Abstract
Prompt antiviral treatment has the potential to reduce influenza virus transmission to close contacts, but rigorous data on the magnitude of treatment effects on transmission are limited. Animal model data indicate that rapid reductions in viral replication after antiviral treatment reduce the risk of transmission. Observational and clinical trial data with oseltamivir and other neuraminidase inhibitors indicate that prompt treatment of household index patients seems to reduce the risk of illness in contacts, although the magnitude of the reported effects has varied widely across studies. In addition, the potential risk of transmitting drug-resistant variants exists with all approved classes of influenza antivirals. A controlled trial examining baloxavir treatment efficacy to reduce transmission, including the risk of transmitting virus with reduced baloxavir susceptibility, is currently in progress. If reduced transmission risk is confirmed, modeling studies indicate that early treatment could have major epidemiologic benefits in seasonal and pandemic influenza.
Keywords: influenza, transmission, antiviral, baloxavir, oseltamivir
Prompt antiviral treatment has the potential to reduce influenza virus transmission from infected persons to contacts. This article reviews data addressing this topic, including resistance and the potential role of antiviral treatment in indirectly reducing seasonal and pandemic influenza burden.
Seasonal and pandemic influenza viruses can cause explosive outbreaks of illness leading to considerable morbidity of varying severity. Person-to-person transmission is facilitated by several factors, including high infectious virus titers in the respiratory tract immediately before and at illness onset, a short incubation period, and the potential for spread via multiple routes (eg, respiratory droplets, aerosols, and hand contamination–self inoculation) and in various transmission settings (eg, households, schools, acute, and chronic care facilities) [1]. As with severe acute respiratory syndrome coronavirus 2, many questions remain about the relative importance of different transmission routes, transmission risk from asymptomatic or presymptomatic persons, and the impact of superspreading events.
Currently, the principal strategies to reduce transmission, in addition to virus-specific immunization, are nonpharmaceutical interventions (NPIs) directed at the individual (eg, cough etiquette, hand hygiene, masking, case isolation, and physical distancing) and at the facility or community level (eg, school closures, curtailing mass gatherings, and travel restrictions) [2, 3]. The effectiveness of NPIs in reducing influenza transmission has been debated and often has been limited by poor compliance and delayed application. Household- and dormitory-based trials indicate that prompt, consistent use of masking and hand hygiene may reduce secondary illnesses in close contacts [3, 4], and modeling studies indicate that timely school closures or holidays impact the magnitude of local outbreaks. Of note, wide-scale use of NPIs, including masking and community mitigation strategies like stay-at-home orders, business closures, and travel restrictions in response to the severe acute respiratory syndrome coronavirus 2, pandemic, have markedly diminished the circulation of seasonal influenza viruses in most countries at present [5]. These observations indicate that sustained adherence to social distancing NPIs can reduce seasonal influenza virus transmissibility and that of other respiratory viruses.
Despite the incomplete protection afforded by current influenza vaccines, immunization has the direct benefit of reducing the risk of influenza illness and complications in recipients. Increased vaccine coverage may provide indirect protection in nonimmunized persons and in those with inadequate immune responses to vaccine, especially when targeted to those central to influenza virus transmission like school-aged children. While the extent of seasonal influenza vaccine coverage has been associated with reductions in hospitalizations and deaths, there is little evidence that it has reduced the overall duration of seasonal outbreaks.
Two complementary pharmacologic strategies to reduce respiratory virus transmission to contacts are chemoprophylaxis and rapid antiviral treatment of ill persons to reduce their infectiousness. The rationale for the latter is based on the use of a sufficiently potent antiviral to quickly reduce infectious virus titers in the respiratory tract and also diminish symptoms (eg, coughing, runny nose, sneezing) that contribute to the generation of infectious secretions. To date, 3 classes of inhibitors that target the influenza viral M2 ion channel (adamantanes), neuraminidase (eg, oseltamivir, zanamivir), or cap-dependent endonuclease (baloxavir) have been shown to be effective for both chemoprophylaxis [6, 7] and for treating outpatients with influenza due to susceptible strains, although the magnitude of the antiviral efficacy differs across these agents. This commentary focuses on the body of evidence from studies that have addressed the effects of influenza antiviral treatment on reducing virus transmissibility. We review data from nonclinical, clinical, and modeling studies, and we consider the potential reductions in seasonal and pandemic influenza morbidity and mortality rates associated with increased therapeutic antiviral use.
NONCLINICAL STUDIES
A limited number of animal model studies have investigated the effect of antiviral treatment on reducing viral shedding and virus transmission to contact animals. To our knowledge, only 4 studies have specifically evaluated the effect of antiviral treatment on the likelihood of onward transmission, all of which used either the ferret or the guinea pig model (Table 1). Three of these studies focused on the Food and Drug Administration–approved antivirals oseltamivir and baloxavir [8–10], and 1 evaluated the effect of an investigational anti-hemagglutinin head monoclonal antibody [11]. When this plant-produced human anti-hemagglutinin antibody was administered to guinea pigs 24 hours after infection with A/H1N1pdm09 virus, viral shedding duration was reduced by day 3 after inoculation compared with controls, and onward transmission to cohoused contacts was completely prevented compared with control-treated animals, all of which were infected [11] (Table 1).
Table 1.
