Abstract
Use of all-trans-retinoic acid (ATRA) as a differentiation agent has been limited to acute promyelocytic leukemia (APL) as non-APL leukemias are insensitive to ATRA. We recently demonstrated that the rexinoid, bexarotene, induces differentiation and therapeutic responses in patients with refractory AML. Rexinoids bind and activate retinoid X receptors (RXR), however rexinoids alone are incapable of activating retinoic acid receptor (RAR)/RXR complexes, suggesting that myeloid differentiation can occur independent of RAR. In this study we demonstrate that rexinoid differentiation of AML cells is RAR independent and requires the expression of PU.1. Because of the promiscuousness of RXR with other nuclear receptors, myeloid differentiation by bexarotene with other nuclear receptor ligands was explored. Bexarotene cooperated with ATRA to enhance differentiation in some AML cell lines, however the combination of bexarotene with the PPARγ agonist rosiglitazone did not. In contrast, bexarotene combined with Liver X Receptor (LXR) agonists T0901317 or GW3965 induced potent differentiation and cytotoxicity in AML cell lines and primary human AML cells, but not in normal progenitor cells. These results suggest that RXR/LXR regulated gene expression in normal cells is deregulated in AML cells and identifies a potential role for these agonists in differentiation therapy of non-APL leukemias.
Keywords: myeloid differentiation, acute myeloid leukemia, rexinoids, retinoid X receptors, liver X receptors
Introduction
The great majority of patients with acute promyelocytic leukemia (APL) respond to treatment with all-trans-retinoic acid (ATRA), resulting in a differentiation response of the leukemic clone and complete remission (1). The t(15;17) translocation associated with APL generates an oncogene expressing the fusion of the promyelocytic leukemia (PML) and retinoic acid receptor alpha (RARα) genes (2). When ATRA binds to the RARα region, the consequential degradation of the oncogenic protein leads to expression of genes that regulate normal hematopoiesis and were originally deregulated by the fusion protein (1). Differentiation of leukemic cells by ATRA along with chemotherapy is associated with a high clinical response rate (1). In contrast, therapy for other AML subtypes remains challenging; the cure rate for adults under age 60 is 38% and for older individuals is even lower (3). However, we previously demonstrated that the rexinoid bexarotene stimulates differentiation and clinical responses in AML patients not expressing the t(15;17) translocation (4). This finding led us to examine whether myeloid maturation is regulated independently of genes regulated by RAR.
Retinoid X receptors (RXR) are a common heterodimerization partner for many nuclear receptors and play a role in many physiological processes. The multiple isotypes of RXR (α,β,γ) and other nuclear receptors, as well as cell dependent receptor, coactivator, and corepressor expression allow for specificity in cell specific responses (5). Simplified models of RXR activation and gene regulation describe their function with other nuclear receptor (NR) partners as being permissive or non-permissive according to the ability of the RXR ligand to activate a RXR/NR complex (5). While RAR is thought to be a non-permissive partner to RXR, RXR ligands alone are capable of activating RXR/NR complexes of permissive partners such as Peroxisome Proliferator Activated Receptors (PPAR) and Liver X Receptors (LXR) (5). Curiously, while ATRA is clinically efficacious in APL treatment but not other AML subtypes, bexarotene and other synthetic rexinoids have been shown to induce differentiation in diverse AML cell lines and primary AML patient samples (6, 7). The use of bexarotene as a differentiation agent was not pursued until recently and is currently being tested as a single agent and in combination with demethylating agents for AML (clinical trials.gov). We therefore hypothesized that there are RAR-independent, RXR-dependent complexes that regulate genes involved in myeloid differentiation.
In this study, we determined that RXR specific ligands induce myeloid maturation in a variety of AML cell lines and primary AML cells independent of RAR activation. Although the master hematopoietic regulator PU.1 is known to be downregulated, mutated, or functionally impaired in some AML subtypes and is induced after ATRA treatment in APL, we observed a requirement for PU.1 expression for either RAR or RXR mediated differentiation, suggesting that a critical basal level of PU.1 is required (8-11). In order to identify the binding partner for RXR-induced myeloid differentiation, we tested the effects of bexarotene in combination with other NR ligands for myeloid maturation activity. Liver X receptor ligands consistently enhanced bexarotene effects in AML cell lines and combined induced cytotoxicity or differentiation in primary AML cells but not normal progenitor cells. In addition, loss of PU.1 reduced LXR/RXR induced differentiation, suggesting that PU.1 may be required for differentiation induction through this pathway. These results describe a previously unappreciated RAR independent RXR dependent mechanism of myeloid maturation and suggest that combinations of RXR and LXR agonists may be therapeutically useful in the treatment of AML.
Methods
Cell Culture
HL60, KG1a, MOLM14, NB4 and THP-1 cells were obtained from and grown as previously described (ATCC, Manassas, VA, USA and DSMZ, Braunschweig, DE). PUER cells (a gift from Dr. Celeste Simon (12)) were grown as previously described (13). Charcoal stripped FBS (Invitrogen, Life Technologies, Grand Island, NY, USA) was used in media when testing antagonists. Peripheral blood T cells and monocytes were obtained from the Human Immunology Core (UPENN). Bone marrow mononuclear cells (BMMNC), cord blood (CB) CD34+ progenitor cells and AML leukapheresis samples were obtained from the Stem Cell and Xenograft Core at the University of Pennsylvania Cancer Center (UPCC). Primary cells were obtained at the Hospital of the University of Pennsylvania after informed consent. Leukapheresis samples contained at least 90% blasts (flow cytometry and morphology) at the time of collection. Primary cells were cultured in EGM2 base media (Cambrex, East Rutherford, NJ USA) with 2% FCS, human IL-3 (5 ng/ml), IL-6 (10 ng/ml) and SCF (50 ng/ml) (R&D Systems, Minneapolis, MN, USA) immediately after thawing. Colony forming assays for normal progenitors and AML samples were grown in human enriched methylcellulose based media (R&D systems) for 14 days with indicated cell counts per plate in triplicate as described by the manufacturer. Colonies were quantified using a conventional light microscope.
Compounds
ATRA (Sigma-Aldrich, St Louis, MS, USA), bexarotene (LC labs, Woburn, MA, USA), T0901317 (Cayman Chemical, Ann Arbor, MI, USA), GW3965, rosiglitazone, ER50891 (Tocris Bioscience, Bristol, UK), SR1001 (Biovision, Milpitas, CA, USA) and R041-5253 (Enzo Life Sciences, Farmingdale, NY, USA) were prepared in DMSO,; actinomycin D and 4-hydroxytamoxifen (4-OHT) (Sigma-Aldrich) in ethanol; and cycloheximide (Sigma-Aldrich) in water at stock concentrations to obtain a final concentration of less than 0.1% solvent in media for experiments. LG100268 and LG101208 were prepared in DMSO (gifts from Ligand Pharmaceuticals, La Jolla, CA, USA).
Differentiation and apoptosis analysis of AML cell lines and AML primary cells
Mouse anti-human CD11b PE-Cy7, CD14 APC-Cy7, CD45 V450, CD15 V500, and CD2/3 FITC (BD Biosciences, San Jose, CA, USA) were used to measure differentiation induction of AML cell lines and primary cells. Rat anti-mouse CD11b-PE was used to measure differentiation of PUER cells. Annexin V-APC (Invitrogen, Life Technologies, Grand Island, NY, USA) and 7AAD (BD Biosciences) staining in 1X Annexin V Binding buffer (BD Biosciences) was used per manufacturers instructions. Acquisition of data was obtained on a FACS Canto digital flow cytometer using Diva software (BD Biosciences). Flow plots and expression of myeloid antigens was done following singlet exclusion (FSC-H/FSC-W) on viable (Annexin V-/7AAD-) cell populations using FlowJo software (V 9.6.2 and 10.0.6, TreeStar, Oregon, USA). Primary AML myeloid blasts were characterized for differentiation markers and cell viability. Lymphocytes were excluded by CD2/3 expression. Morphological analysis was determined by cell cytospin preparation onto slides at 600 rpm for 6 minutes followed by Hema3 staining (Fisher Scientific, Middletown, VA, USA). Images were taken at 50× magnification using a Nikon 80i microscope (Nikon Instruments, Melville, NY, USA) coupled to a Leica DFC320 CCD camera and acquired with Leica FireCam 1.4 software (Leica Microsystems, Buffalo Grove, IL, USA).
