Abstract
Two murine monoclonal antibodies (MAb), 2C5-F10 and 8D1-H10, reactive with Escherichia coli O4 and H5 antigens, respectively, were generated and characterized. Enzyme immunoassays and immunoblots demonstrated that MAb 2C5-F10 reacted specifically with lipopolysaccharide O antigen of E. coli O4 isolates, while MAb 8D1-H10 reacted with E. coli strains expressing H5 flagella.
Escherichia coli expressing O4 lipopolysaccharide (LPS) and/or H5 flagellin frequently causes extraintestinal infections in humans and domestic animals (1, 10, 19). E. coli O4 organisms are common etiologic agents of human, canine, and feline genitourinary tract infections, manifested as pyelonephritis, cystitis, prostatitis, urosepsis, and asymptomatic bacteriuria (4, 8, 13, 25, 31). In particular, the E. coli O4:H5 serotype is a potent human uropathogen, especially the disseminated virulent J96 clone (14, 15). E. coli O4 and/or H5 antigens are also associated with enteric disease. Shiga toxin-producing E. coli (STEC) O4:H-negative (26), O4:H5 (11), O4:H10 (29), O2:H5 (5, 27), and O75:H5 (5) strains have caused hemorrhagic colitis and hemolytic uremic syndrome cases, while non-STEC O4 and enteroaggregative O4 have caused pediatric diarrhea outbreaks (6, 7). STEC O4:H-negative (28), O4:H4 (3), O4:H21 (24), O4:H25 (30), and O2:H5 (23) have been isolated from healthy cattle, while STEC O4 and non-STEC O4 have been associated with calves (18), pigs (9), and lambs (2) with diarrhea. While the E. coli O4 and H5 antigens are both markers of strain pathogenic potential, the O4 antigen moiety may itself function as a urovirulence factor (22).
Bacteria reference laboratories usually perform E. coli O and H serotyping using agglutination assays and rabbit hyperimmune antisera against reference O (n = 181) and H (n = 52) antigens (20; R. A. Wilson, personal communication). However, absorbed anti-E. coli O4 polyclonal antibodies (PAb) may cross-react with E. coli possessing O antigens 12, 13, 16, 18, 19, and 102 (20). Furthermore, E. coli expressing H antigens 1, 4, 8, 12, 38, 44, and 56 may cross-react with anti-H5 PAb (R. A. Wilson, personal communication). Anti-E. coli O4 and H5 monoclonal antibodies (MAb) have not been reported but offer potential diagnostic specificity, reagent quality, and typing assay format flexibility advantages over conventional agglutinating PAb. We generated anti-O4 and anti-H5 MAb in order to possess accurate serotyping reagents for use in ongoing and planned epidemiologic surveys for pathogenic and zoonotic E. coli in livestock.
MAb were produced from splenocytes of BALB/c mice immunized with E. coli O4:K3:H5 (E. coli Reference Center [ECRC] U4-41, the O4 and H5 reference strain) (20) whole-bacterium lysate and boosted with semipurified H5 flagellin (12). Immunization, hybridoma and ascites production, and MAb screening, isotyping, and characterization protocols were as previously described (12, 21). One anti-E. coli O4 MAb (2C5-F10; immunoglobulin M [IgM] isotype) and one anti-H5 MAb (8D1-H10; IgG1 isotype) were generated and characterized. MAb diagnostic sensitivity and specificity were estimated by enzyme-linked immunosorbent assay (ELISA) reactivity with whole-bacterium lysate preparations from 350 antigenically diverse gram-negative bacterial (272 E. coli and 78 non-E. coli) strains. The E. coli subset consisted of 8 O4:H5 strains, 4 O4:non-H5 strains, 8 non-O4:H5 strains, and 252 non-O4:non-H5 (including 1 non-O4:H autoagglutinable [HA]) strains of various O:H serotypes including 12 non-O4 isolates reported to react with anti-O4 PAb (O12 [n = 2], O16, O18 [n = 5], O19 [n = 3], O102) and 33 non-H5 isolates reported to react with anti-H5 PAb (H1 [n = 5], H4 [n = 14], H8 [n = 5], H12 [n = 4], H38 [n = 3], H44, and H56). Overall, E. coli isolates possessed 102 different O antigens and 51 different H antigens. Non-E. coli strains consisted of 50 Salmonella spp. and 28 other gram-negative bacteria from 22 genera. For ELISA, bacterial-antigen-coated plates were sequentially incubated with MAb (diluted ascites fluid), horseradish peroxidase (HRP)-conjugated antibody against mouse IgG plus mouse IgM (anti-mouse IgG+IgM), and 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate(6)] solution (ABTS peroxidase substrate) (12). ELISA optical density at dual wavelengths of 405 and 490 nm (OD405/490) was measured, and OD405/490 of >0.200 was considered positive (12). Dot box plots (16) of MAb ELISA OD405/490 values for bacterial antigen subsets were generated (Prism 3.0; Graph Pad Software Inc., San Diego, Calif.), and MAb diagnostic-sensitivity and -specificity point estimates with exact binomial 95% confidence intervals (CI) were calculated (Epi Info 6.0; Centers for Disease Control and Prevention, Atlanta, Ga.). Sensitivity was defined as the number of MAb ELISA-positive isolates per the total number of isolates tested possessing the target (O4 or H5) antigen. Specificity was defined as the number of MAb ELISA-nonreactive isolates per the total number of isolates tested that did not possess the target antigen.
