Abstract
Background:
Bisphenol A (BPA) is a suspected obesogen that has been associated with adiposity in children. Bisphenol S (BPS), a structural analog of BPA, is used as a BPA substitute and may have similar health effects as BPA. However, few studies have examined whether BPS is associated with childhood adiposity.
Methods:
We quantified urinary BPA and BPS concentrations in 212 children age 8 years from the HOME Study, a prospective pregnancy and birth cohort study that enrolled pregnant women in Cincinnati, Ohio (2003–2006). We assessed children’s adiposity by bioelectric impedance at age 8 years (n = 212), and by anthropometry and dual-energy X-ray absorptiometry at age 12 years (n = 181). We measured serum adipocytokine concentrations at age 12 years (n = 155). Using multivariable linear regression, we estimated covariate-adjusted associations of BPA and BPS with adiposity measures at ages 8 and 12 years and adipocytokine concentrations at age 12 years.
Results:
Each 10-fold increase in urinary BPA concentrations were inversely associated with percent body fat at age 8 years [β = −1.2, 95% confidence interval (CI) = −3.4, 1.0] and 12 years (β = −1.6, 95% CI = −4.0, 0.9). In contrast, urinary BPS concentrations were positively associated with percent body fat at age 8 years (β = 1.1, 95% CI = −0.6, 2.7), but not at 12 years (β = 0.1, 95% CI = −1.7, 1.8). Urinary BPA and BPS concentrations were not associated with serum adiponectin or leptin concentrations.
Conclusions:
We did not observe evidence that urinary BPA or BPS concentrations during childhood were associated with greater child adiposity at ages 8 and 12 years in this cohort.
Keywords: Bisphenol A, Bisphenol S, Adolescent, Child, Obesity, DXA
What this study adds
Although in-vitro studies suggest that BPS could have obesogenic effects, this is one of the first cohort studies conducted in the United States focused on childhood exposure to BPA and BPS in relation to adiposity. Our study used various adiposity outcomes across mid-childhood and adolescence, including body mass index (BMI), body fat by bioelectrical impedance (BIA) and dual-energy X-ray absorptiometry (DXA), and adipocytokine concentrations. We found no evidence of a significant association of urinary BPA and BPS concentrations with increased adiposity in children.
Introduction
The prevalence of childhood obesity has increased worldwide.1,2 In the US, the prevalence of obesity among adolescents increased from 6.1% in 1971 to 20.6% in 2016.3 Child and adolescent obesity increases the risk of adult diabetes, heart disease, hypertension, and stroke.4 Therefore, it is essential to identify modifiable determinants of obesity. During early development, exposure to some endocrine-disrupting chemicals, such as polychlorinated biphenyls, bisphenol A (BPA), and phthalates, may increase the risk of obesity later in life by disrupting neuroendocrine systems involved in energy metabolism and eating behaviors.5
BPA, a suspected obesogen, is found in some polycarbonate plastics, food can linings, thermal receipts, and medical equipment; diet is the primary source of BPA exposure for most individuals.6 BPA may affect hormone homeostasis, adipocyte proliferation and differentiation, and DNA methylation to increase the risk of obesity.5,7 Since 2007, manufacturers are gradually replacing BPA with bisphenol S (BPS) and other substitutes in some plastics, food can linings, and personal care products; however, it is not known if BPS is less toxic than BPA.8,9 BPS is chemically similar to BPA, as it is a structural analog (Figure S1; http://links.lww.com/EE/A170), and so may have similar health effects as BPA. BPS, unlike BPA, contains a sulfone group, that makes it more heat resistant and environmentally stable.10 A review of in vivo and in vitro studies found that BPS has similar estrogenic potency as BPA.9 For example, BPS is equipotent to BPA in activating extracellular signal-regulated kinases in rat pituitary cells and subsequently disrupting cell proliferation and death.11 In vitro studies also indicate that BPA and BPS induce adipocyte differentiation.12 BPA may also inhibit adiponectin release from adipocytes.13
Although several epidemiological studies have examined BPA exposure and child adiposity, fewer have examined the potential obesogenic effects of BPS. Cross-sectional studies found that urinary BPA concentrations were significantly associated with increased odds of obesity.14–16 In two cohort studies of 4- and 9-year old children, urinary BPA concentrations were significantly associated with concurrent higher body mass index (BMI) z-scores, with no evidence of effect measure modification by sex.17,18 Other cohort studies with prospective childhood measurements have found null or inverse associations.19–21 In a cross-sectional study of 6- to 17-year-old children, increased odds of obesity were observed among children with higher urinary BPA and BPS concentrations.15 In a recent cohort study, BPA and BPS concentrations at age 6 years were inversely associated with BMI z-scores at age 10 years.22
Given the sparse data on the effects of childhood BPA and BPS exposure, we investigated whether childhood urinary BPA or BPS concentrations at age 8 years were associated with excess adiposity in children at ages 8 and 12 years, and alterations in serum adiponectin and leptin at age 12 years.
Methods
Study participants
We used data from the Health Outcomes and Measures of the Environment (HOME) Study, a prospective pregnancy and birth cohort in Cincinnati, Ohio, established to examine the potential health effects of environmental chemical exposures in children.23,24 Women were recruited between 2003 and 2006 from nine prenatal clinics affiliated with three delivery hospitals in Cincinnati, Ohio. Women were eligible if they were 16 ± 3 weeks pregnant, older than 18 years old, living in a home built in or before 1978, fluent in English, HIV-negative, and not taking medications for seizures and/or thyroid disorders, and had no diagnosis of diabetes, bipolar disorder, schizophrenia, or cancer resulting in radiation treatment or chemotherapy.
All women provided written informed consent for themselves and their children at all visits; children provided written informed assent at the 12-year visit. Study protocols were approved by the institutional review boards (IRBs) of Cincinnati Children’s Hospital Medical Center (CCHMC) and the cooperating delivery hospitals. Brown University and the Centers for Disease Control and Prevention (CDC) IRBs deferred to the CCHMC IRB.
The HOME Study enrolled 468 women, of which 389 women delivered live singleton infants. Study staff completed follow-up on 233 children at age 8 years and 242 children at age 12 years. The present analyses include 212 singleton children with biomarker measures for BPA and BPS, anthropometry, body composition, and covariate data at age 8 years (Figure S2; http://links.lww.com/EE/A170).
