Abstract
Late-onset Pompe disease (LOPD) is a rare, progressive disorder characterized by limb–girdle muscle weakness and/or respiratory insufficiency, caused by acid alpha-glucosidase (GAA) gene mutations and treated with enzyme replacement therapy. We studied isometric muscle strength in eight muscle groups bilaterally using a Biodex® dynamometer, as well as the Medical Research Council sum score (MRC-SS), hand grip strength, 6 min walk distance (6MWD), 10 m walk test (10MWT) and timed up-and-go test (TUG) in 12 adult, ambulatory, treated LOPD patients and 12 age-/gender-matched healthy controls, every 6 months for 2 years. The mean isometric muscle strength showed a significant decline in right and left knee extensors at 12 months in controls (p < 0.014; p < 0.016), at 18 months in patients (p < 0.010; p < 0.007) and controls (only right side, p < 0.030) and at 24 months in both groups (p < 0.035). The mean 6MWD in patients significantly decreased after 24 months, from 451.9 m to 368.1 m (p < 0.003), whereas in controls, the mean 6MWD significantly increased after 6 months (p < 0.045) and 18 months (p < 0.020) (at 24 months p = 0.054). In patients and controls, the MRC-SS, hand grip test, 10MWT and TUG did not show significant changes (p > 0.05). We conclude that the 6MWD is a useful outcome measure to detect motor decline in treated LOPD patients.
Keywords: glycogen storage disease type 2, GSD2, LOPD, 6MWD, muscle strength, Biodex® dynamometer, isometric, longitudinal, ERT, enzyme replacement therapy
1. Introduction
Late-onset Pompe disease (LOPD; also known as glycogen storage disease type 2 or GSD2) is a rare autosomal recessive disorder caused by acid alpha-glucosidase (GAA) deficiency. The lack of the GAA lysosomal enzyme results in the accumulation of glycogen in muscle cells, leading to progressive limb–girdle muscle weakness and respiratory insufficiency [1,2]. The onset and severity of Pompe disease largely depends on the residual GAA enzyme activity: the disease either develops during the first months of life as the classic severe infantile-onset Pompe disease (IOPD) [3], or later in life with a milder phenotype known as late-onset Pompe disease (LOPD) [2]. Current treatment consists of enzyme replacement therapy (ERT) with recombinant human alglucosidase alfa [4], (non-)invasive ventilation and physiotherapy.
In previous studies showing the effect of ERT in LOPD patients, the 6 min walk distance test (6MWD) was mainly used as a consistent positive outcome measure of motor function in this disease [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. In contrast, motor strength as an outcome measure using the manual Medical Research Council sum score (MRC-SS) showed inconsistent results, with a significant improvement in ERT-treated LOPD patients in some studies [17,21], but without amelioration in others [9,15]. More recently, the Biodex® dynamometer has been introduced in the neuromuscular field to assess muscle strength in an objective, quantitative manner, particularly in patients with Duchenne muscular dystrophy [25], hereditary inclusion body myopathy [26] and in a small study with four treated LOPD patients [18].
In this study, we assessed isometric muscle strength measurements in eight muscle groups bilaterally using the Biodex® dynamometer in adult, ambulatory, ERT-treated LOPD patients and in age-/gender-matched controls every 6 months for a duration of 2 years. We also evaluated the MRC-SS, hand grip strength, 6MWD, 10 m walk test (10MWT) and timed up-and-go test (TUG) as outcome measures in this patient group, and compared the data with age- and gender-matched healthy controls.
2. Patients and Methods
2.1. Patients and Controls
We included 12 adult Belgian patients with genetically confirmed and symptomatic (i.e., presence of muscle weakness) LOPD and 12 gender- and age-matched, healthy control individuals. All patients were ambulatory and treated with alglucosidase alfa 20 mg/kg intravenously (Myozyme®, Sanofi-Genzyme, Genzyme Corporation, Cambridge, MA, USA). The Ethical Committee Research of UZ/KU Leuven approved the study (S-60965; date of approval: 20 December 2017). We obtained written informed consent from all study participants.
2.2. Muscle Strength Assessment Using Biodex® Dynamometer
We measured isometric muscle strength using a quantitative Biodex® dynamometer (Biodex System 4, Procare Belgium and Biodex Medical Systems, Shirley, NY, USA) in all patients and controls every 6 months for a study duration of 2 years. All measurements were performed by the same investigator to avoid inter-investigator variability. The isometric muscle strength of knee flexors and extensors was measured in sitting position with the knee at 60°, hip flexors and extensors were assessed in supine position with the hip at 60°, elbow flexors and extensors with the elbow at 60° and shoulder abductors and adductors with the shoulder at 60°. Muscle groups were assessed bilaterally. The order of muscle strength testing was held constant with strength of the knee flexors/extensors assessed first, then elbow, shoulder and lastly, the hip muscles. Prior to the first session, participants were familiarized with the Biodex® dynamometer to avoid confounding strength changes due to exercise training or greater familiarity with the test equipment. There were three five-second contractions performed consecutively by each muscle group with 10 s rests between contractions. The participant was verbally encouraged during the test to perform maximum contraction. The peak torque output for each muscle group (in Newton meters, Nm) was used in the analysis.
2.3. Additional Muscle Strength and Motor Function Assessments
In addition, we assessed muscle strength using the manual 80-point MRC-SS and hand grip strength (in kilograms) of the dominant hand (right hand in all participants) using a Jamar® hand dynamometer (Jamar Technologies, Hatfield, PA, USA). We measured motor function using the 6MWD (in meter), 10MWT (in seconds) and TUG (in seconds).
2.4. Pulmonary Function Tests
In the patients, Forced Vital Capacity (FVC) was measured at study onset (visit 1) and at the end of the study (visit 5), both in sitting and supine position, following standard procedures. FVC was measured in liters (L) and in percent decrease (%) compared to controls matched for age and sex, height and body weight.
2.5. Statistical Analysis
We used MedCalc® for statistical analyses (MedCalc Software, Ostend, Belgium) [27]. Descriptive statistics are stated as averages (minimum–maximum) and percentages. We applied paired t-tests for the comparison of outcome variables between baseline visit (V1) and visits at months 6 (V2), 12 (V3), 18 (V4) and 24 (V5). If assumptions for normality were not met, non-parametric equivalents (Wilcoxon signed-rank test or sign-test) were applied. An unpaired t-test was applied for the comparison between left and right in patients and control subjects. Analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons was applied to analyze the differences between the means at the different visits (V1–V5) for each of the outcome measures in both LOPD patients and controls. Significance level was determined at α = 0.05.
3. Results
3.1. Demographics and Clinical Characteristics of LOPD Patients
In both patient and control groups (n = 12), we included five males (42%) and seven females (58%) (Table 1). The mean age at study entry was 51.3 years (range 22–67) and 50.9 years (range 23–64), respectively. The mean age at symptom onset in LOPD patients was 32.8 years (range 1–52). At the time of study inclusion, all patients were of adult age, ambulatory, symptomatic showing muscle weakness and treated with alglucosidase alfa. The mean disease duration was 18.4 years (range 0.5–36), and the mean duration of ERT therapy at the time of start of the study was 8.8 years (range 0.5–13). Only one patient (i.e., patient 7) had an ERT duration at the time of study inclusion of less than 2 years (i.e., 0.5 years), whereas all the other patients had ERT treatment durations of much longer than 2 years, i.e., between 6 and 13 years. The 6MWD in patient 7 also showed a slight deterioration during the study (data from visit 1 to visit 5: 594 m, 508 m, 486 m, 474 m and 477 m) and did not have an impact on the reported results, significances or conclusions.
Table 1.
