Abstract
High-quality patient information material (PIM) is essential for patients´ informed decision-making, and its quality may influence a care program’s acceptance. In the new psycho-oncological care program, isPO, the initial PIM was developed top-down and required optimization. In this paper, we report on the process and experiences of optimizing PIM’s quality bottom-up by applying a Participatory Health Research (PHR) approach. Cancer-patient representatives of the national peer-support group contributed as co-researchers as part of the optimization team. A mixed-methods design was chosen. First, the quality of the initially utilized PIM was assessed with the newly designed user-friendly instrument UPIM-Check. Next, three Participatory Action Research loops were conducted, including cancers survivors and isPO service providers. The initial isPO PIM’s were assed to be of low quality, limited usability and incomplete. Bottom-up generated optimization suggestions led to the improvement of two initially used PIMs (leaflet, patient information folder) and the design of two new PIMs (poster, study information overview). The optimized PIM facilitates isPO service providers’ care provision and helps newly diagnosed cancer patients in understanding and accepting the new program. PIM optimization benefited from applying PHR. The patient representatives’ contribution and active patient engagement were central for quality assessment and designing needs-driven, mature and complete PIM.
Keywords: cancer, psycho-oncological support, patient information material, quality assessment, optimization process, participatory health research, patient engagement, participation
1. Introduction
1.1. Background
Worldwide, patient information material (PIM) is essential in healthcare [1]. Well-designed high-quality PIM empowers the end-user (e.g., cancer patients) [1]; increases their satisfaction concerning communication with health professionals, especially in complex situations (e.g., with life threatening events) [2]; and augments the openness and motivation to participate in new interventions or research programs [3,4]. PIM can enhance end-users’ program understanding [5] and, therefore, may increase program acceptance [6]. Low-quality PIM may provoke uncertainty, misinterpretation, or patients’ resistance to a program [7,8]. In cancer-care support, PIM’s quality impacts on patients’ anxiety levels, emotional distress, vulnerability, and unfamiliarity with the new situation [9,10].
Different materials (e.g., leaflets or posters) are utilized for different activities and purposes in the healthcare system [11]. High-quality PIM should target two objectives: (1) end-user friendly information transmission and (2) a recommendation for action [7]. PIM should be in a readable and motivational style (e.g., use of positive language) and is considered as most effective if it is perceived as being user-friendly [12,13]. Four PIM quality criteria are considered essential: (1) the correctness and validity of content, (2) the readability in respect to text structure and graphic, (3) the comprehensibility for the end-users, and (4) their utility in the field [7,8,14]. International and national checklists, such as Discern´ [15] or TEMPtEd [16], aim to support the design and optimization of PIM and its quality assessment [8]. One already existing checklist, the PEMAT [13], helps professionals in assessing PIM´s understandability and actionability. Unfortunately, the available assessment instruments were developed with, and for, experts and researchers, whereas end-users (patients) were rarely engaged. Moreover, most instruments appear to be unsuitable for end-users’ application, due to the required literacy level. Consequently, end-users are hardly involved in PIM assessment [4,17,18].
When designing new PIM, end-users are rarely engaged [13,18]. As a result, many materials are of low efficacy, as they contain too much information, include difficult words, or lack recommendations for action [1,17,19,20]. Before utilizing a new PIM, it is recommended to assess its readability and suitability for end-users [1,11]. However, for various reasons, e.g., lack of availability or time, such assessments are rarely conducted [19].
The assessment, optimization, and development of PIM can benefit from key stakeholders´ (e.g., end-users or service providers) active participation [18,21]. The PHR approach aims to actively build on stakeholders’ implicit knowledge and experiences by their continuous involvement and engagement [22,23,24,25]. PHR acknowledges the insider perspective of people living with the health problem (e.g., cancer) as a practical and intuitive source [25].
In order to enable the co-creation of new knowledge, the empowerment and capacity building of the co-researchers is vital [26].
1.2. The Integrated Cross-Sectoral Psycho-Oncological (isPO) Program
In Germany, between 2017 and 2022, the Integrated Cross-Sectoral Psycho-Oncological (isPO) care program was recently designed, implemented, and evaluated; it is financed by the federal innovation fund [27,28]. The 12-month psychosocial and psychotherapeutic isPO program is offered to newly diagnosed adult cancer patients, parallel to their biomedical therapeutic treatment [28]. It aims to impact (1) on an individual-patient level, reducing patients’ depression and anxiety due to the cancer diagnosis; and, (2) on a system level, it seeks to offer a high-quality needs-driven psycho-oncological care program for comprehensive implementation into nationwide cancer care [27]. Since January 2019, isPO has been available free of charge for its target group (newly diagnosed cancer patients) in four especially established care networks in North-Rhine Westphalia, Germany. Due to its stepped care approach, as well as the inclusion of several service providers and different components, isPO is considered to be a complex intervention [29]. Complex interventions work best if they are well understood by their end-users and/or service providers. Consequently, the availability of high-quality PIMs seems to be vital for isPO to achieve its purpose [3,4].
The Institute of Medical Sociology, Health Services Research and Rehabilitation Science (IMVR) at the University of Cologne conducts external formative and summative program evaluation [27]. Moreover, isPO is currently implemented as part of a project and therefore includes both a program (treatment) component and a study (research) component [27]. In the program’s first formal evaluation, the “inclusion of both elements at the same time” was, especially in the beginning of the implementation phase, challenging for the service providers and confusing for the patients, causing some misunderstandings, frustrations, or even resistance to and non-acceptance of the new program [30].
During the program’s development phase (2018), four different isPO PIMs (leaflet, poster, patient information map, and website) were designed with a top-down approach, aiming to inform newly diagnosed cancer patients about this new program. The material was designed by the project leaders that included the German Cancer Society North-Rhine Westphalia (KG-NRW), which is responsible for the isPO care network support during program implementation. Both organizations possess a high level of content competence. Unfortunately, the isPO stakeholder “patient representative”, represented by the House of the Cancer Patient Support Associations of Germany (HKSH-BV), was only requested to provide general feedback to those materials after finalization. Before implementing the PIM in practice (2019), due to time constraints, no PIM quality assessment, end-user comprehensibility, and service-provider usability checks were conducted. Furthermore, no patient-information strategy was developed for a systematic assessment of the completeness of the PIM [31]. When conducting the external formative program evaluation, the evaluators were constantly engaged in different activities (e.g., observations, focus groups, and program quality workshops) with isPO stakeholders (e.g., service providers and network supporters). During the first formative evaluation that was conducted in the early program-implementation phase (June 2019), service providers of all four isPO networks claimed in individual interviews and focus groups that the initial isPO PIM urgently required optimization, e.g., in terms of quality and usability [30]. Furthermore, it turned out that PIM was utilized in a different manner or even partly ignored in the four care networks [30]. In the cross-sectoral isPO quality workshop (September 2019), in addition to the service providers, the German Cancer Society North-Rhine Westphalia (KG-NRW) in its role as network supporter, and the House of the Cancer Patient Support Associations of Germany (HKSH-BV), in its role as “active patient voice”, both emphasized that the currently utilized PIM was better suited to academic project information than as end-user program information. The before-mentioned stakeholders and the external evaluation team suspected that the insufficient quality of the PIM does negatively influence both end-users´ program and study acceptance. Hence, it was agreed by all participants of the workshop that the initial top-down designed four PIM should be assessed concerning their quality and subsequently optimized. In order to address the specific needs, the PIMs’ target groups (end-users and service providers), it was decided to actively involve them in this process.