Among the 242 singleton children who completed follow-up at the 12-year visit, 181 children had BPA and BPS measurements available at age 8 years, dual-energy X-ray absorptiometry (DXA) measurements at age 12 years, and covariate information (Figure S2; http://links.lww.com/EE/A170). Analyses of adiponectin and leptin measurements at age 12 years included 155 children who had available BPA and BPS measurements, adiponectin and leptin measurements, and covariate information. Five to seven children with complete exposure and outcome data were missing covariate information in our analyses (Figure S2; http://links.lww.com/EE/A170). The distribution of BPA and BPS concentrations were similar between participants with and without covariate information (Table S1; http://links.lww.com/EE/A170).
Exposure assessment
Children in the HOME Study provided urine samples at the 8-year visit.
All samples were refrigerated until they were processed, after which they were stored at or below −20oC until shipped on dry ice to the National Center for Environmental Health Laboratories, CDC for analysis. BPA and BPS concentrations were measured using online solid-phase extraction coupled to high-performance liquid chromatography-isotope dilution tandem mass spectrometry with peak focusing.25 The limits of detections (LODs) were 0.1 ng/mL and 0.03 ng/mL for BPA and BPS, respectively. Two samples were below the LOD for BPA, and no samples were below the LOD for BPS.
To account for urine dilution, BPA and BPS concentrations were divided by creatinine and multiplied by 100 to yield units of micrograms BPA and BPS per gram creatinine. The creatinine-standardized urinary BPA and BPS concentrations were log10-transformed in statistical models to reduce the influence of outliers.
Outcome assessment
At age 8 years, trained study staff measured children’s weight, height, body fat percentage via BIA (Tanita body fat scale BF-659), and waist circumference (cm), in triplicate, after standardized protocols.24 We calculated BMI (kg/m2) and age- and sex-specific z-scores using US references from the National Center for Health Statistics.26
At age 12 years, we measured weight, height, and waist circumference, and obtained a whole-body DXA scan (Hologic Horizon) to estimate total and regional adiposity. Our adiposity measures included whole-body fat mass index (FMI) z-scores, whole-body fat mass percent (%), visceral fat area (cm2), android region fat (%), and gynoid region fat (%). We calculated age- and sex-specific FMI (fat mass/height2, kg/m2) z-scores based on the reference values generated using the 1999–2004 National Health and Nutrition Examination Survey (NHANES).27 Visceral fat area (cm2) is the cross-sectional area of fat inside the abdomen. We calculated android fat percent as the [android fat mass (g) in the android region]/(total mass in the android region) × 100. We calculated gynoid fat percent the same way except using the values for the gynoid region. We did not use bioelectrical impedance to measure percent body fat at age 12 years.
We measured serum leptin and adiponectin concentrations in venous blood samples obtained at the 12-year visit using an ELISA sandwich assay (Millipore/Linco, Linco Research, St Charles, MO, Catalog #EZHADP-61K) for adiponectin, and the Millipore/EMD, St. Charles, MO, Catalog EZHL-805K assay for leptin, and BioTeck microtiter ELx 808 plate reader. Trained technicians performed all assays at the CCHMC National Institutes of Health (NIH)-funded Clinical Translational Research Center Core Laboratory. The LODs were 0.8 ng/mL (leptin) and <2 μg/mL (adiponectin). We included reagent blanks and quality control (QC) samples in each analytic batch, with coefficients of variation for repeated QC measurements of approximately 11% and 13% for leptin and adiponectin, respectively. We log10-transformed adipocytokine concentrations for statistical analysis to minimize the influence of outliers and meet the linear regression assumption that the residuals should follow a normal distribution.
Covariates
We selected covariates potentially associated with BPA/BPS exposure and adiposity using a directed acyclic graph and prior knowledge28,29 (Figure S3; http://links.lww.com/EE/A170). Though covariates such as child sex, physical activity, and maternal BMI are not associated with urinary BPA and BPS concentrations, we adjusted for them to reduce residual error and improve the precision of estimates. We collected sociodemographic data, including maternal and child race/ethnicity, age, education, marital status, employment, and insurance using standardized computer-assisted interviews in the second or third trimester. Through the review of medical records, we obtained the mother’s pre-pregnancy weight, height, and parity.
Research assistants collected the frequency of fresh fruit and vegetable consumption at age 8 years using computer-assisted interviews. Physical activity data were collected using the Physical Activity Questionnaire for Older Children (PAQ-C), a validated self-administered, 7-day recall instrument at age 12 years.30 We calculated 2010 Healthy Eating Index scores using data from three 24-hour dietary recalls at age 12 years.31 The 2010 Healthy Eating Index Scores are based on the United States Department of Agriculture’s (USDA) 2010 dietary guidelines and comprises of 12 components, including total fruit, whole fruit, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids, refined grains, sodium, and empty calories.32 We used self-reported Tanner staging to evaluate pubertal development with pubic hair growth in both boys and girls.33
Statistical analysis
We described univariate characeristics of BPA, BPS, adiposity measures, and covariates. We also calculated median urinary BPA and BPS concentrations at age 8 years and mean ± SD, BMI z-scores at age 8 years according to categories of each covariate (Table 1). We calculated Spearman correlation coefficients between log10-transformed BPA and BPS concentrations. In addition, we examined potential nonlinear associations of BPA and BPS concentrations with adiposity measures at ages 8 and 12 years using covariate-adjusted natural cubic splines (Figure S4; http://links.lww.com/EE/A170).
Table 1.