The Effect of Antiviral Treatment on Blocking Onward Influenza Virus Transmission in Animal Models
| Reference | Experimental Setup | Transmission to Contacts | ||
|---|---|---|---|---|
| Inoculation | Donor Antiviral Regimen | Transmission | ||
| Oh et al (2014) [8] | Ferret; intranasal; A/H1N1pdm09 (106 TCID50) | Oseltamivir: 5 mg/kg, BID for 10 d (36 h after inoculation) | Direct contact: donor cohoused with 6 naive contacts (24 h after inoculation) | No difference in oseltamivir vs control group (12/12 infected; both groups) |
| Belser et al (2015) [9] | 1. Ferret; A/H1N1pdm09 (<1 PFUs) via OA inoculation 2. Ferret; A/H7N3 (15–216 PFUs) via OA inoculation 3. Ferret; A/H7N9 (214–520 PFUs) via OA inoculation 4. Ferret; A/H7N9 via OA (116–840 PFUs) plus respiratory-aerosol inoculation (1.2–11 PFUs) inoculation |
Oseltamivir: 12.5 mg/kg, BID for 5 d (2 h after inoculation) | Direct contact: donor cohoused with 1 naive contact (from 24 h after inoculation onward) | 1. Reduced transmission in oseltamivir vs control groupa (1/3 vs 3/3 infected) 2. Reduced transmission in oseltamivir vs control groupb (0/3 vs 1/2 infected) 3. No difference in oseltamivir vs control groupc (2/2 vs 4/4 infected) 4. Reduced transmission in oseltamivir vs control groupc (2/4 vs 4/4 infected) |
| Lee et al (2020) [10] | Ferret; intranasal; A/H1N1pdm09 (1000 PFUs) | Baloxavir: 4 mg/kg, single dose (24 h after inoculation) Oseltamivir: 5 mg/kg, BID for 5 d (24 h after inoculation) |
Direct and indirect contact: donor cohoused with 1 naive direct contact and in a cage adjacent to indirect contacts (24 h after inoculation) | Baloxavir: direct contact, no difference in baloxavir vs control group (4/4 infected; both groups); indirect contact, reduced transmission in baloxavir vs control group (1/4 vs 3/4 infected) Oseltamivir: direct contact, no difference in oseltamivir vs control group (4/4; both groups); indirect contact, no difference in oseltamivir vs control group (3/4; both groups) |
| Ferret; intranasal; A/H1N1pdm09 (102 TCID50) | Baloxavir: 4 mg/kg, single dose (24 h after inoculation) Oseltamivir: 5 mg/kg, BID for 2 d (24 h after inoculation) |
Direct contact: donor cohoused with 1 naive contact (24 h after inoculation) | Baloxavir: reduced transmission in baloxavir vs control group (1/4 vs 4/4 infected) Oseltamivir: no difference in oseltamivir vs control group (4/4; both groups) |
|
| Ferret; intranasal; A/H1N1pdm09 (102 TCID50) | Baloxavir: 4 mg/kg, single dose (24 h after inoculation) Oseltamivir: 5 mg/kg, BID for 3 d (24 h after inoculation) |
Direct contact: donor was cohoused with 1 naive contact (48 h after inoculation) | Baloxavir: reduced transmission in baloxavir vs control group (1/4 vs 4/4 infected) Oseltamivir: no difference in oseltamivir vs control group (4/4; both groups) |
|
| Ferret; intranasal; A/H1N1pdm09 (102 TCID50) | Baloxavir: 4 mg/kg, single dose (48 h after inoculation) Oseltamivir: 5 mg/kg, BID for 2 d (48 h after inoculation) |
Direct contact: donor was cohoused with 1 naive contact (48 h after inoculation) | Baloxavir: reduced transmission in baloxavir vs control group (2/4 vs 4/4 infected) Oseltamivir: no difference in oseltamivir vs control group (4/4; both groups) |
|
| Park et al (2020) [11] | Guinea pig; intranasal; A/H1N1pdm09 (1000 PFUs) | Broadly neutralizing anti-H1 (head) monoclonal antibody 24 h after inoculation | Direct contact: each donor cohoused with 1 naive contact (from 48 h after inoculation for 7 d) | Reduced transmission in anti-H1 vs control group (0/3 vs 3/3 infected) |
Abbreviations: BID, twice daily; OA, ocular-aerosol; PFUs, plaque-forming units; TCID50, tissue culture infectious dose at 50%
aMock-treated donors received OA inoculation with 0.36–0.84 PFUs of A/Mexico/4482/2009 A/H1N1pdm09 virus.
bMock-treated donors received OA inoculation with 94–114 PFUs of A/Mexico/InDRE7298/2012 (H7N3) virus.
cMock-treated donors received OA inoculation with 780–1220 PFUs of A/Anhui/1/2013 (H7N9) virus.
Of the 3 studies evaluating the effect of oseltamivir in ferrets [8–10], only 1 demonstrated reductions in onward transmission [9]. That study examined transmission of A/H1N1pdm09 and zoonotic A/H7N3 and A/H7N9 viruses after infection by ocular-aerosol or ocular-aerosol plus respiratory-aerosol inoculation. Oseltamivir treatment started 2 hours after infection reduced viral replication in ferrets inoculated with a low dose of A/H1N1pdm09 or A/H7N3, but not A/H7N9 viruses. Subsequent direct contact transmissions (ie, through cohousing in the same cage) of A/H1N1pdm09 virus and A/H7N3 virus was prevented [9]. A higher dose of oseltamivir and a lower dose of virus inoculum may have contributed to the protective antiviral effect observed in this study compared with 2 studies that observed no protective effect of oseltamivir [8, 10] (Table 1).
Oseltamivir treatment of donor ferrets had no effect on the likelihood of infecting cohoused sentinel animals, but baloxavir treatment resulted in reduced transmission of A/H1N1pdm09 viruses by direct contact and also by indirect (ie, airborne) spread [10] (Table 1). Higher viral inocula to donor ferrets reduced the efficacy of baloxavir in preventing direct contact transmission. The effect of baloxavir treatment on viral shedding was rapid, such that reduced transmission frequency was observed regardless of whether ferrets were cohoused immediately or 24 hours after treatment. When baloxavir treatment was delayed until 48 hours after infection, some reduction in transmission to contact ferrets was still observed. This may be an important finding given that influenza-infected patients often are delayed in seeking treatment.
