Abstract
Background:
Physical activity may increase the intake of air pollutants due to a higher ventilation rate, which may exacerbate the adverse health effects. This study investigated the combined effects of habitual exercise and long-term exposure to fine particulate matter (PM2.5) on the incidence of dyslipidemia in a large longitudinal cohort in Taiwan.
Methods:
A total of 121,948 adults (≥18 years) who received at least two medical examinations from 2001 to 2016 were recruited, yielding 407,821 medical examination records. A satellite-based spatiotemporal model was used to estimate the 2-year average PM2.5 concentration (i.e., the year of and the year before the medical examination) at each participant’s address. Information on habitual exercise within 1 month before the medical examination was collected using a standard self-administered questionnaire. A Cox regression model with time-dependent covariates was used to investigate the combined effects.
Results:
Compared with inactivity, moderate and high levels of exercise were associated with a lower incidence of dyslipidemia, with hazard ratios (HRs) (95% confidence intervals [CIs]) of 0.91 (0.88, 0.94) and 0.73 (0.71, 0.75), respectively. Participants with a moderate (22.37–25.96 μg/m3) or high (>25.96 μg/m3) level of PM2.5 exposure had a higher incidence of dyslipidemia than those with a low level of PM2.5 exposure (≤22.37 μg/m3), with HRs (95% CIs) of 1.36 (1.32, 1.40), and 1.90 (1.81, 1.99), respectively. We observed a statistically significant, but minor, interaction effect of PM2.5 exposure and exercise on the development of dyslipidemia, with an overall hazard ratios (95% CI) of 1.08 (1.05, 1.10), indicating that an incremental increase in the level of exercise was associated with an 8% increase in the risk of dyslipidemia associated with every 10 μg/m3 increase in PM2.5 exposure. However, the negative association between habitual exercise and dyslipidemia remained, regardless of the level of PM2.5 exposure, suggesting that the benefits of increased habitual exercise outweighed the adverse effects of the increase in PM2.5 intake during exercise.
Conclusions:
Increased levels of exercise and reduced levels of PM2.5 exposures were associated with a lower incidence of dyslipidemia. Although an increase in habitual exercise slightly increased the risk of dyslipidemia associated with PM2.5 exposure, the benefits of the increased habitual exercise outweighed the risks. Our findings suggest that habitual exercise is an effective approach for dyslipidemia prevention, even for people residing in relatively polluted areas.
Keywords: ambient PM2.5, habitual exercise, dyslipidemia, longitudinal cohort, Taiwan
What this study adds
This is the first large-scale cohort study investigating the combined effects of habitual exercise and chronic exposure to fine particulate matter (PM2.5) on dyslipidemia.
Increased levels of exercise and reduced exposure to PM2.5 are associated with a lower incidence of dyslipidemia.
Although the association between habitual exercise and dyslipidemia is slightly attenuated by PM2.5 exposure, the negative associations between dyslipidemia and habitual exercise remains, regardless of the level of PM2.5 exposure.
Habitual exercise is beneficial for dyslipidemia prevention, even for people residing in Taiwan, which has an annual PM2.5 concentration of 26.7 μg/m3.
Introduction
Cardiovascular disease contributed to 17.8 million deaths globally and was the leading cause of death in 2017.1 Cardiovascular-related deaths have increased by 21.1% over the last 10 years.1 Dyslipidemia, which refers to abnormal levels of blood lipids, is regarded as one of the crucial risk factors that can be modified to prevent cardiovascular disease, especially atherosclerotic cardiovascular disease.2
At least 30 minutes of exercise per day is recommended to improve the lipid abnormalities of dyslipidaemic patients.3 Previous studies have also shown that habitual exercise is associated with a better lipid profile in the general population.4,5 Habitual exercise is, therefore, important to improve lipid profiles and prevent cardiovascular disease. However, approximately 1 in 4 adults worldwide do not meet the World Health Organization (WHO) recommendations for physical activity.6 The WHO has proposed a global action plan to reduce the prevalence of insufficient physical activity by 15% by 2030.6
However, air pollution may discourage people from exercising because the higher ventilation rate during exercise may increase the inhalation and deposition of air pollutants. An increasing number of studies have shown that exposure to air pollution is associated with a poor lipid profile and a higher incidence of dyslipidemia.7–9 More than 91% of the global population lives in areas where air pollution levels exceed the WHO guidelines.10 Exposure to air pollution seems unavoidable to most people in the world. Balancing the beneficial health effects of exercise with the potentially harmful effects of the excess inhalation of air pollutants during exercise has become an important public health concern.
There is a lack of evidence for the combined effects of exercise and air pollution on dyslipidemia. We only identified one cross-sectional study in rural Chinese adults that reported no significant interaction effects of exercise and air pollution on metabolic syndrome,11 which includes dyslipidemia as a component. It is necessary to understand whether habitual exercise is beneficial to the lipid profile of people with exposure to high levels of air pollution. Therefore, we conducted a longitudinal cohort study consisting of 121,948 adults with 407,821 medical examination records to investigate the combined effects of habitual exercise and chronic fine particulate matter (PM2.5) exposure on the development of dyslipidemia in Taiwan, where the annual PM2.5 concentration is 2.6 times as high as the WHO guideline.12
Methods
Study design and population
This study was based on an ongoing cohort in Taiwan. Details of this cohort have been described in our previous publications.7,13–15 In brief, since 1994, a private firm, the MJ Health Management Institution, has recruited residents across Taiwan who have paid the membership fees to join the standard medical screening programme.16 Participants were encouraged to visit the institution periodically to receive a series of medical examinations, including anthropometric measurements, blood glucose/lipids measurements, and liver/kidney/lung functions tests during each medical visit. In addition, they were required to complete a standard self-administered questionnaire survey, consisting of questions about demographic information, family medical history, current health status, life style, and dietary habits. Information collected during each medical examination has been computerized since 1996 and has been deidentified for research use.17 From 1996 to 2016, approximately 600,000 Taiwan residents were recruited in the programme and 44% of those recruited had at least two medical visits. Each participant signed an informed consent form before each medical visit authorizing MJ Health Management Institution to release the data for research purposes. Ethical approval for this study has been obtained from the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee.