1.3. Aim
Works in the literature that describe, in detail, how to design, assess, and optimize PIM with patient participation are rare [18]. In health research in Germany, comprehensive patient involvement and engagement (PPI) with a high degree of participation is still rare or even considered as not appropriate (e.g., when conducting evaluations). However, formative evaluation should help minimize a new program’s teething problems by providing optimization suggestions [32]. By applying the critical-friend approach, the isPO evaluation team may serve as an impulse provider, provoking a look at the issue (e.g., PIM) with another perception and may foster development of a co-creative learning process [33].
The specific aim of this paper is to describe the bottom-up quality assessment and optimization process of the initial isPO PIM by applying the PHR approach with constant engagement of the patient representative. First, the evaluation team felt it is crucial to understand how the initial PIMs’ quality is perceived. Second, we aimed to involve the target group (cancer patients) in the PIM optimization process, as we were convinced that, due to their valuable contribution, the optimized PIM will turn out to be end-user friendly, high quality, and complete.
2. Materials and Methods
2.1. Optimization Approach
Participatory health research (PHR), a bottom-up approach, was applied [34] as a supplement to the formative evaluation activities in order to optimize the initial isPO PIM. PHR’s leading principle is shifting power from the “experts” (e.g., professional researchers) to those stakeholders who possess insightful knowledge (e.g., patients) and/or experience (e.g., service providers) by enabling high participation [34]. Cornwall´s participation typology differentiates between six participation degrees: (1) co-option, (2) compliance, (3) consultation, (4) cooperation, (5) co-learning, and (6) collective action. It was utilized to describe the relationship between the researcher and stakeholder during the optimization process [35].
2.2. Roles and Competences
In order to gain a profound understanding of the quality requirements, a temporary PIM optimization team was formed, consisting of eight individuals with different backgrounds (Table 1), including patients´ representatives [36]. The patient representatives and the network support experts were constantly engaged as co-researchers with high participation degrees (degree 4/5). Moreover, respective end-users (exemplified by different German cancer survivors, representing their self-help group cohort) and isPO service providers (e.g., psychotherapists and isPO case managers) contributed with their valuable knowledge during the iterative optimization loops [37].
Table 1.
Composition of the isPO PIM optimization team.
| Affiliation and Number of Participants |
Role during the PIM Optimization Process | Role in IsPO | Overall Expertise |
|---|---|---|---|
| House of the Cancer Patient Support Associations of Germany (HKSH-BV) 2 participants |
Co-researcher patient perspective
|
|
|
| German Cancer Society North-Rhine Westphalia (KG-NRW) 3 participants |
Co-researcher expert perspective
|
|
|
| Institute for Medical Sociology, Health Services Research and Rehabilitation Science (IMVR), University of Cologne 3 participants |
External impulse provider (critical friend approach)
|
|
|
2.3. The PIM Optimization Process
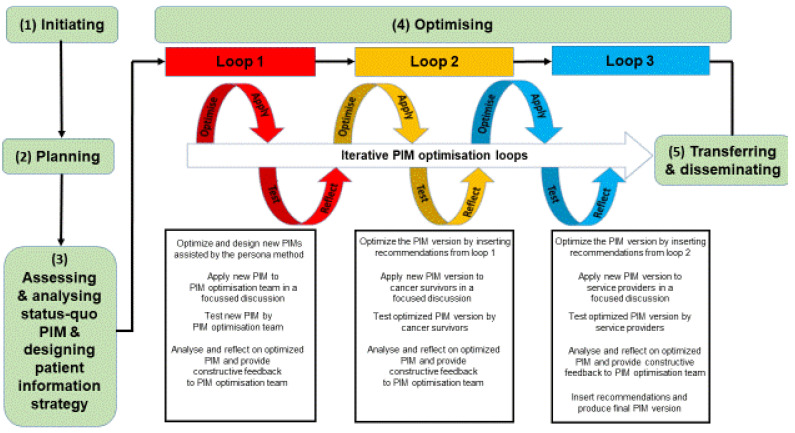
Our PIM optimization process contained five phases: (1) initiating, (2) planning, (3) assessing the status-quo, (4) optimizing, and (5) transferring and disseminating (Figure 1).
Figure 1.
Optimization process of the isPO patient information material (PIM).
Initiation phase: The PIM optimization team was formed, the mutual goal was defined, responsibilities were negotiated, and it was agreed to conduct the optimization process bottom-up with the PHR approach (Figure 1, left side).
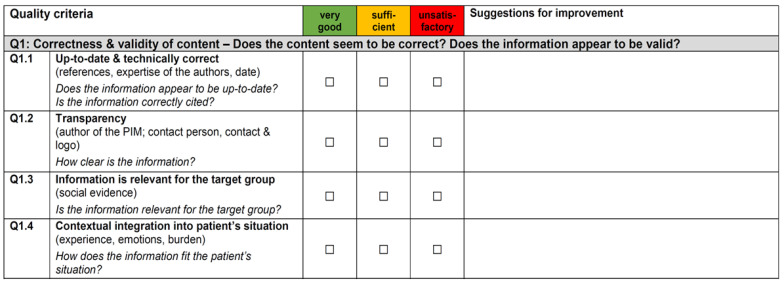
Planning phase: As no German appropriate end-user (patients)-friendly quality assessment instrument was found for our purpose, a new instrument, the User-friendly Patient Information Material Check list (UPIM-Check), was developed in a participatory manner and piloted (Figure 2). The UPIM-Check is divided into two parts: (1) assessment and (2) improvement. A traffic-light system (green = very good, orange = sufficient, and red = unsatisfactory) is used for the quality assessment. It consists of 31 criteria within four categories, namely correctness and validity of content, readability of content, structural readability, and graphical readability. Next, users may insert individual optimization impulses for each item in a free text field (see Figure 2). Details on the UPIM-Check’s development and validity are published elsewhere [38].
Figure 2.
Extract of PIM assessment instrument UPIM-Check.
Moreover, the team discussed and agreed on the general assessment and optimization process, which included groups or numbers of participants, responsibilities, communication lines, resources, and data management. Finally, a plan of action was approved (Figure 1, left side).
