Abstract
Beach sand and water have both shown relevance for human health and their microbiology have been the subjects of study for decades. Recently, the World Health Organization recommended that recreational beach sands be added to the matrices monitored for enterococci and Fungi. Global climate change is affecting beach microbial contamination, via changes to conditions like water temperature, sea level, precipitation, and waves. In addition, the world is changing, and humans travel and relocate, often carrying endemic allochthonous microbiota. Coastal areas are amongst the most frequent relocation choices, especially in regions where desertification is taking place. A warmer future will likely require looking beyond the use of traditional water quality indicators to protect human health, in order to guarantee that waterways are safe to use for bathing and recreation. Finally, since sand is a complex matrix, an alternative set of microbial standards is necessary to guarantee that the health of beach users is protected from both sand and water contaminants. We need to plan for the future safer use of beaches by adapting regulations to a climate-changing world.
Keywords: climate change, global warming, beach sand, FIB, sand, recreational water, bathing water
1. Introduction and Broad Climate Change Projections
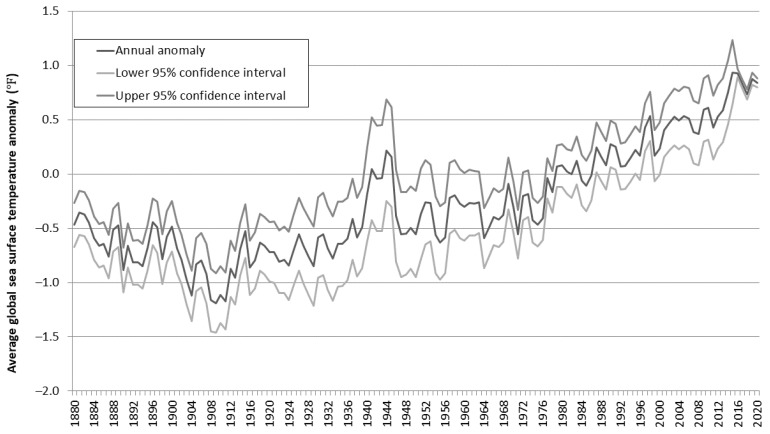
Global surface temperatures are predicted to increase by 1–4 °C by the end of this century (2081–2100) relative to those observed from 1986–2005 [1]. The four climate change scenarios presented in the Intergovernmental Panel on Climate Change (IPCC) Synthesis Reports (RCP2.6, RCP4.5, RCP6.0, and RCP8.5) describe the range of predicted changes in climate from mild (RCP2.6) to extreme (RCP8.5), in response to increasing greenhouse gas emissions. It is important to note that, while showing global trends, the predicted changes are not uniform across all regions [2]. Together with a predicted rise in atmospheric temperature, the surface temperature of both fresh and saltwater is also projected to rise. The average global sea surface temperature (SST) has increased steadily for the last 30 years (Figure 1) [3], and the increase in SST is projected to continue, although at a rate slower than the projected rise in the atmospheric temperature. Depending on the emissions scenarios examined, the global SST rise estimates vary from about 1 °C (RCP2.6) to more than 3 °C (RCP8.5). Moreover, the rise in SST is predicted to be highest in subtropical and tropical regions [2].
Figure 1.
Annual anomaly in average global sea surface temperature in 1880–2020 (adapted from [3]).
In addition to temperatures, the global mean sea level is also predicted to increase. Similar to atmospheric and water temperature projections, the predicted sea-level rise depends on the emissions scenario utilized (Table 1). For scenario RCP8.5, the sea level rise during 2081–2100 is predicted to occur at a rate of 8 to 16 mm/year. However, in some regions such as the northern Baltic Sea coastline, the isostatic land uplift is still ongoing after the last ice age and the race between land uplift and sea-level rise will determine the direction in which the coastline will move [4].
Table 1.
Global mean sea level rise projections for 2100, under varying emissions scenarios [2].
| RCP Emissions Scenario | Range of Projected Sea Level Rise (m) |
|---|---|
| RCP2.6 | 0.26–0.55 |
| RCP4.5 | 0.32–0.63 |
| RCP6.0 | 0.33–0.63 |
| RCP8.5 | 0.45–0.82 |
Sea level rise has also other implications for recreational beach use. For example, dunes and beach sands are dynamic systems and seasonal tides claim and re-deposit sand from the shores. In some instances, water-driven scour and beach sand removal outweighs re-deposition processes, leading to retracting shorelines.
Current global trends of increasing SST and lake surface temperatures [5,6,7], along with increasing wind speeds and wave heights in coastal regions [8,9], might play a significant role in structuring coastal communities such as kelp forests and shellfish beds (e.g., [10]). Moreover, several predicted climate change scenarios suggest the potential for an increase in human exposure to microbial agents from recreational activities throughout the beach environment, including beach sands and water. The fate and transport of microbes in beach sand are driven by physical, chemical, and biological factors [11]. Weiskerger et al. [12] discussed these mechanisms and dynamics in a previous review. Here, the authors aim to expand upon some of the specific climate change implications for microbiota at recreational beaches, and offer perspectives regarding the future of beach safety in the face of climate change. The discussion herein focuses on providing additional specific details of climate change and how they are projected to impact microbial fate and transport at beaches, supplementing the more holistic and big-picture view presented in Weiskerger et al. [12]. Further, this work aims to expand the analysis to include additional details on the supratidal area of the beach and climate change impacts to fungi, an emerging recreational contaminant and important microbial group affecting public health at recreational sites worldwide [13,14,15,16].
1.1. Characterizing Climate Change Impacts on Microbiota
Climate-mediated changes might affect processes controlling inland, estuarine, and coastal concentrations of faecal indicator bacteria (FIB), primarily Escherichia coli (E. coli), enterococci, pathogens, and the frequency and duration of cyanobacterial blooms, including changes in pH, dissolved oxygen (DO) concentration, salinity (S), dissolved organic carbon (DOC), and dissolved organic nitrogen (DON). Nearshore changes in pH and DO have been documented along the west coast of North America due to several chemical contributions, as well as changes in the intensity of upwelling [17]. These types of changes can be attributable to changes in water temperature and subsequent alterations to water density and potential stratification regimes. Additional changes in pH and DO may also be associated with eutrophication, as has been observed in the Gulf of Mexico and the South China Sea [18]. These two may affect the expected behaviour of FIB in coastal waters and their interactions with sediments, which may be controlled in part by pH. These pH alterations, increases in DOC and DON, and also the increase in water temperature can lead to microbial growth, and changes to some phytoplankton communities (cyanobacteria, diatoms, dinoflagellates, green algae, and chalk-coated Coccolithophores) [19]. Subsequently, these changing communities may result in changes to light penetration in the water column, affecting solar-mediated inactivation of indicator organisms and pathogens [20], or perhaps their growth.
The case of cyanobacteria and dinoflagellates is of particular concern since these phytoplanktonic groups are able to develop Harmful Algal Blooms (HABs), as some of their species are able to produce diverse types of toxins, thus causing adverse effects [19,21,22]. These toxins may have a nefarious effect on water associated activities and/or ecology. They can also accumulate in the sand and persist for some time [23], jeopardizing, for instance, recreational activities taking place at those beach environments.
Consequently, both the presence and persistence of waterborne pathogens and indicator microorganisms will likely change in response to climate change. This could have an impact on the concentration of microbiota in near- and foreshore sands. Deposition and scouring processes associated with waves in the nearshore environment [12,24,25] may intensify due to changes in wave dynamics associated with climate change [8,9].
Although not a comprehensive list, Table 2 follows Weiskerger et al. [12] and highlights hypothesized impacts of climate change scenarios on pathogenic microbes in beach sands and adjacent recreational waters. These include: (a) increased temperatures in both fresh and salt waters; (b) altered patterns of precipitation resulting in either flooding or drought; (c) increased wave activity due to rising ocean levels; and (d) an increase in extreme weather events.
Table 2.
