Abstract
Background and Aims
Determining within-species large-scale variation in phenotypic traits is central to elucidate the drivers of species’ ranges. Intraspecific comparisons offer the opportunity to understand how trade-offs and biogeographical history constrain adaptation to contrasted environmental conditions. Here we test whether functional traits, ecological strategies from the CSR scheme and phenotypic plasticity in response to abiotic stress vary along a latitudinal or a center- margins gradient within the native range of Arabidopsis thaliana.
Methods
We experimentally examined the phenotypic outcomes of plant adaptation at the center and margins of its geographic range using 30 accessions from southern, central and northern Europe. We characterized the variation of traits related to stress tolerance, resource use, colonization ability, CSR strategy scores, survival and fecundity in response to high temperature (34 °C) or frost (- 6 °C), combined with a water deficit treatment.
Key Results
We found evidence for both a latitudinal and a center-margins differentiation for the traits under scrutiny. Age at maturity, leaf dry matter content, specific leaf area and leaf nitrogen content varied along a latitudinal gradient. Northern accessions presented a greater survival to stress than central and southern accessions. Leaf area, C-scores, R-scores and fruit number followed a center-margins differentiation. Central accessions displayed a higher phenotypic plasticity than northern and southern accessions for most studied traits.
Conclusions
Traits related to an acquisitive/conservative resource-use trade-off followed a latitudinal gradient. Traits associated with a competition/colonization trade-off differentiated along the historic colonization of the distribution range and then followed a center-margins differentiation. Our findings pinpoint the need to consider the joint effect of evolutionary history and environmental factors when examining phenotypic variation across the distribution range of a species.
Keywords: phenotypic plasticity, CSR strategies, performance, water stress, functional trait, plant trait-based ecology, intraspecific variation, stress resistance-fecundity trade-off
Introduction
The way species deploy various ecological strategies to cope with local abiotic and biotic conditions across their geographical distribution range is critical to understand the evolution of distribution range (Brown, 1984; Banta et al., 2012; Schurr et al., 2012). Surprisingly though, most theoretical developments on the determinants of species’ ranges have focused on biogeographical and evolutionary aspects linked to colonization ability (e.g. Kirkpatrick and Barton, 1997; Sexton et al., 2009; Bridle et al., 2010) while overlooking the divergence of populations in term of eco-physiological traits. Phenotypic adaptations along environmental gradients have been widely recognized both between- and within species in functional and evolutionary ecology (Reich et al., 1997, 2003; Wright et al., 2017; Dong et al., 2020; Kuppler et al., 2020). However, such phenotypic adaptations have been scarcely added to the long list of usual suspects that determine species’ range size and dynamics (Brown and Gibson, 1983; Gaston, 2009; Sexton et al., 2009). The ecological drivers of range size variation remain largely tackled through the comparison of multiple species while considering species’ ecological characteristics as fixed. This is most often implicit in model species distributions studies (Guisan and Thuiller, 2005), and explicit in studies dedicated to the analysis of phenotypic diversity (namely functional diversity) across species and scales (Violle et al., 2014). The lack of consideration of within-species ecological variation might be necessary in biogeography from a pragmatic point of view, but it ignores theoretical expectations of major differences in ecological performances—survival, growth and reproduction—when moving from the center to the margins of the range of a given species (Abeli et al., 2014; Csergő et al., 2017; Salguero-Gómez et al., 2018).
Plant trait-based ecology has long investigated the variability of phenotypic features (functional traits hereafter) among species, and has linked it to the environments and communities they live in (Violle et al., 2007; Garnier et al., 2016). The joint analysis of the variability of multiple traits further led to the identification of plant ecological strategies that are expected to reflect the phenotypic outcome of natural selection at a given place (Westoby et al., 2002). Notably, based on the combination of a limited number of plant functional traits, the CSR scheme (Grime, 1977, 1988; Hodgson et al., 1999; Pierce et al., 2013, 2017) depicts alternative ecological strategies displayed by any plant species within a triangle whose three summits represent plants completely invested in either competitive strategies (C), stress-tolerant strategies (S), or ruderal strategies (R). Despite its simplicity, the CSR scheme has successfully been used to describe plant community gradients across broad environmental clines (Cerabolini et al., 2010; Rosenfield et al., 2019). CSR strategies are tightly linked to the slow-fast economic spectrum (Wright et al., 2004, Dayrell et al., 2018, Grime & Pierce, 2012, Reich, 2014). Stress-tolerant plants invest resources in long-lived structures, leading to a higher leaf dry matter content (LDMC), and are then associated with a conservative strategy (Dayrell et al., 2018, Wright et al., 2005). At the opposite, ruderal plants invest resources in a fast growth, captured by high values of specific leaf areas and are then associated to the acquisition part of the leaf economics spectrum (Dayrell et al., 2018). Competitive plants maximize their vegetative growth and their organ size, notably through larger leaf area, and are then associated with a resource acquisitive strategy (Dayrell et al., 2018). However, this classification remains silent regarding its underlying adaptive causes, although this was a prerequisite of plant functional ecology at its infancy (Calow, 1987). The lack of consideration of the adaptive value of functional strategies is partly due to a negligence of intraspecific trait variation in functional ecology (Albert et al., 2010, 2011, 2012; Violle et al., 2012). Recent efforts have emphasized noticeable variations of plant functional strategies across ecotypes of a given species, and demonstrated their adaptive value (Vasseur, et al., 2018a). The exploration of functional trait variation across species’ range is promising since they can reveal the ability of populations to adapt to local, potentially stressful, conditions through functional specialization.
It is expected by definition that the populations at the edges of a species distribution experience the most extreme environmental conditions the species can tolerate (Central-Periphery Hypothesis (CPH); Brown, 1984; Holt, 2009; discussed in Pironon et al., 2017). The contrasted environmental conditions throughout the species’ distribution area are expected to select for differential values of functional traits that reflect physiological tolerance and plant performance as a whole, but also for different levels of phenotypic plasticity. Theoretical considerations predict higher adaptive phenotypic plasticity at the margins of the distribution than at the center (Chevin and Lande, 2011) as a flexible adaptive response to stressful conditions (Chevin and Lande, 2010). Strikingly, the few empirical studies that explicitly quantify plastic divergence across the range draw divergent conclusions, depending on the trait and on the species. In some cases, phenotypic plasticity was found to be higher at the margins than at the center, which was interpreted as an adaptation to more stressful and fluctuating climatic conditions (Volis et al., 1998, 2001, 2015; Lázaro-Nogal et al., 2015; Carvajal et al., 2017; Molina-Montenegro & Naya, 2012). Conversely, some studies highlighted lower plasticity at the margins of plant species’ distribution compared to the center (eg., Mägi et al., 2011), which was explained by a higher cost of maintaining environment sensors in stressful conditions (van Kleunen and Fischer, 2005). Testing hypotheses linking variation and plasticity of functional traits with geography will thus be key to understanding the emergence of species distribution ranges.
The model species Arabidopsis thaliana (L.) Heynh., for which both functional traits (Lasky et al., 2012; Vile et al., 2012; Vasseur et al., 2018ab; Sartori et al., 2019; Exposito-Alonso, 2020) as well as biogeographic history (Lee et al., 2017; Hsu et al., 2019) are well studied, presents a unique opportunity to test above hypotheses (Takou et al., 2019). The native distribution of this annual selfing species extends latitudinally from north Africa to the north of Norway and thus its populations experience dramatically-different environmental conditions (Hoffmann, 2002). Thanks to an international effort of sampling, seeds from more than a thousand of fully sequenced accessions are available (1001 Genomes Consortium, 2016). Taking advantage of this unique genomic database, Lee et al. (2017) reconstructed the recent history of colonization of Europe of A. thaliana. They showed that the majority of actual European lineages originate from the recolonization of a single lineage of Europe, from central Europe to the south and to the north since the last glacial event (Lee et al., 2017). This central lineage then became admixed with southern and northern populations. Thus, comparing northern and southern margins allows us to compare adaptations to very contrasted climates in a similar demographic context (Lee et al., 2017). Moreover, single-nucleotide polymorphisms (SNP) analyses suggested that the post-glacial recolonization of Europe may have involved adaptations to the contrasted European climates (Méndez-Vigo et al., 2011; Lasky et al., 2012). A temperature gradient (5 °C—25 °C) should impose an intense selective strength throughout the latitudinal distribution of A. thaliana (Kaplan et al., 2004; Swindell et al., 2007; Vile et al., 2012). Furthermore, some similarities in water availability (due to summer drought or winter frost) may suggest similar strategies for water use on the two opposite margins (Exposito-Alonso et al., 2018). Then, the geographical (Hoffman, 2002), ecological (1001 Genome consortium, 2016; Supplementary data Fig S1) and historical (1001 Genome consortium, 2016; Lee et al., 2017) centers of the distribution of Arabidopsis thaliana are overlapping in central Europe. In this context, it makes sense to compare groups of accessions originating from the two opposite margins to the center of the distribution in order to test specifically if adaptations to contrasted climates in Europe relate to a latitudinal gradient or to a center-to-margins differentiation. Interestingly, genomic and functional ecology studies provided contrasting evidence in A. thaliana. On the one hand, alleles conferring resistance to extreme drought are maintained at both geographical margins (Exposito-Alonso et al., 2018). On the other hand, the “S” (stress-resistance) strategy seems to be displayed by northern accessions only (Vasseur et al., 2018a). Again, the lack of consideration for phenotypic plasticity in functional ecology, and its role in local adaptation, impedes a comprehensive understanding of variation in plant ecological strategies throughout a species distribution range. Here we asked: (i) How do functional traits and strategies vary across the distribution range of A. thaliana? (ii) Do the plasticity of traits and strategies differ between the center and the margins of its distribution range? (iii) Are the accessions from the margins more resistant to abiotic stresses than those from the center? To answer these questions, we compared geographical groups in order to specifically test the center-margins and latitudinal differentiation hypothesis in the species’ native range. We analyzed functional traits used to quantify CSR strategies as well as performance variations of 30 accessions from the south, the center and the north of Europe, grown in controlled conditions under different temperature and water availability treatments.
