Abstract
Background and Aims
Balanophoraceae is one of the most bizarre and biologically interesting plant clades. It groups species with peculiar features that offers an opportunity for investigating several aspects of parasite plant development and morphogenesis. We analysed the development and the mature vegetative body of Lathrophytum peckoltii Eichler, focusing on the formation of the host–parasite interface. Additionally, we analysed how this parasitic interaction causes modifications to the anatomy of Paullinia uloptera Radlk and Serjania clematidifolia Cambess host roots.
Methods
Vegetative bodies of the parasite at different developmental stages were collected while infesting the roots of Sapindaceae vines. Non-parasitized host roots were also collected for comparison. Light, epifluorescence, confocal and scanning electron microscopy were used for the analysis.
Key Results
The initial cells of the vegetative axis divide repeatedly, originating a parenchymatous matrix, which occupies the space from the cortex to the vascular cylinder of the host’s root. In the peripheral layers of the matrix, located near the xylem of the host’s roots, a few cells initiate the process of wall lignification, originating the parasitic bundle. The vascular cambium of the host’s root changes the division plane and becomes composed of fusiform initials, forming the vascular bundle. The vegetative axis presents a dermal tissue similar to a phellem, a parenchymatous matrix and a vascular system with different origins.
Conclusion
The parasite reproduces by endophytic development, in a manner similar to that observed for endoparasites. The strategy of late cell differentiation could aid the parasite in avoiding early detection and triggering of defence responses by the host. This development causes changes to the host root cambial activity, leading to the establishment of direct, vessel to vessel connection between host and parasite. We associate these changes with the cambium modularity and an influx of parasite-derived hormones into the host cambium.
Keywords: Balanophoraceae, endophyte, haustorium, holoparasite, Lathrophytum peckoltii Eichler, parasitic plant, plant development, Santalales, Sapindaceae, tribe Paullineae, woody vines
INTRODUCTION
Balanophoraceae is one of the most bizarre and biologically interesting plant clades (Kuijt, 1969). Species in this family are devoid of chlorophyll and obtain all of their resources by living as parasites upon the roots of a wide range of host species (Hansen, 2015). Similar to other parasitic plants, interface with a host is promoted by the haustorium, a specialized organ that acts in the attachment, penetration and connection to the host vasculature (Joel et al., 2013). Unlike most parasites, however, the haustorium of Balanophoraceae species often develops into large, underground tuber-like structures (Kuijt and Bruns, 1987; Cardoso, 2014). These large attachment structures contrast with the remarkable reductions, modifications and losses observed in species in this family at both the molecular and the morphological level (Su et al., 2015; Nickrent, 2020). Indeed, such reductions and losses have hampered robust phylogenetic positioning and led to persisting controversies regarding the status of Balanophoraceae sensu lato (The Angiosperm Phylogeny Group, 2016; Nickrent, 2020).
On the other hand, the combination of such peculiar features offers an intriguing opportunity for investigating several aspects of plant development and morphogenesis (Gonzalez and Mauseth, 2010). For instance, nearly all Balanophoraceae species lack proper roots, stems, leaves, apical meristems and a true epidermis, and even stomata could be entirely absent in the family (Kuijt and Bruns, 1987; Kuijt and Dong, 1990; Mauseth et al., 1992; Kuijt and Hansen, 2015). Among the constitutive organs, flowers are some of the smallest among angiosperms (Eberwein et al., 2009), and the tuber-like structure that constitutes the host–parasite interface forms a chimeric structure in at least three genera (Mangenot, 1947; Hsiao et al., 1995). This type of interface, in which host and parasite cells are mixed in the same vascular bundle, contrasts with a simpler, but no less puzzling, type of interface in which there is clear separation between parasite and host vascular tissues (Moore, 1940; Holzapfel, 2001).
The literature also describes three types of parasite–host interface (Fig. 1A): (1) simple – when the vessel elements from both species are in physical contact; (2) intermediate – when a ‘woodrose’ is formed by the proliferation of secondary xylem in the host; and (3) complex – when parasitic cells are mixed in the middle of the host’s conductive tissue (Gonzalez and Mauseth, 2010).
Fig. 1.
(A) Schematic representation of Balanophoraceae interface types as described in the literature. Type 1: the interface between host and parasite is sinuous, and there is no mixture of host and parasite cells. Type 2: woodrose, a coralloid interface caused by localized proliferation of host wood. The interface is inside of the host’s body. Type 3: elaborate chimeral interfaces, by composite bundles growing within the tuber with parasite transfer cells located within the host vascular tissue. (B) Development of Balanophoraceae in the host plant as described in the literature. Stage 1: parasite endosperm cells produce tubular, slender extensions for anchorage of the fruit to the young host rootlets. Stage 2: by repeated divisions of parasite embryo, the tuber emerges out of the pericarp above the ground and the cells of the central cylinder of the host rootlet become active in the region of contact. Stage 3: the tuber continues to grow at the same time as the host’s vascular tissue enters the tuber and originate composite bundles inside which parasite transfer cells are located. Layout inspired by Shivamurthy et al. (1981), Mauseth et al. (1992), Hsiao et al. (1993, 1994, 1995) and Gonzalez and Mauseth (2010).
