Abstract
Cerebral blood flow and perfusion are tightly maintained through autoregulation despite changes in transmural pressure. Oxidative stress impairs cerebral blood flow, precipitating cerebrovascular events. Phosphorylation of the adaptor protein p66Shc increases mitochondrial-derived oxidative stress. The effect of p66Shc gain or loss of function in nonhypertensive rats is unclear. We hypothesized that p66Shc gain of function would impair autoregulation of cerebral microcirculation under physiological and pathological conditions. Three previously established transgenic [salt-sensitive (SS) background] p66Shc rats were used, p66-Del/SS (express p66Shc with a nine-amino acid deletion), p66Shc-knockout (KO)/SS (frameshift premature termination codon), and p66Shc signaling and knock-in substitution of Ser36Ala (p66Shc-S36A)/SS (substitution of Ser36Ala). The p66Shc-Del were also bred on Sprague-Dawley (SD) backgrounds (p66-Del/SD), and a subset was exposed to a hypertensive stimulus [NG-nitro-l-arginine methyl ester (l-NAME)] for 4 wk. Active and passive diameters to increasing transmural pressure were measured and myogenic tone was calculated in all groups (SS and SD). Myogenic responses to increasing pressure were impaired in p66Shc-Del/SS rats relative to wild-type (WT)/SS and knock-in substitution of Ser36Ala (S36A; P < 0.05). p66-Del/SD rats did not demonstrate changes in active/passive diameters or myogenic tone relative to WT/SD but did demonstrate attenuated passive diameter responses to higher transmural pressure relative to p66-Del/SS. Four weeks of a hypertensive stimulus (l-NAME) did not alter active or passive diameter responses to increasing transmural pressure (P = 0.86–0.99), but increased myogenic responses relative to p66-Del/SD (P < 0.05). Collectively, we demonstrate the functional impact of p66Shc within the cerebral circulation and demonstrate that the genetic background of p66Shc rats largely drives changes in cerebrovascular function.
NEW & NOTEWORTHY We demonstrate that the modulation of p66Shc signaling impairs cerebral artery myogenic tone in a low renin model of hypertension. This impairment is dependent upon the genetic background, as modulated p66Shc signaling in Sprague-Dawley rats does not impair cerebral artery myogenic tone.
Keywords: myogenic tone, oxidative stress, p66Shc
INTRODUCTION
Cerebral blood flow regulation is multifaceted, including autoregulation, mechanosensitive (flow), and neurogenic mechanisms (1). Within the cerebral circulation, resistance arteries such as the middle cerebral artery (MCA) contribute to the maintenance of autoregulation and vascular resistance (1, 2). Autoregulation acts to maintain adequate perfusion of cerebral tissue, attenuating the effects of arterial blood pressure excursions. Myogenic mechanisms are key to autoregulation, whereby cerebral blood flow is maintained across a window of arterial pressure. Under physiological conditions, increases in pressure beyond this window elicit vasodilation, whereas pressure below this window results in vasoconstriction. Endothelial function has been demonstrated to play a key role in cerebral autoregulation and myogenic tone. Oxidative stress has been demonstrated to contribute to the development of endothelial dysfunction within the cerebrovasculature (3, 4). Although reactive oxygen species (ROS) can act as signaling mechanisms, elevated/prolonged exposure to ROS precipitates endothelial dysfunction (5). Important to the cerebral circulation, elevations in blood pressure enhance oxidative stress and can impair microvascular myogenic tone (6, 7).