Assessing and analyzing phase: First, the eight individual PIM optimization team members (Table 1) assessed the initial isPO PIM’s quality independently, using the UPIM-check (Figure 1, left side). Patients’ perspective was represented by the two members of the House of the Cancer Patient Support Associations of Germany (HKSH-BV). Next, eight isPO service providers (two from each of the four networks) assessed the material with the UPIM-check. Results were collected, merged into one overarching table, and distributed amongst the optimization team. Outcomes were thematically analyzed separately, distinguishing by PIM team, service providers, and end-users [39]. During two workshops (3 hours each), the optimization team conducted further analyses on the initial isPO PIM, distinguishing its strengths and weaknesses and summarizing the crucial points for improvement by focusing on its end-user friendliness and usability (Table 4 and Table A2) [17,20,40]. As the initial PIM was considered as being incomplete and unstructured in its utilization, the team developed a patient-information strategy.
Optimizing phase: Based on the PIM assessment’s findings, the iterative optimization process started (Figure 1, center). Participatory Action Research (PAR) in three overarching optimization loops was conducted [37]. Each loop contained 4 steps: optimize, apply, test, and reflect [41].
The first PAR loop was conducted by the PIM optimization team (Table 1) itself. First, two isPO PIM elements were improved, and two elements were newly designed, according to the patient information strategy (Phase 3), with the aim to adequately address the end-users’ (cancer patients and service providers) needs (optimize). For that purpose, the first author (belonging to the external impulse provider group) applied the persona method, resulting in an objective creation and design of potential isPO end-users types (adult cancer patients) [42]. Second, together with these personas, the new and optimized material was presented to the entire PIM optimization team (apply). They critically assessed the quality of the optimized PIM regarding its end-user friendliness and usability (test), and finally provided valuable recommendations for their improvement (reflect). Moreover, they reassessed its correctness, validity of content, and completeness according to the patient-information strategy.
During the second PAR loop, the potential program´s end-users (cancer patients) were engaged during a three-hour focus-group discussion with a high degree of participation (co-learning, degree 5). Four cancer survivors, representing different cohorts of cancer self-help groups, and the federal consultant of the support associations (member of the PIM optimization team; see Table 1) participated. They concentrated on assessing and optimizing the end-user-friendly comprehensibility of content (e.g., understandability and terminology) and readability concerning structure and graphics (e.g., font, layout, and design) [7].
In the third loop, eight isPO service providers (e.g., case manager and psychotherapist) were engaged in a 90-minute focused discussion. The optimization concentrated on the usability of the PIM in the daily routine of the service providers (e.g., program information, and counseling) and its completeness.
Transferring and disseminating phase: In March 2020, the optimized and completed PIM was made accessible to the four isPO networks. Since then, this optimized PIM is utilized within all four isPO networks in the same manner and intensity for program information and orientation (Figure 2, right side).
2.4. Ethical Considerations
During the entire optimization process, co-researchers´ (Table 1) and participants’ participation was on a voluntary basis; no incentives were offered. Ethical principles, such as mutual respect, equality and inclusion, democratic participation, active learning, and personal integrity, were considered during this PHR project [34]. All participants provided a written informed consent before data collection.
3. Results
The bottom-up optimization of the isPO PIM lasted five months (09/2019–02/2020). Four PIM outputs that build on each other were achieved by considering high quality, user-friendliness, usability, and completeness. First, we present the results of the process outputs: (1) assessment of initial materials, (2) development of a patient information strategy, (3) optimization of initial materials, and (4) design of new PIM. Finally, a pre–post example of an optimized PIM is given.
3.1. Assessment of the Initial IsPO-PIM
During the assessment phase of the initial isPO-PIM (Figure 2, phase 3), the entire PIM optimization team (Table 1) worked in a co-creative process together, shared their knowledge, and created a new understanding towards the required needs (participation degree five—co-learning) [35]. It became apparent that not all materials were utilized in each network by the isPO service providers for patient program information and orientation. Table 2 gives an overview of the four PIM elements and their imbalanced utilization.
Table 2.
Utilization of the initial isPO-PIM in the four care networks.
| Initial IsPO PIM Elements | Care Network 1 | Care Network 2 | Care Network 3 | Care Network 4 |
|---|---|---|---|---|
| Leaflet | X | X | X | X |
| Patient information folder | X | X | X | X |
| Poster | X | |||
| Website of the care network (program subpage) | X |
As only the leaflet and patient-information folder were universally utilized, in the following quality-assessment process, we focused on these two PIM elements. The initial isPO leaflet was assessed by 18 individuals with the UPIM-Check (Figure 2) from three perspectives: the PIM optimization team (n = 8), isPO service providers (n = 8), and end-users (n = 2), represented by cancer survivors. The initial isPO leaflet was assed as “unsatisfactory” in 11 subcriteria that were spread over all four quality criteria (Table 3, upper part). The key recommendations of the 18 individual assessments are summarized in Table 3, lower part). The assessment of the patient-information folder was conducted by the same participants (N = 18). Many redundancies among leaflet and information folder became visible, and weaknesses in all four quality categories were detected (see Table A1 for the recommendations given by the participants).
Table 3.
Multi-perspective outcomes of the quality-assessment process of the initial program leaflet.
| Leaflet Weaknesses Listed by the Four Quality Criteria of the UPIM-Check (Part 1 UPIM-Check) | ||||
| Quality Criteria | Criteria that Were Assessed with Red (=Unsatisfactory) within the UPIM-Check | PIM Optimization Team * | Service Providers * |
End-Users
(Cancer Survivors) * |
| Correctness and validity of content | Contextual integration into patient´s situation | 8 | 2 | |
| Relevance of the information | 6 | 2 | ||
| Recommendation for action | 8 | 2 | ||
| Motivation and increase of self-efficiency | 8 | 2 | ||
| Readability of content | Aim for the patient identifiable | 6 | 8 | 2 |
| Simple, clear language | 8 | 8 | 2 | |
| Use of empowering words | 8 | 2 | ||
| Structural readability | Appropriate sentence complexity | 8 | 8 | 2 |
| Graphically readability | Layout/overall visual appearance | 7 | 2 | |
| Appealing “eye catcher” functioning as a “door opener” for recruitment | 8 | 8 | ||
| Illustrations | 7 | 2 | ||
| ||||
| Perspective | Summary of Key Optimization Recommendations | |||
| PIM optimization team |
|
|||
| isPO service providers (psychotherapist, case manager) |
|
|||
| End-users (cancer survivors) |
|
|||
* Number of assessments by the respective groups (PIM optimization team, service providers, end-users).
3.2. Development of the Patient Information Strategy
The isPO PIM’s assessment showed that the initial four PIM elements (Table 2) appeared to be incomplete, that information was partly redundant, and that its information provision was unsystematic and unstructured. Hence, the PHR team co-created an isPO patient-information strategy (Table 4) during the first PIM optimization workshop (see Figure 1, left side) whilst working in a co-learning atmosphere (participation degree 5) [35]. The strategy aimed to clarify (1) which isPO PIM components were essential, (2) the respective PIM’s target group, (3) the purpose, (4) the moment of utilization, and (5) the information specification. The co-creative process led to the decision that the complete isPO patient-information strategy should contain five PIM elements: a poster, a leaflet, a patient information folder, a one-pager (study information overview), and an end-user friendly website.
Table 4.