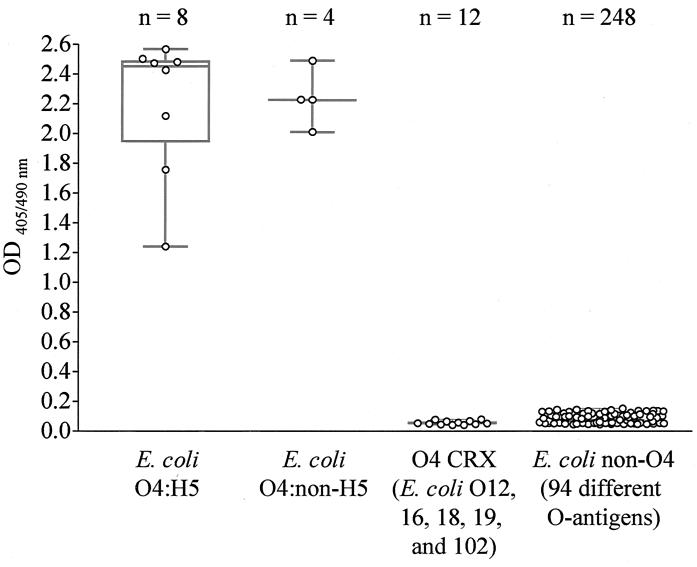
MAb 2C5-F10 was ELISA reactive with 12 E. coli O4 isolates (sensitivity, 100%; 95% CI, 73.5 to 100) and nonreactive with 260 non-O4 E. coli and 78 non-E. coli isolates (specificity, 100%; 95% CI, 98.9 to 100) (Fig. 1). Significantly, there was no MAb ELISA reactivity with 12 bacteria that cross-react with anti-O4 PAb.
FIG. 1.
Dot box plot of ELISA reactivities of 272 whole-bacterium lysates with anti-E. coli O4 MAb 2C5-F10. ELISA plates coated with whole-bacterium antigens were incubated sequentially with MAb 2C5-F10 (ascites fluid; 1:32,000), anti-mouse IgG+IgM-HRP conjugate, and ABTS peroxidase substrate. A MAb ELISA OD405/490 of >0.200 was considered positive. Dots represent means of duplicate OD values. The bottom and top edges of the superimposed box plots are the 25th and 75th distribution percentiles, respectively; the central horizontal line represents the median (50th percentile), and the central vertical lines extend from the box as far as the data extend (range). CRX, non-E. coli O4 bacteria known to cross-react with anti-E. coli O4 PAb. Data for MAb ELISA reactivity with 78 non-E. coli isolates are not shown.
