Abstract
Background
As in most land plants, the roots of orchids (Orchidaceae) associate with soil fungi. Recent studies have highlighted the diversity of the fungal partners involved, mostly within Basidiomycotas. The association with a polyphyletic group of fungi collectively called rhizoctonias (Ceratobasidiaceae, Tulasnellaceae and Serendipitaceae) is the most frequent. Yet, several orchid species target other fungal taxa that differ from rhizoctonias by their phylogenetic position and/or ecological traits related to their nutrition out of the orchid roots (e.g. soil saprobic or ectomycorrhizal fungi). We offer an evolutionary framework for these symbiotic associations.
Scope
Our view is based on the ‘Waiting Room Hypothesis’, an evolutionary scenario stating that mycorrhizal fungi of land flora were recruited from ancestors that initially colonized roots as endophytes. Endophytes biotrophically colonize tissues in a diffuse way, contrasting with mycorrhizae by the absence of morphological differentiation and of contribution to the plant’s nutrition. The association with rhizoctonias is probably the ancestral symbiosis that persists in most extant orchids, while during orchid evolution numerous secondary transitions occurred to other fungal taxa. We suggest that both the rhizoctonia partners and the secondarily acquired ones are from fungal taxa that have broad endophytic ability, as exemplified in non-orchid roots. We review evidence that endophytism in non-orchid plants is the current ecology of many rhizoctonias, which suggests that their ancestors may have been endophytic in orchid ancestors. This also applies to the non-rhizoctonia fungi that were secondarily recruited by several orchid lineages as mycorrhizal partners. Indeed, from our review of the published literature, they are often detected, probably as endophytes, in extant rhizoctonia-associated orchids.
Conclusion
The orchid family offers one of the best documented examples of the ‘Waiting Room Hypothesis’: their mycorrhizal symbioses support the idea that extant mycorrhizal fungi have been recruited among endophytic fungi that colonized orchid ancestors.
Keywords: Ectomycorrhizal fungi, endophytism, mixotrophy, mycoheterotrophy, rhizoctonias, saprobic fungi
INTRODUCTION
As in most other plants, orchid roots symbiotically associate with soil fungi. The dual organ formed, the mycorrhiza, plays a major role in plant protection and mineral nutrition (Smith and Read, 2008; van der Heijden et al., 2015; Li et al., 2021). In orchids, this symbiosis displays several features: (1) it occurs from germination onwards, where the fungus also brings carbon resources to the germinating seed, which is almost devoid of reserves; (2) the fungal hyphae cross the wall of some cells in germinating seeds and in roots and, without disrupting the plasma membrane, form a hyphal coil called a peloton (Fig. 1B); and (3) the association is frequently more specific than in other mycorrhizal plants (Rasmussen, 1995; Dearnaley et al., 2016). We deal here with the evolution of this mycorrhizal association.
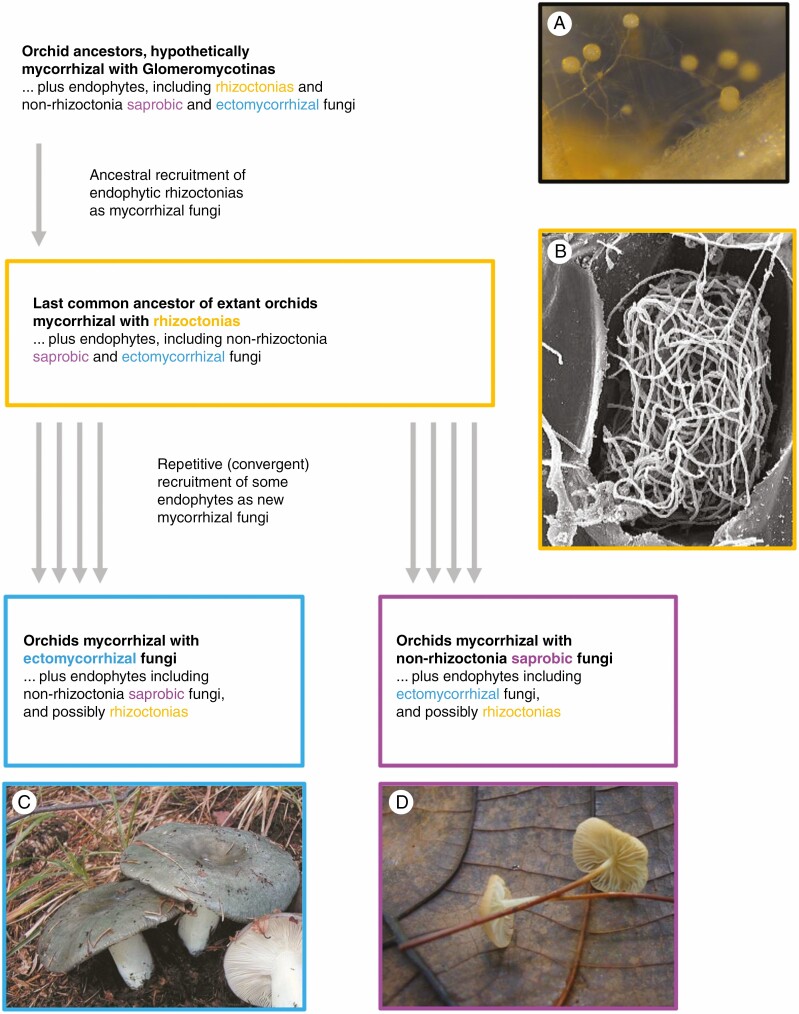
Figure 1.
A summary of the evolution of mycorrhizal partners in orchids, emphasizing that they may have been recruited from fungi that were ancestrally endophytic (in orchids, fungi that are ectomycorrhizal on other plants can be endophytic or orchid-mycorrhizal, but for ease of reading, they are collectively qualified as ‘ectomycorrhizal’ fungi). (A) Hyphae and spores of Rhizophagus irregularis (courtesy Jelle van Creij, University of Wageningen). (B) Electron micrograph of an unknown rhizoctonia peloton in a Dactylorhiza majalis orchid mycorrhiza. (C) Fruiting bodies of ectomycorrhizal Russula cyanoxantha. (D) Fruiting bodies of litter-decaying Mycena spp.
Most orchids associate with rhizoctonias (Fig. 1), a polyphyletic grouping of three Basidiomycota lineages (Dearnaley et al., 2012), namely Ceratobasidiaceae and Tulasnellaceae (in the Cantharellales; Smith and Read, 2008) as well as Serendipitaceae (in the Sebacinales; Weiss et al., 2016). These taxa are not mycorrhizal in other plants, with the exception of a few of their sub-clades (see below).
Yet, many orchids from unrelated genera associate with diverse non-rhizoctonia fungi from the Basidiomycota (mostly in Agaricomycetes), and more rarely from the Ascomycota (Dearnaley et al., 2012; Fig. 1). These fungal taxa are saprobic (decomposing plant residues) or ectomycorrhizal, i.e. they normally form a mycorrhizal association with trees and shrubs (Smith and Read, 2008).
Finally, beyond mycorrhizal fungi, orchid roots harbour diverse fungi that do not entail morphological symptoms (as pathogenic fungi would do) nor differentiation of one or the other partner (e.g. no mycorrhizal morphogenesis of hyphae, such as pelotons, nor developmental change of root tissues). Such biotrophic fungi occur in all plant roots and are called endophytes (Wilson, 1995; Rodriguez et al., 2009). Compared to mycorrhizal fungi, endophytes produce no specific morphological features, are often less abundant in plant tissues and do not strongly contribute to host nutrition. In the current text, to facilitate readability, we restrictively mean by ‘endophytes’ fungi that (1) colonize roots (we do not consider endophytes that colonize shoots and leaves) and (2) simultaneously colonize soil around the roots (we do not consider endophytes that strictly develop in host tissues). Endophytic fungi have been well characterized and studied in orchids, by isolation and molecular methods (Sarsaiya et al., 2019).
Here, we put the diversity of orchid mycorrhizal fungi into an evolutionary perspective. We suggest that (1) an ancestral mycorrhizal association with rhizoctonias (most probably Cantharellales) and (2) repeated evolutionary shifts to other fungal lineages (sometimes allowing a new ecology) occurred through the same basic evolutionary process, namely the selection of fungi that already occupied roots as endophytes. This phenomenon has been previously described as the ‘Waiting Room Hypothesis’ (WRH; Selosse et al., 2009; box 1 and Fig. 2). We developed this scenario for fungal lineages (van der Heijden et al., 2015; Selosse et al., 2018; Schneider-Maunoury et al., 2018, 2020) and, in this review, we tentatively apply it to orchids. Evidence that supports this scenario comes from an increasing trend to report the whole fungal communities detected in roots, in part thanks to next-generation sequencing. Cessation of screening data and of reporting of only the taxa known, or expected, to be mycorrhizal (Selosse et al., 2010) as well as interest in describing microbiota diversity more comprehensively have enhanced knowledge of endophytic fungi and the development of new working hypotheses.
Box 1. The Waiting Room Hypothesis.
The Waiting Room Hypothesis (WRH) was first proposed by Selosse et al. (2009) in a study revealing widespread endophytic abilities in the Sebacinales. The latter order encompasses many sub-clades within its two families, Serendipitaceae and Sebacinaceae, that evolved different mycorrhizal associations on various hosts, including orchids (see main text). It was suggested that root endophytism in Sebacinales was a predisposition for mycorrhizal evolution, and that such an evolutionary trajectory could apply to other fungal taxa (Selosse et al., 2018).