MATERIAL AND METHODS
Plant material
We chose thirty natural accessions of Arabidopsis thaliana, randomly selected among three geographical groups (Fig. 1, Supplementary data Table S1). Ten accessions came from Iberian Peninsula and from Cape Verde, ten accessions from central Europe and ten accessions from Scandinavia (namely hereafter South, Center and North, respectively). All the seeds originated from multiplication realized at the Center of Evolutionary and Functional Ecology (CEFE, Montpellier, France) from original stocks of the 1001 Genome Project (1001 Genome consortium, 2016). These accessions covered a large range of climatic conditions where A. thaliana can grow (Supplementary data Fig. S1). This set of thirty accessions represents 86.4% of allelic diversity of A. thaliana and is representative of the Eurasian genetic diversity (Supplementary data Fig. S2).
Fig. 1.
Geographical origin of the 30 accessions of Arabidopsis thaliana. The southern group is represented with orange dots, the central group in green and the northern group in purple. Accessions were chosen randomly in each geographical group.
Experimental design
Seeds of the 30 accessions were sown in November 2018. We used 25 alveolate culture plates containing 120 individual pots of 130mL each filled with peat soil (Neuhaus Humin substrat N2). Each accession was replicated four times in every plate and distributed randomly within and among plates (n = 100 replicates per accession). We stratified seeds by placing plates at 4 °C for four days. Then, the plates were placed in a greenhouse at 10 °C average temperature during 40 days for vernalization. During the vernalization period, we sub-irrigated the pots for 30 minutes once a week. Thereafter, we settled the temperature at 15 °C until the end of January 2019. We then applied five environmental conditions during two weeks (Table 1). These five environmental conditions were composed of five culture plates each, for a total of 20 individuals per accession and per condition (n = 600 individuals in each condition). The control condition consisted of a temperature of 15 °C day and night without any water limitation. These conditions are considered as non-stressful for A. thaliana. The cold (LT) treatment consisted of a nocturnal temperature of -6 °C and 15 °C during the day. We set up the nocturnal temperature in a refrigeration enclosure where temperature was homogenous inside (Platinium PLAT7BT, Franstal, France). The Hot (HT) treatment consisted of a daily temperature of 35 °C and of 15 °C during the night. For this treatment, we moved the plants in another compartment at 35 °C. Light and air humidity were kept identical both in LT and HT treatments. In each temperature condition, half of the plates was sub-irrigated at field capacity once a week for 30 minutes (WW) while the second half was not watered during 15 days (WD) (Table 1). At the end of the two weeks of the five differential treatments, temperature was settled back at 15 °C day and night and all pots were sub-irrigated during 30 minutes once a week until the end of the experiment when plants reproduced and completed their life cycle or otherwise died.
Table 1.
Environmental treatments and their mean effects on plant traits and CSR scores. Traits mean and standard deviation over the 30 accessions are presented for each treatment. SLA: specific leaf area; LA: leaf area; LDMC: leaf dry matter content; C: Competitive; S: Stress-tolerance; R: Ruderal. WW: well-watered; WD: water deficit; HT: hot temperature; LT: low temperature. Temp.: mean air temperature
| Treatment | Temp. (°C) | Water deficit | LA (mm²) | SLA (mm²/mg) | LDMC (mg/g) | LNC (%) | C (%) | S (%) | R (%) | Age at maturity (days) | Fruit number |
|---|---|---|---|---|---|---|---|---|---|---|---|
| control | 15 | 138.44 ± 54.78 | 53.1 ± 20.45 | 85.57 ± 19.94 | 5.65 ± 1.18 | 8.05 ± 2.32 | 0 ± 0 | 91.04 ± 3.57 | 114.34 ± 10.23 | 76.71 ± 37.01 | |
| WW-HT | 35 | 142.33 ± 62.54 | 54.76 ± 12.84 | 84.92 ± 18.96 | 5.55 ± 1.23 | 7.89 ± 2.28 | 0 ± 0 | 91.11 ± 2.99 | 114.37 ± 10.45 | 77.57 ± 35.8 | |
| WD-HT | 35 | x | 148.76 ± 55 | 53.43 ± 14.38 | 85.96 ± 19.29 | 5.52 ± 1.19 | 8.02 ± 2.21 | 0 ± 0 | 91.19 ± 2.89 | 114.25 ± 10.28 | 78.01 ± 36.04 |
| WW-LT | -6 | 149.81 ± 65.85 | 56.04 ± 23.01 | 86.61 ± 19.31 | 5.52 ± 1.13 | 7.97 ± 2.41 | 0 ± 0 | 90.9 ± 3.73 | 115.34 ± 10.26 | 77.23 ± 38.81 | |
| WD-LT | -6 | x | 139.24 ± 59.12 | 52.97 ± 16.49 | 84.91 ± 18.29 | 5.54 ± 1.1 | 8.05 ± 2.32 | 0 ± 0 | 91.06 ± 3.28 | 115.21 ± 10.14 | 77.74 ± 37.01 |
Survival measurement
We estimated survival directly after the temperature treatments. An individual was considered as alive if at least the center of its rosette was still green. We estimated pre-treatment mortality by analyzing pictures of the plate the day before treatment settlement. Individuals that did not germinate or died before the treatments were discarded from the analysis.
Leaf trait measurements and CSR scores
Two days after the end of the temperature and watering treatments, we selected 270 leaves among living individuals (2 individuals per accession and per treatment). Each leaf was rehydrated during 24 hours at 4 °C in demineralized water then weighted (Balco ME2355, France) and scanned (Epson Perfection V800, 300dpi). Then leaves were dried in an oven at 60 °C for three days and leaf dry weight determined using a balance (10–5g resolution, Balco ME2355, France). We measured the leaf area (LA, mm²) from leaf scans using ImageJ (Schneider et al., 2012). LA is a key component of the capacity for an individual to intercept light (Wright et al., 2004). We calculated specific leaf area (SLA, mm².g-1) as the ratio of leaf area to leaf dry mass. SLA is closely associated with relative growth rate and the rate of photosynthesis per unit biomass (Pérez-Harguindeguy et al., 2013). We calculate the leaf dry mass content (LDMC, mg.g-1) as the ratio of leaf dry mass to leaf rehydrated mass (Pérez-Harguindeguy et al., 2013). LDMC is closely associated with tolerance to water stress (Rigano et al., 2016). SLA and LDMC shape the main axis of the leaf economics spectrum (Wright et al., 2004), with a fast growth strategy associated with high values of SLA and low values of LDMC, whereas a slow growth strategy is merely associated with high values of LDMC and low values of SLA (Reich, 2014). From these three leaf traits, we calculated the CSR scores using the algorithm provided by Pierce et al (2017). This algorithm locates every species within a triangle whose three summits correspond to the extremes of CSR strategies. C-scores, R-scores and S-scores are tightly correlated with LA, SLA and LDMC respectively. Their calculations were calibrated on more than 30 000 individuals using a regression of leaf traits against a principal component analysis (PCA). Calculation of CSR scores from new leaf traits measurements can be performed from an open access Excel spreadsheet built from Pierce’s interspecific calibration (see Pierce et al., 2017 for details). The CSR scores obtained through this method is in accordance with scores using more traits (see Pierce et al., 2017).