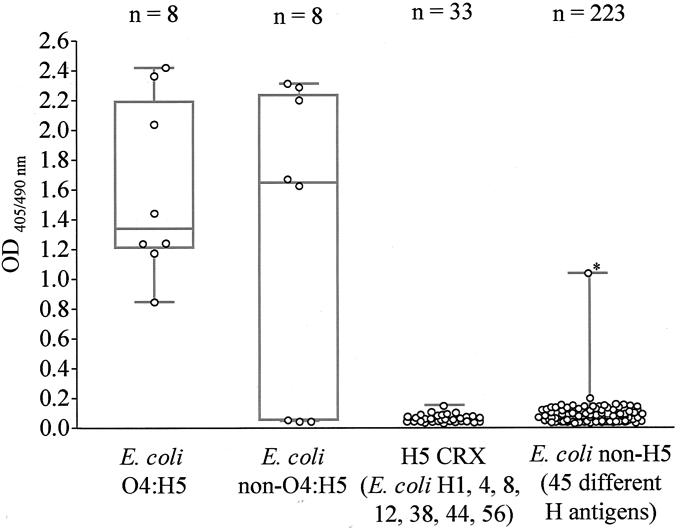
MAb 8D1-H10 reacted with 13 of 16 E. coli H5 isolates (sensitivity, 81.3%; 95% CI, 54.4 to 96.0) (Fig. 2); the three nonreactive strains were E. coli O75:H5 isolates. In spite of repeated attempts to induce and select for motility using flagellum broth and semisolid motility agar (12), these three strains remained nonmotile in our laboratory both macroscopically in media and microscopically under phase-contrast microscopy. This suggests that these E. coli O75:H5 strains may have been nonreactive with the anti-H5 MAb due to lack of expression of flagellar antigens (12). This MAb did not react with 78 non-E. coli strains but reacted with 1 of 255 non-H5 E. coli (specificity, 99.6%; 95% CI, 97.9 to 99.9) strains (Fig. 2). The cross-reactive strain was E. coli O2:HA (ECRC 94-0609), which possesses an unknown H-antigen.
FIG. 2.
Dot box plot of ELISA reactivities of 272 whole-cell bacterium lysates with anti-E. coli H5 MAb 8D1-H10. ELISA plates coated with whole-bacterium antigens were incubated sequentially with MAb 8D1-H10 (ascites fluid; 1:50,000), IgG+IgM-HRP, and ABTS solution. OD values represent means of duplicate wells. For dot box plot interpretation, see the legend for Fig. 1. CRX, non-E. coli H5 bacteria known to cross-react with anti-E. coli H5 PAb; *, datum point for E. coli O2:HA (autoagglutinable) 92–0609, which was presumptively H5 based on Western immunoblotting (Fig. 4). Data for MAb ELISA reactivity with 78 non-E. coli isolates are not shown.
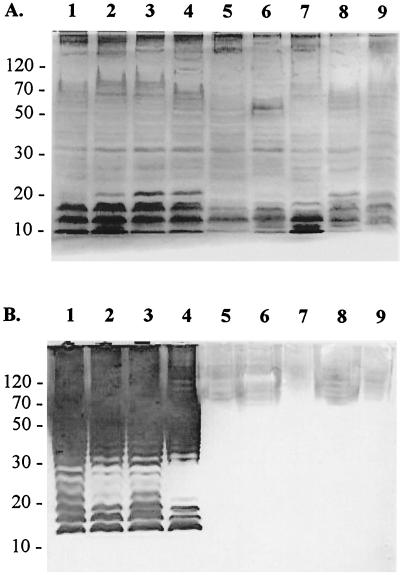
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western immunoblotting using whole-bacterium lysates, semipurified flagellin, and LPS preparations from E. coli O4 and H5 and non-O4 and non-H5 antigens confirmed MAb O4 and H5 specificity (12, 21) (Fig. 3 and 4). Reactive bands from gels transferred onto polyvinylidene difluoride membranes and incubated with MAb were revealed with HRP-conjugated rabbit anti-mouse IgG+IgM and diaminobenzidine substrate. The ladder pattern characteristic of LPS was observed on silver-stained gels and membranes incubated with anti-O4 MAb 2C5-F10 (Fig. 3), which reacted only with E. coli O4:H5 (lanes 1 to 3) and O4:H32 (lane 4), but not with four other whole-bacterium preparations (Fig. 3B). Similar results were obtained using semipurified LPS as antigen (data not shown).
FIG. 3.