A crucial regulator of oxidative stress is the adaptor protein p66Shc (Src homologous and collagen). We have previously demonstrated the crucial role of p66Shc within the renal microvasculature in regulating vascular tone (8), whereas others have demonstrated that age-associated cerebrovascular dysfunction is mediated in part by p66Shc (9). The adaptor protein p66Shc regulates oxidative stress via posttranslational modifications such that phosphorylation of p66Shc, which increases mitochondrial-derived oxidative stress (10) via oxidation of cytochrome-c, rendering the kidneys susceptibility to diabetic or hypertensive nephropathy (11–13). Furthermore, p66Shc was shown to regulate spontaneous Ca2+ oscillations controlling renal arterial spontaneous motion, which could contribute to age-related cardiorenal pathologies (14). Notably, animal models of diabetic and hypertensive nephropathy were generated on a genetic background of Dahl salt-sensitive (SS) rat, an established model of low-renin hypertension (15). However, the effect of p66Shc gain or loss of function in nonhypertensive rats has not been studied. The goal of the present study was to evaluate how a potentially constitutively active mutant version of p66Shc contributes to the regulation of cerebral vascular tone in normal rats (Sprague-Dawley, SD) compared with genetic (SS) or pharmacological models of hypertension [SD + chronic administration of nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME)]. We hypothesized that p66Shc gain of function would impair autoregulation of cerebral microcirculation under different pathological conditions that may be sufficient to impair cerebral vascular tone under physiological conditions.
METHODS
All animal procedures were conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. All animal procedures were submitted to and approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. Male and female 14–15-wk-old SpragueDawley rats were used. Transgenic p66Shc rats were established on Dahl salt-sensitiveMCW (SS) background using zinc-finger nucleases (ZFN) directed at the CH2 domain of SHC1, frameshift leading to premature termination codon [termed p66Shc-knockout (KO)/SS; n = 4], an in-frame deletion of nine amino acids including serine-36 (termed p66Shc-Del; n = 7), which removes phosphorylation-dependent regulation of p66Shc signaling and knock-in substitution of Ser36Ala substitution (p66Shc-S36A/SS; n = 3). Rats negative for ZFN modification at the SHC1 locus were determined to be wild type (WT/SS; n = 3; 12). To generate p66Shc-Del (n = 3) on a nonpathological background, male rats carrying mutated p66Shc-Del were backcrossed to Sprague-Dawley (SD) females for three generations. All rats were fed a normal chow diet (Mod LabDiet 5001; <0.4% NaCl content). Rats were given drinking water ad libitum. For tissue collection, rats were euthanized by CO2 overdose or isoflurane + thoracotomy.
Induction of Hypertension
Hypertension was induced in SD rats (n = 3–5) by supplementing drinking water with the nitric oxide inhibitor l-NAME (12.5 mg/L) for 4 wk (∼4 mg/kg/day). This dose has previously been successfully used to induce an increase in mean arterial pressure (16).
Isolation of Middle Cerebral Artery
Brains were rapidly extracted and placed in a dissecting dish with cold HEPES solution. A 1–2-mm segment of the middle cerebral artery was carefully dissected and cannulated on glass pipettes of matching impedance, secured with nylon ties in Krebs buffer in an organ chamber heated to 37°C. The arteries were allowed to equilibrate at 60 cmH2O for 60 min. Internal diameters of cannulated arteries were viewed and measured with an inverted microscope and television monitor with on-screen callipers. Active tone was calculated as (ΔD/Dmax) × 100, where ΔD is the diameter increase from rest in response to Ca2+-free Krebs and Dmax is the maximum diameter measured at the equilibration pressure in Ca2+-free Krebs.
Myogenic Responses
Changes in artery diameter in response to increased transmural pressure (no flow) were measured. After the 60-min equilibration period, the pressure was reduced to 0 cmH2O. Pressure was then increased to 30 cmH2O in a single pressure step and then 15 cmH2O increments thereafter (cessation at 150 cmH2O). Following each increment in pressure, internal diameters were recorded after 5 min. Each artery underwent two rounds of pressure increments. The first round was in standard Krebs buffer (containing 2.5 mM Ca2+) and then the second in Ca2+-free Krebs (equal molar EGTA substituted for EDTA). Myogenic tone was determined by subtracting the steady-state diameter at any given pressure in Krebs from the passive diameter (Ca2+-free Krebs) at that same pressure increment.
Statistics
Data are expressed as means ± SD. Statistical significance was determined using an ANOVA and defined as P < 0.05. Differences between the groups to increasing pressures were tested by two-way ANOVA for repeated measures with Tukey’s post hoc test. Statistical analyses were made with GraphPad Prism 9.2.
