Abstract
Background:
Psychological attitudes reflecting expectations about the future (optimism, pessimism) and people (cynical hostility) independently predict incident cardiovascular disease and possibly diabetes, but underlying biologic pathways are incompletely understood. Herein we examined the cross-sectional relationship between optimism, pessimism, and cynicism and biomarkers of metabolic function in the Women’s Health Initiative.
Methods:
Among 3443 postmenopausal women, biomarkers of metabolic function (fasting insulin [FINS] and glucose) were measured at baseline and used to calculate insulin resistance (homeostasis model assessment of insulin resistance [HOMA-IR]) and pancreatic β-cell activity (homeostasis model assessment of β-cell function [HOMA-B]). Psychological attitudes were assessed by the Life Orientation Test, Revised (full scale, and optimism and pessimism subscales) and the Cook–Medley cynicism subscale. Multivariable linear regression modeled the association of psychological attitudes with biomarker levels, adjusting for sociodemographics, health conditions, and health behaviors. Because obesity promotes insulin resistance and obese individuals tend to report higher levels of pessimism and cynical hostility, an interaction with body mass index (BMI) was explored.
Results:
In fully adjusted models, only pessimism remained independently associated with higher FINS and insulin resistance (HOMA-IR). Scoring 1 point higher on the pessimism subscale was associated with a 1.2% higher FINS, whereas scoring 1 SD higher was associated with a 2.7% higher FINS (P = 0.03); results were similar for HOMA-IR. An interaction term with BMI was not significant.
Conclusions:
In multivariable models, higher dispositional pessimism was associated with worse metabolic function; these findings were not modified by obesity status. Results extend prior work by linking pessimism to an objective biomarker of insulin resistance in elderly women.
Keywords: cynical hostility, diabetes, insulin resistance, optimism, pessimism
Introduction
Psychological attitudes, health and disease
Psychological attitudes about the future (optimism, or positive future expectation)1 and about other people (cynical hostility, or strong mistrust of others)2 are prospectively associated with morbidity and mortality in postmenopausal women.3 Among participants of the Women’s Health Initiative (WHI), individuals who scored highest on a standard optimism scale1 had a lower incidence of coronary heart disease (CHD), CHD-related mortality, and all-cause mortality after adjustment for important potential confounders, including depressive symptoms.3 Further, women who strongly endorsed attitudes of cynical hostility had a higher incidence of total and cancer-related mortality than women who did not endorse these attitudes. Effects were attenuated after controlling for health behaviors and lifestyle factors, but remained clinically and statistically significant.3
A large body of literature demonstrates similar findings in other populations,4–11 supporting theoretical and conceptual models of how psychological attitudes, which develop early in life, indirectly and directly affect health and disease over time (see also Fig. 1).12–15 Individuals with lower trait optimism, higher trait pessimism (negative future expectation), or cynical hostility are more likely to smoke,16 have poorer dietary habits,8,9,17 be obese,3,10 and be less likely to adhere to physician advice.18 Attitudes are also longitudinally related to incident high blood pressure, subclinical and clinical atherosclerosis, and stroke.3,19–22 In addition, direct pathways, such as the experience of stress, with resultant activation of the sympathetic–adrenal–medullary system,23 inflammatory and endothelial cascades24,25 (so called “inflammaging”) and neuroendocrine pathways,26 may mediate the observed relationship between psychological attitudes and health outcomes. For example, ambulatory blood pressure is higher in individuals endorsing lower levels of optimism and greater daily experience of stress.28
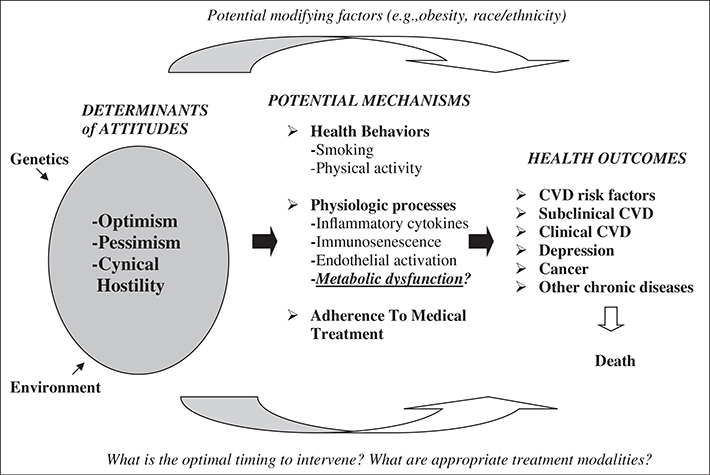
Figure 1.
How psychological attitudes may influence health and disease over time. Understanding the biological pathways between psychological attitudes and disease is limited. This is a critical gap in knowledge, because psychological attitudes, which form early in life, may affect physiology in myriad ways, including via direct and indirect pathways. Optimism (positive future expectation), pessimism (negative future expectation), and cynical hostility (mistrust of other people) form early in life and may be considered early “risk factors” for cardiovascular and metabolic conditions. This paper specifically focuses on the relationship between psychological attitudes and biomarkers of metabolic function. CVD, cardiovascular disease. (Adapted from Tindle et al.12)
Cynical hostility and optimism have also been studied in association with diabetes and metabolic syndrome. In a subsample of WHI participants, the highest tertile of cynical hostility, compared with the lowest, was associated with a higher risk of diabetes over a 1-year period, yet statistical significance was marginal.29 For worsening metabolic syndrome, odds were 27% higher among women in the highest (vs lowest) tertile of cynical hostility.29 In another study of British men and women, optimism (as assessed by a single-item measure) was unrelated to an objective measure of incident diabetes.30 Neither study included an assessment of pessimism or fasting insulin levels.