The vegetative body development is understudied for Balanophoraceae. Study has been detailed for Balanophora abbreviata Blum and B. fungosa J.R.Forst. & G.Forst. ssp. indica (Arn.) Hansen var. indica (Shivamurthy et al., 1981; Johri et al., 1992) (Fig. 1B). Lathrophytum peckoltii Eichler is the sole species of the monospecific genus Lathrophytum, subfamily Lophophytoideae, Balanophoraceae s.str., Santalales (Kuijt and Hansen, 2015; Su et al., 2015). Lathrophytum peckoltii is a root holoparasite, according to Těšitel (2016), following the functional characteristics known for the family, with neotropical distribution (Delprete, 2004; Cardoso, 2014; Santamaría-Aguilar and Fernández, 2017). Based on the excavation for the identification and sampling of L. peckoltii hosts in the Atlantic Forest, Cardoso (2014) refers the parasitism in roots of lianas from Sapindaceae, Paullinia racemosa Wawra and Paullinia sp. as hosts.
The tuber’s anatomy and the alterations resulting from parasitism in the host’s roots were observed between Lophophytum leandri Eichler and Parapiptadenia rigida (Benth.) Brenan, and between L. mirabile subsp. bolivianum (Wedd.) B. Hansen and Anadenanthera colubrina var. cebil (Griseb.) Altschul (Gonzalez and Mauseth, 2010; González and Sato, 2016). Both hosts are trees belonging to the Fabaceae.
There are no references in the literature to anatomical studies of liana roots as hosts to species of Balanophoraceae. The roots in lianescent Sapindaceae present the typical characteristics of the lianescent vascular syndrome, such as vessel dimorphism and banded parenchyma (Bastos et al., 2016). This can lead to different responses from the host whenever infested by Balanophoraceae. Therefore, this study aims to describe the anatomy of the development of the vegetative body in L. peckoltii, detailing the formation of the parasite–host interface. Furthermore, we aim to analyse the changes resulting from parasitism in the roots of Paullinia uloptera Radlk and Serjania clematidifolia Cambess, paving the way for future studies in the ecology and evolution in root holoparasitic plants.
MATERIALS AND METHODS
Plant material
Specimens of L. peckoltii and its host roots were manually excavated during two field expeditions. The first expectation was carried out in December 2012 at Santa Maria Madalena, Rio de Janeiro, Brazil (21°57′43′′S, 42°0′54′′W), and resulted in the collection of 27 samples at different developmental stages. At this location, the parasite was sampled on roots of P. uloptera (Sapindaceae). The second expedition took place in November 2016 at the Parque Natural Municipal da Prainha, Rio de Janeiro, Brazil (23°2′39′′S, 43°30′48′′W), and resulted in the collection of three samples fully developed with inflorescences, which were on roots of S. clematidifolia (Sapindaceae). On both occasions, we also collected non-parasitized host roots, which ranged from 3 to 5 mm in diameter. Branches of host species were additionally collected as voucher material and incorporated into the collections of the Jardim Botânico do Rio de Janeiro Herbarium (RB 616545; RB 668557).
Sample preparation
During fieldwork, small vegetative axes and fragments of large specimens were fixed in a solution of 2.5 % glutaraldehyde and 4.0 % formaldehyde in sodium cacodylate buffer solution (1 L, 0.05 mol in pH 7.2) (Barros and Miguens, 1998). Fully developed vegetative bodies were fixed in formaldehyde, acetic acid, 70 % ethanol (FAA) and later stored in 70 % ethanol (Bukatsch, 1972). Non-parasitized roots were also fixed in FAA 70 and analysed to provide a comparison with parasitized host roots. During preparation, samples were arranged in transversal and tangential positions, based either on the insertion of the inflorescence in relation to the vegetative body, or on the position of the host root in relation to the parasite. A total of 30 specimens, at least five of each different stage of vegetative development, were analysed.
Light field microscopy
Samples were dehydrated in a graded ethanol series and embedded in methacrylate resin (Historesin, Leica, Nussloch, Heidelberg, Germany) (Gerrits and Smid, 1983), sectioned with a Leica RM2245 semi-automated rotary microtome (Wetzlar, Germany) (5–9 µm average thickness) and stained with 0.05 % toluidine blue (Feder and O’Brien, 1968). Larger samples were embedded in 1500 polyethylene glycol (Rupp, 1964), sectioned with a Leica SM2010 R sliding microtome (Wetzlar, Germany) (15–30 μm average thickness) with adhesive tape and a solution of polystyrene foam (Barbosa et al., 2010), and finally double stained with 1 % Astra blue and 1 % safranin (Bukatsch, 1972).
For histochemical tests, unstained sections of the layer of dermal tissue, parenchymatous tissues and the vascular system of L. peckoltii were stained using the following reagents: 5 % Sudan III and 5 % Sudan IV (Foster, 1942), for the detection of lipids; 10 % iron chloride (Johansen, 1940), for the detection of phenolic compounds; Lugol’s iodine solution (Johansen, 1940), for the detection of starch grains; and 18 % acid phloroglucinol (Sass, 1958), for the detection of lignin. All anatomical slides were analysed and imaged using an Olympus BX-50 optical microscope coupled with a DP73 digital camera (Olympus, Melville, USA).
Epifluorescence and confocal microscopy
For the analysis of autofluorescence, semi-permanent slides were mounted in 50 % glycerin water with sections obtained from both small and large samples (Purvis et al., 1964). The autofluorescence documentation was conducted with an Olympus DP73 digital camera, attached to the Olympus BX50 epifluorescence microscope.