Quantitative PCR
RNA was isolated from cell pellets using RNeasy kits per manufacturers instructions (Qiagen, Valencia, CA, USA). cDNA was synthesized using iScript reverse transcriptase synthesis kit (Bio-Rad, Hercules, CA, USA). SYBR green primers, Taqman Probes and PCR conditions are listed in Tables S1, S2, S3 and Supplemental Methods. Fold changes in gene expression were calculated using the 2-ΔΔCT method (14).
Statistical analysis
Error bar values are taken from the mean +/- the standard deviation or standard error of the mean. Statistical significance for single treatment of compounds compared to vehicle treated control was calculated using an unpaired Student's t test. Mixed models of linear regression taking into account observations per experimental group were used to determine the significance of the interaction of two drugs compared to either drug alone (Stata Software, V 11.0, Stata Corp, College Station, TX, USA). For gene expression analysis, we used the z-score as the test statistic, and constructed those z statistics for contrasts (zc) using means and standard errors from output. P-values were two-tailed, calculated as p=2*Pr(z>|zc|) using the standard normal distribution. P values < 0.05 were considered significant.
Complete methods described in Supplemental Methods Tables and Legends.
Results
Bexarotene induces myeloid maturation of AML cell lines
To determine if bexarotene induces myeloid differentiation of AML cells in vitro, AML cell lines from different FAB subtypes were studied (Table S4). Bexarotene induced myeloid differentiation comparable to ATRA in most AML subtypes as demonstrated by the significant induction of myelo-monocytic markers CD11b and CD14 compared to vehicle treated control (Figures 1 A-B)(14-16). In addition, bexarotene and ATRA induced apoptosis (7AAD negative, Annexin V positive) and modification of cell growth differed in a cell-specific manner suggesting compounds have unique mechanisms of actions in different cell lines (Figure S1 A-B). Although cell lines varied in G2/M or G1/G0 arrest following treatment, cell lines that differentiated were reduced in BrdU incorporation over time compared to DMSO treated control (Figure S2), a feature associated with terminal differentiation. The appearance of multi-lobed nuclei associated with mature myeloid cells correlated with induction of myelomonocytic markers, supporting their use in analyzing bexarotene-induced differentiation in vitro (Figure 1C). The lack of a differentiation response of KG1a cells (as reported previously, (17)) compared with differences in apoptosis and cell cycle arrest among responsive cells demonstrate that bexarotene differentiation responses vary among AML cell lines from distinct FAB subtypes, and correlate with diverse responses seen in vivo(4).
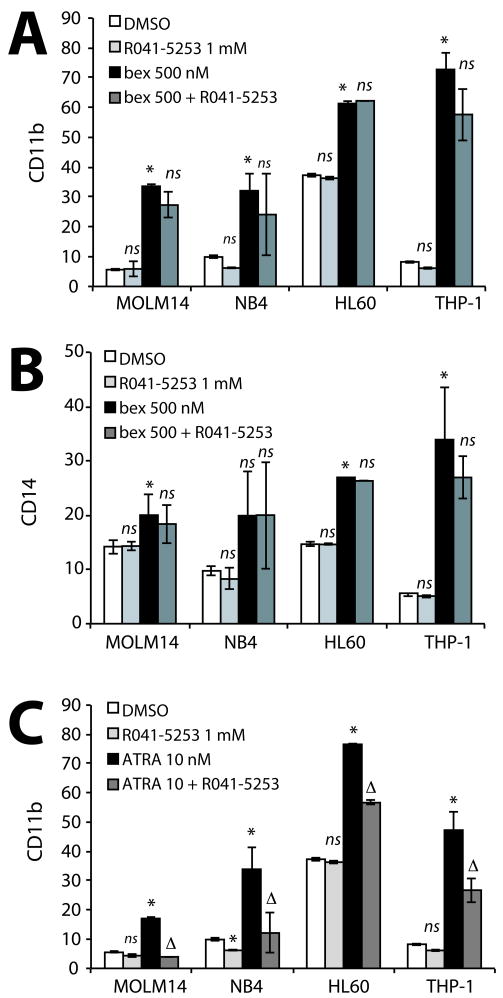
Figure 1. Bexarotene induced myeloid maturation of AML cell lines is RXR dependent.
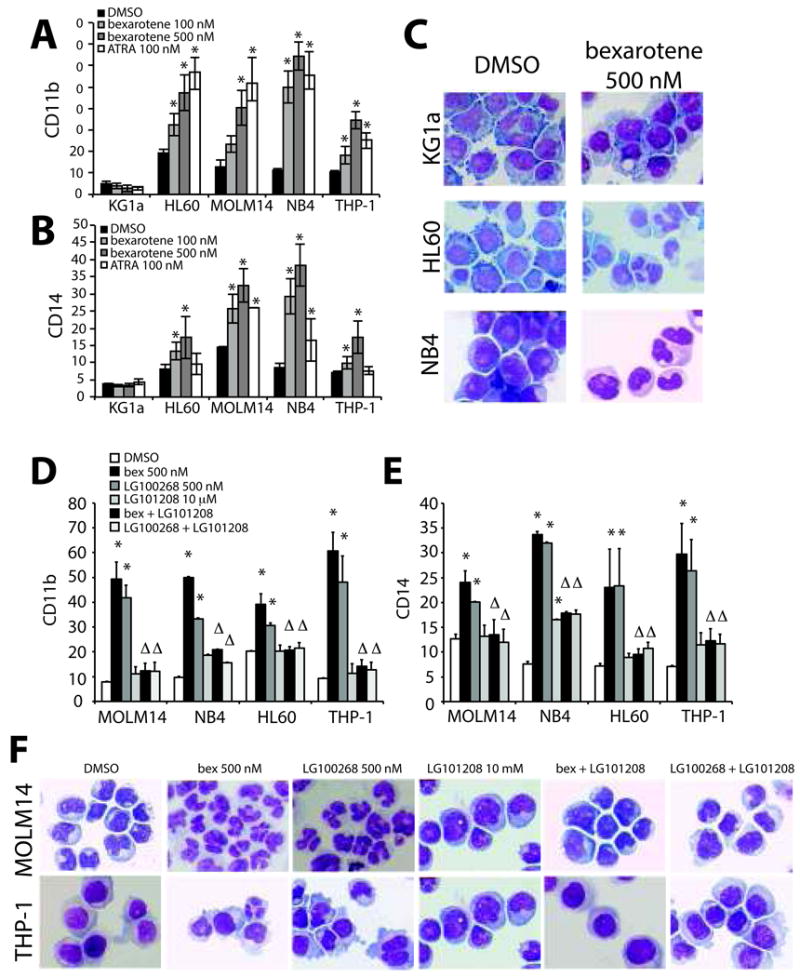
(A-C) AML cell lines treated with increasing concentrations of bexarotene or with ATRA for 4 days compared to vehicle (DMSO) treated cells. (A) CD11b and (B) CD14 expression was significantly higher than vehicle treated control alone (* P<.05). (C). Changes in cellular morphology associated with differentiated myeloid cells correlates with induction of differentiation markers. A representative example of AML cell lines treated with DMSO or bexarotene prepared by Hema3 staining are shown for comparison. (D-F) AML cell lines were treated with bexarotene, the pure RXR agonist LG100268, the pan-RXR antagonist LG101208 and the combination of agonist and antagonist for 4 days. (D) CD11b and (E) CD14 expression was significantly increased for the pure RXR agonists (* P<0.05). In the majority of samples treatment with LG101208 alone did not significantly increase expression of CD11b or CD14 in AML cells. However, the combination of LG101208 with either bexarotene or LG100268 significantly blocked CD11b and CD14 expression by either drug alone. Linear regression analysis demonstrates that the interaction of agonist (bexarotene or LG100268) with antagonist (LG101208) leads to significantly less CD11b and CD14 expression than with either drug alone (Δ P<.05). (F) Cellular morphology of AML cells by Hema3. Bar graphs represent the mean +/- standard error of the mean and are representative of at least 4-6 independent experiments.
Bexarotene requires RXR but not RAR activation to induce myeloid differentiation
In vitro binding assays have demonstrated a low binding affinity of bexarotene for RAR isoforms and a high affinity for RXR isoforms, however, transactivation assays have shown activity with RAR isoforms despite being more potent at activating RXR isoforms (18). To determine if bexarotene-induced differentiation is RXR specific, AML cell lines were cultured in charcoal-treated serum (to exclude hormones in serum that may enhance differentiation) with the pure RXR agonist LG100268. LG100268 is greater than 1000-fold more specific for RXR relative to RAR with an EC50 at least ten fold lower than pan RAR/RXR agonist 9-cis-retinoic acid (19). Although levels of CD11b and CD14 were reduced compared to previous experiments, suggesting serum enhances differentiation, LG100268 induced myeloid differentiation was similar to bexarotene (Figure 1 D-E). This is supported by the appearance of cells with mature myeloid features (Figure 1F). Co-treatment with the RXR antagonist LG101208 further demonstrated differentiation was specific to RXR activation (Figures 1 D-F). Of note, all experiments measuring antagonist function are cultured with media containing charcoal-treated serum to limit our interpretation of results to the ligands assayed.