Optimism and pessimism may further be considered as separate constructs rather than bipolar31,32 opposites. These theoretical considerations are supported by findings that optimism and pessimism differentially predict health-related outcomes. For example, we have found that optimism, but not pessimism, predicts lower rehospitalization and recovery from depression after coronary artery bypass grafting,18 whereas other research has linked pessimism, but not optimism, with higher ambulatory blood pressure33 and higher markers of thrombosis.34
Psychological attitudes and biomarkers
To further understand how psychological attitudes such as optimism, pessimism, and hostility may affect health at the cellular level, a growing body of literature has examined their relationship with biomarkers of inflammation and endothelial cell dysfunction.34–37 These inflammatory biomarkers are measurable in the blood and are associated with metabolic conditions such as insulin resistance,38,39 as well as cardiovascular disease (CVD).40 Hostility has also been linked to biomarkers of inflammation and endothelial dysfunction, with the totality of evidence supporting a positive correlation.41–43 Leukocyte telomere length and telomerase activity are additional biomarkers of cellular aging in that they are vulnerable to the effects of chronic stress and associated increases in inflammation.44,45 Pessimism has been independently associated with shorter leukocyte telomere length in US military veterans46 and older women.35 In another group of US veterans, hostility was associated with shorter telomere length,47 and in Whitehall II, high levels of hostility in men predicted both shorter telomeres and high telomerase activity.48
However, there remains a notable gap in the literature regarding the relationship between psychological attitudes and biomarkers of metabolic function, including insulin, insulin resistance (homeostasis model assessment of insulin resistance [HOMA-IR]), and pancreatic islet cell function (homeostasis model assessment of β-cell function [HOMA-B]).49,50 Furthermore, certain conditions, such as obesity, could interact with psychological attitudes to explain some of the observed relationships between higher levels of pessimism, hostility, and poor health. Obesity is not only positively correlated with pessimism and hostility, but also confers a proinflammatory state leading to serious morbidity and mortality.51–53 Adipose tissue has been characterized as an endocrine organ54,55 that actively secretes inflammatory cytokines,56 leading to insulin resistance57 and ultimately diabetes.58,59 Although the interaction of optimism, pessimism, and cynical hostility with obesity has not been closely examined with regard to biomarkers of metabolic function, Rius-Ottenheim et al.60 found an interaction with dispositional optimism and obesity for C-reactive protein levels. Furthermore, both depression and anger have been shown to interact with obesity in diabetes.61–65
Objectives
The overarching aim of the present study was to determine the extent to which optimism, pessimism, and cynical hostility are associated with biomarkers of metabolic function in older postmenopausal women. We hypothesized that lower optimism, higher pessimism, and higher cynical hostility would be associated with less favorable profiles of higher fasting insulin and glucose, higher insulin resistance (HOMA-IR), and lower pancreatic β-cell activity (HOMA-B). Based on prior research discussed above, we further predicted that when optimism and pessimism were considered as unipolar traits, pessimism, rather than optimism, would be independently related to poor metabolic function. Finally, because of the complex relationships between obesity, diabetes, and other psychological conditions, such as depression and anger,61,65 we explored whether findings would be more pronounced among obese women.
Methods
Study sample
The WHI recruited 161 808 postmenopausal women aged 50–79 years from diverse racial/ethnic, geographic, and socioeconomic backgrounds into one of two longitudinal study branches between 1994 and 1998: (i) the clinical trial (CT; n = 68 133) or the observational study (OS; n = 93 676).66 Exclusion criteria relevant to the current study included substance abuse (except alcohol or cigarettes); mental illness, including severe depression, dementia, or life expectancy <3 years; participation in other randomized trials; and plans to relocate within 3 years. Further restrictions have been described elsewhere.66
For the WHI study, institutional review board approval was obtained at each clinical center and all participants provided written informed consent.
Characteristics of women in the present case-control ancillary study to the WHI have been described previously.67 Briefly, Liu et al.67 analyzed blood that was collected at the baseline visit of the WHI. Stored blood was analyzed from 1584 women who developed diabetes during almost 6 years of follow-up in the main WHI OS (cases) and from 2198 study participants who remained free of diabetes and CVD during that time period (controls). Eligible cases were WHI OS participants who provided adequate blood specimens, who were free of reported CVD or diabetes at baseline, and who subsequently reported new diabetes treatment with oral hypoglycemic drugs or insulin or hospitalization for diabetes during a median follow-up period of 5.9 years (mean 5.5 years). Following the principles of risk-set sampling,68 for each new case, controls were selected randomly from women who remained free of CVD and/or diabetes at the time the case was identified during follow-up. Identical exclusion or inclusion criteria were applied to eligible controls who were WHI OS participants, free of reported CVD and/or diabetes at baseline, and provided baseline blood specimens. Controls were further matched to the cases by age (±2.5 years), racial/ethnic group (White, Black, Hispanic, and Asian/Pacific Islander), clinical center (geographic location), time of blood draw (±0.10 h), and length of follow-up. The study included four races/ethnicities: Caucasian, African American, Hispanic, and Asian/Pacific Islander. In the overall ancillary study, Liu et al.67 found that relative risks of incident diabetes among individuals with highest (vs lowest) quartiles of inflammatory biomarkers were elevated, and models including all inflammatory markers revealed that some remained significant independent predictors of diabetes. However, when incorporated into a prediction model, these biomarkers did not add predictive value above and beyond known risk factors for diabetes.69 Thus, the present analyses combine cases and controls from the ancillary study sample.
Biomarkers of metabolic function
Biomarkers of metabolic function (fasting insulin, fasting glucose), insulin resistance (HOMA-IR), and pancreatic β-cell activity (HOMA-B)50 were examined.
According to a standardized protocol, fasting blood specimens were collected from each participant at baseline and processed locally into separate aliquots containing serum, plasma, and buffy coat. The aliquots were frozen and then shipped to a central repository, where they were kept for long-term storage at −70°C. All biochemical assays were performed by laboratory staff blinded to case or control status. Blood samples from cases and their matched controls were handled identically, shipped in the same batch, and assayed in random order in the same analytical run to reduce systematic bias and interassay variation. The coefficients of variation for the assays were 1.7% for glucose and 5.8% for insulin. As a surrogate measure of insulin resistance, homeostasis model assessment, the product of basal fasting glucose (mmol/mL) and insulin levels (μIU/mL) divided by 22.5, was used to estimate baseline insulin resistance (HOMA-IR). To evaluate pancreatic β-cell activity, HOMA-B was calculated using the following formula:70
Psychological attitudes (assessed at baseline enrollment into the WHI)
Life orientation test, revised full scale (optimism/ pessimism as a bipolar trait)
An individual’s degree of optimism or pessimism is considered a personality trait and may be assessed by several validated scales. The Life Orientation Test, Revised (LOT-R)1 measures optimism and pessimism, and contains six scored items. Item ratings are summed to yield a total score ranging from 6 to 30, with higher scores reflecting greater optimism and lower scores reflecting greater pessimism. In the present study, LOT-R scores were categorized into quartiles in unadjusted analyses, but for adjusted models scores were considered as a continuous variable to assess associations of a 1-point difference on the scale with biomarkers. To aid interpretability of adjusted models, we also report associations of metabolic biomarkers with a 1-SD increment in each attitudinal scale score.