RESULTS
Diameter Differences at Rest (60 mmHg) Active
MCA diameter and myogenic responses at 60 mmHg are shown in Table 1. There were no differences in active, passive, or myogenic responses across groups at 60 cmH2O. The average spontaneous tone across all groups was 28.8 ± 13.3%. Interestingly, arteries from p66-Del/SS and p66-Del/SD rats tended to develop less active tone compared with the arteries from WT counterparts (17.3 ± 10% and 19.3 ± 6.7%, respectively; P = 0.1–0.16).
Table 1.
Middle cerebral artery active and passive tone (60 cmH2O)
| Salt Sensitive |
Sprague-Dawley |
Sprague-Dawley + 4-wk l-NAME |
||||||
|---|---|---|---|---|---|---|---|---|
| WT | p66Shc-Del | p66Shc KO | p66Shc-S36A | WT | p66Shc-Del | WT | p66Shc-Del | |
| Active, µm | 146 ± 7 | 129 ± 26 | 153 ± 26 | 108 ± 17 | 99 ± 44 | 111 ± 42 | 102 ± 29 | 104 ± 45 |
| Passive, µm | 221 ± 20 | 154 ± 32 | 190 ± 20 | 149 ± 6 | 161 ± 58 | 105 ± 23 | 131 ± 39 | 154 ± 50 |
| Myogenic tone, % | 36 ± 5 | 17 ± 10 | 24 ± 7 | 36 ± 8 | 35 ± 2 | 19 ± 7 | 30 ± 8 | 32 ± 19 |
Values are means ± SD. KO, knockout; l-NAME, NG-nitro-l-arginine methyl ester; S36A, knock-in substitution of Ser36Ala; WT, wild type.
Active, Passive Diameters, and Myogenic Tone
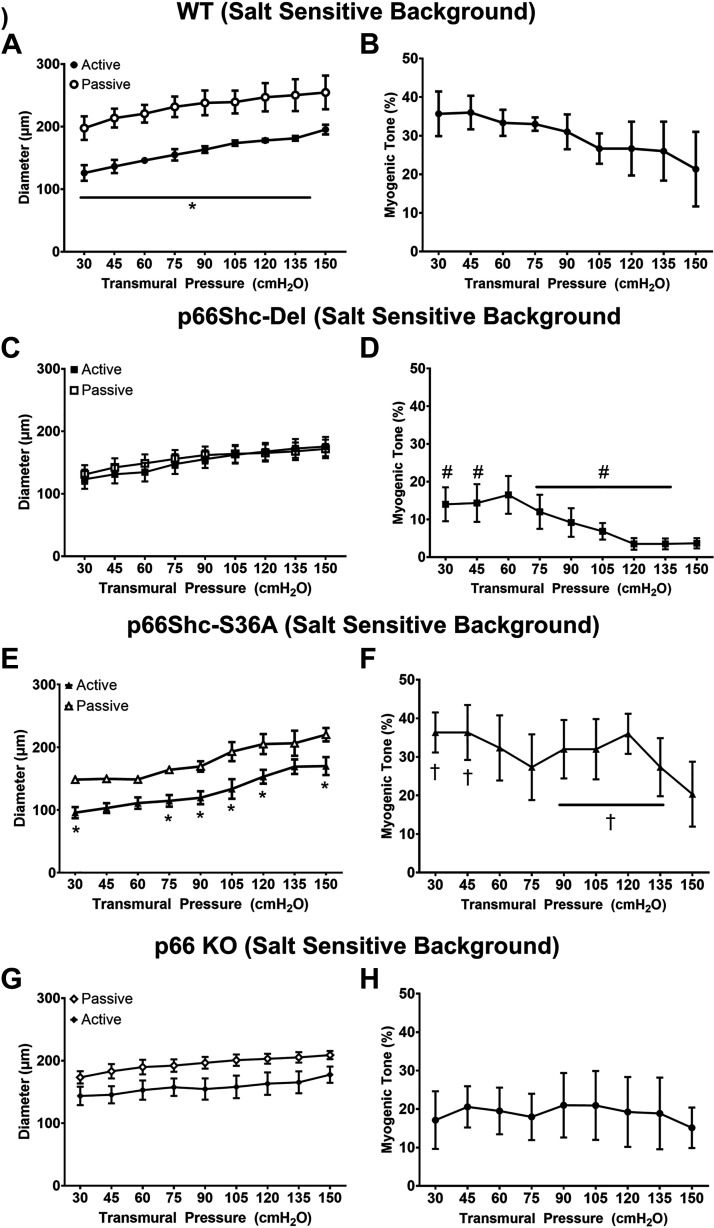
Active and passive diameters, along with myogenic tone in response to increasing transmural pressure in rats bred on the salt-sensitive background (WT/SS, p66Shc-Del/SS, p66Shc-S36A/SS, and p66Shc-KO/SS), are shown in Fig. 1. Active diameters were not different across groups (WT/SS, p66-Del/SS, p66-KO/SS, and p66-S36A/SS), whereas passive diameter (Ca2+-free Krebs) responses were reduced in p66-Del/SS relative to WT/SS (30–150 cmH2O, P < 0.05). Knock-in substitution of S36A elicited reduced passive diameter responses relative to WT/SS at lower transmural pressures (30–90 cmH2O, P < 0.05) but were similar at higher pressures (105–150 cmH2O, P = 0.21–0.48). These changes in passive diameter responses led to differences in myogenic tone in p66-Del/SS rats relative to WT/SS rats across pressures (Fig. 1; P < 0.05) and in p66-Del/SS relative to S36A knock-in substitution (30–45 and 90–135 cmH2O, P < 0.05).
Figure 1.
