Abstract
Despite significant improvements in reperfusion strategies, acute coronary syndromes all too often culminate in a myocardial infarction (MI). The consequent MI can, in turn, lead to remodeling of the left ventricle (LV), the development of LV dysfunction, and ultimately progression to heart failure (HF). Accordingly, an improved understanding of the underlying mechanisms of MI remodeling and progression to HF is necessary. One common approach to examine MI pathology is with murine models that recapitulate components of the clinical context of acute coronary syndrome and subsequent MI. We evaluated the different approaches used to produce MI in mouse models and identified opportunities to consolidate methods, recognizing that reperfused and nonreperfused MI yield different responses. The overall goal in compiling this consensus statement is to unify best practices regarding mouse MI models to improve interpretation and allow comparative examination across studies and laboratories. These guidelines will help to establish rigor and reproducibility and provide increased potential for clinical translation.
Keywords: cardiac, ischemia-reperfusion, myocardial infarction, rigor and reproducibility, rodent
INTRODUCTION
Coronary artery disease continues to be a leading cause of morbidity and mortality, with the most significant consequence being an abrupt reduction in blood flow through a major coronary vessel, causing myocardial ischemia, clinically termed acute coronary syndrome (1, 2). Due to various risk factors and other influences, ischemia can range from low flow to no flow, and the period of ischemia can extend from a very short duration in time to permanent occlusion of the vessel. When the reduction in flow is of sufficient duration to cause irreversible damage to the myocardium (>30 min in mice), cardiomyocyte necrosis ensues to generate myocardial infarction (MI) (3). Clinically, the current optimal therapy is reperfusion to restore blood flow through the artery. Approximately 25% of patients will not be reperfused due to many reasons, including not seeking medical attention early enough or lack of success in reperfusion (4). In addition, up to 30% of those given reperfusion therapy will experience no-reflow, which is a state of myocardial tissue hypoperfusion due to impaired microvascular flow that occurs in the presence of a patent epicardial coronary artery (5). This also contributes to adverse cardiac remodeling and heart failure (3).
Although both reperfused and nonreperfused coronary artery occlusion can result in an MI, the underlying pathology, remodeling, and subsequent progression to dysfunction of the left ventricle (LV) can be distinctly different (4, 6–8). The pathophysiological distinction between MI types when induced in animal models is sometimes unintentionally disregarded or ignored. This lack of distinction can cause confusion when evaluating new therapeutic strategies or assessing comparative studies among laboratories. The overall goal of this consensus document is to provide guidance to establish uniformity in terms of MI models in mice. As both reperfused and nonreperfused MI models hold clinical relevance, this perspective is not intended to promulgate the superiority of one MI animal model. Instead, we will provide recommendations on how the cardiovascular physiology research community can standardize approaches and best evaluate primary response variables in the proper context.
There is a wide range of MI animal models that span from rodents to large animals. There are distinct advantages and disadvantages associated with all animal models. Large animals may recapitulate a higher fidelity of the clinical phenotype, whereas rodent models allow for examination of gene effects and offer higher throughput. These guidelines will examine murine models of MI and summarize approaches and biological and physiological response variables that are translationally important. There is a growing awareness in the cardiovascular physiology research community and funding agencies that provide financial support for this area of research for the importance of studies that exhibit strong experimental rigor and reproducibility. The outcome from this consensus document is to put forward a set of uniform strategies for inducing and evaluating reperfused or nonreperfused MI, to establish benchmarks regarding LV remodeling and function that arise as a response to MI, and to provide an extensive reference list that may serve as a useful guide for those interested in designing murine MI studies.
EVALUATION OF CURRENT LITERATURE
To perform a focused assessment of current practices in using MI models in mice and determine where improvements may be needed, we evaluated recent articles published by the American Journal of Physiology-Heart and Circulatory Physiology (AJP-Heart and Circ). The search included all research articles on mouse MI published in 2019 and 2020. Articles were identified from two searches of the journal website (accessed April 7, 2021 by M.L.L.) using the terms: myocardial infarction, mice, reperfusion, heart or myocardial infarction, and coronary artery occlusion. The first search identified 24 original research articles, of which 10 were excluded primarily for not using mice. The second search identified 30 articles, of which an additional 17 articles were included. A third search was performed (accessed April 22, 2021) by a second investigator (K.Y.D.P.) using the terms myocardial infarction, ischemia, and ischemia reperfusion, which identified one additional article that had not been previously included. Of the 32 articles remaining, 7 articles were duplicates and 6 articles were excluded during analysis for not meeting search criteria. This left 19 articles for evaluation (9–27). Four authors evaluated the articles (M.L.L., K.Y.D.P., G.V.H., and Z.K.).
A summary of the evaluation results is provided in Table 1. The use of reperfused versus nonreperfused MI was approximately a 60% reperfused/40% nonreperfused split. None of the articles reported the rationale for why the selected model was used. For reperfused MI studies, both the duration of ischemia and when the hearts were examined (time of reperfusion) were highly variable. Almost every study showed a different duration of ischemia and reperfusion. For the nonreperfused MI studies, there was more uniformity, with most studies evaluating end points at 7 days, 4 wk, or 12 wk, although the rationale for time of end point selected was often not provided.
Table 1.
Evaluation of publications on MI published by the American Journal of Physiology-Heart and Circulatory Physiology in 2019–2020
| Criteria | Results and Comments |
|---|---|
| Reperfused vs. Nonreperfused MI | Type: 11 reperfused (58%); 8 nonreperfused (42%) |
| Rationale provided for model selection | None |
| Duration of ischemia and reperfusion | Highly variable Ischemia times: 30’ (n = 5), 40’ (n = 1), 45’ (n = 3), 50’ (n = 1), 60’ (n = 1) Reperfusion times: 2.5 h (n = 1), 1 day (n = 2), 2 days (n = 1), 3 days (n = 3), 2 wk (n = 1), 4 wk (n = 3), 35 days (n = 1) |
| Nonreperfused MI time of evaluation | Focused on four times: 7 day (n = 2), 8 days and 21 days (n = 1); 4 wk (n = 4), 12 wk (n = 1) |
| Sex, age, strain detailed | Most provide details (only 1 did not report sex, 3 did not report age, 1 did not report strain); 53% used males only; wide variation in ages used (range 7 wk to 1 yr old); 89% used C57/BL6 (82% did not clarify J or N) and 11% used mixed strain |
| Sample sizes clear | All provided; not always clear the n/group; 9 studies had samples size for at least 1 group in at least 1 experiment that was n < 5, may have power issues with this analysis |
| Surgery detailed, inclusion/exclusion criteria provided (1-no; 5-very detailed) | Mean = 2.5; highly variable in the information provided; 47% did not confirm occlusion/ischemia (of those that did, 5 used EKG, 3 used visual inspection, 1 used both, and 1 did not state); Of 11 nonreperfused MI, 8 (73%) did not state whether reperfusion was confirmed (of those that did, 1 used EKG and 3 used visual confirmation) |
| Anesthesia/analgesics reported | 79% reported anesthesia use and 37% reported analgesics use; for the 79% who reported what anesthetic was used, 80% used isoflurane. Sevoflurane, ketamine, and pentobarbital each reported n = 1. For analgesic use, 86% of those reporting used buprenorphine. Carprofen was used in one study. |
| Infarct sizes/area at risk reported | Reported for 74% (n = 14); details variable, with some simply indicating it was done, some missing when it was done; for n = 2, one used Masson’s trichrome staining as a surrogate for infarct sizing and one used hematoxylin & eosin staining as surrogate for area at risk |
| Cardiac physiology measured | 79% performed echocardiography; 94% measured cardiac physiology by echocardiography, MRI, or PV loops; wide variation in time of evaluation and details on methods used |
| Recommendations from analysis to improve standardization |
|
n = 19 publications analyzed. MI, myocardial infarction.
There was overall good reporting of sex, age, and strain used. Recent efforts to include both sexes in rodent studies had an observable effect, with 47% of the studies using male and female mice (53% used males only, no study used females only). In 2018, there was an evaluation of mouse echocardiography articles published in AJP-Heart and Circ between January 1, 2016 and July 21, 2017 (28). In that evaluation, 84% provided details on sex (vs. 95% in the current evaluation), with 21% of studies using male and female mice (55% used males only, 8% used females only). Sample sizes were reported to varying degrees, making it unclear how many mice were used in each group or experiment. Half of the studies included sample sizes for at least one group in at least one experiment of n < 5, which may indicate a potential issue with statistical power (29).
The details provided in the methods to describe the surgical procedure were highly variable, with some articles reporting minimal technical information and others providing extensive details. In terms of rigor, the number of studies that did not confirm ischemia or reperfusion at the time of surgery was high [47% did not confirm ischemia by electrocardiogram (ECG) or blanching and 73% did not confirm reperfusion by resolution of ST segment elevation on ECG), as was the number of studies that did not calculate area at risk (for reperfused MI) or infarct size (for nonreperfused MI). There was adequate reporting of cardiac physiology, with 94% of the studies using either echocardiography, magnetic resonance imaging, hemodynamics by pressure-volume loops, or a combination of these approaches to document effects of MI on organ-level physiology. Based on these analyses, we developed a list of recommendations to focus efforts for improvement in experimental design and reporting on areas where gaps were identified.
REPERFUSED VERSUS NONREPERFUSED MI
Although both reperfused and nonreperfused MI models share an underlying initial ischemic insult, the type of myocardial injury is distinct and the models should not be used interchangeably. When deciding between models, one should first consider what aspect of the myocardial response to ischemic injury is to be studied. This section briefly describes the clinical situations modeled by reperfused and nonreperfused MI, summarizing the key similarities and differences. For a brief review on these two models of MI and which model may be most appropriate for a given study, we point the reader to a recent perspective on this topic (4).
As indicated in the most recent American Heart Association Guidelines for managing patients with ST-elevation MI (STEMI) (30), once a STEMI has been indicated by electrocardiogram, how to achieve reperfusion should be decided within 10 min. Regardless of reperfusion strategy, the goal is to restore flow to avoid cell death as rapidly as possible within 60–90 min (31). Because of increased emphasis on patient education to identify a heart attack and the need for rapid, effective reperfusion, the initial mortality from this acute coronary syndrome has declined (32). Thus, from a clinical perspective, an MI model that simulates a period of coronary occlusion followed by reperfusion is the most representative of the current clinical scenario.
A portion of MI patients are not reperfused; however, for various reasons, including medical decisions (inaccessible anatomy), a delay or no access to care (silent MI, misdiagnosis, or distance from the hospital), or ineffective reperfusion due to thrombotic microvascular obstruction (33). Patients in whom optimal reperfusion (thrombolysis in MI, TIMI 3 flow) is not achieved are at the highest risk of developing heart failure with reduced ejection fraction (HFrEF). Thus, rapid decompensation to HF is best modeled with the nonreperfused MI model. Patients undergoing reperfusion are also at risk of developing HFrEF, albeit at a lower rate (34–36). Thus, there is overlap in the phenotypes of these two animal models in the clinical situation. Plus, it is important to remember that the clinical situation is rarely binary (reperfused or nonreperfused MI), as the severity of both initial insult (lack of perfusion) and efficacy of reperfusion are highly variable, resulting in a gradient of perfusion outcomes in patients (37). In the clinical setting, all patient groups (i.e., those who are successfully and optimally reperfused, those who are not, and for everyone in between) are prone to progressive heart failure and recurrent cardiovascular events (35, 38, 39).
It is important to note that distinct cellular and molecular pathways can be activated in response to ischemia alone versus in response to ischemia and reperfusion. For reperfused MI, the duration of ischemia is an important determinant of the overall tissue response and extent of LV remodeling. Although ischemia tolerance is strain dependent (40), ischemia typically varies from 30–90 min in reperfused MI animal models. Rather than list an exhaustive inventory of differences between reperfused versus nonreperfused MI, which would be at the same time enormous and certainly incomplete, we focus here on the major differences as they apply to animal models (Fig. 1). The most obvious differences arise from the LV remodeling that occurs (43). The reperfused heart shows minimal changes in mass, size, shape, and function for ischemic intervals <45 min (44, 45). The Calvert laboratory and others have found that extended periods of ischemia (60–90 min) before reperfusion can induce large changes in LV dilation and function (46). Indeed, ischemia periods of >60–90 min are considered irreversible, with similar responses to nonreperfused MI (47).
Figure 1.
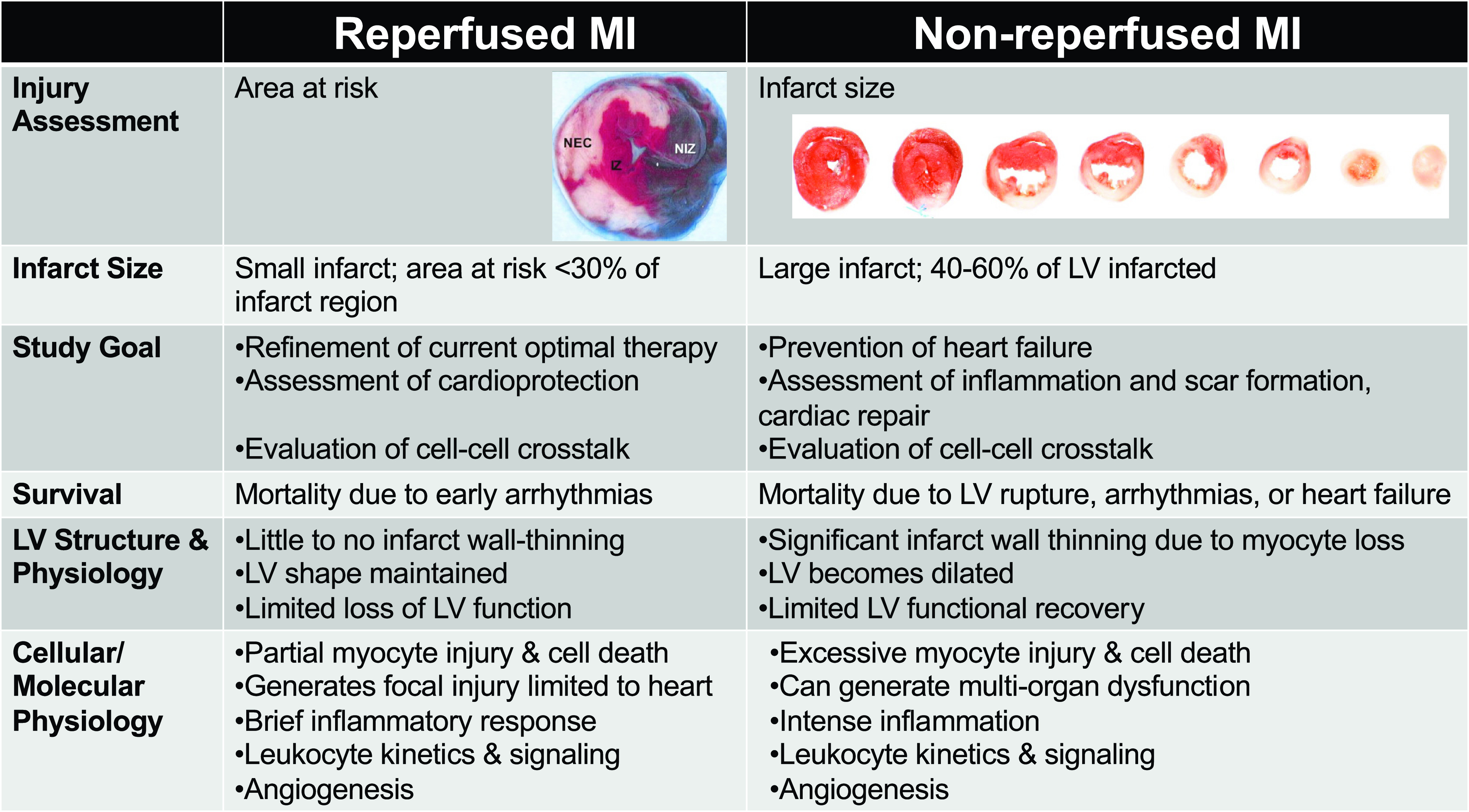
Key differences between reperfused myocardial infarction (MI) and nonreperfused MI, including differences in infarct sizes, study goals, structural and physiological responses, survival, and cell and molecular signaling. For the reperfused heart, the mid-myocardial section was stained with Evans blue (in vivo) and 1% triphenyltetrazolium chloride (ex vivo) to show the necrotic zone (NEC; white), nonischemic zone (NIZ; blue), and ischemic zone (IZ; red and white) (41). For the nonreperfused heart, the ventricles were sectioned from base (left) to apex (right) and stained with 1% triphenyltetrazolium chloride, which stains viable myocardium red and infarct is white (42). Images are reproduced with permission from the American Journal of Physiology-Heart and Circulatory Physiology.
