Abstract
Ageing is an unavoidable multi-factorial process, characterised by a gradual decrease in physiological functionality and increasing vulnerability of the organism to environmental factors and pathogens, ending, eventually, in death. One of the most elaborated ageing theories implies a direct connection between ROS-mediated mtDNA damage and mutations. In this review, we focus on the role of mitochondrial metabolism, mitochondria generated ROS, mitochondrial dynamics and mitophagy in normal ageing and pathological conditions, such as inflammation. Also, a chronic form of inflammation, which could change the long-term status of the immune system in an age-dependent way, is discussed. Finally, the role of inflammaging in the most common neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, is also discussed.
Keywords: mitophagy, ageing, inflammation, inflammaging
1. Ageing Theories
Ageing is a continuous multi-factorial process characterised by a gradual decrease in physiological functionality, resulting in gradually increasing vulnerability of the organism to environmental factors and, eventually, in death. Usually, ageing correlates with a higher risk to different pathogens and diseases (mental, neurodegenerative, heart) cancer. Many theories have been suggested to explain the ageing process [1,2], most of it, in a broad overview, could be sorted into three main groups: programmed, damage, and vicious cycles. Programmed theory suggests a genetically-based limitation of the lifespan that depends on the regulation of different systems (DNA methylation [3,4], autophagy [5,6], telomeres [7,8], autoimmunity [9,10] and others); damage theory suggests that internal and external (environmental) factors affect the organism at various levels, and that results in the shortening of the lifespan (DNA damage [11], free-radicals [12], transposable elements [13] and others); vicious cycles theory suggests the activity of the positive feedback loops between different age-related diseases (in particular, atherosclerosis, hypertension, diabetes, Alzheimer’s (AD) and Parkinson’s), thus supporting and promoting each other [14,15]. The main difference of the inflammaging concept is a chronic progressive increase in the proinflammatory status. This phenomenon combines a decline in adaptive immunity (immunosenescence) and a rise in proinflammation [16]. This review is dedicated to the role of mitochondrial mutation and mitophagy in inflammaging. For the other aspects of the inflammaging theories, we wish to redirect readers to the cited papers.
1.1. Mitochondria Functions, Quality Control and Turnover
The primary function of mitochondria is to supply cells with ATP, produced by oxidative phosphorylation of various substrates [17]. Additionally, mitochondria store a high concentration of calcium, thus participating in calcium homeostasis: interplay with endoplasmic reticulum Ca2+ storage [18], activation of the secondary messengers’ signalling systems [19], cell cycle [20], proliferation [21], regulation of the respiratory bioenergetics [22]. Mitochondria also participate in steroid biosynthesis [23], hormonal signalling [24], apoptosis programmed cell death [25], immune signalling [26] and other processes.
During normal functioning, mitochondria are exposed to molecular damage due to protein oxidation; with time, damaged proteins are accumulated and cause loss of membrane potential. Mitochondrial quality control (MQC) pathways are several important cellular mechanisms keeping mitochondria functional:
-
-
Mitochondrial biogenesis is a process of mitochondrial self-replication. Mitochondrial biogenesis could be activated by numerous developmental signals, different types of cellular stress or in response to environmental stimuli. PGC-1α (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha) is the central regulator of mitochondrial biogenesis [27].
-
-
Fission and fusion, the most basic mechanism, seems to be conserved from yeast to mammals [28]. Mitochondrial morphology is dynamic and regulated by complex protein machinery-fission and fusion. The fusion of the outer membrane is regulated by mitofusin 1 and 2, and the inner membrane-by the dynamin-like GTPase (OPA1) [29].
-
-
Mitophagy is a specialised form of autophagy, selectively degrading damaged and malfunctioning mitochondria. Mitophagy plays an essential role in many cellular processes, such as mitochondrial turnover, cell differentiation and embryonic development, apoptosis and inflammation, adjusting mitochondrial numbers to actual cellular metabolic needs. Impaired mitophagy is associated with many neurodegenerative diseases, cancer, ageing and other pathological conditions [30].
-
-
The next pathway of the MQC acts when mtDNA damage has occurred and includes several repairing mechanisms. Base excision repair (BER) is the main mitochondrial DNA repair pathway; it repairs DNA damage during normal functioning (caused by alkylation, deamination or oxidation). In the first step, substrate-specific DNA glycosylases recognise and remove modified bases. The second step generates an abasic site, further processed by an apurinic/apyrimidinic endonuclease (APE1). In the next step, DNA polymerase γ incorporates a single nucleotide into the gap (short-patch BER) or two-seven nucleotides (long-patch BER). On the final step, 5′ flap is cleaved by FEN-1 protein and ligated with DNA ligase III. Mitochondrial BER significantly contributes to longevity determination in mammals [31].
-
-
Damaged mitochondrial proteins could be repaired and refolded back to their native state. Several heat shock proteins (HSP) located in the mitochondrial matrix (for example, Hsp22, Hsp60 and Hsp70) have been connected with extended lifespan and increased stress resistance [32,33].
-
-
Mitochondrial unfolded protein response (UPRmt) activates upon an accumulation of the critical amount of misfolded or damaged proteins. UPRmt requires active bidirectional communication and proteins exchange between nucleus and mitochondria. ATFS-1/ATF5 is the best characterised UPRmt pathway. Normal transport of the leucine zipper protein ATFS-1 is interrupted by mitochondria stress and redirected to the nucleus, where it could interact with DVE-1 and UBL-5. UPRmt acts through two main mechanisms: chromatin remodeling and stimulating the expression of several mitochondrial chaperones (hsp-60, hsp-6 and protease clpp-1) [34].
-
-
Too damaged proteins that could not be efficiently repaired or refolded, irreversible degraded from the cell by proteolysis [35]. There are several mitochondrial protease systems with specific localisation of its activity: cytosolic (ubiquitin/26S proteasome system (UPS)), inner membrane internal protease (AAA-protease complex) [36] and matrix protease (Lon) [37].
1.2. Mitochondrial Genome and Heteroplasmy
To understand the role of mtDNA’s mutations in inflammaging, it is necessary to describe the basic features of the mitochondrial genome. MtDNA is mostly maternally-inherited, with the size of the double-stranded circular genome about ~16.4 kb. Most mitochondrial proteins are encoded in the nucleus, folded and transported into the mitochondria by the specialised importing pathway. MtDNA encodes only a limited number of molecules: 11 mRNAs, 22 tRNA, two rRNAs, and translates 13 subunits of the OXPHOS system (oxidative phosphorylation) [38]. Because mtDNA lacks histones, nuclear-encoded nucleoprotein complexes protects mtDNA from the generated oxidants damage, where mitochondrial transcription factor A plays the leading role [39].
Different cells, tissues and organs have genetically diverse populations of mitochondria. Mitochondria are dynamic, continuously fusing and fissioning. Hundreds of mtDNA could be proliferated independently and, even, as a part of the mitochondrial network, move to the neighbouring cells. The number of mtDNA copies per individual mitochondrion varies and could reach 10 nucleoids [40]. This polypoid nature of the mtDNA allows unique mutations to arise or be inherited by an independent cellular lineage. Heteroplasmy is the presence of both mtDNA genomes (wild-type and mutant) within one cell. Recent research suggests that even different cells of one organ could have a distinct set of nucleotide mutations [41]. During cytokinesis, mitochondria are unequally distributed between daughter cells. The precise molecular mechanism of the mitotic segregation of mtDNA mutations is unknown, while cells, to inherit healthier organelles, could clearly distinguish and avoid aged or damaged organelles [42,43].
The intercellular mitochondrial transfer has been shown on many non-lineage-related cells and tissues [44]. It is presumed that the main purpose of this process is to replace malfunctional mitochondria in recipient cells [45], but with a broad cross-cell-type compatibility of mitochondrial transfer, the same pathway could also be used to spread mtDNA mutations into new cells and tissues [46]. Interestingly, some mutant mtDNA genotypes could expand faster, especially when they have some advantage over wild-type mtDNA. For example, mtDNA genotypes with a large deletion would have a shorter genome and replicate more quickly [47]. The highest level of positive selection of the mutant mtDNA could be observed in intensely proliferating and spreading cancer cells [48,49]. Additionally, different mtDNA polymorphism variants could have an advantage in particular cell types, nuclear, or environmental contexts [50].
