altınyazar C., Koca R., Tekin N. Oxidative stress in acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2009;23:135–146. doi: 10.1111/j.1468-3083.2009.03505.x. [DOI] [PubMed] [Google Scholar]
- 2.Bojar R.A., Holland K.T. Acne and Propionibacterium acnes. Clin. Dermatol. 2004;22:375–379. doi: 10.1016/j.clindermatol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Dreno B., Pecastaings S., Corvec S., Veraldi S., Khammari A., Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018;32:5–14. doi: 10.1111/jdv.15043. [DOI] [PubMed] [Google Scholar]
- 4.Mclaughlin J., Watterson S., Layton A.M., Bjourson A.J., Barnard E., Mcdowell A. Propionibacterium acnes and acne vulgaris: New insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms. 2019;7:128. doi: 10.3390/microorganisms7050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keshari S., Kumar M., Balasubramaniam A., Chang T.W., Tong Y., Huang C.M. Prospects of acne vaccines targeting secreted virulence factors of Cutibacterium acnes. Expert Rev. Vaccines. 2019;18:433–437. doi: 10.1080/14760584.2019.1593830. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., Hata T.R., Tong Y.L., Kao M.S., Zouboulis C.C., Gallo R.L., Huang C.M. The anti-inflammatory activities of Propionibacterium acnes CAMP Factor-Targeted acne vaccines. J. Investig. Dermatol. 2018;138:2355–2364. doi: 10.1016/j.jid.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Vora J., Srivastava A., Modi H. Antibacterial and antioxidant strategies for acne treatment through plant extracts. Inform. Med. Unlocked. 2018;13:128–132. doi: 10.1016/j.imu.2017.10.005. [DOI] [Google Scholar]
- 8.Soleymani S., Farzaei M.H., Zargaran A., Niknam S., Rahimi R. Promising plant-derived secondary metabolites for treatment of acne vulgaris: A mechanistic review. Arch. Dermatol. Res. 2020;312:5–23. doi: 10.1007/s00403-019-01968-z. [DOI] [PubMed] [Google Scholar]
- 9.Mahdy K., Shaker O., Wafay H., Nassar Y., Hassan H., Hussein A. Effect of some medicinal plant extracts on the oxidative stress status in Alzheimer’s disease induced in rats. Eur. Rev. Med. Pharmacol. Sci. 2021;16:31–42. [PubMed] [Google Scholar]
- 10.Chang C.-S., Jeon J.I. Leaf flavonoids in Cotoneaster wilsonii (Rosaceae) from the island Ulleung-do, Korea. Biochem. Syst. Ecol. 2003;31:171–179. doi: 10.1016/S0305-1978(02)00064-9. [DOI] [Google Scholar]
- 11.Mahmutović-Dizdarević I., Dizdar M., Čulum D., Vidic D., Dahija S., Jerković-Mujkić A., Bešta-Gajević R. Phenolic composition, antioxidant and antimicrobial activity of Cotoneaster Medik. species from Bosnia and Herzegovina. Bull. Chem. Technol. Bosnia Herzeg. 2020;54:1–6. doi: 10.35666/ghtbh.2020.54.01. [DOI] [Google Scholar]
- 12.Swati S., Manjula R.R., Sowjanya K., Vennela Y., Tanuja K. A phyto pharmacological review on Cotoneaster microphyllus species. J. Pharm. Sci. Res. 2018;10:2166–2168. [Google Scholar]
- 13.Zengin G., Ferrante C., Menghini L., Orlando G., Brunetti L., Recinella L., Chiavaroli A., Leone S., Ronci M., Aumeeruddy M.Z. Protective effects of Cotoneaster integerrimus on in vitro and ex-vivo models of H2O2-induced lactate dehydrogenase activity in HCT116 cell and on lipopolysaccharide-induced inflammation in rat colon. J. Food Biochem. 2019;43:e12766. doi: 10.1111/jfbc.12766. [DOI] [PubMed] [Google Scholar]
