Abstract
PURPOSE:
For many patients with cancer, the frequency of surveillance after primary treatment depends on the risk for cancer recurrence or progression. Lack of risk-aligned surveillance means too many unnecessary surveillance procedures for low-risk patients and not enough for high-risk patients. Using bladder cancer as an example, we examined whether practice determinants differ between Department of Veterans Affairs sites where risk-aligned surveillance was more (risk-aligned sites) or less common (need improvement sites).
METHODS:
We used our prior quantitative data to identify two risk-aligned sites and four need improvement sites. We performed semistructured interviews with 40 Veterans Affairs staff guided by the Tailored Implementation for Chronic Diseases framework that were deductively coded. We integrated quantitative data (risk-aligned site v need improvement site) and qualitative data from interviews, cross-tabulating salient determinants by site type.
RESULTS:
There were 14 participants from risk-aligned sites and 26 participants from need improvement sites. Irrespective of site type, we found a lack of knowledge on guideline recommendations. Additional salient determinants at need improvement sites were a lack of resources (“the next available without overbooking is probably seven to eight weeks out”) and an absence of routines to incorporate risk-aligned surveillance (“I have my own guidelines that I've been using for 35 years”).
CONCLUSION:
Knowledge, resources, and lack of routines were salient barriers to risk-aligned bladder cancer surveillance. Implementation strategies addressing knowledge and resources can likely contribute to more risk-aligned surveillance. In addition, reminders for providers to incorporate risk into their surveillance plans may improve their routines.
INTRODUCTION
Surveillance after primary cancer treatment is an important component of cancer care to assure timely detection of recurrence or progression. Surveillance and long-term treatment represent approximately three quarters of oncology visits.1 For many patients, the frequency of surveillance depends on characteristics of the cancer they had at the time of diagnosis, eg, stage, grade, and other details of the primary neoplastic lesion.
However, there is little research on how to effectively implement risk-aligned cancer surveillance. Our recent work as well as the work of others has shown that despite guideline recommendations, risk-aligned surveillance does not regularly occur.2-6 We used non–muscle-invasive bladder cancer within the Department of Veterans Affairs (VA)—where it is the third most prevalent noncutaneous cancer7—as an important model to study risk-aligned surveillance. The most common risk these patients face is recurrence within the bladder. To monitor for recurrence, patients undergo cystoscopic surveillance at regular intervals, where the provider visually examines the urethra and bladder via an endoscope. The frequency with which this surveillance occurs should be aligned with each patient's risk for recurrence of disease, ranging from every 3-4 months for high-risk patients to approximately yearly for low-risk patients, per current guideline recommendations.8,9
Lack of risk-aligned surveillance has several negative consequences for patients and the health care system. For low-risk patients, undergoing unnecessary surveillance is associated with discomfort, travel and opportunity costs, and more invasive procedures such as biopsies, with their risk for more complications.10-12 For high-risk patients, not undergoing enough surveillance could lead to delayed cancer care, which is important because delays in diagnosis and treatment are associated with increased mortality.13,14 For the health care system, providing too much surveillance for low-risk patients competes for limited resources and increases cost.
For these reasons, we embarked on systematically identifying practice determinants of risk-aligned surveillance. It is essential to understand these practice determinants, because this understanding is the foundation for the subsequent development of implementation strategies to improve risk-aligned cancer surveillance.
