Abstract
Cold stress limits plant geographical distribution and influences plant growth, development, and yields. Plants as sessile organisms have evolved complex biochemical and physiological mechanisms to adapt to cold stress. These mechanisms are regulated by a series of transcription factors and proteins for efficient cold stress acclimation. It has been established that the ICE-CBF-COR signaling pathway in plants regulates how plants acclimatize to cold stress. Cold stress is perceived by receptor proteins, triggering signal transduction, and Inducer of CBF Expression (ICE) genes are activated and regulated, consequently upregulating the transcription and expression of the C-repeat Binding Factor (CBF) genes. The CBF protein binds to the C-repeat/Dehydration Responsive Element (CRT/DRE), a homeopathic element of the Cold Regulated genes (COR gene) promoter, activating their transcription. Transcriptional regulations and post-translational modifications regulate and modify these entities at different response levels by altering their expression or activities in the signaling cascade. These activities then lead to efficient cold stress tolerance. This paper contains a concise summary of the ICE-CBF-COR pathway elucidating on the cross interconnections with other repressors, inhibitors, and activators to induce cold stress acclimation in plants.
Keywords: cold stress, Inducer of CBF Expression, C-repeat Binding Factor, cold response genes, transcription factors, plant
1. Introduction
Cold stress diminishes plant growth, development, yield, and the geographical distribution of crops, liable for ~40% harvest reduction of crops in temperate regions [1]. It is estimated that extreme cold stress causes between 51–82% of annual crop yield losses globally [2]. Cold stress has been categorized into chilling stress (0–15 °C) and freezing stress (<0 °C) depending on plant effects [3] Cold receptors localized in the plant plasma membrane perceive cold stress stimulus. Instantly, a progression of cell reactions and sub-atomic system adjustments are triggered, remodeling plant physiological, biochemical, and molecular mechanisms for cold stress tolerance through the regulatory actions of numerous transcription factors [4,5,6]. The three main cold-responsive genes in plants are Inducer of CBF Expression (ICE), C-repeat Binding Factors (CBFs), and the Cold-Regulated genes (CORs) [7]. These three forenamed key players, ICE, CBF, and COR genes, model an imperative signaling pathway, the ICE-CBF-COR cascade, a cold response pathway that alleviates cold stress in plants [8,9,10,11,12]. Usually, plant cold stress tolerance is characterized by a decrease in plant water losses, reduced plant growth, decreased photoperiod, and other physiological changes [13]. To date, several plant species genomes have been characterized and the ICE-CBF-COR cascade has been identified in rice [14], wheat [10,15], and tea [16].
The ICE acts upstream to induce and regulate the expression of the C-repeat Binding Factor (CBF) [8,9,10,11]. Consequently, the CBFs otherwise known as the DREB1 genes, regulate cold stress by binding to the cold and dehydration regulatory elements (CRT/DRE) in the promoter regions of COR genes to induce their expression; for instance, COR15A [17,18] and RD29A [19] in Arabidopsis. Thus, CBFs trigger and regulate the expression of COR genes under cold stress. Amongst these three aforementioned genes, perhaps CBF genes are the most vital cold response factors in plants, other researchers have also published diverse roles and responses in different plant species played by the C-repeat Binding Factor/dehydration-responsive element-binding 1 (CBF/DREB1) genes [10,12]. It is established that CBFs (CBF1, CBF2, and CBF3) have different roles under cold stress due to their several modifications in their individual protein sequences, although they have similar sequence structures and binding properties [1,10,20]. Two homologs of ICE genes (ICE1 and ICE2) have been characterized in many plant species and their cold tolerance roles were deduced [21]. The activity of the ICE1 is mainly regulated at the protein level by post-transcriptional and/or post-translational modifications (PTMs). Recent research has shown the importance of PTMs in regulating the ICE-CBF cascade pathway during cold stress [22,23,24]. Several PTMs have been shown to increase the stability and binding efficiency of ICE genes to downstream genes for instance: phosphorylation, ubiquitination, and SUMOylation [25]. Phosphorylation is one of the most vital post-translational modifications of ICE genes, regulating the cold stress tolerance through the actions of the OPEN STOMATA 1 (OST1) and other various transcription factors. The OST1 mediates the ICEs and CBFs in various ways. OST1 has been demonstrated to phosphorylate the ICE1 in Arabidopsis and rice for stability, by binding to the HIGH EXPRESION OF OSMOTICALLY RESPONSIVE GENE 1 (HOS1) to prevent the degradation of ICE1 by HOS1. Furthermore, the OST1 regulates CBF gene expression by phosphorylating BASIC TRANSCRIPTION FACTOR 3 (BTF3), a binding substrate to CBF genes [24]. Moreover, kinases within the MITOGEN-ACTIVATED PROTEIN KINASE (MAPK) cascade play an essential role in the phosphorylation of ICE genes; ICE1 is phosphorylated at the Ser403 for stability and CBF regulation activation in the MAPK cascade [26]. In potatoes, SaMMK2, a constitutive kinase, was recently shown to positively promote the expression of SaCBF under cold stress, leading to cold stress tolerance through expression activation of COR genes [27].
Ubiquitination-regulated turnover of the ICE-CBF proteins improves cold stress tolerances in plants [28]. Little has been shown recently on the ubiquitination mechanism regulating the ICE-CBF-COR cascade. However, a PUTATIVE U-BOX type E3 ligase gene in grapevine, VpPUB25/26, was shown to promote the accumulation of VpICE1 and suppress the expression of VpHOS1 [29]. PUB25/26 was demonstrated to degrade the MYB15, an inhibitor of the ICE-CBF pathway during cold stress, thereby increasing the expression of ICE1 [30]. Additionally, SINA, a ubiquitin ligase in bananas was also reported to increase the stability of MaICE1 and to improve transcriptional activation of the CBF regulon [31].
In addition to PTMS, the ICE-CBF is also regulated by the hormonal responses of jasmonates (JA), ethylene, brassinosteroids (BR) [32], gibberellin (GA) [33], auxin, and salicylic acid (SA). Numerous auxin-related genes have been thoroughly discussed which include auxin biosynthetic genes (CYP79B3 and CYP83B1) and auxin carrier genes (LAX1/2), and their down-regulatory effect in CBF expression [34]. Interestingly, exogenous treatment of several hormones on plants during cold stress has also been demonstrated to relieve the cold stress in plants. For instance, in the GA-CBF crosstalk, exogenous application of GA has been shown to regulate the over-expression of CBFs in dwarf plants, while underlying mechanisms still require more research. Other phytohormones are discussed in detail below.
Accumulating evidence has shown that most of the cold stress tolerances are due to the targets of CBFs, the COR genes. In Arabidopsis, more than 200 COR genes are either activated or repressed by the actions of the CBF1/2/3 [11]. A myriad of COR genes has been identified and demonstrated to increase cold stress tolerance directly or indirectly in plants. These include the plant Dehydrins (Dhns), late-embryogenesis-abundant (LEA) proteins, low-temperature induced proteins (LTIs) and their products: anti-freeze proteins [35] osmo-regulators [36], chaperones, functional proteins, and kinases [17,18].
This review paper sums up recent studies and findings on the ICE-CBF-COR cold signaling pathway, discussing how plants continue to evolve for cold stress acclimation. These insights will enrich the plant stress response knowledge base, providing vital information on how to ameliorate plant losses due to cold stress in the wake of global climate change.
2. Conserved Motifs and Their Functionality in CBF, ICE, and COR Genes
2.1. C-Repeat Binding Factor/Dehydration-Responsive Elements Binding 1 (CBF/DREB1)
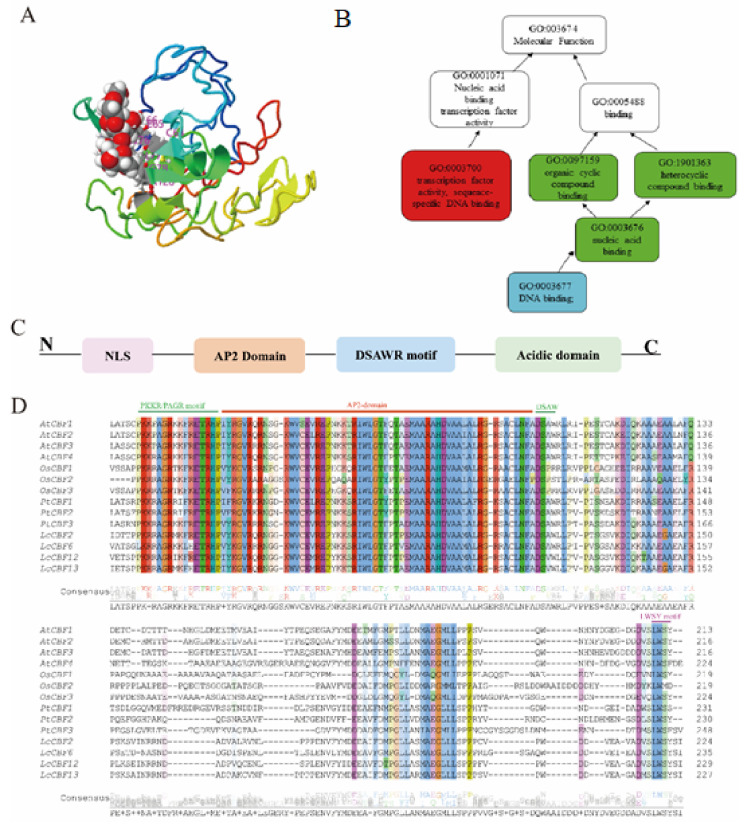
CBF transcription factors are involved in the cold signaling pathway in plants [24,37]. They were first discovered by Jofuku et al. [37] in Arabidopsis thaliana as plant-specific transcription factor types triggered by cold stress and/or the ICE [38]. CBFs belong to the superfamily of APETALA2/Ethylene Responsive (AP2/ERF) transcription factors, composed of c. 60 amino acid residues, and conferring a three-dimensional (3D) conformation arranged into a layer of three antiparallel β-sheets followed by a parallel α-helix sheet [39]. The 3D structure protein prediction analysis (Figure 1A) exposed Arg- and Try- residues within the β-sheet that link nucleotides of the binding site in the key groove of the DNA. Moreover, these key residues are well conserved in the AP2/ERF family [40]. The Dehydration Responsive Binding Factor/C-repeat Binding Factor (DREB1/CBF) family is distinguished by PKK/KPAGRxKFxETRHP, DSAWR sequence signatures, and an LSWY motif, schematically shown in Figure 1C. Medina et al. [41] first sequenced the CBF gene structure in Arabidopsis and revealed that CBF1/2/3 genes are clustered on chromosome IV, with CBF2 and CBF3 located 3 and 7 kb downstream of CBF1, respectively [41]. In addition, they showed the presence of several regulatory sequences: the core CANNTG-consensus motif, the CACGTC-, and TACGTG-related sequences in their promoter regions [42]. CBFs (CBF1/DREB1B, CBF2/DREB1C, and CBF3/DREB1A) are known to bind to the C-repeat/Dehydration Responsive Element (CRT/DRE) sequence (TACCGCAT) in the promoters of COR genes for their transcription activation. Gene ontology (GO) analysis (Figure 1B) of CBFs revealed that their main molecular function is in the binding to cold-responsive genes for cold stress tolerance, through the CRT/DRE binding domains [43]. Recent reports have exhibited several CBF amino acid sequences from other plant species with a higher homology, carrying similarly conserved motifs (Figure 1D) [10,44,45]. Additionally, Novillo et al. [46] paraded a negative feedback mechanism of the CBF/DREB1 transcription factors, that CBF2/DREBIC negatively regulates the expression of CBF1/DREB1B and CBF3/DREB1A in Arabidopsis. Likewise, overexpressed CBF1/DREB1B inhibits the accumulation of CBF3/DREB1A transcripts. However, mutational changes in CBF2 (cbf2) enhance the collection of CBF1/DREB1B and CBF3/DREB1B transcripts leading to cold stress tolerance through the expression of COR genes. However, this negative feedback is essential for the accurate expression of cold regulatory genes in response to cold stress.