Median child urinary BPA and BPS concentrations and body mass index z-scores in study participants at age 8 years according to maternal and child covariates: The HOME Study.
| Variable | n (%) | Median BPA μg/L (25th, 75th) | Median BPS μg/L (25th, 75th) | BMI z-score (Mean ± SD) |
|---|---|---|---|---|
| Overall | 212 | 1.6 (1, 3.6) | 0.4 (0.2, 0.7) | 0.5 ± 1.2 |
| Maternal age at delivery (years) | ||||
| <25 | 56 (26.4) | 2.1 (1.3, 3.8) | 0.4 (0.2, 1.0) | 0.6 ± 1.3 |
| 25–35 | 125 (59.0) | 1.6 (1.0, 3.4) | 0.4 (0.2, 0.6) | 0.6 ± 1.2 |
| >35 | 31 (14.6) | 1.2 (0.9, 2.0) | 0.4 (0.2, 0.5) | 0.4 ± 1.1 |
| Pre-pregnancy BMI | ||||
| Underweight-normal (<25) | 111 (52.4) | 1.6 (1.1, 3.2) | 0.4 (0.2, 0.6) | 0.2 ± 1.2 |
| Overweight (25–<30) | 55 (25.9) | 1.5 (0.8, 3.4) | 0.4 (0.2, 0.8) | 0.6 ± 1.0 |
| Obese (≥30) | 46 (21.7) | 2.2 (1.0. 4.0) | 0.4 (0.2, 0.8) | 1.2 ± 1.3 |
| Maternal marital status | ||||
| Married | 141 (66.5) | 1.5 (0.9, 3.3) | 0.4 (0.2, 0.6) | 0.3 ± 1.1 |
| Unmarried, cohabitating | 27 (12.7) | 2.3 (1.4 4.0) | 0.6 (0.2, 2.3) | 0.8 ± 1.1 |
| Unmarried, living alone | 44 (20.8) | 1.8 (1.3, 3.4) | 0.4 (0.2, 0.8) | 1.0 ± 1.4 |
| Maternal education | ||||
| High school or less | 36 (17.0) | 1.8 (1.2, 3.6) | 0.6 (0.3, 1.0) | 0.9 ± 1.2 |
| Some college | 70 (33.0 | 2.3 (1.2, 4.1) | 0.4 (0.2, 1.0) | 0.5 ± 1.3 |
| Bachelors or more | 106 (50.0) | 1.4 (0.9, 2.2) | 0.3 (0.2, 0.6) | 0.5 ± 1.1 |
| Insurance | ||||
| Private | 140 (66.0) | 1.5 (0.9, 3.2) | 0.4 (0.2, 0.6) | 0.4 ± 1.1 |
| Public/uninsured | 72 (34.0) | 2.1 (1.3, 4.0) | 0.4 (0.2, 1.0) | 0.9 ± 1.3 |
| Maternal race | ||||
| Black and other | 83 (39.2) | 2.1 (1.2, 4) | 0.5 (0.2. 1) | 0.8 ± 1.3 |
| Non-Hispanic White | 129 (60.8) | 1.5 (0.9, 3) | 0.3 (0.2, 0.6) | 0.4 ± 1.1 |
| Child race | ||||
| Black and other | 89 (42.0) | 2.1 (1.2, 4.0) | 0.5 (0.2, 0.9) | 0.8 ± 1.3 |
| Non-Hispanic White | 123 (58.0) | 1.4 (0.8, 3.0) | 0.3 (0.2, 0.6) | 0.4 ± 1.1 |
| Child sex | ||||
| Female | 116 (54.7) | 1.6 (1.0, 3.7) | 0.4 (0.2, 0.9) | 0.6 ± 1.2 |
| Male | 96 (45.3) | 1.6 (1.0, 3.3) | 0.4 (0.2, 0.7) | 0.4 ± 1.2 |
| Child fruit/vegetable consumption | ||||
| Daily | 117 (55.2) | 1.5 (1.0, 2.9) | 0.3 (0.2, 0.6) | 0.5 ± 1.3 |
| Weekly | 85 (40.1) | 1.8 (1.0, 3.9) | 0.4 (0.2, 0.7) | 0.6 ± 1.1 |
| Monthly | 10 (4.7) | 3.5 (1.5, 4.1) | 0.8 (0.5, 2.5) | 0.4 ± 1.3 |
BMI indicates body mass index; BPA, bisphenol A; BPS, bisphenol S.
We used multivariable linear regression models to estimate covariate-adjusted cross-sectional associations of creatinine-standardized log10-transformed urinary BPA and BPS concentrations with BMI z-score, body fat percentage, and waist circumference at age 8 years. These models adjusted for child race (Black and other race, Non-Hispanic White), maternal education (high school or less, some college, bachelor’s or more), maternal marital status (married, unmarried and cohabitating, unmarried and living alone), and insurance status (public/uninsured and private) at the 8-year visit. Other covariates include maternal age (continuous, years) at delivery and maternal pre-pregnancy BMI (continuous, kg/m2). Additionally, we adjusted for fresh fruit and vegetable consumption at the 8-year visit (daily, weekly, and monthly) as a measure of diet quality. Body fat percentage and waist circumference were further adjusted for child sex (boys, girls) and age (continuous, years)
Using multivariable linear regression models, we estimated covariate-adjusted prospective associations of creatinine-standardized log10-transformed BPA and BPS concentrations at age 8 years with BMI z-scores, whole-body fat mass percent, waist circumference, whole-body FMI z-scores, visceral fat area, android fat percent, gynoid fat percent, log10-transformed serum adiponectin, and log10-transformed serum leptin concentrations, and the ratio between log10-transformed adiponectin and leptin concentrations at age 12 years. These models adjusted for child race, maternal education, maternal marital status, insurance, maternal age at delivery, and maternal pre-pregnancy BMI. Instead of fresh fruit and vegetable consumption, we adjusted for Total Healthy Eating Scores (continuous) as a measure of diet quality. We also adjusted for physical activity scores (continuous). We adjusted all models, except for whole-body FMI z-scores, for child sex and age.
Secondary and sensitivity analyses
Given that some previous studies reported sex-specific associations between BPA and adiposity,15,16 we evaluated effect measure modification by child sex using stratified models and interaction terms between BPA, BPS, and child sex. We chose to use stratified models because confounders such as diet and physical activity may have sex-specific patterns.34 Previous studies have found that girls tend to consume more fruits and vegetables than boys, whereas physical activity is more common in boys.35
We further examined potential synergism or antagonism by modeling adiposity measures at ages 8 and 12 years as a function of BPA and BPS terciles, with an interaction term of BPA × BPS tercile. Finally, we adjusted for puberty, but acknowledge that it may be a causal intermediate between BPA/BPS exposure and adiposity-related outcomes.
We performed all analyses using R version 3.6.3 (R Core Team, Vienna, Austria).
Results
The mean ages of the 212 children at the 8- and 12-year visits were 8.1 years (SD: 0.6, n = 212) and 12.3 years (SD: 0.6, n = 181), respectively. Most mothers in our analytic sample were Non-Hispanic White (61%), married (67%), and college-educated (50%) (Table 1). Mothers of the study participants at age 12 years had demographic characteristics similar to mothers of study participants at age 8 years (Table S2; http://links.lww.com/EE/A170).