As RXR is an obligate partner of RAR and previous studies have emphasized that RXR signaling requires the activation of RAR (6, 20), we next studied if bexarotene and ATRA cooperated to induce differentiation. Using physiologically attainable concentrations of ATRA or bexarotene (21, 22), a statistically significant combinatorial effect on differentiation was only seen in HL60 cells as previously reported (Figure S3 A-C) (6). Cytotoxic effects were again variable demonstrating that RAR and RXR activity is heterogeneous among AML cells (Figure S3D). However, co-treatment of AML cells with bexarotene and the pan RAR antagonist RO41-5253 (Figure 2 A-B and Figure S4) or the RARα antagonist ER50891 (Figure S5A) did not significantly reduce bexarotene-induced myeloid differentiation in AML cell lines tested. RAR antagonist activity of both compounds was confirmed by reduced ATRA-mediated differentiation (Figure 2C). Together these experiments demonstrate that bexarotene induces differentiation in AML cell lines through a RAR-independent mechanism and suggests that RAR activation is not required for the induction of myeloid differentiation by rexinoids.
Figure 2. Rexinoid mediated Myeloid Maturation does not require activation of RAR.
In contrast to previous reports suggesting RXR requires RAR for differentiation induction, RAR independent activation by RXR was confirmed using the pan-RAR antagonist R041-5253, which had no effect on (A) CD11b or (B) CD14 induction by bexarotene compared to either drug alone. R041-5253 antagonist activity was confirmed by significantly reduced ATRA induced differentiation of AML cells when compared to either drug alone (Figure 2C). Bar graphs represent the mean +/- standard error of the mean and are representative of at least 3 independent experiments. A positive significant interaction between combination drug treatment is demonstrated by (Δ P<.05) while a negative interaction is shown by (Δ- P<0.05). Significance of single treatment compared to DMSO (* P<0.5), ns = not significant.
Bexarotene induced gene expression is unique from ATRA, however, both require PU.1 for myeloid differentiation induction
The data above suggest that discrete signaling pathways in myeloid cells through which RXR agonists enhance differentiation exist, one involving RAR activation and another involving an alternate RAR-independent pathway. To study these mechanisms on a molecular level, we examined the ATRA or bexarotene mediated induction of the endogenous expression of RARβ and C/EBPε, two genes whose expression has been reported to be regulated by an RXR/RAR complex via a retinoic response element in their promoters (23). As previously reported, ATRA significantly induced expression of RARβ and C/EBPε in HL60 cells in a dose dependent manner (Figure 3 A-B). Consistent with the absence of rexinoid activity on RXR/RAR complexes, bexarotene did not induce RARβ expression. In contrast, C/EBPε expression was modestly but significantly induced within 6 hours of activation by bexarotene or ATRA (Figure 3B). This data initially suggested that the myeloid transcription factor C/EBPε could be a direct target and modulator of differentiation induction by ATRA or bexarotene. However, inhibition of protein synthesis or RNA translation preceding ATRA or bexarotene treatment dramatically reduced the expression of C/EBPε demonstrating that it is not a direct target of RAR or RXR activation (Figure 3C). Taken together, these results demonstrate that rexinoids can regulate genes also regulated by retinoids (C/EBPε) but also have distinct differences in regulating others.
Figure 3. Bexarotene induced gene expression is unique from ATRA however both require PU.1 for myeloid differentiation induction.
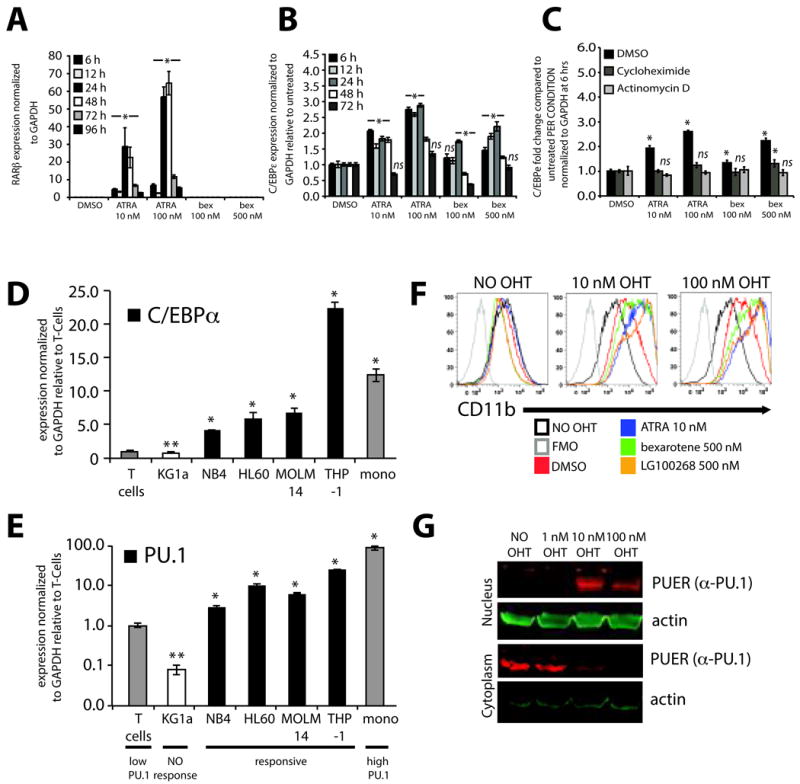
(A) Endogenous expression and induction of the canonical RARβ gene is activated by ATRA in a dose dependent manner but not by bexarotene. Data shows expression of RARβ normalized to GAPDH following treatment with ATRA or bexarotene as measured by PCR using Taqman Gene expression system for RARβ. RARβ expression is reported relative to a standard curve for RARβ expression of NB4 cells treated with 1 μM ATRA as HL-60 cells do not express RARβ. (B) Endogenous expression of C/EBPε using SYBR GREEN based real-time PCR is significantly induced by ATRA or bexarotene in a dose dependent manner compared to vehicle treated control (DMSO). (C) Inhibition of protein (cycloheximide, 200 μg/ml) and RNA synthesis (actinomycin D, 5 μg/ml) 1 hour before treatment with ATRA or bexarotene demonstrate that C/EBPε is not a direct target of RXR or RAR containing complexes. Significance of single treatment compared to DMSO or DMSO vs cycloheximide or actinomycin D (* P<0.5), ns = not significant. (D) Basal levels of C/EBPα or (E) PU.1 using SYBR GREEN based real-time PCR in rexinoid unresponsive (white bar) and responsive (black bars) AML cells normalized to endogenous PU.1 in normal T cells (gray bar) and compared to PU.1 in monocytes (mono, gray bar). C/EBPα and PU.1 expression was significantly less in unresponsive AML cells compared to T cells (** P<.05) while expression was significantly greater in all responsive AML cell lines (* P<.05). Bar graphs represent the mean +/- standard error of the mean of triplicates per condition and are representative of at least 3 independent experiments. (F). PUER cells were treated for 4 days with increasing OHT concentrations and analyzed for changes in CD11b expression with the addition of RXR and RAR agonists. CD11b expression of PUER cells not treated with OHT (first panel) following ATRA (blue histogram), bexarotene (green histogram) or LG100268 (orange histogram) did not increase suggesting that nuclear PU.1 expression was necessary for RAR and RXR mediated differentiation. However, co-treatment of PUER cells with 10 nM or 100 nM OHT differentiated in response to No OHT (black histogram, in 10 nM and 100 nM OHT overlayed histogram plots, respectively). Differentiation was further enhanced by the addition of ATRA, bexarotene, and LG100268 (second and third panels) demonstrating that expression of nuclear PU.1 is required for differentiation induction by RAR and RXR agonists. Fluorescence minus 1 control (FMO, gray histogram) of CD11b was included to demonstrate basal CD11b levels (black histogram, No OHT) above non-specific fluorescence in all three panels. Bar graphs represent the mean +/- standard deviation and all panels (A-F) are representative of at least 3 independent experiments. (G) Nuclear and cytoplasmic fractionated PUER cells at 18 hrs after OHT treatment demonstrate PUER translocation from the cytoplasm to the nucleus when higher concentrations of OHT (10 nM and 100 nM) but not lower concentrations of OHT (1 nM) are used. PUER was detected by a rabbit polyclonal PU.1 antibody followed by α-rabbit secondary IR680DL dye. beta-actin was detected using a mouse mono-clonal antibody to beta-actin followed by α-mouse antibody conjugated to IR800. Images were acquired using the Odyssey Infrared Imaging System.
