Abstract
Questing Ixodes ricinus (Acari: Ixodidae) adult and nymphal ticks collected in various parts of Slovenia were tested for the presence of babesial parasites with a PCR assay based on the nuclear small subunit rRNA gene (nss-ribosomal DNA [rDNA]). Thirteen of 135 ticks were found to contain babesial DNA. Sequence determination and analysis of amplified portions of nss-rDNA revealed their identity with Babesia microti and a high degree of homology with Babesia odocoilei and Babesia divergens. The results of this study represent the first genetic evidence of B. microti and B. divergens-like parasites in I. ricinus ticks in Europe.
Babesiosis, caused by infection with intraerythrocytic parasites of the genus Babesia, is a well-recognized disease of veterinary importance in cattle, horses, and dogs. However, babesiosis is gaining increasing attention as an emerging tick-borne disease in humans (8). The first confirmed case of human babesiosis was documented in the former Yugoslavia in 1957—approximately 100 km from the study sites described in this report (17). Since then, three distinctly different babesial parasites have been recognized as the primary agents of human disease. In Europe, 31 cases of human babesial infection were mainly caused by Babesia divergens (4). Since 1982, over 200 cases due to Babesia microti have been reported in the eastern United States (23). In the western United States, seven cases in humans were attributed to the WA1 type (12). Babesial parasites require both a competent vertebrate host and nonvertebrate host to maintain transmission cycles. All babesial parasites described to date are transmitted by ixodid ticks to their vertebrate hosts (6).
Ixodes ricinus is the most prevalent and widely distributed tick in Slovenia. As in other European countries, I. ricinus is the main vector of the causative agents of Lyme borreliosis, tick-borne encephalitis, and the recently described agent of human granulocytic ehrlichiosis in Slovenia (13, 18; D. Tovornik, data presented at Symposium of Tick-Borne Encephalitis, Slovenian Medical Society, Celje, Slovenia, 1973). The aim of this study was to determine whether I. ricinus ticks collected in Slovenia were infected with babesial parasites.
In the summer of 1997, 69 nymphs and 70 adult I. ricinus ticks were collected by flagging vegetation in the Prealpine and Dinaric regions of Slovenia. The species, stage, and gender were determined by a professional entomologist. DNA was extracted from a single tick by using the QIAamp DNA Mini kit (Qiagen, GmbH, Hilden, Germany). The efficiency of DNA extraction was confirmed by PCR assay, which amplifies the mitochondrial 16S rRNA gene (ribosomal DNA [rDNA]) of tick origin (3). Each sample was tested with the primers PIRO-A and PIRO-B, which were designed to amplify 407-, 408-, and 435-bp fragments of the nuclear small subunit rRNA gene (nss-rDNA) of B. odocoilei, B. divergens, and B. microti, respectively (2, 10). All samples that demonstrated a positive reaction with the PIRO-A and -B primer set were additionally tested with BAB-1 and -4 primers, specific for nss-rDNA of B. microti, as described by Persing et al. (11).
The mitochondrial 16S rDNA of tick origin was amplified in 135 (97.1%) of the 139 samples tested. Four samples in which we were unable to amplify tick DNA were excluded from further analysis. Among 135 I. ricinus ticks, 13 (9.6%; 4 adults and 9 nymphs) were positive when tested with the PIRO-A and -B primer set. Ten of them (7.4%; 3 adults and 7 nymphs), with DNA sizes of 435 bp, were additionally positive when tested with BAB-1 and -4 primers.
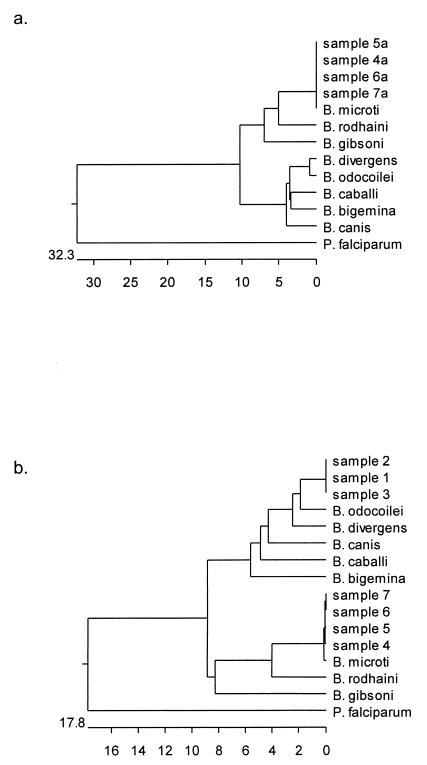
To provide an objective and precise means of identification, amplicons were further characterized by sequence analysis (1) (Fig. 1). Both strand sequences were determined for seven amplicons (three 407 bp, four 435 bp) obtained with PIRO-A and -B primers and for four amplicons of 238 bp obtained with the BAB-1 and -4 primers.