Children’s median urinary BPA and BPS concentrations were 1.6 µg/L (IQR: 1, 3.6) and 0.4 µg/L (IQR: 0.2, 0.7), respectively (Table 1). Children whose mothers were less than 25 years old at delivery, were obese pre-pregnancy, or unmarried and cohabitating had higher median BPA concentrations. Children who consumed fresh fruits and vegetables weekly or monthly tended to have higher urinary BPA and BPS concentrations (Table 1). BPA and BPS were moderately correlated with each other (Spearman correlation coefficient = 0.4, P-value <0.001).
BMI z-scores at age 8 years were higher in children whose mothers were overweight or obese before pregnancy, were unmarried, or had less than high school education. Black children and girls also had higher mean BMI z-scores at age 8 years compared with Non-Hispanic White children and boys (Table 1). FMI z-scores at age 12 years were higher in Black children, girls, and children whose mothers were overweight or obese before pregnancy. We observed similar patterns for visceral fat area, android fat percent, and gynoid fat percent (Table S3; http://links.lww.com/EE/A170).
Generally, urinary BPA concentrations at age 8 years were inversely associated with adiposity measures at ages 8 and 12 years (Table 2). However, the 95% confidence intervals (CIs) for these estimates included the null, and the point estimates were often close to the null. For instance, after adjusting for covariates, each 10-fold increase in urinary BPA concentrations was associated with lower body fat percent at both 8 (β: −1.2; 95% CI = −3.4, 1.0) and 12 years (β: −1.6; 95% CI = −4.0, 0.9), but the associations for BMI z-scores were null (β’s = −0.1 and −0.2).
Table 2.
Unadjusted and adjusted difference in measures of overall and regional adiposity for a 10-fold increase in creatinine-standardized child urinary BPA and BPS concentrations: The HOME Study.
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Outcome | BPA β (95% CI ) | BPS β (95% CI ) | BPA β (95% CI ) | BPS β (95% CI ) |
| Age 8 Year (n = 212) | ||||
| BMI z-score a | −0.1 (−0.6, 0.3) | 0.1 (−0.2, 0.4) | −0.1 (−0.6, 0.3) | 0.1 (−0.2, 0.4) |
| Body fat (%) | −1.2 (−3.5, 1.1) | 1.0 (−0.7, 2.8) | −1.2 (−3.4, 1.0) | 1.1 (−0.6, 2.7) |
| Waist circumference (cm) | −1.2 (−4.3, 1.9) | 0.3 (−2.1, 2.6) | −0.6 (−3.6, 2.4) | 0.5 (−1.8, 2.8) |
| Age 12 Year DXA (n = 181) | ||||
| BMI z-score b | − 0.1 (−0.5, 0.2) | 0.2 (−0.2, 0.5) | −0.2 (−0.7, 0.2) | 0.1 (−0.2, 0.4) |
| Body fat (%) | −1.1 (−3.6, 1.4) | 0.3 (−1.5, 2.2) | −1.6 (−4.0, 0.9) | 0.1 (−1.7, 1.8) |
| Waist circumference (cm) | −1.7 (−6.8, 3.3) | 1.0 (−2.8, 4.8) | −2.5 (−7.4, 2.3) | 1.0 (−2.6, 4.5) |
| Whole body FMI z-score b | −0.1 (−0.5, 0.2) | 0.1 (−0.1, 0.3) | −0.2 (−0.5, 0.1) | 0.1 (−0.2, 0.3) |
| Visceral fat area (cm2) | −2.4 (−10.9, 6.0) | 0.6 (−5.7, 6.8) | −2.4 (−11.0, 6.1) | 0.8 (−5.4, 7.0) |
| Android fat (%) | −1.7 (−4.9, 1.4) | 0.6 (−1.8, 2.9) | −2.2 (−5.3, 0.9) | 0.3 (−2.0, 2.6) |
| Gynoid fat (%) | −1.2 (−3.5, 1.1) | 0.3 (−1.3, 2.0) | −1.4 (−3.7, 0.8) | 0.1 (−1.5, 1.7) |
a Age 8 year outcomes adjusted for child race (Black and other race, Non-Hispanic White), maternal education (high school or less, some college, bachelor’s or more) at the 8-year visit, maternal marital status (married, unmarried and cohabitating, unmarried and living alone) at the 8-year visit, insurance (public/uninsured, private) at the 8-year visit, maternal age (continuous, years) at delivery, maternal pre-pregnancy BMI (continuous, kg/m2), fresh fruit and vegetable consumption at the 8-year visit (daily, weekly, monthly)..Body fat percentage and waist circumference further adjusted for child sex (boys, girls) and age (continuous, years).
b Age 12 year outcomes adjusted for child race, maternal education at the 8-year visit, maternal marital status at the 8-year visit, insurance at the 8-year visit, maternal age at delivery, maternal pre-pregnancy BMI, total healthy eating scores (continuous), and physical activity scores (continuous). Body fat percentage, waist circumference, visceral fat area, android fat, and gynoid fat were further adjusted for child sex and age.
BMI indicates body mass index; BPA, bisphenol A; BPS, bisphenol S; CI, confidence interval; DXA, dual-energy X-ray absorptiometry; FMI, fat mass index.
We observed null or weak positive associations of urinary BPS concentrations at age 8 years with adiposity measures at both ages 8 and 12 years (Table 2). The 95% CIs for these associations included the null value in all cases. After adjusting for covariates, each 10-fold increase in urinary BPS concentrations was associated with modestly higher body fat percent at age 8 years (β: 1.1; 95% CI = −0.6, 2.7). We did not observe evidence of associations of BPA and BPS with adiponectin, leptin, or adiponectin to leptin ratio (Table 3).
Table 3.
Unadjusted and adjusted percent difference in adiponectin and leptin for a 10-fold increase in creatinine-standardized child urinary BPA and BPS concentrations: The HOME Study.