In reperfused MI, inflammation is accelerated and amplified as leukocytes infiltrate and accumulate in the previously ischemic myocardium through the patent vessel. Infarcted regions in reperfused hearts sometimes present diffuse and patchy necrosis, making it difficult to quantify the true size of the infarct region because surviving myocytes are interspersed. The nonreperfused heart, in contrast, shows extensive LV dilation, infarct wall thinning, and LV dysfunction in response to a substantial amount of cell death (48). Because of considerable wall thinning (∼50% of the infarct wall), the structural and functional signs of HFrEF are rapidly reflected in the nonreperfused MI model. Therefore, rupture-related mortality is only appreciable in the nonreperfused MI model (49). Based on extensive wall thinning and heightened infiltration of leukocytes (neutrophils and monocytes), there is considerable mortality ranging from 10% to 40% due to ischemic events or congestive heart failure-related mortality in the nonreperfused MI model. The mortality range for both reperfused and nonreperfused MI is dependent both on sex (female mice have lower mortality) and on infarct size (50). Overall, if survival outcomes from an investigational therapy are of interest, the nonreperfused MI model is more appropriate (51). Due to accentuated heart failure, multiorgan inflammation and dysfunction (e.g., involving kidney and spleen) are typically more common in the nonreperfused MI (52, 53). These key points provide a means to compare the suitability of the specific models and tailor use to the unique research hypothesis.
MI MODELS IN MICE
The Evolution of the MI Model
The sophistication of MI models in mice have advanced over the last 25 yr. After decades of experimentation in large animals (including nonhuman primates, dogs, cats, pigs, sheep, and goats) and in rats and guinea pigs (54–85), mouse models of reperfused and nonreperfused MI were developed (86) and extensively characterized (6). Although large animal models may better recapitulate the clinical phenotype of ischemic injury regarding indices of cardiac remodeling and arrhythmogenesis, rodent studies, in particular mice, allow for basic mechanistic insight. Through the use of transgenesis, murine studies allow dissection of cellular and molecular mechanisms. In addition, with standardized approaches (the focus of this article), high throughput analyses of a consistent ischemic injury can be achieved.
One initial issue with establishing a protocol for MI surgery in mice was the heterogeneity of the coronary anatomy, which made artery visualization inconsistently difficult (87–89). Despite the technical challenges related to the small size of the animal and the difficulties in visualization of the coronary artery, MI in mice is highly reproducible in laboratories with experienced microsurgeons (1, 90). Because of the rapid progress in the development of genetic targeting approaches in mice and availability of a wide range of reagents, the mouse model of MI has emerged as a tractable tool for mechanistic dissection of cellular events and molecular pathways that may be involved in the injury, repair, and remodeling of the infarcted heart. The first iteration of MI in mice included intubation, followed by a sternal thoracotomy in which approximately three ribs were cut from the sternum and cauterized to gain access to the heart, then ligation of the left coronary artery (LCA) (86). This surgical approach generated a significant injury response that potentially masked early MI-induced inflammation (91).
Over the last 20 yr, significant technical advances have further expanded the range of capabilities of mouse models of MI. First was the development of a closed-chest model of coronary ischemia and reperfusion, in which the coronary artery could be occluded and reperfused 7–10 days after initial surgery through an exteriorized suture, allowing sufficient recovery time such that the study of inflammatory changes following MI could occur without the confounding effects of thoracotomy (91). The closed-chest model of ischemia-reperfusion can also be used to induce repetitive ischemic insults that may recapitulate aspects of chronic ischemic coronary disease (92–94).
Second was the development of a rapid approach for coronary artery ligation surgery by manually exteriorizing the heart outside the chest cavity, which reduced the duration of the surgical procedure by not requiring intubation and significantly decreased perioperative mortality and periprocedural inflammation (91, 95, 96). Even less invasive, ultrasound-guided approaches have been developed in mice, including transthoracic puncture and LCA ligation (97), as well as a novel needle-based electrocautery technique to rapidly coagulate and clot the LCA (98). This method by design does not allow for reperfusion. To date, these procedures have not been widely adopted. Third was later refinement using a minimally invasive intercostal thoracotomy (typically the 4th intercostal space) that does not involve cutting the ribs and was shown to have minimal adverse effects compared with unoperated controls (99). The minimally invasive surgery is recommended to prevent excessive inflammation due to surgery and eliminates the need for sham controls (99).
Additional murine variations on this approach include recurrent MI, which can now be studied in mice (100). As the ischemic event is a direct consequence of advanced atherosclerosis arising from vascular defects (endothelial dysfunction and lipoprotein retention) that are present in humans for years to decades before the MI, this pathological milieu should be considered (101). This model can be accomplished by individually ligating on separate days, first the LCA, followed later by the left circumflex artery distal to the first branching point of the LCA. These are challenging major survival microsurgeries when performed sequentially, and controls should be included to monitor the degree of first infarction, such as blood troponin levels. As another variation, MI has also been reported in the right ventricle using right coronary artery ligation in mice (102). In this model, right ventricle heart failure is induced and is associated with the development of LV diastolic dysfunction. This model provides a means to study mechanisms and treatments of the combination of right and left ventricular dysfunction after MI.
Ablation Models
In contrast to traditional ischemia-induced infarcts, cryoablation is used in mice to produce infarcts, with precisely controlled size, shape, and location (103–107). In this approach, a precooled probe or handheld liquid nitrogen probe is applied to the epicardial surface at a specific location for a precise amount of time, resulting in consistent infarcts that are independent of variations in individual coronary anatomy (98). Because of the controlled shape, size, and degree of transmurality of infarcts with this approach, it can be useful for studies of infarct mechanics and long-term LV structural and functional remodeling (107). With potentially lower animal-to-animal variability, cryoablation may increase statistical power to detect treatment effects. Like prolonged ischemia, cryoablation results in widespread necrosis that involves complete cell death in the injured region. Because of the differential modes of cell death, ablative infarcts are less appropriate for studying early mechanisms of infarct-induced inflammation, neovascularization, and scar formation and may be suitable for testing tissue-engineered, stem cell, or other regenerative or rejuvenating therapies (108, 109).
Neonatal MI and Regenerative Medicine Aspects
In contrast to adults, MI in neonatal mice can lead to cardiac regeneration (110, 111). Neonatal cardiac regeneration is reproducible only if the cardiac injury is induced by coronary artery ligation on the first postnatal day after birth (112). Neonatal mice (before P8) have the capability to proliferate myocytes and show self-renewal of resident macrophages (113, 114). The potential importance of macrophages for this response was demonstrated using a targeted cell depletion model, which prevented regeneration of the neonatal myocardium and reduced angiogenesis following MI (115). Cardiac fibroblasts switch from STAT3 to TLR and NF-κB signaling early postnatally, which is also believed to play a role in how a very young versus adult heart will respond to an ischemic event (116, 117). Although neonatal rodents tolerate low temperatures, their body temperature is considerably sensitive to ambient temperature. Thus, the standardized control of body heat is key for reproducible results (118). Initial inhaled isoflurane, combined with induced hypothermia, is advised for anesthesia (119). The reduced blood flow of hypothermia may make it difficult to visualize the LCA, which is critical for consistent degrees of cardiac injury. The length of time that pups are away from the mother can affect maternal cannibalization, and this time should be minimized. Particular attention should be paid to noting sex of the pup. The cardiac metabolic response to ischemia may be distinct in neonates, which may affect degree of ischemic injury and repair (120).
Computational Models Using Mouse Big Data and Artificial Intelligence
One advantage of having uniform evaluation times is the ability to combine results across studies for meta-analysis examination (121). Comparing adult and neonatal MI using a big data approach can yield nonintuitive insights. The Engler laboratory used such an approach to define cross talk mechanisms that occur between macrophages and cardiac fibroblasts in a regenerative versus nonregenerative heart (116). Using genomics data from multiple data sets, they were able to identify key regulators that are changing with age and contributing to LV remodeling after MI injury. In a similar study, Lindsey et al. demonstrated the strength of using a large data set to determine markers of adverse remodeling (122). By querying the mouse Heart Attack Research Tool (mHART) 1.0 (90), a data set collected from a single site laboratory with multiple investigators, the authors were able to identify a phenotype of extreme LV dilation that was not due to infarct size and identify plasma proteins and LV infarct inflammatory cells and genes that explain the differences in dilation. Using mHART in addition to proteomic evaluation of plasma from participants in the Jackson Heart Study, DeLeon-Pennell and colleagues (123) demonstrated a sex-dependent response to MI and development of heart failure. These studies highlight the strength of translating findings by confirming mouse findings in samples from humans.
RECOMMENDATIONS FOR DESIGNING EXPERIMENTS AND REPORTING RESULTS
Experimental Design Details
The MI surgery procedure in mice has been detailed extensively (52, 124–126), and Table 2 provides a summary of recommendations that include consideration for the Animals in Research: Reporting in vivo Experiments (ARRIVE) Guidelines (127). The American Physiological Society has made available guidelines for transparent reporting that it recommends all authors follow (https://www.physiology.org/author-info.promoting-transparent-reporting). We include recommendations to authors to use the ARRIVE guidelines and suggest that journals adopt the ARRIVE or similar reporting guidelines to improve transparency and reproducibility (128). The quality of MI is optimally assessed by measuring infarct size (for nonreperfused MI) and additionally area at risk (for reperfused MI). For detailed staining methodologies, please see Refs. 48 and 129–133. In mice, permanently ligating the coronary artery around 1–2 mm distal to the left atrium will consistently generate large infarcts (35%–60% of total LV) or large areas at risk (47, 134, 135). Small infarcts in the nonreperfused MI model may reflect technical issues in missing the coronary artery, with damage and localized fibrosis resulting from the suture rather than reflecting the intended MI. Ligating more than 3 mm from the atria may result in high MI variability due to branching of the coronary artery (88).
Table 2.
Recommendations for MI studies
| Model | Recommendations |
|---|---|
| All models | • Follow ARRIVE guidelines |
| Reperfused MI (MI/R) | • Measure both infarct size and area-at-risk • Measure blood pressure and heart rate during ischemia (note that especially the heart rate during ischemia significantly influences the infarct size; if necessary, include appropriate control groups) • Body temperature must be tightly regulated • Use to study impacts on infarct size (cardioprotection) • Use to study interventions that require reperfusion and relationship to clinical scenario of reperfusion • Use to study regeneration, repair, or remodeling • Use MI/R test interventions in a model clinically relevant to the reperfused patient |
| Nonreperfused MI | • Measure infarct size within 3 days of MI to determine whether the intervention/mutation impacts acute infarct size; differences are highly improbable in mice because of lack of functional collaterals • Use to study regeneration, repair, or remodeling • Use nonreperfused MI to test interventions in a robust remodeling model and to test interventions in a model clinically relevant to the nonreperfused/very late reperfused patient |
| Cryoinjury | • Use to control size, shape, or location of infarct, but experimental protocol (e.g., contact duration, probe temperature) must be standardized to enhance consistency • Use to achieve uniform cell death in target region, but extent of transmural damage should be determined (early and late)Use for relevance to ablation used in humans • Not ideal for studying pathophysiology of MI |
ARRIVE, animals in research: Reporting in vivo experiments; MI/R, myocardial ischemia-reperfusion; MI, myocardial infarction.
In addition to monitoring the LV for visual changes at the time of surgical MI induction (i.e., blanching and akinesis), ECG monitoring for ST segment elevation to confirm ischemia is also recommended. Cardiac troponin I or other cardiac injury markers can also be measured in plasma or serum as surrogates for infarct size. Technical ischemia success should be confirmed for both MI models by confirming ST segment elevation and QTc prolongation. These alterations should normalize with timely reperfusion (136), and a lack of ECG changes during reperfusion can indicate permanent ligation (perhaps due to vessel damage). Telemetry can also be useful to monitor postsurgical intervention ECG in conscious and unrestrained mice. Noninvasive two-dimensional short and long-axis echocardiography can be used to estimate the degree of infarction by akinesis, wall thickness, and chamber dimensions (24). Cardiac output with a deformed LV free wall can be measured by Doppler echocardiography of pulmonary flow (125). In vivo infarct size can be noninvasively measured with late gadolinium enhancement MRI and independently corroborated by histology (42, 69). For terminal endpoints, LV pressure-volume catheterization is useful to determine load-dependent and load-independent conditions of systolic and diastolic function (137, 138). For nonreperfused MI, infarct sizes ≤30% (% infarct to total LV size) are typically excluded because of the absence of remodeling. If included, small and large infarcts should be grouped separately to reduce possible type II statistical errors. A necropsy is strongly recommended for all mice that die prematurely to determine the cause of death and assess early deaths due to technical issues for improvement. Perioperative death within 24 h of MI in nonreperfused mice is usually due to surgical errors (e.g., excessive bleeding or lung injury) or very large infarct sizes and arrhythmia. In mice with nonreperfused MI, postoperative deaths typically occur during days 3–7 after MI and are most often due to cardiac rupture, acute heart failure, or arrhythmias. Table 3 lists suggested inclusion and exclusion criteria for reperfused and nonreperfused MI experiments. We recommend that studies be randomized and blinded, with operators remaining blinded during treatment, data acquisition (e.g., functional diagnostic by echocardiography), and analysis (139–141).
Table 3.
