Abstract
Gut microbiota encompasses a wide variety of commensal microorganisms consisting of trillions of bacteria, fungi, and viruses. This microbial population coexists in symbiosis with the host, and related metabolites have profound effects on human health. In this respect, gut microbiota plays a pivotal role in the regulation of metabolic, endocrine, and immune functions. Bacterial metabolites include the short chain fatty acids (SCFAs) acetate (C2), propionate (C3), and butyrate (C4), which are the most abundant SCFAs in the human body and the most abundant anions in the colon. SCFAs are made from fermentation of dietary fiber and resistant starch in the gut. They modulate several metabolic pathways and are involved in obesity, insulin resistance, and type 2 diabetes. Thus, diet might influence gut microbiota composition and activity, SCFAs production, and metabolic effects. In this narrative review, we discuss the relevant research focusing on the relationship between gut microbiota, SCFAs, and glucose metabolism.
Keywords: intestine, bacteria, metabolome, fiber, diet, glucose homeostasis
1. Introduction
The gastrointestinal tract hosts a large interface between the external environment and the human body. The gut surface is the largest of human body and extends for roughly 200–300 m2 [1]. At this level, the gut microbiota represents a complex polymicrobial ecology and a dynamic ecosystem. More than 100 trillion bacteria in the human gut operate symbiotically with the host and with diverse external stimuli. Products of gut microbial degradation of nutrients are bioactive metabolites that bind target receptors, activate signaling cascades, and modulate several metabolic pathways with local and systemic effects [2].
Short chain fatty acids (SCFAs) are among the important class of gut microbiota bio-products, produced mainly from fermentation of non-digestible carbohydrates, including dietary fiber, that become available to the gut microbiota [3]. Although fiber fermentation plays an essential role in the modulation of gut microbiota physiology and composition, SCFAs also impact on host health at cellular, tissue, and organs levels by mechanisms related to gut barrier function, glucose homeostasis, immunomodulation, and obesity [4]. Thus, the gut microbiota plays a critical role in maintaining homeostasis and health during the whole lifespan. By contrast, an imbalance in the ecological composition of the gut microbiota, often termed “dysbiosis”, paves the way to several diseases, including metabolic disorders which involve glucose homeostasis.
With the worldwide increasing burden of diseases involving glucose homeostasis and insulin resistance (including pre-diabetes and type 2 diabetes—T2D), the knowledge about the relationships between gut microbiota-generated SCFAs and glucose homeostasis can offer new tools for clinical management and prevention. In this regard, in this review we assessed the latest evidence linking SCFAs with host metabolic health with special focus on the interplay between intestinal microbiota, dietary fiber, generation of SCFAs from fiber, and the effect of SCFAs on glucose homeostasis, as confirmed by clinical studies.
2. Methodology
In this narrative review, we explored the scientific literature by PubMed analysis (PubMed (nih.gov)), scanning for international papers in English language (both observational and interventional studies) exploring the relationships between gut microbiota, SCFAs, and glucose metabolism, including pre-diabetes and type 2 diabetes (T2D).
3. The Gut, the Microbiota, Fiber, and SCFAs
3.1. Microbiota and Intestinal Barrier
Gut microbiota are part of the complex and dynamic machinery known as the “intestinal barrier”, functionally generated by the interplay between different layers (Table 1).
Table 1.
Different physical and anatomical layers contributing to the intestinal barrier.
|
Adapted from Portincasa et al. [5].
Microbes, which include bacteria, viruses, fungi, bacteriophages, and protists, spread across the human intestine and form the microbial barrier. In health, microbes grow within the central mucin layer far from the surface of the enterocytes [6]. The human gut microbiota consists of trillions of these microbes which form a complex ecosystem that ranges between 103–104 per gram in the stomach, 105–106 in the jejunum, 108–109 in the terminal ileum, and about 1012–1014 bacteria per gram of gut content in the colon [7], containing at least 1000 different species of known bacteria, and carries 150 times more microbial genes than the human genome [8]. The second layer of the intestinal barrier is the mucin, consisting of heavily glycosylated proteins secreted by the intestinal goblet cells. A third “functional” layer is the combination of gastrointestinal motility involving the stomach, the gallbladder, and intestine, plus the secretion of gastric acid, hepatic bile, and pancreatic juice. A fourth layer is the epithelial barrier, consisting of the enterocytes, the Paneth cells, secreting antimicrobial peptides, and the goblet cells, secreting mucin.
Enterocytes are sealed by specific junctional proteins consisting of tight junctions found at the apical end of junctional complexes. Tight junctions are complex and dynamic structures, contacting neighboring intestinal epithelial cells comprising multiple protein families, such as occludins, claudins, and junctional adhesion molecules (JAMs). They bind intracellular membrane proteins, the zonula occludens (ZOs), which connects the transmembrane tight junction to the actin skeleton.
Adherens junctions are found below the tight junction and are composed of transmembrane proteins, as follows: E-cadherin and nectin, linked to the cytoskeleton by scaffolding proteins, and catenin and afadin, connected to actin filaments.
Desmosomes are transmembrane liner glycoproteins, desmoglein and democollin, which are cadherin proteins linked to intermediate keratin filaments [9].
In the context of the paracellular barrier, gap junctions are complex structures forming clusters of intercellular channels allowing direct diffusion of ions and small molecules between adjacent epithelial cells through multiple pathways, such as a pore pathway and a leak pathway.
A fifth layer is the immune barrier, which consists of antimicrobial peptides, Toll-like receptors (TLRs), microbiota- and pathogen-associated molecular patterns (MAMPs and PAMPs, respectively), lymph nodes, and B/T lymphocytes. A sixth barrier is the gut vascular barrier consisting of endothelium associated with pericytes and enteric glial cells, tight junctions, and adherens junctions. The last barrier is the liver barrier, consisting of hepatic macrophagic Kupffer cells and stellate cells [6].
In this multilayer, inter-dependent, complex, and dynamic intestinal environment, the main functions of microbiota include the synthesis of micronutrients, the digestion and metabolism of nutrients, such as carbohydrates, fat, proteins, and vitamins, and the biotransformation of hepatic primary bile acids to secondary intestinal bile acids that play a key role in fat digestion and act as potent signaling hormone-like agonists. The host develops a natural immunity and tolerance towards the microbiota. This interaction influences the development and maturation of several cells within lymphoid tissues of the intestinal immune system [10,11]. In general, interventions with prebiotics, probiotics, and diet modify the intestinal microbiota. Notably, the bioavailability of substrates into the intestinal lumen drives the shape and the activity of gut microbes harboring or suppressing the presence of specific bacterial patterns at equilibrium with the resulting metabolites [12,13]. Fecal communities cluster into enterotypes, distinguished primarily by levels of Bacteroides and Prevotella. Enterotypes are strongly associated with long-term diets, particularly protein and animal fat (Bacteroides), versus carbohydrates (Prevotella) [14].
3.2. Fiber
Dietary nutrients are in close contact with the intestinal polymicrobial community and dietary fibers work as key substrates to generate SCFAs [15,16]. Fibers are carbohydrate polymers and oligomers encompassing monosaccharides, linked themselves to final molecules of different sizes. Other chemical groups or molecules, such as acetyl- and methyl-, can be linked to the carbon chains. Fibers are characterized by a large structure heterogeneity and various physical properties in terms of solubility or viscosity. The main food groups and fiber varieties according to solubility appear in Table 2. Fibers can be classified as dietary from whole food fibers and synthesized functional fibers [17]. Dietary fibers derive from grains, fruits, vegetables, and legumes [18]. The insoluble dietary fibers include cellulose, some hemicellulose, and lignin [19], which act mainly as mass-forming agents in gut transit [20]. Soluble fibers include wheat dextrin, pectin, gums, β-glucan, psyllium, fructans, and some hemicellulose [19].
Table 2.
Main food groups and fiber varieties according to solubility.
| Food Group | Soluble Fibers | Insoluble Fibers |
|---|---|---|
| Cereals and grains | Nonstarch polysaccharides | Nonstarch polysaccharides |
| Hemicellulose Arabinoxylan β-glucan |
Hemicellulose Cellulose Lignin |
|
| Resistant oligosaccharides | Resistant starch | |
| Inulin | ||
| Fruits and vegetables | Nonstarch polysaccharides | Nonstarch polysaccharides |
| Hemicellulose Pectin |
Hemicellulose Cellulose Pectin |
|
| Resistant oligosaccharides | Lignin | |
| Inulin | Resistant starch | |
| Legumes and pulses | Nonstarch polysaccharides | Nonstarch polysaccharides |
| Hemicellulose Pectin Gum |
Hemicellulose Pectin |
|
| Lignin | ||
| Resistant starch |
3.3. Fiber as a Source of SCFAs in Health
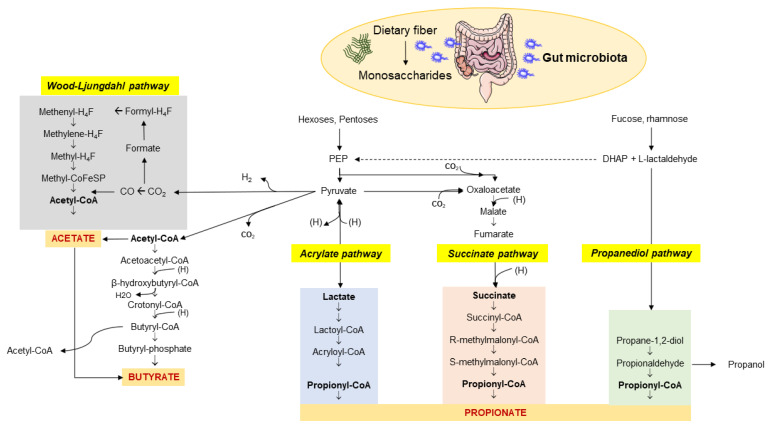
The enzymatic profile of the human gut is not sufficient to completely metabolize dietary fiber. Soluble fiber, resistant to digestion by the host, represent the microbiota-accessible carbohydrates (MACs) [21]. The microbiota has a great gene-encoding power and is essential to guarantee the complete fiber digestion through the cecal and colonic fermentation [22]. This capacity of digestion depends on the richness in glycoside hydrolases (more than 260) of the huge microbial population, compared with only 17 enzymes in humans [23], which break down various types of carbohydrates [24]. This process involves more than 100 trillion bacteria. An MACs-rich diet in humans is associated with an increased content of colonic and fecal SCFAs [3,25,26,27,28,29,30], which are microbial metabolites and major products from bacterial anaerobic fermentation of dietary fiber and resistant starches in the intestine. Major SCFAs include acetic, propionic, butyric, valeric, and caproic acids, accounting for between two and six carbon (C) units. Among them, acetate (C2), propionate (C3), and butyrate (C4) are the most abundant SCFAs in the human body and the most abundant anions in the colon. Their synthesis involves different pathways, which include the Wood–Ljungdahl pathway for acetate, two molecules of acetate for butyrate, and the acrylate, succinate, and propanediol pathways for propionate (Figure 1) [31].