Clinical and genetic features of the LOPD patients included in the study.
| ID | Gender | Age at Symptom Onset (y) |
Symptoms at Onset |
GAA Mutations |
Age at Study Inclusion (y) |
Disease Duration at Inclusion (y) |
Duration of ERT (y) |
NIV at Night (Y/N), Age at Start NIV (y) |
FVC Sitting Visit 1 (0 Months) (%) |
FVC Supine Visit 1 (0 Months) (%) |
FVC Sitting Visit 5 (24 Months) (%) |
FVC Supine Visit 5 (24 Months) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 42 | LW | c.-32-13T>G; c.482_483delCC | 48 | 6 | 6 | N | 101 | 97 | 96 | 94 |
| 2 | F | 1 | LW | c.-32-13T>G; c.923A>C |
22 | 21 | 13 | N | 106 | 91 | 106 | 107 |
| 3 | M | 39 | R | c.-32-13T>G; c.258dupC |
61 | 22 | 11 | Y (40) | 33 | ND | 30 | ND |
| 4 | F | 35 | LW | c.-32-13T>G; c.1548G>A |
49 | 14 | 12 | Y (36) | 48 | 33 | 51 | 38 |
| 5 | M | 17 | LW | c.-32-13T>G; c.482_483del |
43 | 26 | 8 | N | 73 | 67 | 76 | 62 |
| 6 | F | 27 | hyperCK, F | c.-32-13T>G; c.525delT |
40 | 13 | 8 | N | 114 | 93 | 121 | 105 |
| 7 | M | 42 | LW | c.-32-13T>G; c.2608C>T |
54 | 12 | 0.5 | Y (53) | 76 | 45 | 84 | 45 |
| 8 | M | 45 | LW | c.-32-13T>G; c.1681_1699dup19 | 67 | 22 | 11 | Y (60) | 101 | 49 | 105 | 59 |
| 9 | F | 44 | LW | c.-32-13T>G; del exon 18 |
62 | 18 | 10 | N | 70 | 43 | 64 | 32 |
| 10 | F | 52 | LW | c.-32-13T>G; c.258dupC |
63 | 11 | 8 | N | 108 | 78 | 73 | 56 |
| 11 | F | 25 | LW | c.-32-13T>G; c.186dup11 |
61 | 36 | 9 | N | 84 | 64 | 79 | 62 |
| 12 | M | 25 | LW | c.-32-13T>G; c.1075G>A |
45 | 20 | 9 | N | 80 | 62 | 79 | 56 |
| 32.8 (1–52) |
51.3 (22–67) |
18.4 (0.5–36) |
8.8 (0.5–13) |
ID, patient number; y, years; GAA, acid alpha-glucosidase gene; ERT, enzyme replacement therapy; NIV, non-invasive ventilation; FVC, forced vital capacity; F, female; M, male; LW, limb–girdle weakness; R, respiratory weakness; hyperCK, increased creatine kinase in blood; F, fatigue; Y, yes; N, No; ND, not done.
At the time of the study, one third of the patients (4/12) were non-invasively ventilated during the night.
Statistical analyses in the patients (n = 12) did not show significant changes in FVC for the study duration of two years, both in the sitting and in supine position (p > 0.05). In one patient, supine FVC measurements were not possible due to respiratory insufficiency in the supine position (patient 3, Table 1). Therefore, since in our study group there was no progressive respiratory insufficiency for the duration of the study, the decline in the 6MWD cannot be explained by changes in respiratory function.
3.2. Results of Biodex® Dynamometer and Other Outcome Measures in LOPD Patients
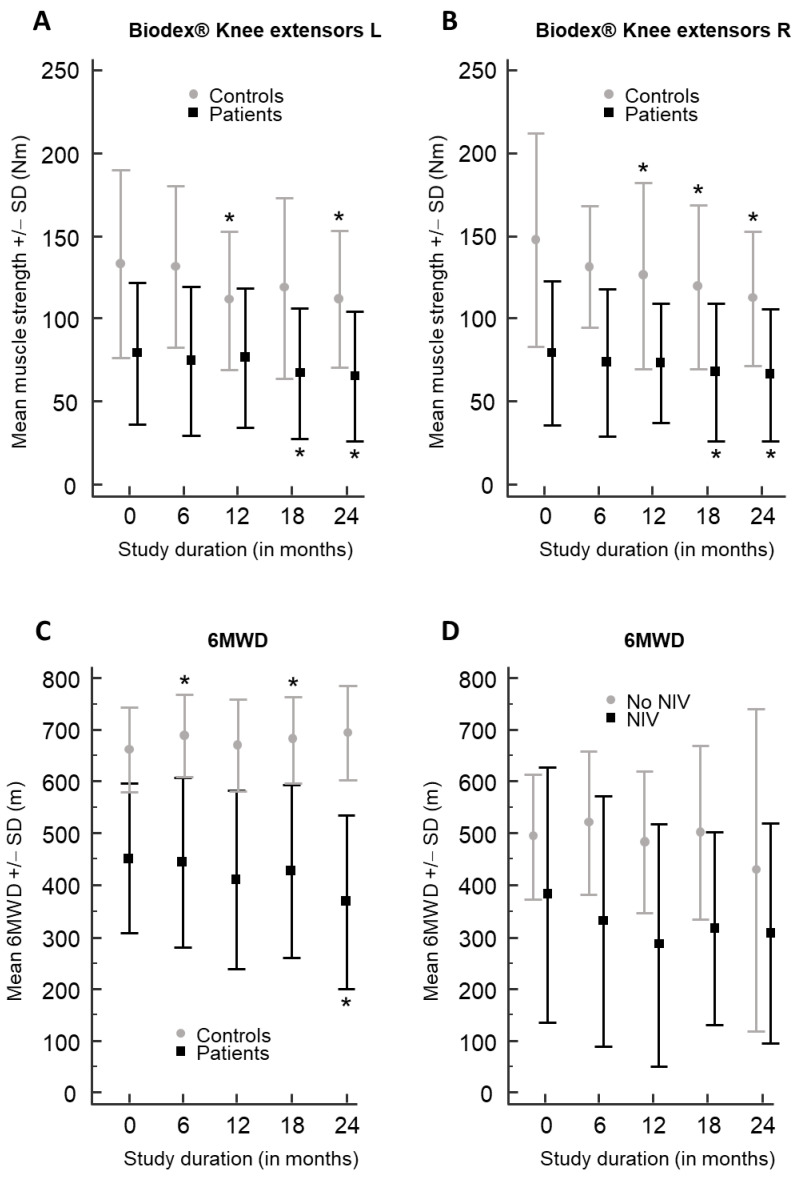
In LOPD patients, the mean isometric muscle strength measured using a Biodex® dynamometer showed a significant deterioration in the knee extensors bilaterally at 18 months (right: p < 0.010; left p < 0.007) and 24 months (right: p < 0.002; left: p < 0.017) compared to baseline (Table 2; Figure 1A,B). At the baseline visit, the mean muscle strength at the knee extensors was 79.5 Nm ± 43.4 (right) and 79.3 Nm ± 42.6 (left), whereas after 18 months, the mean muscle strength significantly decreased to 67.7 Nm ± 41.3 (right) and 67.1 Nm ± 39.6 (left), and after 24 months, to 66.2 Nm ± 39.6 (right) and 65.1 Nm ± 39.0 (left) (Table 2). The mean isometric muscle strength in the hip flexors on the right side showed a significant change after 18 months (p < 0.031), but this effect did not sustain after 24 months and was not present at the left side. No significant decrease in the mean isometric muscle strength was measured for the study duration of 24 months in any of the other muscle groups (hip extensors, knee flexors, shoulder abductors and adductors and elbow extensors and flexors) using the Biodex® dynamometer (p > 0.05). There were no significant differences between the mean isometric muscle strength in the different muscle groups measured at the left and right side in patients (p > 0.05).
Table 2.