The WRH is an evolutionary process where endophytism, a biotrophic interaction that by definition does not harm the host (Wilson, 1995), is an ecological niche predisposing to evolve tighter associations with the host (Fig. 1). The WRH acknowledges that most evolutionary pathways or new anatomical structures evolve from pre-existing structures (Shubin et al., 2009). Mycorrhizal associations imply, for both partners, (1) morphological modification to build the mycorrhiza, and (2) more elaborated and intense nutrient exchanges than in endophytism. Thus, endophytes are predisposed to evolve mycorrhizal abilities, but this is only a possibility since not every endophyte is called out of the waiting room. Of course, endophytes predisposed to become mycorrhizal are exclusively those that (1) colonize roots (we do not consider here endophytes that colonize shoots and leaves) and (2) simultaneously colonize soil around the roots (we do not consider here endophytes that strictly develop in host tissues). By endophytes, we mean in this review fungi with the above-mentioned specific traits.
When exiting the waiting room, the new mycorrhizal lineage may add up to existing partners, especially when the association is generalist, such as the ectomycorrhizal symbiosis. It may alternatively displace some or all existing symbionts, as probably happened in the more specific orchid mycorrhizal symbiosis (Fig. 1). In terms of physiological effects on the host, trophic and protective effects observed in the mycorrhizal association may arise during the transition to mycorrhizal status, or even before: some endophytic fungi have a positive effect on their hosts, such as the dark septate endophytes (Newsham, 2011) and more generally some endophytic Helotiales (Almario et al., 2017) and Sebacinales (Weiss et al., 2016).
The WRH predicts patterns of interaction at ecological and/or phylogenetic levels:
- First, the fungus that is mycorrhizal in a given lineage may have retained endophytic abilities in other plants (and thus have a dual niche; Selosse et al., 2018; Fig. 2). Additionally, its endophytic presence is especially expected in phylogenetically related plants. Of course, endophytism can be secondarily lost in mycorrhizal species but then the following pattern may be retained. Here, we report this for orchid mycorrhizal fungi, rhizoctonias (see main text) and non-rhizoctonias (Tables 1 and 2).
- Second, the mycorrhizal lineage arises phylogenetically among earlier-diverging lineages that are endophytic, such as mycorrhizal sub-clades in Sebacinales (Weiss et al., 2016) or in Hymenochaetales (Korotkin et al., 2018). Here again, these endophytic ‘basal’ lineages may be missing because they went extinct or changed their ecology. For example, this limits the discussion of the WRH for Glomeromycotinas, the ancient mycorrhizal symbionts that diverged early from other fungi (Smith and Read, 2008; Strullu-Derrien et al., 2018).
Thus, the two previous patterns may not be observed but, whenever they are observed, they are expected under the WRH.
The WRH is sometimes viewed as an alternative to the idea that mycorrhizal fungi repetitively evolved from saprobic ancestors (Floudas et al., 2012; Kohler et al., 2015). The latter idea, which received strong support from fungal phylogenomics, is in no way contradictory: First, the cited studies did not consider endophytism as an ecological niche for fungi, so that they cannot evaluate the WRH; second, the WRH suggests that endophytism is the missing link between saprobic and mycorrhizal life in an evolutionary trajectory between saprobic and mycorrhizal lifestyles (Fig. 1A). Indeed, several fungi have a dual, saprobic and endophytic niche (Selosse et al., 2007; Thoen et al., 2020; Fig. 1B–D). They illustrate the first part of such a trajectory, if not the second part in the case of Mycena, saprobic fungi that are endophytic and even mycorrhizal in some orchids (see main text).
Finally, the WRH does not exclude other evolutionary trajectories leading to a mycorrhizal lifestyle.
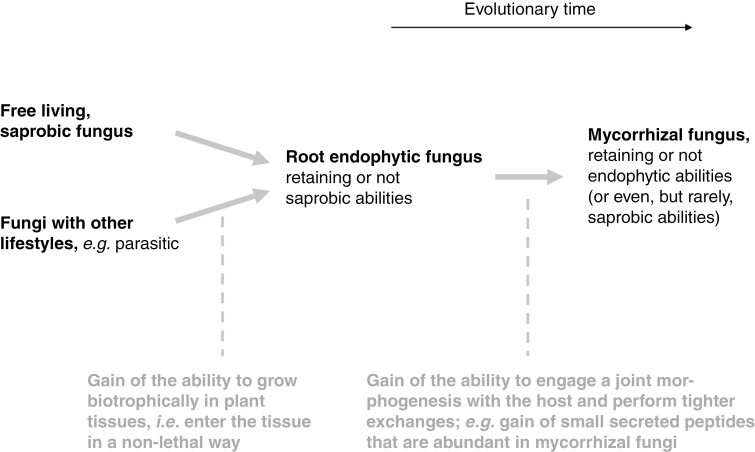
Figure 2.
The evolutionary trajectory of the Waiting Room Hypothesis (WRH) on the fungal side. A general pathway where mycorrhizal partners arise from saprobic fungi by way of endophytism.
The single ancestral transition to rhizoctonias
The name rhizoctonias in orchid research can be traced back to the pioneering work of Noël Bernard who attributed the orchid symbionts to the genus Rhizoctonia (Selosse et al., 2017), using a larger, now outdated definition of this genus (Oberwinkler et al., 2013). Nevertheless, the guild of mycorrhizal fungi that associate with most orchids, namely Serendipitaceae, Ceratobasidiaceae and Tulasnellaceae, is still commonly called rhizoctonias (Dearnaley et al., 2012).
Orchids belong to Asparagales (Smith and Read, 2008), which, like most land plants, form arbuscular mycorrhizas with Glomeromycotinas, a mycorrhizal partnership that is ancestral in land plants (Strullu-Derrien et al., 2018; van der Heijden et al., 2015). It can therefore be speculated that the ancestors of orchids formed arbuscular mycorrhizas, although we are not aware of any support for this from the fossil record. Glomeromycotinas are infrequently reported in molecular barcoding of orchid roots (e.g. Abadie et al., 2006; May et al., 2020). On the one hand, the primers used often disfavour their detection, but on the other hand, we are not aware of any morphological data indicating that they form arbuscular mycorrhizal associations in extant orchids.
As present members of Apostasioideae, the most anciently diverging orchid lineage, associate with rhizoctonias, notably Cantharellales (Kristiansen et al., 2004; Yukawa et al., 2009; Suetsugu and Matsubayashi, 2021a), the last common ancestor of extant orchids was most probably already mycorrhizal with rhizoctonias (Dearnaley et al., 2012; Fig. 1), as also suggested by a reconstruction of ancestral states by Wang et al. (2021). The acquisition of the rhizoctonia partners may have occurred in several steps. For example, the absence of Serendipitaceae in Apostasioideae (Kristiansen et al., 2004; Yukawa et al., 2009; Suetsugu and Matsubayashi, 2021a) suggests that this family may have been acquired after the divergence of this lineage. Yet, a loss or a hitherto undetected presence of Serendipitaceae in Apostasioideae cannot be excluded.
Thus, all extant orchids studied up till now abandoned Glomeromycotinas and shifted to a mycorrhizal association with rhizoctonias (with exception of some secondarily evolved species described below).
Endophytism in non-orchids may have predisposed rhizoctonias to become mycorrhizal
Despite very limited data on their in situ ecology, rhizoctonias are often considered to live as saprobes in soil around the roots (Smith and Read, 2008), or on the tree bark around epiphytic orchids (Petrolli et al., 2021). This is supported by their cultivability in vitro (Rasmussen, 1995), and by genome data evidencing large enzymatic abilities (Kohler et al., 2015; Weiss et al., 2016; Miyauchi et al., 2020); recently, a Serendipitaceae fungus was even suggested to access dead organic matter by way of 14C dating its carbon (Suetsugu et al., 2021a). Yet, some rhizoctonias are also endophytes of non-orchid plants (see text below). Unfortunately, up to now, endophytism has not been correlated with any known fingerprint in the fungal genome, so that genomic studies cannot help detect this status.
First, endophytism is a general feature of Serendipitaceae (Selosse et al., 2009; Weiss et al., 2016): for example, Serendipita (= Piriformospora) vermifera is a famous and well-studied model of a plant endophyte (Zuccaro et al., 2011; Gill et al., 2016) that is also orchid-mycorrhizal (Oliveira et al., 2014). Second, members of Tulasnellaceae are endophytic, e.g. in Euphorbia spp. (Crous et al., 2015) and Bromus erectus (Girlanda et al., 2011). In the latter case, the endophytic individual is also orchid-mycorrhizal (Girlanda et al., 2011). Unfortunately, Tulasnellaceae are absent from most descriptions of endophytic fungal communities since the primers used in barcoding for PCR (polymerase chain reaction) amplification of ribosomal DNA present mismatches with their genomic sequences (Vogt-Schilb et al., 2020). Third, although many Ceratobasidiaceae are parasitic on non-orchid roots, some strains are symptomless and endophytic (Sen et al., 1999; Likar et al., 2008; Yuan et al., 2010b; Girlanda et al., 2011); yet, the endophytic abilities of orchid mycorrhizal sub-clades remain unclear (Veldre et al., 2013).
Thus, rhizoctonias are saprobic and endophytic in non-orchid roots (Selosse and Martos, 2014). Here, endophytism should not be opposed to saprophytism, and rhizoctonias simply have a broad ecological niche encompassing these two facets (Selosse et al., 2018). As expected under the WRH (box 1), this permissive ecology may be a predisposition to evolve mycorrhizal abilities, not only in the ancestors of orchids but also in other plant groups. Indeed, some rhizoctonia lineages were also recruited in other mycorrhizal types. Ectomycorrhizal associations arose in at least two sub-clades of Tulasnellaceae (Tedersoo and Smith, 2013), two sub-clades of Ceratobasidiaceae (Veldre et al., 2013) and one sub-clade of Serendipitaceae (Hynson et al., 2013; Tedersoo and Smith, 2013). Members of Serendipitaceae were also recruited as mycorrhizal fungi of crown Ericaceae, where they (and other mycorrhizal fungi) form intracellular coils similar to those formed in orchid mycorrhizae (Selosse et al., 2007). Thus, endophytism in rhizoctonias would have allowed at least five transitions to ectomycorrhizas and four transitions to coil-forming mycorrhizae, i.e. one in Ericaceae and three in orchids (the latter, counting conservatively, assumes one transition to orchid mycorrhiza per rhizoctonia family, but repeated acquisition may have occurred within each family; Veldre et al., 2013; Weiss et al., 2016). Such repeated transitions make the rhizoctonias excellent models for the WRH and for the endophytic-to-mycorrhizal transition.