2. Key Roles of Mitochondria in the Current Ageing Theory
2.1. ROS-Mediated Accumulation of mtDNA Mutations
One of the most elaborated ageing theories implies a direct connection between ROS-mediated mtDNA damage and mutations [51]. Normal ageing is accompanied by a gradual decline in activity of mitochondrial enzymes, phosphocreatine recovery time and average respiratory capacity per mitochondria, while ROS production is increased [52]. In turn, increased ROS production makes more severe damage to mtDNA with a higher number of mutations, and thus, closing a so-called vicious cycle because such damaged mitochondria release more ROS.
A low-level of mutant mitochondrial genomes is usually inherited by a child from a mother and seems normal, while the number of mutations grows with age and accumulates [53]. Because of the multiple copies of mitochondrial genomes per cell, detecting and characterising a single mutation is rather difficult [54]. A particular threshold, where the mutation level is so high as to interrupt normal functioning, was not defined. Mutant mtDNA levels should be around 60–90% [55] to exceed repair mechanism capacity [56]. Most probably, this threshold is specific for every mutation type and cell-type, tissue, organs, or even person [57,58,59,60]. Many experiments have proved a direct connection between mtDNA mutations load, lifespan and rate of ageing [61,62,63]. The same mechanism was also identified in humans, where it was shown to be involved in developing Parkinson’s disease [64,65].
2.2. Mitophagy Dysregulation and Defects
In addition to the ROS-mediated damage of the mtDNA and proteins, the age-dependent decline in the removal of malfunction mitochondria influences the normal functioning of mitochondria. Selective removal of damaged proteins and mitochondria parts occurs through mitophagy [66]. Damaged proteins are ubiquitinated and accumulated on the outer mitochondrial membrane. Ubiquitination is catalysed by the E3-ubiquitin ligase Parkin, activated by PTEN-inducible putative kinase 1 (PINK1) [67]. Further, PINK1/Parkin-mediated mitophagy acts as a positive feedback amplification cascade [68].
Malfunction mitophagy and mitochondria dynamics have been connected to many human diseases, most importantly neurodegenerative diseases like Parkinson’s disease (PD), Alzheimer’s disease (AD), and Huntington’s disease (HD) [69,70]. Not surprisingly, the first connection between mitophagy and PD was established when mutations in the mitophagy-regulating genes were found. The primary hereditary form of PD has mutations in the PARK6 and PARK2 genes, encoding PINK1 and Parkin, respectively [71]. Interestingly, deletion of the autophagy genes leads to the accumulation of abnormal mitochondria and a rise in ROS production [72]. Additionally, a positive correlation between mitophagy rate, mitochondrial health and longevity have been established on many model systems. Thus, stimulation of mitophagy (chemically or with genetic manipulation) leads to increased longevity in C. elegans [73], Drosophila [74,75], and humans [76].
Similarly, chemical treatment was studied on zebrafish retina (resveratrol [77]), rat hippocampus explant cultures, SKNSH human neuroblastoma cells (Kisspeptin-10 [78]), mice heart (Kanglexin [79]), and C. elegans (tomatidine [80]). Urolithin A has shown promising results in C. elegans, rodents [81] and human [82] studies.
Thus, these studies imply that mitophagy is a target for many mutations, leading to the development of a life-threatening disease; and specific treatment could influence mitophagy to provide positive effects on age-related diseases and improve the quality of life of older people.
2.3. Criticism: The Connection between Lifespan and ROS; Beneficial Effects of Mild Stress
The main criticism of the ROS-mediated theory of mitochondria damage and subsequent ageing is based on the experiments breaking the connection between ROS level and lifespan. In particular, overexpression of the CuZn superoxide dismutase does not prolong the lifespan of many species by reducing ROS [83,84], but through a more complex pathway that includes ER-stress response factors [85]. Similarly, the reduction of antioxidant enzymes increased MtDNA damage but did not influence lifespan [86,87,88]. Also, a high level of oxidative stress was noticed in some long-living species [89,90]. Altogether, this suggests that a mild level of oxidative stress could be beneficial for organisms, while, in the broader scope, the ROS-mediated theory of ageing requires further research to update [91].
3. Inflammaging
Inflammaging theory is not new; many aspects of the relations between inflammation, development of age-related diseases and ageing have been suggested by many researchers before, while in a modern form, this theory was postulated in the 1990s [reviewed in [92]. This specific role was also given to the status of the immune system, which, due to the influence of age-related processes (immunosenescence), was unable to timely deactivate the inflammation and transmit it to the chronic form [93]. However, the proper terminology is still a matter of discussion while scientists mainly accept the main principle of inflammaging: it is an age-dependent increase in one’s pro-inflammatory status that influences lifespan and has associated pathological processes (diseases) [16]. Furthermore, we will characterise the most crucial aspects in more detail.
Pathogen attack, infection, or tissue injury could trigger normal inflammation. A fine-tuned signalling network regulates the shift from a pro-inflammatory state to a highly active inflammation state. Usually, inflammatory responses vanish as only threat or injury is removed (so-called resolved inflammation). In the case of non-resolved inflammation, some unknown factors are keeping on, providing low-level and long-lasting stimulation of the inflammation process in the cell or tissue [94].
3.1. Shift from Inflammation to Chronic Inflammation
Pro-inflammatory cytokines are the main factors responsible for a shift from normal inflammation to chronic inflammation and subsequent pathological inflammaging [95]. Experimental evidence from animals suggests an involvement of IL-1β, IL-4, IL-10, IL-17, IFN-γ, NF-κB, TNF-α into the chronic inflammation development [96,97,98]. It was shown in elderly people that high levels of IL-6 and TNF-α are associated with disabilities and mental and physiological disorders [99,100]. As a result of several large-scale studies, the serum level of IL-6 was suggested as a reliable general marker of inflammaging and some specific diseases and infections like COVID-19 and cancer [101,102]. The promoter regions of cytokines are polymorphic in the human population that could cause a regional difference in reaction and susceptibility to the development of age-related diseases, inflammaging and lifespan [103,104,105]. 174 G > C polymorphism in the IL-6 gene is associated with a high risk of heart failure in patients with obesity and heart diseases [106]. A study of the Chinese Han population suggests higher susceptibility to the development of knee osteoarthritis for individuals with IL-6 rs12700386 polymorphism. Adverse environmental factors (smoking and drinking) in combination with the rs12700386 genotype have shown even higher osteoarthritis risk, establishing a solid connection between the gene and environment [107]. As was demonstrated in another study, IL-6-174 G/C polymorphism was not connected to the ophthalmic diseases, while the GC genotype of IL-6-174 G/C was associated with proliferative diabetic retinopathy. Also, a higher intraocular level of IL-6 was defined among ocular disease patients [108]. The polymorphism of four genes examined in the North-Italian population (IL-6 (G > C, rs1800796), IL-10-1082 (G > A, rs1800896), TNF-α-308 (G > A, rs1800629), and TGFβ1 codon 10 (T > C, rs1800471)) was connected with an increased duration of inflammation and cancer risk [109].
Several pro-inflammatory cytokines, like TNF-α and interferons, were shown to cause cellular senescence and enhance inflammaging. Such senescence was studied in many cell-types [110,111], including stem cells [112,113], and driven by different ROS-based pathways (such as NF-κB [114], JAK/STAT [115], TGFβ/Smad [116]), launching a positive feedback loop that supports the further release of ROS and some cytokines [115].
From a general point of view, an imbalance of pro-inflammatory/anti-inflammatory cytokines could explain the inflammaging-mediated ageing. On the other side, several large-cohort studies suggested that longevity could be associated with the inflammaging process [117,118,119]. However, while those results have been obtained on relatively closed centenarians’ groups (Japanese, Italians and Greeks, respectively), the exact molecular mechanism is not proven and requires further investigation.
3.2. Role of mtDNA Mutations in Inflammation
In addition to the role of mitochondrial mutations and mitophagy in many life-threatening diseases, discussed in Section 2.1, the same violations could also influence other cell types (including immune cells [120] and alter their functionality, with further effect on inflammation and inflammaging processes.
Mutation m.3243A > G in mtTL1 gene, encoding tRNALeu-UUR, was identified in 80% of patients with MELAS maternally inherited mitochondrial disorder. MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) is a rare genetic disorder characterised by the brain, nervous system and muscles symptoms (temporary muscle weakness, hallucinations, seizures, abdominal pain, difficulty understanding, thinking or speaking and many others) with no cure available. Malfunction of the tRNALeu-UUR leads to the disassembly of the electron transport chain, subsequent defective oxidative phosphorylation, and energy production. At the final stages, those events reduce the production of ATP and accumulate lactate, causing lactic acidosis. Such events are incredibly stressful for energy-demanding cells, like neurons and myocytes [121]. Recently it was shown on endothelial cells delivered from MELAS syndrome patients that they have a high level of ROS and ox-LDL (oxidised-low-density lipoprotein). Also, the basal level of isoform b of VCAM-1 was high [122]. VCAM-1 is a crucial inflammation gene responsible for leucocyte-endothelial cell adhesion before leucocytes transmigrate through vascular walls [123].