Figure 1.
Pathways involved in the biosynthesis of SCFAs from dietary fiber and carbohydrate fermentation by the colonic microbiota. The three major SCFAs are: (1) acetate which originates via the Wood–Ljungdahl pathway or acetyl-CoA; (2) butyrate synthesized from two molecules of acetyl-CoA; (3) propionate from PEP involving the acrylate pathway or the succinate pathway or the propanediol pathway after microbial transformation of fucose and rhamnose. Abbreviations: PEP—phosphoenolpyruvate; DHAP—dihydroxyacetone phosphate. Adapted from Kho et al. [31].
Acetate, propionate, and butyrate act as post-biotic molecules [32] and in the colon they exist in a molar ratio of 60:20:20, respectively; although, the relative proportion of each SCFAs depends on the substrate, the microbiota composition, and the gut transit time [33,34] (Figure 2 and Table 3). About 500–600 mM of SCFAs is produced in the large intestine daily, depending on the dietary fiber content. Psyllium and gums are not included into this process.
Figure 2.
Chemical formula, molecular weight, and 3D structure of the three main short chain fatty acids acetate (C2), propionate (C3), and butyrate (C4). In the 3D structure, atoms appear as hydrogen in white color, carbon as grey color, and oxygen as red color. https://pubchem.ncbi.nlm.nih.gov/ (accessed 18 January 2022).
Table 3.
Dietary source, fiber substrates, and SCFA-producing bacteria.
| Dietary Source | Substrates | Fermenting Genera |
|---|---|---|
| Cashew, green banana, white beans, oat, and potato | Resistant starch | Ruminococcus, Bacteroides |
| Seaweed and cereal bran | Cellulose | Bacteroides, Ruminococcus |
| Cereal bran | Hemi-celluloses (xylan and arabinoxylan) | Bacteroides, Roseburia, Prevotella |
| Apples, apricots, cherries, oranges, and carrots | Pectin | Eubacterium, Bacteroides, Fecalibacterium |
| Asparagus, leek, onions, banana, wheat, garlic, chicory, and artichoke | Fructans (inulin and fructooligosaccharides) | Bacteroides, Fecalibacterium |
| Breast milk | Milk oligosaccharides | Bifidobacterium |
| Milk, yogurt, buttermilk, and cheese | Lactose (only in lactose-intolerant people) | Bifidobacterium |
| Oat, barley, wheat, rye, mushrooms, and seaweed | β-Glucan | Eubacterium, Atopobium, Enterococcus, Lactobacillus, Prevotella, Clostridium cluster XIVa |
| Acacia tree and prepared food additive | Gum arabic | Bifidobacterium, Lactobacillus, Ruminococcus |
| Guar bean and prepared food additive | Guar gum | Bifidobacterium, Ruminococcus |
| Seaweed | Laminarin | Prevotella |
| Artichoke, beans, beetroot, broccoli, chickpeas, fennel, lentils, lettuce, radicchio, and onion | Galacto-oligosaccharides | Bifidobacterium |
| Cottonseed flour, soy flour, onions, chickpeas, beans, peas, and lentils | Raffinose and stachyose | Bifidobacterium, Lactobacillus |
The three SCFAs are produced in small amount (<1%) from amino acid metabolism [38]. Fecal excretion of SCFAs accounts for about 5% of total SCFAs. Microbial species are involved in shaping the SCFAs profile (Table 3). Bacteria species intake lactate and succinate and convert them into propionates [37]. Abundance of Bacteroides spp. is associated with production of propionate [39] and acetate [40], while butyrate is produced mainly by the Firmicutes phylum [40]. Colonic fermentation of fiber to SCFAs decreases pH levels, increases fecal acidification, and increases the growth and diversity of the gut microbiota taxa [41].
SCFAs act as mediators in several pathways, which include local, immune, endocrine effects, and microbiota–gut–brain communication.
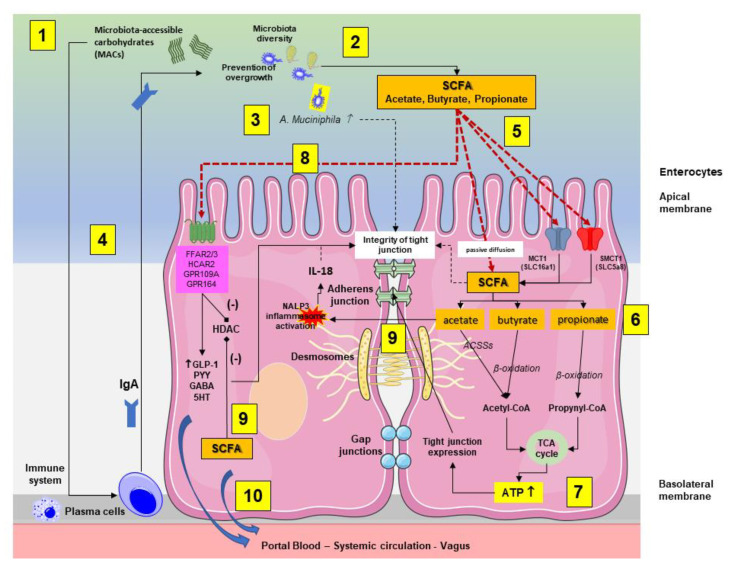
As reported in Figure 3, following intestinal bacteria digestion of fermentable microbiota-accessible carbohydrates (MACs) consisting of fiber and starches, SCFAs are absorbed by colonocytes via passive diffusion or via active transport, mediated by H+-dependent monocarboxylate transporters (MCTs), previously known as solute carrier family (SLC) transporters. MCT1 (also known as SLC16a1) transports SCFAs in an H+-dependent, electroneutral manner. The SCFA anion transport occurs via the electrogenic, sodium-dependent monocarboxylate transporter 1 (SMCT1; also known as SLC5A8) [42].
Figure 3.
Interplay between the microbiota-accessible carbohydrates (MACs), the gut microbiota, the production short chain fatty acids (SCFAs), and the enterocytes (mainly colonocytes). The main pathways involved are summarized in the two enterocytes. (1) Following initial digestion and intestinal transit, the dietary MACs meet the gut microbiota, which is characterized by high and physiological diversity and no bacterial overgrowth. (2) SCFA-producing bacteria, mainly in the colon, will digest MACs and increase the luminal content of SCFAs. (3) In this environment, the abundance of A. muciniphila increases and is associated with protective effects on mucin and tight junction integrity. (4) In addition, a diet enriched in MACs will positively stimulate the immune system, leading to plasma cell-mediated production of immunoglobulins A (IgA) with further control on microbiota function, diversity, and prevention of overgrowth. (5) In the colonocyte, SCFAs are absorbed by colonocytes via passive diffusion or via active transport mediated by H+-dependent monocarboxylate transporters (MCTs). (6) The SCFAs acetate, butyrate, and propionate are converted to acetyl-CoA or propynyl-CoA by pathways involving the acetyl-CoA carboxylases (ACSSs) and beta oxidation. (7) This step produces ATP, which contributes to the maintenance of cell homeostasis, including the function of tight junctions. (8) Via stimulation of receptors at the apical membrane, SCFAs promote the secretion of gut hormones, such as glucagon-like peptide 1 (GLP1) and peptide YY (PYY), γ-aminobutyric acid (GABA), and serotonin (5-HT). At this level, butyrate inhibits (-) histone deacetylases (HDACs) with consequent anti-inflammatory effect by reducing NF-κB-induced pro-inflammatory mediators, such as TNF-α, IL-6, IL-12, and iNOS [43]. (9) Intracellular SCFAs contribute to inhibition (-) of HDAC. Acetate activates the inflammasome nucleotide-binding oligomerization domain 3 (NLRP3) with secretion of the protective IL-18 from epithelial cells, which maintains the tight junction’s function. (10) Colon-derived SCFAs reach the systemic circulation promoting anti-inflammatory and immunomodulatory effects as well as increasing insulin secretion, maintaining energy homeostasis, and improving liver and brain function.
At a cellular level, SCFAs promote complex and integrated effects, modulating the homeostasis and function of intestinal epithelial cell (Table 4).
Table 4.
Effects of SCFAs on intestinal epithelial cell homeostasis and function.
|
In the enterocyte, acetate, butyrate, and propionate are converted to acetyl-CoA or propynyl-CoA via acetyl-CoA carboxylases (ACSSs) or β-oxidation to produce ATP via the citric acid cycle. This pathway contributes to maintain cell homeostasis including the function of the apical tight junctions [23]. SCFAs are also involved in cellular metabolism and immunity. In vitro, the three SCFAs promote intracellular permeability and the mechanism involved the modification of tight junction expression or distribution and zonulin (ZO-1) [44]. SCFAs potently stimulate ANGPTL4 (fasting-induced adipose factor—Fiaf) in human colon cell lines via PPARγ [45].
Butyrate provides the energy source to the gut mucosa. Propionate contributes to the liver gluconeogenesis and acetate achieves the highest systemic serum concentrations. Both propionate and butyrate provide the strongest inhibition of histone deacetylases [38].
Acetate in the gut epithelial cells of the animal model directly activates the nucleotide-binding oligomerization domain 3 (NLRP3) inflammasome, which increases the release of IL-18 [46], engaging the epithelial IL-18 receptor and promoting intestinal barrier integrity [47].
Butyrate provides energy, maintains the integrity of colonocytes, enhances the barrier function [48], and increases mucin expression, via MUC2 gene expression [49]. The protective effect on tight junction likely involves the activation of AMP-activated protein kinase (AMPK) [50] or the downregulation of claudin 2 expression [51]. This latter pathway involves the hypoxia-inducible factor-1, which modulates the efficiency of epithelial tight junction CLDN1, the gene coding for claudin-1 [52,53]. Butyrate induces Treg differentiation and plays a role in the control of inflammation [54]. Notably, administration of butyrate to ulcerative colitis patients decreases fecal calprotectin, a marker of gut inflammation [55].
The protective effects of propionate are also evident on the intestinal barrier. In the mice model fed with dextran sulfate sodium (DSS), propionate 1% counteracted the inflammatory changes due to DSS-induced colitis manifesting by weight loss, colonic damage, the increase in FITC-dextran in serum and the decrease in zonula occludens-1 (ZO-1), occludin, and E-cadherin expression in the colonic tissue. In addition, propionate inhibited the expression of interleukin (IL)-1β, IL-6, and tumor necrosis factor-alpha (TNF-alpha) mRNA and phosphorylation of signal transducer, the activator of transcription 3 (STAT3), reduced the myeloperoxidase level, and increased the superoxide dismutase and catalase level in the colon [56].