Examples of how predicted variability of environmental/climatic factors may affect pathogenic microbial exposure burdens and human health risks at the nearshore environment.
| Hydrometeorological Variable | Projected Change in Variable | Impacts on Beach Microbes and Public Health | References |
|---|---|---|---|
| Air Temperature | Increased Air Temperature | Direct Effects: | [26,27,28] |
| |||
| Indirect Effects: | [29,30] | ||
| |||
| Water Temperature | Increased Water Temperature | Direct Effects: | [5,6,7,31,32,33,34,35,36,37] |
| |||
|
[36,38,39,40,41,42,43,44] | ||
|
[19,22,31,32,45] | ||
| Indirect Effects: | [46] | ||
| |||
| Precipitation | Increased Frequency and Intensity of Storm Events | Direct Effects: | [47,48,49,50,51] |
| |||
|
[52,53,54] | ||
|
[47,55,56] | ||
| Indirect Effects: | [20,49,57] | ||
| |||
|
[50,58] | ||
| Increased Drought Conditions | Direct Effects: | [59,60] | |
| |||
|
[15,61] | ||
| Sea Level | Sea Level Rise | Direct Effects: | [62,63] |
| |||
| Indirect Effects: | [64] | ||
| |||
|
[12,65] | ||
| Waves | Increased Wave Activity | Direct Effects: | [54,66,67] |
| |||
|
[54,68] |
Both empirical data collection and mechanistic modelling of FIB are useful tools to help understand the relative importance of the various faecal sources, fate processes, and transport pathways, and for making predictions concerning possible climate change generated phenomena. However, it is important to note that while FIB have been useful to monitor faecal contamination of waterways, there may be differential responses of different microbiota to climate-induced environmental changes, especially once we look into the backshore. FIB may in fact persist or even multiply in the water–sand interface for a longer time than some faecal pathogens [69]. In particular, FIB are unable to predict the growth potential of opportunistic pathogens such as those Vibrio species thriving in the water environments [70,71]. Accordingly, models also need to incorporate data from specific pathogens likely to cause human illnesses. For example, Topić et al. [72] describe an outbreak of impetigo in bathers in 2015, linked to the Croatian Vodice beach, due to Staphylococcus aureus in water, which was not accompanied by an increase in the levels of FIB. In this paper, 258 locations in Primorje-Gorski Kotar County were investigated for the presence of S. aureus in water, as a follow-up of the outbreak, and the results found were that the bacterium was present in between 2.2 and 36.3% of the 2867 samples collected between mid-May and the end of September. The presence of the bacterium correlates with the intensity of human use of the beach. Croatia has very few beaches with sand, but should that be the case, sand could have added to the intensity of the outbreak. Nonetheless, given incomplete information and knowledge of nearshore systems, both empirical and mechanistic models and analyses are needed to illustrate climate change impacts and inform management decisions in the context of environmental and public health in nearshore zones.
1.2. Temperature Increases
Rising temperature is a direct response to anthropogenic activities leading to climate change. Moreover, the temperature has a direct effect on the survival and persistence of specific microbial species in both water and sand. Water temperature scenarios are tightly coupled to nearshore hydrodynamics such as stratification, thermal bars, and density-driven currents. An average rise in global atmospheric temperature will result in increases in the temperature of both fresh and marine surface waters, which can directly affect the survival kinetics and growth rates of microbes in both nearshore and upstream tributary environments [73]. However, while it is generally thought that increases in temperature enhance microbial growth, many environmental microbes prefer mesophilic growth temperatures, and elevated temperatures may inhibit the growth of many species. Conversely, rising temperatures may also increase the occurrence of other pathogenic microbes which are capable of multiplying in water environments near body temperature [5,6,7,31,32]. This is especially relevant for fungi when considering the possible selective effect of heatwaves on their potential to infect warm-blooded animals, as described by Casadevall and Kontoyiannis [13] on the emerging Candida auris, a multi-resistant yeast of currently high nosocomial impact in multiple regions and whose original habitat is thought to be aquatic [14]. Parasitic protozoa may also benefit from rising temperatures, as described in the new WHO guidelines, namely Acanthamoeba and Naegleria fowleri [74]. Conversely, the abundance of faecal microbes would decrease in response to many climate change models since faecal microbes generally degrade faster when temperatures increase (i.e., bacterial die-off processes are enhanced) [33,34,35]. For example, Viau et al. [36] and Hokajärvi et al. [37] reported a negative association of the faecal pathogen Campylobacter with temperature. Rising temperatures may also increase the occurrence of other pathogenic microbes, which are capable of multiplying in water environments, including in sand, potentially changing the local microbial community [5,6,7,31,32].
Changes in temperature impact physical and biological properties of water in many ways. For example, changing temperatures affect water and contaminant plume buoyancy and dynamics [46]. Changes to relative densities of water and contamination plumes, due to temperature changes in the water column, may lead to increases in surface FIB plumes that are susceptible to solar inactivation. Conversely, changes in relative density may lead to increases in sinking or settling plumes that are more resistant to solar inactivation, yielding potentially higher survival and persistence of contaminants in the environment.
The potential for increased microbial proliferation, in combination with the expansion of the geographic range and seasonality of various tropical pathogens, could pose a significant risk of increases in human exposure. For example, the presence of Salmonella spp. in Hawaiian coastal streams has a positive correlation with water temperature [36]. Likewise, the presence and persistence of Vibrio spp. is closely and positively correlated with water temperatures [38,39,40]. It has been further suggested that increasing temperatures would allow for increased range and extended seasonality of Leptospira spp. [41,42,43]. The effects of increased temperatures also have the potential to alter the persistence of pathogens in beach sand, including methicillin-resistant Staphylococcus aureus (MRSA) [44], allergenic fungi, and antifungal resistant fungal species [16].
Another concern is associated with toxic algal blooms, which are also predicted to increase over the next century with the rise of SST and global average lake surface temperature [31,32]. These blooms, along with any microbes that they may be carrying, may be deposited at the sand–water continuum during high tides and high waves, providing an additional input of contaminants to this continuum [45].
In addition to direct changes in the microbial dynamics in the beach ecosystem, temperature rise may have significant indirect effects on environmental conditions and associated microbial loads at beaches. FIB contamination inputs in coastal areas are predicted to change significantly and subsequently may increase adverse health effects for beach users as a result of climate change [75]. Changes in human behaviour may thus challenge beach management paradigms. Examples have been reported in shallow freshwater lakes, where norovirus outbreaks have been associated with a sudden increase in the number of beach users [26]. High temperatures lead to increased beach use, for heat relief, especially if exceptional or extreme weather events occur [27,28]. Such contamination events might have a lesser role in riverine and marine conditions with water flow and larger water volume, respectively. There may also be expanded recreational usage as a result of a growing interest in water sports, ranging from triathlon swims to surfing and wind sailing. Expansion of such recreational use may be particularly prominent at those beaches in proximity to large urban centres. Consequently, direct human inputs may temporarily increase via shedding, leading to increased pathogen loading in beach environments [29,30], or even outbreaks of gastrointestinal illnesses. High temperatures may also attract people to congregate at nearby shores, often at locations that do not comply with recreational water safety standards.
Increased urbanization and climate change may also alter migratory bird patterns, impacting the numbers, species, and behaviour of birds associated with the beach environment. Populations of adaptable bird species, like gulls and Canada geese, have been growing in many urban settings around the world [76], and particularly around the Great Lakes [77]. Urbanisation has been reported as a factor for the presence of pigeon-borne Cryptococcus spp. both in sand and in water, which are yeasts responsible for fungal meningitis [16].
Resident populations of birds, particularly shorebirds, can be expected to continue their role as sources of direct animal faecal deposition in the sand. In fact, their removal leads to a dramatic improvement in water quality, as has been reported for example for gulls [78]. Bird population increase in beach settings is expected to lead to a potential increase in human exposure risk to pathogens such as Salmonella, Campylobacter and Chlamydia if populations continue growing in response to climate change [79]. These pathogens, in addition to other zoonotic disease-associated microbiota such as West Nile Virus, Aspergillus, Staphylococcus, and a variety of antimicrobial-resistant bacteria have been documented in gulls, terns, and barnacle geese [80,81,82]. These microbiota may be transmitted directly via deposition from the birds to coastal environments (sand and water), where they may accumulate and present human health risks. These risks may further be amplified as human beach usage may increase in response to climate change and increasing air temperatures.
1.3. Precipitation Increases
Altered precipitation patterns can also greatly influence pathogen exposures at the beach. The intensification of storms, extreme precipitation, and other severe weather events like drought, flooding, storm surge, or even damaging cyclones [1] could cause nearshore inundation, coastal erosion and run-off, introducing pathogenic microbes into coastal waters and on beaches [47,48,49,50,51,52,53,54,57,83,84,85]. Heavy precipitation events, predicted to increase in both frequency and intensity, can cause the resuspension of FIB from beach sand into the water column as well as increases in the dissolved organic matter that can influence solar/UV inactivation of microbial contaminants. These impacts may have subsequent effects on water quality lasting up to 5–7 days after the precipitation event [20,49,57]. Changes in global patterns of precipitation are also predicted to amplify existing direct and anthropogenic impacts of microbiota in coastal environments [11,30]. Increased frequency and intensity of precipitation events can lead to the breakdown of already taxed wastewater infrastructure, resulting in increased point sources of faecal contamination of beaches, such as Combined Sewer Overflow (CSO) events or wastewater effluent [50,58]. Stormwater outfalls frequently present the greatest direct source of faecal contamination, including pathogens, for adjacent surface waters, even in areas with mixed land use [86,87,88]. Further, runoff from impervious surfaces (such as parking lots), CSO discharge events, and stormwater outfall discharges located at or in proximity to beaches can directly contribute to faecal contamination of beaches [55,56].