The data above suggests that C/EBPε may be an important mediator of bexarotene and ATRA induced myeloid differentiation. Because C/EBPε is essential for terminal differentiation of committed granulocytic progenitors (24), we analyzed the basal expression of C/EBPα and PU.1, transcription factors known to be critical for myeloid development and regulating the expression of C/EBPε (25, 26). Expression of either gene was normalized to their expression in T cells, in which they are silenced prior to (C/EBPα) or downregulated (PU.1) after T cell commitment (27) and compared to their abundant expression in monocytes (28). Both C/EBPα and PU.1 gene expression was significantly lower in unresponsive KG1a cells than T cells, however expression was significantly higher in all responsive AML cell lines (Figure 3D-E). This result suggested to us that PU.1 and or C/EBPα may be required for bexarotene or ATRA induced differentiation. As C/EBPα is known to regulate the expression of PU.1, we chose to study the role of PU.1 in retinoid or rexinoid induced differentiation. To address this question, we utilized an IL-3 dependent PU.1-/- fetal liver progenitor cell line transduced with a fusion protein of PU.1 with the estrogen receptor binding domain (PUER) whose nuclear localization is regulated by 4-hydroxy-tamoxifen (OHT) (13). CD11b expression following ATRA, bexarotene or LG100268 treatment was unchanged by no or low (1 nM) OHT concentrations (Figure 3F and S6A). Lack of differentiation under these conditions correlated with low levels of nuclear PUER protein available to induce differentiation (Figure 3G and S6B). However, co-treatment of PUER cells with higher doses of OHT (10 nM or 100 nM) with ATRA, bexarotene or LG100268 enhanced differentiation relative to that seen by 10nM or 100 nM OHT alone (Figure 3F, agonists listed in order of magnitude of activity). Differentiation data correlated with an increase of PUER in the nucleus (Figure 3G and S6B). Together, these data suggest that RAR and RXR require a threshold of expression of PU.1 to induce differentiation.
To determine the role of PU.1 expression on rexinoid-induced differentiation in human AML cells, we retrovirally transduced PU.1 shRNA expressed in the context of a mir30 backbone into THP-1 cells. Expression of two previously described PU.1 shRNA sequences (28) demonstrated reduced mRNA and protein levels in sorted GFP+ THP-1 cells (Figure 4A). Differentiation induction by ATRA was significantly reduced in both PU.1 clones compared to control vector, suggesting that efficient differentiation induction requires adequate levels of PU.1 (Figure 4B). Differentiation was further reduced following use of bexarotene and LG100268 (Figure 4B, 2nd and 3rd rows), suggesting that PU.1 may have a more significant role in RXR-mediated differentiation of AML cells. Together these data suggest that threshold levels of PU.1 are necessary for efficient differentiation of PUER and AML cells through RAR and RXR pathways.
Figure 4. Suppression of PU.1 expression by shRNA results in reduced retinoid and rexinoid induced differentiation of THP-1 cells.
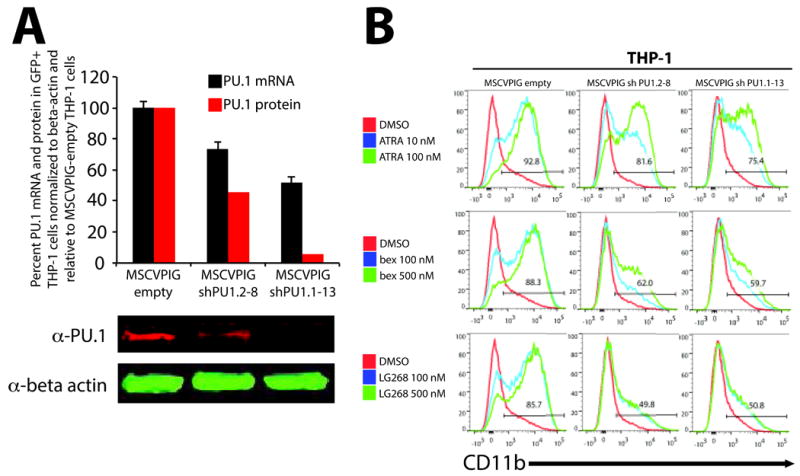
(A) THP-1 cells were transduced with VSV-G pseudotyped MSCVPIG (MSCV-puro-ires-GFP) retroviruses expressing PU.1 shRNA in the context of the mir30 microRNA backbone to enhance shRNA efficiency (MSCVPIG shPU1.2-8 and MSCVPIG shPU1.1-13) and compared to transduced cells with empty vector (MSCVPIG empty). Four days after infection, transduced cells were sorted for GFP expression and analyzed for PU.1 mRNA expression (Taqman Gene Assays for PU.1 and beta-actin) and PU.1 protein expression (PU.1 and beta-actin antibodies) normalized to beta-actin. (A) Suppression of PU.1 by MSCVPIG shPU1.2-8 and MSCVPIG shPU1.1-13 significantly reduced mRNA and protein levels when compared to MSCVPIG-empty transduced THP-1 cells. (B) Viable and GFP+ MSCVPIG empty, MSCVPIG shPU1.2-8, and MSCVPIG shPU1.1-13 transduced THP-1 cells were analyzed for CD11b expression 4 days after treatment with ATRA, bexarotene, and LG100268. CD11b induction by ATRA, bexarotene, and LG100268 was robust in MSCVPIG empty THP-1 cells consistent with previous data using parental THP-1 cells. However, CD11b induction by ATRA in MSCVPIG shPU.1.2-8 and MSCVPIG shPU1.1-13 was reduced compared to MSCVPIG-empty, suggesting a role in mediating efficient differentiation by RAR. Suppression of PU.1 had a greater effect on bexarotene and LG100268 CD11b induction than ATRA, suggesting that RXR mediated differentiation is more sensitive to PU.1 levels than ATRA and may play a more prominent role in RXR mediated differentiation of AML cells than RAR.
Bexarotene cooperates with LXR but not PPARγ agonists to induce myeloid differentiation
Having shown that rexinoids can induce myeloid differentiation independent of RAR, we explored the role of permissive nuclear receptors PPAR and LXR in cooperating with rexinoids to induce myeloid differentiation. Previous studies have shown that co-stimulation of PPARγ/RXR heterodimers enhances rexinoid-induced differentiation of leukemic cells(29). While in some cell lines (MOLM14 and NB4) there was a modest effect on CD11b and CD14 induction by rosiglitazone alone (Figure 5 A-B), the combination with bexarotene resulted in no or negative induction of CD11b and CD14, suggesting that PPARγ/RXR complex activation is not involved in rexinoid-mediated differentiation. This result is in contrast to published reports and suggests that PPARγ activation does not consistently enhance bexarotene-induced differentiation.
Figure 5. Rexinoids cooperate with LXR but not PPARγ agonists to induce myeloid differentiation.