Active and passive diameters and myogenic responses in response to increasing transmural pressure in rats bred on salt-sensitive (SS) genetic background. Wild-type (WT) salt-sensitive (A and B), p66Shc-Del/SS (C and D), single amino acid substitution of serine-36 (S36A/SS; E and F), and p66Shc-KO/SS (G and H). Two-way repeated-measures ANOVA. *P < 0.05 vs. passive diameter; #P < 0.05 vs. WT/SS; †P < 0.05 vs. p66-Del/SS; n = 3–7 rats/group. KO, knockout; S36A, knock-in substitution of Ser36Ala.
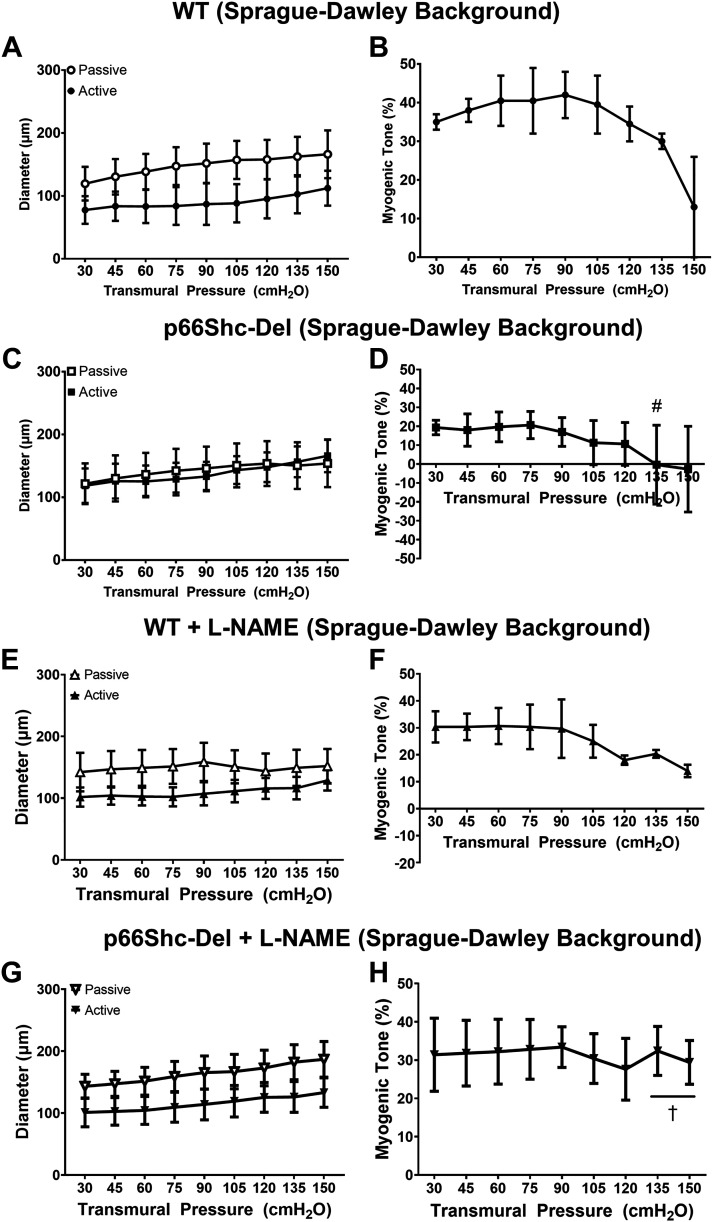
To examine whether the p66Shc-Del phenotype was sufficient to drive the observed phenotype or whether the phenotype was a result of genetic background, rats expressing p66Shc-Del were backcrossed onto an SD background for three generations (p66Shc-Del/SD; Fig. 2). There were no differences in active and passive diameters or myogenic tone between WT/SD and p66-Del/SD groups (Fig. 2, A–D). Relative to the p66-Del/SS group, the p66-Del/SD rats demonstrated reduced passive diameter responses to higher transmural pressure (135 and 150 cmH2O, P < 0.05). Myogenic tone was higher in WT/SD rats relative to p66-Del/SS rats (75–135 cmH2O, P < 0.05) and p66-Del/SD rats (135 cmH2O, P < 0.05).
Figure 2.
Active and passive diameters and myogenic responses in response to increasing transmural pressure in rats bred onto a Sprague-Dawley (SD) background. Wild-type (WT/SD; A and B), p66Shc-Del/SD (C and D), WT/SD + NG-nitro-l-arginine methyl ester (l-NAME; E and F), and p66Shc-Del/SD + NG-nitro-l-arginine methyl ester (l-NAME; G and H). Two-way repeated-measures ANOVA. #P < 0.05 vs. WT/SD; †P < 0.05 vs. p66-Del/SD; n = 3–5 rats/group.
To examine whether a “second hit” was responsible to uncover the p66Shc-Del phenotype observed within the SS background, WT/SD and p66Shc-Del/SD rats were given a hypertensive stimulus (l-NAME, a NOS inhibitor) in drinking water for 4 wk (Fig. 2, E–H). This hypertensive stimulus did not alter active or passive diameter responses during increasing transmural pressure (P = 0.86–0.99; Fig. 2, E and G). The addition of a hypertensive stimulus elevated myogenic responses at higher pressures (135–150 cmH2O) in p66-Del/SD + l-NAME relative to p66-Del/SD (P < 0.05; Fig. 2H).
DISCUSSION