- 14.Khan S., Yasmeen S., Afza N., Malik A., Iqbal L., Lateef M. Cotonoates A and B, new aromatic esters from Cotoneaster racemiflora. Z. Naturforsch. B. 2008;63:1219–1222. doi: 10.1515/znb-2008-1013. [DOI] [Google Scholar]
- 15.Khan S., Wang Z., Wang R., Zhang L. Horizontoates A–C: New cholinesterase inhibitors from Cotoneaster horizontalis. Phytochem. Lett. 2014;10:204–208. doi: 10.1016/j.phytol.2014.09.007. [DOI] [Google Scholar]
- 16.Akbar S. Handbook of 200 Medicinal Plants. Springer; New York, NY, USA: 2020. Cotoneaster nummularius Fisch. & C.A.Mey. (Rosaceae) [DOI] [Google Scholar]
- 17.Polish Pharmaceutical Society . Polish Pharmacopoeia IX, PTFarm. Polish Pharmaceutical Society; Warsaw, Poland: 2011. p. 150. [Google Scholar]
- 18.Szewczyk K., Bogucka-Kocka A., Vorobets N., Grzywa-Celińska A., Granica S. Phenolic composition of the leaves of Pyrola rotundifolia L. and their antioxidant and cytotoxic activity. Molecules. 2020;25:1749. doi: 10.3390/molecules25071749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo J.T., Lee H.L., Chiang S.H., Lin H.I., Chang C.Y. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J. Food Drug Anal. 2001;9:96–101. doi: 10.38212/2224-6614.2795. [DOI] [Google Scholar]
- 20.Nowak R., Szewczyk K., Gawlik-Dziki U., Rzymowska J., Komsta Ł. Antioxidative and cytotoxic potential of some Chenopodium L. species growing in Poland. Saudi J. Biol. Sci. 2016;23:15–23. doi: 10.1016/j.sjbs.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liyanaarachchi G.D., Samarasekera J.K.R.R., Mahanama K.R.R., Hemalal K.D.P. Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind. Crop. Prod. 2018;111:597–605. doi: 10.1016/j.indcrop.2017.11.019. [DOI] [Google Scholar]
- 22.Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 23.Szewczyk K., Pietrzak W., Klimek K., Miazga-Karska M., Firlej A., Flisiński M., Grzywa-Celińska A. Flavonoid and phenolic acids content and in vitro study of the potential anti-aging properties of Eutrema japonicum (Miq.) Koidz cultivated in Wasabi Farm Poland. Int. J. Mol. Sci. 2021;22:6219. doi: 10.3390/ijms22126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. Clinical Laboratory Standards Institute; Wayne, PA, USA: 2008. Eighteenth International Supplement M7-MIC. [Google Scholar]
- 25.O’Donnell F., Smyth T.J.P., Ramachandran V.N., Smyth W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents. 2010;35:30–38. doi: 10.1016/j.ijantimicag.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 26.Chrząszcz M., Miazga-Karska M., Klimek K., Granica S., Tchórzewska D., Ginalska G., Szewczyk K. Extracts from Cephalaria uralensis (Murray) Roem. & Schult. and Cephalaria gigantea (Ledeb.) Bobrov as potential agents for treatment of acne vulgaris: Chemical characterization and in vitro biological evaluation. Antioxidants. 2020;9:796. doi: 10.3390/antiox9090796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kicel A., Michel P., Owczarek A., Marchelak A., Zyzelewicz D., Budryn G., Oracz J., Olszewska M.A. Phenolic profile and antioxidant potential of leaves from selected Cotoneaster Medik. species. Molecules. 2016;21:688. doi: 10.3390/molecules21060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kicel A., Kołodziejczyk-Czepas J., Owczarek A., Rutkowska M., Wajs-Bonikowska A., Granica S., Nowak P., Olszewska M.A. Multifunctional phytocompounds in Cotoneaster fruits: Phytochemical profiling, cellular safety, anti-inflammatory and antioxidant effects in chemical and human plasma models in vitro. Oxid. Med. Cell. Longev. 