Patient-information strategy applied to PIM elements and hierarchical order.
| PIM Elements (o or n) * |
Target Group | Purpose | Moment of Utilization | Information Specification |
|---|---|---|---|---|
| Poster n | All patients | Display/present the existence and purpose of isPO (“door opener”, motivator) |
Broad (waiting room area, general hospital area) |
General information concerning isPO Focus on available support and resources |
| Leaflet o | Potential isPO-patients | Specific (first introduction to isPO, multiplication factor) |
Soon after the first cancer diagnosis | isPO-specific and end-user oriented information (clarification of the benefits) Relevant elements: |
| ||||
| Patient information folder o |
Suitable isPO patients | Briefing the patient | During the introductory conversation (intake) | Crucial isPO program and study details |
| One-pager n | Patients that should be enrolled in the isPO study | Enrolment (to provide a comprehensible overview regarding the study and all ethical aspects and informed consent) |
Enrolment | Overview and orientation (e.g., reference to pages in the ethical consideration paper) |
| Website n | All patients and other interested persons (e.g., researchers) | Broad (to increase motivation, to raise awareness for psycho-oncology and isPO) |
Various (when individually needed for patient information during research) |
isPO-specific and needs driven information |
Note: * o = optimized PIM, n = newly designed PIM.
The website was considered as important for augmenting the program´s transparency, dissemination, and to potentially increase the level of awareness beyond the current catchment areas. However, as designing an end-user-friendly isPO website (with several subpages) is a complex process that requires sufficient resources, it was decided that this work package needed to be separated from the other four elements. Thus, the website design’s experiences and outcomes are published elsewhere.
3.3. Optimizing the Initial IsPO PIM
When optimizing the initial PIM, experts by experience (cancer survivors representing their self-help peer group cohort) and isPO service providers contributed with their insightful knowledge and experiences (from participation degree four = cooperation to participation degree five = co-learning) [35].
The external impulse providers (see Table 1) facilitated and stimulated the entire PHR research process, structured and managed the three PAR loops, and inserted the optimization requirements in the different PIM elements. The three potential isPO end-users types (personas), created by the persona method [43], supported the external impulse providers in assessing the suitability of the optimized product in each loop. One isPO persona example is offered in Table A2.
Following the logic of the patient information strategy (Table 4), the leaflet builds on the information of the poster. Therefore, a corporate design was chosen to help patients recognize that both materials belong to the same program (e.g., a tree image with strong roots and an “encouraging” green for headings and subheadings). In its user-friendly wording and design, the new leaflet complements the poster and clearly addresses end-users’ needs (Table 4). It includes easy-to-understand elementary information about the isPO program and an empowering recommendation for action that is applicable in all four care networks. Easy-to-find contact details (e.g., case manager) are placed directly on the leaflet. As specifically recommended by cancer survivors in the assessment phase, Arial font size 12 was used to improve end-user-friendliness. The language was improved with the use of positive and resource-oriented words (e.g., “isPO can support you with your fight against cancer”) and completely avoiding technical terms. Finally, it was highlighted that isPO is “free of charge” and supportive for the biomedical cancer trajectory (Figure 3).
Figure 3.
Example of the outcome optimization process on two elements of the isPO PIM.
The optimization of the patient-information folder resulted in significantly fewer pages (from five to three pages). It appears as one document that personally addresses the end-user (Table 4 and Table A1). Its wording and layout were tailored (e.g., sentence structure and subheadings) to the end-users’ (cancer patients) needs. Redundancies and over-complex information regarding the contextual and legal frameworks of the isPO program (e.g., legislative information or paragraphs) were deleted. The benefit of participating in the isPO program and study was emphasized by using neutral wording. Furthermore, the point was stressed that isPO is offered to all newly diagnosed cancer patients, regardless of the degree of perceived emotional or social burden. The recommendation for action was formulated with empowering words and stands out graphically.
3.4. Designing the New IsPO PIM
The design process of the new PIM was achieved in a co-creative manner (participation degree five, co-learning), as all PIM optimization team members shared their knowledge and experiences for this purpose [35]. Moreover, cancer survivors and isPO service providers also contributed as co-researchers. We designed two new components: the poster and a one-pager (see Table 4).
According to the patient information strategy (Table 4), the poster is the first PIM element that will inform about isPO in the care networks. Instead of the red “attention calling” color theme, an “encouraging” green was used (Figure 3). In the center of the poster, a tree image is placed as an eye-catcher and, simultaneously, as a reassuring and encouraging element. In the treetop the four general isPO care-support offers are formulated as possible actions. Furthermore, initial information on the stepped psycho-oncological care approach is provided. Considering the new corporate design, the poster includes a clear recommendation for action: “patients should ask their doctor in charge about isPO and should seek support”.
The one-pager aims to give an overview on the isPO study (Table 4). It was designed to transfer the compulsory study information (a twelve-page informed consent form) into a user-friendly format and language. It contains an easy-to-understand graphical representation of the isPO timeline (12 months) and shows the course of events within the isPO support program (e.g., intake) and its study (e.g., timing and type of data collection). Moreover, crucial points of the study consent form are summarized and highlighted, and references are provided to the in-depth information and its corresponding page reference in the informed-consent form.
After completing the third optimization loop (Figure 1), no further optimization suggestions were made by participants of the PAR process. Hence, the PIM optimization team was convinced that the four PIM elements (outputs) consider end-users’ (newly diagnosed cancer patients and isPO service providers) needs adequately. Therefore, the optimization process resulted in the availability of four end-user friendly isPO PIM (outcome). These PIM meets the quality criteria, as well as the specific needs of its end-users (e.g., correctness and validity of content, readability, and comprehensibility) and isPO service providers (e.g., usability). Figure 3 gives an example on the outputs pre–post. The new four PIM elements and patient information strategy were introduced to all care networks (network coordinator and head of psycho-oncological care) during the regularly occurring isPO quality workshop (February 2020). Since then, the optimized PIM have been similarly applied and utilized in the four care networks. During further external formative program evaluation rounds in 2020, including focus groups and interviews with end-users and service providers, it became evident that the optimized PIM positively assists service providers in their isPO care provision and helps newly diagnosed cancer patients to understand and accept the new program [43]. Their user-friendly approach turned out to be vital for the program’s stability, since, due to the Corona pandemic, in March 2020, face-to-face intakes were forbidden for some weeks. During this time, PIM were sent via e-Mail or letter before the intake. The feedback of the service providers and patients was very positive, especially for the leaflet and the one-pager [43].
4. Discussion
This article reports on the experiences of optimizing the quality of PIM for the new complex German psycho-oncological care program isPO by applying participatory health research (PHR). Hereby, the patient perspective was constantly included in the process, as two members of the House of the Cancer Patient Support Association, representing the active voice of “experts by experience” [44], participated as co-researchers as an integral part of the PIM optimization team (Table 1). Furthermore, during the assessment and optimization process, six respective end-users (cancer survivors) and eight isPO service providers contributed with their knowledge with a high degree of participation (Figure 1; participation degree 4 = cooperation/participation degree 5 = co-learning) [35]. By involving PIM’s end-users, we made a very positive difference to the initial PIM development process, where material was developed by the project managers and experts for the program’s end-users, but without engaging them (participation degree 2 = compliance) [35].