METHODS
Overview of Study Design
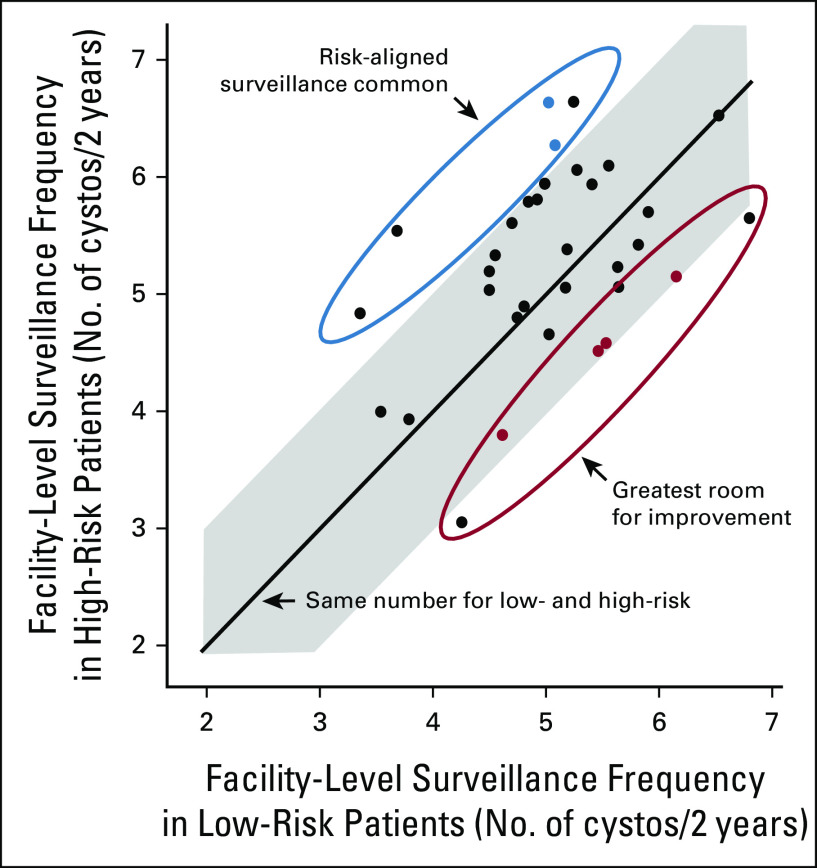
This was a sequential mixed-methods design to assess provider- and facility-level determinants of risk-aligned surveillance.15 We leveraged our prior work with quantitative data from VA Medical Centers to purposefully sample two sites where risk-aligned surveillance was common (risk-aligned sites [RA]) and four sites where risk-aligned surveillance was less common (need improvement sites [IMP]).4 Risk-aligned sites were those that performed low-frequency surveillance for low-risk and high-frequency surveillance for high-risk patients (ie, positive deviance sites); need improvement sites were those that performed low-frequency surveillance for high-risk and high-frequency surveillance for low-risk patients (ie, negative deviance sites; Fig 1).4 Six sites representing a broad range of facility size and geography were selected before submission of the grant application funding this project, as previous qualitative research projects achieved their goals of thematic saturation with inclusion of five to six sites (see the Data Supplement, online only for further information).16-18 We then conducted semistructured interviews with providers, physician and nurse leadership, nurses, and schedulers, and compared and contrasted determinants of risk-aligned surveillance between risk-aligned sites and need improvement sites.19
FIG 1.
Sampling of sites. We previously evaluated facility-level surveillance for low- versus high-risk bladder cancer patients in VA. Each dot represents one site. The line represents the same cystoscopy frequency for low- and high-risk patients. The shaded area represents sites where low-risk and high-risk patients undergo cystoscopy at comparable rates (ie, absolute difference of < 1 cystoscopy over 2 years). For the current project, we purposefully sampled two sites where risk-aligned surveillance was common (risk-aligned sites, blue dots) and four sites where risk-aligned surveillance was less common (need improvement sites, red dots). VA, Veterans Affairs. Adapted from eFigure shown in the study by Schroeck et al.4
Implementation Science Framework
Our interview guide development, interviews, and analyses were guided by the Tailored Implementation for Chronic Diseases (TICD) framework. The TICD is an implementation science framework that incorporates 12 prior frameworks based on a systematic review of the literature.20 It was developed to guide data collection for projects that focus on identification of the most important practice determinants affecting care delivery and is meant to guide efforts to improve care delivery.20,21 The TICD includes 57 determinants of practice grouped in seven domains. Figure 2 shows the seven domains with example determinants relevant to our data.
FIG 2.
