Abstract
Calcium signaling plays important roles in physiological and pathological conditions, including cutaneous melanoma, the most lethal type of skin cancer. Intracellular calcium concentration ([Ca2+]i), cell membrane calcium channels, calcium related proteins (S100 family, E-cadherin, and calpain), and Wnt/Ca2+ pathways are related to melanogenesis and melanoma tumorigenesis and progression. Calcium signaling influences the melanoma microenvironment, including immune cells, extracellular matrix (ECM), the vascular network, and chemical and physical surroundings. Other ionic channels, such as sodium and potassium channels, are engaged in calcium-mediated pathways in melanoma. Calcium signaling serves as a promising pharmacological target in melanoma treatment, and its dysregulation might serve as a marker for melanoma prediction. We documented calcium-dependent endoplasmic reticulum (ER) stress and mitochondria dysfunction, by targeting calcium channels and influencing [Ca2+]i and calcium homeostasis, and attenuated drug resistance in melanoma management.
Keywords: calcium, melanoma, progression, melanoma microenvironment, mitochondria
1. Introduction
Cutaneous melanoma, one of the most malignant skin cancers, emerges from pigmented melanocytes. Although melanoma accounts for <2% of malignant skin tumors, it is the most aggressive form of skin cancer [1]. Calcium is a messenger molecule that plays several important roles in different physiological and pathological functions in cells, including melanoma cells. Calcium channels are widely expressed on several biological membranes, such as the mitochondrial, endoplasmic reticulum (ER), and plasma membranes. These channels regulate calcium flux and concentration under normal physiological conditions.
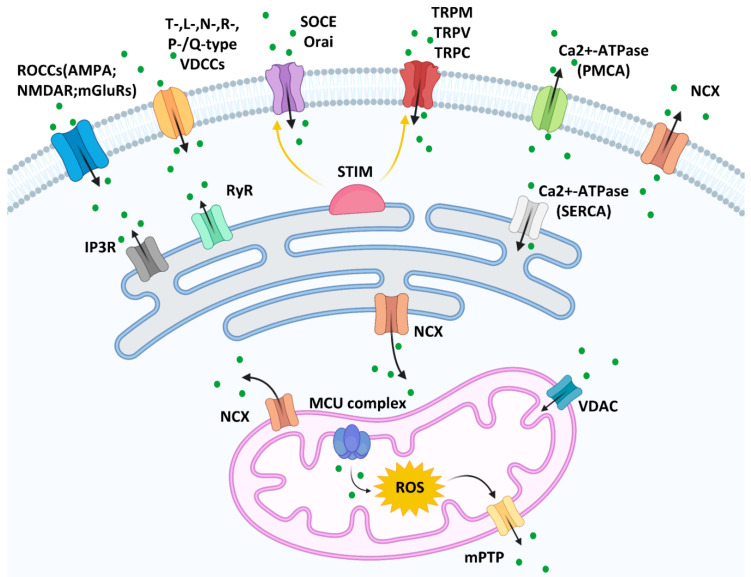
The calcium entry channels can be divided into (but are not limited to) receptor-operated calcium channels (ROCCs), voltage-dependent calcium channels (VDCCs), and store-operated calcium entry (SOCE) on the plasma membrane [2]. Glutamate receptor-mediated calcium channels, such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-d-aspartate receptor (NMDAR), and metabotropic glutamate receptors (mGluRs), are ROCCs and have been widely studied in nerve cells [3]. However, their roles have been described in other cell types, including melanoma. It has been documented that blocking the NMDA receptor inhibits melanoma proliferation [4]. Furthermore, mGluR1 and mGluR5 expression is related to melanoma development [5,6]. VDCCs are located on the plasma membrane and are activated by electrical potential changes across the membrane. VDCCs can be classified into T-, L-, N-, R-, and P-/Q-subtypes, the expression of which varies among different cell types. Melanoma and melanocytes express high voltage-activated Ca(v)1 (L-types) and Ca(v)2 channels (N, P/Q, or R-types), while low voltage-activated Ca(v)3 channels (T-type) only exist in melanoma [7]. The depletion of Ca2+ is detected by the ER membrane protein STIM, which activates SOCE on the plasma membrane (Orai channels) and transient receptor potential calcium channels, including transient receptor potential melastatin (TRPM), transient receptor potential vanilloid (TRPV), and transient receptor potential canonical (TRPC), to allow Ca2+ influx [8,9]. Calcium efflux is supported by Ca2+-ATPase pump and sodium calcium exchanger (NCX); the latter is not only in the plasma membrane but in the mitochondria and ER membrane.
The ER is one of the largest membrane-bound cellular calcium storage organelles. The ER transmembrane ryanodine receptor (RyR) and inositol 1,4,5-trisphosphate receptor (IP3R) channels mediate calcium release from the ER into the cytosol. Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) facilitates calcium pumping from the cytosol into the ER in an ATPase-dependent manner [10].
Mitochondria also play an important role in calcium homeostasis. Voltage-dependent anion channels (VDACs) located in the outer mitochondrial membrane increase the Ca2+ uptake into the intermembrane space [11]. Mitochondrial calcium uniporter (MCU) complex, which contains the major protein MCU and regulatory subunits MICU1/2, EMRE, and MCUb, is the major mediator of mitochondrial Ca2+ uptake on the mitochondrial inner membrane [12]. High matrix concentrations of Ca2+ with reactive oxygen species (ROS) in the mitochondria trigger mitochondrial permeability transition pore (mPTP) opening and release the Ca2+ into the cytoplasm [13]. (Figure 1).
Figure 1.
Calcium channels on the membranes of plasma, mitochondria, and the endoplasmic reticulum [14].
2. Calcium Signaling in Melanogenesis and Melanoma Tumorigenesis
2.1. Calcium Signaling in Melanogenesis
Calcium signaling plays a pivotal role in melanogenesis, which has effects on melanoma tumorigenesis and therapeutic outcomes [15]. Pigmentation can be regulated by membrane voltage changes mediated by modulating calcium channels with increased cytosolic Ca2+ influx. Cytosolic Ca2+ transports into melanosomes to increase tyrosinase activity, trigger melanin transfer, or regulate organelle interaction by activating PKCβ [16]. For example, repression of TRPM1 expression results in reduced intracellular Ca2+ and decreased uptake of extracellular Ca2+, accompanied by attenuated activity of the melanogenic enzyme tyrosinase and melanin pigment [17]. Sun et al. found that the stimulation of melanogenesis by synaptotagmin-4 is mediated by regulating Ca2+ influx through TRPM1 [18]. In addition, the release of internal Ca2+ stores through the Orai/STIM pathway increases tyrosinase activity and melanin content; this is triggered by solar ultraviolet radiation-induced endothelin-1 release [19]. Two-pore channel (TPC) located on the lysosomes, late endosomes, and melanosomes was reported to increase the risk of developing skin cancer by decreasing the melanin production and regulating melanosome maturation modulated by mTOR [16,20].
2.2. Calcium Signaling in Melanoma Tumorigenesis
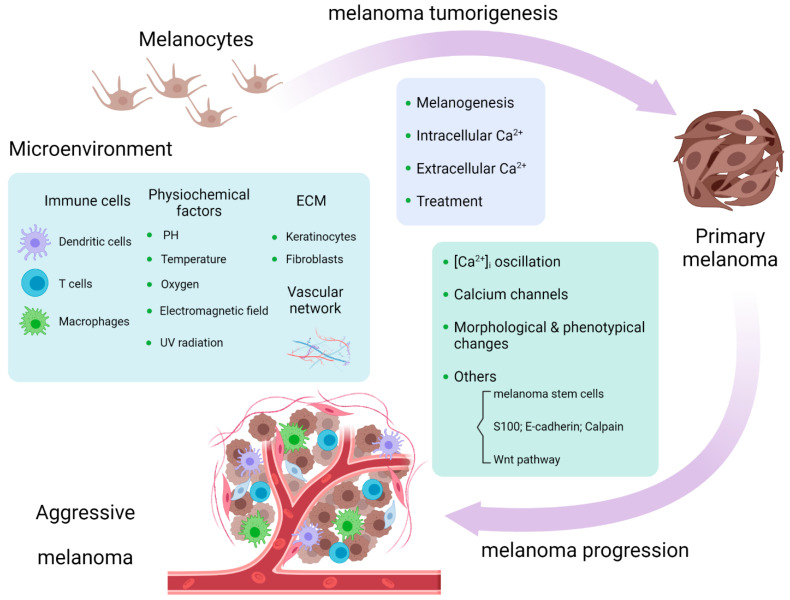
Melanoma tumorigenesis is a process whereby a benign melanocyte transforms into a primary melanoma in which the calcium influx across multiple cellular compartments is a key controller of the process [21]. Aside from the role in melanogenesis, calcium-related pathways are involved in the tumorigenesis of melanoma. IP3-mediated Ca2+ release from intracellular stores activates non-phosphorylated PKC isoforms which act as tumor promoters and are linked to carcinogenesis; some isoforms especially, PKC α and β, represent a malignant phenotype in melanoma [22,23]. PAR1 signaling accelerates calcium mobilization. The downstream pathways of PAR1 signaling, such as the activation of MAPKs, are involved in melanoma tumorigenesis [24]. Although there is no direct evidence that PAR1-induced Ca2+ flux affects melanoma tumorigenesis, it is worthy of further investigations. Extracellular Ca2+ regulators play important roles in melanoma tumorigenesis. Robert et al. documented that the extracellular Ca2+-binding matricellular glycoprotein SPARC promotes early transformation of melanocytes by mediating E-cadherin suppression and Snail induction [25]. PRP4 blocks the Ca2+ influx through desensitization of the extracellular calcium sensing receptor (CaSR), with the involvement of TRP cation channel subfamily C member 1, which is the promoting factor of skin carcinogenesis [26]. Altering Ca2+ homeostasis by targeting lipid rafts, the cholesterol-enriched membrane microdomains in melanoma cells, abolishes activated PKB, rendering melanoma susceptible to apoptosis and attenuating its tumorigenicity; this can act as a therapeutic target in melanoma prevention [27]. From the glimpse of the role of calcium signaling in melanoma tumorigenesis, we conclude that calcium flux controls melanoma tumorigenesis mainly through calcium-related pathways, which requires further investigations about the direct impact of Ca2+ on the carcinogenesis of melanoma. (Figure 2).
3. Calcium Signaling in Melanoma Progression
Melanoma progression happens when the primary melanoma progresses to a metastatic melanoma with a migrating and invading capacity. Intracellular calcium concentration ([Ca2+]i) and its multiple channels function as regulators of melanoma progression that serve as mechanistic targets for control of melanoma growth and management of metastasis.