Figure 1.
Structure, GO analysis, and sequence alignment of CBF in plants. (A) 3D prediction of AtCBF1 secondary structure showing different domains denoted by different colors. (B) Gene ontology (GO) analysis of CBF1 in Arabidopsis. (C) The schematic presentation of the AP2 structure, shows PKK/KPAGRxKFxETRHP, DSAWR, and LWSY motifs located upstream and downstream respectively, from the AP2 domain. These sequences contribute to the DNA binding specificity of CBFs to COR genes. (D) Multiple alignments of the amino acid sequences of CBF/DREB1 proteins from different plant species. Different color schemes in the background show conserved amino acid sequences within the conserved AP2 DNA-binding domains, PKKR/PAGR, DASW, and LWSY, motifs. Characterized sequences include AtCBF (Arabidopsis thaliana), OsCBF (Oryza sativa), LcCBF (Liriodendron chinense), and PtCBF (Populus trichocarpa).
Further Hannah et al. [47] demonstrated that there is an inherited relationship between the total number and expression levels of CBFs and cold stress tolerance [48,49] and that some CBFs are specific to dicots, while others are specific to monocots and exhibit different response patterns during cold stress [50]. Therefore, there is a shred of cumulative evidence on the functional roles of the CBFs in plants. Furthermore, CBFs have been expressed in several transgenic plants, and their effect revealed. Table 1 summarizes some of the CBFs that augmented cold stress in transgenic plants. Moreover, previous studies have demonstrated the functional roles of CBF1/2/3 in Arabidopsis to bind to the promoters of target COR genes (COR15A, COR47, COR78, KIN1, and LTI78), inducing their expression for cold stress regulation. They all concurred that CBFs are induced by MYC-like bHLH and AtICE2, via the AtCAMTA3 promoter [51,52]. A recent study in Longan (Dimocarpus longan) has identified three novel CBF genes, DlCBF1/2/3, that bind the CRT/DRE cis-elements, inducing the expression of AtRD29A, AtCOR15A, AtCOR47, and AtKIN1 consequentially in plant cold acclimation [53].
Table 1.
Transgenic plants developed by the overexpression of CBF genes.
| Gene | Species | Transgenic Technique | Transgenic Plant | Effect | References |
|---|---|---|---|---|---|
| DlCBF1-3 | D. longan | Agrobacterium-mediated transfer | A. thaliana | cold stress tolerance | [53] |
| PpyCBF1-3 | P. pyrifolia | Agrobacterium-mediated transfer | A. thaliana | cold tolerance | [54] |
| IbCBF3 | Sweet potato | Agrobacterium-mediated transfer | S. tuberosum | cold tolerance | [55] |
| AtCBF3 | A. thaliana | Agrobacterium-mediated transfer | S. melongena L. | cold stress tolerance | [56] |
| EgCBF3 | E. guineensi | Agrobacterium-mediated transfer | L. esculenta | freezing tolerance | [57] |
| PpCBF3 | P. pratensis L. | Agrobacterium-mediated transfer | A. thaliana | freezing tolerance | [58] |
| GmDREB1B | G. max | Agrobacterium-mediated transfer | G. max | cold tolerance | [59] |
| DaCBF7 | D. antarctica | Agrobacterium-mediated transfer | O. sativa | cold tolerance | [60] |
| PpCBF1V | P. pratensis L. | Agrobacterium-mediated transfer | M. domestica | cold tolerance | [61] |
| AtCBF1 |
A. thaliana
|
Agrobacterium-mediated transfer | S. lycopersicum | freezing tolerance cold tolerance |
[62] |
| OsDREB1B | O. sativa | Agrobacterium-mediated transfer | N. tabacum | cold tolerance | [63] |
| HvCBF4 | H. vulgare | Agrobacterium-mediated transfer | O. sativa | Regulates cold stress | [64] |
| TaDREB2 | T. aestivum | Agrobacterium-mediated transfer | Hordeum vulgare | freezing tolerance | [65] |
| BnCBF5/17 | B. napus | Agrobacterium-mediated transfer | Brassica napus | freezing tolerance | [66] |
A few reports have stated the equal importance of CBF1/2/3 in Arabidopsis for cold tolerance, while other researchers have proposed that only AtCBF2/3 play significant roles in cold stress tolerance [56,57,58]. Salvo et al. [67] also revealed the importance of CBF1 in cold induced (CI) citrus cultivars, participating in natural cold stress tolerance by triggering the expression of downstream COR genes. They concluded that CBF1 is essential for cold tolerance in citrus fruits. A recent study in Asian pears (Pyrus pyrifolia) has shown the functional roles of PpyCBF3 for cold tolerance. They showed that expressed PpyCBF2/3 were linked to the expression of PpyCOR genes (PpyCOR47, PpyCOR15, PpyRD29A, and PpyKIN). They expressed PpyCBF 2/3 genes in transgenic Arabidopsis and augmented cold tolerance through the lowering of ROS species, and antioxidant gene activities, suggesting that PpyCBF2 and PpyCBF3 were responsible for the expression of COR genes [54]. Nevertheless, it can be inferred that the importance of CBF proteins depends on the plant species and all CBFs are vital and unique in function for cold tolerance.
2.2. Inducer of CBF Expression (ICE)
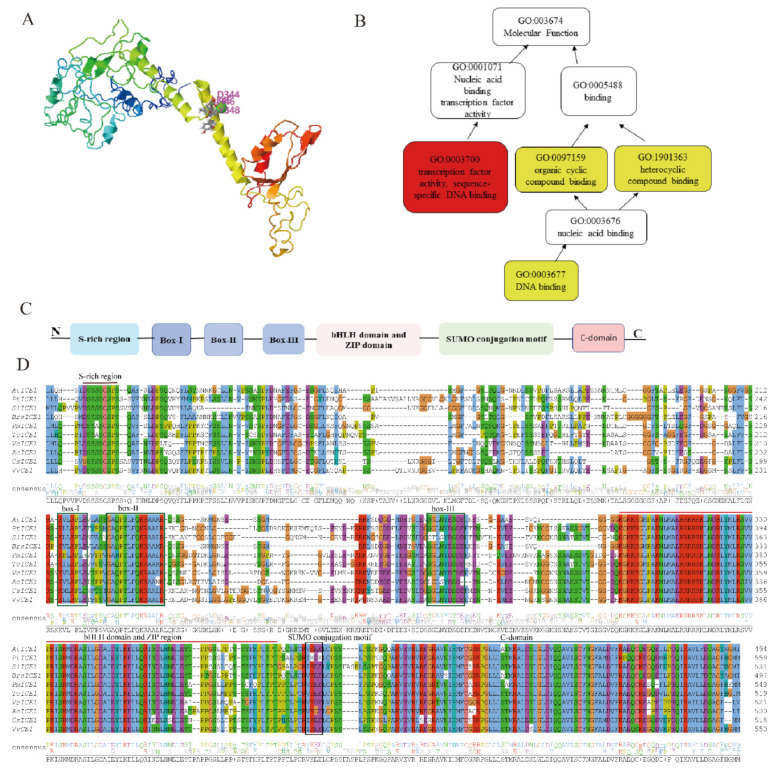
The ICE is a forerunner in the cold acclimation process that acts upstream of the cold response cascade [68,69]. It belongs to the basic Helix-Loop-Helix (bHLH) family. The bHLH transcription factors regulate the expression of cold regulatory genes; they contain conserved bHLH binding domains at C-terminals, as shown in Figure 2C, for specific interactions with downstream cold regulatory genes. The ICE was reported to carry the bHLH binding domain, and its amino acid sequence in the basic region is highly similar to other bHLH proteins. The ICE proteins bind to the canonical MYC cis-elements (CANNTG) in the CBF3/DREB1A promoter, leading to the induction of CBF/DREB1 regulon [70,71]. Two isoforms of the ICE protein have been identified in Arabidopsis, ICE1, and ICE2, consisting of 494 and 450 amino acids, respectively. Distinguished by the presence of an additional amino acid box in ICE2, towards the end of Box II (Figure 2C,D), modifying the conserved LPPT sequence, and also the absence of Box I in the ICE1 genes [72]. The Glu- and Leu-rich regions of the ICE2 localized in the exon part, form additional alpha-helices in the secondary structure (Figure 2A). Additionally, the structure of ICE2 has more phosphorylation sites than ICE1, otherwise, their secondary structures are similar, and they both include four exons and three introns [73]. Gene ontology (GO) enrichment analysis of AtICE1 (Figure 2B) showed that the ICE1 binding sites are enriched in several categories including nucleic acid binding (GO:0001071), an organic cyclic compound binding site (GO: 0097159), and heterocyclic compound binding (GO: 1901363) [71]. Thus, its main molecular function is for binding downstream of CBF genes.
Figure 2.
Structure, GO analysis, and sequence alignment of ICE genes in plants. (A) 3D prediction of AtICE1 secondary structure, showing different domains denoted by different colors. (B) Gene ontology (GO) analysis of ICE1 in Arabidopsis. (C) A schematic presentation of AtICE1, depicting conserved binding domains and motifs. (D) Multiple alignments of ICE amino acid. Different color schemes in the background show conserved amino acid sequences within the conserved DNA-binding domains, the S-rich region, bHLH domain, the ZIP region, and the SUMO-conjugated motif in the ICE1 proteins. Shown sequences have been characterized from AtICE1 (A. thaliana), SlICE1 (S. lycopersicum), PtICE1 (P. trifoliata), PmICE1 (P. mume), VvICE1 (V. vinera), and CsICE1 (C. sinensis).
Badawi et al. [15] demonstrated that the ICE1 is specific to monocots and ICE2 is specific to eudicots. However, other ICE1-like proteins are also present in dicots and they show high homology in the C-terminus region [69]. Many different types of ICE-like genes with homologous conserved domains have been recently revealed and expressed in various transgenic plants for tolerance investigation to cold stress (Table 2). Recently, Kashyap et al. [73] showed an ICE homolog, BjICE53, to be involved in the cold signaling pathway in Brassica juncea. They revealed conserved domains and motifs that bind to the CRT/DRE motifs of BjCBF for the expression of downstream cold-regulatory genes [74,75,76]. Another study in Chrysanthemum morifolium, “Jinba”, demonstrated that overexpression of CmICE2 in transgenic Arabidopsis induces the expression of downstream cold regulatory genes (AtCBF1/2, AtCOR6.6a/414, and AtKIN1), leading to cold stress tolerance through increased proline contents, superoxide dismutase (SOD) activities, and elevating catalase (CAT) levels [77]. Zuo et al. [78] also revealed the biological roles of ICE1 in Zoysia japonica (ZjICE1) to positively regulate the cold response signaling pathway. They disclosed that the overexpression of ZjICE1 triggers the expression of cold regulatory genes (ZjCBF1-3 and ZjCOR47A).
Table 2.