Additionally, novel mycorrhizal associations have been reported in some orchid species that involve Basidiomycotas. For example, tropical orchids are mycorrhizal with Atractiellales (Pucciniomycotina; Kottke et al., 2010) and possibly with Trechisporales (Martos et al., 2012); and Nervilia japonica associates with an unknown Basidiomycota taxon (Nomura et al., 2013). Although the ancestral niche for the latter fungal taxon is unclear, the WRH may apply to the other two. Atractiellales encompass saprobic species (Bauer et al., 2006), some of which may have evolved endophytism before mycorrhizal abilities; and Trechisporales have endophytic abilities, from which not only orchid mycorrhizas, but also ectomycorrhizas may have evolved (Henry et al., 2017; Vanegas-León et al., 2019). For orchids, these partners are often observed in the company of rhizoctonias and in a limited set of species and ecological conditions. Such associations probably evolved late in orchid diversification.
The multiple, convergent transitions to ectomycorrhizal fungi
Another category of fungi that are frequently mycorrhizal in orchids belongs to taxa that are also known to form ectomycorrhizas (Dearnaley et al., 2012; Wang et al., 2021). In orchids, they form typical orchid mycorrhizas and pelotons (Fig. 1B), but, due to the fact that they are mainly (and historically known as) ectomycorrhizal (Fig. 1C), they are collectively qualified as ‘ectomycorrhizal’ fungi. Their presence in orchids was recognized when molecular identifications became available, because these fungi are poorly cultivable and therefore escaped cultivation attempts (Rasmussen, 1995).
The symbiosis with ectomycorrhizal fungi in orchids is linked to a physiological ability to extract carbon compounds from the fungus. Some of these orchids use the fungus as an exclusive carbon source and are therefore achlorophyllous (the so-called mycoheterotrophic orchids; Merckx et al., 2013). Some others still retain photosynthesis and use fungal carbon in addition to autotrophy: these chlorophyllous orchids are called mixotrophic (Julou et al., 2005; Selosse and Roy, 2009; Jacquemyn and Merckx, 2019). This probably represents secondary shifts to ectomycorrhizal fungi, which is congruent with the fact that orchids arose ca. 112 million years ago (Givnish et al., 2015), while most ectomycorrhizal fungal clades arose 30–90 million years later (Hibbett and Matheny, 2009). Such transitions of partners and nutritional physiology occurred convergently in orchid evolution (e.g. Wang et al., 2021). For example, the evolution to full mycoheterotrophy with the replacement of associated symbionts occurred two to three times in Vanilloideae, nine or more times in Orchidoideae and ≥14 times in Epidendroideae (Merckx, 2013). Yet, mycoheterotrophy often evolved in ancestors that were already mixotrophic with ectomycorrhizal fungi (Selosse and Roy, 2009; Jacquemyn and Merckx, 2019), and therefore several mycoheterotrophic taxa may derive from a single ancestral shift to mixotrophy. Thus, the exact number of mycorrhizal partner transitions may be smaller than predicted from the number of shifts to mycoheterotrophy. Yet, it is difficult to assess the number of transitions to mixotrophy, which is less obvious and less well documented. Nevertheless, given the diverse range of orchid sub-families where mixotrophy can be found, an intriguing parallel convergent evolution is almost certain (Wang et al., 2021; Fig. 1).
Along with a dominance of ectomycorrhizal fungi, mixo- and mycoheterotrophic orchids often harbour a few rhizoctonias (e.g. Julou et al., 2005; Abadie et al., 2006; Jacquemyn et al., 2021), but it is unknown if such rhizoctonias are endophytic or mycorrhizal (i.e. form true functional pelotons). There is even a growing awareness, thanks to next-generation sequencing, that a continuum exists between rhizoctonia-associated orchids and those associated with ectomycorrhizal fungi, so that the evolutionary transition may occur with some coexistence of both fungal guilds (Jacquemyn et al., 2017).
Most importantly, ectomycorrhizal fungi are frequently present at low to very low frequencies in fully photosynthetic rhizoctonia-associated orchids as a ‘background’ noise (Table 1). In a literature survey, we found this scenario in at least 54 orchid species throughout the phylogeny of this family, based on 42 studies (Table 1). Ectomycorrhizal fungi are found in a few samples and/or in a minor amount among clones (in PCR cloning) or among next-generation sequencings reads so that the orchids are considered as rhizoctonia-associated in these studies (see references in Table 1). In the last decade, this possibility has triggered the call for reporting of all fungal species found, even if unexpected (Selosse et al., 2010). Of course, next-generation sequencing has prompted larger reports of the diversity encountered, but well before these methods arose, evidence had already been obtained from cloning the barcoding PCR products, or even from direct Sanger sequencing (Table 1).
Table 1.
Ectomycorrhizal non-rhizoctonia fungi present, possibly as endophytes, in rhizoctonia-associated green orchids
| Orchid species | Fungi | Method | Reference |
|---|---|---|---|
| Terrestrial | |||
| Vanilloideae | |||
| Vanilleae | |||
| Vanillia planifolia | Scleroderma | PCR (pel.) | González-Chávez et al. (2018) |
| Scleroderma, Inocybe, Russula, Tuber | NGS | Johnson et al. (2021) | |
| Cypripedioideae | |||
| Cypripedium acaule | Russula, Lactarius | PCR | Bunch et al. (2013) |
| Russula | Cloning | Shefferson et al. (2007) | |
| Cypripedium candidum | Thelephoraceae | Cloning | Shefferson et al. (2005) |
| Cypripedium debile | Sebacinaceae | Cloning | Suetsugu et al. (2021c) |
| Cypripedium fasciculatum | Sebacinaceae | Cloning | Shefferson et al. (2005) |
| Russula, Tomentalla | Cloning | Whitridge and Southworth ( 2005) | |
| Cypripedium flavum | Boletaceae | Cloning | Yuan et al. (2010a) |
| Cypripedium formosanum | Russula | Cloning | Shefferson et al. (2007) |
| Cypripedium montanum | Thelephoraceae | Cloning | Shefferson et al. (2005) |
| Cypripedium parviflorum | Russula | Cloning | Shefferson et al. (2005) |
| Paphiopedilum spicerianum | Tricholoma, Thelephoraceae | NGS | Han et al. (2016) |
| Orchidoideae | |||
| Cranichideae | |||
| Aa achalensis | Pezizaceae | Isolat. | Sebastian et al. (2014) |
| Goodyera foliosa | Sistotrema | Cloning | Shefferson et al. (2010) |
| Goodyera procera | Tomentella | Cloning | Shefferson et al. (2010) |
| Goodyera repens | Russula, Lactarius | PCR | Liebel et al. (2015) |
| Russula, Tomentella | PCR | Voronina et al. (2018) | |
| Goodyera velutina | Russula, Clavulina | Cloning | Shefferson et al. (2010) |
| Russula, Tuber, Thelephoraceae | Cloning | Suetsugu et al. (2019) | |
| Pterostylis nutans | Russulaceae, Tricholoma | Cloning | Irwin et al. (2007) |