Inclusion and exclusion criteria for reperfused and nonreperfused MI experiments
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Reperfused | Confirmed ligation by electrocardiogram (ECG, ST segment elevation) and visual inspection (blanching and akinesis downstream of ligation) Confirmed reperfusion by ECG (resolution of ST segment elevation) and visual inspection (return of coloration and movement) |
Failure to confirm ischemia or reperfusion Inconsistent ischemia period Death due to a technical error |
| Nonreperfused | Confirmed ligation by ECG (ST segment elevation), visual inspection (blanching and akinesis downstream of ligation), or echocardiography assessment after ligation (wall motion abnormalities and reduced ejection fraction) Infarct size ≥30% measured in the acute phase (i.e., MI days 1–3) |
Perioperative death (within 24 h of ligation, which generally indicates a technical error) Infarct size <30% Ejection fraction >40% or fractional shortening >20% at MI day 1 |
These criteria are indicated for control wild-type mice, and adjustments may need to be considered for mice that are genetically modified or treated. MI, myocardial infarction.
When selecting optimal time points for evaluation, know that timing is everything. We noticed from our evaluation that there was a wide range of options for both duration of ischemia and time of end point assessment. For reperfused MI studies, ischemia times ranged from 30 to 60 min and evaluation time ranged from 2.5 h to 35 days after reperfusion, with no uniformity across studies. For nonreperfused MI studies, the evaluation time ranged from 7 days to 4 wk to 12 wk and showed more uniformity across studies. The optimal design of studies using mouse models of reperfused or nonreperfused MI requires an understanding of the temporal sequence of the events associated with injury and repair (1, 142–147).
Traditional concepts on the time course of ischemic myocardial injury, derived from seminal studies in the canine model, suggest that cardiomyocyte death progresses with increased duration of the ischemic insult as a wavefront of necrosis from the more susceptible subendocardium to the less vulnerable subepicardium (148, 149). A similar wavefront of cardiomyocyte death likely occurs in the mouse model of coronary occlusion (6). This wavefront leads to expansion of the infarct zone with increasing duration of ischemia, from a thin area of dead cardiomyocytes in the subendocardium and mid-myocardium after 30 min of coronary occlusion to a more extensive infarct that becomes transmural throughout the area at risk after 4–6 h of ischemia (1). Studies using the reperfused MI model to dissect pathways regulating cardiomyocyte survival in response to injury typically use ischemia intervals between 45 and 60 min, thus allowing evaluation of interventions that may either accelerate or delay the wavefront of death. Shorter intervals of ischemia (<45 min) are associated with very small infarcts and may hamper documentation of cardioprotection or cardiac repair interventions. With long coronary occlusion intervals (>2 h), the infarct involves almost the entire area at risk, precluding evaluation of interventions that may accentuate injury. Some investigators have used varying times of ischemia to differentiate genotypes with increased susceptibility from those with protective genotypes (e.g., 30 vs. 60 min), and shorter durations can be useful to reveal interventions that increase cardiac susceptibility to injury or dysfunction (150–153). The scientific question to be addressed is the driving aspect of the experimental design, and the exact time of ischemia or reperfusion should be determined based on that question. As a general rule for reperfused MI, starting out with ischemia of 45 min is recommended. For evaluation times for both reperfused and nonreperfused MI, any time in the first week is appropriate to determine how the inflammatory and healing responses are progressing, with days 1, 3, 5, and 7 being common times of evaluation in the current literature. For chronic evaluation, 4 wk is a common end point, with 2, 8, and 12 wk also being represented (12, 13, 154–162).
In adult mammals, repair of the infarcted heart is dependent on the formation of a collagen-based scar and requires sequential infiltration of the infarcted heart with phagocytic leukocytes, as well as activation of reparative myofibroblasts and vascular cells (163, 164). These cellular events can be studied using either reperfused or nonreperfused MI model. Because inflammation and repair are accelerated upon reperfusion, early time points need to be studied when using the reperfused MI model (56). In nonreperfused MI, the inflammatory response is initiated early upon ischemic injury and peaks between days 1 and 3 after coronary occlusion. Transcription of proinflammatory cytokine and chemokine genes exhibits an early peak that is followed by transient neutrophil infiltration peaking at MI days 1–3 (131, 165, 166). This is overlapped by monocyte recruitment (166–168), prolonged accumulation of macrophages (113, 169, 170), and activation of α-smooth muscle actin (α-SMA)-expressing myofibroblasts at MI days 3–7 (171). As the scar matures (beginning around MI day 7), infarct myofibroblasts transition to matrifibrocytes, which are specialized fibroblast-like cells that do not express α-SMA but may be involved in scar maintenance (172). Infarct neovessels acquire a coat comprised of mural cells, which may stabilize the vasculature and inhibit angiogenesis (144, 173, 174). Lymphatics also help to modulate inflammation resolution after MI (175). Healing of the infarct zone is associated with remodeling of the noninfarcted remote myocardium, as viable cardiomyocytes become hypertrophic and the chamber progressively dilates, presumably through the activation of matrix-degrading proteases and matricellular proteins in the cardiac interstitium (176). Optimal study of chronic dilative remodeling of the infarcted heart requires assessing chamber dimensions and function for at least 4 wk after coronary occlusion. Ultimately, the experimental question will drive the time point(s) selected. We recommend 28 days for a universal chronic time point, as this is the most common time of chronic evaluation in the literature (12, 13, 154–161). For exercise protocols that start at 4 wk after MI, the 12-wk time point is often used, and we refer to recent guidelines on using exercise protocols (177–179). The major reason for conforming times is to evaluate reproducibility of findings. In addition, compiling datasets across studies and teams that follow the same protocols will allow the use of big data tools to provide conserved observations, generate new hypotheses, and assess variability across laboratories.
Anesthesia and Analgesics
The majority of studies evaluated (80%) used the volatile anesthetic isoflurane for the MI surgery while postoperative analgesics varied. Several of these agents may influence the biology of the MI through effects on inflammation that should be considered (180). For example, isoflurane is often used as a volatile anesthetic that can systemically reduce levels of inflammatory cytokines and impact diastolic function, heart rate, and body temperature; therefore, prolonged exposure is not recommended (137, 181). The anesthetic ketamine can exhibit anti-inflammatory properties that directly ameliorate ischemia-reperfusion injury. Anesthetics and analgesics can also affect myocardial oxygen consumption and influence infarct size. Buprenorphine, one of the most used analgesics in MI models, has also been associated with suppression of inflammation in rodents (182). Carprofen is a nonsteriodal anti-inflammatory drug, and pretreatment of carprofen in a mouse nonreperfused MI model impairs the initiation of the inflammatory phase and delays resolution response to promote development of the cardiorenal syndrome (180, 183). The analgesic meloxicam can reduce infarct size during cerebral injury (184). Lidocaine has well-known anti-inflammatory properties and is rarely used in mice (184). Because anesthesia and analgesics are necessary components of the surgical protocol, it is important to consider the possible influence these agents may have on results and to consistently monitor for respiration, heart rate, and body temperature to ensure time and exposures are applied similarly across comparison groups.
Sex as a Biological Variable
In our evaluation, 47% of studies included both males and females, up from 21% found in an evaluation done in 2018 for studies using echocardiography (28). For nonreperfused MI, female mice survive better and have a lower incidence of rupture in nonsurvivors (15, 50, 123, 185). Women who present with MI are not reperfused as quickly as men and have lower rates of guideline-directed medical care (32). Unadjusted 5- and 10-yr MI mortality was reported to be higher in women than men, and sex-dependent differences became less prominent when corrected for age, comorbidities, and treatments (186). A recent study from Spain reported that women with STEMI had higher mortality than men, underwent invasive intervention procedures less often than men, and had higher in-hospital mortality when admitted for STEMI (187). Another recent study from India similarly reported worse in-hospital outcomes in women because they did not receive guideline-directed management (188), whereas women undergoing percutaneous coronary intervention showed better outcomes (lower all-cause death) compared with men (189). In patients with type 2 diabetes, the risk of death following MI was lower in women than men (186–190). When systems were implemented to remove sex disparities in care, the differences in outcomes resolved (191), indicating the increased MI mortality in females is not due to underlying biological differences. Therefore, our understanding of MI responses across sexes/genders is still rudimentary and future studies on this important topic are warranted (192).
Because MI is prevalent in humans regardless of sex or gender, we recommend using both male and female mice. The National Institutes of Health requires that consideration be given to sex as a biological variable, and AJP-Heart and Circ has recently published an editorial on journal expectations for use of sex and gender (192). There was a lack of consensus by the writing team regarding whether sexes or genders should be combined for analysis. Using both sexes in a combined analysis will allow identification of pathways and targets that affect both sexes equally. At the same time, the best way to formulate a therapeutic approach for sexes may entail identifying similarities and differences with tailored intervention by sex/gender. Evaluating by gender in humans and sex in mice allowed the elucidation of the role of LXR/RXR signaling in female versus male neutrophil responses to MI (123). Initial concerns that female mice may show high variability has not stood up in the data; in fact, female mice often have lower standard deviations than male counterparts (90, 193–195). Although male and female mouse hearts remodel after MI, the exact underlying mechanisms may vary. Furthermore, even if no overt sex differences are observed with MI in wild-type mice or control settings, there could be sex differences in response to specific treatments, particularly when interactions with G protein-coupled receptors or other hormone-related receptors are involved (196–199) Together, the existing data indicate a strong reason to evaluate MI remodeling in both sexes/genders.
If there is no a priori rationale to segregate or bias to one sex, then equal numbers of both sexes should be included under the assumption of nonvariation (e.g., n = 8, 4 M/4 F). The aggregated data should be reported with indication within the group as to sex of each individual. If there is an a priori rationale to segregate (e.g., evaluating LV rupture as a primary outcome of interest), this should be stated and cited in the methods to justify separate sex analysis. If a mouse breeding colony is being maintained for study enrollment, there is an ethical responsibility to ensure both sexes are responsibly used to avoid the unnecessary generation of animals (200, 201).
Time of Day Considerations
Circadian rhythm is essential for healthy cardiovascular physiology, and occurrence of MI exhibits a diurnal rhythm with increased incidence of patients presenting early in the morning compared with any other time of the day (202, 203). In studies when MI was induced in mice during wake versus sleep hours, higher mortality was reported in the sleep-time MI cohort (204). The Steffens laboratory has demonstrated that mice show larger infarct size and greater neutrophil infiltration and more adverse remodeling when coronary ligation is performed in the evening, compared with the morning (204). In addition, the time of MI-triggered expression of a unique set of genes with higher inflammatory and cytokine expression following sleep-time MI (205). Diurnal fluctuation in blood monocyte count has been reported in humans (206). Differences are reported to be more relevant to permanent MI, whereas reperfused MI may not exhibit a circadian rhythm (207). Alternating group randomization during the operational window is recommended (i.e., do not work on all males, followed by all females, nor a series of controls, followed by a series of treatment groups). We recommend that similar time of day (e.g., 8:00 AM to 12:00 PM or 11:00 AM to 3:00 PM) be used across your experimental protocol and reported in methods, such that group differences influenced by circadian rhythms are mitigated.
Comorbidities
In addition to considering biological variables (i.e., age, sex, and circadian rhythm), the presence of comorbidities that replicate the human scenario more closely have been used with MI models in mice. At 3–6 mo of age, mice are homogenous in physiological maturation, including body weight (208–210), and MI has been reported in adult mice ranging in age from 6 wk to 36 mo (186–190). In this context, it is relevant to note that increased age in humans reduces sex-specific associations between risk factors and MI (211). Mouse MI models have been used in various experimental contexts that mimic different clinical scenarios to enhance our understanding of the role of comorbidities in the response of the heart to injury.
In addition to aging (212–215), other comorbidities examined in MI models include dietary interventions with or without aging (216, 217), preexposure to lipopolysaccharide from Porphyromonas gingivalis (218, 219), preexisting atherosclerosis (220), and other comorbid conditions prevalent in patients with coronary disease that may contribute to coronary events (41, 221). In both MI models, coexistence of morbidities such as obesity, metabolic syndrome, lifestyle factor modifications (sleep, stress, exercise), or aging increases the translational relevance of the study, as these are often associated with MI in humans. MI in diabetic or hyperlipidemic mice can provide information on the impact of common comorbid conditions on ischemia-induced cell death, repair, remodeling, and fibrosis, thus contributing to our understanding of the highly heterogeneous spectrum of clinical responses (41, 222). In addition, other comorbidities can be induced by MI itself to worsen outcomes; one example is cardiac dyssynchrony (223).
To summarize our recommendations for experimental design, we suggest using mice of similar age, of both sexes, and performing MI surgery and tissue collection at a consistent time of day for all mice, preferably within a 4-h window. All these factors should be considered when designing, analyzing, and reporting experimental studies.
Reporting Details
Over the last decade, there has been an increasing recognition for the necessity of established standard operating procedures and guidelines related to the rigor and reproducibility of reported scientific data. Several reports highlighted the lack of reproducibility in preclinical animal research, particularly from findings presented in high-profile journals (224–228). Lack of rigor or reproducibility affects the rate of basic research discovery and the success of clinical translation (225). To avoid biased, false, or incorrect reporting, rigor and reproducibility must be included within the study design and reporting. Table 4 provides a checklist for reporting MI study results that give consideration for rigor and reproducibility.
Table 4.
Reporting checklist, modified from the Animals in Research: reporting in vivo Experiments (ARRIVE) Guidelines
| Item | Details |
|---|---|
| Abstract: report of relevant details | • Provide summary of research background, rationale, objectives • Give details to the experimental approach, report animal species and the animal model used |
| Ethical statements | • Institutional Animal Care and Use Committee approval, Guide for the Care and Use of Laboratory Animals, welfare assessments and interventions |
| Animal description, housing, husbandry, animal care, and monitoring | • Species, strain, source, age (mean and range), sex, genotypes, body weight (mean and range), health/immune status, housing • Type (specific pathogen free), cage type, bedding material, number of cage companions, light-dark cycle, room • Temperature and humidity, food type, food, and water access • Report interventions/steps to reduce pain, suffering and distress and report animal monitoring (if necessary) • Report anesthesia and analgesic use and pain monitoring; surgical procedure details and monitoring, method of euthanasia • Report expected and unexpected adverse events |
| Study design | • Define model used • Define groups; matching, randomization, and blinding protocols; order of treatment and assessment of groups; method to confirm model success • Report inclusion/exclusion criteria (e.g., hemodynamics or lower limit for infarct size) • Report daily monitoring to record time and cause of death • Define timing of perioperative and postoperative phases for survival analysis, flow chart for complex designs |
| Interpretation and translation | • Comment on study limitations, limitations to the animal model used • Comment on the relevance to human myocardial infarction |
| Experimental procedures | • Define area examined (e.g., infarct, remote, both regions) • Define positive and negative controls; for drugs: formulation, dose, site, and route of administration • Record and report the recorded data (e.g., electrocardiogram, heart rate, and anesthesia depth) • Report time of day performed |
| Sample sizes | • Report sample size calculation • Report number of animals used per group for each experiment • List any animals or samples removed from analysis with reason |
| Variables measured and data access | • Report primary and secondary end points • Provide a statement describing if and where data are available |
| Statistics | • Methods used for each analysis, test for assumptions • Report a measure of variability (e.g., means ± standard deviation or median and range) |
For each study, a hypothesis should be stated (as appropriate), and inclusion and exclusion experimental criteria should be predefined to determine true outliers before experiments are initiated (229). Details on the rationale for specific ischemia and reperfusion times for reperfused MI must be provided in the methods. Finally, sample size and outcome measures should be determined a priori (230, 231). Power analyses are warranted to determine the sample size that can detect a relevant treatment effect based upon the primary outcome measure evaluated. Several statistical programs allow for these calculations and, in general, include four basic components: 1) type I error (α, commonly fixed at 0.05), which measures the probability that the differences found are likely to happen; 2) type II error (β, commonly set at 0.20), which accounts for false negatives and represents the power of a study (i.e., a β of 0.20 represents 0.80 power or 80% probability of avoiding a false negative conclusion); 3) the smallest effect of interest, which represents the minimal difference between the studied groups that the investigator wishes to detect (often referred to as the minimal difference physiologically/biologically relevant or effect size); and 4) variability, for which preliminary or published data can be used to estimate the standard deviation of a given outcome variable (232). For more details on use of statistics, please see the guidelines Statistical Considerations in Reporting Cardiovascular Research (29).
