Abstract
Simple Summary
Bone marrow disorders such as leukemia and myelodysplastic syndromes are characterized by abnormal healthy blood cells production and function. Uncontrolled growth and impaired differentiation of white blood cells hinder the correct development of healthy cells in the bone marrow. One of the most frequent alterations that appear to initiate this deregulation and persist in leukemia patients are mutations in epigenetic regulators such as TET2. This review summarizes the latest molecular findings regarding TET2 functions in hematopoietic cells and their potential implications in blood cancer origin and evolution. Our goal was to encompass and interlink up-to-date discoveries of the convoluted TET2 functional network to provide a more precise overview of the leukemic burden of this protein.
Abstract
Cytosine methylation (5mC) of CpG is the major epigenetic modification of mammalian DNA, playing essential roles during development and cancer. Although DNA methylation is generally associated with transcriptional repression, its role in gene regulation during cell fate decisions remains poorly understood. DNA demethylation can be either passive or active when initiated by TET dioxygenases. During active demethylation, transcription factors (TFs) recruit TET enzymes (TET1, 2, and 3) to specific gene regulatory regions to first catalyze the oxidation of 5mC to 5-hydroxymethylcytosine (5hmC) and subsequently to higher oxidized cytosine derivatives. Only TET2 is frequently mutated in the hematopoietic system from the three TET family members. These mutations initially lead to the hematopoietic stem cells (HSCs) compartment expansion, eventually evolving to give rise to a wide range of blood malignancies. This review focuses on recent advances in characterizing the main TET2-mediated molecular mechanisms that activate aberrant transcriptional programs in blood cancer onset and development. In addition, we discuss some of the key outstanding questions in the field.
Keywords: DNA methylation, TET2, chromatin, transcription factors, gene regulation, blood malignancies
1. Introduction
CpG methylation is the most common DNA modification found in the mammalian genome [1], playing an essential role in development and cancer [2,3]. DNA demethylation can be either passive, by dilution of DNA methylation after each cell division, or active when initiated by Tet dioxygenases [4]. During active demethylation, the Ten-eleven-translocation (TET) family of enzymes first catalyze the iterative oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) and subsequently to higher oxidized derivatives, 5-formylcytosine (5fC) and 5-carboxycytosine (5CaC) [5,6,7]. These higher oxidized forms of cytosine, in turn, can either be lost during replication or enzymatically removed, restoring unmodified cytosine and alleviating transcriptional repression typically associated with 5mC residues [4]. However, 5hmC and higher oxidized derivatives should be considered not only as mere transient states in the DNA demethylation path but as bona fide epigenetic marks as illustrated by their complex network of specialized readers [8].
In the context of the hematopoietic system, TET family members have been involved in naturally occurring and experimentally induced cell fate decisions [9,10,11,12,13,14]. Among the TETs, TET2 is the most broadly expressed and frequently mutated gene in blood malignancies [15,16,17,18,19,20]. TET2 mutational profiles show inactivating mutations occurring along the whole gene coding region and are not only restricted at the 3′ region where the catalytic domain is located [15,17,21]. Therefore, TET2 epigenetic regulation during blood differentiation might be mediated not only by its catalytic activity but also by its association with critical partners [22,23].
Here we review the current understanding of TET2 functions in normal and malignant hematopoiesis, providing an extensive overview of the intricate molecular mechanisms controlling gene expression, protein stability and function, and enzyme’s genome recruitment.
2. Mechanisms of TET2 Protein/Enzymatic Regulation
Several mechanisms regulating TET2 expression and activity have been elucidated in the last decade. Here we summarize main control systems, encompassing basal post/transcriptional regulation, direct protein modulation through post-translation modifications (PTMs), and enzymatic substrate availability.
2.1. Transcriptional Regulation
Some transcriptional factors have been defined as direct regulators of TETs’ gene expression. In mouse embryonic stem cells (ESCs), Tet1 and Tet2 are positively regulated by the pluripotency TF Oct4 that binds to conserved non-coding sequences in both genes [24]. Accordingly, upon ESC differentiation, Tet1 and Tet2 levels decrease due to Oct4 depletion [24].
A similar regulatory mechanism was described for the CXXC-DNA binding domain protein Rinf (CXXC5), whose depletion leads to decreased Tet1 and Tet2 expression [25]. During myeloid cell fate commitment, Tet2 expression is boosted by the action of the myeloid transcription factor CEBPα, which binds to Tet2 enhancer regions [26]. CEBPα might exert its transcriptional control by recruiting Tet2 protein itself to Tet2 gene’s distal regulatory regions leading to their demethylation and activation [10]. Of note, a mutant form of CEBPα (Brm2), recapitulating naturally occurring mutations in AML patients [27], failed to demethylate the Tet2 enhancers [10]. In addition, histone deacetylase 4 (HDAC4) protein has been recently described as a positive regulator of TET2 expression in the context of MDS and AML [28]. Finally, in regulatory T cells (Treg), Tet1 and Tet2 are regulated in response to hydrogen sulfide (H2S) through the action of the sulfhydrating nuclear transcription factor Y subunit beta (NFYB) [29].
2.2. Post-Transcriptional Regulation
miRNAs regulatory networks targeting TET2 mRNAs have been proposed as the primary post-transcriptionally regulatory mechanism during blood differentiation and in myeloid malignancies. High-throughput screens identified a large subset of TET2 3′UTR targeting miRNAs with different efficiencies. Induced expression of those led to an array of leukemic traits such as myeloid lineage bias, phenocopying to an extent, a direct TET2 loss [30]. In a related study, miR-22 (also targeting TET2 mRNA) was detected overexpressed in MDS patients. Mechanistically, miR-22 overexpression leads to reduced genome-wide levels of 5hmC, increased self-renewal, and myeloid skewing [31]. Also, in MDS, miR-9 and miR-34a indirectly control TET2 by post-transcriptionally regulating SIRT1 levels, which affect the TET2 protein function at a post-translational level (See Section 2.3) [32].
2.3. Post-Translational Regulation
Although TET2 protein levels are mainly regulated via transcriptional mechanisms, TET2 post-translational modifications might be involved in rapidly fine-tuning protein levels in response to external cues.
During ESC differentiation, the CXXC-DNA binding domain protein IDAX (CXXC4) recruits TET2 to DNA, activating caspases that cleave the TET2-IDAX complex leading to TET2 protein depletion [33]. Similarly, TET proteins have been described as direct substrates of the calpain family of proteases [34]. Calpain1 regulates the degradation of Tet1 and Tet2 in mouse pluripotent ESCs and calpain2 of Tet3 during ESCs neural differentiation. These negative regulatory mechanisms ensure correct global 5hmC level maintenance and expression of lineage-specific genes in ESCs [34].
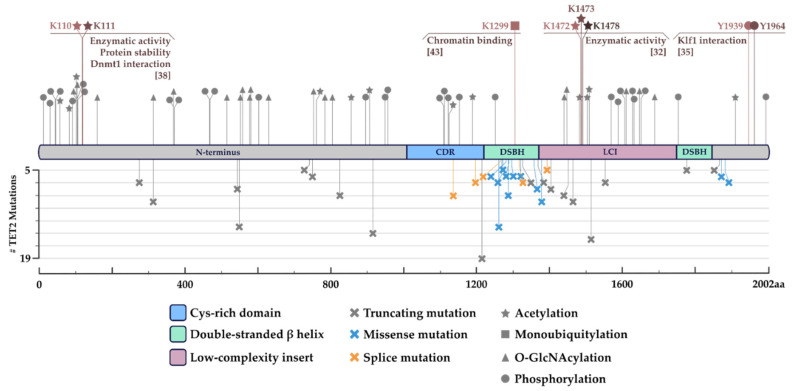
In addition, TET2 protein can be largely post-translationally modified (PTM) by (de)phosphorylation, (de)acetylation, O-GlcNAcylation, or ubiquitylation, among others [32,35,36,37,38] (Figure 1). The specific output driven by particular PTMs on TET2 activity is cell type and amino acid residue-specific. For instance, cytokine receptor-associated JAK2 (in response to FLT3 or EPO/SCF signaling in blood progenitor cells) phosphorylates TET2 at tyrosine residues 1939 and 1964, leading to enhanced enzymatic activity [35] (Figure 1). However, TET2 tyrosine residues 1939 and 1964 are not the only phosphorylatable residues since the whole N-terminus region of the protein (constituting a large disordered domain) is usually highly phosphorylated in ESCs [36] (Figure 1). Interestingly, the O-linked N-acetylglucosaminyltransferase (OGT), a strong TET interactor [36,39,40,41,42], adds O-GlcNAcylation groups to serine and threonine residues of TET2, thereby reducing the number of available phosphorylation sites and their site occupancy [36]. Once again, the described phosphorylation vs. O-GlcNAcylation mechanism highlights the fine-tuning TET proteins might undergo for correct localization and activity according to external signals [36].
Figure 1.
Schematic representation of TET2 post-translational modifications (PTMs) and most frequently found TET2 mutations. In the upper half, compiled PTM data based on mass spectrometry (MS) and in silico predictions [35,36,38,43,44]. In grey, proposed modifications with an undefined function. In color, fully characterized PTMs and their impact on TET2 activity. In the bottom half, a compilation of TET2′s most frequent mutations and their type (truncating, missense, or splice mutations). Filtering was done from combined 12,845 samples from 35 studies where the mutation was found in >5 different patients. Data from ‘Myeloid’ dataset from cBioPortal (https://www.cbioportal.org/, accessed on 19 January 2022) [45,46].
TET2 activity can also be regulated through protein (de)acetylases. This is the case of the NAD-dependent deacetylase sirtuin-1 (SIRT1) that removes acetylation at TET2 specific lysine residues K1472, K1473, and K1478, increasing protein’s enzymatic activity [32]. Consequently, reduced SIRT1 activity in human HSPCs leads to the onset of an MDS-like disease recapitulating the phenotype observed in TET2-mutated MDS patients [32]. Whereas TET2 global deacetylation mediated by histone deacetylases, 1 and 2 (HDAC1 and 2) leads to reduced enzymatic activity triggering the emergence of abnormal DNA methylation profiles typically associated with cancer [38]. Zhang and co-workers also studied the effects on TET2 stability/activity mediated by histone acetyltransferase p300 (enzymatic counterpart of the HDAC1/2 enzymes). p300 was shown to acetylate the TET2 N-terminus region leading to increased protein activity, stability, and partnering with other proteins such as DNMT1 [38]. TET2/DNMT1 complex might prevent abnormal promoter methylation typically observed upon exposure to oxidative stress [38].
