Abstract
Cryptococcus neoformans var. neoformans (serotype D) and C. neoformans var. grubii (serotype A) differ in geographic prevalence and dermatotropism, with C. neoformans var. neoformans strains being more prevalent among isolates from temperate countries as well as from skin infections. Analysis of 19 strains from each serotype revealed wide variation in thermal susceptibility, with C. neoformans var. neoformans strains being more susceptible, on average, to heat killing. The results suggest a consistent explanation for the geographic differences between serotype A and D strains and for the dermatotropism of serotype D strains.
Cryptococcus neoformans is an opportunistic fungus that is a relatively common cause of life-threatening meningoencephalitis in individuals with impaired immunity (reviewed in reference 5). Approximately 6 to 8% of patients with AIDS develop cryptococcal infections (7). C. neoformans was originally classified into two varieties, C. neoformans var. neoformans (serotypes A and D) and C. neoformans var. gattii (serotypes B and C), based on biochemical, morphological, and genetic characteristics (2, 16, 17). These four serotype classifications were based on antigenic differences resulting from structural variation of the major capsular polysaccharide glucuronoxylomannan (4, 6, 20). On the basis on genetic differences, it was proposed that C. neoformans var. neoformans be further subdivided into two varieties: C. neoformans var. grubii (serotype A) and C. neoformans var. neoformans (serotype D) (11).
There are differences in the geographic distribution of serotype A and D isolates from both clinical and environmental sources (1, 18). Serotype A predominates among clinical and environmental sources in most areas of the world with the exception of certain northern European countries (reviewed in reference 19). Geographic differences in the prevalence of serotypes A and D of C. neoformans may reflect climatic tolerances and/or virulence (9). Another interesting difference between serotypes A and D is the association between serotype D infection and skin involvement (9). Given the higher prevalence of serotype D isolates in temperate countries and its relative predilection for skin tissue, we hypothesized that serotype D strains were more susceptible to high temperatures than serotype A strains. To validate or refute this hypothesis, we compared the susceptibilities of serotypes A and D to killing by heat.
The 19 clinical strains of C. neoformans serotype A utilized in this study (J4, J8, J9B, J10, J11, J20, J28, J40, J41, J43, J45, J48, J51, J52, J53, M5, M10, M14, and M16) were recovered from patients in two New York City hospitals, Jacobi Medical Center (J strains) and Montefiore Medical Center (M strains) (19). Eighteen strains of C. neoformans serotype D utilized in this study (CDCY4889, CDCY59392, CDCY59492, CDCY60092, NIH 12, NIH 426, NIH 430, NIH 433, NIH 1152, NIH 1154, NIH 1153, NIH 2256, NIH 1157, MMRU 625, MMRU 751, MMRU 757, MMRU 1077, and MMRU 1078) were acquired from Laurie Watt (BioMérieux, Marcy l'Etoile, France). Strain 24067 (serotype D) of C. neoformans was acquired from the American Type Culture Collection (Rockville, Md.). For some strains, serotype assignment was confirmed by two methods: factor-serum agglutination with the Crypto-Check kit (Iatron Inc., Tokyo, Japan) and indirect immunofluorescence with monoclonal antibody 13F1 (19).