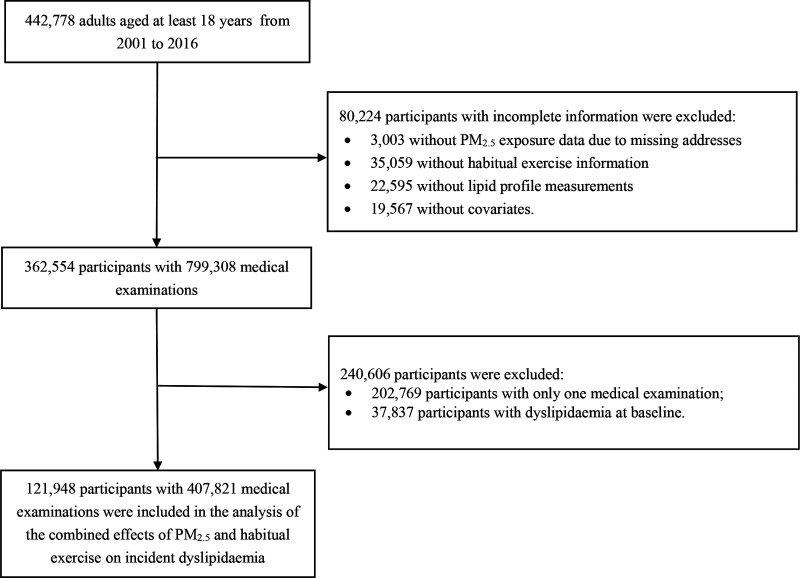
Figure 1 shows the participant selection procedure of. We targeted 442,778 participants aged at least 18 years who were recruited from 2001 to 2016, when satellites data were available for air pollution assessment. We excluded 80,224 participants due to their incomplete information (3,003 with incomplete PM2.5 exposure data due to missing addresses, 35,059 with missing habitual exercise data, 22,595 with missing lipid profiles, and 19,567 with missing covariates data). We further excluded 202,769 participants who had only one medical examination and 37,837 participants who had dyslipidemia at baseline (defined as total cholesterol [TC] concentration ≥240 mg/L, triglyceride [TG] concentration ≥200 mg/dL, high density lipoprotein-cholesterol [HDL-C] concentration <40 mg/dL, or low density lipoprotein-cholesterol [LDL-C] concentration ≥160 mg/dL).2,18,19 Finally, 121,948 participants with 407,821 medical measurements were selected for data analysis.
Figure 1.
Flow chart of participant selection.
Compared with the 121,948 participants included in the data analysis, the 320,830 excluded participants had similar distributions for most characteristics (eTable 1; http://links.lww.com/EE/A172). However, the excluded group comprised a higher percentage of males (50.0% vs. 43.4%) and inactivity (52.7% vs. 45.4%). The excluded group also had a higher TC concentration (mean: 194.9 mg/dL vs. 182.6 mg/dL), a higher TG concentration (mean: 122.4 mg/dL vs. 86.2 mg/dL), and a higher LDL-C concentration (mean: 116.5 mg/dL vs. 105.8 mg/dL).
PM2.5 exposure assessment
The relevant details about the assessment of ambient PM2.5 concentration have been described in previous studies.20,21 Briefly, an atmospheric optical depth (AOD)-PM2.5 model was developed to estimate ground-level PM2.5 concentration based on satellite data at a 1-km spatial resolution combined with ground-level measurements (i.e., meteorological and particulate matter data).20,21 The AOD data were derived from Terra and Aqua, the two moderate resolution imaging spectroradiometer (MODIS) instruments aboard the National Aeronautics and Space Administration Earth Observing System satellites.20,21 This method also accounted for the effects of aerosol characteristics (hygroscopic growth, particle mass extinction efficiency, and size distribution) on the AOD-PM2.5 relationship, which were generally not considered in previous observation-based methods.20,21 To validate our AOD-PM2.5 model, we obtained ground-based PM2.5 concentrations from more than 70 ground stations across Taiwan from 2005 to 2014 and compared them with the satellite-retrieved PM2.5 concentrations.15 The correlation coefficients for the two measurements ranged from 0.72 to 0.83.15
The address of each participant was geocoded into latitudinal and longitudinal data, and the estimated PM2.5 concentrations were matched with individuals’ addresses to determine their exposure to ambient PM2.5. We calculated the 2-year average PM2.5 concentrations based on the yearly average of PM2.5 concentrations for the year of the medical examination and the previous year. This value was then used as the indicator of long-term exposure to ambient PM2.5 air pollution. Both continuous (per 10 μg/m3) and categorical (participants were grouped into three categories based on the tertile cutoff points of PM2.5, i.e. low: ≤22.37; moderate: 22.37–25.96; and high: >25.96 μg/m3) PM2.5 concentration data were used for data analysis.
Habitual exercise assessment
Information on habitual leisure-time exercise was collected and assessed as described elsewhere.13,14,22,23 Briefly, information on habitual exercise was collected using a standard self-administrated questionnaire, in which participants were asked about the intensity level and duration of weekly exercise they completed in the previous month. The intensity level of weekly exercise was classified into the following four categories, with an example list of the types of exercise for each category, respectively: light (e.g., walking), moderate (e.g., brisk walking), medium-vigorous (e.g., jogging), and high-vigorous (e.g., rope skipping). Each level of exercise was assigned one of the following specific metabolic equivalent of task (MET; 1 MET = 1 kcal per hour per kilogram of bodyweight) values: 2.5 (light), 4.5 (moderate), 6.5 (medium-vigorous), and 8.5 (high-vigorous).24,25 If participants engaged in more than one intensity level of exercise, a weighted MET was assigned based on the time spent at each level. Therefore, the weekly exercise volume (MET-h) of each participant was calculated as the product of the intensity (MET) and duration (hours) of exercise. The participants were then classified into three physical activity groups for data analysis, based on the following rough tertile cutoff points of exercise-volume (MET-h): inactive (0 MET-h), moderate (0.01–8.75 MET-h), and high (> 8.75 MET-h). We did not use the continuous MET-h variable for data analysis because 0 MET-h was assigned to all participants in the inactive group.
Outcome ascertainment
Participants without dyslipidemia at baseline (n = 121,948) were followed up, and incident dyslipidemia was identified at subsequent follow-up visits for medical examinations. Incident dyslipidemia was defined as a TC concentration ≥ 240 mg/L, a TG concentration ≥ 200 mg/dL, an HDL-C concentration < 40 mg/dL, or an LDL-C concentration ≥ 160 mg/dL.2,18,19 Overnight fasting blood samples were drawn in the morning, and lipid profiles and fasting plasma glucose concentration were measured using an automated biochemical analyzer (HITACHI 7150 [Hitachi, Tokyo, Japan] before 2005 or TOSHIBA C8000 [Toshiba, Tokyo, Japan] since 2005). The end point was defined as the first occurrence of dyslipidemia or the final visit if dyslipidemia was not detected over the study period.
Covariates
The details of the data collection and quality-control measures have been described in the Technical Reports of the MJ Health Research Foundation and in previous studies.7,16,26 The weight and height of each participant were measured by trained staffs using an autoanthropometer (KN-5000A; Nakamura, Tokyo, Japan). Body mass index (BMI) was then calculated as the weight (kg) divided by the square of the height (m). Seated blood pressure including systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using a computerized automercury sphygmomanometer (CH-5000; Citizen, Tokyo, Japan). In addition, the participants’ demographic characteristics, behavioral and lifestyle factors, and medical history were collected through a standard self- administered questionnaire.
