Abstract
Tomato spotted wilt virus impacts negatively on a wide range of economically important plants, especially tomatoes. When plants facing any pathogen attack or infection, increase the transcription level of plant genes that are produced pathogenesis-related (PR) proteins. The aim of this study is a genome-wide identification of PR-10 superfamily and comparative analysis of PR-10 and Sw-5b gene functions against tomato responses to biotic stress (TSWV) to systemic resistance in tomato. Forty-five candidate genes were identified, with a length of 64–210 amino acid residues and a molecular weight of 7.6–24.4 kDa. The PR-10 gene was found on ten of the twelve chromosomes, and it was determined through a genetic ontology that they were involved in six biological processes and molecular activities, and nine cellular components. Analysis of the transcription level of PR-10 family members showed that the PR-10 gene (Solyc09g090980) has high expression levels in some parts of the tomato plant. PR-10 and Sw-5b gene transcription and activity in tomato leaves were strongly induced by TSWV infection, whereas H8 plants having the highest significantly upregulated expression of PR-10 and Sw-5b gene after the inoculation of TSWV, and TSWV inoculated in M82 plants showed significantly upregulated expression of PR-10 gene comparatively lower than H8 plants. There was no significant expression of Sw-5b gene of TSWV inoculated in M82 plants and then showed highly significant correlations between PR-10 and Sw-5b genes at different time points in H8 plants showed significant correlations compared to M82 plants after the inoculation of TSWV; a heat map showed that these two genes may also participate in regulating the defense response after the inoculation of TSWV in tomato.
Keywords: PR-10 protein, resistance, Sw-5b, tomato, TSWV
1. Introduction
When pathogens infect a plant, pathogenesis-related proteins are produced. They are now categorized into 17 groups based on structural and functional features, and they play a key role in plant growth and development under a variety of stressors [1]. Through their enzymatic activity, PR proteins can directly impact pathogen integrity and/or create signal molecules, which act as a trigger to activate other plant defense processes [2]. PR-10 is a naturally occurring acidic protein with a molecular weight of 16–19 kDa and three-dimensional β-sheet topologies covered by a compact, bipartite framework held together by hydrophobic interactions. PR-10 proteins have antimicrobial action and are involved in a variety of biological processes, including ribonuclease and other secondary metabolism enzymatic functions, along with plant defense against biotic and abiotic stressors [3]. The majority of PR-10 proteins are made up of two domains: Bet_ v _1 and P-loop. Bet_ v _1 is a conserved domain of the PR-10 protein family that serves as a defense against pathogen infection. The RNase activity of these proteins contains a P-loop motif, such as GxGGxGxxK, which serves as a nucleotide-binding site [4].
Tomatoes (Solanum lycopersicum) are the most widely grown commercial vegetable crop in the world. The tomato plant, on the other hand, is susceptible to a variety of diseases. More than 136 viral species that are detrimental to tomatoes have been discovered so far [5]. Tomato spotted wilt virus (TSWV) is one of the most dangerous. TSWV cause significant reductions in tomato output and market value, as well as mortality in certain cases [6]. Usually, the entire plant is dwarfed, with necrotic streaks and dark brown flecks on the leaves, stems, and fruits. The first symptoms of seedlings inhibited the growth points and young leaves turn into copper-colored rolls, and subsequently form many small dark brown flecks, the leaves’ veins will be purple. Furthermore, the virus causes growth points leaf necrosis and drooping, and the stem end will have brown necrotic streaks. The plant grows only halfway or is completely dwarfed and wilted, and causes chlorotic rings on the green fruit [7].
The Sw-5 gene is a single dominant quality of resistance gene that protects a wide range of Tospoviruses [8], originating from L. peruvianum, and has been identified and introgressed in the fresh tomato cultivar (Lycopersicon esculentum). Sw-5 was discovered near the telomeric area of chromosome 9, between the CT71 and CT220 RFLP markers [9], and more closely linked to the CT220 marker (about within 65 kb) [10]. Sw-5 locus is part of a loosely clustered gene family that includes six homologous paralog genes: Sw-5a, Sw-5b, Sw-5c, Sw-5d, Sw-5e, and Sw-5f are all potential variations [11]. Sw-5a and Sw-5b genes are strongly homologous (95%), and only Sw-5b has universal various isolates of TSWV [12]. Sw-5b was also linked to Tospovirus resistance to TCSV (tomato chlorotic spot virus), TZSV (tomato zonate spot virus), and GRSV (groundnut ringspot virus) [10].
TSWV is an RNA globular virus with an envelope structure and an outer membrane with a continuous protrusion layer. Its genome consists of three separate genomic RNA strands: long (L) RNA, medium (M) RNA, and short (S) RNA, all of which code for five different proteins. RdRp (RNA-dependent RNA polymerase) is a replication-related protein that interacts with host encoding factors. L RNA is a negative-sense RNA that encodes RdRp (RNA-dependent RNA polymerase) [13]. M RNA is an antisense RNA that codes for the amino acid and carboxy-terminal location of the glycoprotein precursor (GnGc), which is required for virions to assemble, mature, and release into their host [14]. The viral nonstructural proteins (NSm), which are encoded by sense RNA, are the main promoters of TSWV infection [15]. S RNA also contains a double sense RNA, with antisense RNA encoding nucleocapsid proteins (N) and sense RNA encoding nonstructural proteins (NSs) [16]; both proteins have a crucial function in the TSWV infection cycle [17]. Tomato spotted wilt virus (TSWV) is one of the most important plant viruses in the world [18]. TSWV belongs to the species Tospovirus that infect only plants, the genus Orthotospovirus, the family Tospoviridae, and the order Bunyaviridae [19]. TSWV caused widespread reductions in tomato yield and commercial value, together with plant mortality. Furthermore, the fast proliferation of TSWV-carrying Frankliniella occidentalis (Western flower thrips) has affected tomato agriculture and output severely [6]. As a result, tomato faces a major threat from this virus. We speculated on which genes are used to defend this virus, and considered the utilization of resistant/tolerant tomato cultivars to remedy the problem. These findings may lead to a better understanding of how the PR-10 protein and the Sw-5b gene act in the generation of systemic necrosis by the tomato spotted wilt tospovirus, which could aid in the future development of new antiviral approaches.
2. Results
2.1. Genome-Wide Identification and Analysis of Solanum Lycopersicum PR-10 Genes
From a genome-wide investigation of tomatoes, 45 putative PR-10 encoding gene candidates were discovered. There are no chromosomal locations for the two PR-10 gene ID numbers. Table 1 contains basic information on the PR-10 genes, such as protein sequence length, isoelectric points, and molecular weight. PR-10 proteins varied in length from 64 to 210 amino acids, with molecular weights ranging from 7.6 to 24.4 kDa. The bulk of PR-10 proteins was classified as acidic based on theoretical isoelectric point (pI) values. The severe acidic or basic characteristics of each PR-10 gene may contribute to different activities. In addition, all of the PR-10 protein sequences in tomatoes were shown to have the CDS, molecular weight, pI, instability index, aliphatic index, GRAVY, and subcellular localization (Table 1).