3.1. [Ca2+]i Oscillation Influences Melanoma Progression
Evidence documents that increased intracellular calcium stores are associated with highly metastatic melanoma cells [28]. Calcium released from ER facilitates melanoma cell migration. Epac1 activated by cAMP induces calcium elevation from ER via the PLC/IP3 receptor pathway and facilitates cell migration with the involvement of actin assembly, which is inhibited by mSIRK, a Gβγ-activating peptide, activating calcium influx from the extracellular space [29,30]. The expression of cGMP phosphodiesterase PDE5A is downregulated by oncogenic BRAF in BRAFV600E mutated melanoma by the extracellular-signal-regulated kinase (ERK) pathway, which induces an increase in [Ca2+]i, stimulating melanoma cell invasion and short-term and long-term lung colonization [31]. Y-box binding protein 1 is an unfavorable prognostic marker secreted from melanoma depending on [Ca2+]i and ATP levels, the expression of which increases in primary and metastatic melanoma, compared to benign melanocytic nevi. Conversely, elevated Y-box binding protein 1 secretion stimulates melanoma cell migration, invasion, and tumorigenicity [32]. Paradoxically, increased [Ca2+]i was reported to decrease melanoma progression. Olfactory receptor 51E2 activated by its ligand β-ionone suppresses the migration of vertical-growth phase melanoma cells by increasing [Ca2+]i [33].
3.2. Calcium Channels Are Involved in Melanoma Progression
Since [Ca2+]i plays an important mechanistic role in melanoma progression, the role of calcium channels cannot be neglected. Basically, NMDAR calcium channel function is weak in melanoma cells but strongly contributes to cell proliferation and invasion when its encoding gene GRIN2A is mutated at certain sites, such as G762E, with less glutamate supplementation [34]. Another glutamate receptor calcium channel mGluR5 was proved to have a profound effect on melanoma progression in vivo by triggering the phosphorylation of ERK [5]. The ERK pathway is also implicated in SOCE-mediated melanoma progression. Inhibition of SOCE by knockdown of STIM1 or Orai or by SOCE inhibitors suppresses melanoma cell proliferation and migration, while induction of SOCE activates ERK, which is inhibited by calmodulin kinase II or Raf-1 inhibitors [35]. TPC2 influences melanoma progression via SOCE. Downregulation of TPC2 expression in metastatic melanoma leads to a decrease of Orai1 expression and an increase of YAP/TAZ activity, which is responsible for melanoma’s aggressive property [36]. In BRAF mutant melanoma—the BRAFV600E mutation in particular—the expression of Ca2+-ATPase isoform 4b (PMCA4b) on the plasma membrane is low compared with benign nevi and is markedly elevated by vemurafenib (BRAF inhibitor) or selumetinib (MEK inhibitor) treatment, which indicates crosstalk between PMCA4b and the MAPK pathway. Activation of p38 MAPK induces the degradation of PMCA4b, while suppression of p38 MAPK by increasing the abundance of PMCA4b promotes the [Ca2+]i clearance and inhibits the migration of melanoma cells [37,38]. Moreover, SERCA on the ER membrane, controlled by the interaction between calcium-modulating cyclophilin ligand and basigin, was reported to have an effect on invasion and metastasis by regulating [Ca2+]i and matrix metalloproteinase (MMP)-9 activity in A375 cells [39]. Unlike Ca2+-ATPase, T-type VDCCs drive migration and invasion in BRAF mutant melanoma cells depending on Snail1 levels, suggesting therapeutic strategies by blocking T-type VDCCs to inhibit progression of melanoma [40]. Other ion channels are implicated in melanoma progression through calcium signaling. Nav1.6 sodium channel promotes melanoma cell (WM266 and WM115) invasion and proliferation by mTOR-mediated Na+/Ca2+ exchange [41]. KCa3.1 potassium channel was reported to promote melanoma cell migration by controlling the secretion of melanoma inhibitory activity proteins depending on [Ca2+]i [42].
3.3. Ca2+ Signaling Influences Melanoma Progression through the Change of Morphological and Phenotypical Changes
Ca2+ signaling also leads to cell morphological and phenotypical changes, including the elongated cell axonal- and mesenchymal-like shape, formulation of invadopodia, and altered cytoskeleton structure, making cancer cells become more deformable and more invasive. Except for the role in melanogenesis, synaptotagmin-4 is thought to have a relationship with the growth and metastasis of melanoma by influencing axonal elongation [43]. Orai- and STIM1-mediated Ca2+ oscillation signals were reported to facilitate invadopodium assembly and thus promote melanoma invasion by regulating the recycling of membrane-bound MT1-MMP and extracellular matrix (ECM) remodeling [18,44]. The effect of the β2-adrenergic–Ca2+–actin axis on cancer invasion was reported in melanoma and other cancer types. β-adrenergic receptor (βAR) signaling triggers actin remodeling and reorganization to enhance cell contractility and promote cell invasion. β-adrenergic receptor-induced Ca2+ acts as a regulator of cytoskeletal actin by directly binding to actin or binding to filamin, the crosslinker of actin [45]. Meghnani et al. reported the upregulated expression of receptor for advanced glycation end products (RAGE) in melanoma patients in late metastatic stages. Overexpression of RAGE induced melanoma cells to become more metastatic by triggering cells into mesenchymal-like morphologies, which is associated with the upregulation of its ligand S100B, a calcium-binding protein [46].
3.4. Calcium-Related Pathways Participate in Melanoma Progression
Other factors (melanoma stem cells), other proteins (S100 family, E-cadherin, and calpain), and the Wnt/Ca2+ pathway influence melanoma progression through calcium signaling. Ca2+ released through IP3R in melanoma cells is crucial for the function of cancer stem cells. IP3R impairment leads to a diminution in the population of melanoma stem cells and reduced melanoma growth [47]. A network analysis of the expression of Ca2+ signaling and stem cell pluripotency-related genes (e.g., GSTP1, SMAD4, CTNNB1, MAPK3, GNAQ, PPP1CC, GSK3B, and PRKACA) showed some candidates that may contribute to the melanoma metastatic transformation and potential therapeutic biomarkers for metastatic melanoma [48].
S100A4 is a metastasis-promoting protein in melanoma cells which acts by targeting metabolic reprogramming, that is, the suppression of mitochondrial respiration and the activation of aerobic glycolysis [49]. Upregulation of S100P, ezrin, and RAGE improves the malignancy of melanoma [50]. E-cadherin has extracellular Ca2+-binding domains whose functions are dependent on Ca2+ and is essential for melanogenesis and melanoma suppression. E-cadherin silencing is related to melanoma metastatic dissemination and poor prognosis [51,52]. The decreasing expression of E-cadherin by overexpression of T-box transcription factors Tbx2 and Tbx3 is associated with enhanced melanoma invasiveness [53]. Promoter methylation by activating E-cadherin expression represents its therapeutic role in the treatment of melanoma [51]. Evidence in vitro and in vivo showed that inhibition of calpain, whose activity is promoted by calcium signaling, blunts melanoma growth, allows melanoma cells to escape from anti-tumor immunity, and increases metastatic dissemination by accelerating the migration process and reducing apoptosis [54].
Wnt5a was found to be expressed in highly aggressive melanoma and was able to increase melanoma invasive potential by activating PKC and raising [Ca2+]i in a transfected model [55]. Interestingly, Wnt5a signaling was engaged into melanoma cell movement, rendering them more aggressive. Wnt5a leads to the remodeling of the cytoskeleton and increases melanoma motility by activating calpain-1, leading to the cleavage of filamin A [56]. The assembly of the “Wnt-receptor-actin-myosin-polarity” structure, which is promoted by Wnt5a, promotes actomyosin contractility and substrate detachment for membrane retraction, mediated by the recruitment of cortical ER and elevation of Ca2+ [57]. (Figure 2).
Figure 2.
Calcium signaling is involved in melanoma tumorigenesis and progression and melanoma microenvironment [58].
4. Calcium Signaling in Melanoma Microenvironment
The tumor microenvironment, including surrounding immune cells and other cells, signaling molecules, blood vessels, and ECM, is closely related to and constantly interactive with melanoma cells, playing pivotal roles in melanoma generation, progress, and prognosis. Calcium signaling influences the altered microenvironment to change the fate of the melanoma by influencing the function of innate and adaptive immune cells, regulating ECM and tumor vascularization, and adapting to different physical and chemical surroundings.
4.1. Immune Cells
In T cell-based tumor immunosurveillance, cytotoxic T lymphocytes (CTLs) kill tumor cells by recognizing their specific T cell receptor. It was proved that CTLs-mediated cytotoxic function in melanoma and other cancers depends on a SOCE-mediated [Ca2+]I rise by regulating the degranulation of CTLs, the production of TNFα and IFNγ, and the expression of Fas ligand both in vivo and in vitro [59]. CD4+CD25+Foxp3+ regulatory T cells cause effector T cell death and suppress activation of T cells to induce immunosuppression through TGFβ-induced inhibition of IP3 production with a decrease in intracellular Ca2+ flux. Accordingly, Kim et al. increased IFNγ production and activated T cells in vitro and reduced melanoma growth in vivo through highly selective optical control of Ca2+ signaling in CTLs [60]. EGR4, a member of the zinc finger transcription factor family, was reported as a key regulator of T cell differentiation. Knocking out EGR4 in T cells triggers an enhanced Ca2+ response and increased IFNγ production in vitro and leads to regulatory T cells loss, Th1 bias, and CTL generation in a mouse melanoma lung colonization model [61]. Histamine and its H4 receptor induce the chemotaxis and migratory properties of γδ T cells through Gi protein-dependent [Ca2+]i increase in the microenvironment of melanoma cells [62].