Additionally, sections obtained from both small and large samples were also prepared for confocal laser scanning microscopy. To do so, sections were washed with phosphate buffer solution for 1 h to remove autofluorescence. Auramine-O and white calcium fluoride fluorophores were used. Slides were analysed by confocal laser scanning microscopy using a 450–480 nm laser to stimulate the auramine-O fluorophore, which emitted fluorescence at 515 nm, and a 360–370 nm laser to stimulate the white calcium fluoride fluorophores emitting at 420 nm (Herth and Schnepf, 1980). The images were captured by direct acquisition with a 3.36 μm Piezo stage Z, generating 15 optical sections in the LAS AF LITE software (version 2.6.0, Leica Microsystems) in a confocal laser scanning microscope Leica TCS SPE (Wetzlar, Germany).
Scanning electron microscopy
Paradermic sections of the vegetative body dermal tissue were dehydrated in an ascending ethanol series (Barros and Miguens, 1998), and critical point dried with the use of CO2 in a Leica EM CPD030 dryer. Sections were then placed in a stub and coated with 20 nm of gold using a Emitech K550X sputter coater (Quorum Technologies Ltd, UK). Samples were observed using a Zeiss EVO 040 (Carl Zeiss Microscopy, Germany) scanning electron microscope.
Analysis and terminology
The vegetative body analysis and description of the surface of L. peckoltii under the scanning electron microscope followed Koch et al. (2009). The anatomical description followed the terminology used by Gonzalez and Mauseth (2010) and Teixeira-Costa and Ceccantini (2018). The analysis and description of host roots followed the IAWA Committee (1989) and Angyalossy et al. (2016).
RESULTS
Lathrophytum peckoltii vegetative body morphology
The vegetative body had a brown colour and a sub-spherical shape with a glabrous, warty surface. Parasitic plants were up to 16 cm tall from the base of the host–parasite interface to the apex of the inflorescence (Fig. 2A). Each vegetative body produced both runners, which were thin and non-persistent, and a single inflorescence, which emerged above ground level. Seed germination and seedling development were not observed during fieldwork excavations. Nevertheless, several vegetative bodies of the parasite were found on the roots of a single host plant (Fig. 2B), thus increasing the possibility of capturing multiple developmental stages in the sampling. Inflorescences were observed emerging from vegetative bodies of different sizes, suggesting that the size of each underground body was not a reliable hallmark for the progression of parasite growth. A detailed understanding of parasite development was only accomplished through the analyses of multiple vegetative structures of the same overall size.
Fig. 2.
Latrophytum peckoltii parasitizing the roots of different host species: (A) in the field site, on the roots of S. clematidifolia (Sapindaceae) and (B) on the roots of P. uloptera (Sapindaceae). (C) Longitudinal section through an infested host root. Note the different developmental stages of L. peckoltii, the parasite cells demarcated by the white outline. Pi = parasite inflorescence; Hr = host root; white arrow = vegetative axis.
Development of the parasite vegetative body
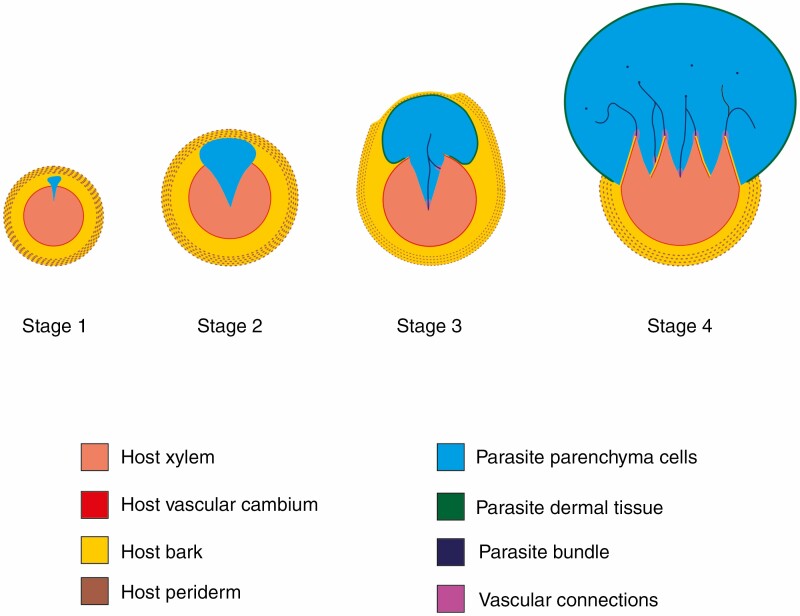
During the morphological examination of the host plant root system, multiple small and warty protuberances were detected in association with fine roots. Anatomical sections revealed these protuberances to be composed of parasite cells shrouded by parasite bark (Fig. 2C). Distinction between parasite and host parenchyma cells was based on the initial analysis of non-parasitized plants (see Supplementary Information Files) and on the presence of large and prominent nuclei on parasitic cells. This observation then motivated us to analyse the finest host roots available, which were about 1 mm across. In these root segments, the vegetative body of L. peckoltii was observed as clusters or filaments of parenchyma cells embedded within host tissues (Fig. 3). These groupings of parenchyma cells were dispersed among cells of the host dermal and xylem tissues (Fig. 4A, B). We identified these cell groupings as the earliest developmental stage of the parasite body.
