Abstract
Restriction fragment length polymorphism (RFLP) analysis of 209 Mycobacterium tuberculosis clinical isolates obtained from newly detected pulmonary tuberculosis patients (151 male and 58 female; mean age, 41 years) in Estonia during 1994 showed that 61 isolates (29%) belonged to a genetically closely related group of isolates, family A, with a predominant IS6110 banding pattern. These strains shared the majority of their IS6110 DNA-containing restriction fragments, representing a predominant banding pattern (similarity, >65%). This family A comprised 12 clusters of identical isolates, and the largest cluster comprised 10 strains. The majority (87.5%) of all multidrug-resistant (MDR) isolates, 67.2% of all isolates with any drug resistance, but only 12% of the fully susceptible isolates of M. tuberculosis belonged to family A. These strains were confirmed by spoligotyping as members of the Beijing genotype family. The spread of Beijing genotype MDR M. tuberculosis strains was also frequently seen in 1997 to 1999. The members of this homogenous group of drug-resistant M. tuberculosis strains have contributed substantially to the continual emergence of drug-resistant tuberculosis all over Estonia.
An increase in tuberculosis (TB) morbidity accompanied by an appearance of multidrug-resistant (MDR) TB has been documented in Estonia since the early 1990s. After a decline in incidence from 417 per 100,000 population in 1953 to 26 per 100,000 in 1992, the incidence showed a steady increase, which reached 52 per 100,000 in 1999 (26). This twofold increase in morbidity was accompanied by an increase in drug-resistant TB and particularly in MDR TB (i.e., TB that is resistant to at least isoniazid and rifampin) and has become a serious problem in Estonia. In 1994 and 1998, MDR TB comprised 10% (13) and 14% (25) of the new pulmonary cases detected. This places Estonia among the countries with the highest MDR TB rates in the world (24).
To understand the epidemiology of tuberculosis, molecular methods such as restriction fragment length polymorphism (RFLP) analyses of the infecting organisms have proven to be very powerful tools (11, 20, 27). The RFLP method can detect genotypic variations between strains by using repetitive DNA sequences as probes in Southern hybridization analyses (12).
In outbreak investigations (1, 14, 16), mutual association of IS6110 RFLP patterns and resistance profiles has often been described. However, in population-based studies, the molecular epidemiology and drug resistance pattern of the M. tuberculosis isolates (constituting a genetically closely related group of bacteria) have been studied only in very few geographical settings (23).
In the epidemiology of drug-resistant disease, molecular epidemiological studies of resistant strains could help identify current and past failures in TB control and allow tracking of the transfer path of the resistant strains (6). In this study we explore the spread of M. tuberculosis strains in Estonia by molecular epidemiological methods and demonstrate that transmission of several highly related clones of drug-resistant M. tuberculosis strains contributes to the emergence of drug-resistant TB all over Estonia.
MATERIALS AND METHODS
The Estonian TB Register was the source of demographic data and clinical information on the patients. The majority of TB patients were diagnosed and treated at either of two lung hospitals, Tartu University Lung Hospital in Tartu (South Estonia) or Kivimäe Hospital in Tallinn (North Estonia). In 1994, 518 patients (355 men and 163 women; mean age, 41 years) were reported to have newly detected TB cases, i.e., with no previous history of tuberculosis. Of the 518 newly detected pulmonary TB patients, 299 (63%) had cases that were smear and/or culture positive.
Patients.
In 1994, 209 (70%) of 299 isolates were available for IS6110 RFLP typing. The mean age of the 209 patients who were included in the study was 41 years; 151 (72%) were male (mean age, 42 years), and 58 (28%) were female (mean age, 39 years). There was no significant difference in age and sex distribution between these 209 patients with pulmonary TB and the 518 patients reported as the total of newly detected cases in 1994.
Characterization of mycobacterial isolates.
The analyzed M. tuberculosis strains were isolated at the Tuberculosis Laboratory in Tallinn (central laboratory for the North Estonia) and the National Reference Laboratory (NRL) in Tartu. The species identification of the isolates was based on standard microbiological tests: colony morphology, acid-fast staining, and biochemical tests (10). It was confirmed by a DNA-RNA hybridization technique (AccuProbe; GenProbe Inc., San Diego, Calif.) at the Swedish Institute for Infectious Disease Control (SMI) in Stockholm, Sweden.
Drug susceptibility testing.