We experienced that the PIM optimization team composition, containing patient representatives, experts, and researchers (Table 1), was crucial for gaining a multi-perspective comprehensive understanding of the real needs of the end-users, as also experienced by other researchers in different settings [21,45]. By incorporating both experiences and skills, we also felt that three issues were crucial: (1) to choose the team members carefully, (3) to constantly monitor the team composition, and (3) to be open for necessary adjustments [36]. Moreover, all team members (co-researchers and external impulse providers) benefited from the open, power sharing, and co-creative working atmosphere [46].
Before implementation, investing in a systematic PIM quality assessment seems to be imperative, as is also highlighted in other studies [16]. Even though patients possess a good understanding of their informational needs [47,48], only recently their perspectives got actively included in PIM quality assessments [17,47,49]. After reviewing the existing literature, the PIM team perceived the existing PIM assessment instruments as unpromising for end-users’ application, e.g., by not considering end-user’s health-literacy level. To the best of our knowledge, previously “no suitable” end-user friendly quality assessment instrument existed in Germany. We developed and tested our UPIM-check instrument in a co-creative manner with a strong contribution from the patient representatives [39]. In this context, we declared suitability according to four of the six categories defined as follows [20]: content, graphics, layout/typography, and learning stimulation and motivation for the decision-making process. After the UPIM-check utilization, the involved experts, researchers, service providers, and end-users perceived the instrument as “suitable”, “surprisingly end-user friendly” and “resource saving in its application”. End-users (cancer patients and service providers), in particular, welcomed the UPIM-check as the instrument empowered them to actively participate in the scientific work. This, in turn, prepares them in the long term to contribute to the optimization processes on its highest level of participation degree 6 = collective action [35]. UPIM-Check turned out to be helpful to identify the shortcomings and limitations of the initial isPO PIM from three perspectives: experts, patients, and service providers. The need for gathering a multi-perspective understanding was also considered as important by other researchers [50]. As suggestions for its optimization were offered by the three different groups, we were confident to address several important quality criteria when preparing the material for the first PAR loop. Regardless, it still needed three loops until we were confident that the maturity of all PIM (high quality and user-friendly) was fully achieved. During further formative evaluations (2021), it became evident that optimized material helped potential end-users to better comprehend the new program, leading to better program acceptance [43]. Moreover, service providers highlighted that, especially the “one pager (Table 4), was very helpful for informing newly diagnosed cancer patients about the program. Even with the Corona pandemic, the PIM turned out to be appropriate for the program orientation and intake [43]. If the program is rolled-out to nationwide care, it might be helpful to invest in an APP, e.g., as a platform for PIM. However, developing an APP is a complex process itself that needs sufficient resources (e.g., time, design thinking, financing, or knowledge). Moreover, in the development of an end-user-friendly APP, we recommend the constant engagement of end-users.
Due to time constraints, in the beginning of the isPO program implementation, the importance of a so-called “patient information strategy” was undervalued. However, after investing sufficient resources into its development, it helped to define, structure, navigate, and complete the analogue PIM for our new and complex program. Due to the different informational needs, we propose to develop such a strategy as part of the implementation strategy, as also suggested by Huynh et al. [51].
4.1. Designing and Optimizing High-Quality PIM Bottom-Up
In Germany, PHR, patient involvement and engagement in research is still rare [52,53,54]. By engaging co-researchers early, proactively, and with high participation degrees 23 during the PIM optimization and designing process, an innovative shift in power towards the co-researchers occurred. Our PIM benefited from these conditions, as also promoted or experienced by other researchers [18,21]. Additionally, we experienced that the criteria correctness and validity of content, readability in respect to text structure and graphics, comprehensibility for the end-users, and their utility in practice were crucial to achieving the high quality of our PIM. However, we believe that these criteria can only be accomplished if a program’s end-users (e.g., cancer patients or service providers) are invited to participate in the PIM design or optimization process. In our case, end-users´ recommendations had a high impact on the readability and comprehensibility, whereas the feedback of the service providers reflected on the utility, as also highlighted by Cook and colleagues [55]. We are certain that the optimized PIM addresses the needs of the program’s end-users, because it underwent serval pilot loops via PAR, as recommended [37,55], before its dissemination to the field. By prioritizing high participation with so-called experts by experience (e.g., farmer cancer patients), we perceived PHR also as bridging the gap between theory and practice [47,56].
We demonstrated that designing a high-quality PIM requires time, resources, in-depth contextual understanding, and an appropriate approach [3,21,57]. The effect of a detailed understanding of end-users’ needs (e.g., comprehensibility) on the program´s acceptance by those end-users was underestimated by the program designers when developing the PIM top-down [50]. We chose PHR, a bottom-up approach, as it is perceived as a strategy to overcome the gap between academic researchers or experts and end-users (e.g., patients) in practice [23,46]. Hereby, both the co-creation of new practical-based knowledge and the empowerment of co-researchers was achieved by a high degree of participation [34,35,58]. Overall, our entire PIM optimization team (Table 1) and the different participants of the study experienced the high degree of participation as empowering. However, they varied in regard to their specific role and earlier engagement experiences between “advantageous and practicable” (experts), “expedient and impulse giving” (academic researchers), “innovative and empowering” (isPO service providers), and “democratic and highly welcoming” (patient representatives) [30].
4.2. Strengths and limitations
Optimizing PIM with the PHR approach requires the availability of resources (e.g., time, skills, and staff) and an appropriate access to the end-user group [59,60]. In our case, both were available. PHR requires constant openness for power sharing and respect for accepting democratic rules from all participants [34]. Seven of the eight members of the PIM-optimization team were novices in applying PHR. We experienced that, despite the increased attention for participatory approaches, in the German health-research domain, the benefit of participative approaches remains largely undiscovered. The lack of experience of most members of the team required intense methodological guidance in order to hold the team on the “PHR road”. Hereby, the methodological experience of the external impulse provider as a facilitator was imperative. The fact that all participants were committed to the PHR approach was also helpful. To set clear mutual expectations and agree on conflict-resolution mechanisms right in the beginning of a PHR process seemed to be crucial [36]. Overall, the PIM´s optimization with the PHR approach was perceived as communication intensive, as also experienced by others [61]. It required strong communication and mediation skills from the facilitator (external impulse provider, first author). However, investing constantly in knowledge transfer and skills training resulted in co-researchers´ empowerment [61]. Finally, it was more time-consuming than expected (2 months longer). We learned that, beforehand, sufficient resources (e.g., time and staff) should be calculated for PIM [36,61].