Near-infrared spectra predictions
At the end of the treatment, we acquired spectra of near-infrared reflectance of green leaves, non-destructively, using a portable spectrometer (ASD LabSpec, Malvern Panalytical, Holland, wavelength range: [780; 2500 nm]). Spectra were taken on leaves dedicated for leaf traits measurements just before harvesting and on additional individuals in order to get 12 spectra per accession and per treatment. Acquired spectra were used to predict SLA, LDMC, leaf nitrogen concentration (LNC, %), R scores and C scores for 2160 individuals. LNC is a key component of the investment in the photosynthetic machinery (Tantray et al., 2020). Predictive models based on convolutional neural networks (CCNs) were developed using an independent database gathering more than 20 000 spectra and their respective reference. We evaluated the robustness of our predictions by testing their correlation with values obtained with the traditional destructive methods (R² between 0.84 and 0.92 for internal validations; [Supplementary Material S1; Table S2, Fig. S3]). Afterward, we considered predicted values superior or inferior to three median absolute values as outliers for each trait, each accession, in every treatment (Hampel, 1974). Final dataset contains traits values for 6 to 12 individuals of each of the 30 accessions in each treatment.
Phenology and fecundity measurements
We monitored the 2365 surviving individuals from germination to the date of the first mature and dehiscent fruit. The age at maturity was calculated as the number of days from germination to the date at which the first fruit became dehiscent. The duration of the life cycle is closely associated with CSR strategies (Grime,1977; Hodgson et al., 1999). At this date, we took a picture of the inflorescence of every individual to estimate the number of fruits. We took all the pictures at the same distance from the floral stem. Based on Vasseur et al. (2018c), we first segmented the images and then shrank them in lines of crossed pixels (“skeleton”) using ImageJ (Schneider et al., 2012). Thanks to nine variables describing these skeletons and automatically measured by ImageJ, we built a linear model to estimate fecundity (n = 100, R² = 0.92). This method detects aborted or non-fecundated fruits from mature and fecundated fruits (Vasseur et al., 2018c).
Statistical analyses
We compared means of traits observed in control condition and coefficients of variation of NIRS-predicted values of traits in all treatments thanks to Tukey tests (‘Multcomp’ package, Hothorn et al., 2008). We compared cross-treatment plasticity of geographical groups through Tukey tests comparisons of the coefficient of variation across all treatments. The coefficient of variation (CV) is calculated as the total standard deviation of traits of each group across treatments divided by the cross-treatment mean. We analyzed trait plasticity in response to the treatments using linear mixed-effects models that test log-response ratios (log ratios hereafter) of traits and CSR scores as a function of geographical groups, treatments, and their interactions. Accession identity and plate identity were considered as random effects in the models. Log ratios were calculated as the logarithm of the ratio of an individual value in a given treatment and the mean value of its accession in control condition. To compare Log ratios of geographical groups within the treatments to the control, we compared Log ratios to zero through Student tests, with a Holm’s correction.
We analyzed the variability of performance traits (survival and number of fruits) using generalized mixed models (‘lme4’ package, Bates et al., 2015). We performed a binomial regression for survival models (logit as a link function) and a Poisson regression for fecundity models (log as a link function). We considered three fixed effects in these models: geographical origin (3 levels), treatment effect (5 levels) and their interaction. Two random effects were considered: accession identity and plate. Only one plant died in the control condition. Because most values were at the extreme of the binomial distribution in this condition, the model suffered from convergence issues. Consequently, this treatment was not compared to the others in the survival analysis.
In every model, we calculated means and standard errors of estimates with the ‘emmeans’ package (Lenth et al., 2019). We compared means between groups and between treatments with Tukey post-hoc tests (‘Multcomp’ package, Hothorn et al., 2008). We analyzed the relationship between survival and fruit production using a linear model with mean values per accessions.
RESULTS
Variation of functional traits and ecological strategies across the geographical range
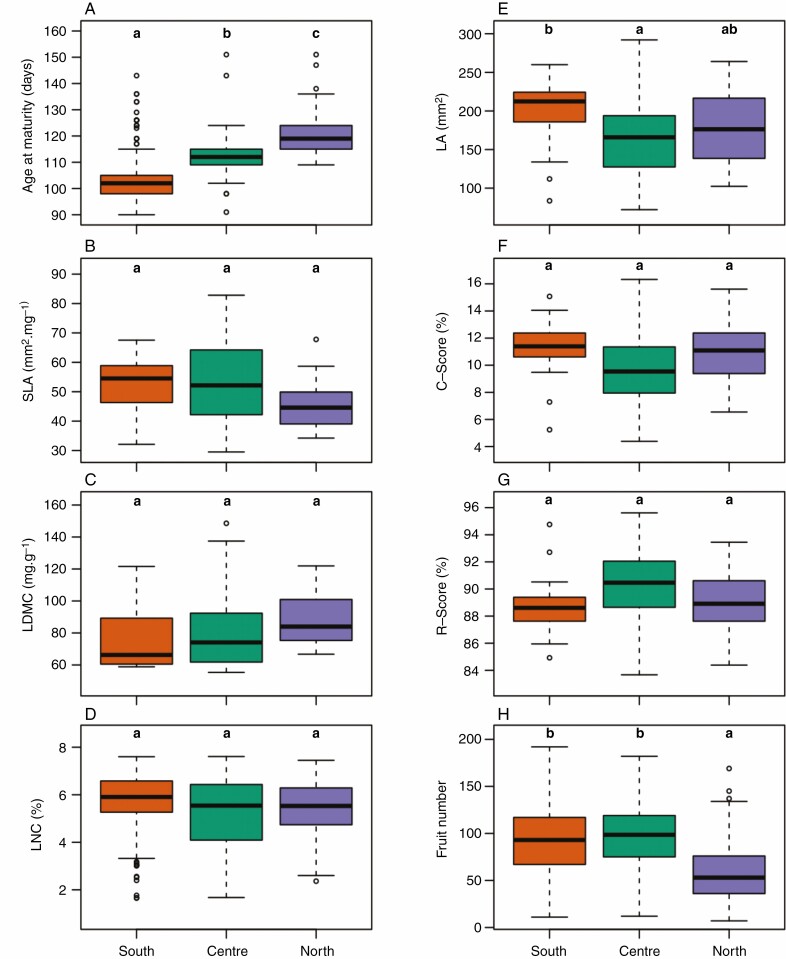
Under control conditions, traits can be categorized into two groups: those that tend to exhibit a latitudinal gradient and those that tend to exhibit a center-margins gradient. Within the former group, geographical origin had a significant effect on age at maturity: southern accessions had a shorter lifespan than central and northern accessions (both P < 0.001), while northern accessions had a longer lifespan than central accessions (P < 0.001, Fig. 2A). A trend for a latitudinal gradient existed for SLA, LDMC and LNC, but no significant differences were found across geographical groups for these traits (Fig. 2BCD). Central accessions had significantly smaller leaves than southern accessions (P = 0.046), and non-significantly smaller leaves than northern accessions (Fig. 2E). Consistent with their lower leaf area, central accessions tended to exhibit smaller C-scores and higher R-scores than southern and northern accessions, but these variations were not significant (Fig. 2FG). All accessions had a null S-score. Central and southern accessions produced on average more fruits than northern accessions, (P < 0.001; Fig. 2H). The differences between geographical groups were qualitatively similar with the variation of mean traits of accessions across latitudes (Supplementary data Fig. S4).
Fig. 2.
Phenotypic variation in Control condition across the distribution range of A. thaliana. Different letters indicate significant differences between geographical groups following Tukey tests at P < 0.05. The southern group is represented with orange dots, the central group in green and the northern group in purple.