Table 1.
The sequence features and physicochemical properties of tomato PR-10 proteins.
| SL. No. | Chr | Start | END | Strand | Number of Amino Acids | CDS | Molecular Weight | pI | Instability Index |
Aliphatic Index |
GRAVY | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ch00 | 14,149,228 | 14,151,711 | reverse | 158 | 441 | 18,196.91 | 5.03 | 38.70 | 99.94 | −0.218 | Cytoplasmic |
| 2 | ch00 | 21,749,238 | 21,751,331 | forward | 210 | 633 | 24,401.45 | 5.15 | 43.03 | 86.62 | −0.417 | Cytoplasmic |
| 3 | ch01 | 9,342,757 | 9,343,038 | reverse | 93 | 282 | 11,233.13 | 7.65 | 50.57 | 90.11 | −0.134 | Cytoplasmic |
| 4 | ch01 | 80,454,898 | 80,456,153 | forward | 76 | 231 | 8763.32 | 6.71 | 30.32 | 86.97 | −0.051 | Cytoplasmic |
| 5 | ch03 | 66,611,609 | 66,612,183 | forward | 159 | 480 | 17,608.98 | 5.02 | 32.81 | 82.20 | −0.35 | Cytoplasmic |
| 6 | ch03 | 66,614,237 | 66,614,792 | forward | 159 | 480 | 17,636.95 | 4.90 | 34.90 | 79.12 | −0.401 | Cytoplasmic |
| 7 | ch04 | 455,936 | 457,664 | reverse | 147 | 444 | 16,774.40 | 6.29 | 35.91 | 86.19 | −0.334 | Cytoplasmic |
| 8 | ch04 | 737,430 | 738,426 | forward | 146 | 441 | 16,613.22 | 5.96 | 33.78 | 99.38 | −0.168 | Cytoplasmic |
| 9 | ch04 | 1,363,357 | 1,364,224 | forward | 95 | 288 | 10,838.61 | 9.64 | 21.65 | 109.58 | −0.226 | Cytoplasmic |
| 10 | ch04 | 1,429,473 | 1,430,430 | forward | 147 | 444 | 16,734.98 | 5.59 | 31.71 | 94.76 | −0.318 | Cytoplasmic |
| 11 | ch04 | 1,435,905 | 1,437,368 | forward | 146 | 441 | 16,583.08 | 6.03 | 25.97 | 98.01 | −0.2 | Cytoplasmic |
| 12 | ch04 | 1,456,588 | 1,458,908 | reverse | 147 | 444 | 16,595.16 | 5.96 | 42.21 | 106.05 | −0.162 | Cytoplasmic |
| 13 | ch04 | 1,466,509 | 1,468,357 | forward | 148 | 447 | 17,017.30 | 5.62 | 19.96 | 90.88 | −0.403 | Cytoplasmic |
| 14 | ch04 | 1,474,832 | 1,476,779 | forward | 147 | 444 | 16,824.05 | 5.63 | 17.31 | 88.10 | −0.407 | Cytoplasmic |
| 15 | ch04 | 1,496,628 | 1,497,874 | reverse | 147 | 444 | 16,770.01 | 5.72 | 35.06 | 87.41 | −0.365 | Cytoplasmic |
| 16 | ch04 | 1,505,284 | 1,506,417 | forward | 134 | 405 | 15,762.00 | 5.64 | 56.47 | 92.84 | −0.653 | Cytoplasmic |
| 17 | ch04 | 49,064,622 | 49,065,920 | reverse | 147 | 444 | 17,152.77 | 5.90 | 36.51 | 91.43 | −0.351 | Cytoplasmic |
| 18 | ch05 | 58,320,136 | 58,321,632 | reverse | 150 | 453 | 17,596.99 | 5.22 | 50.34 | 96.00 | −0.467 | Cytoplasmic |
| 19 | ch05 | 58,346,771 | 58,347,729 | reverse | 150 | 453 | 17,698.46 | 5.47 | 51.29 | 86.93 | −0.456 | Cytoplasmic |
| 20 | ch05 | 58,352,298 | 58,352,492 | reverse | 64 | 195 | 7600.66 | 5.18 | 43.07 | 83.75 | −0.502 | Cytoplasmic |
| 21 | ch05 | 58,352,813 | 58,353,016 | reverse | 67 | 204 | 7959.23 | 7.06 | 33.19 | 92.99 | −0.058 | Cytoplasmic |
| 22 | ch05 | 58,415,437 | 58,415,727 | reverse | 96 | 291 | 11,409.16 | 5.04 | 54.80 | 89.38 | −0.431 | Cytoplasmic |
| 23 | ch05 | 58,420,502 | 58,420,723 | reverse | 73 | 222 | 8461.61 | 7.93 | 30.44 | 77.26 | −0.458 | Cytoplasmic |
| 24 | ch05 | 64,304,632 | 64,306,501 | reverse | 162 | 489 | 18,181.90 | 4.78 | 37.17 | 89.63 | −0.188 | Cytoplasmic |
| 25 | ch06 | 1,757,744 | 1,758,229 | forward | 161 | 486 | 17,717.56 | 5.23 | 33.57 | 106.40 | 0.131 | Cytoplasmic |
| 26 | ch07 | 288,360 | 289,609 | reverse | 149 | 450 | 16,770.27 | 4.73 | 25.47 | 99.33 | −0.013 | Cytoplasmic |
| 27 | ch07 | 291,108 | 292,521 | forward | 158 | 477 | 17,981.36 | 5.23 | 37.64 | 87.47 | −0.285 | Cytoplasmic |
| 28 | ch07 | 3,683,904 | 3,685,158 | forward | 159 | 480 | 18,422.81 | 5.31 | 32.72 | 75.91 | −0.613 | Cytoplasmic |
| 29 | ch08 | 26,737,023 | 26,738,299 | reverse | 148 | 447 | 17,074.62 | 6.50 | 16.91 | 88.92 | −0.42 | Cytoplasmic |
| 30 | ch09 | 296,718 | 297,754 | reverse | 148 | 453 | 17,074.62 | 6.50 | 16.91 | 88.92 | −0.42 | Cytoplasmic |
| 31 | ch09 | 301,066 | 301,598 | reverse | 136 | 411 | 15,514.72 | 5.09 | 22.29 | 95.96 | −0.171 | Cytoplasmic |
| 32 | ch09 | 304,321 | 305,404 | reverse | 150 | 453 | 17,083.46 | 4.88 | 28.19 | 96.07 | −0.289 | Cytoplasmic |
| 33 | ch09 | 339,412 | 340,474 | forward | 151 | 456 | 17,425.73 | 5.10 | 40.68 | 94.77 | −0.438 | Cytoplasmic |
| 34 | ch09 | 6,149,317 | 6,151,058 | reverse | 150 | 453 | 17,100.71 | 5.57 | 25.66 | 92.87 | −0.223 | Cytoplasmic |
| 35 | ch09 | 6,153,756 | 6,154,061 | reverse | 101 | 306 | 11,770.43 | 8.44 | 34.15 | 73.27 | −0.716 | Cytoplasmic |
| 36 | ch09 | 6,176,738 | 6,177,747 | reverse | 152 | 459 | 17,414.09 | 5.47 | 24.29 | 88.29 | −0.266 | Cytoplasmic |
| 37 | ch09 | 6,195,377 | 6,197,648 | reverse | 74 | 225 | 8310.73 | 8.80 | 24.74 | 75.00 | −0.176 | Cytoplasmic |
| 38 | ch09 | 6,221,354 | 6,222,253 | reverse | 152 | 459 | 17,423.11 | 5.62 | 31.78 | 90.26 | −0.248 | Cytoplasmic |
| 39 | ch09 | 70,346,085 | 70,347,506 | reverse | 155 | 468 | 17,235.60 | 5.79 | 22.44 | 84.84 | −0.497 | Cytoplasmic |
| 40 | ch09 | 70,350,133 | 70,352,154 | reverse | 160 | 483 | 17,368.77 | 5.44 | 30.62 | 94.38 | −0.128 | Cytoplasmic |
| 41 | ch09 | 70,355,642 | 70,357,036 | reverse | 160 | 483 | 17,914.20 | 5.34 | 32.81 | 88.25 | −0.426 | Cytoplasmic |
| 42 | ch09 | 70,360,338 | 70,361,437 | forward | 155 | 468 | 17,352.86 | 5.67 | 27.16 | 86.06 | −0.389 | Cytoplasmic |
| 43 | ch10 | 2,466,093 | 2,467,665 | reverse | 149 | 450 | 17,272.9 | 8.74 | 25.91 | 72.01 | −0.606 | Cytoplasmic |
| 44 | ch10 | 43,660,724 | 43,661,266 | reverse | 146 | 441 | 16,685.34 | 6.37 | 13.79 | 85.48 | −0.305 | Cytoplasmic |
| 45 | ch12 | 65,664,355 | 65,665,560 | forward | 158 | 477 | 18,196.91 | 5.03 | 38.7 | 99.94 | −0.218 | Cytoplasmic |
2.2. Phylogenetic Analysis, Conserved Motifs, and Gene Structures
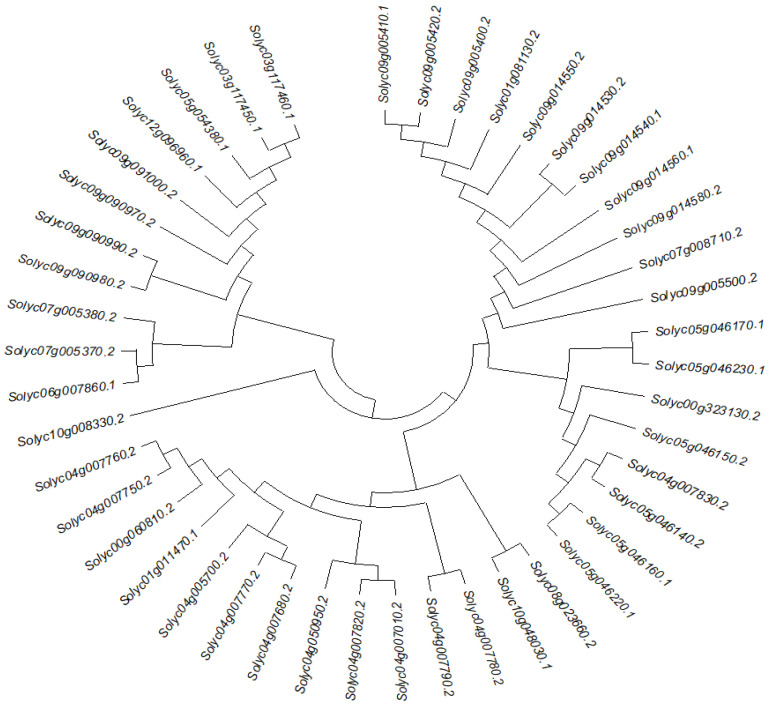
To further characterize and identify potential functional relationships between the PR-10 proteins of tomato, a phylogenetic tree was constructed and 45 gene IDs of PR-10 protein were identified (Figure 1). The neighbor-joining method, with 1000 bootstrap reconstruction and completed deletion gaps/missing data, yielded five known subfamilies.
Figure 1.
Phylogenetic tree of 45 genes of PR-10 protein in tomato. Sequence alignment was done by ClustalW and Phylogenetic tree generated by MEGA 5.1 with maximum likelihood (ML) method for 1000 replicates as the bootstrap value.
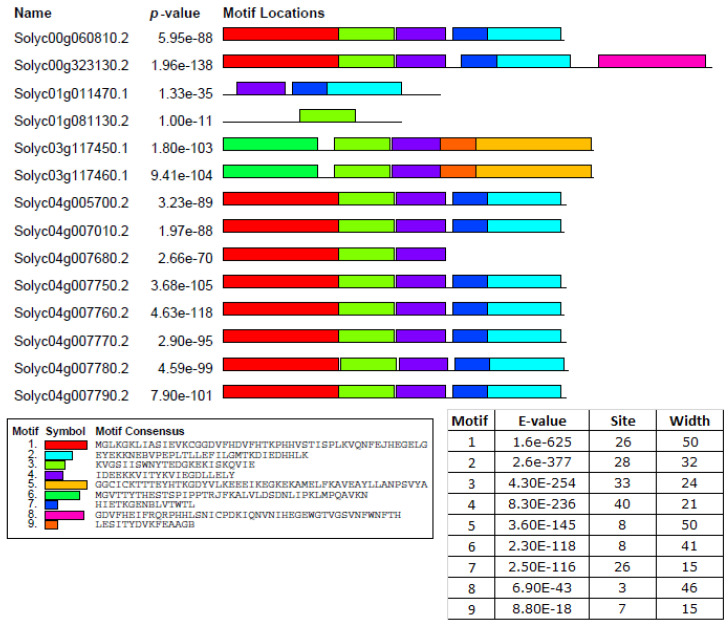
A total of nine conserved motifs were identified using the MEME server (Figure 2), and only motif 1 and motif 6 were found to be associated with the Bet_ V_ 1 (PF000407) domain. These two motifs (motif 1 and 6) were found in all SlPR-10 proteins, as expected. Remarkably, motif 9 was found only in the Solyc03g117450.1 and Solyc03g117460.1 protein sequence. The fewest motifs were identified as in Solyc01g011470.1 and Solyc01g081130.2 protein sequences. These variations of the protein motifs in number, types, and positions may be related to molecular function and roles in different tomato metabolic pathways.
Figure 2.
Motif locations of PR-10 protein. Each motif is represented using a box with different colors. Motif 1, red; Motif 2, violet; Motif 3, light green; Motif 4, light blue; Motif 5, yellow; Motif 6, green; Motif 7, blue; Motif 8, purple; Motif 9, orange.