Drug susceptibility testing of all isolates was performed both in Estonia and in Sweden. In Estonia it was done by conventional culturing on solid media using the proportion method (4), and at SMI it was done using radiometric respirometry with the Bactec system (18). This method is in good agreement with the resistance ratio method on Löwenstein-Jensen medium (9). The TB laboratory at SMI is part of the network of Supranational Reference Laboratories for drug susceptibility testing of M. tuberculosis, initiated by the World Health Organization (15), and serves as an external reference laboratory for Estonia. The drugs tested included streptomycin (4 mg/liter), isoniazid (0.2 mg/liter), ethambutol (5 mg/liter), and rifampin (2 mg/liter).
RFLP analyses.
Extraction of DNA from mycobacterial strains and DNA fingerprinting with IS6110 as a probe were performed by standardized methods (20) at SMI. In brief, after 3 to 4 weeks of growth on Löwenstein-Jensen medium, the bacteria were harvested and heat killed (80°C for 20 min). DNA was extracted and digested with PvuII. After electrophoresis of the digested DNA on an agarose gel, the 245-bp sequence of IS6110 was chemiluminescence labeled. The gels were scanned and the results were analyzed by computer with the Gelcompar software (Applied Maths, Kortrijk, Belgium) as described previously (7).
Similarity matrices were generated to visualize the relatedness between the banding patterns of all isolates. Isolates with banding patterns whose similarity coefficients were ≥65% (sharing more than two-thirds of the IS-containing PvuII fragments) (12, 20) are defined as belonging to a family of strains. Clinical isolates with identical IS6110 RFLP pattern constituted one cluster.
Spoligotyping.
The spoligotyping method, with a slightly lower level of discrimination than that of IS6110-RFLP typing (1, 12), was used as an additional tool for determining relationships among the isolates belonging to family A (21). The DR locus in the M. tuberculosis complex genome contains multiple, highly conserved 36-bp direct repeats (DR). The repeats are separated by 35- to 41-bp spacer sequences, which are variable. The analysis of this locus by spoligotyping is based on the fact that the separate spacer sequences are unique and can be hybridized to synthetic spacer oligonucleotides that are bound to a membrane. The hybridization pattern will then show which spacer oligonucleotides are present in each strain (11). The DR-based fingerprint is a good secondary marker to support or rule out strain clustering. A total of 37 isolates with different RFLP patterns were selected for spoligotyping, according to the similar distribution of different families, clones, and nonrelated strains in the investigated samples. Purified chromosomal DNA from RFLP typing was available. Membranes for spoligotyping were obtained from Isogen, Bioscience BV, Utrecht, The Netherlands. The membranes contained oligonucleotides derived from the spacer DNA sequences, interspersed with the directly repeated sequences in the DR region of M. tuberculosis strain H37RV and M. bovis BCG. The presence or absence of these spacers in M. tuberculosis complex strains can be detected by hybridization of the amplified DNA of these spacer regions by using primers complementary to the DR. PCR and hybridization were performed as previously described (11).
Statistics.
Data analysis was performed using the Statistica program. Subject variables were examined using the χ2 test of association for categorical variables and t test for continuous variables. A P value of <0.05 was used to indicate statistical significance.
RESULTS
IS6110 fingerprints in the study population.
The 209 M. tuberculosis isolates were characterized by IS6110 RFLP analysis. The number of IS6110 copies per isolate varied between 5 and 19 (mean, 12 bands). The majority, i.e., 195 (93%) of the isolates, contained 8 to 17 copies, with a mean of 12 bands.
M. tuberculosis isolates of 102 of the 209 patients (49%) belonged to different clusters, while 107 (51%) clinical isolates presented unique (individual) RFLP fingerprint patterns.
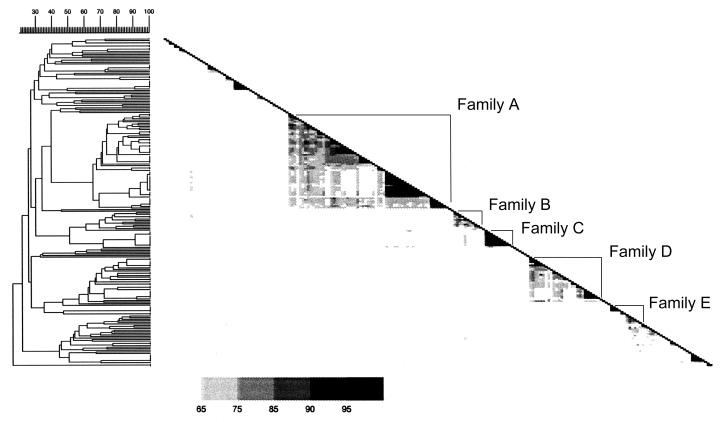
A total of 140 isolates (67%) (44 with unique patterns and 96 with clustered ones) could be allocated into five major families on the basis of their RFLP patterns (≥65% similarity), families A to E (Fig. 1). The largest family, family A, comprised 61 isolates in 12 clusters, with largest cluster comprising 10 closely related or identical isolates (Table 1). Of the 209 patients, 47 (22%) were infected with isolates belonging to families B to E, and 34 (16%) isolates belonged to 12 clusters outside the five major families (Fig. 1; Table 1).