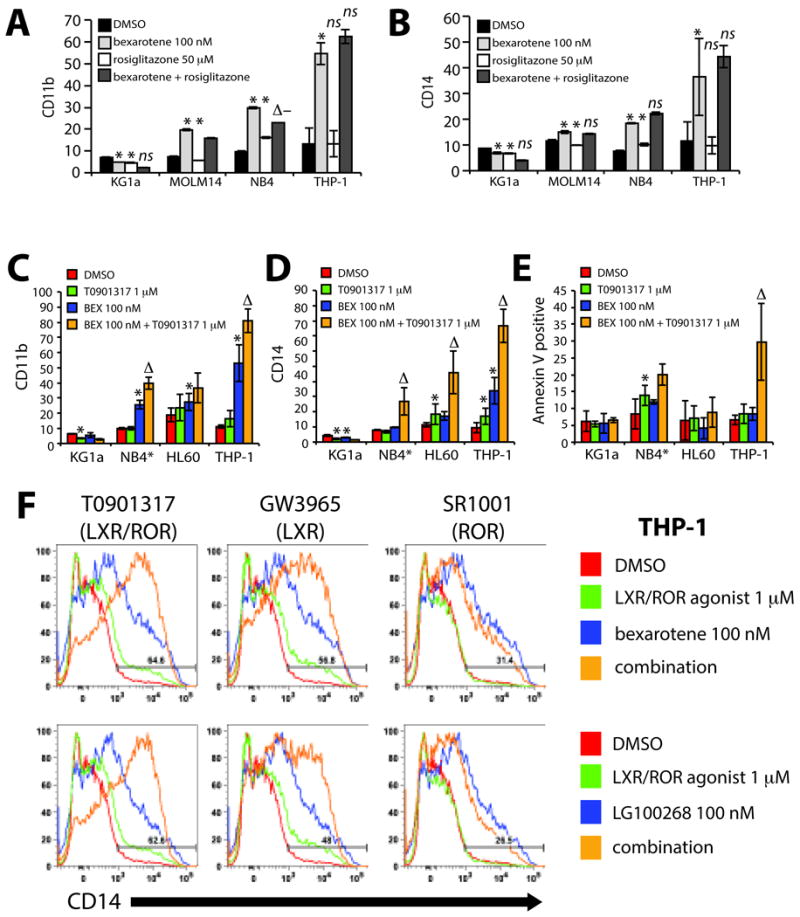
As measured by (A) CD11b and (B) CD14, bexarotene does not cooperate with the PPARγ agonist rosiglitazone in all AML cell lines tested compared to either drug alone. Bexarotene cooperates with the LXR α/β agonist T0901317 in differentiation responsive cell lines as measured by (C) CD11b and (D) CD14 induction compared to either drug alone. (D) Cytotoxicity measured by the percentage of Annexin V+ 7AAD+/- cells is increased by the combination of bexarotene with T0901317 in 2 of the 4 cell lines tested (NB4 and THP-1). CD11b, CD14 and Annexin V results for NB4 cells (*) are shown at Day 2 because of the > 80% increase in necrotic cells by Day 4. (F) LXR/RXR activation specificity is supported by differentiation induction of THP-1 cells using the pure RXR agonist LG100268 combined with T0901317 or the pure LXR α/β agonist GW3965. Off target effects of ROR α/γ suppression by T0901317 do not contribute the combination effects seen with rexinoids as demonstrated by the co-incubation of rexinoids with SR1001, a compound that suppresses ROR α/γ activity. Bar graphs represent the mean +/- standard deviation and all panels (A-F) and are representative of at least 3 independent experiments. Linear regression analysis demonstrate that the interaction of agonist (bexarotene or LG100268) with LXR agonists (T0901317, GW3965) is significantly greater than with either drug alone (ΔP<.05). A negative significant interaction between combination drug treatment with the ROR α/γ inverse agonist (SR1001) is demonstrated by (Δ− P<.05). The significance of a single treatment compared to DMSO (* P<0.5), ns = not significant.
As LXR α/β increases during monocyte to macrophage differentiation and LXRα transcript is overexpressed in AML cells, we explored its role in mediating myeloid differentiation(30, 31). Treatment of differentiation responsive AML cell lines with the LXR α/β agonist T0901317 and bexarotene resulted in a significant increase in differentiation (except CD11b induction of HL60) compared to either drug alone (Figure 5 C-D). Cytotoxicity significantly increased in THP-1 and NB4 cells, demonstrating the diverse response between cell lines. (Figure 5E). Note that NB4 induced differentiation and cytotoxicity on Day 2 is shown due to the greater than 80% cell death on Day 4 (data not shown). Although T0901317 is a potent LXR α/β agonist it also acts as an inverse agonist of retinoic acid related orphan receptors RORα and RORγ by suppressing their transcriptional activity (32). To exclude this off-target effect, we combined bexarotene with the pure LXR α/β agonist GW3965 or SR1001, a compound devoid of LXR α/β activity but retaining ROR α/γ activity (33). Bexarotene together with GW3965 induced differentiation similar to T0901317 while no combined effects were seen with SR1001, demonstrating that differentiation induction is specific to activation of an LXR/RXR complex (Figure 5F). Similar results were seen with LG100268 and T0901317 or GW3965 excluding the possible activation of RAR by bexarotene (Figure 5F), which was confirmed by lack of RARbeta induction by LXR and RXR agonist combinations (Figure S7A). Cytotoxicity elicited from the combination of LXR/RXR stimulation was also specific to LXR/RXR activation (Figure S7B).
To further confirm the role of a proposed activated LXR/RXR complex in inducing myeloid differentiation, we demonstrate that the RXR antagonist LG101208 completely inhibited CD11b/CD14 and cytotoxicity induced by co-treatment with LG100268 and T0901317 (Figure 6 A-B). The gradual increase in CD11b and CD14 expression at Day 2 without cytotoxicity (Figure 6 A-B) demonstrates that differentiation induction precedes cell death. Similar results were seen with bexarotene and T0901317 (Figure S8 A-B). As predicted, adequate levels of PU.1 expression were necessary for efficient LXR/RXR mediated differentiation induction as low levels PU.1 had a significant inhibitory effect on differentiation by bexarotene or LG100268 alone (Figure 6C and S8C). Importantly, LXR agonists alone had minimal effects on differentiation induction and cytotoxicity. These results suggest that LXR/RXR complexes regulating myeloid differentiation are distinct from permissive LXR/RXR complexes described in other cell systems and only the combined use of LXR and RXR agonists can induce potent myeloid differentiation and cytotoxicity in AML cell lines.
Figure 6. Differentiation induction and apoptosis by combined LXR/RXR activation in AML cells is specific to activation of RXR and requires threshold levels of PU.1.
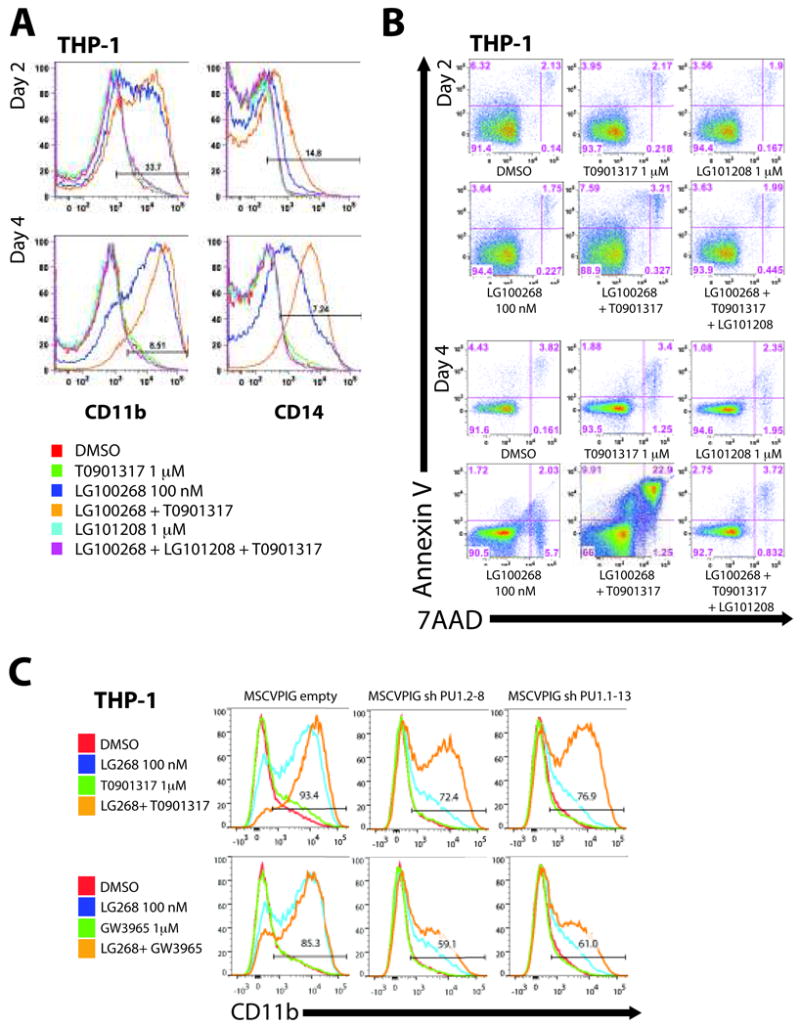
A time course of LXR/RXR activation by the pure RXR agonist LG100268 (blue histogram) and T0901317 (green histogram) demonstrates a gradual induction of (A) differentiation markers CD11b and CD14 followed by an increase in (B) apoptotic cells by Day 4, suggestive of terminal differentiation pathways following maturation and not drug induced cytotoxicity. Use of the pan RXR antagonist LG101208 (alone - blue histogram) combined with LG100268 and T0901317 (purple histogram) completely inhibited CD11b and CD14 expression and apoptosis induced by the combination of LG100268 and T0901317 (orange histogram) compared to DMSO control (red histogram) demonstrating that potent myeloid differentiation induction and apoptosis is specific to RXR activation. (C) GFP+ THP-1 PU.1 shRNA cells were treated for 3 days with combinations of LG100268 and T0901317 or GW3965 and assayed for CD11b expression to determine if PU.1 was also important in LXR/RXR mediated differentiation. LG100268 induced differentiation of THP-1 cells was significantly reduced with PU.1 shRNA suppression (MSCVPIG shPU1.2-8 and MSCVPIG shPU1.1-13 compared to MSCVPIG empty). As expected CD11b induction by the combination of LG100268 with T0901317 was reduced but to a lesser extent than seen with the combination of LG100268 and GW3965. As GW3965 is a pure LXR α/β agonist, the significant reduction of CD11b induction by suppressing PU.1 in THP-1 cells suggests a more prominent role for PU.1 in LXR/RXR mediated differentiation. All panels (A-B) are representative of at least 3 independent experiments.