Finally, ubiquitylation of TET2 has been described to regulate its chromatin association [43]. CRL4 (VprBP) E3 ligase, a member of the ubiquitin ligase complex, has been shown to interact with the cysteine-rich domain of TET2 and promote K1299 monoubiquitylation, enhancing its chromatin association [43]. On the contrary, USP15-dependent K1299 deubiquitylation leads to decreased TET2 activity [44].
2.4. Enzymatic Regulation
Regarding catalytic activity, TET enzymes are Fe2+/α-KG-dependent dioxygenases. Metabolite and cofactor availability constitute another relevant layer of protein regulation potentially influencing hematopoiesis and leukemic development. Interestingly, experimentally-induced ascorbate (VitC) depletion leads to increased HSC function and compartment expansion, resembling the aberrant self-renewal phenotype typically observed upon Tet2 depletion in HSCs [47,48,49]. In addition, Cimmino and co-workers elegantly demonstrated the potential of VitC treatment to rescue an aberrant self-renewal phenotype initiated upon Tet2 in vivo depletion [50]. On the contrary, 2-Hydroxyglutarate (2-HG), an oncometabolite produced in IDH1/2 mutated patient cells, competitively inhibits TET2 catalytic activity [50,51], resulting in genome-wide DNA hypermethylation and impaired myeloid differentiation [52]. Similarly, mutations in other metabolic players such as fumarate hydratase (FH) and succinate dehydrogenase (SDH) lead to fumarate and succinate accumulation that inhibit Tet enzymatic activity even with stable α-KG levels [53]. The interplay between these metabolic intermediaries and TET2 might also be directly relevant in clinics as mutations in iron and 2-oxoglutarate-binding sites have been reported in AML patients [15,54]. Finally, although essential for TET catalytic activity, oxygen has been described as a minor direct regulator of TET function in physiological settings [55]. However, low oxygen levels might indirectly regulate TET2 expression and activity in leukemic cells through a mechanism involving the activation of the hypoxia-inducible factor 1α (HIF1α) [56].
3. Partner-Instructed Tet2 Genomic Recruitment during Development and Cancer
Regulation of TET activity by controlling enzymes’ genomic distribution allows surgically modifying DNA methylation at particular genomic loci and only in specific cell types. Since TET2 lacks the low-affinity (CXXC) DNA binding domain, which is present in TET1 and TET3 [33], the enzyme must always be recruited to specific genomic locations through a ‘partner’ protein such as transcription factors.
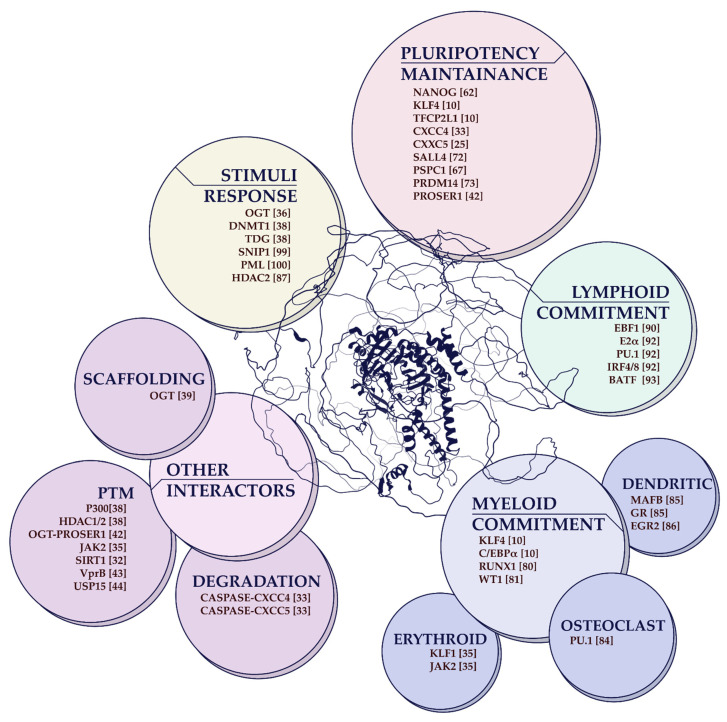
Here we present an elaborated list of potential Tet2 interactors/recruiters playing relevant roles in different biological settings (Figure 2).
Figure 2.
Graphical overview of TET2 protein partners/interactors. In the center, the human TET2 AlphaFold Database predicted protein structure (last updated on 1 July 2021 with AlphaFold v2.0) [57,58]. Encompassing the TET2 protein structure, color-coded bubbles cluster TET2 interactors based on previously described cell fate implications and physiological responses when cooperating with TET2. GR: glucocorticoid receptor.
3.1. During Embryonic Stem Cell Fate Decisions
Tet2 is considered an important pluripotency regulator playing critical roles during experimentally-induced pluripotency establishment [10,59,60,61,62,63,64] and in pluripotency maintenance [65,66,67]. In addition, Tet enzymes are essential for proper pluripotency exit during embryo development [68,69] and in ES cell differentiation [70,71].
Molecular mechanisms underlying the Tet2 pluripotency-related functions have recently been partially uncovered by systematically identifying and characterizing critical Tet2 interactors in ESCs. The naïve pluripotency TF Nanog was the first pluripotency-related protein identified interacting with Tet2 and Tet1 [62]. Thus, explaining Tet2 and Tet1 bound at regulatory regions of pluripotency genes in ESCs [62,66]. However, Tet2 is also functionally associated with other pluripotency-related TF at these regions, including Sall4 [72] and Prdm14 [73]. Sall4 acts in concert with Tet1 to firstly oxidize 5mC CpG residues into 5hmC and later with Tet2 to further oxidize them into 5fC and 5caC residues. However, no direct physical interaction between Sall4 and Tet2 was observed in ESCs [72]. Thus, suggesting the involvement of additional factors in this functional interplay. On the contrary, Prdm14, through direct association with Tet2, drives active demethylation at pluripotency and germline-associated genes such as Tcl1, Tcfap2c, and Spo11, Sycp3, respectively [73].
Additionally, Tet2 has been described to interact with a handful of non-canonical pluripotency TFs equally playing essential roles in regulating its functions in ESCs. The latter includes the CXXC-DNA binding domain proteins Idax (CXXC4) and Rinf (CXXC5), potentially influencing Tet2 functions in differentiation [33] and pluripotency [25], respectively. Rinf co-occupies with Tet2 and Nanog distal regulatory regions of relevant pluripotency genes (such as Oct3/4, Sox2, and Nanog itself), positively regulating their transcription. The RNA-binding protein PSPC1 associates with Tet2 and targets the enzyme to the MERVL endogenous retroviral elements in ESCs [67]. Of note, these retroviral elements have been described to regulate the expression of 2C-genes in ESCs [74,75]. Once recruited to MERVL elements, Tet2 contributes to their transcriptional and epitranscriptomic regulation by recruiting HDACs to chromatin and oxidizing MERVL transcripts, respectively [67].
As mentioned above, Tet2 is also required during experimentally induced pluripotency establishment. To uncover factors associated with Tet2 in this biological setting, we analyzed DNA (hydroxy)methylation dynamics during rapid and highly efficient iPSC reprogramming systems [59,60,76]. Here, Tet2 is recruited by the Klf4 and Tfcp2lƒ1 transcription factors to drive active enhancer demethylation of chromatin and pluripotency-related genes to reprogram cell fate [10].
Of note, a prominent study has recently identified common molecular mechanisms controlling DNA methylation during embryo development and in the leukemic transformation [77]. Thus, highlighting the potential health implications of studying Tet-mediated DNA demethylation in embryonic stem cells.
3.2. During Blood Cell Fate Decisions
Tet2 role in hematopoiesis and its influence on the DNA methylome has also been widely explored in the context of physiological and pathological conditions. Several studies have described how Tet2 protein deficiency can lead to aberrant cell fate decisions by extensively altering the DNA methylome during hematopoiesis. Here we recapitulate the role of Tet2 recruitment to specific genomic regions in different hematopoietic developmental pathways:
3.2.1. During Myeloid Cell Fate Decisions
Tet2-mediated epigenetic gene regulation is crucial during myeloid cell development. C/EBPα is an essential factor in the differentiation process from HSPCs to GMPs [78]. C/EBPα alone, through its pioneer activity, or in concert with PU.1, binds regulatory regions of myeloid genes to establish the myeloid cell fate [79]. To this end, C/EBPα directly activates Tet2 expression [26] and, through direct interaction with the enzyme, targets it to regulatory regions of myeloid genes such as Klf4 or Chd7, driving their active DNA demethylation and subsequent enhancer activation [10]. RUNX1, another key hematopoietic transcription factor, has also been described to interact with TET2 [80]. Thus, it potentially leads to the DNA demethylation observed at RUNX1 binding sites (including promoters of PTPN22, RUNX1, and RUNX3, among others) during hematopoietic differentiation [80]. Wilm’s tumor (WT1) gene encodes a sequence-specific transcription factor found mutated in a mutually exclusive manner with TET2 in AML patients [20]. Interestingly, WT1 directly associates with TET2 [81] to regulate WT1-target genes (including Wnt and MAPK signaling-related genes such as BTRC, DACT1, and TBL1X), preventing AML onset [81,82].
However, TET2 activity is not only relevant during early myeloid commitment but also in myeloid terminal differentiation [83]. In this regard, during monocyte-to-osteoclast differentiation, the master myeloid TF PU.1 recruits TET2 to promoters of key osteoclast-genes (including ACP5, CTSK, and TM7SF4), leading to their demethylation and cell fate transition [84]. Tolerogenic dendritic cells (tolDCs) are also terminally differentiated myeloid cells with potent immunosuppressive properties. Mechanistically, the tolerogenic phenotype is acquired through a synergistic interplay between the glucocorticoid receptor (GR) and the specific myeloid transcription factor MAFB. Both TFs target TET2 at genomic loci exclusively demethylated in tolDCs [85]. In addition, EGR2, an essential transcription factor during IL4/GM-CSF-driven monocyte (MO) to monocyte-derived DCs (moDCs) differentiation, has also been described to interact with TET2 and initiate DNA demethylation at both EGR2 stable and transient binding sites [86]. However, TET2 genomic recruitment is not always associated with positive regulation of gene expression in dendritic cells. For instance, Zhang and co-workers identified that IκBζ-dependent Tet2 targeting the Il6 locus leads to Hdac2 recruitment. Thus, finally leading to Il6 gene repression and inflammation resolution [87].