SCFAs protect the intestinal barrier by additional mechanisms which involve plasma membrane cholesterol-rich microdomain in T84 and Caco-2 cell cultures challenged with physiological concentrations of SCFAs [57]. In addition, SCFAs interact with the epithelial Toll-like receptors (TLRs) and activate the nuclear factor-κB signaling pathway which regulates the integrity of gut epithelial cells [46]. In colonic organoid from human colonic mucosal biopsies, the fermentable substrate 2′-O-fucosyllactose caused the increase in bifidobacteria and butyrate, with upregulation of claudin-5, a marker of enhanced barrier function [58]. Studies in humans showed that shift workers are prone to circadian oscillation and have decreased gut-derived plasma SCFAs, a finding which correlates with increased colonic permeability [59].
Several systemic effects of SCFAs originate from the intracellular effects of SCFAs which bind the G protein-coupled receptors (GPCRs), such as the free fatty acid receptor 2 (FFAR2, earlier known as GPR43) and FFAR3 (earlier known as GPR42), as well as GPR109A/HCAR2 (hydrocarboxylic acid receptor) and GPR164. These receptors are expressed in colonocytes, enterocytes, and in several other cells in the body, including neural cells. At the level of enteroendocrine L cells, the SCFAs-mediated stimulation of receptors is associated with the release of two anorexic hormones, glucagon-like peptide-1 (GLP-1) and the peptide YY (PYY). Notably, this pathway is independent of the bile acid pathway involving the ileal membrane receptor G protein-coupled bile acid receptor 1 (GPBAR-1) [6,11,26]. The SCFA-related pathways of hormone release are associated with several effects on appetite and food intake via systemic circulation or vagal afferents [60,61]. In addition, the incretin GLP-1 slows gastric emptying and gut transit, helps energy absorption [62], and enhances glucose-dependent insulin release [63]. Additional effects on gut motility likely involve the SCFAs-induced serotonin release from enterochromaffin cells [64].
Intracellular SCFAs have epigenetic effects, mainly through an inhibitory effect on histone deacetylases (HDAC) and an hyperacetylation of histones in lysine residues present in nucleosome [65]. This pathway is expressed in the gut, the associated immune tissue [66,67], and in the peripheral nervous system and the CNS [68,69].
SCFAs by acting on signaling pathways HDAC and GPR4 can influence the function of intestinal immune T cells 3 [70,71].
Although further studies are required, there is evidence suggesting additional roles for SCFAs in activation of brown adipose tissue and appetite control [72], body energy homeostasis [73], and even regulation of mitochondrial function [74].
In the colonocytes, SCFAs are partly oxidized to CO2, which can enter the portal blood. SCFAs which are not metabolized in the colonocytes enter the portal circulation at a concentration of about 260 μM (acetate), 30 μM (propionate), and 30 μM (butyrate). Once in the liver, SCFAs become substrates for energy production of hepatocytes via oxidation [75]. Acetate is also used for the synthesis of fatty acids and cholesterol [76]. Propionate can become a substrate for gluconeogenesis in the liver [77,78,79].
Only a minor fraction of the colon-derived acetate (36%), propionate (9%), and butyrate (2%) is transferred to the systemic circulation and peripheral tissues [80], with plasma concentrations of 25–250 μM, 1.4–13.4 μM, and 0.5–14.2 μM for acetate, propionate, and butyrate, respectively [81]. Acetate is also converted into acetyl-CoA in peripheral muscles for lipogenesis or oxidation.
4. Manipulation of Intestinal Microbiota, SCFA and Effect on Glucose Homeostasis
The literature clearly points to a pivotal involvement of microbes in SCFAs’ metabolism [38,82]. A high microbiota diversity is associated with digestion of many types of complex carbohydrates with production of several SCFAs and additional gut microbiota diversity [26]. The interaction between gut microbiota and diet governs the qualitative and quantitative production of SCFAs.
Potential tools to manipulate the intestinal microbiota include the ingestion of live beneficial bacteria as probiotics [83,84] or prebiotics as substrates selectively utilized by host microorganisms, conferring a health benefit [85]. The intestinal microbiota will ferment the prebiotic into SCFAs. In addition, diet will provide substrates able to manipulate the microbial composition. As mentioned earlier, plant-based foods, which are typical of the Mediterranean diet, will increase the availability of fermentable substrates by specific bacteria leading to SCFAs formation [86]. The intestinal microbiota is amenable to modulation by prebiotics [31,81,87,88,89], probiotics [66,90], or dietary regimens, which include the adherence to a Mediterranean diet [91,92,93]. Such approaches can substantially increase the colonic production of SCFAs. The ultimate clinical outcome of such approaches requires further studies.
In addition, following dietary changes, including habitual fiber intake, can select fiber-fermenting microbes, which, in turn, will inhibit other harmful species [94]. Production of SCFAs will influence different metabolic pathways [95] and therefore will contribute to prevent and modulate T2DM [2,96]. Further evidence is required in this field; therefore, herein, we discuss the main human studies addressing the relationship between the composition of the gut microbiota, concentration of SCFAs, and glucose metabolism.
Among humans undergoing a controlled-feeding study, a high-fat/low-fiber or low-fat/high-fiber diet within 24 h were associated positively with Bacteroidetes and Actinobacteria in fat and a negative association between Firmicutes and Proteobacteria [14]. By contrast, an unbalanced microbiota, unable (or less capable) to metabolize dietary fiber, negatively impacts the hosts’ overall health. The reduced microbiota diversity decreased the availability of digestible carbohydrates, and certain SCFAs, such as propionate, can abnormally increase, and several functions, including metabolic effects, will decrease or become impaired.
Results from animal models suggest that the composition of the gut microbiota can influence metabolic disorders, such as obesity, insulin resistance, and T2DM. In accordance with this hypothesis, prediabetic or T2DM patients often show intestinal dysbiosis, compared with healthy subjects [97,98]; although, specific microbial clusters are still lacking. The profile of gut microbiota plays an important role in T2DM [99] and steps include the maintenance of integrity of intestinal barrier. This step implies decreased chance for bacteria translocation and endotoxemia-dependent systemic inflammation, two changes likely involved during the early stage of disease. Patients with T2DM have reduced abundance of butyrate-producing bacteria [100]. SCFA supplementation in patients with T2DM increased the abundance of butyrate-producing bacteria and increased GLP-1 and hemoglobin A1c levels [101].
In line with the above-mentioned background, ongoing research is focusing on strategies able to modulate the human gut microbiota, since this approach represents a potential tool to prevent both metabolic and inflammatory diseases. Where genetic factors play a limited role in shaping the microbiota composition [102], environmental factors, which include the short-term effect of fecal transplantation [103] and the more stable effects of dietary habits [94], are potent tools in this respect.
5. Clinical Studies on the Effects of Microbiota Profile and Dietary Manipulations in the Maintenance of Glucose Homeostasis and in T2DM
A series of observational studies spanning from 2010 to 2020 show that the host metabolic state can profoundly influence the gut microbiota profile [86,91,92,100,104,105,106,107,108,109] (Table 5), affecting the microbiota/SCFA relationships, both in terms of maintenance of glucose homeostasis and pathological conditions.
Table 5.
Observational studies pointing to the role of SCFAs in the maintenance of glucose homeostasis, in pre-diabetes and T2DM.
| Author, Year | Study Groups | Number (M/F) | BMI (Kg/m2) | Major Findings |
|---|---|---|---|---|
| Wu et al., 2020 [105] 2020 |
Healthy individuals | 206 (106/100) |
28.2 | In pre-diabetes and patients with T2DM ↓ abundance of butyrate-producing bacteria. Findings are independent of fasting glucose or treatment with metformin treatment. |
| Pre-diabetes | 220 (107/113) |
28.3 | ||
| T2DM | 58 (32/27) |
31.6 | ||
| Sanna et al., 2019 [107] | Healthy individuals selected on the basis of SCFAs and genome, fecal metagenomic sequences. | 952 | - | Butyrate-producing bacteria have a protective role against T2DM. |
| Garcia-Mantrana et al., 2018 [86] | Healthy individuals | 27 (11/16) |
19–28 | Evaluation by food frequency questionnaire. High adherence to Mediterranean diet associated with ↑ abundance of Bifidobacteria, ↑ Bacteroidetes. ↓ abundance of Firmicutes:Bacteroidates ratio. ↑ Fecal SCFAs and propionate. High animal protein intake associated with ↓ abundance of Bacteroidetes, ↑ Firmicutes:Bacteroidates ratio. |
| Mitsou et al., 2017 [109] | Healthy individuals | 116 (61/55) |
25–30 | Evaluation by food frequency questionnaire. High adherence to Mediterranean diet associated with ↑ fecal SCFAs. |
| Gutierrez-Diaz et al., 2016 [91] | Healthy individuals with low adherence to Mediterranean diet. | 10 (2/8) |
21.2–31.2 | Evaluation by food frequency questionnaire. High adherence to Mediterranean diet associated with ↑ abundance of fecal SCFAs, ↑ faecal propionate and butyrate. |
| Healthy individuals with high adherence to Mediterranean diet. | 21 (8/13) |
21.6–31.0 | ||
| De Filippis et al., 2016 [92] | Vegetarian individuals | 51 | 19.4–24.4 | Vegetarian diet, vegan diet, and omnivore; high adherence to Mediterranean diet associated with ↑ abundance of Prevotella and ↑ fecal propionate. |
| Vegan individuals | 51 | 19.1–23.5 | ||
| Omnivore individuals | 51 | 20.1–24.1 | ||
| Zhang et al., 2013 [104] | Healthy individuals | 44 | 23.4 | In healthy individuals ↑ abundance of butyrate-producing bacteria (Akkermansia muciniphila ATCCBAA-835, and Fecalibacterium prausnitzii L2-6). In patients with pre-T2DM ↓ abundance of Bacteroides and Verrucomicroniae. |
| Pre-diabetes | 64 | 24.9 | ||
| T2DM | 13 | 26.5 | ||
| Karlsson et al., 2013 [106] | Healthy individuals | 43 (0/43) |
20–40 |
In healthy individuals ↑ abundance of Roseburia ↑ Fecalibacterium prausnitzii. In patients with T2DM ↓ decreased Roseburia ↓ Fecalibacterium prausnitzii. |
| Pre-diabetes | 49 (0/49) |
|||
| T2DM | 53 (0/53) |
|||
| Quin et al., 2012 [100] | Healthy individuals | 182 | 18–40 | In healthy individuals ↑ abundance of Roseburia and ↑ Fecalibacterium prausnitzii. In patients with T2DM ↓ abundance of Roseburia and ↓ Fecalibacterium prausnitzii in individuals with T2DM. |
| T2DM | 183 | |||
| De Filippo et al., 2010 [108] | African children (1–6 yrs) | 14 (9/5) |
Evaluation by food frequency questionnaire. Plant-based diet associated with ↑ abundance of Prevotella and ↑ Xylanibacter ↓ Firmicutes. Animal-based diet associated with ↓ abundance of Prevotella ↓ Xylanibacter ↑ Firmicutes. |
|
| Italian children (1–6 yrs) | 15 (9/6) |
Abbreviations: ↓—significant decrease; ↑—significant increase; BMI—body mass index; SCFAs— short chain fatty acids; T2DM— type 2 diabetes.