FIG. 1.
Similarity matrix showing the relatedness between the RFLP banding patterns of all isolates. The different RFLP patterns divided the isolates into five major families (families A, B, C, D, and E). The value of the similarity coefficient between values of 65 and 100% is depicted by the five different grey tones in the matrices. The diagonal is formed by the 100% similarity coefficient values of the corresponding strains.
TABLE 1.
Drug resistance patterns of 140 M. tuberculosis isolates clustered or belonging to family A to E
| Family | Cluster | No. of isolates | Drug resistance patternb | Susc. | Any res. | MDR |
|---|---|---|---|---|---|---|
| A | A1 | 2 | AB I + AB II | 1 | 1 | 0 |
| A2 | 2 | AB I | 2 | 0 | 0 | |
| A3 | 2 | AB I | 2 | 0 | 0 | |
| A4 | 2 | AB II | 0 | 2 | 0 | |
| A5 | 2 | AB IV | 0 | 2 | 2 | |
| A6 | 4 | AB IV | 0 | 4 | 4 | |
| A7 | 5 | AB III + AB V | 0 | 5 | 0 | |
| A8 | 2 | AB I | 2 | 0 | 0 | |
| A9 | 3 | AB III + AB IV | 0 | 3 | 2 | |
| A10 | 10 | AB I + AB III + AB IV + AB V | 1 | 9 | 4 | |
| A11 | 3 | AB IV + AB V | 0 | 3 | 2 | |
| A12 | 4 | AB II + AB IV | 0 | 4 | 1 | |
| Aa (SC 65–99%) | 20 | AB I + AB II + AB III + AB IV | 10 | 10 | 6 | |
| Total | 61 | 18 | 43 | 21 | ||
| B | B1 | 2 | AB II + AB IV | 0 | 2 | 1 |
| Ba (SC 69–92%) | 9 | AB I + AB II + AB III | 6 | 3 | 0 | |
| Total | 11 | 6 | 5 | 1 | ||
| C | C1 | 3 | AB I | 3 | 0 | 0 |
| C2 | 7 | AB I | 7 | 0 | 0 | |
| Total | 10 | 10 | 0 | 0 | ||
| D | D1 | 2 | AB I + AB II | 1 | 1 | 0 |
| D2 | 3 | AB I | 3 | 0 | 0 | |
| D3 | 2 | AB I | 2 | 0 | 0 | |
| D4 | 2 | AB I | 2 | 0 | 0 | |
| D5 | 2 | AB I | 2 | 0 | 0 | |
| Da (SC 66–86%) | 6 | AB I + AB II | 5 | 1 | 0 | |
| Total | 17 | 15 | 2 | 0 | ||
| E | E1 | 2 | AB I + AB IV | 1 | 1 | 1 |
| E2 | 5 | AB I + AB II | 3 | 2 | 0 | |
| Ea (SC 92%) | 2 | AB I + AB II | 1 | 1 | 0 | |
| Total | 9 | 5 | 4 | 1 | ||
| Other | F1 | 2 | AB I | 2 | 0 | 0 |
| F2 | 2 | AB I + AB III | 1 | 1 | 0 | |
| F3 | 2 | AB I + AB II | 1 | 1 | 0 | |
| F4 | 3 | AB I | 3 | 0 | 0 | |
| F5 | 2 | AB I | 2 | 0 | 0 | |
| F6 | 6 | AB I + AB II | 5 | 1 | 0 | |
| F7 | 4 | AB I | 4 | 0 | 0 | |
| F8 | 2 | AB I | 2 | 0 | 0 | |
| F9 | 2 | AB II | 0 | 2 | 0 | |
| F10 | 5 | AB I | 5 | 0 | 0 | |
| F11 | 2 | AB I | 2 | 0 | 0 | |
| Total | 32 | 27 | 5 | 0 |
Contains isolates which did not cluster but belonged to a family having a similarity coefficient of ≥65%.
AB I, susceptible isolates; AB II, monoresistant isolates; AB III, isoniazid- plus streptomycin-resistant isolates; AB IV, MDR isolates; AB V, isoniazid- plus streptomycin- plus ethanbutol-resistant isolates.