| Spiranthes spiralis | Sebacinaceae, Thelephoraceae, | NGS | Duffy et al. (2019) |
| Inocybaceae, Russulaceae | NGS | Calevo et al. (2021) | |
| Orchideae | |||
| Anacamptis laxiflora | Pezizaceae | Culture | Multu et al. (2020) |
| Anacamptis morio | Terfezia | Cloning | Ercole et al. (2015) |
| Pezizaceae | NGS | Voyron et al. (2017) | |
| Thelephoraceae, Cortinarius | NGS | Jacquemyn et al. (2014) | |
| Anacamptis papilionacea | Thelephoraceae, Cortinarius, Russula | NGS | Jacquemyn et al. (2014) |
| Corycium excisum, crispum, orobanchoides, ingeanum, nigrescens and bicolorum | Peziza | Cloning | Waterman et al. (2011) |
| Dactylorhiza spp. | Cortinarius, Clavulina, Inocybe, Thelephoraceae | NGS | Jacquemyn et al. (2016b) |
| Dactylorhiza fuchsii | Cenococcum, Cortinarius, Hebeloma, Inocybe, Sebacina, Tomentella | NGS | Jacquemyn et al. (2017) |
| Dactylorhiza incarnata | Peziza, Cortinarius, Inocybe, Tomentella | NGS | Jacquemyn et al. (2017) |
| Dactylorhiza majalis | Laccaria | PCR (pel.) | Kristiansen et al. (2001) |
| Craterellus, Hydnum | PCR | Schweiger et al. (2018) | |
| Dactylorhiza praetermissa | Cenococcum, Cortinarius, Hebeloma, Inocybe, Sebacina, Tomentella | NGS | Jacquemyn et al. (2017) |
| Gymnadenia conopsea | Terfezia, Russula, Lactarius, Cenococcum, Geopixis, Tomentella | Cloning | Stark et al. (2009) |
| Terfezia | PCR | Tesitelova et al. (2013) | |
| Tomentella | Cloning | Vogt-Schilb et al. (2020) | |
| Herminium monorchis | Cenococcum, Helvella, Cortinarius, Hebeloma, Inocybe, Sebacina, Tomentella | NGS | Jacquemyn et al. (2017) |
| Peziza | PCR | Schiebold et al. (2018) | |
| Orchis anthropophora | Thelephoraceae, Hebeloma | Cloning | Jacquemyn et al. (2010) |
| Orchis canariensis | Russula | Cloning | Liebel et al. (2010) |
| Orchis galilaea | Thelephoraceae, Russula | Cloning | Jacquemyn et al. (2011) |
| Ophrys sicula | Cortinarius, Russula | NGS | Jacquemyn et al. (2014) |
| Orchis simia | Hebeloma | Cloning | Jacquemyn et al. (2011) |
| Ophrys sphegodes | Pezizaceae, Tomentella | NGS | Voyron et al. (2017) |
| Piperia unalascensis | Sistotrema | Isolat. | Currah and Sherburne (1992) |
| Platanthera bifolia | Cortinarius, Helvella, Inocybe, Peziza, Sebacina, Suillus, Thelephora, Tuber | NGS | Esposito et al. (2016) |
| Platanthera chlorantha | Cortinarius, Sebacina, SuillusThelephora | NGS | Esposito et al. (2016) |
| Platanthera cooperi | Thelephoraceae, Sebacinaceae | NGS | Kaur et al. (2021) |
| Pterygodium halli and inversum | Peziza | Cloning | Waterman et al. (2011) |
| Pterygodium leucanthum | Sebacinaceae | Cloning | Waterman et al. (2011) |
| Serapias bergonii | Thelephoraceae, Cortinarius | NGS | Jacquemyn et al. (2014) |
| Epidendroideae | |||
| Calypsoeae | |||
| Tipularia discolor | Tomentella | PCR (pel.) | McCormick et al. (2004) |
| Malaxideae | |||
| Liparis loeselii | Thelephoraceae, Russulaceae, | NGS | Waud et al. (2017) |
| Tricholomataceae, Inocybaceae, Cortinariaceae, Sebacinaceae | Jacquemyn et al. (2017) | ||
| Neottieae | |||
| Epipactis palustris | Thelephora, Inocybe, Geopora, Cortinarius, Leptodontidium, Exophiala, Hebeloma | NGS | Jacquemyn et al. (2016a) |
| Jacquemyn et al. (2017) | |||
| Epiphytic | |||
| Epidendroideae | |||
| Arethuseae | |||
| Coelogyne viscosa | Tomentella | Cloning | Xing et al. (2014) |
| Epidendreae | |||
| Epidendrum armeniacum | Pisolithus | NGS | Petrolli et al. (2021) |
| Epidendrum firmum | Thelephoraceae, Russulaceae, Scleroderma, etc. | Cloning | Kartzinel et al. (2013) |
| Isochilus linearis | Pisolithus | NGS | Petrolli et al. (2021) |
Cloning: cloning of PCR products; NGS, next-generation sequencing; PCR, direct sequencing of polymerase chain reaction products; PCR (Pel.) direct sequencing of polymerase chain reaction products performed on isolated peloton.
Ectomycorrhizal fungi in rhizoctonia-associated orchids may be endophytic or truly mycorrhizal. On the one hand, in three cases, ectomycorrhizal taxa were identified from DNA isolated from pelotons [see PCR (Pel.) in Table 1]. Although co-isolation of endophytic hyphae is possible, this suggests that these fungi formed pelotons in a plant otherwise massively mycorrhizal with rhizoctonias. On the other hand, endophytism is a reasonable option in most cases because there are growing reports that some ectomycorrhizal fungi colonize the roots of non-ectomycorrhizal plants as endophytes (Selosse et al., 2018). This is demonstrated for the genus Tuber, whose endophytism is supported in non-ectomycorrhizal plants by fluorescence in situ hybridization (FISH; Schneider-Maunoury et al., 2020; Fig. 3). Endophytism has also been suggested for ectomycorrhizal Sebacinaceae (Selosse et al., 2009; Weiss et al., 2011;, 2016), and it may extend to various other lineages, at least Scleroderma and Thelephoraceae (Schneider-Maunoury et al., 2018, 2020), as well as Inocybe (Yung et al., 2021). Indeed, all these taxa are well represented, among other ectomycorrhizal fungi, in rhizoctonia-associated orchids (Table 1).
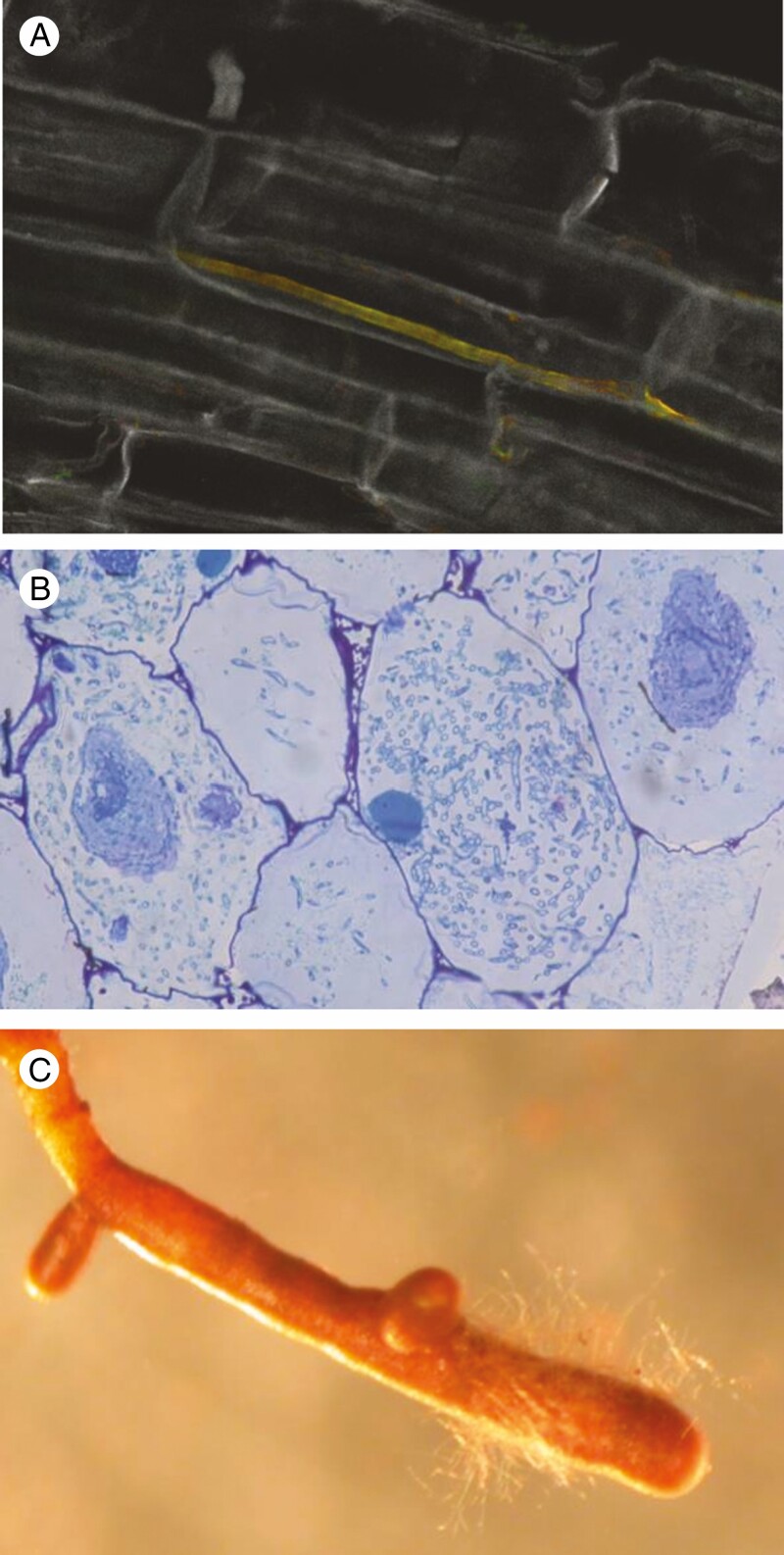
Figure 3.
The truffles (Tuber spp.) are demonstrated examples of mycorrhizal fungi that also are endophytic in roots of plants where they do not form mycorrhizae. (A) An endophytic Tuber melanosporum hypha (orange) in a Geranium sp. root (grey background), coloured by fluorescence in situ labelling as in Schneider-Maunoury et al. (2020). (B) Mycorrhizal pelotons of T. aestivum in an Epipactis microphylla orchid mycorrhiza (thin section under light microscopy, prepared as in Selosse et al., 2004). (C) An ectomycorrhiza of T. melanosporum, with hyphae covering the tip of a Quercus pubescens root (courtesy François Le Tacon, INRAe).
These data support that repeated recruitment of ectomycorrhizal fungi as orchid mycorrhizal partners is well explained under the WRH. With the permanent opportunity to shift to orchid-mycorrhizal, some of these ectomycorrhizal fungi are endophytic, and some others may already form pelotons and achieve the first steps of evolution to orchid mycorrhizal status (Fig. 1). In other words, the orchid/ectomycorrhizal fungi coevolution displays various stages of the WRH process. We note that some taxa (such as Sebacinaceae and Thelephoraceae; Table 1) are frequently present in rhizoctonia-associated orchids, which may reflect why they are often involved in secondarily evolved mixo- and mycoheterotrophic orchids (Merckx, 2013). The reason for this bias is not known, but it is possible that not all ectomycorrhizal taxa retained the ancestral endophytic abilities to the same degree (Fig. 2). Finally, an unexpected observation is that some rhizoctonia-associated epiphytic orchids display ectomycorrhizal taxa (Table 1). The surprising occurrence of such taxa on tree bark in forests dominated by arbuscular mycorrhizal trees suggests that the ecological niche of ectomycorrhizal fungi still hides some unknown aspects (Selosse et al., 2018).