On the other hand, areas experiencing increased drought may observe increases in microbial exposure in depleted waters, resulting from a higher accumulated concentration and diversity of microbial communities, including pathogens in depleted water supplies [59,60]. Drought conditions may also improve habitat for specific pathogens, including the xerotolerant opportunistic fungal group Candida, which can preferentially survive in dry sands [15,61]. Climate change is likely to reduce snowfall amounts and change the timing of snowmelt events, shifting them to earlier in the season, ultimately decreasing snowmelt in the spring, and perpetuating drought conditions in some areas [89].
1.4. Wave Activity and Sea Level Rise
Temperature and precipitation changes are two environmental conditions that have been frequently considered as hallmarks of climate change in the literature. Wave activity is not as commonly explored [90] and the effects of waves on the transfer and accumulation of human pathogens in the sand, and their subsequent transfer to recreational water, needs to be studied. We include wave activity here because the sand–water continuum involves dynamic exchanges of microbes between sand and water [12]. Potential climate-induced changes to microbial interactions between water and sand could have important implications on altered exposure conditions [91]. Direct faecal deposition from birds or dogs, or direct runoff onto sand may be distributed over a greater area of beach with increased human activity and extreme weather events, including increased wave activity [54,66,67,92,93,94]. Similarly, periodic tidal rewetting enables FIB and pathogens deposited into dry sands to persist for longer periods [54,68]. Moreover, if ocean depth rises, the tides will edge further inland, allowing for the persistence and exchange of FIB at the sand–water continuum closer to densely populated areas.
Changes in the coastline could also have significant negative impacts on human exposure to pathogens as there has been a global shift towards urbanization with a tendency for cities to develop along coastlines. It has been estimated that nearly half of the world’s population lives within a few hundred kilometres of a coast [95]. The combination of rising sea levels, increasing rainfall amounts and intensity, and increasing urbanization means that the risk of exposure to microbial contamination along the coast will continue to rise. An increase in sea level can also lead to the development of new microbial communities in the sand–water continuum of more inland areas [62,63]. This may precipitate exposure to more diverse microbial contaminants at beaches.
Further, climate changes could result in an increase in gently-sloping coastal areas that could be classified as low-energy for wave action due to sea-level rise. For example, Florida is characterized by flat terrain, which extends outward into the oceans. This platform is composed of a limestone aquifer, which is very shallow in coastal areas. Many beaches and offshore regions are often considered calm and of relatively low energy. As sea levels rise and the coast migrates landward, the topography of terrain similar to south Florida’s could potentially create more areas of low energy, assuming that the platform maintains its integrity as sea level rises [64]. Because of this flat terrain, flooding events will be followed by stranded water, creating low-energy surface water bodies. The increase in flood-prone areas, along with disruptions or overflow of sanitary services, could potentially create pockets of contaminated waters that would not have the benefit of dilution, as is the case for beaches directly connected to the ocean. This, coupled with biofilm dynamics and the influence of low energy waves, could result in changes to how FIB and pathogens are retained along the coast in response to sea-level rise [65].
1.5. Economic Impact
There are both direct and indirect costs associated with climate change-induced increases in the number of harmful bacteria and fungi and resulting exposures to humans. While some are obvious, such as loss of wages due to illness, others are more intangible but no less severe. For example, current costs of recreational waterborne illnesses in the US alone approaches $3 billion annually (range of $2.2–$3.7 billion), with moderate to severe illnesses accounting for ~73% of the economic burden [96]. These costs will likely drastically increase due to climate change-induced rises in the number of pathogenic bacteria and fungi present in recreational waters. Moreover, climate-induced increases in temperature, precipitation, sea level, and storm intensities worldwide will likely lead to more disasters and increases in human exposure to pathogens in waterways [12], increases in contact of humans and animals, as well as a greater prevalence of antibiotic and heavy metal resistant bacteria and fungi, primarily due to the greater runoff [97]. These costs are in addition to those required to maintain and enhance infrastructure in a changing environment [98,99].
2. Recommendations for the Future
The popularity of human recreational beach activities, combined with predicted climate change scenarios, could amplify the risk of human exposure to pathogens and increase the incidence of illnesses. It has been estimated that 4 billion surface water recreation events occur annually in the US alone [96,100], and the use of water for recreational activities is growing worldwide [101]. If climate variability increases the microbial burden of beach environments, it is likely to result in increased human exposure and resultant disease through recreational contact with water via swimming, watersports, activities at the sand–water continuum [102], and contact with beach sand (expert review by Solo-Gabriele et al. [84]). In addition, human behaviours at the beach can influence the degree and severity of outcomes associated with exposure, especially among sensitive sub-populations (i.e., immune-compromised patients, elderly people, or children) who experience enhanced susceptibility to adverse health effects [11,103]. In fact, evidence suggests that beach sand can harbour higher levels of FIB than the adjacent water, and that sand contact can elevate the risk of human disease outcomes [104,105,106,107,108,109]. As hand-to-mouth behaviours are typical in beachgoers who often eat, drink, and play in the sand or at the sand–water continuum, it is crucial to consider microbial exposures among sensitive sub-groups as well as the general population, within the context of climate-induced pathogen variability predictions and mitigation actions now and into the future. Moreover, disease occurrence is not limited to direct sand and water contact, since strong winds and water activities can generate and propagate aerosolized particles carrying microbiota from what is in the water surface [110] and in the sand [74]. These particles may be inhaled but can also deposited on any exposed skin and mucosal surface or penetrate nostrils and ears, which is particularly relevant for some bacterial and fungal pathogens such as Mucoraceae [111] and Aspergillus section Nigri [112], respectively.
The traditional microbiological parameters used to assess water and sand quality, are unable to indicate the biological origin of a faecal contamination source. Microbial Source Tracking (MST) is a method that has been used and improved during the last two decades [113], which allows the establishment of an association between some faecal microorganisms with a particular animal host [114]. The association between the faecal contamination detected and the host enables the management authorities to take the most suitable measures to mitigate the problem and thus allow the safeguarding of public health. This methodology was used, for example, in an episode where sand contamination was detected and several biological sources (including canine) were identified [115].
Quantitative Microbial Risk Assessment (QMRA) [116,117] is a widely used tool for determining the health effects of waterborne pathogens. It has been used, to assess the extent of microbial contamination in food [118] and water [119,120,121], as well as in beach environments for calculating infection risks to recreational water users [122,123,124,125,126]. This approach takes into consideration microbial exposure levels, which might change in response to a changing climate [48,120,127]. QMRA has helped advance the field beyond FIB to an informed understanding of the contribution of specific pathogens to enteric diseases associated with recreational beach activities, and to fill knowledge gaps that remain from studying FIB levels alone [122,128,129,130,131]. This framework can assess the public health impacts of a changing microbiome at beaches and may enable more accurate management actions to prevent potential climate change-driven health impacts. With the emergence of new contaminants of human health concern, as well as projected effects of climate change on microbial communities in beach systems, the development and use of QMRA will depend on additional epidemiological and exposure pathway data collection. Therefore, it is important that future research in this area account for these emerging and changing conditions in order to effectively model and manage health risks at beaches.
While a statistical relationship between waterborne diarrheal diseases and severe weather-related events has been observed in several studies [132,133,134,135,136], the literature lacks a comprehensive investigation of long-term microbial exposure trends associated with climate, especially in the context of beach sand-related microbial exposure. While there have been some longer-term change detection studies that can aid in the understanding of climate change impacts on the environment, these can be time and cost-intensive and are therefore rare [137,138,139,140]. Without these longer-term analyses, it can be difficult to characterize change, let alone determine how to respond to it. Now that the effects of climate change are becoming clearer, it is time to focus on utilizing empirical and mechanistic modelling to fill in data gaps and optimally enhance understanding of climate change impacts and how to mitigate them. Climate change projection scenarios can be used in modelling sources of disease-related microbes, and fate and transport processes in coastal waters, to assess the effects of climate change on human health [141]. Modelling and QMRA can aid in understanding the risk level of microbial exposure in a changing environment [142]. Standardized surveillance and monitoring are still necessary to protect the health and safety of beachgoers on a daily basis. These data can also be used to supplement existing research, better inform, and drive models and shed light on climate change implications and management into the future.
Implementation of mitigation actions such as beach maintenance, informing beach users of risks associated with waterborne illness, and land use management will play a key role in protecting human health at beaches in the future.