Subscales of LOT-R (optimism and pessimism as unipolar traits)
To disaggregate optimism from pessimism, we also considered each subscale of the LOT-R separately. Evidence to date, for example, suggests that pessimism may be associated with unfavorable biomarker profiles as well as higher risk of CVD and mortality,34,35,71,72 whereas optimism has also been independently associated with favorable health outcomes.18 The optimism subscale was the sum of the three positively worded questions to yield a total score ranging from 3 to 15, with higher scores indicating greater optimism and lower scores indicating neutral attitudes about the future (i.e., neither expecting good things nor bad things). A sample positively worded question is, “In uncertain times, I usually expect the best.” The pessimism subscale was the sum of responses to the three negatively worded questions to yield a total score ranging from 3 to 15, with higher scores indicating greater pessimism and lower scores indicating neutral expectations of the future. A sample negatively worded question is, “If something can go wrong for me, it will.” Both these subscales were treated as continuous measures.
Cynical hostility
Cynical hostility was assessed by the cynicism subscale of the Cook–Medley Hostility Scale,2 which contains 13 true/false items, with higher scores indicating higher levels of cynical hostility. For descriptive purposes, cynical hostility scores were categorized into quartiles; in multivariable models, hostility was considered a continuous variable, as well as according to a 1-SD difference.
Additional covariates (measured or reported at baseline enrollment in the WHI)
Age was considered a continuous variable. Sociodemographic variables included race (Caucasian, African American, Hispanic, Asian/Pacific Islander), education (less than high school, high school or equivalent, any college or higher), income (US$; <20 000, 20 000–49 999, 50 000–74 999; >75 000), health conditions (history of hypertension at baseline [yes/no], history of high cholesterol [yes/no], depressive symptoms [assessed using the eight-item Center for Epidemiology Studies Depression Scale {CES-D}73 as a continuous score with ≥5 as a cut-off, as well as the Burnam algorithm74 with >0.06 as a cut-off], and body mass index [BMI; <30, ≥30 kg/m2], calculated using self-reported height and weight). Although the sample was free of diabetes at baseline, some women self-reported a history of diabetes outside of pregnancy, thus we additionally adjusted for this self-reported measure of history of diabetes. Health behaviors included smoking (current, former, never), alcohol consumption (current, former, never), and exercise habits (metabolic equivalents [MET]/week). Health-related quality of life was also assessed via the Short Form (SF)-12.75
Statistical analysis
Biomarker, demographic, clinical, and behavioral data were collected on 3713 WHI participants at their baseline visit. From these 3713 women, 267 who were missing LOT-R scores or Cook–Medley cynical hostility subscale scores were excluded, as were two women with glucose measures below 63 mg/dL so that HOMA-B could not be accurately calculated, as well as an additional woman with an unspecified race/ethnicity. Thus, the final sample included 3443 women. Although 71% of the sample had complete data on all variables, the remaining 29% were missing data on one or more variables. To handle the missingness in analyses involving the biomarkers under investigation, 10 complete datasets were created through multiple imputation using fully conditional specification methods. Specifically, continuous variables were imputed through predictive mean matching in order to produce biologically plausible imputed values, and the discriminant function with a non-informative Jeffreys prior was used to impute categorical and binary variables.
Using the observed (non-imputed) data, overall descriptive statistics were first produced and analysis of variance (ANOVA) and Chi-squared tests were then used for continuous and categorical characteristics, respectively, to determine whether the baseline characteristics differed across quartiles of the full LOT-R and the Cook–Medley cynical hostility subscale. Pearson correlation coefficients and ANOVA were performed to quantify the association between continuous LOT-R and participant characteristics; this was also repeated for the LOT-R optimism subscale, the LOT-R pessimism subscale, and the continuous cynical hostility subscale. Using imputed data, similar bivariate methods were used to relate the measured biomarkers with the LOT-R, its subscales, and the cynical hostility subscale. Finally, multiple linear regression was performed with the biomarkers serving as the dependent variables (separate models for each biomarker) and the psychological constructs serving as the independent variable of interest. Three separate groups of covariates were used in the multiple regression models. The association between biomarkers and psychological constructs was first adjusted for demographic characteristics (age, race/ethnicity, education, and income). Models were then additionally adjusted for clinical measures, including history of hypertension, history of high cholesterol, self-reported history of diabetes, obesity (BMI ≥30 kg/m2), presence of depressive symptoms, and overall sense of well being. Finally, behavioral characteristics (smoking status, alcohol consumption, and physical activity) were added to the list of covariates. In addition to considering obesity as a potential confounder, the presence of an interaction with each psychological trait was explored in the fully adjusted models (separate models for each trait).
In all analyses, biomarkers were log transformed in order to satisfy the normality and homoscedasticity assumptions for ANOVA and multiple linear regression. Due to the number of covariates in the multiple linear regression models, multicollinearity was assessed via the variance inflation factor, but none was detected. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Psychological attitudes and baseline characteristics
Optimism and pessimism (as assessed by the full scale LOT-R; Table 1) and cynical hostility (Table 2) were associated with a number of baseline characteristics, including sociodemographic conditions, health conditions, and health behaviors. Quartiles (Q) of scores for optimism (full scale LOT-R) were as follows: Q1, <21; Q2, 21–22; Q3, 23–24; and Q4, ≥25. Quartiles for cynical hostility scores (Cook–Medley) were as follows: Q1, <2; Q2, 2–3; Q3, 4–5; and Q4, ≥6. Overall, women who endorsed more optimistic attitudes about the future reported higher levels of education and income and generally exhibited more favorable health characteristics than those who were more pessimistic. In contrast, women who endorsed more cynically hostile attitudes, compared with those who did not, demonstrated profiles that mirrored those of the most pessimistic women. Women who scored in the lowest quartile of optimism (i.e. most “pessimistic”) and those who scored in the highest quartile of cynical hostility were more likely to be obese.
Table 1.