3.2.2. Erythroid Lineage
HSPC commitment towards erythroid lineage also correlates with 5hmC accumulation and increased expression of erythroid-specific genes such as EPOR, GATA1, and HBB [88]. 5hmC accumulation might be mediated by Klf1-dependent Tet2 recruitment at erythroid-specific genes. Of note, both factors have been recently described to interact upon Jak2-driven Tet2 phosphorylation [35].
3.2.3. B-Cell Lineage
B cell differentiation is tightly regulated at the epigenetic level. B cell maturation is characterized by extensive reshaping of the cellular methylome [89]. Tet2 might contribute to the process by interacting with B-cell master regulators such as EBF1 [90], IRF4/8, E2A, and PU.1 or BATF and driving focal demethylation at regulatory regions of key B-cell loci (including IgK or Aicda) [91,92,93].
3.2.4. T-Cell Lineage
5hmC is accumulated at genes encoding key regulators of T cell identity, development, and differentiation [94]. However, what factors recruit Tet enzymes to the T-cell key regulatory regions is poorly known. In regulatory T cells (Tregs), Tet1 and Tet2 are upregulated in response to the sulfhydrating nuclear transcription factor Y subunit beta (NFYB) [29]. Tet1 and Tet2 are targeted by the activated forms of Smad3 and Stat5 to the Foxp3 promoter favoring its hypomethylation and stable gene expression [29].
3.3. In Response to External Stimuli
Tet2 has also been implicated in genome stability. Upon exposure to oxidative stress (OS), protein complexes containing DNMTs and TET2 are recruited to the specific genomic regions to repair the damaged DNA properly. TET2 depletion leads to reduced 5hmC levels at the damaged regions and impaired repair [95,96,97,98]. As a result of these exciting findings, several epigenetic regulatory mechanisms that tie Tet2 to protection against abnormal DNA methylation during stress have been proposed. For instance, Zhang and co-workers showed that upon OS, TET2 interacts with the thymidine glycosylase (TDG), an enzyme involved in the DNA active demethylation pathway. Then, both proteins are recruited to chromatin in a DNMT1 dependent manner, a process that is enhanced when TET2 is acetylated [38]. Therefore, the latter mechanism is suggested to protect against the acquisition of abnormal DNA methylation at typically unmethylated gene regulatory regions.
Another Tet2 interactor in the context of DNA damage response is the SMAD nuclear interacting protein 1 (SNIP1) that regulates the expression of relevant c-MYC target genes involved in apoptosis. Therefore, reduced SNIP1 levels lead to diminished 5hmC levels and gene expression at c-MYC target genes [99].
Finally, chemotherapeutic drugs have also been described to trigger hydroxymethylation changes in a mechanism mediated by the interaction between the promyelocytic leukemia protein (PML) and TET2, linking the protein to direct clinical applications [100].
4. TET2 Loss of Function in Blood Malignancies
DNA methylation aberrations are considered hallmarks of cancer onset and progression [101,102,103]. TET2 loss of function mutations are frequently found in patients suffering from myeloid malignancies such as acute myeloid leukemia, myeloproliferative neoplasms, or myelodysplastic syndromes [15,20,104] (Table 1). However, these mutations are also commonly observed in other blood malignancies, including B and T lymphomas [105,106,107,108,109], such as the angioimmunoblastic T-cell lymphoma (AITL), showing the highest incidence of TET2 mutations (roughly 80%) among all blood cancer types (Table 1). The broad TET2 mutational profile observed in the hematopoietic system has awakened great interest in the field to unravel the molecular mechanisms underlying TET2 involvement in the onset of preleukemic and leukemic diseases.
Table 1.
List of TET2 mutation frequencies in hematologic malignancies and their prognostic value. Combined 12845 samples from 35 studies were analyzed according to cancer type and detailed classification based on WHO Classification of Hematologic Malignancies or The French–American–British (FAB) classification. Data from ‘Lymphoid’ and ‘Myeloid’ datasets from cBioPortal (https://www.cbioportal.org/, accessed on 19 January 2022) [45,46] and selected referenced studies [108,110,111,112]. AML = Acute myeloid leukemia, AMML = Acute Myelomonocytic Leukemia, AML-M5 = Acute Monoblastic/Monocytic Leukemia, CML = Chronic Myelogenous Leukemia, MPN = Myeloproliferative Neoplasms, MDS = Myelodysplastic Syndromes, CMML = Chronic Myelomonocytic Leukemia, CLL/SLL = Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, DLBCL = Diffuse Large B-Cell Lymphoma, AITL = Angioimmunoblastic T-cell lymphoma, NOS = Not Otherwise Specified, N/A = Not Available.
| Cancer Type | Detailed Cancer Type | Frequency (%) | Prognosis |
|---|---|---|---|
| AML (399/4014) 10% | AML (unspecified) | 9 | Unfavorable [113] |
| AML, NOS | 17 | Unfavorable [113] | |
| AML with Biallelic Mutations of CEBPA | 24 | Unfavorable [114] | |
| AML with inv (3) (q21.3q26.2) or t(3;3) (q21.3;q26.2); GATA2, MECOM | 8 | N/A | |
| AML with mutated NPM1 | 18 | Unfavorable [19] | |
| AML with Myelodysplasia Related Changes | 8 | Unaffected [115] | |
| AML with Recurrent Genetic Abnormalities | 5 | N/A | |
| AML with t(8;21) (q22;q22.1); RUNX1-RUNX1T1 | 13 | N/A | |
| AMML | 8 | N/A | |
| AML-M5 | 15 | N/A | |
| MDS/MPN (1023/2700) 38% | MDS (unspecified) | 25 | Favorable [116] |
| MDS, Unclassifiable | 13 | Favorable [116] | |
| MDS with Excess Blasts (unspecified) | 22 | Favorable [116] | |
| MDS with excess blasts-1 | 21 | Favorable [116] | |
| MDS with excess blasts-2 | 16 | Favorable [116] | |
| MDS with isolated del(5q) | 16 | Unaffected [117] | |
| MDS with Multilineage Dysplasia | 33 | Favorable [116] | |
| MDS with Single Lineage Dysplasia | 20 | N/A | |
| MDS/MPN with Ring Sideroblasts and Thrombocytosis | 28 | N/A | |
| MPN | 22 | Unaffected [118] | |
| CMML | 56 | Favorable [118] | |
| CML | 30 | Unaffected [119] | |
| Essential thrombocythemia | 9 | Unaffected [54] | |
| Polycythemia Vera | 28 | Unaffected [54] | |
| Primary myelofibrosis | 26 | Unaffected [54] | |
| Histiocytic and Dendritic Cell Neoplasms | 2 | N/A | |
| B/T-cell neoplasms (343/3712) 9% | Burkitt Lymphoma | 4 | N/A |
| DLBCL, NOS | 6 | N/A | |
| DLBCL (unspecified) | 11 | Favorable [120] | |
| DLBCL, Germinal Center B-Cell Type | 7 | N/A | |
| DLBCL, Activated B-cell Type | 1 | N/A | |
| Follicular Lymphoma | 4 | N/A | |
| High-Grade B-Cell Lymphoma, NOS | 5 | N/A | |
| Mantle Cell Lymphoma | 3 | N/A | |
| Marginal Zone Lymphoma | 4 | N/A | |
| Mature B-Cell Neoplasms | 16 | N/A | |
| AITL | 78 | Unaffected [108] | |
| CLL/SLL | 1 | N/A | |
| Sezary Syndrome | 12 | N/A | |
| Therapy-Related Neoplasms (12/115) 10% |
Therapy-Related Myeloid Neoplasms (unspecified) | 8 | N/A |
| Therapy-Related Myelodysplastic Syndrome | 27 | N/A |
4.1. Preleukemic Conditions
HSCs, like any other somatic cells, slowly accumulate mutations during the whole life of an individual [121]. TET2 mutated HSC clones are frequently found among healthy aged individuals characterized by an increased risk of cancer onset and a higher propensity to develop cardiovascular diseases [18,122,123,124]. This condition is named clonal hematopoiesis of indeterminate potential (CHIP). Mechanistically, TET2 loss of function mutations appear to enhance HSCs’ expansion capabilities and lead to a myeloid bias [48,52,125,126,127]. Additionally, TET2 mutant cells show an enhanced response to pro-inflammatory cytokines such as IL-6 and TNF-α [128], potentially representing a competitive advantage in response to inflammatory environments. Moreover, inflammation-based preleukemic myeloproliferation has been linked to TET2 activity [129,130], where Il6 expression might be negatively regulated by Tet2-mediated Hdac2 recruitment to its promoter [87].
Regardless, HSCs with altered TET2 activity acquire a competitive clonal advantage due to an altered DNA methylation landscape that finally allows aberrant gene expression. However, the precise order of events leading from CHIP to the development of myeloid malignancies is not yet fully uncovered, nor is the CHIP potential as a clinical predictor.
4.2. Leukemic Conditions
As previously mentioned, TET2 loss of function mutations are frequently found in patients with myeloid malignancies, myeloproliferative disorders (MPN), or myelodysplastic syndromes (MDS) (Table 1). These conditions are typically considered as an early event in myeloid cancer development [15,131] and are characterized by presenting aberrant DNA methylation profiles due to incorrect TET2 function [52,103]. This is the case, for instance, of chronic myelomonocytic leukemia (CMML), a mixed MDS/MPN neoplasm where TET2 mutations are present in up to 60% of patients [16,132] (Table 1). In CMML cells, aberrant methylation was observed at promoters of genes involved in neoplastic transformation, WNT and PDGF signaling pathways; inflammation; and apoptosis [133]. Of note, similar gene sets were observed aberrantly methylated and expressed in TET2-mutated AML patients, including genes coding for tumor suppressors (PDZD2); transcription modulators (ZNF667, ZNF582, PIAS2, CDK8); nuclear import receptors (TNPO3, IPO8); and myeloid cytokines (CSDA) [103].