Results of Biodex® dynamometer and other outcome measures in LOPD patients.
| Outcome | Visit 1 (0 Months) |
Visit 2 (6 Months) |
Visit 3 (12 Months) |
Visit 4 (18 Months) |
Visit 5 (24 Months) |
ANOVA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Mean | ±SD | Mean | ±SD | V2 vs. V1 | 95%CI | p | Mean | ±SD | V3 vs. V1 | 95%CI | p | Mean | ±SD | V4 vs. V1 | 95%CI | p | Mean | ±SD | V5 vs. V1 | 95%CI | p | p |
| Hip ext. R | 54.1 | 19.8 | 62.0 | 29.6 | 7.7 | −0.4 to 15.8 | 0.061 | 59.6 | 24.7 | 6.0 | −3.6 to 15.7 | 0.191 | 59.4 | 27.1 | 5.3 | −2.5 to 13.2 | 0.162 | 61.5 | 28.5 | 7.4 | −2.5 to 17.3 | 0.126 | 0.959 |
| Hip ext. L | 55.5 | 26.4 | 62.3 | 31.1 | 6.7 | −2.1 to 15.5 | 0.119 | 59.1 | 30.3 | 5.6 | −4.8 to 16.0 | 0.253 | 60.1 | 28.4 | 4.6 | −4.3 to 13.5 | 0.274 | 57.8 | 24.3 | 2.3 | −9.5 to 14.1 | 0.677 | 0.986 |
| Hip flex. R | 19.2 | 14.8 | 22.5 | 14.2 | 3.7 | −1.8 to 9.2 | 0.162 | 23.9 | 16.6 | 4.2 | −1.9 to 10.4 | 0.156 | 24.9 | 11.5 | 5.8 | 0.6 to 10.9 | 0.031 | 26.4 | 15.6 | 7.3 | −1.0 to 15.5 | 0.079 | 0.813 |
| Hip flex. L | 17.9 | 12.4 | 20.9 | 13.8 | 3.3 | −1.5 to 8.2 | 0.153 | 22.0 | 15.4 | 3.5 | −2.7 to 9.7 | 0.233 | 23.1 | 14.2 | 5.2 | −1.6 to 11.9 | 0.119 | 22.1 | 12.8 | 4.2 | −2.1 to 10.5 | 0.170 | 0.921 |
| Knee ext. R | 79.5 | 43.4 | 73.5 | 44.5 | −3.3 | −8.5 to 2.0 | 0.194 | 73.2 | 35.9 | −6.4 | −18.1 to 5.3 | 0.256 | 67.7 | 41.3 | −11.8 | −20.2 to −3.5 | 0.010 | 66.2 | 39.6 | −13.3 | −20.5 to −6.2 | 0.002 | 0.937 |
| Knee ext. L | 79.3 | 42.6 | 74.8 | 44.8 | −2.4 | −7.7 to 2.9 | 0.332 | 76.7 | 42.0 | −2.6 | −13.3 to 8.2 | 0.609 | 67.1 | 39.6 | −12.1 | −20.3 to −4.0 | 0.007 | 65.1 | 39.0 | −14.1 | −25.2 to −3.1 | 0.017 | 0.901 |
| Knee flex. R | 52.6 | 24.7 | 51.9 | 25.7 | −1.3 | −4.6 to 1.9 | 0.378 | 44.3 | 21.6 | −8.2 | −16.6 to 0.2 | 0.055 | 48.1 | 24.6 | −4.4 | −9.7 to 0.9 | 0.095 | 47.6 | 23.9 | −5.0 | −11.0 to 1.0 | 0.093 | 0.919 |
| Knee flex. L | 47.4 | 21.3 | 48.2 | 24.8 | 0.4 | −3.2 to 4.0 | 0.807 | 42.3 | 20.4 | −5.1 | −11.0 to 0.7 | 0.081 | 46.6 | 22.0 | −0.9 | −4.2 to 2.5 | 0.577 | 44.9 | 22.2 | −2.5 | −6.2 to 1.2 | 0.167 | 0.970 |
| Shoulder abd. R | 18.8 | 5.0 | 18.6 | 6.1 | 0.4 | −3.4 to 4.2 | 0.821 | 18.3 | 8.7 | 0.4 | −4.2 to 5.1 | 0.839 | 17.7 | 6.7 | −0.4 | −2.9 to 2.1 | 0.726 | 16.8 | 6.1 | −1.2 | −3.4 to 0.9 | 0.223 | 0.953 |
| Shoulder abd. L | 16.8 | 6.8 | 16.9 | 7.8 | 1.1 | −2.4 to 4.5 | 0.498 | 17.5 | 6.9 | 0.8 | −1.5 to 3.2 | 0.437 | 16.2 | 7.0 | −0.7 | −3.6 to 2.2 | 0.596 | 17.3 | 7.1 | 0.6 | −2.1 to 3.2 | 0.646 | 0.993 |
| Shoulder add. R | 42.3 | 14.4 | 40.6 | 14.5 | −1.2 | −3.5 to 1.2 | 0.291 | 40.1 | 14.8 | −2.1 | −6.2 to 1.9 | 0.269 | 41.2 | 15.0 | −0.6 | −3.9 to 2.7 | 0.696 | 41.6 | 15.3 | −0.3 | −3.4 to 2.8 | 0.853 | 0.997 |
| Shoulder add. L | 47.3 | 14.9 | 44.7 | 13.9 | −2.2 | −6.0 to 1.7 | 0.236 | 46.5 | 13.7 | −1.0 | −5.2 to 3.3 | 0.627 | 45.5 | 14.0 | −1.7 | −6.3 to 3.0 | 0.443 | 47.3 | 14.5 | −0.1 | −5.1 to 4.8 | 0.952 | 0.991 |
| Elbow ext. R | 40.8 | 13.8 | 40.9 | 13.8 | 1.8 | −4.4 to 8.0 | 0.534 | 42.1 | 14.9 | 1.2 | −4.0 to 6.4 | 0.614 | 43.3 | 14.3 | 2.5 | −3.4 to 8.4 | 0.378 | 40.0 | 13.0 | −0.9 | −7.0 to 5.3 | 0.765 | 0.982 |
| Elbow ext. L | 44.8 | 16.9 | 45.7 | 13.1 | 3.2 | −1.5 to 8.0 | 0.159 | 43.2 | 16.3 | −1.6 | −6.0 to 2.8 | 0.446 | 44.8 | 15.5 | 0.0 | −4.7 to 4.7 | 0.988 | 43.1 | 13.7 | −1.7 | −7.0 to 3.7 | 0.506 | 0.993 |
| Elbow flex. R | 22.6 | 10.9 | 21.0 | 10.2 | −1.4 | −6.0 to 3.1 | 0.506 | 22.0 | 12.2 | −0.6 | −6.0 to 4.8 | 0.810 | 22.2 | 11.2 | −0.5 | −4.7 to 3.7 | 0.805 | 22.7 | 11.7 | 0.0 | −5.2 to 5.2 | 0.995 | 0.997 |
| Elbow flex. L | 22.2 | 14.1 | 22.3 | 12.3 | 2.1 | −1.1 to 5.4 | 0.178 | 22.7 | 12.1 | 0.5 | −4.0 to 4.9 | 0.816 | 22.9 | 12.0 | 0.7 | −2.3 to 3.7 | 0.626 | 23.1 | 11.2 | 0.9 | −3.2 to 4.9 | 0.646 | 1.000 |
| Hand grip R(kg) | 37.3 | 10.7 | 39.3 | 8.9 | 1.1 | −1.3 to 3.5 | 0.323 | 37.4 | 7.9 | −0.8 | −5.1 to 3.5 | 0.680 | 37.5 | 9.3 | −0.5 | −4.0 to 3.0 | 0.743 | 36.8 | 7.7 | −2.2 | −7.1 to 2.7 | 0.328 | 0.974 |
| MRC-SS (/80) | 67.2 | 8.2 | 70.4 | 7.7 | 1.0 | −5.5 to 7.5 | 0.733 | 71.1 | 8.3 | 1.8 | −4.9 to 8.5 | 0.556 | 70.4 | 8.3 | 1.2 | −5.8 to 8.1 | 0.709 | 71.3 | 8.1 | 2.3 | −5.3 to 9.8 | 0.506 | 0.785 |
| 6MWD (m) | 451.9 | 143.3 | 443.9 | 163.6 | −19.1 | −58.8 to 20.6 | 0.305 | 410.6 | 172.2 | −41.3 | −86.9 to 4.3 | 0.071 | 427.6 | 167.0 | −28.5 | −63.9 to 6.9 | 0.102 | 368.1 | 167.6 | −60.6 | −92.0 to −29.1 | 0.003 | 0.826 |
| 10MWT (s) | 8.4 | 2.8 | 8.6 | 2.8 | 0.2 | −0.7 to 1.2 | 0.626 | 9.4 | 3.7 | 1.0 | −0.3 to 2.4 | 0.127 | 9.0 | 3.8 | 0.7 | −0.4 to 1.7 | 0.214 | 9.1 | 3.5 | 0.8 | −1.3 to 2.9 | 0.414 | 0.960 |
| TUG (s) | 7.4 | 4.7 | 7.8 | 5.8 | 0.2 | −1.0 to 1.3 | 0.779 | 8.2 | 5.4 | 0.6 | −0.5 to 1.7 | 0.265 | 7.8 | 5.4 | 1.1 | −0.3 to 2.5 | 0.112 | 9.0 | 6.2 | 1.3 | −1.4 to 4.0 | 0.289 | 0.981 |
Biodex® measurements in Newton meters (Nm); SD, standard deviation; V, visit; V1, baseline visit (0 months); 95%CI, 95% confidence interval; p, p-value; ANOVA, analysis of variance; L, left; R, right; ext., extensors; flex., flexors; abd., abductors; add., adductors; hand grip, hand grip test (in kilograms); MRC-SS, Medical Research Council sum score (on a maximum of 80 points); 6MWD, 6 min walk distance (in meter); 10MWT, 10 m walk test (in seconds); TUG, timed up-and-go test (in seconds). Significant values are underlined and indicated in bold.
