Abstract
Hemorrhagic fever with renal syndrome (HFRS), caused by different hantaviruses, is a distinct clinical syndrome endemic in several parts of Asia and Europe. However, the clinical picture can sometimes be indistinguishable from that of other infectious or noninfectious diseases. In this report we describe a clinical case, which is a rare occurrence but is a prime example of the difficulties in the diagnosis of HFRS in areas with a low prevalence of the disease.
Hemorrhagic fever with renal syndrome (HFRS) is a disease caused by viruses of the family Bunyaviridae, genus Hantavirus. Worldwide, several different human pathogenic hantaviruses are known (2, 9, 15). The subtypes Hantaan (HTN), Dobrova (DOB), and Seoul (SEO) cause moderate to severe HFRS in Asia and Europe, whereas Puumala (PUU) causes a mild form of HFRS in central Europe and Scandinavia also referred to as nephropathia epidemica (13). There are also several New World hantaviruses, the prototype of which is Sin Nombre virus, that cause hantavirus pulmonary syndrome in the Americans (1). Hantaviruses are rodent-borne pathogens and are normally transmitted to humans via aerosols generated from feces, urine, and saliva of infected rodents. In Germany two different serotypes of hantaviruses are known to be endemic. The predominant virus is PUU virus, mainly found in southwest Germany, which is carried by the bank vole (Clethrionomys glareolus) (5, 14, 15, 17; J. Pilaski, C. Ellerich, T. Kreutzer, A. Lang, W. Benik, A. Pohl-Koppe, L. Bode, E. Vanek, I. B. Autenrieth, K. Bigos, and H. W. Lee, Letter, Lancet 337:111, 1991). Recently several cases of DOB virus infection in Northeast Germany have been described (12, 16). In this part of Germany, DOB virus is found in Apodemus agrarius and seems to be less pathogenic to humans than Apodemus flavicollis-derived DOB virus which is found in the Balkans (16). In Germany, PUU and DOB viruses usually cause a mild form of HFRS with occasional severe complications such as acute renal failure and bleeding (14).
With the exception of known areas of endemicity, such as the Schwaebische Alp (Pilaski et al., Letter), clinical HFRS cases occur relatively infrequently in most parts of Germany. Consequently, hantavirus infections are not always considered in the initial differential diagnosis of infectious and/or febrile diseases. In addition, the use of commercial diagnostic tests is limited and sometimes unreliable. The following clinical case description demonstrates the general difficulties associated with the diagnosis of hantavirus infections in areas having low disease frequency.
Clinical case report and laboratory findings.
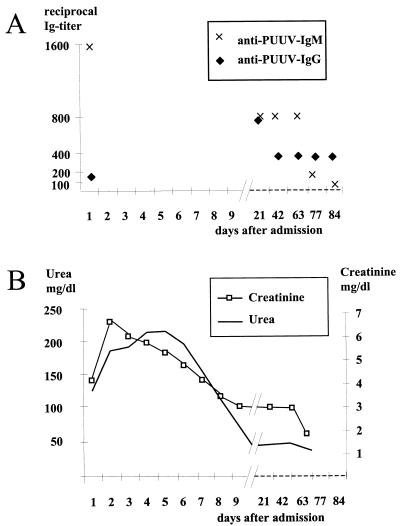
A 25-year old plumber was admitted to a small hospital in Hessen, Germany, with a 39°C fever that had developed over the previous days. Based on the clinical presentation, history, and laboratory parameters, acute appendicitis was diagnosed and surgery was performed. In situ the appendix appeared normal, but 800 ml of intraperitoneal fluid was observed. Shortly after surgery the patient became oliguric and was transferred to the intensive care unit of a nearby university hospital. The physical examination on admission showed a febrile (39.9°C) male patient who was somnolent but awake and well orientated. The patient suffered from diffuse abdominal pain, but without any classical signs of peritonitis, and presented bilateral pleural effusions, a reduction in pulmonary ventilation, and tachycardia (120/min). Conspicuous laboratory results were as follows: leukocytosis, 35,000/ml; thrombocytopenia, 38,000/ml; creatinine, 3.8 mg/dl; urea, 134 mg/dl; C-reactive protein, 115 mg/liter; and serum lactate, 3.2 mmol/liter. The blood coagulation parameters suggested a disseminated intravascular coagulopathy. Urine analysis displayed a nonselective proteinuria with a total loss of 3.52 g/liter over 24 h and an osmolarity of 274 mosmol/kg. Autoantibodies were not detected, and a primary hematological disease was also excluded. Three days after admission the patient developed large diffuse hemorrhages in the thighs, the back, and the intra-abominal area of the surgery. In order to stop the intra-abdominal bleeding, a second surgical