Fig. 3.
Schematic representation of endophytic development of L. peckoltii. Stage 1: clusters of parasite parenchyma cells embedded within host tissues. Stage 2: the parasite’s parenchyma cells divide and form a wedge-shaped parenchymatous mass within host tissues. Stage 3: the ruptures of the periderm starts and cambium cells of the host root change their plane of division. The differentiation of the dermal parasite system and parasite bundles begins. Stage 4: the new vegetative body becomes exposed, and there is an increase of the development of the vascular system of the host and parasite bundles and establishment of multiple direct connections.
Fig. 4.
Cross-sections of infested host roots. (A and B) Parenchyma cells of L. peckoltii (white outline) interspersed with host xylem and bark cells. (C and D) Median section through the vegetative axis of the parasite at early developmental stages. (D) Autofluorescence of the host root phellem and xylem. Hr = host root; Hpe = host phellem; Pp = parasite parenchyma.
The development of parasitic cell groupings was observed to culminate in the formation of new vegetative bodies, which indicated that the ontogenesis of the vegetative body has an initial endomorphic development. The establishment of a new vegetative body of L. peckoltii was then initiated by cell division. This created a wedge-shaped parenchymatous mass that occupied part of both the cortex and the vascular cylinder of the host root, which then became initially lobed (Fig. 4C). At this developmental stage, the cells of L. peckoltii exhibit no sign of autofluorescence (Fig. 4D).
Following development of the vegetative body, ruptures in the host periderm were observed, leading to exposure of the host root cortex (Fig. 5A). At this stage, the peripheral layers of the parasitic parenchymatous tissue were observed to differentiate into a rudimentary dermal tissue covering the vegetative body (Fig. 5B). The fully differentiated dermal system was easily recognized by the autofluorescence of its suberized cell walls (Fig. 5C). We did not observe phellogen differentiation in the parasite dermal layers. At the same time, in the innermost region of the parasite parenchymatous tissue, next to the host xylem, the process of cell wall lignification was observed to begin in some of the parasite cells (Fig. 5D). These cells differentiated from cells with non-lignified walls (Fig. 5E) into small vessel elements with helicoid to scalariform pits, secondary walls with ingrowths, and simple perforation plates (Fig. 5F). Simultaneous with the enlargement of the parasite vegetative body, the host root cambium zone was detected to change its plane of cell division, from predominantly periclinal to predominantly anticlinal to the root surface (Fig. 5D).
Fig. 5.
Cross-section of infested host roots at the median section of the parasite vegetative axis. (A) Initial developmental of L. peckoltii dermal tissues and rupture of the host root periderm (Hpe) exposing (arrow) cortex cells (Hc). (B) Detail showing the pattern of periclinal cell divisions (arrow) in the dermal tissue of L. peckoltii below the host cortex (Hc) and phellem (Hpe). (C) Autofluorescence of the cells in both the host root phellem (Hpl) and the dermal tissue of the parasite (arrow). (D) Early stages of vascular system differentiation in L. peckoltii. Note the change in host cambial activity (dotted line). (E) Calcofluor fluorescence outlining the cellulose structure of parasite vessel elements prior to lignification. (F) Auramin O fluorescence indicating ingrowths in two vessel elements of L. peckoltii. Hr = host root; Pp = parasite parenchyma.
Ontogenesis of parasite–host vascular connections
The continuous growth of the new vegetative body caused it to become exposed to the outside as the host dermal tissue was shed (Fig. 6A). At this stage, as the host secondary growth progresses, the formation of a vascular tissue exclusively composed of fusiform initials becomes evident. The absence of radial initials leads to the development of new vascular tissue without ray cells (Fig. 6A). In addition to the lack of ray cells, this newly formed host vascular tissue was observed to differentiate towards the parasite vegetative body (Fig. 6B). As the host vasculature branched out among cells of the vegetative body, parasite vessel element differentiation increased, leading to the establishment of multiple direct connections between vessel elements of the two plants (Fig. 6C). Sieve tubes were not detected among the parasite vascular cells, and only parenchyma cells were observed near the host phloem tissue (Fig. 6D). For a comparison with the anatomy of non-parasitized host roots, see Supplementary data Fig. S1.
Fig. 6.
Longitudinal section of the host root in the median section of the parasite vegetative axis. (A) Parasite dermal tissue (Pdt) exposed by the growth of the parasite parenchymal matrix. Note the change in host cambium modularity (white outline). (B) Mature vegetative axis with both parasite and host vascular cells. Note a parenchymatous sheath of parasite cells (arrow) around the host xylem; note also direct vessel–vessel connections between parasite and hosts (insert). (C) Terminal portion of the host vascular tissue within the parasite vegetative axis forming direct vessel–vessel connections between the two plants. (D) Terminal portion of the host phloem (Hph) within the vegetative axis of the parasite, among cells of the parenchymatous matrix. The insert shows sieve tube elements at greater magnification. Hr = host root; Hx = host xylem; Pb = parasite vascular bundle; Pp = parasite parenchyma.