FIG. 1.
Phylogenetic relationships among Babesia species and tested samples inferred from multiple sequence alignment of 190 and 364 bp (primer sequences were removed) of nss-rDNA. (a) Samples 4a, 5a, 6a, and 7a were obtained by amplification with the BAB-1 and -4 primers. (b) Samples 1, 2, 3, 4, 5, 6, and 7 were obtained by amplification with the PIRO-A and -B primers. The sequences obtained were aligned with those of B. divergens (GenBank accession no. U07885), B. odocoilei (U16369), B. caballi (Z15104), B. bigemina (X59607), B. canis (L19079), B. microti (M93660, U09833), B. gibsoni (AF158702), B. rodhaini (M87565), and Plasmodium falciparum (M19172). Units at the bottom of the phylogenetic tree indicate the percentage of nucleotide substitutions.
Three sequences have shown a high degree of homology with B. odocoilei (U16369) (94.5%) and B. divergens (U07885) (93.7%), respectively. Four gene sequences obtained from 364-bp fragments (PIRO-A and -B primers) showed 99.7% identity with B. microti (U09833) and 100% identity with B. microti (M93660) when amplicons obtained with BAB-1 and -4 primers were compared.
In Europe, I. ricinus ticks serve as a vector for many tick-transmitted pathogens (i.e., borreliae, ehrlichiae, tick-borne encephalitis virus, and babesiae), although this has not been proven by molecular techniques for any Babesia sp. (4, 8). Most human infections with babesiae in Europe are believed to be caused by B. divergens. Even though we did not find an exactly identical sequence of B. divergens, experimental data showed that the human strain of B. divergens can be transmitted to cattle and gerbils by I. ricinus (9). However, we should note that in all European human infections described to date, researchers used light microscopy for identification of the implicated Babesia species. There is no deposited sequence of B. divergens causing disease in humans or animals in mainland Europe. Human cases of babesiosis caused by B. microti in Europe are rarely described and were most likely acquired abroad (4). B. microti has also been documented as a parasite of rodents in different parts of Europe (5, 7, 22). The tick that is probably involved in maintenance of B. microti in rodent populations in Europe is Ixodes trianguliceps. It is a nest-dwelling tick that does not readily bite humans (14–16). Walter has described isolation of B. microti from free-living nymphs of I. ricinus by xenodiagnosis with golden hamsters (21). The results of our study indicate for the first time that I. ricinus can indeed carry different babesiae. The obtained prevalence rate (7.4%) for B. microti determined in our study is high and is comparable to the prevalence rate for B. microti in Ixodes scapularis in the United States (19, 20). From these findings, important questions arise. Why are there no indigenous human cases of babesiosis caused by B. microti reported from Europe? Our findings do not support the explanation that this is caused by the selective feeding behavior of the proposed vector I. trianguliceps (8).
In conclusion, our results represent the first study in Europe in which B. microti and B. divergens-like parasites were directly identified in nymphs and adult I. ricinus ticks by PCR and subsequent sequence analysis.
Nucleotide sequence accession numbers.
The unique sequences determined in this study were deposited in GenBank and may be accessed under accession no. AF373331, AF373332, and AF373333.
