Abstract
In this work, we report in-depth computational studies of three plausible tautomeric forms, generated through the migration of two acidic protons of the N4-hydroxylcytosine fragment, of molnupiravir, which is emerging as an efficient drug to treat COVID-19. The DFT calculations were performed to verify the structure of these tautomers, as well as their electronic and optical properties. Molecular docking was applied to examine the influence of the structures of the keto-oxime, keto-hydroxylamine and hydroxyl-oxime tautomers on a series of the SARS-CoV-2 proteins. These tautomers exhibited the best affinity behavior (−9.90, −7.90, and −9.30 kcal/mol, respectively) towards RdRp-RTR and Nonstructural protein 3 (nsp3_range 207–379-MES).
Keywords: COVID-19, SARS-CoV-2, molnupiravir, virus, computational study, DFT, molecular docking
1. Introduction
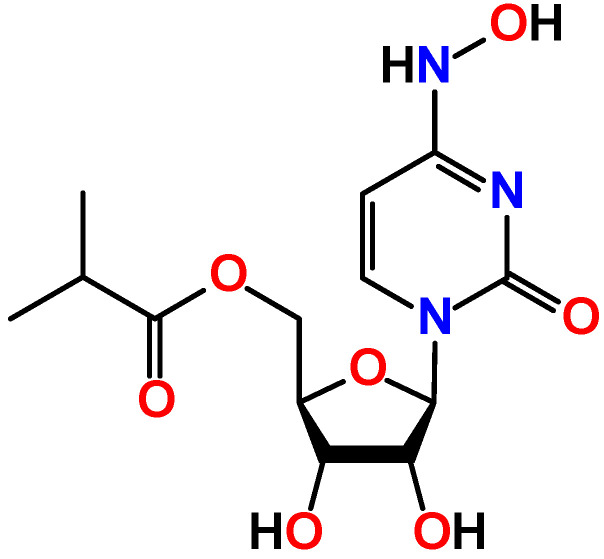
Molnupiravir, which is known under the trademark Lagevrio, is a first oral antiviral for COVID-19 approved by Medicines and Healthcare products Regulatory Agency (MHRA) [1]. It is an N4-hydroxycytidine derivative, where the ribose residue is bonded to the isobutyric acid ester group (Figure 1). Molnupiravir was obtained at University of Emory (USA) but was refused due to mutagenicity. Later, rights on molnupiravir were purchased by a biotechnology company Ridgeback Biotherapeutics, which, in turn, partnered with Merck & Co, an American multinational pharmaceutical company, to perform clinical trials with molnupiravir in humans to treat COVID-19 [2]. Initially, molnupiravir was developed for the treatment of influenza [3], acting through integration into the replication process of the viral RNA. As a result, accumulation of a number of mutations does not allow the virus to maintain its own population [4,5].
Figure 1.
Diagram of molnupiravir.
Nowadays, coronavirus is one of the most discussed and actively investigated viruses. To be said, coronaviruses are a large family of viruses, which may cause illness in animals or humans. In humans, several coronaviruses are known to cause respiratory infections ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The most recently discovered coronavirus causes coronavirus disease COVID-19 [6]. Since the time when this disease was recognized, it has rapidly spread, and the World Health Organization (WHO) announced a pandemic in March 2020 [7]. As the causative agent of COVID-19, was found the betacoronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
To date, the beginning of 2022, about 310 million infections were confirmed with about 5.5 million deaths [8]. A steady upward trend in this disease has been observed. Unfortunately, the situation with COVID-19 remains very complicated due new strains, of which variants of concern are alpha, beta, gamma, delta and omicron. The latter strain was first discovered in November 2021.
The dire need to search for antiviral agents to combat COVID-19 has led to the emergence of studies on the effectiveness of molnupiravir against SARS-CoV-2 [9,10,11,12,13,14,15,16]. As of October 2021, it was established that oral administration of molnupiravir reduces the risk of severe disease by about 50% in comparison to placebo in patients with mild to moderate disease. Furthermore, molnupiravir was found to be more efficient in comparison to other drugs against COVID-19 [17,18]. In addition, molnupiravir was also established to be effective against the omicron strain, since it interferes with how the virus replicates, a process that isn’t altered across variants [19,20].
All this dictates that molnupiravir is currently in the limelight of research and under an ever-growing interest. Thus, deeper properties of molnupiravir are revealed as a more powerful weapon against viruses, including COVID-19.
With all this in mind, as well as in continuation of our ongoing interest in in silico studies of bioactive compounds [21,22,23,24] we have directed our attention to molnupiravir. Theoretical calculations based on density functional theory (DFT) were performed to examine electronic and optical properties of its three tautomers. The global chemical reactivity descriptors were estimated from the energy of the HOMO and LUMO orbitals to examine the relative reactivity of the molecules. Using an in silico molecular docking method, we have explored the binding modes and interactions of each tautomer with binding sites of a series nonstructural proteins and the structural protein (Spike protein, RBD) of the SARS-CoV-2 as targets.
2. Results and Discussion
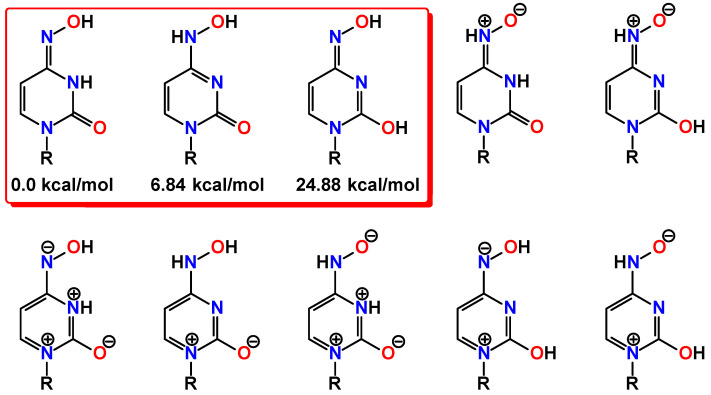
Molnupiravir can conventionally be considered as a molecule constructed from the two main structural fragments, namely the substituted ribose and N4-hydroxylcytosine (Figure 1). Due to the hydroxylamine group, the latter fragment can, in general, exhibit two tautomeric forms of either the hydroxylamine or oxime structure, of which the latter can further generate the amide-iminol tautomerism (Figure 2). Furthermore, two nitrone forms can also be highlighted as plausible tautomeric forms of molnupiravir also dictated by the amide-iminol transformation (Figure 2). Finally, a series of ionic aromatic forms are further tautomers of molnupiravir (Figure 2). Thus, two acidic protons of the N4-hydroxylcytosine fragment are of great importance and play a pivotal role in a rich library of plausible tautomeric forms of molnupiravir. Notably, to the best of our knowledge, the crystal structure of molnupiravir has not been reported so far. This can also be explained by intertautomer transformation in solutions.
Figure 2.
Diagrams of the plausible tautomers of the N4-hydroxylcytosine fragment in the molecule of molnupiravir (R = residue of the molecule of molnupiravir).
Among the variety of tautomers of molnupiravir, herein, we have directed our attention to three forms without any charged centers, namely keto-oxime, keto-hydroxylamine, and hydroxyl-oxime (Figure 2). We have applied the DFT calculations to shed light on fine features of these tautomers. Each structure was first optimized in gas phase at 298.15 K, and the energies and thermodynamic parameters are given in Table 1.
Table 1.
Thermodynamic parameters of the optimized structures of the keto-oxime, keto-hydroxylamine, and hydroxyl-oxime tautomers of molnupiravir, obtained by using the DFT/B3LYP/6-311++G(d,p) method.
| Thermodynamic Parameter | Keto-oxime | Keto-hydroxylamine | Hydroxyl-oxime |
|---|---|---|---|
| Self-consistent field energy (a.u.) | −1197.962 | −1197.951 | −1197.922 |
| Total energy (thermal) (kcal mol−1) | 225.112 | 224.768 | 224.572 |
| Electronic energy (thermal) (kcal mol−1) | 0.000 | 0.000 | 0.000 |
| Translational energy (thermal) (kcal mol−1) | 0.889 | 0.889 | 0.889 |
| Rotational energy (thermal) (kcal mol−1) | 0.889 | 0.889 | 0.889 |
| Vibrational energy (thermal) (kcal mol−1) | 223.335 | 222.990 | 222.794 |
| Total heat capacity (thermal) (cal mol−1 K−1) | 85.340 | 84.962 | 86.088 |
| Electronic heat capacity (thermal) (cal mol−1 K−1) | 0.000 | 0.000 | 0.000 |
| Translational heat capacity (thermal) (cal mol−1 K−1) | 2.981 | 2.981 | 2.981 |
| Rotational heat capacity (thermal) (cal mol−1 K−1) | 2.981 | 2.981 | 2.981 |
| Vibrational heat capacity (thermal) (cal mol−1 K−1) | 79.379 | 79.001 | 80.127 |
| Total entropy (thermal) (cal mol−1 K−1) | 164.370 | 163.370 | 165.868 |
| Electronic entropy (thermal) (cal mol−1 K−1) | 0.000 | 0.000 | 0.000 |
| Translational entropy (thermal) (cal mol−1 K−1) | 43.269 | 43.269 | 43.269 |
| Rotational entropy (thermal) (cal mol−1 K−1) | 35.425 | 35.425 | 35.428 |
| Vibrational entropy (thermal) (cal mol−1 K−1) | 85.676 | 84.677 | 87.170 |
| Zero-point vibrational energy (thermal) (kcal mol−1) | 210.837 | 210.592 | 210.174 |
| Rotational constants (GHz) | |||
| A | 0.38535 | 0.39715 | 0.41586 |
| B | 0.12511 | 0.12266 | 0.11980 |
| C | 0.10302 | 0.10192 | 0.09932 |
It was established that the keto-oxime tautomer is the most energetically stable, followed by the keto-hydroxylamine tautomer, which is about 7 kcal/mol less favorable, while the hydroxyl-oxime tautomer is completely unfavorable (Figure 2). The calculated bond lengths between the non-hydrogen atoms are gathered in Table 2. Notably, all tautomers exhibit very similar bond lengths within the substituted ribose fragment, while differ in their N4-hydroxylcytosine parts (Table 2).