New context-specific bottom-up knowledge was gathered from multiple perspectives (e.g., patients, isPO service providers, and experts). Therefore, we are certain that PIM should fit the real needs of service providers (e.g., utility) and patients (e.g., understandability) in practice [18,21]. However, the optimized PIM is limited to (1) the geographic coverage of our program, (2) end-users (cancer patients) needs, (3) German reading skills, and (4) a moderate health-literacy level. Therefore, a generalization of the optimized PIM is restricted.
The optimization process was started by assessing the initially used isPO-PIM. Our instrument (UPIM-Check) that was developed for this purpose was perceived as a “highly valuable instrument”. End-users, isPO service providers, and the PIM optimization team experienced it as user-friendly during assessment and resource saving by generating recommendations for optimizations from the same perspective. Currently, this instrument is validated and accessible free of charge to the German-speaking society only [38,62]. However, an English version is currently being piloted with self-help organizations in the UK, USA, Canada, and Australia.
5. Conclusions
The optimized PIM facilitates isPO service providers’ care provision and helps newly diagnosed cancer patients in understanding and accepting the new psycho-oncological care program (isPO). The correctness and validity of content, readability, comprehensibility, and utility of the PIM fit to end-users’ needs and, therefore, simplify isPO service providers’ work.
PIM optimization benefited from applying PHR, besides the fact that the bottom-up approach required resources. The patient representatives’ contribution and active patient engagement were central for quality assessment and designing needs-driven, mature, and complete PIM.
Critical reflection on research and power sharing stimulated the entire PIM optimization team. Finally, both the PIM team and co-researchers were empowered through the mutual learning process that may positively impact further program implementation.
Acknowledgments
The authors strongly appreciate the participants of the diverse German cancer self-help groups and the isPO service providers in the University Hospital Cologne, Clinic Maria Hilf and Clinic St. Franziskus Mönchengladbach, Johanna-Etienne-Clinic Neuss, and GFO clinics Troisdorf for their active contribution, openness, and engagement.
Appendix A
Table A1.
Multi-perspective recommendations for optimizations of the initial program patient information folder.
| Patient Information Folder: Key Optimization Recommendations (Part 2 of UPIM-Check) | |
|---|---|
| Perspective | Summary of Key Optimization Recommendations |
|
isPO service providers (psychotherapist and case manager) |
|
|
End-users (Cancer survivors) |
|
Table A2.
Persona example developed to support the isPO PIM optimization.
|
Target Group: middle-aged man
Individual Characteristics: | ||
| ||
|
Name: Albert |
Age: 56 Years |
Family status: married, 3 adult children |
|
Gender: masculine |
Occupation: Head of car dealership, car mechanic (master) |
Education level: secondary school leaving certificate |
Living environment:
| ||
Interests and habits:
| ||
Personal goals:
| ||
Frustration and Limitation due to cancer diagnosis:
| ||
Author Contributions
T.K., conceptualization, methodology, validation, formal analysis, investigation, resources, writing—original draft, visualization, and project administration; S.S., conceptualization, formal analysis, investigation, resources, writing—review and editing; A.D., supervision, validation, resources, and writing—review and editing; A.A., investigation, formal analysis, resources, and writing—review and editing; A.G., investigation, formal analysis, resources, writing —review and editing; K.S., investigation, formal analysis, resources, and writing—review and editing; S.H., investigation, formal analysis, resources, and writing—review and editing; A.D., supervision, validation, and writing—review and editing; H.P., validation and writing—review and editing; N.C., conceptualization, methodology, validation, formal analysis, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors. The isPO project itself is funded by the German Innovation Fund of the Federal Joint Committee, the G-BA (01NVF17022).
Institutional Review Board Statement
The study was performed according to the Declaration of Helsinki. The ethics committee of the Medical Faculty of the University of Cologne has approved the isPO project and its study design (No. 18-092). The relevant national and European data-protection regulations were considered for data collection. The isPO project study is registered in the German Clinical Trials Register (No. DRKS00015326).
Informed Consent Statement
All participants received oral and written information regarding the aim of the study and its voluntary nature and provided written consent to participate.
Data Availability Statement
The data are not publicly available due to ethical and legal restrictions, as participants of this study did not agree for their data to be shared publicly. Upon reasonable request, the data presented in this study are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funding organization was not involved in the design of the study, data collection and analyses, interpretation of data, writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Protheroe J., Estacio E.V., Saidy-Khan S. Patient information materials in general practices and promotion of health literacy: An observational study of their effectiveness. Br. J. Gen. Pract. 2015;65:e192–e197. doi: 10.3399/bjgp15X684013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sustersic M., Tissot M., Tyrant J., Gauchet A., Foote A., Vermorel C., Bosson J.L. Impact of patient information leaflets on doctor-patient communication in the context of acute conditions: A prospective, controlled, before-after study in two French emergency departments. BMJ Open. 2019;9:e024184. doi: 10.1136/bmjopen-2018-024184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann T., Worrall L. Designing effective written health education materials: Considerations for health professionals. Disabil. Rehabil. 2004;26:1166–1173. doi: 10.1080/09638280410001724816. [DOI] [PubMed] [Google Scholar]
- 4.Sacristán J.A., Aguarón A., Avendaño-Solá C., Garrido P., Carrion J., Gutierrez A., Kroes R., Flores A. Patient involvement in clinical research: Why, when, and how. Patient Prefer. Adherence. 2016;10:631–640. doi: 10.2147/PPA.S104259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthur V.A. Written patient information: A review of the literature. J. Adv. Nurs. 1995;21:1081–1086. doi: 10.1046/j.1365-2648.1995.21061081.x. [DOI] [PubMed] [Google Scholar]
- 6.Alexander J.W., Karantanis E., Turner R.M., Faasse K., Watt C. Patient attitude and acceptance towards episiotomy during pregnancy before and after information provision: A questionnaire. Int. Urogynecol. J. 2020;31:521–528. doi: 10.1007/s00192-019-04003-x. [DOI] [PubMed] [Google Scholar]