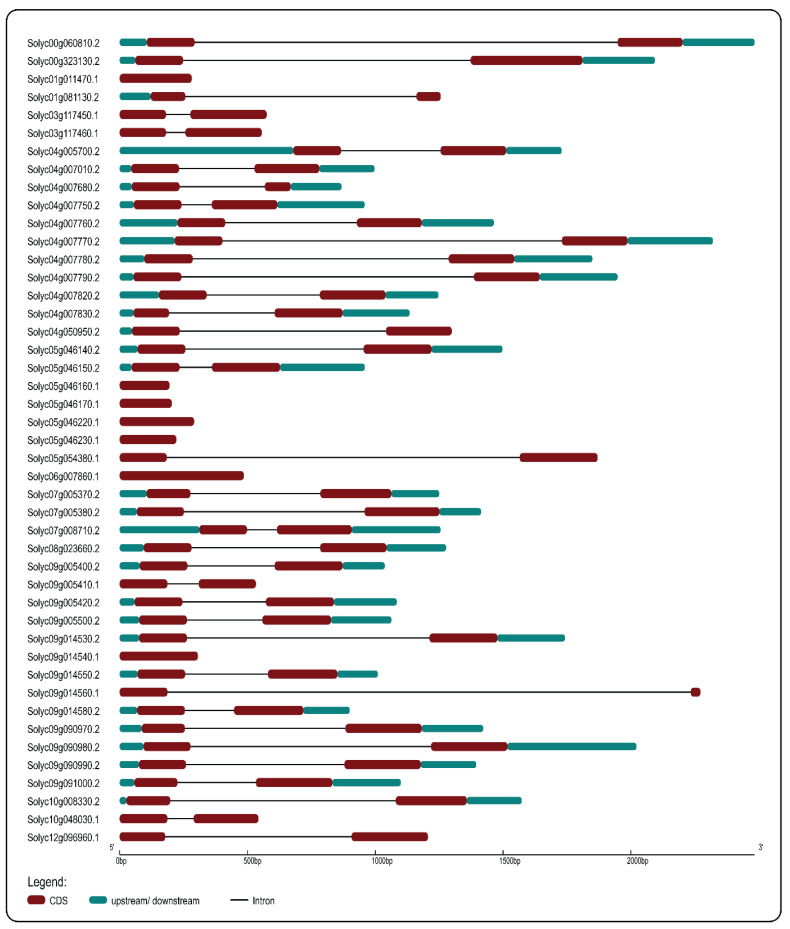
The distribution of the intronic phase and the location of exons/introns are key features for gene structure study. Introns are divided into three phases: phase 0 introns are found between two consecutive codons, phase 1 introns are found between a codon’s first and second nucleotide, and phase 2 introns are found between a codon’s second and third nucleotide [20]. The exon–intron and intron phase organization of tomato PR-10 genes is shown in Figure 3. The location and length of introns–exons organization in tomato PR-10 genes are shown in Table 1.
Figure 3.
Gene structure of 45 genes of PR-10 protein in tomato. Exon, intron, upstream, and downstream regions are indicated as dark red boxes, black lines, and light blues boxes, respectively.
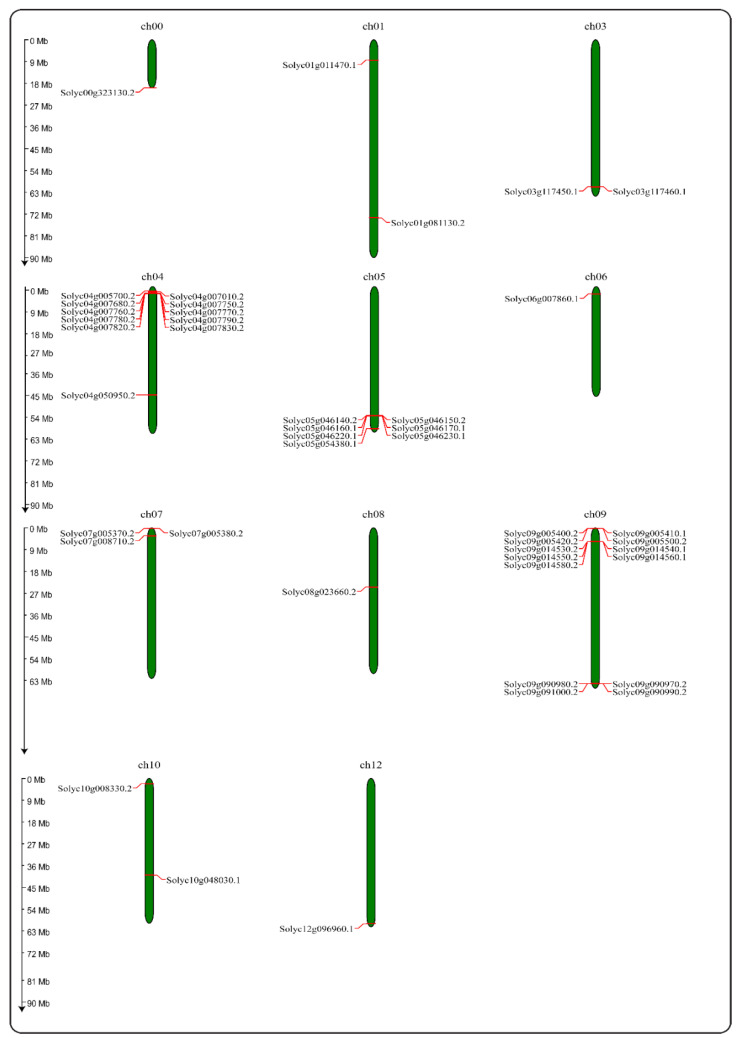
2.3. Chromosome Mapping of the PR-10 Gene
CDD (Conserved domains database) from NCBI was used to compare the domain architecture of PR-10 gene sequences. This study’s findings include the presence of conserved domains in their sequences, indicating that they are homologs. In silico chromosomal mapping of tomato PR-10 genes are shown in Figure 4. Tomato PR-10 genes have been discovered on ten of the twelve chromosomes. There are 11 genes on chromosome 4 and 13 genes on chromosome 9 in tomatoes; however, PR-10 genes were not discovered on chromosomes 2 and 11.
Figure 4.
Chromosome mapping of the PR-10 gene of tomato.
2.4. GO Analysis
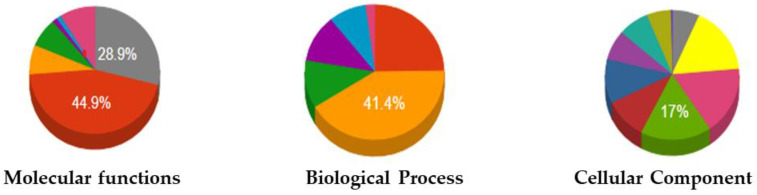
Six biological processes and molecular activities and nine cellular components were identified via a gene ontology (GO) study, shown in Figure 5. Most PR-10 genes have a role in defensive responses and a reaction to abiotic stimulus in a biological function, according to the GO enrichment study. It exhibited protein kinase activity together with adenyl nucleotide and purine ribonucleoside binding activities at the molecular level. In the extracellular area, the cellular component had a function (Table 2).
Figure 5.
The gene ontology (GO) term distribution of PR-10 proteins of tomato.
Table 2.
The gene ontology (GO) term distribution of PR-10 proteins of tomato.
| Molecular Functions | Biological Process | Cellular Component |
|---|---|---|
| Protein binding | Immune system process | Cytosol |
| Lipid binding 44.9% | Response to stress 41.4% | Nucleus |
| Lyase activity | Reproduction | Organelle |
| Ion binding | Anatomical structure development | Vacuole |
| Nuclease activity | Cellular nitrogen compound metabolic process | Cytoplasmic-membrane-bounded vesicle |
| Hydrolase activity | Signal transduction | Cytoplasm 17% |
| N/A 28.9% | Cell | |
| Intercellular | ||
| N/A |
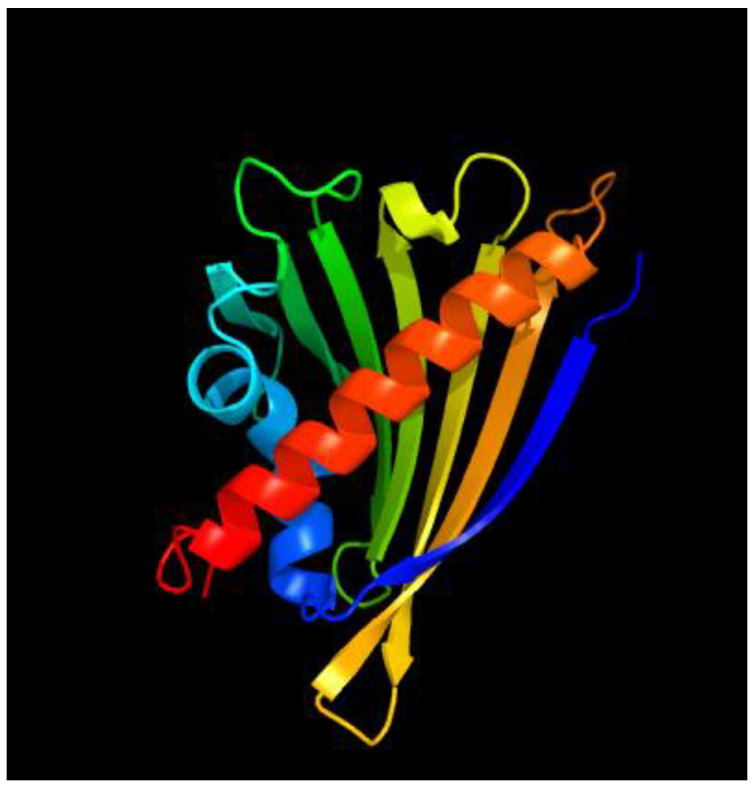
2.5. Three-Dimensional Structure of the PR-10 Protein
There was a difference in the percentage of structural attributes that changed among the 45 PR-10 gene IDs. Despite the 3D structural variances revealed among tomato PR-10 proteins, all 45 candidates have the binding proteins essential for protein interaction. For PR-10 gene cloning and plant transformation, we chose the Solyc09g090980 gene ID of PR-10 protein, which is a phenolic, oxidative coupling protein with a length of 1424 bp and is situated on chromosome 9, as shown in Figure 6.
Figure 6.
The predicted 3D structures of Solyc09g090980 of PR-10 protein, generated using the Phyre2 server, and binding pockets identified by the CASTp 3.0 server (shown in red).
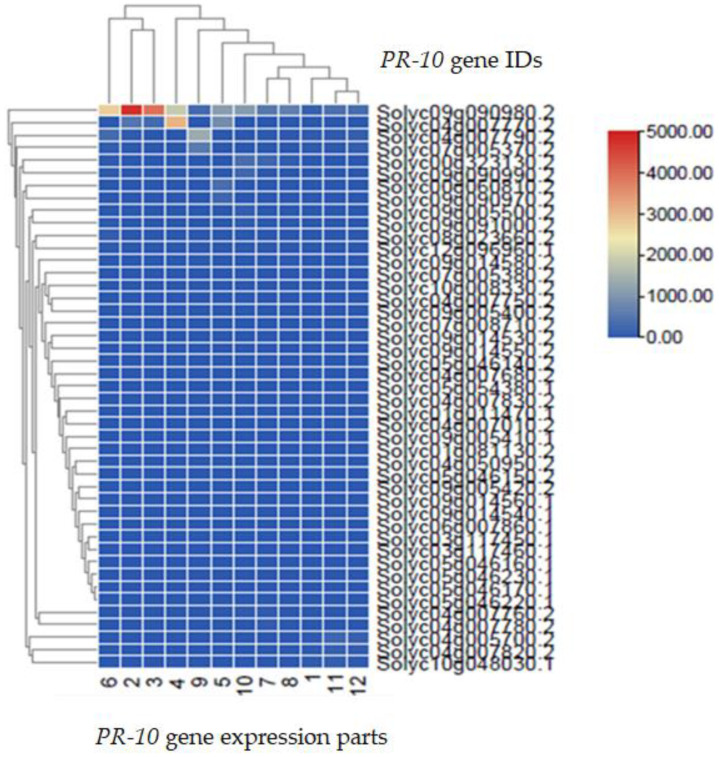
2.6. Transcriptomic Analysis of the PR-10 Protein of Tomato
The root, stem, branch, tendon, male flower, female flower, and fully opened young leaf were all subjected to spatial expression profiling of all PR-10 genes in tomatoes. The PR-10 genes were expressed differently in different plant tissues, according to the heatmap analysis. However, most of the genes in tomatoes had a relatively low expression level. Furthermore, the highest level of PR-10 expression of the Solyc09g090980 gene compared to the other tissues (Figure 7).