Table 2.
Selected bond lengths (Å) in the optimized structures of the keto-oxime, keto-hydroxylamine, and hydroxyl-oxime tautomers of molnupiravir, obtained by using the DFT/B3LYP/6-311++G(d,p) method.
| Bond | Keto-oxime | Keto-hydroxylamine | Hydroxyl-oxime |
|---|---|---|---|
| C1–C4 | 1.525 | 1.526 | 1.529 |
| C1–C9 | 1.528 | 1.527 | 1.527 |
| C2–C3 | 1.551 | 1.551 | 1.546 |
| C3–C4 | 1.539 | 1.540 | 1.538 |
| C6–C7 | 1.344 | 1.358 | 1.341 |
| C7–C8 | 1.443 | 1.426 | 1.451 |
| C10–C11 | 1.528 | 1.528 | 1.527 |
| C11–C12 | 1.544 | 1.544 | 1.544 |
| C11–C13 | 1.534 | 1.534 | 1.534 |
| C1–O1 | 1.451 | 1.452 | 1.452 |
| C2–O1 | 1.409 | 1.411 | 1.409 |
| C3–O3 | 1.405 | 1.403 | 1.413 |
| C4–O2 | 1.416 | 1.415 | 1.416 |
| C5–O4 | 1.228 | 1.229 | 1.359 |
| C9–O6 | 1.432 | 1.431 | 1.430 |
| C10–O6 | 1.369 | 1.369 | 1.371 |
| C10–O7 | 1.200 | 1.200 | 1.200 |
| C5–N1 | 1.384 | 1.422 | 1.365 |
| C5–N2 | 1.373 | 1.358 | 1.281 |
| C6–N1 | 1.395 | 1.363 | 1.409 |
| C8–N2 | 1.396 | 1.323 | 1.407 |
| C8–N3 | 1.289 | 1.359 | 1.291 |
| N3–O5 | 1.421 | 1.398 | 1.389 |
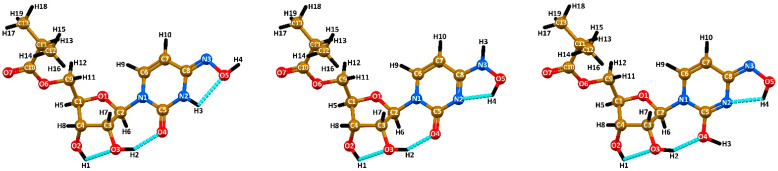
Each tautomeric form is stabilized by three hydrogen bonds. Particularly, the same hydrogen bond O–H∙∙∙O is formed in the tautomers between one of the hydroxyl hydrogen atoms and the next hydroxyl oxygen atom within the ribose cycle (Figure 3, Table 3). The second O–H∙∙∙O hydrogen bond is formed between the other hydroxyl hydrogen atom of the ribose residue and either with the carbonyl oxygen atom, in the keto-oxime and keto-hydroxylamine tautomers, or the third hydroxyl oxygen atom, in the hydroxyl-oxime tautomer, respectively (Figure 3, Table 3). Finally, the third N–H∙∙∙O or O–H∙∙∙N hydrogen bond is formed within the N4-hydroxylcytosine fragment (Figure 3, Table 3). As a result of these non-covalent interactions, each tautomer exhibits two five-membered and one seven-membered hydrogen bonded rings.
Figure 3.
Optimized structures of the keto-oxime (left), keto-hydroxylamine (middle), and hydroxyl-oxime (right) tautomers of molnupiravir, obtained by using the B3LYP/6-311++G(d,p) method. Cyan dashed line = O–H∙∙∙O, O–H∙∙∙N and N–H∙∙∙O hydrogen bonds.
Table 3.
Hydrogen bond lengths (Å) and angles (°) in the optimized structures of the keto-oxime, keto-hydroxylamine and hydroxyl-oxime tautomers of molnupiravir, obtained by using the DFT/B3LYP/6-311++G(d,p) method.
| Tautomer | D–X∙∙∙A | d(D–X) | d(X∙∙∙A) | d(D∙∙∙A) | ∠(DXA) |
|---|---|---|---|---|---|
| keto-oxime | O2–H1∙∙∙O3 | 0.968 | 2.095 | 2.648 | 114.52 |
| O3–H2∙∙∙O4 | 0.974 | 1.843 | 2.718 | 147.92 | |
| N2–H3∙∙∙O5 | 1.012 | 2.163 | 2.537 | 99.62 | |
| keto-hydroxylamine | O2–H1∙∙∙O3 | 0.967 | 2.165 | 2.676 | 111.59 |
| O3–H2∙∙∙O4 | 0.966 | 2.064 | 2.917 | 146.30 | |
| O5–H4∙∙∙N2 | 0.975 | 2.032 | 2.622 | 116.97 | |
| hydroxyl-oxime | O2–H1∙∙∙O3 | 0.967 | 2.165 | 2.676 | 111.59 |
| O3–H2∙∙∙O4 | 0.966 | 2.064 | 2.917 | 146.30 | |
| O5–H4∙∙∙N2 | 0.975 | 2.032 | 2.622 | 116.97 |
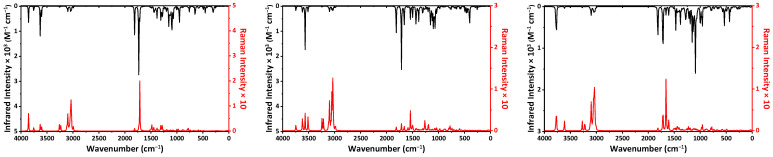
The molecules of the discussed tautomeric forms of molnupiravir contain 42 atoms and, thus, have 120 normal modes (Table 4). All the frequencies were found to be positive, indicating local energy minima for the optimized structure. In general, both the IR and Raman spectra of each tautomer are informative for the C=O, OH, and NH groups (Figure 4). The most intense band in the calculated IR spectra of the keto-oxime and keto-hydroxylamine tautomers is observed at 1733 and 1715 cm−1, respectively (Figure 4, Table 4). In both spectra this band is associated with stretching of the carbonyl group of the cyclic fragment, bending of one of the ribose hydroxyl group together with bending of the amine group in the former spectrum or bending of the hydroxylamine OH group, and one of the CH groups of the dinitrogen containing cycle, respectively (Table 4). In the IR spectrum of the hydroxyl-oxime tautomer the most intense band is observed at 1092 cm−1 and is due to stretching of one of the ribose CO groups, bending of both CH groups of the dinitrogen containing cycle and rocking of the CH2 fragment.
Table 4.
Values of the selected vibrations in the calculated IR and Raman spectra (Figure 4) for the optimized structures of the keto-oxime, keto-hydroxylamine, and hydroxyl-oxime tautomers of molnupiravir, obtained by using the DFT/B3LYP/6-311++G(d,p) method.