Differences in activation of LXR/RXR complexes on proliferation, growth and differentiation of normal progenitors vs primary AML cells
To determine if the activation of an LXR/RXR differentiation program was unique to leukemic cells or was conserved in normal progenitors, we treated normal bone marrow mononuclear cells (BMMNC) or cord blood (CB) derived CD34+ cells with bexarotene, T0901317 or the combination and quantified lineage development and proliferation using methylcellulose colony forming assays. The overall number of colonies and CFU-G, CFU-M, BFU-E and CFU-GM colony types significantly increased with bexarotene treatment (Figure 7A). T0901317 had no significant effect on BMMNC while in CB total colony number and CFU-G colonies decreased significantly. T0901317 with bexarotene offset increases in proliferation and differentiation by bexarotene alone, resulting in similar colony numbers and colony types compared to DMSO. Importantly, this suggested that the combination would not affect proliferation and development of normal bone marrow progenitors.
Figure 7. Effects on colony formation, proliferation, cell growth, and differentiation following activation of LXR/RXR in normal progenitors and primary AML cells.
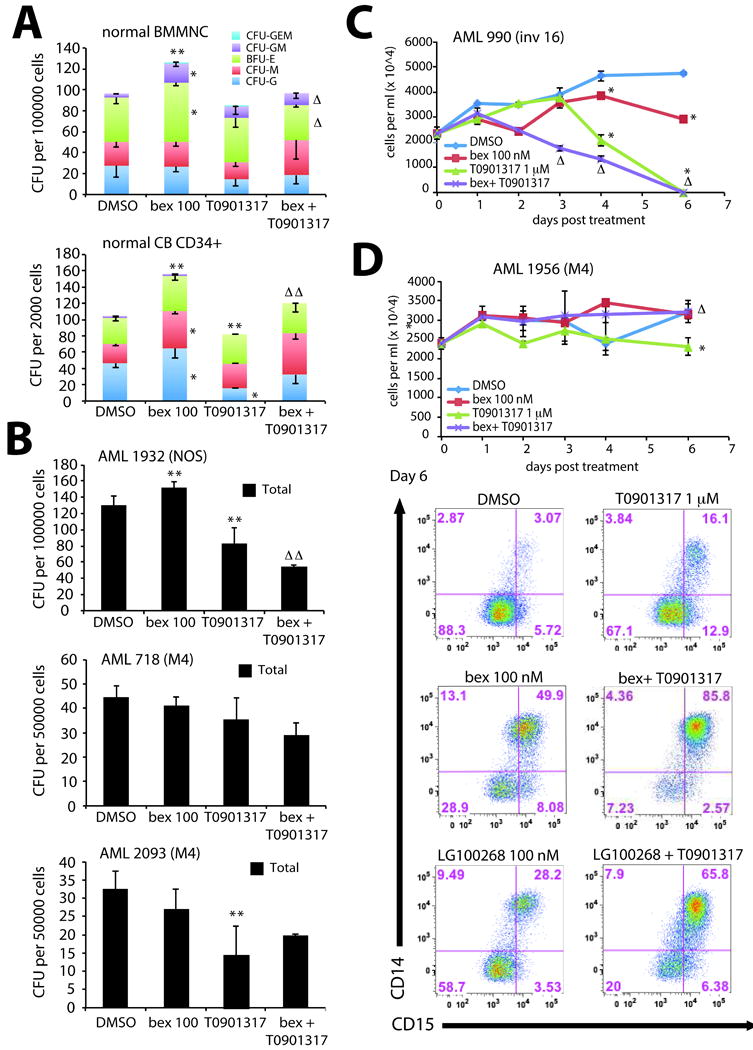
Colony formation of (A) normal bone marrow mononuclear cells (BMMNC) or cord blood (CB) derived CD34+ cells was significantly increased in total colony number (** P<.05) and CFU-G, CFU-M, BFU-E, and CFU-GM colony types (* P<.05) with bexarotene treatment. While treatment with T0901317 significantly reduced total colony number and CFU-G in CB CD34+ cells but had no effects on BMMNC, the combination of bexarotene and T0901317 offset both changes in total colony number (ΔΔ P<.05) and colony types (Δ P<.05) compared to either drug alone. (B) AML sample 1932 (NOS – not otherwise specified), AML 718 (FAB M4), and AML 2093 (FAB M4) following single or combined treatment with bexarotene and T0901317 demonstrates unique differences in colony formation following 14 day incubation. The combination treatment with bexarotene and T0901317 on AML 1932 was significantly reduced than either drug alone (ΔΔ P<.05) but was not statistically in samples AML 718 and AML 2093. (C) A 6 day time course of cell growth of AML sample 990 (inversion 16) by trypan blue exclusion demonstrates significant reduction in growth by bexarotene or T0901317 alone by Day 4 (* P<.05), however combination treatment dramatically reduced growth by Day 3 compared to either drug alone (Δ P<.05) demonstrating a rapid induction of cytotoxicity in this sample. (D) Although growth of AML sample 1956 (FAB M4) was significantly reduced by T0901317 by Day 6 (* P<.05), growth inhibition was offset by the combination with bexarotene (Δ P<.05) demonstrating the heterogeneity in cytotoxic response among AML samples. However, analysis of differentiation by expression of CD14 and CD15 on myeloid blasts on Day 6 demonstrates a more than additive increase with the combined treatment of bexarotene and T0901317 compared to either drug alone. Percentages of cells expressing CD14 and CD15 and combinations of both are indicated in the flow panels. All panels (A-D) are representative of at least 3 independent experiments.
Finally, we tested the effects of LXR and RXR activation on primary AML leukemia samples (Table S5) and compared their colony formation to normal progenitors. Although colonies from sample AML 1932 increased after bexarotene treatment and T0901317 decreased colony formation (similar to normal CB progenitors), the combination significantly reduced total colonies compared to DMSO, bexarotene or T0901317 single treated cultures (Figure 7B). In contrast, differences in colony numbers formed by AML samples 718 and 2093 following combined treatment was not statistically significant. Therefore, similar to results obtained for AML cell lines, cytotoxicity of bexarotene and T0901317 on primary AML samples varied among leukemic samples as assessed by Day 14 colony forming assays.
Because colony formation assays are limited to studying the proliferation and clonal growth of AML cells, we also assessed LXR/RXR differentiation effects of primary AML cells in short-term liquid culture assays. Such cultures allow the demonstration of short-term effects of ligand combinations on cell growth and expression of mature myeloid surface antigens. Similar to results seen with AML 1932, bexarotene and T0901317 was more potent at inhibiting growth of AML 990 than single treatment by bexarotene or T0901317 at day 3 (Figure 7C). By day 4 either compound significantly reduced growth compared to DMSO treated control. In contrast, treatment with bexarotene and T0901317 had no significant cytotoxic effects on AML 1956 compared to DMSO control (Figure 7D). Although T0901317 significantly reduced cell growth by day 6, the combination with bexarotene offset cytotoxic effects similar to that seen in CB CD34+ progenitors (Figure 7D). In contrast, expression of CD11b/CD15 and CD14/CD15 was significantly increased in bexarotene or T0901317 treated cultures (Figure 7D and S9) with a more than additive increase in the combined treatment. Similar results were also seen with LG100268 and T0901317 (Figure 7D and S9). Overall these results demonstrate that combined rexinoid and LXR agonist activation of LXR/RXR complexes results in AML specific cytotoxic effects and differentiation of leukemic cells.