5.1. Effects on Glucose Homeostasis
In the large study carried out by Sanna et al. [107], 952 normoglycemic individuals underwent a genome-wide genotyping, gut metagenomic sequencing, and SCFA analyses. The authors adopted a bidirectional Mendelian randomization (MR) analysis to assess causality and found how the host’s genetically driven increase on butyrate was associated with improved insulin response after an oral glucose-tolerance test. By contrast, abnormalities in the production or absorption of propionate were associated with an increased risk of T2DM, suggesting a causal effect of the gut microbiome on metabolic traits.
A deeper investigation into the field of dietary intake contributes was reached evaluating the degree of adherence to the Mediterranean diet (MD), which usually involves a high-fiber intake. This additional focus was investigated in some studies enrolling healthy individuals with a low compared with high adherence to MD, or vegetarian individuals. These studies evaluated the gut microbiota profiling volunteers split based on the degree of adherence to MD. Studies reporting an higher adherence to MD found a high abundance of Bacteroidetes in light to the detection of Prevotella [86,91], Bifidobacteria [109], or other bacteria commonly metabolizing fiber (e.g., Roseburia, Prevotella, and Lachnospira) [92]. In the same studies, fecal butyrate or fecal propionate concentrations increased in individuals more compliant to MD and, therefore, reporting a higher daily intake of fiber [86,91,92,109]. Following a fiber-enriched diet, changes can be even more complex. As an example, Mitsou et al. [109] found that subjects more compliant to MD had lower Escherichia coli counts, a higher Bifidobacteria:E. coli ratio, increased levels and prevalence of Candida albicans, greater molar ratio of acetate, higher defecation frequency, and a more pronounced gastrointestinal symptomatology compared with those reporting low adherence. The molar ratio of another SCFA to valerate was lower in the case of high adherence to the MD compared with the other two tertiles. By contrast, fast food consumption was characterized by suppressed representation of lactobacilli and butyrate-producing bacteria.
The importance of dietary pattern in shaping the gut microbiota was the aim of the study carried out by De Filippo et al. [108], which compared the composition of gut microbiota in children from Africa and Italy. Notably, Bacteroidetes (mainly Prevotella and Xylanibacter) were increased in African children, while Firmicutes were reduced, in response to a plant-based diet, including minor cereals, such as millet and sorghum, legumes, and vegetables. The enrichment of such bacterial taxa suggested that the rural African diet seems to harbor the growth of fiber-hydrolyzing bacteria (xylans, cellulose). The SFCA profiling carried out onto fecal samples confirmed a higher amount of total SCFAs in African samples according to a significantly higher fecal concentration of acetic, propionic, butyric, and valeric acids. By contrast, Italian children who mainly consumed an animal-based diet, rich in fat and protein, had a decreased abundance of Bacteroidetes, while reporting more Firmicutes. Over this intriguing suggestion, it is important to consider the influence of the genetic background. With a specific focus onto the phylum Bacteroidetes, Italian children mainly accounted in Bacteroides, instead of Prevotella, and Xylanibacter as found for African children, further supporting what was previously found by Arumugam et al. [110].
Even short-term treatments might also provide beneficial effects with some fiber-enriched foods. Kovatcheva-Datchary et al. [111] and Nilsson et al. [112] compared the barley kernel-based bread, containing fiber 37.6 g/day, to wheat bread containing fiber 9.1 g/day in healthy volunteers for only 3 days. A brief consumption of barley kernel-based bread was associated with increased Prevotella and reduced Bacteroides abundance, confirming how these two genera are competitors for the same substrates. Additionally, even if not significantly, the genus Prevotella showed a greater relationship than Bacteroides with other genera recognized for metabolizing polysaccharides (i.e., Dorea and Roseburia) [111]. Moreover, values of correlation with the same taxa were higher than ones resulting from Bacteroides. Concerning the influence of the barley kernel on the host’s metabolism, in both studies, authors documented the reduction in postprandial glucose response [111,112], while only one of them showed increased total serum SCFAs concentration [112].
With the similar purpose to increase evidence about effects linked to short-term consumptions of fiber, David et al. [93] randomized 10 healthy individuals in a cross-over controlled 4-day trial. Subjects followed both a Western diet (enriched in animal foods, such as meat, eggs, and cheese, and a low fiber intake, equal to 9.36 ± 2.1 g/1000 kcal) or a plant-based diet, enriched in wholegrains, legumes, fruits, vegetables, and fiber, equal to 25.6 ± 1.1 g/1000 kcal. Compared with the Western diet, the plant-based diet resulted in increased plant-polysaccharide metabolizing bacteria belonging to Lachnospiraceae and Ruminococcaceae (such as Roseburia, Ruminococcus, and Eubacterium) at the same level of Prevotella among Bacteroidetes. In this study, despite the short term of evaluation, the variation of the above-mentioned taxa (which all increased) resulted in a higher concentration of fecal SCFAs butyrate and acetate.
A similar outcome occurred in the study of Freeland et al. [111,113]. Randomizing hyperinsulinemic subjects (in accordance with fasting plasma insulin > 40 pmol/l) in two arms—a high- fiber wheat cereal diet against a low-fiber cereal diet—the profiles of metabolites were collected every 3 months for 12 months. No significant differences were found until 6 months into the study. Only at 9 months, instead, were acetate and butyrate concentrations higher for participants on the high-fiber than the control diet. Meanwhile, at the end of the trial (i.e., 12 months), authors detected an increase in GLP-1 secretion. Therefore, although without an immediate effect, this evidence suggested a contribution of fiber to a reduced risk for T2D.
5.2. Effects on Pre-Diabetes and T2DM
Wu et al. [105] found that the overall gut microbiota parallelly shifted according to the glycemic status in humans showing insulin resistance (not fasting glucose), and irrespective of diabetes treatment (metformin). In this study, according to KEGG orthologs (KO) annotations resulting from metagenomic analyses of fecal samples, bacterial genes involved in the butyrate metabolism were reduced in pre-diabetes and T2DM.
Zhang et al. [104] and Wu et al. [105] designed two large studies with three subgroups of individuals consisting of subjects with normal glucose tolerance, subjects with pre-diabetes, and T2DM individuals. The body mass index increased in accordance with unhealthy status and the study analyzed the composition of fecal microbiota. Based on collected microbiota profiles, the authors speculated on a higher presence of butyrate-producing bacteria in normal glucose tolerance subjects than individuals with either pre-diabetes or T2DM. This conclusion was supported by the highest abundance of Roseburia (OTU1900), Akkermansia muciniphila ATCCBAA-835, and Fecalibacterium prausnitzii L2-6 in normal glucose tolerance subjects [104]. Nonetheless, Lachnospiraceae, Ruminococcus, Eubacterium, and overall Clostridiales were found at the highest abundance in the T2DM group and even these taxa are known to be within the main butyrate producers, as well as saccharolytic microorganisms [114]. Therefore, considering that no analyses were carried out to determine the SCFAs concentration, these findings were not sufficient to reach an absolute conclusion about those individuals, which were mainly colonized by butyrate-producing bacteria. Differently, a clear trend was observed for Verrucomicrobia that was reduced in prediabetic volunteers and even more in T2DM compared with NGT, to be claimed as a potential microbial marker of T2DM.
Further studies based on metagenomic sequencing found increased Roseburia and Fecalibacterium prausnitzii in normal glucose tolerance individuals differently than patients with T2DM, in which the abundance of these taxa decreased [100,106]. Of note, similar findings were collected in spite of studies sampling feces from subjects with a different ethnicity (in Europe by Karlsson et al. [106] and in China by Quin et al. [100]). Both these studies [100,106] adopted the same statistical methodology to investigate fecal microbial genes. By combining the observations, we learn that metagenomic clusters of bacterial genes identify T2DM patients more accurately than the taxonomic assignment. The analysis of fecal microbiome allowed a better classification of women with impaired glucose tolerance, providing a potential tool to identify individuals at high risk of developing T2DM. Studies suggest the existence of changes in the intestinal environment of T2DM patients, which are likely to change in different geographical areas, even if maintaining a similar core. In T2DM subjects, this conclusion was reached thanks to similar findings in terms of gene contribution but given by different actors, as found for Akkermansia muciniphila by the first study [106], and for lactobacilli and clostridial species in the latter study [100].
Few interventional trials have tested the effect of dietary modulation of microbiota by using high-fiber diets or fiber-rich foods with the intent to ameliorate glucose metabolism [93,101,111,112,115,116,117,118] (Table 6). In general, a wide variability is typical of such studies, in terms of type of design (parallel, crossover), duration of study (from 3 days to 1 year), study groups (healthy individuals, subjects with metabolic syndrome—MetS—or T2DM), and type of intervention (plant-based diets, MD, or other high-fiber diets, etc.).
Table 6.