Moreover, Ca2+ flux was involved in the NK cell-mediated innate immune response to melanoma cells. Although no difference in the formation of metastatic lung lesions was observed, NK cells are hyporesponsive to MHC class I-deficient target cells, with NK cells continuously activating by the Ly49H receptor [63]. Tumor-associated macrophages, especially CD163+ M2 macrophages, are related to immune escape, supporting cancer development [64]. Secreted flavoprotein renalase enhances the function of M2 macrophages to promote melanoma growth through the PMCA4b calcium channel by activating the MAPK and PI3K/AKT pathways [65]. Recently, mesencephalic astrocyte-derived neurotrophic factor, a novel immunoregulator basically secreted from pancreatic beta cells, was found to be secreted from melanoma and other cancer cell lines upon IFNγ-induced ER calcium depletion, which was proved to activate M2 macrophages and promote melanoma growth [66,67]. In addition, macrophages in the melanoma microenvironment are less susceptible to calcium electroporation compared with melanoma cells, but calcium electroporation stimulates the immunogenic capacity of melanoma-conditioned macrophages [68]. Calcium electroporation is a promising method in anti-cancer treatment under clinical trial which utilizes high-voltage electric pulses to introduce calcium flux into cells [69]. Recently, a near-infrared-stimulable optogenetic platform was established to remotely and selectively control Ca2+ oscillations and Ca2+-related gene expression and to modulate immunoinflammatory responses by regulating the functions of T lymphocytes, macrophages, and dendritic cells [70]. What is more, bone marrow-derived mast cells prefer to locate in hypoxic zones of the melanoma microenvironment, inducing CCL-2 synthesis and calcium rise by activating LVDCCs [71].
4.2. ECM and Vascular Network
In melanoma, ECM, molecules, proteins, and stromal cells interacting with Ca2+ signaling influence melanoma development. As we discussed above, Orai1- and STIM1-mediated Ca2+ oscillations regulate melanoma ECM degradation by MT1-MMP [18,44]. Attenuated [Ca2+]i enhances the chemotaxis of melanoma cells to type IV collagen, a member of the ECM proteins, depending on CD47 and integrins α2β1 and ανβ3 [72,73]. Thrombomodulin, an integral membrane glycoprotein on endothelial cells, acts as a Ca2+-dependent molecule controlling melanoma cell adhesion [74]. Kallikrein-related peptidase 6 is detected in neighboring stromal cells and keratinocytes and displays a paracrine function to accelerate melanoma migration and invasion which was proved to depend on protease-activated receptor 1-induced intracellular Ca2+ flux [24]. Skin keratinocytes and fibroblasts in melanoma ECM play important roles in melanoma development. Keratinocytes reduce the expression of TRPC1, 3, and 6 to decrease [Ca2+]i and negatively regulate the N-cadherin levels, a progressive factor in melanoma cells [75]. Keratinocytes can lead to cutaneous malignant lesions, dependent on the loss of calcium channel P2X1–3 and P2Y2 receptors and E-cadherin [76]. N-cadherin can promote melanoma cell migration and metastasis by facilitating the adhesion of melanoma cells to dermal fibroblasts and vascular endothelial cells [77].
The vascular network in the melanoma microenvironment, tightly interacting with ECM, provides nutrients and advantageous conditions for proliferation and metastasis. As we discussed above, the positive effects of Wnt5a on melanoma metastasis also include Ca2+-dependent exosome release, containing the pro-angiogenic and immunosuppressive factors (VEGF, IL-6, and MMP-2), which suppresses endothelial cell branching. Wnt5a expression has a potential relationship with the angiogenesis marker ESAM [78]. Nicotinic acid adenine dinucleotide phosphate, which is capable of triggering Ca2+ release from endosomes and lysosomes by targeting TPCs, was reported to control VEGF-induced angiogenesis in melanoma cells [79]. Moreover, vasculogenic mimicry is specific in less vascularized areas of the tumor microenvironment, providing nutrients and oxygen to facilitate tumor metastasis. Zhang et al. reported the role of the calcium/phospholipid-binding protein myoferlin in the inhibition of vasculogenic mimicry formation in melanoma by inducing mesenchymal-to-epithelial transition and decreasing MMP-2 expression [80]. The reconstitution of vascular mimicry with the combination of VEGFA signaling in ECM contributes to the formation of capillary-like structures in the melanoma microenvironment which is regulated by intracellular and extracellular Ca2+ levels and ανβ3 and ανβ5 integrins [81]. Studies displayed some anti-vascular methods in anti-tumor treatment by targeting Ca2+ signaling. Carboxyamido-triazole, an inhibitor of non-VDCCs, displayed inhibitory effects on melanoma invasion and angiogenesis, disrupting the signaling between melanoma and its microenvironment by suppressing VEGF production and endothelial cell response to VEGF [82]. Calcium electroporation not only directly induced melanoma necrosis and indirectly affected macrophages in the melanoma microenvironment but recently was found to suppress the formation of capillary-like structures in vitro and damage melanoma blood vessels in vivo [83,84]. Particularly, vascular endothelial cadherin is basically specific to endothelia but also presented in some melanomas [85]. Vascular endothelial cadherin-mediated interaction between melanoma and adjacent endothelium plays an important role in tumor metastasis properties. Inhibition of the PLC/IP3 pathway disrupts the melanoma–endothelium junctions by diminishing endothelial [Ca2+]i response [86,87].
4.3. Physical and Chemical Surroundings
The extracellular pH in melanoma is acidic because of the excess amount of anaerobic glucose metabolites [71]. Acidic extracellular pH enhances Ca2+ influx through VDCCs [88]. Noguchi et al. demonstrated therapeutic roles of mitochondrial inhibitors against melanoma accompanied by increasing [Ca2+]i at acidic extracellular pH, but a neutral or alkaline microenvironment enhanced melanoma growth and lung metastasis under the treatment of mitochondrial inhibitors [89]. Consequently, the tumor microenvironment was utilized to improve the treatment of melanoma. Cold atmospheric plasma induced Ca2+ influx in melanoma cells and acidification in the tumor microenvironment, which was thought to be the reason for its anti-cancer effects [90]. Except for low pH in the melanoma microenvironment, hypoxic conditions in melanoma lead to increased adenosine levels and high production of ROS [71]. Physical microenvironment changes, such as confinement, are able to elevate [Ca2+]i and suppress PKA activity via a PDE1-dependent pathway in melanoma cells which affects cell stiffness and locomotion [91]. Exposing melanoma cells to low-intensity, frequency-modulated electromagnetic fields for more than 15 min exhibits cytotoxic effects, with the involvement of VDCCs in an in vitro study [92]. Yu et al. reported the “cold/hot” properties of traditional Chinese medicine, which changes the temperature in A375 cells by TRPV4-mediated intracellular calcium influx [93]. UV radiation is a risk factor of melanoma. The roles of UV radiation in melanoma with calcium signaling involvement occur mainly by influencing vitamin D signaling, mitochondria-related Ca2+ influx, and ORAI1 channel-mediated melanogenesis [94,95,96] (Figure 2).
5. Calcium Signaling and Other Ionic Channels in Melanoma
5.1. Sodium Channels
Other ionic channels, including sodium and potassium channels, are engaged in the calcium transport systems. Normally, NCX transports three sodium ions into the cell and one calcium ion outside (forward mode), which can be performed in the opposite way (reverse mode) under special conditions [97]. Therefore, the function of sodium channels is relevant to the Ca2+ current. Nav 1.6, a voltage-gated sodium channel, is overexpressed in melanoma cells, compared with normal melanocytes. Inactivation of Nav 1.6 by its inhibitor tetrodotoxin suppresses aggressive properties and promotes apoptosis in melanoma cells by reducing mTOR activity and interrupting the translocation of mitochondrial Ca2+ flux [41]. Another study also evidenced the role of Nav 1.6 in regulating invasion by controlling the Ca2+-dependent podosome and invadopodium formation [98]. In melanoma cells with different metastatic capacities, Ca2+ buffering capacities are different. NCX functions in a reverse mode for Ca2+ entry, which leads to a sudden increase in [Ca2+]i in highly metastatic melanoma cells, while the NCX in lowly metastatic melanoma cells is in a forward mode, suggesting the vital role of NCX mode in melanoma metastasis characteristics [97,99]. Additionally, the expression of NCX1 varies between NRASQ61R and BRAFV600E mutated human melanoma cells with different Ca2+ homeostasis and Ca2+-dependent aggressiveness. NRASQ61R mutated (SK-MEL-147) cells contain higher levels of NCX1 expression and exhibit more sensitivity to vemurafenib treatment with NCX inhibition as compared to BRAFV600E mutated (SK-MEL-19) cells [100].
5.2. Potassium Channels
Ca2+-activated K+ (KCa) channels can be divided into three subfamilies: small-conductance K+ (SKCa) channels, intermediate-conductance K+ (IKCa) channels, and big-conductance K+ (BKCa) channels. Voltage-insensitive SKCa and IKCa channels are activated by low [Ca2+]i. In contrast, BKCa channels are activated by voltage and high [Ca2+]i [101]. KCa channels, especially the SKCa channels, are upregulated by hypoxia, which provides the underlying mechanism of enhanced proliferation in melanoma cells under hypoxic conditions [102]. KCa3.1 potassium channels, a subfamily of SKCa/IKCa channels, were found to support the secretion of melanoma inhibitory activity, promoting melanoma cell migration [42]. The disruption of cholesterol rafts proximal to BKCa channels increases the activity of BKCa channels. In human melanoma IGR39 cells, Na+/K+-ATPase in the rafts that control intracellular Na+ levels was reported to influence the efficient functioning of BKCa channels [103]. Filamin A is also necessary for the normal function of BKCa channels, which normally traffic to the plasma membrane in A7 melanoma cells with filamin A but have trouble trafficking in M2 cells without filamin A [104]. Except for KCa channels, Ca2+-inactivated K+ channels were reported to control the proliferation of murine B16 melanoma cells, mediated by endothelin-1 [105].
6. Calcium Signaling in Melanoma Treatment
Taken together, calcium signaling is tightly related to melanogenesis, melanoma tumorigenesis and progression, and the melanoma microenvironment in consideration of its pivotal roles in melanoma growth. As we document above, multiple therapeutic strategies targeting calcium-related pathways were described during melanoma development from benign melanocyte to highly malignant melanoma, from melanoma itself to the surroundings. All in all, targeting calcium signaling in melanoma treatment is basically performed by targeting calcium channels and influencing [Ca2+]i and calcium homeostasis to directly kill melanoma cells or affect relative pathways. Here, we put emphasis on the strategies for melanoma treatment targeting Ca2+-related mitochondrial dysfunction and ER stress to illustrate those in consideration of the essential roles of ER and mitochondria in the regulation of calcium signaling. Additionally, calcium-related treatment can combine with other drugs in melanoma management by attenuating drug resistance in indirect manners.
6.1. Targeting Calcium, Mitochondria, and ER Stress in Melanoma
ER calcium imbalance can induce ER stress due to its capacity to accumulate unfolded proteins and, in turn, enhance Ca2+ efflux from the ER and feed mitochondrial Ca2+ uptake, triggering mitochondrial swelling, cell necrosis, and apoptosis. Specifically, mitochondrial Ca2+ overload triggers the formation of ROS, a decline in mitochondrial membrane potential, and opening of mPTPs with resultant release of the pro-apoptosis factor cytochrome c followed by activation of caspase-dependent and -independent apoptosis pathways [106,107].