Within the current study, we examined the effect of p66Shc on MCA myogenic tone across different p66Shc mutations. We have previously demonstrated a critical role for p66Shc in mediating oxidative stress within the kidneys and representing a link between oxidative stress generation and renal disease (10, 12). Other groups have demonstrated that deletion of p66Shc reduces damage from ischemia-reperfusion within the brain by reducing oxidative stress and restoring nitric oxide bioavailability (17–19). Rats expressing constitutively signaling p66Shc (p66Shc-Del/SS) are characterized by enhanced ROS generation (12). Deletion of nine amino acids in the NH2-terminal portion of p66Shc in p66Shc-Del/SS mutant removes phosphorylation-dependent regulation of p66Shc signaling and could result in constitutive translocation of p66Shc into mitochondria where it facilitates the production of ROS (12). p66Shc-KO/SS rats do not demonstrate the same ROS generation as p66Shc-Del/SS counterparts, and within the current study, p66-Del/SS rats demonstrate some changes in diameter in response to transmural pressure (Fig. 1D).
As p66Shc-Del signaling enhances generation of ROS (12) and decreased myogenic tone, we hypothesized that this was presumably due to the SS background that these rats were bred on. Previous evidence has demonstrated that the severity of endothelial dysfunction differs across genetic backgrounds (20). To circumvent this, we backcrossed the p66Shc-Del rats onto an SD background. No differences for passive or myogenic responses between the two genetic backgrounds were detected. We hypothesized that a “second hit” is needed to uncover the p66Shc phenotype. Chronic ingestion of l-NAME in drinking water reduces endothelial-dependent vasodilation (21), results in cerebral artery remodeling (22, 23), elicits larger infarcts following MCA occlusion (24), and elicits increases in arterial blood pressure (25). The addition of a second stressor failed to replicate these responses when bred on an SD background except at higher transmural pressure (Fig. 2G). This observation is in line with our previously published work that ablation of p66Shc signaling restored renal microvascular responses in hypertensive rats (8).