The multiple, convergent transitions to non-rhizoctonia saprobic fungi
Finally, some orchid taxa stopped interactions with rhizoctonias by shifting to wood- or litter-decaying non-rhizoctonia Basidiomycotas, in a secondary evolution that also implied the transition to full mycoheterotrophy (Ogura-Tsujita et al., 2009; Martos et al., 2009; Merckx, 2013; Hatté et al., 2020; Suetsugu et al., 2020; Wang et al., 2021). For example, the fungal genera Psathyrella, Mycena, Coprinus and Gymnopus were repeatedly involved in such transitions within the orchid lineages (Selosse et al., 2010; Lee et al., 2015). Recently, it was demonstrated that some mixotrophic orchids rely on such non-rhizoctonia fungi (Suetsugu et al., 2021b; Yagame et al., 2021). Based on this, mixotrophy in green orchids associated with Mycena spp. (Fan et al., 1996; Guo et al., 1997; Zhang et al., 2012) now deserves further study.
Thus, parallel transitions to non-rhizoctonia saprobic Basidiomycotas occurred (Fig. 1). Here again, a closer look at published studies indicates that such fungi are strikingly present in rhizoctonia-associated orchids (Table 2), albeit at low to very low frequency (minor amount among clones in PCR cloning or among next-generation sequencings reads; see references in Table 2). In a literature survey, we found this scenario in at least 15 orchid species throughout the phylogeny of this family, based on 15 studies (Table 2). Non-rhizoctonia saprobic Basidiomycotas may have been frequently underreported, since detection of saprobic fungi, which sporulate abundantly, can easily be attributed to contamination. Yet, here again, an endophytic colonization is possible, and we cannot rule out peloton formation in some cases. Interestingly, the ability of some saprobic fungi to live biotrophically in tissues (Fig. 2) has been speculated or demonstrated in aerial parts (Selosse et al., 2007) and has recently been highlighted in the roots of non-orchid plants for the genus Mycena (Thoen et al., 2020).
Table 2.
Saprobic non-rhizoctonia Basidiomycotas present, possibly as endophytes, in rhizoctonia-associated green orchids
| Orchid species | Fungi | Method | Reference |
|---|---|---|---|
| Terrestrial | |||
| Cypripedioideae | |||
| Cypripedium flavum | Tapinellaceae | Cloning | Yuan et al. (2010a) |
| Paphiopedilum micranthum | Psathyrellaceae | Cloning | Yuan et al. (2010a) |
| Orchidoideae | |||
| Cranichideae | |||
| Anoectochilus roxburghi | Mycena | Culture | Guo et al. (1997) |
| Goodyera repens | Mycena, Trametes, Lentinus | PCR | Voronina et al. (2018) |
| Orchideae | |||
| Dactylorhiza spp. | Mycena, Psathyrella, Coprinus, Marasmius | NGS | Jacquemyn et al. (2016b) |
| Gymnadenia conopsea | Serpulaceae | PCR | Tesitelova et al. (2013) |
| Mycena | Cloning | Vogt-Schilb et al. (2020) | |
| Epidendroideae | |||
| Arethuseae | |||
| Bletilla spp. | Mycena, Gymnopus | NGS | Zeng et al. (2021) |
| Pleione spp. | Protomerulius | Cloning | Quin et al. (2019) |
| Calypsoeae | |||
| Calypso bulbosa | Protomerulius | NGS | Suetsugu and Matsubayashi (2021b) |
| Cymbidieae | |||
| Oeceoclades maculata | Psathyrella, Sparassis, Fomes | PCR | Bayman et al. (2016) |
| Malaxideae | |||
| Liparis loeselii | Psathyrellaceae | NGS | Waud et al. (2017) |
| Epiphytic | |||
| Epidendroideae | |||
| Arethuseae | |||
| Coelogyne viscosa | Marasmiaceae, Ganoderma | Cloning | Xing et al. (2014) |
| Cymbidieae | |||
| Cymbidium sinense | Mycena | Culture | Fan et al. (1996) |
| Dendrobieae | |||
| Dendrobium officinale | Mycena | Culture | Zhang et al. (2012) |
| Epidendreae | |||
| Epidendrum firmum | Coprinellus, Polyporales | Cloning | Kartzinel et al. (2013) |
Cloning: cloning of PCR products; NGS, next-generation sequencing; PCR, direct sequencing of polymerase chain reaction products.
Overall, these results suggest that a low level of endophytic presence of non-rhizoctonia saprobic Basidiomycotas in rhizoctonia-associated orchids is a predisposition to evolve mycorrhizal interactions, as expected under the WRH (Fig. 1).
PERSPECTIVES
We support the hypothesis that all orchid mycorrhizal associations, from the ancestral rhizoctonias to the various derived fungal partners, are based on the evolutionary recruitment of endophytes that became mycorrhizal (Fig. 1), according to the WRH (box 1). In the future, we see five ways in which this assertion may be supported.
First, analysis of fungal partners for orchid clades in which some species have shifted to non-rhizoctonia partners may allow us to test whether these partners are also endophytic in phylogenetically related orchids that still associate with rhizoctonias. Such a pattern would show that these partners were also endophytic in the common ancestor of the clade (box 1). Such phylogenetically informed investigations could be carried out in the already well-explored tribe Neottieae (Selosse and Roy, 2009) and genus Cymbidium (Ogura-Tsujita et al., 2012).
Second, more attention is needed to demonstrate that non-rhizoctonia fungi are really present (and are not laboratory contamination) in rhizoctonia-associated orchids, and what their interaction is with the host tissues. Do they really enter the root by crossing the epidermis? Do they sometimes even form pelotons, as suggested in some cases above, or do they simply colonize the roots loosely between cells, as typical endophytes do (Fig. 3A)? As stated above, the two situations, with the first closer to a mycorrhizal status, may exist under the WRH. We need more microscopic investigations, especially using FISH to identify the hyphae (e.g. as in Schneider-Maunoury et al., 2020; Fig. 3A), a kind of approach and savoir faire that remain too rare. Such data may also demonstrate that the molecular detection of these fungi is not artefactual.
Third, the physiological relevance of fungal endophytism in orchids should be addressed: do endophytes have some influence on host nutrition, physiology or defence; are they mutualistic, neutral or slightly detrimental? Obviously, this cannot be examined in the ancestors of the orchids that recruited a given endophyte as a mycorrhizal fungus; yet, the question can be addressed in extant rhizoctonia-associated orchids, where such fungi are currently still endophytic. Transcriptomic expression of genes by the fungus (e.g. as in Schneider-Maunoury et al., 2020) and of the host may be investigated. In particular, in orchids, an important interaction stage is germination, where the fungus provides most nutrients required for plant development (Rasmussen, 1995). A large diversity of isolated endophytes has been tested to support germination, which they often fail to do (e.g. Meng et al., 2019); yet, studies mostly used endophytes related to Ascomycotas that are more easily cultivated than those related to Basidiomycotas (although the latter can be isolated; Table 2), because Ascomycotas grow faster in vitro. Thus, available orchid endophytes do not encompass the ectomycorrhizal and non-rhizoctonia saprobic Basidiomycotas that sometimes became mycorrhizal in orchid evolution. The latter taxa now deserve attention for their potential role in germination of rhizoctonia-associated orchids.
Fourth, an open, intriguing question is why most endophytes belonging to Ascomycotas were never recruited as mycorrhizal partners (with the exception of some ectomycorrhizal Pezizomycetes). Orchids often display Xylariales, Dothideomycetes, Leotiomycetes, etc. (Bayman and Otero, 2006; Sarsaiya et al., 2019), as endophytes, which never act as mycorrhizal in any orchid species. This may be explained by the fact that they are strictly endophytic, without extraradical mycelium enabling a mycorrhizal role. Yet, this restricted ecology deserves confirmation.
Fifth, and more generally, the question of the exact impact of root endophytes extends to the rhizoctonias in the roots of non-orchid plants. How do they grow and interact within these roots? Do they have some influence on their hosts, e.g. a slightly beneficial role placing them on a slope toward mutualism? In the future, inoculation experiments, which are allowed by the in vitro tractability of rhizoctonias, may test this. Indeed, such investigations on Serendipitaceae have already revealed significant and positive physiological outcomes [Fritsche et al., 2021; see Weiss et al. (2016) and Gill et al. (2016) for reviews].
Because of the high rate of symbiotic partner replacement during their evolution, some orchid groups are outstanding ‘laboratories’ for studying the evolution of symbiosis. They offer a window on the evolution that also operates, albeit more slowly, in other more conservative lineages. In a similar way, the photosynthetic Dinoflagellate algae have gained new algal endosymbionts many times, replacing their previous plastids and offering insights into plastid evolution (Waller and Kořený, 2017). Orchids also display a fast dynamic of replacement of mycorrhizal fungi, which occurs in the framework of the WRH. Thus, although the WRH has wider domains of applications, orchids are certainly the most relevant group for its further testing.
ACKNOWLEDGEMENTS
The authors acknowledge laboratory discussions at the Muséum national d’Histoire naturelle and at the University of Gdansk, which allowed the application of the WRH to orchids. We thank David Marsh for correction of the English text, two anonymous referees as well as Kenji Suetsugu and Trude Schwarzacher (review editor) for very helpful comments on previous versions of this paper. We thank Juan Pablo Suárez Chacón, Jane Oja and Hélène Vogt-Schilb for help in establishing Tables 1 and 2; we apologize for the omission of any studies not compiled in these tables. M.A.S. previously published the WRH. The idea behind this review stemmed from research and laboratory discussions with R.P., L.L., B.P.L., J.M. and A.B. To prepare the paper, H.J., M.-I.M. and T.F. reviewed the literature to prepare the tables; M.-A.S., J.G. and F.M. conceived and wrote the first draft. All authors contributed to subsequent drafts and provided comments.