Fully developed vegetative body of L. peckoltii
At the fully developed stage, the vegetative body of L. peckoltii is connected to the host root via a single fragile point (Fig. 6B). The host vascular tissue extends and continues to differentiate deep into the parasite body, leading to a mesh of tissues from both plants (Fig. 6). The vegetative body of the parasite showed dermal tissue similar to a rudimentary phellem, with suberized cells (Fig. 7A). Scanning electron microscopy showed that the outer layer presents an irregular surface with polygonal cells, a convex outer periclinal wall and straight anticlinal walls (Fig. 7B). The parenchymatous matrix that comprised most of the vegetative body showed copious amounts of starch and phenolic compounds (Fig. 7C, D). Immersed in this parenchymatous matrix, we also detected two types of meristems: (1) the reproductive meristem (Fig. 7E); and (2) the meristem of the runner (Fig. 7F).
Fig. 7.
(A and B) Parasite dermal tissues (Pdt). (A) Multiple dermal tissue layers seen in cross-section. (B) Irregular surface of the parasite composed of polygonal cells observed via scanning electron microscopy. Note the insert showing cells at greater magnification. (C) Positive reaction to Lugol’s solution detecting the presence of starch granules in black. (D) Positive reaction to iron chloride, detecting the presence of phenolic compounds in brown to black. (E–G) Endogenous meristems in the parasite vegetative axis. (E) Longitudinal section of the inflorescence meristem. (F) Longitudinal section of the runner apical meristem. Note the insert showing the runner vascular system composed of vessel elements (arrow) and parenchyma cells with a reduced lumen. (G) Cross-section of the runner, composed of epidermis (arrow), cortical parenchyma cells (C) and a variable stele (Vs). Note the insert showing vessel elements (arrows) at greater magnification.
In the reproductive meristem, which later differentiated into the inflorescence, we also observed the formation of individual flower meristems (Fig. 7E). The meristem that will differentiate into the runner showed a tunica–corpus zonation at its apex, with the tunica being composed of one or two cell layers. Below and parallel to the protoderm of this apical meristem, procambium cells with initial differentiation into vessel elements were also observed (Fig. 7F). When fully differentiated, the runner showed an epidermis with cutinized outer walls, cortex composed of parenchyma cells and a variable stele (Fig. 7F). In certain regions, we were able to observe the stele with five xylem poles. The vessel elements of the runner were similar to those observed in the parasite xylem (Fig. 7G).
DISCUSSION
Members of the Balanophoraceae (sensu lato) are often referred to as being unique among parasitic plants for their haustoria composed of an intricate mixture of host and parasite tissue (Kuijt, 1969; Hansen, 1980). These complex structures tend to be described in their fully developed form, probably due to difficulties in sampling early developmental stages. The notable exception to this trend is the genus Balanophora, in which a few species have been analysed from seedling to mature plants (Fagerlind, 1945; Shivamurthy et al., 1981; Weber and Sunaryo, 1990; Johri et al., 1992). Apart from this genus, Dactylanthus taylorii Hook. f. appears to be the only other species for which vegetative body development has been detailed. Here we describe this process for L. peckoltii for the first time by unearthing its endophytic origin and clonal reproduction.
Our results corroborate a hypothesis originally proposed by Kuijt (1969), in which the author suggested that Balanophoraceae could reproduce clonally via endophyte proliferation and differentiation. The suggestion was based on the work of Beccari (1869), who first observed endophyte cells of Balanophora reflexa Becc. in a position approx. 3 cm away from the primary site of infestation. It is noteworthy that while all parasitic plants develop an endophyte, which corresponds to part of the haustorium immersed in host tissues (Heide-Jørgensen, 2008), certain species show extensive endophyte growth leading to a ramifying and anastomosing system (Kuijt, 1977). This is the case of parasites such as Cytinaceae and Rafflesiaceae, in which the external plant body is restricted to reproductive structures derived entirely from the endophytic system (De Vega et al., 2007; Nikolov et al., 2014). Aptly called endoparasites (Těšitel, 2016), these species live as fungal-like filaments of cells embedded within their hosts and only become visible when flowers/inflorescences emerge from the endophyte (Nikolov and Davis, 2017).
Considering the characteristics described above, the ability of L. peckoltii to reproduce vegetatively through endophyte proliferation and differentiation, forming a new underground tuberous body, differs from the lifestyle of endoparasites. Nevertheless, similarities in the developmental process are noteworthy. Comparably with endoparasites that retain embryonic characteristics and delay connection to host vessels (Teixeira-Costa, 2021), L. peckoltii was observed to differentiate vessel elements and tap into the host xylem only at a stage when the new vegetative body begins emerging from within the host. The strategy of late cell differentiation could aid the parasite in avoiding early detection and triggering of defence responses by the host (Nikolov et al., 2014). This form of furtive growth could be related to the severe modifications L. peckoltii, as well as most endoparasites that cause morphogenesis changes to the host vascular cambium. In endoparasites that show early cell differentiation, such as Tristerix aphyllus (D.C.) Barlow & Weins (Loranthaceae) and Viscum minimum Harvey (Santalaceae), host cambium stimulation is not observed (Mauseth et al., 1984; Mauseth and Rezaei, 2013).