| Molecular Vibration 1 | Frequency (cm−1) |
IR Intensity (KM∙mol−1) |
Raman Activity (Å4∙amu−1) |
Force Constant, k (mDyne A−1) |
|---|---|---|---|---|
| keto-oxime | ||||
| νO5–H4 | 3856 | 189.04 | 220.68 | 9.3559 |
| νO2–H3 | 3754 | 73.43 | 60.22 | 8.8462 |
| νO3–H2 | 3629 | 417.06 | 96.23 | 8.2735 |
| νNH | 3605 | 109.93 | 48.16 | 8.2416 |
| νs(C6–H9 + C7–H10) | 3261 | 12.72 | 74.49 | 6.8628 |
| νas(C6–H9 + C7–H10) | 3237 | 6.62 | 66.59 | 6.7326 |
| νC13–H14 | 3120 | 20.70 | 48.34 | 6.3159 |
| νasH14–C12–H15 | 3106 | 15.07 | 37.91 | 6.2675 |
| νC1–H5 | 3102 | 21.15 | 84.32 | 6.1803 |
| νC13–H18 | 3099 | 25.87 | 71.32 | 6.2376 |
| νasH15–C12–H16 | 3095 | 29.03 | 43.98 | 6.2194 |
| νasH11–C9–H12 + νC1–H5 | 3077 | 13.81 | 48.81 | 6.1665 |
| νC11–H13 | 3058 | 14.12 | 130.10 | 5.9645 |
| νC2–H6 | 3046 | 37.37 | 58.52 | 5.9362 |
| νC4–H8 + νC2–H6 | 3043 | 22.81 | 157.47 | 5.9235 |
| νs(C13–H17 + C13–H18 + C13–H19) | 3037 | 22.14 | 204.18 | 5.6303 |
| νs(C12–H14 + C12–H15 + C12–H16) | 3031 | 25.20 | 121.28 | 5.6016 |
| νs(C9–H11 + C9–H12) | 3027 | 19.80 | 43.60 | 5.7009 |
| νC3–H7 | 2994 | 23.23 | 49.13 | 5.7226 |
| νC10=O7 | 1814 | 367.75 | 20.51 | 22.8748 |
| νC5=O4 + βN2–H3 + βO3–H2 | 1733 | 836.72 | 22.71 | 11.9387 |
| νC8=N3 + νC5=O4 + νC6=C7 +βO5–H4 + βN2–H3 + βC6–H9 + βC7–H10 | 1714 | 140.42 | 371.53 | 13.9766 |
| keto-hydroxylamine | ||||
| νO2–H1 | 3745 | 76.60 | 59.88 | 8.8028 |
| νN3–H3 | 3619 | 108.95 | 137.56 | 8.3276 |
| νO3–H2 | 3569 | 526.21 | 126.65 | 8.0039 |
| νO5–H4 | 3512 | 85.58 | 129.66 | 7.7383 |
| νs(C6–H9 + C7–H10) | 3244 | 9.42 | 77.27 | 6.7877 |
| νas(C6–H9 + C7–H10) | 3216 | 0.59 | 82.40 | 6.6483 |
| νC13–H17 + νC13–H18 + νC13–H19 | 3120 | 19.99 | 48.02 | 6.3164 |
| νC12–H14 + νC12–H15 + νC12–H16 | 3106 | 15.40 | 37.56 | 6.2662 |
| νC1–H5 | 3101 | 20.69 | 86.39 | 6.1751 |
| νasH18–C13–H19) | 3099 | 26.15 | 71.95 | 6.2365 |
| νasH15–C12–H16) | 3095 | 28.63 | 44.13 | 6.2181 |
| νasH11–C9–H12 + νC1–H5 | 3075 | 14.97 | 46.70 | 6.1604 |
| νC11–H13 | 3056 | 13.35 | 110.25 | 5.9559 |
| νC2–H6 | 3055 | 21.17 | 105.53 | 5.9716 |
| νC4–H8 | 3041 | 33.92 | 129.47 | 5.9150 |
| νs(C13–H17 + C13–H18 + C13–H19) | 3037 | 22.96 | 204.79 | 5.6307 |
| νs(C12–H14 + C12–H15 + C12–H16) | 3031 | 23.47 | 110.87 | 5.5975 |
| νsH11–C9–H12 | 3025 | 21.21 | 50.87 | 5.6943 |
| νC3–H7 | 2987 | 27.06 | 45.99 | 5.6959 |
| νC10=O7 | 1815 | 365.00 | 19.83 | 22.8983 |
| νC5=O4 + βO5–H4 + βO3–H2 + βC6–H9 | 1715 | 785.23 | 33.24 | 17.0337 |
| νC8=N3 + νC5=O4 + νC6=C7 +βC6–H9 + βC7–H10 + βC2–H6 | 1663 | 291.45 | 25.55 | 10.4411 |
| βN3–H3 + βO5–H4 | 1591 | 20.24 | 20.25 | 2.7871 |
| hydroxyl-oxime | ||||
| νO2–H1 + νO3–H2 + νO4–H3 | 3782 | 94.84 | 36.15 | 8.9787 |
| νO2–H1 + νO4–H3 | 3774 | 27.16 | 36.51 | 8.9377 |
| νO5–H4 | 3615 | 16.28 | 90.73 | 8.2026 |
| νC6–H9 | 3273 | 9.92 | 75.23 | 6.9078 |
| νC7–H10 | 3222 | 2.37 | 85.81 | 6.6768 |
| νC13–H17 + νC13–H18 + νC13–H19 | 3121 | 19.94 | 49.24 | 6.3195 |
| νC12–H14 + νC12–H15 + νC12–H16 | 3106 | 14.34 | 42.24 | 6.2714 |
| νasH18–C13–H19 | 3100 | 23.66 | 67.74 | 6.2426 |
| νC1–H5 + νasH11–C9–H12 | 3099 | 22.56 | 82.80 | 6.1714 |
| νC12–H14 + νC12–H15 + νC12–H16 | 3095 | 30.72 | 47.70 | 6.2155 |
| νC1–H5 + νasH11–C9–H12 | 3077 | 12.97 | 50.18 | 6.1565 |
| νC2–H6 | 3061 | 20.82 | 56.13 | 5.9950 |
| νC11–H13 | 3056 | 14.50 | 140.64 | 5.9601 |
| νC4–H8 | 3047 | 29.86 | 139.46 | 5.9408 |
| ν(C13–H17 + C13–H18 + C13–H19) | 3038 | 21.16 | 199.10 | 5.6333 |
| ν(C12–H14 + C12–H15 + C12–H16) | 3031 | 24.44 | 122.92 | 5.5991 |
| νsH11–C9–H12 | 3025 | 23.18 | 61.34 | 5.7005 |
| νC3–H7 | 3006 | 17.27 | 35.96 | 5.7667 |
| νC10=O7 | 1817 | 363.72 | 20.82 | 22.9946 |
| νC5=N2 + νC6=C7 + βO4–H3 + βC6–H9 + βC7–H10 | 1718 | 556.37 | 146.50 | 12.9824 |
| νC8=N3 + νC5=N2 + νC6=C7 +βO4–H3 + βC7–H10 | 1658 | 71.05 | 288.48 | 13.6289 |
| νC8=N3 + νC5=N2 + νC6=C7 +βO4–H3 + βO5–H4 + βC6–H9 + βC7–H10 | 1609 | 85.65 | 63.89 | 13.3853 |
1 ν—stretching, νs—symmetric stretching, νas—antisymmetric stretching, β—bending.
Figure 4.
The calculated IR (black) and Raman (red) spectra of the keto-oxime (left), keto-hydroxylamine (middle), and hydroxyl-oxime (right) tautomers of molnupiravir, obtained by using the DFT/B3LYP/6-311++G(d,p) method.
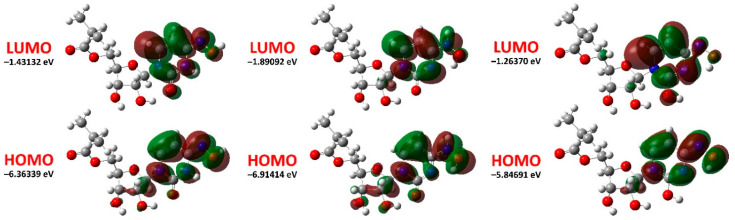
According to the DFT calculations, the energies of the HOMO and LUMO are −6.36339 ÷ −5.84691 and −1.89092 ÷ −1.26370 eV, respectively, with the lowest and highest values corresponding to the keto-hydroxylamine and hydroxyl-oxime tautomers (Table 5). The corresponding energy gap varies from 4.58321 to 5.02322 eV (Table 5).
Table 5.
Frontier molecular HOMO and LUMO orbitals, gap value, and descriptors for the optimized structures of the keto-oxime, keto-hydroxylamine, and hydroxyl-oxime tautomers of molnupiravir, obtained by using the DFT/B3LYP/6-311++G(d,p) method.
| Parameter | Keto-oxime | Keto-hydroxylamine | Hydroxyl-oxime |
|---|---|---|---|
| EHOMO (eV) | −6.36339 | −6.91414 | −5.84691 |
| ELUMO (eV) | −1.43132 | −1.89092 | −1.26370 |
| ΔELUMO−HOMO = ELUMO − EHOMO (eV) | 4.93207 | 5.02322 | 4.58321 |
| Ionization energy, I = −EHOMO (eV) | 6.36339 | 6.91414 | 5.84691 |
| Electron affinity, A = −ELUMO (eV) | 1.43132 | 1.89092 | 1.26370 |
| Electronegativity, χ = (I + A)/2 (eV) | 3.89736 | 4.40253 | 3.55531 |
| Chemical potential, μ = −χ (eV) | −3.89736 | −4.40253 | −3.55531 |
| Global chemical hardness, η = (I − A)/2 (eV) | 2.46604 | 2.51161 | 2.29161 |
| Global chemical softness, S = 1/(2η) (eV−1) | 0.20275 | 0.19908 | 0.21819 |
| Global electrophilicity index, ω = μ2/(2η) (eV) | 3.07972 | 3.85854 | 2.75794 |
| Maximum additional electric charge, ΔNmax = −μ/ƞ | 1.58041 | 1.75287 | 1.55145 |
We have also visualized HOMO and LUMO for the discussed tautomers. It was found that both orbitals are mainly delocalized over the substituted dinitrogen fragment with some contribution from the ribose fragment for HOMO (Figure 5).
Figure 5.
Energy levels and views on the electronic isosurfaces of the HOMO and LUMO of the optimized structures of the keto-oxime (left), keto-hydroxylamine (middle), and hydroxyl-oxime (right) tautomers of molnupiravir, obtained by using the B3LYP/6-311++G(d,p) method.