The following covariates were selected based on previous studies:7-9 age (years); sex (male or female); education status (lower than high school [<10 years], high school [10–12 years], college or university [13–16 years], or postgraduate [>16 years]); smoking status (never, ever [smoked at least once but quit later] or current [more than once a week]); alcohol consumption (seldom [<once/week], occasional [1–3 times/week], or regular [>3 times/week]); vegetable intake (seldom [<1 serving/day], moderate [1–2 servings/day], or frequent [>2 servings/day]); fruit intake (seldom [<1 serving/day], moderate [1–2 servings/day], or frequent [>2 servings/day]); occupational exposure to dust or solvents in the workplace (yes or no); physical labor at work (sedentary jobs [e.g., clerk], jobs that require approximately half sedentary and half standing/walking [e.g., nurse], jobs that mostly require walking and standing [e.g., retail salesperson], or jobs that require vigorous physical activity [e.g., porter]); BMI (kg/m2); diabetes (defined as fasting blood glucose concentration ≥ 126 mg/dL or self-reported physician-diagnosed diabetes); hypertension (defined as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or self-reported physician-diagnosed hypertension); self-reported cardiovascular disease, including stroke (yes or no); any self-reported form of cancer (yes or no); and the season (spring [March to May], summer [June to August], autumn [September to November], or winter [December to February]) and calendar year of baseline visit.
Data analysis
A time-varying Cox regression model with a random participant-level intercept was used to investigate the associations between exercise or PM2.5 concentration and incident dyslipidemia. A city-level random intercept was added to the model to adjust for the correlation between individuals living within the same city. The follow-up time was used as the time scale in the model. The level of habitual exercise, PM2.5 concentration, and all covariates (except for sex and baseline year) were treated as time-varying variables in the analysis, to account for changes in the variables during the study period. Three models with the gradual addition of the aforementioned covariates were developed to investigate the main effects of exercise or PM2.5 exposure level. The Crude Model had no adjustments for covariates. Model 1 was adjusted for age, sex, educational level, season, baseline year, smoking status, alcohol consumption, vegetable intake, fruit intake, occupational exposure, and physical labor at work. Model 2 was further adjusted for BMI, diabetes, hypertension, self-reported cardiovascular disease, and self-reported cancer. A trend test was performed across exercise and PM2.5 categories, with the corresponding category treated as a numerical variable (an ordinal variable code of 1–3). HRs with 95% CIs for exercise are presented with the inactive-exercise group as the reference. HRs with 95% CIs for PM2.5 were estimated for every 10 μg/m3 increase in PM2.5 concentration or with the low-PM2.5 exposure group as the reference.
Mutual adjustments for exercise and PM2.5 concentration were performed for comparison (i.e., we further included PM2.5 concentration in the model to assess the main effect of exercise, or exercise in the model to assess the main effect of PM2.5 concentration). The interaction term, “exercise (inactive, moderate, and high) × continuous PM2.5 concentration (every 10 μg/m3)” was added to Model 2 to examine the potential interaction effects of exercise and PM2.5 concentration. We also used a likelihood ratio test to explore the potential interaction effect for comparison.
In addition, subgroup analyses were performed through the stratification of PM2.5 concentration or exercise categories, separately. Finally, to examine the combined effects of exercise and PM2.5 exposure, the participants were classified into nine groups based on their exercise and PM2.5 concentration categories, and those who were inactive and had a high level of PM2.5 exposure served as the reference group.
A series of sensitivity analyses were performed. First, we excluded participants with baseline diabetes, hypertension, obesity (defined as BMI ≥30), cardiovascular diseases, or cancer and those using lipid-lowering, antidiabetic or antihypertensive drugs to eliminate potential comorbidity effects. Second, we used the annual average PM2.5 concentration from the year before the medical visit to examine the stability of the PM2.5 exposure effects. Third, we excluded participants who enrolled before 2005, whose lipid profiles were measured using the HITACHI 7150 analyzer, to eliminate potential measurement bias due to the use of different equipment. Fourth, we excluded the participants who were followed-up for less than 2 years in the analysis, as dyslipidemia is a chronic disease. Fifth, we grouped the participants into four categories using the following exercise cutoff values: inactive (≤3.75 MET-h), low (3.75–7.49 MET-h), moderate (7.49–16.49 MET-h), and high (> 16.49 MET-h) based on the Physical Activity Guidelines for Americans, to determine whether using different cutoff points would result in different findings from the main findings.27 Sixth, we further examined the interaction of habitual exercise and PM2.5 exposure in different types of dyslipidemia (hypercholesterolemia, defined as a TC concentration ≥ 240 mg/dL); hypertriglyceridemia, defined as a TG concentration ≥ 200 mg/dL; hypoalphalipoproteinaemia, defined as an HDL-C concentration < 40 mg/dL; and hyperbetalipoproteinaemia, defined as an LDL-C concentration ≥ 160 mg/dL). Seventh, we compared the results of the association between PM2.5 exposure and incident dyslipidemia using model 2 with the results obtained using the same model, but with the city-level random intercept excluded.
Statistical analyses were performed using R 3.2.5 (R Core Team, Vienna, Austria). A two-tailed P < 0.05 was used to define statistical significance.
Results
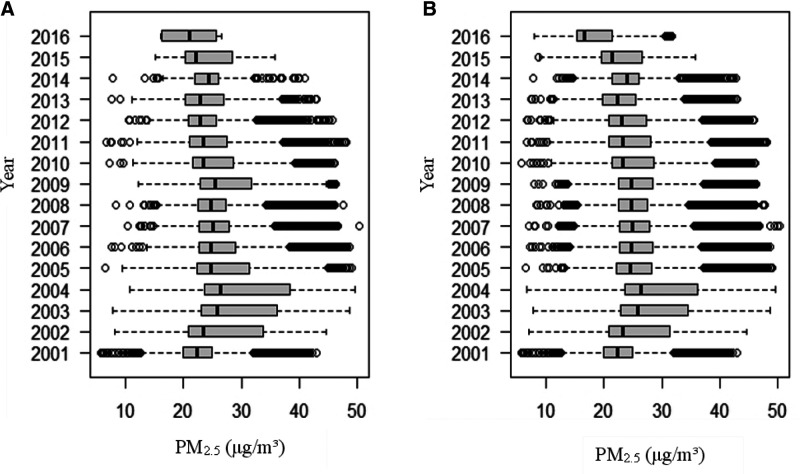
In total, 121,948 participants with 407,821 medical records were included in the data analysis. The mean follow-up duration was 4.6 years with a standard deviation of 3.4 years. During the study period, 26,669 participants developed dyslipidemia, leading to an incidence rate of 4.7 per 100 person-years. The baseline characteristics of all participants and the 26,669 participants who developed dyslipidemia during the study period are shown in Table 1. The mean age of the participants at baseline was 37.8 years, and 43.4% of them were male. Most of the participants were well educated, nonsmokers, and light drinkers. More than half of the participants had a sedentary job and approximately 45% of them were physically inactive. The PM2.5 concentrations were similar across different exercise groups. The temporal distribution of PM2.5 concentration by year is presented in Figure 2. PM2.5 concentration peaked in 2004 and then declined gradually.