SDS-PAGE and Western blots of MAb 2C5-F10 (anti-E. coli O4) on whole-bacterium antigens (25 μg per well). Lane 1, E. coli O4:H5 U4-41; lane 2, E. coli O4:H5 J96; lane 3, E. coli O4:H5 CA002; lane 4, O4:H32 90-1870; lane 5, E. coli O-negative:NM CDC 3377-85; lane 6, O-negative:H-negative 90-1870; lane 7, E. coli O18:H5 94-0061; lane 8, E. coli O12:H6 H9; and lane 9, E. coli O13:H11 SU 4321-41. After electrophoresis, one gel was silver stained (A). The other gel was transferred onto a polyvinylidene difluoride membrane and incubated with MAb 2C5-F10 (cell culture medium; 1:2) (B). MAb-reactive bands were revealed with rabbit anti-mouse IgG+IgM-HRP (1:1,000) and diaminobenzidine substrate. Molecular masses (in kilodaltons) are indicated on the left.
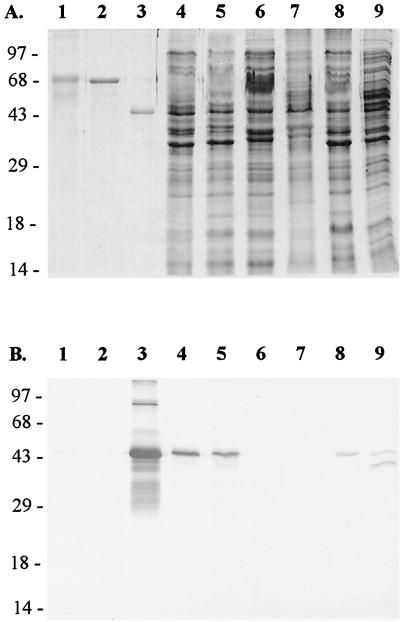
FIG. 4.
SDS-PAGE and Western blots of MAb 8D1-H10 (anti-E. coli H5) on partially purified flagellins (lanes 1 to 3; 2 μg per well) and whole-bacterium antigens (lanes 4 to 9; 24 μg per well). Lane 1, E. coli O157:H7 43895; lane 2, E. coli O9:H12 Bi316-42; lane 3, E. coli O4:H5 U4-41; lane 4, E. coli O4:H5 J96; lane 5, E. coli O75:H5 CL35; lane 6, E. coli O166:H-negative 94-0287; lane 7, E. coli O139:H56 SN3N/1; lane 8, E. coli O4:H5 91-1750; and lane 9, E. coli O2:HA 94-0609. After electrophoresis, one gel was Coomassie brilliant blue stained (A). The other gel was transferred onto a polyvinylidene difluoride membrane and incubated with MAb 8D1-H10 (cell culture medium; 1:1,000) (B). MAb-reactive bands were revealed with rabbit anti-mouse IgG+IgM-HRP (1:1,000) and diaminobenzidine substrate. Molecular masses (in kilodaltons) are indicated on the left.
Specificity of MAb 8D1-H10 for H5 antigen was confirmed by SDS-PAGE of semipurified flagellins and whole-bacterium preparations followed by immunoblotting (Fig. 4). MAb 8D1-H10 reacted predominantly with purified flagellins (lane 3) and whole-bacterium preparations (lanes 4, 5, and 8) of E. coli H5 at the expected molecular mass of 46 kDa (17). MAb 8D1-H10 immunoblotting of ELISA-reactive E. coli O2:HA 94-0609 produced a doublet of reactive bands at Mr of ≈46 and ≈37 kDa. The 46-kDa reactive band of this autoagglutinable strain was of a size consistent with H5 flagellin; the 37-kDa band could be an H5 degradation product or a non-H5 flagellin.
To validate MAb performance, a second panel of 37 E. coli O4, H5, non-O4, and non-H5 human clinical isolates (kindly provided by J. R. Johnson, University of Minnesota, St. Paul) was tested blindly for ELISA reactivity separately with each MAb (Table 1). The anti-O4 MAb reacted with 11 of 12 E. coli O4 strains and with 0 of 25 non-O4 strains. The nonreactive E. coli O4:H-multiple strain Z was serotyped as O82:H-multiple when submitted to the ECRC after MAb ELISA testing. The anti-H5 MAb reacted only with the four E. coli H5 strains.
TABLE 1.