Randomized clinical trials pointing to the role of SCFAs in the maintenance of glucose homeostasis, in metabolic syndrome, and in type 2 diabetes.
| Author Year |
Study Groups | Number (M/F) |
BMI Kg/m2 |
Study Design | Duration | Intervention | Major Findings |
|---|---|---|---|---|---|---|---|
| Vitale et al., 2021 [115] | At least one criterion of MetS (overweight/obesity) |
29 (14/15) |
25–35 | Parallel | 8 weeks | Mediterranean diet consisting of fiber 19.3 g/1000 kcal compared with control diet consisting of fiber 8.1 g/1000 kcal) |
↑ Intestinimonas butyriciproducens ↑ Akkermansia muciniphila ↑ Plasma butyric acid ↓ Postprandial glucose ↓ Postprandial insulin ↑ Oral glucose insulin sensitivity |
| Zhao et al., 2018 [101] | Type 2 DM | 43 | 25–35 | Parallel | 12 weeks | High-fiber diet consisting of fiber 37.1 g compared with control diet consisting of fiber 16.1 g | ↑ Fecal butyrate ↓ HbA1c ↓ Fasting glucose |
| Haro et al., 2016 [116] | MetS (insulin sensitivity/obesity) |
20 (20/0) |
30–40 | Parallel | 1 year | Mediterranean diet consisting of fiber: 12.9 ± 0.2 g/Kcal mainly from vegetables compared with high-fiber diet consisting of fiber: 14.1 ± 0.2 g/1000 kcal, mainly form wholegrains | ↑ Roseburia ↓ Prevotella ↑ Insulin sensitivity index High-fib diet ↓ Roseburia ↑ Prevotella |
| Hald et al., 2016 [117] | MetS | 19 | 25.9–41 | Crossover | 4 weeks | Diet enriched with Arabinoxylan and Resistant starch consisting of fiber 64 g compared with Western diet consisting of fiber 17.6 g | ↑ Bifidobacteria ↑ Fecal SCFAs ↑ Fecal butyrate |
| Vetrani et al., 2016 [118] | MetS (overweight/obesity and type 2 diabetes) |
40 (16/24) |
25–35 | Parallel | 12 weeks | Wholegrain diet consisting of total fiber 40 g with fiber from cereal 28.9 g, compared with refined cereal diet consisting of total fiber 22.1 g with fiber from cereal 11.8 g | ↑ Plasma propionate ↓ Postprandial insulin |
| Kovatcheva-Datchary et al., 2015 [111] | Healthy individuals | 39 (6/33) |
18–28 | Parallel | 3 days | Barley kernel-based bread consisting of fiber 37.6 g compared with white wheat bread consisting of fiber 9.1 g | ↑ Prevotella:Bacteroides ↓ Postprandial glucose |
| Nilsson et al., 2015 [112] | Healthy individuals | 20 (3/17) |
18–28 | Crossover | 3 days | Barley kernel-based bread consisting of fiber 37.6 g compared with white wheat bread consisting of fiber 9.1 g | ↑ Plasma SCFAs ↓ Glucose ↓ Insulin |
| David et al., 2014 [93] | Healthy individuals | 10 (5/5) |
19–32 | Crossover | 4 days | Plant-based diet consisting of fiber 26 g/1000 kcal compared with Western diet consisting of fiber 9.3 g/1000 kcal | ↑ Prevotella ↑ Roseburia ↑ Fecal butyrate Western diet ↓ Prevotella ↑ Bacteroides |
| Freeland et al., 2010 [114] | Hyperinsulinaemic individuals | 28 | 24–27 | Parallel | 1 year | High-wheat fiber cereal consisting of 24 g fiber/day compared with low-fiber cereal | ↑ Acetate and butyrate concentrations ↑ Plasma GLP-1 |
Abbreviations: ↓—significant decrease; ↑—significant increase; BMI—body mass index; HbA1c—glycated hemoglobin; GLP-1—glucagon-like peptide-1; MetS—metabolic syndrome; SCFAs—short chain fatty acids.
Nevertheless, the emerging concept is that high-fiber diets and fiber-rich foods genuinely improve glucose metabolism through pathways involves gut microbiota and increased SCFAs metabolism.
Vitale et al. [115] enrolled overweight/obese individuals to evaluate the effects of an 8-week MD enriched in fiber (19.3 ± 3.1 g/1000 kcal). The regimen was associated with increased scores of alpha-diversities of fecal microbiota related to an increased abundance of different species, such as Akkermansia muciniphila and Intestinimonas butyriciproducens, and an increased concentration of plasma butyrate, postprandially. Notably, in individuals with high cardiometabolic risk, postprandial glucose and insulin sensitivity also improved when compared with the control diet, consisting of fiber 8.1 ± 2.3 g/1000 kcal. Butyrate concentrations directly correlate with postprandial insulin sensitivity.
Zhao et al. [101] treated T2DM patients with a 12-week high-fiber diet enriched in fiber 37.1 ± 1.9 g. The control diet consisted of half amount of fiber. Compared with baseline, the high-fiber diet caused increased levels of fecal butyrate concentrations associated with a reduced concentration of fasting glucose and HbA1c. The whole genome shotgun of fecal microbiota showed a greater gene-encoding power to metabolize fiber mainly determined by increased abundances of Fecalibacterium prausnitzii and Bifidobacterium pseudocatenulatum. Notably, the fecal microbiota transplant into gnotobiotic recipients (C57BL/6J mice) confirmed the microbiota-related and metabolomic findings obtained from the in vivo evaluation.
Haro et al. [116] studied 20 obese male subjects over 1 year. In a crossover design, authors used a low-fat/high-fiber diet consisting of 14.1 ± 0.2 g/1000 kcal (mainly from wholegrains) and an MD enriched in fiber (12.9 ± 0.2 g/1000 kcal, mainly from vegetables and nuts). No common findings were found in terms of microbiota variations that underlined a strict relationship occurring from specific taxa and the type of diet. Additionally, only few differences were found in plasma metabolites, as well as fecal ones. Focusing on the abundance of the main recognized SCFA-producing bacteria, the genus Roseburia decreased in the low-fat/high-fiber diet, while the MD enhanced Roseburia and Oscillospira genera. A deeper evaluation (at species level) revealed an increase in Fecalibacterium prausnitzii in the low-fat/high-fiber diet only. Therefore, although collected results emphasized a specific relationship between microbes and these two different diets without compromising the hosts’ metabolomic profiles, both regimens improved insulin sensitivity as a result of the significant consumption of fiber.
In a crossover study, Hald et al. [117] investigated, in MetS subjects, the effects of arabinoxylan (from whole grain rye and wheat bran) and resistant starch type 2 (from raw potato starch and maize starch) on intestinal microbiota and also included a specific focus onto SCFAs. Whole grains are of interest because of their high fiber content and fermentative effect, as in the case of cereal fiber arabinoxylans and bran. Collected outcomes were compared against a low-fiber Western-style diet. Volunteers fed the fiber-enriched diet reported a significant decrease in indices of alpha diversity related to fecal microbiota community. This resulted from a decrease in various taxa (e.g., Bacteroides, Odoribacter, Dorea, Lachnospira, Ruminococcus, and Eubacterium), while only Bifidobacterium has been harbored by the fiber-enriched diet. Despite the few differences in microbial taxonomy, the study revealed how the fiber-enriched diet determined a greater fecal concentration of total SCFAs (mainly acetate and butyrate) than the low-fiber Western-style diet. Oppositely, the latter diet determined a greater concentration of brain chain fatty acids (BCFAs), indicating a high intestinal fermentation of proteins (mainly iso-butyrate and iso-valerate).
Vetrani et al. [118] studied 40 MetS individuals during a 12-week whole grain-based diet containing cereal fiber 28.9 ± 1.1 g/day compared with a refined cereal-based diet used as control (cereal fiber: 11.8 ± 0.4 g/day). Starting from no differences in SCFAs concentration at baseline between the two arms, the whole grain-diet was associated with an increased plasma propionate concentration correlating with improved insulin postprandial response.
6. Mechanisms of Action of Dietary Manipulation
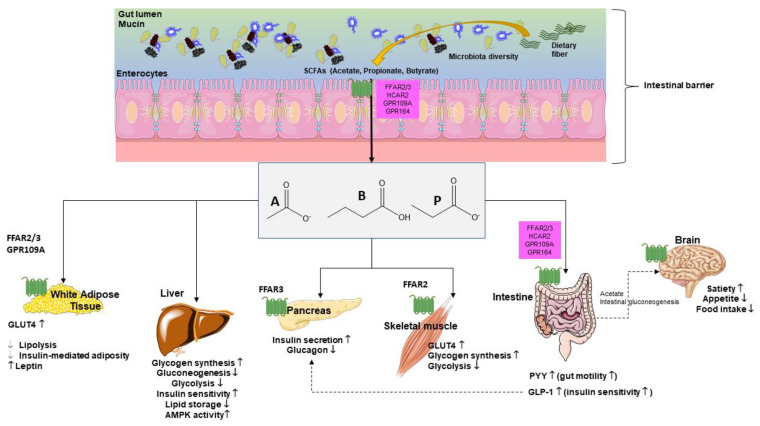
Fiber in diet can influence glucose metabolism as shown in both healthy individuals and T2DM individuals. Fiber viscosity, water solubility, and fermentation rates represent important properties in this respect [119]. In addition, dietary fiber modulates microbial composition and some metabolites, including SCFAs with ultimate effects on glucose metabolism (Figure 4).
Figure 4.
Glucose metabolism is influenced by short chain fatty acids (SCFAs), systemically, at various levels [120]. Main target organs include adipocytes, liver, pancreas, skeletal muscle, intestine, and brain where pathways govern mechanisms, which include receptors, synthesis, hormones, and perception. Abbreviations: GLP-1—glucagon-like peptide-1; GLUT-4—activated glucose transporter protein-4; GPR—G-protein-coupled receptors; PYY—peptide YY; A—acetate; P—propionate; B—butyrate.
SCFAs act as secretagogues for two key intestinal hormones, namely glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). This step appears to increase the satiety feeling through the gut–brain axis. By this pathway, SCFAs indirectly reduce appetite and food intake, a step which prevents body weight gain and, in turn, the risk of T2DM. The SCFAs effect on GLP-1-mediated increase in insulin secretion can regulate blood glucose concentrations [33].
SCFAs can decrease hepatic glycolysis and gluconeogenesis and increase glycogen synthesis. SCFAs also increase long chain fatty acids oxidation [33,121,122,123,124].
In skeletal muscle and adipose tissue SCFAs improve glucose uptake, an effect mediated by increased expression of GLUT4, through AMP kinase (AMPK) activity. In the skeletal muscle, SCFAs reduce glycolysis, a step associated with accumulation of glucose-6-phosphate and increased glycogen synthesis [121,122,123,124,125,126,127].
These studies are not conclusive and deserve confirmation; however, further evidence suggests that daily supplementation with 10 g inulin-propionate is associated with increased GLP-1 and PYY and reduced food intake [128,129]. In addition, daily supplementation with 4 g sodium butyrate improves insulin sensitivity; however, the effect is evident in lean subjects and not in subjects with metabolic syndrome [130].