Correlation between IS6110 RFLP patterns and drug resistance.
Of the 209 M. tuberculosis isolates, 64 (31%) were resistant to one or more of the drugs tested while 24 (11.5%) of them were multidrug resistant (MDR), i.e., resistant to both isoniazid and rifampin.
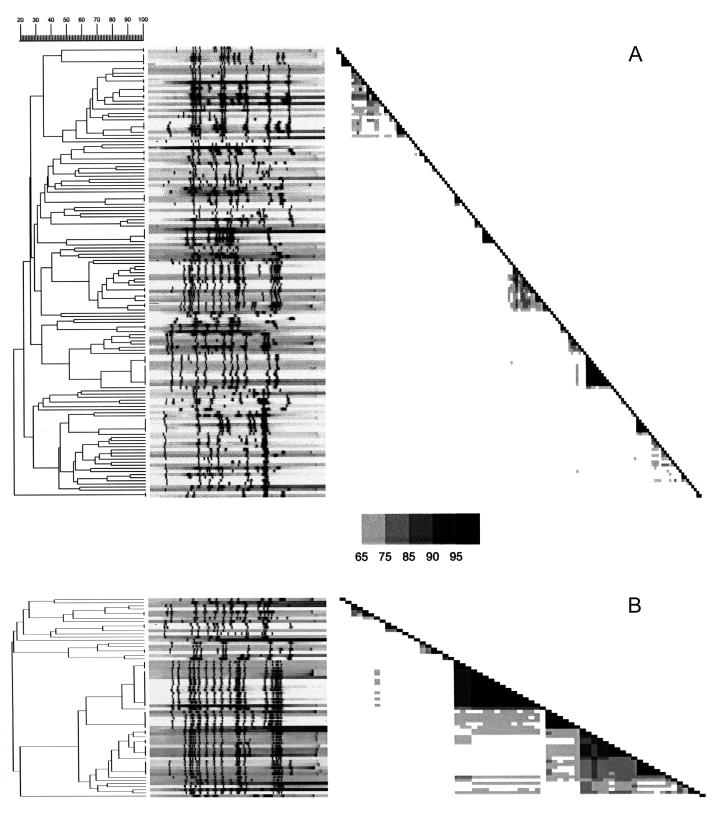
There was a remarkable homogeneity in RFLP banding patterns in drug-resistant isolates, with the majority belonging to family A (Fig. 2). Family A (n = 61) comprised 21 MDR isolates and 43 isolates with any drug resistance. The other families contained only 2 of 24 MDR isolates (8.3%) and 10 of 64 isolates with any drug resistance (15.6%) (Table 1). Among isolates that were not clustered or did not belong to any of the five families, only 1 of 24 MDR (4.2%) and 64 of 145 fully susceptible (44%) isolates were found.
FIG. 2.
IS6110 fingerprint patterns of the 145 pansensitive (A) and 64 drug-resistant (B) isolates and the corresponding dendrogram and similarity matrixes. Banding patterns are ordered by similarity. The position of each IS6110 band is normalized so that the banding patterns of all isolates are mutually comparable. Band positions were determined by using the peak finder function of the Gelcompar software and were controlled manually by comparison with the original IS6110 autoradiogram. The fingerprint patterns were analyzed for similarity by using the Dice coefficient, and a dendorogram was calculated with the unweighted pair group method using average linkage as specified by the supplier.
Genetic homogenity of family A confirmed by spoligotyping.
The 12 strains from family A showed an identical and unusual spoligopattern, lacking spacers 1 to 34. This pattern is consistent with the spoligotype pattern of the so-called Beijing genotype M. tuberculosis strains (21). The spoligotypes of the non-Beijing family strains were highly diverse (Table 2).
TABLE 2.