ELISA reactivities of MAb 2C5-F12 (anti-O4) and 8D1-H10 (anti-H5) to 37 E. coli whole-bacterium antigens
| Isolate group | O-H serotype | Strain | ELISA reactivity of:
|
|
|---|---|---|---|---|
| MAb 2C5-F12 (anti-O4) | MAb 8D1-H10 (anti-H5) | |||
| O4:non-H5 | O4:NM | Bos 021 | + | − |
| O4:NM | Bos 038 | + | − | |
| O4:NM | Bos 046 | + | − | |
| O4:NM | Bos 105 | + | − | |
| O4:NM | PM8 | + | − | |
| O4:H1 | Afr 015 | + | − | |
| O4:H1 | Bos 040 | + | − | |
| O4:H1 | V31 | + | − | |
| O4:H-multiplea | Z | − | − | |
| O4:H5 | O4:H5 | CP9 | + | + |
| O4:H5 | R28 | + | + | |
| O4:H5 | 518 | + | + | |
| non-O4:H5 | O85/157:H5 | Bos 023 | − | + |
| non-O4:non-H5 | Ox7w:H16 | Y | − | − |
| O15:H1 | 2H17 | − | − | |
| O15/40:H1 | V32 | − | − | |
| O17:H8 | V11 | − | − | |
| O18ac:H7 | 2H25 | − | − | |
| O18:Haggeb | V | − | − | |
| O18:H23 | C | − | − | |
| O19:H27 | G | − | − | |
| O36:ntc | U | − | − | |
| O59:nt | B | − | − | |
| O63w:H6 | R | − | − | |
| O64:H21 | PM1 | − | − | |
| O73w:nt | F | − | − | |
| O75:NM | N | − | − | |
| O82:H12 | X | − | − | |
| O84:H- | V12 | − | − | |
| O86:H12 | M | − | − | |
| O113:H- | E | − | − | |
| O143:H- | 2V5 | − | − | |
| O149w:H8 | I | − | − | |
| O152:Hnt | IA | − | − | |
| O169w:H8 | L | − | − | |
| Ont:H7/24 | D | − | − | |
| Ont:H16 | A | − | − | |
Serotyped as O82:H-multiple on resubmission to ECRC after MAb testing.
agge, autoaggregative.
nt, nontypeable.
In conclusion, we produced MAb against the E. coli O4 and H5 antigens that appeared to be sensitive and specific as assessed by ELISA and immunoblotting. These MAb have potential clinical use as immunodiagnostic or serotyping reagents, especially if used in combination with other phenotypic or genotypic pathogenicity markers. They may be of particular utility for experimental or epidemiologic studies of extraintestinal E. coli O4:H5 infections.
Acknowledgments
We thank R. A. Wilson, ECRC, Pennsylvania State University, University Park, and J. R. Johnson, University of Minnesota, St. Paul, for providing bacterial strains. We also acknowledge S. Fryda-Bradley, R. Mlejnek, and S. Yang for technical assistance and J. Rosch for manuscript preparation.