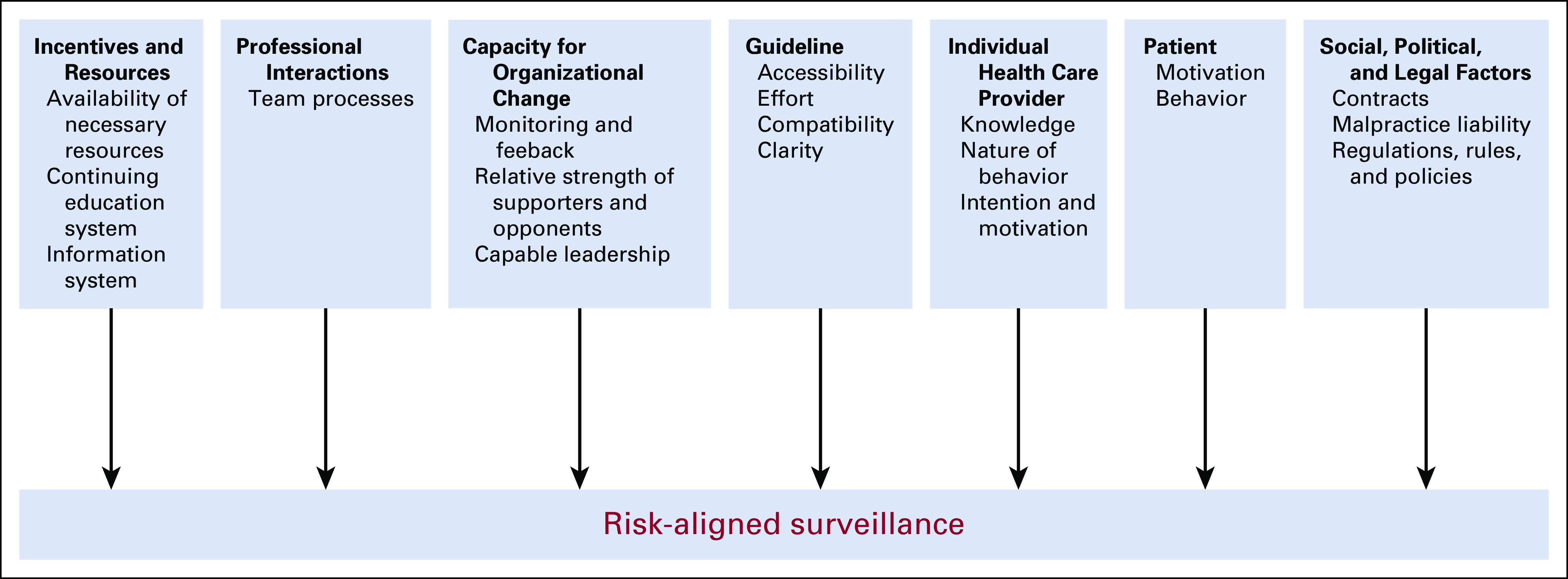
Schematic of the TICD framework as applied to the current project. The TICD is based on a systematic review of the literature, incorporating 12 prior frameworks and meant to guide efforts to improve care.20,21 It includes 57 determinants of practice grouped in seven domains as applied to our study. TCID, Tailored Implementation for Chronic Diseases.
Recruitment
Potential participants were identified by asking staff at each site who is clinically or administratively involved in bladder cancer surveillance (ie, snow-ball sampling) and from administrative data stored within the VA Corporate Data Warehouse. Potential participants were contacted first via e-mail and then via telephone. We sent initial e-mails to 79 potential participants. We got a response from 49, of whom 6 declined participation and three initially agreed to participate, but never confirmed an interview date. Thus, 40 were interviewed via telephone for a median length of 27 minutes (range, 11-50 minutes). Patients were not included in the current work as their perspectives were evaluated in our prior work.22
Data Collection
Quantitative data included whether the participants worked at a risk-aligned site versus a need improvement site. In addition, we collected demographic data at the end of interviews via a brief series of questions (age, sex, self-identified race or ethnicity, years spent in their current role, years spent working at VA, and providers' level of training).
Qualitative data were collected through audio-recorded semistructured telephone interviews. The interviews were conducted by professional neutral interviewers from the VA Salt Lake City qualitative research core (G.N.P., J.Y., S. Yang, and P. Galyean; three female and one male). Interview guides were developed covering domains and determinants from the TICD framework and were pilot tested before their use (see the Data Supplement for guides).
Analyses of Qualitative Data
All interviews were recorded, transcribed, and iteratively coded. We used a priori codes from the TICD framework and allowed for additional codes as they emerged. Atlas.ti software program (Version 8, Scientific Software Development GmbH, Berlin, Germany) was used to facilitate coding. We assured validity by (1) assuring that thematic saturation was reached, (2) including a urologist (F.R.S.), an implementation scientist (L.Z.), and qualitative analysts during analyses (G.N.P. and J.Y.), increasing validity by providing perspectives from researchers with different backgrounds (triangulation),23 and (3) searching and accounting for disconfirming evidence.23 After 18 provider, five nurse, five scheduler, and 12 leadership interviews, we had reached thematic saturation, and no new concepts were identified.
Based on this qualitative analytic process, we identified salient determinants of risk-aligned bladder cancer surveillance. Salient determinants were those mentioned by participants most frequently in response to our open-ended questions (measured by calculating the average code count per interview), as well as those that were conceptually important as agreed upon by the research team.24
Analysis of Knowledge for Providers and Nurses
We asked providers and nurses, “What do you think is the surveillance recommendation for low-, intermediate-, and high-risk non-muscle-invasive bladder cancer?” Responses to this question were dichotomized into having or not having a knowledge deficit of risk-aligned bladder cancer surveillance (see the Data Supplement for details).