For transparent reporting, we recommend researchers adhere to the ARRIVE Guidelines (127). These guidelines were initially developed in 2010 to improve the reporting of in vivo research to ensure a comprehensive and transparent description; thus, better enabling others to evaluate and reproduce the study. The guidelines were recently updated, and information was reorganized to facilitate better use in practice (233).
DISCUSSION
The approach selected (reperfused vs. nonreperfused MI) will depend on the questions being asked. Likewise, the inputs examined, outputs measured, and time(s) of evaluation are dependent on study goals. There is no one gold standard to use for experimental design of MI studies. The focus instead should be on the design that most appropriately addresses the specific hypothesis being tested. Both reperfused and nonreperfused MI models are well suited to study repair and remodeling. Reperfused MI is mandatory for studying cardioprotection, and ischemia must be of sufficient severity and duration to induce ischemic injury and remodeling. Consideration should be given to the question being asked and to prospectively plan the study design around that question. This includes determining the best controls, randomization, blinding schemes, and analysis using appropriate statistics before experiments commence.
MI models are essential for translational research. Only under in vivo conditions can the effect of an intervention by adequately studied to assess the full interaction among different cell and molecular components to influence overall LV remodeling or healing after MI. Several examples highlight the translational potential of animal models. A classic example of a bench to bedside translation is the use of ACE inhibitors as standard treatment of MI; these studies were initially undertaken in a rodent permanent occlusion MI model by Janice Pfeffer and colleagues (234). There are clear limitations to clinical translation that include the small size of the mouse, which makes arrhythmias difficult to detect; different healing patterns (frequent LV rupture after nonreperfused MI); the predominant use of young adult animals without comorbidities; a lack of genetic variations in the mouse strains used; and cleaner working conditions (lack of regular infections in animal facilities and specific diets). Another caveat that must be considered is that in the murine models described, there is no inciting thrombotic event causing myocardial ischemia, which makes it difficult to determine the impact of platelet activation and release of numerous inflammatory mediators that contribute to microvascular obstruction. Add to this list the potential variability in experimental conditions across laboratories and differences can be observed between preclinical research and the actual clinical practice. The last point can be avoided and is a prime motivation for the writing of this article.
In conclusion, these Guidelines provide best practice details to assist investigators in the planning and executing studies involving MI in mice. This consensus is part of ongoing efforts by AJP-Heart and Circ to provide guidelines articles that help to improve the rigor and reproducibility of cardiovascular physiology studies (28, 29, 47, 179, 235, 236).
GRANTS
This work was funded by National Institutes of Health Grants GM115458 and HL137319 (to M. L. Lindsey); AA027625, HL136737, and HL147570 (to J. A. Kirk); HL076246, HL085440, and HL149407 (to N. G. Frangogiannis); HL111600 (to C. M. Ripplinger); HL122309 (to E. B. Thorp); HL147844, GM127607, and HL078825 (to S. P. Jones); HL132989 and HL144788 (to G. V. Halade); HL145817 (to K. Y. DeLeon-Pennell); HL130972 (to F. G. Spinale); HL127442 (to R. J. Gumina); DK115213 and HL136915 (to J. W. Calvert); and HL152297 (to L. E. C. Brás); Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Grants BX000168 (to F. G. Spinale), BX000505 (to M. L. Lindsey), and BX003922 (to K. Y. DeLeon-Pennell); American Heart Association Innovator Project IPA35260039 (KYD-P); United States Department of Defense Grant PR181464 (to N. G. Frangogiannis); Natural Sciences Engineering Research Council Grants RGPIN-05520 and RGPIN-04878 (to K. R. Brunt); Canadian Institutes of Health Research Grants PJT-37522 and PJT-153306 (to Z. Kassiri) and PJT-421341 and PJo-413883 (to K. R. Brunt); Canadian Foundation for Innovation Grant 31708 (to K. R. Brunt); Heart and Stroke Foundation of Canada Grants G-19–0026282 (to Z. Kassiri) and G-16–00014524 (to K. R. Brunt); Heart and Stroke Foundation of New Brunswick (to K. R. Brunt); American Heart Association Grants 18AIREA33960311 (to L. E. C. Brás) and TPA2035490150 (to D. P. Del Re.); National Health and Medical Research Council of Australia Grant APP1158013 (to R. H. Ritchie); and New Brunswick Health Research and Innovation Foundations (to K. R. Brunt).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies or the American Physiological Society. All authors have reviewed and approved the article.
DISCLOSURES
M. L. Lindsey, K. R. Brunt, J. A. Kirk, P. Kleinbongard, C. M. Ripplinger, and Z. Kassiri are editors of AJP-Heart and Circ. Given their roles, none had any involvement in the peer review of this article outside of being authors and had no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Jason Carter.
AUTHOR CONTRIBUTIONS
M.L.L., J.A.K., and P.K. conceived and designed research; M.L.L., L.E.d.C.B., and K.Y.D.-P. analyzed data; M.L.L., L.E.d.C.B., and K.Y.D.-P. interpreted results of experiments; M.L.L., K.R.B., J.A.K., P.K., J.W.C., L.E.d.C.B., K.Y.D.-P., D.P.D.R., N.G.F. S.F., R.J.G., G.V.H., S.P.J., R.H.R., F.G.S., E.B.T., C.M.R., and Z.K., drafted manuscript; M.L.L., K.R.B., J.A.K., P.K., J.W.C., L.E.d.C.B., K.Y.D.-P., D.P.D.R., N.G.F. S.F., R.J.G., G.V.H., S.P.J., R.H.R., F.G.S., E.B.T., C.M.R., and Z.K., edited and revised manuscript; M.L.L., K.R.B., J.A.K., P.K., J.W.C., L.E.d.C.B., K.Y.D.-P., D.P.D.R., N.G.F., S.F., R.J.G., G.V.H., S.P.J., R.H.R., F.G.S., E.B.T., C.M.R., and Z.K. approved final version of manuscript.
ACKNOWLEDGMENTS
M. L. Lindsey is a Stokes-Shackleford Professor at University of Nebraska Medical Center. R. J. Gumina is the James Hay and Ruth Jansson Wilson Professor in Cardiology and recipient of the Robert J. Anthony Fund for Cardiovascular Disease Research at The Ohio State University Wexner Medical Center. Z. Kassiri holds a Canada Research Chair (Tier 1) in Cardiovascular Matrix Remodeling and is a member of the Royal Society of Canada: College of New Scholars. K. R. Brunt is a Translational Scientist at the New Brunswick Heart Centre.
REFERENCES
- 1.Frangogiannis NG. Pathophysiology of myocardial infarction. Compr Physiol 5: 1841–1875, 2015. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics-2021 Update: a report from the American Heart Association. Circulation 143: e254–e743, 2021. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 3.Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet 389: 197–210, 2017. [Erratum in Lancet 389: 156, 2017]. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 4.Lindsey ML, de Castro Brás LE, DeLeon-Pennell KY, Frangogiannis NG, Halade GV, O'Meara CC, Spinale FG, Kassiri Z, Kirk JA, Kleinbongard P, Ripplinger CM, Brunt KR. Reperfused vs. nonreperfused myocardial infarction: when to use which model. Am J Physiol Heart Circ Physiol 321: H208–H213, 2021. doi: 10.1152/ajpheart.00234.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation 105: 656–662, 2002. doi: 10.1161/hc0502.102867. [DOI] [PubMed] [Google Scholar]
- 6.Christia P, Bujak M, Gonzalez-Quesada C, Chen W, Dobaczewski M, Reddy A, Frangogiannis NG. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem 61: 555–570, 2013. doi: 10.1369/0022155413493912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamasaki S, Kusachi S, Moritani H, Kondo J, Hirohata S, Tamura A, Tsuji T. Reperfusion hastens appearance and extent of distribution of type I collagen in infarct zone: immunohistochemical study in rat experimental infarction. Cardiovasc Res 30: 763–768, 1995. [PubMed] [Google Scholar]
- 8.Vandervelde S, van Amerongen MJ, Tio RA, Petersen AH, van Luyn MJ, Harmsen MC. Increased inflammatory response and neovascularization in reperfused vs. non-reperfused murine myocardial infarction. Cardiovasc Pathol 15: 83–90, 2006. doi: 10.1016/j.carpath.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Alter C, Ding Z, Flögel U, Scheller J, Schrader J. A2bR-dependent signaling alters immune cell composition and enhances IL-6 formation in the ischemic heart. Am J Physiol Heart Circ Physiol 317: H190–H200, 2019. doi: 10.1152/ajpheart.00029.2019. [DOI] [PubMed] [Google Scholar]
- 10.Audam TN, Nong Y, Tomlin A, Jurkovic A, Li H, Zhu X, Long BW, Zheng YW, Weirick T, Brittian KR, Riggs DW, Gumpert A, Uchida S, Guo Y, Wysoczynski M, Jones SP. Cardiac mesenchymal cells from failing and nonfailing hearts limit ventricular dilation when administered late after infarction. Am J Physiol Heart Circ Physiol 319: H109–H122, 2020. doi: 10.1152/ajpheart.00114.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conklin DJ, Guo Y, Nystoriak MA, Jagatheesan G, Obal D, Kilfoil PJ, Hoetker JD, Guo L, Bolli R, Bhatnagar A. TRPA1 channel contributes to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 316: H889–H899, 2019. doi: 10.1152/ajpheart.00106.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBerge M, Yu S, Dehn S, Ifergan I, Yeap XY, Filipp M, Becker A, Luo X, Miller S, Thorp EB. Monocytes prime autoreactive T cells after myocardial infarction. Am J Physiol Heart Circ Physiol 318: H116–H123, 2020. doi: 10.1152/ajpheart.00595.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duerr GD, Wu S, Schneider ML, Marggraf V, Weisheit CK, Velten M, Verfuerth L, Frede S, Boehm O, Treede H, Dewald O, Baumgarten G, Kim S-C. CpG postconditioning after reperfused myocardial infarction is associated with modulated inflammation, less apoptosis, and better left ventricular function. Am J Physiol Heart Circ Physiol 319: H995–H1007, 2020. doi: 10.1152/ajpheart.00269.2020. [DOI] [PubMed] [Google Scholar]
- 14.Dugaucquier L, Feyen E, Mateiu L, Bruyns TAM, Keulenaer GWD, Segers VFM. The role of endothelial autocrine NRG1/ERBB4 signaling in cardiac remodeling. Am J Physiol Heart Circ Physiol 319: H443–H455, 2020. doi: 10.1152/ajpheart.00176.2020. [DOI] [PubMed] [Google Scholar]
- 15.Hanna A, Shinde AV, Frangogiannis NG. Validation of diagnostic criteria and histopathological characterization of cardiac rupture in the mouse model of nonreperfused myocardial infarction. Am J Physiol Heart Circ Physiol 319: H948–H964, 2020. doi: 10.1152/ajpheart.00318.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, Guo Y, Xia Y, Guo Y, Wang R, Zhang F, Guo L, Liu Y, Yin T, Gao C, Gao E, Li C, Wang S, Zhang L, Yan W, Tao L. Resistin promotes cardiac homing of mesenchymal stem cells and functional recovery after myocardial ischemia-reperfusion via the ERK1/2-MMP-9 pathway. Am J Physiol Heart Circ Physiol 316: H233–H244, 2019. doi: 10.1152/ajpheart.00457.2018. [DOI] [PubMed] [Google Scholar]
- 17.Ilatovskaya DV, Pitts C, Clayton J, Domondon M, Troncoso M, Pippin S, DeLeon-Pennell KY. CD8+ T-cells negatively regulate inflammation post-myocardial infarction. Am J Physiol Heart Circ Physiol 317: H581–H596, 2019. doi: 10.1152/ajpheart.00112.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaminski AR, Moore ET, Daseke MJ 2nd, Valerio FM, Flynn ER, Lindsey ML. The compendium of matrix metalloproteinase expression in the left ventricle of mice following myocardial infarction. Am J Physiol Heart Circ Physiol 318: H706–H714, 2020. doi: 10.1152/ajpheart.00679.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konhilas JP, Sanchez JN, Regan JA, Constantopoulos E, Lopez-Pier M, Cannon DK, Skaria R, McKee LA, Chen H, Lipovka Y, Pollow D, Brooks HL. Using 4-vinylcyclohexene diepoxide as a model of menopause for cardiovascular disease. Am J Physiol Heart Circ Physiol 318: H1461–H1473, 2020. doi: 10.1152/ajpheart.00555.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koppinger MP, Lopez-Pier MA, Skaria R, Harris PR, Konhilas JP. Lactobacillus reuteri attenuates cardiac injury without lowering cholesterol in low-density lipoprotein receptor-deficient mice fed standard chow. Am J Physiol Heart Circ Physiol 319: H32–H41, 2020. doi: 10.1152/ajpheart.00569.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philip JL, Murphy TM, Schreier DA, Stevens S, Tabima DM, Albrecht M, Frump AL, Hacker TA, Lahm T, Chesler NC. Pulmonary vascular mechanical consequences of ischemic heart failure and implications for right ventricular function. Am J Physiol Heart Circ Physiol 316: H1167–H1177, 2019. doi: 10.1152/ajpheart.00319.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren X, Roessler AE, Lynch TL, Haar L, Mallick F, Lui Y, Tranter M, Ren MH, Xie WR, Fan G-C, Zhang J-M, Kranias EG, Anjak A, Koch S, Jiang M, Miao Q, Wang Y, Cohen A, Rubinstein J, Weintraub NL, Jones WK. Cardioprotection via the skin: nociceptor-induced conditioning against cardiac MI in the NIC of time. Am J Physiol Heart Circ Physiol 316: H543–H553, 2019. doi: 10.1152/ajpheart.00094.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scofield SLC, Dalal S, Lim KA, Thrasher PR, Daniels CR, Peterson JM, Singh M, Singh K. Exogenous ubiquitin reduces inflammatory response and preserves myocardial function 3 days post-ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 316: H617–H628, 2019. doi: 10.1152/ajpheart.00654.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Olivas A, Stuart SDF, Tapa S, Blake MR, Woodward WR, Habecker BA, Ripplinger CM. Cardiac sympathetic nerve transdifferentiation reduces action potential heterogeneity after myocardial infarction. Am J Physiol Heart Circ Physiol 318: H558–H565, 2020. doi: 10.1152/ajpheart.00412.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, Zhu T, Chen L, Luo W, Chao J. MCP-1 mediates ischemia-reperfusion-induced cardiomyocyte apoptosis via MCPIP1 and CaSR. Am J Physiol Heart Circ Physiol 318: H59–H71, 2020. doi: 10.1152/ajpheart.00308.2019. [DOI] [PubMed] [Google Scholar]