3. Conclusions
Understanding the most critical climatic factors that influence changes in the microbiota of beach sands and recreational water, will aid in recommending best management practices to minimize the exposure risk and illness rates of the millions of individuals, who visit beaches to recreate each year. Some of these visitors may be immunocompromised at some level, which generates higher susceptibility to opportunistic microbes. If this is currently a reality, in a future reshaped by climate change, this type of knowledge may be much more relevant. Planning the future of recreational water use should consider sand and water, as well as the influences of climate changes in their microbiota.
Author Contributions
Conceptualization, J.B., C.W. and M.J.S.; investigation, J.B., C.W., E.V., T.P., P.M., L.A. and C.D.H.; writing—original draft preparation, J.B., C.W., E.V., T.P., P.M., L.A. and C.D.H.; writing—review and editing, J.B., C.W., E.V., T.P., P.M., L.A., C.D.H. and M.J.S.; All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from CESAM (UID/AMB/50017-POCI-01-0145-FEDER-007638) and CITAB (UID/AGR/04033/2019), via FCT/MCTES, from national funds (PIDDAC), cofounded by FEDER, (PT2020 Partnership Agreement and Compete 2020).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IPCC . In: Climate Change Synthesis Report 2014. Pachauri R.K., Meyer L., editors. Intergovernmental Panel on Climate Change; New York, NY, USA: 2014. [Google Scholar]
- 2.IPCC . In: Climate Change the Physical Science Basis 2013. Stocker T.F., Qin D., editors. Intergovernmental Panel on Climate Change; New York, NY, USA: 2013. [Google Scholar]
- 3.NOAA Extended Reconstructed Sea Surface Temperature (ERSST.v5) [(accessed on 10 December 2021)];2021 Available online: https://www.epa.gov/climate-indicators/climate-change-indicators-sea-surface-temperature.
- 4.Johansson M.M., Pellikka H., Kahma K.K., Ruosteenoja K. Global sea level rise scenarios adapted to the Finnish coast. J. Mar. Syst. 2014;129:35–46. doi: 10.1016/j.jmarsys.2012.08.007. [DOI] [Google Scholar]
- 5.O’Reilly C.M., Sharma S., Gray D.K., Hampton S.E., Read J.S., Rowley R.J., Schneider P., Lenters J.D., McIntyre P.B., Kraemer B.M., et al. Rapid and highly variable warming of lake surface waters around the globe. Geophys. Res. Lett. 2015;42:10773–10781. doi: 10.1002/2015GL066235. [DOI] [Google Scholar]
- 6.Sharma S., Gray D.K., Read J.S., O’Reilly C.M., Schneider P., Qudrat A., Gries C., Stefanoff S., Hampton S.E., Hook S., et al. A global database of lake surface temperatures collected by in situ and satellite methods from 1985–2009. Sci. Data. 2015;2:19. doi: 10.1038/sdata.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu L.X., Cai W.J., Zhang L.P., Nakamura H., Timmermann A., Joyce T., McPhaden M.J., Alexander M., Qiu B., Visbecks M., et al. Enhanced warming over the global subtropical western boundary currents. Nat. Clim. Chang. 2012;2:161–166. doi: 10.1038/nclimate1353. [DOI] [Google Scholar]
- 8.Young I.R., Zieger S., Babanin A.V. Global trends in wind speed and wave height. Science. 2011;332:451–455. doi: 10.1126/science.1197219. [DOI] [PubMed] [Google Scholar]
- 9.Tokinaga H., Xie S.P. Wave and anemometer-based sea surface wind (WASWind) for climate change analysis. J. Clim. 2011;24:267–285. doi: 10.1175/2010JCLI3789.1. [DOI] [Google Scholar]
- 10.Byrnes J.E., Reed D.C., Cardinale B.J., Cavanaugh K.C., Holbrook S.J., Schmitt R.J. Climate-driven increases in storm frequency simplify kelp forest food webs. Glob. Chang. Biol. 2011;17:2513–2524. doi: 10.1111/j.1365-2486.2011.02409.x. [DOI] [Google Scholar]
- 11.Stewart J.R., Gast R.J., Fujioka R.S., Solo-Gabriele H.M., Meschke J.S., Amaral-Zettler L.A., del Castillo E., Polz M.F., Collier T.K., Strom M.S., et al. The coastal environment and human health: Microbial indicators, pathogens, sentinels and reservoirs. Environ. Health. 2008;7:S3. doi: 10.1186/1476-069X-7-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiskerger C.J., Brandao J., Ahmed W., Aslan A., Avolio L., Badgley B.D., Boehm A.B., Edge T.A., Fleisher J.M., Heaney C.D., et al. Impacts of a changing earth on microbial dynamics and human health risks in the continuum between beach water and sand. Water Res. 2019;162:456–470. doi: 10.1016/j.watres.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Casadevall A., Kontoyiannis D.P., Robert V. On the emergence of Candida auris: Climate change, azoles, swamps, and birds. mBio. 2019;10:e01397-19. doi: 10.1128/mBio.01397-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arora P., Singh P., Wang Y., Yadav A., Pawar K., Singh A., Padmavati G., Xu J., Chowdhary A. Environmental isolation of Candida auris from the coastal wetlands of Andaman Islands, India. mBio. 2021;12:e03181-20. doi: 10.1128/mBio.03181-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabino R., Veríssimo C., Cunha M., Wergikoski B., Ferreira F., Rodrigues R., Parada H., Falcão L., Rosado L., Pinheiro C., et al. Pathogenic fungi: An unacknowledged risk at coastal resorts? New insights on microbiological sand quality in Portugal. Mar. Pollut. Bull. 2011;62:1506–1511. doi: 10.1016/j.marpolbul.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Brandão J., Gangneux J.P., Arikan-Akdagli S., Barac A., Bostanaru A.C., Brito S., Bull M., Çerikçioğlu N., Chapman B., Efstratiou M.A., et al. Mycosands: Fungal diversity and abundance in beach sand and recreational waters—Relevance to human health. Sci. Total Environ. 2021;781:146598. doi: 10.1016/j.scitotenv.2021.146598. [DOI] [PubMed] [Google Scholar]
- 17.Byrne R.H., Mecking S., Feely R.A., Liu X.W. Direct observations of basin-wide acidification of the North Pacific Ocean. Geophys. Res. Lett. 2010;37:5. doi: 10.1029/2009GL040999. [DOI] [Google Scholar]
- 18.Cai W.J., Hu X.P., Huang W.J., Murrell M.C., Lehrter J.C., Lohrenz S.E., Chou W.C., Zhai W.D., Hollibaugh J.T., Wang Y.C., et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011;4:766–770. doi: 10.1038/ngeo1297. [DOI] [Google Scholar]
- 19.Henson S.A., Cael B.B., Allen S.R., Dutkiewicz S. Future phytoplankton diversity in a changing climate. Nat. Commun. 2021;12:1–8. doi: 10.1038/s41467-021-25699-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson C.E., Madronich S., Lal A., Zepp R.G., Lucas R.M., Overholt E.P., Rose K.C., Schladow S.G., Lee-Taylor J. Climate change-induced increases in precipitation are reducing the potential for solar ultraviolet radiation to inactivate pathogens in surface waters. Sci. Rep. 2017;7:12. doi: 10.1038/s41598-017-13392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Environmental Protection Agency Impacts of Climate Change on the Occurrence of Harmful Algal Blooms. (2013, May) [(accessed on 17 November 2021)]; Available online: https://www.epa.gov/sites/default/files/documents/climatehabs.pdf.