Spoligopatterns of 37 M. tuberculosis strains showing representative examples of families A to E and other clustered and nonclustered strains
| Isolatea | Spoligotypeb | IS6110 clusterc | No. of IS6110 elements |
|---|---|---|---|
| 355/94 | xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx....xxxxxxx | F1 | 11 |
| 3734/94 | x.xxxxxxxxxxx.xxxxxxxxxxxxxxxxxx....xxxxxxx | F2 | 9 |
| 740/94 | xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx....xxxxxxx | NC | 17 |
| 704/94 | xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx....xxxxxxx | F4 | 9 |
| 3775/94 | xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx....xxxxxxx | F4 | 9 |
| 1155/94 | xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx....xxxxxxx | NC | 10 |
| 2937/94 | xxxxxxx.xxxxxxxxxxxxxxxxxxxxxxxx....xxxxxxx | F5 | 10 |
| 3748/94 | xxxxxxxxxxxx.xxxxxxxxxxxxxxxxxxx....xxxxxxx | NC | 9 |
| 3505/94 | xxxxxxxxxxxxxxxxxxxxxxxxx......x....xxxxxxx | NC | 9 |
| 2661/94 | xxxxxx.xxxxxxxxxxxxxxxxxxxxxxxxx....xxxxxxx | NC | 11 |
| 461/94 | ..................................xxxxxxxxx | NC (A) | 14 |
| 1832/94 | ..................................xxxxxxxxx | A1 | 15 |
| 1716/94 | ..................................xxxxxxxxx | A6 | 15 |
| 2403/94 | ..................................xxxxxxxxx | A6 | 15 |
| 1277/94 | ..................................xxxxxxxxx | A7 | 17 |
| 1550/94 | ..................................xxxxxxxxx | A7 | 17 |
| 3032/94 | ..................................xxxxxxxxx | A7 | 17 |
| 2935/94 | ..................................xxxxxxxxx | A7 | 17 |
| 3942/94 | ..................................xxxxxxxxx | NC (A) | 17 |
| 904/94 | ..................................xxxxxxxxx | A9 | 16 |
| 1001/94 | ..................................xxxxxxxxx | A9 | 16 |
| 2356/94 | ..................................xxxxxxxxx | NC (A) | 18 |
| 1004/94 | xxxxxxx..xxxxxxxxxxxxxxxxxxx...x....xxx.xxx | NC (B) | 10 |
| 2011/94 | xxxxxxx..xxxxxxxxxxxxxxxxxxx...x....xxxxxxx | NC (B) | 12 |
| 3754/94 | xxxxxxxxxxxxxxxxxxxx....xxxxxxxx....xxxxxxx | NC | 13 |
| 205/94 | xxxxxxx..xxxxxxxxxxxxxxxxxxx...x.....xxxxxx | C2 | 10 |
| 1221/94 | xxxxxxxxxxxx.xxxxxxxxxxxxxxx...x....xxx.xxx | NC | 6 |
| 631/94 | xxxxxxxxxxxx.xxxxxxxxxxxxxxx...x....xxxxxxx | NC | 8 |
| 3674/94 | xxxxxxxxxxxxx.xxxxxx....xxxxxxxx....xxxxxxx | D2 | 10 |
| 1084/94 | xxxxxxxxxxxxxxxxxxxx....xxxxxxxx....xxxxxxx | D2 | 10 |
| 1485/94 | xxxxxxxxxxxxx...........xxxxxxxx....xxxxxxx | D3 | 9 |
| 696/94 | xxxxxxxxxxxxxx..........xxxxxxxx....xxx.xxx | D4 | 7 |
| 795/94 | xxxxxxxxxxxxxxxxxxxx....xxxxxxxx....xxxxxxx | F1 | 11 |
| 917/94 | x..xxxxxxxxxxxxxxxxx....xxxxxxxx....xxxxxxx | F4 | 11 |
| 865/94 | xxxxxxxxxxxxxxxxxxxxxxxxxxxxxx.x....xxxxxxx | NC | 8 |
| 1667/94 | xxxxxxxxxxxxxxxxx.xxxxxxxxxxxx.x....xxxxxxx | NC | 12 |
| 2900/94 | xxxx.xxxxxxxxxxxxxxxxxxxxx.....x....xxxxxxx | NC | 11 |
The isolates are sorted in the same order in which they were sorted by their IS6110 RFLP patterns in Fig. 2.
x, positive hybridization signals; ., lack of hybridization.
NC, nonclustered strains.
Patient characteristics in relation to isolate characteristics.
There was no statistically significant relationship between the gender or age of the patients and the RFLP patterns of the isolates. Of 33 clusters, only 6 smaller clusters (two or three isolates per cluster) represented patients who belonged to the same age group. In general, there was no clear correlation between RFLP patterns and the geographical origin of the isolates. Thus, the predominant family A was made of clusters which represent patient groups from both Northern and Southern part of Estonia (Table 3).
TABLE 3.
Characteristics of 209 TB patients and their isolates in relation to IS6110 RFLP patterns of isolates
| Patients | No. (%) | No of isolates/no of clusters
|
||
|---|---|---|---|---|
| 100% similarity | 90% similarity | 65% similaritya | ||
| Male | 151 (72) | 79/32 | 92/32 | 81 |
| Female | 58 (28) | 24/15 | 28/16 | 28 |
| Agea (mean, 41 yr) | ||||
| ≤ 41 yr | 103 (54) | 53/26 | 63/26 | 53 |
| > 41 yr | 88 (46) | 39/26 | 46/26 | 44 |
| From: | ||||
| North Estonia | 140 (67) | 65/29 | 78/29 | 74 |
| South Estonia | 69 (33) | 36/22 | 42/21 | 35 |
In 18 cases the ages of the patients were not known.