Animal models have also been used to assess the replication and transmission fitness of antiviral-resistant influenza virus variants. Early studies in ferrets (before 2007) showed that the oseltamivir-resistant variant R292K (A/H3N2) did not readily transmit compared with wild-type (WT) viruses [12, 13], although H275Y (A/H1N1) and E119V (A/H3N2) variants were able to transmit among ferrets by direct contact [12, 13]. Between 2007 and 2009, seasonal A/H1N1 viruses with the oseltamivir-resistant H275Y variant became widespread globally due to the acquisition of several compensatory mutations [14], and were confirmed to transmit efficiently in animal models [15, 16]. Although these oseltamivir-resistant variants were ultimately displaced by oseltamivir-sensitive pandemic A/H1N1pdm09 virus, clusters of oseltamivir-resistant A/H1N1pdm09 virus have been identified [17], and ferret models suggested that these variants can replicate and transmit efficiently [18]. Likewise, some baloxavir-resistant viruses with polymerase acidic/I38X substitutions also appear to transmit with similar efficiency as WT viruses in ferrets [19, 20], although competitive fitness experiments evaluating the effect of the polymerase acidic/I38T substitution in A/H1N1pdm09 and A/H3N2 viruses detected a minor reduction in fitness compared with their respective WT viruses [21].
CLINICAL STUDIES
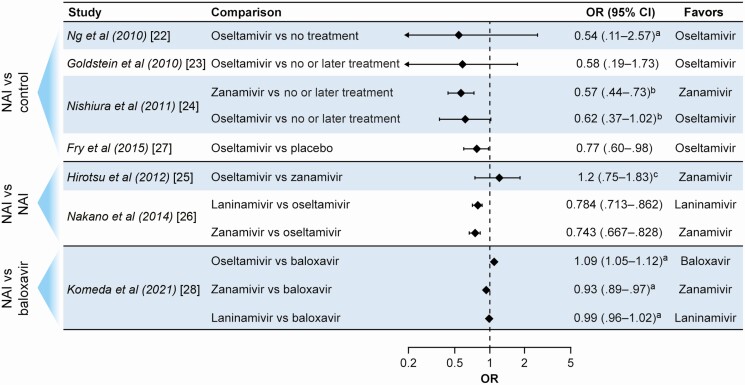
Households are important sites for transmission of influenza viruses, and randomized controlled trials (RCTs) have demonstrated that timely use of antiviral postexposure prophylaxis (PEP) is highly effective in reducing illness risk in contacts [6, 7]. Several household-based studies have attempted to determine whether antiviral treatment of ill index patients (IPs) is associated with reduced influenza virus transmission to close contacts (Table 2 and Figure 1).
Table 2.
Representative Clinical and Epidemiologic Studies on Influenza Virus Transmission to Household Contacts of Index Patients Treated With Neuraminidase Inhibitor or Baloxavir
| Reference | Study Design | Study Location (Years) | No. of IPs/No. of Contacts (or Households) | Secondary Illness Definition | Influenza Outcomesa | Commentsa |
|---|---|---|---|---|---|---|
| Ng et al (2010) [22] | Prospective, observational, cohort | Hong Kong (2007–2008) | 384/989; Oseltamivir in 90 IPs | Household contacts were followed up during 3–4 home visits to monitor symptoms and collect nose and throat swab samples over 7 d (2007) or 10 d (2008) | Overall SAR: 8.1% (6.5%–10%); contacts of IPs who had taken oseltamivir ≤24 h of onset had nonsignificantly numerically lower risks of laboratory-confirmed infection (adjusted OR 0.54; .11–2.57) or clinical illness (0.52; .25–1.08) compared with contacts of IPs who did not take oseltamivir | IPs who took oseltamivir ≤24 h after symptom onset reduced the time to symptom alleviation (adjusted acceleration factor, 0.56; 95% CI, .42–.76), compared with later or no treatment; oseltamivir treatment was not associated with significant reductions in the duration of viral shedding |
| Goldstein et al (2010) [23] | Prospective, observational, cohort | United States (2009) | 135/411; Oseltamivir in 91 IPs | RT-PCR–confirmed illness, ILI (fever with cough or sore throat), or ≥2 listed symptoms | Overall SAR: 13.4%; oseltamivir treatment of IP within 1 d of onset associated with lower odds (OR, 0.58; .19–1.73) of ≥1 secondary infection in a household and of secondary infection in individual contacts (OR, 0.50; .17–1.46) vs later or no treatment | Younger household contacts were at higher risk of infection (OR, 2.79, 1.50–5.20); larger household size (≥4 members) was associated with a higher risk of transmission; small number of households with early oseltamivir treatment in IP |
| Nishiura et al (2011) [24] | Retrospective, observational, survey | Japan (2009–2010) | Total: 1547/4689; antivirals in 83.6% of IPs |
RT-PCR or rapid antigen confirmation or development of ILI (fever with cough and/or sore throat) within 7 d after onset in IP | Overall SAR: 11.4% (10.5%–12.3%); zanamivir treatment of IP within 24 h (OR, 0.57; .44–.73 or within 24–48 h (0.58; .38–.86) associated with lower odds of secondary illness in contacts compared with delayed or no treatment; oseltamivir treatment of IP within 24 h (OR, 0.62; .37–1.02) or 24–48 h (1.10; .56–2.05) was not associated with lower odds of secondary illness |
SAR highest in contacts of IPs aged 0–4 y (19.4%; 15.4%–24.2%) and in contacts aged 0–4 y (29.6%; 24.8%–34.9%); antiviral prophylaxis with zanamivir or oseltamivir also used |
| Hirotsu et al (2012) [25] | Observational, cohort | Japan (2009–2010) | Total: 591/1629; oseltamivir in 295 and zanamivir in 296 IPs |
Clinic visit and positive result for influenza A by rapid diagnostic test within 7 d of onset in IP | Overall SAR: 7.3% (6.1%–8.7%); oseltamivir: 9.7%; zanamivir: 5.0%; differences in antiviral medication were not significantly associated with household transmission in a multivariate analysis |
Transmission associated with IPs aged ≤12 y and adults aged ≥30 y with children, ≥5 persons in the household, and antiviral treatment initiated ≥48 h after fever onset in IP |