Figure 7.
Transcriptomic analysis of the PR-10 protein in tomato. The data presented are based on at least three separate replicates. The gene expression within each family is represented using hierarchical clustering.
2.7. Incidence and Disease Index Level of TSWV in Each Group of Tomato Plants at Each Observation Time Point
Tomato-sensitive cultivar M82 and TSWV-resistant cultivar H8 were evaluated for their response to TSWV infections. As previously stated, plants were manually infected with TSWV and kept in an insect-proof greenhouse [21]. M82 and H8 plants treated plants with TSWV virus inoculum compared with M82 and H8 CK plants. It was found that there were no obvious symptoms of TSWV after 7 days of inoculation. After 14 days, the plants showed obvious dwarfing, and the growing point of the plant was purple; all M82 treatment groups showed purple spots in growth and wilting after 21 days. Incidence of TSWV in each CK and treatment (TR) group of M82 tomato plants showed the highest TSWV incidence rate compared to H8 plants (Table 3) after 28 days.
Table 3.
Disease incidence and index level of TSWV in the CK and treatment (TR) groups of M82 and H8 tomato cultivars (highly resistance (0 < disease index ≤ 5); medium resistance (5 < disease index ≤ 20); resistance (20 < disease index ≤ 40); highly sensitive (60 < disease index); sensitive (40 < disease index ≤ 60)).
| Post Inoculation of TSWV | CK Group | TR Group | ||
|---|---|---|---|---|
| cv. M82 (Sensitive) | cv. H8 (Resistant) | cv. M82 (Sensitive) | cv. H8 (Resistant) | |
| 14 days | 10.0% c 5.3 d |
3.33% c 0.0 e |
16.67% c 12.5 d |
10.0% c 3.0 f |
| 21 days | 26.67% b 46.66 b |
10.0% c 5.4 d |
36.67% b 52.3 b |
10.0% c 8.3 e |
| 28 days | 40.0% a 58.88 a |
26.66% b 14.0 c |
100% a 88.88 a |
33.33% c 18.33 c |
Note: Same letters represent no significant difference, while different letters represent significant difference between CK and TR groups of M82 and H8 plants of TSWV disease incidence and disease index level, after TSWV inoculation at 14, 21 and 28 days. TSWV disease incidence expressed in percentages (%) and disease index express HR, MR, S, and HS respectively. Means ± SD at p < 0.05 using Tukey’s test with three biological replicates.
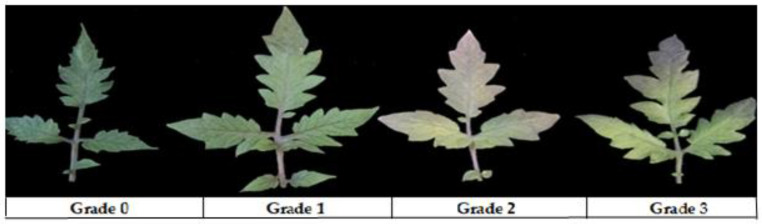
The severity of TSWV symptoms was scored on a scale of 0–4 (Table 4) for each plant at 14, 21 and 28 days post inoculation (dpi) (Table 3) to calculate the TSWV disease incidence rate and disease index level.
Table 4.
Plant disease index level parameters.
| Grade Series | TSWV Symptoms |
|---|---|
| 0 | Asymptomatic |
| 1 | Leaves are less obvious bronze (black) small spots |
| 2 | Bronze (black) necrosis spots spread in small area of leaves |
| 3 | Plant leaves on the film even into pieces, nearly 2/3 leaf area dry necrosis |
| 4 | Whole plant necrosis |
On the other hand, the plant disease index level of TSWV in each group of CK and TR; M82 showed the highest sensitivity compared to H8 plants, and H8 plants showed resistance phenotype after 28 days of infection of TSWV (Table 3).
After inoculation of TSWV, M82 and H8 plants are (shown in Figure 8) TSWV disease severity showed after 28 days of infection; the plant’s leaves were purple and dark brown patches in M82 plants (shown in Figure 9).
Figure 8.
TSWV inoculate of M82 and H8 plants of tomato; (a) TSWV inoculation of M82 plant, (b) TSWV inoculation of H8 plant, (c) TSWV inoculated leaves, (d) TSWV inoculate of M82 plants after 28 days, and (e) TSWV inoculate of H8 plants after 28 days.
Figure 9.
M82 plant leaves show typical symptoms of TSWV: Grade 0, no disease; Grade 1, slightly infected; Grade 2, 50% infected; and Grade 3, 75% infected leaves.
2.8. PR-10 Expression in M82 and H8 Plants
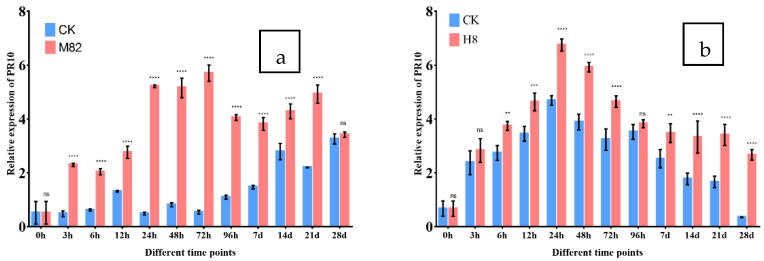
The purpose of this study was to determine how PR-10 protein expression affected infection in M82 and H8 plants following TSWV virus inoculation. Both CK and TR plants were sampled at various time intervals, including 0, 3, 6, 12, 24, 48, 72, 96 h and 7, 14, 21, 28 days. The expression pattern of PR-10 in different tissues of leaves of the tomato M82 (sensitive) and H8 (resistant) plants after the TSWV virus inoculation follows: M82 inoculated plants showed a wide range of response of PR-10 protein (5.70-fold relative expression at 72 h) compared to CK and H8 inoculated plants, which showed a wide range of response of PR-10 protein relative expression compared to CK (6.74-fold at 24 h) and significantly upregulated in the leaves after the TSWV inoculation (Figure 10).
Figure 10.
PR-10 expression in M82 and H8 plants. The relative transcription levels of TSWV-infected tomato M82 and H8 plants were normalized to the control gene Actin, as measured by qRT-PCR of PR-10 protein. At 0, 3, 6, 12, 24, 48, 72, and 96 h pi (hours post infection) and 7, 14, 21 and 28 dpi (days post infection), the expression levels in the leaf tissues were assessed (days post infection). The CK plants are shown by blue bars, while the M82 and H8 plants are represented by red bars. Three biological replicates’ mean results (±SD) are shown as relative changes. (a) PR-10 relative expression in M82 plants vs. CK (not inoculated with TSWV) and (b) PR-10 relative expression in H8 plants compared to CK. Asterisk indicates statistically significant; ** p = 0.01, *** p = 0.001, **** p = 0.0001, and ns = non-significant are the p values.
2.9. TSWV-cp Expression in M82 and H8 Plants
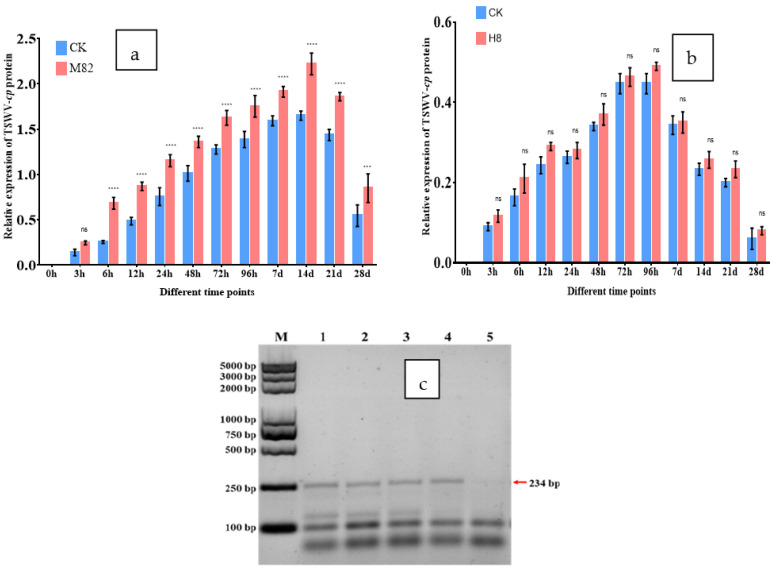
To examine the effect of TSWV-cp protein expression after the TSWV virus inoculation in M82 and H8 plants. Leaf samples were taken at different time points such as 0, 3, 6, 12, 24, 48, 72, 96 h and 7, 14, 21, 28 days both CK and TR plants. The expression pattern of TSWV-cp in different tissues of leaves in the tomato M82 and H8 plants after the TSWV virus inoculation, M82 infected plants showed a wide range of response of TSWV-cp compared to CK (2.22-fold relative expression at 14D) were significantly upregulated, and H8 infected plants showed a narrow range of response of TSWV-cp compared to CK were non-significantly upregulated in leaves after the TSWV inoculation in Figure 11.
Figure 11.
TSWV-cp expression in M82 and H8 plants. Tomato M82 and H8 plants infected with TSWV inoculum, and relative transcript levels normalized to the control gene Actin. At 0, 3, 6, 12, 24, 48, 72, and 96 hpi (hours post infection) and 7, 14, 21 and 28 dpi (days post infection), the expression levels in the leaf tissues were assessed (days post infection). The CK plants are shown by blue bars, while the M82 and H8 plants are represented by red bars. The mean values (±SD) from three biological replicates are provided as relative changes: (a) TSWV-cp relative expression in M82 plants compared to CK; (b) TSWV-cp relative expression in H8 plants compared to CK; and (c) TSWV-cp confirmation in Yangling, China, isolates 234 bp. Asterisk indicates statistically significant; *** p = 0.001, **** p = 0.0001, and ns = non-significant are the p values.
2.10. Expression of Sw-5b in M82 and H8 Plants
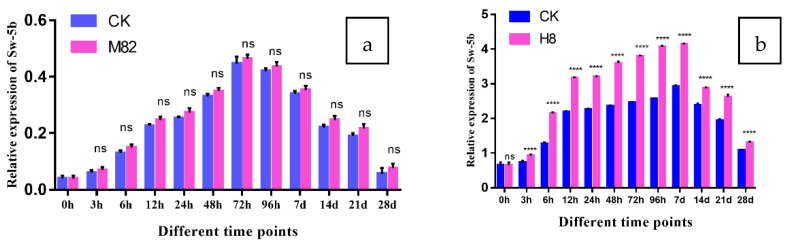
The purpose of this study was to determine how Sw-5b expression changed following TSWV virus inoculation in M82 and H8 plants. Tomato leaf samples were taken from CK and TR plants at various times, including 0, 3, 6, 12, 24, 48, 72, 96 h and 7, 14, 21, 28 days. Figure 12 shows the Sw-5b expression pattern in different tissues of tomato M82 and H8 plants’ leaves after TSWV virus inoculation; M82-infected plants showed no significant expression of Sw-5b, while H8-infected plants showed a wide range of relative expression of Sw-5b (4.14-fold at 7D) and significantly upregulated in leaves after TSWV inoculation.