Changes in host cambium activity are also common in several other Balanophoraceae–host associations (Shivamurthy et al., 1981; Weber, 1987; Mauseth et al., 1992; Hsiao et al., 1993; Holzapfel, 2001; Gonzalez and Mauseth, 2010). Here, in the case of L. peckoltii, we associate these changes in cambium modularity (Tomescu and Groover, 2019) with the pressure exerted by the growing endophyte at the beginning of the host root secondary growth. At the same time, an influx of parasite-derived hormones into the host cambium could also lead to the pattern of alterations observed here. In other associations, cytokinins produced by the parasite were shown to control host root/stem anatomy and development (Hu et al., 2017; Spallek et al., 2017). Manipulation of host vascular differentiation also occurs in cases when the parasite acts as either a sink (Bar-Nun et al., 2007) or a source (Aloni, 2015) of auxin. In either case, an increased auxin concentration in the host cambium could antagonize ethylene, thus inhibiting local differentiation of radial cambium initials (Lev-Yadun and Aloni, 1991). These hormonal interactions could lead to the pattern of absent radial parenchyma at the interface formed by L. peckoltii, as well as by other Balanophoraceae species (Gonzalez and Mauseth, 2010; González and Sato, 2016).
Interestingly, similar forms of alterations to host cambium activity are reported in Balanophoraceae species that form different types of interface with their host plants. Species in the genera Balanophora, Langsdorffia and Thonningia develop a complex, or chimeric, interface that includes composite vascular bundles, characterized by parasite transfer cells abutting host vessels with mixture of both parasite and host meristematic cells (Mangenot, 1947; Gedalovich-Shedletzky and Kuijt, 1990; Hsiao et al., 1994, 1995). In other genera, including Dactylanthus, Helosis, Lophophytum and Ombrophytum, despite the intricacy of the parasite–host connections, the interface is usually termed ‘simple’, as vascular tissues have either parasite or host origin, with no blend of meristematic cells (Mauseth et al., 1992; Hsiao et al., 1993; Holzapfel, 2001; Gonzalez and Mauseth, 2010). Furthermore, contrary to what is observed in a chimeric interface, species with the simple type of interface show direct vessel–vessel connections between parasite and host, without the presence of transfer cells (Hsiao et al., 1995). Our results place L. peckoltii among species with the simple type of interface. Still, taking the developmental process of the interface of L. peckoltii into account, we consider the terminology proposed by González and Mauseth (2010), which designates this interface as simple, to be at odds with the complexity of the structure.
In this context, Lophophytum and Ombrophytum species have been regarded as having conspicuous similarities (Harms, 1935; Cardoso, 2014), possibly due to a shared evolutionary history (Su et al., 2015). There are remarkable similarities between observations reported for these two clades and the results presented here, including the two vascular systems and the type of parasite–host interface. Additionally, the presence of an apical meristem was not observed in the tuberous organ, as noticed in the runners. Therefore, the dermal tissue of L. peckoltii cannot have originated from the protodermis, similar to what occurs in Ombrophytum subterraneum, which also does not have an epidermis in the traditional sense (Mauseth et al., 1992).
Teixeira-Costa and Ceccantini (2018) suggest using ‘tuberous’ as an adjective when referring to the haustorium morphology in Balanophoraceae and Orobanchaceae. Anatomically, it was not possible to determine the cauline origin of the studied structure. Also, the parasite bundles are made up solely of xylem, and there is no gradient in size in the tracheary elements – the protoxylem and metaxylem. Thus, for this work, we chose to name the studied structure as the vegetative body.
Our results highlight the need for further studies on Balanophoraceae, especially regarding the ontogeny of the parasite–host interface. As indicated by Kuijt (1969), such studies should provide a better understanding of the life history of these parasites by resolving questions of annual vs. perennial strategies. In combination with new phylogenetic analyses, developmental studies can shed light on the evolution of the peculiar vegetative organs of Balanophoraceae species and the different types of interface these parasites form with their hosts.
The anatomy of non-parasitized roots P. uloptera and S. clematidifolia was analysed and described, for comparison with the anatomy of parasitized host roots (see Supplementary data File S1).
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. File S1: anatomy of Paullinia uloptera and Serjania clematidifolia roots without parasitism. Figure S1: anatomy of roots without parasitism.
ACKNOWLEDGEMENTS
We are grateful for our friends and staff at the Laboratório de Botânica Estrutural of the Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. This research was part of the first author’s PhD thesis at the Graduate Program in Botany-Botanical Garden Research Institute of Rio de Janeiro. We also thank the anonymous reviewers who spend hours of their time to review papers without any other remuneration other than the advancement of science.
FUNDING
We thank CAPES for awarding the PhD scholarship to the first author. C.F.B. received grants from the CNPq council.