Calcium channel dynamics are implicated in melanoma treatment targeting mitochondria/ER stress. Although SOCE-mediated Ca2+ responses are critical for melanoma proliferation and apoptosis [108], drugs with anti-melanoma effects, such as diallyl trisulfide [109], to induce mitochondrial Ca2+ overload, ROS production, and caspase activation are mediated not by SOCEs but VDCCs. Rouaud et al. reported ER stress in melanoma induced by a NADPH analog, NS1, relying on TRPM2 and Ca2+-activated K+ channels [110]. A combination of ER transmembrane protein selenoprotein K and ER enzyme DHHC6 can palmitoylate IP3R and stabilize Ca2+ flux. Impairing selenoprotein K promotes ER stress for melanoma progression [111,112]. In addition, mitochondrial Ca2+ overload contributes to the apoptosis-promoting effect of metformin and lectin, purified from Bothrops leucurus snake venom, on melanoma through mPTP opening [113,114]. Ribosomal protein S3 acts as a potential therapeutic target for melanoma treatment on account of its regulatory effects on mitochondrial Ca2+ and cascading apoptosis by mPTP and MICU1 [115]. Except for calcium signaling-related apoptosis, Raimondi et al. revealed that δ- tocotrienol triggered paraptosis, the nonapoptotic programmed cell death, caused by Ca2+ overload and ROS-associated mitochondrial dysfunction in melanoma cells. Additionally, δ-tocotrienol treatment also reduced mitochondrial membrane potential, oxygen consumption, and the expression of mitochondrial complex I. The mitochondrial Ca2+ overload was thought to be mediated by IP3R and VDAC [116].
Since calcium homeostasis is pivotal in ER stress and mitochondria-mediated cell death, several studies applied Ca2+-induced cell death to cancer treatment, including melanoma treatments such as luteolin, N-acetyl-S-(p-chlorophenylcarbamoyl) cysteine (NACC), and sanguinarine [107,117,118], which mechanically revealed their potential pathways. In particular, aripiprazole is not only an antipsychotic drug but a compound capable of depleting ER calcium in melanoma, thereby leading to activation of the unfolded protein response via protein kinase R-like ER kinase (PERK) and inositol-requiring enzyme 1 [119]. Another study found that the anti-tumor effects of polyphenols was also mediated by PERK-directed Ca2+ release [120]. Some molecules that are cytotoxic to melanoma cells, for example, digitoxin and MEK inhibitors, alter mitochondrial membrane potential and trigger mitochondrial calcium dysregulation, intracellular acidification, and ATP depletion by disrupting ion gradients and reducing ERK phosphorylation, respectively [121]. Imiquimod, a toll-like receptor (TLR) agonist, was demonstrated to induce ER stress and Ca2+ depletion followed by mitochondrial membrane potential loss and cytochrome c release, independently of TLR7 and TLR8, to trigger the apoptosis of melanoma cells, which was associated with Kinase 1/c-Jun-N-terminal kinase/p38 pathways. Apoptosis protein antagonists and NF-κB inhibitors can improve the effectiveness of imiquimod in melanoma treatment [122,123]. The underlying mechanism is the reduction of SOCE and mitochondrial Ca2+ loading as well as fragmentation, clustering, and swelling in mitochondria [124]. Recently, a study demonstrated that pulsed focused ultrasound induced DNA damage in melanoma cells by superoxide and H2O2 formation caused by Ca2+ homeostasis change [125]. Interestingly, photodynamic therapy can directly kill melanoma cells by triggering Ca2+-related ROS formation; it was proved to have “bystander effects” on nearby cells that are not exposed to light. Ca2+ released from the ER in a single exposed melanoma cell is capable of promoting mitochondrial O2−. formation in its bystander cells [126].
6.2. Drug Resistance and Combination Treatment
In some conditions, targeting calcium signaling is able to render melanomas more susceptible to conventional therapy, preventing the development of drug resistance and providing novel ideas for combination treatment. Molecular targeting T-type VDCCs is a promising solution for melanoma chemoresistance, since the Ca(v)3.1 isoform is high expressed in vemurafenib-resistant BRAFV600E mutated melanoma. Mibefradil, a T-type VDCCs blocker, can restore the sensitivity of de-differentiated murine melanoma cells to MAPK inhibitors [127,128] and can reduce the motility and invasion capacity of BRAFV600E mutants [40]. Silencing of Ca(v)3.1 or Ca(v)3.2 reduced the invasiveness of melanoma cells with BRAFV600E mutation [129]. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising anticancer drug, while some melanomas are resistant to TRAIL treatment. Studies demonstrated that Ca2+ dynamics are a promising approach to overcome TRAIL resistance. Mitochondrial Ca2+ removal increases TRAIL efficacy against melanoma through mitochondrial hyperfusion [130]. Interestingly, mitochondrial Ca2+ overload results in selective sensitization to TRAIL cytotoxicity by increasing mitochondrial fragmentation [131]. Cold plasma-stimulated medium exhibits its tumor-selective cytotoxicity in the treatment of TRAIL-resistant melanoma cells, as evidenced by mitochondrial network abnormalities, disrupting Ca2+ homeostasis and caspase-independent cell death [132]. Co-treatment with autophagy inhibitors and TRAIL displays promising therapeutic effects. NCX inhibitors altering Ca2+ flux sensitize NRASQ61R mutated melanoma cells to vemurafenib [96]. K2[B3O3F4OH] exhibits its cytotoxic effects on melanoma cells but not melanocytes only under low Ca2+ concentrations, suggesting the therapeutic effects of the combination of K2[B3O3F4OH] and methods for Ca2+ depletion [133]. Therefore, with the developing perception of calcium signaling in melanoma, it will provide more options for melanoma treatments and expand the pharmacological arsenal in the future.
6.3. S100 Protein Family in Melanoma Prediction
In addition to its therapeutic roles, calcium signaling is presenting potential diagnostic biomarkers for melanoma. We mainly document the role of the S100 protein family in the diagnosis of melanoma and prediction of prognosis. The S100 protein family, consisting of a Ca2+-binding EF-hand structure, is an important biomarker in serum that has been well studied in melanoma. S100B levels reflect the stage and prognosis in melanoma, due to its stage-dependent secretion [134]; in particular, it is considered as a biomarker of tumor load and progression in stage IV melanoma patients [135]. During the first week of anti-PD-1 therapy, S100B levels can also serve as a biomarker to predict the overall survival and response to the treatment and help to guide treatment decisions [136]. Other clinical studies revealed its predictive function in melanoma patients with BRAF inhibitor or CTLA-4 inhibitor treatment [137,138]. Nordlinger et al. proved that poor patient prognoses are correlated with high S100A4 expression levels [139]. In addition, high serum levels of heterodimer S100A8/S100A9 in early stages of melanoma patients with ipilimumab treatment predict worse response [1]. S100A13 is upregulated in melanoma, cooperating with VEGFA in supporting angiogenesis, leading the shift from radial to vertical growth [140]. Moreover, other calcium-associated biomarkers are being studied as well. For instance, a cross-sectional study showed that high levels of albumin-corrected serum calcium may predict the progression of malignant melanoma [141]. The expression of T-type VDCCs is increased in BRAFV600E mutated cells, especially in those resistant to MAPK inhibitors, and this can serve as valuable prognostic markers in melanoma [129].
Acknowledgments
Author Contributions
Conceptualization, D.J.H. and A.Z.; writing—original draft preparation, H.Z.; writing—review and editing, A.A.G. and D.J.H.; visualization: Z.C.; funding acquisition, D.J.H.; supervision, A.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received intramural and philanthropic funding, including support from the Marvy Finger Family Foundation Distinguished Chair to D.J.H.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allgower C., Kretz A.L., von Karstedt S., Wittau M., Henne-Bruns D., Lemke J. Friend or Foe: S100 Proteins in Cancer. Cancers. 2020;12:2037. doi: 10.3390/cancers12082037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brini M., Cali T., Ottolini D., Carafoli E. Neuronal calcium signaling: Function and dysfunction. Cell Mol. Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan Y., Deng Y.L., Qing H. Calcium channel blockers and Alzheimer’s disease. Neural Regen. Res. 2012;7:137–140. doi: 10.3969/j.issn.1673-5374.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro M.P.C., Nunes-Correia I., Santos A.E., Custodio J.B.A. The combination of glutamate receptor antagonist MK-801 with tamoxifen and its active metabolites potentiates their antiproliferative activity in mouse melanoma K1735-M2 cells. Exp. Cell Res. 2014;321:288–296. doi: 10.1016/j.yexcr.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Choi K.Y., Chang K., Pickel J.M., Badger J.D., 2nd, Roche K.W. Expression of the metabotropic glutamate receptor 5 (mGluR5) induces melanoma in transgenic mice. Proc. Natl. Acad. Sci. USA. 2011;108:15219–15224. doi: 10.1073/pnas.1107304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu L.J., Wall B.A., Wangari-Talbot J., Chen S. Metabotropic glutamate receptors in cancer. Neuropharmacology. 2017;115:193–202. doi: 10.1016/j.neuropharm.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das A., Pushparaj C., Bahi N., Sorolla A., Herreros J., Pamplona R., Vilella R., Matias-Guiu X., Marti R.M., Canti C. Functional expression of voltage-gated calcium channels in human melanoma. Pigment Cell Melanoma Res. 2012;25:200–212. doi: 10.1111/j.1755-148X.2012.00978.x. [DOI] [PubMed] [Google Scholar]
- 8.Serwach K., Gruszczynska-Biegala J. Target Molecules of STIM Proteins in the Central Nervous System. Front. Mol. Neurosci. 2020;13:617422. doi: 10.3389/fnmol.2020.617422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinsen A., Dessy C., Morel N. Regulation of calcium channels in smooth muscle: New insights into the role of myosin light chain kinase. Channels (Austin) 2014;8:402–413. doi: 10.4161/19336950.2014.950537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ushioda R., Miyamoto A., Inoue M., Watanabe S., Okumura M., Maegawa K.I., Uegaki K., Fujii S., Fukuda Y., Umitsu M., et al. Redox-assisted regulation of Ca2+ homeostasis in the endoplasmic reticulum by disulfide reductase ERdj5. Proc. Natl. Acad. Sci. USA. 2016;113:E6055–E6063. doi: 10.1073/pnas.1605818113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao Y., Hao Y., Chen H., He Q., Yuan Z., Cheng J. Mitochondrial calcium uniporter protein MCU is involved in oxidative stress-induced cell death. Protein Cell. 2015;6:434–442. doi: 10.1007/s13238-015-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feno S., Butera G., Vecellio Reane D., Rizzuto R., Raffaello A. Crosstalk between Calcium and ROS in Pathophysiological Conditions. Oxid. Med. Cell. Longev. 2019;2019:9324018. doi: 10.1155/2019/9324018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briston T., Roberts M., Lewis S., Powney B., Staddon J.M., Szabadkai G., Duchen M.R. Mitochondrial permeability transition pore: Sensitivity to opening and mechanistic dependence on substrate availability. Sci. Rep. 2017;7:10492. doi: 10.1038/s41598-017-10673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adapted from “TGFb Signaling Pathway”, by BioRender.com. [(accessed on 19 November 2021)]. Available online: https://app.biorender.com/biorender-templates.