Figure 12.
Expression of Sw-5b in M82 and H8 plants. Sw-5b relative transcription levels were assessed by qRT-PCR; tomato M82 and H8 plants were inoculated with TSWV, and relative transcript levels were normalized to the control gene Actin. At 0, 3, 6, 12, 24, 48, 72, and 96 hpi (hours post infection) and 7, 14, 21 and 28 dpi (days post infection), the expression levels in the leaf tissues were assessed (days post infection). The CK plants are shown by blue bars, while the M82 and H8 plants are represented by red bars. Three biological replicates’ mean results (±SD) are reported as relative changes. (a) Sw-5b expression in M82 plants in comparison to CK, and (b) Sw-5b expression in H8 plants in comparison to CK. Asterisk indicates statistically significant; **** p = 0.0001, and ns = non-significant are the p values.
2.11. Expression of Different Index Levels of Leaves in M82 and H8 Plants
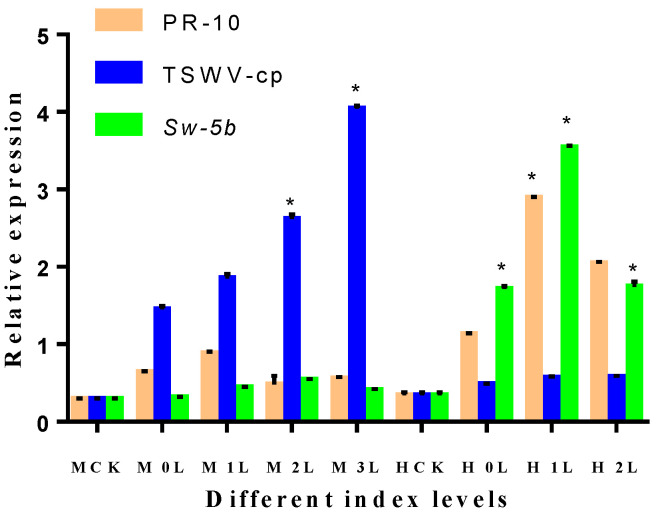
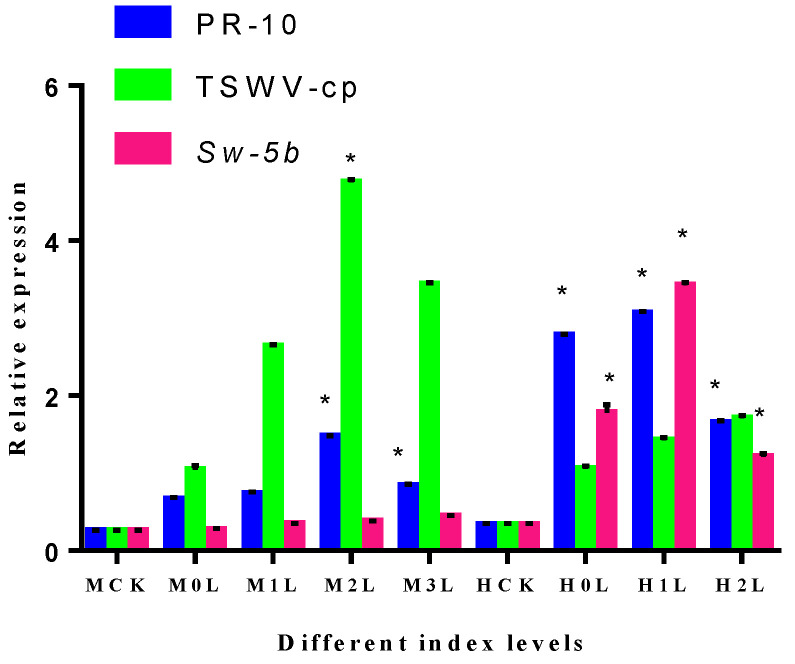
The purpose of this study was to determine how PR-10, TSWV-cp, and Sw-5b expression affected different disease index levels of M82 and H8 tomato leaves following TSWV inoculation. After 28 days, tomato leaf samples were obtained from both CK and TR plant leaves. In the Section 4, different disease index levels are explained. Different disease index levels show various types of expression of PR-10, TSWV-cp, and Sw-5b in M82 and H8 plants. H8-infected plants showed a higher range of PR-10 protein compared to M82 plants. On the other hand, M82-infected plants showed a wide range of TSWV-cp (4.02-fold relative expression at level 3) compared to H8 plants. Additionally, M82 plants showed no significant expression of Sw-5b, but H8 plants showed 3.53-fold relative expression of Sw-5b, as shown in Figure 13.
Figure 13.
Expression of different index levels of leaves in M82 and H8 plants. After the TSWV inoculum was applied to tomato M82 and H8 plants, the relative transcript expression levels of PR-10 protein, TSWV-cp protein, and Sw-5b were determined using qRT-PCR, and the relative transcript levels were normalized to the control gene Actin. The expression levels in the leaf tissues were measured at different disease index levels. Light yellow bars represent the PR-10 proteins, blue bars represent the TSWV-cp protein, and light green bars represent the Sw-5b. The mean values (±SD) from three biological replicates are presented as relative changes. The least significant difference (LSD) test was used to determine if a gene showed significant induction as compared to the transcript abundance of the control group (MCK represents the M82, CK plant; MTR represents the M82-treated plant; HCK represents the H8, CK plant; and HTR represents the H8-treated plant). Asterisk indicates statistically significant; * p = 0.05, and ns = non-significant are the p values.
2.12. Expression of Different Index Levels of the Tomato Plants Root
To examine the effect of PR-10, TSWV-cp, and Sw-5b expression of different index levels of M82 and H8 tomato plant roots, tomato plant root samples were taken after 28 days of CK and TR plants. Different index levels show various types of expression levels of PR-10, TSWV-cp, and Sw-5b in M82 and H8 plants. H8-infected plants showed a higher range of PR-10 protein compared to M82 plants. Additionally, M82-infected plants showed a wide range of response of TSWV-cp (4.72-fold relative expression at level 2) and significantly upregulated, and H8 plants showed relatively lower expression of TSWV-cp. M82 plants also showed no significant expression of Sw-5b, but H8 plants showed 3.40-fold relative expression that was significantly upregulated (Figure 14).
Figure 14.
Expression of different index levels of plant roots in M82 and H8. Relative transcription levels determined by qRT-PCR of PR-10 protein, TSWV-cp protein, and Sw-5b; tomato M82 and H8 plants were infected with TSWV inoculum and the relative transcription levels were normalized to the control gene Actin. The expression levels in the root tissues were measured at different disease index levels. Blue bars represent the PR-10 proteins, light green bars represent the TSWV-cp protein, and purple bars represent Sw-5b. The mean values (±SD) from three biological replicates are presented as relative changes. The least significant difference (LSD) test was used to determine if a gene showed significant induction as compared to the transcript abundance of the control group (MCK represents the M82 CK plant; M represents the M82-treated plant; HCK represents the H8, CK plant; and H represents the H8-treated plant). Asterisk indicates statistically significant; * p = 0.05 and ns = non-significant are the p values.
2.13. Correlation between PR-10 and Sw-5b Gene Expression after TSWV Inoculation
Table 5 shows the results of a correlation study between the PR-10 and Sw-5b gene expressions following TSWV inoculation. At multiple time intervals, such as 3, 6, 12, 24, 48, 72, 96 h and 7, 14, 21, 28 days, the studies revealed extremely significant correlations between PR-10 and Sw-5b genes. M82 and H8 tomato plants exhibited extremely significant correlations in this study; however, H8 plants showed significant correlations when compared to M82 plants.
Table 5.
The correlation coefficient between PR-10 protein and Sw-5b at different time points after TSWV inoculation.
| Index | CK | 3 h | 6 h | 12 h | 24 h | 48 h | 72 h | 96 h | 7 D | 14 D | 21 D | 28 D |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TR | 1.000 | |||||||||||
| 3 h | 0.495 | 1.000 | ||||||||||
| 6 h | 0.550 | 0.926 ** | 1.000 | |||||||||
| 12 h | 0.460 | 0.952 ** | 0.960 ** | 1.000 | ||||||||
| 24 h | 0.123 | 0.692 ** | 0.489 | 0.606 ** | 1.000 | |||||||
| 48 h | 0.516 | 0.850 ** | 0.959 ** | 0.923 ** | 0.341 | 1.000 | ||||||
| 72 h | 0.154 | 0.764 ** | 0.590 ** | 0.713 ** | 0.975 ** | 0.456 | 1.000 | |||||
| 96 h | 0.363 | 0.869 ** | 0.853 ** | 0.902 ** | 0.785 ** | 0.820 ** | 0.841 ** | 1.000 | ||||
| 7 D | 0.314 | 0.839 ** | 0.847 ** | 0.820 ** | 0.527 | 0.822 ** | 0.602 ** | 0.777 ** | 1.000 | |||
| 14 D | 0.322 | 0.821 ** | 0.799 ** | 0.752 ** | 0.480 | 0.717 ** | 0.565 | 0.636 ** | 0.926 ** | 1.000 | ||
| 21 D | 0.200 | 0.616 ** | 0.651 ** | 0.659 ** | 0.479 | 0.720 ** | 0.545 | 0.782 ** | 0.799 ** | 0.672 * | 1.000 | |
| 28 D | 0.323 | 0.581 ** | 0.718 ** | 0.675 ** | 0.374 | 0.772 ** | 0.447 | 0.777 ** | 0.715 ** | 0.587 * | 0.923 ** | 1.000 |
* Correlation is significant at the 0.05 level; ** correlation is significant at the 0.01 level.
2.14. Heat Map of PR-10 Protein and Sw-5b Gene Expression at Different Time Points
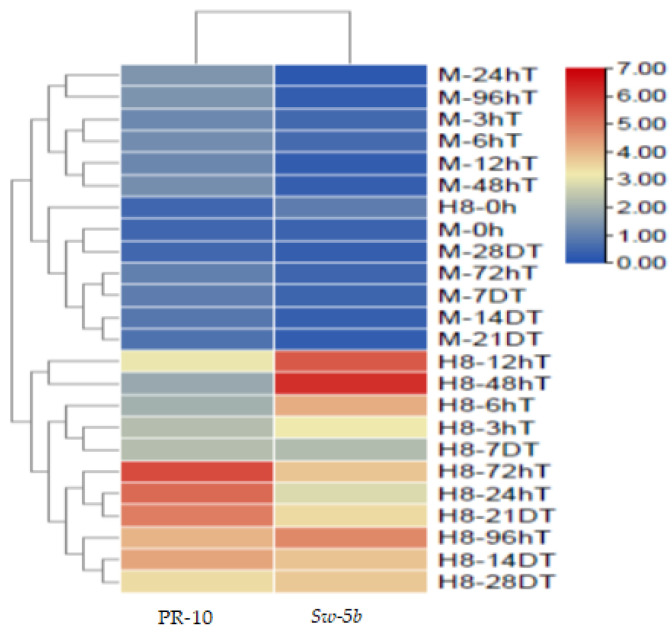
PR-10 protein and Sw-5b gene expression at various time periods following TSWV inoculation. TB tools software was used to create the heat map, which was then applied to two clades of M82 and H8 plants. Figure 15 shows that PR-10 protein and Sw-5b gene expression patterns were clustered; H8 plants demonstrated resistance to TSWV of various colors with high PR-10 protein and Sw-5b gene expression, whereas M82 plants showed susceptibility to TSWV. We have a comprehensive understanding of the PR-10 protein and Sw-5b, both of which play an equal role in TSWV resistance following TSWV inoculation. H8 plants were more resistant to Sw-5b than M82 plants because M82 plants lack Sw-5b resistant genes, but H8 plants have the resistant genes.