- 26.Zhuang L, Li C, Chen Q, Jin Q, Wu L, Lu L, Yan X, Chen K. Fatty acid-binding protein 3 contributes to ischemic heart injury by regulating cardiac myocyte apoptosis and MAPK pathways. Am J Physiol Heart Circ Physiol 316: H971–H984, 2019. doi: 10.1152/ajpheart.00360.2018. [DOI] [PubMed] [Google Scholar]
- 27.Nawaito SA, Sahadevan P, Clavet-Lanthier M-É, Pouliot P, Sahmi F, Shi Y, Gillis M-A, Lesage F, Gaestel M, Sirois MG, Calderone A, Tardif J-C, Allen BG. MK5 haplodeficiency decreases collagen deposition and scar size during post-myocardial infarction wound repair. Am J Physiol Heart Circ Physiol 316: H1281–H1296, 2019. [Erratum in Am J Physiol Heart Circ Physiol 321: H161, 2021]. doi: 10.1152/ajpheart.00532.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol 314: H733–H752, 2018. doi: 10.1152/ajpheart.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindsey ML, Gray GA, Wood SK, Curran-Everett D. Statistical considerations in reporting cardiovascular research. Am J Physiol Heart Circ Physiol 315: H303–H313, 2018. doi: 10.1152/ajpheart.00309.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK; American College of Cardiology, American Heart Association Task Force on Practice Guidelines, and Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the management of patients with acute myocardial infarction). Circulation 110: e82–e292, 2004. [PubMed] [Google Scholar]
- 31.Armstrong PW, Collen D, Antman E. Fibrinolysis for acute myocardial infarction: the future is here and now. Circulation 107: 2533–2537, 2003. doi: 10.1161/01.CIR.0000072930.64775.DC. [DOI] [PubMed] [Google Scholar]
- 32.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 Update: a report from the American Heart Association. Circulation 141: e139–e596, 2020. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 33.Ribichini F, Ferrero V, Wijns W. Reperfusion treatment of ST-elevation acute myocardial infarction. Prog Cardiovasc Dis 47: 131–157, 2004. doi: 10.1016/j.pcad.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Cahill TJ, Kharbanda RK. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: mechanisms, incidence and identification of patients at risk. World J Cardiol 9: 407–415, 2017. doi: 10.4330/wjc.v9.i5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert L, Brown K, Segal E, Brophy J, Rodes-Cabau J, Bogaty P. Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA 303: 2148–2155, 2010. doi: 10.1001/jama.2010.712. [DOI] [PubMed] [Google Scholar]
- 36.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 37.Bagai A, Dangas GD, Stone GW, Granger CB. Reperfusion strategies in acute coronary syndromes. Circ Res 114: 1918–1928, 2014. doi: 10.1161/CIRCRESAHA.114.302744. [DOI] [PubMed] [Google Scholar]
- 38.Nair R, Johnson M, Kravitz K, Huded C, Rajeswaran J, Anabila M, Blackstone E, Menon V, Lincoff AM, Kapadia S, Khot UN. Characteristics and outcomes of early recurrent myocardial infarction after acute myocardial infarction. J Am Heart Assoc 10: e019270, 2021. doi: 10.1161/JAHA.120.019270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta RH, Harjai KJ, Cox D, Stone GW, Brodie B, Boura J, O'Neill W, Grines CL. Clinical and angiographic correlates and outcomes of suboptimal coronary flow inpatients with acute myocardial infarction undergoing primary percutaneous coronary intervention. J Am Coll Cardiol 42: 1739–1746, 2003. doi: 10.1016/j.jacc.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Guo Y, Flaherty MP, Wu WJ, Tan W, Zhu X, Li Q, Bolli R. Genetic background, gender, age, body temperature, and arterial blood pH have a major impact on myocardial infarct size in the mouse and need to be carefully measured and/or taken into account: results of a comprehensive analysis of determinants of infarct size in 1,074 mice. Basic Res Cardiol 107: 288, 2012. doi: 10.1007/s00395-012-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones SP, Girod WG, Granger DN, Palazzo AJ, Lefer DJ. Reperfusion injury is not affected by blockade of P-selectin in the diabetic mouse heart. Am J Physiol Heart Circ Physiol 277: H763–H769, 1999. doi: 10.1152/ajpheart.1999.277.2.H763. [DOI] [PubMed] [Google Scholar]
- 42.Bohl S, Lygate CA, Barnes H, Medway D, Stork LA, Schulz-Menger J, Neubauer S, Schneider JE. Advanced methods for quantification of infarct size in mice using three-dimensional high-field late gadolinium enhancement MRI. Am J Physiol Heart Circ Physiol 296: H1200–H1208, 2009. doi: 10.1152/ajpheart.01294.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81: 1161–1172, 1990. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 44.Tejada T, Tan L, Torres RA, Calvert JW, Lambert JP, Zaidi M, Husain M, Berce MD, Naib H, Pejler G, Abrink M, Graham RM, Lefer DJ, Naqvi N, Husain A. IGF-1 degradation by mouse mast cell protease 4 promotes cell death and adverse cardiac remodeling days after a myocardial infarction. Proc Natl Acad Sci USA 113: 6949–6954, 2016. doi: 10.1073/pnas.1603127113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimizu Y, Nicholson CK, Lambert JP, Barr LA, Kuek N, Herszenhaut D, Tan L, Murohara T, Hansen JM, Husain A, Naqvi N, Calvert JW. Sodium sulfide attenuates ischemic-induced heart failure by enhancing proteasomal function in an Nrf2-dependent manner. Circ Heart Fail 9: e002368, 2016. doi: 10.1161/CIRCHEARTFAILURE.115.002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimizu Y, Nicholson CK, Polavarapu R, Pantner Y, Husain A, Naqvi N, Chin LS, Li L, Calvert JW. Role of DJ-1 in modulating glycative stress in heart failure. J Am Heart Assoc 9: e014691, 2020. doi: 10.1161/JAHA.119.014691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindsey ML, Bolli R, Canty JM, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Longacre LS, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314: H812–H838, 2018. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeap XY, Dehn S, Adelman J, Lipsitz J, Thorp EB. Quantitation of acute necrosis after experimental myocardial infarction. Methods Mol Biol 1004: 115–133, 2013. doi: 10.1007/978-1-62703-383-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res 112: 675–688, 2013. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pullen AB, Kain V, Serhan CN, Halade GV. Molecular and cellular differences in cardiac repair of male and female mice. J Am Heart Assoc 9: e015672, 2020. doi: 10.1161/JAHA.119.015672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin TG, Myers VD, Dubey P, Dubey S, Perez E, Moravec CS, Willis MS, Feldman AM, Kirk JA. Cardiomyocyte contractile impairment in heart failure results from reduced BAG3-mediated sarcomeric protein turnover. Nat Commun 12: 2942, 2021. doi: 10.1038/s41467-021-23272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halade GV, Kain V, Ingle KA. Heart functional and structural compendium of cardiosplenic and cardiorenal networks in acute and chronic heart failure pathology. Am J Physiol Heart Circ Physiol 314: H255–H267, 2018. doi: 10.1152/ajpheart.00528.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gowda SB, Gowda D, Kain V, Chiba H, Hui SP, Chalfant CE, Parcha V, Arora P, Halade GV. Sphingosine-1-phosphate interactions in the spleen and heart reflect extent of cardiac repair in mice and failing human hearts. Am J Physiol Heart Circ Physiol 321: H599–H611, 2021. doi: 10.1152/ajpheart.00314.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hood WB Jr, McCarthy B, Lown B. Myocardial infarction following coronary ligation in dogs. Hemodynamic effects of isoproterenol and acetylstrophanthidin. Circ Res 21: 191–199, 1967. doi: 10.1161/01.res.21.2.191. [DOI] [PubMed] [Google Scholar]
- 55.Michael LH, Ballantyne CM, Zachariah JP, Gould KE, Pocius JS, Taffet GE, Hartley CJ, Pham TT, Daniel SL, Funk E, Ml E. Myocardial infarction and remodeling in mice: effect of reperfusion. Am J Physiol 277: H660–H668, 1999. doi: 10.1152/ajpheart.1999.277.2.H660. [DOI] [PubMed] [Google Scholar]
- 56.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 164: 665–677, 2004. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva KAS, Emter CA. Large animal models of heart failure: a translational bridge to clinical success. JACC Basic Transl Sci 5: 840–856, 2020. doi: 10.1016/j.jacbts.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romagnuolo R, Masoudpour H, Porta-Sanchez A, Qiang B, Barry J, Laskary A, Qi X, Masse S, Magtibay K, Kawajiri H, Wu J, Valdman Sadikov T, Rothberg J, Panchalingam KM, Titus E, Li RK, Zandstra PW, Wright GA, Nanthakumar K, Ghugre NR, Keller G, Laflamme MA. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Reports 12: 967–981, 2019. doi: 10.1016/j.stemcr.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heusch G, Skyschally A, Schulz R. The in-situ pig heart with regional ischemia/reperfusion - ready for translation. J Mol Cell Cardiol 50: 951–963, 2011. doi: 10.1016/j.yjmcc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 60.Shirakawa Y, Sawa Y, Takewa Y, Tatsumi E, Kaneda Y, Taenaka Y, Matsuda H. Gene transfection with human hepatocyte growth factor complementary DNA plasmids attenuates cardiac remodeling after acute myocardial infarction in goat hearts implanted with ventricular assist devices. J Thorac Cardiovasc Surg 130: 624–632, 2005. doi: 10.1016/j.jtcvs.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 61.Gomoll AW. Cardioprotection associated with preconditioning in the anesthetized ferret. Basic Res Cardiol 91: 433–443, 1996. doi: 10.1007/BF00788724. [DOI] [PubMed] [Google Scholar]
- 62.McKean T, Mendenhall W. Comparison of the responses to hypoxia, ischaemia and ischaemic preconditioning in wild marmot and laboratory rabbit hearts. J Exp Biol 199: 693–697, 1996. [DOI] [PubMed] [Google Scholar]
- 63.Ritchie DM, Kelliher GJ, MacMillan A, Fasolak W, Roberts J, Mansukhani S. The cat as a model for myocardial infarction. Cardiovasc Res 13: 199–206, 1979. doi: 10.1093/cvr/13.4.199. [DOI] [PubMed] [Google Scholar]
- 64.Reynolds RD, Kelliher GJ, Ritchie DM, Roberts J, Beasley AB. Comparison of the arrhythmogenic effect of myocardial infarction in the cat and dog. Cardiovasc Res 13: 152–159, 1979. doi: 10.1093/cvr/13.3.152. [DOI] [PubMed] [Google Scholar]
- 65.Liu S, Li K, Wagner Florencio L, Tang L, Heallen TR, Leach JP, Wang Y, Grisanti F, Willerson JT, Perin EC, Zhang S, Jf M. Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction. Sci Transl Med 13, 2021. doi: 10.1126/scitranslmed.abd6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538: 388–391, 2016. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 67.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510: 273–277, 2014. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang DH, Kim TH, Choi JW, Kim ST, Yoon JH, Shin JH, Seo JB, Lim TH. Manganese dipyridoxyl diphosphate (MnDPDP)-enhanced magnetic resonance imaging of acute reperfused myocardial injury in a cat model: part II: comparison with cine magnetic resonance imaging. Invest Radiol 40: 56–61, 2005. [PubMed] [Google Scholar]
- 69.Lee SS, Goo HW, Park SB, Lim CH, Gong G, Seo JB, Lim TH. MR imaging of reperfused myocardial infarction: comparison of necrosis-specific and intravascular contrast agents in a cat model. Radiology 226: 739–747, 2003. doi: 10.1148/radiol.2263011473. [DOI] [PubMed] [Google Scholar]
- 70.Verdouw PD, van den Doel MA, de Zeeuw S, Duncker DJ. Animal models in the study of myocardial ischaemia and ischaemic syndromes. Cardiovasc Res 39: 121–135, 1998. doi: 10.1016/S0008-6363(98)00069-8. [DOI] [PubMed] [Google Scholar]
- 71.Holt WW, Wendland MF, Derugin N, Wolfe C, Saeed M, Higgins CB. Effects of nicardipine, a calcium antagonist, on myocardial salvage and high energy phosphate stores in reperfused myocardial injury. J Am Coll Cardiol 16: 1736–1744, 1990. doi: 10.1016/0735-1097(90)90328-m. [DOI] [PubMed] [Google Scholar]
- 72.Silvis MJM, van Hout GPJ, Fiolet ATL, Dekker M, Bosch L, van Nieuwburg MMJ, Visser J, Jansen MS, Timmers L, de Kleijn DPV. Experimental parameters and infarct size in closed chest pig LAD ischemia reperfusion models; lessons learned. BMC Cardiovasc Disord 21: 171, 2021. doi: 10.1186/s12872-021-01995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lock MC, Tellam RL, Darby JRT, Soo JY, Brooks DA, Seed M, Selvanayagam JB, Morrison JL. Identification of novel miRNAs involved in cardiac repair following infarction in fetal and adolescent sheep hearts. Front Physiol 11: 614, 2020. doi: 10.3389/fphys.2020.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castro L, Geertz B, Reinsch M, Aksehirlioglu B, Hansen A, Eschenhagen T, Reichenspurner H, Weinberger F, Pecha S. Implantation of hiPSC-derived cardiac-muscle patches after myocardial injury in a Guinea pig model. J Vis Exp, 2019. doi: 10.3791/58810. [DOI] [PubMed] [Google Scholar]
- 75.Dasagrandhi D, R ASK, Muthuswamy A, Lennox AM, Jayavelu T, Devanathan V, Kesavan Swaminathan J. Ischemia/reperfusion injury in male guinea pigs: an efficient model to investigate myocardial damage in cardiovascular complications. Biomed Pharmacother 99: 469–479, 2018. doi: 10.1016/j.biopha.2018.01.087. [DOI] [PubMed] [Google Scholar]
- 76.Chandrakala AN, Kwiatkowski P, Sai-Sudhakar CB, Sun B, Phillips A, Parthasarathy S. Induction of early biomarkers in a thrombus-induced sheep model of ischemic heart failure. Tex Heart Inst J 40: 511–520, 2013. [PMC free article] [PubMed] [Google Scholar]
- 77.Long M, Yang L, Huang G, Liu L, Dong Y, Du Z, Tang A, Hu C, Gu R, Gao X, Tang L. Thrombin and its receptor enhance ST-segment elevation in acute myocardial infarction by activating the KATP channel. Mol Med 16: 322–332, 2010. doi: 10.2119/molmed.2010.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jardine DL, Charles CJ, Frampton CM, Richards AM. Cardiac sympathetic nerve activity and ventricular fibrillation during acute myocardial infarction in a conscious sheep model. Am J Physiol Heart Circ Physiol 293: H433–H439, 2007. doi: 10.1152/ajpheart.01262.2006. [DOI] [PubMed] [Google Scholar]