Figure 1.
Isometric muscle strength results using Biodex® in knee extensors and 6MWD in patients and controls. Results of the mean isometric muscle strength (±1 standard deviation, SD) in Newton meters (Nm) of the left (A) and right (B) knee extensors measured using the Biodex® dynamometer are shown for LOPD patients (black) and controls (gray) for the study duration of 24 months. In (C), the mean 6MWD (±1 standard deviation, SD) in meters (m) is presented for patients (black) and control individuals (gray) for the study duration of 24 months. In (D), the mean 6MWD (±1 standard deviation, SD) in meters (m) is presented for patients with non-invasive ventilation (NIV) (black) and those without NIV (gray). In panels (A–C), an asterisk (*) indicates a significant difference with the baseline value (0 months).
In patients with LOPD, the mean 6MWD significantly decreased after 24 months (p < 0.003), from 451.9 m at baseline to 368.1 m after 2 years, corresponding to a mean decline of 83.8 m (Table 2; Figure 1C). We compared the 6MWD between the LOPD patients with non-invasive ventilation (n = 4) and those without non-invasive ventilation (n = 8), and there was no significant difference in the 6MWD between the two groups (p > 0.05) and no difference in the 6MWD trend for the study duration (Table 1; Figure 1D). All patients remained ambulatory during the study. The MRC-SS, hand grip test, 10MWT and TUG did not show a significant change during the study (p > 0.05; Table 2). There were no significant differences between the means at the different visits (V1–V5) for each of the outcome measures in LOPD patients (ANOVA, p > 0.05; Table 2).
3.3. Results of Biodex® Dynamometer and Other Outcome Measures in Controls
Similarly to LOPD patients, in the age- and gender-matched control individuals, the mean isometric muscle strength measured using a Biodex® dynamometer also showed a significant decline in the knee extensors at 12 months at both sides (right: p < 0.014; left: p < 0.016), at 18 months only at the right side (p < 0.030) and at 24 months at both sides (right: p < 0.007; left: p < 0.035) compared to baseline (Table 3; Figure 1A,B). At the baseline visit, the mean muscle strength at the knee extensors was 147.7 Nm ± 64.5 (right) and 133.0 Nm ± 56.7 (left), whereas after 12 months, the mean muscle strength significantly decreased to 125.9 Nm ± 56.5 (right) and 111.0 Nm ± 41.7 (left), and after 24 months to 112.2 Nm ± 40.2 (right) and 111.8 Nm ± 41.3 (left) (Table 3).
Table 3.
Results of Biodex® dynamometer and other outcome measures in controls.
| Outcome | Visit 1 (0 Months) |
Visit 2 (6 Months) |
Visit 3 (12 Months) |
Visit 4 (18 Months) |
Visit 5 (24 Months) |
ANOVA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Mean | ±SD | Mean | ±SD | V2 vs. V1 | 95%CI | p | Mean | ±SD | V3 vs. V1 | 95%CI | p | Mean | ±SD | V4 vs. V1 | 95%CI | p | Mean | ±SD | V5 vs. V1 | 95%CI | p | p |
| Hip ext. R | 107.7 | 31.8 | 115.6 | 41.2 | 3.1 | −14.7 to 20.9 | 0.705 | 110.4 | 47.5 | 2.7 | −24.4 to 29.7 | 0.833 | 108.3 | 42.8 | −2.1 | −23.4 to 19.3 | 0.833 | 109.5 | 36.3 | −7.1 | −25.9 to 11.6 | 0.407 | 0.992 |
| Hip ext. L | 100.1 | 33.3 | 115.4 | 38.1 | 12.1 | −8.9 to 33.0 | 0.229 | 102.3 | 35.1 | 2.2 | −21.3 to 25.6 | 0.843 | 105.9 | 39.9 | 3.6 | −21.3 to 28.5 | 0.755 | 107.2 | 32.1 | −1.8 | −24.6 to 21.1 | 0.864 | 0.872 |
| Hip flex. R | 52.1 | 22.6 | 51.7 | 19.3 | −3.9 | −10.4 to 2.6 | 0.207 | 45.5 | 16.0 | −6.6 | −15.9 to 2.6 | 0.142 | 42.7 | 19.5 | −8.8 | −19.6 to 2.1 | 0.102 | 46.0 | 17.8 | −5.2 | −12.6 to 2.2 | 0.141 | 0.723 |
| Hip flex. L | 47.1 | 22.5 | 48.8 | 22.6 | −1.2 | −5.6 to 3.3 | 0.570 | 45.1 | 21.7 | −2.1 | −10.8 to 6.7 | 0.610 | 46.6 | 24.6 | −0.8 | −13.8 to 12.3 | 0.896 | 42.7 | 19.8 | −5.6 | −20.7 to 9.6 | 0.422 | 0.980 |
| Knee ext. R | 147.7 | 64.5 | 131.4 | 36.4 | −23.1 | −49.7 to 3.5 | 0.082 | 125.9 | 56.5 | −21.8 | −38.3 to −5.3 | 0.014 | 119.1 | 49.4 | −29.7 | −55.8 to −3.6 | 0.030 | 112.2 | 40.2 | −32.4 | −53.2 to −11.7 | 0.007 | 0.557 |
| Knee ext. L | 133.0 | 56.7 | 131.7 | 48.8 | −8.1 | −24.2 to 8.0 | 0.289 | 111.0 | 41.7 | −22.0 | −39.0 to −5.0 | 0.016 | 118.4 | 54.5 | −16.4 | −35.5 to 2.8 | 0.086 | 111.8 | 41.3 | −21.6 | −41.3 to −2.0 | 0.035 | 0.725 |