M.3243A > G mutation showed a high level of autophagy and a deficit of mitophagy on MELAS-patient delivered induced pluripotent stem cells (iPS). Under oxidative stress challenge, bulk macroautophagy was increased, with further accumulation of toxic autophagosomes and autolysosomes and subsequent lower cell viability [124].
Rett syndrome, a crucial neurodevelopmental disorder, is associated with mutations in the MECP2 (methyl-CpG-binding domain-containing-protein 2) gene, clearly linked with abnormal mitochondrial dynamics, morphology and mitophagy [125]. Mitochondria malfunction leads to imbalanced redox status, high cytokine production and aberrant immune response, resulting in chronic inflammation [126].
Recent experiments on a mice model have identified age-related V338Y amino acid exchange in the MRPS5 (a mitochondrial ribosomal protein) as the ram (ribosomal ambiguity) mutation, leading to ribosomal proteins mistranslation. In the skeletal muscle, MrpS5 V338Y mutation resulted in impaired oxidative phosphorylation, higher ROS and bioactive lipids production, and enhanced inflammation [127].
Recently, mitochondrial tRNAThr 15927G > A mutation was linked to the pathogenesis of coronary artery disease. M.15927G > A mutation causes lower respiration efficiency, diminished membrane potential and higher ROS production on a cybrid cell culture. On the organism level, such mutation might be responsible for the inflammatory vascular reactions leading to cardiovascular diseases [128]. A novel mitochondrial tRNAGln m.4349C > T mutation was identified in the blood, urinary sediments and muscle samples of a patient with encephalopathy, epilepsy, and deafness. Similarly to other mitochondrial tRNA mutations, tRNAGln m.4349C > T has lower efficiency of the respiratory chain complex, high ROS level and decreased mitochondrial membrane potential, leading to premature cell senescence [129].
T6459C mutation of the mtCO1 gene, identified in the Chinese Han population, was linked to the higher genetic susceptibility to sepsis. mtCO1 encodes a functional subunit of COX protein that can fix ROS. Under normal conditions, biochemical parameters were close to the non-mutant group. However, after 6 h of lipopolysaccharide stimulation (simulation of the pathogen infection environment), the mutation-currying group has shown higher ROS production, lower levels of ATP and mitochondrial membrane potential, higher apoptosis rate. In total, those results suggest the prolonged release of the pro-inflammation mediators due to mitochondria injury and increased production of ROS [130].
Thus, the mitochondrial mutation, high ROS level, unbalanced nutrient supply and pathogens could affect mitophagy, directly and indirectly, engaging many physiological pathways and influencing the inflammation and shifting it to chronic status.
3.3. The Theory of Oxidation-Inflammation
A close correlation between mitochondria functionality, generated oxidative stress, the status of the immune system, severity of inflammation and ageing has been known to exist for a long time [131]. The modern theory, combining those processes, isoxidation-stress-mediated inflammaging [132]. Mitochondria play the primary role in this theory, where the original oxidative stress appears and leads to a further disturbance on the cellular level. A positive point of this theory, supported by many researchers, suggests that an adequate supply of cells with antioxidants may normalise the redox state, improve the immune system’s efficiency, extend lifespan, and lower the disease burden. Considerable research data has suggested that calorie restriction is the simplest type of intervention, with proven positive effects. Many aspects of calorie restriction (CR) were studied, and we can redirect readers to a specific topic of their interest: nutrition habits and inflammation status [133], the role of CR in tumour progression and inflammaging [134], autophagy and inflammation [135], microbiota and inflammation [136], cognition [137] and inflammaging [138]. A newly emerging area in the inflammaging research implies the application of artificial and natural compounds that mimic calorie restriction [139,140].
Chronic oxidative stress impacts every cell type, whereas the most pronounced effect can be observed in active, energy-demanding cells responsible for regulation and homeostasis, such as immune, nervous and endocrine cells [141,142,143]. Not surprisingly, many chronic inflammation-based diseases manifest in elderly people, significantly decreasing lifespan, quality of life, and increasing susceptibility to many accompanying diseases (Summarised in Figure 1). In the next section, we will update the role of inflammaging in the most common neurodegenerative diseases such as Alzheimer’s and Parkinson’s.
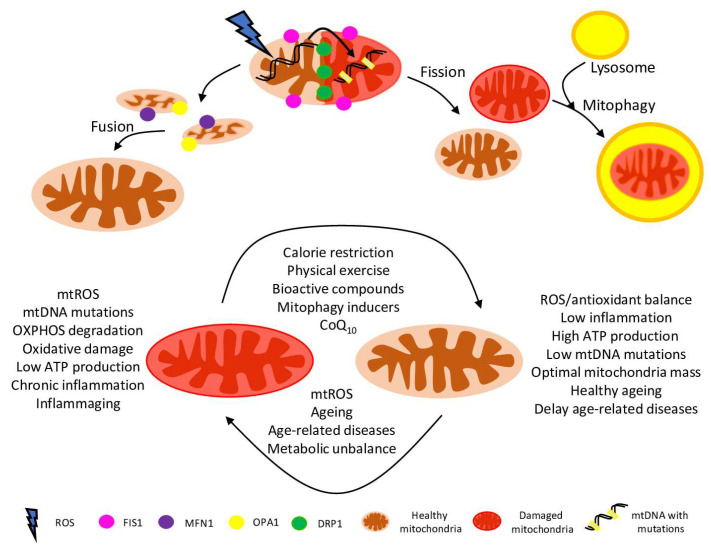
Figure 1.
Schematic representation of the role of mitochondria in healthy ageing. Mitochondrial dynamics are the coordinated fusion/fission events driven by complex machinery, involving Mfn, OPA1, Drp1, Fis1 and many other proteins. Mitochondrial fusion (interconnecting organelles) promotes mtDNA mixing and enhances bioenergetic efficiency. Mitochondrial fission is organelle segregation: equal distribution between daughter cells, healthy and damaged parts separation. Aged, damaged, and defective mitochondria are targeted for degradation via mitophagy. Mitochondrial health is crucial for delaying ageing and age-related diseases, including many metabolic disorders. Also, dysfunctional mitochondrial release many damage signals to the cytosol, which are leading to the release of inflammatory cytokines and causing chronic inflammation associated with ageing and age-related diseases. However, some activities, compounds and procedures (such as CoQ10 supply, calorie restriction and physical exercise) could reverse the damage and ameliorate mitochondria via mitophagy and de novo biogenesis.
3.4. Noncanonical mtDNA-Mediated Inflammation
MtDNA itself is a circular molecule of double-stranded DNA that is recognised as “foreign” by the immune system and that triggers various inflammatory pathways. The hypomethylated status of mtDNA could explain such an effect, where unmethylated CpG motifs would be similar to bacterial DNA and could potentially activate pattern recognition receptors such as TLR9 (Toll-Like Receptor 9) [144]. Additionally, the key cytosolic DNA sensor cGAS (Cyclic GMP-AMP Synthase) could recognise RNA:DNA hybrids and long stretches of single-stranded DNA, typical for mitochondrial DNA replication and transcription processes [145].
The release of mtDNA from mitochondria and subsequent activation of TLR9, cGAS and inflammasomes could occur during many cellular processes, including infection, neurodegeneration and cell death. The pioneering research in 2004 [146] demonstrated that local inflammation and arthritis could be experimentally induced by the injection of mtDNA into mice joints. Also, induced inflammation depended on the presence of oxidatively damaged bases in the mtDNA.
Nowadays, mtDNA is associated with the pathogenesis of several inflammatory diseases, such as diabetes, sickle cell disease, non-alcoholic steatohepatitis, lupus-like disease, Aicardi–Goutières syndrome, myocardial infarction and Parkinson’s disease. Therefore, we wish to redirect interested readers to the recent reviews [147,148].