Table 1.
Baseline characteristics of the participants
| All participants at baselinea | Dyslipidemia caseb | |||
|---|---|---|---|---|
| Characteristic | (n = 121,948) | (n = 26,669) | ||
| Age (year) | 37.8 | (11.7) | 40.6 | (11.8) |
| Male (n, %) | 52,967 | (43.4) | 11,095 | (41.6) |
| Educational level (n, %) | ||||
| Lower than high school | 14,161 | (11.6) | 4,170 | (15.6) |
| High school | 23,760 | (19.5) | 5,402 | (20.3) |
| College or university | 68,754 | (56.4) | 13,993 | (52.5) |
| Postgraduate | 15,273 | (12.5) | 3,104 | (11.6) |
| Smoking status (n, %) | ||||
| Never | 96,474 | (79.1) | 19,101 | (71.6) |
| Former | 5,934 | (4.9) | 1,613 | (6.1) |
| Current | 19,540 | (16.0) | 5,955 | (22.3) |
| Alcohol consumption (n, %) | ||||
| Seldom | 106,825 | (87.6) | 22,326 | (83.7) |
| Occasional | 10,390 | (8.5) | 2,915 | (10.9) |
| Regular | 4,733 | (3.9) | 1,428 | (5.4) |
| Physical labor at work (n, %) | ||||
| Sedentary job | 79,247 | (65.0) | 16,598 | (62.2) |
| Job that requires approximately half sedentary and half standing/walking | 31,392 | (25.7) | 7,290 | (27.3) |
| Job that mostly requires walking and standing | 9,355 | (7.7) | 2,241 | (8.4) |
| Job that requires vigorous physical activity | 1,954 | (1.6) | 540 | (2.0) |
| Habitual exercise (n, %) | ||||
| Inactive (0 MET-h) | 55,299 | (45.4) | 11,581 | (43.4) |
| Moderate (0.01–-8.75 MET-h) | 36,867 | (30.2) | 8,051 | (30.2) |
| High (>8.75 MET-h) | 29,782 | (24.4) | 7,037 | (26.4) |
| Vegetable intake (n, %) | ||||
| Seldom | 16,216 | (13.3) | 3,548 | (13.3) |
| Moderate | 72,744 | (59.7) | 16,079 | (60.3) |
| Frequent | 32,988 | (27.1) | 7,042 | (26.4) |
| Fruit intake (n, %) | ||||
| Seldom | 39,508 | (32.4) | 8,302 | (31.1) |
| Moderate | 67,081 | (55.0) | 14,951 | (56.1) |
| Frequent | 15,359 | (12.6) | 3,416 | (12.8) |
| Occupational exposurec (n, %) | 9,791 | (8.0) | 2,424 | (9.1) |
| BMI (kg/m2) | 22.3 | (3.4) | 23.5 | (3.3) |
| Total cholesterol concentration (mg/dL) | 182.6 | (26.6) | 196.3 | (26.4) |
| Triglyceride concentration (mg/dL) | 86.2 | (37.3) | 109.5 | (40.7) |
| High-density lipoprotein cholesterol concentration (mg/dL) | 59.8 | (13.6) | 54.9 | (13.1) |
| Low-density lipoprotein cholesterol concentration (mg/dL) | 105.8 | (24.6) | 119.7 | (23.4) |
| Diabetes (n, %) | 2,819 | (2.3) | 942 | (3.5) |
| Hypertension (n, %) | 12,990 | (10.7) | 4,116 | (15.4) |
| Cardiovascular disease (n, %) | 2,688 | (2.2) | 704 | (2.6) |
| Cancer (n, %) | 1,235 | (1.0) | 331 | (1.2) |
| PM2.5 concentration (μg/m3) d | 26.7 | (7.7) | 26.7 | (7.8) |
| PM2.5 concentration by exercise category (μg/m3) | ||||
| Inactive (0 MET-h) | 26.8 | (7.8) | 26.8 | (7.8) |
| Moderate (0.01–8.75 MET-h) | 26.7 | (7.7) | 26.8 | (7.8) |
| High (>8.75 MET-h) | 26.6 | (7.7) | 26.6 | (7.8) |
| Season (n, %) | ||||
| Spring | 30,380 | (24.9) | 6,704 | (25.1) |
| Summer | 34,017 | (27.9) | 7,326 | (27.5) |
| Autumn | 32,602 | (26.7) | 7,134 | (26.8) |
| Winter | 24,949 | (20.5) | 5,505 | (20.6) |
Values are presented as mean (standard deviation) for continuous variables and count (%) for categorical variables.
aBaseline characteristics of the 121,948 participants without dyslipidemia at baseline.
bBaseline characteristics of the 26,669 participants who developed dyslipidemia during the study period.
cClassified as exposure to dust or organic solvents in the workplace, as established by asking, ‘Are there any occupational hazards in your workplace?’
dRefers to the average PM2.5 concentration levels of the year of the visit and the year before the visit.
BMI, body mass index; MET, metabolic equivalent of task.
Figure 2.
The temporal distribution of the 2-year average of PM2.5 concentration by year in Taiwan. (A, B) represent the temporal distributions of the 2-year average PM2.5 concentrations by year. Boxes cover the 25th–75th percentiles (IQR) with center lines indicating the median concentration. Whiskers extend to the highest observed concentrations within the three IQRs of the box, with more extreme observations shown as circles. Panel A illustrates the distribution of baseline PM2.5 concentrations from 121,948 participants. (B) shows the distribution of PM2.5 exposure of 407,821 medical visits from the 121,948 participants. IQR, interquartile range.
Table 2 shows the main effects of habitual exercise and PM2.5 on incident dyslipidemia. A higher level of habitual exercise was associated with a lower incidence of dyslipidemia, with fully adjusted HRs (95% CI) of 0.91 (0.88, 0.94) and 0.73 (0.71, 0.75) for moderate and high levels of exercise, respectively (Model 2). In contrast, a higher level of PM2.5 exposure was associated with a higher incidence of dyslipidemia, with fully adjusted HRs (95% CI) of 1.36 (1.32, 1.40) and 1.90 (1.81, 1.99) for moderate and high levels of PM2.5 exposure, respectively (model 2). Mutual adjustment for PM2.5 concentration or exercise did not affect the associations. We also found significant exposure-response associations for the categories of exercise and PM2.5 exposure level. The interaction between habitual exercise and ambient PM2.5 concentration was statistically significant, with HRs (95% CI) of 1.09 (1.05, 1.14) and 1.16 (1.11, 1.21) for interactions of PM2.5 concentration with moderate- and high-level of exercise, respectively (results not shown). The HR (95% CI) for the overall interaction test was 1.08 (1.05, 1.10), and the likelihood ratio test also showed that the interaction effect was significant (P < 0.001; results not shown).