The fine interplay between host and microbiota includes intestinal aspects involving production of microbial metabolites and preservation of intestinal integrity. If mechanisms fail, dysbiosis, leaky gut, and endotoxemia become pathological aspects. Evidence suggests that lactic acid bacteria (LAB), as well as other taxa, such as Akkermansia muciniphila and Bifidobacteria, can reduce intestinal permeability and inflammation [95,131]. In this scenario, SCFAs are bacterial post-biotics made from fermentation of dietary fiber. In line with this evidence, a diet enriched in fiber, following a prebiotic effect, drives the gut microbiota mainly harboring SCFA-producing microbial groups (e.g., LAB [31]) and taxa, such as Roseburia, Blautia, Fecalibacterium prausnitzii, and Prevotella [132,133,134]. Moreover, evidence suggests that the gut microbiota is involved in the regulation of glucose metabolism, and fiber intake is one protective factor. These mechanisms can therefore play a role with respect to the risk of developing T2DM [135].
Fiber intake might become an additional “natural” tool used for the prevention and management of metabolic disorders. Studies need to check if soluble and insoluble fiber may affect microbiota in a different way. Fiber in diet include β-glucan and arabinoxylans from wholegrains, pectins from fruit, vegetables, and legumes, and resistant starch. Therefore, the gut microbiota could distinctively process fiber and influence glucose homeostasis. In this respect, soluble, readily fermented fiber should be more effective than other types of fiber [119]. The effect of insoluble fiber on T2DM risk might differ and involve additional mechanisms, such as control of body weight gain, and increased fecal excretion of glucose [119,136]. Thus, the consumption of wholegrain, legumes, fruit, and vegetables should be increased in patients with pre-diabetes or diabetes, since this approach will increase dietary fiber intake.
By contrast, there is no evidence for fiber supplements or SCFA-based formulations.
Whether special—rather than traditional—dietary regimens provide similar or different effects on the fiber–microbiota–SCFA axis is still under debate. For example, short-term carbohydrate-restricted diets are associated with a reduction in SCFA-producing microbial species (Bifidobacteria) and SCFAs [137,138]. Ketogenic diets might also dramatically influence the gut microbiota profile [139]. By literature review, Rondanelli et al. [140] suggests that the very low calorie ketogenic diet preserved the core fecal microbiome but altered the composition of fecal microbial populations in relation to the plasma metabolome and fecal bile acid composition. The weight loss resulted in a reduction in E. rectale and Roseburia and an increase in Christensenellaceae and Akkermansia. Not all studies found a decrease in Fecalibacterium prausnitzii; however, in this field of research, it is important to emphasize the great impact of the adopted sequencing methods [141].
7. Conclusions
An increasing number of studies are actively investigating how diet and other external stimuli drive the gut microbiota composition and activity. Quality and quantity of dietary fiber play a pivotal role and act as microbiota-accessible carbohydrates and substrates for production of SCFAs, namely acetate, butyrate, and propionate. SCFAs, in turn, produce effects both at the local intestinal level and at a systemic level, acting through epigenetic mechanisms and via interaction with several receptors and tissues involved in the maintenance of glucose homeostasis, in pre-diabetes and in T2DM.
Specific links have been identified between microbiota diversity, SCFAs production, and glucose homeostasis, with possible implications in terms of management and prevention of altered glucose metabolism and T2DM. In this context, several clinical studies show the beneficial effect of fiber-enriched diets on health and in patients with pre-diabetes or T2DM, mainly in terms of increased insulin sensitivity, reduced fasting, and postprandial glucose.
The exclusion from the diet of whole grains, such as pasta and bakery products, may not be beneficial for healthy microbiota patterns. Thus, an increased daily fiber intake might be a valid tool to improve microbiota composition and activity, and to prevent metabolic disorders. In addition, the role of prebiotics, probiotics, and SCFAs, per se, in improving abnormalities in the maintenance of glucose homeostasis is a matter of active research.
Acknowledgments
We acknowledge the technical expertise of Paola De Benedictis, Rosa De Venuto, and Domenica Di Palo.
Author Contributions
Conceptualization, P.P. and A.D.C.; writing—original draft preparation, P.P. and L.B.; writing—review and editing, M.V., M.S., M.D.A., I.F., E.L. and M.K.; supervision P.P. and D.Q.-H.W.; funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
The present paper is written in the context of the project FOIE GRAS where P.P. has received funding from the European Union’s Horizon 2020 Research and Innovation program under the Marie Sklodowska-Curie Grant Agreement No. 722619. P.P. and M.D.A. are recipient of grant FEVER APULIAE from Region Apulia (Rare Diseases—all’art. 6 della l.r. n. 15 del 2018), 2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dommett R., Zilbauer M., George J.T., Bajaj-Elliott M. Innate immune defence in the human gastrointestinal tract. Mol. Immunol. 2005;42:903–912. doi: 10.1016/j.molimm.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Abdul Rahim M.B.H., Chilloux J., Martinez-Gili L., Neves A.L., Myridakis A., Gooderham N., Dumas M.-E. Diet-induced metabolic changes of the human gut microbiome: Importance of short-chain fatty acids, methylamines and indoles. Acta Diabetol. 2019;56:493–500. doi: 10.1007/s00592-019-01312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers E.S., Preston T., Frost G., Morrison D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018;7:198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portincasa P., Bonfrate L., Khalil M., Angelis M.D., Calabrese F.M., D’Amato M., Wang D.Q.-H., Di Ciaula A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines. 2022;10:83. doi: 10.3390/biomedicines10010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Ciaula A., Baj J., Garruti G., Celano G., De Angelis M., Wang H.H., Di Palo D.M., Bonfrate L., Wang D.Q.-H., Portincasa P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020;9:2648. doi: 10.3390/jcm9082648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohajeri M.H., Brummer R.J.M., Rastall R.A., Weersma R.K., Harmsen H.J.M., Faas M., Eggersdorfer M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018;57:1–14. doi: 10.1007/s00394-018-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGhee J.R., Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012;10:e1001397. doi: 10.1371/journal.pbio.1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammad S., Thiemermann C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2020;11:594150. doi: 10.3389/fimmu.2020.594150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makki K., Deehan E.C., Walter J., Backhed F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Di Ciaula A., Garruti G., Baccetto R.L., Molina-Molina E., Bonfrate R.L., Wang D.Q.-H., Portincasa P. Bile Acid Physiology. Ann. Hepatol. 2017;16:S4–S14. doi: 10.5604/01.3001.0010.5493. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Guryn K., Hubert N., Frazier K., Urlass S., Musch M.W., Ojeda P., Pierre J.F., Miyoshi J., Sontag T.J., Cham C.M., et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe. 2018;23:458–469. doi: 10.1016/j.chom.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocchetti M.T., Di Iorio B.R., Vacca M., Cosola C., Marzocco S., di Bari I., Calabrese F.M., Ciarcia R., De Angelis M., Gesualdo L. Ketoanalogs’ Effects on Intestinal Microbiota Modulation and Uremic Toxins Serum Levels in Chronic Kidney Disease (Medika2 Study) J. Clin. Med. 2021;10:840. doi: 10.3390/jcm10040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Angelis M., Ferrocino I., Calabrese F.M., De Filippis F., Cavallo N., Siragusa S., Rampelli S., Di Cagno R., Rantsiou K., Vannini L., et al. Diet influences the functions of the human intestinal microbiome. Sci. Rep. 2020;10:4247. doi: 10.1038/s41598-020-61192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Q., Liang Q., Balakrishnan B., Belobrajdic D.P., Feng Q.J., Zhang W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients. 2020;12:381. doi: 10.3390/nu12020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of Medicine . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. National Academies Press; Washington, DC, USA: 2005. Dietary, functional, and total fiber. [Google Scholar]
- 18.Swann O.G., Kilpatrick M., Breslin M., Oddy W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020;78:394–411. doi: 10.1093/nutrit/nuz072. [DOI] [PubMed] [Google Scholar]
- 19.Soliman G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients. 2019;11:1155. doi: 10.3390/nu11051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Titgemeyer E.C., Bourquin L.D., Fahey G.C., Jr., Garleb K.A. Fermentability of various fiber sources by human fecal bacteria in vitro. Am. J. Clin. Nutr. 1991;53:1418–1424. doi: 10.1093/ajcn/53.6.1418. [DOI] [PubMed] [Google Scholar]
- 21.Sonnenburg E.D., Smits S.A., Tikhonov M., Higginbottom S.K., Wingreen N.S., Sonnenburg J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamaker B.R., Tuncil Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014;426:3838–3850. doi: 10.1016/j.jmb.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 23.Usuda H., Okamoto T., Wada K. Leaky Gut: Effect of Dietary Fiber and Fats on Microbiome and Intestinal Barrier. Int. J. Mol. Sci. 2021;22:7613. doi: 10.3390/ijms22147613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantarel B.L., Lombard V., Henrissat B. Complex carbohydrate utilization by the healthy human microbiome. PLoS ONE. 2012;7:e28742. doi: 10.1371/journal.pone.0028742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pham V.T., Calatayud M., Rotsaert C., Seifert N., Richard N., Van den Abbeele P., Marzorati M., Steinert R.E. Antioxidant Vitamins and Prebiotic FOS and XOS Differentially Shift Microbiota Composition and Function and Improve Intestinal Epithelial Barrier In Vitro. Nutrients. 2021;13:1125. doi: 10.3390/nu13041125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnenburg J.L., Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schirmer M., Smeekens S.P., Vlamakis H., Jaeger M., Oosting M., Franzosa E.A., Ter Horst R., Jansen T., Jacobs L., Bonder M.J., et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell. 2016;167:1125–1136. doi: 10.1016/j.cell.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rios-Covian D., Ruas-Madiedo P., Margolles A., Gueimonde M., De Los Reyes-Gavilan C.G., Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rios-Covian D., Gueimonde M., Duncan S.H., Flint H.J., de los Reyes-Gavilan C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015;362:fnv176. doi: 10.1093/femsle/fnv176. [DOI] [PubMed] [Google Scholar]
- 30.Mahowald M.A., Rey F.E., Seedorf H., Turnbaugh P.J., Fulton R.S., Wollam A., Shah N., Wang C., Magrini V., Wilson R.K., et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA. 2009;106:5859–5864. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 32.Peluzio M.D.C.G., Martinez J.A., Milagro F.I. Postbiotics: Metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci. Technol. 2021;108:11–26. doi: 10.1016/j.tifs.2020.12.004. [DOI] [Google Scholar]