Number of isolates belonging to families A to E.
However, on a cluster level, there was a certain correlation with the geographical origin of the isolates. A comparison of the geographical origin of the isolates in family A shows that the isolates belonging to cluster A10 were scattered throughout the community while all isolates in cluster A7 and A12 were more geographically focused. All of the isolates from cluster A7 originated from Tartu (Southern Estonia), and all isolates in cluster A12 originated from North Estonia.
DISCUSSION
In this study we analyzed the drug resistance patterns of the M. tuberculosis isolates from newly detected and culture-verified pulmonary TB patients in Estonia during 1994 in relation to their RFLP patterns obtained with the insertion IS6110 as probe.
This is, to our knowledge, the first time a large group of closely related drug-resistant M. tuberculosis isolates with a limited number of different RFLP banding patterns in a human immunodeficiency virus-(HIV)-negative population has been demonstrated. A high proportion (49%) of all strains tested appeared in clusters. A predominant basic 15- to 19-band RFLP pattern was seen among 29% of all isolates, making up the family A. This family A comprised 12 clusters of identical isolates where the largest cluster comprised 10 identical isolates. The majority of all MDR isolates and isolates with any drug resistance belonged to family A.
A study on the population structure of M. tuberculosis strains in Ethiopia, Tunisia, and The Netherlands suggested that there are a small number of predominant families of genetically related strains in Ethiopia and Tunisia whereas no such distinct groupings predominate in The Netherlands (8, 22). Van Soolingen et al. have reinforced the idea that the predominance success of particular clones is related to a high incidence of TB (21). The current epidemiological situation place Estonia between the high-risk, resource-poor communities (like Ethiopia and Tunisia) and low-risk, developed communities (like The Netherlands).
The investigation of the population structure of M. tuberculosis in Estonia suggests that one-third of the isolates belonged to a remarkably homogeneous family of strains (family A). This family of strains may have contributed to the recent steady increase in TB morbidity since 1992 and, even more importantly, may have contributed to the emergence of MDR TB in the region (24–26).
The high similarity between the drug-resistant isolates indicates that in Estonia these strains are spreading rapidly, in fact more rapidly than the drug-susceptible strains, although the forces that contributed to the selection and dissemination of strains of family A are likely to be multifactorial. One reason for this rapid spread is probably that patients with drug-resistant strains are difficult to cure (19) and therefore will be contagious for a longer period.
In other studies where resistant strains were found in clusters, it was clearly demonstrated that transmission of MDR strains had occurred among HIV-infected patients (2, 16). In 1994, coinfection with tuberculosis and HIV was not reported in Estonia. Transmission of MDR TB is thus not limited to HIV-seropositive patients in an institutional setting but occurs within a community.
The strains included in family A were confirmed by spoligotyping as Beijing genotype strains. Van Soolingen et al. have shown that M. tuberculosis isolates grouped into the Beijing family by their IS6110 fingerprints and spoligopatterns also have a similar grouping pattern when other genetic markers are used. These characteristics are not generally shared with other strains outside the Beijing family (21). Recent observations suggest that strains of the Beijing family constitute a genetically closely related group of bacteria. Strains belonging to this genotype family possibly share particular phenotypic properties, such as antigens and virulence factors, which may be expressed as distinct manifestations in the pathology and the epidemiology of tuberculosis (12).
The Beijing genotype is the predominant clone of M. tuberculosis isolates in China (21), and isolates belonging to the Beijing family have been reported from four continents during the last few years (5, 21). This is the first countrywide study showing a correlation between the Beijing family and drug resistance.
The majority of isolates belonging to family A. i.e., the Beijing family. were drug resistant, but there was heterogeneity in resistance patterns among the isolates, ranging from full susceptibility to resistance to all four drugs tested. Of 12 clusters, 3 small clusters had isolates that were uniformly fully susceptible in their antibiotic resistance pattern. This fact indicates that drug resistance developed recently and in different clones among strains belonging to this family of strains.
Alito et al. have shown that some genotypes of MDR M. tuberculosis strains may have a higher mutation frequency and that the instability of IS6110 RFLP (highly similar but not identical RFLP patterns and with identical spoligotype) may be somehow related to the selective pressure of a combination of drugs used in therapy (1).