Table 2.
Associations of incident dyslipidemia with habitual exercise and PM2.5 exposure in Taiwanese adults
| Model | Without mutual adjustment | Mutual adjustmenta | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Main effects of exercise | ||||
| Crude model | ||||
| Inactive (0 MET-h) | Reference | – | Reference | – |
| Moderate (0.01–8.75 MET-h) | 0.96 (0.93, 0.99) | 0.008 | 0.97 (0.94, 1.00) | 0.04 |
| High (> 8.75 MET-h) | 0.87 (0.85, 0.90) | <0.001 | 0.89 (0.86, 0.91) | <0.001 |
| Test for trend b | 0.93 (0.92, 0.95) | <0.001 | 0.94 (0.93, 0.96) | <0.001 |
| Model 1 | ||||
| Inactive (0 MET-h) | Reference | – | Reference | – |
| Moderate (0.01–8.75 MET-h) | 0.90 (0.87, 0.93) | <0.001 | 0.90 (0.87, 0.93) | <0.001 |
| High (> 8.75 MET-h) | 0.71 (0.69, 0.74) | <0.001 | 0.72 (0.69, 0.74) | <0.001 |
| Test for trendb | 0.84 (0.83, 0.86) | <0.001 | 0.85 (0.83, 0.86) | <0.001 |
| Model 2 | ||||
| Inactive (0 MET-h) | Reference | – | Reference | – |
| Moderate (0.01–8.75 MET-h) | 0.91 (0.88, 0.93) | <0.001 | 0.91 (0.88, 0.94) | <0.001 |
| High (>8.75 MET-h) | 0.72 (0.70, 0.75) | <0.001 | 0.73 (0.71, 0.75) | <0.001 |
| Test for trendb | 0.85 (0.84, 0.87) | <0.001 | 0.86 (0.84, 0.87) | <0.001 |
| Main effects of PM2.5 exposure | ||||
| Crude Model | ||||
| Low (≤ 22.37 μg/m3) | Reference | – | Reference | – |
| Moderate (22.37–25.96 μg/m3) | 1.22 (1.18, 1.26) | <0.001 | 1.21 (1.18, 1.25) | <0.001 |
| High(> 25.96 μg/m3) | 1.52 (1.45, 1.58) | <0.001 | 1.50 (1.44, 1.57) | <0.001 |
| Test for trendb | 1.23 (1.20, 1.25) | <0.001 | 1.22 (1.20, 1.25) | <0.001 |
| Per 10 μg/m3 | 1.46 (1.41, 1.51) | <0.001 | 1.44 (1.39, 1.49) | <0.001 |
| Model 1 | ||||
| Low (≤ 22.37 μg/m3) | Reference | – | Reference | – |
| Moderate (22.37–25.96 μg/m3) | 1.35 (1.31, 1.39) | <0.001 | 1.34 (1.30, 1.39) | <0.001 |
| High(> 25.96 μg/m3) | 1.88 (1.79, 1.96) | <0.001 | 1.86 (1.77, 1.95) | <0.001 |
| Test for trendb | 1.37 (1.34, 1.40) | <0.001 | 1.36 (1.33, 1.39) | <0.001 |
| Per 10 μg/m3 | 2.04 (1.96, 2.12) | <0.001 | 2.01 (1.93, 2.09) | <0.001 |
| Model 2 | ||||
| Low (≤ 22.37 μg/m3) | Reference | – | Reference | – |
| Moderate (22.37–25.96 μg/m3) | 1.37 (1.32, 1.41) | <0.001 | 1.36 (1.32, 1.40) | <0.001 |
| High(> 25.96 μg/m3) | 1.92 (1.83, 2.01) | <0.001 | 1.90 (1.81, 1.99) | <0.001 |
| Test for trendb | 1.38 (1.35, 1.41) | <0.001 | 1.37 (1.34, 1.41) | <0.001 |
| Per 10 μg/m3 | 2.09 (2.01, 2.18) | <0.001 | 2.07 (1.99, 2.15) | <0.001 |
Crude Model: without adjustment; Model 1: adjusted for age, sex, educational level, season, baseline calendar year, physical labor at work, smoking status, alcohol consumption, occupational exposure, vegetable intake, and fruit intake; Model 2 further adjusted for BMI, hypertension, diabetes, self-reported cardiovascular disease, and self-reported cancer.
aFurther adjusted for PM2.5 concentration (for the association between exercise and incident dyslipidemia) or exercise (for the association between PM2.5 concentration and incident dyslipidemia).
bThe trend test was performed by entering 1, 2, and 3 as continuous variables in the models for inactive- exercise level/low—PM2.5 concentration, moderate—exercise level/ moderate-PM2.5 concentration, and high-exercise level/high-PM2.5 concentration, respectively.
95% CI, 95% confidence interval; HR, hazard ratio.
Subgroup analyses revealed that a higher level of habitual exercise was significantly associated with a lower incidence of dyslipidemia in all PM2.5 categories, although the associations were slightly attenuated for higher concentrations of PM2.5. Positive associations between PM2.5 exposure and incident dyslipidemia were observed for each exercise level, with stronger associations seen for higher levels of exercise (Table 3).
Table 3.
Results of stratified analyses by habitual exercise or PM2.5 concentration quartiles in Taiwanese adults
| Subgroup | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Effects of exercise: stratified by PM2.5 concentration | Low—PM2.5 concentration | Moderate—PM2.5 concentration | High—PM2.5 concentration | ||||||
| Inactive (0 MET-h) | Reference | Reference | Reference | ||||||
| Moderate (0.01–8.75 MET-h) | 0.87 | (0.83, 0.92) | <0.001 | 0.90 | (0.86, 0.95) | <0.001 | 0.95 | (0.90, 1.00) | 0.05 |
| High (>8.75 MET-h) | 0.68 | (0.64, 0.72) | <0.001 | 0.75 | (0.71, 0.80) | <0.001 | 0.77 | (0.73, 0.81) | <0.001 |
| Test for trend a | 0.82 | (0.80, 0.85) | <0.001 | 0.87 | (0.85, 0.89) | <0.001 | 0.88 | (0.86, 0.90) | <0.001 |
| Effects of PM 2.5 : stratified by exercise | Inactive | Moderate-exercise level | High – exercise level | ||||||
| Low (≤22.37 μg/m3) | Reference | Reference | Reference | ||||||
| Moderate (22.37–25.96 μg/m3) | 1.27 | (1.21, 1.34) | <0.001 | 1.31 | (1.24, 1.39) | <0.001 | 1.42 | (1.34, 1.50) | <0.001 |
| High (>25.96 μg/m3) | 1.63 | (1.52, 1.75) | <0.001 | 1.83 | (1.70, 1.97) | <0.001 | 2.01 | (1.85, 2.18) | <0.001 |
| Test for trend a | 1.28 | (1.23, 1.32) | <0.001 | 1.34 | (1.30, 1.39) | <0.001 | 1.42 | (1.36, 1.47) | <0.001 |
| Per 10 μg/m3 increment | 1.74 | (1.63, 1.86) | <0.001 | 1.92 | (1.80, 2.05) | <0.001 | 2.16 | (2.02, 2.30) | <0.001 |
All results were fully adjusted for age, sex, educational level, season, baseline calendar year, physical labor at work, smoking status, alcohol consumption, occupational exposure, vegetable intake, fruit intake, BMI, hypertension, diabetes, self-reported cardiovascular disease, and self-reported cancer.
aThe trend test was performed by entering 1, 2, and 3 as continuous variables in the models for inactive exercise level/low PM2.5 concentration, moderate exercise level/moderate PM2.5 concentration, and high exercise level/high PM2.5 concentration, respectively.