| Nakano et al (2014) [26] | Retrospective, insurance database, active control | Japan (2010–2011) | Oseltamivir: 12 142/(12 142); laninamivir: 6362/(6362); zanamivir: 6222/(6222) (no. of IPs = no. of households) |
Prescription for NAI in non-IP family member 3–8 d after treatment of IP | Household secondary infection: oseltamivir, 14.3%; laninamivir, 11.0%; zanamivir, 11.6%; laninamivir (OR, 0.784; .713– .862; P < .001) and zanamivir (0.743; .667–.828; P < .001) associated with lower risk than oseltamivir |
Inhaled NAI treatment appeared more effective in reducing SAR than oseltamivir for IPs aged ≤15 y but not in older ones |
| Fry et al (2015) [27] | Double-blind, placebo-controlled randomized trial (1:1 ratio) of oseltamivir treatment in IPs aged ≥1 y | Bangladesh (2008–2010) | Total: 1190/4694; oseltamivir: 598/2402; placebo: 592/2292 |
Household secondary illness due to clinical illness and household secondary RT-PCR–confirmed illness | Overall SAR for illness: 9% of household members; household SAR for illness was lower in the oseltamivir group (8%) than in the placebo group (10%) (OR 0.77; .60–.98; P = .03); RT-PCR–confirmed influenza illness did not differ (4% vs 5%; OR, 0.84; .59–1.19) between the groups |
Trial conducted in crowded, low-income setting; median age (IQR) of IPs was 5 (3–10) y; the highest SAR risk was in household contacts aged <5 y; 4 treatment-emergent cases of oseltamivir resistance in A/H1N1pdm09 infections were detected (2 without ill contacts, 2 with ill contacts not sampled) |
| Komeda et al (2021) [28] | Retrospective, insurance database, active control | Japan (2018–2019) | Baloxavir: 84 672/(84 672); oseltamivir: 62 004/(62 004); zanamivir: 14 085/(14 085); laninamivir: 47 464/(47 464) |
Influenza diagnosed in any non-IP family member 3−8 d after treatment of IP | Household SAR: 17.98% for baloxavir vs 24.16% for oseltamivir (adjusted OR for oseltamivir vs baloxavir, 1.09; 1.05–1.12); vs zanamivir, 18.41% (0.93; .89–.97); vs laninamivir, 17.43% (adjusted OR, 0.99; .96–1.02) | Incidence of household transmission was especially high when the IP was aged ≤12 y and generally lower for influenza B than for influenza A virus |
Abbreviations: CI, confidence interval; ILI, influenzalike illness; IP, index patient; IQR, interquartile range; NAI, neuraminidase inhibitor; OR, odds ratio; RT-PCR, reverse-transcription polymerase chain reaction; SAR, secondary attack rate.
aRanges provided with ORs and SARs represent 95% CIs.
Figure 1.
Summary of main outcomes in observational studies and 1 published randomized trial examining the effects of antiviral treatment on influenza infection risk in household contacts. See Table 2 for details of study design and findings. Abbreviations: CI, confidence interval; NAI, neuraminidase inhibitor; OR, odds ratio. Footnotes: aAdjusted OR. bTreatment within 24 hours. cMultivariate analysis.
An early RCT gave all household members with influenza illness an adamantane (amantadine or rimantadine) or placebo. The authors concluded that “We evaluated the overall frequency of infection among families…; it was ~30% lower among those given an antiviral agent, a result suggesting that early treatment may not only be effective for ameliorating symptoms of influenza but also for reducing spread (unpublished data)” [29]. However, they cautioned that the data were pooled from 3 locations and 3 years of study and “must not be regarded as definitive.” A subsequent randomized placebo-controlled trial in which IPs and their contacts within a household were given either rimantadine or placebo found no reductions in secondary attack rates (SARs) in the rimantadine households because of the emergence and transmission of adamantane-resistant variants from treated IPs to their contacts [30].
Four subsequent RCTs of neuraminidase inhibitors (NAIs) (inhaled zanamivir and oseltamivir in 2 RCTs each), testing the efficacy of PEP, provided data that confirmed high prophylactic efficacy against illness in household contacts with both antivirals [31]. IPs were treated with the same antiviral used for PEP in 2 of the studies, and this modeling analysis of pooled data estimated that the effect on reducing infectiousness was 19% (95% confidence interval, −160% to 75%) for zanamivir and 80% (43%–93%) for oseltamivir. However, these trials were conducted in different seasons and with differing study designs, meaning that the large effect found with oseltamivir might have been due to these differences and other unidentified confounding factors between the trials.
One prospective observational study included 384 antigen-positive outpatients and their household contacts during periods of influenza activity in 2007–2008 [22]. Among the 331 households with a single IP, influenza confirmed by reverse-transcription polymerase chain reaction or viral culture developed in 8.1% of 989 contacts. Compared with contacts of IPs who did not take oseltamivir, contacts of IPs taking oseltamivir within 24 hours of symptom onset had a nonsignificantly lower risk of laboratory-confirmed infection (adjusted odds ratio [OR], 0.54) and clinical influenza (adjusted OR, 0.52) (Table 2). Lesser effects were found in contacts of IPs starting treatment later. Of note, oseltamivir treatment in IPs did not significantly decrease viral shedding in this study.