Figure 15.
Heat map of PR-10 protein and Sw-5b at different time points after TSWV inoculation. PR-10 and Sw-5b genes are highly expressed in H8 plants compared to M82 plants.
3. Discussion
To confirm that the PR-10 proteins found in tomatoes are related to pathogenesis-related proteins from the PR-10 family, a general NCBI-BLAST was performed using their deduced amino acid (a.a) sequence as the query of FASTA search. PR-10 protein sequences are near in molecular weight (17–18 kDa) and amino acid length (157–166 a.a) with a pI value of 4.69–6.17 and a Bet_ V_ 1 (PF000407) domain structure. The subcellular localization of the PR-10 protein was predicted to be cytoplasmic (Table 1).
To characterize and identify potential functional relationships between the PR-10 proteins of tomato, a phylogenetic tree was constructed and 45 gene IDs of PR-10 protein identified. The neighbor-joining method, with 1000 bootstrap reconstruction and completed deletion gaps/missing data, yielded five known subfamilies. The subsequent phylogenetic analysis of all forty-five PR-10 nucleotide and protein sequences were carried out using the maximum-likelihood method with 1000 bootstraps (Figure 1).
Although PR-10 proteins are mostly recognized for their roles in plant defense in response to biotic and abiotic stress, the overall biological function of many PR-10 members are yet unknown. We investigated the PR-10 genes to better understand the evolutionary relationship between tomato genes and proteins. The findings of this work serve as a theoretical foundation for future research into the structural relationships that define pathogenesis-related proteins [22]. To fight broad pathogenic activity, plants have evolved several defensive mechanisms. Among other strategies, they create antibiotic chemicals such as phytoalexins [23]; and multiple pathogens such as viruses, bacteria, and fungi induce the expression of several genes, as well as the formation of ethylene and salicylic acid in the plant after pathogen infection, resulting in a stress response [24]. The induced genes inform the plants to generate pathogenesis-related (PR) proteins, which are involved in a defensive mechanism [25]. The level at which new cases of a disease appear in a plant population within a given period is referred to as incidence and the disease intensity is calculated using an interval scale measure, which has been used to quantify a disease severity index (DSI) on a percentage basis [26]. We measured different types of diseased leaf samples at different time points and estimated disease incidence and index level (Table 3) with the information contained in Table 4.
The PR-10 family is a critical element of pathogenesis-related proteins, which aid plant defense against biotic and abiotic stress. For example, several tobacco PRs have been identified as chitinases and β-1-3 glucanases with antifungal activity [27]. Multigene families code for PR-10 proteins. This may be the reason for the multifunctional existence of these ancient proteins, which spent time in a phase known as protein promiscuity for mutations and functions [28]. The cAMP-dependent protein kinase, casein kinase II, and protein kinase C also have phosphorylation sites on several of the PR-10 proteins [29].
Tomato spotted wilt virus (TSWV) is transmitted by thrips and has become one of the most important viral vector complexes for agriculture and food defense [30]. One of the most effective approaches to decrease viral diseases is to grow virus resistant cultivars. This technique’s effectiveness is dependent on the presence of resistant genes in either cultivated or wild relatives. The most prevalent mechanism of natural plant resistance to viral infection is the hypersensitive response [31]. HR causes viral invasion-associated cells to die quickly, reducing viral cell-to-cell multiplication and the virus’s subsequent spread throughout the plant. The hypersensitive reaction is caused by the virus’s exact identification, which is based on comparable plant and viral gene products (HR). In Tospoviruses, particularly TSWV, the dominant genes of Sw-5 are the principal sources of HR-based resistance [30]. Sw-5 clustered proteins were discovered to be part of the resistance (R) gene family, encoded by the amino-terminal Solanaceae domain (SD) and coiled-coil domain (CC), a core nucleotide binding-adapter shared by APAF-1, R proteins, and a CED-4 (NB-ARC), and a leucine-rich repeat (LRR) domain [32]. Multiple homologs have been found in the tomato genome; nevertheless, Sw-5b provides broad and long-lasting resistance and has been extensively researched because of this functionality [11]. The NSm encoded in the TSWV M segment is the TSWV product that triggers the resistance response (Avr Determinant) of Sw-5b-mediated resistance [11,32,33]. Sw-5 containing tomato cultivar have L and S segments, as well as the M segment form a TSWV resistance-inducing (RI) isolate and a TSWV resistance-breaking (RB) isolate [34]. Because H8 plants had Sw-5b resistant genes, PR-10 protein expression was higher in H8 plants compared to M82 plants (Figure 10); nevertheless, TSWV-cp protein expression was lower in H8 plants, as seen in Figure 11. However, due to these resistance genes (Sw-5b) (Figure 12), Sw-5b was substantially expressed in H8 plants.
The viral small interfering RNAs (vsiRNAs) originating from the TSWV genome have been discovered to potentially target host genes involved in a variety of processes, including plant–pathogen interactions [35]. As a result, they experience much plant protection and autoimmunity. Plant pattern-recognition receptors (PRRs) sense the virus that has infected the plants by recognizing pathogen-associated molecular patterns (PAMPs). The plants’ first line of defense against an immunological response is PAMP-triggered immunity (PTI). On the other hand, rapid pathogen effectors hinder the PTI response. PAMP-triggered immunity (PTI) and effector-triggered immunity (ETI) are two types of immune systems found in plants. PTI is elicited by pathogen/microbe-associated molecular patterns (PAMPs/MAMPs), while ETI is elicited by disease resistance plant proteins (R), which are highly effective for disease resistance reactions upon precise identification of pathogen effectors, and both PTI and ETI cause local immune responses. During virus invasion, nucleotide-binding leucine-rich repeat receptors (NLRs) recognize specific pathogen effectors and induce effector-triggered immunity (ETI), which triggers a long-distance protective mechanism known as SAR (systemic acquired resistance) in plants [36].
NLRs (nucleotide-binding leucine-rich repeat receptors) identify unique pathogen effectors during virus invasion and cause effector-triggered immunity (ETI) in the plant [37]. Because of their distinct N-terminal configurations, plant NLRs are known as coiled–coil (CC)-NLRs (CNLs) or Toll/interleukin-1 (TIR)-NLRs (TNLs) [38]. The NLRs specifically or implicitly recognize pathogen effectors and initiate a hypersensitive cell death response (HR) aimed at limiting TSWV infection to the site of infection [39]. TSWV-cp protein was highly expressed in M82 sensitive plants, but PR-10 protein and Sw-5b were highly expressed in H8 plants leave and roots in different types of disease index levels (Figure 13 and Figure 14), and PR-10 protein and Sw-5b are highly correlated for TSWV disease resistance in the plants (Figure 15). The tomato Sw-5b belongs to the coiled–coil leucine-rich repeats (CNLs) and also auto-activated. Sw-5b is a typical class of proteins, CC-NB-ARC protein, and Sw-5b has broad-spectrum resistance that its SD domain can specifically recognize a 21- amino acid (21-aa) in NSm of TSWV. The region of NSm is highly conserved in the American-type Tospoviruses only, and not in Euro-Asian-type Tospoviruses [39]. At the moment, the Sw-5b genes have been discovered in various tomato germplasm materials by genome sequencing, and the sequence variations between the genes of different tomato plants are very large [40]. TSWV-specific NSm attaches to the expanded SD domain of the Sw-5b protein, activating the switch that triggers the receptor and HR, resulting in a vigorous defensive reaction against Tospoviruses [40]. The CNLs (including Sw-5b protein) from tomato, on the other hand, detect NSm and NSs with robust effector-triggered immunity (ETI) and activate the hypersensitive cell death reaction (HR). Salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) play critical roles in PTI and ETI immunity, along with helping plants to establish systemic acquired resistance (SAR) [41]. The SA signaling pathway is essential in tomato plants for basal protection against TSWV [42]. The SA accumulates in the contaminated regions, where it then triggers the rapid activation of transcriptional resistance (R) genes [43]. These findings suggest that PR-10 protein and its involvement with Sw-5b have a resistance mechanism against TSWV to protect against these devastating viruses in tomatoes, which also establish systemic acquired resistance (SAR) in the plants.
4. Materials and Methods
4.1. Materials
Forty-five amino acid sequences of PR-10 encoding genes were used to analyze the genome-wide identification of the PR-10 superfamily. M82 and H8 tomato seeds were used in this experiment as a sensitive and resistant material because M82 was included as a TSWV susceptible cultivar, as reported by [44], and H8 was included as a TSWV resistant cultivar, as reported by the Laboratory of Tomato Quality and Stress Tolerance Regulation Mechanism and Genetic Improvement, College of Horticulture, Northwest A and F University, China.
4.2. Methods
4.2.1. Identification and Sequence Analysis
The PR-10 protein amino acid sequences were used as a reference point for a number of database searches against the Phytozome database proteome files. (https://phytozome-next.jgi.doe.gov/) accessed on 23 May 2021. The National Center for Biotechnology Information (NCBI) provided isolated versions of BLASTP, which were utilized with an e-value threshold of 1e−10 [45]. The candidate sequences were further screened by searching for the PR-10 domain in tomato by using PFAM (http://pfam.xfam.org/) accessed on 23 May 2021 and SMART (http://smart.embl-heidelberg.de/) accessed on 23 May 2021. The characteristic of PR-10 protein, including protein length, isoelectric point (pI), molecular weight (MW), and grand average of hydropathicity (GRAVY) were predicted by ExPASy ProtParam (http://web.expasy.org/protparam/) accessed on 23 May 2021. The signal peptides and transmembrane (TM) domains were predicted with SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) accessed on 23 May 2021.
4.2.2. Phylogenetic Analysis
To compare the evolutionary relationships and identify the subfamilies of PR-10, proteins were used to construct the phylogenetic tree using MEGA-X with the neighbor-joining (NJ) method [46]. The phylogenetic tree was then visualized by iTol (https://itol.embl.de/) accessed on 24 May 2021.