Global methylation analysis also revealed a shared hypermethylation signature in a subset of AML patients carrying IDH1/2 mutations [52,134], a well-known TET2 inhibitor and mutually exclusive mutated gene (See Section 2.4). The latter highlights the importance of TET2 enzymatic deregulation.
Similarly, mutations in the IDH1/2-TET2-WT1 network, which collectively appear in 30-50% of AML cases [20,81,131], also present an apparent hypermethylated phenotype as a consequence of deficient TET2 activity [82,135]. Several ongoing clinical trials aim to restore TET2 activity within the IDH1/2-TET2-WT1 mutated network by combining hypomethylating agents with ascorbate treatment [50,51,135,136,137] (ClinicalTrials.gov identifier: NCT03999723, NCT03682029).
In the lymphoid lineage, aberrant B cell differentiation and chronic lymphocytic leukemia (CLL) have been associated with DNA hypomethylation [138,139,140]. Hypomethylation at gene bodies and enhancer regions correlates with gene expression differences in CLL samples compared to normal B cells [89,139,141]. However, available data do not support a primary role of TET enzymes in CLL, but perhaps an accessory role in establishing leukemia-specific patterns of 3D chromatin conformation. The latter might be accomplished, for instance, by modulating CTCF binding to the chromatin, a DNA methylation-sensitive mechanism [142].
TET2 has also been studied in the context of lymphoproliferative diseases. Diffuse large B-cell lymphoma (DLBCL) patients have shown specific hypermethylation signatures on promoters of tumor suppressor genes involved in cell fate and cell cycle changes [109]. Germinal center analysis of B cells in Tet2 deficient mice showed promoter hypermethylation and defective transcription factor binding at essential B-cell pathway genes. Tet2 knockout mice partially phenocopied DLBCL patients, characterized by downregulation of antigen presentation genes/interferon pathway, lymphoma-like transcriptional profiles similar to CREBBP-mutant patients, and a failure at the germinal center exit. Therefore, this data suggests that TET2 is relevant in B cell lymphoma development and how acquiring mutations in HSCs might influence B-cell maturation and cancer development [105,106].
5. Summary and Conclusions
The last decade of research has shed light on the complex biological functions of TET2 in normal hematopoiesis and blood cancer development. However, as described in this manuscript, a holistic approach is required to deconvolute the intricated regulatory networks determining TET2 function in a cell type-specific manner. Precisely, TET2 activity is controlled by: (1) TET2 transcriptional regulation (through Oct4, C/EBPα, Rinf or HDAC4); (2) post-transcriptional regulation (by miR-22, miR-9, or miR-34a); (3) post-translational regulation (by a whole array of PTMs (Figure 1); (4) enzymatic regulation (by VitC, α-KG/2-HG, FH, SDH or oxygen/HIF1α) and (5) genomic localization (through interactions with an extensive network of partners (Figure 2).
C/EBPα regulating Tet2 gene expression by directly recruiting Tet2 enzyme to demethylate its own enhancers during myeloid cell fate specification [10] perfectly illustrates the complexity of the Tet2 regulatory networks. Another interesting example of multilayer regulation is the miR-9/miR-34a-SIRT1-TET2 network. Sun and co-workers identified miR-9/miR-34a overexpression within a subset of TET2-WT MDS patients displaying a TET2 loss of function phenotype [32]. miR-9/miR-34a post-transcriptionally regulates SIRT1, which modulates TET2 activity by mediating deacetylation at key lysine residues of the enzyme [32].
About TET2 post-translational regulation, a comprehensive characterization of all potential PTMs described (Figure 1) is needed to fully determine their physiological relevance. Enhanced knowledge of TET2′s PTM-ome might be particularly relevant to fully elucidate the functions mediated by well-known TET2 interactors such as SIRT1 or OGT. Factors that can modify TET2 enzymatic capabilities and modulate its interaction with other proteins [32,37,38,39,40,42,43] (Figure 1).
Substrate availability is the primary mechanism of enzymatic regulation. Therefore, TET2 activity is tightly regulated by the availability of α-KG and 2-HG produced by IDH1/2-WT or -mutated enzymes, respectively [50,51]. First studies conducted in AML patient cells harboring 2-HG-producing IDH1/2 mutations uncovered an altered cellular epigenetic landscape and a myeloid bias characteristic of AML patients with TET2 loss of function [52,90,134,143]. However, such myeloid bias has not been observed when analyzing the multilayer differentiation potential of Idh2 mutated cells through a single-cell approach [144]. The apparent discrepancy observed in the phenotype might be explained by species-specific differences, variations in 2-HG levels produced; differences in 2-HG subtype (D-2-HG or L-2-HG) produced; or a side-effect of 2-HG-mediated inactivation of other α-KG-dependent dioxygenases such as the Jumonji-C domain histone demethylase family.
The rapidly increasing list of partners/interactors identified highlights the idea that TET2 has been promiscuously recruited to the genome by sequence-specific transcription factors (also by other epigenetic regulators including HDAC1/2 or DNMT1) to drive active DNA demethylation in a cell state-specific manner. TET2 promiscuous behavior might be encoded in its nucleotide sequence where only part of the catalytic domain (coding for the cysteine-rich domain and the first double-stranded β-helix) is structured and ordered (Figure 1 and Figure 2 and in silico prediction). Whereas the N-terminus protein region is unstructured, therefore, constituting a large intrinsically disordered domain (IDR) that might favor most TET2 described interactions through a mechanism of liquid-liquid phase separation (LLPS). Of note, IDR-mediated LLPS events have been recently described for the TET2 recruiter KLF4 and its associated pluripotency transcription factor OCT4 [145,146].
To sum up, fine dissection of the molecular mechanisms underlying TET2 activity is essential to fully uncover its contribution to clonal expansion and malignant transformation.
Author Contributions
A.L. and J.L.S. planned the manuscript outline, wrote the first draft, and prepared figures. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been funded by Worldwide Cancer Research (grant reference no. 20-0269). J.L.S was supported by ISCIII (CP19/00176), co-funded by ESF, “Investing in your future.” We thank CERCA Programme / Generalitat de Catalunya for institutional support.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 2.Baylin S.B., Jones P.A. A decade of exploring the cancer epigenome—Biological and translational implications. Nat. Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hackett J.A., Surani M.A. Regulatory Principles of Pluripotency: From the Ground State Up. Cell Stem Cell. 2014;15:416–430. doi: 10.1016/j.stem.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Wu X., Zhang X.W.Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017;18:517–534. doi: 10.1038/nrg.2017.33. [DOI] [PubMed] [Google Scholar]
- 5.Tahiliani M., Koh K.P., Shen Y., Pastor W.A., Bandukwala H., Brudno Y., Agarwal S., Iyer L.M., Liu D.R., Aravind L., et al. Conversion of 5-Methylcytosine to 5-Hydroxymethylcytosine in Mammalian DNA by MLL Partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito S., D’Alessio A.C., Taranova O.V., Hong K., Sowers L.C., Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito S., Shen L., Dai Q., Wu S.C., Collins L.B., Swenberg J.A., He C., Zhang Y. Tet Proteins Can Convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spruijt C.G., Gnerlich F., Smits A.H., Pfaffeneder T., Jansen P.W.T.C., Bauer C., Munzel M., Wagner M., Muller M., Khan F., et al. Dynamic readers for 5-(hydroxy) methylcytosine and its oxidized derivatives. Cell. 2013;152:1146–1159. doi: 10.1016/j.cell.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Kunimoto H., Fukuchi Y., Sakurai M., Takubo K., Okamoto S., Nakajima H. Tet2-mutated myeloid progenitors possess aberrant in vitro self-renewal capacity. Blood. 2014;123:2897–2899. doi: 10.1182/blood-2014-01-552471. [DOI] [PubMed] [Google Scholar]
- 10.Sardina J.L., Collombet S., Tian T., Gómez A., Di Stefano B., Berenguer C., Brumbaugh J., Stadhouders R., Morales C.S., Gut M., et al. Transcription Factors Drive Tet2-Mediated Enhancer Demethylation to Reprogram Cell Fate. Cell Stem Cell. 2018;23:727–741.e9. doi: 10.1016/j.stem.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Tsagaratou A., Lio C.W.J., Yue X., Rao A. TET Methylcytosine Oxidases in T Cell and B Cell Development and Function. Frontiers in Immunol. 2017;8:220. doi: 10.3389/fimmu.2017.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Z., Chen L., Dawlaty M.M., Pan F., Weeks O., Zhou Y., Cao Z., Shi H., Wang J., Lin L., et al. Combined Loss of Tet1 and Tet2 Promotes B Cell, but Not Myeloid Malignancies, in Mice. Cell Rep. 2015;13:1692–1704. doi: 10.1016/j.celrep.2015.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimmino L., Dawlaty M.M., Ndiaye-Lobry D., Yap Y.S., Bakogianni S., Yu Y., Bhattacharyya S., Shaknovich R., Geng H., Lobry C., et al. TET1 is a tumor suppressor of hematopoietic malignancy. Nat. Immunol. 2015;16:653–662. doi: 10.1038/ni.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poole C.J., Lodh A., Choi J.-H., Van Riggelen J. MYC deregulates TET1 and TET2 expression to control global DNA (hydroxy)methylation and gene expression to maintain a neoplastic phenotype in T-ALL. Epigenet. Chromatin. 2019;12:1–20. doi: 10.1186/s13072-019-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delhommeau F., Dupont S., Della Valle V., James C., Trannoy S., Massé A., Kosmider O., Le Couedic J.-P., Robert F., Alberdi A., et al. Mutation inTET2in Myeloid Cancers. N. Engl. J. Med. 2009;360:2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 16.Kosmider O., Gelsi-Boyer V., Ciudad M., Racoeur C., Jooste V., Vey N., Quesnel B., Fenaux P., Bastie J.-N., Beyne-Rauzy O., et al. TET2 gene mutation is a frequent and adverse event in chronic myelomonocytic leukemia. Haematologica. 2009;94:1676–1681. doi: 10.3324/haematol.2009.011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langemeijer S.M.C., Kuiper R.P., Berends M., Knops R., Aslanyan M.G., Massop M., Stevens-Linders E., Van Hoogen P., Van Kessel A.G., Raymakers R.A.P., et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat. Genet. 2009;41:838–842. doi: 10.1038/ng.391. [DOI] [PubMed] [Google Scholar]