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Outcome | n | BPA β (95% CI ) | BPS β (95% CI ) | BPA β (95% CI ) | BPS β (95% CI ) |
| Adiponectin a | 155 | 16 (−10, 49) | 11 (−8, 34) | 24 (−5, 60) | 14 (−6, 37) |
| Leptin a | 155 | −3 (−39, 53) | −4 (−32, 34) | −18 (−48, 30) | −11 (−36, 23) |
| Adiponectin: Leptin Ratio a | 155 | −1 (−12, 11) | −2 (−10, 7) | −6 (−16, 6) | −3 (−11, 5) |
a Adjusted for child sex (boys, girls), child race (Black and other race, Non-Hispanic White), child age (continuous, years), maternal education (high school or less, some college, bachelor’s or more) at the 8-year visit, maternal marital status (married, unmarried and cohabitating, unmarried and living alone) at the 8-year visit, insurance (public/uninsured, private) at the 8-year visit, maternal age (continuous, years) at delivery, maternal pre-pregnancy BMI (continuous, kg/m2), total healthy eating scores (continuous), and physical activity scores (continuous).
BPA indicates bisphenol A; BPS, bisphenol S; CI, confidence interval.
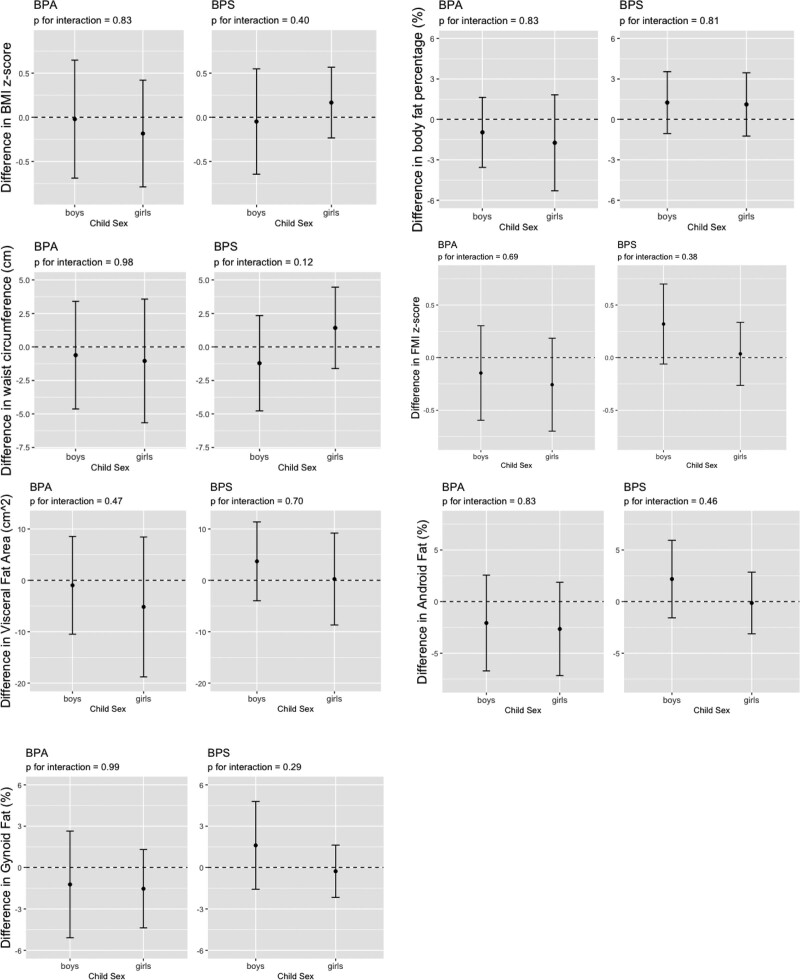
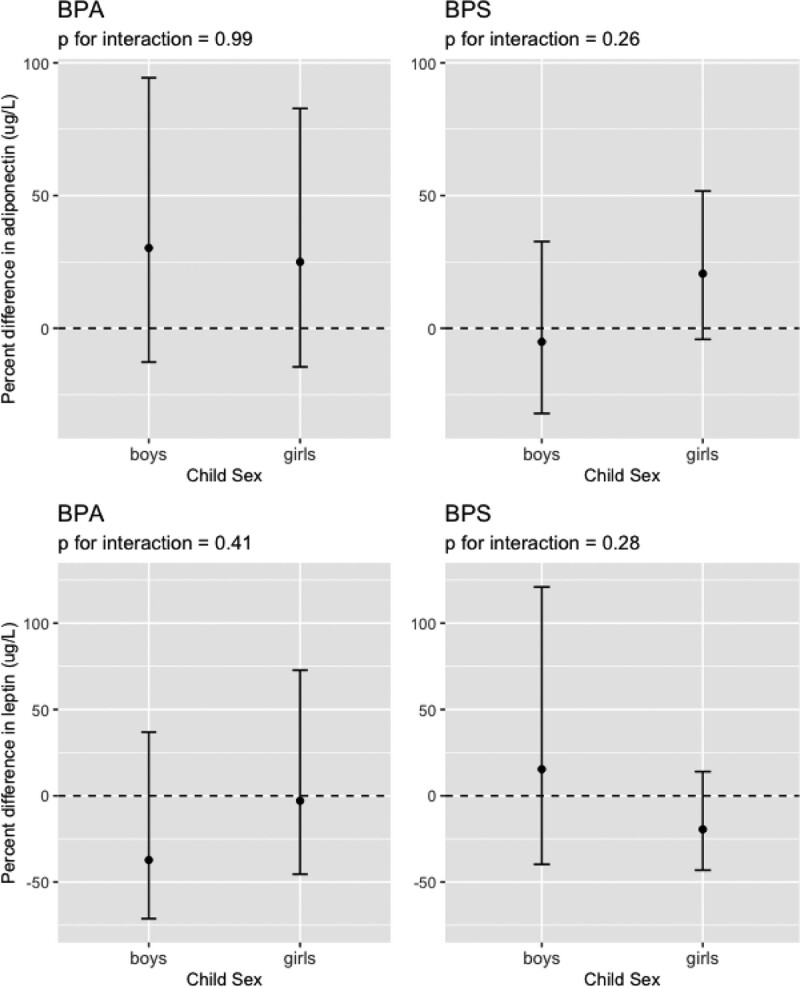
In secondary analyses, we did not observe strong evidence that child sex modified the association of BPA or BPS with child adiposity outcomes, as well as leptin and adiponectin concentrations (Figures 1 and 2, Figure S5; http://links.lww.com/EE/A170). The most notable sex-specific findings were between urinary BPS concentrations and waist circumference at age 8 years; where we observed inverse association in boys (β = −1.2, 95% CI = −4.8, 2.3, N = 96) and a positive association in girls (β = 1.4, 95% CI = −1.6, 4.5, N = 116) (BPS × sex interaction term P-value = 0.12). The stratified estimates did not differ from the sex-specific estimates from the interaction models (Table S4; http://links.lww.com/EE/A170).