- 79.Schulz R, Post H, Vahlhaus C, Heusch G. Ischemic preconditioning in pigs: a graded phenomenon: its relation to adenosine and bradykinin. Circulation 98: 1022–1029, 1998. doi: 10.1161/01.cir.98.10.1022. [DOI] [PubMed] [Google Scholar]
- 80.Behrends M, Schulz R, Post H, Alexandrov A, Belosjorow S, Michel MC, Heusch G. Inconsistent relation of MAPK activation to infarct size reduction by ischemic preconditioning in pigs. Am J Physiol Heart Circ Physiol 279: H1111–1119, 2000. doi: 10.1152/ajpheart.2000.279.3.H1111. [DOI] [PubMed] [Google Scholar]
- 81.Aikawa T, Kariya T, Yamada KP, Miyashita S, Bikou O, Tharakan S, Fish K, Ishikawa K. Impaired left ventricular global longitudinal strain is associated with elevated left ventricular filling pressure after myocardial infarction. Am J Physiol Heart Circ Physiol 319: H1474–H1481, 2020. doi: 10.1152/ajpheart.00502.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hobby ARH, Sharp TE 3rd, Berretta RM, Borghetti G, Feldsott E, Mohsin S, Houser SR. Cortical bone-derived stem cell therapy reduces apoptosis after myocardial infarction. Am J Physiol Heart Circ Physiol 317: H820–H829, 2019. doi: 10.1152/ajpheart.00144.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skyschally A, Amanakis G, Neuhäuser M, Kleinbongard P, Heusch G. Impact of electrical defibrillation on infarct size and no-reflow in pigs subjected to myocardial ischemia-reperfusion without and with ischemic conditioning. Am J Physiol Heart Circ Physiol 313: H871–H878, 2017. doi: 10.1152/ajpheart.00293.2017. [DOI] [PubMed] [Google Scholar]
- 84.Fomovsky GM, Holmes JW. Evolution of scar structure, mechanics, and ventricular function after myocardial infarction in the rat. Am J Physiol Heart Circ Physiol 298, 2010. doi: 10.1152/ajpheart.00495.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wheeler DG, Joseph ME, Mahamud SD, Aurand WL, Mohler PJ, Pompili VJ, Dwyer KM, Nottle MB, Harrison SJ, d'Apice AJ, Robson SC, Cowan PJ, Gumina RJ. Transgenic swine: expression of human CD39 protects against myocardial injury. J Mol Cell Cardiol 52: 958–961, 2012. doi: 10.1016/j.yjmcc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol 269: H2147–H2154, 1995. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- 87.Kumar D, Hacker TA, Buck J, Whitesell LF, Kaji EH, Douglas PS, Kamp TJ. Distinct mouse coronary anatomy and myocardial infarction consequent to ligation. Coron Artery Dis 16: 41–44, 2005. doi: 10.1097/00019501-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 88.Chen J, Ceholski DK, Liang L, Fish K, Hajjar RJ. Variability in coronary artery anatomy affects consistency of cardiac damage after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 313: H275–H282, 2017. doi: 10.1152/ajpheart.00127.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahn D, Cheng L, Moon C, Spurgeon H, Lakatta EG, Talan MI. Induction of myocardial infarcts of a predictable size and location by branch pattern probability-assisted coronary ligation in C57BL/6 mice. Am J Physiol Heart Circ Physiol 286: H1201–H1207, 2004. doi: 10.1152/ajpheart.00862.2003. [DOI] [PubMed] [Google Scholar]
- 90.DeLeon-Pennell KY, Iyer RP, Ma Y, Yabluchanskiy A, Zamilpa R, Chiao YA, Cannon PL, Kaplan A, Cates CA, Flynn ER, Halade GV, de Castro Brás LE, Lindsey ML. The Mouse Heart Attack Research Tool 1.0 database. Am J Physiol Heart Circ Physiol 315: H522–H530, 2018. doi: 10.1152/ajpheart.00172.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nossuli TO, Lakshminarayanan V, Baumgarten G, Taffet GE, Ballantyne CM, Michael LH, Entman ML. A chronic mouse model of myocardial ischemia-reperfusion: essential in cytokine studies. Am J Physiol Heart Circ Physiol 278: H1049–H1055, 2000. doi: 10.1152/ajpheart.2000.278.4.H1049. [DOI] [PubMed] [Google Scholar]
- 92.Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, Taffet G, Michael LH, Crapo JD, Welz A, Entman ML. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci USA 100: 2700–2705, 2003. doi: 10.1073/pnas.0438035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 115: 584–592, 2007. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 94.Lavine KJ, Kovacs A, Weinheimer C, Mann DL. Repetitive myocardial ischemia promotes coronary growth in the adult mammalian heart. J Am Heart Assoc 2: e000343, 2013. doi: 10.1161/JAHA.113.000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res 107: 1445–1453, 2010. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Entman ML, Nossuli TO, Lakshminarayanan V, Michael LH. For want of a few good shams. Am J Physiol Heart Circ Physiol 278: H1017–H1018, 2000. doi: 10.1152/ajpheart.2000.278.4.H1017. [DOI] [PubMed] [Google Scholar]
- 97.Sun Q, Wang KK, Pan M, Zhou JP, Qiu XT, Wang ZY, Yang Z, Chen Y, Shen H, Gu QL, Fang LH, Zhang GG, Bai YP. A minimally invasive approach to induce myocardial infarction in mice without thoracotomy. J Cell Mol Med 22: 5208–5219, 2018. doi: 10.1111/jcmm.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sicklinger F, Zhang Y, Lavine KJ, Simon N, Bucher V, Jugold M, Lehmann L, Konstandin MH, Katus HA, Leuschner F. A minimal-invasive approach for standardized induction of myocardial infarction in mice. Circ Res 127: 1214–1216, 2020. doi: 10.1161/CIRCRESAHA.120.317794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iyer RP, de Castro Brás LE, Cannon PL, Ma Y, DeLeon-Pennell KY, Jung M, Flynn ER, Henry JB, Bratton DR, White JA, Fulton LK, Grady AW, Lindsey ML. Defining the sham environment for post-myocardial infarction studies in mice. Am J Physiol Heart Circ Physiol 311: H822–H836, 2016. doi: 10.1152/ajpheart.00067.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cremer S, Schloss MJ, Vinegoni C, Foy BH, Zhang S, Rohde D, Hulsmans M, Fumene Feruglio P, Schmidt S, Wojtkiewicz G, Higgins JM, Weissleder R, Swirski FK, Nahrendorf M. Diminished reactive hematopoiesis and cardiac inflammation in a mouse model of recurrent myocardial infarction. J Am Coll Cardiol 75: 901–915, 2020. doi: 10.1016/j.jacc.2019.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pullen AB, Jadapalli JK, Rhourri-Frih B, Halade GV. Re-evaluating the causes and consequences of non-resolving inflammation in chronic cardiovascular disease. Heart Fail Rev 25: 381–391, 2020. doi: 10.1007/s10741-019-09817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sicard P, Jouitteau T, Andrade-Martins T, Massad A, GRd A, David H, Miquerol L, Colson P, Richard S. Right coronary artery ligation in mice: a novel method to investigate right ventricular dysfunction and biventricular interaction. Am J Physiol Heart Circ Physiol 316: H684–H692, 2019. doi: 10.1152/ajpheart.00573.2018. [DOI] [PubMed] [Google Scholar]
- 103.Duerr GD, Elhafi N, Bostani T, Ellinger J, Swieny L, Kolobara E, Welz A, Dewald O. Comparison of myocardial remodeling between cryoinfarction and reperfused infarction in mice. J Biomed Biotechnol 2011: 961298, 2011. doi: 10.1155/2011/961298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Strungs EG, Ongstad EL, O’Quinn MP, Palatinus JA, Jourdan LJ, Gourdie RG. Cryoinjury models of the adult and neonatal mouse heart for studies of scarring and regeneration. Methods Mol Biol 1037: 343–353, 2013. doi: 10.1007/978-1-62703-505-7_20. [DOI] [PubMed] [Google Scholar]
- 105.Grisel P, Meinhardt A, Lehr HA, Kappenberger L, Barrandon Y, Vassalli G. The MRL mouse repairs both cryogenic and ischemic myocardial infarcts with scar. Cardiovasc Pathol 17: 14–22, 2008. doi: 10.1016/j.carpath.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 106.van den Bos EJ, Mees BM, de Waard MC, de Crom R, Duncker DJ. A novel model of cryoinjury-induced myocardial infarction in the mouse: a comparison with coronary artery ligation. Am J Physiol Heart Circ Physiol 289: H1291–H1300, 2005. doi: 10.1152/ajpheart.00111.2005. [DOI] [PubMed] [Google Scholar]
- 107.Fomovsky GM, Rouillard AD, Holmes JW. Regional mechanics determine collagen fiber structure in healing myocardial infarcts. J Mol Cell Cardiol 52: 1083–1090, 2012. doi: 10.1016/j.yjmcc.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Amerongen MJ, Harmsen MC, Petersen AH, Popa ER, van Luyn MJ. Cryoinjury: a model of myocardial regeneration. Cardiovasc Pathol 17: 23–31, 2008. doi: 10.1016/j.carpath.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 109.Ma N, Ladilov Y, Moebius JM, Ong L, Piechaczek C, David A, Kaminski A, Choi YH, Li W, Egger D, Stamm C, Intramyocardial SG. Intramyocardial delivery of human CD133+ cells in a SCID mouse cryoinjury model: bone marrow vs. cord blood-derived cells. Cardiovasc Res 71: 158–169, 2006. doi: 10.1016/j.cardiores.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 110.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science 331: 1078–1080, 2011. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haubner BJ, Schneider J, Schweigmann U, Schuetz T, Dichtl W, Velik-Salchner C, Stein JI, Penninger JM. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ Res 118: 216–221, 2016. doi: 10.1161/CIRCRESAHA.115.307017. [DOI] [PubMed] [Google Scholar]
- 112.Mahmoud AI, Porrello ER, Kimura W, Olson EN, Sadek HA. Surgical models for cardiac regeneration in neonatal mice. Nat Protoc 9: 305–311, 2014. doi: 10.1038/nprot.2014.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L, Wong A, Zaman R, Barbu I, Besla R, Lavine KJ, Razani B, Ginhoux F, Husain M, Cybulsky MI, Robbins CS, Epelman S. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol 20: 29–39, 2019. doi: 10.1038/s41590-018-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA 111: 16029–16034, 2014. [Erratum in Proc Natl Acad Sci USA 113: E1414, 2016]. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest 124: 1382–1392, 2014. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Whitehead AJ, Engler AJ. Regenerative cross talk between cardiac cells and macrophages. Am J Physiol Heart Circ Physiol 320: H2211–H2221, 2021. doi: 10.1152/ajpheart.00056.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Giudice J, Xia Z, Wang ET, Scavuzzo MA, Ward AJ, Kalsotra A, Wang W, Wehrens XH, Burge CB, Li W, Cooper TA. Alternative splicing regulates vesicular trafficking genes in cardiomyocytes during postnatal heart development. Nat Commun 5: 3603, 2014. doi: 10.1038/ncomms4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blom JN, Lu X, Arnold P, Feng Q. Myocardial infarction in neonatal nice, a model of cardiac regeneration. J Vis Exp, 2016. doi: 10.3791/54100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Villiers C, Riley PR. A refined protocol for coronary artery ligation in the neonatal mouse. Curr Protoc 1: e66, 2021. doi: 10.1002/cpz1.66. [DOI] [PubMed] [Google Scholar]
- 120.Wittnich C, Quaglietta D, Tan L, Belanger MP. Sex differences in newborn myocardial metabolism and response to ischemia. Pediatr Res 70: 148–152, 2011. doi: 10.1203/PDR.0b013e3182218c6c. [DOI] [PubMed] [Google Scholar]
- 121.van Hout GP, Jansen Of Lorkeers SJ, Wever KE, Sena ES, Kouwenberg LH, van Solinge WW, Macleod MR, Doevendans PA, Pasterkamp G, Chamuleau SA, Ie H. Translational failure of anti-inflammatory compounds for myocardial infarction: a meta-analysis of large animal models. Cardiovasc Res 109: 240–248, 2016. doi: 10.1093/cvr/cvv239. [DOI] [PubMed] [Google Scholar]
- 122.Lindsey ML, Ma Y, Flynn ER, Winniford MD, Hall ME, DeLeon-Pennell KY. Identifying the molecular and cellular signature of cardiac dilation following myocardial infarction. Biochim Biophys Acta Mol Basis Dis 1865: 1845–1852, 2019. doi: 10.1016/j.bbadis.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DeLeon-Pennell KY, Mouton AJ, Ero OK, Ma Y, Padmanabhan Iyer R, Flynn ER, Espinoza I, Musani SK, Vasan RS, Hall ME, Fox ER, Lindsey ML. LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res Cardiol 113: 40, 2018. doi: 10.1007/s00395-018-0699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zamilpa R, Zhang J, Chiao YA, de Castro Brás LE, Halade GV, Ma Y, Hacker SO, Lindsey ML. Cardiac wound healing post-myocardial infarction: a novel method to target extracellular matrix remodeling in the left ventricle. Methods Mol Biol 1037: 313–324, 2013. doi: 10.1007/978-1-62703-505-7_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Platt MJ, Huber JS, Brunt KR, Simpson JA. Pulmonary flow as an improved method for determining cardiac output in mice after myocardial infarction. J Am Soc Echocardiogr 30: 612–623, 2017. doi: 10.1016/j.echo.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 126.Takawale A, Zhang P, Azad A, Wang W, Wang X, Murray AG, Kassiri Z. Myocardial overexpression of TIMP3 after myocardial infarction exerts beneficial effects by promoting angiogenesis and suppressing early proteolysis. Am J Physiol Heart Circ Physiol 313: H224–H236, 2017. doi: 10.1152/ajpheart.00108.2017. [DOI] [PubMed] [Google Scholar]
- 127.Kilkenny C, Altman DG. Improving bioscience research reporting: ARRIVE-ing at a solution. Lab Anim 44: 377–378, 2010. doi: 10.1258/la.2010.0010021. [DOI] [PubMed] [Google Scholar]
- 128.Yosten GLC, Adams JC, Bennett CN, Bunnett NW, Scheman R, Sigmund CD, Yates BJ, Zucker IH, Samson WK. Revised guidelines to enhance the rigor and reproducibility of research published in American Physiological Society journals. Am J Physiol Regul Integr Comp Physiol 315: R1251–R1253, 2018. doi: 10.1152/ajpregu.00274.2018. [DOI] [PubMed] [Google Scholar]