Optimism and pessimism by baseline characteristics of women
| LOT-R full scale scores (quartiles) |
||||||
|---|---|---|---|---|---|---|
| Characteristic* | Total sample (n = 3443) | Highest (n = 1128) | Mid-high (n = 869) | Mid-low (n = 656) | Lowest (n = 790) | P-value |
| Age (years) | 62.5 ± 7.0 | 62.5 ± 6.9 | 62.4 ± 7.0 | 62.7 ± 7.0 | 62.5 ± 7.1 | 0.9111 |
| Race/ethnicity | <0.0001 | |||||
| White | 1826 (53) | 624 (55) | 509 (59) | 338 (52) | 355 (45) | |
| Black | 1011 (29) | 372 (33) | 224 (26) | 197 (30) | 218 (28) | |
| Hispanic | 375 (11) | 79 (7) | 90 (10) | 75 (11) | 131 (17) | |
| Asian/Pacific Islander | 231 (7) | 53 (5) | 46 (5) | 46 (7) | 86 (11) | |
| Education | <0.0001 | |||||
| Less than high school graduate | 298 (9) | 50 (4) | 50 (6) | 57 (9) | 141 (18) | |
| High school graduate or GED | 554 (16) | 124 (11) | 133 (15) | 114 (18) | 183 (23) | |
| More than high school | 2561 (75) | 942 (84) | 682 (79) | 477 (74) | 460 (59) | |
| Income (US$) | <0.0001 | |||||
| <20 000 | 659 (20) | 144 (13) | 130 (16) | 148 (23) | 237 (32) | |
| 20 000–49 999 | 1415 (43) | 430 (40) | 367 (44) | 311 (49) | 307 (41) | |
| 50 000–74 999 | 628 (19) | 250 (23) | 179 (22) | 98 (15) | 101 (13) | |
| ≥75 000 | 463 (14) | 212 (20) | 126 (15) | 60 (9) | 65 (9) | |
| Does not know | 121 (4) | 34 (3) | 24 (3) | 23 (4) | 40 (5) | |
| History of hypertension | 1451 (42) | 446 (40) | 368 (42) | 289 (45) | 348 (45) | 0.0999 |
| History of high cholesterol | 502 (15) | 153 (14) | 113 (13) | 105 (16) | 131 (17) | 0.0728 |
| Depressive symptoms | 399 (12) | 60 (5) | 72 (8) | 81 (13) | 186 (25) | <0.0001 |
| Diabetes | 273 (8) | 68 (6) | 74 (9) | 61 (9) | 70 (9) | 0.0348 |
| Obesity (BMI ≥30 kg/m2) | 1341 (39) | 393 (35) | 332 (39) | 266 (42) | 350 (45) | 0.0002 |
| Smoking | 0.1798 | |||||
| Current | 249 (7) | 77 (7) | 62 (7) | 43 (7) | 67 (9) | |
| Former | 1333 (39) | 470 (42) | 329 (38) | 248 (38) | 286 (37) | |
| Never | 1825 (54) | 567 (51) | 471 (55) | 357 (55) | 430 (55) | |
| Alcohol consumption | <0.0001 | |||||
| Current | 2048 (60) | 717 (64) | 542 (63) | 385 (59) | 404 (52) | |
| Former | 815 (24) | 247 (22) | 192 (22) | 164 (25) | 212 (27) | |
| Never | 555 (16) | 156 (14) | 129 (15) | 105 (16) | 165 (21) | |
| Exercise (MET/week) | 11.6 ± 13.6 | 13.8 ± 14.9 | 11.5 ± 12.6 | 10.7 ± 12.8 | 9.4 ± 12.8 | <0.0001 |
| Health-related QoL | 77.9 ± 15.4 | 84.0 ± 11.9 | 79.9 ± 12.9 | 75.7 ± 14.3 | 68.5 ± 18.4 | <0.0001 |
Categorical variables are presented as n (%), whereas continuous variables are presented as the mean ± SD.
Values for the following characteristics were partially missing on the sample (n missing): education (n = 30); income (n = 157); history of hypertension (n = 23); history of high cholesterol (n = 90); depressive symptoms (n = 98); obesity (n = 47); smoking (n = 36); alcohol (n = 25); exercise (n = 46); and quality of life (QoL; n = 57);
BMI, body mass index; GED, General Educational Development; LOT-R, Life Orientation Test, Revised; MET, metabolic equivalents.
Table 2.
Cynical hostility by baseline characteristics of women
| Cook–Medley cynicism subscale scores (quartiles) |
||||||
|---|---|---|---|---|---|---|
| Characteristic* | Total sample (n = 3443) | Highest (n = 1103) | Mid-high (n = 833) | Mid-low (n = 798) | Lowest (n = 709) | P-value |
| Age (years) | 62.5 ± 7.0 | 62.0 ± 7.1 | 62.7 ± 6.9 | 62.9 ± 6.9 | 62.7 ± 6.9 | 0.0387 |
| Race/ethnicity | <0.0001 | |||||
| White | 1826 (53) | 454 (41) | 452 (54) | 473 (59) | 447 (63) | |
| Black | 1011 (29) | 440 (40) | 250 (30) | 190 (24) | 131 (18) | |
| Hispanic | 375 (11) | 154 (14) | 80 (10) | 72 (9) | 69 (10) | |
| Asian/Pacific Islander | 231 (7) | 55 (5) | 51 (6) | 63 (8) | 62 (9) | |
| Education | <0.0001 | |||||
| Less than high school graduate | 298 (9) | 175 (16) | 50 (6) | 47 (6) | 26 (4) | |
| High school graduate or GED | 554 (16) | 210 (19) | 146 (18) | 102 (13) | 96 (14) | |
| More than high school | 2561 (75) | 705 (65) | 633 (76) | 644 (81) | 579 (83) | |
| Income (US$) | <0.0001 | |||||
| <20 000 | 659 (20) | 308 (29) | 169 (21) | 100 (13) | 82 (12) | |
| 20 000–49 999 | 1415 (43) | 441 (42) | 335 (42) | 349 (46) | 290 (43) | |
| 50 000–74 999 | 628 (19) | 142 (14) | 164 (20) | 159 (21) | 163 (24) | |
| ≥75 000 | 463 (14) | 107 (10) | 104 (13) | 124 (16) | 128 (19) | |
| Does not know | 121 (4) | 52 (5) | 30 (4) | 25 (3) | 14 (2) | |
| History of hypertension | 1451 (42) | 520 (48) | 330 (40) | 318 (40) | 283 (40) | 0.0007 |
| History of high cholesterol | 502 (15) | 150 (14) | 135 (17) | 127 (16) | 90 (13) | 0.1181 |
| Depressive symptoms | 399 (12) | 194 (18) | 84 (10) | 80 (10) | 41 (6) | <0.0001 |
| Diabetes | 273 (8) | 88 (8) | 65 (8) | 68 (9) | 52 (7) | 0.8617 |
| Obesity (BMI ≥30 kg/m2) | 1341 (39) | 525 (48) | 309 (38) | 272 (34) | 235 (34) | <0.0001 |
| Smoking | 0.0514 | |||||
| Current | 249 (7) | 89 (8) | 71 (9) | 53 (7) | 36 (5) | |
| Former | 1333 (39) | 397 (36) | 330 (40) | 322 (41) | 284 (41) | |
| Never | 1825 (54) | 606 (55) | 425 (51) | 418 (53) | 376 (54) | |
| Alcohol consumption | <0.0001 | |||||
| Current | 2048 (60) | 569 (52) | 532 (64) | 481 (61) | 466 (66) | |
| Former | 815 (24) | 314 (29) | 179 (22) | 192 (24) | 130 (18) | |
| Never | 555 (16) | 208 (19) | 117 (14) | 121 (15) | 109 (15) | |
| Exercise (MET/week) | 11.6 ± 13.6 | 9.6 ± 12.9 | 11.6 ± 13.1 | 12.6 ± 13.9 | 13.6 ± 14.3 | <0.0001 |
| Health-related QoL | 77.9 ± 15.4 | 73.5 ± 17.4 | 78.6 ± 14.4 | 79.8 ± 14.1 | 81.6 ± 13.3 | <0.0001 |
Categorical variables are presented as n (%), whereas continuous variables are presented as the mean ± SD.