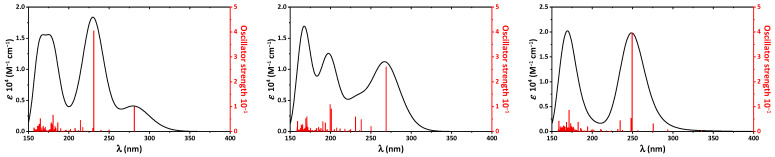
The calculated UV-vis spectra of all tautomers exhibit bands exclusively in the UV region. Particularly, the spectrum of the keto-oxime tautomer contains bands at 168, 175, 230, and 280 nm (Figure 6), which mainly correspond to the transitions at 164.8, 178.3, 180.6, 186.4, 231.0, and 281.3 nm, respectively (Table 6). The latter two low-energy transitions are due to HOMO → LUMO and HOMO → LUMO+2 (Table 6). The spectrum of the keto-hydroxylamine tautomer exhibits bands centered at 168 (transitions at 159.1, 169.3, and 170.7 nm), 198 (transitions at 190.6, 199.6, and 201.1 nm), and 267 (transition at 267.0 nm) nm accompanied with a shoulder at about 235 (transitions at 231.0 and 238.0 nm) nm (Figure 6). The latter two low-energy transitions are mainly due to HOMO → LUMO, HOMO → LUMO+1 and HOMO → LUMO+2 (Table 6). The calculated absorption spectrum of the hydroxyl-oxime tautomer exhibits only two clearly distinguished bands at 169 (transitions at 158.7, 171.3, 173.4, 182.4, and 182.6 nm) and 248 (transitions at 234.6, 248.0, 249.6, and 275.5 nm) nm (Figure 6). The low-energy band is assigned to HOMO → LUMO+1÷7 (Table 6).
Figure 6.
The calculated UV-vis spectra of the keto-oxime (left), keto-hydroxylamine (middle), and hydroxyl-oxime (right) tautomers of molnupiravir, obtained by using the TD-DFT/B3LYP/6-311++G(d,p) method.
Table 6.
Values of the calculated UV-vis spectra (Figure 6) for the optimized structures of the keto-oxime, keto-hydroxylamine, and hydroxyl-oxime tautomers of molnupiravir, obtained by using the TD-DFT/B3LYP/6-311++G(d,p) method.
| λmax (nm) | Osc. Strength | Transition | λmax (nm) | Osc. Strength | Transition |
|---|---|---|---|---|---|
| keto-oxime | |||||
| 164.8 | 0.0514 | HOMO−7 → LUMO+3 (32.5%) | 186.4 | 0.0353 | HOMO−8 → LUMO (16.5%) |
| HOMO−6 → LUMO+5 (5.8%) | HOMO−2 → LUMO+2 (11.6%) | ||||
| HOMO−5 → LUMO+5 (7.2%) | HOMO−1 → LUMO+2 (47.8%) | ||||
| 178.3 | 0.0351 | HOMO−3 → LUMO+2 (21.2%) | 214.5 | 0.0445 | HOMO−5 → LUMO (8.9%) |
| HOMO → LUMO+15 (11.6%) | HOMO−2 → LUMO (13.4%) | ||||
| HOMO → LUMO+16 (27.3%) | HOMO−1 → LUMO (36.5%) | ||||
| 180.6 | 0.0655 | HOMO−4 → LUMO+1 (8.0%) | HOMO → LUMO+6 (18.4%) | ||
| HOMO−3 → LUMO+1 (14.4%) | 231.0 | 0.4040 | HOMO → LUMO (15.2%) | ||
| HOMO−2 → LUMO+2 (43.7%) | HOMO → LUMO+2 (64.7%) | ||||
| HOMO−1 → LUMO+2 (9.5%) | 281.3 | 0.0987 | HOMO → LUMO (79.3%) | ||
| HOMO → LUMO+2 (17.5%) | |||||
| keto-hydroxylamine | |||||
| 159.1 | 0.0406 | HOMO−15 → LUMO (7.8%) | 199.6 | 0.0340 | HOMO−1 → LUMO+3 (29.8%) |
| HOMO−13 → LUMO (9.0%) | HOMO−1 → LUMO+4 (28.8%) | ||||
| HOMO−9 → LUMO+1 (16.9%) | HOMO → LUMO+6 (13.5%) | ||||
| HOMO−9 → LUMO+2 (34.7%) | 0.1085 | HOMO−1 → LUMO+2 (23.1%) | |||
| 169.3 | 0.0516 | HOMO−10 → LUMO (19.8%) | HOMO → LUMO+5 (25.1%) | ||
| HOMO−3 → LUMO+7 (9.2%) | HOMO → LUMO+6 (14.2%) | ||||
| HOMO−2 → LUMO+7 (10.6%) | HOMO → LUMO+7 (9.8%) | ||||
| HOMO−1 → LUMO+10 (7.3%) | 201.1 | 0.0902 | HOMO−1 → LUMO+2 (50.2%) | ||
| 170.7 | 0.0589 | HOMO−10 → LUMO (23.3%) | HOMO → LUMO+5 (20.6%) | ||
| HOMO−2 → LUMO+7 (7.7%) | 231.0 | 0.0581 | HOMO → LUMO+2 (70.8%) | ||
| HOMO−1 → LUMO+10 (12.0%) | HOMO → LUMO+4 (9.9%) | ||||
| 190.6 | 0.0396 | HOMO−5 → LUMO+2 (8.0%) | 238.0 | 0.0473 | HOMO → LUMO+1 (73.5%) |
| HOMO−3 → LUMO+2 (8.5%) | HOMO → LUMO+2 (9.9%) | ||||
| HOMO−2 → LUMO+1 (22.9%) | 267.0 | 0.2593 | HOMO → LUMO (85.7%) | ||
| HOMO−2 → LUMO+2 (35.1%) | |||||
| hydroxyl-oxime | |||||
| 158.7 | 0.0413 | HOMO−9 → LUMO+2 (8.7%) | 182.4 | 0.0330 | HOMO−5 → LUMO (8.7%) |
| HOMO → LUMO+32 (10.7%) | HOMO−4 → LUMO+1 (50.6%) | ||||
| HOMO → LUMO+34 (10.2%) | HOMO−3 → LUMO+2 (11.7%) | ||||
| 168.1 | 0.0366 | HOMO−9 → LUMO (22.2%) | 182.6 | 0.0374 | HOMO−5 → LUMO (38.7%) |
| HOMO−8 → LUMO+1 (7.5%) | HOMO−3 → LUMO+2 (15.6%) | ||||
| HOMO−1 → LUMO+7 (10.3%) | HOMO−2 → LUMO+3 (16.5%) | ||||
| HOMO → LUMO+30 (8.7%) | 234.6 | 0.0434 | HOMO → LUMO+6 (7.1%) | ||
| 171.3 | 0.0855 | HOMO−9 → LUMO (15.4%) | HOMO → LUMO+7 (77.8%) | ||
| HOMO−8 → LUMO+1 (16.6%) | 248.0 | 0.0521 | HOMO → LUMO+3 (9.0%) | ||
| HOMO−5 → LUMO+2 (10.9%) | HOMO → LUMO+4 (8.4%) | ||||
| HOMO−4 → LUMO+3 (7.7%) | HOMO → LUMO+5 (76.9%) | ||||
| 173.4 | 0.0315 | HOMO−7 → LUMO+1 (12.8%) | 249.6 | 0.3958 | HOMO → LUMO+1 (10.6%) |
| HOMO−6 → LUMO+1 (16.9%) | HOMO → LUMO+2 (8.3%) | ||||
| HOMO−5 → LUMO+1 (8.3%) | HOMO → LUMO+3 (55.2%) | ||||
| HOMO−4 → LUMO+3 (30.5%) | HOMO → LUMO+5 (10.4%) | ||||
| 275.5 | 0.0308 | HOMO → LUMO+2 (61.6%) | |||
| HOMO → LUMO+3 (23.7%) | |||||
The ionization potential (I) and the electron affinity (A) value of the molecule, determined as I = −EHOMO and A = −ELUMO (Table 5) [25], are large indicating that the reported tautomers exhibit low electron donating and high electron accepting properties. Notably, the highest and lowest values of I and A were found for the keto-hydroxylamine and hydroxyl-oxime tautomers, respectively (Table 5).
We have further established values of the so-called global chemical reactivity descriptors. Chemical potential (μ) for the discussed tautomers varies from −4.40253 to −3.55531 eV, indicating electron accepting ability and the low donating ability, which is supported by the corresponding high value of electronegativity, χ (Table 5). The electrophilicity index (ω), which is denoted as the energy of stabilization to accept electrons [25], is 2.75794–3.85854 eV, indicating the pronounced electrophilic nature of the tautomers. Finally, the reported tautomers of molnupiravir can accept about 1.55–1.75 electrons as evidenced from the corresponding ΔNmax values (Table 5).