Discussion
Previous studies have focused on understanding the mechanism of RXR/RAR signaling in hematopoiesis because of the differentiation of APL cells in response to ATRA (34). However, recent studies have more clearly defined the role of ATRA in APL treatment and have demonstrated that differentiation alone is insufficient to eliminate APL leukemia initiating cells and may explain the failure to successfully treat other myeloid malignancies (1, 35). Hematopoietic specific knockdowns of RAR or RXR isoforms result in modest hematopoietic deficiencies, implying a modest role of these nuclear receptors in normal hematopoiesis (36, 37). However, clinical studies have demonstrated that bexarotene induces differentiation responses in non-APL leukemias (4). We demonstrate here that leukemic cell lines and primary AML cells have an RAR independent, RXR dependent mechanism of myeloid differentiation regulation. This pathway is dependent on PU.1 expression and likely involves an RXR/LXR heterodimeric complex. Together, these data suggest that RXR activation may play a central role in regulating myeloid differentiation and self-renewal pathways independent of RAR activation (4).
Due to the heterogeneity of AML, rexinoid-induced differentiation and cytotoxicity varied among AML cells and primary AML samples. Although differentiation was characterized by morphological changes associated with mature myeloid cells or expression or CD11b and CD14, it is important to note that AML cells are not normal cells and therefore these observations represent aberrant myeloid differentiation induction by activating pathways that may be regulated in normal cells. Molecularly, rexinoids and retinoids demonstrate unique and common induction of genes, as shown by the activation of RARβ by ATRA and not bexarotene, but their common induction of the myeloid transcription factor C/EBPε. Despite previous reports demonstrating that the C/EBPε promoter encodes a functional RARE, it was not a direct target of RAR or RXR in our studies (Figure 3C). These results suggest in vitro studies may not represent physiologically relevant gene regulation and emphasizes the importance of characterizing pathways within the cell of interest. Although induction of C/EBPε may play a role in orchestrating a myeloid differentiation program, direct targets of ATRA or bexarotene in mediating myeloid differentiation are yet to be determined.
As predicted, graded expression of PU.1 correlated with a RAR- or RXR-mediated differentiation response, while no or low levels or PU.1 abrogated this effect, indicating that threshold levels of PU.1 are required for RAR and RXR mediated differentiation. In the reciprocal experiment, constitutive knockdown of PU.1 in THP-1 cells reduced ATRA differentiation induction and more significantly differentiation by bexarotene and LG100268, suggesting that RXR mediated differentiation in AML cells is more sensitive to PU.1 levels (Figure 4B). These data are novel in that previous studies have reported the restoration of PU.1 to promote differentiation induction by ATRA and did not address its role in RXR mediated myeloid differentiation (8, 38).
Co-activation of LXR/RXR complexes (and not activation of PPARγ/RXR or suppression of ROR α/γ) induced a potent differentiation response in multiple AML cell lines and primary AML cells (Figure 5-7). Of note, studies demonstrating PPARγ responses with rexinoids pretreated cells with PMA, which was later found to increase PPARγ levels (39-41). This suggests that altered nuclear receptor expression in AML cells predetermines sensitivity to differentiation by receptor specific agonists and previous experiments may not be physiologically relevant. PU.1 may also contribute to LXR/RXR mediated differentiation, as the combination with the pure LXR α/β agonist GW3965 with LG100268 (Figure 6) or bexarotene (Figure S8) significantly reduced CD11b induction in THP-1 cells suppressed for PU.1 expression. Differentiation was sometimes accompanied by cytotoxicity that was specific to activation of LXR/RXR, however this was not seen in normal hematopoietic progenitors suggesting that LXR/RXR complexes may be uniquely deregulated in leukemic cells (Figure 7). Recent studies have demonstrated elevated levels of LXRα and the aberrant expression of LXR regulated genes such as ABCA1 and ABCG1 in primary AML cells compared to normal bone marrow progenitors (31). Interestingly, knockout models of ABCA1 and ABCG1 display a transplantable myeloproliferative disorder and a dramatic expansion of stem and progenitor cell populations in the bone marrow (42). Together these data suggest that deregulation of LXR/RXR regulated cholesterol homeostasis may be associated with increased proliferation and self-renewal properties of AML.
Considering our studies in the context of recent results suggests a novel hypothesis about the role of cholesterol homeostasis and RXR/LXR activation in myelomonocytic differentiation and its deregulation in AML. We hypothesize that LXR/RXR activation can enhance myelomonocytic differentiation, but the activation of LXR and RXR containing complexes must be tightly regulated. Similar to results seen in normal mouse BM cells, treatment of CB CD34+ cells with T0901317 alone was sufficient to reduce proliferation and colony formation (Figure 7A) (42). In contrast, bexarotene-induced proliferation and differentiation of normal progenitors was offset by T0901317 (Figure 7A). This suggests that discrete complexes may exist whose activation may be restricted by a limited cellular pool of RXR in normal progenitors. In contrast, as LXRα expression has been reported to be elevated in leukemic cells, we can speculate there may be an imbalance and deregulation of LXR containing complexes (perhaps through an excess of co-repressor containing complexes) that regulate proliferation and differentiation. Further studies in normal and leukemic myeloid cells defining LXR/RXR regulated gene targets will more clearly define how these complexes are deregulated in AML.
Supplementary Material
Acknowledgments
The authors thank the Stem Cell and Xenograft Core, the Human Immunology Core, the Flow Cytometry Core, the Bioinformatics Core at the Perelman School of Medicine, UPENN Cancer Center, and the Carroll lab for advice and assistance. This study was supported by K01-CA-129151 (P.V.S.), 1R01CA149566 (M.C.), the Perelman School of Medicine at the University of Pennsylvania and the US Veterans Administration (M.C.)
Footnotes
Conflict of Interest: M.C. receives research support from Glaxo Smith Kline, Sanofi Aventis Corporation and Tetralogic Pharmaceuticals.
Supplementary information is available at Leukemia's website.