Susceptibility to heat killing was determined by exposing a suspension of C. neoformans to the desired temperature for a defined time interval followed by measuring the percentage of cells that survived, as indicated by colony counts relative to those of a control suspension maintained at room temperature. Each C. neoformans strain was grown in Sabouraud dextrose broth (Difco Laboratories, Detroit, Mich.) for 48 h at 30°C in a rotary shaker at 150 rpm (to early stationary phase). Cells were then collected by centrifugation, washed twice with distilled water, counted using a hematocytometer, and suspended at a density of 106 cells per ml in distilled water. A 0.1-ml aliquot of this suspension was placed in a microcentrifuge tube and inserted into a prewarmed water bath at either 45 or 47°C for 30 and 60 min. These temperatures were selected after preliminary studies revealed that they resulted in significant killing for strain 24067 (see below). After incubation in the water bath, the cell suspension was plated on Sabouraud dextrose agar (Difco Laboratories) and incubated for 48 h at 30°C. The percent survival was determined by counting the number of CFU (1 colony = 1 CFU) in suspensions of cells exposed to the various temperatures and comparing these to the number of colonies in an equivalent suspension of cells not exposed to heat. To establish the ability of each strain to grow at various temperatures, cells were streaked on the surface of Sabouraud dextrose agar using a loop and the plate was incubated for 72 h at temperatures ranging from 37 to 45°C. Each strain was observed daily, and growth was considered to have occurred when colonies were visible with the unaided eye. CFU data were analyzed by both Student's t test and Kruskal-Wallis statistical analysis. Chi-square (χ2) analysis was used to determine the significance of the correlation between the growth of the strains of serotypes A and D of C. neoformans and the various temperatures. P values lower than 0.05 were considered significant. Statistical analysis was done by using Primer of Statistics—The Program (a software program; McGraw Hill Co., New York, N.Y.).
The susceptibility of C. neoformans 24067 cells to thermal killing was determined by incubating cells at various temperatures followed by plating assays to determine the percentage of cells that survived. This strain was selected because it is a well- characterized standard serotype D strain (10). Cells of C. neoformans 24067 were heated for 1 h at 37, 40, or 45°C to determine the appropriate temperature to study heat killing. At temperatures below 40°C, there was no significant killing of 24067 cells after incubation for 1 h (data not shown). In contrast, incubation of 24067 cells at 45°C resulted in the killing of most cells after 30 min (data not shown). Hence, we selected 45°C as the temperature to begin the study.
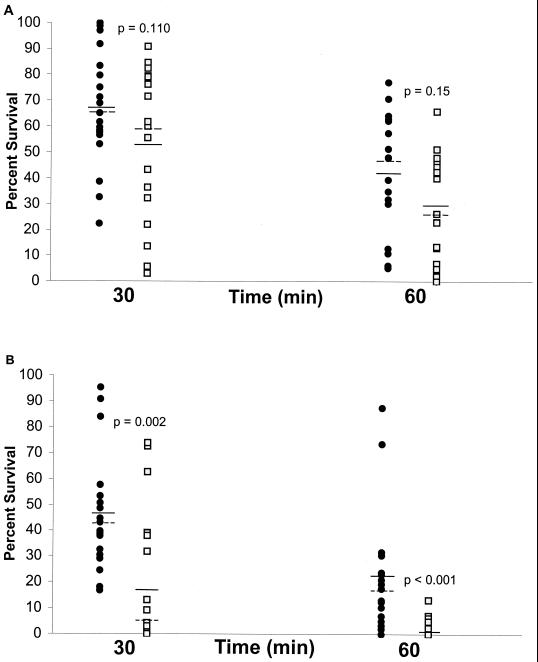
There was no significant difference between serotype A and D strains in the average percent survival after incubation at 45°C for 30 or 60 min (Fig. 1A) even though, on average, cells from serotype A strains demonstrated higher survival percentages than serotype D strains (P = 0.110 for 30 min and P = 0.15 for 60 min; Student's t test). C. neoformans cells from both serotypes demonstrated significant variation in the percent survival after exposure to a temperature of 45°C. Incubation at 47°C resulted in a considerable increase in killing for cells of both serotypes (Fig. 1B). However, at this temperature, the average percent survival of serotype A strains was significantly higher than that for serotype D strains (P was 0.002 for 30 min and <0.001 for 60 min; Student's t test). Next, we determined the highest temperature that was permissive for growth for the serotype A and D strains (Table 1). From 37 to 40°C, all strains of both serotypes exhibited growth after plating on agar. At 41 and 42°C, more serotype A strains exhibited growth than serotype D strains but the difference was not significant by χ2 analysis (Table 1). At 43°C, 16 of 19 strains of serotype A grew while only 6 of 19 strains of serotype D grew, demonstrating a significant difference in permissive growth temperature between the two serotypes of C. neoformans (Table 1). At 44 and 45°C, there was no growth from any of the strains tested.