Values for the following characteristics were partially missing on the sample (n missing): education (n = 30); income (n = 157); history of hypertension (n = 23); history of high cholesterol (n = 90); depressive symptoms (n = 98); obesity (n = 47); smoking (n = 36); alcohol (n = 25); exercise (n = 46); quality of life (QoL; n = 57).
BMI, body mass index; GED, General Educational Development; MET, metabolic equivalents.
Optimism and biomarkers
In unadjusted analyses, optimism (full LOT-R scale) and the optimism and pessimism subscales were associated with metabolic biomarkers (Table 3). Fasting insulin and glucose, as well as markers of pancreatic β-cell activity, were associated with optimism (full LOT-R scale) and the pessimism subscale, whereas the optimism subscale was only associated with insulin resistance. In adjusted analyses, the association of fasting insulin and HOMA-IR with pessimism remained significant (Table 4). In contrast, associations of metabolic biomarkers with optimism (full LOT-R scale) were significant only after adjustment for sociodemographic factors, losing significance after adjustment for clinical characteristics. Associations with the optimism subscale lost statistical significance after adjustment for sociodemographic factors (data not shown).
Table 3.
Metabolic biomarkers by level of optimism (unadjusted)
| LOT-R full scale scores (quartiles) |
LOT-R full scale, continuous |
LOT-R optimism subscale, continuous |
LOT-R pessimism subscale, continuous |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total sample (n = 3443) | Highest (n = 1128) | Mid-high (n = 869) | Mid-low (n = 656) | Lowest (n = 790) | P-value | r | P-value | r | P-value | r | P-value |
| FINS (μIU/mL) | 8.3 [5.2, 13.8] | 2.05 ± 0.02 | 2.12 ± 0.02 | 2.16 ± 0.03 | 2.27 ± 0.03 | <0.0001 | −0.1158 | <0.0001 | −0.0448 | 0.0115 | 0.1373 | <0.0001 |
| Fasting glucose (mg/dL) | 98.0 [90.0, 117.0] | 4.65 ± 0.01 | 4.67 ± 0.01 | 4.68 ± 0.01 | 4.69 ± 0.01 | 0.0132 | −0.0657 | 0.0003 | −0.0356 | 0.0510 | 0.0695 | 0.0003 |
| HOMA-IR | 2.1 [1.2, 4.0] | 0.69 ± 0.03 | 0.79 ± 0.03 | 0.84 ± 0.03 | 0.95 ± 0.03 | <0.0001 | −0.1166 | <0.0001 | −0.0484 | 0.0074 | 0.1356 | <0.0001 |
| HOMA-B | 79.6 [51.6, 119.9] | 4.31 ± 0.02 | 4.35 ± 0.02 | 4.34 ± 0.03 | 4.44 ± 0.03 | 0.0010 | −0.0582 | 0.0014 | −0.0150 | 0.3930 | 0.0752 | <0.0001 |
Data are presented as the median [interquartile range], the mean ± SD of the log-transformed biomarker, or as correlation coefficients (r) between Life Orientation Test, Revised (LOT-R) scores and log-transformed biomarkers.
FINS, fasting insulin; HOMA-B, homeostasis model assessment of β-cell function; HOMA-IR, homeostasis model assessment of insulin resistance.
Table 4.
Multivariable-adjusted linear regression modeling of metabolic biomarkers by pessimism (Life Orientation Test, Revised [LOT-R] subscale)
| Characteristic | β estimate (SE) | 95% CI | P-value |
|---|---|---|---|
| LOT-R Pessimism Subscale scores | |||
| Adjusted for demographic characteristics | |||
| Fasting insulin | 0.03 (0.006) | 0.02, 0.04 | <0.0001 |
| Fasting glucose | 0.006 (0.002) | 0.002, 0.01 | 0.0067 |
| HOMA-IR | 0.03 (0.007) | 0.02, 0.05 | <0.0001 |
| HOMA-B | 0.01 (0.006) | 0.003, 0.03 | 0.0147 |
| Adjusted for demographic and clinical characteristics | |||
| Fasting insulin | 0.01 (0.005) | 0.003, 0.02 | 0.0131 |
| Fasting glucose | 0.002 (0.002) | −0.002, 0.007 | 0.3626 |
| HOMA-IR | 0.02 (0.007) | 0.003, 0.03 | 0.0190 |
| HOMA-B | 0.008 (0.006) | −0.003, 0.02 | 0.1559 |
| Adjusted for demographic, clinical, and behavioral characteristics | |||
| Fasting insulin | 0.01 (0.005) | 0.001, 0.02 | 0.0311 |
| Fasting glucose | 0.002 (0.002) | −0.003, 0.006 | 0.4306 |
| HOMA-IR | 0.01 (0.007) | 0.0006, 0.03 | 0.0405 |
| HOMA-B | 0.007 (0.006) | −0.005, 0.02 | 0.2333 |
CI, confidence interval; HOMA-B, homeostatic model assessment of β-cell function; HOMA-IR, homeostasis model assessment of insulin resistance.
Cynical hostility and biomarkers
In analyses adjusted for sociodemographics only, cynical hostility was related to fasting insulin and glucose, yet these associations were no longer significant after adjustment for clinical characteristics (data not shown).
Interaction of attitudes and obesity on biomarkers
There was no evidence of a significant interaction between obesity and any of the psychological constructs.