2018;2018:3482521. doi: 10.1155/2018/3482521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kicel A., Kolodziejczyk-Czepas J., Owczarek A., Marchelak A., Sopinska M., Ciszewski P., Nowak P., Olszewska M.A. Polyphenol-rich extracts from Cotoneaster leaves inhibit pro-inflammatory enzymes and protect human plasma components against oxidative stress in vitro. Molecules. 2018;23:2472. doi: 10.3390/molecules23102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoo N.H., Kim H.K., Lee C.O., Park J.H., Kim M.J. Comparison of antioxidant and anti-inflammatory activities of methanolic extracts obtained from different parts of Cotoneaster wilsonii Nakai. Korean J. Med. Crop Sci. 2019;27:194–201. doi: 10.7783/KJMCS.2019.27.3.194. [DOI] [Google Scholar]
- 31.Holzer V.M.D., Lower-Nedza A.D., Nandintsetseg M., Batkhuu J., Brantner A.H. Antioxidant constituents of Cotoneaster melanocarpus Lodd. Antioxidants. 2013;2:265–272. doi: 10.3390/antiox2040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palme E., Bilia A.R., de Feo V., Morelli I. Flavonoid glycosides from Cotoneaster thymaefolia. Phytochemistry. 1994;35:1381–1382. doi: 10.1016/S0031-9422(06)80133-0. [DOI] [Google Scholar]
- 33.Kicel A., Owczarek A., Gralak P., Ciszewski P., Olszewska M.A. Polyphenolic profile, antioxidant activity, and pro-inflammatory enzymes inhibition of leaves, flowers, bark and fruits of Cotoneaster integerrimus: A comparative study. Phytochem. Lett. 2019;30:349–355. doi: 10.1016/j.phytol.2019.02.027. [DOI] [Google Scholar]
- 34.Ekin H.N., Gokbulut A., Aydin Z.U., Donmez A.A. Insight into anticholinesterase and antioxidant potential of thirty-four Rosaceae samples and phenolic characterization of the active extracts by HPLC. Ind. Crops Prod. 2016;91:104–113. doi: 10.1016/j.indcrop.2016.06.029. [DOI] [Google Scholar]
- 35.Les F., López V., Caprioli G., Iannarelli R., Fiorini D., Innocenti M., Bellumori M., Maggi F. Chemical constituents, radical scavenging activity and enzyme inhibitory capacity of fruits from Cotoneaster pannosus Franch. Food Funct. 2017;8:1775–1784. doi: 10.1039/C7FO00330G. [DOI] [PubMed] [Google Scholar]
- 36.Kicel A., Owczarek A., Kapusta P., Kolodziejczyk-Czepas J., Olszewska M.A. Contribution of individual polyphenols to antioxidant activity of cotoneaster bullatus and Cotoneaster zabelii leaves—Structural relationships, synergy effects and application for quality control. Antioxidants. 2020;9:69. doi: 10.3390/antiox9010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odontuya G. Phytochemicals in leaves of Cotoneaster mongolica, their antioxidative, and acetylcholinesterase inhibitory activity. Mong. J. Chem. 2019;20:1–6. [Google Scholar]
- 38.El-Mousallamy A.M., Hussein S.A., Merfort I., Nawwar M.A. Unusual phenolic glycosides from Cotoneaster orbicularis. Phytochemistry. 2000;53:699–704. doi: 10.1016/S0031-9422(99)00598-1. [DOI] [PubMed] [Google Scholar]
- 39.Palme E., Bilia A.R., Morelli I. Flavonols and isoflavones from Cotoneaster simonsii. Phytochemistry. 1996;42:903–905. doi: 10.1016/0031-9422(95)00023-2. [DOI] [Google Scholar]
- 40.Cooke R.G., Fletcher R.A.H. Isoflavonoids. III. Constituents of Cotoneaster species. Aust. J. Chem. 1974;27:1377–1379. doi: 10.1071/CH9741377. [DOI] [Google Scholar]