References
- 1.Ablain J, de The H. Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood. 2011 Jun 2;117(22):5795–5802. doi: 10.1182/blood-2011-02-329367. [DOI] [PubMed] [Google Scholar]
- 2.Alcalay M, Zangrilli D, Pandolfi PP, Longo L, Mencarelli A, angelo G, et al. Translocation Breakpoint of Acute Promyelocytic Leukemia Lies Within the Retinoic Acid Receptor {alpha} Locus. PNAS %R 101073/pnas8851977. 1991 March 1;88(5):1977–1981. doi: 10.1073/pnas.88.5.1977. 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juliusson G, Karlsson K, Lazarevic V, Wahlin A, Brune M, Antunovic P, et al. Hematopoietic stem cell transplantation rates and long-term survival in acute myeloid and lymphoblastic leukemia: real-world population-based data from the Swedish Acute Leukemia Registry 1997-2006. Cancer. 2011 Sep 15;117(18):4238–4246. doi: 10.1002/cncr.26033. [DOI] [PubMed] [Google Scholar]
- 4.Tsai DE, Luger SM, Andreadis C, Vogl DT, Kemner A, Potuzak M, et al. A phase I study of bexarotene, a retinoic X receptor agonist, in non-M3 acute myeloid leukemia. Clin Cancer Res. 2008 Sep 1;14(17):5619–5625. doi: 10.1158/1078-0432.CCR-07-5185. [DOI] [PubMed] [Google Scholar]
- 5.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001 Jul;81(3):1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 6.Kizaki M, Dawson MI, Heyman R, Elster E, Morosetti R, Pakkala S, et al. Effects of novel retinoid X receptor-selective ligands on myeloid leukemia differentiation and proliferation in vitro. Blood. 1996 Mar 1;87(5):1977–1984. [PubMed] [Google Scholar]
- 7.Altucci L, Rossin A, Hirsch O, Nebbioso A, Vitoux D, Wilhelm E, et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res. 2005 Oct 1;65(19):8754–8765. doi: 10.1158/0008-5472.CAN-04-3569. [DOI] [PubMed] [Google Scholar]
- 8.Mueller BU, Pabst T, Fos J, Petkovic V, Fey MF, Asou N, et al. ATRA resolves the differentiation block in t(15;17) acute myeloid leukemia by restoring PU.1 expression. Blood. 2006 Apr 15;107(8):3330–3338. doi: 10.1182/blood-2005-07-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbauer F, Wagner K, Kutok JL, Iwasaki H, Le Beau MM, Okuno Y, et al. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nature genetics. 2004 Jun;36(6):624–630. doi: 10.1038/ng1361. [DOI] [PubMed] [Google Scholar]
- 10.Vangala RK, Heiss-Neumann MS, Rangatia JS, Singh SM, Schoch C, Tenen DG, et al. The myeloid master regulator transcription factor PU.1 is inactivated by AML1-ETO in t(8;21) myeloid leukemia. Blood. 2003 Jan 1;101(1):270–277. doi: 10.1182/blood-2002-04-1288. [DOI] [PubMed] [Google Scholar]
- 11.Mueller BU, Pabst T, Osato M, Asou N, Johansen LM, Minden MD, et al. Heterozygous PU.1 mutations are associated with acute myeloid leukemia. Blood. 2003 Mar 1;101(5):2074. doi: 10.1182/blood-2002-12-3903. [DOI] [PubMed] [Google Scholar]
- 12.Dahl R, Walsh JC, Lancki D, Laslo P, Iyer SR, Singh H, et al. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPalpha ratio and granulocyte colony-stimulating factor. Nature immunology. 2003 Oct;4(10):1029–1036. doi: 10.1038/ni973. [DOI] [PubMed] [Google Scholar]
- 13.Walsh JC, DeKoter RP, Lee HJ, Smith ED, Lancki DW, Gurish MF, et al. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity. 2002 Nov;17(5):665–676. doi: 10.1016/s1074-7613(02)00452-1. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001 Dec;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Ross AC. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Experimental cell research. 2004 Jul 1;297(1):68–81. doi: 10.1016/j.yexcr.2004.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iijima K, Honma Y, Niitsu N. Granulocytic differentiation of leukemic cells with t(9;11)(p22;q23) induced by all-trans-retinoic acid. Leuk Lymphoma. 2004 May;45(5):1017–1024. doi: 10.1080/1042819031000163887. [DOI] [PubMed] [Google Scholar]
- 17.Tagliafico E, Tenedini E, Manfredini R, Grande A, Ferrari F, Roncaglia E, et al. Identification of a molecular signature predictive of sensitivity to differentiation induction in acute myeloid leukemia. Leukemia. 2006 Oct;20(10):1751–1758. doi: 10.1038/sj.leu.2404358. [DOI] [PubMed] [Google Scholar]
- 18.Howell SR, Shirley MA, Grese TA, Neel DA, Wells KE, Ulm EH. Bexarotene metabolism in rat, dog, and human, synthesis of oxidative metabolites, and in vitro activity at retinoid receptors. Drug metabolism and disposition: the biological fate of chemicals. 2001 Jul;29(7):990–998. [PubMed] [Google Scholar]
- 19.Perez E, Bourguet W, Gronemeyer H, de Lera AR. Modulation of RXR function through ligand design. Biochimica et biophysica acta. 2011 Apr 16; doi: 10.1016/j.bbalip.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Shiohara M, Dawson MI, Hobbs PD, Sawai N, Higuchi T, Koike K, et al. Effects of novel RAR- and RXR-selective retinoids on myeloid leukemic proliferation and differentiation in vitro. Blood. 1999 Mar 15;93(6):2057–2066. [PubMed] [Google Scholar]
- 21.van de Merbel NC, van Veen JH, Wilkens G, Loewen G. Validated liquid chromatographic method for the determination of bexarotene in human plasma. Journal of chromatography. 2002 Aug 5;775(2):189–195. doi: 10.1016/s1570-0232(02)00291-x. [DOI] [PubMed] [Google Scholar]
- 22.Kizaki M, Ueno H, Matsushita H, Takayama N, Muto A, Awaya N, et al. Retinoid resistance in leukemic cells. Leuk Lymphoma. 1997 May;25(5-6):427–434. doi: 10.3109/10428199709039029. [DOI] [PubMed] [Google Scholar]
- 23.Park DJ, Chumakov AM, Vuong PT, Chih DY, Gombart AF, Miller WH, Jr, et al. CCAAT/enhancer binding protein epsilon is a potential retinoid target gene in acute promyelocytic leukemia treatment. The Journal of clinical investigation. 1999 May 15;103(10):1399–1408. doi: 10.1172/JCI2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lekstrom-Himes JA. The role of C/EBP(epsilon) in the terminal stages of granulocyte differentiation. Stem cells (Dayton, Ohio) 2001;19(2):125–133. doi: 10.1634/stemcells.19-2-125. [DOI] [PubMed] [Google Scholar]
- 25.Friedman AD. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007 Oct 15;26(47):6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida H, Ichikawa H, Tagata Y, Katsumoto T, Ohnishi K, Akao Y, et al. PML-retinoic acid receptor alpha inhibits PML IV enhancement of PU.1-induced C/EBPepsilon expression in myeloid differentiation. Molecular and cellular biology. 2007 Aug;27(16):5819–5834. doi: 10.1128/MCB.02422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kueh HY, Rothenberg EV. Regulatory gene network circuits underlying T cell development from multipotent progenitors. Wiley interdisciplinary reviews Systems biology and medicine. 2011 Oct 4; doi: 10.1002/wsbm.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novershtern N, Subramanian A, Lawton LN, Mak RH, Haining WN, McConkey ME, et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell. 2011 Jan 21;144(2):296–309. doi: 10.1016/j.cell.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konopleva M, Elstner E, McQueen TJ, Tsao T, Sudarikov A, Hu W, et al. Peroxisome proliferator-activated receptor gamma and retinoid X receptor ligands are potent inducers of differentiation and apoptosis in leukemias. Mol Cancer Ther. 2004 Oct;3(10):1249–1262. [PubMed] [Google Scholar]
- 30.Kohro T, Nakajima T, Wada Y, Sugiyama A, Ishii M, Tsutsumi S, et al. Genomic structure and mapping of human orphan receptor LXR alpha: upregulation of LXRa mRNA during monocyte to macrophage differentiation. Journal of atherosclerosis and thrombosis. 2000;7(3):145–151. doi: 10.5551/jat1994.7.145. [DOI] [PubMed] [Google Scholar]
- 31.Peeters SD, van der Kolk DM, de Haan G, Bystrykh L, Kuipers F, de Vries EG, et al. Selective expression of cholesterol metabolism genes in normal CD34+CD38- cells with a heterogeneous expression pattern in AML cells. Experimental hematology. 2006 May;34(5):622–630. doi: 10.1016/j.exphem.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, et al. The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluorometh yl)ethyl]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Molecular pharmacology. 2010 Feb;77(2):228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011 Apr 28;472(7344):491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins SJ. The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia. 2002 Oct;16(10):1896–1905. doi: 10.1038/sj.leu.2402718. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher RE. Retinoic acid resistance in acute promyelocytic leukemia. Leukemia. 2002 Oct;16(10):1940–1958. doi: 10.1038/sj.leu.2402719. [DOI] [PubMed] [Google Scholar]
- 36.Kastner P, Lawrence HJ, Waltzinger C, Ghyselinck NB, Chambon P, Chan S. Positive and negative regulation of granulopoiesis by endogenous RARalpha. Blood. 2001 Mar 1;97(5):1314–1320. doi: 10.1182/blood.v97.5.1314. [DOI] [PubMed] [Google Scholar]
- 37.Ricote M, Snyder CS, Leung HY, Chen J, Chien KR, Glass CK. Normal hematopoiesis after conditional targeting of RXRalpha in murine hematopoietic stem/progenitor cells. Journal of leukocyte biology. 2006 Oct;80(4):850–861. doi: 10.1189/jlb.0206097. [DOI] [PubMed] [Google Scholar]
- 38.Durual S, Rideau A, Ruault-Jungblut S, Cossali D, Beris P, Piguet V, et al. Lentiviral PU.1 overexpression restores differentiation in myeloid leukemic blasts. Leukemia. 2007 May;21(5):1050–1059. doi: 10.1038/sj.leu.2404645. [DOI] [PubMed] [Google Scholar]
- 39.Szanto A, Nagy L. Retinoids potentiate peroxisome proliferator-activated receptor gamma action in differentiation, gene expression, and lipid metabolic processes in developing myeloid cells. Molecular pharmacology. 2005 Jun;67(6):1935–1943. doi: 10.1124/mol.104.006445. [DOI] [PubMed] [Google Scholar]
- 40.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998 Apr 17;93(2):229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 41.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998 Apr 17;93(2):241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 42.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010 Jun 25;328(5986):1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.