Integrating Quantitative and Qualitative Data in Mixed-Methods Analyses
We assessed whether determinants for risk-aligned bladder cancer surveillance differed by site type by integrating the quantitative and qualitative data in a tabular joint display (Table 1).25 The study was approved by the VA Central Institutional Review Board (No.19-01) and is reported according to the COnsolidated criteria for REporting Qualitative research Checklist.19
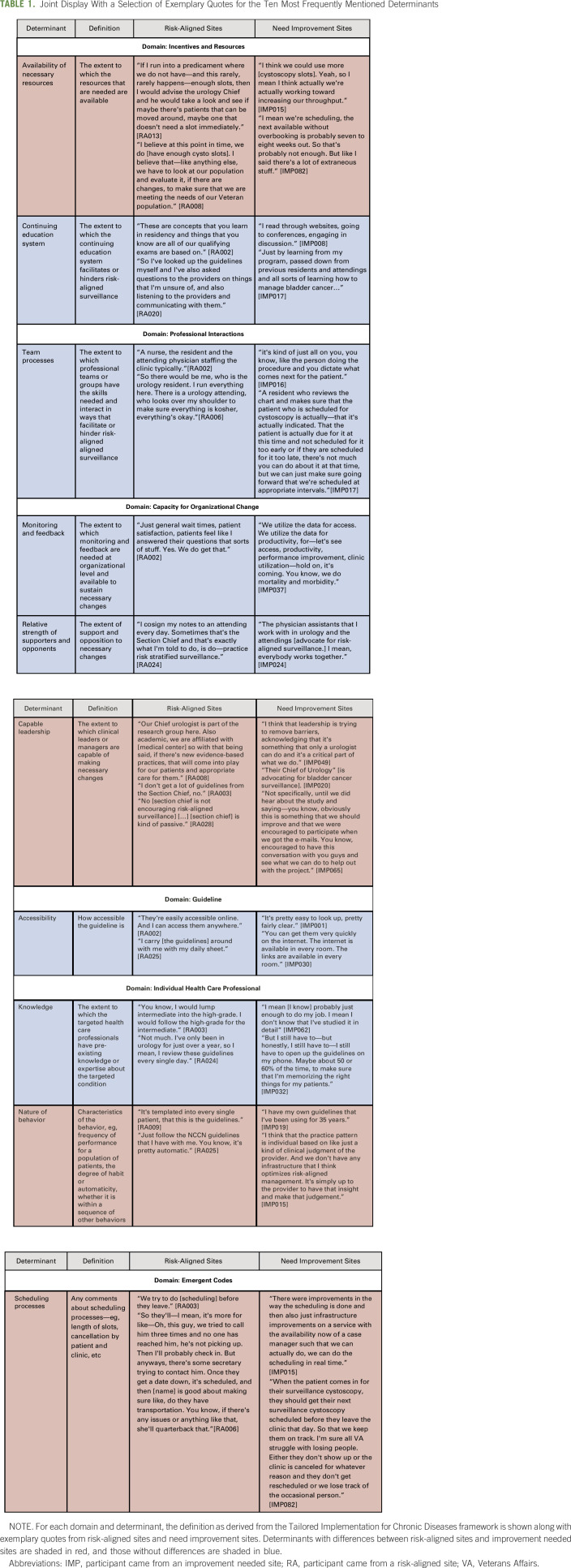
TABLE 1.
Joint Display With a Selection of Exemplary Quotes for the Ten Most Frequently Mentioned Determinants
Participants in the semistructured telephone interviews received an information sheet attached to the invitation to participate in the study. At the beginning of each interview, the interviewer read a standardized script (see Interview guide in the Data Supplement) and verified and recorded the participant's consent to be recorded.
RESULTS
Overview
There were 14 participants from the two risk-aligned sites and 26 participants from the four need improvement sites. Demographic characteristics of the participants are summarized in the Data Supplement.
In the following sections, we first report differences in determinants found when comparing risk-aligned sites to need improvement sites. Second, we summarize select salient determinants by TICD framework domain where no differences were seen by site type. Data on additional determinants are provided in the Data Supplement. Third, we present emergent codes that are conceptually important to this work, but not captured by the TICD framework.
Differences Between Risk-Aligned Sites and Need Improvement Sites
The joint display in Table 1 shows exemplary quotes for the 10 most frequently mentioned determinants, stratified by risk-aligned sites versus need improvement sites. There were several notable differences. Among the 14 participants from risk-aligned sites, a substantial proportion (n = 5 of 14; 36%) stated that enough cystoscopy slots are available. This was contrasted by 42% of participants from need improvement sites (n = 11), who felt that more cystoscopy slots are needed (availability of necessary resources in Table 1).