95% CI, 95% confidence interval; HR, hazard ratio.
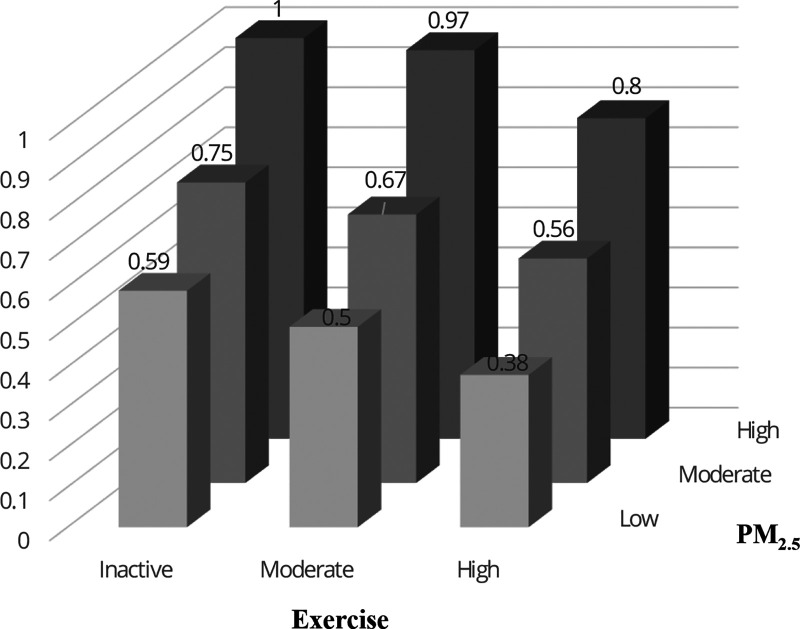
Figure 3 shows the combined effects of exercise and PM2.5 concentration on incident dyslipidemia. Participants with a high level of exercise and a low level of PM2.5 exposure had the lowest incidence of dyslipidemia, whereas physically inactive participants with a high level of exposure to PM2.5 had the highest incidence of dyslipidemia. The trend patterns of associations of dyslipidemia with PM2.5 and exercise were prominent in each PM2.5 concentration and exercise stratum. The corresponding estimated HRs are shown in eTable 2; http://links.lww.com/EE/A172.
Figure 3.
Combined effects of habitual exercise and PM2.5 exposure on incident dyslipidemia in adults in Taiwan. The results are fully adjusted for age, sex, educational level, season, baseline calendar year, physical labor at work, smoking status, alcohol consumption, occupational exposure, vegetable intake, fruit intake, BMI, hypertension, diabetes, self-reported cardiovascular disease, and self-reported cancer. Combined effects were analyzedwith participants classified into nine groups according to exercise and PM2.5 exposure categories, with the inactive-exercise group exposed to a high-PM2.5 concentration comprising the reference group. The inactive, moderate, and high levels of exercise were 0, 0.01–8.75, and > 8.75 MET-h, respectively. The low, moderate, and high level of PM2.5 were ≤22.37, 22.37–25.96, and >25.96 μg/m3, respectively. MET, metabolic equivalent of task.
The results of sensitivity analyses showed that the associations remained robust after excluding participants with baseline chronic conditions (eTable 3; http://links.lww.com/EE/A172), using the average annual PM2.5 concentration of the year before the medical examinations (eTable 4; http://links.lww.com/EE/A172); excluding participants who enrolled before 2005 (eTable 5; http://links.lww.com/EE/A172); excluding participants who were followed up for less than 2 years (eTable 6; http://links.lww.com/EE/A172); and using the exercise categories listed in the Physical Activity Guidelines for Americans27 (eTable 7; http://links.lww.com/EE/A172). The associations between habitual exercise and PM2.5 concentration were also robust among the four types of dyslipidemias (see eTable 8; http://links.lww.com/EE/A172). The association between PM2.5 exposure and incident dyslipidemia was slightly stronger after including a city-level random intercept in the model (eTable 9; http://links.lww.com/EE/A172).
Discussion
We found that a higher level of habitual exercise was associated with a lower incidence of dyslipidemia at all levels of PM2.5 exposure, although the negative associations with habitual exercise were slightly attenuated at higher levels of PM2.5 exposure. However, a higher level of PM2.5 exposure was associated with a higher incidence of dyslipidemia at different levels of habitual exercise. This association was slightly stronger when participants engaged in a higher level of exercise. We detected a statistically significant, but minor, interaction effect of PM2.5 exposure and exercise on the development of dyslipidemia, with an overall HR (95% CI) of 1.08 (1.05, 1.10), which indicates that an incremental level of exercise was associated with an 8% increase in the risk of dyslipidemia associated with every 10 μg/m3 increase in PM2.5 exposure.
Our findings of the inverse association between dyslipidemia and exercise confirm those of previous studies.4,28,29 A positive association between PM2.5 exposure and the incidence of dyslipidemia has also been reported in previous studies.7,8,30 However, information on the combined effects of habitual exercise and chronic exposure to air pollution on dyslipidemia is scarce. Previous cohort studies that have investigated the combined effects of exercise and air pollutants have mainly focused on lung function, cardiovascular disease, diabetes, and mortality.31–36 Most of these studies found no statistically significant interaction between exercise and air pollution,31–35 but some found evidence of a reduction in the beneficial effects of exercise on specific outcomes in relatively highly polluted areas.32,34–36 A study of the Danish Diet, Cancer, and Health Cohort found that the inverse association between exercise and respiratory mortality was more pronounced in people with low levels of NO2.32 Similar findings have been reported in a cohort study of children, showing that the development of asthma is only associated with exercise in children living in areas with high ozone levels.36 A study of including 66,820 Hong Kong elderly individuals reported that the cardiovascular benefits of walking are attenuated in people with exposure to a higher level of exposure to air pollution.34 In a Korean cohort, the negative association between exercise and stroke was found to be stronger in people with exposed to a low or moderate concentration of PM2.5.35 Although statistically significant interaction results were observed in the aforementioned studies, the magnitudes of the interactions were generally small, which is consistent with our results (HR: 1.08, 95% CI = 1.05, 1.10). Inconsistencies in the statistically significant interactions in different studies may be due to the differences in the sample sizes and outcomes measured. The heterogeneity of study populations and regions may also contribute to the inconsistent findings. Similarly, our previous studies based on the same cohort revealed a statistically significant interaction of habitual exercise and PM2.5 exposure on lung function but not on hypertension, diabetes, and inflammation.14,22,23,37 However, previous studies have demonstrated that habitual exercise does more good than harm in people exposed to air pollution regardless of the statistically significant interactions between the two factors.14,32
We have previously examined the association between long-term exposure to PM2.5 and the development of dyslipidemia previously in the same cohort.7 The association between dyslipidemia and PM2.5 in this study was slightly stronger than in the previous study. This phenomena may be because our previous study did not consider the correlation between individuals living within the same city. A previous study showed that a statistical model adjusting for the correlation between individuals living within the same city may affect the associations between air pollution and mortality,38 but we were not aware of this phenomenon at that time. Our sensitivity analysis (eTable 9; http://links.lww.com/EE/A172) also showed that the effect of PM2.5 exposure on dyslipidemia was slightly stronger after including the city-level random intercept. In addition, the sample size in the present study was larger in the present study than in our previous study (121,948 vs. 66,702 participants at the baseline visit), which increased the robustness and stability of our results.