There are several experimental considerations that warrant discussion. First, we present the effects of p66Shc on cerebral artery diameter changes to increased transmural pressure and the myogenic responses on two distinct genetic backgrounds and in response to a hypertensive stimulus. These changes could be due to cerebral artery remodeling and biomechanical changes within the arterial wall, particularly in response to our hypertensive stimulus (26, 27). Hypertension, a primary risk factor for stroke, causes alterations in cerebrovascular structure and function. Chronic elevations in blood pressure result in arterial remodeling, which changes vascular resistance (26). Arterial wall remodeling is an adaptive process in an attempt to protect vulnerable downstream arterioles and capillaries. Hypertension is also associated with elevations in ROS, stemming from a multitude of factors including the renin-angiotensin system and production of ROS via NADPH oxidase (28). Elevations in ROS impair endothelial function, which in turn can reduce the ability to regulate cerebrovascular myogenic activity (reviewed in Refs. 27, 29). We performed studies examining functional responses to changes in pressure, and we did not examine the effects of p66Shc and l-NAME on cerebral artery remodeling. Second, p66Shc has been demonstrated to enhance ROS production in both the renal circulation and cerebrovasculature (8, 9, 12). ROS production via NADPH oxidase (high expression within cerebrovasculature) within the cerebral circulation is a potent mediator of vascular tone and contributes to the development of cerebrovascular diseases, and inactivation of p66Shc dramatically reduces superoxide content within basilar arteries of aged mice (3, 9, 30, 31). Whether constitutively active p66Shc-mediated increases in ROS are responsible for the reductions in myogenic tone observed within these experiments is unclear and an area for future investigation. As inactivation of p66Shc (p66Shc-KO/SS) or mutation of the phosphorylation site of p66Shc (p66Shc-S36A/SS) did not demonstrate similar reductions in myogenic tone, this hypothesis may be substantiated. Third, although we have previously reported changes in blood pressure (BP) on the p66Shc-Del/SS group in response to a high-salt diet (8), we did not collect BP data on p66Shc-Del/SD or l-NAME groups. Within the current study, we cannot determine if this is also observed when these rats are backcrossed with an SD background. We backcrossed p66Shc-Del/SS rats with SD rats for three generations. According to Mendelian genetics, this should result in an SD background of ∼87.5%. Backcrossing for an additional two to three generations would not necessarily have a greater effect. Finally, although our experiments focused on the MCA, these vascular beds alone do not constitute the entirety of the cerebral circulation, nor are the mechanisms governing autoregulation uniform across cerebral vascular beds (32).
These experimental considerations do not detract from the current study. In fact, we present for the first time the role of p66Shc on cerebrovascular myogenic activity and open new areas of investigation. For example, whether activation/inactivation of p66Shc results in cerebral arterial remodeling and how activation/inactivation regulates cerebrovascular ROS production are unclear and are two intriguing areas for future investigation. Furthermore, the role of p66Shc in hypertension-induced arterial remodeling within the cerebrovasculature remains to be explored.
Conclusions
The current study demonstrates the functional impact of p66Shc within the cerebral circulation. We demonstrated that signaling by p66Shc-Del decreases myogenic responses. This was in part due to the genetic background (Dahl salt sensitive) of the rats used as pharmacological means to elevate systemic pressure (l-NAME-induced hypertension) on a physiological genetic background that failed to induce the same phenotype. Collectively, these results highlight that p66Shc is critically involved in vascular dysfunction; however, the phenotype seems to be associated with other genetic components and not directly related to the presence of systemic hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL147976 (to A. Sorokin) and R01HL133029 (to A. M. Beyer) and American Heart Association Grant 20POST35050017 (to W. E. Hughes).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.M., A.S., and A.M.B. conceived and designed research; W.E.H. and J.H. performed experiments; W.E.H. and A.S. analyzed data; W.E.H., B.M., A.S., and A.M.B. interpreted results of experiments; W.E.H. prepared figures; W.E.H. drafted manuscript; W.E.H., B.M., A.S., and A.M.B. edited and revised manuscript; W.E.H., B.M., A.S., and A.M.B. approved final version of manuscript.