REFERENCES
- 1.Allsopp M T, Cavalier-Smith T, De Waal D T, Allsopp B A. Phylogeny and evolution of the piroplasms. Parasitology. 1994;108:147–152. doi: 10.1017/s0031182000068232. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong P M, Katavolos P, Caporale D A, Smith R P, Spielman A, Telford S R. Diversity of Babesia infecting deer ticks (Ixodes dammini) Am J Trop Med Hyg. 1998;58:739–742. doi: 10.4269/ajtmh.1998.58.739. [DOI] [PubMed] [Google Scholar]
- 3.Black W C, Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci USA. 1994;91:10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorenflot A, Moubri K, Precigout E, Carcy B, Schetters T P. Human babesiosis. Ann Trop Med Parasitol. 1998;92:489–501. doi: 10.1080/00034989859465. [DOI] [PubMed] [Google Scholar]
- 5.Healing T D. Infections with blood parasites in the small British rodents Apodemus sylvaticus, Clethrionomys glareolus and Microtus agrestis. Parasitology. 1981;83:179–189. doi: 10.1017/s0031182000050149. [DOI] [PubMed] [Google Scholar]
- 6.Homer M J, Aguilar-Delfin I, Telford III S R, Krause P J, Persing D H. Babesiosis. Clin Microbiol Rev. 2000;13:451–469. doi: 10.1128/cmr.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karbowiak G, Stanko M, Rychlik L, Nowakowski W. The new data about zoonotic reservoir of Babesia microtiin small mammals in Poland. Acta Parasitol. 1999;44:142–144. [Google Scholar]
- 8.Kjemtrup A M, Conrad P A. Human babesiosis: an emerging tick-borne disease. Int J Parasitol. 2000;30:1323–1337. doi: 10.1016/s0020-7519(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 9.Lewis D, Young E R. The transmission of a human strain of Babesia divergens by Ixodes ricinusticks. J Parasitol. 1980;66:359–360. [PubMed] [Google Scholar]
- 10.Olmeda A S, Armstrong P M, Rosenthal B M, Valladares B, del Castillo A, de Armas F, Miguelez M, Gonzalez A, Rodriguez J A, Spielman A, Telford S R. A subtropical case of human babesiosis. Acta Trop. 1997;67:229–234. doi: 10.1016/s0001-706x(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 11.Persing D H, Mathiesen D, Marshall W F, Telford S R, Spielman A, Thomford J W, Conrad P A. Detection of Babesia microtiby polymerase chain reaction. J Clin Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persing D H, Herwaldt B L, Glaser C, Lane R S, Thomford J W, Mathiesen D, Krause P J, Philip D F, Conrad P A. Infection with a babesia-like organism in northern California. N Engl J Med. 1995;332:298–303. doi: 10.1056/NEJM199502023320504. [DOI] [PubMed] [Google Scholar]
- 13.Petrovec M, Sumner J W, Nicholson W L, Childs J E, Strle F, Barlič J, Lotrič-Furlan S, Avšič-Županc T. Identity of ehrlichial DNA sequences derived from Ixodes ricinusticks with those obtained from patients with human granulocytic ehrlichiosis in Slovenia. J Clin Microbiol. 1999;37:209–210. doi: 10.1128/jcm.37.1.209-210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randolph S E. Density-dependent acquired resistance to ticks in natural hosts, independent of concurrent infection with Babesia microti. Parasitology. 1994;108:413–419. doi: 10.1017/s003118200007596x. [DOI] [PubMed] [Google Scholar]
- 15.Randolph S E. Patterns of distribution of the tick Ixodes triangulicepsBirula on its hosts. J Anim Ecol. 1975;44:425–429. [Google Scholar]
- 16.Randolph S E. Quantifying parameters in the transmission of Babesia microti by the tick Ixodes trianguliceps amongst voles (Clethrionomys glareolus) Parasitology. 1995;110:287–295. doi: 10.1017/s0031182000080872. [DOI] [PubMed] [Google Scholar]
- 17.Skrabalo Z, Deanovic Z. Piroplasmosis in man. Doc Med Geogr Trop. 1957;9:11–16. [PubMed] [Google Scholar]
- 18.Strle F, Cheng Y, Nelson J A, Picken M M, Bouseman J K, Picken R N. Infection rate of Ixodes ricinus ticks with Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferisensu stricto in Slovenia. Eur J Clin Microbiol Infect Dis. 1995;14:994–1001. doi: 10.1007/BF01691382. [DOI] [PubMed] [Google Scholar]
- 19.Telford S R, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varde S, Beckley J, Schwartz I. Prevalence of tick-borne pathogens in Ixodes scapularisin a rural New Jersey county. Emerg Infect Dis. 1998;4:97–99. doi: 10.3201/eid0401.980113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter G. Isolation of Babesia microti (Franca 1912) from free-living nymphs of Ixodes ricinus(Linnaeus 1758) Acta Trop. 1981;38:187–188. . (Author's translation.) [PubMed] [Google Scholar]
- 22.Walter G, Liebisch A. Studies of the ecology of some blood protozoa of wild small mammals in North Germany. Acta Trop. 1980;37:31–40. . (Author's translation.) [PubMed] [Google Scholar]
- 23.White D J, Talarico J, Chang H G. Human babesiosis in New York State: review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med. 1998;158:2149–2154. doi: 10.1001/archinte.158.19.2149. [DOI] [PubMed] [Google Scholar]