Discussion
Herein we report that attitudes of optimism, pessimism, and cynical hostility were associated with biomarkers that reflect metabolic function. Only the LOT-R pessimism subscale remained significantly associated with fasting insulin and HOMA-IR after adjustment for important covariates. To put this relationship into another context, for every 1-point higher score on the pessimism subscale, an individual’s fasting insulin levels were 1.2% higher, on average, all else held constant. Similarly, for each 1-SD increase on the pessimism scale, an individual’s fasting insulin level was approximately 3% higher. Although there is no clear threshold of fasting insulin known to confer increased risk of CVD in healthy adults, in general higher levels confer higher risk.76,77 Thus, individuals who score extremely high on the pessimism subscale (i.e., 2 SD) could exhibit fasting insulin levels at least 6% higher than those without pessimistic tendencies. The fact that pessimism, and not optimism, remained independently related to higher fasting insulin levels is interesting, and in keeping with other research demonstrating similar findings linking pessimism to inflammatory biomarkers.34
The mechanism underlying the link between higher pessimism and insulin resistance is not clear. Findings could be explained, in part, by higher stress levels that have been documented among pessimistic individuals, with resultant sustained activation of stress effector systems.28 Although acute episodes of psychological stress have not been found to affect metabolic control in type 1 diabetics,78 chronic, sustained stress could, for example, potentially disrupt the homeostatic mechanisms that govern insulin levels among nondiabetics and diabetics alike.79 In addition, stress and anger, which are related to high pessimism, have been found to affect the development of the metabolic syndrome in women.80 However, this remains speculative, because the WHI was not designed to conduct laboratory-based or longitudinal ambulatory assessments that would be required to understand dynamic, ongoing stress responses. In addition, health behaviors almost certainly play a role, because individuals who endorse strong pessimistic tendencies also tend to smoke,81 have a poorer diet,8,9 be physically inactive and overweight or obese, and exhibit other unhealthy behaviors.3 Nevertheless, pessimism remained associated with elevated insulin levels even after correcting for important health behaviors.
In contrast with our hypotheses, we did not detect an interaction with obesity. This could be due to lack of adequate sample size for detection, which is a common limitation in investigations of potential interactions. Further research is needed in a larger sample to clarify this potential relationship.
Also in contrast with our hypotheses, after adjustment for clinical factors, hostility was not independently related to metabolic biomarkers in this sample. Although the WHI was limited by a relatively low prevalence of women with high levels of cynical hostility, a prior study (also in the WHI) found a positive association between high cynical hostility and worsening metabolic syndrome, as well as the suggestion of an increased risk in self-reported diabetes over 1 year.29 In contrast with the present study, insulin resistance was not measured in those participants. Thus, a future examination of incident diabetes in the present sample could shed additional light on mechanisms linking hostility with incident diabetes.
The present analysis had a number of strengths, including measured biomarker data nested within the well-established and rich WHI dataset and a relatively large and representative sample of older postmenopausal women representing multiple races and ethnicities. The WHI further supports the ability to assess pessimism and cynical hostility independently from depressive symptoms, optimism, and a number of other important health conditions and behaviors that may affect insulin resistance. Further separating pessimism from optimism through LOT-R subscale analysis is also important to probe mechanisms of observed relationships between these traits and health outcomes, as well as to lay groundwork for considering future clinical interventions designed to shift attitudes. For example, it may be that interventions designed to modify pessimistic attitudes could potentially lead to improved health. This issue requires further investigation.
The present analysis also had a number of limitations, including the sample size that, although large by some standards, was only a fraction of the size of the larger WHI, thus limiting statistical power to detect interactions. In addition, the cross-sectional nature of the analysis precludes any determination of a causal relationship between pessimism and poor metabolic function. The cross-sectional design could also have misclassified individuals with prediabetes. A longitudinal analysis of optimism, pessimism, cynical hostility, and incident diabetes in the WHI is beyond the scope of the present analysis, but is feasible for future research.
Understanding how psychological factors relate to clinically relevant biomarkers elucidates the biological mechanisms by which these psychological traits are associated with health and disease. Optimism, pessimism, and cynical hostility, which form relatively early in life and are associated with the development of CVD risk factors even in younger populations, may represent some of the earliest health risk factors. Preliminary evidence suggests that these traits could be modifiable and, as such, they may be an important target for future preventive interventions,82 such as promoting greater attainment of ideal cardiac and metabolic health.83–86
The present study extends prior research by documenting, in a large sample of postmenopausal women, that higher levels of pessimism were associated with objective measures of worse metabolic function. A future longitudinal study in this population should probe the development of overt diabetes, which could clarify whether pessimism and hostility predict incident diabetes in post-menopausal women.
Acknowledgements
HAT thanks the University of Pittsburgh, where she was on faculty, for support during the initial phases of this study. The authors acknowledge Stephen M. KING and Vanessa C. GATSKIE (Vanderbilt University Medical Center, Nashville, TN, USA) for help with the preparation of this manuscript. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), and US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN 268201100002C, HHSN268201100003C, HHSN26820 1100004C, and HHSN271201100004C. The research reported in this paper was supported by the National Institute of Mental Health of the NIH under Award no. T32MH019733. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This manuscript was prepared in collaboration with investigators of the WHI, and has been reviewed and approved by the Women’s Health Initiative (WHI). The short list of WHI investigators can be found at: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf (accessed 18 July 2017).
Footnotes
Disclosure
None declared.