4. Alzheimer’s Disease
AD is a common chronic neurodegenerative disease affecting approximately 50 million people in 2020 [149], with the most prominent symptoms similar to dementia and behavioural and memory problems. Despite more than 100 years of intensive studies (Alois Alzheimer described the first case in 1907), the understanding of the cause of AD is still not certain. Frontal and limbic cortices microglia inflammation is the common source of pro-inflammatory and oxidative stress, and is mostly associated with neurofibrillary tangles (NFT) and amyloid beta peptide (Aβ) deposits [149]. At the latter stages, AD is associated with higher and wider Aβ deposition, dysregulation of T-cell responses, increased lipid peroxidation and lower levels of plasma antioxidants [150]. Also, AD patients without dementia histories have a high level of Aβ/NFT but much lower levels of pro-inflammatory markers [151]. On the other side, the level of anti-inflammation cytokines is also crucial for the disease progress and could be connected to a particular genotype and degree of the immune system’s efficiency [152,153]. We could also redirect readers interested in the specific role of mitophagy in AD development to a recent review [154].
Several studies suggest a connection between pro-inflammatory cytokines and the Aβ peptide level. For example, injections of IFNγ up-regulate Atg5 and Atg7 expression, thus enhancing autophagy and, subsequently, lowering Aβ toxicity and decreasing the Aβ plaque load in the cortex and hippocampus [155]. Similarly, IL-10 and IL-12 have been defined as protective cytokines in another study [156], where systemic inflammation was found to be associated with hippocampal atrophy and cerebrospinal fluid protein levels in AD patients. One study defines IL-9 as a reliable marker for AD-related changes in African Americans but not Caucasians [157], while the exact genetic or environmental reasons and consequences for those observations are now known and require further investigation. Systemic levels of the cytokines IL-6 and IL-10 were higher in AD patients, while other cytokines did not correlate with neuroinflammation [158]. IL-10 is one of the major cytokines, playing a crucial role in many neurodegenerative diseases [159]. IL-33 is one of the minor cytokines, most likely playing an anti-inflammatory role and preserving cognitive functions in non-AD patients, while AD patients have a lower level of IL-33 and cognitive decline [160].
Effective mitophagy in microglia cells plays a crucial role in the removal of neurotoxic components, regulation of the pro-inflammation/anti-inflammatory cytokines balance, and general immune reactions. As was recently shown on the APP/PS1 mouse AD model [161], PINK1- and parkin-dependent mitophagy in microglia is responsible for the elimination of pro-inflammatory cytokines and intracellular Aβ. The revealed mechanism suggests adverse AD effects not only on neural cells but also on peripheral tissues. Mitophagy stimulation reverses cognitive symptoms and decreases extracellular Aβ plaques and the degree of neuroinflammation in several Aβ and tau AD animal model systems, suggesting mitophagy as a potential therapeutic target [161].
Similarly, aged mutant APP mouse (an AD model, expressing transgenic human amyloid-beta precursor protein (APP)), has shown altered levels of mitochondrial fission/fusion proteins, decreased levels of autophagy and mitophagy marker proteins [162]. Closer examination of APP mice hippocampal tissues revealed higher mitochondrial numbers but lower mitochondrial length, accompanied by defective mitochondria biogenesis, structure and dynamics, and cognitive degradation.
Recent results obtained in sporadic and familial AD patients suggest a tighter connection between ER and mitochondria contact sites (MERCS) in brain tissue and primary neurons [163]. Also, in AD animal models, increased levels of Aβ was associated with altered mitochondria functions and autophagosome formation. Altogether, this suggests a new role of MERCS as a self-cleaning mechanism aimed at removing toxic Aβ aggregates by quicker autophagosome formation and increased mitochondrial energy production [163].
As shown on the mouse AD model, accumulation of mutant APP and Aβ in hippocampal neurons leads to higher mitochondrial numbers, reduced mitochondrial length and a general decline of cell survival. Also, mitochondrial biogenesis, dynamics, mitophagy rate and general functions have been reduced [164].
5. Parkinson’s Disease
Parkinson’s disease (PD) is the second (after AD) most prevalent neurodegenerative disease; approximately 1% of the worlds’ population over the age of 60 have been diagnosed with it [165]. Slowly emerging symptoms include mainly motor functions (tremor, slowness, walking, rigidity), but cognitive and behavioural indications are also possible [166]. Despite almost two centuries of intensive study, the exact cause for PD is unknown, although it is likely that both inherited and environmental factors are involved [167]. Several recent excellent reviews described the close connection between mitochondria functions, effective mitophagy and autophagy in PD development, its pathogenesis, and therapy [168,169,170]. Furthermore, we will highlight the most important research in line with the inflammaging theory, mtDNA mutations and mitophagy.
Recent data further supported the role of age-dependent neuroinflammation. Two important features were associated with biallelic parkin/PINK1 mutations in German and Italian cohort patients. Firstly, such patients have higher levels of IL-6, which suggests using IL-6 as a reliable disease progression marker. Secondly, this mutation was linked with a high level of circulating cell-free mtDNA in serum, causing further progress and spreading neuroinflammation. Those data suggest that the application of NSAIDs (general unspecific therapy) and targeted anti-IL-6 antibodies (specialised treatment) may have a positive effect on Parkinson’s disease progress [171].
Insufficient PINK1-mediated mitophagy in the brain causes a lower level of dopamine and the release of cytokines (like TNF-α, IL-1β) by astrocytes and microglia [172]. A sufficient level of PINK1 may restrict neuroinflammation, ROS production and cell death, suggesting PINK1 protection from proteolysis as a promising strategy in PD treatment. Recently, a small molecule, BC1464, has been shown to limit PINK1 degradation by the ubiquitin-proteasome system, thus preventing mitochondrial damage. Neuroprotective properties of BC1464 have been confirmed on human primary cortical neurons, neuroblastoma cells and several PD patient-derived cell cultures, suggesting its application in PD treatment [173]. The application of the anticancer drug gemcitabine, which acts presumably via the MUL1 (mitochondrial ubiquitin ligase) pathway, leads to PINK1 stabilisation [174].
Degraded dopaminergic neurons in the substantia nigra pars compacta are the main diagnostic trait of PD. The recent paper also describes the implication of PD development in the cerebellum, which is responsible for some motor-related functions [175]. We have found increased levels of pro- (I-309, TNF and IL-1β) and anti-inflammatory (IL-1ra) cytokines in cerebellar mitochondria. This paper suggests that the pathophysiological manifestation of PD is much broader and not limited only to the substantia nigra brain regions.
In total, we could conclude that mitochondria health plays a crucial role in AD and PD development, closely connecting inflammation status, redox/antioxidants balance, cell energy demand and toxin removal.
6. Conclusions
Inflammaging is a modern concept of the ageing process based on long-lasting system inflammation. Inflammaging defines the pace of ageing, lifespan and quality of life of an aged population and is highly related to major human diseases, such as AD, PD, heart diseases, multiple sclerosis, atherosclerosis, cancer, type II diabetes, and many others [176]. Chronic, subclinical inflammation may be used as a marker for screening of risk groups and particular cytokinesas a therapeutic target when the specific disease is defined.
Despite intensive research, the exact molecular mechanism of inflammaging is still not known. Here we have discussed some therapeutic interventions that could be promising in combatting inflammaging. For example, mitophagy, as the basic cellular process, could be sped-up and improved by genetic manipulations and medicines. Cytokine-specific antibodies applied in particular tissues or organs could appose chronic inflammation. In a very optimistic scenario, such intervention could serve as a universal tool, amending many diseases and prolonging lifespan. For now, unfortunately, such a miracle tool is still missing; however, as we discussed, some effective methods have been found, such ascalorie restriction and regular physical exercise.
Thus, we could conclude that healthy mitochondria are crucial for proper cellular functioning. Further research on the fundamental molecular level and clinically controlled pharmacologic modulation therapy are required.