- 129.Bohl S, Medway DJ, Schulz-Menger J, Schneider JE, Neubauer S, Lygate CA. Refined approach for quantification of in vivo ischemia-reperfusion injury in the mouse heart. Am J Physiol Heart Circ Physiol 297: H2054–H2058, 2009. doi: 10.1152/ajpheart.00836.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hadi AM, Mouchaers KT, Schalij I, Grunberg K, Meijer GA, Vonk-Noordegraaf A, van der Laarse WJ, Beliën JA. Rapid quantification of myocardial fibrosis: a new macro-based automated analysis. Cell Oncol (Dordr) 34: 343–354, 2011. doi: 10.1007/s13402-011-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Qin CX, May LT, Li R, Cao N, Rosli S, Deo M, Alexander AE, Horlock D, Bourke JE, Yang YH, Stewart AG, Kaye DM, Du XJ, Sexton PM, Christopoulos A, Gao XM, Ritchie RH. Small-molecule-biased formyl peptide receptor agonist compound 17b protects against myocardial ischaemia-reperfusion injury in mice. Nat Commun 8: 14232, 2017. doi: 10.1038/ncomms14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schipke J, Brandenberger C, Rajces A, Manninger M, Alogna A, Post H, C M. Assessment of cardiac fibrosis: a morphometric method comparison for collagen quantification. J Appl Physiol (1985) 122: 1019–1030, 2017. doi: 10.1152/japplphysiol.00987.2016. [DOI] [PubMed] [Google Scholar]
- 133.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol 89: 397–410, 1994. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- 134.Muthuramu I, Lox M, Jacobs F, De Geest B. Permanent ligation of the left anterior descending coronary artery in mice: a model of post-myocardial infarction remodelling and heart failure. J Vis Exp e52206, 2014. doi: 10.3791/52206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu Z, Alloush J, Beck E, Weisleder N. A murine model of myocardial ischemia-reperfusion injury through ligation of the left anterior descending artery. J Vis Exp (86): e51329, 2014. doi: 10.3791/51329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Preda MB, Burlacu A. Electrocardiography as a tool for validating myocardial ischemia-reperfusion procedures in mice. Comp Med 60: 443–447, 2010. [PMC free article] [PubMed] [Google Scholar]
- 137.Ogilvie LM, Edgett BA, Huber JS, Platt MJ, Eberl HJ, Lutchmedial S, Brunt KR, Simpson JA. Hemodynamic assessment of diastolic function for experimental models. Am J Physiol Heart Circ Physiol 318: H1139–H1158, 2020. doi: 10.1152/ajpheart.00705.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shioura KM, Geenen DL, Goldspink PH. Assessment of cardiac function with the pressure-volume conductance system following myocardial infarction in mice. Am J Physiol Heart Circ Physiol 293: H2870–H2877, 2007. doi: 10.1152/ajpheart.00585.2007. [DOI] [PubMed] [Google Scholar]
- 139.Psaty BM, Prentice RL. Minimizing bias in randomized trials: the importance of blinding. JAMA 304: 793–794, 2010. doi: 10.1001/jama.2010.1161. [DOI] [PubMed] [Google Scholar]
- 140.Bridgman S, Engebretsen L, Dainty K, Kirkley A, Maffulli N, Committee IS; ISAKOS Scientific Committee. Practical aspects of randomization and blinding in randomized clinical trials. Arthroscopy 19: 1000–1006, 2003. [Erratum in Arthroscopy 20: A14, 2004]. doi: 10.1016/j.arthro.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 141.Karanicolas PJ, Farrokhyar F, Bhandari M. Practical tips for surgical research: blinding: who, what, when, why, how? Can J Surg 53: 345–348, 2010. [PMC free article] [PubMed] [Google Scholar]
- 142.Becirovic-Agic M, Chalise U, Daseke MJ 2nd, Konfrst S, Salomon JD, Mishra PK, Lindsey ML. Infarct in the heart: what's MMP-9 got to do with it? Biomolecules 11: 491, 2021. doi: 10.3390/biom11040491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Daseke MJ, Tenkorang MAA, Chalise U, Konfrst SR, Lindsey ML. Cardiac fibroblast activation during myocardial infarction wound healing: fibroblast polarization after MI. Matrix Biol 91–92: 109–116, 2020. doi: 10.1016/j.matbio.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mouton AJ, Ma Y, Rivera Gonzalez OJ, Daseke MJ 2nd, Flynn ER, Freeman TC, Garrett MR, DeLeon-Pennell KY, Lindsey ML. Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic Res Cardiol 114: 6, 2019. doi: 10.1007/s00395-019-0715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Daseke MJ 2nd, Valerio FM, Kalusche WJ, Ma Y, DeLeon-Pennell KY, Lindsey ML. Neutrophil proteome shifts over the myocardial infarction time continuum. Basic Res Cardiol 114: 37, 2019. doi: 10.1007/s00395-019-0746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, Flynn ER, Freeman TC, Saucerman JJ, Garrett MR, Ma Y, Harmancey R, Lindsey ML. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol 113: 26, 2018. doi: 10.1007/s00395-018-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen B, Frangogiannis NG. Chemokines in myocardial infarction. J Cardiovasc Transl Res 14: 35–52, 2021. doi: 10.1007/s12265-020-10006-7. [DOI] [PubMed] [Google Scholar]
- 148.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 56: 786–794, 1977. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 149.Boatwright RB, Downey HF, Bashour FA, Crystal GJ. Transmural variation in autoregulation of coronary blood flow in hyperperfused canine myocardium. Circ Res 47: 599–609, 1980. doi: 10.1161/01.res.47.4.599. [DOI] [PubMed] [Google Scholar]
- 150.Zhang B, Novitskaya T, Wheeler DG, Xu Z, Chepurko E, Huttinger R, He H, Varadharaj S, Zweier JL, Song Y, Xu M, Harrell FE Jr, Su YR, Absi T, Kohr MJ, Ziolo MT, Roden DM, Shaffer CM, Galindo CL, Wells QS, Gumina RJ. Kcnj11 ablation is associated with increased nitro-oxidative stress during ischemia-reperfusion injury: implications for human ischemic cardiomyopathy. Circ Heart Fail 10: e003523, 2017. doi: 10.1161/CIRCHEARTFAILURE.116.003523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cai M, Huttinger ZM, He H, Zhang W, Li F, Goodman LA, Wheeler DG, Druhan LJ, Zweier JL, Dwyer KM, He G, d'Apice AJ, Robson SC, Cowan PJ, Gumina RJ. Transgenic over expression of ectonucleotide triphosphate diphosphohydrolase-1 protects against murine myocardial ischemic injury. J Mol Cell Cardiol 51: 927–935, 2011. doi: 10.1016/j.yjmcc.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mital R, Zhang W, Cai M, Huttinger ZM, Goodman LA, Wheeler DG, Ziolo MT, Dwyer KM, d'Apice AJ, Zweier JL, He G, Cowan PJ, Gumina RJ. Antioxidant network expression abrogates oxidative posttranslational modifications in mice. Am J Physiol Heart Circ Physiol 300: H1960–H1970, 2011. doi: 10.1152/ajpheart.01285.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smith SB, Xu Z, Novitskaya T, Zhang B, Chepurko E, Pu XA, Wheeler DG, Ziolo M, Gumina RJ. Impact of cardiac-specific expression of CD39 on myocardial infarct size in mice. Life Sci 179: 54–59, 2017. doi: 10.1016/j.lfs.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 154.Sciarretta S, Yee D, Nagarajan N, Bianchi F, Saito T, Valenti V, Tong M, Del Re DP, Vecchione C, Schirone L, Forte M, Rubattu S, Shirakabe A, Boppana VS, Volpe M, Frati G, Zhai P, Sadoshima J. Trehalose-induced activation of autophagy improves cardiac remodeling after myocardial infarction. J Am Coll Cardiol 71: 1999–2010, 2018. doi: 10.1016/j.jacc.2018.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chau VQ, Salloum FN, Hoke NN, Abbate A, Kukreja RC. Mitigation of the progression of heart failure with sildenafil involves inhibition of RhoA/Rho-kinase pathway. Am J Physiol Heart Circ Physiol 300: H2272–H2279, 2011. doi: 10.1152/ajpheart.00654.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Du Y, Wang X, Li L, Hao W, Zhang H, Li Y, Qin Y, Nie S, Christopher TA, Lopez BL, Lau WB, Wang Y, Ma XL, Wei Y. miRNA-mediated suppression of a cardioprotective cardiokine as a novel mechanism exacerbating post-MI remodeling by sleep breathing disorders. Circ Res 126: 212–228, 2020. doi: 10.1161/CIRCRESAHA.119.315067. [DOI] [PubMed] [Google Scholar]
- 157.Desjardins JF, Pourdjabbar A, Quan A, Leong-Poi H, Teichert-Kuliszewska K, Verma S, Parker TG. Lack of S100A1 in mice confers a gender-dependent hypertensive phenotype and increased mortality after myocardial infarction. Am J Physiol Heart Circ Physiol 296: H1457–H1465, 2009. doi: 10.1152/ajpheart.00088.2008. [DOI] [PubMed] [Google Scholar]
- 158.Nambu H, Takada S, Maekawa S, Matsumoto J, Kakutani N, Furihata T, Shirakawa R, Katayama T, Nakajima T, Yamanashi K, Obata Y, Nakano I, Tsuda M, Saito A, Fukushima A, Yokota T, Nio-Kobayashi J, Yasui H, Higashikawa K, Kuge Y, Anzai T, Sabe H, Kinugawa S. Inhibition of xanthine oxidase in the acute phase of myocardial infarction prevents skeletal muscle abnormalities and exercise intolerance. Cardiovasc Res 117: 805–819, 2021. doi: 10.1093/cvr/cvaa127. [DOI] [PubMed] [Google Scholar]
- 159.Whitehurst KS, Chan VA, Estes HK, Valsaraj S, Kent S, Sharma UM, Chase RC, Bhuiyan M, Virag JAI. EphrinA1-Fc attenuates ventricular remodeling and dysfunction in chronically nonreperfused WT but not EphA2-R-M mice. Int J Mol Sci 21, 2020. doi: 10.3390/ijms21165811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kholmukhamedov A, Logdon C, Hu J, McKinney RA, Spinale FG, Lemasters JJ, Mukherjee R. Cyclosporin A in left ventricular remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol 306: H53–H59, 2014. doi: 10.1152/ajpheart.00079.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Daniel LL, Scofield SL, Thrasher P, Dalal S, Daniels CR, Foster CR, Singh M, Singh K. Ataxia telangiectasia-mutated kinase deficiency exacerbates left ventricular dysfunction and remodeling late after myocardial infarction. Am J Physiol Heart Circ Physiol 311: H445–H452, 2016. doi: 10.1152/ajpheart.00338.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cellini A, Höfler D, Arias-Loza PA, Bandleon S, Langsenlehner T, Kohlhaas M, Maack C, Bauer WR, Eder-Negrin P. The α2-isoform of the Na(+)/K(+)-ATPase protects against pathological remodeling and β-adrenergic desensitization after myocardial infarction. Am J Physiol Heart Circ Physiol 321: H650–H662, 2021. doi: 10.1152/ajpheart.00808.2020. [DOI] [PubMed] [Google Scholar]
- 163.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119: 91–112, 2016. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113: 1004–1012, 2013. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, Henry J, Cates CA, Deleon-Pennell KY, Lindsey ML. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res 110: 51–61, 2016. doi: 10.1093/cvr/cvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qin CX, Rosli S, Deo M, Cao N, Walsh J, Tate M, Alexander AE, Donner D, Horlock D, Li R, Kiriazis H, Lee MKS, Bourke JE, Yang Y, Murphy AJ, Du XJ, Gao XM, Rh R. Cardioprotective actions of the annexin-A1 N-terminal peptide, Ac(2-26), against myocardial infarction. Front Pharmacol 10: 269, 2019. doi: 10.3389/fphar.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 115: 284–295, 2014. doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qin CX, Finlayson SB, Al-Sharea A, Tate M, De Blasio MJ, Deo M, Rosli S, Prakoso D, Thomas CJ, Kiriazis H, Gould E, Yang YH, Morand EF, Perretti M, Murphy AJ, Du XJ, Gao XM, Ritchie RH. Endogenous annexin-A1 regulates haematopoietic stem cell mobilisation and inflammatory response post myocardial infarction in mice in vivo. Sci Rep 7: 16615, 2017. [Erratum in Sci Rep 8: 7185, 2018]. doi: 10.1038/s41598-017-16317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen B, Huang S, Su Y, Wu YJ, Hanna A, Brickshawana A, Graff J, Frangogiannis NG. Macrophage Smad3 protects the infarcted heart, stimulating phagocytosis and regulating inflammation. Circ Res 125: 55–70, 2019. doi: 10.1161/CIRCRESAHA.119.315069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Virag JI, Murry CE. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am J Pathol 163: 2433–2440, 2003. doi: 10.1016/S0002-9440(10)63598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, Sargent MA, Prasad V, Valiente-Alandi I, Blaxall BC, Molkentin JD. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest 128: 2127–2143, 2018. doi: 10.1172/JCI98215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol 48: 2315–2323, 2006. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 174.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem 50: 71–79, 2002. doi: 10.1177/002215540205000108. [DOI] [PubMed] [Google Scholar]
- 175.Le Bras A. Cardiac lymphatics mediate the resolution of inflammation. Nat Rev Cardiol 15: 583, 2018. doi: 10.1038/s41569-018-0067-z. [DOI] [PubMed] [Google Scholar]
- 176.Kirk JA, Cingolani OH. Thrombospondins in the transition from myocardial infarction to heart failure. J Mol Cell Cardiol 90: 102–110, 2016. doi: 10.1016/j.yjmcc.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Souza LM, Okoshi MP, Gomes MJ, Gatto M, Rodrigues EA, Pontes THD, Damatto FC, Oliveira LRS, Borim PA, Lima ARR, Zornoff LAM, Okoshi K, Pagan LU. Effects of late aerobic exercise on cardiac remodeling of rats with small-sized myocardial infarction. Arq Bras Cardiol 116: 784–792, 2021. doi: 10.36660/abc.20190813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lund JS, Aksetøy IA, Dalen H, Amundsen BH, Støylen A. Left ventricular diastolic function: Effects of high-intensity exercise after acute myocardial infarction. Echocardiography 37: 858–866, 2020. doi: 10.1111/echo.14750. [DOI] [PubMed] [Google Scholar]
- 179.Poole DC, Copp SW, Colburn TD, Craig JC, Allen DL, Sturek M, O'Leary DS, Zucker IH, Musch TI. Guidelines for animal exercise and training protocols for cardiovascular studies. Am J Physiol Heart Circ Physiol 318: H1100–H1138, 2020. doi: 10.1152/ajpheart.00697.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Halade GV, Kain V, Wright GM, Jadapalli JK. Subacute treatment of carprofen facilitate splenocardiac resolution deficit in cardiac injury. J Leukoc Biol 104: 1173–1186, 2018. doi: 10.1002/JLB.3A0618-223R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee YM, Song BC, Yeum KJ. Impact of volatile anesthetics on oxidative stress and inflammation. Biomed Res Int 2015: 242709, 2015. doi: 10.1155/2015/242709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Volker D, Bate M, Gentle R, Garg M. Oral buprenorphine is anti-inflammatory and modulates the pathogenesis of streptococcal cell wall polymer-induced arthritis in the Lew/SSN rat. Lab Anim 34: 423–429, 2000. doi: 10.1258/002367700780387732. [DOI] [PubMed] [Google Scholar]