FUNDING
This work was financially supported by a grant from the National Science Center, Poland (project No: 2015/18/A/NZ8/00149). This paper is humbly dedicated to the memory of the late Sally Smith, who is much missed by the mycorrhizal community.
LITERATURE CITED
- Abadie JC, Püttsepp Ü, Gebauer G, Faccio A, Bonfante P, Selosse MA. 2006. Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and nonphotosynthetic individuals. Canadian Journal of Botany 84: 1462–1477. [Google Scholar]
- Almario J, Jeena G, Wunder J, et al. . 2017. Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition. Proceedings of the National Academy of Sciences 114: E9403–E9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Begerow D, Sampaio JP, Weiß M, Oberwinkler F. 2006. The simple-septate basidiomycetes: a synopsis. Mycological Progress 5: 41–66. [Google Scholar]
- Bayman P, Otero JT. 2006. Microbial endophytes of orchid roots. In: Schulz BJE, Boyle C, Sieber TN, eds. Microbial root endophytes. New York: Springer, 153–178. [Google Scholar]
- Bayman P, Mosquera-Espinosa AT, Saladini-Aponte CM, Hurtado-Guevara NC, Viera-Ruiz NL. 2016. Age-dependent mycorrhizal specificity in an invasive orchid, Oeceoclades maculata. American Journal of Botany 103: 1880–1889. [DOI] [PubMed] [Google Scholar]
- Bunch WD, Cowden CC, Wurzburger N, Shefferson RP. 2013. Geography and soil chemistry drive the distribution of fungal associations in lady’s slipper orchid, Cypripedium acaule. Botany 91: 850–856. [Google Scholar]
- Calevo J, Voyron S, Adamo M, Alibrandi P, Perotto S, Girlanda M. 2021. Can orchid mycorrhizal fungi be persistently harbored by the plant host? Fungal Ecology 53: 101071. [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. . 2015. Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currah RS, Sherburne R. 1992. Septal ultrastructure of some fungal endophytes from boreal orchid mycorrhizas. Mycological Research 96: 583–587. [Google Scholar]
- Dearnaley JDW, Martos F, Selosse MA. 2012. Orchid mycorrhizas: molecular ecology, physiology, evolution and conservation aspects. In: Hock B, ed. Fungal associations, 2nd Edition. The Mycota IX. Berlin: Springer, 207–230. [Google Scholar]
- Dearnaley J, Perotto S, Selosse MA. 2016. Structure and development of orchid mycorrhizas. In: Martin F, ed. Molecular mycorrhizal symbiosis. Hoboken: John Wiley & Sons, 63–86. [Google Scholar]
- Duffy KJ, Waud M, Schatz B, Petanidou T, Jacquemyn H. 2019. Latitudinal variation in mycorrhizal diversity associated with a European orchid. Journal of Biogeography: 46: 968–980. [Google Scholar]
- Ercole E, Adamo M, Rodda M, Gebauer G, Girlanda M, Perotto S. 2015. Temporal variation in mycorrhizal diversity and carbon and nitrogen stable isotope abundance in the wintergreen meadow orchid Anacamptis morio. New Phytologist 205: 1308–1319. [DOI] [PubMed] [Google Scholar]
- Esposito F, Jacquemyn H, Waud M, Tyteca D. 2016. Mycorrhizal fungal diversity and community composition in two closely related Platanthera (orchidaceae) species. PLoS ONE 11: e0164108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Guo SX, Cao WQ, Xiao PG, Xu JT. 1996. Isolation, culture, identification and biological activity of Mycena orchidicola sp. nov. in Cymbidium sinense (Orchidaceae). Acta Mycologica Sinica 15: 251–255. [Google Scholar]
- Floudas D, Binder M, Riley R, et al. . 2012. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336: 1715–1719. [DOI] [PubMed] [Google Scholar]
- Fritsche Y, Lopes ME, Selosse M-A, et al. . 2021. Serendipita restingae sp. nov. (Sebacinales): an orchid mycorrhizal agaricomycete with wide host range. Mycorrhiza 31: 1–15. [DOI] [PubMed] [Google Scholar]
- Gill SS, Gill R, Trivedi DK, et al. . 2016. Piriformospora indica: potential and significance in plant stress tolerance. Frontiers in Microbiology 7: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girlanda M, Segreto R, Cafasso D, et al. . 2011. Photosynthetic Mediterranean meadow orchids feature partial mycoheterotrophy and specific mycorrhizal associations. American Journal of Botany 98: 1148–1163. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Spalink D, Ames M, et al. . 2015. Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proceedings of the Royal Society B 282: 20151553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Chávez M del CA, Torres-Cruz TJ, Sánchez SA, Carrillo-González R, Carrillo-López LM, Porras-Alfaro A. 2018. Microscopic characterization of orchid mycorrhizal fungi: Scleroderma as a putative novel orchid mycorrhizal fungus of Vanilla in different crop systems. Mycorrhiza 28: 147–157. [DOI] [PubMed] [Google Scholar]
- Guo SX, Fan L, Cao WQ, Xu JT, Xiao PG. 1997. Mycena anoectochila sp. nov. isolated from mycorrhizal roots of Anoectochilus roxburghii from Xishuangbanna, China. Mycologia 89: 952–954. [Google Scholar]
- Han JY, Xiao HF, Gao JY. 2016. Seasonal dynamics of mycorrhizal fungi in Paphiopedilum spicerianum (Rchb. f) Pfitzer - A critically endangered orchid from China. Global Ecology and Conservation 6: 327–338. [Google Scholar]
- Hatté C, Zazzo A, Selosse MA. 2020. The radiocarbon age of mycoheterotrophic plants. New Phytologist 227: 1284–1288. [DOI] [PubMed] [Google Scholar]
- Henry C, Raivoarisoa JF, Razafimamonjy A, et al. . 2017. Transfer to forest nurseries significantly affects mycorrhizal community compositions of Asteropeia macphersonii wildings. Mycorrhiza 27: 321–330. [DOI] [PubMed] [Google Scholar]
- Hibbett DS, Matheny PB. 2009. The relative ages of ectomycorrhizal mushrooms and their plant hosts estimated using Bayesian relaxed molecular clock analyses. BMC Biology 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynson NA, Madsen TP, Selosse MA, et al. . 2013. The physiological ecology of mycoheterotrophy. In: Merckx V, ed. Mycoheterotrophy: the biology of plants living on fungi. Berlin: Springer, 297–342. [Google Scholar]
- Irwin MJ, Bougoure JJ, Dearnaley JDW. 2007. Pterostylis nutans (Orchidaceae) has a specific association with two Ceratobasidium root-associated fungi across its range in eastern Australia. Mycoscience 48: 231–239. [Google Scholar]
- Jacquemyn H, Brys R, Merckx VSFT, Waud M, Lievens B, Wiegand T. 2014. Coexisting orchid species have distinct mycorrhizal communities and display strong spatial segregation. New Phytologist 202: 616–627. [DOI] [PubMed] [Google Scholar]
- Jacquemyn H, Brys R, Waud M, Evans A, Figura T, Selosse MA. 2021. Mycorrhizal communities and isotope signatures in two partially mycoheterotrophic orchids. Frontiers in Plant Science 12: 618140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemyn H, Honnay O, Cammue BPA, Brys R, Lievens B. 2010. Low specificity and nested subset structure characterize mycorrhizal associations in five closely related species of the genus Orchis. Molecular Ecology 19: 4086–4095. [DOI] [PubMed] [Google Scholar]
- Jacquemyn H, Merckx VSFT. 2019. Mycorrhizal symbioses and the evolution of trophic modes in plants. Journal of Ecology 107: 1567–1581. [Google Scholar]
- Jacquemyn H, Merckx V, Brys R, et al. . 2011. Analysis of network architecture reveals phylogenetic constraints on mycorrhizal specificity in the genus Orchis (Orchidaceae). New Phytologist 192: 518–528. [DOI] [PubMed] [Google Scholar]
- Jacquemyn H, Waud M, Brys R, et al. . 2017. Mycorrhizal associations and trophic modes in coexisting orchids: an ecological continuum between auto- and mixotrophy. Frontiers in Plant Science 8: 1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemyn H, Waud M, Lievens B, Brys R. 2016a. Differences in mycorrhizal communities between Epipactis palustris, E. helleborine and its presumed sister species E. neerlandica. Annals of Botany 118: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemyn H, Waud M, Merckx VSFT, et al. . 2016. b. Habitat-driven variation in mycorrhizal communities in the terrestrial orchid genus Dactylorhiza. Scientific Reports 6: 37182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LJAN, Gónzalez-Chávez M del CA, Carrillo-González R, Porras-Alfaro A, Mueller GM. 2021. Vanilla aerial and terrestrial roots host rich communities of orchid mycorrhizal and ectomycorrhizal fungi. Plants, People, Planet 3: 541–552. [Google Scholar]
- Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse MA. 2005. Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytologist 166: 639–653. [DOI] [PubMed] [Google Scholar]
- Kartzinel TR, Trapnell DW, Shefferson RP. 2013. Critical importance of large native trees for conservation of a rare neotropical epiphyte. Journal of Ecology 101: 1429–1438. [Google Scholar]
- Kaur J, Phillips C, Sharma J. 2021. Host population size is linked to orchid mycorrhizal fungal communities in roots and soil, which are shaped by microenvironment. Mycorrhiza 30: 17–30. [DOI] [PubMed] [Google Scholar]