Abbreviations
| Aβ | Amyloid Beta Peptide |
| AD | Alzheimer’s disease |
| APE1 | apurinic/apyrimidinic endonuclease |
| APP | amyloid-beta precursor protein |
| BER | base excision repair |
| COX | cytochrome C oxidase |
| CR | calorie restriction |
| HD | Huntington’s disease |
| HSP | heat shock proteins |
| IFN-γ | Interferon-gamma |
| IL-4 | Interleukin 4 |
| iPS | induced pluripotent stem cells |
| JAK | Janus Kinase |
| MECP2 | methyl-CpG-binding domain-containing-protein 2 |
| MELAS | Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes |
| MERCS | mitochondria and ER contact sites |
| MRPS5 | mitochondrial ribosomal protein |
| MQC | mitochondrial quality control |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NFT | neurofibrillary tangles |
| NSAIDs | non-steroid-anti-inflammatory-drugs |
| OXPHOS | oxidative phosphorylation system |
| PD | Parkinson’s disease |
| PINK1 | PTEN-inducible putative kinase 1 |
| ram | ribosomal ambiguity |
| ROS | reactive oxygen species |
| STAT | signal transducers and activators of transcription |
| TGF-β | Transforming growth factor-beta |
| TNF-α | tumour necrosis factor-alpha |
| UPRmt | mitochondrial unfolded protein response |
| UPS | ubiquitin/26S proteasome system |
| VCAM-1 | vascular cell adhesion molecule-1 |
Author Contributions
S.A.D. and A.N.O. conceptualized the manuscript; S.A.D. wrote the manuscript text; N.G.N., A.D.Z. and N.A.O. reviewed the text; N.A.O. and A.V.G. methodology; A.D.Z. and A.V.G. formal analysis; N.G.N. and A.N.O. obtained funding and supervised. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (Grant # 20-15-00337).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Partridge L., Barton N.H. Optimally, Mutation and the Evolution of Ageing. Nature. 1993;362:305–311. doi: 10.1038/362305a0. [DOI] [PubMed] [Google Scholar]
- 2.Williams G.C. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution. 1957;11:398. doi: 10.1111/j.1558-5646.1957.tb02911.x. [DOI] [Google Scholar]
- 3.Ross K.M., Carroll J.E., Horvath S., Hobel C.J., Coussons-Read M.E., Dunkel Schetter C. Epigenetic Age and Pregnancy Outcomes: GrimAge Acceleration Is Associated with Shorter Gestational Length and Lower Birthweight. Clin. Epigenet. 2020;12:120. doi: 10.1186/s13148-020-00909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szymczak S., Dose J., Torres G.G., Heinsen F.-A., Venkatesh G., Datlinger P., Nygaard M., Mengel-From J., Flachsbart F., Klapper W., et al. DNA Methylation QTL Analysis Identifies New Regulators of Human Longevity. Hum. Mol. Genet. 2020;29:1154–1167. doi: 10.1093/hmg/ddaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmona-Gutierrez D., Zimmermann A., Kainz K., Pietrocola F., Chen G., Maglioni S., Schiavi A., Nah J., Mertel S., Beuschel C.B., et al. The Flavonoid 4,4′-Dimethoxychalcone Promotes Autophagy-Dependent Longevity across Species. Nat. Commun. 2019;10:651. doi: 10.1038/s41467-019-08555-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z., Wang Y., Wang H., Wu J., Tan Y. Rapamycin-Preactivated Autophagy Enhances Survival and Differentiation of Mesenchymal Stem Cells After Transplantation into Infarcted Myocardium. Stem Cell Rev. Rep. 2020;16:344–356. doi: 10.1007/s12015-020-09952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chilton W., O’Brien B., Charchar F. Telomeres, Aging and Exercise: Guilty by Association? Int. J. Mol. Sci. 2017;18:2573. doi: 10.3390/ijms18122573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q., Zhan Y., Pedersen N.L., Fang F., Hägg S. Telomere Length and All-Cause Mortality: A Meta-Analysis. Ageing Res. Rev. 2018;48:11–20. doi: 10.1016/j.arr.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Hughes J.W., Bao Y.K., Salam M., Joshi P., Kilpatrick C.R., Juneja K., Nieves D., Bouhairie V., Jordan O.J., Blustein E.C., et al. Late-Onset T1DM and Older Age Predict Risk of Additional Autoimmune Disease. Diabetes Care. 2019;42:32–38. doi: 10.2337/dc18-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vadasz Z., Haj T., Kessel A., Toubi E. Age-Related Autoimmunity. BMC Med. 2013;11:94. doi: 10.1186/1741-7015-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M., Chen J., Chen F., Liu Q., Sun Y., Yan C., Yang T., Bao Y., Hu Y.-P. Rejuvenating Strategies of Tissue-Specific Stem Cells for Healthy Aging. Aging Dis. 2019;10:871. doi: 10.14336/AD.2018.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos A.L., Sinha S., Lindner A.B. The Good, the Bad, and the Ugly of ROS: New Insights on Aging and Aging-Related Diseases from Eukaryotic and Prokaryotic Model Organisms. Oxidative Med. Cell. Longev. 2018;2018:1–23. doi: 10.1155/2018/1941285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sturm Á., Ivics Z., Vellai T. The Mechanism of Ageing: Primary Role of Transposable Elements in Genome Disintegration. Cell. Mol. Life Sci. 2015;72:1839–1847. doi: 10.1007/s00018-015-1896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belikov A.V. Age-Related Diseases as Vicious Cycles. Ageing Res. Rev. 2019;49:11–26. doi: 10.1016/j.arr.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Franceschi C., Garagnani P., Morsiani C., Conte M., Santoro A., Grignolio A., Monti D., Capri M., Salvioli S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018;5:61. doi: 10.3389/fmed.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn M.G., Markofski M.M., Carrillo A.E. Elevated Inflammatory Status and Increased Risk of Chronic Disease in Chronological Aging: Inflamm-Aging or Inflamm-Inactivity? Aging Dis. 2019;10:147. doi: 10.14336/AD.2018.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt-Rohr K. Oxygen Is the High-Energy Molecule Powering Complex Multicellular Life: Fundamental Corrections to Traditional Bioenergetics. ACS Omega. 2020;5:2221–2233. doi: 10.1021/acsomega.9b03352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Depaoli M.R., Hay J.C., Graier W.F., Malli R. The Enigmatic ATP Supply of the Endoplasmic Reticulum: ER ATP Supply. Biol. Rev. 2019;94:610–628. doi: 10.1111/brv.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabassi A., Miragoli M. Altered Mitochondrial Metabolism and Mechanosensation in the Failing Heart: Focus on Intracellular Calcium Signaling. Int. J. Mol. Sci. 2017;18:1487. doi: 10.3390/ijms18071487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao Y.-J., Chan J.-F., Hsu Y.-H.H. Chemotherapy Drug Induced Discoordination of Mitochondrial Life Cycle Detected by Cardiolipin Fluctuation. PLoS ONE. 2016;11:e0162457. doi: 10.1371/journal.pone.0162457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoque S.A.M., Kawai T., Zhu Z., Shimada M. Mitochondrial Protein Turnover Is Critical for Granulosa Cell Proliferation and Differentiation in Antral Follicles. J. Endocr. Soc. 2019;3:324–339. doi: 10.1210/js.2018-00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordmann C., Strokin M., Schönfeld P., Reiser G. Putative Roles of Ca2+ -Independent Phospholipase A 2 in Respiratory Chain-Associated ROS Production in Brain Mitochondria: Influence of Docosahexaenoic Acid and Bromoenol Lactone. J. Neurochem. 2014;131:163–176. doi: 10.1111/jnc.12789. [DOI] [PubMed] [Google Scholar]
- 23.Miller W.L. Disorders in the Initial Steps of Steroid Hormone Synthesis. J. Steroid Biochem. Mol. Biol. 2017;165:18–37. doi: 10.1016/j.jsbmb.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Klinge C.M. Estrogenic Control of Mitochondrial Function. Redox Biol. 2020;31:101435. doi: 10.1016/j.redox.2020.101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dadsena S., King L.E., García-Sáez A.J. Apoptosis Regulation at the Mitochondria Membrane Level. Biochim. Biophys. Acta (BBA)-Biomembr. 2021;1863:183716. doi: 10.1016/j.bbamem.2021.183716. [DOI] [PubMed] [Google Scholar]