Figure 1.
Estimated differences and 95% CIs in BMI z-score, body fat percentage, waist circumference at age 8 years and whole-body fat mass index (FMI) z-score, visceral fat area, android fat percent, and gynoid fat percent at age 12 years for a 10-fold increase in creatinine-standardized child bisphenol A (BPA) and bisphenol S (BPS) urinary concentrations by child sex. BMI z-score was adjusted for child race (Black and other, Non-Hispanic White), maternal education (high school or less, some college, bachelor’s or more) at the 8-year visit, maternal marital status (married, unmarried and cohabitating, unmarried and living alone) at the 8-year visit, insurance (public/uninsured, private) at the 8-year visit, maternal age (continuous, years) at delivery, maternal pre-pregnancy BMI (continuous, kg/m2), fresh fruit and vegetable consumption at the 8-year visit (daily, weekly, monthly). Models for body fat percentage and waist circumference further adjust for child sex (boys, girls) and age (continuous, years). Whole-body FMI z-score was adjusted for child race, maternal education at the 8-year visit, maternal marital status at the 8-year visit, insurance at the 8-year visit, maternal age at delivery, maternal pre-pregnancy BMI, total healthy eating scores (continuous), and physical activity scores (continuous). Visceral fat area, android fat, and gynoid fat were further adjusted for child age (continuous, years).
Figure 2.
Percent difference and 95% CIs in adiponectin and leptin at age 12 years for a 10-fold increase in creatinine-standardized child bisphenol A (BPA) and bisphenol S (BPS) urinary concentrations by child sex. Adiponectin and leptin were adjusted for child race (Black and other race, Non-Hispanic White), maternal education (high school or less, some college, bachelor’s or more) at the 8-year visit, maternal marital status (married, unmarried and cohabitating, unmarried and living alone) at the 8-year visit, insurance (private, public/uninsured) at the 8-year visit, maternal age (continuous, years) at delivery, maternal pre-pregnancy body mass index (continuous, kg/m2), total healthy eating scores (continuous), physical activity scores (continuous), and child age (continuous, years).
We found no evidence of a statistical interaction between BPA and BPS with adiposity outcomes at age 8 years (continuous BPA × BPS interaction term P-values >0.3). However, we found some evidence of a statistical interaction between BPA and BPS with adiposity outcomes at age 12 years. Specifically, we found some evidence of antagonism between BPA and BPS for body fat percent (continuous BPA × BPS interaction P-value = 0.03), FMI z-score (continuous BPA × BPS interaction P-value = 0.02), and gynoid fat at age 12 years (continuous BPA × BPS interaction P-value = 0.01) (Table S5; http://links.lww.com/EE/A170). We further examined these antagonistic associations for FMI z-score, body fat percentage, and gynoid fat at age 12 years using BPA and BPS terciles (Table S6; http://links.lww.com/EE/A170). Although the BPA × BPS tercile interaction term was nonsignificant (P = 0.13) (Table S6; http://links.lww.com/EE/A170), there was evidence of a positive association between BPS and gynoid fat among children in the lowest BPA tercile (3rd vs. 1st difference: 2.6; 95% CI = −0.7, 5.9), but an inverse association of BPS with gynoid fat among children in the highest BPA tercile (3rd vs. 1st difference: −3.5; 95% CI = −8.3, 1.1). We included BPA and BPS in the primary models to estimate the independent associations, which were similar but less precise (Table 2, Table S7; http://links.lww.com/EE/A170).
Adjusting for puberty did not meaningfully change the primary results (Figure S3; http://links.lww.com/EE/A170) or sex-specific associations between BPA and BPS with child adiposity outcomes at age 12 years (Table S8; http://links.lww.com/EE/A170).
Discussion
In this cohort, urinary concentrations of BPA and BPS at age 8 years were not significantly associated with elevated childhood adiposity at age 8 or 12 years. Moreover, child sex did not modify these associations. The associations between BPA and BPS concentrations with adiponectin and leptin at age 12 years were null. We found some evidence for antagonistic interaction between BPA and BPS and some measures of body fat at age 12 years.
The direction of our associations is consistent with those observed in some prior cohort studies examining BPA and adiposity in early childhood or adolescence.19,20 Specifically, in a prior study from this cohort, BPA concentrations in the first 2 years of life were inversely associated with BMI z-scores in 285 children at ages 2 to 5 years.20 Among 298 children from New York City, BPA concentrations at ages 3 and 5 years were inversely associated with differences in BMI z-score from 5 to 7 years.19 However, in the present and prior studies, the 95% CI of these point estimates includes the null value. In contrast, BPA concentrations at age 3 years were positively associated with BMI z-scores at age 7 years among 412 children recruited from the Jiangsu province, China.21 In the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) Study in California, higher BPA concentrations in 290 children at 9 years old were significantly associated with higher BMI z-score, waist circumference, and body fat percentage in a cross-sectional analysis.17 In the Rhea cohort study in Greece, higher BPA concentrations at age 4 years were significantly associated with higher concurrent BMI z-score and waist circumference.18 Another cohort study among 8- to 14-year-old children in Mexico City36 found positive associations between BPA and concurrent BMI z-scores.
The lack of consistency between the associations for BPA and adiposity in previous studies may be owing to inaccurate measures of BPA. There is high within-subject variation in urinary BPA concentrations owing to the short half-life of BPA, episodic nature of exposure, and variations in the timing of urine sample collection relative to exposures during the day.37,38 Thus, relying on one biospecimen for BPA may lead to exposure misclassification, attenuated effect estimates, and inconsistent estimates across studies.
The differences in adjustment for confounding may also contribute to the lack of consistency. Residual confounding from social and dietary factors associated with both BPA exposure and adiposity may be present in the previous studies. Many food frequency questionnaires may not adequately incorporate potential sources of BPA exposure that are also associated with adiposity, such as canned foods, so adjusting for measures of dietary exposure may still lead to confounded associations between BPA or BPS and adiposity in cross-sectional analyses. Future studies should use multiple biomarker measures and adjust for specific dietary sources of BPA.