intervention became necessary. Extensive capillary leakage led to multiple edema and pleural effusions, the latter of which had to be treated by punctuation. Hantavirus serology was ordered 2 days after admission to the intensive care unit. Immunoglobulin M (IgM) antibodies specific to PUU virus were detected using a μ-capture enzyme-linked immunosorbent assay (ELISA). Subsequently, IgM and IgG titers and specific parameters of kidney function were monitored over the next 3 months (Fig. 1). Reverse transcription-PCR for hantaviruses on RNA isolated from blood (whole blood, blood clots, and serum) and urine remained negative. On the second and third days after admission, intravenous substitution of 9,000 ml of fluid was necessary to sustain blood circulation. Until day 5 postadmission, the patient had displayed oliguria with urine volumes less than 70 ml/h; however, dialysis was not necessary. On day 6, polyuria started, with a urine volume of 250 ml/h. Leukocytes and thrombocytes recovered to normal levels on days 5 and 11 postadmission, respectively. The patient was dismissed from the hospital in good physical condition on day 18 postadmission.
FIG. 1.
Progression of selected laboratory parameters. (A) Hantavirus-specific antibodies. ELISAs were performed as described previously (3, 8, 14; Gerke et al., letter). (B) Creatinine and urea.
Diagnostic assays.
Hantavirus-specific IgM and IgG antibodies were detected using previously described ELISAs (3, 8, 14). Briefly, for IgM detection the serum antibodies were captured with goat anti-human IgM μ chain adsorbed to the wells of microtiter plates. The captured IgM was then allowed to react with viral antigen, and bound antigen was measured by the use of hyperimmune rabbit serum and appropriate enzyme conjugate and substrate. The antigens for this ELISA were extracted from uninfected and infected Vero cells (here, PUU and SEO) by several cycles of freeze-thawing followed by sonication and inactivation with 5 × 106 rads from a 60Co source. The IgG ELISA was performed by coating microtiter plates overnight with basic buffer detergent extracts (borate saline, pH 9.0, with 1% Triton X-100) of uninfected or infected Vero cells (here, PUU and SEO) previously inactivated with 2 × 106 rads from a 60Co source. Standard methodology was applied, with uninfected antigen controls run for each serum. For all ELISAs, optical densities at 410 nm were recorded and the optical density of the corresponding negative control antigen was substracted to yield the adjusted value (adOD). The cutoffs of the ELISAs were determined by using a panel of positive and negative control sera and defined as an adOD of 0.100. Serum titers of ≥1:400 were considered seropositive if the added adOD of positively reacting serum dilutions exceeded 1.0 (P. Gerke, D. Wichmann, U. Schonermarck, M. Schutt, H. Feldmann, T. G. Ksiazek, P. M. Rob, and W. L. Gross, Letter, Rheumatology 39:1424–1425, 2000).
PCR was performed using protocols established earlier and commonly used in routine diagnostics for hantaviruses (6, 14). Briefly, RNA was extracted from heparinized whole blood, blood clots, serum, and urine using different RNeasy kits (Qiagen, Düsseldorf, Germany). For urine, 30 ml collected over 24 h was pelleted at ∼107,000 × g for 2 h (SW41 Beckman rotor) prior to RNA extraction. Amplification of RNA was done by reverse transcription-PCR in a single-step, single-tube reaction using oligonucleotides previously described (6, 14).
Discussion.
The above-described clinical case may be rare, but it is a prime example of the difficulties in diagnosing HFRS in areas where the disease is not endemic and cases are sporadic. Hantavirus infections can appear clinically uncharacteristic and mimic syndromes such as an acute abdomen. Unnecessary surgeries with sometimes life-threatening complications can be the consequence of misinterpreted symptoms. Similar cases have occurred in Scandinavian countries and Russia, especially when HFRS was not generally recognized (4). Increasing awareness of hantavirus infections in Scandinavia has drastically reduced unnecessary surgical interventions. This may be more difficult to achieve in Western and Central Europe, since HFRS case numbers are much smaller.