The response of transgenic plants developed by overexpression of ICE homologous.
| Gene | Species | Transgenic Technique | Transgenic Plant | Effect | References |
|---|---|---|---|---|---|
| SiICE1/2 | S. involucrata | Agrobacterium-mediated transfer | Arabidopsis | cold tolerance | [75] |
| AtICE1 | A. thaliana | Agrobacterium-mediated transfer | Indica rice | cold regulation | [21] |
| CmICE2 | C. morifolium | Agrobacterium-mediated transfer | Arabidopsis | cold tolerance | [77] |
| BjICE46/53 | B. juncea | Agrobacterium-mediated transfer | Arabidopsis | cold tolerance | [73] |
| HbICE1/2 | H. brasiliens | Agrobacterium-mediated transfer | Arabidopsis | cold tolerance | [79] |
| ZjICE2 | Z. japonica | Agrobacterium-mediated transfer | Arabidopsis | cold tolerance | [78] |
| RsICE1 | R. sativus | Agrobacterium-mediated transfer | Rice | cold tolerance | [80] |
| OsICE1/2 | O. sativa | Agrobacterium-mediated transfer | Arabidopsis | cold tolerance | [81] |
| ZmmICE1 | Z. mays | Agrobacterium-mediated transfer | Arabidopsis | freezing tolerance | [82] |
| SlICE1a | S. lycopersicum | Agrobacterium-mediated transfer | Tobacco | cold tolerance | [83] |
| TaICE41/87 | T. aestivum | Agrobacterium-mediated transfer | Arabidopsis | freezing tolerance | [20] |
2.3. Cold Regulated (COR) Genes
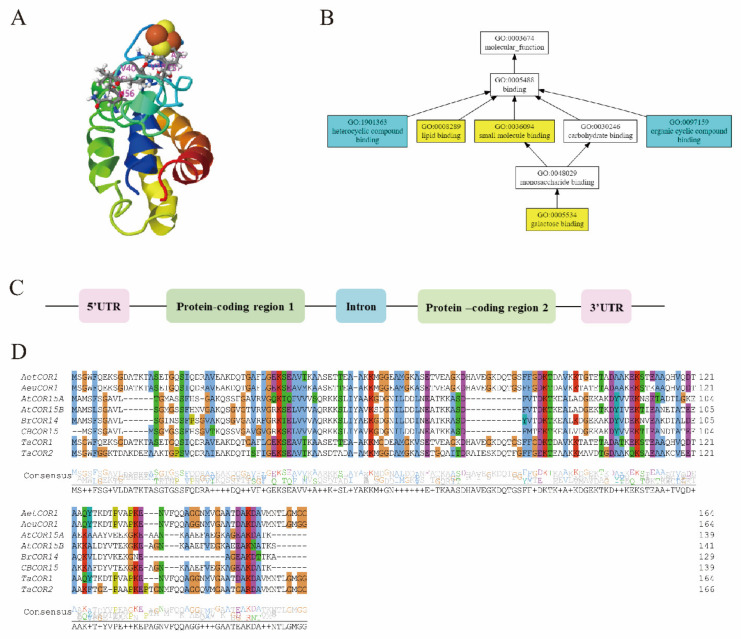
Several reports have shown that cold-inducible genes designated as Cold-responsive or Cold Regulated Genes (COR genes), ABA-inducible protein-coding (KIN1 and KIN2) [84], Responsive to Desiccation (RD), and Low-Temperature-Induced (LTI) genes carry the CRT/DRE cis-acting element augmenting cold stress tolerance through the CBF-dependent pathway [11]. CBFs bind to the C-repeat (CRT/DRE) cis-elements located in the promoters of COR genes denoted by a CCGAC sequence, further activating their transcription [85]. About 10–20% of the total COR genes in Arabidopsis are estimated to be directly regulated by CBFs [86]. Most studied COR gene structures are flanked by exons (protein-coding regions) localized both in the 5′UTR and 3′UTR with a central intron, schematically shown in Figure 3C. However, different COR gene families are distinguished by specific motifs, but all share a conserved CRT/DRE binding site that binds upstream of CBF genes for their expression [87]. Several COR genes in the cold signaling pathway have been characterized and some of their amino acid sequences are shown in Figure 3D, showing several conserved domains within the COR genes. Therefore, plants respond to cold stress in three discrete phases depending on the temperature range, that is, pre-hardening, hardening, and plant recovery [88]. Specific COR genes act to stabilize both membrane phospholipids, proteins, and cytoplasmic proteins, maintaining hydrophobic interactions, ion homeostasis, and scavenging ROS, depending on the temperature range [89,90].
Figure 3.
Structure, GO analysis, and sequence alignment of COR genes in plants. (A) 3D prediction of AtCOR15A secondary structure, showing different domains denoted by different colors and helices formed by different interactions of domains. (B) Gene ontology (GO) analysis of AtCOR15A. (C) The schematic presentation of plant COR genes with two flanking exons in the 5′UTR and the 3′UTR and a central intron. (D) Multiple amino acid sequence alignments of different COR genes. Different color schemes in the background show conserved amino acid sequences in different COR genes. Aligned sequences include: AetCOR1, AeuCOR1, AtCRO15A/B, BrCOR15, and TaCOR1/2.
Previously, different targets of the CBF genes were discussed fully. We will partially discuss a few COR gene families in this section. The COR413 family has two distinct groups, COR413-plasma membrane (COR413pm), COR413-inner membrane 1 (COR413im1), and COR413-thylakoid membrane (COR413tm) [91]. It is known that low temperature influences the structure of the plasma membrane by reducing the fluidity and increasing rigidity, with these changes leading to the expression of COR413pm genes. Recent studies have revealed that cold-induced PsCOR413Pm2 [92] and AtCOR413pm [93] carry similarly conserved binding domains in their promoter regions. The COR413pm genes regulate cold stress through enhancing the Ca2+ influx and the expression of stress-related COR genes (COR6.6, KIN2, COR15A, COR15B, COR47, and COR78/RD29) and CBF (CBF2 and CBF3) genes in Arabidopsis. These results suggest the interconnection with the cold-responsive genes, concurring with the ICE-CBF-COR cascade. While the COR413im localized in the inner-membrane was shown to activate the cold-expression of COR15A and COR15B in Arabidopsis, their expression mechanism still remains a mystery to be unrevealed [92].
Dehydrins (DHNs) are a subgroup of the Late-Embryogenesis-Abundant (LEA) proteins in angiosperms. They are characterized by high hydrophilicity and a diverse combination of typical domains. Most notably, the K-segment (EKKGIMDKIKEKLPG) sequence near the C- terminus. They accumulate in plants in response to cold stress, particularly, the SKn type, which protects the membrane from freeze desiccation by potential dehydration-induced demixing of membrane lipids, acting as molecular chaperones or ion sequestration [94]. For instance, the AtCOR15A with its secondary structure (Figure 3A), is suited for binding to other proteins and acts as a chaperone protecting the membrane from freeze desiccation. GO analysis of COR15A (Figure 3B) has provided supporting evidence on the molecular binding function of AtCOR15A to lipids, carbohydrates, heterocyclic compounds, and other small molecules [95]. Previous research evidenced dehydrins (OsDhn1, lip5, and lip9) to regulate cold stress through the CBF pathway in rice, and their homologs Wcor410 and AtCOR47, which are both known to be regulated by CBF1/DREB1B. Apart from these aforementioned dehydrins, several DHN proteins have been shown to regulate cold stress through the CBF pathway including, Wcs120, COR47, and RD17. Recently, research has evidenced that CBF1 identifies the consensus sequence (CCGAC) of the CRT/DRE elements from Dehydrins in Vitis vinera and Triticeae species [96].
Low-Temperature Induced proteins (LTIs) enable plants to acclimate during low but non-freezing temperatures. Two LTIs have been shown, LTI78 and LTI 65 in Arabidopsis, to regulate cold stress and carry a 9 bp conserved sequence (TACCGACAT) in their promoter regions, termed the dehydration-responsive element (DRE) (Figure 3D) [35]. COR genes have also been demonstrated to act as regulators of other cold regulatory genes. Recently, the COR27/28 genes were reported to regulate the COP1-HY5 regulatory hub influencing the freezing tolerance and the circadian clock. These genes interact directly with HY5 promoters and regulate negatively the transcription of other COR genes promoting hypocotyl elongation in Arabidopsis [97]. Several COR genes have been expressed in different transgenic plants and their regulatory effect revealed. Table 3 below summarizes reports of different COR genes that were expressed in other transgenic plants.
Table 3.
The transgenic plants developed by overexpression of COR genes.
| Gene | Species | Transgenic Technique | Transgenic Plant | Effect | References |
|---|---|---|---|---|---|
| LeCOR413PM2 | L. esculanta | A. tumefaciens | Tomato | cold tolerance | [97] |
| AtCOR27/28 | A. thaliana | A. tumefaciens | Arabidopsis | freezing tolerance | [11] |
| MfLEA3 | M. falcata | A. tumefaciens | Tobacco | cold tolerance | [98] |
| SikCOR413PM1 | S. involucrate | A. tumefaciens | Tobacco | cold tolerance | [99] |
| SiDHN | S. involucrata | A. tumefaciens | Tomato | cold tolerance | [100] |
| PsCOR413PM2 | P. subulate | A. tumefaciens | Arabidopsis | cold tolerance | [91] |
| RcDhn5 | R. catawbiense | A. tumefaciens | Arabidopsis | freezing tolerance | [101] |
3. Mitogen-Activated Protein Kinase (MAPK) Cascade and Hormonal Responses Regulating the ICE-CBF-COR
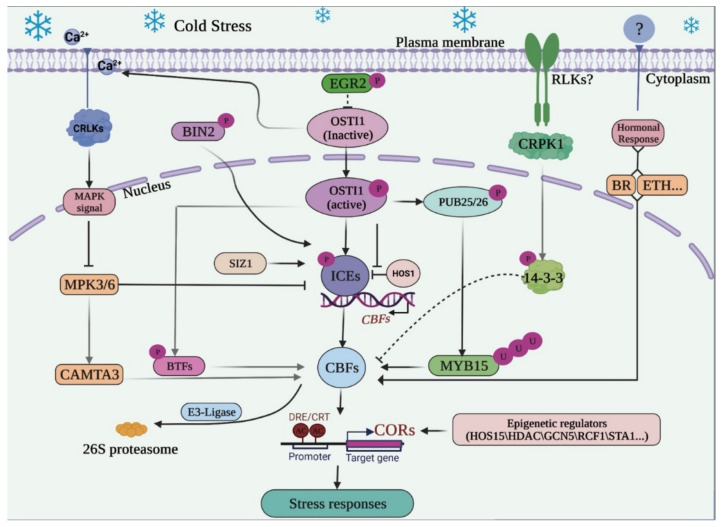
Putative sensors embedded in the plasma membrane such as the OsCGNC14/16 in rice [102] and AtNN1 in Arabidopsis [103] trigger Ca2+ influx in the cytosol and other cell organelles through Ca2+ channels as a secondary response to cold stress. Secondary messengers, Ca2+-dependent proteins, retort to cold stress, playing an imperative role in intracellular signal transduction [104]. They bind to several proteins (TFs, protein kinases, ion channels, and other enzymes) including calmodulins to execute their regulatory functions. Therefore, Ca2+/CaM-dependent proteins have been reported in various plants including: Vitis vinifera [105], Zea mays L [106], soybean [107], Brassica napus [108], Populus trichocarpa [109], citrus trees [110], and other Gossypium species [111]. Kinases and their profile expressions under cold stress have been recently reported in Brassica napus [112], Jatropha curcas [113], and Common vetch [114]. The Mitogen-Activated Protein Kinase (MAPK) cascade regulates cold stress through the binding roles of Calcium/Calmodulin-Regulated Receptors Kinase-Like 1 (CRKL1) [115]. Research on the MAPK cascade in Arabidopsis has demonstrated that CRLK1/2 interacts with the MEKK1, a MAPK module responding to lower temperatures [116]. Then, the MEKK1 sequentially phosphorylates the MKK2, in turn activating MPK4/6 [117], formulating a pathway upstream, CRLK1-MEKK1-MKK2-MPK4-MPK3/6, that enhances the expression of CBF genes [118]. Previous studies reported on an MPK3/6-CBF enhancing substrate, the calmodium-binding transcriptional activator 3 (CAMTA3), a putative MPK3/6 substrate with five phosphopeptides and MAPK phosphorylation sites, that activates MAPKs in the MPK3/6-CAMTA3 module. The CAMTA3 binds to the CBF2 promoters to induce the expression of COR genes in Arabidopsis [119]. Put together, these entities model a series of phosphorylation reactions after the Ca2+ influx, the Ca2+/CaM-CRLK1/2-MEKK1-MKK2-MKK2-MPK3/6-CAMTA3-CBF2, to enhance the expression of CBF2 and consequent downstream COR genes (Figure 4). Early research showed that the overexpression of CAMTA3 induces the expression of RD29A and COR6.6 through CBF regulations [120]. Likewise, a comprehensive set of experiments has shown the importance of this pathway in the induction of the AtCBF2, by proving that mutants of mpk3, mpk5, and camta3 are freezing sensitive [121]. Meanwhile, another pathway, the CRLK1/2-MKK4/5-MPK3/6, negatively regulates cold stress tolerance by reducing CBF expression through inhibiting the ICE transcription (Figure 4). The MPK3 binds to the promoters of ICE1, promoting its degradation and consequently reducing the transcription of the CBFs [122,123]. Nonetheless, both mpk3 and mpk6 mutants have been shown to increase CBF expression leading to cold stress resistance in plants [84]. Wholly, these two regulatory pathways may be viewed as a single negative feedback mechanism that regulates the expression of cold regulatory genes. In Arabidopsis, phosphorylated MPK6 mediates the negative expression of CBF3 by activating a negative regulator, MYB15 [122]. On the contrary, MPK4/6 activates CBF expression by inhibiting the MKK4/5-MPK3/6 pathway [112]
Figure 4.