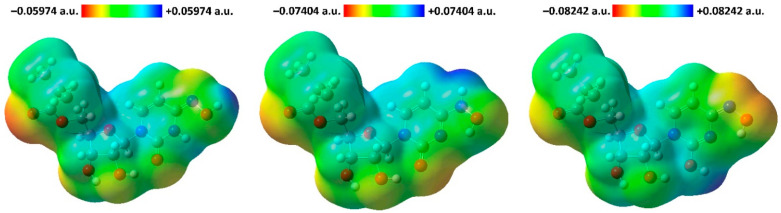
The electrophilic and nucleophilic sites in the discussed tautomers of molnupiravir were examined using the molecular electrostatic potential (MEP) analysis. The red and blue colours of the MEP surface correspond to electron-rich (nucleophilic) and electron-deficient (electrophilic) regions, respectively. On the MEP surface of the keto-oxime and keto-hydroxylamine tautomers the most pronounced nucleophilic centers are located on the carbonyl oxygen atom of the ester fragment followed by the carbonyl oxygen atom of the dinitrogen cycle and hydroxyl oxygen atoms (Figure 7). As the most electrophilic region in the former tautomer the hydroxyl hydrogen atom of the oxime fragment can be highlighted, while in the latter tautomer, the amine hydrogen atom is the most electrophilic site (Figure 7). Interestingly, in the hydroxyl-oxime tautomer the most pronounced nucleophilic centers are located on the nitrogen and oxygen atoms of the oxime fragments, followed by the carbonyl oxygen atom of the ester fragment, while the most electrophilic site was found on the hydrogen atom of the hydroxyl group attached to the dinitrogen cycle (Figure 7).
Figure 7.
View of the molecular electrostatic potential surfaces of the optimized structures of the keto-oxime (left), keto-hydroxylamine (middle), and hydroxyl-oxime (right) tautomers of molnupiravir, obtained by using the B3LYP/6-311++G(d,p) method.
The calculated 1H NMR spectra of the reported tautomers of molnupiravir each contain a set of signals, characteristic for protons of a certain nature. Particularly, the CH3 and CH protons of the isopropyl group and CH2 protons are observed at 0.71–1.62, 2.38–2.46, and 3.53–4.05 ppm, respectively (Table 7). The signals for the CH protons of the ribose and dinitrogen cycles are found at 3.77–5.59 and 5.24–7.80 ppm, respectively (Table 7). Furthermore, while the signals for the H1 hydroxyl protons are shown almost in the same region at 2.33–2.90 ppm, the signals for the other hydroxyl protons, together with the signals for the NH protons, vary from 2.87 to 7.88 ppm (Table 7). Notably, the calculated 1H NMR spectra of both the keto-oxime and hydroxyl-oxime tautomers agree with the experimental one [26]. Since the experimental spectrum was recorded in methanol-d4, thus, vanishing plausible signals from the hydroxyl and amine hydrogens, it is impossible to clearly attribute the exact tautomer of the mentioned two. However, since the keto-oxime tautomer is much more energetically favorable in comparison to the hydroxyl-oxime tautomer, we can tentatively assign the experimental 1H NMR spectrum to the former tautomer.
Table 7.
Signals for the calculated 1H NMR spectra of the ground states of the optimized structures of the keto-oxime, keto-hydroxylamine, and hydroxyl-oxime tautomers of molnupiravir, obtained by using the DFT/GIAO/B3LYP/6-311++G(2d,p) method (see Figure 3 for atoms labelling).
| Hydrogen | Keto-oxime | Keto-hydroxylamine | Hydroxyl-oxime |
|---|---|---|---|
| H1 | 2.74 | 2.90 | 2.33 |
| H2 | 5.47 | 6.24 | 2.87 |
| H3 | 7.54 | 6.35 | 4.89 |
| H4 | 5.29 | 7.88 | 6.82 |
| H5 | 4.59 | 4.61 | 4.54 |
| H6 | 5.39 | 5.55 | 5.59 |
| H7 | 3.82 | 3.77 | 3.82 |
| H8 | 4.40 | 4.38 | 4.35 |
| H9 | 7.07 | 7.80 | 6.51 |
| H10 | 5.39 | 5.24 | 5.84 |
| H11 | 3.54 | 3.53 | 3.59 |
| H12 | 3.96 | 3.95 | 4.05 |
| H13 | 2.41 | 2.38 | 2.46 |
| H14 | 0.96–1.06 | 1.02–1.06 | 1.03–1.06 |
| H15 | 0.96–1.06 | 0.94–0.95 | 1.03–1.06 |
| H16 | 0.96–1.06 | 0.94–0.95 | 0.96 |
| H17 | 1.59 | 1.60 | 1.62 |
| H18 | 0.73 | 0.71 | 0.76 |
| H19 | 0.96–1.06 | 1.02–1.06 | 1.03–1.06 |
To examine the potential nonlinear optical properties of three discussed tautomers of molnupiravir, parameters of the dipole moment (μ), polarizability (α), anisotropy of polarizability (Δα), and first-order hyperpolarizability (β) [27,28] were computed using the B3LYP/6-311++G(d,p) method (Table 8). The calculated dipole moment significantly increases from the hydroxyl-oxime through the keto-hydroxylamine to the keto-oxime tautomer. Such pronounced dipole moments for the latter two tautomers are due to the overall imbalance in the charge from one side of a molecule to the other side, which is also supported by the corresponding MEP surfaces (Figure 7). Thus, the presence of the keto-fragment formed within the dinitrogen cycle plays a pivotal role to increase the dipole moment of molnupiravir. Notably, an absolute value of the μy component exhibits the highest magnitude for the same two tautomers. Values for the calculated polarizability and first-order hyperpolarizability parameters for the three tautomers of molnupiravir are about 8.0 and 6.0–12.1 times higher in comparison to those of urea (Table 8), which is commonly used as a refernece for studying the nonlinear optical (NLO) properties of the molecular systems [29]. Thus, molnupiravir is of potential interest for future studies of its NLO properties.
Table 8.
Nonlinear optical parameters for the ground state of the optimized structure of the keto-oxime, keto-hydroxylamine and hydroxyl-oxime tautomers of molnupiravir, and urea [29], obtained by using the DFT/B3LYP/6-311++G(d,p) method 1.
| Parameter | Keto-oxime | Keto-hydroxylamine | Hydroxyl-oxime | Urea [29] |
|---|---|---|---|---|
| μx (Debye) | −2.7182 | −0.6997 | 1.0585 | |
| μy (Debye) | −4.9139 | −7.5858 | 1.0487 | |
| μz (Debye) | −0.0544 | −1.3843 | 0.7004 | |
| μD (Debye) | 5.6159 | 7.7428 | 1.6465 | |
| αxx (a.u.) | 256.320 | 254.188 | 272.451 | |
| αyy (a.u.) | 213.808 | 216.526 | 212.955 | |
| αzz (a.u.) | 153.182 | 152.609 | 144.264 | |
| αxy (a.u.) | 26.209 | 22.665 | 22.940 | |
| αxz (a.u.) | −14.113 | −12.219 | −12.450 | |
| αyz (a.u.) | 6.759 | 9.801 | 1.162 | |
| α (a.u.) | 207.770 | 207.774 | 208.89 | |
| α (esu) | 30.792 × 10−24 | 30.792 × 10−24 | 31.106 × 10−24 | 3.8312 × 10−24 |
| αtautomer/αurea | 8.0 | 8.0 | 8.1 | |
| Δα (a.u.) | 104.189 | 100.937 | 119.970 | |
| Δα (esu) | 15.441 × 10−24 | 14.959 × 10−24 | 17.780 × 10−24 | |
| βxxx (a.u.) | −263.723 | −47.212 | 16.843 | |
| βyyy (a.u.) | −5.671 | −49.597 | 46.796 | |
| βzzz (a.u.) | 2.192 | 0.908 | 0.007 | |
| βxyy (a.u.) | −0.326 | −25.778 | 53.700 | |
| βxxy (a.u.) | −86.296 | −66.014 | 130.374 | |
| βxxz (a.u.) | 23.622 | −18.584 | 6.807 | |
| βxzz (a.u.) | 9.757 | 13.287 | 11.574 | |
| βyzz (a.u.) | −4.432 | −2.785 | 5.898 | |
| βyyz (a.u.) | 5.210 | −7.786 | 4.231 | |
| βxyz (a.u.) | 18.340 | −1.679 | −11.407 | |
| β (a.u.) | 273.715 | 135.020 | 200.946 | |
| β (esu) | 2.365 × 10−30 | 1.166 × 10−30 | 1.736 × 10−30 | 0.1947 × 10−30 |
| βtautomer/βurea | 12.1 | 6.0 | 8.9 |
1 For α 1 a.u. = 0.1482 × 10−24 esu, for β 1 a.u. = 8.6393 × 10−33 esu.
Molnupiravir is known to be mutagenic [30]. Interestingly, using the OSIRIS Property Explorer software [31], we have established that while the keto-hydroxylamine tautomer is indeed mutagenic, its keto-oxime and hydroxyl-oxime derivatives do not possess mutagenic properties. Furthermore, the latter two tautomeric forms of molnupiravir exhibit more potent drug-likeness and drug-scores (−0.065 and 0.410 for keto-oxime, and −1.284 and 0.336 for hydroxyl-oxime, respectively) in comparison to those of the former tautomer (−2.529 and 0.178, respectively). Thus, the tautomeric form of molnupiravir is of importance in terms of drug safety.