- 18.Busque L., Patel J.P., Figueroa M.E., Vasanthakumar A., Provost S., Hamilou Z., Mollica L., Li J., Viale A., Heguy A., et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat. Genet. 2012;44:1179–1181. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metzeler K.H., Maharry K., Radmacher M.D., Mrózek K., Margeson D., Becker H., Curfman J., Holland K.B., Schwind S., Whitman S.P., et al. TET2 Mutations Improve the New European LeukemiaNet Risk Classification of Acute Myeloid Leukemia: A Cancer and Leukemia Group B Study. J. Clin. Oncol. 2011;29:1373–1381. doi: 10.1200/JCO.2010.32.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Cancer Genome Atlas Research Network Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu L., Li Z., Cheng J., Rao Q., Gong W., Liu M., Shi Y.G., Zhu J., Wang P., Xu Y. Crystal Structure of TET2-DNA Complex: Insight into TET-Mediated 5mC Oxidation. Cell. 2013;155:1545–1555. doi: 10.1016/j.cell.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Montagner S., Leoni C., Emming S., Della Chiara G., Balestrieri C., Barozzi I., Piccolo V., Togher S., Ko M., Rao A., et al. TET2 Regulates Mast Cell Differentiation and Proliferation through Catalytic and Non-catalytic Activities. Cell Rep. 2016;15:1566–1579. doi: 10.1016/j.celrep.2016.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito K., Lee J., Chrysanthou S., Zhao Y., Josephs K., Sato H., Teruya-Feldstein J., Zheng D., Dawlaty M.M., Ito K. Non-catalytic Roles of Tet2 Are Essential to Regulate Hematopoietic Stem and Progenitor Cell Homeostasis. Cell Rep. 2019;28:2480–2490.e4. doi: 10.1016/j.celrep.2019.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh K.P., Yabuuchi A., Rao S., Huang Y., Cunniff K., Nardone J., Laiho A., Tahiliani M., Sommer C.A., Mostoslavsky G., et al. Tet1 and Tet2 Regulate 5-Hydroxymethylcytosine Production and Cell Lineage Specification in Mouse Embryonic Stem Cells. Cell Stem Cell. 2011;8:200–213. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravichandran M., Lei R., Tang Q., Zhao Y., Lee J., Ma L., Chrysanthou S., Lorton B.M., Cvekl A., Shechter D., et al. Rinf Regulates Pluripotency Network Genes and Tet Enzymes in Embryonic Stem Cells. Cell Rep. 2019;28:1993–2003.e5. doi: 10.1016/j.celrep.2019.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kallin E.M., Rodríguez-Ubreva J., Christensen J., Cimmino L., Aifantis I., Helin K., Ballestar E., Graf T. Tet2 Facilitates the Derepression of Myeloid Target Genes during CEBPα-Induced Transdifferentiation of Pre-B Cells. Mol. Cell. 2012;48:266–276. doi: 10.1016/j.molcel.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasemann M.S., Damgaard I., Schuster M.B., Theilgaard-Mönch K., Sorensen A., Mršić A., Krugers T., Ylstra B., Pedersen F.S., Nerlov C., et al. Mutation of C/EBPα predisposes to the development of myeloid leukemia in a retroviral insertional mutagenesis screen. Blood. 2008;111:4309–4321. doi: 10.1182/blood-2007-06-097790. [DOI] [PubMed] [Google Scholar]
- 28.Huang F., Sun J., Chen W., He X., Zhu Y., Dong H., Wang H., Li Z., Zhang L., Khaled S., et al. HDAC4 inhibition disrupts TET2 function in high-risk MDS and AML. Aging. 2020;12:16759–16774. doi: 10.18632/aging.103605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang R., Qu C., Zhou Y., Konkel J.E., Shi S., Liu Y., Chen C., Liu S., Liu D., Chen Y., et al. Hydrogen Sulfide Promotes Tet1- and Tet2-Mediated Foxp3 Demethylation to Drive Regulatory T Cell Differentiation and Maintain Immune Homeostasis. Immunity. 2015;43:251–263. doi: 10.1016/j.immuni.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng J., Guo S., Chen S., Mastriano S.J., Liu C., D’Alessio A.C., Hysolli E., Guo Y., Yao H., Megyola C.M., et al. An Extensive Network of TET2-Targeting MicroRNAs Regulates Malignant Hematopoiesis. Cell Rep. 2013;5:471–481. doi: 10.1016/j.celrep.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song S.J., Ito K., Ala U., Kats L., Webster K., Sun S.M., Jongen-Lavrencic M., Manova-Todorova K., Teruya-Feldstein J., Avigan D.E., et al. The Oncogenic MicroRNA miR-22 Targets the TET2 Tumor Suppressor to Promote Hematopoietic Stem Cell Self-Renewal and Transformation. Cell Stem Cell. 2013;13:87–101. doi: 10.1016/j.stem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J., He X., Zhu Y., Ding Z., Dong H., Feng Y., Du J., Wang H., Wu X., Zhang L., et al. SIRT1 Activation Disrupts Maintenance of Myelodysplastic Syndrome Stem and Progenitor Cells by Restoring TET2 Function. Cell Stem Cell. 2018;23:355–369.e9. doi: 10.1016/j.stem.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko M., An J., Bandukwala H.S., Chavez L., Äijö T., Pastor W.A., Segal M.F., Li H., Koh K.P., Lähdesmäki H., et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature. 2013;497:122–126. doi: 10.1038/nature12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Zhang Y. Regulation of TET Protein Stability by Calpains. Cell Rep. 2014;6:278–284. doi: 10.1016/j.celrep.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong J.J., Gu X., Nie J., Sundaravel S., Liu H., Kuo W.-L., Bhagat T.D., Pradhan K., Cao J., Nischal S., et al. Cytokine-Regulated Phosphorylation and Activation of TET2 by JAK2 in Hematopoiesis. Cancer Discov. 2019;9:778–795. doi: 10.1158/2159-8290.CD-18-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauer C., Göbel K., Nagaraj N., Colantuoni C., Wang M., Müller U., Kremmer E., Rottach A., Leonhardt H. Phosphorylation of TET Proteins Is Regulated via O-GlcNAcylation by the O-Linked N-Acetylglucosamine Transferase (OGT) J. Biol. Chem. 2015;290:4801–4812. doi: 10.1074/jbc.M114.605881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etchegaray J.-P., Chavez L., Huang Y., Ross K.N., Choi J., Martinez-Pastor B., Walsh R.M., Sommer C.A., Lienhard M., Gladden A., et al. The histone deacetylase SIRT6 controls embryonic stem cell fate via TET-mediated production of 5-hydroxymethylcytosine. Nat. Cell Biol. 2015;17:545–557. doi: 10.1038/ncb3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y.W., Wang Z., Xie W., Cai Y., Xia L., Easwaran H., Luo J., Yen R.-W.C., Li Y., Baylin S.B. Acetylation Enhances TET2 Function in Protecting against Abnormal DNA Methylation during Oxidative Stress. Mol. Cell. 2017;65:323–335. doi: 10.1016/j.molcel.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deplus R., Delatte B., Schwinn M.K., Defrance M., Méndez J., Murphy N., Dawson M.A., Volkmar M., Putmans P., Calonne E., et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J. 2013;32:645–655. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vella P., Scelfo A., Jammula S., Chiacchiera F., Williams K., Cuomo A., Roberto A., Christensen J., Bonaldi T., Helin K., et al. Tet Proteins Connect the O-Linked N-acetylglucosamine Transferase Ogt to Chromatin in Embryonic Stem Cells. Mol. Cell. 2013;49:645–656. doi: 10.1016/j.molcel.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Chen Q., Chen Y., Bian C., Fujiki R., Yu X. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature. 2012;493:561–564. doi: 10.1038/nature11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X., Rosikiewicz W., Sedkov Y., Martinez T., Hansen B.S., Schreiner P., Christensen J., Xu B., Pruett-Miller S.M., Helin K., et al. PROSER1 mediates TET2 O-GlcNAcylation to regulate DNA demethylation on UTX-dependent enhancers and CpG islands. Life Sci. Alliance. 2021;5:e202101228. doi: 10.26508/lsa.202101228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakagawa T., Lv L., Nakagawa M., Yu Y., Yu C., D’Alessio A.C., Nakayama K., Fan H.-Y., Chen X., Xiong Y. CRL4VprBP E3 Ligase Promotes Monoubiquitylation and Chromatin Binding of TET Dioxygenases. Mol. Cell. 2014;57:247–260. doi: 10.1016/j.molcel.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L.-L., Smith M.D., Lv L., Nakagawa T., Li Z., Sun S.-C., Brown N.G., Xiong Y., Xu Y.-P. USP15 suppresses tumor immunity via deubiquitylation and inactivation of TET2. Sci. Adv. 2020;6:eabc9730. doi: 10.1126/sciadv.abc9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quivoron C., Couronné L., Della Valle V., Lopez C.K., Plo I., Wagner-Ballon O., Cruzeiro M.D., Delhommeau F., Arnulf B., Stern M.-H., et al. TET2 Inactivation Results in Pleiotropic Hematopoietic Abnormalities in Mouse and Is a Recurrent Event during Human Lymphomagenesis. Cancer Cell. 2011;20:25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Moran-Crusio K., Reavie L., Shih A., Abdel-Wahab O., Ndiaye-Lobry D., Lobry C., Figueroa M.E., Vasanthakumar A., Patel J., Zhao X., et al. Tet2 Loss Leads to Increased Hematopoietic Stem Cell Self-Renewal and Myeloid Transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ko M., Bandukwala H.S., An J., Lamperti E.D., Thompson E.C., Hastie R., Tsangaratou A., Rajewsky K., Koralov S.B., Rao A. Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc. Natl. Acad. Sci. USA. 2011;108:14566–14571. doi: 10.1073/pnas.1112317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cimmino L., Dolgalev I., Wang Y., Yoshimi A., Martin G.H., Wang J., Ng V., Xia B., Witkowski M.T., Mitchell-Flack M., et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell. 2017;170:1079–1095.e20. doi: 10.1016/j.cell.2017.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agathocleous M., Meacham C.E., Burgess R.J., Piskounova E., Zhao Z., Crane G., Cowin B.L., Bruner E., Murphy M.M., Chen W., et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature. 2017;549:476–481. doi: 10.1038/nature23876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figueroa M.E., Abdel-Wahab O., Lu C., Ward P.S., Patel J., Shih A., Li Y., Bhagwat N., VasanthaKumar A., Fernandez H.F., et al. Leukemic IDH1 and IDH2 Mutations Result in a Hypermethylation Phenotype, Disrupt TET2 Function, and Impair Hematopoietic Differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao M., Yang H., Xu W., Ma S., Lin H., Zhu H., Liu L., Liu Y., Yang C., Xu Y., et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tefferi A., Pardanani A., Lim K.-H., Abdel-Wahab O., Lasho T.L., Patel J., Gangat N., Finke C.M., Schwager S., Mullally A., et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia. 2009;23:905–911. doi: 10.1038/leu.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laukka T., Mariani C.J., Ihantola T., Cao J.Z., Hokkanen J., Kaelin W.G., Jr., Godley L.A., Koivunen P. Fumarate and Succinate Regulate Expression of Hypoxia-inducible Genes via TET Enzymes. J. Biol. Chem. 2016;291:4256–4265. doi: 10.1074/jbc.M115.688762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He P., Lei J., Zou L.-X., Zhou G.-Z., Peng L., Deng Q., Liu X.-L. Effects of hypoxia on DNA hydroxymethylase Tet methylcytosine dioxygenase 2 in a KG-1 human acute myeloid leukemia cell line and its mechanism. Oncol. Lett. 2021;22:1–12. doi: 10.3892/ol.2021.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., Yuan D., Stroe O., Wood G., Laydon A., et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2021;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Stefano B., Collombet S., Jakobsen J.S., Wierer M., Sardina J.L., Lackner A., Stadhouders R., Morales C.S., Francesconi M., Limone F., et al. C/EBPα creates elite cells for iPSC reprogramming by upregulating Klf4 and increasing the levels of Lsd1 and Brd4. Nat. Cell Biol. 2016;18:371–381. doi: 10.1038/ncb3326. [DOI] [PubMed] [Google Scholar]
- 60.Di Stefano B., Sardina J.L., van Oevelen C., Collombet S., Kallin E.M., Vicent G.P., Lu J., Thieffry D., Beato M., Graf T. C/EBPα poises B cells for rapid reprogramming into induced pluripotent stem cells. Nature. 2013;506:235–239. doi: 10.1038/nature12885. [DOI] [PubMed] [Google Scholar]
- 61.Piccolo F.M., Bagci H., Brown K.E., Landeira D., Soza-Ried J., Feytout A., Mooijman D., Hajkova P., Leitch H.G., Tada T., et al. Different Roles for Tet1 and Tet2 Proteins in Reprogramming-Mediated Erasure of Imprints Induced by EGC Fusion. Molecular Cell. 2013;49:1023–1033. doi: 10.1016/j.molcel.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa Y., Ding J., Theunissen T.W., Faiola F., Hore T.A., Shliaha P.V., Fidalgo M., Saunders A., Lawrence M., Dietmann S., et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature. 2013;495:370–374. doi: 10.1038/nature11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu X., Zhang L., Mao S.-Q., Li Z., Chen J., Zhang R.-R., Wu H.-P., Gao J., Guo F., Liu W., et al. Tet and TDG Mediate DNA Demethylation Essential for Mesenchymal-to-Epithelial Transition in Somatic Cell Reprogramming. Cell Stem Cell. 2014;14:512–522. doi: 10.1016/j.stem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Doege C.A., Inoue K., Yamashita T., Rhee D.B., Travis S., Fujita R., Guarnieri P., Bhagat G., Vanti W., Shih A., et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fidalgo M., Huang X., Guallar D., Sanchez-Priego C., Valdes V.J., Saunders A., Ding J., Wu W.-S., Clavel C., Wang J. Zfp281 Coordinates Opposing Functions of Tet1 and Tet2 in Pluripotent States. Cell Stem Cell. 2016;19:355–369. doi: 10.1016/j.stem.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pantier R., Tatar T., Colby D., Chambers I. Endogenous epitope-tagging of Tet1, Tet2 and Tet3 identifies TET2 as a naïve pluripotency marker. Life Sci. Alliance. 2019;2:e201900516. doi: 10.26508/lsa.201900516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guallar D., Bi X., Pardavila J.A., Huang X., Saenz C., Shi X., Zhou H., Faiola F., Ding J., Haruehanroengra P., et al. RNA-dependent chromatin targeting of TET2 for endogenous retrovirus control in pluripotent stem cells. Nat. Genet. 2018;50:443–451. doi: 10.1038/s41588-018-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dai H.-Q., Wang B.-A., Yang L., Chen J.-J., Zhu G.-C., Sun M.-L., Ge H., Wang R., Chapman D., Tang F., et al. TET-mediated DNA demethylation controls gastrulation by regulating Lefty–Nodal signalling. Nature. 2016;538:528–532. doi: 10.1038/nature20095. [DOI] [PubMed] [Google Scholar]
- 69.Bogdanovic O., Smits A.H., de la Calle-Mustienes E., Tena J.J., Ford E., Williams R., Senanayake U., Schultz M.D., Hontelez S., Van Kruijsbergen I., et al. Active DNA demethylation at enhancers during the vertebrate phylotypic period. Nat. Genet. 2016;48:417–426. doi: 10.1038/ng.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Charlton J., Jung E.J., Mattei A.L., Bailly N., Liao J., Martin E.J., Giesselmann P., Brändl B., Stamenova E.K., Müller F.-J., et al. TETs compete with DNMT3 activity in pluripotent cells at thousands of methylated somatic enhancers. Nat. Genet. 2020;52:819–827. doi: 10.1038/s41588-020-0639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hon G.C., Song C.-X., Du T., Jin F., Selvaraj S., Lee A.Y., Yen C.-A., Ye Z., Mao S.-Q., Wang B.-A., et al. 5mC Oxidation by Tet2 Modulates Enhancer Activity and Timing of Transcriptome Reprogramming during Differentiation. Mol. Cell. 2014;56:286–297. doi: 10.1016/j.molcel.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiong J., Zhang Z., Chen J., Huang H., Xu Y., Ding X., Zheng Y., Nishinakamura R., Xu G.-L., Wang H., et al. Cooperative Action between SALL4A and TET Proteins in Stepwise Oxidation of 5-Methylcytosine. Mol. Cell. 2016;64:913–925. doi: 10.1016/j.molcel.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 73.Okashita N., Kumaki Y., Ebi K., Nishi M., Okamoto Y., Nakayama M., Hashimoto S., Nakamura T., Sugasawa K., Kojima N., et al. PRDM14 promotes active DNA demethylation through the Ten-eleven translocation (TET)-mediated base excision repair pathway in embryonic stem cells. Development. 2014;141:269–280. doi: 10.1242/dev.099622. [DOI] [PubMed] [Google Scholar]
- 74.Eckersley-Maslin M.A., Svensson V., Krueger C., Stubbs T., Giehr P., Krueger F., Miragaia R.J., Kyriakopoulos C., Berrens R.V., Milagre I., et al. MERVL/Zscan4 Network Activation Results in Transient Genome-wide DNA Demethylation of mESCs. Cell Rep. 2016;17:179–192. doi: 10.1016/j.celrep.2016.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Macfarlan T.S., Gifford W.D., Driscoll S., Lettieri K., Rowe H.M., Bonanomi D., Firth A., Singer O., Trono D., Pfaff S.L. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature. 2012;487:57–63. doi: 10.1038/nature11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bar-Nur O., Brumbaugh J., Verheul C., Apostolou E., Pruteanu-Malinici I., Walsh R.M., Ramaswamy S., Hochedlinger K. Small molecules facilitate rapid and synchronous iPSC generation. Nat. Chem. Biol. 2014;11:1170–1176. doi: 10.1038/nmeth.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith Z.D., Shi J., Gu H., Donaghey J., Clement K., Cacchiarelli D., Gnirke A., Michor F., Meissner A. Epigenetic restriction of extraembryonic lineages mirrors the somatic transition to cancer. Nature. 2017;549:543–547. doi: 10.1038/nature23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang D.-E., Zhang P., Wang N.-D., Hetherington C.J., Darlington G.J., Tenen D.G. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein -deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Oevelen C., Collombet S., Vicent G., Hoogenkamp M., Lepoivre C., Badeaux A., Bussmann L., Sardina J.L., Thieffry D., Beato M., et al. C/EBPα Activates Pre-existing and De Novo Macrophage Enhancers during Induced Pre-B Cell Transdifferentiation and Myelopoiesis. Stem Cell Rep. 2015;5:232–247. doi: 10.1016/j.stemcr.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suzuki T., Shimizu Y., Furuhata E., Maeda S., Kishima M., Nishimura H., Enomoto S., Hayashizaki Y., Suzuki H. RUNX1 regulates site specificity of DNA demethylation by recruitment of DNA demethylation machineries in hematopoietic cells. Blood Adv. 2017;1:1699–1711. doi: 10.1182/bloodadvances.2017005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y.-P., Xiao M., Chen X., Chen L., Xu Y., Lv L., Wang P., Yang H., Ma S., Lin H., et al. WT1 Recruits TET2 to Regulate Its Target Gene Expression and Suppress Leukemia Cell Proliferation. Mol. Cell. 2015;57:662–673. doi: 10.1016/j.molcel.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rampal R., Akalin A., Madzo J., Vasanthakumar A., Pronier E., Patel J., Li Y., Ahn J., Abdel-Wahab O., Shih A., et al. DNA Hydroxymethylation Profiling Reveals that WT1 Mutations Result in Loss of TET2 Function in Acute Myeloid Leukemia. Cell Rep. 2014;9:1841–1855. doi: 10.1016/j.celrep.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alvarez-Errico D., Vento-Tormo R., Sieweke M., Ballestar E. Epigenetic control of myeloid cell differentiation, identity and function. Nat. Rev. Immunol. 2014;15:7–17. doi: 10.1038/nri3777. [DOI] [PubMed] [Google Scholar]