A trial conducted during the 2008–2009 season, comparing combined oseltamivir and inhaled zanamivir treatment with each respective monotherapy in 267 IPs, found no significant difference in the frequencies of secondary illness in household contacts between the arms (12.5% overall) [32]. However, when the analysis was limited to 136 IPs treated within 24 hours after symptom onset, a significantly lower proportion of contacts developed illness in the combination arm (4%) than in the oseltamivir (17%) or zanamivir (15%) arms. A large placebo-controlled trial of oseltamivir treatment in household IPs aged ≥1 year in Bangladesh during 2008–2010 found that illness was somewhat less frequent in the contacts of oseltamivir-treated IPs (8%) than in those of placebo recipients (10%) (OR, 0.77 [95% confidence interval, .60–.98]; P = .03), although no significant difference in reverse-transcription polymerase chain reaction–confirmed influenza was found (4% vs 5%), perhaps because of limited contact sampling [27]. Of note, this trial documented significant reductions in the frequency of infectious virus detection in oseltamivir-treated children [27].
Some evidence on the effect of antiviral treatment on household transmission comes from studies during the 2009 pandemic. A retrospective survey of 1547 households in Japan found a contact SAR of 11.4% overall [24]. Multivariable analysis showed that inhaled zanamivir treatment within 24 and 24–48 hours after illness onset, primarily administered to teenagers, significantly reduced the risk of household transmission to 0.57 and 0.58, respectively, compared with the effect of receiving delayed or no treatment, whereas the corresponding effects of oseltamivir on transmission risk were nonsignificant (Table 2). An observational study in 135 households found that oseltamivir treatment on either day of illness onset or the next day was associated with a nonsignificant 42% reduction in the odds of ≥1 secondary infections or influenzalike illness in a household and a 50% reduction in individual contacts, compared with later or no treatment [23].
Two studies using a large, Japanese health insurance claims database have compared household influenza transmission rates among families where the IP was treated with oseltamivir or an inhaled NAI [26] during the 2010–2011 season or with baloxavir or an NAI during the 2018–2019 season [28]. The first study found a significantly lower proportion of families with household transmission when the IP was treated with inhaled zanamivir (11.6%) or inhaled laninamivir (11.0%) compared with oseltamivir (14.3%) (Table 2). In the more recent study, baloxavir treatment was associated with lower household transmission than oseltamivir (17.98% vs 24.16%) (Table 2). In families in which the IP was treated with the inhaled zanamivir or laninamivir, household transmission was similar to that of baloxavir, at 18.41% and 17.43%, respectively. In both studies, comparisons were likely complicated by known (different IP ages or administration routes) and unknown confounding factors. A small retrospective study in Japan assessing the effect of IP treatment with baloxavir or oseltamivir on household transmission found a SAR of 9.0% in the baloxavir and 13.5% in the oseltamivir households (P = .34) (Table 2), indicating that baloxavir was at least as effective as oseltamivir in reducing transmission [33].
Taken together, the available studies suggest that prompt NAI or baloxavir treatment of household IPs likely reduces the risk of secondary illness in close contacts, albeit to a limited extent. However, most studies have not been designed to answer this question rigorously, and the magnitude of reported reductions are inconsistent across reports. The best contemporary data derive from the oseltamivir trial in Bangladesh which included a large number of pediatric IPs but may have underestimated the effects of treatment owing to the crowded housing conditions and sometimes delayed antiviral use [27]. Moreover, the risk of transmission of resistant variants from treated IPs, especially young children, to close contacts is an important question that has not been addressed adequately in most human influenza antiviral studies to date. More data are needed in household and other higher transmission-risk settings such as long-term care facilities, including studies of antiviral combinations.
Because of the more rapid and greater antiviral treatment efficacy of baloxavir compared with oseltamivir [34–36], baloxavir might exert greater effects on virus transmission. However, the higher frequency of treatment-emergent viruses with reduced baloxavir susceptibility compared with NAIs, particularly in younger children, raises concern about the transmission of such variants to contacts [37, 38]. An ongoing, randomized placebo-controlled phase III trial is assessing baloxavir efficacy in reducing onward transmission of influenza virus from IPs aged 5–64 years to their household contacts (NCT03969212) and may give important evidence on the value of this strategy. The primary end point is secondary transmission based on viral RNA detection in contacts up to day 5, and secondary end points include monitoring for baloxavir variants with reduced susceptibility and illness occurrence.
MODELING STUDIES
Multiple modeling studies have addressed the potential societal benefits of antiviral treatment in reducing healthcare utilization, deaths, and the economic consequences of seasonal and pandemic influenza [39] and the potential negative consequences of antiviral resistance emergence [40]. Several have concluded that mass targeted chemoprophylaxis might extinguish an emerging novel influenza virus [41] and that targeted chemoprophylaxis in households and schools could reduce transmission [41], but these are beyond the scope of this review.
Epidemiologic models primarily work on the assumption that patient infectiousness is equated with the presence of virus shedding [42], and exploring different relationships between viral load and infectiousness can be used to infer the impact of antiviral drugs. For example, infectiousness may be assumed to be constant over the time that viral load exceeds a defined threshold, and antivirals that shorten this interval would be predicted to reduce infectiousness. Alternatively, infectiousness can be assumed to be proportional to viral load (usually on a log or natural scale); this can be affected by antivirals that alter this time course. Another assumption is where the time dependence of infectiousness is ignored and instead antivirals are assumed to cause an overall reduction of transmission, which may be estimated from clinical studies.