Regarding capable leadership (Capacity for Organizational Change Domain), participants at risk-aligned sites more commonly stated that they “don't get a lot of guidelines from the Section Chief” [RA003] compared with those from need improvement sites (n = 6 of 14, 43% v n = 5 of 26, 19%).
Comparing workflow (nature of behavior in Table 1), routine use of National Comprehensive Cancer Network guidelines was more common in risk-aligned sites (n = 6 of 14 [43%] v n = 7 of 26 [27%]). Participants from risk-aligned sites less commonly used their own clinical judgment to determine plans for surveillance than those from need improvement sites (n = 1 of 14 [7%] v n = 5 of 26 [19%]). At risk-aligned sites, four participants stated that risk-aligned surveillance is routinely provided, and no participants discussed a default approach that did not include risk-aligned surveillance. This was in contrast to need improvement sites, where two providers stated that risk-aligned surveillance is routinely provided, but three providers mentioned default approaches that did not include risk-aligned surveillance.
Regarding scheduling processes, seven of 26 participants from need improvement sites (27%) discussed that they schedule a surveillance cystoscopy before the patient has left the facility, which participants felt facilitates risk-aligned surveillance. However, at risk-aligned sites, many (n = 6 of 14, 43%) mentioned that scheduling is primarily done over the telephone.
Salient Determinants by TICD Framework Domain
The following sections summarize select salient determinants by TICD framework domain, where no differences were seen between site types.
Incentives and Resources Domain
Availability of necessary resources.
This determinant was the most frequently mentioned. Almost half of the participants (n = 16 of 40) confirmed that there is no shortage of necessary equipment. There was consensus among many participants (n = 16) that “staffing is a major hurdle” [IMP030]. Participants had several suggestions as to how to improve availability of resources, including freeing up more space (n = 3), training more physician assistants (n = 2), hiring more staff (n = 2), opening a Saturday clinic (n = 2), and adding a case manager (n = 1).
Information system.
Almost half of the participants (n = 17 of 40) stated that the information to assign bladder cancer risk is readily accessible in the medical record. Nine participants felt that “making sure that there's uniform […] documentation so that any person looking at a patient's chart […] can easily identify the patient's risk stratification without any confusion” [IMP017] and that “giving the whole history […] in the procedure note [will help] the next person who is doing [surveillance]” [IMP016].
Capacity for Organizational Change Domain
Almost all participants (n = 34 of 40) indicated that staff is not receiving any feedback on whether or not they are providing risk-aligned bladder cancer surveillance. However, about a third of participants (n = 14) were familiar with data feedback for other care, including “[clinic] access data and […] clinic utilization” [IMP037], “data with regard to […] mortalities, morbidities” [IMP030], or “how long [patients] stay in the hospital” [IMP081]. Almost half of the participants (n = 16) felt that data feedback would be helpful (Individual Health Care Provider domain, self-monitoring & feedback).
Guideline Domain
The majority of participants (n = 27 of 40) felt that the guidelines are accessible, for example, because they are posted in the clinic or on cheat sheets (n = 9) or because they are online (n = 7). Participants had several suggestions to facilitate accessibility, including use of cheat sheets (n = 5), use of a mobile app to access the guidelines (n = 4), or incorporating the guidelines into a resident physician orientation packet handed out at the start of their rotation (n = 1).
Individual Health Care Provider Domain
A knowledge deficit was present among the majority of providers (n = 12 of 18) and among all five nurses interviewed, with no substantial difference between risk-aligned sites and need improvement sites. Among providers, three stated that they had limited or no knowledge of risk-aligned surveillance recommendations, whereas 15 indicated they had at least some knowledge. Among those 15, the majority (n = 9) had a knowledge deficit despite their self-perception: six provided incorrect surveillance frequencies for low- or high-risk patients, one only had a general concept of less frequent surveillance for low-risk and more frequent surveillance for high-risk, and two provided no response when asked to identify the surveillance recommendations.
Among nurses, three indicated they have limited or no knowledge of risk-aligned surveillance recommendations. Two nurses indicated they had some knowledge, but when asked about recommended surveillance frequencies, their responses were incorrect.
Emergent Code—Nurses' Lack of Integration
Three nurse leaders, one physician leader, and two nurses commented that the nurses are “just going by what the cysto[scopy] team is telling them to do” [IMP020], that nurses “don't treat or diagnose [patients and] don't have conversations with the patient [about] the actual bladder cancer [or] the pathology” [RA019], and that “providers are communicating amongst themselves and they don't pass the plan on to the nurses” [IMP037]. Thus, nurses were perceived as lacking engagement or integration into the surveillance process.