References
- 1.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994; 67: 1063–78. [DOI] [PubMed] [Google Scholar]
- 2.Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954; 38: 414–8. [Google Scholar]
- 3.Tindle HA, Chang Y-F, Kuller LH et al. Optimism, cynical hostility, and incident coronary heart disease and mortality in the Women’s Health Initiative. Circulation. 2009; 120: 656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen HN, Scheier MF, Greenhouse JB. Optimism and physical health: A meta-analytic review. Ann Behav Med. 2009; 37: 239–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehm JK, Kubzansky LD. The heart’s content: The association between positive psychological well-being and cardiovascular health. Psychol Bull. 2012; 138: 655–91. [DOI] [PubMed] [Google Scholar]
- 6.Giltay EJ, Geleijnse JM, Zitman FG, Hoekstra T, Schouten EG. Dispositional optimism and all-cause and cardiovascular mortality in a prospective cohort of elderly Dutch men and women. Arch Gen Psychiatry. 2004; 61: 1126–35. [DOI] [PubMed] [Google Scholar]
- 7.Giltay EJ, Kamphuis MH, Kalmijn S, Zitman FG, Kromhout D. Dispositional optimism and the risk of cardiovascular death: The Zutphen Elderly Study. Arch Intern Med. 2006; 166: 431–6. [DOI] [PubMed] [Google Scholar]
- 8.Tinker LF, Rosal MC, Young AF et al. Predictors of dietary change and maintenance in the Women’s Health Initiative Dietary Modification Trial. J Acad Nutr Diet. 2007; 107: 1155–65. [DOI] [PubMed] [Google Scholar]
- 9.Hingle MD, Wertheim BC, Tindle HA et al. Optimism and diet quality in the Women’s Health Initiative. J Acad Nutr Diet. 2014; 114: 1036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabi H, Kivimaki M, Sabia S et al. Hostility and trajectories of body mass index over 19 years: The Whitehall II Study. Am J Epidemiol. 2009; 169: 347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: A meta-analytic review of prospective evidence. J Am Coll Cardiol. 2009; 53: 936–46. [DOI] [PubMed] [Google Scholar]
- 12.Tindle H, Davis E, Kuller L. Attitudes and cardiovascular disease. Maturitas. 2010; 67: 108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen S, Pressman SD. Positive affect and health. Curr Dir Psychol Sci. 2006; 15: 122–5. [Google Scholar]
- 14.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: Do negative emotions play a role? Psychol Bull 2003; 129: 10–51. [DOI] [PubMed] [Google Scholar]
- 15.Matthews KA, Shumaker SA, Bowen DJ et al. Women’s health initiative. Why now? What is it? What’s new? Am Psychol. 1997; 52: 101–16. [DOI] [PubMed] [Google Scholar]
- 16.Peterson C, Seligman ME. Explanatory style and illness. J Pers. 1987; 55: 237–65. [DOI] [PubMed] [Google Scholar]
- 17.Giltay EJ, Geleijnse JM, Zitman FG, Buijsse B, Kromhout D. Lifestyle and dietary correlates of dispositional optimism in men: The Zutphen Elderly study. J Psychosom Res 2007; 63: 483–90. [DOI] [PubMed] [Google Scholar]
- 18.Tindle H, Belnap BH, Houck PR et al. Optimism, response to treatment of depression, and rehospitalization after coronary artery bypass graft surgery. Psychosom Med. 2012; 74: 200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan LL, Liu K, Matthews KA, Daviglus ML, Ferguson TF, Kiefe CI. Psychosocial factors and risk of hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2003; 290: 2138–48. [DOI] [PubMed] [Google Scholar]
- 20.Iribarren C, Sidney S, Bild DE et al. Association of hostility with coronary artery calcification in young adults: The CARDIA study. Coronary Artery Risk Development in Young Adults. JAMA. 2000; 283: 2546–51. [DOI] [PubMed] [Google Scholar]
- 21.Kim ES, Park N, Peterson C. Dispositional optimism protects older adults from stroke. Stroke. 2011; 42: 2855–9. [DOI] [PubMed] [Google Scholar]
- 22.Nabi H, Koskenvuo M, Sing-Manoux A et al. Low pessimism protects against stroke: The Health and Social Support (HeSSup) prospective cohort study. Stroke. 2010; 41: 187–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007; 298: 1685–7. [DOI] [PubMed] [Google Scholar]
- 24.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun. 2007; 21: 901–12. [DOI] [PubMed] [Google Scholar]
- 25.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014; 69 (Suppl. 1): S4–9. [DOI] [PubMed] [Google Scholar]
- 26.Endrighi R, Hamer M, Steptoe A. Associations of trait optimism with diurnal neuroendocrine activity, cortisol responses to mental stress, and subjective stress measures in healthy men and women. Psychosom Med 2011; 73: 672–8. [DOI] [PubMed] [Google Scholar]
- 27.Demaree HA, Harrison DW. Physiological and neuropsychological correlates of hostility. Neuropsychologia. 1997; 35: 1405–11. [DOI] [PubMed] [Google Scholar]
- 28.Raikkonen K, Matthews KA, Flory JD, Owens JF, Gump BB. Effects of optimism, pessimism, and trait anxiety on ambulatory blood pressure and mood during everyday life. J Pers Soc Psychol. 1999; 76: 104–13. [DOI] [PubMed] [Google Scholar]
- 29.Wylie-Rosett J, Aragaki AK, Cochrane B, Perri MG, Rosal MC, Rapp SR. Cynicism: Incident diabetes and worsening of metabolic syndrome in postmenopausal women. Diabetes Metab Syndr Clin Res Rev. 2010; 4: 187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boehm JK, Trudel-Fitzgerald C, Kivimaki M, Kubzansky LD. The prospective association between positive psychological well-being and diabetes. Health Psychol. 2015; 34: 1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubzansky LD, Kubzansky PE, Maselko J. Optimism and pessimism in the context of health: Bipolar opposites or separate constructs? Pers Soc Psychol Bull. 2004; 30: 943–56. [DOI] [PubMed] [Google Scholar]
- 32.Herzberg PY, Glaesmer H, Hoyer J. Separating optimism and pessimism: A robust psychometric analysis of the revised Life Orientation Test (LOT-R). Psychol Assess. 2006; 18: 433–8. [DOI] [PubMed] [Google Scholar]
- 33.Raikkonen K, Matthews KA. Do dispositional pessimism and optimism predict ambulatory blood pressure during school days and nights in adolescents? J Pers 2008; 76: 605–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy B, Diez-Roux AV, Seeman T, Ranjit N, Shea S, Cushman M. The association of optimism and pessimism with inflammation and hemostasis in the Multi-Ethnic Study of Atherosclerosis (MESA). Psychosom Med 2010; 72: 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donovan A, Lin J, Tillie J et al. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009; 23: 446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranjit N, Diez-Roux AV, Shea S et al. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007; 167: 174–81. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda A, Schwartz J, Peters JL et al. Optimism in relation to inflammation and endothelial dysfunction in older men: The VA normative aging study. Psychosom Med. 2011; 73: 664–71. [DOI] [PubMed] [Google Scholar]
- 38.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005; 115: 1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbatecola AM, Ferrucci L, Grella R et al. Diverse effect of inflammatory markers on insulin resistance and insulin-resistance syndrome in the elderly. J Am Geriatr Soc. 2004; 52: 399–404. [DOI] [PubMed] [Google Scholar]
- 40.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003; 107: 391–7. [DOI] [PubMed] [Google Scholar]
- 41.Mwendwa DT, Ali MK, Sims RC et al. Dispositional depression and hostility are associated with inflammatory markers of cardiovascular disease in African Americans. Brain Behav Immun. 2013; 28: 72–82. [DOI] [PubMed] [Google Scholar]
- 42.Stewart JC, Janicki-Deverts D, Muldoon MF, Kamarck TW. Depressive symptoms moderate the influence of hostility on serum interleukin-6 and C-reactive protein. Psychosom Med. 2008; 70: 197–204. [DOI] [PubMed] [Google Scholar]