- 41.Chumbalov T.K., Pashinina L.T., Shukenova R.Z. Flavonoids of Cotoneaster oligantha. Chem. Nat. Comp. 1978;14:166–172. [Google Scholar]
- 42.Huang W.C., Tsai T.H., Huang C.J., Li Y.Y., Chyuan J.H., Chuang L.T., Tsai P.J. Inhibitory effects of wild bitter melon leaf extract on Propionibacterium acnes-induced skin inflammation in mice and cytokine production in vitro. Food Funct. 2015;6:2550–2560. doi: 10.1039/C5FO00550G. [DOI] [PubMed] [Google Scholar]
- 43.Khan S., Rehman A.U., Riaz N., Afza N., Malik A. Isolation studies on Cotoneaster racemiflora. J. Chem. Soc. Pak. 2007;29:620–623. [Google Scholar]
- 44.Khan S., Riaz N., Afza N., Malik A., Rehman A.U., Iqbal L., Lateef M. Antioxidant constituents from Cotoneaster racemiflora. J. Asian Nat. Prod. Res. 2009;11:44–48. doi: 10.1080/10286020802435745. [DOI] [PubMed] [Google Scholar]
- 45.Al-Snafi A.E. Pharmacological activities of Cotoneaster racemiflorus—A review. Pharm. Chem. J. 2016;3:98–104. [Google Scholar]
- 46.Fakhri M., Davoodi A., Parviz M., Ghadi Z.S., Mousavinasab S.N., Farhadi R., Azadbakht M. Characterization and HPLC analysis of manna from some Cotoneaster species. Int. J. Pharm. Sci. 2017;8:5360–5366. [Google Scholar]
- 47.Nahrstedt A. Cyanogenesis in Cotoneaster-arten. Phytochemistry. 1973;12:1539–1542. doi: 10.1016/0031-9422(73)80364-4. [DOI] [Google Scholar]
- 48.Sokkar N., El-Gindi O., Sayed S., Mohamed S., Ali Z., Alfishawy I. Antioxidant, anticancer and hepatoprotective activities of Cotoneaster horizontalis Decne extract as well as tocopherol and amygdalin production from in vitro culture. Acta Physiol. Plant. 2013;35:2421–2428. doi: 10.1007/s11738-013-1276-z. [DOI] [Google Scholar]
- 49.Dudonné S., Poupard P., Coutiere P., Woillez M., Richard T., Mérillon J.M., Vitrac X. Phenolic composition and antioxidant properties of poplar bud (Populus nigra) extract: Individual antioxidant contribution of phenolics and transcriptional effect on skin aging. J. Agric. Food Chem. 2011;59:4527–4536. doi: 10.1021/jf104791t. [DOI] [PubMed] [Google Scholar]
- 50.Pluemsamran T., Onkoksoong T., Panich U. Caffeic acid and ferulic acid inhibit UVA-induced matrix metalloproteinase-1 through regulation of antioxidant defense system in keratinocyte HaCaT cells. Photochem. Photobiol. 2012;88:961–968. doi: 10.1111/j.1751-1097.2012.01118.x. [DOI] [PubMed] [Google Scholar]
- 51.Działo M., Mierziak J., Korzun U., Preisner M., Szopa J., Kulma A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016;17:160. doi: 10.3390/ijms17020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang X.X., Lu J.Y., Lai H.F., Wei L.Q. F33O4 magnetic nanoparticles for cinnamic acid extraction from Ramulus cinnamomi. J. Chin. Chem. Soc. 2015;62:52–58. doi: 10.1002/jccs.201400180. [DOI] [Google Scholar]
- 53.Wittenauer J., Mäckle S., Sußmann D., Schweiggert-Weisz U., Carle R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia. 2015;101:179–187. doi: 10.1016/j.fitote.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Lee C., Chien Y., Chiu T., Huang W., Lu C., Chiang J., Yang J. Apoptosis triggered by vitexin in U937 human leukemia cells via a mitochondrial signaling pathway. Oncol. Rep. 2012;28:1883–1888. doi: 10.3892/or.2012.2000. [DOI] [PubMed] [Google Scholar]
- 55.Mohamed S.A., Sokkar N.M., El-Gindi O., Ali Z.Y., Alfishawy I.A. Phytoconstituents investigation, anti-diabetic and anti-dyslipidemic activities of Cotoneaster horizontalis Decne cultivated in Egypt. Life Sci. J. 2012;9:394–403. [Google Scholar]