The ICE-CBF-COR response pathway to cold stress initiates at the plasma membrane to plant cold tolerance. Cold sensors localized in the plasma membrane sense cold stress and an influx of Ca2+ ions trigger the calcium downstream effector, CRLKs in the calcium response channel. Consecutively, triggering the MAPK cascade, through the activities of MPK3/6, and directly inhibiting the ICE2 and/or activating the CBF genes through the CAMTA3. Resulting in enhanced expression of COR genes and cold tolerance. Another receptor, RLK phosphorylates the 14-3-3, stabilizing it for translocation into the nucleus, and inhibiting the CBF transcripts expression. Hormonal responses in the ethylene, BR, and JA hormones directly enhance the expression of CBFs through triggering various TFs. The ICE genes are further phosphorylated through several PTMs to regulate the expression of CBFs, sequentially regulating the expression of COR genes and cold stress response.
Likewise, the hormonal response controls vital biochemical regulatory processes in the ICE-CBF-COR cascade during plant cold stress. Important plant hormones in the cold signaling pathway are brassinosteroids (BR), jasmonates (JA), ethylene (Eth), and Abscisic acid (ABA) (which will not be discussed in this review). Jasmonate including its derivatives, methyl jasmonate (MeJA) and jasmonic acid are called jasmonates (JA). Cold stress in plants has been established to elevate endogenous jasmonates (JAs) biosynthesis. In the same manner, JAs also increase cold stress tolerance by interfering in the inhibitory effect of JASMONATE ZIM-DOMAIN 1/2 (JAZ 1/2) proteins on the transcriptional activity of CBFs [124]. Recently, An et al. [125] demonstrated the role of MdBBX37, that is its binding effect to the MdCBF promoters activating the expression of MdCBF in the BBX37-ICE1-CBF module. Further analysis of this pathway in the rubber tree also exhibited that exogenous treatment with methyl jasmonate (MeJA) weaken the inhibition of JAZ1/2 on the HbICE2 transcriptional hub, resulting in the upregulation of HbCBF1, HbCBF2, and HbCOR47. These findings suggest that the relieved and expressed HbICE2 prompt the expression of HbCBF1 and consequently HbCOR47. In the rubber tree, JAZ1/2 proteins bind to the F-box protein receptor (COI1), a ubiquitin ligase of the SCF complex, inhibiting the activation of ICE2 and downstream genes, and in apple trees, MdJAZ1/2 inhibit the binding of MdBBX37 to MdCBF1/4 reducing CBF expression [79,126]. However, cold-elevated endogenous jasmonic-acid levels in apple plants relieve the repressive effect of JA-repressors (JAZ1-2) on the MdBBX37. Exogenous application of MeJA and its regulating effect on cold stress has been demonstrated in several plant species including C. annuum [126], Musca acuminate [127], and Arabidopsis [124]. Previous studies showed the interaction of jasmonate with other hormones such as auxins and ethylene to regulate the ICE-CBF-COR cold signaling pathway [128,129]. For instance, in the JA-auxin crosstalk, IAA29 a type of auxin interferes with the ICE-CBF-COR pathway by inhibiting the inhibitory action of JAZs proteins on the ICE2 and CBF1 [124].
Like the jasmonates, cold alters the endogenous levels of ethylene, although the regulating effect of ethylene on the cold stress is inconsistent with various plant species. Ethylene has been demonstrated to alleviate cold stress in G. max [130], tomato [131], and grapevine [132], while in Arabidopsis [133] and M. truncatula [134], ethylene reduces cold stress tolerance [128]. Additional analyses in the ethylene response signaling pathway have suggested that ethylene regulates CBF/DREB expression through the action of EIN3, a transcription factor that binds the consensus sequence ATGYATNY [130,135]. In G. max, EIN3 binds to the promoters of CBFs in the absence of ethylene reducing its transcriptional activity and expression of downstream COR genes [130]. However, exogenous treatment with an ethylene precursor (1-aminocyclopropane-1-carboxylate) and an ethylene biosynthesis inhibitor (amino-ethoxy vinyl glycine) were shown to increase and decrease cold tolerance, respectively. 1-aminocyclopropane-1-carboxylate augments the expression of MdCBF1 through the mediating roles of ethylene response factors (ERFs) in the MdERF1B-MdCIgHLH1-MdCBF1 pathway [135]. ERFs are known to bind COR genes (CORLTRECOREATCOR15 and MYBCORE) cis-elements, enhancing freezing tolerance by reducing ROS species, and increasing SOD and POD levels [136]
As steroid hormones, brassinosteroids (BR) are synthesized from mass sterol campesterol through multiple hydroxylations and oxidations, further catalyzed with various cytochrome P450 enzymes, including DWARF4, CPD, ROT3, and the CYP85A2 BR6ox2 steroid, cumulatively known as BR-biosynthetic genes. BRs induce a multidirectional response in plants that include the regulation of cold-responsive genes (ICEs, CBFs, and CORs) and other hormonal cross-talks (ABA and JA) [137]. Nevertheless, cold treatment downregulates these BR-biosynthetic genes [32]. BRASSINOSTEROID INSENSITIVE 2 (BIN2), a GSK3-like protein kinase form of brassinosteroids, a repressor and regulator in the BR-signaling is also known to target the bHLH-type proteins including the ICE genes. Ye et al. [138] recently showed that BIN2 phosphorylates the ICE1, thereby reducing its stability and transcription of the CBF regulon. Further downstream the BIN2 activities are controlled by acetylation roles of histone deacetylase 6 (HDA6 discussed below). The phosphorylated ICE1 interacts with HOS15 at the C-terminus further degrading ICE1 and attenuating CBF expression. Additional studies evidenced that BIN2 activities are down-regulated in the early stages of cold stress by HDA6 and later restored as a regulatory measure for CBF expression and levels [138]. Cold-induced BR also directly participates in the regulation of basal cold tolerance by increasing the expression levels of CBF1/2/3, COR15A, and COR47-like transcripts in A. thaliana [139]. Consistent with these findings, studies in tomatoes have suggested a BR component, brassinazole-resistant 1 (BRZ1), that inducts the expression of CBFs. They proposed that cold induces BR and BRZ1 abundancies, then BRZ1 binds to the E-box (CANNTG) and BRRE (CGTGT/CG) motifs in their promoters and increases the expression of downstream genes through the resultant RBHO1 and hence cold stress tolerance. Further analysis demonstrated that RBOH1 enhances CBF expression by altering the cold- and BR-induced accumulation in the redox-dependent system. The significance of the BR component, BRZ1, in the ICE-CBF-COR signaling pathway has been verified through the overexpression of mutant brz1, resulting in cold stress reduction and low expression levels of the CBF transcripts [140,141]. Furthermore, CBF1 has been related to positive relief of chilling injury during post-harvest storage of tomato expressing BRI1 and to decrease chilling injury tolerances in mutant BR synthesis CPD. Cold-induced BR biosynthetic gene in tomato, SLCTP90B3, has been established to bind the promoters of CBF1 and induce its expression through the activation of the ICE1 transcription hub [142].
4. Post-Transcriptional Regulations and Post-Translational Modification
Cold stress induces extensive post-transcriptional and post-translational-modifications (PTM) in several plants, affecting the quality and quantity of the mRNA and ultimately cold stress tolerance [143]. Thus, post-transcriptional regulations and PTMs regulate the expression of the entities in the ICE-CBF-COR signaling pathway. Two protein families regulate the developmental steps of post-transcription, the RNA binding proteins (RBPs) [144] and the RNA helicases [145]. The RBPs function as molecular chaperones, regulating alternative splicing (AS). AS events produce multiple transcripts from a single RNA and they transpire in specific mRNAs families of genes, affecting their normal gene transcription. Previously, AS was demonstrated to modulate WDREB2 in wheat [146] and MYB48/59 in Arabidopsis [147] affecting their binding efficiency to downstream COR genes. A recent study in tea (Camellia sinensis) has explored the impact of AS events on the ICE-CBF-COR genes. They reported that AS induces the expression of genes involved in the cold response signaling and their regulators including CsbHLH1/2, CsMYBs, and other COR genes, alleviating cold stress through the CBF-dependent pathways [148]. Although the mechanism by which ICE-CBF-COR genes are induced is still unclear. Chromatin remodeling changes the transcriptional activities of several COR genes during cold stress, rendering it more or less accessible to the transcriptional machinery [22]. Chromatin modification of histone deacetylase 6/9 (HDAC 6/9) during cold stress links directly to the transcriptional activities and negatively regulates COR gene expressions [149,150,151]. Studies in rice have shown that O. sativa HADCs functional proteins positively regulate cold stress tolerance by activating OsDREB1 expression, thereby enhancing cold stress through expression activation of downstream COR genes by the CBFs [152]. Epigenetic switches from a repressed state in chromatin models also regulate the expression of COR genes. HOS15, a WD40-repeat protein degrades histone deacetylation 2C (HD2C), modulating a complex (HOS15-H2DC) that deacetylases COR gene chromatin to repress gene expression. The HOS15-H2DC complex binds to the promoters of cold-responsive genes, for instance, COR15 and COR47 [153], and activates their expression, resulting in cold acclimation through the cold regulatory roles of these COR genes.
During post-translational modifications (PTMs), several genes and TFs interact with the ICE, CBF, and COR genes to modify their activity, conformation, localization, and stability. Phosphorylation, ubiquitination, and SUMO conjugations are major PTMs in plants regulating the cold stress response pathway [154]. Phosphorylation plays a significant role in plant cold acclimatization and is a reversible protein modification, with a high dependence on kinases and phosphatases. The most common phosphatase, open stomata 1 (OST1) appertain for the SNF1-related protein kinase family and phosphorylates the entities in the ICE-CBF response pathway. The OST1 interacts with E3-ubiquitin ligase (HOS1), thereby phosphorylating the ICE1, increasing its stability, and alleviating cold stress through inducing the activities of CBF genes [155]. Furthermore, variants of the mature polypeptide-associated complex of OST1 phosphorylate the BASIC TRANSCRIPTION FACTOR 3 (BTF3) proteins, promoting their interaction with CBF proteins, and consequently increasing the stability of CBFs for efficient binding to COR genes downstream [123,156]. OST1 has also been shown to interact with PUB25/26 in the OST1-PUB25/26-MYB15 pathway and to upregulate the expression of CBFs in Arabidopsis. The two U-box type ubiquitin ligases (PUB25/26) degrade MYB15, an inhibitor of CBF, thereby increasing the expression of CBF and COR genes [30]. A plasma membrane-localized receptor-like cytoplasmic kinase, cold-responsive protein kinase 1 (CRPK1) phosphorylates the 14-3-3 genes, promoting their significance in the nucleus from the cytosol, coherently interacting with the CBF proteins, and reducing cold tolerance through destabilizing their binding affinity to COR genes [157].