LITERATURE CITED
- Aloni R. 2015. Ecophysiological implications of vascular differentiation and plant evolution. Trees 29: 1–16. [Google Scholar]
- Angyalossy V, Pace MR, Evert RF, et al. 2016. IAWA list of microscopic bark features. IAWA Journal 37: 517–615. [Google Scholar]
- Barbosa ACF, Pace MR, Witovisk L, Angyalossy V. 2010. A new method to obtain good anatomical slides of heterogeneous plant parts. IAWA Journal 31: 373–383. [Google Scholar]
- Bar-Nun N, Sachs T, Mayer AM. 2007. A role for IAA in the infection of Arabidopsis thaliana by Orobanche aegyptiaca. Annals of Botany 101: 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros CF, Miguens FC. 1998. Ultrastructure of the epidermal cells of Beilshmiedia rigida (Mez) Kosterm (Lauraceae). Acta Microscopica 7: 1–11. [Google Scholar]
- Bastos CL, Tamaio N, Angyalossy V. 2016. Unravelling roots of lianas: a case study in Sapindaceae. Annals of Botany 118: 733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccari O. 1869. Nuovo Giornale Botanico Italiano, Vol. 1. https://hdl.handle.net/2027/umn.31951d00248583z [Google Scholar]
- Bukatsch F. 1972. Bermerkungen zur Doppelf€arbung Astrablau-Safranin. Mikrokosmos 61: 255. [Google Scholar]
- Cardoso LJT. 2014. Balanophoraceae no Brasil. PhD Thesis, Escola Nacional de Botânica Tropical, Rio de Janeiro. [Google Scholar]
- Delprete PG. 2004. A new species of lophophytum and the first report of lathrophytum (Balanophoraceae) from the State of Goias, Central Brazil. Kew Bulletin 59: 291. [Google Scholar]
- De Vega C, Ortiz PL, Arista M, Talavera S. 2007. The endophytic system of Mediterranean Cytinus (cytinaceae) developing on five host cistaceae species. Annals of Botany 100: 1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberwein R, Nickrent DL, Weber A. 2009. Development and morphology of flowers and inflorescences in Balanophora papuana and B. elongata (Balanophoraceae). American Journal of Botany 96: 1055–1067. [DOI] [PubMed] [Google Scholar]
- Fagerlind F. 1945. Blüte und Blütenstand der Gattung Balanophora. Botaniska Notiser 4: 330–350. [Google Scholar]
- Feder N, O’Brien T. 1968. Plant microtechnique: some principles and new methods. American Journal of Botany 55: 123–142. [Google Scholar]
- Foster AS. 1942. Practical plant anatomy. Toronto: David Van Nostrand. [Google Scholar]
- Gedalovich-Shedletzky E, Kuijt J. 1990. An ultrastructural study of the tuber strands of Balanophora (Balanophoraceae). Canadian Journal of Botany 68: 1271–1279. [Google Scholar]
- Gerrits PO, Smid L. 1983. A new, less toxic polymerization system for the embedding of soft tissues in glycol methacrylate and subsequent preparing of serial sections. Journal of Microscopy 132: 81–85. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Mauseth JD. 2010. Morphogenesis is highly aberrant in the vegetative body of the holoparasite Lophophytum leandrii (Balanophoraceae): all typical vegetative organs are absent and many tissues are highly modified. International Journal of Plant Sciences 171: 499–508. [Google Scholar]
- González AM, Sato HA. 2016. Anatomía vegetativa de Lophophytum mirabile subsp. bolivianum (Balanophoraceae) y efecto de su parasitismo en la anatomía de las raíces de su hospedante Anadenanthera colubrina var. cebil. Anales del Jardin Botanico de Madrid 73. [Google Scholar]
- Hansen B. 1980. Flora neotropica Balanophoraceae. Flora Neotropica 23: 1–80. [Google Scholar]
- Hansen B. 2015. Balanophorales. In: Kubitzki K, ed. The families and genera of vascular plants. Volume XII flowering plants. Eudicots. Berlin Heidelberg: Springer, 192–208. [Google Scholar]
- Harms H. 1935. Balanophoraceae. In: Engler A,, Prantl K, eds. Die Natürlichen Planzenfamilien. Liepzig: Engelmann, 296–339. [Google Scholar]
- Heide-Jørgensen HS. 2008. Parasitic flowering plants. Boston: Brill. [Google Scholar]
- Herth W, Schnepf E. 1980. The fluorochrome, calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma 105: 129–133. [Google Scholar]
- Holzapfel S. 2001. Studies of the New Zealand root-parasite Dactylanthus taylorii (Balanophoraceae). Englera 22: 7–176. [Google Scholar]
- Hsiao S, Mauseth JD, Gómez LD. 1993. Growth and anatomy of the vegetative body of the parasitic angiosperm Helosis cayennensis (Balanophoraceae). Bulletin of the Torrey Botanical Club 120: 295. [Google Scholar]
- Hsiao S-C, Mauseth JD, Gomez LD. 1994. Growth and anatomy of the vegetative body of the parasitic angiosperm Langsdorffia hypogaea (Balanophoraceae). Bulletin of the Torrey Botanical Club 121: 24. [Google Scholar]
- Hsiao S-C, Mauseth JD, Peng C-I. 1995. Composite bundles, the host/parasite interface in the holoparasitic angiosperms Langsdorffia and Balanophora (Balanophoraceae). American Journal of Botany 82: 81–91. [Google Scholar]
- Hu B, Sakakibara H, Takebayashi Y, et al. 2017. Mistletoe infestation mediates alteration of the phytohormone profile and anti-oxidative metabolism in bark and wood of its host Pinus sylvestris. Tree Physiology 37: 676–691. [DOI] [PubMed] [Google Scholar]
- IAWA Committee . 1989. IAWA list of microscopic features for hardwood identification. Iawa Bulletin 19: 219–232. [Google Scholar]