REFERENCES
- 1.Blanco J E, Blanco J, Blanco M, Alonso M P, Jansen W H. Serotypes of CNF1-producing Escherichia coli strains that cause extraintestinal infections in humans. Eur J Epidemiol. 1994;10:707–711. doi: 10.1007/BF01719286. [DOI] [PubMed] [Google Scholar]
- 2.Blanco J, Cid D, Blanco J E, Blanco M, Ruiz Santa Quiteira J A, de la Fuente R. Serogroups, toxins and antibiotic resistance of Escherichia coli strains isolated from diarrhoeic lambs in Spain. Vet Microbiol. 1996;49:209–217. doi: 10.1016/0378-1135(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 3.Blanco M, Blanco J, Blanco J E, Gonzalez E A, Gomes T A, Zerbini L F, Yano T, de Castro A F. Genes coding for Shiga-like toxins in bovine verotoxin-producing Escherichia coli (VTEC) strains belonging to different O:K:H serotypes. Vet Microbiol. 1994;42:105–110. doi: 10.1016/0378-1135(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 4.Blanco M, Blanco J E, Alonso M P, Blanco J. Virulence factors and O groups of Escherichia coli isolates from patients with acute pyelonephritis, cystitis and asymptomatic bacteriuria. Eur J Epidemiol. 1996;12:191–198. doi: 10.1007/BF00145506. [DOI] [PubMed] [Google Scholar]
- 5.Bockemuhl J, Aleksic S, Karch H. Serological and biochemical properties of shiga-like toxin (verocytotoxin)-producing strains of Escherichia coli, other than O-group 157, from patients in Germany. Zentbl Bakteriol. 1992;276:189–195. doi: 10.1016/s0934-8840(11)80005-8. [DOI] [PubMed] [Google Scholar]
- 6.Cobeljic M, Miljkovic-Selimovic B, Paunovic-Todosijevic D, Velickovic Z, Lepsanovic Z, Zec N, Savic D, Ilic R, Konstantinovic S, Jovanovic B, Kostic V. Enteroaggregative Escherichia coli associated with an outbreak of diarrhoea in a neonatal nursery ward. Epidemiol Infect. 1996;117:11–16. doi: 10.1017/s0950268800001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colonna B, Ranucci L, Fradiani P A, Casalino M, Calconi A, Nicoletti M. Organization of aerobactin, hemolysin, and antibacterial resistance genes in lactose-negative Escherichia coli strains of serotype O4 isolated from children with diarrhea. Infect Immun. 1992;60:5224–5233. doi: 10.1128/iai.60.12.5224-5231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donneberg M S, Welch R A. Virulence determinants of uropathogenic Escherichia coli. In: Mobley H L T, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington D.C.: American Society for Microbiology; 1996. pp. 135–174. [Google Scholar]
- 9.Garabal J I, Gonzalez E A, Vazquez F, Blanco J, Blanco M, Blanco J E. Serogroups of Escherichia coli isolated from piglets in Spain. Vet Microbiol. 1996;48:113–123. doi: 10.1016/0378-1135(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 10.Gransden W R, Eykyn S J, Phillips L, Rowe B. Bacteremia due to Escherichia coli: a study of 861 episodes. Rev Infect Dis. 1991;12:1008–1018. doi: 10.1093/clinids/12.6.1008. [DOI] [PubMed] [Google Scholar]
- 11.Gunzburg S, Gracey M, Forbes D, Hewitt L, Bettelheim K. Haemolytic-uraemic syndrome and verocytotoxigenic Esch. coli. Med J Aust. 1988;149:54–55. doi: 10.5694/j.1326-5377.1988.tb120495.x. [DOI] [PubMed] [Google Scholar]
- 12.He Y, Keen J E, Westerman R B, Littledike E T, Kwang J. Monoclonal antibodies for detection of the H7 antigen of Escherichia coli. Appl Environ Microbiol. 1996;62:3325–3332. doi: 10.1128/aem.62.9.3325-3332.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson J R, Russo T A, Scheutz F, Brown J J, Zhang L, Palin K, Rode C, Bloch C, Marrs C F, Foxman B. Discovery of disseminated J96-like strains of uropathogenic Escherichia coli O4:H5 containing genes for both PapGJ96 (class I) and PrsGJ96 (class III) Gal(α1-4) Gal-binding adhesin. J Infect Dis. 1997;175:983–988. doi: 10.1086/514006. [DOI] [PubMed] [Google Scholar]
- 15.Johnson J R, Stapleton A E, Russo T A, Scheutz F, Brown J J, Maslow J N. Characteristics and prevalence within serogroup O4 of a J96-like clonal group of uropathogenic Escherichia coli O4:H5 containing the class I and class III alleles of papG. Infect Immun. 1997;65:2153–2159. doi: 10.1128/iai.65.6.2153-2159.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieg A F, Beck J R, Bongiovanni M B. The dot plot: a starting point for evaluating test performance. JAMA. 1988;260:3309–3312. doi: 10.1001/jama.260.22.3309. [DOI] [PubMed] [Google Scholar]