The impact of antiviral treatment on population-level influenza transmission was examined using a hierarchical mathematical model to link within-host viral replication dynamics to between-host transmission [43]. Using data from the phase 3 baloxavir treatment trial [34] to characterize the model, the impact of initiating antiviral treatment at various time points after symptom onset was predicted, on the assumption of a logarithmic relationship between viral load and patient infectiousness. The model predicted that treatment within 48 hours of symptom onset would cause baloxavir-treated patients to be noninfectious within 2 days of treatment, whereas oseltamivir-treated patients would remain infectious for 4–5 days. When scaled up and applied to existing epidemiologic data from the 2017–2018 influenza season in the United States, it predicted substantial reductions in infections and deaths averted depending on the extent and timing of antiviral treatment levels (Table 3). Of note, this model did not consider the possible impact of treatment-emergent variants with reduced antiviral susceptibility [43].
Table 3.
Estimates of the Potential Impact of Antiviral Treatment on Clinical Outcome Measures Based on the 2017–2018 US Influenza Seasona
|
Outcome by Timing of Treatmentb |
Treatment Impactc | |
|---|---|---|
| Oseltamivir | Baloxavir | |
| Antiviral treatment ≤24 h after symptom onset in 30% of patients | ||
| Infections (63.3 million) | Reduced by 23% (16%–30%) or 14.3 million (10.4–18.8 million) | Reduced by 33% (26%–41%) or 21.1 million (16.4–26.2 million) |
| Hospitalizations (>900 000) | Reduced by 13% (10%–17%) or 119 500 (87 100–157 100) | Reduced by 20% (15%–24%) or 176 000 (137 200–218 500) |
| Deaths (>79 000) | Reduced by 5% (4%–7%) or 3978 (2899–5228) | Reduced by 7% (6%–9%) or 5858 (4567–7274) |
| Antiviral treatment ≤48 h after symptom onset in 30% of patients | ||
| Infections (63.3 million) | Reduced by 20% (15%–28%) or 12.9 million (9.6–17.5 million) | Reduced by 31% (23%–40%) or 19.3 million (14.3–25.2 million) |
| Hospitalizations (>900 000) | Reduced by 12% (9%–16%) or 107 400 (80 100–145 800) | Reduced by 18% (13%–23%) or 161 400 (119 800–210 400) |
| Deaths (>79 000) | Reduced by 5% (3%–6%) or 3576 (2666–4854) | Reduced by 7% (5%–9%) or 5373 (3988–7005) |
aThe estimated impact of antiviral treatment is based on the model described by Du et al (2020) [43]. This model does not take into account the possible effects of changes in social interactions or the possible effects of treatment emergence and transmission of influenza virus variants with reduced antiviral susceptibility.
bEstimated numbers for the 2017–2018 US influenza season from the US Centers for Disease Control and Prevention.
cParenthetical ranges represent 95% credible intervals.
Another study examined the correlation between viral shedding and patient infectiousness using 3 separate epidemiologic models, which assume that infectiousness is proportional to either natural or logarithmic viral titers, or to a semimechanistic dose-response transformation of viral titer that is intermediate between the natural and logarithmic models [44]. Pairing of either the logarithmic or dose-response models with clinical pharmacokinetic–pharmacodynamic parameters led to a predicted reduction of approximately 40% in household transmission with baloxavir versus oseltamivir treatment, if treatment was given within 24 hours of symptom onset. This reduction dropped to approximately 20% if treatment took place within 24–48 hours. Importantly, these modeling simulations were correlated closely with observations from the phase 3 RCT of baloxavir PEP [6], and post hoc estimates, comparing baloxavir with NAIs, found that transmission was reduced by 47% if treatment was within 24 hours and by 18% if it was within 24–48 hours [44].
Results from the ongoing baloxavir trial will help refine such models. In addition, studies need to examine the potential of “infection blocking” antivirals, for example, routinely giving PEP to asymptomatic contacts of case patients to prevent presymptomatic transmission and determining whether a “herd antiviral” effect might be possible with sufficient treatment coverage in an outbreak.
Conclusions
Definitive data on the effect of antiviral treatment on virus transmission during seasonal or pandemic influenza are currently lacking, but an ongoing study of baloxavir will address this important question. The available animal model data indicate that more rapid reductions in viral shedding with antiviral treatment correlate with a lower likelihood of transmission to susceptible contacts. Observational and clinical trial data confirm that early antiviral treatment of IPs can reduce infection risk and illness in household contacts, although the magnitude of the effect has varied widely across studies and is highly dependent on timing. Epidemiologic models indicate that prompt antiviral treatment could have major indirect benefits in reducing virus transmission. Validation and refinement of current models, including incorporation of the effects of treatment-emergent antiviral resistance, will improve their predictive value in various settings, including seasonal and pandemic influenza scenarios.
Notes
Acknowledgments. The authors thank John Bett, PhD, of Ashfield MedComms, an Ashfield Health company, who provided medical writing assistance for portions of the manuscript, under the direction of the authors with funding from F. Hoffmann-La Roche.
Financial support. This work was supported by F. Hoffmann-La Roche Ltd. (funding for medical writing support and journal fee) and by a seed fund for basic research for new staff from the University of Hong Kong (202009185062 to Z. D.).