FIG. 1.
Survival of serotype A (•) or D (▫). C. neoformans strains after exposure to 45°C (A) or 47°C (B) for 30 and 60 min. Solid and dashed lines denote mean and median values, respectively, for the distribution shown. This experiment was done twice with similar results.
TABLE 1.
Growth of C. neoformans strains of serotypes A and D at various temperatures
| Temperature (°C) | Serotype | No. of strains displaying:
|
P valuea | |
|---|---|---|---|---|
| Growth | No growth | |||
| 40 | A | 19 | 0 | |
| D | 19 | 0 | 1.0 | |
| 41 | A | 19 | 0 | |
| D | 15 | 4 | 0.113 | |
| 42 | A | 17 | 2 | |
| D | 12 | 7 | 0.124 | |
| 43 | A | 16 | 3 | |
| D | 6 | 13 | 0.003 | |
| 44 | A | 0 | 19 | |
| D | 0 | 19 | 1.0 | |
Calculated by χ2 statistical analysis.
Our results indicate that, on average, cells of serotype D strains are more susceptible to killing by high temperature than are cells of serotype A strains. This observation suggests a consistent explanation for the higher prevalence of serotype D strains among northern European countries with temperate climates. The higher thermal tolerance of serotype A isolates could confer a survival advantage in warmer climates. Furthermore, the dermatotropism associated with serotype D isolates may reflect a preference for growth in the cooler tissues of the skin. This suggestion can be tested in future studies by comparing the thermal susceptibility of isolates from infections involving skin to those that involve only the central nervous system, without skin involvement. Although we did not observe a difference in average thermal susceptibility at 37°C between the serotypes in our in vitro experimental conditions, it is conceivable that the thermal susceptibility of serotype D isolates is greater in infected tissues, where yeast cells would be expected to be in a different nutritional milieu and under attack by the immune system. In this regard, there is evidence from animal studies that body temperature can affect the course and outcome of cryptococcal infection. The survival of mice infected with C. neoformans is longer when the mice are kept at room temperatures of 35 to 36°C than it is when they are exposed to cooler temperatures (14, 15). The survival of chicken embryos infected with C. neoformans is enhanced if these are incubated at temperatures above 39°C (12). Rabbits are notoriously resistant to infection with C. neoformans, possibly as a result of their higher core body temperature (15). Systemic infection in rabbits leads to a higher infection burden in the testis which may reflect a lower temperature in the scrotum (3). In humans, there is a case report of an individual with simultaneous malaria and cryptococcal infection who had clearance of yeast cells in the cerebrospinal fluid during febrile episodes caused by relapsing malaria (13). Heat-sensitive C. neoformans strains have been reported to be rhinotropic, which presumably reflects a tropism of the cooler tissues of the nose (8). Our findings also suggest the possibility that the predominance of serotype A strains among clinical isolates could also reflect some selection against serotype D strains by the temperatures associated with high fever. The results of the present study add to the increasing body of data indicating major differences between serotype A and D isolates and leading to the proposal that a different varietal status be assigned to each serotype (11).
Acknowledgments
L.R.M. is supported by the Bridge to Doctorate LIU-AECOM Program grant NIH-NIGMS 2R25GM56630-02. J.G.-R. is supported by grant T32-AI07501. A.C. is supported by NIH awards AI33774, AI3342, and HL-59842-01 and is a recipient of a Burroughs Wellcome Development Therapeutics Award.