| Knee flex. R | 98.4 | 34.9 | 100.8 | 40.4 | −1.1 | −11.1 to 9.0 | 0.819 | 89.2 | 35.3 | −9.2 | −17.5 to −0.8 | 0.034 | 92.2 | 43.7 | −4.1 | −15.4 to 7.1 | 0.431 | 86.1 | 27.4 | −9.5 | −17.1 to −2.0 | 0.020 | 0.880 |
| Knee flex. L | 94.4 | 39.1 | 98.0 | 45.0 | −1.5 | −11.8 to 8.8 | 0.754 | 86.7 | 38.5 | −7.7 | −16.9 to 1.6 | 0.095 | 89.6 | 42.7 | −4.0 | −15.9 to 7.9 | 0.474 | 86.6 | 28.8 | −8.9 | −22.0 to 4.1 | 0.153 | 0.951 |
| Shoulder abd. R | 32.4 | 21.1 | 32.0 | 21.2 | −0.4 | −3.9 to 3.2 | 0.827 | 29.6 | 21.4 | −2.8 | −7.0 to 1.4 | 0.167 | 31.0 | 22.7 | −1.1 | −5.5 to 3.3 | 0.593 | 27.3 | 12.5 | −1.8 | −8.3 to 4.7 | 0.535 | 0.981 |
| Shoulder abd. L | 29.8 | 16.8 | 30.1 | 16.7 | 0.3 | −2.6 to 3.1 | 0.843 | 28.2 | 18.0 | −1.6 | −5.3 to 2.0 | 0.345 | 27.8 | 16.7 | −1.3 | −4.1 to 1.4 | 0.297 | 24.9 | 12.7 | −1.5 | −4.4 to 1.4 | 0.265 | 0.958 |
| Shoulder add. R | 78.2 | 37.5 | 74.2 | 31.6 | −4.0 | −12.6 to 4.6 | 0.333 | 70.6 | 25.5 | −7.6 | −18.0 to 2.9 | 0.140 | 73.2 | 31.9 | −4.3 | −16.2 to 7.7 | 0.444 | 67.9 | 24.5 | −7.7 | −22.3 to 6.8 | 0.256 | 0.954 |
| Shoulder add. L | 73.3 | 31.9 | 69.9 | 27.8 | −3.4 | −8.6 to 1.8 | 0.180 | 69.3 | 22.6 | −4.0 | −13.0 to 5.0 | 0.348 | 69.5 | 28.3 | −3.3 | −13.7 to 7.0 | 0.490 | 64.1 | 18.6 | −5.4 | −21.1 to 10.2 | 0.446 | 0.959 |
| Elbow ext. R | 44.6 | 19.4 | 44.3 | 19.6 | −0.3 | −3.6 to 3.1 | 0.864 | 43.8 | 17.2 | −0.7 | −4.7 to 3.2 | 0.693 | 43.6 | 18.8 | 0.2 | −3.5 to 4.0 | 0.892 | 40.3 | 13.0 | −2.4 | −10.2 to 5.4 | 0.500 | 0.986 |
| Elbow ext. L | 46.3 | 19.2 | 45.5 | 21.1 | −0.8 | −3.1 to 1.5 | 0.470 | 44.9 | 13.9 | −1.4 | −6.3 to 3.6 | 0.552 | 44.5 | 18.2 | −0.5 | −5.8 to 4.9 | 0.853 | 43.5 | 14.5 | 0.2 | −5.1 to 5.5 | 0.937 | 0.998 |
| Elbow flex. R | 37.2 | 20.8 | 36.2 | 20.1 | −1.0 | −2.7 to 0.8 | 0.249 | 36.0 | 17.0 | −1.1 | −4.5 to 2.2 | 0.470 | 32.8 | 18.3 | −4.1 | −11.3 to 3.1 | 0.230 | 32.8 | 16.2 | −2.1 | −6.6 to 2.3 | 0.298 | 0.971 |
| Elbow flex. L | 32.4 | 18.6 | 34.6 | 18.2 | 2.2 | −1.0 to 5.4 | 0.159 | 31.8 | 15.2 | −0.6 | −4.6 to 3.4 | 0.742 | 31.0 | 16.5 | −0.9 | −4.9 to 3.1 | 0.623 | 29.3 | 11.4 | 0.7 | −4.3 to 5.7 | 0.752 | 0.964 |
| Hand grip R(kg) | 36.5 | 9.6 | 34.9 | 9.5 | −1.6 | −4.5 to 1.2 | 0.233 | 34.7 | 12.2 | −1.8 | −5.8 to 2.1 | 0.331 | 36.2 | 12.7 | −0.3 | −3.5 to 2.8 | 0.814 | 35.4 | 11.5 | −0.9 | −3.2 to 1.4 | 0.401 | 0.993 |
| MRC−SS (/80) | 80.0 | 0.0 | 80.0 | 0.0 | 0.0 | 0.0 to 0.0 | 1.000 | 80.0 | 0.0 | 0.0 | 0.0 to 0.0 | 1.000 | 80.0 | 0.0 | 0.0 | 0.0 to 0.0 | 1.000 | 80.0 | 0.0 | 0.0 | 0.0 to 0.0 | 1.000 | 1.000 |
| 6MWD (m) | 661.3 | 81.8 | 688.2 | 78.7 | 16.0 | 0.5 to 31.5 | 0.045 | 670.4 | 88.8 | 9.2 | −9.2 to 27.5 | 0.295 | 680.2 | 82.9 | 17.7 | 3.5 to 32.0 | 0.020 | 694.6 | 91.0 | 23.3 | −0.4 to 47.1 | 0.054 | 0.896 |
| 10MWT (s) | 4.2 | 1.4 | 4.7 | 1.4 | 0.5 | −0.4 to 1.3 | 0.242 | 4.4 | 1.2 | 0.2 | −0.5 to 0.9 | 0.486 | 4.5 | 1.1 | 0.3 | −0.6 to 1.1 | 0.502 | 4.2 | 0.9 | 0.2 | −0.7 to 1.1 | 0.702 | 0.867 |
| TUG (s) | 1.8 | 0.3 | 1.8 | 0.5 | 0.0 | −0.2 to 0.2 | 0.927 | 1.9 | 0.9 | 0.2 | −0.4 to 0.7 | 0.540 | 2.1 | 1.0 | 0.2 | −0.3 to 0.8 | 0.357 | 1.8 | 0.5 | 0.0 | −0.3 to 0.3 | 1.000 | 0.799 |
Biodex® measurements in Newton meters (Nm); SD, standard deviation; V, visit; V1, baseline visit (0 months); 95%CI, 95% confidence interval; p, p-value; ANOVA, analysis of variance; L, left; R, right; ext., extensors; flex., flexors; abd., abductors; add., adductors; hand grip, hand grip test (in kilograms); MRC-SS, Medical Research Council sum score (on a maximum of 80 points); 6MWD, 6 min walk distance (in meter); 10MWT, 10 m walk test (in seconds); TUG, timed up-and-go test (in seconds). Significant values are underlined and indicated in bold.
The mean isometric muscle strength in the knee flexors on the right side showed a significant decrease after 12 months (p < 0.034) and 24 months (p < 0.020), but this effect was not measured at 18 months and was not present at the left side (p > 0.05). No significant decrease in the mean isometric muscle strength was measured for the study duration of 24 months in any of the other muscle groups (hip extensors, hip flexors, shoulder abductors and adductors and elbow extensors and flexors) using the Biodex® dynamometer (p > 0.05). In comparison to the patient group, there were no significant differences between the mean isometric muscle strength in the different muscle groups measured at the left and right side in controls (p > 0.05).