- 15.Davis L., Tarduno A., Lu Y.C. Neoantigen-Reactive T Cells: The Driving Force behind Successful Melanoma Immunotherapy. Cancers. 2021;13:6061. doi: 10.3390/cancers13236061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellono N.W., Oancea E.V. Ion transport in pigmentation. Arch. Biochem. Biophys. 2014;563:35–41. doi: 10.1016/j.abb.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devi S., Kedlaya R., Maddodi N., Bhat K.M., Weber C.S., Valdivia H., Setaluri V. Calcium homeostasis in human melanocytes: Role of transient receptor potential melastatin 1 (TRPM1) and its regulation by ultraviolet light. Am. J. Physiol. Cell Physiol. 2009;297:C679–C687. doi: 10.1152/ajpcell.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J., Lu F., He H., Shen J., Messina J., Mathew R., Wang D., Sarnaik A.A., Chang W.C., Kim M., et al. STIM1- and Orai1-mediated Ca(2+) oscillation orchestrates invadopodium formation and melanoma invasion. J. Cell. Biol. 2014;207:535–548. doi: 10.1083/jcb.201407082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanisz H., Stark A., Kilch T., Schwarz E.C., Muller C.S., Peinelt C., Hoth M., Niemeyer B.A., Vogt T., Bogeski I. ORAI1 Ca(2+) channels control endothelin-1-induced mitogenesis and melanogenesis in primary human melanocytes. J. Investig. Dermatol. 2012;132:1443–1451. doi: 10.1038/jid.2011.478. [DOI] [PubMed] [Google Scholar]
- 20.Alharbi A.F., Parrington J. Endolysosomal Ca(2+) Signaling in Cancer: The Role of TPC2, From Tumorigenesis to Metastasis. Front. Cell Dev. Biol. 2019;7:302. doi: 10.3389/fcell.2019.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X., Liu H., Xu Y., Xie J., Zhu D., Amos C.I., Fang S., Lee J.E., Li X., Nan H., et al. Genetic variants in the calcium signaling pathway genes are associated with cutaneous melanoma-specific survival. Carcinogenesis. 2019;40:279–288. doi: 10.1093/carcin/bgy188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dissanayake S.K., Weeraratna A.T. Detecting PKC phosphorylation as part of the Wnt/calcium pathway in cutaneous melanoma. Methods Mol. Biol. 2008;468:157–172. doi: 10.1007/978-1-59745-249-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oka M., Kikkawa U. Protein kinase C in melanoma. Cancer Metastasis Rev. 2005;24:287–300. doi: 10.1007/s10555-005-1578-8. [DOI] [PubMed] [Google Scholar]
- 24.Krenzer S., Peterziel H., Mauch C., Blaber S.I., Blaber M., Angel P., Hess J. Expression and function of the kallikrein-related peptidase 6 in the human melanoma microenvironment. J. Investig. Dermatol. 2011;131:2281–2288. doi: 10.1038/jid.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert G., Gaggioli C., Bailet O., Chavey C., Abbe P., Aberdam E., Sabatie E., Cano A., Garcia de Herreros A., Ballotti R., et al. SPARC represses E-cadherin and induces mesenchymal transition during melanoma development. Cancer Res. 2006;66:7516–7523. doi: 10.1158/0008-5472.CAN-05-3189. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed M.B., Islam S.U., Lee Y.S. PRP4 Promotes Skin Cancer by Inhibiting Production of Melanin, Blocking Influx of Extracellular Calcium, and Remodeling Cell Actin Cytoskeleton. Int. J. Mol. Sci. 2021;22:6992. doi: 10.3390/ijms22136992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fedida-Metula S., Elhyany S., Tsory S., Segal S., Hershfinkel M., Sekler I., Fishman D. Targeting lipid rafts inhibits protein kinase B by disrupting calcium homeostasis and attenuates malignant properties of melanoma cells. Carcinogenesis. 2008;29:1546–1554. doi: 10.1093/carcin/bgn146. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Zaguilan R., Martinez G.M., Gomez A., Hendrix M.J.C., Gillies R.J. Distinct regulation of pH(in) and [Ca2+](in) in human melanoma cells with different metastatic potential. J. Cell Physiol. 1998;176:196–205. doi: 10.1002/(SICI)1097-4652(199807)176:1<196::AID-JCP21>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Baljinnyam E., Umemura M., De Lorenzo M.S., Xie L.H., Nowycky M., Iwatsubo M., Chen S., Goydos J.S., Iwatsubo K. Gbetagamma subunits inhibit Epac-induced melanoma cell migration. BMC Cancer. 2011;11:256. doi: 10.1186/1471-2407-11-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baljinnyam E., De Lorenzo M.S., Xie L.H., Iwatsubo M., Chen S., Goydos J.S., Nowycky M.C., Iwatsubo K. Exchange protein directly activated by cyclic AMP increases melanoma cell migration by a Ca2+-dependent mechanism. Cancer Res. 2010;70:5607–5617. doi: 10.1158/0008-5472.CAN-10-0056. [DOI] [PubMed] [Google Scholar]
- 31.Arozarena I., Sanchez-Laorden B., Packer L., Hidalgo-Carcedo C., Hayward R., Viros A., Sahai E., Marais R. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 32.Kosnopfel C., Sinnberg T., Sauer B., Niessner H., Muenchow A., Fehrenbacher B., Schaller M., Mertens P.R., Garbe C., Thakur B.K., et al. Tumour Progression Stage-Dependent Secretion of YB-1 Stimulates Melanoma Cell Migration and Invasion. Cancers. 2020;12:2328. doi: 10.3390/cancers12082328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gelis L., Jovancevic N., Bechara F.G., Neuhaus E.M., Hatt H. Functional expression of olfactory receptors in human primary melanoma and melanoma metastasis. Exp. Dermatol. 2017;26:569–576. doi: 10.1111/exd.13316. [DOI] [PubMed] [Google Scholar]
- 34.D’Mello S.A., Joseph W.R., Green T.N., Leung E.Y., During M.J., Finlay G.J., Baguley B.C., Kalev-Zylinska M.L. Selected GRIN2A mutations in melanoma cause oncogenic effects that can be modulated by extracellular glutamate. Cell Calcium. 2016;60:384–395. doi: 10.1016/j.ceca.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Umemura M., Baljinnyam E., Feske S., De Lorenzo M.S., Xie L.H., Feng X., Oda K., Makino A., Fujita T., Yokoyama U., et al. Store-operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS ONE. 2014;9:e89292. doi: 10.1371/journal.pone.0089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Amore A., Hanbashi A.A., Di Agostino S., Palombi F., Sacconi A., Voruganti A., Taggi M., Canipari R., Blandino G., Parrington J., et al. Loss of Two-Pore Channel 2 (TPC2) Expression Increases the Metastatic Traits of Melanoma Cells by a Mechanism Involving the Hippo Signalling Pathway and Store-Operated Calcium Entry. Cancers. 2020;12:2391. doi: 10.3390/cancers12092391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hegedus L., Garay T., Molnar E., Varga K., Bilecz A., Torok S., Padanyi R., Paszty K., Wolf M., Grusch M., et al. The plasma membrane Ca(2+) pump PMCA4b inhibits the migratory and metastatic activity of BRAF mutant melanoma cells. Int. J. Cancer. 2017;140:2758–2770. doi: 10.1002/ijc.30503. [DOI] [PubMed] [Google Scholar]
- 38.Naffa R., Vogel L., Hegedus L., Paszty K., Toth S., Kelemen K., Singh N., Remenyi A., Kallay E., Cserepes M., et al. P38 MAPK Promotes Migration and Metastatic Activity of BRAF Mutant Melanoma Cells by Inducing Degradation of PMCA4b. Cells. 2020;9:1209. doi: 10.3390/cells9051209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long T., Su J., Tang W., Luo Z., Liu S., Liu Z., Zhou H., Qi M., Zeng W., Zhang J., et al. A novel interaction between calcium-modulating cyclophilin ligand and Basigin regulates calcium signaling and matrix metalloproteinase activities in human melanoma cells. Cancer Lett. 2013;339:93–101. doi: 10.1016/j.canlet.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 40.Maiques O., Barcelo C., Panosa A., Pijuan J., Orgaz J.L., Rodriguez-Hernandez I., Matas-Nadal C., Tell G., Vilella R., Fabra A., et al. T-type calcium channels drive migration/invasion in BRAFV600E melanoma cells through Snail1. Pigment Cell Melanoma Res. 2018;31:484–495. doi: 10.1111/pcmr.12690. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y., Luo Z., Hao Y., Ba W., Wang R., Wang W., Ding X., Li C. mTOR-mediated Na(+)/Ca(2+) exchange affects cell proliferation and metastasis of melanoma cells. Biomed. Pharmacother. 2017;92:744–749. doi: 10.1016/j.biopha.2017.05.104. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt J., Friebel K., Schonherr R., Coppolino M.G., Bosserhoff A.K. Migration-associated secretion of melanoma inhibitory activity at the cell rear is supported by KCa3.1 potassium channels. Cell Res. 2010;20:1224–1238. doi: 10.1038/cr.2010.121. [DOI] [PubMed] [Google Scholar]