- 7.Herm K., Linden M. Qualitätssicherung von schriftlichen Patienteninformationen. Psychother. Psychosom. Med. Psychol. 2013;63:176–184. doi: 10.1055/s-0032-1330012. [DOI] [PubMed] [Google Scholar]
- 8.Steckelberg A., Berger B., Köpke S., Heese C., Mühlhauser L. Kriterien evidenzbasierter Patienteninformationen. Z. Ärztl. Fortbild. Qual. 2005;99:343–351. [PubMed] [Google Scholar]
- 9.Estey A., Musseau A., Keehn L. Patient’s understanding of health information: A multihospital comparison. Patient Educ. Couns. 1994;24:73–78. doi: 10.1016/0738-3991(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 10.Rozmovits L., Ziebland S. What do patients with prostate or breast cancer want from an Internet site? A qualitative study of information needs. Patient Educ. Couns. 2004;53:57–64. doi: 10.1016/S0738-3991(03)00116-2. [DOI] [PubMed] [Google Scholar]
- 11.Rudd R.E. Health literacy skills of US adults. Am. J. Health Behav. 2007;31((Suppl. 1)):S8–S18. doi: 10.5993/AJHB.31.s1.3. [DOI] [PubMed] [Google Scholar]
- 12.Finnie R.K.C., Felder T.M., Linder S.K., Mullen P.D. Beyond reading level: A systematic review of the suitability of cancer education print and Web-based materials. J. Cancer Educ. 2010;25:497–505. doi: 10.1007/s13187-010-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoemaker S.J., Wolf M.S., Brach C. Development of the Patient Education Materials Assessment Tool (PEMAT): A new measure of understandability and actionability for print and audiovisual patient information. Patient Educ. Couns. 2014;96:395–403. doi: 10.1016/j.pec.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lühnen J., Albrecht M., Mühlhauser I., Steckelberg A. Leitlinie Evidenzbasierte Gesundheitsinformation. 2017. [(accessed on 15 April 2020)]. Available online: http://www.ebm-netzwerk.de/was-wir-tun/publikationen/
- 15.Charnock D., Shepperd S., Needham G., Gann R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J. Epidemiol. Community Health. 1999;53:105–111. doi: 10.1136/jech.53.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clayton L.H. TEMPtEd: Development and psychometric properties of a tool to evaluate material used in patient education. J. Adv. Nurs. 2009;65:2229–2238. doi: 10.1111/j.1365-2648.2009.05049.x. [DOI] [PubMed] [Google Scholar]
- 17.Demir F., Ozsaker E., Ilce A.O. The quality and suitability of written educational materials for patients*. J. Clin. Nurs. 2008;17:259–265. doi: 10.1111/j.1365-2702.2007.02044.x. [DOI] [PubMed] [Google Scholar]
- 18.van Beusekom M.M., Kerkhoven A.H., Bos M.J.W., Guchelaar H.-J., van den Broek J.M. The extent and effects of patient involvement in pictogram design for written drug information: A short systematic review. Drug Discov. Today. 2018;23:1312–1318. doi: 10.1016/j.drudis.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Williams A.M., Muir K.W., Rosdahl J.A. Readability of patient education materials in ophthalmology: A single-institution study and systematic review. BMC Ophthalmol. 2016;16:133. doi: 10.1186/s12886-016-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doak L.G., Doak C.C., Meade C.D. Strategies to improve cancer education materials. Oncol. Nurs. Forum. 1996;23:1305–1312. [PubMed] [Google Scholar]
- 21.Jewitt N., Hope A.J., Milne R., Le L.W., Papadakos J., Abdelmutti N., Catton P., Giuliani M.E. Development and Evaluation of Patient Education Materials for Elderly Lung Cancer Patients. J. Cancer Educ. 2016;31:70–74. doi: 10.1007/s13187-014-0780-1. [DOI] [PubMed] [Google Scholar]
- 22.Spinuzzi C. The Methodology of Participatory Design. Tech. Commun. 2005;52:162–174. [Google Scholar]
- 23.Cornwall A.J.R. What is participatory research? Soc. Sci. Med. 1995;41:1667–1676. doi: 10.1016/0277-9536(95)00127-S. [DOI] [PubMed] [Google Scholar]
- 24.Bergold J., Thomas S. Participatory Research Methods: A Methodological Approach in Motion. [(accessed on 16 December 2021)];Forum Qual. Soc. Res. 2012 13:30. Available online: http://www.qualitative-research.net/index.php/fqs/article/view/1801/3334. [Google Scholar]
- 25.van den Muijsenbergh M., Teunissen E., van Weel-Baumgarten E., van Weel C. Giving voice to the voiceless: How to involve vulnerable migrants in healthcare research. Br. J. Gen. Pract. 2016;66:284–285. doi: 10.3399/bjgp16X685321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unger H. Community-based participatory health research: Principles and practice. Eur. J. Public Health. 2019;29((Suppl. 4)):ckz185.762. doi: 10.1093/eurpub/ckz185.762. [DOI] [Google Scholar]
- 27.Jenniches I., Lemmen C., Cwik J.C., Kusch M., Labouvie H., Scholten N., Gerlach A., Stock S., Samel C., Hagemeier A., et al. Evaluation of a complex integrated, cross-sectoral psycho-oncological care program (isPO): A mixed-methods study protocol. BMJ Open. 2020;10:e034141. doi: 10.1136/bmjopen-2019-034141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusch M., Labouvie L., Schiewer V., Talalaev N., Cwik J.C., Gerlach A.L., Krieger T., Dresen A., Pfaff H., Lemmen C., et al. Integrated, cross-sectoral psycho-oncology (isPO): A new form of care for newly diagnosed cancer patients in Germany. BMJ Open. 2021. in preparation . [DOI] [PMC free article] [PubMed]
- 29.Craig P., Dieppe P., Macintyre S., Michie S., Nazareth I., Petticrew M. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieger T., Salm S., Cecon N., Pfaff H., Dresen A. Ergebnisbericht der Ersten Externen Formativen Evaluation des Projekts IsPO (FE 1.0): Forschungsbericht. IMVR; Cologne, Germany: 2020. Veröffentlichungsreihe des Instituts für Medizinsoziologie, Versorgungsforschung und Rehabilitationswissenschaft. [Google Scholar]
- 31.Commodore-Mensah Y., Himmelfarb C.R.D. Patient education strategies for hospitalized cardiovascular patients: A systematic review. J. Cardiovasc. Nurs. 2012;27:154–174. doi: 10.1097/JCN.0b013e318239f60f. [DOI] [PubMed] [Google Scholar]
- 32.Moore G.F., Audrey S., Barker M., Bond L., Bonell C., Hardeman W., Moore L., O’Cathain A., Tinati T., Wight D., et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015;350:h1258. doi: 10.1136/bmj.h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balthasar A. Fremd- und Selbstevaluation kombinieren: Der ‚Critical Friend Approach’ als Option. Z. Eval. 2012;11:173–198. [Google Scholar]
- 34.International Collaboration for Participatory Health Research . International Collaboration for Participatory Health Research; 2013. [(accessed on 15 April 2020)]. What Is Participatory Health Research?: Position Paper No. 1. Available online: http://www.icphr.org/uploads/2/0/3/9/20399575/ichpr_position_paper_1_defintion-_version_may_2013.pdf. [Google Scholar]
- 35.Cornwall A. Towards Participatory Practice: Participatory Rural Appraisal and the Participatory Process. In: de Koning K., Martin M., editors. Participatory Research in Health: Issues and Experiences. Vistaar Publications; New Delhi, India: 1996. [Google Scholar]
- 36.Cvitanovic C., Howden M., Colvin R.M., Norström A., Meadow A.M., Addison P.F.E. Maximising the benefits of participatory climate adaptation research by understanding and managing the associated challenges and risks. Environ. Sci. Policy. 2019;94:20–31. doi: 10.1016/j.envsci.2018.12.028. [DOI] [Google Scholar]