- 43.Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Cynical hostility, depressive symptoms, and the expression of inflammatory risk markers for coronary heart disease. J Behav Med. 2003; 26: 501–15. [DOI] [PubMed] [Google Scholar]
- 44.Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci (Lond) 2001; 101: 185–92. [PubMed] [Google Scholar]
- 45.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003; 100: 9090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikeda A, Schwartz J, Peters JL et al. Pessimistic orientation in relation to telomere length in older men: The VA normative aging study. Psychoneuroendocrinology. 2014; 42: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watkins LE, Harpaz-Rotem I, Sippel LM, Krystal JH, Southwick SM, Pietrzak RH. Hostility and telomere shortening among U.S. military veterans: Results from the National Health and Resilience in Veterans Study. Psychoneuroendocrinology. 2016; 74: 251–7. [DOI] [PubMed] [Google Scholar]
- 48.Brydon L, Lin J, Butcher L et al. Hostility and cellular aging in men from the Whitehall II cohort. Biol Psychiatry. 2012; 71: 767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radziuk J Homeostastic model assessment and insulin sensitivity/resistance. Diabetes. 2014; 63: 1850–4. [DOI] [PubMed] [Google Scholar]
- 50.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28: 412–9. [DOI] [PubMed] [Google Scholar]
- 51.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer: View-point of the IARC Working Group. N Engl J Med. 2016; 375: 794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. 2013; 309: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckel RH. Obesity and heart disease. A statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1997; 96: 3248–50. [DOI] [PubMed] [Google Scholar]
- 54.Kershaw EE, Flier JS. Adipose tissue as an endocrineorgan. J Clin Endocrinol Metab. 2004; 89: 2548–56. [DOI] [PubMed] [Google Scholar]
- 55.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998; 22: 1145–58. [DOI] [PubMed] [Google Scholar]
- 56.Wajchenberg BL. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr Rev. 2000; 21: 697–738. [DOI] [PubMed] [Google Scholar]
- 57.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993; 259: 87–91. [DOI] [PubMed] [Google Scholar]
- 58.Özcan U, Cao Q, Yilmaz E et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004; 306: 457–61. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009; 460: 534–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rius-Ottenheim N, de Craen AJ, Geleijnse JM et al. C-reactive protein haplotypes and dispositional optimism in obese and nonobese elderly subjects. Inflamm Res. 2012; 61: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsenkova VK, Carr D, Coe CL, Ryff CD. Anger, adiposity, and glucose control in nondiabetic adults: Findings from MIDUS II. J Behav Med 2014; 37: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Surwit RS, Williams RB, Lane JD, Feinglos MN, Kuhn CM, Georgiades A. Plasma epinephrine predicts fasting glucose in centrally obese African-American women. Obesity (Silver Spring). 2010; 18: 1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmitz N, Deschenes SS, Burns RJ et al. Depression and risk of type 2 diabetes: The potential role of metabolic factors. Mol Psychiatry. 2016; 21: 1726–32. [DOI] [PubMed] [Google Scholar]
- 64.Tsenkova VK, Karlamangla AS, Ryff CD. Parental history of diabetes, positive affect, and diabetes risk in adults: Findings from MIDUS. Ann Behav Med 2016; 50: 836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsenkova VK, Karlamangla A. Depression amplifies the influence of central obesity on 10-year incidence of diabetes: Findings from MIDUS. PLoS ONE. 2016; 11: e0164802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hays J, Hunt JR, Hubbell FA et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol 2003; 13 (Suppl.): S18–77. [DOI] [PubMed] [Google Scholar]
- 67.Liu S, Tinker L, Song Y et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007; 167: 1676–85. [DOI] [PubMed] [Google Scholar]
- 68.Prentice RL, Moolgavkar SH, Farewell VT. Biostatistical issues and concepts in epidemiologic research. J Chronic Dis. 1986; 39: 1169–83. [DOI] [PubMed] [Google Scholar]
- 69.Chao C, Song Y, Cook N et al. The lack of utility of circulating biomarkers of inflammation and endothelial dysfunction for type 2 diabetes risk prediction among postmenopausal women: The Women’s Health Initiative Observational Study. Arch Intern Med. 2010; 170: 1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song Y, Manson JE, Tinker L et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: The Women’s Health Initiative Observational Study. Diabetes Care. 2007; 30: 1747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tindle HA, Chang YF, Matthews KA, Kuller LH et al. Istrait optimism or pessimism more important for incident chd and mortality? Psychosom Med 2010; 73: A-81–2 (Abstract). Available from: http://www.psychosomaticmedicine.org/misc/abstractbooklet2010.pdf (accessed 16 June 2010). [Google Scholar]
- 72.Robinson-Whelen S, Kim C, MacCallum RC, Kiecolt-Glaser JK. Distinguishing optimism from pessimism in older adults: Is it more important to be optimistic or not to be pessimistic? J Pers Soc Psychol. 1997; 73: 1345–53. [DOI] [PubMed] [Google Scholar]
- 73.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measur. 1977; 1: 385–401. [Google Scholar]
- 74.Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Medical care. 1988; 26(8): 775–89. [DOI] [PubMed] [Google Scholar]
- 75.Ware JE, Keller SD, Kosinski M. SF-12: How to Score the SF-12 Physical and Mental Helath Summary Scales. The Health Institute, New England Medical Center, Boston, 1995. [Google Scholar]
- 76.Xun P, Wu Y, He Q, He K. Fasting insulin concentrations and incidence of hypertension, stroke, and coronary heart disease: A meta-analysis of prospective cohort studies. Am J Clin Nutr. 2013; 98: 1543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang F, Han L, Hu D. Fasting insulin, insulin resistance and risk of hypertension in the general population: A meta-analysis. Clin Chim Acta. 2017; 464: 57–63. [DOI] [PubMed] [Google Scholar]
- 78.Kemmer FW, Bisping R, Steingruber HJ et al. Psychological stress and metabolic control in patients with type I diabetes mellitus. N Engl J Med. 1986; 314: 1078–84. [DOI] [PubMed] [Google Scholar]
- 79.Lloyd C, Smith J, Weinger K. Stress and diabetes: A review of the links. Diabetes Spectr. 2005; 18: 121–7. [Google Scholar]
- 80.Raikkonen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: Antecedent or consequence? Metabolism. 2002; 51: 1573–7. [DOI] [PubMed] [Google Scholar]
- 81.Progovac AM, Chang YF, Chang CH et al. Are optimism and cynical hostility associated with smoking cessation in older women? Ann Behav Med 2017: Epub ahead of print. 10.1007/s12160-016-9873-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rozanski A. Optimism and other sources of psychological well-being: A new target for cardiac disease prevention. Circ Heart Fail. 2014; 7: 385–7. [DOI] [PubMed] [Google Scholar]
- 83.Lloyd-Jones DM, Hong Y, Labarthe D et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010; 121: 586–613. [DOI] [PubMed] [Google Scholar]
- 84.Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003; 107: 1562–6. [DOI] [PubMed] [Google Scholar]
- 85.Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community-based population: The heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011; 123: 850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011; 57: 1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]