- 84.De La Rica L., Rodríguez-Ubreva J., García M., Islam A.B.M.M.K., Urquiza J.M., Hernando H., Christensen J., Helin K., Gómez-Vaquero C., Ballestar E. PU. 1 target genes undergo Tet2-coupled demethylation and DNMT3b-mediated methylation in monocyte-to-osteoclast differentiation. Genome Biol. 2013;14:1–21. doi: 10.1186/gb-2013-14-9-r99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morante-Palacios O., Ciudad L., Micheroli R., de la Calle-Fabregat C., Li T., Barbisan G., Houtman M., Edalat S.G., Frank-Bertoncelj M., Ospelt C., et al. Coordinated glucocorticoid receptor and MAFB action induces tolerogenesis and epigenome remodeling in dendritic cells. Nucleic Acids Res. 2021;50:108–126. doi: 10.1093/nar/gkab1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mendes K., Schmidhofer S., Minderjahn J., Glatz D., Kiesewetter C., Raithel J., Wimmer J., Gebhard C., Rehli M. The epigenetic pioneer EGR2 initiates DNA demethylation in differentiating monocytes at both stable and transient binding sites. Nat. Commun. 2021;12:1–15. doi: 10.1038/s41467-021-21661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Q., Zhao K., Shen Q., Han Y., Gu Y., Li X., Zhao D., Liu Y., Wang C., Zhang X., et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525:389–393. doi: 10.1038/nature15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madzo J., Liu H., Rodriguez A., Vasanthakumar A., Sundaravel S., Caces D.B.D., Looney T.J., Zhang L., Lepore J.B., Macrae T., et al. Hydroxymethylation at Gene Regulatory Regions Directs Stem/Early Progenitor Cell Commitment during Erythropoiesis. Cell Rep. 2013;6:231–244. doi: 10.1016/j.celrep.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kulis M., Merkel A., Heath S., Queiros A., Schuyler R.P., Castellano G., Beekman R., Raineri E., Esteve-Codina A., Clot G., et al. Whole-genome fingerprint of the DNA methylome during human B cell differentiation. Nat. Genet. 2015;47:746–756. doi: 10.1038/ng.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guilhamon P., Eskandarpour M., Halai D., Wilson G.A., Feber A., Teschendorff A.E., Gómez V., Hergovich A., Tirabosco R., Amary M.F., et al. Meta-analysis of IDH-mutant cancers identifies EBF1 as an interaction partner for TET2. Nat. Commun. 2013;4:2166. doi: 10.1038/ncomms3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lio C.-W.J., Yuita H., Rao A. Dysregulation of the TET family of epigenetic regulators in lymphoid and myeloid malignancies. Blood. 2019;134:1487–1497. doi: 10.1182/blood.2019791475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lio C.-W., Zhang J., González-Avalos E., Hogan P.G., Chang X., Rao A. Tet2 and Tet3 cooperate with B-lineage transcription factors to regulate DNA modification and chromatin accessibility. eLife. 2016;5:e18290. doi: 10.7554/eLife.18290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lio C.-W.J., Shukla V., Samaniego-Castruita D., González-Avalos E., Chakraborty A., Yue X., Schatz D.G., Ay F., Rao A. TET enzymes augment activation-induced deaminase (AID) expression via 5-hydroxymethylcytosine modifications at the Aicda superenhancer. Sci. Immunol. 2019;4:eaau7523. doi: 10.1126/sciimmunol.aau7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsangaratou A., Äijö T., Lio J., Yue X., Huang Y., Jacobsen S.E., Lähdesmäki H., Rao A. Dissecting the dynamic changes of 5-hydroxymethylcytosine in T-cell development and differentiation. Proc. Natl. Acad. Sci. USA. 2014;111:E3306–E3315. doi: 10.1073/pnas.1412327111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ding N., Bonham E.M., Hannon B.E., Amick T.R., Baylin S.B., O’Hagan H.M. Mismatch repair proteins recruit DNA methyltransferase 1 to sites of oxidative DNA damage. J. Mol. Cell Biol. 2016;8:244–254. doi: 10.1093/jmcb/mjv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O’Hagan H.M., Wang W., Sen S., DeStefanoShields C., Lee S., Zhang Y.W., Clements E.G., Cai Y., Van Neste L., Easwaran H., et al. Oxidative Damage Targets Complexes Containing DNA Methyltransferases, SIRT1, and Polycomb Members to Promoter CpG Islands. Cancer Cell. 2011;20:606–619. doi: 10.1016/j.ccr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kafer G.R., Li X., Horii T., Suetake I., Tajima S., Hatada I., Carlton P.M. 5-Hydroxymethylcytosine Marks Sites of DNA Damage and Promotes Genome Stability. Cell Rep. 2016;14:1283–1292. doi: 10.1016/j.celrep.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 98.An J., González-Avalos E., Chawla A., Jeong M., López-Moyado I.F., Li W., Goodell M., Chavez L., Ko M., Rao A. Acute loss of TET function results in aggressive myeloid cancer in mice. Nat. Commun. 2015;6:10071. doi: 10.1038/ncomms10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen L.-L., Lin H.-P., Zhou W.-J., He C.-X., Zhang Z.-Y., Cheng Z.-L., Song J.-B., Liu P., Chen X.-Y., Xia Y.-K., et al. SNIP1 Recruits TET2 to Regulate c-MYC Target Genes and Cellular DNA Damage Response. Cell Rep. 2018;25:1485–1500.e4. doi: 10.1016/j.celrep.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song C., Wang L., Wu X., Wang K., Xie D., Xiao Q., Li S., Jiang K., Liao L., Yates J.R., et al. PML Recruits TET2 to Regulate DNA Modification and Cell Proliferation in Response to Chemotherapeutic Agent. Cancer Res. 2018;78:2475–2489. doi: 10.1158/0008-5472.CAN-17-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rasmussen K.D., Jia G., Johansen J.V., Pedersen M.T., Rapin N., Bagger F.O., Porse B.T., Bernard O.A., Christensen J., Helin K. Loss of TET2 in hematopoietic cells leads to DNA hypermethylation of active enhancers and induction of leukemogenesis. Genes Dev. 2015;29:910–922. doi: 10.1101/gad.260174.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tulstrup M., Soerensen M., Hansen J.W., Gillberg L., Needhamsen M., Kaastrup K., Helin K., Christensen K., Weischenfeldt J., Grønbæk K. TET2 mutations are associated with hypermethylation at key regulatory enhancers in normal and malignant hematopoiesis. Nat. Commun. 2021;12:1–10. doi: 10.1038/s41467-021-26093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Figueroa M.E., Lugthart S., Li Y., Erpelinck-Verschueren C., Deng X., Christos P.J., Schifano E., Booth J., van Putten W., Skrabanek L., et al. DNA Methylation Signatures Identify Biologically Distinct Subtypes in Acute Myeloid Leukemia. Cancer Cell. 2010;17:13–27. doi: 10.1016/j.ccr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ko M., Huang Y., Jankowska A.M., Pape U.J., Tahiliani M., Bandukwala H.S., An J., Lamperti E.D., Koh K.P., Ganetzky R., et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–843. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dominguez P.M., Ghamlouch H., Rosikiewicz W., Kumar P., Béguelin W., Fontan L., Rivas M.A., Pawlikowska P., Armand M., Mouly E., et al. TET2 deficiency causes germinal center hyperplasia, impairs plasma cell differentiation and promotes B-cell lymphomagenesis. Cancer Discov. 2018;8:1632–1653. doi: 10.1158/2159-8290.CD-18-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rosikiewicz W., Chen X., Dominguez P.M., Ghamlouch H., Aoufouchi S., Bernard O.A., Melnick A., Li S. TET2 deficiency reprograms the germinal center B cell epigenome and silences genes linked to lymphomagenesis. Sci. Adv. 2020;6:eaay5872. doi: 10.1126/sciadv.aay5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Couronné L., Bastard C., Bernard O.A. TET2andDNMT3AMutations in Human T-Cell Lymphoma. N. Engl. J. Med. 2012;366:95–96. doi: 10.1056/NEJMc1111708. [DOI] [PubMed] [Google Scholar]
- 108.Odejide O., Weigert O., Lane A.A., Toscano D., Lunning M.A., Kopp N., Kim S.S., Van Bodegom D., Bolla S., Schatz J., et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123:1293–1296. doi: 10.1182/blood-2013-10-531509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Asmar F., Punj V., Christensen J., Pedersen M.T., Pedersen A., Nielsen A.B., Hother C., Ralfkiaer U., Brown P., Ralfkiaer E., et al. Genome-wide profiling identifies a DNA methylation signature that associates with TET2 mutations in diffuse large B-cell lymphoma. Haematologica. 2013;98:1912–1920. doi: 10.3324/haematol.2013.088740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coltro G., Mangaonkar A.A., Lasho T.L., Finke C.M., Pophali P., Carr R., Gangat N., Binder M., Pardanani A., Fernandez-Zapico M., et al. Clinical, molecular, and prognostic correlates of number, type, and functional localization of TET2 mutations in chronic myelomonocytic leukemia (CMML)—A study of 1084 patients. Leukemia. 2020;34:1407–1421. doi: 10.1038/s41375-019-0690-7. [DOI] [PubMed] [Google Scholar]
- 111.Lewis N.E., Petrova-Drus K., Huet S., Epstein-Peterson Z.D., Gao Q., Sigler A.E., Baik J., Ozkaya N., Moskowitz A.J., Kumar A., et al. Clonal hematopoiesis in angioimmunoblastic T-cell lymphoma with divergent evolution to myeloid neoplasms. Blood Adv. 2020;4:2261–2271. doi: 10.1182/bloodadvances.2020001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yao W., Wu F., Zhang W., Chuang S., Thompson J.S., Chen Z., Zhang S., Clipson A., Wang M., Liu H., et al. Angioimmunoblastic T-cell lymphoma contains multiple clonal T-cell populations derived from a common TET2 mutant progenitor cell. J. Pathol. 2019;250:346–357. doi: 10.1002/path.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang R., Gao X., Yu L. The prognostic impact of tet oncogene family member 2 mutations in patients with acute myeloid leukemia: A systematic-review and meta-analysis. BMC Cancer. 2019;19:389. doi: 10.1186/s12885-019-5602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Konstandin N.P., Pastore F., Herold T., Dufour A., Rothenberg-Thurley M., Hinrichsen T., Ksienzyk B., Tschuri S., Schneider S., Hoster E., et al. Genetic heterogeneity of cytogenetically normal AML with mutations of CEBPA. Blood Adv. 2018;2:2724–2731. doi: 10.1182/bloodadvances.2018016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kosmider O., Delabesse E., Mas V.M.-D., Cornillet-Lefebvre P., Blanchet O., Delmer A., Recher C., Raynaud S., Bouscary D., Viguié F., et al. TET2 mutations in secondary acute myeloid leukemias: A French retrospective study. Haematologica. 2011;96:1059–1063. doi: 10.3324/haematol.2011.040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kosmider O., Gelsi-Boyer V., Cheok M., Grabar S., Della-Valle V., Picard F., Viguié F., Quesnel B., Beyne-Rauzy O., Solary E., et al. TET2 mutation is an independent favorable prognostic factor in myelodysplastic syndromes (MDSs) Blood. 2009;114:3285–3291. doi: 10.1182/blood-2009-04-215814. [DOI] [PubMed] [Google Scholar]