Potential conflicts of interest. F. G. H. reports the following: meeting travel support from the Bill & Melinda Gates Foundation, Shionogi, and F. Hoffmann-La Roche; charitable donations from Shionogi for consulting time; textbook royalties from the American Society for Microbiology; honoraria for scientific advisory board work from the University of Alabama Antiviral Drug Discovery and Development Program (includes influenza antivirals); honoraria/payment for the invited lectures to the Hong Kong Society for Infectious Diseases 24th Annual Meeting (2021), Second Huaxia Clinical Microbiology and Infection Congress and Eighth Peking-Hong Kong Infection and Clinical Microbiology Congress (2019), University of Tennessee–Memphis (2019), and the Going Viral Symposium, University of North Carolina (2018); serving on data and safety monitoring boards for Vaccitech, CytoDyn, Celltrion, and Enanta (fees to the University of Virginia for all) and for Imperial College and Oxford University (no compensation); serving as chair of the Program Committee for Virtual Conferences on COVID-19 Therapeutics (October 2020) and cochair for the International Society for Influenza and other Respiratory Viruses–World Health Organization Virtual Conference on COVID-19, Influenza, and RSV (October 2021); and serving as an unpaid consultant to Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV), UK COVID-19 Therapeutics Advisory Panel, and many companies involved in developing influenza therapeutics or vaccines (Arcturus, Cidara, Enanta, FujiFilm/Toyama, Gilead, GlaxoSmithKine, Janssen/Johnson & Johnson, MedImmune, Merck, Pfizer, Primmune, Regeneron, resTORbio, Ridgeback, Roche/Genentech, SAB Biotherapeutics, Shionogi, Vir, Visterra) and charitable donations for consulting from Cidara, Enanta, resTORbio, and Shionogi, all outside the submitted work. J. A. reports serving as contractor for the Office of the Assistant Secretary for Preparedness and Response, US Department of Health and Human Services, outside the conduct of the study (no funds used for the preparation of this manuscript). B. J. C. consults for or has received personal payments and honoraria for presentations and participation in advisory board meetings from F. Hoffmann-La Roche, GlaxoSmithKline, Moderna, AstraZeneca, and Sanofi Pasteur. A. C. H., K. K., and A. L. D. are employees of F. Hoffmann-La Roche and report holding stock in Roche, which is a manufacturer of influenza antivirals, including Tamiflu and Xofluza. H. I. has received lecture fees and travel support from Shionogi and personal payments or honoraria for educational events, speaking, or manuscript writing from Shionogi, Daiichi-Sankyo, and F. Hoffmann-La Roche, outside the submitted work. L. A. M. has received honoraria from F. Hoffmann-La Roche, outside the submitted work. P. A. P. reports personal fees (consultancy) from F. Hoffmann-La Roche (work in the area of influenza) and grants from Shionogi (in the area of respiratory virus diagnostics), outside the submitted work. T. T. has received research grants from Shionogi (Japan), outside the submitted work. H. L. Y. reports research support from the National Institute of Allergy and Infectious Diseases (contract HHSN272201400006C), GRF, European Commission/Research Grants Council Collaboration Scheme, CRF, TRS, Research Grant Council, Hong Kong, China, HMRF, Food and Health Bureau, Hong Kong, China, Neoleukin, Therapeutics (contract research), and Saiba, Switzerland (contract research); and travel support from the World Health Organization, outside the submitted work. A. S. M. has been a consultant and received fees from F. Hoffmann-La Roche, Sanofi, and Seqirus. Z. D. reports no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hayden FG, Palese P. Influenza virus. In: Richman DD, Whitley RJ, Hayden FG, eds. Clinical virology. 4th ed. Washington, DC: American Society for Microbiology, 2017:1009–58. [Google Scholar]
- 2. Xiao J, Shiu EYC, Gao H, et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings—personal protective and environmental measures. Emerg Infect Dis 2020; 26:967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fong MW, Gao H, Wong JY, et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings—social distancing measures. Emerg Infect Dis 2020; 26:976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 2020; 26:676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laurie KL, Rockman S. Which influenza viruses will emerge following the SARS-CoV-2 pandemic? Influenza Other Respir Viruses doi:10.1111/irv.12866. Published 6 May 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ikematsu H, Hayden FG, Kawaguchi K, et al. Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med 2020; 383:309–20. [DOI] [PubMed] [Google Scholar]
- 7. Okoli GN, Otete HE, Beck CR, Nguyen-Van-Tam JS. Use of neuraminidase inhibitors for rapid containment of influenza: a systematic review and meta-analysis of individual and household transmission studies. PLoS One 2014; 9:e113633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oh DY, Lowther S, McCaw JM, et al. Evaluation of oseltamivir prophylaxis regimens for reducing influenza virus infection, transmission and disease severity in a ferret model of household contact. J Antimicrob Chemother 2014; 69:2458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belser JA, Maines TR, Creager HM, Katz JM, Tumpey TM. Oseltamivir inhibits influenza virus replication and transmission following ocular-only aerosol inoculation of ferrets. Virology 2015; 484:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee LYY, Zhou J, Frise R, et al. Baloxavir treatment of ferrets infected with influenza A(H1N1)pdm09 virus reduces onward transmission. PLoS Pathog 2020; 16:e1008395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park JG, Ye C, Piepenbrink MS, et al. A broad and potent H1-specific human monoclonal antibody produced in plants prevents influenza virus infection and transmission in guinea pigs. Viruses 2020; 12:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herlocher ML, Carr J, Ives J, et al. Influenza virus carrying an R292K mutation in the neuraminidase gene is not transmitted in ferrets. Antiviral Res 2002; 54:99–111. [DOI] [PubMed] [Google Scholar]
- 13. Herlocher ML, Truscon R, Elias S, et al. Influenza viruses resistant to the antiviral drug oseltamivir: transmission studies in ferrets. J Infect Dis 2004; 190:1627–30. [DOI] [PubMed] [Google Scholar]