- Joel DM, Gressel J, Musselman LJ. 2013. Parasitic Orobanchaceae. Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Johansen DA. 1940. Plant microtechnique. New York and London: McGraw-Hill Book Company. [Google Scholar]
- Johri BM, Ambegaokar KB, Srivastava PS. 1992. Comparative embryology of angiosperms. Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Koch K, Bhushan B, Barthlott W. 2009. Multifunctional surface structures of plants: an inspiration for biomimetics. Progress in Materials Science 54: 137–178. [Google Scholar]
- Kuijt J. 1969. The biology of parasitic flowering plants. Berkeley: University of California Press. [Google Scholar]
- Kuijt J. 1977. Haustoria of phanerogamic parasites. Annual Review of Phytopathology 15: 91–118. [Google Scholar]
- Kuijt J, Bruns D. 1987. Roots in Corynaea (Balanophoraceae). Nordic Journal of Botany 7: 539–542. [Google Scholar]
- Kuijt J, Dong W-X. 1990. Surface features of the leaves of Balanophoraceae? A family without stomata? Plant Systematics and Evolution 170: 29–35. [Google Scholar]
- Kuijt J, Hansen B. 2015. Flowering plants. Eudicots. Cham: Springer International Publishing. [Google Scholar]
- Lev-Yadun S, Aloni R. 1991. Polycentric vascular rays in Suaeda monoica and the control of ray initiation and spacing. Trees 5: 22–29. [Google Scholar]
- Mangenot MG. 1947. Sur les galles de Thonningia coccinea. Comptes rendus de l’Académie des Sciences 224: 665–666. [Google Scholar]
- Mauseth JD, Rezaei K. 2013. Morphogenesis in the parasitic plant Viscum minimum (Viscaceae) is highly altered, having apical meristems but lacking roots, stems, and leaves. International Journal of Plant Sciences 174: 791–801. [Google Scholar]
- Mauseth JD, Montenegro G, Walckowiak AM. 1984. Studies of the holoparasite Tristerix aphyllus (Loranthaceae) infecting Trichocereus chilensis (Cactaceae). Canadian Journal of Botany 62: 847–857. [Google Scholar]
- Mauseth JD, Hsiao S-C, Montenegro G. 1992. vegetative body of the parasitic angiosperm Ombrophytum subterraneum (Balanophoraceae). Bulletin of the Torrey Botanical Club 119: 407. [Google Scholar]
- Moore LB. 1940. The structure and life history of the root parasite Dactylanthus taylori Hook. New Zealand Journal of Science and Technology Section B 21: 206–224. [Google Scholar]
- Nickrent DL. 2020. Parasitic angiosperms: how often and how many? Taxon 69: 5–27. [Google Scholar]
- Nikolov LA, Davis CC. 2017. The big, the bad, and the beautiful: biology of the world’s largest flowers. Journal of Systematics and Evolution 55: 516–524. [Google Scholar]
- Nikolov LA, Tomlinson PB, Manickam S, Endress PK, Kramer EM, Davis CC. 2014. Holoparasitic Rafflesiaceae possess the most reduced endophytes and yet give rise to the world’s largest flowers. Annals of Botany 114: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis MJ, Collier DC, Walls D. 1964. Laboratory techniques in botany. London: Butterworths. [Google Scholar]
- Rupp P. 1964. Polyglykol als Einbettungsmedium zum Schneiden botanischer Präparate. Mikrokosmos 53: 123–128. [Google Scholar]
- Santamaría-Aguilar D, Fernández RA. 2017. Hirtella crusa (Chrysobalanaceae), una especie nueva de la Península de Osa, Costa Rica. Phytoneuron 74: 1–9. [Google Scholar]
- Sass JE. 1958. Botanical microtechnique. Iowa City, IA: The Iowa State College Press. [Google Scholar]
- Shivamurthy GR, Arekal GD, Swamy BGL. 1981. Establishment, structure and morphology of the tuber of Balanophora. Annals of Botany 47: 735–745. [Google Scholar]
- Spallek T, Melnyk CW, Wakatake T, et al. 2017. Interspecies hormonal control of host root morphology by parasitic plants. Proceedings of the National Academy of Sciences, USA 114: 5283–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H-J, Hu J-M, Anderson FE, Der JP, Nickrent DL. 2015. Phylogenetic relationships of Santalales with insights into the origins of holoparasitic Balanophoraceae. Taxon 64: 491–506. [Google Scholar]
- Teixeira-Costa L. 2021. A living bridge between two enemies: haustorium structure and evolution across parasitic flowering plants. Brazilian Journal of Botany 44: 165–178. [Google Scholar]
- Teixeira-Costa L, Ceccantini G. 2018. Extensor: glossário ilustrado sobre plantas parasitas e a problemática das homologias das estruturas de conexão parasita–hospedeira. Boletim de Botânica - Revistas USP 36: 103–120. [Google Scholar]
- Těšitel J. 2016. Functional biology of parasitic plants: a review. Plant Ecology and Evolution 149: 5–20. [Google Scholar]
- The Angiosperm Phylogeny Group, Chase MW, Christenhusz MJM, et al. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Tomescu AMF, Groover AT. 2019. Mosaic modularity: an updated perspective and research agenda for the evolution of vascular cambial growth. New Phytologist 222: 1719–1735. [DOI] [PubMed] [Google Scholar]
- Weber HC. 1987. Evolution of the secondary haustoria to a primary haustorium in the parasitic Scrophulariaceae/Orobanchacea. Plant Systematics and Evolution 156: 127–131. [Google Scholar]
- Weber HC, Sunaryo. 1990. Spadix-bearing parasites (Balanophoraceae): extreme flowering plants with fungal character. Biologische Rundschau 28: 83–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.