- 56.Jayawardena T.U., Kim H.S., Sanjeewa K.K.A., Kim S.Y., Rho J.R., Jee Y., Ahn G., Jeon Y.J. Sargassum horneri and isolated 6-hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one (HTT); LPS-induced inflammation attenuation via suppressing NF-κB, MAPK and oxidative stress through Nrf2/HO-1 pathways in RAW 264.7 macrophages. Algal Res. 2019;40:101513. doi: 10.1016/j.algal.2019.101513. [DOI] [Google Scholar]
- 57.Zengin G., Uysal A., Gunes E., Aktumsek A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE. 2014;9:e113527. doi: 10.1371/journal.pone.0113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016;15:71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lončarić M., Strelec I., Moslavac T., Šubarić D., Pavić V., Molnar M. Lipoxygenase inhibition by plant extracts. Biomolecules. 2021;11:152. doi: 10.3390/biom11020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinha P., Srivastava S., Mishra N., Yadav N.P. New perspectives on anti acne plant drugs: Contribution to modern therapeutics. Biomed Res. Int. 2014;301:4. doi: 10.1155/2014/301304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferreres F., Lopes G., Gil-Izquierdo A., Andrade P.B., Sousa C., Mouga T., Valentão P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs. 2012;10:2766–2781. doi: 10.3390/md10122766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bralley E., Greenspan P., Hargrov J.L., Hartle D.K. Inhibition of hyaluronidase activity by Vitis rotundifolia. (Muscadine) Berry seeds and skins. Pharm. Biol. 2007;45:667–673. doi: 10.1080/13880200701545018. [DOI] [Google Scholar]
- 63.Alberto M.R., Zampini I.C., Isla M.I. Inhibition of cyclooxygenase activity by standardized hydroalcoholic extracts of four Asteraceae species from the Argentine Puna. Braz. J. Med. Biol. Res. 2009;42:787–790. doi: 10.1590/S0100-879X2009000900003. [DOI] [PubMed] [Google Scholar]
- 64.Sati S.C., Sati M., Sharma A., Joshi M. Isolation and characterisation of phenolics from the roots of Cotoneaster acuminatus and determination of their antimicrobial activity. Int. J. Pharm. Pharm. Sci. 2010;2:58–60. [Google Scholar]
- 65.Tsai T.H., Wu W.H., Tseng J.T.P., Tsai P.J. In vitro antimicrobial and anti-inflammatory effects of herbs against Propionibacterium acnes. Food Chem. 2010;119:964–968. doi: 10.1016/j.foodchem.2009.07.062. [DOI] [Google Scholar]
- 66.Norimoto H., Nakajima K., Yomoda S., Morimoto Y. Testostrone 5-alphareductase inhibitory consituents from the fruits of Rosa multiflora. Thunb. J. Trad. Med. 2010;27:90–95. [Google Scholar]
- 67.Azimi H., Fallah-Tafti M., Khakshur A.A., Abdollahi M. A review of phytotherapy of acne vulgaris: Perspective of new pharmacological treatments. Fitoterapia. 2012;83:1306–1317. doi: 10.1016/j.fitote.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 68.Soto M., Falqué E., Domínguez H. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics. 2015;2:259–276. doi: 10.3390/cosmetics2030259. [DOI] [Google Scholar]
- 69.Lim Y.H., Kim I.H., Seo J.J. In vitro activity of kaempferol isolated from Impatiens balsamina alone and in combination with erythromycin or clindamycin against Propionibacterium acnes. J. Microbiol. 2007;45:473–477. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request.