- 33.Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 34.Macfarlane S., Macfarlane G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 35.Joseph J., Depp C., Shih P.-A.B., Cadenhead K.S., Schmid-Schonbein G. Modified Mediterranean Diet for Enrichment of Short Chain Fatty Acids: Potential Adjunctive Therapeutic to Target Immune and Metabolic Dysfunction in Schizophrenia? Front. Neurosci. 2017;11:155. doi: 10.3389/fnins.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basson A., Trotter A., Rodriguez-Palacios A., Cominelli F. Mucosal Interactions between Genetics, Diet, and Microbiome in Inflammatory Bowel Disease. Front. Immunol. 2016;7:290. doi: 10.3389/fimmu.2016.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 38.Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 39.Salonen A., Lahti L., Salojarvi J., Holtrop G., Korpela K., Duncan S.H., Date P., Farquharson F., Johnstone A.M., Lobley G.E., et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8:2218–2230. doi: 10.1038/ismej.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baothman O.A., Zamzami M.A., Taher I., Abubaker J., Abu-Farha M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016;15:108. doi: 10.1186/s12944-016-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker A.W., Duncan S.H., McWilliam Leitch E.C., Child M.W., Flint H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005;71:3692–3700. doi: 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim C.H., Park J., Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–288. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parada Venegas D., De la Fuente M.K., Landskron G., Gonzalez M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Y., Wang Y., Wang P., Huang Y., Wang F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell. Physiol. Biochem. 2018;49:190–205. doi: 10.1159/000492853. [DOI] [PubMed] [Google Scholar]
- 45.Alex S., Lange K., Amolo T., Grinstead J.S., Haakonsson A.K., Szalowska E., Koppen A., Mudde K., Haenen D., Al-Lahham S., et al. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor gamma. Mol. Cell. Biol. 2013;33:1303–1316. doi: 10.1128/MCB.00858-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macia L., Tan J., Vieira A.T., Leach K., Stanley D., Luong S., Maruya M., Ian McKenzie C., Hijikata A., Wong C., et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 47.Nowarski R., Jackson R., Gagliani N., de Zoete M.R., Palm N.W., Bailis W., Low J.S., Harman C.C., Graham M., Elinav E., et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell. 2015;163:1444–1456. doi: 10.1016/j.cell.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathewson N.D., Jenq R., Mathew A.V., Koenigsknecht M., Hanash A., Toubai T., Oravecz-Wilson K., Wu S.R., Sun Y., Rossi C., et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016;17:505–513. doi: 10.1038/ni.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaudier E., Jarry A., Blottiere H.M., de Coppet P., Buisine M.P., Aubert J.P., Laboisse C., Cherbut C., Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G1168–G1174. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- 50.Peng L., Li Z.-R., Green R.S., Holzman I.R., Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang X., Oshima T., Tomita T., Fukui H., Miwa H. Butyrate Alleviates Cytokine-Induced Barrier Dysfunction by Modifying Claudin-2 Levels. Biology. 2021;10:205. doi: 10.3390/biology10030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saeedi B.J., Kao D.J., Kitzenberg D.A., Dobrinskikh E., Schwisow K.D., Masterson J.C., Kendrick A.A., Kelly C.J., Bayless A.J., Kominsky D.J., et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol. Biol. Cell. 2015;26:2252–2262. doi: 10.1091/mbc.E14-07-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J., Wilson K.E., Glover L.E., Kominsky D.J., Magnuson A. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vernero M., De Blasio F., Ribaldone D.G., Bugianesi E., Pellicano R., Saracco G.M., Astegiano M., Caviglia G.P. The Usefulness of Microencapsulated Sodium Butyrate Add-On Therapy in Maintaining Remission in Patients with Ulcerative Colitis: A Prospective Observational Study. J. Clin. Med. 2020;9:3941. doi: 10.3390/jcm9123941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tong L.-C., Wang Y., Wang Z.-B., Liu W.-Y., Sun S., Li L., Su D.-F., Zhang L.-C. Propionate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Front. Pharmacol. 2016;7:253. doi: 10.3389/fphar.2016.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki T., Yoshida S., Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 2008;100:297–305. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- 58.Suligoj T., Vigsnaes L.K., Abbeele P.V.D., Apostolou A., Karalis K., Savva G.M., McConnell B., Juge N. Effects of Human Milk Oligosaccharides on the Adult Gut Microbiota and Barrier Function. Nutrients. 2020;12:2808. doi: 10.3390/nu12092808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanson G.R., Siskin J., Gorenz A., Shaikh M., Raeisi S., Fogg L., Forsyth C., Keshavarzian A. Disrupted diurnal oscillation of gut-derived Short chain fatty acids in shift workers drinking alcohol: Possible mechanism for loss of resiliency of intestinal barrier in disrupted circadian host. J. Lab. Clin. Med. 2020;221:97–109. doi: 10.1016/j.trsl.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sam A.H., Troke R.C., Tan T.M., Bewick G.A. The role of the gut/brain axis in modulating food intake. Neuropharmacology. 2012;63:46–56. doi: 10.1016/j.neuropharm.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Freeland K.R., Wolever T.M.S. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br. J. Nutr. 2010;103:460–466. doi: 10.1017/S0007114509991863. [DOI] [PubMed] [Google Scholar]
- 62.Musso G., Gambino R., Cassader M. Obesity, diabetes, and gut microbiota: The hygiene hypothesis expanded? Diabetes Care. 2010;33:2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Svegliati-Baroni G., Saccomanno S., Rychlicki C., Agostinelli L., De Minicis S., Candelaresi C., Faraci G., Pacetti D., Vivarelli M., Nicolini D., et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–1297. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 64.Fukumoto S., Tatewaki M., Yamada T., Fujimiya M., Mantyh C., Voss M., Eubanks S., Harris M., Pappas T.N., Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 65.Marchix J., Goddard G., Helmrath M.A. Host-Gut Microbiota Crosstalk in Intestinal Adaptation. Cell. Mol. Gastroenterol. Hepatol. 2018;6:149–162. doi: 10.1016/j.jcmgh.2018.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Derrien M., van Hylckama Vlieg J.E.T. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23:354–366. doi: 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Derrien M., Van Baarlen P., Hooiveld G., Norin E., Muller M., de Vos W.M. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Front. Microbiol. 2011;2:166. doi: 10.3389/fmicb.2011.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kien C.L., Peltier C.P., Mandal S., Davie J.R., Blauwiekel R. Effects of the in vivo supply of butyrate on histone acetylation of cecum in piglets. JPEN J. Parenter. Enter. Nutr. 2008;32:51–56. doi: 10.1177/014860710803200151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stilling R.M., van de Wouw M., Clarke G., Stanton C., Dinan T.G., Cryan J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 70.Park J., Kim M., Kang S.G., Jannasch A.H., Cooper B., Patterson J., Kim C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim M.H., Kang S.G., Park J.H., Yanagisawa M., Kim C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 72.Li Z., Yi C.-X., Katiraei S., Kooijman S., Zhou E., Chung C.K., Gao Y., van den Heuvel J.K., Meijer O.C., Berbee J.F.P., et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67:1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- 73.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Backhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 74.Mollica M.P., Mattace Raso G., Cavaliere G., Trinchese G., De Filippo C., Aceto S., Prisco M., Pirozzi C., Di Guida F., Lama A., et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes. 2017;66:1405–1418. doi: 10.2337/db16-0924. [DOI] [PubMed] [Google Scholar]
- 75.Schoenfeld P., Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016;57:943–954. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boets E., Gomand S.V., Deroover L., Preston T., Vermeulen K., De Preter V., Hamer H.M., Van den Mooter G., De Vuyst L., Courtin C.M., et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017;595:541–555. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leung C., Rivera L., Furness J.B., Angus C.L.P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 78.Brussow H., Parkinson S.J. You are what you eat. Nat. Biotechnol. 2014;32:243–245. doi: 10.1038/nbt.2845. [DOI] [PubMed] [Google Scholar]
- 79.Subramanian S., Goodspeed L., Wang S., Kim J., Zeng L., Ioannou G.N., Haigh W.G., Yeh M.M., Kowdley K.V., O’Brien K.D., et al. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J. Lipid Res. 2011;52:1626–1635. doi: 10.1194/jlr.M016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boets E., Deroover L., Houben E., Vermeulen K., Gomand S.V., Delcour J.A., Verbeke K. Quantification of in Vivo Colonic Short Chain Fatty Acid Production from Inulin. Nutrients. 2015;7:8916–8929. doi: 10.3390/nu7115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cummings J.H., Pomare E.W., Branch W.J., Naylor C.P., Macfarlane G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu Y., Zhu Y., Li X., Sun B. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends Food Sci. Technol. 2020;100:118–130. doi: 10.1016/j.tifs.2020.02.026. [DOI] [Google Scholar]
- 83.Sanders M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008;46((Suppl. 2)):S58–S61. doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- 84.LeBlanc J.G., Chain F., Martin R., Bermudez-Humaran L.G., Courau S., Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017;16:79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 86.Garcia-Mantrana I., Selma-Royo M., Alcantara C., Collado M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018;9:890. doi: 10.3389/fmicb.2018.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verbeke K.A., Boobis A.R., Chiodini A., Edwards C.A., Franck A., Kleerebezem M., Nauta A., Raes J., van Tol E.A., Tuohy K.M. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr. Res. Rev. 2015;28:42–66. doi: 10.1017/S0954422415000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roberfroid M., Gibson G.R., Hoyles L., McCartney A.L., Rastall R., Rowland I., Wolvers D., Watzl B., Szajewska H., Stahl B., et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010;104((Suppl. 2)):S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 89.Macfarlane G.T., Macfarlane S. Fermentation in the human large intestine: Its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 2011;45:S120–S127. doi: 10.1097/MCG.0b013e31822fecfe. [DOI] [PubMed] [Google Scholar]
- 90.Sakata T., Kojima T., Fujieda M., Takahashi M., Michibata T. Influences of probiotic bacteria on organic acid production by pig caecal bacteria in vitro. Proc. Nutr. Soc. 2003;62:73–80. doi: 10.1079/PNS2002211. [DOI] [PubMed] [Google Scholar]