We have further applied a molecular docking approach for all the three tautomers of molnupiravir against a series of SARS-CoV-2 proteins. The molecular docking aids in visualization and explication of the interaction between a small compound as ligand and biomolecule(s) as target(s) [32]. This application is one of the most broadly exerted technique to examine the structure-activity relationship and biological activity in the drug discovery [33]. Docking is the best option to diminish the time and cost of synthesis and to increase the influences of the medicines. In addition, it is considered as a current and advantageous method to have insight information of the possible binding site of the ligand in the protein [34].
In this study, molecular docking was employed to rationalize the three tautomers of molnupiravir in the SARS-CoV-2 targets. The target structures were primarily selected in accordance with the structural features of the virus [35,36] as well as based on biological mechanisms and functions that can be utilized to reduce, prevent, or treat the virus [37] (Table 9).
Table 9.
The best poses of the keto-oxime, keto-hydroxylamine, and hydroxyl-oxime tautomers of molnupiravir inside the binding sites of the listed proteins.
| Protein | PDB Code | Keto-oxime | Keto-hydroxylamine | Hydroxyl-oxime |
|---|---|---|---|---|
| Main protease (Mpro) | 6LU7 | −6.60 | −7.00 | −7.30 |
| Papain-like protease (PLpro) | 6WUU | −7.50 | −7.40 | −7.40 |
| Nonstructural protein 3 (Nsp3_range 207–379-AMP) | 6W6Y | −6.90 | −7.20 | −6.90 |
| Nonstructural protein 3 (Nsp3_range 207–379-MES) | 6W6Y | −8.10 | −7.90 | −7.80 |
| Helicase (Nsp13)-adp | 6JYT | −6.30 | −6.50 | −6.20 |
| Helicase (Nsp13)-ncb | 6JYT | −6.80 | −6.90 | −6.60 |
| RdRp-RTP | 7BV2 | −9.90 | −7.50 | −9.30 |
| RdRp-RNA | 7BV2 | −7.00 | −6.60 | −6.80 |
| Nsp14 (ExoN) | 5C8S | −6.80 | −7.00 | −6.60 |
| Nsp14 (N7-MTase) | 5C8S | −7.50 | −7.80 | −7.80 |
| Nsp15 (endoribonuclease) | 6WLC | −6.30 | −6.40 | −6.50 |
| Nsp16 (GTA site) | 6WVN | −7.70 | −7.70 | −7.60 |
| Nsp16 (MGP site) | 6WVN | −6.30 | −6.10 | −6.10 |
| Nsp16 (SAM site) | 6WVN | −7.60 | −7.30 | −7.40 |
| N protein (NCB site) | 6WXD | −6.90 | −7.20 | −6.80 |
| Spike protein, RBD (Native) | 6M0J | −5.75 | −5.91 | −5.18 |
| Spike protein, RBD (Mutated) | 6M0J | −5.88 | −5.85 | −5.74 |
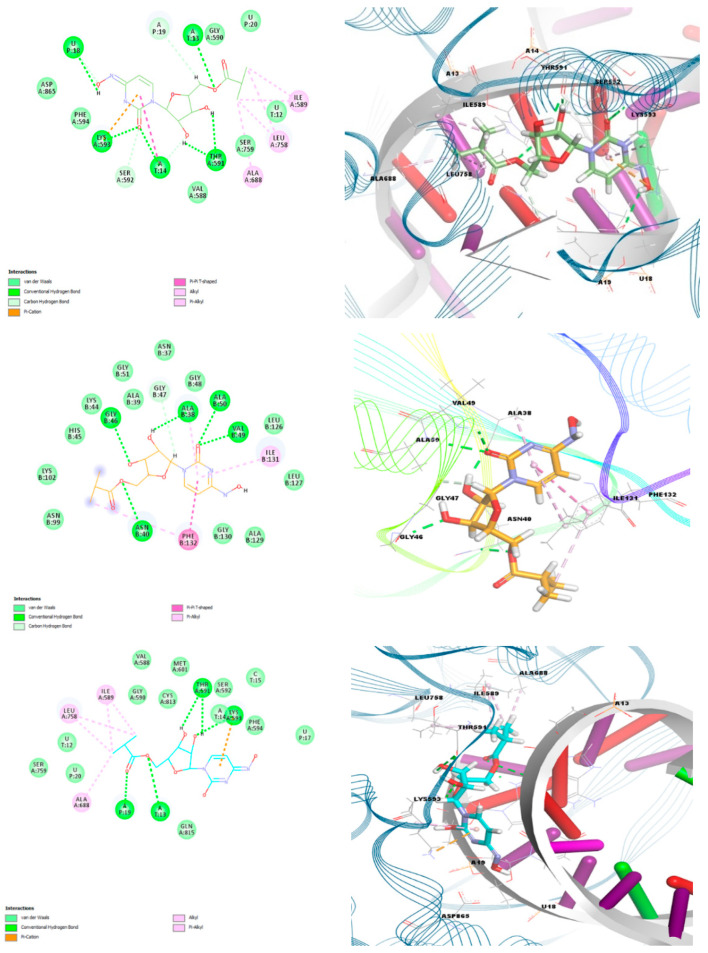
According to the docking analyses results, both the keto-oxime and hydroxyl-oxime tautomers show the best binding affinity with the RdRp-RTR protein, while the keto-hydroxylamine tautomer is more efficient towards the nonstructural protein 3 (Nsp3_range 207–379-MES) (Figure 8, Table 9).
Figure 8.
Two-dimensional (left) and 3D (right) views on the interaction of the keto-oxime (top), keto-hydroxylamine (middle), and hydroxyl-oxime (bottom) tautomers of molnupiravir with (from top to bottom) RdRp-RTR, Nonstructural protein 3 (Nsp3_range 207–379-MES) and RdRp-RTR.
Complex of the keto-oxime tautomer with RdRp-RTP is described with the following interactions: eleven hydrogen bonds with LYS593, T:A13, T:A14, P:U18, THR591, SER592, and P:A19; one π-system∙∙∙cation interaction with LYS593; one T-shaped π∙∙∙π interaction with T:A14; five alkyl interactions with ALA688, ILE589, and LEU758; and one π-system∙∙∙alkyl interaction with LYS593 (Figure 8, Table S1 in the Supplementary Materials). The hydroxyl-oxime tautomer of molnupiravir forms a complex with the same protein through five hydrogen bonds with LYS593, P:A19, T:A13, and THR591; one π-system∙∙∙cation interaction with LYS593; five alkyl interactions with ALA688, ILE589, and LEU758; and one π-system∙∙∙alkyl interaction with LYS593 (Figure 8, Table S1 in the Supplementary Materials). Thus, these two tautomeric forms of monupiravir exhibit similar docking properties with the RdRp-RTP protein. For the keto-hydroxylamine tautomer of molnupiravir the most efficient interaction was found with the Nonstructural protein 3 (Nsp3_range 207–379-MES) through six hydrogen bonds with ASN40, GLY46, VAL49, ALA38, ALA50, and GLY47; one T-shaped π∙∙∙π interaction with PHE132; and three π-system∙∙∙alkyl interactions with PHE132, ALA38, and ILE131 (Figure 8, Table S1 in the Supplementary Materials).
Although none of the reported tautomers of molnupiravir showed superior binding scores with the main protease, Mpro (Table 9), this protein is a potential important drug target for coronavirus infections due to its essential role in processing the polyproteins that are translated from the viral RNA [38]. It was established that the keto-oxime tautomer of molnupiravir interacts with Mpro through seven hydrogen bonds formed with GLY143, SER144, CYS145, HIS163, LEU141, and GLN186; two alkyl interactions with MET165; and two π-system∙∙∙alkyl interactions with HIS41 and CYS145 (Table S2 in the Supplementary Materials). The keto-hydroxylamine forms complex with Mpro due to ten hydrogen bonds with GLY143, HIS163, GLU166, LEU141, SER144, MET165, and GLN198; two alkyl interactions with MET49 and MET165; and three π-system∙∙∙alkyl interactions with HIS41 and CYS145 (Table S2 in the Supplementary Materials). Finally, the hydroxyl-oxime tautomer of molnupiravir, which is the least efficiently bound to Mpro among the three reported tautomers (Table 9), interacts with the main protease via four hydrogen bonds with CYS145, LEU141, and PHE140; two alkyl interactions with MET49 and MET165; and three π-system∙∙∙alkyl interactions with HIS41 and CYS145 (Table S2 in the Supplementary Materials). Interestingly, one of the π-system∙∙∙alkyl interactions for all the tautomers with Mpro is formed by the π-system of the ligands (Figure S1 and Table S2 in the Supplementary Materials).