- 22.Rastogi R.P., Madamwar D., Incharoensakdi A. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front. Microbiol. 2015;6:1254. doi: 10.3389/fmicb.2015.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richer R., Banack S.A., Metcalf J.S., Cox P.A. The persistence of cyanobacterial toxins in desert soils. J. Arid Environ. 2015;112:134–139. doi: 10.1016/j.jaridenv.2014.01.023. [DOI] [Google Scholar]
- 24.Phillips M.C., Feng Z., Vogel L.J., Reniers A.J.H.M., Haus B.K., Enns A.A., Zhang Y., Hernandez D.B., Solo-Gabriele H.M. Microbial release from seeded beach sediments during wave conditions. Mar. Pollut. Bull. 2014;79:114–122. doi: 10.1016/j.marpolbul.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogel L.J., O’Carroll D.M., Edge T.A., Robinson C.E. Release of Escherichia coli from foreshore sand and pore water during intensified wave conditions at a recreational beach. Environ. Sci. Technol. 2016;50:5676–5684. doi: 10.1021/acs.est.6b00707. [DOI] [PubMed] [Google Scholar]
- 26.Kauppinen A., Al-Hello H., Zacheus O., Kilponen J., Maunula L., Huusko S., Lappalainen M., Miettinen I., Blomqvist S., Rimhanen-Finne R. Increase in outbreaks of gastroenteritis linked to bathing water in Finland in summer 2014. Eurosurveillance. 2017;22:13–20. doi: 10.2807/1560-7917.ES.2017.22.8.30470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno A., Amelung B., Santamarta L. Linking beach recreation to weather conditions: A case study in Zandvoort, Netherlands. Tour. Mar. Environ. 2009;5:111–119. doi: 10.3727/154427308787716758. [DOI] [Google Scholar]
- 28.Smith K. The influence of weather and climate on recreation and tourism. Weather. 1993;48:398–404. doi: 10.1002/j.1477-8696.1993.tb05828.x. [DOI] [Google Scholar]
- 29.Shuval H. Estimating the global burden of thalassogenic diseases: Human infectious diseases caused by wastewater pollution of the marine environment. J. Water Health. 2003;1:53–64. doi: 10.2166/wh.2003.0007. [DOI] [PubMed] [Google Scholar]
- 30.Perkins T.L., Clements K., Baas J.H., Jago C.F., Jones D.L., Malham S.K., McDonald J.E. Sediment composition influences spatial variation in the abundance of human pathogen indicator bacteria within an estuarine environment. PLoS ONE. 2014;9:9. doi: 10.1371/journal.pone.0112951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brookes J.D., Carey C.C. Resilience to blooms. Science. 2011;334:46–47. doi: 10.1126/science.1207349. [DOI] [PubMed] [Google Scholar]
- 32.Rigosi A., Hanson P., Hamilton D.P., Hipsey M., Rusak J.A., Bois J., Sparber K., Chorus I., Watkinson A.J., Qin B., et al. Determining the probability of cyanobacterial blooms: The application of Bayesian networks in multiple lake systems. Ecol. Appl. 2015;25:186–199. doi: 10.1890/13-1677.1. [DOI] [PubMed] [Google Scholar]
- 33.Bussi G., Whitehead P.G., Thomas A.R.C., Masante D., Jones L., Cosby B.J., Emmett B.A., Malham S.K., Prudhomme C., Prosser H. Climate and land-use change impact on faecal indicator bacteria in a temperate maritime catchment (the River Conwy, Wales) J. Hydrol. 2017;553:248–261. doi: 10.1016/j.jhydrol.2017.08.011. [DOI] [Google Scholar]
- 34.Noble R.T., Lee I.M., Schiff K.C. Inactivation of indicator micro-organisms from various sources of faecal contamination in seawater and freshwater. J. Appl. Microbiol. 2004;96:464–472. doi: 10.1111/j.1365-2672.2004.02155.x. [DOI] [PubMed] [Google Scholar]
- 35.Sokolova E., Astrom J., Pettersson T.J.R., Bergstedt O., Hermansson M. Decay of Bacteroidales genetic markers in relation to traditional fecal indicators for water quality modeling of drinking water sources. Environ. Sci. Technol. 2012;46:892–900. doi: 10.1021/es2024498. [DOI] [PubMed] [Google Scholar]
- 36.Viau E.J., Goodwin K.D., Yamahara K.M., Layton B.A., Sassoubre L.M., Burns S.L., Tong H.I., Wong S.H.C., Lu Y.A., Boehm A.B. Bacterial pathogens in Hawaiian coastal streams—Associations with fecal indicators, land cover, and water quality. Water Res. 2011;45:3279–3290. doi: 10.1016/j.watres.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Hokajärvi A.M., Pitkanen T., Siljanen H.M.P., Nakari U.M., Torvinen E., Siitonen A., Miettinen I.T. Occurrence of thermotolerant Campylobacter spp. and adenoviruses in Finnish bathing waters and purified sewage effluents. J. Water Health. 2013;11:120–134. doi: 10.2166/wh.2012.192. [DOI] [PubMed] [Google Scholar]
- 38.Baker-Austin C., Trinanes J.A., Taylor N.G.H., Hartnell R., Siitonen A., Martinez-Urtaza J. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Chang. 2013;3:73–77. doi: 10.1038/nclimate1628. [DOI] [Google Scholar]
- 39.Huehn S., Eichhorn C., Urmersbach S., Breidenbach J., Bechlars S., Bier N., Alter T., Bartelt E., Frank C., Oberheitmann B., et al. Pathogenic vibrios in environmental, seafood and clinical sources in Germany. Int. J. Med. Microbiol. 2014;304:843–850. doi: 10.1016/j.ijmm.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Sterk A., Schets F.M., Husman A.M.D., de Nijs T., Schijven J.F. Effect of climate change on the concentration and associated risks of Vibrio spp. in Dutch recreational waters. Risk Anal. 2015;35:1717–1729. doi: 10.1111/risa.12365. [DOI] [PubMed] [Google Scholar]
- 41.Desvars A., Jego S., Chiroleu F., Bourhy P., Cardinale E., Michault A. Seasonality of human Leptospirosis in Reunion Island (Indian Ocean) and its association with meteorological data. PLoS ONE. 2011;6:10. doi: 10.1371/journal.pone.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sumi A., Telan E.F.O., Chagan-Yasutan H., Piolo M.B., Hattori T., Kobayashi N. Effect of temperature, relative humidity and rainfall on dengue fever and leptospirosis infections in Manila, the Philippines. Epidemiol. Infect. 2017;145:78–86. doi: 10.1017/S095026881600203X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J., Liao J.S., Huang X., Wang Y.P., Ren J.H., Wang X.Y., Ding F. Mapping risk of leptospirosis in China using environmental and socioeconomic data. BMC Infect. Dis. 2016;16:10. doi: 10.1186/s12879-016-1653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodwin K.D., McNay M., Cao Y.P., Ebentier D., Madison M., Griffith J.F. A multi-beach study of Staphylococcus aureus, MRSA, and enterococci in seawater and beach sand. Water Res. 2012;46:4195–4207. doi: 10.1016/j.watres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Maghsoudi E., Prevost M., Duy S.V., Sauve S., Dorner S. Adsorption characteristics of multiple microcystins and cylindrospermopsin on sediment: Implications for toxin monitoring and drinking water treatment. Toxicon. 2015;103:48–54. doi: 10.1016/j.toxicon.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Akar P.J., Jirka G.H. Lateral spreading with ambient current advection: Processus d’étalement en surface dans le transport et le mélange de polluants lere partie: Etalement lateral dans un écoulement ambiant. J. Hydraul. Res. 1994;32:815–831. doi: 10.1080/00221689409498692. [DOI] [Google Scholar]
- 47.Hellberg R.S., Chu E. Effects of climate change on the persistence and dispersal of foodborne bacterial pathogens in the outdoor environment: A review. Crit. Rev. Microbiol. 2016;42:548–572. doi: 10.3109/1040841X.2014.972335. [DOI] [PubMed] [Google Scholar]
- 48.Hofstra N. Quantifying the impact of climate change on enteric waterborne pathogen concentrations in surface water. Curr. Opin. Environ. Sustain. 2011;3:471–479. doi: 10.1016/j.cosust.2011.10.006. [DOI] [Google Scholar]
- 49.Curriero F.C., Patz J.A., Rose J.B., Lele S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am. J. Public Health. 2001;91:1194–1199. doi: 10.2105/AJPH.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patz J.A., Vavrus S.J., Uejio C.K., McLellan S.L. Climate change and waterborne disease risk in the Great Lakes region of the US. Am. J. Prev. Med. 2008;35:451–458. doi: 10.1016/j.amepre.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 51.Mimura N. Sea-level rise caused by climate change and its implications for society. Proc. Japan Acad. Ser. B-Phys. Biol. Sci. 2013;89:281–301. doi: 10.2183/pjab.89.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steets B.M., Holden P.A. A mechanistic model of runoff-associated fecal coliform fate and transport through a coastal lagoon. Water Res. 2003;37:589–608. doi: 10.1016/S0043-1354(02)00312-3. [DOI] [PubMed] [Google Scholar]