- 43.Jia Q., Hu S., Jiao D., Li X., Qi S., Fan R. Synaptotagmin-4 promotes dendrite extension and melanogenesis in alpaca melanocytes by regulating Ca(2+) influx via TRPM1 channels. Cell Biochem. Funct. 2020;38:275–282. doi: 10.1002/cbf.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun J., Lin S., Keeley T., Yang S. Disseminating Melanoma Cells Surf on Calcium Waves. Mol. Cell. Oncol. 2015;2:e1002714. doi: 10.1080/23723556.2014.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim T.H., Gill N.K., Nyberg K.D., Nguyen A.V., Hohlbauch S.V., Geisse N.A., Nowell C.J., Sloan E.K., Rowat A.C. Cancer cells become less deformable and more invasive with activation of beta-adrenergic signaling. J. Cell Sci. 2016;129:4563–4575. doi: 10.1242/jcs.194803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meghnani V., Vetter S.W., Leclerc E. RAGE overexpression confers a metastatic phenotype to the WM115 human primary melanoma cell line. Biochim. Biophys. Acta. 2014;1842:1017–1027. doi: 10.1016/j.bbadis.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Terrie E., Coronas V., Constantin B. Role of the calcium toolkit in cancer stem cells. Cell Calcium. 2019;80:141–151. doi: 10.1016/j.ceca.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Neves de Oliveira B.H., Dalmaz C., Zeidan-Chulia F. Network-Based Identification of Altered Stem Cell Pluripotency and Calcium Signaling Pathways in Metastatic Melanoma. Med. Sci. 2018;6:23. doi: 10.3390/medsci6010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bettum I.J., Gorad S.S., Barkovskaya A., Pettersen S., Moestue S.A., Vasiliauskaite K., Tenstad E., Oyjord T., Risa O., Nygaard V., et al. Metabolic reprogramming supports the invasive phenotype in malignant melanoma. Cancer Lett. 2015;366:71–83. doi: 10.1016/j.canlet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Zhu L., Ito T., Nakahara T., Nagae K., Fuyuno Y., Nakao M., Akahoshi M., Nakagawa R., Tu Y., Uchi H., et al. Upregulation of S100P, receptor for advanced glycation end products and ezrin in malignant melanoma. J. Dermatol. 2013;40:973–979. doi: 10.1111/1346-8138.12323. [DOI] [PubMed] [Google Scholar]
- 51.Venza M., Visalli M., Catalano T., Biondo C., Beninati C., Teti D., Venza I. DNA methylation-induced E-cadherin silencing is correlated with the clinicopathological features of melanoma. Oncol. Rep. 2016;35:2451–2460. doi: 10.3892/or.2016.4618. [DOI] [PubMed] [Google Scholar]
- 52.Wu L., Zhu L., Li Y., Zheng Z., Lin X., Yang C. LncRNA MEG3 promotes melanoma growth, metastasis and formation through modulating miR-21/E-cadherin axis. Cancer Cell Int. 2020;20:12. doi: 10.1186/s12935-019-1087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez M., Aladowicz E., Lanfrancone L., Goding C.R. Tbx3 represses E-cadherin expression and enhances melanoma invasiveness. Cancer Res. 2008;68:7872–7881. doi: 10.1158/0008-5472.CAN-08-0301. [DOI] [PubMed] [Google Scholar]
- 54.Raimbourg Q., Perez J., Vandermeersch S., Prignon A., Hanouna G., Haymann J.P., Baud L., Letavernier E. The calpain/calpastatin system has opposing roles in growth and metastatic dissemination of melanoma. PLoS ONE. 2013;8:e60469. doi: 10.1371/journal.pone.0060469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weeraratna A.T. A wnt-er wonderland—The complexity of wnt signaling in melanoma. Cancer Metast. Rev. 2005;24:237–250. doi: 10.1007/s10555-005-1574-z. [DOI] [PubMed] [Google Scholar]
- 56.O’Connell M.P., Fiori J.L., Baugher K.M., Indig F.E., French A.D., Camilli T.C., Frank B.P., Earley R., Hoek K.S., Hasskamp J.H., et al. Wnt5A activates the calpain-mediated cleavage of filamin A. J. Investig. Dermatol. 2009;129:1782–1789. doi: 10.1038/jid.2008.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Witze E.S., Connacher M.K., Houel S., Schwartz M.P., Morphew M.K., Reid L., Sacks D.B., Anseth K.S., Ahn N.G. Wnt5a directs polarized calcium gradients by recruiting cortical endoplasmic reticulum to the cell trailing edge. Dev. Cell. 2013;26:645–657. doi: 10.1016/j.devcel.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adapted from “Tumor Microenvironment”, by BioRender.com. [(accessed on 19 December 2021)]. Available online: https://app.biorender.com/biorender-templates.
- 59.Singh K., Rosenberg P. Anti-tumour activity and store operated calcium entry: New roles in immunology. EMBO Mol. Med. 2013;5:1297–1299. doi: 10.1002/emmm.201303129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim K.D., Bae S., Capece T., Nedelkovska H., de Rubio R.G., Smrcka A.V., Jun C.D., Jung W., Park B., Kim T.I., et al. Targeted calcium influx boosts cytotoxic T lymphocyte function in the tumour microenvironment. Nat. Commun. 2017;8:15365. doi: 10.1038/ncomms15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mookerjee-Basu J., Hooper R., Gross S., Schultz B., Go C.K., Samakai E., Ladner J., Nicolas E., Tian Y., Zhou B., et al. Suppression of Ca(2+) signals by EGR4 controls Th1 differentiation and anti-cancer immunity in vivo. EMBO Rep. 2020;21:e48904. doi: 10.15252/embr.201948904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Truta-Feles K., Lagadari M., Lehmann K., Berod L., Cubillos S., Piehler S., Herouy Y., Barz D., Kamradt T., Maghazachi A., et al. Histamine modulates gammadelta-T lymphocyte migration and cytotoxicity, via Gi and Gs protein-coupled signalling pathways. Br. J. Pharmacol. 2010;161:1291–1300. doi: 10.1111/j.1476-5381.2010.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Key P.N., Germino J., Yang L., Piersma S.J., Tripathy S.K. Chronic Ly49H Receptor Engagement in vivo Decreases NK Cell Response to Stimulation Through ITAM-Dependent and Independent Pathways Both in vitro and in vivo. Front. Immunol. 2019;10:1692. doi: 10.3389/fimmu.2019.01692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 65.Hollander L., Guo X., Velazquez H., Chang J., Safirstein R., Kluger H., Cha C., Desir G.V. Renalase Expression by Melanoma and Tumor-Associated Macrophages Promotes Tumor Growth through a STAT3-Mediated Mechanism. Cancer Res. 2016;76:3884–3894. doi: 10.1158/0008-5472.CAN-15-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hakonen E., Chandra V., Fogarty C.L., Yu N.Y., Ustinov J., Katayama S., Galli E., Danilova T., Lindholm P., Vartiainen A., et al. MANF protects human pancreatic beta cells against stress-induced cell death. Diabetologia. 2018;61:2202–2214. doi: 10.1007/s00125-018-4687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peled M., Bar-Lev T.H., Talalai E., Aspitz H.Z., Daniel-Meshulam I., Bar J., Kamer I., Ofek E., Mor A., Onn A. Mesencephalic astrocyte-derived neurotrophic factor is secreted from interferon-gamma-activated tumor cells through ER calcium depletion. PLoS ONE. 2021;16:e0250178. doi: 10.1371/journal.pone.0250178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tremble L.F., Heffron C., Forde P.F. The effect of calcium electroporation on viability, phenotype and function of melanoma conditioned macrophages. Sci. Rep. 2020;10:20645. doi: 10.1038/s41598-020-77743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Falk H., Matthiessen L.W., Wooler G., Gehl J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018;57:311–319. doi: 10.1080/0284186X.2017.1355109. [DOI] [PubMed] [Google Scholar]
- 70.He L., Zhang Y., Ma G., Tan P., Li Z., Zang S., Wu X., Jing J., Fang S., Zhou L., et al. Near-infrared photoactivatable control of Ca(2+) signaling and optogenetic immunomodulation. Elife. 2015;4:e10024. doi: 10.7554/eLife.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramirez-Moreno I.G., Ibarra-Sanchez A., Castillo-Arellano J.I., Blank U., Gonzalez-Espinosa C. Mast Cells Localize in Hypoxic Zones of Tumors and Secrete CCL-2 under Hypoxia through Activation of L-Type Calcium Channels. J. Immunol. 2020;204:1056–1068. doi: 10.4049/jimmunol.1801430. [DOI] [PubMed] [Google Scholar]
- 72.Shahan T.A., Fawzi A., Bellon G., Monboisse J.C., Kefalides N.A. Regulation of tumor cell chemotaxis by type IV collagen is mediated by a Ca(2+)-dependent mechanism requiring CD47 and the integrin alpha(V)beta(3) J. Biol. Chem. 2000;275:4796–4802. doi: 10.1074/jbc.275.7.4796. [DOI] [PubMed] [Google Scholar]
- 73.Hodgson L., Dong C. [Ca2+](i) as a potential downregulator of alpha(2)beta(1)-integrin-mediated A2058 tumor cell migration to type IV collagen. Am. J. Physiol.-Cell Physiol. 2001;281:C106–C113. doi: 10.1152/ajpcell.2001.281.1.C106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang H.C., Shi G.Y., Jiang S.J., Shi C.S., Wu C.M., Yang H.Y., Wu H.L. Thrombomodulin-mediated cell adhesion: Involvement of its lectin-like domain. J. Biol. Chem. 2003;278:46750–46759. doi: 10.1074/jbc.M305216200. [DOI] [PubMed] [Google Scholar]
- 75.Chung H., Jung H., Jho E.H., Multhaupt H.A.B., Couchman J.R., Oh E.S. Keratinocytes negatively regulate the N-cadherin levels of melanoma cells via contact-mediated calcium regulation. Biochem. Biophys. Res. Commun. 2018;503:615–620. doi: 10.1016/j.bbrc.2018.06.050. [DOI] [PubMed] [Google Scholar]
- 76.Slater M., Scolyer R.A., Gidley-Baird A., Thompson J.F., Barden J.A. Increased expression of apoptotic markers in melanoma. Melanoma. Res. 2003;13:137–145. doi: 10.1097/00008390-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Li G., Satyamoorthy K., Meier F., Berking C., Bogenrieder T., Herlyn M. Function and regulation of melanoma-stromal fibroblast interactions: When seeds meet soil. Oncogene. 2003;22:3162–3171. doi: 10.1038/sj.onc.1206455. [DOI] [PubMed] [Google Scholar]
- 78.Ekstrom E.J., Bergenfelz C., von Bulow V., Serifler F., Carlemalm E., Jonsson G., Andersson T., Leandersson K. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol. Cancer. 2014;13:88. doi: 10.1186/1476-4598-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Favia A., Pafumi I., Desideri M., Padula F., Montesano C., Passeri D., Nicoletti C., Orlandi A., Del Bufalo D., Sergi M., et al. NAADP-Dependent Ca(2+) Signaling Controls Melanoma Progression, Metastatic Dissemination and Neoangiogenesis. Sci. Rep. 2016;6:18925. doi: 10.1038/srep18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang W., Zhou P., Meng A., Zhang R., Zhou Y. Down-regulating Myoferlin inhibits the vasculogenic mimicry of melanoma via decreasing MMP-2 and inducing mesenchymal-to-epithelial transition. J. Cell. Mol. Med. 2018;22:1743–1754. doi: 10.1111/jcmm.13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vartanian A., Stepanova E., Grigorieva I., Solomko E., Belkin V., Baryshnikov A., Lichinitser M. Melanoma vasculogenic mimicry capillary-like structure formation depends on integrin and calcium signaling. Microcirculation. 2011;18:390–399. doi: 10.1111/j.1549-8719.2011.00102.x. [DOI] [PubMed] [Google Scholar]