- Kohler A, Kuo A, Nagy LG, et al. . 2015. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics 47: 410–415. [DOI] [PubMed] [Google Scholar]
- Korotkin HB, Swenie RA, Miettinen O, et al. . 2018. Stable isotope analyses reveal previously unknown trophic mode diversity in the Hymenochaetales. American Journal of Botany 105: 1869–1887. [DOI] [PubMed] [Google Scholar]
- Kottke I, Garnica S, Herrera P, et al. . 2010. Atractiellomycetes belonging to the ‘rust’ lineage (Pucciniomycotina) form mycorrhizae with terrestrial and epiphytic neotropical orchids. Proceedings of the Royal Society B 277: 1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen KA, Freudenstein JV, Rasmussen FN, Rasmussen HN. 2004. Molecular identification of mycorrhizal fungi in Neuwiedia veratrifolia (Orchidaceae). Molecular Phylogenetics and Evolution 33: 251–258. [DOI] [PubMed] [Google Scholar]
- Kristiansen KA, Taylor DL, Kjøller R, Rasmussen HN, Rosendahl S. 2001. Identification of mycorrhizal fungi from single pelotons of Dactylorhiza majalis (Orchidaceae) using single-strand conformation polymorphism and mitochondrial ribosomal large subunit DNA sequences. Molecular Ecology 10: 2089–2093. [DOI] [PubMed] [Google Scholar]
- Lee YI, Yang CK, Gebauer G. 2015. The importance of associations with saprotrophic non-Rhizoctonia fungi among fully mycoheterotrophic orchids is currently under-estimated: novel evidence from sub-tropical Asia. Annals of Botany 116: 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Yang W, Shimao W, Selosse MA, Gao J. 2021. Progress and prospects of mycorrhizal fungal diversity in orchids. Frontiers in Plant Science 12: 646325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebel HT, Bidartondo MI, Gebauer G. 2015. Are carbon and nitrogen exchange between fungi and the orchid Goodyera repens affected by irradiance? Annals of Botany 115: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebel HT, Bidartondo MI, Preiss K, et al. . 2010. C and N stable isotope signatures reveal constraints to nutritional modes in orchids from the Mediterranean and Macaronesia. American Journal of Botany 97: 903–912. [DOI] [PubMed] [Google Scholar]
- Likar M, Bukovnik U, Kreft I, Chrungoo NK, Regvar M. 2008. Mycorrhizal status and diversity of fungal endophytes in roots of common buckwheat (Fagopyrum esculentum) and tartary buckwheat (F. tataricum). Mycorrhiza 18: 309–315. [DOI] [PubMed] [Google Scholar]
- Martos F, Dulormne M, Pailler T, et al. . 2009. Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytologist 184: 668–681. [DOI] [PubMed] [Google Scholar]
- Martos F, Munoz F, Pailler T, Kottke I, Gonneau C, Selosse MA. 2012. The role of epiphytism in architecture and evolutionary constraint within mycorrhizal networks of tropical orchids. Molecular Ecology 21: 5098–5109. [DOI] [PubMed] [Google Scholar]
- May M, Jąkalski M, Novotná A, et al. . 2020. Three-year pot culture of Epipactis helleborine reveals autotrophic survival, without mycorrhizal networks, in a mixotrophic species. Mycorrhiza 30: 51–61. [DOI] [PubMed] [Google Scholar]
- McCormick MK, Whigham DF, O’Neill J. 2004. Mycorrhizal diversity in photosynthetic terrestrial orchids. New Phytologist 163: 425–438. [DOI] [PubMed] [Google Scholar]
- Meng YY, Zhang WL, Selosse MA, Gao JY. 2019. Are fungi from adult orchid roots the best symbionts at germination? A case study. Mycorrhiza 29: 541–547. [DOI] [PubMed] [Google Scholar]
- Merckx VSFT, ed.2013. Mycoheterotrophy: the biology of plants living on fungi. Berlin: Springer. [Google Scholar]
- Miyauchi S, Kiss E, Kuo Aet al. . 2020. Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nature Communications 11: 5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu VA, Kömpe YÖ. 2020. Mycorrhizal fungi of some Orchis species of Turkey. Pakistan Journal of Botany 52: 687–695. [Google Scholar]
- Newsham KK. 2011. A meta-analysis of plant responses to dark septate root endophytes. New Phytologist 190: 783–793. [DOI] [PubMed] [Google Scholar]
- Nomura N, Ogura-Tsujita Y, Gale SWet al. . 2013. The rare terrestrial orchid Nervilia nipponica consistently associates with a single group of novel mycobionts. Journal of Plant Research 126: 613–623. [DOI] [PubMed] [Google Scholar]
- Oberwinkler F, Riess K, Bauer R, Kirschner R, Garnica S. 2013. Taxonomic re-evaluation of the Ceratobasidium–Rhizoctonia complex and Rhizoctonia butinii, a new species attacking spruce. Mycological Progress 12: 763–776. [Google Scholar]
- Ogura-Tsujita Y, Gebauer G, Hashimoto T, Umata H, Yukawa T. 2009. Evidence for novel and specialized mycorrhizal parasitism: the orchid Gastrodia confusa gains carbon from saprotrophic Mycena. Proceedings of the Royal Society B 276: 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura-Tsujita Y, Yokoyama J, Miyoshi K, Yukawa T. 2012. Shifts in mycorrhizal fungi during the evolution of autotrophy to mycoheterotrophy in Cymbidium (Orchidaceae). American Journal of Botany 99: 1158–1176. [DOI] [PubMed] [Google Scholar]
- Oliveira SF, Bocayuva MF, Veloso TGRet al. . 2014. Endophytic and mycorrhizal fungi associated with roots of endangered native orchids from the Atlantic Forest, Brazil. Mycorrhiza 24: 55–64. [DOI] [PubMed] [Google Scholar]
- Petrolli R, Vieira CA, Jakalski M, et al. . 2021. A fine-scale spatial analysis of fungal communities on tropical tree bark unveils the epiphytic rhizosphere in orchids. New Phytologist 231: 2002–2014. [DOI] [PubMed] [Google Scholar]
- Qin J, Zhang W, Ge ZW, Zhang SB. 2019. Molecular identifications uncover diverse fungal symbionts of Pleione (Orchidaceae). Fungal Ecology 37: 19–29. [Google Scholar]
- Rasmussen HN. 1995. Terrestrial orchids - from seed to mycotrophic plant. Cambridge: Cambridge University Press. [Google Scholar]
- Rodriguez RJ, White JF, Arnold AE, Redman RS. 2009. Fungal endophytes: diversity and functional roles. New Phytologist 182: 314–330. [DOI] [PubMed] [Google Scholar]
- Sarsaiya S, Shi J, Chen J. 2019. A comprehensive review on fungal endophytes and its dynamics on Orchidaceae plants: current research, challenges, and future possibilities. Bioengineered 10: 316–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebold JMI, Bidartondo MI, Lenhard F, Makiola A, Gebauer G. 2018. Exploiting mycorrhizas in broad daylight: partial mycoheterotrophy is a common nutritional strategy in meadow orchids. Journal of Ecology 106: 168–178. [Google Scholar]
- Schneider-Maunoury L, Deveau A, Moreno M, et al. . 2020. Two ectomycorrhizal truffles, Tuber melanosporum and T. aestivum, endophytically colonise roots of non-ectomycorrhizal plants in natural environments. New Phytologist 225: 2542–2556. [DOI] [PubMed] [Google Scholar]
- Schneider-Maunoury L, Leclercq S, Clément C, et al. . 2018. Is Tuber melanosporum colonizing the roots of herbaceous, non-ectomycorrhizal plants? Fungal Ecology 31: 59–68. [Google Scholar]
- Schweiger JMI, Bidartondo MI, Gebauer G. 2018. Stable isotope signatures of underground seedlings reveal the organic matter gained by adult orchids from mycorrhizal fungi. Functional Ecology 32: 870–881. [Google Scholar]
- Sebastián F, Vanesa S, Eduardo F, Graciela T, Silvana S. 2014. Symbiotic seed germination and protocorm development of Aa achalensis Schltr., a terrestrial orchid endemic from Argentina. Mycorrhiza 24: 35–43. [DOI] [PubMed] [Google Scholar]
- Selosse MA, Dubois MP, Alvarez N. 2009. Do Sebacinales commonly associate with plant roots as endophytes? Mycological Research 113: 1062–1069. [DOI] [PubMed] [Google Scholar]
- Selosse MA, Martos F. 2014. Do chlorophyllous orchids heterotrophically use mycorrhizal fungal carbon? Trends in Plant Science 19: 683–685. [DOI] [PubMed] [Google Scholar]
- Selosse MA, Martos F, Perry BA, Padamsee M, Roy M, Pailler T. 2010. Saprotrophic fungal mycorrhizal symbionts in achlorophyllous orchids: finding treasures among the ‘molecular scraps’? Plant Signaling and Behavior 5: 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selosse MA, Minasiewicz J, Boullard B. 2017. An annotated translation of Noël Bernard’s 1899 article ‘On the germination of Neottia nidus-avis’. Mycorrhiza 27: 611–618. [DOI] [PubMed] [Google Scholar]
- Selosse MA, Roy M. 2009. Green plants that feed on fungi: facts and questions about mixotrophy. Trends in Plant Science 14: 64–70. [DOI] [PubMed] [Google Scholar]
- Selosse MA, Scappaticci G, Faccio A, Bonfante P, 2004. Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microbial Ecology 47: 416–426. [DOI] [PubMed] [Google Scholar]
- Selosse MA, Schneider-Maunoury L, Martos F. 2018. Time to re-think fungal ecology? Fungal ecological niches are often prejudged. New Phytologist 217: 968–972. [DOI] [PubMed] [Google Scholar]
- Selosse MA, Setaro S, Glatard F, Richard F, Urcelay C, Weiß M. 2007. Sebacinales are common mycorrhizal associates of Ericaceae. New Phytologist 174: 864–878. [DOI] [PubMed] [Google Scholar]