REFERENCES
- 1.Bennett J E, Kwon-Chung K J, Howard D H. Epidemiologic differences among serotypes of Cryptococcus neoformans. Am J Epidemiol. 1977;105:582–586. doi: 10.1093/oxfordjournals.aje.a112423. [DOI] [PubMed] [Google Scholar]
- 2.Bennett J E, Kwon-Chung K J, Theodore T S. Biochemical differences between serotypes of Cryptococcus neoformans. Sabouraudia. 1978;16:167–174. [PubMed] [Google Scholar]
- 3.Bergman F. Effect of temperature on intratesticular cryptococcal infection in rabbits. Sabouraudia. 1966;5:54–58. doi: 10.1080/00362176785190101. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharjee A K, Bennett J E, Glaudemans C P J. Capsular polysaccharides of Cryptococcus neoformans. Rev Infect Dis. 1984;6:619–624. doi: 10.1093/clinids/6.5.619. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C.: American Society for Microbiology; 1998. [Google Scholar]
- 6.Cherniak R, Sundstrom J B. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994;62:1507–1512. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currie B P, Casadevall A. Estimation of the prevalence of cryptococcal infection among HIV infected individuals in New York City. Clin Infect Dis. 1994;19:1029–1033. doi: 10.1093/clinids/19.6.1029. [DOI] [PubMed] [Google Scholar]
- 8.Dixon D M, Polak A. In vivo and in vitro studies with an atypical, rhinotrophic isolate of Cryptococcus neoformans. Mycopathologia. 1986;96:33–40. doi: 10.1007/BF00467683. [DOI] [PubMed] [Google Scholar]
- 9.Dromer F, Mathoulin S, Dupont B, Letenneur L, Ronin O. Individual and environmental factors associated with infection due to Cryptococcus neoformans serotype D. Clin Infect Dis. 1996;23:91–96. doi: 10.1093/clinids/23.1.91. [DOI] [PubMed] [Google Scholar]
- 10.Franzot S P, Mukherjee J, Cherniak R, Chen L, Hamdan J S, Casadevall A. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect Immun. 1998;66:89–97. doi: 10.1128/iai.66.1.89-97.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franzot S P, Salkin I F, Casadevall A. Cryptococcus neoformans var. grubii: separate variety status for Cryptococcus neoformans serotype A isolates. J Clin Microbiol. 1999;37:838–840. doi: 10.1128/jcm.37.3.838-840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kligman A M, Crane A P, Norris R F. Effect of temperature on survival of chick embryos infected intravenously with Cryptococcus neoformans (Torula histolytica) Am J Med Sci. 1951;221:273–278. doi: 10.1097/00000441-195103000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Kligman A M, Weidman F D. Experimental studies on treatment of human torulosis. Arch Derm Syph. 1949;60:726–741. doi: 10.1001/archderm.1949.01530050088008. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn L R. Experimental cryptococcic infection. Arch Pathol. 1939;27:803–803. [Google Scholar]
- 15.Kuhn L R. Growth and viability of Cryptococcus hominis at mouse and rabbit body temperatures. Proc Soc Exp Biol Med. 1939;41:573–574. [Google Scholar]
- 16.Kwon-Chung K J. A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia. 1975;67:1197–1200. [PubMed] [Google Scholar]
- 17.Kwon-Chung K J. A new species of Filobasidiella, the sexual state of Cryptococcus neoformans B and C serotypes. Mycologia. 1976;68:942–946. [PubMed] [Google Scholar]
- 18.Kwon-Chung K J, Bennett J E. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol. 1984;120:123–130. doi: 10.1093/oxfordjournals.aje.a113861. [DOI] [PubMed] [Google Scholar]
- 19.Steenbergen J N, Casadevall A. Prevalence of Cryptococcus neoformans var. neoformans (serotype D) and Cryptococcus neoformans var. grubii (serotype A) isolates in New York City. J Clin Microbiol. 2000;38:1974–1976. doi: 10.1128/jcm.38.5.1974-1976.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson D E, Bennett J E, Bailey J W. Serologic grouping of Cryptococcus neoformans. Proc Soc Exp Biol Med. 1968;127:820–823. doi: 10.3181/00379727-127-32812. [DOI] [PubMed] [Google Scholar]