Our findings indicated that exercising in an area with a higher concentration of PM2.5 may slightly attenuate the benefits of habitual exercise in the prevention of dyslipidemia, but the benefits of exercise remained. Thus, the benefits of exercise outweighed the adverse effects of excess inhalation of PM2.5 due to the exercise. Therefore, our results suggest that people should exercise in areas with lower PM2.5 concentrations when possible. However, exercise in a polluted area has more health benefits than staying inactive. Our study reinforces the importance of air pollution mitigation to maximize the benefits of exercise.
Indeed, habitual exercise is known to have an antiinflammatory effect by increasing the production of anti-inflammatory cytokines (e.g., IL-10) and suppressing the production of the proinflammatory cytokines (e.g., TNF-α).39 In addition, exercise improves lipid profiles through the activation of the enzyme, lipoprotein lipase.39,40 Conversely, exposure to PM2.5 has been shown to induce inflammation and lipid oxidation, which increases the risk of dyslipidemia.41 However, the mechanism responsible for these interaction effects on dyslipidemia remains unclear. We observed significant benefits for all the PM2.5 exposure categories and the modifying effects of PM2.5 exposure were minor. It may be that the harmful effects of excess inhaled air pollution during exercise do not completely offset the long-term beneficial health effects of habitual exercise.32 Moreover, the additional air pollutants inhaled, while performing exercise may account for only a small fraction of the total inhaled air pollutants.42
This study has several important strengths. First, this is the first large-scale cohort study investigating the combined effects of habitual exercise and chronic exposure to PM2.5 on dyslipidemia. Second, the large sample size provided sufficient power to detect the combined effects of habitual exercise and PM2.5 exposure, resulting in more stable and precise estimates. The large sample size also enabled us to conduct a series of subgroup and sensitivity analyses to test the robustness of the estimates. Third, the repeated measurements allowed us to consider the effects of the changes in exercise level, PM2.5 exposure, and other covariates over the study period. Finally, the satellite-based spatiotemporal model used in our study enabled us to overcome the limited spatial coverage and resolution that generally occur when exclusively using data from monitoring stations.
This study had several limitations that should be noted. First, we did not collect information on whether the exercises were performed indoors or outdoors. However, a series of national surveys reported that more than 86% of Taiwanese residents chose outdoor exercise as their most frequent form of exercise from 2005 to 2016.43 Therefore, the majority of habitual exercise in this study was likely to be outdoor exercise. Second, the information on habitual exercise was collected using a self-administered questionnaire, which may be imprecise at evaluating the amount of exercise. However, the validity and reliability of the questionnaire have been confirmed previously.25 The assessment of habitual exercise based on a questionnaire is commonly used in large-scale epidemiologic studies because formal exercise testing is not practical. Third, information on indoor air pollution was not available in this study. Instead, we accounted for smoking in our analysis, which is the major source of indoor air pollution in developed economies. Fourth, we did not consider the effects of other air pollutants, such as SO2 and NO2, because this information was not available. However, the collinear relationship between pollutants suggests that we should analyze the pollutants separately. Finally, the participants of this cohort study were relatively healthy and well educated. Therefore, we should be cautious when generalizing the results to other populations.
In conclusion, a high level of habitual exercise, combined with a low level of PM2.5 exposure, were associated with a lower incidence of dyslipidemia, whereas a low level of exercise, combined with a high level of PM2.5 exposure, were associated with a higher incidence of dyslipidemia. We found that habitual exercise reduced the incidence of dyslipidemia, regardless of the levels of chronic PM2.5 exposure, although the benefits of exercise were slightly attenuated at high levels of PM2.5 exposure. Our findings suggest that people should exercise in areas with lower PM2.5 concentrations. However, if this is not possible, exercising in polluted areas is better than staying inactive. Our results reinforce the importance of air pollution mitigation to maximize the beneficial effects of habitual exercise. More research is warranted to confirm our findings in areas with higher air pollution levels.
Acknowledgments
The authors declare that they have no conflicts of interest with regard to the content of this report.
X.Q.L. conceived and designed the study. L.Y.C., A.K.H.L., C.Q.L., T.T., and X.Q.L. acquired the data. Y.Q.Z., C.G., C.Q.L., and Y.C.B. searched the literature. Y.Q.Z. performed data analysis. Y.Q.Z., M.C.S.W., and X.Q.L. interpreted the data. Y.Q.Z. wrote the first draft of the article. All authors critically revised the article. X.Q.L. acquired the funding. L.Y.C., A.K.H.L., T.T., and X.Q.L. supervised this study. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy. We would like to thank MJ Health Research Foundation for the authorization of using MJ health data (authorization code MJHR2019006A). Any interpretation or conclusion related to this article does not represent the views of MJ Health Research Foundation.
Supplementary Material
Footnotes
Published online 5 January 2022
This work was supported by RGC-General Research Fund (14603019) of University Grant Committee of Hong Kong. Miss Yi Qian Zeng is supported by the PhD Studentship of the Chinese University of Hong Kong. Dr Cui Guo is supported by the RGC Postdoctoral Fellowship Scheme of the University Grants Committee of Hong Kong. Dr Yacong Bo is in part supported by the Faculty Postdoctoral Fellowship Scheme of the Faculty of Medicine of the Chinese University of Hong Kong.
Availability of data and materials: The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 3.Reiner Ž, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 4.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. [DOI] [PubMed] [Google Scholar]
- 5.Shaw KA, Gennat HC, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006:CD003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Global action plan on physical activity 2018–2030: more active people for a healthier world. 2018. Available at: https://www.who.int/ncds/prevention/physical-activity/global-action-plan-2018-2030/en/. Accessed July 20, 2020.