- 26.de Breda C.N.S., Davanzo G.G., Basso P.J., Saraiva Câmara N.O., Moraes-Vieira P.M.M. Mitochondria as Central Hub of the Immune System. Redox Biol. 2019;26:101255. doi: 10.1016/j.redox.2019.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popov L.-D. Mitochondrial Biogenesis: An Update. J. Cell. Mol. Med. 2020;24:4892–4899. doi: 10.1111/jcmm.15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao S., Hu J. Mitochondrial Fusion: The Machineries In and Out. Trends Cell Biol. 2021;31:62–74. doi: 10.1016/j.tcb.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Youle R.J., van der Bliek A.M. Mitochondrial Fission, Fusion, and Stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onishi M., Yamano K., Sato M., Matsuda N., Okamoto K. Molecular Mechanisms and Physiological Functions of Mitophagy. EMBO J. 2021;40:e104705. doi: 10.15252/embj.2020104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gredilla R., Sánchez-Román I., Gómez A., López-Torres M., Barja G. Mitochondrial Base Excision Repair Positively Correlates with Longevity in the Liver and Heart of Mammals. GeroScience. 2020;42:653–665. doi: 10.1007/s11357-020-00158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dabbaghizadeh A., Morrow G., Amer Y.O., Chatelain E.H., Pichaud N., Tanguay R.M. Identification of Proteins Interacting with the Mitochondrial Small Heat Shock Protein Hsp22 of Drosophila Melanogaster: Implication in Mitochondrial Homeostasis. PLoS ONE. 2018;13:e0193771. doi: 10.1371/journal.pone.0193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu W., Lai L., Xie M., Qiu H. Insights of Heat Shock Protein 22 in the Cardiac Protection against Ischemic Oxidative Stress. Redox Biol. 2020;34:101555. doi: 10.1016/j.redox.2020.101555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muñoz-Carvajal F., Sanhueza M. The Mitochondrial Unfolded Protein Response: A Hinge Between Healthy and Pathological Aging. Front. Aging Neurosci. 2020;12:581849. doi: 10.3389/fnagi.2020.581849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravanelli S., den Brave F., Hoppe T. Mitochondrial Quality Control Governed by Ubiquitin. Front. Cell Dev. Biol. 2020;8:270. doi: 10.3389/fcell.2020.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Botham A., Coyaud E., Nirmalanandhan V.S., Gronda M., Hurren R., Maclean N., St-Germain J., Mirali S., Laurent E., Raught B., et al. Global Interactome Mapping of Mitochondrial Intermembrane Space Proteases Identifies a Novel Function for HTRA2. Proteomics. 2019;19:1900139. doi: 10.1002/pmic.201900139. [DOI] [PubMed] [Google Scholar]
- 37.Zeinert R.D., Baniasadi H., Tu B.P., Chien P. The Lon Protease Links Nucleotide Metabolism with Proteotoxic Stress. Mol. Cell. 2020;79:758–767.e6. doi: 10.1016/j.molcel.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace D.C. Mitochondrial Genetic Medicine. Nat. Genet. 2018;50:1642–1649. doi: 10.1038/s41588-018-0264-z. [DOI] [PubMed] [Google Scholar]
- 39.Bonekamp N.A., Larsson N.-G. SnapShot: Mitochondrial Nucleoid. Cell. 2018;172:388–388.e1. doi: 10.1016/j.cell.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 40.Wiesner R.J., Rüegg J.C., Morano I. Counting Target Molecules by Exponential Polymerase Chain Reaction: Copy Number of Mitochondrial DNA in Rat Tissues. Biochem. Biophys. Res. Commun. 1992;183:553–559. doi: 10.1016/0006-291X(92)90517-O. [DOI] [PubMed] [Google Scholar]
- 41.Maeda R., Kami D., Maeda H., Shikuma A., Gojo S. High Throughput Single Cell Analysis of Mitochondrial Heteroplasmy in Mitochondrial Diseases. Sci. Rep. 2020;10:10821. doi: 10.1038/s41598-020-67686-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katajisto P., Dohla J., Chaffer C.L., Pentinmikko N., Marjanovic N., Iqbal S., Zoncu R., Chen W., Weinberg R.A., Sabatini D.M. Asymmetric Apportioning of Aged Mitochondria between Daughter Cells Is Required for Stemness. Science. 2015;348:340–343. doi: 10.1126/science.1260384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarraf S.A., Sideris D.P., Giagtzoglou N., Ni L., Kankel M.W., Sen A., Bochicchio L.E., Huang C.-H., Nussenzweig S.C., Worley S.H., et al. PINK1/Parkin Influences Cell Cycle by Sequestering TBK1 at Damaged Mitochondria, Inhibiting Mitosis. Cell Rep. 2019;29:225–235.e5. doi: 10.1016/j.celrep.2019.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berridge M.V., McConnell M.J., Grasso C., Bajzikova M., Kovarova J., Neuzil J. Horizontal Transfer of Mitochondria between Mammalian Cells: Beyond Co-Culture Approaches. Curr. Opin. Genet. Dev. 2016;38:75–82. doi: 10.1016/j.gde.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Jiang D., Chen F.-X., Zhou H., Lu Y.-Y., Tan H., Yu S.-J., Yuan J., Liu H., Meng W., Jin Z.-B. Bioenergetic Crosstalk between Mesenchymal Stem Cells and Various Ocular Cells through the Intercellular Trafficking of Mitochondria. Theranostics. 2020;10:7260–7272. doi: 10.7150/thno.46332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin T.-K., Chen S.-D., Chuang Y.-C., Lan M.-Y., Chuang J.-H., Wang P.-W., Hsu T.-Y., Wang F.-S., Tsai M.-H., Huang S.-T., et al. Mitochondrial Transfer of Wharton’s Jelly Mesenchymal Stem Cells Eliminates Mutation Burden and Rescues Mitochondrial Bioenergetics in Rotenone-Stressed MELAS Fibroblasts. Oxidative Med. Cell. Longev. 2019;2019:9537504. doi: 10.1155/2019/9537504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arbeithuber B., Hester J., Cremona M.A., Stoler N., Zaidi A., Higgins B., Anthony K., Chiaromonte F., Diaz F.J., Makova K.D. Age-Related Accumulation of de Novo Mitochondrial Mutations in Mammalian Oocytes and Somatic Tissues. PLoS Biol. 2020;18:e3000745. doi: 10.1371/journal.pbio.3000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pérez-Amado C.J., Tovar H., Gómez-Romero L., Beltrán-Anaya F.O., Bautista-Piña V., Dominguez-Reyes C., Villegas-Carlos F., Tenorio-Torres A., Alfaro-Ruíz L.A., Hidalgo-Miranda A., et al. Mitochondrial DNA Mutation Analysis in Breast Cancer: Shifting From Germline Heteroplasmy Toward Homoplasmy in Tumors. Front. Oncol. 2020;10:572954. doi: 10.3389/fonc.2020.572954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strakova A., Nicholls T.J., Baez-Ortega A., Ní Leathlobhair M., Sampson A.T., Hughes K., Bolton I.A.G., Gori K., Wang J., Airikkala-Otter I., et al. Recurrent Horizontal Transfer Identifies Mitochondrial Positive Selection in a Transmissible Cancer. Nat. Commun. 2020;11:3059. doi: 10.1038/s41467-020-16765-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lechuga-Vieco A.V., Latorre-Pellicer A., Johnston I.G., Prota G., Gileadi U., Justo-Méndez R., Acín-Pérez R., Martínez-de-Mena R., Fernández-Toro J.M., Jimenez-Blasco D., et al. Cell Identity and Nucleo-Mitochondrial Genetic Context Modulate OXPHOS Performance and Determine Somatic Heteroplasmy Dynamics. Sci. Adv. 2020;6:eaba5345. doi: 10.1126/sciadv.aba5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harman D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 52.Barbouti A., Vasileiou P.V.S., Evangelou K., Vlasis K.G., Papoudou-Bai A., Gorgoulis V.G., Kanavaros P. Implications of Oxidative Stress and Cellular Senescence in Age-Related Thymus Involution. Oxidative Med. Cell. Longev. 2020;2020:7986071. doi: 10.1155/2020/7986071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lujan S.A., Longley M.J., Humble M.H., Lavender C.A., Burkholder A., Blakely E.L., Alston C.L., Gorman G.S., Turnbull D.M., McFarland R., et al. Ultrasensitive Deletion Detection Links Mitochondrial DNA Replication, Disease, and Aging. Genome Biol. 2020;21:248. doi: 10.1186/s13059-020-02138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Hara R., Tedone E., Ludlow A., Huang E., Arosio B., Mari D., Shay J.W. Quantitative Mitochondrial DNA Copy Number Determination Using Droplet Digital PCR with Single-Cell Resolution. Genome Res. 2019;29:1878–1888. doi: 10.1101/gr.250480.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thelen M.P., Wirth B., Kye M.J. Mitochondrial Defects in the Respiratory Complex I Contribute to Impaired Translational Initiation via ROS and Energy Homeostasis in SMA Motor Neurons. Acta Neuropathol. Commun. 