DISCUSSION
We conducted a mixed-methods study of determinants of risk-aligned bladder cancer surveillance guided by the TICD framework. A salient determinant at need improvement sites was an absence of routines to incorporate risk-aligned surveillance into clinical workflow. Irrespective of site type, we found a lack of knowledge of guideline recommendations among both providers and nurses. Participants suggested that risk-aligned surveillance could be facilitated by data feedback, clear documentation of bladder cancer risk and surveillance schedules in the electronic health record, and increased accessibility of guideline recommendations via cheat sheets or mobile apps.
Our findings are consistent with previous work indicating that the individual health care provider domain explains important barriers to guideline concordant care. For example, lack of knowledge was a key barrier in a recent study evaluating barriers to thrombolysis among stroke patients presenting to the emergency room.26 Similar findings were also noted in one of the original TICD projects, where a key determinant of practice was individual health care providers' knowledge about clinical recommendations.27 However, lack of knowledge does not automatically translate into lack of risk-aligned surveillance, as several participants commented that they regularly look up the guideline recommendations online or in mobile apps. Thus, lack of knowledge can be mitigated via readily accessible guideline recommendations. Similarly, it is conceivable that not all clinicians need to have knowledge of the guidelines. For example, it may be feasible and appropriate for nurses to support risk-aligned surveillance even if they do not know the guideline recommendations.
Lack of capable leadership support was more commonly mentioned in risk-aligned sites than in need improvement sites, which seems counterintuitive. There are several potential reasons for this: (1) this finding may have been driven by findings at a single risk-aligned site; (2) individual providers at sites can be successful with implementation of risk-aligned surveillance even without strong leadership support; (3) lack of strong leadership may foster a sense of autonomy and allow individual providers to retain more ownership over their patients—causing reliance on frequently checking guidelines rather than relying on leadership; or (4) leadership support on its own is not sufficient for risk-aligned surveillance at need improvement sites.
There are several important limitations to note. First, code counts depend on the specific questions asked. Although we systematically organized our interview guides according to the TICD framework, it was not feasible to explicitly ask all participants about all 57 determinants across seven domains. Thus, determinants we did not explicitly ask about likely had lower code counts. However, our participants had ample opportunity to answer open-ended questions and we integrated conceptually important determinants into our main findings even if they had lower code counts. Thus, it is unlikely that our systematic evaluation missed any highly relevant determinants. Second, as in any study relying on interviews, there may have been selection bias among staff volunteering for an interview. Third, our work focused on risk-aligned surveillance, which conceptually entails both avoiding overuse among low-risk and underuse among high-risk patients. However, some of our findings are more about avoiding underuse among high-risk patients, especially those regarding lack of available resources (eg, cystoscopy slot availability, staffing, or freeing up space). Nevertheless, implementation strategies improving risk-aligned surveillance have the potential to address this lack of resources, as resources can be made available by reducing overuse among low-risk patients, thus freeing up resources for high-risk patients.
Despite these limitations, our current study has several important strengths. First, we conducted a systematic assessment of determinants of risk-aligned bladder cancer surveillance using the TICD framework and thereby covered a comprehensive set of explanatory domains. Second, we interviewed many different role types involved in risk-aligned surveillance, including urologists, advanced practice providers, nurses, schedulers, and leaders, thus obtaining views from multiple perspectives. Third, we used a sequential mixed-methods approach, leading with quantitative data to select risk-aligned sites and need improvement sites. This sampling approach allowed us to identify differences in determinants across site types, such as routine surveillance approaches that incorporate National Comprehensive Cancer Network guidelines at risk-aligned sites versus providers following their own guidelines at need improvement sites.
To our knowledge, this study is the first to systematically evaluate practice determinants for risk-aligned cancer surveillance and has several important implications. Our work,4 as well as the work of others,5 has shown that risk is not routinely used to select bladder cancer surveillance schedules. Rather, a one-size-fits-all approach is more prevalent. Risk-aligned surveillance not only maximizes benefit and minimizes harm for patients with bladder cancer,28 but it is also the clinical approach recommended for other common neoplasms, such as prostate, lung, and colorectal cancer.29-31 Some of our findings are likely specific to bladder cancer, such as lack of cystoscopy slots. However, other findings may extend to other cancer types. These may include barriers, such as lack of knowledge and routine provider approaches not incorporating risk. Risk-aligned surveillance in other settings may also be facilitated by clear documentation as well as the use of cheat sheets or mobile apps to increase guideline accessibility.