4.2.3. Gene Structure, Conserved Motifs, and Chromosome Mapping Analysis
Gene Structure Display Server 2.0 software (http://gsds.cbi.pku.edu.cn/) accessed on 23 May 2021 was used to investigate the exon–intron organizations of PR-10 genes based on their information given in the Phytozome database. The novel motifs of PR proteins were searched using MEME 5.0.3 (http://meme-suite.org/tools/meme) accessed on 23 May 2021. The parameters were set as follows: the site distribution was any number of repetitions (anr); the number of motifs was 20; the width of the motif was limited to between 10 and 30, and other optional parameters remained as the default. The combination of gene structures, motifs, and the phylogenetic tree was then generated using the iTol tool. The distributions of PR-10 genes on tomato chromosome mapping were illustrated with MapInspect 1.0 (http://mapinspect.software.informer.com/) accessed on 24 May 2021.
4.2.4. GO Analysis
Biological processes, cellular components, and molecular function are the three categories of gene ontology (GO). Using the PANNZER2 web server, the PR-10 genes were investigated for their role in GO (http://ekhidna2.biocenter.helsinki.fi/sanspanz/) accessed on 23 May 2021.
4.2.5. Three-Dimensional Structure of the PR-10 Protein
A Protein Homology/Analogy Recognition Engine v2 (Phyre2) server was used to generate the anticipated 3D structures. (http://www.sbg.bio.ic.ac.uk/~phyre2/html/page.cgi?id=index) accessed on 23 May 2021.
4.2.6. Transcriptomic Analysis
Genome-wide expression data from tomatoes were utilized to reveal the expression patterns of the PR-10 protein family in different tissues and developmental periods. Transcriptomic data from several different plant tissues, including leaves, stems, roots, and various developmental stages of tomato fruit, were downloaded from the Sol genomics network (https://solgenomics.net/) accessed on 30 May 2021, and then TB tools software was used to analyze the expression levels of PR-10 genes in various tissues of tomato plants by using 45 gene IDs of PR-10 protein.
4.2.7. TSWV Virus Solution Stock Preparation
TSWV virus solution stock was prepared according to the method of [30]. A 0.5 g diseased leaf sample was ground in a mortar and pestle and mixed with a buffer solution (pH 7.0) of 10 mL (0.1 mol/LH24 PO4)/Na2HPO4; 2% poly-vinyl pyrolidon; and 0.2% Na2SO3. The TSWV virus solution was kept at −80 °C prior to further analysis.
4.2.8. Plant Materials and Growth Conditions
To minimize pests and disease incidence during seedling growth and development, the experiment was conducted in the greenhouse at the College of Horticulture Northwest Agriculture and Forestry University in Yangling, China. The seeds were germinated for 3 days on moist filter paper in Petri dishes at 28 °C in the dark. The seedlings were transported to a growth chamber with a 16 h light/8 h dark photoperiod and a temperature cycle of 25/16 °C. The plants were moved to a solar greenhouse at Northwest A and F University in Yangling, Shaanxi Province, China, at the four true-leaf stage. The plants were cultivated under a 22/18 °C day/night temperature during the TSWV inoculation.
4.2.9. Virus Inoculation
The TSWV virus was inoculated into six-week-old seedlings. A syringe was used to inject the TSWV virus. The injections were made with a 1.0 mL syringe at three sites on the leaf: border, middle, and base, covering around 0.5 cm2 of leaf area. The presence of the virus was determined visually and confirmed by PCR.
4.2.10. Treatment and Sampling
Inoculated treatment (TR) and non-inoculated (CK) seedlings were used to split tomato cultivars into two groups. At 0, 3, 6, 12, 24, 48, 72, 96 h, and 7, 14, 21, 28 days, samples were taken from the control and treatment groups. TSWV infection of tomato leaves was identified by PCR using TSWV-CP specific primers. At 14, 21, and 28 days after inoculation, the incidence and disease index of TSWV were evaluated, along with the disease symptoms of susceptible and resistant plants, to determine the relative expression of TSWV-cp, PR-10, and Sw-5b. There were 20 plants in each treatment. For this experiment, three biological replicates were used. Viral disease classification standard records disease index level in Table 4 [47].
4.2.11. Investigation of Incidence Rate and Disease Index
The incidence of TSWV was observed at 14, 21, and 28 days after inoculation, and the disease symptoms of susceptible and resistant plants were observed.
Incidence rate (%) = (number of infected plants / total number of treated plants) × 100.
Measurement of Disease index = (number of disease plants at all levels × number of representatives at all levels)/(total number of investigated plants × maximum series)] × 100 (at 14, 21, and 28 days after inoculation).
4.2.12. RNA Extraction and qRT-PCR
Leaf samples were collected from three TSWV-inoculated and three uninoculated plants. The leaves were directly frozen in liquid nitrogen and kept at −80 °C for further analysis. Total RNA was derived from tomato leaves using an Omega plant RNA kit, and cDNA was generated using an M-MuLV reverse transcriptase kit (Thermo Scientific, USA). For TSWV-cp protein confirmation, the cDNA samples were amplified using PCR: 95 °C for 5 min, 35 cycles of 95 °C for 30 s, 53 °C for 30 s, 72 °C for 3.5 min, and 72 °C for 10 min. Another cDNA was also synthesized for qPCR using the Evo M-MLV RT Kit (Accurate Biotechnology, Hunan). PCR was used to amplify the cDNA samples: 42 °C for 2 min, 37 °C for 15 min, and 85 °C for 5 s. Thermocycler iQ5 Real-Time PCR Detection Device (BIO-RAD Corp., Hercules, CA, USA) and SYBR Green Pro Taq HS qPCR (Accurate Biotechnology, Hunan) were then used to operate qRT-PCR according to manufacturer guidance. The PCR conditions were as follows: 95 °C for 30 s, accompanied by 40 cycles of 95 °C for 5 s, 60 °C for 30 s, and 72 °C for 20 s. As an internal control, the tomato actin gene was used. The qRT-PCR data were analyzed using the 2−ΔΔ CT method [48]. The primers are listed in Table S1.
4.2.13. Statistical Analysis
GraphPad Prism version 7.00 for Windows (one-way ANOVA followed by Dunnett test) GraphPad Software, San Diego, California, USA, www.graphpad.com, accessed on 23 May 2021, was used to plot and statistically analyze the graphs. A value of p > 0.05 was regarded as statistically significant; * p = 0.05, ** p = 0.01, *** p = 0.001, **** p = 0.0001, and ns = non-significant. Correlation coefficients were determined by SPSS 25.0 (IBM, Armonk, NY, USA).
5. Conclusions
We found 45 PR-10 protein superfamily candidate genes with lengths ranging from 64 to 210 amino acid residues and molecular weights ranging from 7.6 to 24.4 kDa. The 5 subfamilies of PR-10 protein was discovered, as well as 9 conserved motifs. On ten of the twelve chromosomes, PR-10 genes have been found. Gene ontology research revealed six biological processes and molecular activities, as well as nine cellular components, as well as the greatest level of expression in the Solyc09g090980 PR-10 gene compared to other tomato tissues. TSWV infection strongly induced PR-10 and Sw-5b gene transcription and activity in tomato leaves, H8 plants having the highest significant expression of PR-10 at 24 h (6.74-fold) and Sw-5b gene at 7 days (4.14-fold) compared to control after the TSWV inoculation, TSWV inoculated M82 plants showed significant expression of PR-10 gene at 72 h (5.70-fold) and no significant expression of Sw-5b gene all the time, compared to control. The expressions of PR-10 and Sw-5b genes showed highly significant correlations in H8 plants after the inoculation of TSWV at different time points and also heat map showed that these two genes may participate in regulating the defense response after the inoculation of TSWV in tomatoes. Thus, we conclude that PR-10 and Sw-5b genes have an important role in the defense response after the infection of TSWV.
Acknowledgments
The authors are extremely thankful to the Liang Yan, College of Horticulture, Northwest A and F University, Yangling, Shaanxi, 712100, China, for providing necessary facilities during research work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23031502/s1.