In contrast to LOPD patients, in controls, the mean 6MWD significantly increased after 6 and 18 months, from 661.3 m at baseline to 688.2 m after 6 months (p < 0.045), to 680.2 m after 18 months (p < 0.020), to 694.6 m after 24 months, which just failed to reach significance (p = 0.054), corresponding to a mean amelioration of 33.3 m over 2 years (Table 3; Figure 1C). This increase in the 6MWD in control individuals is probably due to a training effect. Similarly to the patient group, the MRC-SS, hand grip test, 10MWT and TUG did not show a significant change during the study (Table 3; p > 0.05). There were no significant differences between the means at the different visits (V1–V5) for each of the outcome measures in controls (ANOVA, p > 0.05; Table 3).
4. Discussion
Our study showed that the 6MWD is a useful outcome measure to detect motor decline in treated LOPD patients. In contrast, quantitative isometric strength measurement using a Biodex® dynamometer, MRC-SS, hand grip strength, 10MWT and TUG proved not to be suitable outcome measure in this group of patients for a study duration of 2 years.
The 6 min walk distance (6MWD) was originally developed in 2002 as an integrated assessment of pulmonary, cardiac, circulatory and muscular capacity in patients with moderate to severe lung disease and provides a measure of the functional exercise level needed to perform daily physical activities [28]. Since then, several studies have also used the 6MWD in neuromuscular diseases as an endpoint to assess muscular function during disease progression or treatment, such as in Duchenne muscular dystrophy [29,30], hereditary inclusion body myopathy [26], spinal muscular atrophy [31] or metabolic myopathies including LOPD [8,11,12,15,24,32].
In the LOPD patients in our study, the mean baseline 6MWD was 452 m ± 143, whereas other studies reported lower baseline 6MWDs from 246 to 376 m [8,9,11,12,15,32]. These lower values can be explained by differences in age, disease duration or treatment duration at study inclusion. For comparison, the mean baseline 6MWD in our healthy age- and gender-matched controls aged 23–64 years (mean 51 years) was 661 m ± 82, similar to 571 m ± 90 in another study in healthy adult controls aged 40–80 years (median 58 years) that also showed significantly shorter distances in controls above 60 years [33].
After 2 years of treatment, 6MWD significantly declined in LOPD patients with a mean distance of 84 m. A deterioration of 6MWD in treated LOPD patients has been shown in other studies as well after 2–3 years of treatment following an initial improvement [15,21,23,34]. In contrast to these studies, the baseline in our study did not correspond to the start of treatment but represented a mean treatment duration of 8.8 years. Therefore, the mean decline in 6MWD in our study cannot be compared directly with the other reports. Moreover, since the 6MWD at the time point of start of treatment in our LOPD patient group is not known, the 6MWD at 2-year follow up might still be higher than the initial value at start of treatment, similarly to the findings in other studies [15,21,23,34]. In untreated LOPD patients, the 6MWD has been shown to be lower than in treated patients [11]. In our study, we did not include a group of untreated LOPD patients, since this comparison was not the objective of the study, and not treating LOPD patients when a treatment is available would not be acceptable from an ethical point of view.
A change in the mean distance in 6MWD in patients of 83.8 m (representing about 18% of the initial mean value of 451.9 m) represents a clinically meaningful change according to literature data [35]. In controls, a mean change of 33.3 m (which ranged from 3.5 to 32 m at 18 months) over an initial value of 661.3 m represents a 5.0% change (in line with reported SEM% for 6MWD), which does not reach the clinically meaningful change in the 6MWD as established by the literature and as expected in controls. Since the MDC value in meters is expected to change in relation to the condition of patients versus controls, our data in controls suggest that a variation in the 6MWD higher than 5% may be considered, even in patients, as the minimal detectable change and it is likely to reflect a true change rather than a measurement error, while variations < 5% may be measurement errors/training effects and so on. Indeed, the patients show a change in the 6MWD much higher than 5%, even when other motor measures are stable, reflecting the sensibility of this measure and its ability to detect little changes.
The 6MWD is easy to perform, quick and inexpensive, but can only be used in ambulatory patients and depends on motivation, age, sex, height, weight and skeletal problems, which can influence gait and thereby affect the distance walked. However, the 6MWD will usually not be the only endpoint in clinical trials, and in LOPD patients, parameters for respiratory function such as forced vital capacity (FVC) will also be included, as well as patient-reported outcome measures (PROMs), which are becoming more and more important, such as the Rasch-built Pompe-specific Activity (R-Pact) scale, measuring daily-life activities, with a proven positive correlation with physical outcomes and developed specifically for LOPD [36,37].
In contrast to the 6MWD, which we would recommend using as an endpoint in clinical trials in LOPD based on our own data and others, we showed that isometric strength measurement using a Biodex® dynamometer was not a suitable outcome measure in LOPD patients for the study duration of two years. However, we cannot exclude the idea that the muscle strength measurement using Biodex® might be a good outcome measure when a longer observation period would be considered. In most of the tested muscle groups, we did not find a significant change over the study duration of 24 months. Only in the knee extensors was there a significant consistent and symmetrical decline in muscle strength measured over 2 years. In comparison to the literature data, the knee extensors in LOPD patients are better preserved with longer disease duration compared to, e.g., the hip extensors or knee flexors, which are already affected early in the disease course [38]. This can also be seen in our results in LOPD patients in Table 2: at the start of the study, the highest muscle strength can be measured in the knee extensors compared to all other tested muscle groups. The fact that muscle strength in the knee extensors is clearly higher to start with at the onset of the study might explain why, especially in those muscles, a significant decline can still be detected over the duration of the study. However, not only in the patients but also in the controls, a decrease in muscle strength in the knee extensors was measured, which might be related to aging and/or other error sources such as the lack of motivation, the selection of controls, a biased examiner since the assessments were of course not blinded, etc.; this did not influence the functional capacity in the 6MWD, which increased in the controls over the 2 years of study duration. The decreased muscle strength in patients might be explained by disease progression, but the factor of aging might also partially contribute to the decline in isometric strength measurement. Thus, since the muscle strength of the knee extensors using the Biodex® dynamometer not only decreased in patients but also significantly decreased in healthy controls even though their 6MWD increased during the study duration of 2 years, we concluded that these Biodex® measurements are not applicable as a reliable and functionally relevant outcome measure in clinical trials in LOPD patients.
A few other studies in neuromuscular disorders have measured muscle strength using a Biodex® dynamometer; however, most of them were cross-sectional [25,26], in contrast to our longitudinal study design. One study concerning only four treated LOPD patients performed strength measurements using Biodex® and concluded there was a small increase in muscle strength after 2–6 years follow up [18].
We showed that in our study group of LOPD patients, there was no progressive respiratory insufficiency for the duration of the study, both in the sitting and in the supine position (p > 0.05). Therefore, the decline in the 6MWD cannot be explained by changes in respiratory function in our patient group. However, considering that the parameter that deteriorates is the 6MWD and not muscle strength, it is conceivable that the aspect involved might be functional endurance, corresponding to other recent studies [39]. However, it might also still be possible that the 6MWT is more capable of detecting small declines in distinct functions, taken together (endurance, strength, posture, respiration), that perhaps single outcome measures (FVC alone, and so on) cannot catch.
Finally, if the TUG, 10MWT, MRC-SS, dynamometry (for most muscles) did not change over a 2-year period but only the 6MWD declined, it might also be argued that the disease is quite stable over the years with ERT, after a mean ERT treatment duration of 8.8 years (range 0.5–13 years), with the exception of distance walked on the 6MWD. It is noteworthy that the MRC score has an intrinsically low reliability due to substantial inter-rater and intra-rater variability. Furthermore, the TUG and 10MWT are also timed tests like 6MWD, but in these short-timed measures, there is a certain degree of error and less reliability due to the short duration of the tests. A correlation with a disease-specific patient-reported outcome measure (PROM), such as the Rasch-built Pompe-specific Activity (R-Pact) scale, might also have helped to identify a real decline from the patient’s perspective [36,37].