- Sen R, Hietala AM, Zelmer CD. 1999. Common anastomosis and internal transcribed spacer RFLP groupings in binucleate Rhizoctonia isolates representing root endophytes of Pinus sylvestris, Ceratorhiza spp. from orchid mycorrhizas and a phytopathogenic anastomosis group. New Phytologist 144: 331–341. [Google Scholar]
- Shefferson RP, Cowden CC, McCormick MK, Yukawa T, Ogura-Tsujita Y, Hashimoto T. 2010. Evolution of host breadth in broad interactions: mycorrhizal specificity in East Asian and North American rattlesnake plantains (Goodyera spp.) and their fungal hosts. Molecular Ecology 19: 3008–3017. [DOI] [PubMed] [Google Scholar]
- Shefferson RP, Taylor DL, Weiß M, et al. . 2007. The evolutionary history of mycorrhizal specificity among lady’s slipper orchids. Evolution 61: 1380–1390. [DOI] [PubMed] [Google Scholar]
- Shefferson RP, Weiß M, Kull T, Taylor DL. 2005. High specificity generally characterizes mycorrhizal association in rare lady’s slipper orchids, genus Cypripedium. Molecular Ecology 14: 613–626. [DOI] [PubMed] [Google Scholar]
- Shubin N, Tabin C, Carroll S. 2009. Deep homology and the origins of evolutionary novelty. Nature 457: 818–823. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. Cambridge: Elsevier, 2008. [Google Scholar]
- Stark C, Babik W, Durka W. 2009. Fungi from the roots of the common terrestrial orchid Gymnadenia conopsea. Mycological Research 113: 952–959. [DOI] [PubMed] [Google Scholar]
- Strullu-Derrien C, Selosse MA, Kenrick P, Martin FM. 2018. The origin and evolution of mycorrhizal symbioses: from palaeomycology to phylogenomics. New Phytologist 220: 1012–1030. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Haraguchi T, Okada H, Tayasu I. 2021a. Stigmatodactylus sikokianus (Orchidaceae) mainly acquires carbon from decaying litter through association with a specific clade of Serendipitaceae. New Phytologist 231: 1670–1675. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Haraguchi TF, Tayasu I. 2021b. Novel mycorrhizal cheating in a green orchid: Cremastra appendiculata depends on carbon from deadwood through fungal associations. New Phytologist in press. doi: 10.1111/nph.17313. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Matsubayashi J. 2021a. Evidence for mycorrhizal cheating in Apostasia nipponica, an early-diverging member of the Orchidaceae. New Phytologist 229: 2302–2310. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Matsubayashi J. 2021b. Subterranean morphology modulates the degree of mycoheterotrophy in a green orchid Calypso bulbosa exploiting wood-decaying fungi. Functional Ecology 35: 2305–2315. [Google Scholar]
- Suetsugu K, Matsubayashi J, Tayasu I. 2020. Some mycoheterotrophic orchids depend on carbon from dead wood: novel evidence from a radiocarbon approach. New Phytologist 227: 1519–1529. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Yamato M, Matsubayashi J, Tayasu I. 2019. Comparative study of nutritional mode and mycorrhizal fungi in green and albino variants of Goodyera velutina, an orchid mainly utilizing saprotrophic rhizoctonia. Molecular Ecology 28: 4290–4299. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Yamato M, Matsubayashi J, Tayasu I. 2021c. Partial and full mycoheterotrophy in green and albino phenotypes of the slipper orchid Cypripedium debile. Mycorrhiza, 31: 301–312. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Smith ME. 2013. Lineages of ectomycorrhizal fungi revisited: foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biology Reviews 27: 83–99. [Google Scholar]
- Těšitelová T, Jersáková J, Roy M, et al. . 2013. Ploidy-specific symbiotic interactions: divergence of mycorrhizal fungi between cytotypes of the Gymnadenia conopsea group (Orchidaceae). New Phytologist 199: 1022–1033. [DOI] [PubMed] [Google Scholar]
- Thoen E, Harder CB, Kauserud H, et al. . 2020. In vitro evidence of root colonization suggests ecological versatility in the genus Mycena. New Phytologist 227: 601–612. [DOI] [PubMed] [Google Scholar]
- Van Der Heijden MGA, Martin FM, Selosse MA, Sanders IR. 2015. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist 205: 1406–1423. [DOI] [PubMed] [Google Scholar]
- Vanegas-León ML, Sulzbacher MA, Rinaldi AC, Roy M, Selosse MA, Neves MA. 2019. Are Trechisporales ectomycorrhizal or non-mycorrhizal root endophytes? Mycological Progress 18: 1231–1240. [Google Scholar]
- Veldre V, Abarenkov K, Bahram M, et al. . 2013. Evolution of nutritional modes of Ceratobasidiaceae (Cantharellales, Basidiomycota) as revealed from publicly available ITS sequences. Fungal Ecology 6: 256–268. [Google Scholar]
- Vogt-Schilb H, Těšitelová T, Kotilínek M, Sucháček P, Kohout P, Jersáková J. 2020. Altered rhizoctonia assemblages in grasslands on ex-arable land support germination of mycorrhizal generalist, not specialist orchids. New Phytologist 227: 1200–1212. [DOI] [PubMed] [Google Scholar]
- Voronina EY, Malysheva EF, Malysheva VF, Dmitriev GV, Tiunov AV, Kovalenko AE. 2018. A mixotrophy is in question: new data on fungal community associated with photosynthetic terrestrial orchid Goodyera repens. Botanica Pacifica 7: 07106. [Google Scholar]
- Voyron S, Ercole E, Ghignone S, Perotto S, Girlanda M. 2017. Fine-scale spatial distribution of orchid mycorrhizal fungi in the soil of host-rich grasslands. New Phytologist 213: 1428–1439. [DOI] [PubMed] [Google Scholar]
- Waller RF, Kořený L. 2017. Plastid complexity in dinoflagellates: a picture of gains, losses, replacements and revisions. Advances in Botanical Research 84: 105–143. [Google Scholar]
- Wang D, Jacquemyn H, Gomes SIF, Vos RA, Merckx VSFT. 2021. Symbiont switching and trophic mode shifts in Orchidaceae. New Phytologist 231: 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman RJ, Bidartondo MI, Stofberg J, et al. . 2011. The effects of above- and belowground mutualisms on orchid speciation and coexistence. American Naturalist 177: 54–68. [DOI] [PubMed] [Google Scholar]
- Waud M, Brys R, Van Landuyt W, Lievens B, Jacquemyn H. 2017. Mycorrhizal specificity does not limit the distribution of an endangered orchid species. Molecular Ecology 26: 1687–1701. [DOI] [PubMed] [Google Scholar]
- Weiss M, Sýkorová Z, Garnica S, et al. . 2011. Sebacinales everywhere: previously overlooked ubiquitous fungal endophytes. PLoS ONE 6: e16793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M, Waller F, Zuccaro A, Selosse MA. 2016. Sebacinales – one thousand and one interactions with land plants. New Phytologist 211: 20–40. [DOI] [PubMed] [Google Scholar]
- Whitridge H, Southworth D. 2005. Mycorrhizal symbionts of the terrestrial orchid Cypripedium fasciculatum. Selbyana 26: 328–334. [Google Scholar]
- Wilson D. 1995. Endophyte – the evolution of a term, and clarification of its use and definition. Oikos 73: 274–276. [Google Scholar]
- Xing X, Gai X, Liu Q, Hart MM, Guo S. 2014. Mycorrhizal fungal diversity and community composition in a lithophytic and epiphytic orchid. Mycorrhiza 25: 289–296. [DOI] [PubMed] [Google Scholar]
- Yagame T, Lallemand F, Selosse MA, Funabiki E, Yukawa T, 2021. Mycobiont diversity and first evidence of mixotrophy associated with Psathyrellaceae fungi in the chlorophyllous orchid Cremastra variabilis. Journal of Plant Research 134: 1213–1224. [DOI] [PubMed] [Google Scholar]
- Yuan L, Yang ZL, Li SY, Hu H, Huang JL. 2010a. Mycorrhizal specificity, preference, and plasticity of six slipper orchids from South Western China. Mycorrhiza 20: 559–568. [DOI] [PubMed] [Google Scholar]
- Yuan ZL, Zhang CL, Lin FC, Kubicek CP. 2010b. Identity, diversity, and molecular phylogeny of the endophytic mycobiota in the roots of rare wild rice (Oryza granulate) from a nature reserve in Yunnan, China. Applied and Environmental Microbiology 76: 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa T, Ogura-Tsujita Y, Shefferson RP, Yokoyama J. 2009. Mycorrhizal diversity in Apostasia (Orchidaceae) indicates the origin and evolution of orchid mycorrhiza. American Journal of Botany 96: 1997–2009. [DOI] [PubMed] [Google Scholar]
- Yung L, Bertheau C, Tafforeau F, et al. . 2021. Partial overlap of fungal communities associated with nettle and poplar roots when co-occurring at a trace metal contaminated site. Science of the Total Environment 782: 146692. [DOI] [PubMed] [Google Scholar]
- Zeng X, Diao H, Ni Z, et al. . 2021. Temporal variation in community composition of root associated endophytic fungi and carbon and nitrogen stable isotope abundance in two Bletilla species (Orchidaceae). Plants 10: 10010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen J, Lv Y, Gao C, Guo S. 2012. Mycena sp., a mycorrhizal fungus of the orchid Dendrobium officinale. Mycological Progress 11: 395–401. [Google Scholar]
- Zuccaro A, Lahrmann U, Güldener U, et al. . 2011. Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathogens 7: e1002290. [DOI] [PMC free article] [PubMed] [Google Scholar]