In this study, we did not find sex-specific effects for BPA. However, in a sample of 298 boys, ages 9–11 years, in the Environment and Childhood (INMA) cohort study in Granada study in Spain, urinary BPA was significantly associated with higher BMI z-scores in a cross-sectional analysis.16 Additionally, the prior HOME study found an inverse association between childhood urinary BPA concentrations and early childhood BMI z-scores among girls and a positive association among boys.20 A study in the Jiangsu province, China, found positive associations between BPA and BMI z-scores for both boys and girls.21 Though no cohort study found significant evidence of effect modification by sex.
We found some modest positive associations between BPS concentrations and some adiposity outcomes at ages 8 and 12 years, but the 95% CIs of our estimates included the null value. This result is somewhat consistent with a cross-sectional study using NHANES data reporting that BPS was associated with higher odds of obesity in children aged 6 to 17 years.15 In contrast, the Generation R cohort study in the Netherlands found that BPS concentrations at age 6 years were inversely associated with BMI at age 10 years.22
The difference in the direction and magnitude of associations of BPA and BPS with adiposity could be owing to differences in the biological pathways they affect. For instance, BPS may have more specific effects on adipogenesis and lipid accumulation than BPA through differentially expressed genes in preadipocytes.39 However, it is also possible that differential patterns of confounding or exposure misclassification could produce the observed results.
Although previous studies have found that BPA inhibits adiponectin release by antagonizing PPARγ,40 we found null associations of BPA and BPS with adiponectin and leptin at age 12 years. Similarly, in the Rhea cohort study, there were null associations of BPA at age 2.5 years with leptin and adiponectin at age 4 years.18 Fewer studies have examined the effects of BPS on adipocytokine expression; however, one study suggests that human adipose tissue treated with BPS does not result in altered expression of adiponectin or leptin.41 Future studies could examine other cardiometabolic or metabolic endpoints, including glucose-insulin homeostasis and lipid concentrations.
Our study contributes to the emerging literature on the health effects of BPA substitutes. However, this study has some limitations. First, we used a single urine sample to assess BPA and BPS exposure, which is subject to within-person variability of BPA and BPS concentrations. Thus, a single spot urine measurement may cause exposure misclassification and bias our results to the null. Second, our sample size was modest, which may have reduced our statistical power and precision to completely rule out the presence of any associations. Additionally, we cannot account for residual confounding from dietary patterns (e.g., packaged versus fresh food) and the use of consumer goods such as toys and personal care products.42–44 However, we adjusted for 2010 Healthy Eating scores, which assess intake of fruits, vegetables, whole grains, and proteins.32 Further, we did not adjust for other potential chemical obesogens correlated with BPA or BPS, such as per- and polyfluoroalkyl Substances (PFAS) and phthalates,45 but it seems unlikely that this would make our results stronger. We did not have BPS concentrations available during gestation or early childhood. Although BPA has been used in consumer goods since the 1950s, BPS only appeared in thermal paper receipts in 2006.46 Since the introduction of BPS in plastics, thermal paper, and personal care products; BPS has been increasingly detected in urine samples in multiple studies.10,47 Thus, exposure is likely to have been low or nonexistent before age 8 years in our cohort. Finally, we did not correct for multiple testing, which may increase the number of false-positive results.
Despite these limitations, there are several strengths to our study. These include using both cross-sectional and prospective analyses to examine the associations of BPA and BPS concentrations at age 8 years with adiposity at ages 8 and 12 years. Moreover, we used highly detailed measures to assess body composition using DXA at age 12 years and adipocytokine biomarkers.48 Finally, we were also able to account for important confounders, including prepregnancy BMI, physical activity, and diet quality.
Conclusion
We did not find evidence that mid-childhood urinary BPA or BPS concentrations were associated with mid-childhood or adolescence adiposity among children enrolled in the HOME Study. Given the increasing use of BPA substitutes, future studies with larger sample sizes and multiple exposure measures should examine the extent and health effects of these compounds.
Acknowledgments
We are grateful to our participants for the time they have given to our study and to laboratory staff who performed chemical analyses.
Supplementary Material
Footnotes
Published online 20 December 2021
J.M.B. served as an expert witness in litigation related to perfluorooctanoic acid contamination in drinking water in New Hampshire. Any funds he received from this arrangement were/are paid to Brown University and cannot be used for his direct benefit (e.g., salary/fringe, travel, etc.). The remaining authors declare that they have no conflicts of interest with regard to the content of this report.
The HOME Study was funded by National Institutes of Environmental Health Sciences grants R01 ES027224, R01 ES025214, R01 ES028277, R01 ES030078, R01 ES024381, R01 ES020349, P01 ES011261, R01 ES014575, and R01 ES015517.
Interested investigators should contact J.M.B. (joseph_braun_1@brown.edu) and K.Y. (kimberly.yolton@cchmc.org) to discuss opportunities for collaboration.