Ubiquitination defines the rigorous action of three enzymes, E1 > E2 > E3. The E3-ubiquitin ligase plays the most vital role by interacting with the target molecule and providing scaffolding for the ubiquitination reaction. The number of ubiquitin molecules attached to a target molecule determines its fate, that is polyubiquitin, monoubiquitin, and ubiquitin [158]. Cold regulatory genes are affected by E3-ubiquitin ligases (polyubiquitination) that regulate their expression and cold stress tolerance. HOS1, a RING-finger E3 ubiquitin ligase participates in the negative feedback regulation of cold stress by mediating ICE1 degradation at the onset of the cold stress response. However, mutant hos1 expression enhances cold tolerance through loss-of-function [159]. CRISPR/cas9-mediated genomic loss of function studies have also revealed that the hos1 provokes significant fluctuations in the expression of ICE1 in Arabidopsis [160]. HOS15, a ubiquitin ligase interacts with CBFs and modulates their binding to the COR gene promoters through chromatin remodeling [161]. ICE1 in Eucalyptus camaldulnesis interacts with EcaHOS15 in the ubiquitination-proteasome pathway, increasing its binding affinity to EcaHOS1. However, substitutional processes of serine (Ser158) by alanine (Ala) inhibit EcaHOS15-EcaICE1 interaction leading to reduced binding efficiency of CBFs to COR genes. When bound to the ICE1, cold stress tolerance is enhanced through the enhanced expression of the CBFs [162].
SUMOylation a similar process to ubiquitination regulates cold stress through the action of SUMOs [163]. SUMOs are bound to a lysine residue of a target protein in three steps with three SUMO ligase enzymes (E1 > E2 > E3), provoking their interaction with target proteins and disturbing their PPIs with other proteins [154]. SIZ1, an E3 SUMO ligase has been demonstrated to positively increase freezing and cold stress tolerance in Arabidopsis by inhibiting ICE1 ubiquitination. More specifically, SIZ1 sumolyates the ICE1 at position K393, and additional results have proven that this sumoylation has no negative implications on the ICE1 activity, but rather inhibits polyubiquitination of ICE1 by the HOS1, decreasing ICE1 degradation and increasing CBF3 expression. Moreover, the sumoylated ICE1 negatively regulates the repressive actions of MYB15 on CBF3. The loss-of-function of SIZ1 has also been shown to reduce cold tolerance and increase ICE1 ubiquitination, concluding that ICE1 levels are determined by the balance of SUMOylation and ubiquitination processes [164,165].
5. Conclusions and Future Perspectives
The ICE-CBF-COR cascade plays a crucial role in the survival of plants during cold stress. Cold stress is perceived by plant sensors and other organelles: secondary responses induce the expressions of downstream cold-responsive genes. Various regulators, inducers, hormonal responses, post-transcriptional regulations, and/or the post-translational modifications induce the expressions of ICE1/2, and CBF1/2/3 genes which in turn enhance the expression of COR genes. In detail, the OST1, HOS15, MYB15, the MAPK cascade and their direct and/or indirect regulation in the expressions of ICE1 and the CBF1/2/3, cross-interlink and interact with the key players within the ICE-CBF-COR and regulate their expression and consequently cold acclimation. The sum of these mechanisms was discussed in this review, and collected insights concur in the sequential expression of ICEs, CBFs, and COR genes. Therefore, it can be concluded that the ICE-CBF-COR is the central pathway to which different transcription factors, regulators, proteins, physiological factors, and other manipulators interlink to enhance cold stress. Although expression of genes at different response levels may or may not follow the hierarchal steps in response, such as the CBF-independent pathway.
Nonetheless, elaborate mechanisms and other additional regulators still require further analysis, to fully understand the effect of seasonal changes, hormonal imbalances, and gene transcriptional/translational on the expression of ICE, CBF, and CORs. This review summarized cold stress tolerances through the CBF-dependent pathway (Figure 4). Expression of the CBFs has been discussed fully, demonstrating the upstream enhancer ICE genes and their roles, and the roles of the CBF in inducing the expression of downstream COR genes. Nonetheless, cross-links and biochemical interactions within these sequential expressions are not facilely comprehended. Further studies on the CBF-dependent pathway are required to expose all the possible and included response factors. This will be important in gene engineering the cold response genes, to improve cold stress acclimation in cold stress-sensitive plants. However, there are few prospects in understanding the cold stress response in model plants such as Arabidopsis, rice, wheat, and other socio-economic plants. For instance, the identification of the CHILLING-TOLERANCE DIVERGENCE 1 (COLD1) receptor and the G-proteins in Japonica rice (not discussed in this review paper). The knowledge of their interaction with the ICE-CBF-COR cascade has improved the understanding of cold stress signaling in plants. Different transgenic plants have been manipulated to improve the expression of the ICE, CBF genes, and ultimately cold stress tolerance with the introduction of the COLD1 receptor and interaction improvements with G-proteins. Furthermore, techniques such as CRISPR have knocked out inhibitors and reducers, reducing the ICEs, CBFs, and CORs expression, leading to increased plant cold tolerance in plants and understanding of the importance of certain regulators and enhancers, suggesting the importance and urgency of further identification of other molecular factors and pathways that directly or indirectly interact with the ICE-CBF-COR pathway. There is still a need to further the understanding of hormonal responses and their effect on the ICE-CBF-COR, such as ethylene regulating ICE-CBF-COR in other plants, the MAPK cascade and its regulatory behavior in the cold signaling pathway, considering the antagonistic roles of the MPK6/3 with MPK4 in Arabidopsis. Other mechanisms such as the PTMs and post-transcriptional regulations require extensive research to fully understand the impact of alternative splicing, chromatin modifications, and methylation on the transcription and translation of ICE, CBF, and COR genes.
Taking into consideration the impact of global climate change on the overall plant growth and yield, there is still an urgent need for intense research on the ICE-CBF-COR cascade to answer many questions that remain unanswered in the ICE-CBF-COR pathway and how it can be improved to ameliorate cold stress and improve plant yield and growth.
Author Contributions
Conceptualization, D.H., Y.G. and L.Y.; writing—original draft preparation, D.H., and Y.G.; writing—review and editing, B.A. and A.M.; supervision, T.M.; Y.L. and Z.H.; funding acquisition, L.Y. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 31971682, 32071784), Youth Foundation of the Natural Science Foundation of Jiangsu Province (No. BK20210614), The Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Research Startup Fund for High-Level and High-Educated Talents of Nanjing Forestry University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the writing of the manuscript, or in the decision to publish the result.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ritonga F.N., Chen S. Physiological and Molecular Mechanism Involved in Cold Stress Tolerance in Plants. Plants. 2020;9:560. doi: 10.3390/plants9050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oshunsanya S.O., Nwosu N.J., Li Y. Abiotic Stress in Agricultural Crops Under Climatic Conditions. In: Jhariya M.K., editor. Sustainable Agriculture, Forest and Environmental Management. Volume 9. Springer; Berlin/Heidelberg, Germany: 2019. pp. 71–100. [Google Scholar]
- 3.Yang C., Yang H., Xu Q. Comparative metabolomics analysis of the response to cold stress of resistant and susceptible Tibetan hulless barley (Hordeum distichon) Phytochemistry. 2020;174:112. doi: 10.1016/j.phytochem.2020.112346. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y., Sommer M.L., Hochholdinger F. Cold response and tolerance in cereal roots. J. Exp. Bot. 2021;72:7474–7481. doi: 10.1093/jxb/erab334. [DOI] [PubMed] [Google Scholar]
- 5.Wang P., Chen X., Guo Y. Identification of CBF Transcription Factors in Tea Plants and a Survey of Potential CBF Target Genes under Low Temperature. Int. J. Mol. Sci. 2019;20:5137. doi: 10.3390/ijms20205137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng X., Liang Z., Dai Y. Predicting transcriptional responses to cold stress across plant species. Proc. Natl. Acad. Sci. USA. 2021;118:e2026330118. doi: 10.1073/pnas.2026330118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehrotra S., Verma S. Transcriptional regulation and signalling of cold stress response in plants: An overview of current understanding. Environ. Exp. Bot. 2020;180:104243. doi: 10.1016/j.envexpbot.2020.104243. [DOI] [Google Scholar]
- 8.Shu Y., Li W., Zhao J., Zhang S., Xu H., Liu Y., Guo C. Transcriptome sequencing analysis of alfalfa reveals CBF genes potentially playing important roles in response to freezing stress. Genet. Mol. Biol. 2017;40:824–833. doi: 10.1590/1678-4685-gmb-2017-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomashow M.F. Plant Cold Acclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- 10.Guo J., Ren Y., Tang Z. Characterization and expression profiling of the ICE-CBF-COR genes in wheat. Peer J. 2019;7:e8190. doi: 10.7717/peerj.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Liu C., Zhao Z. COR27 and COR28 are Novel Regulators of the COP1–HY5 Regulatory Hub and Photomorphogenesis in Arabidopsis. Plant Cell. 2020;32:1393154. doi: 10.1105/tpc.20.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D.Z., Jin Y.N., Ding X.H. Gene Regulation and Signal Transduction in the ICE-CBF-COR Signaling Pathway during Cold Stress in Plants. Biochemistry. 2017;82:1103–1117. doi: 10.1134/S0006297917100030. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Dang P., He C. Cold acclimation by the CBF–COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019;38:511–519. doi: 10.1007/s00299-019-02376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bremer A., Kent B. Intrinsically Disordered Stress Protein COR15A Resides at the Membrane Surface during Dehydration. Biophys. J. 2017;113:572–579. doi: 10.1016/j.bpj.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badawi M., Reddy Y.M. Structure and functional analysis of wheat ICE (inducer of CBF expression) genes. Plant Cell Physiol. 2008;49:1237–1249. doi: 10.1093/pcp/pcn100. [DOI] [PubMed] [Google Scholar]
- 16.Hao X., Wang L. Stress Physiology of Tea in the Face of Climate Change. Springer; Berlin/Heidelberg, Germany: 2018. Response and adaptation mechanisms of tea plant to low-temperature stress; pp. 39–61. [Google Scholar]