- 17.Lawn A M. Comparison of the flagellins from different morphotypes of Escherichia coli. J Gen Microbiol. 1977;101:121–131. doi: 10.1099/00221287-101-1-121. [DOI] [PubMed] [Google Scholar]
- 18.Orden J A, Ruiz-Santa-Quiteria J A, Cid D, Garcia S, Sanz R, de la Fuente R. Verotoxin-producing Escherichia coli (VTEC) and eae-positive non-VTEC in 1-30-days-old diarrhoeic dairy calves. Vet Microbiol. 1998;63:239–248. doi: 10.1016/s0378-1135(98)00218-1. [DOI] [PubMed] [Google Scholar]
- 19.Ørskov F, Ørskov I. Escherichia coli serotyping and disease in man and animals. Can J Microbiol. 1992;38:699–704. [PubMed] [Google Scholar]
- 20.Ørskov F, Ørskov I. Serotyping of Escherichia coli. Methods Microbiol. 1984;14:43–112. [Google Scholar]
- 21.Rivera-Betancourt M, Keen J E. Murine monoclonal antibodies specific for Escherichia coli O26 and O111. Appl Environ Microbiol. 2000;66:4124–4127. doi: 10.1128/aem.66.9.4124-4127.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo T A, Brown J J, Jodush S T, Johnson J R. The O4 specific antigen moiety of lipopolysaccharide but not the K54 group 2 capsule is important for urovirulence of an extraintestinal isolate of Escherichia coli. Infect Immun. 1996;64:2343–2348. doi: 10.1128/iai.64.6.2343-2348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandhu K S, Clarke R C, McFadden K, Brouwer A, Louie M, Wilson J, Lior H, Gyles C L. Prevalence of the eaeA gene in verotoxigenic Escherichia coli strains from dairy cattle in Southwest Ontario. Epidemiol Infect. 1996;116:1–7. doi: 10.1017/s095026880005888x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suthienkul O, Edward Brown J, Seriwatana J, Tienthongdee S, Sastravaha S, Echeverria P. Shiga-like-toxin-producing Escherichia coli in retail meats and cattle in Thailand. Appl Environ Microbiol. 1990;56:1135–1139. doi: 10.1128/aem.56.4.1135-1139.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terai A, Yamamoto S, Mitsumori K, Okada Y, Kurazono H, Takeda Y, Yoshida O. Escherichia coli virulence factors and serotypes in acute bacterial prostatitis. Int J Urol. 1997;4:289–294. doi: 10.1111/j.1442-2042.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 26.Tzipori S, Wachsmuth K I, Smithers J, Jackson C. Studies in gnotobiotic piglets on non-O157:H7 Escherichia coli serotypes isolated from patients with hemorrhagic colitis. Gastroenterology. 1988;94:590–597. doi: 10.1016/0016-5085(88)90228-4. [DOI] [PubMed] [Google Scholar]
- 27.von Wulffen H, Russmann H, Karch H, Meyer T, Bitzan M, Kohrt T C, Aleksic S. Verocytotoxin-producing Escherichia coli O2:H5 isolated from patients with ulcerative colitis. Lancet. 1989;i:1449–1450. doi: 10.1016/s0140-6736(89)90151-7. [DOI] [PubMed] [Google Scholar]
- 28.Wieler L H, Vieler E, Erpenstein C, Schlapp T, Steinrück H, Bauerfeind R, Byomi A, Baljer G. Shiga toxin-producing Escherichia coli strains from bovines: association of adhesion with carriage of eae and other genes. J Clin Microbiol. 1996;34:2980–2984. doi: 10.1128/jcm.34.12.2980-2984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willshaw G A, Scotland S M, Smith H R, Rowe B. Properties of vero cytotoxin-producing Escherichia coli of human origin of O serogroups other than O157. J Infect Dis. 1992;166:797–802. doi: 10.1093/infdis/166.4.797. [DOI] [PubMed] [Google Scholar]
- 30.Wilson J B, Clarke R C, Renwick S A, Bahn K, Johnson R P, Karmali M A, Lior H, Alves D, Gyles C L, Sandhu K S, McEwen S A, Spika J S. Vero cytotoxigenic Escherichia coli infection in dairy farm families. J Infect Dis. 1996;174:1021–1027. doi: 10.1093/infdis/174.5.1021. [DOI] [PubMed] [Google Scholar]
- 31.Yuri K, Nakata K, Katae H, Tsukamoto T, Hasegawa A. Serotypes and virulence factors of Escherichia coli strains isolated from dogs and cats. J Vet Med. 1999;61:37–40. doi: 10.1292/jvms.61.37. [DOI] [PubMed] [Google Scholar]