In contrast to our findings, Wilson et al. (23) have studied the contribution of recent transmission to the spread of non-Beijing drug-resistant TB in Texas. Based on the low percentage of clustered cases and the small cluster size, the authors concluded that there is no evidence for extensive transmission of drug-resistant TB.
Sources of TB have been suggested to preferentially transmit infection to people close to their own age (3). In the present study, when we analyzed cluster data stratified by age, we found that most of the 33 clusters contained patients of all age groups whereas only 6 smaller clusters comprised patients who belonged to same age group.
In general there was no strict correlation between clones and families with respect to the geographical origin of the isolates. Even if the MDR strains belonged to the same cluster, they were isolated from patients from different geographical areas, indicating that transmission of particular drug-resistant clones of M. tuberculosis contributes to the emergence of drug-resistant TB all over the country.
M. tuberculosis strains belonging to the Beijing genotype family have continued to dominate among new MDR TB patients in Estonia to the present day. There are strong indications that strains belonging to this family are widespread within the former Soviet Union and have even spread other parts of Europe such as Germany (17).
The data obtained during this study will contribute to a more thorough understanding of TB and MDR TB transmission in Estonia. Continued surveillance and immediate therapeutic decisions should be undertaken to prevent the dissemination of TB, and particularly when caused by such resistant strains.
ACKNOWLEDGMENTS
We express our gratitude to Marika Mikelsaar for critical reading and fruitful suggestions relating to the manuscript.
The study was supported by the Karolinska International Research and Training Programme, the Swedish Heart-Lung Association, the Swedish Medical Research Council (grant K99-06X), and the Commission of the European Communities, Directorate General XII, contract BMH1-CT93-1614.
REFERENCES
- 1.Alto A, Morcillo N, Scipioni S, Dolmann A, Romano M I, Cataldi A, van Soolingen D. The IS6110 restriction fragment length polymorphism in particular multidrug-resistant Mycobacterium tuberculosis strains may evolve too fast for reliable use in outbreak investigation. J Clin Microbiol. 1999;37:788–791. doi: 10.1128/jcm.37.3.788-791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bifani P J, Plikaytis B B, Kapur V, et al. Origin and interstate spread of a New York City multidrug-resistant Mycobacterium tuberculosis clone family. JAMA. 1996;275:452–457. [PubMed] [Google Scholar]
- 3.Borgdorff M W, Nagelkerke N J D, van Soolingen D, Broekmans J F. Transmission of tuberculosis between people of different ages in the Netherlands: an analysis using DNA fingerprinting. Int J Tuberc Lung Dis. 1999;3:202–206. [PubMed] [Google Scholar]
- 4.Canetti G, Fox W, Khomenko A, Mahler H T, Menon N K, Mitchison D A, Rist N, Smeley N A. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull W H O. 1969;41:21–43. [PMC free article] [PubMed] [Google Scholar]
- 5.Dale J W, Mat Nor R, Ramayah S, Hock Tang T, Zainuddin Z F. Molecular epidemiology of tuberculosis in Malaysia. J Clin Microbiol. 1999;37:1265–1268. doi: 10.1128/jcm.37.5.1265-1268.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glynn J R, Bauer J, de Boer A S, Borgdorff M W, Fine P E, Godfrey-Faussett P, Vynnycky E. Interpreting DNA fingerprint clusters of Mycobacterium tuberculosis. European Concerted Action on Molecular Epidemiology and Control of Tuberculosis. Int J Tuberc Lung Dis. 1999;3:1055–1060. [PubMed] [Google Scholar]
- 7.Heersma H F K, Kremer K, van Embden J D A. Computer analysis of IS6110 RFLP patterns of Mycobacterium tuberculosis. Methods Mol Biol. 1998;101:395–422. doi: 10.1385/0-89603-471-2:395. [DOI] [PubMed] [Google Scholar]
- 8.Hermans P W M, Messadi F, Guebrexabher H, van Soolingen D, de Haas P E W, Heersma H, de Neeling H, Ayoub A, Portales F, Frommel D, Zribi M, van Embden J D A. Usefulness of DNA typing for global tuberculosis epidemiology. J Infect Dis. 1995;171:1504–1513. doi: 10.1093/infdis/171.6.1504. [DOI] [PubMed] [Google Scholar]
- 9.Hoffner S E, Källenius G. Susceptibility of streptomycin-resistant Mycobacterium tuberculosis strains to amikacin. Eur J Clin Microbiol Infect Dis. 1988;7:188–190. doi: 10.1007/BF01963078. [DOI] [PubMed] [Google Scholar]