Variation of plasticity of functional traits and ecological strategies across the geographical range
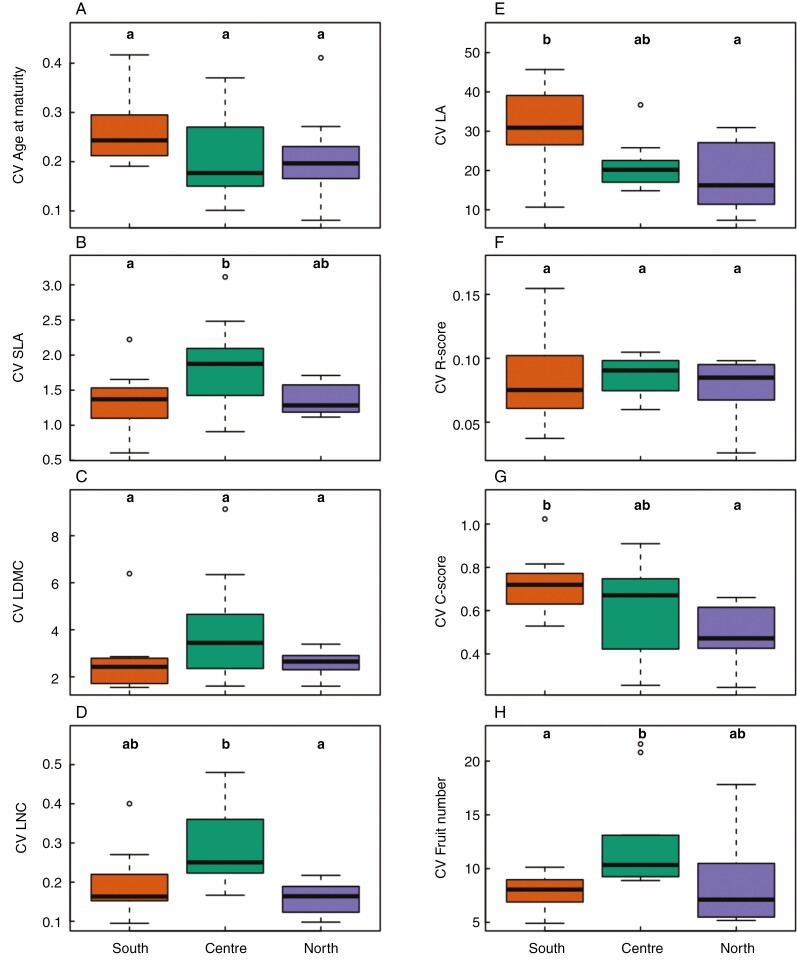
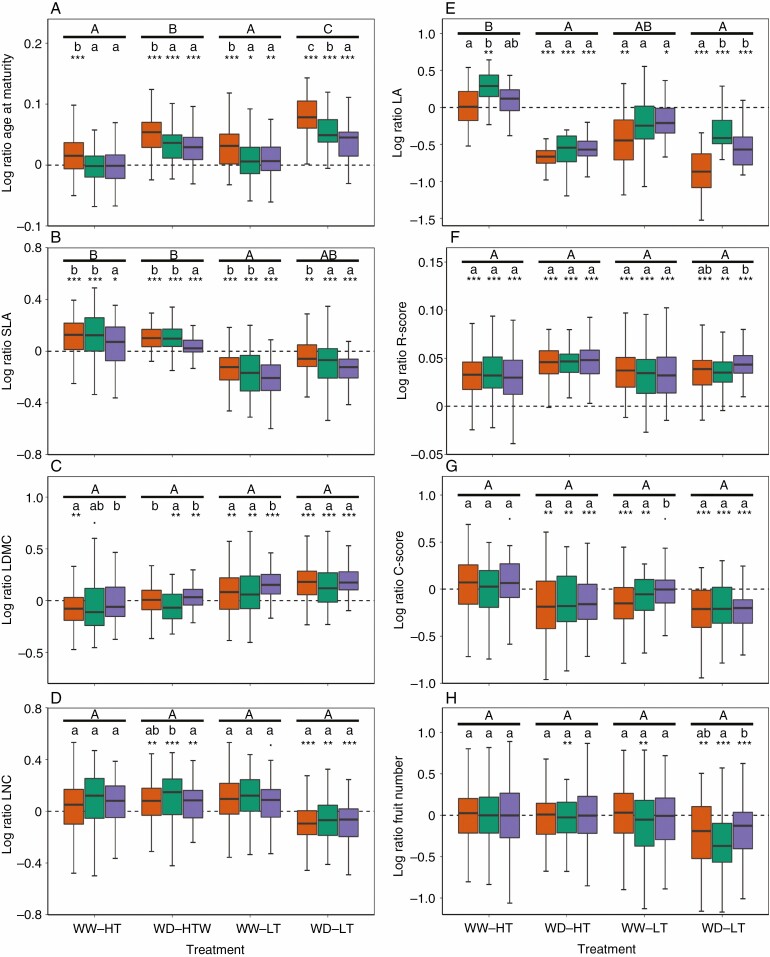
We first estimated cross-treatment trait plasticity with the coefficient of variation across five contrasted environmental conditions (control, WW-HT, WD-HT, WW-LT, WD-LT, Fig. 3). Specific responses of each group in every treatment were then detailed in Fig. 4. The four traits that tended to exhibit a latitudinal gradient for trait values under control condition (age at maturity, SLA, LDMC and LNC) globally had a center-margins differentiation for trait plasticity (Fig. 3ABCD). For instance, central accessions had a higher plasticity of SLA across treatments than southern accessions (P = 0.048) and marginally higher than northern accessions (P = 0.054, Fig. 3B). Yet, the response of SLA to individual treatments exhibited more a latitudinal gradient than a center-margins gradient (Fig. 4B), with both southern and central accessions being more similar in their SLA log-ratio than northern accessions. Northern accessions had a higher decrease in SLA than central and southern accessions in low temperature whereas central and southern accessions had a higher increase in SLA than northern accessions in hot temperature conditions. Cross-treatment plasticity was not significantly different across geographical groups for age at maturity (Fig. 3A). Yet, the response of age at maturity to individual stress displayed a latitudinal gradient with decreasing plasticity toward the north (Fig. 4A). In particular, the three geographical groups differed significantly in plasticity of age at maturity in response to WD-LT. Other traits displayed more a center-margins differentiation than a latitudinal gradient. For instance, central accessions had a higher but not significant cross-treatment plasticity of LDMC (Fig. 3C). Central accessions had a higher coefficient of variation of LNC than northern accessions (P = 0.01) but did not differ significantly with southern accessions for this trait (P = 0.08, Fig. 3D). This center-margins gradient was mainly driven by the response of LNC to WD-HT (Fig. 4D).
Fig. 3.
Coefficient of variation of traits across the distribution range of A. thaliana. Different letters indicate significant differences between geographical groups following Tukey tests at P < 0.05. The southern group is represented with orange dots, the central group in green and the northern group in purple.
Fig. 4.
Plasticity of functional traits and CSR scores across geographical groups and treatments. Difference between mean values of Log Ratios following Tukey tests within and across treatments are indicated with lowercase letters and capital letters, respectively. Log ratios significantly different from zero following Student tests, corrected by Holm’s method, are indicated with stars (*: P < 0.05, **: P < 0.01; ***: P < 0.001). WW: well-watered; WD: Water deficient; HT: Hot temperature; LT: Low temperature. The southern group is represented with orange dots, the central group in green and the northern group in purple.
Among the four traits that tended to exhibit a center-margins gradient in non-stressing conditions (LA, C and R scores, and fruit number), only fruit number also had a center-margins gradient for trait plasticity (Fig. 3H). Central accessions had a significantly higher coefficient of variation of fruit number than southern accessions (P = 0.04) and slightly higher than northern accessions even if not significantly different (P = 0.15). Central accessions are the only accessions to produce less fruits in WD-HT and in WW-LT conditions than in control. (Fig. 4H). In contrast to fruit number, LA and C-scores exhibited a significant latitudinal gradient for cross-treatment plasticity (Fig. 3EG). Northern accessions had a significantly lower plasticity of LA than southern accessions (P = 0.009), central accessions having an intermediate but not significantly different coefficient of variation on this trait (Fig. 3E). This higher cross-treatment plasticity of southern accessions reflected the higher diminution of LA in WD-LT for the southern accessions than central or northern accessions (Fig. 4E). Oppositely, in WW-HT, central accessions exhibited the highest increase in LA among the three geographical groups compared to the control (Fig. 4E). Similar to LA, C-scores had a smaller coefficient of variation in northern than in southern accessions across treatments (P = 0.006), central accessions having an intermediate but not significantly different coefficient of variation for this trait (Fig. 3G). Yet, only northern accessions exhibited a significantly different response of C-scores to WW-LT when looking at individual treatment effect (Fig. 4G). The cross-treatment plasticity of R-scores exhibited no differences between geographical groups (Fig. 3F), although it exhibited significantly different responses of northern accessions to WD-LT (Fig. 4F). All accessions had a null S-score in every treatment. The differences between geographical groups were qualitatively similar to the variation of mean coefficient of variation of accessions across latitudes ([Supplementary Information Fig. S5). The total variance of traits was significantly explained by the effect of the treatments and geographical groups which represented the largest part of the explained variance (Supplementary data Table S3).
Geographical origin effects on survival
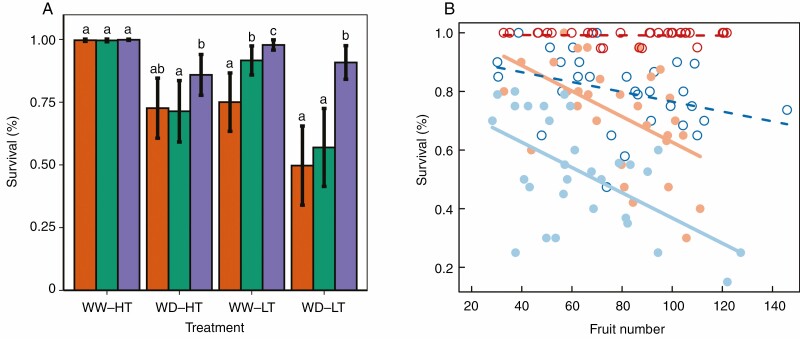
Survival of accessions varied significantly among treatments (P < 0.001). In particular, WW-LT (79.9% of survival), WD-HT (72.2% of survival) and WD-LT (52.4% of survival) were associated with a significantly weaker probability of survival than WW-HT (99.2% of survival) (P = 0.02; P = 0.0003; and P < 0.001 respectively). A single individual died in Control (99.8% of survival), likely unrelated to adaptation to such conditions. Survival of accessions varied significantly across geographical groups (P < 0.001), which globally exhibited a latitudinal gradient. Among all treatments, northern accessions survived significantly more than central (P = 0.01) and southern accessions (P = 0.001), consistent with the significant interaction between geographical group and treatment (P < 0.001). In WW-LT, central accessions survived more than southern accessions (P = 0.007). Northern accessions survived significantly more than the central and southern accessions in the cold treatments (WW-LT and WD-LT, resp. P < 0.001). Moreover, northern accessions survived significantly more than central accessions (P = 0.046) in WD-HT, but they were not different from southern accessions (P = 0.06) (Fig. 5A, [Supplementary data Fig. S6).