- 17.Chandler J.W. Class VIIIb APETALA2 Ethylene Response Factors in Plant Development. Trends Plant Sci. 2018;23:151–162. doi: 10.1016/j.tplants.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y., Ding Y., Yang S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018;23:623–637. doi: 10.1016/j.tplants.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Cho S., Yu S.L., Park J. Accession-Dependent CBF Gene Deletion by CRISPR/Cas System in Arabidopsis. Front. Plant Sci. 2017;8:1910. doi: 10.3389/fpls.2017.01910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin Y., Zhai S., Wang W. Ideentification of genes from the ICE-CBF-COR pathway under cold stress in Aegilops-Triticum composite group and the evolution analysis with those from Triticeae. Physiol. Mol. Biol. Plants. 2018;24:211–229. doi: 10.1007/s12298-017-0495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma R.K., Kumar V.S. Overxpression of Arabidopsis ICE1 enhances yield and multiple abiotic stress tolerance in indica rice. Plant Signal Behav. 2020;15:1814547. doi: 10.1080/15592324.2020.1814547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vyse K., Faivre L. Transcriptional and post-transcriptional regulation and transcriptional memory of chromatin regulators in response to low temperature. Front. Plant Sci. 2020;11:39. doi: 10.3389/fpls.2020.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsz D., Dixon L.E. The Roles of Temperature-Related Post-Transcriptional Regulation in Cereal Floral Development. Plants. 2021;10:2230. doi: 10.3390/plants10112230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin J., Yi H., Chen X. Post-translational modifications of proteins have versatile roles in regulating plant immune responses. Int. J. Mol. Sci. 2019;20:2807. doi: 10.3390/ijms20112807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damaris R.N., Yang P. Protein Phosphorylation Response to Abiotic Stress in Plants. Methods Mol. Biol. 2021;2358:635–674. doi: 10.1007/978-1-0716-1625-3_2. [DOI] [PubMed] [Google Scholar]
- 26.Praat M., De Smet I., van Zanten M. Protein kinase and phosphatase control of plant temperature responses. J. Exp. Bot. 2021;72:7459–7473. doi: 10.1093/jxb/erab345. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y., Chen L. The mitogen-activated protein kinase kinase MKK2 positively regulates constitutive cold resistance in the potato. Environ. Exp. Bot. 2022;19:104702. doi: 10.1016/j.envexpbot.2021.104702. [DOI] [Google Scholar]
- 28.Sharma S., Prasd A. Role of ubiquitination enzymes in abiotic environmental interactions with plants. Int. J. Biol. Macromol. 2021;181:494–507. doi: 10.1016/j.ijbiomac.2021.03.185. [DOI] [PubMed] [Google Scholar]
- 29.Yao W., Wang L., Wang J. VpPUB24, a novel gene from Chinese grapevine, Vitis pseudoreticulata, targets VpICE1 to enhance cold tolerance. J. Exp. Bot. 2017;68:2933–2949. doi: 10.1093/jxb/erx136. [DOI] [PubMed] [Google Scholar]
- 30.Wang X., Ding Y., Li Z. PUB25 and PUB26 Promote Plant Freezing Tolerance by Degrading the Cold Signaling Negative Regulator MYB15. Dev. Cell. 2019;51:222–235. doi: 10.1016/j.devcel.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Fan Z.Q., Chen J.Y., Kuang J.F. The Banana Fruit SINA Ubiquitin Ligase MaSINA1 Regulates the Stability of MaICE1 to be Negatively Involved in Cold Stress Response. Front. Plant Sci. 2017;8:995. doi: 10.3389/fpls.2017.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eremina M., Unterholzner S.J., Rathnayake A.I. Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. USA. 2016;113:e5982–e5991. doi: 10.1073/pnas.1611477113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lantzouni O., Alkofer A., Schwechheimer C. GROWTH-REGULATING FACTORS Interact with DELLAs and Regulate Growth in Cold Stress. Plant Cell. 2020;32:1018–1034. doi: 10.1105/tpc.19.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wi S.D., Lee E.S. Redox-mediated structural and functional switching of C-repeat binding factors enhances plant cold tolerance. New Phytol. 2022;233:1067–1073. doi: 10.1111/nph.17745. [DOI] [PubMed] [Google Scholar]
- 35.Liou Y.C., Daley M.E. Folding and structural characterization of highly disulfide-bonded beetle antifreeze protein produced in bacteria. Protein Expr. Purif. 2000;19:148–157. doi: 10.1006/prep.2000.1219. [DOI] [PubMed] [Google Scholar]
- 36.Ramachandra R.A., Chaitanya K.V., Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Jofuku K.D., den Boer B.G., Van Montagu M. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell. 1994;6:1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamuro J.K., Caster B., Villarroel R. The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA. 1997;94:7076–7081. doi: 10.1073/pnas.94.13.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakano T., Suzuki K., Fujimura T. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006;140:411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen M.D., Yamasaki K. A novel mode of DNA recognition by a beta-sheet revealed by the solution structure of the GCC-box binding domain in complex with DNA. Embo J. 1998;17:5484–5496. doi: 10.1093/emboj/17.18.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medina J., Catalá R., Salinas J. The CBFs: Three arabidopsis transcription factors to cold acclimate. Plant Sci. 2011;180:3–11. doi: 10.1016/j.plantsci.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Warren G.J. Cold stress: Manipulating freezing tolerance in plants. Curr. Biol. 1998;8:514–516. doi: 10.1016/S0960-9822(07)00335-1. [DOI] [PubMed] [Google Scholar]
- 43.Shi Y., Huang J., Sun T. The precise regulation of different COR genes by individual CBF transcription factors in Arabidopsis thaliana. J. Integr. Plant. Biol. 2017;59:118–133. doi: 10.1111/jipb.12515. [DOI] [PubMed] [Google Scholar]
- 44.Hu Z., Ban Q., Hao J. Genome-Wide Characterization of the C-repeat Binding Factor (CBF) Gene Family Involved in the Response to Abiotic Stresses in Tea Plant (Camellia sinensis) Front. Plant Sci. 2020;11:921. doi: 10.3389/fpls.2020.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan Y., Liu S., Wu W. Genome-wide identification and cold stress-induced expression analysis of the CBF gene family in Liriodendron chinense. J. For. Res. 2021;32:2531–2543. doi: 10.1007/s11676-020-01275-8. [DOI] [Google Scholar]
- 46.Novillo F., Alonso J.M., Ecker J.R. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2004;101:3985–3990. doi: 10.1073/pnas.0303029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hannah M.A., Heyer A.G., Hincha D.K. A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Genet. 2005;1:e26. doi: 10.1371/journal.pgen.0010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizoi J., Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta. 2012;1819:86–96. doi: 10.1016/j.bbagrm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Kidokoro S., Hayashi K., Haraguchi H. Post-translational regulation of multiple clock-related transcription factors triggers cold-inducible gene expression in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2021;118:3349234. doi: 10.1073/pnas.2021048118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W., Chen Y., Ye M. Evolutionary history of the C-repeat binding factor/dehydration-responsive element-binding 1 (CBF/DREB1) protein family in 43 plant species and characterization of CBF/DREB1 proteins in Solanum tuberosum. BMC Evol. Biol. 2020;20:142. doi: 10.1186/s12862-020-01710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Achard P., Gong F., Cheminant S. The Cold-Inducible CBF1 Factor–Dependent Signaling Pathway Modulates the Accumulation of the Growth-Repressing DELLA Proteins via Its Effect on Gibberellin Metabolism. Plant Cell. 2008;20:2117–2129. doi: 10.1105/tpc.108.058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang F., Wang F., Wu Z. Components of the Arabidopsis CBF cold-response pathway are conserved in non-heading Chinese cabbage. Plant Mol. Biol. Rep. 2011;29:525–532. doi: 10.1007/s11105-010-0256-3. [DOI] [Google Scholar]
- 53.Yang X., Wang X., Jing H. Three Novel C-Repeat Binding Factor Genes of Dimocarpus longan Regulate Cold Stress Response in Arabidopsis. Front. Plant Sci. 2020;11:1026. doi: 10.3389/fpls.2020.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmad M., Li J., Yang Q. Phylogenetic, molecular, and functional characterization of PpyCBF proteins in Asian pears (Pyrus pyrifolia) Int. J. Mol. Sci. 2019;20:2074. doi: 10.3390/ijms20092074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin R., Kim B.H., Ji C.Y. Overexpressing IbCBF3 increases low temperature and drought stress tolerance in transgenic sweetpotato. Plant Physiol. Biochem. 2017;118:45–54. doi: 10.1016/j.plaphy.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 56.An D., Ma Q., Yan W. Divergent Regulation of CBF Regulon on Cold Tolerance and Plant Phenotype in Cassava Overexpressing Arabidopsis CBF3 Gene. Front. Plant Sci. 2016;7:1866. doi: 10.3389/fpls.2016.01866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebrahimi M., Abdullah S.N. Oil palm EgCBF3 conferred stress tolerance in transgenic tomato plants through modulation of the ethylene signaling pathway. J. Plant Physiol. 2016;202:107–120. doi: 10.1016/j.jplph.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Zhuang L., Yuan X., Chen Y. PpCBF3 from Cold-Tolerant Kentucky Bluegrass Involved in Freezing Tolerance Associated with Up-Regulation of Cold-Related Genes in Transgenic Arabidopsis thaliana. PLoS ONE. 2015;10:e0132928. doi: 10.1371/journal.pone.0132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kidokoro S., Watanabe K., Ohori T. Soybean DREB1/CBF-type transcription factors function in heat and drought as well as cold stress-responsive gene expression. Plant J. 2015;81:505–518. doi: 10.1111/tpj.12746. [DOI] [PubMed] [Google Scholar]
- 60.Byun M.Y., Lee J., Cui L.H. Constutive expression of DaCBF7, an Antarctic vascular plant Deschampsia antarctica CBF homolog, resulted in improved cold tolerance in transgenic rice plants. Plant Sci. 2015;236:61–74. doi: 10.1016/j.plantsci.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 61.Wisniewski M., Norelli J., Bassett C. Ectopic expression of a novel peach (Prunus persica) CBF transcription factor in apple (Malus × domestica) results in short-day induced dormancy and increased cold hardiness. Planta. 2011;233:971–983. doi: 10.1007/s00425-011-1358-3. [DOI] [PubMed] [Google Scholar]
- 62.Pino M.T., Skinner J.S. Ectopic AtCBF1 over-expression enhances freezing tolerance and induces cold acclimation-associated physiological modifications in potato. Plant Cell Env. 2008;31:393–406. doi: 10.1111/j.1365-3040.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 63.Gutha L.R., Reddy A.R. Rice DREB1B promoter shows distinct stress-specific responses, and the overexpression of cDNA in tobacco confers improved abiotic and biotic stress tolerance. Plant Mol. Biol. 2008;68:533–555. doi: 10.1007/s11103-008-9391-8. [DOI] [PubMed] [Google Scholar]
- 64.Oh S.J., Kwon C.W., Choi D.W. Expression of barley HvCBF4 enhances tolerance to abiotic stress in transgenic rice. Plant Biotechnol. J. 2007;5:646–656. doi: 10.1111/j.1467-7652.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- 65.Skinner J.S., von Zitzewitz J., Szucs P. Structural, functional, and phylogenetic characterization of a large CBF gene family in barley. Plant Mol. Biol. 2005;59:533–551. doi: 10.1007/s11103-005-2498-2. [DOI] [PubMed] [Google Scholar]
- 66.Gao M.J., Allard G., Byass L. Regulation and characterization of four CBF transcription factors from Brassica napus. Plant Mol. Biol. 2002;49:459–471. doi: 10.1023/A:1015570308704. [DOI] [PubMed] [Google Scholar]
- 67.Salvo M., Rey F., Arruabarrena A., Gambetta G., Rodrigo M.J., Zacarías L., Lado J. Transcriptional Analysis of C-Repeat Binding Factors in Fruit of Citrus Species with Differential Sensitivity to Chilling Injury during Postharvest Storage. Int. J. Mol. Sci. 2021;22:804. doi: 10.3390/ijms22020804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chinnusamy V., Ohta M., Kanrar S. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003;17:1043–1054. doi: 10.1101/gad.1077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie Y., Chen P., Yan Y. An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol. 2018;218:201–218. doi: 10.1111/nph.14952. [DOI] [PubMed] [Google Scholar]