Serology is still the first choice for the diagnosis of hantavirus infections. Most serological assays are set up to diagnose groups of hantaviruses rather than specific serotypes. Due to serological cross-reactivity between serotypes of such groups (e.g., DOB, SEO, and HTN), a positive result may occur in tests against any of the related antigens. Any laboratory offering hantavirus diagnosis should fulfill minimal requirements for the critical interpretation of their tests and should contact a reference center for advice in critical and questionable cases. Problems with quality control and test evaluation can be exacerbated by the facts that infections are rare and several serotypes may cocirculate. Seropanels might be helpful in determining the proper test antigen for a given geographic location.
Positive serology should be interpreted very cautiously in cases that are based on a single serum sample and where IgG cannot be detected. Independent confirmatory testing should always be attempted. In these cases, PCR detection of viral nucleic acid can be performed on blood samples, but a positive result is to be expected only if the samples were taken within the first days after onset of symptoms. Urine and kidney biopsy materials are sometimes better sources for this particular detection assay but are often not available (6, 7, 14). The first sampling on this case patient was done several days after the appearance of initial clinical symptoms, and therefore, the negative PCR results were not surprising. If clinical material is only useful for serological testing (sampling is performed at a nonviremic stage), serial serum samples are needed to confirm the diagnosis by demonstrating the appearance of IgG-specific antibodies, as was done in the described case. Virus isolation is normally done on Vero cells (American Type Culture Collection, C1008). Since isolation from human material is difficult (10), attempts are routinely made only following positive PCR results.
Areas of hantavirus endemicity in Western and Central Europe are not well defined, and hantavirus infections may be more common than expected. As we have learned from the experience of Scandinavian countries, the problem needs to be widely addressed and discussed with physicians of different specialties. Hantavirus infections should be considered in differential diagnosis along with a series of other acute infectious diseases, especially scrub typhus, murine typhus, spotted fevers, and leptospirosis. Hantavirus infection also needs to be differentiated from hematological diseases, other causes of acute renal failure, acute abdomen, and neurological diseases (11).
Acknowledgments
We thank Mike Drebot and Daryl Dick, Canadian Science Centre for Human and Animal Health, for critical review of the manuscript. The ELISA antigens were kindly provided by the Special Pathogens Branch, Centers for Disease Control and Prevention, Atlanta, Ga. (Thomas G. Ksiazek and Pierre E. Rollin).
Work on hantavirus at the Institute für Virologie, Philipps-Universität, Marburg, Germany, was supported by grants SFB286 and Fe286-5-1 from the Deutsche Forschungsgemeinschaft and grant 72087 from the Volkswagen-Stiftung.
REFERENCES
- 1.Duchin J S, Koster F T, Peters C J, Simpson G L, Tempest B, Zaki S R, Ksiazek T G, Rollin P E, Nichol S T, Umland E T, Moolenaar R L, Reef S E, Nolte K B, Gallaher M M, Butler J C, Breiman R F The Hantavirus Study Group. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N Engl J Med. 1994;330:949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann H. Encyclopedia of life sciences. [Online.] Nature Publishing Group. 2000. Hantaviruses. London, United Kingdom. [Google Scholar]
- 3.Feldmann H, Sanchez A, Morzunov S, Spiropoulou C F, Rollin P E, Ksiazek T G, Peters C J, Nichol S T. Utilization of autopsy RNA for the synthesis of the nucleoprotein antigen of a newly recognized virus associated with hantavirus pulmonary syndrome. Virus Res. 1993;30:351–367. doi: 10.1016/0168-1702(93)90101-r. [DOI] [PubMed] [Google Scholar]