Fig. 5.
A) Survival rate among treatments. Different letters indicate significant differences between geographical groups following Tukey tests at P < 0.0.05 within each treatment. The southern group is represented with orange dots, the central group in green and the northern group in purple. B) Relationship between survival of accessions (n = 30) and fruit production across treatments. Linear regression lines are indicated. Dashed lines and empty points indicate a slope not significantly different from zero. The relationship under control condition was not significant and is not shown. WW-HT is represented in red, WD-HT is represented in orange, WW-LT is represented in dark blue and WD-LT is represented in light blue.
Survival was significantly negatively related to fruit number under WD-HT and WD-LT (R2 = 0.75; P < 0.001) but the slope of the relationship was not significantly different from zero under control, WW-HT and WW-LT. In other words, under WD conditions, accessions with low fecundity survived more than accessions with high fecundity (Fig. 5B, Supplementary data Fig. S7).
Discussion
This study dissects functional variation at the intraspecific level within different environments and across the distribution range of a widespread species. We expected two main types of geographic mean trait variation patterns across A. thaliana distribution; either a latitudinal gradient or a differentiation between the center and the margins of the distribution. The studied traits and their plasticity were correlated but they exhibited various patterns of geographic variation. We discuss the consequences for variation in individual performance and local plant adaptation across the range.
In Europe, Arabidopsis thaliana faces contrasting climates (Hoffmann, 2002), which are expected to constitute strong yet variable natural selection pressures throughout its distribution range (Kaplan et al., 2004; Swindell et al., 2007; Vile et al., 2012). Coherently, part of our results supports a latitudinal gradient in functional variation across the distribution range of A. thaliana. In non-stressful conditions for plant growth, age at maturity, specific leaf area, leaf dry matter content, and leaf nitrogen concentration vary along this latitudinal gradient. These traits are closely associated with the leaf economics spectrum (Wright et al., 2004; Reich, 2014). Our results support a latitudinal gradient in resource-use strategies, from acquisitive resource-use strategy for southern accessions (characterized by short lifespan, thin leaves with high LNC and photosynthetic rate) to conservative resource-use strategy in northern accessions (characterized by long lifespan, thick leaves with low LNC and photosynthetic rate). Abundant literature in A. thaliana supports this functional gradient with latitude in Europe (Stenøien et al., 2002; Stinchcombe et al., 2004; Hopkins et al., 2008; Vasseur et al., 2012; Debieu et al., 2013; Vasseur et al., 2018a; Exposito-Alonso, 2020). In contrast, Sartori et al., (2019) found that both southern and northern accessions displayed a conservative resource-use strategy. Here, we show that northern accessions had a higher survival rate at low temperature than central and southern accessions. This suggests that conservative resource-use strategies selected in cold climates in northern areas of Europe is associated with an optimization of survival to freeze (Sartori et al., 2019). Surprisingly, however, northern accessions had also a higher survival rate than southern and central accessions in the hot temperature treatment combined with water deficit (Fig. 5A). We can hypothesize that the metabolic pathways associated with a increased survival in dehydration caused by freeze could also be co-opted for a increased survival under water deficit conditions (Sanada et al., 2007; Suprasanna et al., 2016; Gillespie and Volaire, 2017; Bristiel et al., 2018). However, the reverse is not true: southern accessions are the most vulnerable to nocturnal freezing. A possible explanation is that stress escaping (Ludlow, 1989) is closely associated with acquisitive resource-use strategies selected in the southern area of the distribution range of A. thaliana. However, in southern populations, the two alternative strategies seem to coexist, as four southern accessions showed higher survival than the other southern accessions (Supplementary data Fig. S6, Fig. S7). Interestingly, these four accessions are from the same genetic lineage: the relict group (Supplementary data Table S1), 1001 Genome consortium, 2016). This ancient genetic lineage is associated with stress-tolerance in Spain (1001 Genome consortium, 2016; Toledo et al., 2020). Modern Spanish accessions present a short life cycle following spring germination (our results; Assmann, 2013; Exposito-Alonso, 2020) that is strongly associated with an acquisitive resource-use strategy. Oppositely in Scandinavia, where low temperatures and short spring season do not allow for a rapid life cycle strategy, plants are selected for high tolerance strategy to resist winter conditions (Bartlett et al., 2014; Delzon, 2015; Exposito-Alonso, 2020). Underpinning this tolerance/avoidance trade-off, southern survivors under stressful conditions increased more their life duration than central and northern accessions. This corroborates the synthesis study of Exposito-Alonso et al. (2020) based on previous datasets (Martínez-Berdeja et al., 2020) who showed that Spanish accessions had a more plastic life cycle than Scandinavian strict winter cycler accessions. These results also corroborate the genetic correlation between water use efficiency and life span found in previous studies (Mckay et al., 2003): increasing water use efficiency through phenotypic plasticity may constrain the life cycle of individuals to be longer.
The center-margins gradient of abiotic stress hypothesis, which posits that less suitable environments occur at the peripheries (Holt, 2009), has been discussed on numerous species (Sexton et al., 2009; Pironon et al., 2017). In A. thaliana’s distribution range, mean annual precipitation exhibits a bell-shaped curve with latitude. Northern and southern populations encountering less precipitations than central populations (Supplementary data Fig. S1). Low precipitations are expected to reduce the variance of phenotypes associated with water-stress resistance and may limit phenotypic plasticity (Valladares et al., 2007; Palacio-López et al., 2015; Stotz et al., 2021). In parallel, northern and southern parts of the distribution range of A. thaliana encounter more seasonal variation of temperature and precipitation (Supplementary data Fig. S1). Fluctuating conditions, when predictable, are expected to select for plastic phenotypes (Lázaro-Nogal et al., 2015; Leung et al., 2020; Stotz et al., 2021). Our results show a general trend for a weaker cross-treatment plasticity in peripheral accessions than central accessions. This could be explained by the cost of phenotypic plasticity, being higher in stressful conditions associated with fewer precipitations (van Kleunen and Fischer, 2005; Molina-Montenegro and Naya, 2012; Nicotra et al., 2015). A weaker or an absence of phenotypic plasticity as well as a stronger genetic determinism independent of the climate at ecological margins may be adaptive (Ghalambor et al., 2007; Murren et al., 2015; Palacio-López et al., 2015; Acasuso-Rivero et al., 2019; Pfennig, 2021). Indeed, only the central populations showed a significant fecundity reduction in stress conditions. This result is also in accordance with Exposito-Alonso et al. (2018) who showed that similar alleles involved in drought resistance are under selection at both latitudinal margins of Europe in A. thaliana. Our results demonstrate stress-tolerance in A. thaliana despite using the classic Pierce et al.’s methodology (2017), all accessions had a null S-score. This result questions the use of such classification at the intraspecific level. Moreover, we showed that leaf traits, on which scores calculation are based, are highly plastic and that accessions responded differently depending on the nature of the stress. We suggest improving the CSR classification by using phenotypic traits and performances actually measured under competition, disturbance and stress conditions.