- 70.Sun X., Wang Y., Sui N. Transcriptional regulation of bHLH during plant response to stress. Biochem. Biophys. Res. Commun. 2018;503:397–401. doi: 10.1016/j.bbrc.2018.07.123. [DOI] [PubMed] [Google Scholar]
- 71.Tang K., Zhao L., Ren Y. The transcription factor ICE1 functions in cold stress response by binding to the promoters of CBF and COR genes. J. Integr. Plant Biol. 2020;62:258–263. doi: 10.1111/jipb.12918. [DOI] [PubMed] [Google Scholar]
- 72.Kurbidaeva A., Ezhova T., Novokreshchenova M. Arabidopsis thaliana ICE2 gene: Phylogeny, structural evolution and functional diversification from ICE1. Plant Sci. 2014;229:10–22. doi: 10.1016/j.plantsci.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 73.Kashyap P., Deswal R. Two ICE isoforms showing differential transcriptional regulation by cold and hormones participate in Brassica juncea cold stress signaling. Gene. 2019;695:32–41. doi: 10.1016/j.gene.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Hu Y., Jiang L., Wang F., Yu D. Jasmonate regulates the INDUCER OF CBF EXPRESSION-C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 CASCADE and freezing tolerance in Arabidopsis. Plant Cell. 2013;25:2907–2924. doi: 10.1105/tpc.113.112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu C.L., Lin L.C.F., Hsu H.C. Saussurea involucrata (Snow Lotus) ICE1 and ICE2 Orthologues Involved in Regulating Cold Stress Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2021;22:10850. doi: 10.3390/ijms221910850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wani U.M., Majeed S.T., Raja V., Wani Z.A., Jan N., Andrabi K.I., John R. Ectopic expression of a novel cold-resistance protein 1 from Brassica oleracea promotes tolerance to chilling stress in transgenic tomato. Sci. Rep. 2021;11:16574. doi: 10.1038/s41598-021-96102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Z., Zhu L., Song A. Chrysanthemum (Chrysanthemum morifolium) CmICE2 conferred freezing tolerance in Arabidopsis. Plant Physiol. Biochem. 2020;146:31–41. doi: 10.1016/j.plaphy.2019.10.041. [DOI] [PubMed] [Google Scholar]
- 78.Zuo Z.F., Kang H.G., Park M.Y. Zoysia japonica MYC type transcription factor ZjICE1 regulates cold tolerance in transgenic Arabidopsis. Plant Sci. 2019;289:110254. doi: 10.1016/j.plantsci.2019.110254. [DOI] [PubMed] [Google Scholar]
- 79.Chen W.J., Wang X., Yan S. The ICE-like transcription factor HbICE2 is involved in jasmonate-regulated cold tolerance in the rubber tree (Hevea brasiliensis) Plant Cell Rep. 2019;38:699–714. doi: 10.1007/s00299-019-02398-x. [DOI] [PubMed] [Google Scholar]
- 80.Man L., Xiang D., Wang L. Stress-responsive gene RsICE1 from Raphanus sativus increases cold tolerance in rice. Protoplasma. 2017;254:945–956. doi: 10.1007/s00709-016-1004-9. [DOI] [PubMed] [Google Scholar]
- 81.Deng C., Ye H., Fan M. The rice transcription factors OsICE confer enhanced cold tolerance in transgenic Arabidopsis. Plant Signal Behav. 2017;12:e1316442. doi: 10.1080/15592324.2017.1316442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu X., Yang L., Yu M. A novel Zea mays ssp. mexicana L. MYC-type ICE-like transcription factor gene ZmmICE1, enhances freezing tolerance in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2017;113:78–88. doi: 10.1016/j.plaphy.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Feng H.L., Ma N.N., Meng X. A novel tomato MYC-type ICE1-like transcription factor, SlICE1a, confers cold, osmotic and salt tolerance in transgenic tobacco. Plant Physiol. Biochem. 2013;73:309–320. doi: 10.1016/j.plaphy.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 84.Ding Y., Shi Y., Yang S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019;222:1690–1704. doi: 10.1111/nph.15696. [DOI] [PubMed] [Google Scholar]
- 85.Leuendorf J.E., Frank M., Schmülling T. Acclimation, priming and memory in the response of Arabidopsis thaliana seedlings to cold stress. Sci. Rep. 2020;10:689. doi: 10.1038/s41598-019-56797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kosová K., Kilma K. COR/LEA Proteins as Indicators of Frost Tolerance in Triticeae: A Comparison of Controlled versus Field Conditions. Plants. 2021;10:789. doi: 10.3390/plants10040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li W., Wang R., Li M. Differential degradation of extraplastidic and plastidic lipids during freezing and post-freezing recovery in Arabidopsis thaliana. J. Biol. Chem. 2008;283:461–468. doi: 10.1074/jbc.M706692200. [DOI] [PubMed] [Google Scholar]
- 88.Welti R., Li W. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- 89.Wang W., Vinocur B., Altman A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta. 2003;218:1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- 90.Okawa K., Nakayama K., Kakizaki T. Identification and characterization of Cor413im proteins as novel components of the chloroplast inner envelope. Plant Cell Environ. 2008;31:1470–1483. doi: 10.1111/j.1365-3040.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 91.Zhou A., Liu E., Li H. PsCor413pm2, a Plasma Membrane-Localized, Cold-Regulated Protein from Phlox subulata, Confers Low Temperature Tolerance in Arabidopsis. Int. J. Mol. Sci. 2018;19:2579. doi: 10.3390/ijms19092579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu X., Liu J., Liu E., Qioa K. Arabidopsis cold-regulated plasma membrane protein Cor413pm1 is a regulator of ABA response. Biochem. Biophys. Res. Commun. 2021;561:88–92. doi: 10.1016/j.bbrc.2021.05.032. [DOI] [PubMed] [Google Scholar]
- 93.Strimbeck R.G. Hiding in plain sight: The F segment and other conserved features of seed plant SK(n) dehydrins. Planta. 2017;245:1061–1066. doi: 10.1007/s00425-017-2679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wan F., Pan Y., Li J. Heterologous expression of Arabidopsis C-repeat binding factor 3 (AtCBF3) and cold-regulated 15A (AtCOR15A) enhanced chilling tolerance in transgenic eggplant (Solanum melongena L.) Plant Cell Rep. 2014;33:1951–1961. doi: 10.1007/s00299-014-1670-z. [DOI] [PubMed] [Google Scholar]
- 95.Vazquez-Hernandez M., Romero I., Escribano I., Merodio C., Sanchez-Ballesta M.T. Deciphering the Role of CBF/DREB Transcription Factors and Dehydrins in Maintaining the Quality of Table Grapes cv. Autumn Royal Treated with High CO(2) Levels and Stored at 0°C. Front Plant Sci. 2017;8:1591. doi: 10.3389/fpls.2017.01591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu W., Zhou H., Lin F. Cold-Regulated Gene27 Integrates Signals from Light and the Circadian Clock to Promote Hypocotyl Growth in Arabidopsis. Plant Cell. 2020;32:3155–3169. doi: 10.1105/tpc.20.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang L., Guo X., Zhang Z. Cold-regulated gene LeCOR413PM2 confers cold stress tolerance in tomato plants. Gene. 2021;764:145097. doi: 10.1016/j.gene.2020.145097. [DOI] [PubMed] [Google Scholar]
- 98.Shi H., He X., Zhao Y. Constitutive expression of a group 3 LEA protein from Medicago falcata (MfLEA3) increases cold and drought tolerance in transgenic tobacco. Plant Cell Rep. 2020;39:851–860. doi: 10.1007/s00299-020-02534-y. [DOI] [PubMed] [Google Scholar]
- 99.Guo X., Zhang L., Dong G. A novel cold-regulated protein isolated from Saussurea involucrata confers cold and drought tolerance in transgenic tobacco (Nicotiana tabacum) Plant Sci. 2019;289:110246. doi: 10.1016/j.plantsci.2019.110246. [DOI] [PubMed] [Google Scholar]
- 100.Guo X., Zhang L., Wang X. Overexpression of Saussurea involucrata dehydrin gene SiDHN promotes cold and drought tolerance in transgenic tomato plants. PLoS ONE. 2019;14:e0225090. doi: 10.1371/journal.pone.0225090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peng Y., Reyes J.L., Wei H. RcDhn5, a cold acclimation-responsive dehydrin from Rhododendron catawbiense rescues enzyme activity from dehydration effects in vitro and enhances freezing tolerance in RcDhn5-overexpressing Arabidopsis plants. Physiol. Plant. 2008;134:583–597. doi: 10.1111/j.1399-3054.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 102.Wang J., Ren Y., Liu X., Luo S., Zhang X. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant. 2021;14:315–329. doi: 10.1016/j.molp.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 103.Liu Q., Ding Y., Shi Y. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. Embo J. 2021;40:e104559. doi: 10.15252/embj.2020104559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iqbal Z., Iqbal M.S. Ca(2+)/Calmodulin Complex Triggers CAMTA Transcriptional Machinery Under Stress in Plants: Signaling Cascade and Molecular Regulation. Front. Plant Sci. 2020;11:598327. doi: 10.3389/fpls.2020.598327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shangguan L., Wang X., Leng X. Identification and bioinformatic analysis of signal responsive/calmodulin-binding transcription activators gene models in Vitis vinifera. Mol. Biol. Rep. 2014;41:2937–2949. doi: 10.1007/s11033-014-3150-5. [DOI] [PubMed] [Google Scholar]
- 106.Zeng R., Li Z. Natural variation in a type-A response regulator confers maize chilling tolerance. Nat. Commun. 2021;12:4713. doi: 10.1038/s41467-021-25001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang G., Zeng H., Hu X. Identification and expression analyses of calmodulin-binding transcription activator genes in soybean. Plant Soil. 2015;386:205–221. doi: 10.1007/s11104-014-2267-6. [DOI] [Google Scholar]
- 108.Rahman H., Xu Y.P., Zhnag X.R. Brassica napus genome possesses extraordinary high number of CAMTA genes and CAMTA3 contributes to PAMP triggered immunity and resistance to Sclerotinia sclerotiorum. Front. Plant Sci. 2016;7:581. doi: 10.3389/fpls.2016.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei M., Xu X., Li C. Identification and expression of CAMTA genes in Populus trichocarpa under biotic and abiotic stress. Sci. Rep. 2017;7:17910. doi: 10.1038/s41598-017-18219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang J., Pan X., Ge T. Genome-wide identification of citrus CAMTA genes and their expression analysis under stress and hormone treatments. J. Hort. Sci. Biotech. 2019;94:331–340. doi: 10.1080/14620316.2018.1504631. [DOI] [Google Scholar]
- 111.Pant P., Iqbal Z., Pandey B. Genome-wide comparative and evolutionary analysis of Calmodulin-binding Transcription Activator (CAMTA) family in Gossypium species. Sci. Rep. 2018;8:5573. doi: 10.1038/s41598-018-23846-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Z., Wan Y., Meng X. Genome-Wide Identification and Analysis of MKK and MAPK Gene Families in Brassica Species and Response to Stress in Brassica napus. Int. J. Mol. Sci. 2021;22:544. doi: 10.3390/ijms22020544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang H., Gong M. Genome-wide Identification of Jatropha curcas MAPK, MAPKK, and MAPKKK Gene Families and Their Expression Profile Under Cold Stress. Sci. Rep. 2018;8:16163. doi: 10.1038/s41598-018-34614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Min X., Liu Z., Wang Y. Comparative transcriptomic analysis provides insights into the coordinated mechanisms of leaves and roots response to cold stress in Common Vetch. Ind. Crops. Prod. 2020;158:112949. doi: 10.1016/j.indcrop.2020.112949. [DOI] [Google Scholar]