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Abarca-Gómez L, Abdeen ZA, Hamid ZA, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999-2016. Pediatrics. 2018;141:e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2015–2016. NCHS Health E-Stats. 2018. Available at: https://www.cdc.gov/nchs/data/hestat/obesity_child_15_16/obesity_child_15_16.htm. Accessed 12 May 2021. [Google Scholar]
- 4.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011;35:891–898. [DOI] [PubMed] [Google Scholar]
- 5.Janesick AS, Blumberg B. Obesogens: an emerging threat to public health. Am J Obstet Gynecol. 2016;214:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikołajewska K, Stragierowicz J, Gromadzińska J. Bisphenol A - Application, sources of exposure and potential risks in infants, children and pregnant women. Int J Occup Med Environ Health. 2015;28:209–241. [DOI] [PubMed] [Google Scholar]
- 7.Desai M, Ferrini MG, Jellyman JK, Han G, Ross MG. In vivo and in vitro bisphenol A exposure effects on adiposity. J Dev Orig Health Dis. 2018;9:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoene M, Dzika E, Gonkowski S, Wojtkiewicz J. Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than bisphenol A: a literature review. Nutrients. 2020;12:E532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochester JR, Bolden AL. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect. 2015;123:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu LH, Zhang XM, Wang F, et al. Occurrence of bisphenol S in the environment and implications for human exposure: a short review. Sci Total Environ. 2018;615:87–98. [DOI] [PubMed] [Google Scholar]
- 11.Viñas R, Watson CS. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect. 2013;121:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed S, Atlas E. Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation. Int J Obes (Lond). 2016;40:1566–1573. [DOI] [PubMed] [Google Scholar]
- 13.Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander W, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from Human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113–1121. [DOI] [PubMed] [Google Scholar]
- 15.Liu B, Lehmler HJ, Sun Y, et al. Association of Bisphenol A and its substitutes, Bisphenol F and Bisphenol S, with obesity in United States Children and Adolescents. Diabetes Metab J. 2019;43:59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustieles V, Casas M, Ferrando-Marco P, et al. Bisphenol A and adiposity measures in peripubertal boys from the INMA-Granada cohort. Environ Res. 2019;173:443–451. [DOI] [PubMed] [Google Scholar]
- 17.Harley KG, Aguilar Schall R, Chevrier J, et al. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect. 2013;121:514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vafeiadi M, Roumeliotaki T, Myridakis A, et al. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ Res. 2016;146:379–387. [DOI] [PubMed] [Google Scholar]
- 19.Hoepner LA, Whyatt RM, Widen EM, et al. Bisphenol A and adiposity in an inner-city birth Cohort. Environ Health Perspect. 2016;124:1644–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun JM, Lanphear BP, Calafat AM, et al. Early-life bisphenol a exposure and child body mass index: a prospective cohort study. Environ Health Perspect. 2014;122:1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo J, Zhang J, Wu C, et al. Urinary bisphenol A concentrations and adiposity measures at age 7 years in a prospective birth cohort. Chemosphere. 2020;251:126340. [DOI] [PubMed] [Google Scholar]
- 22.Silva CCV, Jaddoe VWV, Sol CM, et al. Phthalate and bisphenol urinary concentrations, body fat measures, and cardiovascular risk factors in dutch school-age children. Obesity (Silver Spring). 2021;29:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun JM, Buckley JP, Cecil KM, et al. Adolescent follow-up in the Health Outcomes and Measures of the Environment (HOME) study: cohort profile. BMJ Open. 2020;10:e034838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun JM, Kalloo G, Chen A, et al. Cohort Profile: the Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 2017;46:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, Kramer JP, Calafat AM, Ye X. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;944:152–156. [DOI] [PubMed] [Google Scholar]
- 26.2000 CDC growth charts for the United States. Public Health Service, Centers for Disease Control and Prevention, National Center for Health Statistics; 2002. Available at: https://www.cdc.gov/growthcharts/. Accessed 3 March 2021. [Google Scholar]
- 27.Weber DR, Moore RH, Leonard MB, Zemel BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freemark M. Determinants of risk for childhood obesity. N Engl J Med. 2018;379:1371–1372. [DOI] [PubMed] [Google Scholar]
- 29.Stacy SL, Eliot M, Calafat AM, et al. Patterns, variability, and predictors of urinary bisphenol A concentrations during childhood. Environ Sci Technol. 2016;50:5981–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kowalski K, Crocker P, Donen R, et al. The Physical Activity Questionnaire for Older Children (PAQ-C) and Adolescents (PAQ-A) Manual. College of Kinesiology, University of Saskatchewan; 2004. [Google Scholar]
- 31.Population Ratio Method. EGRP/DCCPS/NCI/NIH. Available at: https://epi.grants.cancer.gov/hei/population-ratio-method.html. Accessed 5 May 2020.
- 32.Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yayah Jones NH, Khoury JC, Xu Y, et al. Comparing adolescent self staging of pubertal development with hormone biomarkers. J Pediatr Endocrinol Metab. 2021;34:1531–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckley JP, Doherty BT, Keil AP, Engel SM. Statistical approaches for estimating sex-specific effects in endocrine disruptors research. Environ Health Perspect. 2017;125:067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darfour-Oduro SA, Buchner DM, Andrade JE, Grigsby-Toussaint DS. A comparative study of fruit and vegetable consumption and physical activity among adolescents in 49 Low-and-Middle-Income Countries. Sci Rep. 2018;8:1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang TC, Peterson KE, Meeker JD, et al. Bisphenol A and phthalates in utero and in childhood: association with child BMI z-score and adiposity. Environ Res. 2017;156:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem Res Toxicol. 2002;15:1281–1287. [DOI] [PubMed] [Google Scholar]
- 38.Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect. 2011;119:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boucher JG, Gagné R, Rowan-Carroll A, Boudreau A, Yauk CL, Atlas E. Bisphenol A and Bisphenol S induce distinct transcriptional profiles in differentiating human primary preadipocytes. PLoS One. 2016;11:e0163318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: implications for the metabolic syndrome. Mol Cell Endocrinol. 2009;304:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed F, Sarsenbayeva A, Katsogiannos P, Aguer C, Pereira MJ. The effects of bisphenol A and bisphenol S on adipokine expression and glucose metabolism in human adipose tissue. Toxicology. 2020;445:152600. [DOI] [PubMed] [Google Scholar]
- 42.Lu S, Yu Y, Ren L, Zhang X, Liu G, Yu Y. Estimation of intake and uptake of bisphenols and triclosan from personal care products by dermal contact. Sci Total Environ. 2018;621:1389–1396. [DOI] [PubMed] [Google Scholar]
- 43.Sharpe RM, Drake AJ. Obesogens and obesity—An alternative view? Obesity 2013;21:1081–1083. [DOI] [PubMed] [Google Scholar]
- 44.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24:139–177. [DOI] [PubMed] [Google Scholar]
- 45.Braun JM. Early life exposure to endocrine disrupting chemicals and childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glausiusz J. Toxicology: the plastics puzzle. Nature. 2014;508:306–308. [DOI] [PubMed] [Google Scholar]
- 47.Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary concentrations of Bisphenol A and three other Bisphenols in convenience samples of U.S. Adults during 2000-2014. Environ Sci Technol. 2015;49:11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindsay RS, Hanson RL, Roumain J, Ravussin E, Knowler WC, Tataranni PA. Body mass index as a measure of adiposity in children and adolescents: relationship to adiposity by dual energy x-ray absorptiometry and to cardiovascular risk factors. J Clin Endocrinol Metab. 2001;86:4061–4067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.