The reduction of plasticity at both margins of the distribution range may also be associated with the evolutionary history of the species. Actual marginal populations of A. thaliana derived from the European colonization of a genetic lineage from central Europe and its admixture with northern and southern populations (Lee et al., 2017). The colonization of margins may have been accompanied with both high cumulative foundation effects and directional selection, limiting thus the phenotypic variability at both opposite margins (Kirkpatrick and Barton, 1997; Sagarin and Gaines, 2002; Bridle and Vines, 2007; Eckert et al., 2008; Sexton et al., 2009; Luo et al., 2015; Pironon et al., 2017; Hämälä et al., 2018). Accordingly, leaf area, C-scores, R-scores and fruit number are more similar among the two opposite margins than with the central accessions. Our results suggest that trait values associated with colonization (number of fruits and R-scores) are higher in central accessions than peripheral ones. Moreover, traits related to competitive ability (leaf area and C-scores) are lower in central accessions than peripheral ones. This result is quite counter-intuitive regarding theoretical expectations that abundance in central populations should be higher than in marginal populations, thus selecting for a higher competition ability at the center than at the margins of a distribution range (Brown, 1984; Brown et al., 1995; Holt, 2009; Sexton et al., 2009; but see Pironon et al., 2017). This may be explained in that central accessions are more ruderal and fit to colonizing disturbed environments, which would explain that they invest more in fecundity than peripheral accessions who invest more in resistance to stress. Grounded on ecological theories regarding the existence of a colonization/competition trade-off (Levins and Culver, 1971; Hastings, 1980; Turnbull et al., 1999; Yu and Wilson, 2001; Cadotte et al., 2006), we can thus hypothesize that central accessions exhibit traits that optimize seed dispersal and colonization, perhaps also at the expense of competitive or stress-coping ability. Indeed, we confirm the classical trade-off described at the interspecific level between survival to stress and fecundity (Muller-Landau, 2010; D’Andrea et al., 2013). It may be thus interesting to experimentally test this competition/colonization ability differentiation across the distribution range in order to better understand how phenotypes evolved through the evolutionary history of A. thaliana (e.g. Lorts and Lasky, 2020).
Considering intraspecific trait variations across distribution ranges has been recently developed in ecology (e.g. Kumordzi et al., 2019; Moran et al., 2016; Ren et al., 2020; Robson et al., 2012; Halbritter et al., 2015; Henn et al., 2018; Marshall et al., 2019; Villellas et al., 2021). However, such studies generally did not take into account evolutionary history to understand how functional traits and ecological strategies are structured across the species’ range. By consequence, non-linear patterns of variations across the distribution range are understudied. Here, we show that both climate variations and evolutionary history shaped the actual phenotypic diversity in this model species, leading to a latitudinal and a center-margins differentiation respectively depending on the nature of the traits. The latitudinal gradient was associated with an acquisition/conservation trade-off in resource-use, tightly linked to a temperature gradient along European latitudes. Oppositely, the center-margins differentiation was more associated with a competition/colonization trade-off explained by the demographic history of this species. More generally, this study points out the need in functional ecology to go beyond a simple description of functional traits, notably through a better understanding of performance and phenotypic plasticity across environments and evolutionary history (Salguero-Gómez et al., 2018; Bohner and Diez, 2020).
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following.
Table S1. List of accessions and their geographical coordinates.
Table S2. Performances of models predicting leaf traits from near infrared spectroscopy predictive models for in-sample and cross-validation sets of. Supplementary Material S1. Predictive models’ development.
Figure S1. Description of climate of origin of the three geographical groups.
Figure S2. Principal Components Analysis of the genetic variations of the Eurasian full-sequenced accessions of Arabidopsis thaliana.
Figure S3. Relationships between predicted traits and observed values for LDMC, SLA, C-scores and R-scores.
Figure S4. Phenotypic variation in Control condition across European latitudes of A. thaliana.
Figure S5. Coefficient of variation of traits across European latitudes of A. thaliana.
Table S3. Partition of variance explained by treatments, geographical groups and the accessions within geographical groups.
Figure S6. Survival rate of accessions among treatments.
Figure S7. Relationship between survival and fruit number of accessions among geographical groups in WD-HT and WD-LT.
Acknowledgements
We are very grateful to Justine Floret and Amelie Emmanuel for their help on measurements. We also thank the technical platform “Plateforme des Terrains d’Expérience du LabEx CeMEB,” (CEFE, CNRS) in Montpellier and particularly Pauline Durbin and Thierry Matthieu for their precious advice. The authors are grateful to two anonymous reviewers for constructive comments and manuscript improvement.
Funding
This work was supported by the European Research Council (ERC) Starting Grant Project “Ecophysiological and biophysical constraints on domestication in crop plants” (Grant ERC-StG-2014- 639706-CONSTRAINTS).
LITERATURE CITED
- 1001 Genomes Consortium. 2016. 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell 166: 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeli T, Gentili R, Mondoni A, Orsenigo S, Rossi G. 2014. Effects of marginality on plant population performance. Journal of Biogeography 41: 239–249. [Google Scholar]
- Acasuso-Rivero C, Murren CJ, Schlichting CD, Steiner UK. 2019. Adaptive phenotypic plasticity for life-history and less fitness-related traits. Proceedings. Biological Sciences 286: 20190653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert CH, de Bello F, Boulangeat I, Pellet G, Lavorel S, Thuiller W. 2012. On the importance of intraspecific variability for the quantification of functional diversity. Oikos 121: 116–126. [Google Scholar]
- Albert CH, Grassein F, Schurr FM, Vieilledent G, Violle C. 2011. When and how should intraspecific variability be considered in trait-based plant ecology? Perspectives in Plant Ecology, Evolution and Systematics 13: 217–225. [Google Scholar]
- Albert CH, Thuiller W, Yoccoz NG, et al. . 2010. Intraspecific functional variability: extent, structure and sources of variation. Journal of Ecology 98: 604–613. [Google Scholar]
- Assmann SM. 2013. Natural variation in abiotic stress and climate change responses in Arabidopsis: implications for twenty-first-century agriculture. International Journal of Plant Sciences 174: 3–26. [Google Scholar]
- Banta JA, Ehrenreich IM, Gerard S, et al. . 2012. Climate envelope modelling reveals intraspecific relationships among flowering phenology, niche breadth and potential range size in Arabidopsis thaliana. Ecology Letters 15: 769–777. [DOI] [PubMed] [Google Scholar]
- Bartlett MK, Zhang Y, Kreidler N, et al. . 2014. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecology Letters 17: 1580–1590. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Bohner T, Diez J. 2020. Extensive mismatches between species distributions and performance and their relationship to functional traits. Ecology Letters 23: 33–44. [DOI] [PubMed] [Google Scholar]
- Bridle JR, Polechová J, Kawata M, Butlin RK. 2010. Why is adaptation prevented at ecological margins? New insights from individual-based simulations. Ecology Letters 13: 485–494. [DOI] [PubMed] [Google Scholar]
- Bridle JR, Vines TH. 2007. Limits to evolution at range margins: when and why does adaptation fail? Trends in Ecology & Evolution 22: 140–147. [DOI] [PubMed] [Google Scholar]
- Bristiel P, Gillespie L, Østrem L, Balachowski J, Violle C, Volaire F. 2018. Experimental evaluation of the robustness of the growth–stress tolerance trade-off within the perennial grass Dactylis glomerata. Functional Ecology 32: 1944–1958. [Google Scholar]
- Brown JH. 1984. On the relationship between abundance and distribution of species. The American Naturalist 124: 255–279. [Google Scholar]
- Brown JH, Gibson AC. 1983. Biogeography. St. Louis, Missouri: Mosby. [Google Scholar]
- Brown JH, Mehlman DW, Stevens GC. 1995. Spatial variation in abundance. Ecology 76: 2028–2043. [Google Scholar]
- Cadotte MW, Mai DV, Jantz S, Collins MD, Keele M, Drake JA. 2006. On testing the competition-colonization trade-off in a multispecies assemblage. The American Naturalist 168: 704–709. [DOI] [PubMed] [Google Scholar]
- Calow P. 1987. Towards a definition of functional ecology. Functional Ecology 1: 57–61. [Google Scholar]
- Carvajal DE, Loayza AP, Rios RS, Gianoli E, Squeo FA. 2017. Population variation in drought-resistance strategies in a desert shrub along an aridity gradient: interplay between phenotypic plasticity and ecotypic differentiation. Perspectives in Plant Ecology, Evolution and Systematics 29: 12–19. [Google Scholar]
- Cerabolini BEL, Brusa G, Ceriani RM, De Andreis R, Luzzaro A, Pierce S. 2010. Can CSR classification be generally applied outside Britain? Plant Ecology 210: 253–261. [Google Scholar]
- Chevin LM, Lande R. 2011. Adaptation to marginal habitats by evolution of increased phenotypic plasticity. Journal of Evolutionary Biology 24: 1462–1476. [DOI] [PubMed] [Google Scholar]