2020;8:223. doi: 10.1186/s40478-020-01101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fontana G.A., Gahlon H.L. Mechanisms of Replication and Repair in Mitochondrial DNA Deletion Formation. Nucleic Acids Res. 2020;48:11244–11258. doi: 10.1093/nar/gkaa804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fendt L., Fazzini F., Weissensteiner H., Bruckmoser E., Schönherr S., Schäfer G., Losso J.L., Streiter G.A., Lamina C., Rasse M., et al. Profiling of Mitochondrial DNA Heteroplasmy in a Prospective Oral Squamous Cell Carcinoma Study. Cancers. 2020;12:1933. doi: 10.3390/cancers12071933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joshi P.R., Baty K., Hopton S., Cordts I., Falkous G., Schoser B., Blakely E.L., Taylor R.W., Deschauer M. Progressive External Ophthalmoplegia Due to a Recurrent de Novo m.15990C>T MT-TP (Mt-TRNAPro) Gene Variant. Neuromuscul. Disord. 2020;30:346–350. doi: 10.1016/j.nmd.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 59.Schlapakow E., Peeva V., Zsurka G., Jeub M., Wabbels B., Kornblum C., Kunz W.S. Distinct Segregation of the Pathogenic m.5667G>A Mitochondrial TRNAAsn Mutation in Extraocular and Skeletal Muscle in Chronic Progressive External Ophthalmoplegia. Neuromuscul. Disord. 2019;29:358–367. doi: 10.1016/j.nmd.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Visuttijai K., Hedberg-Oldfors C., Lindgren U., Nordström S., Elíasdóttir Ó., Lindberg C., Oldfors A. Progressive External Ophthalmoplegia Associated with Novel MT-TN Mutations. Acta Neurol. Scand. 2021;143:103–108. doi: 10.1111/ane.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dobson P.F., Dennis E.P., Hipps D., Reeve A., Laude A., Bradshaw C., Stamp C., Smith A., Deehan D.J., Turnbull D.M., et al. Mitochondrial Dysfunction Impairs Osteogenesis, Increases Osteoclast Activity, and Accelerates Age Related Bone Loss. Sci. Rep. 2020;10:11643. doi: 10.1038/s41598-020-68566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geurts J., Nasi S., Distel P., Müller-Gerbl M., Prolla T.A., Kujoth G.C., Walker U.A., Hügle T. Prematurely Aging Mitochondrial DNA Mutator Mice Display Subchondral Osteopenia and Chondrocyte Hypertrophy without Further Osteoarthritis Features. Sci. Rep. 2020;10:1296. doi: 10.1038/s41598-020-58385-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLaughlin K.L., Kew K.A., McClung J.M., Fisher-Wellman K.H. Subcellular Proteomics Combined with Bioenergetic Phenotyping Reveals Protein Biomarkers of Respiratory Insufficiency in the Setting of Proofreading-Deficient Mitochondrial Polymerase. Sci. Rep. 2020;10:3603. doi: 10.1038/s41598-020-60536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gui Y.-X., Xu Z.-P., Lv W., Zhao J.-J., Hu X.-Y. Evidence for Polymerase Gamma, POLG1 Variation in Reduced Mitochondrial DNA Copy Number in Parkinson’s Disease. Parkinsonism Relat. Disord. 2015;21:282–286. doi: 10.1016/j.parkreldis.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 65.Zheng W., Khrapko K., Coller H.A., Thilly W.G., Copeland W.C. Origins of Human Mitochondrial Point Mutations as DNA Polymerase γ-Mediated Errors. Mutat. Res./Fundam. Mol. Mech. Mutagenesis. 2006;599:11–20. doi: 10.1016/j.mrfmmm.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 66.Burman J.L., Pickles S., Wang C., Sekine S., Vargas J.N.S., Zhang Z., Youle A.M., Nezich C.L., Wu X., Hammer J.A., et al. Mitochondrial Fission Facilitates the Selective Mitophagy of Protein Aggregates. J. Cell Biol. 2017;216:3231–3247. doi: 10.1083/jcb.201612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seabright A.P., Lai Y.-C. Regulatory Roles of PINK1-Parkin and AMPK in Ubiquitin-Dependent Skeletal Muscle Mitophagy. Front. Physiol. 2020;11:608474. doi: 10.3389/fphys.2020.608474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacoupy M., Hamon-Keromen E., Ordureau A., Erpapazoglou Z., Coge F., Corvol J.-C., Nosjean O., Mannoury la Cour C., Millan M.J., Boutin J.A., et al. The PINK1 Kinase-Driven Ubiquitin Ligase Parkin Promotes Mitochondrial Protein Import through the Presequence Pathway in Living Cells. Sci. Rep. 2019;9:11829. doi: 10.1038/s41598-019-47352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burté F., Carelli V., Chinnery P.F., Yu-Wai-Man P. Disturbed Mitochondrial Dynamics and Neurodegenerative Disorders. Nat. Rev. Neurol. 2015;11:11–24. doi: 10.1038/nrneurol.2014.228. [DOI] [PubMed] [Google Scholar]
- 70.Monzio Compagnoni G., Di Fonzo A., Corti S., Comi G.P., Bresolin N., Masliah E. The Role of Mitochondria in Neurodegenerative Diseases: The Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020;57:2959–2980. doi: 10.1007/s12035-020-01926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klein C., Westenberger A. Genetics of Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.García-Prat L., Martínez-Vicente M., Perdiguero E., Ortet L., Rodríguez-Ubreva J., Rebollo E., Ruiz-Bonilla V., Gutarra S., Ballestar E., Serrano A.L., et al. Autophagy Maintains Stemness by Preventing Senescence. Nature. 2016;529:37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 73.Palikaras K., Lionaki E., Tavernarakis N. Coordination of Mitophagy and Mitochondrial Biogenesis during Ageing in C. Elegans. Nature. 2015;521:525–528. doi: 10.1038/nature14300. [DOI] [PubMed] [Google Scholar]
- 74.Rana A., Rera M., Walker D.W. Parkin Overexpression during Aging Reduces Proteotoxicity, Alters Mitochondrial Dynamics, and Extends Lifespan. Proc. Natl. Acad. Sci. USA. 2013;110:8638–8643. doi: 10.1073/pnas.1216197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Si H., Ma P., Liang Q., Yin Y., Wang P., Zhang Q., Wang S., Deng H. Overexpression of Pink1 or Parkin in Indirect Flight Muscles Promotes Mitochondrial Proteostasis and Extends Lifespan in Drosophila Melanogaster. PLoS ONE. 2019;14:e0225214. doi: 10.1371/journal.pone.0225214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang W., Moyzis A.G., Lampert M.A., Diao R.Y., Najor R.H., Gustafsson Å.B. Aging Is Associated with a Decline in Atg9b-mediated Autophagosome Formation and Appearance of Enlarged Mitochondria in the Heart. Aging Cell. 2020;19:e13187. doi: 10.1111/acel.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang N., Luo Z., Jin M., Sheng W., Wang H.-T., Long X., Wu Y., Hu P., Xu H., Zhang X. Exploration of Age-Related Mitochondrial Dysfunction and the Anti-Aging Effects of Resveratrol in Zebrafish Retina. Aging. 2019;11:3117–3137. doi: 10.18632/aging.101966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mattam U., Talari N.K., Paripati A.K., Krishnamoorthy T., Sepuri N.B.V. Kisspeptin Preserves Mitochondrial Function by Inducing Mitophagy and Autophagy in Aging Rat Brain Hippocampus and Human Neuronal Cell Line. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021;1868:118852. doi: 10.1016/j.bbamcr.2020.118852. [DOI] [PubMed] [Google Scholar]
- 79.Li H.-M., Liu X., Meng Z.-Y., Wang L., Zhao L.-M., Chen H., Wang Z.-X., Cui H., Tang X.-Q., Li X.-H., et al. Kanglexin Delays Heart Aging by Promoting Mitophagy. Acta Pharmacol. Sin. 2021 doi: 10.1038/s41401-021-00686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang E.F., Waltz T.B., Kassahun H., Lu Q., Kerr J.S., Morevati M., Fivenson E.M., Wollman B.N., Marosi K., Wilson M.A., et al. Tomatidine Enhances Lifespan and Healthspan in C. Elegans through Mitophagy Induction via the SKN-1/Nrf2 Pathway. Sci. Rep. 2017;7:46208. doi: 10.1038/srep46208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ryu D., Mouchiroud L., Andreux P.A., Katsyuba E., Moullan N., Nicolet-dit-Félix A.A., Williams E.G., Jha P., Lo Sasso G., Huzard D., et al. Urolithin A Induces Mitophagy and Prolongs Lifespan in C. Elegans and Increases Muscle Function in Rodents. Nat. Med. 2016;22:879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 82.Andreux P.A., Blanco-Bose W., Ryu D., Burdet F., Ibberson M., Aebischer P., Auwerx J., Singh A., Rinsch C. The Mitophagy Activator Urolithin A Is Safe and Induces a Molecular Signature of Improved Mitochondrial and Cellular Health in Humans. Nat. Metab. 2019;1:595–603. doi: 10.1038/s42255-019-0073-4. [DOI] [PubMed] [Google Scholar]