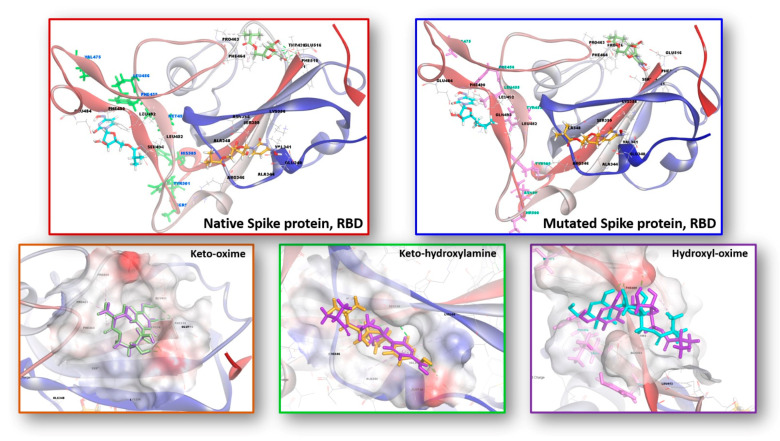
Besides the nonstructural proteins of SARS-CoV-2, spike protein, which is the structural protein, is of importance. The surface spike glycoprotein is consisting of two heterodimers S1 and S2. The receptor binding domain (RBD) is located on the head of S1 and binds the cellular receptor angiotensin-converting enzyme 2 (ACE2), initiating the membrane fusion of the virus and host cell. At this point, eight mutations (Y453F, L455F, F456L, A475V, A475S, T500S, N501Y, and Y505H) in the RBD and hACE2 interaction region (RBD/hACE2) were used to investigate the interaction mechanism of the reported tautomers of molnupiravir tautomers towards Spike protein, RBD as a target [39].
As a result of the calculations, while the binding affinity of the keto-hydroxylamine tautomer towards the mutated spike protein, RBD slightly decreased, the binding affinity of the keto-oxime and hydroxyl-oxime tautomers increased (Table 9). This is obviously explained by a different landscape of noncovalent interactions between the corresponding ligand and the target (Figure 9, Table S3 in the Supplementary Materials). As such, an interesting finding can be highlighted for the interaction of the hydroxyl-oxime tautomer of molnupiravir with the spike protein, RBD. Particularly, this tautomer interacts with the native spike protein, RBD exclusively through a set of hydrogen bonds and alkyl interactions, while π-system∙∙∙alkyl interactions were revealed for binding of the native spike protein, RBD with all the tautomers of molnupiravir, and for binding of the mutated spike protein, RBD with the keto-oxime and keto-hydroxylamine tautomers of molupiravir (Table S3 in the Supplementary Materials).
Figure 9.
Interaction of the keto-oxime (green), keto-hydroxylamine (orange), and hydroxyl-oxime (cyan) tautomers of molnupiravir with the native (top left) and mutated (top right) Spike proteins, RBD. Behaviors of tautomers of molnupiravir towards native and mutated Spike proteins, RBD of SARS-CoV-2. The bottom row depicts the superimposed binding poses of the reported tautomers of molnupiravir with the native (purple) and mutated (green, orange and cyan) Spike proteins, RBD.
3. Methods
3.1. DFT Calculations
The ground state geometries of the keto-oxime, keto-hydroxylamine and hydroxyl-oxime tautomers of molnupiravir were fully optimized without symmetry restrictions. The calculations were performed by means of the GaussView 6.0 molecular visualization program [40] and Gaussian 09, Revision D.01 program package [41] using the density functional theory (DFT) method with Becke-3-parametr-Lee-Yang-Parr (B3LYP) hybrid functional [42,43] and 6-311++G(d,p) [42,44] basis set. The vibration frequencies, as well as nonlinear optical properties (polarizability and first-order hyper-polarizability), were calculated for the optimized structures in gas phase and no imaginary frequencies were obtained. The electronic isosurfaces of the HOMO and LUMO orbitals and MEP surfaces were generated from the fully optimized ground state geometries obtained by using the B3LYP/6-311++G(d,p) method. The absorption and 1H NMR spectra of the fully optimized ground state geometries of the discussed tautomers were simulated at the TD-DFT/B3LYP/6-311++G(d,p) and GIAO/B3LYP/6-311++G(2d,p) levels, respectively.
3.2. Molecular Docking
Molecular docking simulations of the keto-oxime, keto-hydroxylamine, and hydroxyl-oxime tautomers of molnupiravir with a series of the SARS-CoV-2 proteins were carried out with AutoDock Vina [45,46]. The targeted protein structures were acquired via the RCSB PDB database [47], and were pre-treated before the docking, including water removing and inserting hydrogen atoms and missing residues and charges [23]. The ligands were optimized using the DFT/B3LYP/6-311++G(d,p) [42,44] basis set. Autodock Tools 1.5.7 was utilized to define the grid box with the dimensions of 30 × 30 × 30 size. During the docking procedure, 200 conformations for each ligand were left flexible, while the protein was held rigid. The lowest binding energy conformers and two dimensional (2D) interactions were filtered from 10 top ranked poses. Discovery Studio 3.5 [48] was utilized for visualization of the docked conformations and 3D target-ligand interactions.
4. Conclusions
In summary, we report detailed computational analysis of molnupiravir, which is emerging as an efficient drug to treat COVID-19. We have focused on three plausible tautomeric forms of molnupiravir, formed due to two acidic protons of the N4-hydroxylcytosine fragment, namely keto-oxime, keto-hydroxylamine and hydroxyl-oxime. According to the DFT/B3LYP/6-311++G(d,p) calculation results, it was established that the keto-oxime tautomer is the most energetically stable, followed by the keto-hydroxylamine tautomer, which is about 7 kcal/mol less favorable, while the hydroxyl-oxime tautomer is completely unfavorable.
We have also calculated IR, Raman, 1H NMR and absorption spectra, which were fully described and identified. We have also established values of the global chemical reactivity descriptors, which revealed that the discussed tautomers exhibit electron accepting ability and the low donating ability. Furthermore, values for the calculated polarizability and first-order hyperpolarizability parameters for tautomers are remarkably higher in comparison to those of urea, which is commonly used as a reference for studying the nonlinear optical (NLO) properties of the molecular systems. Thus, molnupiravir is of potential interest for future studies of its NLO properties.
In silico molecular docking was applied to probe interactions of the three tautomers of molnupiravir with a series of the SARS-CoV-2 proteins. It was established that both the keto-oxime and hydroxyl-oxime tautomers show the best binding affinity with the RdRp-RTR protein, while the keto-hydroxylamine tautomer is more efficient towards the Nonstructural protein 3 (Nsp3_range 207–379-MES). It was also established that the binding affinity of the keto-hydroxylamine tautomer towards the mutated Spike protein, RBD slightly decreased, while the binding affinity of the keto-oxime and hydroxyl-oxime tautomers increased in comparison to the native Spike protein, RBD.
We hope that the results reported herein will be of value for future design of potential drugs as well as developing new efficient therapies against SARS-CoV-2.
Acknowledgments
The authors thank Esin Akı Yalcin and the research group for technical assistance. We also thank Ural Interregional World-class Scientific and Educational Center «Advanced Production Technologies and Materials» for the support of this work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/1422-0067/23/3/1508/s1.
Author Contributions
Conceptualization, D.A.S. and M.G.B.; software, D.A.S.; validation, D.A.S.; formal analysis, A.V.S., M.G.B., T.T.T. and T.M.B.; investigation, A.V.S., M.G.B., T.T.T. and T.M.B.; resources, D.A.S.; data curation, A.V.S., M.G.B., T.T.T. and T.M.B.; writing—original draft preparation, A.V.S., M.G.B., T.T.T. and D.A.S.; writing—review and editing, A.V.S., M.G.B., T.T.T. and D.A.S.; visualization, M.G.B., T.T.T. and D.A.S.; project administration, D.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by state assignment of the Ministry of Science and Higher Education of the Russian Federation (Project Reg. No. 720000Φ.99.1.БЗ85AA13000).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data supporting the conclusions is included within the manuscript and is available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.First Oral Antiviral for COVID-19, Lagevrio (Molnupiravir), Approved by MHRA. [(accessed on 29 December 2021)]; Available online: https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra.
- 2. [(accessed on 29 December 2021)]. Available online: https://www.indiatoday.in/science/story/merck-covid-19-pill-molnupiravir-efficacy-omicron-vaccine-1889024-2021-12-17.
- 3.Toots M., Plemper R.K. Next-Generation Direct-Acting Influenza Therapeutics. Transl. Res. 2020;220:33–42. doi: 10.1016/j.trsl.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toots M., Yoon J.-J., Cox R.M., Hart M., Sricher Z.M., Makhsous N., Plesker R., Barrena A.H., Reddy P.G., Mitchell D.G., et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 2019;11:eaax5866. doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toots M., Yoon J.-J., Hart M., Natchus M.G., Painter G.R., Plemper R.K. Quantitative efficacy paradigms of the influenza clinical drug candidate EIDD-2801 in the ferret model. Transl. Res. 2020;218:16–28. doi: 10.1016/j.trsl.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. [(accessed on 29 December 2021)]. Available online: https://www.who.int/health-topics/coronavirus#tab=tab_1.