REFERENCES
- 1.Peterson EC, Wang Z, Britz G. Regulation of cerebral blood flow. Int J Vasc Med 2011: 823525, 2011. doi: 10.1155/2011/823525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palomares SM, Cipolla MJ. Vascular protection following cerebral ischemia and reperfusion. J Neurol Neurophysiol 2011: S1-004, 2011. doi: 10.4172/2155-9562.S1-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho C, Moreira PI. Oxidative stress: a major player in cerebrovascular alterations associated to neurodegenerative events. Front Physiol 9: 806, 2018. doi: 10.3389/fphys.2018.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q, Wang Q, Zhu J, Xiao Q, Zhang L. Reactive oxygen species: key regulators in vascular health and diseases. Br J Pharmacol 175: 1279–1292, 2018. doi: 10.1111/bph.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howitt L, Kuo IY, Ellis A, Chaston DJ, Shin HS, Hansen PB, Hill CE. Chronic deficit in nitric oxide elicits oxidative stress and augments T-type calcium-channel contribution to vascular tone of rodent arteries and arterioles. Cardiovasc Res 98: 449–457, 2013. doi: 10.1093/cvr/cvt043. [DOI] [PubMed] [Google Scholar]
- 6.Sweazea KL, Walker BR. Impaired myogenic tone in mesenteric arteries from overweight rats. Nutr Metab (Lond) 9: 18, 2012. doi: 10.1186/1743-7075-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maneen MJ, Hannah R, Vitullo L, DeLance N, Cipolla MJ. Peroxynitrite diminishes myogenic activity and is associated with decreased vascular smooth muscle F-actin in rat posterior cerebral arteries. Stroke 37: 894–899, 2006. doi: 10.1161/01.STR.0000204043.18592.0d. [DOI] [PubMed] [Google Scholar]
- 8.Miller B, Palygin O, Rufanova VA, Chong A, Lazar J, Jacob HJ, Mattson D, Roman RJ, Williams JM, Cowley AW Jr, Geurts AM, Staruschenko A, Imig JD, Sorokin A. p66Shc regulates renal vascular tone in hypertension-induced nephropathy. J Clin Invest 126: 2533–2546, 2016. doi: 10.1172/JCI75079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Savarese G, Perrone-Filardi P, Lüscher TF, Camici GG. Enhanced age-dependent cerebrovascular dysfunction is mediated by adaptor protein p66Shc. Int J Cardiol 175: 446–450, 2014. doi: 10.1016/j.ijcard.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Wright KD, Staruschenko A, Sorokin A. Role of adaptor protein p66Shc in renal pathologies. Am J Physiol Renal Physiol 314: F143–F153, 2018. doi: 10.1152/ajprenal.00414.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122: 221–233, 2005. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Miller B, Palygin O, El-Meanawy A, Mattson DL, Geurts AM, Staruschenko A, Sorokin A. p66Shc-mediated hydrogen peroxide production impairs nephrogenesis causing reduction of number of glomeruli. Life Sci 279: 119661, 2021. doi: 10.1016/j.lfs.2021.119661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller BS, Blumenthal SR, Shalygin A, Wright KD, Staruschenko A, Imig JD, Sorokin A. Inactivation of p66Shc decreases afferent arteriolar K(ATP) channel activity and decreases renal damage in diabetic Dahl SS rats. Diabetes 67: 2206–2212, 2018. doi: 10.2337/db18-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palygin O, Miller BS, Nishijima Y, Zhang DX, Staruschenko A, Sorokin A. Endothelin receptor A and p66Shc regulate spontaneous Ca2+ oscillations in smooth muscle cells controlling renal arterial spontaneous motion. FASEB J 33: 2636–2645, 2019. doi: 10.1096/fj.201800776RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyer AM, Raffai G, Weinberg B, Fredrich K, Lombard JH. Dahl salt-sensitive rats are protected against vascular defects related to diet-induced obesity. Hypertension 60: 404–410, 2012. doi: 10.1161/HYPERTENSIONAHA.112.191551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Cowley AW Jr, Jacob HJ. Chromosomal mapping of the genetic basis of hypertension and renal disease in FHH rats. Am J Physiol Renal Physiol 293: F1905–F1914, 2007. doi: 10.1152/ajprenal.00012.2007. [DOI] [PubMed] [Google Scholar]