- 183.Krishnan V, Booker D, Cunningham G, Jadapalli JK, Kain V, Pullen AB, Halade GV. Pretreatment of carprofen impaired initiation of inflammatory- and overlapping resolution response and promoted cardiorenal syndrome in heart failure. Life Sci 218: 224–232, 2019. doi: 10.1016/j.lfs.2018.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Caracas HC, Maciel JV, Martins PM, de Souza MM, Maia LC. The use of lidocaine as an anti-inflammatory substance: a systematic review. J Dent 37: 93–97, 2009. doi: 10.1016/j.jdent.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 185.DeLeon-Pennell KY, Lindsey ML. Somewhere over the sex differences rainbow of myocardial infarction remodeling: hormones, chromosomes, inflammasome, oh my. Expert Rev Proteomics 16: 933–940, 2019. doi: 10.1080/14789450.2019.1664293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bucholz EM, Butala NM, Rathore SS, Dreyer RP, Lansky AJ, Krumholz HM. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation 130: 757–767, 2014. doi: 10.1161/CIRCULATIONAHA.114.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.de Miguel-Yanes JM, Jimenez-Garcia R, Hernandez-Barrera V, de Miguel-Diez J, Munoz-Rivas N, Mendez-Bailon M, Perez-Farinos N, Lopez-Herranz M, Lopez-de-Andres A. Sex differences in the incidence and outcomes of acute myocardial infarction in Spain, 2016–2018: a matched-pair analysis. J Clin Med 10: 1795, 2021. doi: 10.3390/jcm10081795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Khraishah H, Alahmad B, Alfaddagh A, Jeong SY, Mathenge N, Kassab MB, Kolte D, Michos ED, Albaghdadi M. Sex disparities in the presentation, management and outcomes of patients with acute coronary syndrome: insights from the ACS QUIK trial. Open Heart 8: e001470, 2021. doi: 10.1136/openhrt-2020-001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu SW, Guan CD, Hu FH, Chen J, Dou KF, Yang WX, Wu YJ, Yang YJ, Xu B, Sb Q. Comparison of outcomes for percutaneous coronary intervention in men and women with unprotected left main disease. J Geriatr Cardiol 18: 168–174, 2021. doi: 10.11909/j.issn.1671-5411.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kimenai DM, Lindahl B, Chapman AR, Baron T, Gard A, Wereski R, Meex SJR, Jernberg T, Mills NL, Eggers KM. Sex differences in investigations and outcomes among patients with type 2 myocardial infarction. Heart 107: 1480–1486, 2021. doi: 10.1136/heartjnl-2021-319118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Huded CP, Johnson M, Kravitz K, Menon V, Abdallah M, Gullett TC, Hantz S, Ellis SG, Podolsky SR, Meldon SW, Kralovic DM, Brosovich D, Smith E, Kapadia SR, Khot UN. 4-Step protocol for disparities in STEMI care and outcomes in women. J Am Coll Cardiol 71: 2122–2132, 2018. doi: 10.1016/j.jacc.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 192.Lindsey ML, LeBlanc AJ, Ripplinger CM, Kirk JA, Carter JR, Keehan KH, Brunt KR, Kleinbongard P, Kassiri Z. Reinforcing rigor and reproducibility expectations for use of sex and gender in cardiovascular research. Am J Physiol Heart Circ Physiol 321: H819–H824, 2021. doi: 10.1152/ajpheart.00418.2021. [DOI] [PubMed] [Google Scholar]
- 193.Zajitschek SR, Zajitschek F, Bonduriansky R, Brooks RC, Cornwell W, Falster DS, Lagisz M, Mason J, Senior AM, Noble DW, Nakagawa S. Sexual dimorphism in trait variability and its eco-evolutionary and statistical implications. eLife 9: e63170, 2020. doi: 10.7554/eLife.63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sandberg K, Umans JG; Georgetown Consensus Conference Work Group. Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research. Faseb j 29: 1646–1652, 2015. doi: 10.1096/fj.14-269548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Beery AK. Inclusion of females does not increase variability in rodent research studies. Curr Opin Behav Sci 23: 143–149, 2018. doi: 10.1016/j.cobeha.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Keys JR, Zhou RH, Harris DM, Druckman CA, Eckhart AD. Vascular smooth muscle overexpression of G protein-coupled receptor kinase 5 elevates blood pressure, which segregates with sex and is dependent on Gi-mediated signaling. Circulation 112: 1145–1153, 2005. doi: 10.1161/CIRCULATIONAHA.104.531657. [DOI] [PubMed] [Google Scholar]
- 197.Flores-Monroy J, Ramirez-Hernández D, Samano-Hernández C, Lezama-Martínez D, Sampieri-Cabrera R, Martínez-Aguilar L. Sex differences in vascular reactivity to angiotensin II during the evolution of myocardial infarction. J Cardiovasc Pharmacol 71: 19–25, 2018. doi: 10.1097/FJC.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 198.Le TY, Ashton AW, Mardini M, Stanton PG, Funder JW, Handelsman DJ, Mihailidou AS. Role of androgens in sex differences in cardiac damage during myocardial infarction. Endocrinology 155: 568–575, 2014. doi: 10.1210/en.2013-1755. [DOI] [PubMed] [Google Scholar]
- 199.Phelps T, Snyder E, Rodriguez E, Child H, Harvey P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol Sex Differ 10: 52, 2019. doi: 10.1186/s13293-019-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ruiz-Meana M, Martinson EA, Garcia-Dorado D, Piper HM. Animal ethics in Cardiovascular Research. Cardiovasc Res 93: 1–3, 2012. doi: 10.1093/cvr/cvr289. [DOI] [PubMed] [Google Scholar]
- 201.Brown MJ, Murray KA. Phenotyping of genetically engineered mice: humane, ethical, environmental, and husbandry issues. ILAR J 47: 118–123, 2006. doi: 10.1093/ilar.47.2.118. [DOI] [PubMed] [Google Scholar]
- 202.Willich SN, Lowel H, Lewis M, Arntz R, Baur R, Winther K, Keil U, Schroder R. Association of wake time and the onset of myocardial infarction. Triggers and mechanisms of myocardial infarction (TRIMM) pilot study. TRIMM Study Group. Circulation 84: VI62–V167, 1991. [1683611] [PubMed] [Google Scholar]
- 203.Chen L, Yang G. Recent advances in circadian rhythms in cardiovascular system. Front Pharmacol 6: 71, 2015. doi: 10.3389/fphar.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schloss MJ, Horckmans M, Nitz K, Duchene J, Drechsler M, Bidzhekov K, Scheiermann C, Weber C, Soehnlein O, Steffens S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol Med 8: 937–948, 2016. doi: 10.15252/emmm.201506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bennardo M, Alibhai F, Tsimakouridze E, Chinnappareddy N, Podobed P, Reitz C, Pyle WG, Simpson J, Martino TA. Day-night dependence of gene expression and inflammatory responses in the remodeling murine heart post-myocardial infarction. Am J Physiol Regul Integr Comp Physiol 311: R1243–R1254, 2016. doi: 10.1152/ajpregu.00200.2016. [DOI] [PubMed] [Google Scholar]
- 206.Schloss MJ, Hilby M, Nitz K, Guillamat Prats R, Ferraro B, Leoni G, Soehnlein O, Kessler T, He W, Luckow B, Horckmans M, Weber C, Duchene J, Steffens S. Ly6C(high) monocytes oscillate in the heart during homeostasis and after myocardial infarction-brief report. Arterioscler Thromb Vasc Biol 37: 1640–1645, 2017. doi: 10.1161/ATVBAHA.117.309259. [DOI] [PubMed] [Google Scholar]
- 207.Du Pré B, Van Veen T, Crnko S, Vos M, Deddens J, Doevendans P, Van Laake L. Variation within variation: comparison of 24-h rhythm in rodent infarct size between ischemia reperfusion and permanent ligation. Int J Mol Sci 18: 1670, 2017. doi: 10.3390/ijms18081670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fox J, Barthold S, Davisson M, Newcomer C, Quimby F, Smith A. The Mouse in Biomedical Research. Boston, MA: American College Laboratory Animal Medicine, 2007. [Google Scholar]
- 209.Iyer RP, Patterson NL, Zouein FA, Ma Y, Dive V, de Castro Brás LE, Lindsey ML. Early matrix metalloproteinase-12 inhibition worsens post-myocardial infarction cardiac dysfunction by delaying inflammation resolution. Int J Cardiol 185: 198–208, 2015. doi: 10.1016/j.ijcard.2015.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci 152: 244–248, 2016. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 211.Millett ERC, Peters SAE, Woodward M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ 363: k4247, 2018. doi: 10.1136/bmj.k4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yabluchanskiy A, Ma Y, DeLeon-Pennell KY, Altara R, Halade GV, Voorhees AP, Nguyen NT, Jin YF, Winniford MD, Hall ME, Han HC, Ml L. Myocardial Infarction Superimposed on Aging: MMP-9 Deletion Promotes M2 Macrophage Polarization. J Gerontol A Biol Sci Med Sci 71: 475–483, 2016. doi: 10.1093/gerona/glv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging (Albany NY) 8: 2611–2634, 2016. doi: 10.18632/aging.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gould KE, Taffet GE, Michael LH, Christie RM, Konkol DL, Pocius JS, Zachariah JP, Chaupin DF, Daniel SL, Sandusky GE Jr, Hartley CJ, Ml E. Heart failure and greater infarct expansion in middle-aged mice: a relevant model for postinfarction failure. Am J Physiol Heart Circ Physiol 282: H615–H621, 2002. doi: 10.1152/ajpheart.00206.2001. [DOI] [PubMed] [Google Scholar]
- 215.Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol 51: 1384–1392, 2008. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lopez EF, Kabarowski JH, Ingle KA, Kain V, Barnes S, Crossman DK, Lindsey ML, Halade GV. Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am J Physiol Heart Circ Physiol 308: H269–H280, 2015. doi: 10.1152/ajpheart.00604.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wingard MC, Dalal S, Shook PL, Myers R, Connelly BA, Thewke DP, Singh M, Singh K. Deficiency of ataxia-telangiectasia mutated kinase modulates functional and biochemical parameters of the heart in response to Western-type diet. Am J Physiol Heart Circ Physiol 320: H2324–H2338, 2021. doi: 10.1152/ajpheart.00990.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.DeLeon-Pennell KY, Iyer RP, Ero OK, Cates CA, Flynn ER, Cannon PL, Jung M, Shannon D, Garrett MR, Buchanan W, Hall ME, Ma Y, Lindsey ML. Periodontal-induced chronic inflammation triggers macrophage secretion of Ccl12 to inhibit fibroblast-mediated cardiac wound healing. JCI Insight 2: e94207, 2017. doi: 10.1172/jci.insight.94207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zaidi Y, Corker A, Vasileva VY, Oviedo K, Graham C, Wilson K, Martino J, Troncoso M, Broughton P, Ilatovskaya DV, Lindsey ML, DeLeon-Pennell KY. Chronic Porphyromonas gingivalis lipopolysaccharide induces adverse myocardial infarction wound healing through activation of CD8+ T-cells. Am J Physiol Heart Circ Physiol, 2021. doi: 10.1152/ajpheart.00082.2021.[34597184] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HWM, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature 487: 325–329, 2012. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat Med 20: 754–758, 2014. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Girod WG, Jones SP, Sieber N, Aw TY, Lefer DJ. Effects of hypercholesterolemia on myocardial ischemia-reperfusion injury in LDL receptor-deficient mice. ATVB 19: 2776–2781, 1999. doi: 10.1161/01.ATV.19.11.2776. [DOI] [PubMed] [Google Scholar]
- 223.Kirk JA, Kass DA. Electromechanical dyssynchrony and resynchronization of the failing heart. Circ Res 113: 765–776, 2013. doi: 10.1161/CIRCRESAHA.113.300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 10: 712, 2011. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- 225.Begley CG, Ellis LM. Drug development: raise standards for preclinical cancer research. Nature 483: 531–533, 2012. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 226.Peers IS, Ceuppens PR, Harbron C. In search of preclinical robustness. Nat Rev Drug Discov 11: 733–734, 2012. doi: 10.1038/nrd3849. [DOI] [PubMed] [Google Scholar]
- 227.Begley CG, Ioannidis JP. Reproducibility in science: improving the standard for basic and preclinical research. Circ Res 116: 116–126, 2015. doi: 10.1161/CIRCRESAHA.114.303819. [DOI] [PubMed] [Google Scholar]
- 228.Figtree GA, Broadfoot K, Casadei B, Califf R, Crea F, Drummond GR, Freedman JE, Guzik TJ, Harrison D, Hausenloy DJ, Hill JA, Januzzi JL, Kingwell BA, Lam CSP, MacRae CA, Misselwitz F, Miura T, Ritchie RH, Tomaszewski M, Wu JC, Xiao J, Zannad F. A call to action for new global approaches to cardiovascular disease drug solutions. Eur Heart J 42: 1464–1475, 2021. doi: 10.1093/eurheartj/ehab068. [DOI] [PubMed] [Google Scholar]
- 229.Patino CM, Ferreira JC. Inclusion and exclusion criteria in research studies: definitions and why they matter. J Bras Pneumol 44: 84, 2018. doi: 10.1590/s1806-37562018000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Faber J, Fonseca LM. How sample size influences research outcomes. Dental Press J Orthod 19: 27–29, 2014. doi: 10.1590/2176-9451.19.4.027-029.ebo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Biau DJ, Kernéis S, Porcher R. Statistics in brief: the importance of sample size in the planning and interpretation of medical research. Clin Orthop Relat Res 466: 2282–2288, 2008. doi: 10.1007/s11999-008-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Noordzij M, Tripepi G, Dekker FW, Zoccali C, Tanck MW, Jager KJ. Sample size calculations: basic principles and common pitfalls. Nephrol Dial Transplant 25: 1388–1393, 2010. [Erratum in Nephrol Dial Transplant 25: 3461–3462, 2010]. doi: 10.1093/ndt/gfp732. [DOI] [PubMed] [Google Scholar]
- 233.Percie Du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Physiol 598: 3793–3801, 2020. doi: 10.1113/JP280389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res 57: 84–95, 1985. doi: 10.1161/01.res.57.1.84. [DOI] [PubMed] [Google Scholar]
- 235.Brooks HL, Lindsey ML. Guidelines for authors and reviewers on antibody use in physiology studies. Am J Physiol Heart Circ Physiol 314: H724–H732, 2018. doi: 10.1152/ajpheart.00512.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mishra PK, Adameova A, Hill JA, Baines CP, Kang PM, Downey JM, Narula J, Takahashi M, Abbate A, Piristine HC, Kar S, Su S, Higa JK, Kawasaki NK, Matsui T. Guidelines for evaluating myocardial cell death. Am J Physiol Heart Circ Physiol 317: H891–H922, 2019. [Erratum in Am J Physiol Heart Circ Physiol 317: H1390, 2019]. doi: 10.1152/ajpheart.00259.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