- 7.Bo Y, Chang LY, Guo C, et al. Association of long-term exposure to fine particulate matter and incident dyslipidaemia: a longitudinal cohort study. Environ Res. 2019;173:359–365. [DOI] [PubMed] [Google Scholar]
- 8.Mao S, Chen G, Liu F, et al. Long-term effects of ambient air pollutants to blood lipids and dyslipidemias in a Chinese rural population. Environ Pollut. 2020;256:113403. [DOI] [PubMed] [Google Scholar]
- 9.Yeatts K, Svendsen E, Creason J, et al. Coarse particulate matter (PM2.5-10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ Health Perspect. 2007;115:709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Ambient air pollution: a global assessment of expsure and burden of disease. 2016. Available at: https://www.who.int/phe/publications/air-pollution-global-assessment/en/. Accessed July 20, 2020.
- 11.Hou J, Liu X, Tu R, et al. Long-term exposure to ambient air pollution attenuated the association of physical activity with metabolic syndrome in rural Chinese adults: a cross-sectional study. Environ Int. 2020;136:105459. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Air quality guidelines: global update 2005: particulate matter, ozone, nitrogen dioxide and sulfur dioxide. 2006. Available at: https://apps.who.int/iris/handle/10665/107823/. Accessed July 20, 2020. [PubMed]
- 13.Guo C, Tam T, Bo YC, et al. Habitual physical activity, renal function and chronic kidney disease: a cohort study of nearly 200 000 adults. Br J Sports Med. 2020:bjsports-2019-100989. [DOI] [PubMed] [Google Scholar]
- 14.Guo C, Bo Y, Chan TC, et al. Does fine particulate matter (PM2.5) affect the benefits of habitual physical activity on lung function in adults: a longitudinal cohort study. BMC Med. 2020;18:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Chang LY, Lau AKH, et al. Satellite-based estimates of long-term exposure to fine particulate matter are associated with C-reactive protein in 30 034 Taiwanese adults. Int J Epidemiol. 2017;46:1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang L, Tsai SP, Wang ML. MJ Health Database, MJ Health Research Foundation Technical Report, MJHRF-TR-01. 2016. Available at: http://www.mjhrf.org/main/page/resource/en/#. Accessed July 20, 2020.
- 17.Chuang YC. MJ Health Data information security management guidelines, MJ Health Research Foundation Technical Report, MJHRF-TR-03. 2016. Available at Available at http://www.mjhrf.org/main/page/resource/en/#resource01. Accessed July 20, 2020.
- 18.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143–3412. [PubMed] [Google Scholar]
- 19.Zhao SP, Lu GP, Zhao D, et al. 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CQ, Li Y, Yuan ZB, et al. Using satellite remote sensing data to estimate the high-resolution distribution of ground-level PM2.5. Remote Sens Environ. 2015;156:117–128. [Google Scholar]
- 21.Lin CQ, Liu G, Lau AKH, et al. High-resolution satellite remote sensing of provincial PM2.5 trends in China from 2001 to 2015. Atmos Environ. 2018;180:110–116. [Google Scholar]
- 22.Zhang Z, Hoek G, Chang LY, et al. Particulate matter air pollution, physical activity and systemic inflammation in Taiwanese adults. Int J Hyg Environ Health. 2018;221:41–47. [DOI] [PubMed] [Google Scholar]
- 23.Guo C, Zeng Y, Chang LY, et al. Independent and opposing associations of habitual exercise and chronic PM2.5 exposures on hypertension incidence. Circulation. 2020;142:645–656. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl 9):S498–S504. [DOI] [PubMed] [Google Scholar]
- 25.Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. [DOI] [PubMed] [Google Scholar]
- 26.Wang ML. MJ Health Screening Equipment Use and Replacement Records, MJ Health Research Foundation Technical Report, MJHRF-TR-06. 2016. Available at http://www.mjhrf.org/main/page/resource/en/#resource01. Accessed July 20, 2020.
- 27.US Department of Health Human Services. 2008 Physical Activity Guidelines for Americans. United States, Department of Health and Human Services; 2008. [Google Scholar]
- 28.Costa RR, Buttelli ACK, Fagundes AO, et al. The beneficial effects of a water-based aerobic exercise session on the blood lipids of women with dyslipidemia are independent of their training status. Clinics (Sao Paulo). 2020;75:e1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa R, Buttelli A, Vieira A, et al. Effect of strength training on lipid and inflammatory outcomes: systematic review with meta-analysis and meta-regression. J Phys Activity Health. 2019;16:1–15. [DOI] [PubMed] [Google Scholar]
- 30.Wu XM, Broadwin R, Basu R, et al. Associations between fine particulate matter and changes in lipids/lipoproteins among midlife women. Sci Total Environ. 2019;654:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher JE, Loft S, Ulrik CS, et al. Physical activity, air pollution, and the risk of asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:855–865. [DOI] [PubMed] [Google Scholar]
- 32.Andersen ZJ, de Nazelle A, Mendez MA, et al. A study of the combined effects of physical activity and air pollution on mortality in elderly urban residents: the Danish Diet, Cancer, and Health Cohort. Environ Health Perspect. 2015;123:557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubesch NJ, Therming Jørgensen J, Hoffmann B, et al. Effects of leisure-time and transport-related physical activities on the risk of incident and recurrent myocardial infarction and interaction with traffic-related air pollution: a cohort study. J Am Heart Assoc. 2018;7:e009554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun S, Cao W, Qiu H, et al. Benefits of physical activity not affected by air pollution: a prospective cohort study. Int J Epidemiol. 2020;49:142–152. [DOI] [PubMed] [Google Scholar]
- 35.Kim SR, Choi S, Keum N, Park SM. Combined effects of physical activity and air pollution on cardiovascular disease: a population-based study. J Am Heart Assoc. 2020;9:e013611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McConnell R, Berhane K, Gilliland F, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359:386–391. [DOI] [PubMed] [Google Scholar]
- 37.Guo C, Yang HT, Chang LY, et al. Habitual exercise is associated with reduced risk of diabetes regardless of air pollution: a longitudinal cohort study. Diabetologia. 2021;64:1298–1308. [DOI] [PubMed] [Google Scholar]
- 38.Beelen R, Raaschou-Nielsen O, Stafoggia M, et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383:785–795. [DOI] [PubMed] [Google Scholar]
- 39.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98:1154–1162. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017;16:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulintz L, Sun QH. Ambient particulate matter pollution on lipid peroxidation in cardiovascular diseases. Environ Dis. 2016;1:109–117. [Google Scholar]
- 42.Rojas-Rueda D, de Nazelle A, Tainio M, Nieuwenhuijsen MJ. The health risks and benefits of cycling in urban environments compared with car use: health impact assessment study. BMJ. 2011;343:d4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sports Administration, Ministry of Education, Taiwan. Report of Active Cities. 2016. Available at: https://isports.sa.gov.tw/Index.aspx. Accessed August 9, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.