- Chevin LM, Lande R. 2010. When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution; International Journal of Organic Evolution 64: 1143–1150. [DOI] [PubMed] [Google Scholar]
- Csergő AM, Salguero-Gómez R, Broennimann O, et al. . 2017. Less favourable climates constrain demographic strategies in plants. Ecology Letters 20: 969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea R, Barabás G, Ostling A. 2013. Revising the tolerance-fecundity trade-off; or, on the consequences of discontinuous resource use for limiting similarity, species diversity, and trait dispersion. The American Naturalist 181: E91–101. [DOI] [PubMed] [Google Scholar]
- Dayrell RLC, Arruda AJ, Pierce S, et al. . 2018. Ontogenetic shifts in plant ecological strategies. Functional Ecology 32: 2730–2741. [Google Scholar]
- Debieu M, Tang C, Stich B, et al. . 2013. Co-variation between seed dormancy, growth rate and flowering time changes with latitude in Arabidopsis thaliana. Plos One 8: e61075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzon S. 2015. New insight into leaf drought tolerance. Functional Ecology 29: 1247–1249. [Google Scholar]
- Dong N, Prentice IC, Wright IJ, et al. . 2020. Components of leaf-trait variation along environmental gradients. The New Phytologist 228: 82–94. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Lougheed SC. 2008. Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Molecular Ecology 17: 1170–1188. [DOI] [PubMed] [Google Scholar]
- Exposito-Alonso M. 2020. Seasonal timing adaptation across the geographic range of Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 117: 9665–9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito-Alonso M, Vasseur F, Ding W, Wang G, Burbano HA, Weigel D. 2018. Genomic basis and evolutionary potential for extreme drought adaptation in Arabidopsis thaliana. Nature Ecology & Evolution 2: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier E, Navas M-L, Grigulis K. 2016. Plant functional diversity: Organism traits, community structure, and ecosystem properties. Oxford: Oxford University Press. [Google Scholar]
- Gaston KJ. 2009. Geographic range limits: achieving synthesis. Proceedings. Biological Sciences 276: 1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor CK, Mckay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21: 394–407. [Google Scholar]
- Gillespie LM, Volaire FA. 2017. Are winter and summer dormancy symmetrical seasonal adaptive strategies? The case of temperate herbaceous perennials. Annals of Botany 119: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grime JP. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. The American Naturalist 111: 1169–1194. [Google Scholar]
- Grime JP. 1988. The C-S-R model of primary plant strategies — origins, implications and tests. In: Gottlieb LD, Jain SK. eds. Plant evolutionary biology. Dordrecht: Springer Netherlands, 371–393 [Google Scholar]
- Grime JP, Pierce S. 2012. The evolutionary strategies that shape ecosystems. Oxford: John Wiley & Sons. [Google Scholar]
- Guisan A, Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecology Letters 8: 993–1009. [DOI] [PubMed] [Google Scholar]
- Halbritter AH, Billeter R, Edwards PJ, Alexander JM. 2015. Local adaptation at range edges: comparing elevation and latitudinal gradients. Journal of Evolutionary Biology 28: 1849–1860. [DOI] [PubMed] [Google Scholar]
- Hämälä T, Mattila TM, Savolainen O. 2018. Local adaptation and ecological differentiation under selection, migration, and drift in Arabidopsis lyrata. Evolution 72: 1373–1386. [DOI] [PubMed] [Google Scholar]
- Hampel FR. 1974. The influence curve and its role in robust estimation. Journal of the American Statistical Association 69: 383–393. [Google Scholar]
- Hastings A. 1980. Disturbance, coexistence, history, and competition for space. Theoretical Population Biology 18: 363–373. [Google Scholar]
- Henn JJ, Buzzard V, Enquist BJ, et al. . 2018. Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Frontiers in Plant Science 9: 1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson JG, Wilson PJ, Hunt R, Grime JP, Thompson K. 1999. Allocating C-S-R plant functional types: a soft approach to a hard problem. Oikos 85: 282–294. [Google Scholar]
- Hoffmann MH. 2002. Biogeography of Arabidopsis thaliana (L.) Heynh. (Brassicaceae). Journal of Biogeography 29: 125–134. [Google Scholar]
- Holt RD. 2009. Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proceedings of the National Academy of Sciences of the United States of America 106 Suppl 2: 19659–19665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R, Schmitt J, Stinchcombe JR. 2008. A latitudinal cline and response to vernalization in leaf angle and morphology in Arabidopsis thaliana (Brassicaceae). The New Phytologist 179: 155–164. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical Journal. Biometrische Zeitschrift 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Hsu CW, Lo CY, Lee CR. 2019. On the postglacial spread of human commensal Arabidopsis thaliana: journey to the East. The New Phytologist 222: 1447–1457. [DOI] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, et al. . 2004. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiology 136: 4159–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, Barton NH. 1997. Evolution of a species’ range. The American Naturalist 150: 1–23. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Fischer M. 2005. Constraints on the evolution of adaptive phenotypic plasticity in plants. The New Phytologist 166: 49–60. [DOI] [PubMed] [Google Scholar]
- Kumordzi BB, Aubin I, Cardou F, et al. . 2019. Geographic scale and disturbance influence intraspecific trait variability in leaves and roots of North American understorey plants. Functional Ecology 33: 1771–1784. [Google Scholar]
- Kuppler J, Albert CH, Ames GM, et al. . 2020. Global gradients in intraspecific variation in vegetative and floral traits are partially associated with climate and species richness. Global Ecology and Biogeography 29: 992–1007. [Google Scholar]
- Lasky JR, Des Marais DL, McKay JK, Richards JH, Juenger TE, Keitt TH. 2012. Characterizing genomic variation of Arabidopsis thaliana: the roles of geography and climate. Molecular Ecology 21: 5512–5529. [DOI] [PubMed] [Google Scholar]
- Lázaro-Nogal A, Matesanz S, Godoy A, Pérez-Trautman F, Gianoli E, Valladares F. 2015. Environmental heterogeneity leads to higher plasticity in dry-edge populations of a semi-arid Chilean shrub: insights into climate change responses. Journal of Ecology 103: 338–350. [Google Scholar]
- Lee CR, Svardal H, Farlow A, et al. . 2017. On the post-glacial spread of human commensal Arabidopsis thaliana. Nature Communications 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R, Singmann H, Love J, Buerkner P, Herve M. 2018. Emmeans: Estimated marginal means, aka least-squares means. R package version 1: 3. [Google Scholar]
- Leung C, Rescan M, Grulois D, Chevin LM. 2020. Reduced phenotypic plasticity evolves in less predictable environments. Ecology Letters 23: 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins R, Culver D. 1971. Regional coexistence of species and competition between rare species. Proceedings of the National Academy of Sciences of the United States of America 68: 1246–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorts CM, Lasky JR. 2020. Competition × drought interactions change phenotypic plasticity and the direction of selection on Arabidopsis traits. The New Phytologist 227: 1060–1072. [DOI] [PubMed] [Google Scholar]
- Ludlow MM. 1989. Strategies of Response to Water Stress. In: Kreeeb, KH, Richter, H. and Hinckley, TM, Eds., Structural and Functional Responses to Environmental Stresses, The Hague: SPB Academic Publishing. [Google Scholar]
- Luo Y, Widmer A, Karrenberg S. 2015. The roles of genetic drift and natural selection in quantitative trait divergence along an altitudinal gradient in Arabidopsis thaliana. Heredity 114: 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mägi M, Semchenko M, Kalamees R, Zobel K. 2011. Limited phenotypic plasticity in range-edge populations: a comparison of co-occurring populations of two Agrimonia species with different geographical distributions. Plant Biology (Stuttgart, Germany) 13: 177–184. [DOI] [PubMed] [Google Scholar]
- Marshall MM, Batten LC, Remington DL, Lacey EP. 2019. Natural selection contributes to geographic patterns of thermal plasticity in Plantago lanceolata. Ecology and Evolution 9: 2945–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Berdeja A, Stitzer MC, Taylor MA, et al. . 2020. Functional variants of DOG1 control seed chilling responses and variation in seasonal life-history strategies in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 117: 2526–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JK, Richards JH, Mitchell-Olds T. 2003. Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Molecular Ecology 12: 1137–1151. [DOI] [PubMed] [Google Scholar]
- Méndez-Vigo B, Picó FX, Ramiro M, Martínez-Zapater JM, Alonso-Blanco C. 2011. Altitudinal and climatic adaptation is mediated by flowering traits and FRI, FLC, and PHYC genes in Arabidopsis. Plant Physiology 157: 1942–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Montenegro MA, Naya DE. 2012. Latitudinal patterns in phenotypic plasticity and fitness-related traits: assessing the climatic variability hypothesis (CVH) with an invasive plant species. Plos One 7: e47620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EV, Hartig F, Bell DM. 2016. Intraspecific trait variation across scales: implications for understanding global change responses. Global Change Biology 22: 137–150. [DOI] [PubMed] [Google Scholar]