- 14. Bloom JD, Gong LI, Baltimore D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 2010; 328:1272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bouvier NM, Rahmat S, Pica N. Enhanced mammalian transmissibility of seasonal influenza A/H1N1 viruses encoding an oseltamivir-resistant neuraminidase. J Virol 2012; 86:7268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abed Y, Pizzorno A, Bouhy X, Boivin G. Role of permissive neuraminidase mutations in influenza A/Brisbane/59/2007-like (H1N1) viruses. PLoS Pathog 2011; 7:e1002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Butler J, Hooper KA, Petrie S, et al. Estimating the fitness advantage conferred by permissive neuraminidase mutations in recent oseltamivir-resistant A(H1N1)pdm09 influenza viruses. PLoS Pathog 2014; 10:e1004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imai M, Yamashita M, Sakai-Tagawa Y, et al. Influenza A variants with reduced susceptibility to baloxavir isolated from Japanese patients are fit and transmit through respiratory droplets. Nat Microbiol 2020; 5:27–33. [DOI] [PubMed] [Google Scholar]
- 20. Jones JC, Pascua PNQ, Fabrizio TP, et al. Influenza A and B viruses with reduced baloxavir susceptibility display attenuated in vitro fitness but retain ferret transmissibility. Proc Natl Acad Sci U S A 2020; 117:8593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee LYY, Zhou J, Koszalka P, et al. Evaluating the fitness of PA/I38T-substituted influenza A viruses with reduced baloxavir susceptibility in a competitive mixtures ferret model. PLoS Pathog 2021; 17:e1009527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ng S, Cowling BJ, Fang VJ, et al. Effects of oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin Infect Dis 2010; 50:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goldstein E, Cowling BJ, O’Hagan JJ, et al. Oseltamivir for treatment and prevention of pandemic influenza A/H1N1 virus infection in households, Milwaukee, 2009. BMC Infect Dis 2010; 10:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nishiura H, Oshitani H. Household transmission of influenza (H1N1-2009) in Japan: age-specificity and reduction of household transmission risk by zanamivir treatment. J Int Med Res 2011; 39:619–28. [DOI] [PubMed] [Google Scholar]
- 25. Hirotsu N, Wada K, Oshitani H. Risk factors of household transmission of pandemic (H1N1) 2009 among patients treated with antivirals: a prospective study at a primary clinic in Japan. PLoS One 2012; 7:e31519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakano T, Shiosakai K. Spread of viral infection to family members from influenza patients treated with a neuraminidase inhibitor. J Infect Chemother 2014; 20:401–6. [DOI] [PubMed] [Google Scholar]
- 27. Fry AM, Goswami D, Nahar K, et al. Effects of oseltamivir treatment of index patients with influenza on secondary household illness in an urban setting in Bangladesh: secondary analysis of a randomised, placebo-controlled trial. Lancet Infect Dis 2015; 15:654–62. [DOI] [PubMed] [Google Scholar]
- 28. Komeda T, Takazono T, Hosogaya N, et al. Comparison of household transmission of influenza virus from index patients treated with baloxavir marboxil or neuraminidase inhibitors: a health insurance claims database study. Clin Infect Dis 2021; 72:e859–e67. [DOI] [PubMed] [Google Scholar]
- 29. Couch RB, Kasel JA, Glezen WP, et al. Influenza: its control in persons and populations. J Infect Dis 1986; 153:431–40. [DOI] [PubMed] [Google Scholar]
- 30. Hayden FG, Belshe RB, Clover RD, Hay AJ, Oakes MG, Soo W. Emergence and apparent transmission of rimantadine-resistant influenza A virus in families. N Engl J Med 1989; 321:1696–702. [DOI] [PubMed] [Google Scholar]
- 31. Halloran ME, Hayden FG, Yang Y, Longini IM Jr, Monto AS. Antiviral effects on influenza viral transmission and pathogenicity: observations from household-based trials. Am J Epidemiol 2007; 165:212–21. [DOI] [PubMed] [Google Scholar]
- 32. Carrat F, Duval X, Tubach F, et al. ; BIVIR study group. Effect of oseltamivir, zanamivir or oseltamivir-zanamivir combination treatments on transmission of influenza in households. Antivir Ther 2012; 17:1085–90. [DOI] [PubMed] [Google Scholar]
- 33. Umemura T, Mutoh Y, Kawamura T, et al. Efficacy of baloxavir marboxil on household transmission of influenza infection. J Pharm Health Care Sci 2020; 6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayden FG, Sugaya N, Hirotsu N, et al. ; Baloxavir Marboxil Investigators Group. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913–23. [DOI] [PubMed] [Google Scholar]
- 35. Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis 2020; 20:1204–14. [DOI] [PubMed] [Google Scholar]
- 36. Baker J, Block SL, Matharu B, et al. Baloxavir marboxil single-dose treatment in influenza-infected children: a randomized, double-blind, active controlled phase 3 safety and efficacy trial (miniSTONE-2). Pediatr Infect Dis J 2020; 39:700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takashita E, Ichikawa M, Morita H, et al. Human-to-human transmission of influenza A(H3N2) virus with reduced susceptibility to baloxavir, Japan, February 2019. Emerg Infect Dis 2019; 25:2108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takashita E, Kawakami C, Ogawa R, et al. Influenza A(H3N2) virus exhibiting reduced susceptibility to baloxavir due to a polymerase acidic subunit I38T substitution detected from a hospitalised child without prior baloxavir treatment, Japan, January 2019. Euro Surveill 2019; 24:1900170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamal MA, Smith PF, Chaiyakunapruk N, et al. Interdisciplinary pharmacometrics linking oseltamivir pharmacology, influenza epidemiology and health economics to inform antiviral use in pandemics. Br J Clin Pharmacol 2017; 83:1580–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lipsitch M, Cohen T, Murray M, and Levin B. . Antiviral Resistance and the Control of Pandemic Influenza. PLoS Med 2007; 4:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferguson NM, Cummings DA, Cauchemez S, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 2005; 437:209–14. [DOI] [PubMed] [Google Scholar]
- 42. Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol 2008; 167:775–85. [DOI] [PubMed] [Google Scholar]
- 43. Du Z, Nugent C, Galvani AP, Krug RM, Meyers LA. Modeling mitigation of influenza epidemics by baloxavir. Nat Commun 2020; 11:2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Asher J, Lemenuel-Diot A, Clay M, et al. Novel model to determine how influenza viral shedding correlates with infectiousness and to predict the impact of antivirals on reduction of influenza transmission. Presented at: The 7th ESWI Influenza Conference, 6–9 December 2020. [Google Scholar]