- 82.Oliver V.K., Patton A.M., Desai S., Lorang D., Libutti S.K., Kohn E.C. Regulation of the pro-angiogenic microenvironment by carboxyamido-triazole. J. Cell. Physiol. 2003;197:139–148. doi: 10.1002/jcp.10350. [DOI] [PubMed] [Google Scholar]
- 83.Frandsen S.K., Gissel H., Hojman P., Tramm T., Eriksen J., Gehl J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012;72:1336–1341. doi: 10.1158/0008-5472.CAN-11-3782. [DOI] [PubMed] [Google Scholar]
- 84.Staresinic B., Jesenko T., Kamensek U., Krog Frandsen S., Sersa G., Gehl J., Cemazar M. Effect of calcium electroporation on tumour vasculature. Sci. Rep. 2018;8:9412. doi: 10.1038/s41598-018-27728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boda-Heggemann J., Regnier-Vigouroux A., Franke W.W. Beyond vessels: Occurrence and regional clustering of vascular endothelial (VE-)cadherin-containing junctions in non-endothelial cells. Cell Tissue Res. 2009;335:49–65. doi: 10.1007/s00441-008-0718-1. [DOI] [PubMed] [Google Scholar]
- 86.Peng H.H., Hodgson L., Henderson A.J., Dong C. Involvement of phospholipase C signaling in melanoma cell-induced endothelial junction disassembly. Front Biosci. 2005;10:1597–1606. doi: 10.2741/1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peng H.H., Dong C. Systemic Analysis of Tumor Cell-Induced Endothelial Calcium Signaling and Junction Disassembly. Cell. Mol. Bioeng. 2009;2:375–385. doi: 10.1007/s12195-009-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kato Y., Ozawa S., Tsukuda M., Kubota E., Miyazaki K., St-Pierre Y., Hata R. Acidic extracellular pH increases calcium influx-triggered phospholipase D activity along with acidic sphingomyelinase activation to induce matrix metalloproteinase-9 expression in mouse metastatic melanoma. FEBS J. 2007;274:3171–3183. doi: 10.1111/j.1742-4658.2007.05848.x. [DOI] [PubMed] [Google Scholar]
- 89.Noguchi F., Inui S., Fedele C., Shackleton M., Itami S. Calcium-Dependent Enhancement by Extracellular Acidity of the Cytotoxicity of Mitochondrial Inhibitors against Melanoma. Mol. Cancer Ther. 2017;16:936–947. doi: 10.1158/1535-7163.MCT-15-0235. [DOI] [PubMed] [Google Scholar]
- 90.Schneider C., Gebhardt L., Arndt S., Karrer S., Zimmermann J.L., Fischer M.J.M., Bosserhoff A.K. Acidification is an Essential Process of Cold Atmospheric Plasma and Promotes the Anti-Cancer Effect on Malignant Melanoma Cells. Cancers. 2019;11:671. doi: 10.3390/cancers11050671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hung W.C., Yang J.R., Yankaskas C.L., Wong B.S., Wu P.H., Pardo-Pastor C., Serra S.A., Chiang M.J., Gu Z., Wirtz D., et al. Confinement Sensing and Signal Optimization via Piezo1/PKA and Myosin II Pathways. Cell Rep. 2016;15:1430–1441. doi: 10.1016/j.celrep.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buckner C.A., Buckner A.L., Koren S.A., Persinger M.A., Lafrenie R.M. Inhibition of cancer cell growth by exposure to a specific time-varying electromagnetic field involves T-type calcium channels. PLoS ONE. 2015;10:e0124136. doi: 10.1371/journal.pone.0124136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu S., Li C., Ding Y., Huang S., Wang W., Wu Y., Wang F., Wang A., Han Y., Sun Z., et al. Exploring the ‘cold/hot’ properties of traditional Chinese medicine by cell temperature measurement. Pharm. Biol. 2020;58:208–218. doi: 10.1080/13880209.2020.1732429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nam J.H., Lee D.U. Foeniculum vulgare extract and its constituent, trans-anethole, inhibit UV-induced melanogenesis via ORAI1 channel inhibition. J. Dermatol. Sci. 2016;84:305–313. doi: 10.1016/j.jdermsci.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 95.Slominski A.T., Brozyna A.A., Zmijewski M.A., Jozwicki W., Jetten A.M., Mason R.S., Tuckey R.C., Elmets C.A. Vitamin D signaling and melanoma: Role of vitamin D and its receptors in melanoma progression and management. Lab. Investig. 2017;97:706–724. doi: 10.1038/labinvest.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kleszczynski K., Bilska B., Stegemann A., Flis D.J., Ziolkowski W., Pyza E., Luger T.A., Reiter R.J., Bohm M., Slominski A.T. Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int. J. Mol. Sci. 2018;19:3786. doi: 10.3390/ijms19123786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chovancova B., Liskova V., Babula P., Krizanova O. Role of Sodium/Calcium Exchangers in Tumors. Biomolecules. 2020;10:1257. doi: 10.3390/biom10091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carrithers M.D., Chatterjee G., Carrithers L.M., Offoha R., Iheagwara U., Rahner C., Graham M., Waxman S.G. Regulation of podosome formation in macrophages by a splice variant of the sodium channel SCN8A. J. Biol. Chem. 2009;284:8114–8126. doi: 10.1074/jbc.M801892200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sennoune S.R., Santos J.M., Hussain F., Martinez-Zaguilan R. Sodium calcium exchanger operates in the reverse mode in metastatic human melanoma cells. Cell Mol. Biol. 2015;61:40–49. [PubMed] [Google Scholar]
- 100.Esteves G.N.N., Ferraz L.S., Alvarez M.M.P., Costa C.A.D., Lopes R.M., Tersariol I., Rodrigues T. BRAF and NRAS mutated melanoma: Different Ca(2+) responses, Na(+)/Ca(2+) exchanger expression, and sensitivity to inhibitors. Cell Calcium. 2020;90:102241. doi: 10.1016/j.ceca.2020.102241. [DOI] [PubMed] [Google Scholar]
- 101.Gueguinou M., Chantome A., Fromont G., Bougnoux P., Vandier C., Potier-Cartereau M. KCa and Ca(2+) channels: The complex thought. Biochim. Biophys. Acta. 2014;1843:2322–2333. doi: 10.1016/j.bbamcr.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 102.Tajima N., Schonherr K., Niedling S., Kaatz M., Kanno H., Schonherr R., Heinemann S.H. Ca2+-activated K+ channels in human melanoma cells are up-regulated by hypoxia involving hypoxia-inducible factor-1alpha and the von Hippel-Lindau protein. J. Physiol. 2006;571:349–359. doi: 10.1113/jphysiol.2005.096818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tajima N., Itokazu Y., Korpi E.R., Somerharju P., Kakela R. Activity of BK(Ca) channel is modulated by membrane cholesterol content and association with Na+/K+-ATPase in human melanoma IGR39 cells. J. Biol. Chem. 2011;286:5624–5638. doi: 10.1074/jbc.M110.149898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim E.Y., Ridgway L.D., Dryer S.E. Interactions with filamin A stimulate surface expression of large-conductance Ca2+-activated K+ channels in the absence of direct actin binding. Mol. Pharmacol. 2007;72:622–630. doi: 10.1124/mol.107.038026. [DOI] [PubMed] [Google Scholar]
- 105.Ma L.J., Liu L.Y., Jiao S., Wei S.M., Mei Y.A. Ca2+-inactivated K+ current is modulated by endothelin-1 in B-16 murine melanoma cells. Pigm. Cell Res. 2003;16:463–469. doi: 10.1034/j.1600-0749.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 106.Bahar E., Kim H., Yoon H. ER Stress-Mediated Signaling: Action Potential and Ca(2+) as Key Players. Int. J. Mol. Sci. 2016;17:1558. doi: 10.3390/ijms17091558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen W., Jiang Z., Zhang X., Feng J., Ling Y. Nacetyl-S-(p-chlorophenylcarbamoyl)cysteine induces mitochondrial-mediated apoptosis and suppresses migration in melanoma cells. Oncol. Rep. 2015;34:2547–2556. doi: 10.3892/or.2015.4267. [DOI] [PubMed] [Google Scholar]
- 108.Feldman B., Fedida-Metula S., Nita J., Sekler I., Fishman D. Coupling of mitochondria to store-operated Ca(2+)-signaling sustains constitutive activation of protein kinase B/Akt and augments survival of malignant melanoma cells. Cell Calcium. 2010;47:525–537. doi: 10.1016/j.ceca.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 109.Nakagawa C., Suzuki-Karasaki M., Suzuki-Karasaki M., Ochiai T., Suzuki-Karasaki Y. The Mitochondrial Ca(2+) Overload via Voltage-Gated Ca(2+) Entry Contributes to an Anti-Melanoma Effect of Diallyl Trisulfide. Int. J. Mol. Sci. 2020;21:491. doi: 10.3390/ijms21020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rouaud F., Boucher J.L., Slama-Schwok A., Rocchi S. Mechanism of melanoma cells selective apoptosis induced by a photoactive NADPH analogue. Oncotarget. 2016;7:82804–82819. doi: 10.18632/oncotarget.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marciel M.P., Hoffmann P.R. Molecular Mechanisms by Which Selenoprotein K Regulates Immunity and Cancer. Biol. Trace Elem Res. 2019;192:60–68. doi: 10.1007/s12011-019-01774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marciel M.P., Khadka V.S., Deng Y., Kilicaslan P., Pham A., Bertino P., Lee K., Chen S., Glibetic N., Hoffmann F.W., et al. Selenoprotein K deficiency inhibits melanoma by reducing calcium flux required for tumor growth and metastasis. Oncotarget. 2018;9:13407–13422. doi: 10.18632/oncotarget.