- 53.Evanson M., Ambrose R.F. Sources and growth dynamics of fecal indicator bacteria in a coastal wetland system and potential impacts to adjacent waters. Water Res. 2006;40:475–486. doi: 10.1016/j.watres.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 54.Halliday E., Gast R.J. Bacteria in beach sands: An emerging challenge in protecting coastal water quality and bather health. Environ. Sci. Technol. 2011;45:370–379. doi: 10.1021/es102747s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rippy M.A., Stein R., Sanders B.F., Davis K., McLaughlin K., Skinner J.F., Kappeler J., Grant S.B. Small drains, big problems: The impact of dry weather runoff on shoreline water quality at enclosed beaches. Environ. Sci. Technol. 2014;48:14168–14177. doi: 10.1021/es503139h. [DOI] [PubMed] [Google Scholar]
- 56.Edge T.A., Crowe A., Hill S., Seto P., Marsalek J. Surveillance for Potential Sources of E. coli at Toronto’s Bluffer’s Park Beach 2005–2006. Environment Canada; Burlington, ON, Canada: 2007. [Google Scholar]
- 57.Ackerman D., Weisberg S. Relationship between rainfall and beach bacterial concentrations on Santa Monica Bay beaches. J. Water Health. 2003;1:85–89. doi: 10.2166/wh.2003.0010. [DOI] [PubMed] [Google Scholar]
- 58.McLellan S.L., Salmore A.K. Evidence for localized bacterial loading as the cause of chronic beach closings in a freshwater marina. Water Res. 2003;37:2700–2708. doi: 10.1016/S0043-1354(03)00068-X. [DOI] [PubMed] [Google Scholar]
- 59.O’Dwyer J., Downes M.M., Adley C.C. The impact of meteorology on the occurrence of waterborne outbreaks of vero cytotoxin-producing Escherichia coli (VTEC): A logistic regression approach. J. Water Health. 2016;14:39–46. doi: 10.2166/wh.2015.016. [DOI] [PubMed] [Google Scholar]
- 60.Vermeij W., van der Wiele J., van Moorselaar I., van der Grinten E. Impact of Climate Change on Water Quality in The Netherlands. National Institute for Public Health and Environment; Bilthoven, The Netherlands: 2010. [Google Scholar]
- 61.Shah A.H., Abdelzaher A.M., Phillips M., Hernandez R., Solo-Gabriele H.M., Kish J., Scorzetti G., Fell J.W., Diaz M.R., Scott T.M., et al. Indicator microbes correlate with pathogenic bacteria, yeasts and helminthes in sand at a subtropical recreational beach site. J. Appl. Microbiol. 2011;110:1571–1583. doi: 10.1111/j.1365-2672.2011.05013.x. [DOI] [PubMed] [Google Scholar]
- 62.Lemonte J.J., Stuckey J.W., Sanchez J.Z., Tappero R., Rinklebe J., Sparks D.L. Sea Level Rise Induced Arsenic Release from Historically Contaminated Coastal Soils. Environ. Sci. Technol. 2017;51:5913–5922. doi: 10.1021/acs.est.6b06152. [DOI] [PubMed] [Google Scholar]
- 63.Powers N.C., Pinchback J., Flores L., Huang Y., Wetz M.S., Turner J.W. Long-term water quality analysis reveals correlation between bacterial pollution and sea level rise in the northwestern Gulf of Mexico. Mar. Pollut. Bull. 2021;166:112231. doi: 10.1016/j.marpolbul.2021.112231. [DOI] [PubMed] [Google Scholar]
- 64.Phillips M.C., Solo-Gabriele H.M., Piggot A.M., Klaus J.S., Zhang Y. Relationships between sand and water quality at recreational beaches. Water Res. 2011;45:6763–6769. doi: 10.1016/j.watres.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piggot A.M., Klaus J.S., Johnson S., Phillips M.C., Solo-Gabriele H.M. Relationship between enterococcal levels and sediment biofilms at recreational beaches in south Florida. Appl. Environ. Microbiol. 2012;78:5973–5982. doi: 10.1128/AEM.00603-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonilla T.D., Nowosielski K., Cuvelier M., Hartz A., Green M., Esiobu N., McCorquodale D.S., Fleisher J.M., Rogerson A. Prevalence and distribution of fecal indicator organisms in South Florida beach sand and preliminary assessment of health effects associated with beach sand exposure. Mar. Pollut. Bull. 2007;54:1472–1482. doi: 10.1016/j.marpolbul.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 67.Heaney C.D., Exum N.G., Dufour A.P., Brenner K.P., Haugland R.A., Chern E., Schwab K.J., Love D.C., Serre M.L., Noble R., et al. Water quality, weather and environmental factors associated with fecal indicator organism density in beach sand at two recreational marine beaches. Sci. Total Environ. 2014;497:440–447. doi: 10.1016/j.scitotenv.2014.07.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamahara K.M., Sassoubre L.M., Goodwin K.D., Boehm A.B. Occurrence and persistence of bacterial pathogens and indicator organisms in beach sand along the California coast. Appl. Environ. Microbiol. 2012;78:1733–1745. doi: 10.1128/AEM.06185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tiwari A., Oliver D.M., Bivins A., Sherchan S.P., Pitkänen T. Bathing water quality monitoring practices in europe and the United States. Int. J. Environ. Res. Public Health. 2021;18:5513. doi: 10.3390/ijerph18115513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lutz C., Erken M., Noorian P., Sun S., McDougald D. Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Front. Microbiol. 2013;4:375. doi: 10.3389/fmicb.2013.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tiwari A., Kauppinen A., Pitkänen T. Decay of Enterococcus faecalis, vibrio cholerae and MS2 coliphage in a laboratory mesocosm under brackish beach conditions. Front. Public Health. 2019;7:269. doi: 10.3389/fpubh.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Topić N., Cenov A., Jozić S., Glad M., Mance D., Lušić D., Kapetanović D., Mance D., Lušić D.V. Staphylococcus aureus—An additional parameter of bathing water quality for crowded urban beaches. Int. J. Environ. Res. Public Health. 2021;18:5234. doi: 10.3390/ijerph18105234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu L., Phanikumar M.S., Molloy S.L., Whitman R.L., Shively D.A., Nevers M.B., Schwab D.J., Rose J.B. Modeling the transport and inactivation of E. coli and enterococci in the near-shore region of Lake Michigan. Environ. Sci. Technol. 2006;40:5022–5028. doi: 10.1021/es060438k. [DOI] [PubMed] [Google Scholar]
- 74.WHO . WHO Guidelines on Recreational Water Quality: Volume 1: Coastal and Fresh Waters. Volume 1. WHO; Geneva, Switzerland: 2021. [PubMed] [Google Scholar]
- 75.Moore S.K., Trainer V.L., Mantua N.J., Parker M.S., Laws E.A., Backer L.C., Fleming L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health. 2008;7:12. doi: 10.1186/1476-069X-7-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tryjanowski P., Sparks T.H., Kuźniak S., Czechowski P., Jerzak L. Bird Migration Advances More Strongly in Urban Environments. PLoS ONE. 2013;8:e63482. doi: 10.1371/journal.pone.0063482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conover M.R. Population growth and movements of Canada geese in New Haven County, Connecticut, during a 25-year period. Waterbirds. 2011;34:412–421. doi: 10.1675/063.034.0403. [DOI] [Google Scholar]
- 78.Converse R.R., Kinzelman J.L., Sams E.A., Hudgens E., Dufour A.P., Ryu H., Santo-Domingo J.W., Kelty C.A., Shanks O.C., Siefring S.D. Dramatic improvements in beach water quality following gull removal. Environ. Sci. Technol. 2012;46:10206–10213. doi: 10.1021/es302306b. [DOI] [PubMed] [Google Scholar]
- 79.Navarro J., Grémillet D., Afán I., Miranda F., Bouten W., Forero M.G., Figuerola J. Pathogen transmission risk by opportunistic gulls moving across human landscapes. Sci. Rep. 2019;9:1–5. doi: 10.1038/s41598-019-46326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hubálek Z. Pathogenic microorganisms associated with gulls and terns (Laridae) J. Vertebr. Biol. 2021;70 doi: 10.25225/jvb.21009. [DOI] [Google Scholar]
- 81.Zeballos-Gross D., Rojas-Sereno Z., Salgado-Caxito M., Poeta P., Torres C., Benavides J.A. The Role of Gulls as Reservoirs of Antibiotic Resistance in Aquatic Environments: A Scoping Review. Front. Microbiol. 2021;12:1–15. doi: 10.3389/fmicb.2021.703886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kurittu P., Khakipoor B., Brouwer M.S.M., Heikinheimo A. Plasmids conferring resistance to extended-spectrum beta-lactamases including a rare IncN+IncR multireplicon carrying blaCTX-M-1 in Escherichia coli recovered from migrating barnacle geese (Branta leucopsis) Open Res. Eur. 2021;1:46. doi: 10.12688/openreseurope.13529.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fowler A.M., Hennessy K.J. Potential impacts of global warming on the frequency and magnitude of heavy precipitation. Nat. Hazards. 1995;11:283–303. doi: 10.1007/BF00613411. [DOI] [Google Scholar]