- 37.Baum F., MacDougall C., Smith D. Participatory action research. J. Epidemiol. Community Health. 2006;60:854–857. doi: 10.1136/jech.2004.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salm S., Mollenhauer J., Hornbach C., Cecon N., Dresen A., Houwaart S., Arning A., Göttel A., Schwickerath K., Pfaff H., et al. Participatory development and explorative validation of the User-friendly Patient Information Material Checklist (UPIM-Check) Int. J. Environ. Res. Public Health. 2021;18:8773. doi: 10.3390/ijerph18168773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke V., Braun V. Thematic analysis. J. Posit. Psychol. 2017;12:297–298. doi: 10.1080/17439760.2016.1262613. [DOI] [Google Scholar]
- 40.Tian C., Champlin S., Mackert M., Lazard A., Agrawal D. Readability, suitability, and health content assessment of web-based patient education materials on colorectal cancer screening. Gastrointest. Endosc. 2014;80:284–290.e2. doi: 10.1016/j.gie.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 41.O’Leary Z. The Essential Guide to Doing Research. SAGE Publications; London, UK: Thousand Oaks, CA, USA: New Delhi, India: 2004. [Google Scholar]
- 42.Nielsen L. Personas—User Focused Design. Springer; London, UK: 2019. [Google Scholar]
- 43.Krieger T., Salm S., Cecon N., Pfaff H., Dresen A. Ergebnisbericht der Zweiten Externen Formativen Evaluation des Projekts IsPO: Forschungsbericht 03-2021. IMVR; Cologne, Germany: 2021. Veröffentlichungsreihe des Instituts für Medizinsoziologie, Versorgungsforschung und Rehabilitationswissenschaft. [Google Scholar]
- 44.Houwaart S., Salm S., Krieger T. Was ist Partizipative Gesundheitsforschung und welche Chancen bietet sie für die organisierte Selbsthilfe? Selbsthilfegruppenjahrbuch. 2021;23:108–120. [Google Scholar]
- 45.Muhammad M., Wallerstein N., Sussman A.L., Avila M., Belone L., Duran B. Reflections on Researcher Identity and Power: The Impact of Positionality on Community Based Participatory Research (CBPR) Processes and Outcomes. Crit. Sociol. 2015;41:1045–1063. doi: 10.1177/0896920513516025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minkler M., Wallerstein N. Community-Based Participatory Research for Health: From Process to Outcomes. 2nd ed. Jossey-Bass; Hoboken, NJ, USA: 2008. [Google Scholar]
- 47.Tomisa G., Horváth A., Dombai B., Tamási L. Characteristics of an optimized patient information material for elderly patients with obstructive pulmonary diseases based on patients’ and experts’ assessment. Multidiscip. Respir. Med. 2017;12:6. doi: 10.1186/s40248-017-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfaff H., Schulte H. Der onkologische Patient der Zukunft. Onkologe. 2012;18:127–133. doi: 10.1007/s00761-011-2201-y. [DOI] [Google Scholar]
- 49.Ladegaard Grønkjær L., Berg K., Søndergaard R., Møller M. Assessment of Written Patient Information Pertaining to Cirrhosis and Its Complications: A Pilot Study. J. Patient Exp. 2019;7:499–506. doi: 10.1177/2374373519858025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Cathain A., Croot L., Duncan E., Rousseau N., Sworn K., Turner K.M., Yardley L., Hoddinott P. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open. 2019;9:e029954. doi: 10.1136/bmjopen-2019-029954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huynh A.K., Hamilton A.B., Farmer M.M., Bean-Mayberry B., Stirman S.W., Moin T., Finley E.P. A Pragmatic Approach to Guide Implementation Evaluation Research: Strategy Mapping for Complex Interventions. Front. Public Health. 2018;6:134. doi: 10.3389/fpubh.2018.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Härter M., Müller H., Dirmaier J., Donner-Banzhoff N., Bieber C., Eich W. Patient participation and shared decision making in Germany—History, agents and current transfer to practice. Z. Evid. Fortbild. Qual. Gesundh. 2011;105:263–270. doi: 10.1016/j.zefq.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Härter M., Dirmaier J., Scholl I., Donner-Banzhoff N., Dierks M.-L., Eich W., Müller H., Klemperer D., Koch K., Bieber C. The long way of implementing patient-centered care and shared decision making in Germany. Z. Evid. Fortbild. Qual. Gesundh. 2017;123–124:46–51. doi: 10.1016/j.zefq.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 54.McCarron T.L., Moffat K., Wilkinson G., Zelinsky S., Boyd J.M., White D., Hassay D., Lorenzetti D.L., Marlett N.J., Noseworthy T. Understanding patient engagement in health system decision-making: A co-designed scoping review. Syst. Rev. 2019;8:97. doi: 10.1186/s13643-019-0994-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedman D.B., Hoffman-Goetz L. A systematic review of readability and comprehension instruments used for print and web-based cancer information. Health Educ. Behav. 2006;33:352–373. doi: 10.1177/1090198105277329. [DOI] [PubMed] [Google Scholar]
- 56.Cook T., Boote J., Buckley N., Vougioukalou S., Wright M. Accessing participatory research impact and legacy: Developing the evidence base for participatory approaches in health research. Educ. Action Res. 2017;25:473–488. doi: 10.1080/09650792.2017.1326964. [DOI] [Google Scholar]
- 57.Hutchison C., McCreaddie M. The process of developing audiovisual patient information: Challenges and opportunities. J. Clin. Nurs. 2007;16:2047–2055. doi: 10.1111/j.1365-2702.2006.01758.x. [DOI] [PubMed] [Google Scholar]
- 58.Cargo M., Mercer S.L. The value and challenges of participatory research: Strengthening its practice. Annu. Rev. Public Health. 2008;29:325–350. doi: 10.1146/annurev.publhealth.29.091307.083824. [DOI] [PubMed] [Google Scholar]
- 59.Schaefer I., Kümpers S., Cook T. “Selten Gehörte” für partizipative Gesundheitsforschung gewinnen: Herausforderungen und Strategien. Bundesgesundheitsblatt Gesundh. Gesundh. 2021;64:163–170. doi: 10.1007/s00103-020-03269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dorant E., Krieger T. Contextual Exploration of a New Family Caregiver Support Concept for Geriatric Settings Using a Participatory Health Research Strategy. Int. J. Environ. Res. Public Health. 2017;14:1467. doi: 10.3390/ijerph14121467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright M.T., Hartung S., Bach M., Brandes S., Gebhardt B., Jordan S., Schaefer I., Wihofszky P. Impact and Lessons Learned from a National Consortium for Participatory Health Research: PartKommPlus-German Research Consortium for Healthy Communities (2015–2018) Biomed. Res. Int. 2018;2018:5184316. doi: 10.1155/2018/5184316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krieger T., Salm S., Mollenhauer J., Cecon N., Dresen A., Houwaart S., Schwickerath K., Göttel A., Arning A. UPIM-Check—User-friendly Patient Information Material Checklist. 2021. [(accessed on 16 December 2021)]. Available online: https://www.imvr.de/forschung/upim-check. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to ethical and legal restrictions, as participants of this study did not agree for their data to be shared publicly. Upon reasonable request, the data presented in this study are available from the corresponding author.