In conclusion, we conducted a mixed-methods assessment of determinants of risk-aligned bladder cancer surveillance. The results from this work will inform the development of implementation strategies addressing knowledge and resources, and ultimately may contribute to more risk-aligned surveillance. Given the differences found between risk-aligned sites and need improvement sites, implementation strategies will need to address the current lack of routines to incorporate risk-aligned surveillance into clinical workflow, potentially via reminders or templates to clearly document surveillance plans in the electronic health record.
ACKNOWLEDGMENT
The authors acknowledge assistance with interviewing by Patrick Galyean and Serena Yang.
Florian R. Schroeck
Other Relationship: Sesen Bio
No other potential conflicts of interest were reported.
DISCLAIMER
Opinions expressed in this manuscript are those of the authors and do not constitute official positions of the US Federal Government or the Department of Veterans Affairs. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
SUPPORT
This study was supported using resources and facilities at the White River Junction Department of Veterans Affairs (VA) Medical Center (IIR 18-215, I01HX002780-01), the VA Salt Lake City Health Care System, the Richard L. Roudebush Veterans Affairs Medical Center, and the VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457.
DATA AVAILABILITY STATEMENT
The data sets generated and analyzed during the current study are not publicly available because they contain potentially identifying and sensitive information but are available from the corresponding author on reasonable request. Upon request from a qualified investigator, a limited data set will be created for that investigator's use and shared pursuant to a Data Use Agreement (DUA) appropriately limiting use of the data set and prohibiting the recipient from identifying or reidentifying (or taking steps to identify or reidentify) any individual whose data are included in the data set. Investigators who request to use the data will be required to obtain institutional review board approval and sign the DUA before release of the data. Interested investigators are encouraged to directly contact the corresponding author.
AUTHOR CONTRIBUTIONS
Conception and design: Florian R. Schroeck, Lisa Zubkoff
Financial support: Florian R. Schroeck
Administrative support: Florian R. Schroeck, Steven L. Sanchez, DeRon R. Walker
Provision of study materials or patients: Florian R. Schroeck, A. Aziz Ould Ismail, Steven L. Sanchez, DeRon R. Walker, Susan Zickmund
Collection and assembly of data: Florian R. Schroeck, A. Aziz Ould Ismail, Grace N. Perry, Steven L. Sanchez, DeRon R. Walker, Jeanette Young, Susan Zickmund
Data analysis and interpretation: Florian R. Schroeck, A. Aziz Ould Ismail, Grace N. Perry, David A. Haggstrom, Steven L. Sanchez, DeRon R. Walker, Jeanette Young, Susan Zickmund, Lisa Zubkoff
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Determinants of Risk-Aligned Bladder Cancer Surveillance—Mixed-Methods Evaluation Using the Tailored Implementation for Chronic Diseases Framework
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Florian R. Schroeck
Other Relationship: Sesen Bio
No other potential conflicts of interest were reported.
REFERENCES
- 1.Warren JL, Mariotto AB, Meekins A, et al. Current and future utilization of services from medical oncologists J Clin Oncol 263242–32472008 [DOI] [PubMed] [Google Scholar]
- 2.Cooper GS, Kou TD, Reynolds HL.Receipt of guideline-recommended follow-up in older colorectal cancer survivors Cancer 1132029–20372008 [DOI] [PubMed] [Google Scholar]
- 3. Salz T, Weinberger M, Ayanian JZ, et al. Variation in use of surveillance colonoscopy among colorectal cancer survivors in the United States. BMC Health Serv Res. 2120;10:256. doi: 10.1186/1472-6963-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schroeck FR, Lynch KE, Chang JW, et al. Extent of risk-aligned surveillance for cancer recurrence among patients with early-stage bladder cancer. JAMA Netw Open. 2018;1:e183442. doi: 10.1001/jamanetworkopen.2018.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matulay JT, Tabayoyong W, Duplisea JJ, et al. Variability in adherence to guidelines based management of nonmuscle invasive bladder cancer among Society of Urologic Oncology (SUO) members Urol Oncol 38796.e1–796.e62020 [DOI] [PubMed] [Google Scholar]
- 6.Sehdev A, Sherer EA, Hui SL, et al. Patterns of computed tomography surveillance in survivors of colorectal cancer at Veterans Health Administration facilities Cancer 1232338–23512017 [DOI] [PubMed] [Google Scholar]
- 7.Moye J, Schuster JL, Latini DM, et al. The future of cancer survivorship care for Veterans Fed Pract 2736–432010 [PMC free article] [PubMed] [Google Scholar]
- 8.The National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer Version 1. 2020. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SS, Boorjian SA, Chou R, et al. Non-muscle Invasive Bladder Cancer: American Urological Association/SUO Guideline. 2016. https://www.auanet.org/education/guidelines/non-muscle-invasive-bladder-cancer.cfm [Google Scholar]
- 10.Pruthi RS, Baldwin N, Bhalani V, et al. Conservative management of low risk superficial bladder tumors J Urol 17987–902008 [DOI] [PubMed] [Google Scholar]