- 84.Mearns L.O., Giorgi F., McDaniel L., Shields C. Analysis of daily variability of precipitation in a nested regional climate model-comparison with observations and doubled CO2 results. Glob. Planet. Change. 1995;10:55–78. doi: 10.1016/0921-8181(94)00020-E. [DOI] [Google Scholar]
- 85.Trenberth K.E. Conceptual framework for changes of extremes of the hydrological cycle with climate change. Clim. Change. 1999;42:327–339. doi: 10.1023/A:1005488920935. [DOI] [Google Scholar]
- 86.McBride G.B., Stott R., Miller W., Bambic D., Wuertz S. Discharge-based QMRA for estimation of public health risks from exposure to stormwater-borne pathogens in recreational waters in the United States. Water Res. 2013;47:5282–5297. doi: 10.1016/j.watres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 87.Paule-Mercado M.A., Ventura J.S., Memon S.A., Jahng D., Kang J.H., Lee C.H. Monitoring and predicting the fecal indicator bacteria concentrations from agricultural, mixed land use and urban stormwater runoff. Sci. Total Environ. 2016;550:1171–1181. doi: 10.1016/j.scitotenv.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 88.Selvakumar A., Borst M. Variation of microorganism concentrations in urban stormwater runoff with land use and seasons. J. Water Health. 2006;4:109–124. doi: 10.2166/wh.2006.0009. [DOI] [PubMed] [Google Scholar]
- 89.Adam J.C., Hamlet A.F., Lettenmaier D.P. Implications of global climate change for snowmelt hydrology in the twenty-first century. Hydrol. Process. 2009;23:962–972. doi: 10.1002/hyp.7201. [DOI] [Google Scholar]
- 90.Lo Iacono G., Armstrong B., Fleming L.E., Elson R., Kovats S., Vardoulakis S., Nichols G.L. Challenges in developing methods for quantifying the effects of weather and climate on water-associated diseases: A systematic review. PLoS Negl. Trop. Dis. 2017;11:35. doi: 10.1371/journal.pntd.0005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solo-Gabriele H.M., Harwood V.J., Kay D., Fujioka R.S., Sadowsky M.J., Whitman R.L., Wither A., Canica M., Da Fonseca R.C., Duarte A., et al. Beach sand and the potential for infectious disease transmission: Observations and recommendations. J. Mar. Biol. Assoc. UK. 2016;96:101–120. doi: 10.1017/S0025315415000843. [DOI] [Google Scholar]
- 92.Lu J.R., Ryu H.D., Hill S., Schoen M., Ashbolt N., Edge T.A., Domingo J.S. Distribution and potential significance of a gull fecal marker in urban coastal and riverine areas of southern Ontario, Canada. Water Res. 2011;45:3960–3968. doi: 10.1016/j.watres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 93.Alm E.W., Daniels-Witt Q.R., Learman D.R., Ryu H., Jordan D.W., Gehring T.M., Santo Domingo J. Potential for gulls to transport bacteria from human waste sites to beaches. Sci. Total Environ. 2018;615:123–130. doi: 10.1016/j.scitotenv.2017.09.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rytkönen A., Tiwari A., Hokajärvi A.M., Uusheimo S., Vepsäläinen A., Tulonen T., Pitkänen T. The Use of Ribosomal RNA as a Microbial Source Tracking Target Highlights the Assay Host-Specificity Requirement in Water Quality Assessments. Front. Microbiol. 2021;12:1–16. doi: 10.3389/fmicb.2021.673306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Neumann B., Vafeidis A.T., Zimmermann J., Nicholls R.J. Future coastal population growth and exposure to sea-level rise and coastal flooding—A global assessment. PLoS ONE. 2015;10:e0131375. doi: 10.1371/journal.pone.0118571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeFlorio-Barker S., Wing C., Jones R.M., Dorevitch S. Estimate of incidence and cost of recreational waterborne illness on United States surface waters. Environ. Health. 2018;17:3. doi: 10.1186/s12940-017-0347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burnham J.P. Climate change and antibiotic resistance: A deadly combination. Ther. Adv. Infect. Dis. 2021;8:1–7. doi: 10.1177/2049936121991374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simpkins G. The costs of infrastructure adaptation. Nat. Rev. Earth Environ. 2021;2:661. doi: 10.1038/s43017-021-00222-3. [DOI] [Google Scholar]
- 99.Neumann J.E., Chinowsky P., Helman J., Black M., Fant C., Strzepek K., Martinich J. Climate effects on US infrastructure: The economics of adaptation for rail, roads, and coastal development. Clim. Change. 2021;167:1–23. doi: 10.1007/s10584-021-03179-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Collier S.A., Deng L., Adam E.A., Benedict K.M., Beshearse E.M., Blackstock A.J., Bruce B.B., Derado G., Edens C., Fullerton K.E., et al. Estimate of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United States. Emerg. Infect. Dis. 2021;27:140–149. doi: 10.3201/eid2701.190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ritchie H., Roser M. Water Use and Stress 2017 Published Online at OurWorldInData.org. [(accessed on 10 November 2021)]. Available online: https://ourworldindata.org/water-use-stress.
- 102.Mannocci A., La Torre G., Spagnoli A., Solimini A.G., Palazzo C., De Giusti M. Is swimming in recreational water associated with the occurrence of respiratory illness? A systematic review and meta-analysis. J. Water Health. 2016;14:590–599. doi: 10.2166/wh.2016.266. [DOI] [PubMed] [Google Scholar]
- 103.Gerba C.P., Rose J.B., Haas C.N. Sensitive populations: Who is at the greatest risk? Int. J. Food Microbiol. 1996;30:113–123. doi: 10.1016/0168-1605(96)00996-8. [DOI] [PubMed] [Google Scholar]
- 104.Yamahara K.M., Layton B.A., Santoro A.E., Boehm A.B. Beach sands along the California coast are diffuse sources of fecal bacteria to coastal waters. Environ. Sci. Technol. 2007;41:4515–4521. doi: 10.1021/es062822n. [DOI] [PubMed] [Google Scholar]
- 105.Zampieri B.D.B., Oliviera R.S.D., Pinto A.B., Andrade V.D.C., Barbieri E., Chinellato R.M., Oliviera A.J.F.C.D. Comparison of bacterial densities and resistance in different beach compartments: Should water by our main concern? O Mundo Saúde São Paulo. 2017;40A:461–482. doi: 10.15343/0104-7809.201740A461482. [DOI] [Google Scholar]
- 106.Whitman R.L., Nevers M.B. Foreshore sand as a source of Escherichia coli in nearshore water of a Lake Michigan beach. Appl. Environ. Microbiol. 2003;69:5555–5562. doi: 10.1128/AEM.69.9.5555-5562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heaney C.D., Sams E., Wing S., Marshall S., Brenner K., Dufour A.P., Wade T.J. Contact with beach sand among beachgoers and risk of illness. Am. J. Epidemiol. 2009;170:164–172. doi: 10.1093/aje/kwp152. [DOI] [PubMed] [Google Scholar]
- 108.Heaney C.D., Sams E., Dufour A.P., Brenner K.P., Haugland R.A., Chern E., Wing S., Marshall S., Love D.C., Serre M., et al. Fecal Indicators in Sand, Sand Contact, and Risk of Enteric Illness Among Beachgoers. Epidemiology. 2012;23:95–106. doi: 10.1097/EDE.0b013e31823b504c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sabino R., Rodrigues R., Costa I., Carneiro C., Cunha M., Duarte A., Faria N., Ferreira F.C., Gargate M.J., Julio C., et al. Routine screening of harmful microorganisms in beach sands: Implications to public health. Sci. Total Environ. 2014;472:1062–1069. doi: 10.1016/j.scitotenv.2013.11.091. [DOI] [PubMed] [Google Scholar]
- 110.Graham K.E., Prussin A.J., Marr L.C., Sassoubre L.M., Boehm A.B. Microbial community structure of sea spray aerosols at three California beaches. FEMS Microbiol. Ecol. 2018;94:10. doi: 10.1093/femsec/fiy005. [DOI] [PubMed] [Google Scholar]
- 111.Singh V.P., Bansal C., Kaintura M. Sinonasal Mucormycosis: A to Z. Indian J. Otolaryngol. Head Neck Surg. Off. Publ. Assoc. Otolaryngol. India. 2019;71:1962–1971. doi: 10.1007/s12070-018-1384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kamali Sarvestani H., Seifi A., Falahatinejad M., Mahmoudi S. Black aspergilli as causes of otomycosis in the era of molecular diagnostics, a mini-review. J. Mycologie Medicale. 2021;32:101240. doi: 10.1016/j.mycmed.2021.101240. [DOI] [PubMed] [Google Scholar]