- 10.Isenberg H D, editor. Clinical microbiology procedures handbook. Vol. 1. Washington, D.C.: American Society for Microbiology; 1992. pp. 3.11.1–3.12.27. [Google Scholar]
- 11.Kamerbeek J, Schouls L, Kolk A, van Agderveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J D A. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kremer K, van Soolingen D, Frothingham R, Haas W H, Hermans P W M, Martin C, Palittapongarnpim P, Plikaytis B B, Riley L W, Yakrus M A, Musser M J, van Embden J D A. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol. 1999;37:2607–2618. doi: 10.1128/jcm.37.8.2607-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krüüner A, Sillastu H, Danilovitsh M, Levina K, Svenson S B, Källenius G, Hoffner S E. Drug resistant tuberculosis in Estonia. Int J Tuberc Lung Dis. 1998;2:130–133. [PubMed] [Google Scholar]
- 14.Kubin M, Havelkova M, Hyncicova I, Svecova Z, Kaustova J, Kremer K, van Soolingen D. The first occurrence of a multi-drug resistant tuberculosis epidemic in the Czech Republic caused by genetically closely related Mycobacterium tuberculosis strains. Centr Eur J Publ Health. 2000;8:24–27. [PubMed] [Google Scholar]
- 15.Laszlo A, Rahman M, Raviglione M, Bustreo F WHO/IUATLD Network of Supranational Reference Laboratories. Quality assurance programme for drug susceptibility testing of Mycobacterium tuberculosis in the WHO/IUATLD supranational laboratory network: first round of proficiency testing. Int J Tuberc Lung Dis. 1997;1:231–238. [PubMed] [Google Scholar]
- 16.Moro M I, Errante I, Infuso A, Sodano L, Gori A, Orcese C A, Salamina G, D'Amico C, Besozzi G, Caggese L. Effectiveness of infection control measures in controlling a nosocomial outbreak of multidrug-resistant tuberculosis among HIV patients in Italy. Int J Tuberc Lung Dis. 2000;4:61–68. [PubMed] [Google Scholar]
- 17.Niemann S, Rüsch-Gerdes S, Richter E. IS6110 fingerprinting of drug-resistant Mycobacterium tuberculosis strains isolated in Germany during 1995. J Clin Microbiol. 1997;35:3015–20. doi: 10.1128/jcm.35.12.3015-3020.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqi S H, Libonati J P, Middlebrook G. Evaluation of a rapid radiometric method for drug susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol. 1981;13:908–913. doi: 10.1128/jcm.13.5.908-912.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turett G S, Telzak E E, Torian L V, Blum S, Alland D, Weisfuse I, et al. Improved outcomes for patients with multidrug-resistant tuberculosis. Clin Infect Dis. 1995;21:1238–1244. doi: 10.1093/clinids/21.5.1238. [DOI] [PubMed] [Google Scholar]
- 20.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P W M, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Soolingen D, Qian L, de Haas P E W, Douglas J T, Traore H, Portaels F, Qing H Z, Enkhsaikan D, Nymadawa P, van Embden J D A. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J Clin Microbiol. 1995;33:3234–3238. doi: 10.1128/jcm.33.12.3234-3238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Soolingen D, Borgdorff M W, de Haas P E W, Sebek M M G G, Veen J, Dessens M, Kremer K, van Embden J D A. Molecular epidemiology of tuberculosis in The Netherlands: a nationwide study from 1993 through 1997. J Infect Dis. 1999;180:726–736. doi: 10.1086/314930. [DOI] [PubMed] [Google Scholar]
- 23.Wilson R W, Yang Z, Kelley M, Cave M D, Pogoda J M, Wallace R J, Jr, Cegielski J P, Dunbar D F, Bergmire-Sweat D, Elliott L B, Barnes P F. Evidence from molecular fingerprinting of limited spread of drug-resistant tuberculosis in Texas. J Clin Microbiol. 1999;37:3255–3259. doi: 10.1128/jcm.37.10.3255-3259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Anti-tuberculosis drug resistance in the world. The WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance 1994–1996. WHO/TB/97.229. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 25.World Health Organization. Anti-tuberculosis drug resistance in the world. The WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance 1996–1999. WHO/CDS/TB/2000.278. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 26.World Health Organization. Global tuberculosis control. WHO report 2000. WHO/CDS/TB/2000.275. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 27.Yang Z, Barnes P F, Chaves F, Eisenach K D, Weis S E, Bates J H, Cave M D. Diversity of DNA fingerprints of Mycobacterium tuberculosis isolates in the United States. J Clin Microbiol. 1998;36:1003–1007. doi: 10.1128/jcm.36.4.1003-1007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]