We conclude that the 6MWD is a useful outcome measure to detect motor changes in treated ambulatory late-onset Pompe disease patients and should be included as an endpoint in clinical trials in LOPD. Further studies are needed to also analyze the proper outcome measure for non-ambulatory LOPD patients.
Acknowledgments
We thank the patients and control individuals for their participation in the study. We are grateful to Heleen Adams of the Department of Physical Medicine and Rehabilitation at University Hospitals Leuven for the technical introduction to the Biodex® system. K.G.C. is Chairholder of the Emil von Behring Chair for Neuromuscular and Neurodegenerative Disorders by CSL Behring. K.G.C. is a member of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO-NMD) and of the European Reference Network for Rare Neurological Diseases (ERN-RND).
Author Contributions
Conceptualization, K.G.C. and C.E.D.; Methodology, K.G.C., K.P. and C.E.D.; Software, not applicable; Validation, K.G.C., A.D. and C.E.D.; Formal Analysis, K.G.C., L.F. and C.E.D.; Investigation, K.G.C., A.D. and C.E.D.; Resources, K.G.C. and K.P.; Data Curation, K.G.C., A.D., L.F. and C.E.D.; Writing—Original Draft Preparation, K.G.C., L.F. and C.E.D.; Writing—Review and Editing, K.G.C., A.D., L.F., K.P. and C.E.D.; Visualization, K.G.C., L.F. and C.E.D.; Supervision, K.G.C. and C.E.D.; Project Administration, K.G.C.; Funding Acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive funding for this project.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethical Committee Research of UZ/KU Leuven (S-60965; date of approval: 20 December 2017).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results can be obtained from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schoser B. Pompe disease: What are we missing? Ann. Transl. Med. 2019;7:1–7. doi: 10.21037/atm.2019.05.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toscano A., Rodolico C., Musumeci O. Multisystem late onset Pompe disease (LOPD): An update on clinical aspects. Ann. Transl. Med. 2019;7:284. doi: 10.21037/atm.2019.07.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahn A., Schänzer A. Long-term outcome and unmet needs in infantile-onset Pompe disease. Ann. Transl. Med. 2019;7:1–10. doi: 10.21037/atm.2019.04.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van der Ploeg A.T., Kruijshaar M.E., Toscano A., Laforêt P., Angelini C., Lachmann R.H., Pascual S.I., Roberts M., Rösler K., Stulnig T., et al. European consensus for starting and stopping enzyme replacement therapy in adult patients with Pompe disease: A 10-year experience. Eur. J. Neurol. 2017;24:768.e31. doi: 10.1111/ene.13285. [DOI] [PubMed] [Google Scholar]
- 5.Angelini C., Semplicini C., Tonin P., Filosto M., Pegoraro E., Sorarù G., Fanin M. Progress in Enzyme Replacement Therapy in Glycogen Storage Disease Type II. Ther. Adv. Neurol. Disord. 2009;2:143–153. doi: 10.1177/1756285609103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merk T., Wibmer T., Schumann C., Krüger S. Glycogen storage disease type II (Pompe disease)—influence of enzyme replacement therapy in adults. Eur. J. Neurol. 2009;16:274–277. doi: 10.1111/j.1468-1331.2008.02377.x. [DOI] [PubMed] [Google Scholar]
- 7.Bembi B., Pisa F.E., Confalonieri M., Ciana G., Fiumara A., Parini R., Rigoldi M., Moglia A., Costa A., Carlucci A., et al. Long-term observational, non-randomized study of enzyme replacement therapy in late-onset glycogenosis type II. J. Inherit. Metab. Dis. 2010;33:727–735. doi: 10.1007/s10545-010-9201-8. [DOI] [PubMed] [Google Scholar]
- 8.Ravaglia S., Pichiecchio A., Ponzio M., Danesino C., Saeidi Garaghani K., Poloni G.U., Toscano A., Moglia A., Carlucci A., Bini P., et al. Changes in skeletal muscle qualities during enzyme replacement therapy in late-onset type II glycogenosis: Temporal and spatial pattern of mass vs. strength response. J. Inherit. Metab. Dis. 2010;33:737–745. doi: 10.1007/s10545-010-9204-5. [DOI] [PubMed] [Google Scholar]
- 9.Strothotte S., Strigl-Pill N., Grunert B., Kornblum C., Eger K., Wessig C., Deschauer M., Breunig F., Glocker F.X., Vielhaber S., et al. Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J. Neurol. 2010;257:91–97. doi: 10.1007/s00415-009-5275-3. [DOI] [PubMed] [Google Scholar]
- 10.Van Capelle C.I., van der Beek N.A., Hagemans M.L., Arts W.F., Hop W.C., Lee P., Jaeken J., Frohn-Mulder I.M., Merkus P.J., Corzo D., et al. Effect of enzyme therapy in juvenile patients with Pompe disease: A three-year open-label study. Neuromuscul. Disord. 2010;20:775–782. doi: 10.1016/j.nmd.2010.07.277. [DOI] [PubMed] [Google Scholar]
- 11.Van der Ploeg A.T., Clemens P.R., Corzo D., Escolar D.M., Florence J., Groeneveld G.J., Herson S., Kishnani P.S., Laforet P., Lake S.L., et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N. Engl. J. Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 12.Angelini C., Semplicini C., Ravaglia S., Bembi B., Servidei S., Pegoraro E., Moggio M., Filosto M., Sette E., Crescimanno G., et al. Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years. J. Neurol. 2012;259:952–958. doi: 10.1007/s00415-011-6293-5. [DOI] [PubMed] [Google Scholar]
- 13.Ishigaki K., Murakami T., Nakanishi T., Oda E., Sato T., Osawa M. Close monitoring of initial enzyme replacement therapy in a patient with childhood-onset Pompe disease. Brain Dev. 2012;34:98–102. doi: 10.1016/j.braindev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Ravaglia S., De Filippi P., Pichiecchio A., Ponzio M., Saeidi Garaghani K., Poloni G.U., Bini P., Danesino C. Can genes influencing muscle function affect the therapeutic response to enzyme replacement therapy (ERT) in late-onset type II glycogenosis? Mol. Genet. Metab. 2012;107:104–110. doi: 10.1016/j.ymgme.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Regnery C., Kornblum C., Hanisch F., Vielhaber S., Strigl-Pill N., Grunert B., Müller-Felber W., Glocker F.X., Spranger M., Deschauer M., et al. 36 months observational clinical study of 38 adult Pompe disease patients under alglucosidase alfa enzyme replacement therapy. J. Inherit. Metab. Dis. 2012;35:837–845. doi: 10.1007/s10545-012-9451-8. [DOI] [PubMed] [Google Scholar]
- 16.Van der Ploeg A.T., Barohn R., Carlson L., Charrow J., Clemens P.R., Hopkin R.J., Kishnani P.S., Laforêt P., Morgan C., Nations S., et al. Open-label extension study following the Late-Onset Treatment Study (LOTS) of alglucosidase alfa. Mol. Genet. Metab. 2012;107:456–461. doi: 10.1016/j.ymgme.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Anderson L.J., Henley W., Wyatt K.M., Nikolaou V., Waldek S., Hughes D.A., Lachmann R.H., Logan S. Effectiveness of enzyme replacement therapy in adults with late-onset Pompe disease: Results from the NCS-LSD cohort study. J. Inherit. Metab. Dis. 2014;37:945–952. doi: 10.1007/s10545-014-9728-1. [DOI] [PubMed] [Google Scholar]