- 4.Gantser S K, Zagidullin S Z, Gantseva K K, Ataev M P. Errors in the diagnosis of hemorrhagic fever with nephrotic syndrome in surgical practice. Klin Med (Moscow) 1989;67:42–44. . (In Russian.) [PubMed] [Google Scholar]
- 5.Heiske A, Anheier B, Pilaski J, Volchkov V E, Feldmann H. A new Clethrionomys-derived hantavirus from Germany: evidence for distinct genetic sublineages of Puumala viruses in Western Europe. Virus Res. 1999;61:101–112. doi: 10.1016/s0168-1702(99)00024-6. [DOI] [PubMed] [Google Scholar]
- 6.Heiske A, Anheier B, Pilaski J, Klenk H D, Gröne H J, Feldmann H. Polymerase chain reaction detection of Puumala virus RNA in formaldehyde-fixed biopsy material. Kidney Int. 1999;55:2062–2069. doi: 10.1046/j.1523-1755.1999.00421.x. [DOI] [PubMed] [Google Scholar]
- 7.Hjelle B, Spiropoulou C F, Torrez-Martinez N, Morzunov S, Peters C J, Nichol S T. Detection of Muerto Canyon virus RNA in peripheral blood mononuclear cells in patients with hantavirus pulmonary syndrome. J Infect Dis. 1994;170:1013–1017. doi: 10.1093/infdis/170.4.1013. [DOI] [PubMed] [Google Scholar]
- 8.Ksiazek T G, Peters C J, Rollin P E, Zaki S, Nichol S T, Spiropoulou C F, Morzunov S, Sanchez A, Feldmann H, Khan A S, Wachsmuth K, Butler J C. Identification of a new north American hantavirus that causes acute pulmonary insufficiency. Am J Trop Med Hyg. 1995;52:117–123. doi: 10.4269/ajtmh.1995.52.117. [DOI] [PubMed] [Google Scholar]
- 9.Lee, H. W., C. Calisher, and C. Schmaljohn. (ed.). 1999. Manual of hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. WHO Collaborating Center for Virus Reference and Research (Hantaviruses), Asan Institute for Life Sciences, Seoul, Korea.
- 10.Lee H W. Virus isolation. In: Lee H W, Calisher C, Schmaljohn C, editors. Manual of hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. WHO Collaborating Center for Virus Reference and Research (Hantaviruses) Seoul, Korea: Asan Institute for Life Sciences; 1999. pp. 74–79. [Google Scholar]
- 11.Lee J S, Lähdevirta J, Koster F, Levey H. Clinical manifestations and treatment of HFRS and HPS. In: Lee H W, Calisher C, Schmaljohn C, editors. Manual of hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. WHO Collaborating Center for Virus Reference and Research (Hantaviruses) Seoul, Korea: Asan Institute for Life Sciences; 1999. pp. 17–38. [Google Scholar]
- 12.Meisel, Lundkvist H A, Gantzer K, Bar W, Sibold C, Krueger D H. First case of infection with hantavirus Dobrava in Germany. Eur J Clin Microbiol Infect Dis. 1998;17:884–885. doi: 10.1007/s100960050214. [DOI] [PubMed] [Google Scholar]
- 13.Papadimitriou M. Hantavirus nephropathy. Kidney Int. 1995;48:887–902. doi: 10.1038/ki.1995.365. [DOI] [PubMed] [Google Scholar]
- 14.Pilaski J, Feldmann H, Morzunov S, Rollin P E, Ruo S L, Lauer B, Peters C J, Nichol S T. Genetic identification of a new Puumala virus strain causing severe hemorrhagic fever with renal syndrome in Germany. J Infect Dis. 1994;170:1456–1472. doi: 10.1093/infdis/170.6.1456. [DOI] [PubMed] [Google Scholar]
- 15.Schmaljohn C S, Hjelle B. Hantaviruses: a global disease problem. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sibold C, Ulrich R, Labuda M, Lundkvist A, Martens H, Schutt M, Gerke P, Leitmeyer K, Meisel H, Krüger D H. Dobrava hantavirus causes hemorrhagic fever with renal syndrome in central Europe and is carried by two different Apodemus mice species. J Med Virol. 2001;63:158–167. [PubMed] [Google Scholar]
- 17.Zöller L, Faulde M, Meisel H, Ruh B, Kimmig P, Schelling U, Zeier M, Kulzer P, Becker C, Roggendorf M, Bautz E K F, Krüger D H, Darai G. Seroprevalence of hantavirus antibodies in Germany as determined by a new recombinant enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 1995;14:305–313. doi: 10.1007/BF02116523. [DOI] [PubMed] [Google Scholar]