- 17.Spescha RD, Shi Y, Wegener S, Keller S, Weber B, Wyss MM, Lauinger N, Tabatabai G, Paneni F, Cosentino F, Hock C, Weller M, Nitsch RM, Lüscher TF, Camici GG. Deletion of the ageing gene p66(Shc) reduces early stroke size following ischaemia/reperfusion brain injury. European Heart J 34: 96–103, 2013. doi: 10.1093/eurheartj/ehs331. [DOI] [PubMed] [Google Scholar]
- 18.Spescha RD, Glanzmann M, Simic B, Witassek F, Keller S, Akhmedov A, Tanner FC, Lüscher TF, Camici GG. Adaptor protein p66Shc mediates hypertension-associated, cyclic stretch-dependent, endothelial damage. Hypertension 64: 347–353, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02129. [DOI] [PubMed] [Google Scholar]
- 19.Pérez H, Finocchietto PV, Alippe Y, Rebagliati I, Elguero ME, Villalba N, Poderoso JJ, Carreras MC. p66Shc inactivation modifies RNS production, regulates Sirt3 activity, and improves mitochondrial homeostasis, delaying the aging process in mouse brain. Oxid Med Cell Longev 2018: 8561892, 2018. doi: 10.1155/2018/8561892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Silva TM, Modrick ML, Dabertrand F, Faraci FM. Changes in cerebral arteries and parenchymal arterioles with aging: role of rho kinase 2 and impact of genetic background. Hypertension 71: 921–927, 2018. doi: 10.1161/HYPERTENSIONAHA.118.10865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardiner SM, Kemp PA, Bennett T, Palmer RM, Moncada S. Nitric oxide synthase inhibitors cause sustained, but reversible, hypertension and hindquarters vasoconstriction in Brattleboro rats. Eur J Pharmacol 213: 449–451, 1992. doi: 10.1016/0014-2999(92)90636-i. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh NK, Wang JY, Liu JC, Lee WH, Chen HI. Structural changes in cerebral arteries following nitric oxide deprivation: a comparison between normotensive and hypertensive rats. Thromb Haemost 92: 162–170, 2004. doi: 10.1160/TH03-10-0610. [DOI] [PubMed] [Google Scholar]
- 23.Baumbach GL, Sigmund CD, Faraci FM. Structure of cerebral arterioles in mice deficient in expression of the gene for endothelial nitric oxide synthase. Circ Res 95: 822–829, 2004. doi: 10.1161/01.RES.0000146279.11923.14. [DOI] [PubMed] [Google Scholar]
- 24.Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab 16: 981–987, 1996. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro MO, Antunes E, de Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthesis. a new model of arterial hypertension. Hypertension 20: 298–303, 1992. doi: 10.1161/01.hyp.20.3.298. [DOI] [PubMed] [Google Scholar]
- 26.Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension 13: 968–972, 1989. doi: 10.1161/01.hyp.13.6.968. [DOI] [PubMed] [Google Scholar]
- 27.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol 304: H1598–H1614, 2013. doi: 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM. Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol 31: 631–641, 2015. doi: 10.1016/j.cjca.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Presa JL, Saravia F, Bagi Z, Filosa JA. Vasculo-neuronal coupling and neurovascular coupling at the neurovascular unit: impact of hypertension. Front Physiol 11: 584135, 2020. doi: 10.3389/fphys.2020.584135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int 60: 208–212, 2012. doi: 10.1016/j.neuint.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Miller AA, Drummond GR, Schmidt HH, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res 97: 1055–1062, 2005. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- 32.Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res 49: 375–389, 2012. doi: 10.1159/000338747. [DOI] [PMC free article] [PubMed] [Google Scholar]