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Loubiere C., Clavel S., Gilleron J., Harisseh R., Fauconnier J., Ben-Sahra I., Kaminski L., Laurent K., Herkenne S., Lacas-Gervais S., et al. The energy disruptor metformin targets mitochondrial integrity via modification of calcium flux in cancer cells. Sci. Rep. 2017;7:5040. doi: 10.1038/s41598-017-05052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aranda-Souza M.A., Rossato F.A., Costa R.A., Figueira T.R., Castilho R.F., Guarniere M.C., Nunes E.S., Coelho L.C., Correia M.T., Vercesi A.E. A lectin from Bothrops leucurus snake venom raises cytosolic calcium levels and promotes B16-F10 melanoma necrotic cell death via mitochondrial permeability transition. Toxicon. 2014;82:97–103. doi: 10.1016/j.toxicon.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 115.Tian Y., Qin L.J., Qiu H.J., Shi D.B., Sun R., Li W.B., Liu T.Z., Wang J.S., Xu T.T., Guo W., et al. RPS3 regulates melanoma cell growth and apoptosis by targeting Cyto C/Ca2+/MICU1 dependent mitochondrial signaling. Oncotarget. 2015;6:29614–29625. doi: 10.18632/oncotarget.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raimondi M., Fontana F., Marzagalli M., Audano M., Beretta G., Procacci P., Sartori P., Mitro N., Limonta P. Ca(2+) overload- and ROS-associated mitochondrial dysfunction contributes to delta-tocotrienol-mediated paraptosis in melanoma cells. Apoptosis. 2021;26:277–292. doi: 10.1007/s10495-021-01668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim J.K., Kang K.A., Ryu Y.S., Piao M.J., Han X., Oh M.C., Boo S.J., Jeong S.U., Jeong Y.J., Chae S., et al. Induction of Endoplasmic Reticulum Stress via Reactive Oxygen Species Mediated by Luteolin in Melanoma Cells. Anticancer Res. 2016;36:2281–2289. [PubMed] [Google Scholar]
- 118.Burgeiro A., Bento A.C., Gajate C., Oliveira P.J., Mollinedo F. Rapid human melanoma cell death induced by sanguinarine through oxidative stress. Eur. J. Pharmacol. 2013;705:109–118. doi: 10.1016/j.ejphar.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 119.Forno F., Maatuf Y., Boukeileh S., Dipta P., Mahameed M., Darawshi O., Ferreira V., Rada P., Garcia-Martinez I., Gross E., et al. Aripiprazole Cytotoxicity Coincides with Activation of the Unfolded Protein Response in Human Hepatic Cells. J. Pharmacol. Exp. Ther. 2020;374:452–461. doi: 10.1124/jpet.119.264481. [DOI] [PubMed] [Google Scholar]
- 120.Prieto K., Cao Y., Mohamed E., Trillo-Tinoco J., Sierra R.A., Uruena C., Sandoval T.A., Fiorentino S., Rodriguez P.C., Barreto A. Polyphenol-rich extract induces apoptosis with immunogenic markers in melanoma cells through the ER stress-associated kinase PERK. Cell Death Discov. 2019;5:134. doi: 10.1038/s41420-019-0214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eskiocak U., Ramesh V., Gill J.G., Zhao Z., Yuan S.W., Wang M., Vandergriff T., Shackleton M., Quintana E., Johnson T.M., et al. Synergistic effects of ion transporter and MAP kinase pathway inhibitors in melanoma. Nat. Commun. 2016;7:12336. doi: 10.1038/ncomms12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.El-Khattouti A., Selimovic D., Hannig M., Taylor E.B., Abd Elmageed Z.Y., Hassan S.Y., Haikel Y., Kandil E., Leverkus M., Brodell R.T., et al. Imiquimod-induced apoptosis of melanoma cells is mediated by ER stress-dependent Noxa induction and enhanced by NF-kappaB inhibition. J. Cell. Mol. Med. 2016;20:266–286. doi: 10.1111/jcmm.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nyberg W.A., Espinosa A. Imiquimod induces ER stress and Ca(2+) influx independently of TLR7 and TLR8. Biochem. Biophys. Res. Commun. 2016;473:789–794. doi: 10.1016/j.bbrc.2016.03.080. [DOI] [PubMed] [Google Scholar]
- 124.Onoe-Takahashi A., Suzuki-Karasaki M., Suzuki-Karasaki M., Ochiai T., Suzuki-Karasaki Y. Autophagy inhibitors regulate TRAIL sensitivity in human malignant cells by targeting the mitochondrial network and calcium dynamics. Int J Oncol. 2019;54:1734–1746. doi: 10.3892/ijo.2019.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rosenblatt R.B., Frank J.A., Burks S.R. Cytosolic Ca(2+) transients during pulsed focused ultrasound generate reactive oxygen species and cause DNA damage in tumor cells. Theranostics. 2021;11:602–613. doi: 10.7150/thno.48353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nardin C., Peres C., Mazzarda F., Ziraldo G., Salvatore A.M., Mammano F. Photosensitizer Activation Drives Apoptosis by Interorganellar Ca(2+) Transfer and Superoxide Production in Bystander Cancer Cells. Cells. 2019;8:1175. doi: 10.3390/cells8101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Granados K., Huser L., Federico A., Sachindra S., Wolff G., Hielscher T., Novak D., Madrigal-Gamboa V., Sun Q., Vierthaler M., et al. T-type calcium channel inhibition restores sensitivity to MAPK inhibitors in de-differentiated and adaptive melanoma cells. Br. J. Cancer. 2020;122:1023–1036. doi: 10.1038/s41416-020-0751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barcelo C., Siso P., Maiques O., Garcia-Mulero S., Sanz-Pamplona R., Navaridas R., Megino C., Felip I., Urdanibia I., Eritja N., et al. T-Type Calcium Channels as Potential Therapeutic Targets in Vemurafenib-Resistant BRAF(V600E) Melanoma. J. Investig. Dermatol. 2020;140:1253–1265. doi: 10.1016/j.jid.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 129.Alza L., Visa A., Herreros J., Canti C. The rise of T-type channels in melanoma progression and chemotherapeutic resistance. Biochim. Biophys. Acta Rev. Cancer. 2020;1873:188364. doi: 10.1016/j.bbcan.2020.188364. [DOI] [PubMed] [Google Scholar]
- 130.Takata N., Ohshima Y., Suzuki-Karasaki M., Yoshida Y., Tokuhashi Y., Suzuki-Karasaki Y. Mitochondrial Ca2+ removal amplifies TRAIL cytotoxicity toward apoptosis-resistant tumor cells via promotion of multiple cell death modalities. Int. J. Oncol. 2017;51:193–203. doi: 10.3892/ijo.2017.4020. [DOI] [PubMed] [Google Scholar]
- 131.Ohshima Y., Takata N., Suzuki-Karasaki M., Yoshida Y., Tokuhashi Y., Suzuki-Karasaki Y. Disrupting mitochondrial Ca2+ homeostasis causes tumor-selective TRAIL sensitization through mitochondrial network abnormalities. Int. J. Oncol. 2017;51:1146–1158. doi: 10.3892/ijo.2017.4096. [DOI] [PubMed] [Google Scholar]
- 132.Tokunaga T., Ando T., Suzuki-Karasaki M., Ito T., Onoe-Takahashi A., Ochiai T., Soma M., Suzuki-Karasaki Y. Plasma-stimulated medium kills TRAIL-resistant human malignant cells by promoting caspase-independent cell death via membrane potential and calcium dynamics modulation. Int. J. Oncol. 2018;52:697–708. doi: 10.3892/ijo.2018.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ivankovic S., Stojkovic R., Maksimovic M., Galic B., Milos M. Impact of calcium ion on cytotoxic effect of the boroxine derivative, K2[B3O3F4OH] J. Enzym. Inhib. Med. Chem. 2016;31:70–74. doi: 10.1080/14756366.2016.1204611. [DOI] [PubMed] [Google Scholar]
- 134.Sedaghat F., Notopoulos A. S100 protein family and its application in clinical practice. Hippokratia. 2008;12:198–204. [PMC free article] [PubMed] [Google Scholar]
- 135.Deckers E.A., Kruijff S., Brouwers A.H., van der Steen K., Hoekstra H.J., Thompson J.F., Vallez Garcia D., Wevers K.P. The association between active tumor volume, total lesion glycolysis and levels of S-100B and LDH in stage IV melanoma patients. Eur. J. Surg. Oncol. 2020;46:2147–2153. doi: 10.1016/j.ejso.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 136.Wagner N.B., Forschner A., Leiter U., Garbe C., Eigentler T.K. S100B and LDH as early prognostic markers for response and overall survival in melanoma patients treated with anti-PD-1 or combined anti-PD-1 plus anti-CTLA-4 antibodies. Br. J. Cancer. 2018;119:339–346. doi: 10.1038/s41416-018-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gassenmaier M., Lenders M.M., Forschner A., Leiter U., Weide B., Garbe C., Eigentler T.K., Wagner N.B. Serum S100B and LDH at Baseline and During Therapy Predict the Outcome of Metastatic Melanoma Patients Treated with BRAF Inhibitors. Target Oncol. 2021;16:197–205. doi: 10.1007/s11523-021-00792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Felix J., Cassinat B., Porcher R., Schlageter M.H., Maubec E., Pages C., Baroudjian B., Homyrda L., Boukouaci W., Tamouza R., et al. Relevance of serum biomarkers associated with melanoma during follow-up of anti-CTLA-4 immunotherapy. Int. Immunopharmacol. 2016;40:466–473. doi: 10.1016/j.intimp.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 139.Nordlinger A., Dror S., Elkahloun A., Del Rio J., Stubbs E., Golan T., Malcov H., Pricket T.D., Cronin J.C., Parikh S., et al. Mutated MITF-E87R in Melanoma Enhances Tumor Progression via S100A4. J. Investig. Dermatol. 2018;138:2216–2223. doi: 10.1016/j.jid.2018.03.1524. [DOI] [PubMed] [Google Scholar]
- 140.Massi D., Landriscina M., Piscazzi A., Cosci E., Kirov A., Paglierani M., Di Serio C., Mourmouras V., Fumagalli S., Biagioli M., et al. S100A13 is a new angiogenic marker in human melanoma. Mod. Pathol. 2010;23:804–813. doi: 10.1038/modpathol.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Datta M., Savage P., Lovato J., Schwartz G.G. Serum calcium, albumin and tumor stage in cutaneous malignant melanoma. Future Oncol. 2016;12:2205–2214. doi: 10.2217/fon-2016-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.