- 7. [(accessed on 29 December 2021)]. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 8. [(accessed on 15 January 2022)]. Available online: https://covid19.who.int/
- 9.Abdelnabi R., Foo C.S., Kaptein S.J.F., Zhang X., Do T.N.D., Langendries L., Vangeel L., Breuer J., Pang J., Williams R., et al. The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model. EBioMedicine. 2021;72:103595. doi: 10.1016/j.ebiom.2021.103595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahl A., Gralinski L.E., Johnson C.E., Yao W., Kovarova M., Dinnon K.H., III, Liu H., Madden V.J., Krzystek H.M., De C., et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–457. doi: 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashemian S.M.R., Pourhanifeh M.H., Hamblin M.R., Shahrzad M.K., Mirzaei H. RdRp inhibitors and COVID-19: Is molnupiravir a good option? Biomed. Pharmacother. 2022;146:112517. doi: 10.1016/j.biopha.2021.112517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox R.M., Wolf J.D., Plemper R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat. Microbiol. 2021;6:11–18. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenke K., Hansen F., Schwarz B., Feldmann F., Haddock E., Rosenke R., Meade-White K., Okumura A., Leventhal S., Hawman D.W., et al. Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model. Nat. Commun. 2021;12:2295. doi: 10.1038/s41467-021-22580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabinger F., Stiller C., Schmitzová J., Dienemann C., Kokic G., Hillen H.S., Höbartner C., Cramer P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021;28:740–746. doi: 10.1038/s41594-021-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer W.A., Eron J.J., Holman W., Cohen M.S., Fang L., Szewczyk L.G., Sheahan T.P., Baric R., Mollan K.R., Wolfe C.R., et al. A Phase 2a clinical trial of Molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci. Transl. Med. 2022;14:eabl7430. doi: 10.1126/scitranslmed.abl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malone B., Campbell E.A. Molnupiravir: Coding for catastrophe. Nat. Struct. Mol. Biol. 2021;28:706–708. doi: 10.1038/s41594-021-00657-8. [DOI] [PubMed] [Google Scholar]
- 17. [(accessed on 29 December 2021)]. Available online: https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat.
- 18.The Emergence of Powerful Oral Anti-COVID-19 Drugs in the Post-Vaccine Era. [(accessed on 29 December 2021)]. Available online: https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(21)00278-0/fulltext. [DOI] [PMC free article] [PubMed]
- 19. [(accessed on 29 December 2021)]. Available online: https://www.indiatoday.in/science/story/covid-19-pills-drug-omicron-pfizer-molnupiravir-paxlovid-ronapreve-1889039-2021-12-17.
- 20. [(accessed on 29 December 2021)]. Available online: https://www.reuters.com/world/us/merck-pfizer-covid-19-pills-effective-against-omicron-us-fda-official-2021-12-23/
- 21.Shiryaev A.A., Goncharenko A.N., Burkhanova T.M., Alkhimova L.A., Babashkina M.G., Chandrasekaran R., Safin D.A. A chiral (1R,2R)-N,N′-bis-(salicylidene)-1,2-diphenyl-1,2-ethanediamine Schiff base dye: Synthesis, crystal structure, Hirshfeld surface analysis, computational study, photophysical properties and in silico antifungal activity. J. Iran. Chem. Soc. 2021;18:2897–2911. doi: 10.1007/s13738-021-02237-5. [DOI] [Google Scholar]
- 22.Babashkina M.G., Frontera A., Kertman A.V., Saygideger Y., Murugavel S., Safin D.A. Favipiravir: Insight into the crystal structure, Hirshfeld surface analysis and computational study. J. Iran. Chem. Soc. 2022;19:85–94. doi: 10.1007/s13738-021-02285-x. [DOI] [Google Scholar]
- 23.Alkhimova L.E., Babashkina M.G., Safin D.A. Computational analysis of aspirin. J. Mol. Struct. 2022;1251:131975. doi: 10.1016/j.molstruc.2021.131975. [DOI] [Google Scholar]
- 24.Burkhanova T.M., Babashkina M.G., Taskin-Tok T., Sharov A.V., Safin D.A. Naphthalene-based bis-N-salicylidene aniline dyes: Crystal structures, Hirshfeld surface analysis, computational study and molecular docking with the SARS-CoV-2. J. Iran. Chem. Soc. 2022 doi: 10.1007/s13738-021-02438-y. [DOI] [Google Scholar]
- 25.Geerlings P., De Proft F., Langenaeker W. Conceptual Density Functional Theory. Chem. Rev. 2003;103:1793–1874. doi: 10.1021/cr990029p. [DOI] [PubMed] [Google Scholar]
- 26.Paymode D.J., Vasudevan N., Ahmad S., Kadam A.L., Cardoso F.S.P., Burns J.M., Cook D.W., Stringham R.W., Snead D.R. Toward a Practical, Two-Step Process for Molnupiravir: Direct Hydroxyamination of Cytidine Followed by Selective Esterification. Org. Process Res. Dev. 2021;25:1822–1830. doi: 10.1021/acs.oprd.1c00033. [DOI] [Google Scholar]
- 27.Abraham J.P., Sajan D., Joe I.H., Jayakumar V.S. Molecular structure, spectroscopic studies and first-order molecular hyperpolarizabilities of p-amino acetanilide. Spectrochim. Acta Part A. 2008;71:355–367. doi: 10.1016/j.saa.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Karamanis P., Pouchan C., Maroulis G. Structure, stability, dipole polarizability and differential polarizability in small gallium arsenide clusters from all-electron ab initio and density-functional-theory calculations. Phys. Rev. A. 2008;77:013201–013203. doi: 10.1103/PhysRevA.77.013201. [DOI] [Google Scholar]
- 29.Eme A., Sağdınç S.M. Spectroscopic (FT–IR, FT–Raman, UV–Vis) analysis, conformational, HOMO-LUMO, NBO and NLO calculations on monomeric and dimeric structures of 4–pyridazinecarboxylic acid by HF and DFT methods. J. Mol. Struct. 2017;1147:322–334. doi: 10.1016/j.molstruc.2017.06.110. [DOI] [Google Scholar]
- 30.Cully M. A tale of two antiviral targets—And the COVID-19 drugs that bind them. Nat. Rev. Drug Discov. 2022;21:3–5. doi: 10.1038/d41573-021-00202-8. [DOI] [PubMed] [Google Scholar]
- 31. [(accessed on 29 December 2021)]. Available online: https://www.organic-chemistry.org/prog/peo/
- 32.Meng X.-Y., Zhang H.-X., Mezei M., Cui M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comp.-Aid. Drug. 2011;7:146–157. doi: 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jorgensen W.L. The many roles of computation in drug discovery. Science. 2004;303:1813–1818. doi: 10.1126/science.1096361. [DOI] [PubMed] [Google Scholar]
- 34.Li H., Wang H.-Y., Kang S., Silverman R.B., Poulos T.L. Electrostatic Control of Isoform Selective Inhibitor Binding in Nitric Oxide Synthase. Biochemistry. 2016;55:3702–3707. doi: 10.1021/acs.biochem.6b00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tok T.T., Tatar G. Structures and functions of coronavirus proteins: Molecular modeling of viral nucleoprotein. Int. J. Virol. Infect. Dis. 2017;2:001–007. [Google Scholar]
- 36.Tok T.T., Gowder S.J.T. An Updated Review on COVID-19 with Special Reference to Structural Elucidation and Functional Properties. Biomed. J. Sci. Tech. Res. 2020;31:24345–24351. doi: 10.26717/BJSTR.2020.31.005131. [DOI] [Google Scholar]
- 37.Shamsi A., Mohammad T., Anwar S., Amani S., Khan M.S., Husain F.M., Rehman M.T., Islam A., Hassan M.I. Potential drug targets of SARS-CoV-2: From genomics to therapeutics. Int. J. Biol. Macromol. 2021;177:1–9. doi: 10.1016/j.ijbiomac.2021.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ullrich S., Nitsche C. The SARS-CoV-2 main protease as drug target. Bioorg. Med. Chem. Let. 2020;30:127377. doi: 10.1016/j.bmcl.2020.127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding X.-C., He J., Zhang X., Jiang C., Sun Y., Zhang Y., Chen Q., He H., Li W., Xie J., et al. Crucial Mutations of Spike Protein on SARS-CoV-2 Evolved to Variant Strains Escaping Neutralization of Convalescent Plasmas and RBD-Specific Monoclonal Antibodies. Front. Immunol. 2021;12:693775. doi: 10.3389/fimmu.2021.693775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennington R., Keith T.A., Millam J.M. GaussView. Semichem Inc.; Shawnee Mission, KS, USA: 2016. Version 6.0. [Google Scholar]
- 41.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. Gaussian 09. Gaussian, Inc.; Wallingford, CT, USA: 2016. Revision D.01. [Google Scholar]
- 42.Krishnan R., Binkley J.S., Seeger R., Pople J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980;72:650–654. doi: 10.1063/1.438955. [DOI] [Google Scholar]
- 43.Becke A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993;98:5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- 44.Frisch M.J., Pople J.A., Binkley J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984;8:3265–3269. doi: 10.1063/1.447079. [DOI] [Google Scholar]
- 45.Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eberhardt J., Santos-Martins D., Tillack A.F., Forli S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Modeling. 2021;61:3891–3898. doi: 10.1021/acs.jcim.1c00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rose Y., Duarte J.M., Lowe R., Segura J., Bi C., Bhikadiya C., Chen L., Rose A.S., Bittrich S., Burley S.K., et al. RCSB protein data bank: Architectural advances towards integrated searching and efficient access to macromolecular structure data from the PDB archive. J. Mol. Biol. 2021;433:166704. doi: 10.1016/j.jmb.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Accelrys Software Inc. Discovery Studio Modeling Environment. Accelrys Software Inc.; San Diego, CA, USA: 2013. Release 3.5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the conclusions is included within the manuscript and is available on request from the corresponding authors.