- 18.Andreassen C.S., Schlütter J.M., Vissing J., Andersen H. Effect of enzyme replacement therapy on isokinetic strength for all major muscle groups in four patients with Pompe disease—A long-term follow-up. Mol. Genet. Metab. 2014;112:40–43. doi: 10.1016/j.ymgme.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Montagnese F., Barca E., Musumeci O., Mondello S., Migliorato A., Ciranni A., Rodolico C., De Filippi P., Danesino C., Toscano A. Clinical and molecular aspects of 30 patients with late-onset Pompe disease (LOPD): Unusual features and response to treatment. J. Neurol. 2015;262:968–978. doi: 10.1007/s00415-015-7664-0. [DOI] [PubMed] [Google Scholar]
- 20.Van der Ploeg A., Carlier P.G., Carlier R.Y., Kissel J.T., Schoser B., Wenninger S., Pestronk A., Barohn R.J., Dimachkie M.M., Goker-Alpan O., et al. Prospective exploratory muscle biopsy, imaging, and functional assessment in patients with late-onset Pompe disease treated with alglucosidase alfa: The EMBASSY Study. Mol. Genet. Metab. 2016;119:115–123. doi: 10.1016/j.ymgme.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Kuperus E., Kruijshaar M.E., Wens S.C.A., de Vries J.M., Favejee M.M., van der Meijden J.C., Rizopoulos D., Brusse E., van Doorn P.A., van der Ploeg A.T., et al. Long-term benefit of enzyme replacement therapy in Pompe disease: A 5-year prospective study. Neurology. 2017;89:2365–2373. doi: 10.1212/WNL.0000000000004711. [DOI] [PubMed] [Google Scholar]
- 22.Witkowski G., Konopko M., Rola R., Ługowska A., Ryglewicz D., Sienkiewicz-Jarosz H. Enzymatic replacement therapy in patients with late-onset Pompe disease—6-Year follow up. Neurol. Neurochir. Pol. 2018;52:465–469. doi: 10.1016/j.pjnns.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Semplicini C., De Antonio M., Taouagh N., Béhin A., Bouhour F., Echaniz-Laguna A., Magot A., Nadaj-Pakleza A., Orlikowski D., Sacconi S., et al. Long-term benefit of enzyme replacement therapy with alglucosidase alfa in adults with Pompe disease: Prospective analysis from the French Pompe Registry. J. Inherit. Metab. Dis. 2020;43:1219–1231. doi: 10.1002/jimd.12272. [DOI] [PubMed] [Google Scholar]
- 24.Vanherpe P., Fieuws S., D’Hondt A., Bleyenheuft C., Demaerel P., De Bleecker J., Van den Bergh P., Baets J., Remiche G., Verhoeven K., et al. Late-onset Pompe disease (LOPD) in Belgium: Clinical characteristics and outcome measures. Orphanet J. Rare Dis. 2020;15:83. doi: 10.1186/s13023-020-01353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckon C., Sienko S., Bagley A., Sison-Williamson M., Fowler E., Staudt L., Heberer K., McDonald C.M., Sussman M. Can Quantitative Muscle Strength and Functional Motor Ability Differentiate the Influence of Age and Corticosteroids in Ambulatory Boys with Duchenne Muscular Dystrophy? PLoS Curr. 2016;8 doi: 10.1371/currents.md.1ced64dff945f8958221fddcd4ee60b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plewa J., Surampalli A., Wencel M., Milad M., Donkervoort S., Caiozzo V.J., Goyal N., Mozaffar T., Kimonis V. A cross-sectional analysis of clinical evaluation in 35 individuals with mutations of the valosin-containing protein gene. Neuromuscul. Disord. 2018;28:778–786. doi: 10.1016/j.nmd.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoonjans F., Zalata A., Depuydt C.E., Comhaire F.H. MedCalc: A new computer program for medical statistics. Comput. Methods Programs Biomed. 1995;48:257–262. doi: 10.1016/0169-2607(95)01703-8. [DOI] [PubMed] [Google Scholar]
- 28.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: Guidelines for the 6-min walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 29.Goemans N.M., Tulinius M., van den Akker J.T., Burm B.E., Ekhart P.F., Heuvelmans N., Holling T., Janson A.A., Platenburg G.J., Sipkens J.A., et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N. Engl. J. Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 30.McDonald C.M., Campbell C., Torricelli R.E., Finkel R.S., Flanigan K.M., Goemans N., Heydemann P., Kaminska A., Kirschner J., Muntoni F., et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1489–1498. doi: 10.1016/S0140-6736(17)31611-2. [DOI] [PubMed] [Google Scholar]
- 31.De Wel B., Goosens V., Sobota A., Van Camp E., Geukens E., Van Kerschaver G., Jagut M., Claes K., Claeys K.G. Nusinersen treatment significantly improves hand grip strength, hand motor function and MRC sum scores in adult patients with spinal muscular atrophy types 3 and 4. J. Neurol. 2021;268:923–935. doi: 10.1007/s00415-020-10223-9. [DOI] [PubMed] [Google Scholar]
- 32.Wokke J.H., Escolar D.M., Pestronk A., Jaffe K.M., Carter G.T., van den Berg L.H., Florence J.M., Mayhew J., Skrinar A., Corzo D., et al. Clinical features of late-onset Pompe disease: A prospective cohort study. Muscle Nerve. 2008;38:1236–1245. doi: 10.1002/mus.21025. [DOI] [PubMed] [Google Scholar]
- 33.Casanova C., Celli B.R., Barria P., Casas A., Cote C., de Torres J.P., Jardim J., Lopez M.V., Marin J.M., de Oca M., et al. Six Minute Walk Distance Project (ALAT). The 6-min walk distance in healthy subjects: Reference standards from seven countries. Eur. Respir. J. 2011;37:150–156. doi: 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 34.Harlaar L., Hogrel J.Y., Perniconi B., Kruijshaar M.E., Rizopoulos D., Taouagh N., Canal A., Brusse E., van Doorn P.A., van der Ploeg A.T., et al. Large variation in effects during 10 years of enzyme therapy in adults with Pompe disease. Neurology. 2019;93:e1756–e1767. doi: 10.1212/WNL.0000000000008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohannon R.W., Crouch R. Minimal clinically important difference for change in 6-min walk test distance of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2017;23:377–381. doi: 10.1111/jep.12629. [DOI] [PubMed] [Google Scholar]
- 36.Van der Beek N.A., Hagemans M.L., van der Ploeg A.T., van Doorn P.A., Merkies I.S. The Rasch-built Pompe-specific activity (R-PAct) scale. Neuromuscul. Disord. 2013;23:256–264. doi: 10.1016/j.nmd.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Yuan M., Andrinopoulou E.R., Kruijshaar M.E., Lika A., Harlaar L., van der Ploeg A.T., Rizopoulos D., van der Beek N.A.M.E. Positive association between physical outcomes and patient-reported outcomes in late-onset Pompe disease: A cross sectional study. Orphanet J. Rare Dis. 2020;15:232. doi: 10.1186/s13023-020-01469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Beek N.A., de Vries J.M., Hagemans M.L., Hop W.C., Kroos M.A., Wokke J.H., de Visser M., van Engelen B.G., Kuks J.B., van der Kooi A.J., et al. Clinical features and predictors for disease natural progression in adults with Pompe disease: A nationwide prospective observational study. Orphanet J. Rare Dis. 2012;7:88. doi: 10.1186/1750-1172-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diaz-Manera J., Kishnani P.S., Kushlaf H., Ladha S., Mozaffar T., Straub V., Toscano A., van der Ploeg A.T., Berger K.I., Clemens P.R., et al. Safety and efficacy of avalglucosidase alfa versus alglucosidase alfa in patients with late-onset Pompe disease (COMET): A phase 3, randomised, multicentre trial. Lancet Neurol. 2021;20:1012–1026. doi: 10.1016/S1474-4422(21)00241-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting reported results can be obtained from the corresponding author on reasonable request.