- 11.Koo K, Zubkoff L, Sirovich BE, et al. The burden of cystoscopic bladder cancer surveillance: Anxiety, discomfort, and patient preferences for decision making Urology 108122–1282017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeck FR, Lynch KE, Li Z, et al. The impact of frequent cystoscopy on surgical care and cancer outcomes among patients with low-risk, non–muscle-invasive bladder cancer Cancer 1253147–31542019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Bosch S, Witjes J.Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: A systematic review Eur Urol 60493–5002011 [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni GS, Urbach DR, Austin PC, et al. Longer wait times increase overall mortality in patients with bladder cancer J Urol 1821318–13242009 [DOI] [PubMed] [Google Scholar]
- 15.Fetters MD, Curry LA, Creswell JW.Achieving integration in mixed methods designs-principles and practices Health Serv Res 482134–21562013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beehler GP, Wray LO. Behavioral health providers' perspectives of delivering behavioral health services in primary care: A qualitative analysis. BMC Health Serv Res. 2012;12:337. doi: 10.1186/1472-6963-12-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damschroder LJ, Goodrich DE, Robinson CH, et al. A systematic exploration of differences in contextual factors related to implementing the MOVE! weight management program in VA: A mixed methods study. BMC Health Serv Res. 2011;11:248. doi: 10.1186/1472-6963-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose AJ, Petrakis BA, Callahan P, et al. Organizational characteristics of high- and low-performing anticoagulation clinics in the Veterans Health Administration Health Serv Res 471541–15602012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong A, Sainsbury P, Craig J.Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups Int J Qual Health Care 19349–3572007 [DOI] [PubMed] [Google Scholar]
- 20. Flottorp SA, Oxman AD, Krause J, et al. A checklist for identifying determinants of practice: A systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci. 2013;8:35. doi: 10.1186/1748-5908-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wensing M, Oxman A, Baker R, et al. Tailored Implementation for Chronic Diseases (TICD): A project protocol. Implement Sci. 2011;6:103. doi: 10.1186/1748-5908-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeck FR, St. Ivany A, Lowrance WT, et al. Patient perspectives on the implementation of risk-aligned bladder cancer surveillance: Systematic evaluation using the Tailored Implementation for Chronic Diseases framework JCO Oncol Pract 18e668–e6772020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patton MQ.Enhancing the quality and credibility of qualitative analysis Health Serv Res 341189–12081999 [PMC free article] [PubMed] [Google Scholar]
- 24.Guest G, Namey EE, Mitchell ML. Collecting Qualitative Data: A Field Manual for Applied Research. ed 1. Thousand Oaks, CA: SAGE; 2012. [Google Scholar]
- 25.Guetterman TC, Fetters MD, Creswell JW.Integrating quantitative and qualitative results in health science mixed methods research through joint displays Ann Fam Med 13554–5612015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skolarus LE, Neshewat GM, Evans L, et al. Understanding determinants of acute stroke thrombolysis using the tailored implementation for chronic diseases framework: A qualitative study. BMC Health Serv Res. 2019;19:182. doi: 10.1186/s12913-019-4012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jäger C, Freund T, Steinhäuser J, et al. Impact of a tailored program on the implementation of evidence-based recommendations for multimorbid patients with polypharmacy in primary care practices—Results of a cluster-randomized controlled trial. Implement Sci. 2017;12:8. doi: 10.1186/s13012-016-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassouf W, Traboulsi SL, Schmitz-Dräger B, et al. Follow-up in non–muscle-invasive bladder cancer—International Bladder Cancer Network recommendations Urol Oncol 34460–4682016 [DOI] [PubMed] [Google Scholar]
- 29.The National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Non-small Cell Lung Cancer. Version 5. 2021. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf [Google Scholar]
- 30.The National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 2. 2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf [DOI] [PubMed] [Google Scholar]
- 31.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer Gastroenterology 143844–8572012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and analyzed during the current study are not publicly available because they contain potentially identifying and sensitive information but are available from the corresponding author on reasonable request. Upon request from a qualified investigator, a limited data set will be created for that investigator's use and shared pursuant to a Data Use Agreement (DUA) appropriately limiting use of the data set and prohibiting the recipient from identifying or reidentifying (or taking steps to identify or reidentify) any individual whose data are included in the data set. Investigators who request to use the data will be required to obtain institutional review board approval and sign the DUA before release of the data. Interested investigators are encouraged to directly contact the corresponding author.