- Muller-Landau HC. 2010. The tolerance–fecundity trade-off and the maintenance of diversity in seed size. Proceedings of the National Academy of Sciences 107: 4242–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murren CJ, Auld JR, Callahan H, et al. . 2015. Constraints on the evolution of phenotypic plasticity: limits and costs of phenotype and plasticity. Heredity 115: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra AB, Segal DL, Hoyle GL, Schrey AW, Verhoeven KJ, Richards CL. 2015. Adaptive plasticity and epigenetic variation in response to warming in an Alpine plant. Ecology and Evolution 5: 634–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio-López K, Beckage B, Scheiner S, Molofsky J. 2015. The ubiquity of phenotypic plasticity in plants: a synthesis. Ecology and Evolution 5: 3389–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. . 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167. [Google Scholar]
- Pfennig DW. 2021. Phenotypic plasticity & evolution: causes, consequences, controversies. Boca Raton: Taylor & Francis. [Google Scholar]
- Pierce S, Brusa G, Vagge I, Cerabolini BEL. 2013. Allocating CSR plant functional types: the use of leaf economics and size traits to classify woody and herbaceous vascular plants. Functional Ecology 27: 1002–1010. [Google Scholar]
- Pierce S, Negreiros D, Cerabolini BEL, et al. . 2017. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Functional Ecology 31: 444–457. [Google Scholar]
- Pironon S, Papuga G, Villellas J, Angert AL, García MB, Thompson JD. 2017. Geographic variation in genetic and demographic performance: new insights from an old biogeographical paradigm: the centre-periphery hypothesis. Biological Reviews 92: 1877–1909. [DOI] [PubMed] [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1997. From tropics to tundra: global convergence in plant functioning. Proceedings of the National Academy of Sciences of the United States of America 94: 13730–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, et al. . 2003. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences 164: S143–S164. [Google Scholar]
- Reich PB. 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102: 275–301. [Google Scholar]
- Ren L, Guo X, Liu S, et al. . 2020. Intraspecific variation in Phragmites australis: clinal adaption of functional traits and phenotypic plasticity vary with latitude of origin. Journal of Ecology 108: 2531–2543. [Google Scholar]
- Rigano MM, Arena C, Di Matteo A, Sellitto S, Frusciante L, Barone A. 2016. Eco-physiological response to water stress of drought-tolerant and drought-sensitive tomato genotypes. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology 150: 682–691. [Google Scholar]
- Robson TM, Sánchez-Gómez D, Cano FJ, Aranda I. 2012. Variation in functional leaf traits among beech provenances during a Spanish summer reflects the differences in their origin. Tree Genetics & Genomes 8: 1111–1121. [Google Scholar]
- Rosenfield MF, Müller SC, Overbeck GE. 2019. Short gradient, but distinct plant strategies: The CSR scheme applied to subtropical forests. Journal of Vegetation Science 30: 984–993. [Google Scholar]
- Sagarin RD, Gaines SD. 2002. The ‘abundant centre’ distribution: to what extent is it a biogeographical rule? Ecology Letters 5: 137–147. [Google Scholar]
- Salguero-Gómez R, Violle C, Gimenez O, Childs D. 2018. Delivering the promises of trait-based approaches to the needs of demographic approaches, and vice versa. Functional Ecology 32: 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada Y, Takai T, Yamada T. 2007. Ecotypic variation of water-soluble carbohydrate concentration and winter hardiness in cocksfoot (Dactylis glomerata L.). Euphytica 153: 267–280. [Google Scholar]
- Sartori K, Vasseur F, Violle C, et al. . 2019. Leaf economics and slow-fast adaptation across the geographic range of Arabidopsis thaliana. Scientific Reports 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr FM, Pagel J, Cabral JS, et al. . 2012. How to understand species’ niches and range dynamics: a demographic research agenda for biogeography. Journal of Biogeography 39: 2146–2162. [Google Scholar]
- Sexton JP, McIntyre PJ, Angert AL, Rice KJ. 2009. Evolution and ecology of species range limits. Annual Review of Ecology, Evolution, and Systematics 40: 415–436. [Google Scholar]
- Stenøien HK, Fenster CB, Kuittinen H, Savolainen O. 2002. Quantifying latitudinal clines to light responses in natural populations of Arabidopsis thaliana (Brassicaceae). American Journal of Botany 89: 1604–1608. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JR, Weinig C, Ungerer M, et al. . 2004. A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proceedings of the National Academy of Sciences of the United States of America 101: 4712–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz GC, Salgado-Luarte C, Escobedo VM, Valladares F, Gianoli E. 2021. Global trends in phenotypic plasticity of plants. Ecology Letters 24: 2267–2281. [DOI] [PubMed] [Google Scholar]
- Suprasanna P, Nikalje GC, Rai AN. 2016. Osmolyte accumulation and implications in plant abiotic stress tolerance. In: Iqbal N, Nazar R, Khan NA, eds. Osmolytes and plants acclimation to changing environment: emerging omics technologies. New Delhi, India: Springer, 1–12. [Google Scholar]
- Swindell WR, Huebner M, Weber AP. 2007. Plastic and adaptive gene expression patterns associated with temperature stress in Arabidopsis thaliana. Heredity 99: 143–150. [DOI] [PubMed] [Google Scholar]
- Takou M, Wieters B, Kopriva S, Coupland G, Linstädter A, De Meaux J. 2019. Linking genes with ecological strategies in Arabidopsis thaliana. Journal of Experimental Botany 70: 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantray AY, Bashir SS, Ahmad A. 2020. Low nitrogen stress regulates chlorophyll fluorescence in coordination with photosynthesis and Rubisco efficiency of rice. Physiology and Molecular Biology of Plants: An International Journal of Functional Plant Biology 26: 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo B, Marcer A, Méndez-Vigo B, Alonso-Blanco C, Picó FX. 2020. An ecological history of the relict genetic lineage of Arabidopsis thaliana. Environmental and Experimental Botany 170: 103800. [Google Scholar]
- Turnbull LA, Rees M, Crawley MJ. 1999. Seed mass and the competition/colonization trade-off: a sowing experiment. Journal of Ecology 87: 899–912. [Google Scholar]
- Valladares F, Gianoli E, Gómez JM. 2007. Ecological limits to plant phenotypic plasticity. The New Phytologist 176: 749–763. [DOI] [PubMed] [Google Scholar]
- Vasseur F, Exposito-Alonso M, Ayala-Garay OJ, et al. . 2018. Adaptive diversification of growth allometry in the plant Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 115: 3416–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseur F, Sartori K, Baron E, et al. . 2018. Climate as a driver of adaptive variations in ecological strategies in Arabidopsis thaliana. Annals of Botany 122: 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseur F, Bresson J, Wang G, Schwab R, Weigel D. 2018. Image-based methods for phenotyping growth dynamics and fitness components in Arabidopsis thaliana. Plant Methods 14: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseur F, Violle C, Enquist BJ, Granier C, Vile D. 2012. A common genetic basis to the origin of the leaf economics spectrum and metabolic scaling allometry. Ecology Letters 15: 1149–1157. [DOI] [PubMed] [Google Scholar]
- Vile D, Pervent M, Belluau M, et al. . 2012. Arabidopsis growth under prolonged high temperature and water deficit: independent or interactive effects? Plant, Cell & Environment 35: 702–718. [DOI] [PubMed] [Google Scholar]
- Villellas J, Ehrlén J, Crone EE, et al. . 2021. Phenotypic plasticity masks range-wide genetic differentiation for vegetative but not reproductive traits in a short-lived plant. Ecology Letters 24: 2378–2393. [DOI] [PubMed] [Google Scholar]
- Violle C, Enquist BJ, McGill BJ, et al. . 2012. The return of the variance: intraspecific variability in community ecology. Trends in Ecology & Evolution 27: 244–252. [DOI] [PubMed] [Google Scholar]
- Violle C, Navas M-L, Vile D, et al. . 2007. Let the concept of trait be functional! Oikos 116: 882–892. [Google Scholar]
- Violle C, Reich PB, Pacala SW, Enquist BJ, Kattge J. 2014. The emergence and promise of functional biogeography. Proceedings of the National Academy of Sciences of the United States of America 111: 13690–13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volis S, Mendlinger S, Olsvig-Whittaker L, Safriel UN, Orlovsky N. 1998. Phenotypic variation and stress resistance in core and peripheral populations of Hordeum spontaneum. Biodiversity and Conservation 7: 799–813. [Google Scholar]
- Volis S, Mendlinger S, Orlovsky N. 2001. Variability in phenotypic traits in core and peripheral populations of wild barley Hordeum spontaneum Koch. Hereditas 133: 235–247. [DOI] [PubMed] [Google Scholar]
- Volis S, Ormanbekova D, Yermekbayev K. 2015. Role of phenotypic plasticity and population differentiation in adaptation to novel environmental conditions. Ecology and Evolution 5: 3818–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33: 125–159. [Google Scholar]
- Wright IJ, Dong N, Maire V, et al. . 2017. Global climatic drivers of leaf size. Science (New York, N.Y.) 357: 917–921. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. . 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Cornelissen JHC, et al. . 2005. Modulation of leaf economic traits and trait relationships by climate. Global Ecology and Biogeography 14: 411–421. [Google Scholar]
- Yu DW, Wilson HB. 2001. The competition-colonization trade-off is dead; long live the competition-colonization trade-off. The American Naturalist 158: 49–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.