- 115.Wei X., Liu S., Sun C. Convergence and Divergence: Signal Perception and Transduction Mechanisms of Cold Stress in Arabidopsis and Rice. Plants. 2021;10:1864. doi: 10.3390/plants10091864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin L., Wu J., Wang Y. Plant Mitogen-Activated Protein Kinase Cascades in Environmental Stresses. Int. J. Mol. Sci. 2021;22:1543. doi: 10.3390/ijms22041543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang T., Chaudhuri S., Yang L. A calcium/calmodulin-regulated member of the receptor-like kinase family confers cold tolerance in plants. J. Biol. Chem. 2010;285:7119–7126. doi: 10.1074/jbc.M109.035659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Teige M., Scheikl E., Euglem T., Dóczi R., Ichimura K., Shinozaki K., Dangl J.L., Hirt H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell. 2004;15:141–152. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 119.Jiang X., Hoehenwarter W., Scheel D. Phosphorylation of the CAMTA3 Transcription Factor Triggers Its Destabilization and Nuclear Export. Plant Physiol. 2020;184:1056–1071. doi: 10.1104/pp.20.00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Townley H.E., Knight M.R. Calmodulin as a potential negative regulator of Arabidopsis COR gene expression. Plant Physiol. 2002;128:1169–1172. doi: 10.1104/pp.010814. [DOI] [PubMed] [Google Scholar]
- 121.Zhao C., Wang P. MAP Kinase Cascades Regulate the Cold Response by Modulating ICE1 Protein Stability. Dev. Cell. 2017;43:618–629. doi: 10.1016/j.devcel.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li H., Ding Y. MPK3-and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell. 2017;43:630–642. doi: 10.1016/j.devcel.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 123.Ding Y., Jia X., Shi Y. OST 1-mediated BTF 3L phosphorylation positively regulates CBF s during plant cold responses. EMBO J. 2018;37:e98228. doi: 10.15252/embj.201798228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hu Y., Jiang Y., Han X. Jasmonate regulates leaf senescence and tolerance to cold stress: Crosstalk with other phytohormones. J. Exp. Bot. 2017;68:1361–1369. doi: 10.1093/jxb/erx004. [DOI] [PubMed] [Google Scholar]
- 125.An J.P., Wang X.F., Zhang X. W Apple B-box protein BBX37 regulates jasmonic acid mediated cold tolerance through the JAZ-BBX37-ICE1-CBF pathway and undergoes MIEL1-mediated ubiquitination and degradation. New Phytol. 2021;229:2707–2729. doi: 10.1111/nph.17050. [DOI] [PubMed] [Google Scholar]
- 126.Fu A., Zheng Y., Lv Y. Multi-omics analysis reveals specific modifications associated with reduced chilling injury in bell pepper fruit by methyl jamonate. Postharvest Biol. Technol. 2022;185:111799. doi: 10.1016/j.postharvbio.2021.111799. [DOI] [Google Scholar]
- 127.ZHAO M.L., Wang J.N. Induction of jasmonate signalling regulators MaMYC2s and their physical interactions with MaICE1 in methyl jasmonate-induced chilling tolerance in banana fruit. Plant Cell Enviro. 2013;36:30–51. doi: 10.1111/j.1365-3040.2012.02551.x. [DOI] [PubMed] [Google Scholar]
- 128.Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015;20:219–229. doi: 10.1016/j.tplants.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 129.Zhu Z., An F., Feng Y. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:12539–12544. doi: 10.1073/pnas.1103959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Robison J.D., Yamasaki Y., Randall S.K. The ethylene signaling pathway negatively impacts CBF/DREB-regulated cold response in soybean (Glycine max) Front. Plant Sci. 2019;10:21. doi: 10.3389/fpls.2019.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Z., Huang R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 2010;73:241–249. doi: 10.1007/s11103-010-9609-4. [DOI] [PubMed] [Google Scholar]
- 132.Sun X., Zhao T., Gan S. Ethylene positively regulates cold tolerance in grapevine by modulating the expression of ETHYLENE RESPONSE FACTOR 057. Sci. Rep. 2016;6:24066. doi: 10.1038/srep24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shi Y., Tian S., Hou L. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell. 2012;24:2578–2595. doi: 10.1105/tpc.112.098640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhao M., Liu W., Xia X. Cold acclimation-induced freezing tolerance of Medicago truncatula seedlings is negatively regulated by ethylene. Physi. Plant. 2014;152:115–129. doi: 10.1111/ppl.12161. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y., Jiang H., Mao Z. Ethylene increases the cold tolerance of apple via the MdERF1B-MdCIbHLH1 regulatory module. Plant J. 2021;106:379–393. doi: 10.1111/tpj.15170. [DOI] [PubMed] [Google Scholar]
- 136.Lv K., Zhao K., Chen S. Overexpression of an AP2/ERF family gene, BpERF13, in birch enhances cold tolerance through upregulating CBF genes and mitigating reactive oxygen species. Plant Sci. 2020;292:110375. doi: 10.1016/j.plantsci.2019.110375. [DOI] [PubMed] [Google Scholar]
- 137.Sadura I., Janeczko A. Brassinosteroids and the Tolerance of Cereals to Low and High Temperature Stress: Photosynthesis and the Physicochemical Properties of Cell Membranes. Int. J. Mol. Sci. 2021;23:342. doi: 10.3390/ijms23010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ye K., Li H., Ding Y. Brassinosteroid-Insensitive2 negatively regulates the stability of transcription factor ICE1 in response to cold stress in Arabidopsis. Plant Cell. 2019;31:2682–2696. doi: 10.1105/tpc.19.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fang P., Wang Y., Wang M. Crosstalk between Brassinosteroid and Redox Signaling Contributes to the Activation of CBF Expression during Cold Responses in Tomato. Antioxidants. 2021;10:509. doi: 10.3390/antiox10040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moon J., Park Y.J., Son S.H. Brassinosteroids signaling via BZR1 down-regulates expression of ACC oxidase 4 to control growth of Arabidopsis thaliana seedlings. Plant Signal Behav. 2020;15:1734333. doi: 10.1080/15592324.2020.1734333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chi C., Li X., Fang P. Brassinosteroids act as a positive regulator of NBR1-dependent selective autophagy in response to chilling stress in tomato. J. Exp. Bot. 2020;71:1092–1106. doi: 10.1093/jxb/erz466. [DOI] [PubMed] [Google Scholar]
- 142.Ohnishi T., Watanabe B. CYP724B2 and CYP90B3 function in the early C-22 hydroxylation steps of brassinosteroid biosynthetic pathway in tomato. Biosci. Biotechnol. Biochem. 2006;70:2071–2080. doi: 10.1271/bbb.60034. [DOI] [PubMed] [Google Scholar]
- 143.Calixto C.P.G., Guo W. Rapid and Dynamic Alternative Splicing Impacts the Arabidopsis Cold Response Transcriptome. Plant Cell. 2018;30:1424–1444. doi: 10.1105/tpc.18.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim J.Y., Park S.J. Functional characterization of a glycine-rich RNA-binding protein 2 in Arabidopsis thaliana under abiotic stress conditions. Plant J. 2007;50:439–451. doi: 10.1111/j.1365-313X.2007.03057.x. [DOI] [PubMed] [Google Scholar]
- 145.Owttrim G.W. RNA helicases and abiotic stress. Nucleic Acids Res. 2006;34:3220–3230. doi: 10.1093/nar/gkl408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Egawa C., Kobayashi E. Differential regulation of transcript accumulation and alternative splicing of a DREB2 homolog under abiotic stress conditions in common wheat. Genes Genet. Syst. 2006;81:77–91. doi: 10.1266/ggs.81.77. [DOI] [PubMed] [Google Scholar]
- 147.Li J., Li X., Guo L. A subgroup of MYB transcription factor genes undergoes highly conserved alternative splicing in Arabidopsis and rice. J. Exp. Bot. 2006;57:1263–1273. doi: 10.1093/jxb/erj094. [DOI] [PubMed] [Google Scholar]
- 148.Li Y., Mi X., Zhao S. Comprehensive profiling of alternative splicing landscape during cold acclimation in tea plant. BMC Genom. 2020;21:65. doi: 10.1186/s12864-020-6491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen X., Ding A.B., Zhong X. Functions and mechanisms of plant histone deacetylases. Sci. China Life Sci. 2020;63:206–216. doi: 10.1007/s11427-019-1587-x. [DOI] [PubMed] [Google Scholar]
- 150.Bhadouriya S.L., Mehrotra S., Basantani M.K. Role of Chromatin Architecture in Plant Stress Responses: An Update. Front. Plant Sci. 2020;11:603380. doi: 10.3389/fpls.2020.603380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.De Rooij P.G.H., Perrella G. The diverse and unanticipated roles of histone deacetylase 9 in coordinating plant development and environmental acclimation. J. Exp. Bot. 2020;71:6211–6225. doi: 10.1093/jxb/eraa335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu X., Meng X., Liu Y., Wang X., Wang T.J., Zhang A., Li N., Qi X., Liu B., Xu Z.Y. The chromatin remodeler ZmCHB101 impacts alternative splicing contexts in response to osmotic stress. Plant Cell Rep. 2019;38:131–145. doi: 10.1007/s00299-018-2354-x. [DOI] [PubMed] [Google Scholar]
- 153.Park J., Lim C.J., Shen M. Epigenetic switch from repressive to permissive chromatin in response to cold stress. Proc. Natl. Acad. Sci. USA. 2018;115:e5400–e5409. doi: 10.1073/pnas.1721241115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miura K., Jin J.B., Hasegawa P.M. Sumoylation, a post-translational regulatory process in plants. Curr. Opin. Plant Biol. 2007;10:495–502. doi: 10.1016/j.pbi.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 155.Ding Y., Li H., Zhang X. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell. 2015;32:278–289. doi: 10.1016/j.devcel.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 156.Tang X., Wang Q., Yuan H. Chilling-induced DNA Demethylation is associated with the cold tolerance of Hevea brasiliensis. BMC Plant Biol. 2018;18:70. doi: 10.1186/s12870-018-1276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu Z., Jia Y., Ding Y. Plasma membrane CRPK1-mediated phosphorylation of 14–3-3 proteins induces their nuclear import to fine-tune CBF signaling during cold response. Mol. Cell. 2017;66:117–128. doi: 10.1016/j.molcel.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 158.Mazzucotelli E., Belloni S., Marone D. The e3 ubiquitin ligase gene family in plants: Regulation by degradation. Curr. Genom. 2006;7:509–522. doi: 10.2174/138920206779315728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dong C.H., Agarwal M., Zhang Y. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA. 2006;103:8281–8286. doi: 10.1073/pnas.0602874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shimatani Z., Kashojiya S. Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat. Biotechnol. 2017;35:441–443. doi: 10.1038/nbt.3833. [DOI] [PubMed] [Google Scholar]
- 161.Lim C.J., Park J., Shen M. The Histone-Modifying Complex PWR/HOS15/HD2C Epigenetically Regulates Cold Tolerance. Plant Physiol. 2020;184:1097–1111. doi: 10.1104/pp.20.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cheng L., Zhang W., Hu J. Characterization of the key region and putative phosphorylation sites of EcaICE1 in its molecular interaction with the EcaHOS1 protein in Eucalyptus camaldulensis. Plant Biol. 2021;23:400–406. doi: 10.1111/plb.13205. [DOI] [PubMed] [Google Scholar]
- 163.Castro P.H., Tavares R.M., Bejarano E.R. SUMO, a heavyweight player in plant abiotic stress responses. Cell Mol. Life Sci. 2012;69:3269–3283. doi: 10.1007/s00018-012-1094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Miura K., Jin B.J., Lee J. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell. 2007;19:1403–1414. doi: 10.1105/tpc.106.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wisniewski M., Nassuth A., Arora R. Cold hardiness in trees: A mini-review. Front. Plant Sci. 2018;9:1394. doi: 10.3389/fpls.2018.01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.