Author Contributions
Conceptualization, M.M.I. and Y.L.; writing and original draft preparation, M.M.I.; editing, S.Q., S.Z., B.A., V.Y., A.H.E.-S. and F.Z.; supervision, Y.L.; project administration, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of Stem Cell and Translational Research (2016YFD0101703) and the Shaanxi Province Key Research and Development Plan Project (2019ZDLNY03-05).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ali S., Mir Z.A., Tyagi A., Bhat J.A., Chandrashekar N., Papolu P.K., Rawat S., Grover A. Identification and comparative analysis of Brassica juncea pathogenesis-related genes in response to hormonal, biotic and abiotic stresses. Acta Physiol. Plant. 2017;39:268. doi: 10.1007/s11738-017-2565-8. [DOI] [Google Scholar]
- 2.Boccardo N.A., Segretin M.E., Hernandez I., Mirkin F.G., Chacón O., Lopez Y., Borrás-Hidalgo O., Bravo-Almonacid F.F. Expression of pathogenesis-related proteins in transplastomic tobacco plants confers resistance to filamentous pathogens under field trials. Sci. Rep. 2019;9:2791. doi: 10.1038/s41598-019-39568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha R.K., Verma S.S., Rastogi A. Role of Pathogen-Related Protein 10 (PR 10) under abiotic and biotic stresses in plants. Phyton. 2020;89:167. doi: 10.32604/phyton.2020.09359. [DOI] [Google Scholar]
- 4.Jain S., Kumar A. The pathogenesis related class 10 proteins in plant defense against biotic and abiotic stresses. Adv. Plants Agric. Res. 2015;3:00077. doi: 10.15406/apar.2015.02.00077. [DOI] [Google Scholar]
- 5.Hanssen I.M., Lapidot M., Thomma B.P. Emerging viral diseases of tomato crops. Mol. Plant Microbe Interact. 2010;23:539–548. doi: 10.1094/MPMI-23-5-0539. [DOI] [PubMed] [Google Scholar]
- 6.Sevik M.A., Arli-Sokmen M. Estimation of the effect of Tomato spotted wilt virus (TSWV) infection on some yield components of tomato. Phytoparasitica. 2012;40:87–93. doi: 10.1007/s12600-011-0192-2. [DOI] [Google Scholar]
- 7.Newman S., Pottorff L. Recognizing Tomato Problems. Colorado State University Libraries; Fort Collins, CO, USA: 2013. [Google Scholar]
- 8.Padmanabhan C., Ma Q., Shekasteband R., Stewart K.S., Hutton S.F., Scott J.W., Fei Z., Ling K.-S. Comprehensive transcriptome analysis and functional characterization of PR-5 for its involvement in tomato Sw-7 resistance to tomato spotted wilt tospovirus. Sci. Rep. 2019;9:7673. doi: 10.1038/s41598-019-44100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens M., Lamb E., Rhoads D. Mapping the Sw-5 locus for tomato spotted wilt virus resistance in tomatoes using RAPD and RFLP analyses. Theor. Appl. Genet. 1995;90:451–456. doi: 10.1007/BF00221989. [DOI] [PubMed] [Google Scholar]
- 10.Spassova M.I., Prins T.W., Folkertsma R.T., Klein-Lankhorst R.M., Hille J., Goldbach R.W., Prins M. The tomato gene Sw5 is a member of the coiled coil, nucleotide binding, leucine-rich repeat class of plant resistance genes and confers resistance to TSWV in tobacco. Mol. Breed. 2001;7:151–161. doi: 10.1023/A:1011363119763. [DOI] [Google Scholar]
- 11.De Oliveira A.S., Boiteux L.S., Kormelink R., Resende R.O. The Sw-5 gene cluster: Tomato breeding and research toward orthotospovirus disease control. Front. Plant Sci. 2018;9:1055. doi: 10.3389/fpls.2018.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roselló S., Díez M.J., Nuez F. Genetics of tomato spotted wilt virus resistance coming from Lycopersicon peruvianum. Eur. J. Plant Pathol. 1998;104:499–509. doi: 10.1023/A:1008622128504. [DOI] [Google Scholar]
- 13.Kim J.-H., Kim Y.-S., Jang S.-W., Jeon Y.-H. Complete genome sequence of Tomato spotted wilt virus from paprika in Korea. Int. J. Phytopathol. 2013;2:121–136. doi: 10.33687/phytopath.002.03.0378. [DOI] [Google Scholar]
- 14.Gupta R., Kwon S.-Y., Kim S.T. An insight into the tomato spotted wilt virus (TSWV), tomato and thrips interaction. Plant Biotechnol. Rep. 2018;12:157–163. doi: 10.1007/s11816-018-0483-x. [DOI] [Google Scholar]
- 15.Leventhal S.S., Wilson D., Feldmann H., Hawman D.W. A Look into Bunyavirales Genomes: Functions of Non-Structural (NS) Proteins. Viruses. 2021;13:314. doi: 10.3390/v13020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedil M., Kormelink R. Viral RNA silencing suppression: The enigma of bunyavirus NSs proteins. Viruses. 2016;8:208. doi: 10.3390/v8070208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y., Liu B., Ding Z., Li G., Liu M., Zhu D., Sun Y., Dong S., Lou Z. Distinct mechanism for the formation of the ribonucleoprotein complex of tomato spotted wilt virus. J. Virol. 2017;91:23. doi: 10.1128/JVI.00892-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rybicki E.P. A Top Ten list for economically important plant viruses. Arch. Virol. 2015;160:17–20. doi: 10.1007/s00705-014-2295-9. [DOI] [PubMed] [Google Scholar]
- 19.Adams M.J., Lefkowitz E.J., King A.M., Harrach B., Harrison R.L., Knowles N.J., Kropinski A.M., Krupovic M., Kuhn J.H., Mushegian A.R. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017) Arch. Virol. 2017;162:2505–2538. doi: 10.1007/s00705-017-3358-5. [DOI] [PubMed] [Google Scholar]
- 20.Kerényi Z., Mérai Z., Hiripi L., Benkovics A., Gyula P., Lacomme C., Barta E., Nagy F., Silhavy D. Inter-kingdom conservation of mechanism of nonsense-mediated mRNA decay. EMBO J. 2008;27:1585–1595. doi: 10.1038/emboj.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anfoka G.H., Abhary M., Stevens M.R. Occurrence of Tomato spotted wilt virus (TSWV) in Jordan. EPPO Bull. 2006;36:517–522. doi: 10.1111/j.1365-2338.2006.01052.x. [DOI] [Google Scholar]
- 22.Swoboda I., Hoffmann-Sommergruber K., O’Ríordáin G., Scheiner O., Heberle-Bors E., Vicente O. Bet v 1 proteins, the major birch pollen allergens and members of a family of conserved pathogenesis-related proteins, show ribonuclease activity in vitro. Physiol. Plant. 1996;96:433–438. doi: 10.1111/j.1399-3054.1996.tb00455.x. [DOI] [Google Scholar]
- 23.Hofius D., Tsitsigiannis D.I., Jones J.D., Mundy J. Inducible Cell Death in Plant Immunity, Seminars in Cancer Biology, 2007. Elsevier; Amsterdam, The Netherlands: 2007. pp. 166–187. [DOI] [PubMed] [Google Scholar]
- 24.Hammond-Kosack K.E., Jones J. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marković-Housley Z., Degano M., Lamba D., von Roepenack-Lahaye E., Clemens S., Susani M., Ferreira F., Scheiner O., Breiteneder H. Crystal structure of a hypoallergenic isoform of the major birch pollen allergen Bet v 1 and its likely biological function as a plant steroid carrier. J. Mol. Biol. 2003;325:123–133. doi: 10.1016/S0022-2836(02)01197-X. [DOI] [PubMed] [Google Scholar]
- 26.Yu H., Zhang Q., Sun P., Song C. Impact of droughts on winter wheat yield in different growth stages during 2001–2016 in Eastern China. Int. J. Disaster Risk Sci. 2018;9:376–391. doi: 10.1007/s13753-018-0187-4. [DOI] [Google Scholar]
- 27.Takahashi M., Shigeto J., Izumi S., Yoshizato K., Morikawa H. Nitration is exclusive to defense-related PR-1, PR-3 and PR-5 proteins in tobacco leaves. Plant Signal. Behav. 2016;11:e1197464. doi: 10.1080/15592324.2016.1197464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tokuriki N., Tawfik D.S. Stability effects of mutations and protein evolvability. Curr. Opin. Struct. Biol. 2009;19:596–604. doi: 10.1016/j.sbi.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Ziadi S.L., Poupard P., Brisset M.-N., Paulin J.-P., Simoneau P. Characterization in apple leaves of two subclasses of PR-10 transcripts inducible by acibenzolar-S-methyl, a functional analogue of salicylic acid. Physiol. Mol. Plant Pathol. 2001;59:33–43. doi: 10.1006/pmpp.2001.0343. [DOI] [Google Scholar]
- 30.Olaya C., Fletcher S.J., Zhai Y., Peters J., Margaria P., Winter S., Mitter N., Pappu H.R. The Tomato spotted wilt virus (TSWV) Genome is Differentially Targeted in TSWV-Infected Tomato (Solanum lycopersicum) with or without Sw-5 Gene. Viruses. 2020;12:363. doi: 10.3390/v12040363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldbach R., Bucher E., Prins M. Resistance mechanisms to plant viruses: An overview. Virus Res. 2003;92:207–212. doi: 10.1016/S0168-1702(02)00353-2. [DOI] [PubMed] [Google Scholar]
- 32.De Oliveira A.S., Koolhaas I., Boiteux L.S., Caldararu O.F., Petrescu A.J., Oliveira Resende R., Kormelink R. Cell death triggering and effector recognition by Sw-5 SD-CNL proteins from resistant and susceptible tomato isolines to Tomato spotted wilt virus. Mol. Plant Pathol. 2016;17:1442–1454. doi: 10.1111/mpp.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallwass M., De Oliveira A.S., de Campos Dianese E., Lohuis D., Boiteux L.S., Inoue-Nagata A.K., Resende R.O., Kormelink R. The T omato spotted wilt virus cell-to-cell movement protein (NSM) triggers a hypersensitive response in S w-5-containing resistant tomato lines and in N icotiana benthamiana transformed with the functional S w-5b resistance gene copy. Mol. Plant Pathol. 2014;15:871–880. doi: 10.1111/mpp.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann K., Qiu W., Moyer J. Overcoming host-and pathogen-mediated resistance in tomato and tobacco maps to the M RNA of Tomato spotted wilt virus. Mol. Plant Microbe Interact. 2001;14:242–249. doi: 10.1094/MPMI.2001.14.2.242. [DOI] [PubMed] [Google Scholar]
- 35.Ramesh S.V., Williams S., Kappagantu M., Mitter N., Pappu H.R. Transcriptome-wide identification of host genes targeted by tomato spotted wilt virus-derived small interfering RNAs. Virus Res. 2017;238:13–23. doi: 10.1016/j.virusres.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Poltronieri P., Brutus A., Reca I.B., Francocci F., Cheng X., Stigliano E. Applied Plant Biotechnology for Improving Resistance to Biotic Stress. Elsevier; Amsterdam, The Netherlands: 2020. Engineering plant leucine rich repeat-receptors for enhanced pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) pp. 1–31. [Google Scholar]
- 37.Panigrahi G.K., Sahoo A., Satapathy K.B. Insights to Plant Immunity: Defense Signaling to Epigenetics. Physiol. Mol. Plant Pathol. 2020:101568. doi: 10.1016/j.pmpp.2020.101568. [DOI] [Google Scholar]
- 38.Saile S.C., Jacob P., Castel B., Jubic L.M., Salas-Gonzalez I., Bäcker M., Jones J.D., Dangl J.L., El Kasmi F. Two unequally redundant “helper” immune receptor families mediate Arabidopsis thaliana intracellular “sensor” immune receptor functions. PLoS Biol. 2020;18:e3000783. doi: 10.1371/journal.pbio.3000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu M., Jiang L., Bai B., Zhao W., Chen X., Li J., Liu Y., Chen Z., Wang B., Wang C. The intracellular immune receptor Sw-5b confers broad-spectrum resistance to tospoviruses through recognition of a conserved 21-amino acid viral effector epitope. Plant Cell. 2017;29:2214–2232. doi: 10.1105/tpc.17.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Huang H., Zhu M., Huang S., Zhang W., Dinesh-Kumar S.P., Tao X. A plant immune receptor adopts a two-step recognition mechanism to enhance viral effector perception. Mol. Plant. 2019;12:248–262. doi: 10.1016/j.molp.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Klessig D.F., Choi H.W., Dempsey D.M.A. Systemic acquired resistance and salicylic acid: Past, present, and future. Mol. Plant Microbe Interact. 2018;31:871–888. doi: 10.1094/MPMI-03-18-0067-CR. [DOI] [PubMed] [Google Scholar]
- 42.Zheng X., Chen Y., Zhao L., Chen Y., Zheng L., Zheng K., Mu Y., Zhao X., Gao Y., Zhang J. Tripartite interactions between jasmonic/salicylic acid pathways, western flower thrips, and thrips-transmitted tomato zonate spot virus infection in Capsicuum annuum. Arthropod Plant Interact. 2019;13:289–297. doi: 10.1007/s11829-019-09683-2. [DOI] [Google Scholar]
- 43.Yang L., Li B., Zheng X.-y., Li J., Yang M., Dong X., He G., An C., Deng X.W. Salicylic acid biosynthesis is enhanced and contributes to increased biotrophic pathogen resistance in Arabidopsis hybrids. Nat. Commun. 2015;6:7309. doi: 10.1038/ncomms8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee H.J., Kim B., Bae C., Kang W.-H., Kang B.-C., Yeam I., Oh C.-S. Development of a single-nucleotide polymorphism marker for the Sw-5b gene conferring disease resistance to Tomato spotted wilt virus in tomato. Hortic. Sci. Technol. 2015;33:730–736. doi: 10.7235/hort.2015.15025. [DOI] [Google Scholar]
- 45.Altschul S.F., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mo Y., Bao J., Zhu H., Zhao K. Advance in research on resistance breeding of tomato against Tomato spotted wilt virus. J. Yunnan Agric. Univ. 2016;31:733–737. [Google Scholar]
- 48.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.