- 117.Mallo M., Del Rey M., Ibáñez M., Calasanz M.J., Arenillas L., Larráyoz M.J., Pedro C., Jerez A., Maciejewski J., Costa D., et al. Response to lenalidomide in myelodysplastic syndromes with del(5q): Influence of cytogenetics and mutations. Br. J. Haematol. 2013;162:74–86. doi: 10.1111/bjh.12354. [DOI] [PubMed] [Google Scholar]
- 118.Coltro G., Antelo G., Lasho T.L., Bs C.M.F., Pardanani A., Gangat N., Carr R.M., Binder M., Mangaonkar A.A., Ketterling R., et al. Phenotypic correlates and prognostic outcomes of TET2 mutations in myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes: A comprehensive study of 504 adult patients. Am. J. Hematol. 2020;95 doi: 10.1002/ajh.25721. [DOI] [PubMed] [Google Scholar]
- 119.Makishima H., Jankowska A.M., McDevitt M.A., O’Keefe C., Dujardin S., Cazzolli H., Przychodzen B., Prince C., Nicoll J., Siddaiah H., et al. CBL, CBLB, TET2, ASXL1, and IDH1/2 mutations and additional chromosomal aberrations constitute molecular events in chronic myelogenous leukemia. Blood. 2011;117:e198–e206. doi: 10.1182/blood-2010-06-292433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lacy S.E., Barrans S.L., Beer P.A., Painter D., Smith A.G., Roman E., Cooke S.L., Ruiz C., Glover P., Van Hoppe S.J.L., et al. Targeted sequencing in DLBCL, molecular subtypes, and outcomes: A Haematological Malignancy Research Network report. Blood. 2020;135:1759–1771. doi: 10.1182/blood.2019003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Welch J.S., Ley T.J., Link D.C., Miller C.A., Larson D.E., Koboldt D.C., Wartman L.D., Lamprecht T.L., Liu F., Xia J., et al. The Origin and Evolution of Mutations in Acute Myeloid Leukemia. Cell. 2012;150:264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jaiswal S., Fontanillas P., Flannick J., Manning A., Grauman P.V., Mar B.G., Lindsley R.C., Mermel C.H., Burtt N., Chavez A., et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fuster J.J., MacLauchlan S., Zuriaga M.A., Polackal M.N., Ostriker A.C., Chakraborty R., Wu C.-L., Sano S., Muralidharan S., Rius C., et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xie M., Lu C., Wang J., McLellan M.D., Johnson K.J., Wendl M.C., McMichael J.F., Schmidt H.K., Yellapantula V., Miller C.A., et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 2014;20:1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li Z., Cai X., Cai C.-L., Wang J., Zhang W., Petersen B.E., Yang F.-C., Xu M. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011;118:4509–4518. doi: 10.1182/blood-2010-12-325241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ostrander E.L., Kramer A.C., Mallaney C., Celik H., Koh W.K., Fairchild J., Haussler E., Zhang C.R., Challen G.A. Divergent Effects of Dnmt3a and Tet2 Mutations on Hematopoietic Progenitor Cell Fitness. Stem Cell Rep. 2020;14:551–560. doi: 10.1016/j.stemcr.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rasmussen K.D., Berest I., Keβler S., Nishimura K., Simón-Carrasco L., Vassiliou G.S., Pedersen M.T., Christensen J., Zaugg J.B., Helin K. TET2 binding to enhancers facilitates transcription factor recruitment in hematopoietic cells. Genome Res. 2019;29:564–575. doi: 10.1101/gr.239277.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cai Z., Kotzin J.J., Ramdas B., Chen S., Nelanuthala S., Palam L.R., Pandey R., Mali R.S., Liu Y., Kelley M.R., et al. Inhibition of Inflammatory Signaling in Tet2 Mutant Preleukemic Cells Mitigates Stress-Induced Abnormalities and Clonal Hematopoiesis. Cell Stem Cell. 2018;23:833–849.e5. doi: 10.1016/j.stem.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cull A.H., Snetsinger B., Buckstein R., Wells R.A., Rauh M.J. Tet2 restrains inflammatory gene expression in macrophages. Exp. Hematol. 2017;55:56–70.e13. doi: 10.1016/j.exphem.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 130.Meisel M., Hinterleitner R., Pacis A., Chen L., Earley Z.M., Mayassi T., Pierre J.F., Ernest J.D., Galipeau H.J., Thuille N., et al. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature. 2018;557:580–584. doi: 10.1038/s41586-018-0125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Papaemmanuil E., Gerstung M., Bullinger L., Gaidzik V.I., Paschka P., Roberts N.D., Potter N.E., Heuser M., Thol F., Bolli N., et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016;374:2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Elena C., Galli’ A., Such E., Meggendorfer M., Germing U., Rizzo E., Cervera J., Molteni E., Fasan A., Schuler E., et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood. 2016;128:1408–1417. doi: 10.1182/blood-2016-05-714030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Palomo L., Malinverni R., Cabezón M., Xicoy B., Arnan M., Coll R., Pomares H., García O., Fuster-Tormo F., Grau J., et al. DNA methylation profile in chronic myelomonocytic leukemia associates with distinct clinical, biological and genetic features. Epigenetics. 2018;13:8–18. doi: 10.1080/15592294.2017.1405199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wilson E.R., Helton N.M., Heath S.E., Fulton R.S., Payton J.E., Welch J.S., Walter M.J., Westervelt P., DiPersio J.F., Link D.C., et al. Focal disruption of DNA methylation dynamics at enhancers in IDH-mutant AML cells. Leukemia. 2021:1–11. doi: 10.1038/s41375-021-01476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Das A., Kakadia P.M., Wojcik D., Pemberton L., Browett P.J., Bohlander S.K., Vissers M.C.M. Clinical remission following ascorbate treatment in a case of acute myeloid leukemia with mutations in TET2 and WT1. Blood Cancer J. 2019;9:1–5. doi: 10.1038/s41408-019-0242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao H., Zhu H., Huang J., Zhu Y., Hong M., Zhu H., Zhang J., Li S., Yang L., Lian Y., et al. The synergy of Vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leuk. Res. 2018;66:1–7. doi: 10.1016/j.leukres.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 137.Mingay M., Chaturvedi A., Bilenky M., Cao Q., Jackson L., Hui T., Moksa M., Heravi-Moussavi A., Humphries R.K., Heuser M., et al. Vitamin C-induced epigenomic remodelling in IDH1 mutant acute myeloid leukaemia. Leukemia. 2017;32:11–20. doi: 10.1038/leu.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kulis M., Heath S., Bibikova M., Queirós A.C., Navarro A., Clot G., Martínez-Trillos A., Castellano G., Brun-Heath I., Pinyol M., et al. Epigenomic analysis detects widespread gene-body DNA hypomethylation in chronic lymphocytic leukemia. Nat. Genet. 2012;44:1236–1242. doi: 10.1038/ng.2443. [DOI] [PubMed] [Google Scholar]
- 139.Oakes C.C., Seifert M., Assenov Y., Gu L., Przekopowitz M., Ruppert A.S., Wang Q., Imbusch C.D., Serva A., Koser S.D., et al. DNA methylation dynamics during B cell maturation underlie a continuum of disease phenotypes in chronic lymphocytic leukemia. Nat. Genet. 2016;48:253–264. doi: 10.1038/ng.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beekman R., Chapaprieta V., Russiñol N., Vilarrasa-Blasi R., Verdaguer N., Martens J., Duran-Ferrer M., Kulis M., Serra F., Javierre B.M., et al. The reference epigenome and regulatory chromatin landscape of chronic lymphocytic leukemia. Nat. Med. 2018;24:868–880. doi: 10.1038/s41591-018-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vilarrasa-Blasi R., Soler-Vila P., Verdaguer-Dot N., Russiñol N., Di Stefano M., Chapaprieta V., Clot G., Farabella I., Cuscó P., Kulis M., et al. Dynamics of genome architecture and chromatin function during human B cell differentiation and neoplastic transformation. Nat. Commun. 2021;12:1–18. doi: 10.1038/s41467-020-20849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maurano M.T., Wang H., John S., Shafer A., Canfield T., Lee K., Stamatoyannopoulos J.A. Role of DNA Methylation in Modulating Transcription Factor Occupancy. Cell Rep. 2015;12:1184–1195. doi: 10.1016/j.celrep.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 143.Sasaki M., Knobbe C.B., Munger J.C., Lind E.F., Brenner D., Bruestle A., Harris I.S., Holmes R., Wakeham A., Haight J., et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488:656–659. doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Izzo F., Lee S.C., Poran A., Chaligne R., Gaiti F., Gross B., Murali R.R., Deochand S.D., Ang C., Jones P.W., et al. DNA methylation disruption reshapes the hematopoietic differentiation landscape. Nat. Genet. 2020;52:378–387. doi: 10.1038/s41588-020-0595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharma R., Choi K.-J., Quan M.D., Sharma S., Sankaran B., Park H., LaGrone A., Kim J.J., MacKenzie K.R., Ferreon A.C.M., et al. Liquid condensation of reprogramming factor KLF4 with DNA provides a mechanism for chromatin organization. Nat. Commun. 2021;12:1–17. doi: 10.1038/s41467-021-25761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boija A., Klein I.A., Sabari B.R., Dall’Agnese A., Coffey E.L., Zamudio A.V., Li C.H., Shrinivas K., Manteiga J.C., Hannett N.M., et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell. 2018;175:1842–1855.e16. doi: 10.1016/j.cell.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]