- 91.Gutierrez-Diaz I., Fernandez-Navarro T., Sanchez B., Margolles A., Gonzalez S. Mediterranean diet and faecal microbiota: A transversal study. Food Funct. 2016;7:2347–2356. doi: 10.1039/C6FO00105J. [DOI] [PubMed] [Google Scholar]
- 92.De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L., Serrazanetti D.I., Di Cagno R., Ferrocino I., Lazzi C., et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 93.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 95.Salgaco M.K., Oliveira L.G.S., Costa G.N., Bianchi F., Sivieri K. Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus. Appl. Microbiol. Biotechnol. 2019;103:9229–9238. doi: 10.1007/s00253-019-10156-y. [DOI] [PubMed] [Google Scholar]
- 96.Kappel B.A., Federici M. Gut microbiome and cardiometabolic risk. Rev. Endocr. Metab. Disord. 2019;20:399–406. doi: 10.1007/s11154-019-09533-9. [DOI] [PubMed] [Google Scholar]
- 97.Kreznar J.H., Keller M.P., Traeger L.L., Rabaglia M.E., Schueler K.L., Stapleton D.S., Zhao W., Vivas E.I., Yandell B.S., Broman A.T., et al. Host Genotype and Gut Microbiome Modulate Insulin Secretion and Diet-Induced Metabolic Phenotypes. Cell Rep. 2017;18:1739–1750. doi: 10.1016/j.celrep.2017.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ley R.E., Backhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Federici M. Gut microbiome and microbial metabolites: A new system affecting metabolic disorders. J. Endocrinol. Investig. 2019;42:1011–1018. doi: 10.1007/s40618-019-01022-9. [DOI] [PubMed] [Google Scholar]
- 100.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 101.Zhao L., Zhang F., Ding X., Wu G., Lam Y., Wang X., Fu H., Xue X., Lu C., Ma J., et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 102.Rothschild D., Weissbrod O., Barkan E., Kurilshikov A., Korem T., Zeevi D., Costea P.I., Godneva A., Kalka I.N., Bar N., et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 103.Lynch S.V., Pedersen O. The Human Intestinal Microbiome in Health and Disease. New Engl. J. Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 104.Zhang X., Shen D., Fang Z., Jie Z., Qiu X., Zhang C., Chen Y., Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE. 2013;8:e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu H., Tremaroli V., Schmidt C., Lundqvist A., Olsson L.M., Kramer M., Gummesson A., Perkins R., Bergstrom G., Backhed F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020;32:379–390. doi: 10.1016/j.cmet.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 106.Karlsson F.H., Tremaroli V., Nookaew I., Bergstrom G., Behre C.J., Fagerberg B., Nielsen J., Backhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 107.Sanna S., van Zuydam N., Mahajan A., Kurilshikov A., Vich Vila A., Võsa U., Mujagic Z., Masclee A., Jonkers D., Oosting M. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019;51:600–605. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., Collini S., Pieraccini G., Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mitsou E.K., Kakali A., Antonopoulou S., Mountzouris K.C., Yannakoulia M., Panagiotakos D.B., Kyriacou A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017;117:1645–1655. doi: 10.1017/S0007114517001593. [DOI] [PubMed] [Google Scholar]
- 110.Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R., Fernandes G.R., Tap J., Bruls T., Batto J.M., et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kovatcheva-Datchary P., Nilsson A., Akrami R., Lee Y.S., De Vadder F., Arora T., Hallen A., Martens E., Bjorck I., Backhed F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 112.Nilsson A.C., Johansson-Boll E.V., Bjorck I.M.E. Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: A randomised cross-over study in healthy middle-aged subjects. Br. J. Nutr. 2015;114:899–907. doi: 10.1017/S0007114515002524. [DOI] [PubMed] [Google Scholar]
- 113.Freeland K.R., Wilson C., Wolever T.M. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br. J. Nutr. 2010;103:82–90. doi: 10.1017/S0007114509991462. [DOI] [PubMed] [Google Scholar]
- 114.Vacca M., Celano G., Calabrese F.M., Portincasa P., Gobbetti M., De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020;8:573. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vitale M., Giacco R., Laiola M., Della Pepa G., Luongo D., Mangione A., Salamone D., Vitaglione P., Ercolini D., Rivellese A.A. Acute and chronic improvement in postprandial glucose metabolism by a diet resembling the traditional Mediterranean dietary pattern: Can SCFAs play a role? Clin. Nutr. 2021;40:428–437. doi: 10.1016/j.clnu.2020.05.025. [DOI] [PubMed] [Google Scholar]
- 116.Haro C., Montes-Borrego M., Rangel-Zuniga O.A., Alcala-Diaz J.F., Gomez-Delgado F., Perez-Martinez P., Delgado-Lista J., Quintana-Navarro G.M., Tinahones F.J., Landa B.B., et al. Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population. J. Clin. Endocrinol. Metab. 2016;101:233–242. doi: 10.1210/jc.2015-3351. [DOI] [PubMed] [Google Scholar]
- 117.Hald S., Schioldan A.G., Moore M.E., Dige A., Laerke H.N., Agnholt J., Bach Knudsen K.E., Hermansen K., Marco M.L., Gregersen S., et al. Effects of Arabinoxylan and Resistant Starch on Intestinal Microbiota and Short-Chain Fatty Acids in Subjects with Metabolic Syndrome: A Randomised Crossover Study. PLoS ONE. 2016;11:e0159223. doi: 10.1371/journal.pone.0159223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vetrani C., Costabile G., Luongo D., Naviglio D., Rivellese A.A., Riccardi G., Giacco R. Effects of whole-grain cereal foods on plasma short chain fatty acid concentrations in individuals with the metabolic syndrome. Nutrition. 2016;32:217–221. doi: 10.1016/j.nut.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 119.Bozzetto L., Costabile G., Della Pepa G., Ciciola P., Vetrani C., Vitale M., Rivellese A.A., Annuzzi G. Dietary Fibre as a Unifying Remedy for the Whole Spectrum of Obesity-Associated Cardiovascular Risk. Nutrients. 2018;10:943. doi: 10.3390/nu10070943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salamone D., Rivellese A.A., Vetrani C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021;58:1131–1138. doi: 10.1007/s00592-021-01727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.He J., Zhang P., Shen L., Niu L., Tan Y., Chen L., Zhao Y., Bai L., Hao X., Li X., et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020;21:6356. doi: 10.3390/ijms21176356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li X., Chen H., Guan Y., Li X., Lei L., Liu J., Yin L., Liu G., Wang Z. Acetic acid activates the AMP-activated protein kinase signaling pathway to regulate lipid metabolism in bovine hepatocytes. PLoS ONE. 2013;8:e67880. doi: 10.1371/journal.pone.0067880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li H., Gao Z., Zhang J., Ye X., Xu A., Ye J., Jia W. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes. 2012;61:797–806. doi: 10.2337/db11-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fushimi T. Ph.D. Thesis. Nagoya University; Nagoya, Japan: 2004. Acetic acid feeding enhances glycogen repletion in liver and skeletal muscle of rats. [DOI] [PubMed] [Google Scholar]
- 125.Yamashita H., Maruta H., Jozuka M., Kimura R., Iwabuchi H., Yamato M., Saito T., Fujisawa K., Takahashi Y., Kimoto M., et al. Effects of acetate on lipid metabolism in muscles and adipose tissues of type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol. Biochem. 2009;73:570–576. doi: 10.1271/bbb.80634. [DOI] [PubMed] [Google Scholar]
- 126.Yamashita H. Biological Function of Acetic Acid-Improvement in Obesity and Glucose Tolerance by Acetic Acid in Type 2 Diabetic Rats. Crit. Rev. Food Sci. Nutr. 2016;56((Suppl. 1)):S171–S175. doi: 10.1080/10408398.2015.1045966. [DOI] [PubMed] [Google Scholar]
- 127.Gao Z., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M., Cefalu W.T., Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Byrne C.S., Chambers E.S., Preston T., Tedford C., Brignardello J., Garcia-Perez I., Holmes E., Wallis G.A., Morrison D.J., Frost G.S. Effects of Inulin Propionate Ester Incorporated into Palatable Food Products on Appetite and Resting Energy Expenditure: A Randomised Crossover Study. Nutrients. 2019;11:861. doi: 10.3390/nu11040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chambers E.S., Viardot A., Psichas A., Morrison D.J., Murphy K.G., Zac-Varghese S.E., MacDougall K., Preston T., Tedford C., Finlayson G.S., et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bouter K.E., Bakker G.J., Levin E., Hartstra A.V., Kootte R.S., Udayappan S.D., Katiraei S., Bahler L., Gilijamse P.W., Tremaroli V., et al. Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects. Clin. Transl. Gastroenterol. 2018;9:155. doi: 10.1038/s41424-018-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bui T.P.N., de Vos W.M. Next-generation therapeutic bacteria for treatment of obesity, diabetes, and other endocrine diseases. Best Pract. Research. Clin. Endocrinol. Metab. 2021;35:101504. doi: 10.1016/j.beem.2021.101504. [DOI] [PubMed] [Google Scholar]
- 132.Carroccio A., Celano G., Cottone C., Di Sclafani G., Vannini L., D’Alcamo A., Vacca M., Calabrese F.M., Mansueto P., Soresi M., et al. WHOLE-meal ancient wheat-based diet: Effect on metabolic parameters and microbiota. Dig. Liver Dis. 2021;53:1412–1421. doi: 10.1016/j.dld.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 133.Fu X., Liu Z., Zhu C., Mou H., Kong Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019;59:S130–S152. doi: 10.1080/10408398.2018.1542587. [DOI] [PubMed] [Google Scholar]
- 134.Hosseini E., Grootaert C., Verstraete W., Van de Wiele T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011;69:245–258. doi: 10.1111/j.1753-4887.2011.00388.x. [DOI] [PubMed] [Google Scholar]
- 135.Reynolds A.N., Akerman A.P., Mann J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020;17:e1003053. doi: 10.1371/journal.pmed.1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barber T.M., Kabisch S., Pfeiffer A.F.H., Weickert M.O. The Health Benefits of Dietary Fibre. Nutrients. 2020;12:3209. doi: 10.3390/nu12103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ang Q.Y., Alexander M., Newman J.C., Tian Y., Cai J., Upadhyay V., Turnbaugh J.A., Verdin E., Hall K.D., Leibel R.L., et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell. 2020;181:1263–1275. doi: 10.1016/j.cell.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mardinoglu A., Wu H., Bjornson E., Zhang C., Hakkarainen A., Rasanen S.M., Lee S., Mancina R.M., Bergentall M., Pietilainen K.H., et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018;27:559–571. doi: 10.1016/j.cmet.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paoli A., Mancin L., Bianco A., Thomas E., Mota J.F., Piccini F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes. 2019;10:534. doi: 10.3390/genes10070534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rondanelli M., Gasparri C., Peroni G., Faliva M.A., Naso M., Perna S., Bazire P., Sajuox I., Maugeri R., Rigon C. The Potential Roles of Very Low Calorie, Very Low Calorie Ketogenic Diets and Very Low Carbohydrate Diets on the Gut Microbiota Composition. Front. Endocrinol. 2021;12:518. doi: 10.3389/fendo.2021.662591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mancabelli L., Milani C., Lugli G.A., Fontana F., Turroni F., van Sinderen D., Ventura M. The Impact of Primer Design on Amplicon-Based Metagenomic Profiling Accuracy: Detailed Insights into Bifidobacterial Community Structure. Microorganisms. 2020;8:131. doi: 10.3390/microorganisms8010131. [DOI] [PMC free article] [PubMed] [Google Scholar]