- 113.Bernhard A.E., Field K.G. Identification of nonpoint sources of fecal pollution in coastal waters by using host-specific 16S ribosomal DNA genetic markers from fecal anaerobes. Appl. Environ. Microbiol. 2000;66:1587–1594. doi: 10.1128/AEM.66.4.1587-1594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harwood V.J., Staley C., Badgley B.D., Borges K., Korajkic A. Microbial source tracking markers for detection of fecal contamination in environmental waters: Relationships between pathogens and human health outcomes. FEMS Microbiol. Rev. 2014;38:1–40. doi: 10.1111/1574-6976.12031. [DOI] [PubMed] [Google Scholar]
- 115.Valério E., Santos M.L., Teixeira P., Matias R., Mendonça J., Ahmed W., Brandão J. Beach sand contaminated by dog walking—A molecular case study. Sci. Total Environ. (submitted) [Google Scholar]
- 116.Haas C.N., Rose J.B., Gerba C.P. Quantitative Microbial Risk Assessment. John Wiley & Sons; Hoboken, NJ, USA: 1999. [Google Scholar]
- 117.WHO . Guidelines for Drinking-Water Quality. 4th ed. WHO; Geneva, Switzerland: 2011. [Google Scholar]
- 118.Mylius S.D., Nauta M.J., Havelaar A.H. Cross-contamination during food preparation: A mechanistic model applied to chicken-borne Campylobacter. Risk Anal. 2007;27:803–813. doi: 10.1111/j.1539-6924.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 119.Ashbolt N.J. Risk analysis of drinking water microbial contamination versus disinfection by-products (DBPs) Toxicology. 2004;198:255–262. doi: 10.1016/j.tox.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 120.Schijven J.F., Teunis P.P.M., Rutjes S.A., Bouwknegt M., Husman A.M.D. QMRAspot: A tool for quantitative microbial risk assessment from surface water to potable water. Water Res. 2011;45:5564–5576. doi: 10.1016/j.watres.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 121.Juntunen J., Merilainen P., Simola A. Public health and economic risk assessment of waterborne contaminants and pathogens in Finland. Sci. Total Environ. 2017;599:873–882. doi: 10.1016/j.scitotenv.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 122.Ashbolt N.J., Schoen M.E., Soller J.A., Rose D.J. Predicting pathogen risks to aid beach management: The real value of quantitative microbial risk assessment (QMRA) Water Res. 2010;44:4692–4703. doi: 10.1016/j.watres.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 123.Shibata T., Solo-Gabriele H.M. Quantitative microbial risk assessment of human illness from exposure to marine beach sand. Environ. Sci. Technol. 2012;46:2799–2805. doi: 10.1021/es203638x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schoen M.E., Ashbolt N.J. Assessing pathogen risk to swimmers at non-sewage impacted recreational beaches. Environ. Sci. Technol. 2010;44:2286–2291. doi: 10.1021/es903523q. [DOI] [PubMed] [Google Scholar]
- 125.Jang C.S., Liang C.P. Characterizing health risks associated with recreational swimming at Taiwanese beaches by using quantitative microbial risk assessment. Water Sci. Technol. 2018;77:534–547. doi: 10.2166/wst.2017.571. [DOI] [PubMed] [Google Scholar]
- 126.Brandão J., Solo-Gabriele H.M., Gordon B., Ferguson A.C. Recreational environment-pathogenic fungi in public places, information gaps in assessing public health risk. In: Viegas C., Pinheiro C., Verissimo C., Sabino R., Brandão J., Viegas S., editors. Environmental Mycology in Public Health. Elsevier; Amsterdam, The Netherlands: 2015. [Google Scholar]
- 127.Sterk A., Schijven J., de Nijs T., Husman A.M.D. Direct and indirect effects of climate change on the risk of infection by water-transmitted pathogens. Environ. Sci. Technol. 2013;47:12648–12660. doi: 10.1021/es403549s. [DOI] [PubMed] [Google Scholar]
- 128.Soller J.A., Schoen M.E., Bartrand T., Ravenscroft J.E., Ashbolt N.J. Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of faecal contamination. Water Res. 2010;44:4674–4691. doi: 10.1016/j.watres.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 129.Soller J.A., Eftim S., Wade T.J., Ichida A.M., Clancy J.L., Johnson T.B., Schwab K., Ramirez-Toro G., Nappier S., Ravenscroft J.E. Use of quantitative microbial risk assessment to improve interpretation of a recreational water epidemiological study. Microb. Risk Anal. 2016;1:2–11. doi: 10.1016/j.mran.2015.04.001. [DOI] [Google Scholar]
- 130.Zhang Q., Gallard J., Wu B., Harwood V.J., Sadowsky M.J., Hamilton K.A., Ahmed W. Synergy between quantitative microbial source tracking (qMST) and quantitative microbial risk assessment (QMRA): A review and prospectus. Environ Int. 2019;130:104703. doi: 10.1016/j.envint.2019.03.051. [DOI] [PubMed] [Google Scholar]
- 131.Weiskerger C.J., Brandão J. Fungal contaminants in water and sand: A new frontier for quantitative microbial risk assessment. Curr. Opin. Environ. Sci. Health. 2020;16:73–81. doi: 10.1016/j.coesh.2020.03.001. [DOI] [Google Scholar]
- 132.Auld H., MacIver D., Klaassen J. Heavy rainfall and waterborne disease outbreaks: The Walkerton example. J. Toxicol. Environ. Health A-Curr. Issues. 2004;67:1879–1887. doi: 10.1080/15287390490493475. [DOI] [PubMed] [Google Scholar]
- 133.Cann K.F., Thomas D.R., Salmon R.L., Wyn-Jones A.P., Kay D. Extreme water-related weather events and waterborne disease. Epidemiol. Infect. 2013;141:671–686. doi: 10.1017/S0950268812001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Herrador B.R.G., de Blasio B.F., MacDonald E., Nichols G., Sudre B., Vold L., Semenza J.C., Nygard K. Analytical studies assessing the association between extreme precipitation or temperature and drinking water-related waterborne infections: A review. Environ. Health. 2015;14:12. doi: 10.1186/s12940-015-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Levy K., Woster A.P., Goldstein R.S., Carlton E.J. Untangling the impacts of climate change on waterborne diseases: A systematic review of relationships between diarrheal diseases and temperature, rainfall, flooding, and drought. Environ. Sci. Technol. 2016;50:4905–4922. doi: 10.1021/acs.est.5b06186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thomas M.K., Charron D.F., Waltner-Toews D., Schuster C., Maarouf A.R., Holt J.D. A role of high impact weather events in waterborne disease outbreaks in Canada, 1975-2001. Int. J. Environ. Health Res. 2006;16:167–180. doi: 10.1080/09603120600641326. [DOI] [PubMed] [Google Scholar]
- 137.Floury M., Usseglio-Polatera P., Ferreol M., Delattre C., Souchon Y. Global climate change in large European rivers: Long-term effects on macroinvertebrate communities and potential local confounding factors. Glob. Chang. Biol. 2013;19:1085–1099. doi: 10.1111/gcb.12124. [DOI] [PubMed] [Google Scholar]
- 138.Groffman P.M., Rustad L.E., Templer P.H., Campbell J.L., Christenson L.M., Lany N.K., Socci A.M., Vadeboncoeur M.A., Schaberg P.G., Wilson G.F., et al. Long-term integrated studies show complex and surprising effects of climate change in the northern hardwood forest. Bioscience. 2012;62:1056–1066. doi: 10.1525/bio.2012.62.12.7. [DOI] [Google Scholar]
- 139.Foley B., Jones I.D., Maberly S.C., Rippey B. Long-term changes in oxygen depletion in a small temperate lake: Effects of climate change and eutrophication. Freshw. Biol. 2012;57:278–289. doi: 10.1111/j.1365-2427.2011.02662.x. [DOI] [Google Scholar]
- 140.USEPA Environmental Monitoring in the Everglades, Everglades REMAP Web Page. 1993–2014 (2021) [(accessed on 12 November 2021)]; Available online: https://www.epa.gov/everglades/environmental-monitoring-everglades.
- 141.Bezirtzoglou C., Dekas K., Charvalos E. Climate changes, environment and infection: Facts, scenarios and growing awareness from the public health community within Europe. Anaerobe. 2011;17:337–340. doi: 10.1016/j.anaerobe.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 142.Wu B., Wang C., Zhang C., Sadowsky M.J., Dzakpasu M., Wang X.C. Source-Associated Gastroenteritis Risk from Swimming Exposure to Aging Fecal Pathogens. Environ. Sci. Technol. 2020;54:921–929. doi: 10.1021/acs.est.9b01188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.