- 83.Huang T.T., Carlson E.J., Gillespie A.M., Shi Y., Epstein C.J. Ubiquitous Overexpression of CuZn Superoxide Dismutase Does Not Extend Life Span in Mice. J. Gerontol.-Biol. Sci. Med. Sci. 2000;55:B5. doi: 10.1093/gerona/55.1.b5. [DOI] [PubMed] [Google Scholar]
- 84.Page M.M., Richardson J., Wiens B.E., Tiedtke E., Peters C.W., Faure P.A., Burness G., Stuart J.A. Antioxidant Enzyme Activities Are Not Broadly Correlated with Longevity in 14 Vertebrate Endotherm Species. AGE. 2010;32:255–270. doi: 10.1007/s11357-010-9131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cabreiro F., Ackerman D., Doonan R., Araiz C., Back P., Papp D., Braeckman B.P., Gems D. Increased Life Span from Overexpression of Superoxide Dismutase in Caenorhabditis Elegans Is Not Caused by Decreased Oxidative Damage. Free Radic. Biol. Med. 2011;51:1575–1582. doi: 10.1016/j.freeradbiomed.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flores L.C., Roman M.G., Cunningham G.M., Cheng C., Dube S., Allen C., Van Remmen H., Hubbard G.B., Saunders T.L., Ikeno Y. Continuous Overexpression of Thioredoxin 1 Enhances Cancer Development and Does Not Extend Maximum Lifespan in Male C57BL/6 Mice. Pathobiol. Aging Age-Relat. Dis. 2018;8:1533754. doi: 10.1080/20010001.2018.1533754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ng L.F., Ng L.T., van Breugel M., Halliwell B., Gruber J. Mitochondrial DNA Damage Does Not Determine, C. Elegans Lifespan. Front. Genet. 2019;10:311. doi: 10.3389/fgene.2019.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Remmen H., Ikeno Y., Hamilton M., Pahlavani M., Wolf N., Thorpe S.R., Alderson N.L., Baynes J.W., Epstein C.J., Huang T.-T., et al. Life-Long Reduction in MnSOD Activity Results in Increased DNA Damage and Higher Incidence of Cancer but Does Not Accelerate Aging. Physiol. Genom. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 89.Andziak B., O’Connor T.P., Qi W., DeWaal E.M., Pierce A., Chaudhuri A.R., Van Remmen H., Buffenstein R. High Oxidative Damage Levels in the Longest-Living Rodent, the Naked Mole-Rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 90.Malod K., Archer C.R., Karsten M., Cruywagen R., Howard A., Nicolson S.W., Weldon C.W. Exploring the Role of Host Specialisation and Oxidative Stress in Interspecific Lifespan Variation in Subtropical Tephritid Flies. Sci. Rep. 2020;10:5601. doi: 10.1038/s41598-020-62538-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pérez V.I., Bokov A., Remmen H.V., Mele J., Ran Q., Ikeno Y., Richardson A. Is the Oxidative Stress Theory of Aging Dead? Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ershler W.B. Interleukin-6: A Cytokine for Gerontolgists. J. Am. Geriatr. Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 93.Franceschi C., Monti D., Barbier D., Salvioli S., Grassilli E., Capri M., Troiano L., Guido M., Bonafe M., Tropea F., et al. Successful Immunosenescence and the Remodelling of Immune Responses with Ageing. Nephrol. Dial. Transplant. 1996;11:18–25. doi: 10.1093/ndt/11.supp9.18. [DOI] [PubMed] [Google Scholar]
- 94.Nathan C., Ding A. Nonresolving Inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 95.Rea I.M., Gibson D.S., McGilligan V., McNerlan S.E., Alexander H.D., Ross O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schnabel C.L., Steinig P., Schuberth H.-J., Koy M., Wagner B., Wittig B., Juhls C., Willenbrock S., Escobar H.M., Jaehnig P., et al. Influences of Age and Sex on Leukocytes of Healthy Horses and Their Ex Vivo Cytokine Release. Vet. Immunol. Immunopathol. 2015;165:64–74. doi: 10.1016/j.vetimm.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 97.Siard M.H., McMurry K.E., Adams A.A. Effects of Polyphenols Including Curcuminoids, Resveratrol, Quercetin, Pterostilbene, and Hydroxypterostilbene on Lymphocyte pro-Inflammatory Cytokine Production of Senior Horses in Vitro. Vet. Immunol. Immunopathol. 2016;173:50–59. doi: 10.1016/j.vetimm.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Taylor J., Reynolds L., Hou L., Lohman K., Cui W., Kritchevsky S., McCall C., Liu Y. Transcriptomic Profiles of Aging in Naïve and Memory CD4+ Cells from Mice. Immun. Ageing. 2017;14:15. doi: 10.1186/s12979-017-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boots E.A., Castellanos K.J., Zhan L., Barnes L.L., Tussing-Humphreys L., Deoni S.C.L., Lamar M. Inflammation, Cognition, and White Matter in Older Adults: An Examination by Race. Front. Aging Neurosci. 2020;12:553998. doi: 10.3389/fnagi.2020.553998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van Hulle C., Jonaitis E.M., Betthauser T.J., Batrla R., Wild N., Kollmorgen G., Andreasson U., Okonkwo O., Bendlin B.B., Asthana S., et al. An Examination of a Novel Multipanel of CSF Biomarkers in the Alzheimer’s Disease Clinical and Pathological Continuum. Alzheimer’s Dement. 2021;17:431–445. doi: 10.1002/alz.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brábek J., Jakubek M., Vellieux F., Novotný J., Kolář M., Lacina L., Szabo P., Strnadová K., Rösel D., Dvořánková B., et al. Interleukin-6: Molecule in the Intersection of Cancer, Ageing and COVID-19. Int. J. Mol. Sci. 2020;21:7937. doi: 10.3390/ijms21217937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tyrrell D.J., Goldstein D.R. Ageing and Atherosclerosis: Vascular Intrinsic and Extrinsic Factors and Potential Role of IL-6. Nat. Rev. Cardiol. 2021;18:58–68. doi: 10.1038/s41569-020-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dai W., Ye Z., Lu H., Su Q., Li H., Li L. Meta-Analysis of the Relationship between Single Nucleotide Polymorphism of IL-10-1082G/A and Rheumatic Heart Disease. Oncotarget. 2018;9:12343–12350. doi: 10.18632/oncotarget.23901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Umapathy D., Krishnamoorthy E., Mariappanadar V., Viswanathan V., Ramkumar K.M. Increased Levels of Circulating (TNF-α) Is Associated with (−308G/A) Promoter Polymorphism of TNF-α Gene in Diabetic Nephropathy. Int. J. Biol. Macromol. 2018;107:2113–2121. doi: 10.1016/j.ijbiomac.2017.10.078. [DOI] [PubMed] [Google Scholar]
- 105.Wei G.-Z., Wang F., Zhao Y.-G., Li S.-S., Shi M.-L., Gao K., Luo Y., Tang W.-R. Association of Longevity with TNF-α G308A and IL-6 G174C Polymorphic Inflammatory Biomarkers in Caucasians: A Meta-Analysis. Z. Zeitschrift Gerontologie Geriatrie. 2016;49:706–713. doi: 10.1007/s00391-015-0992-y. [DOI] [PubMed] [Google Scholar]
- 106.Kravchun P.G., Kadykova O.I., Ryndina N.G., Krapivko S.O., Kozhyn M.I., Zolotaikina V.I. Relationship between Interleukin-6 Gene Polymorphism and Heart Failure in Patients with Coronary Artery Disease and Obesity. Wiadomości Lekarskie. 2020;73:1637–1640. doi: 10.36740/WLek202008109. [DOI] [PubMed] [Google Scholar]
- 107.Yang H., Zhou X., Xu D., Chen G. The IL-6 Rs12700386 Polymorphism Is Associated with an Increased Risk of Developing Osteoarthritis in the Knee in the Chinese Han Population: A Case-Control Study. BMC Med. Genet. 2020;21:199. doi: 10.1186/s12881-020-01139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ulhaq Z.S., Soraya G.V., Budu, Wulandari L.R. The Role of IL-6-174 G/C Polymorphism and Intraocular IL-6 Levels in the Pathogenesis of Ocular Diseases: A Systematic Review and Meta-Analysis. Sci. Rep. 2020;10:17453. doi: 10.1038/s41598-020-74203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruberto S., Santovito A. Association of TGFβ1 codon 10 (T > C) and IL-10 (G > C) cytokine gene polymorphisms with longevity in a cohort of Italian population. Am. J. Hum. Biol. 2021;33:e23491. doi: 10.1002/ajhb.23491. [DOI] [PubMed] [Google Scholar]
- 110.Guo F., Wu R., Xu J. Salicin Prevents TNF-α-Induced Cellular Senescence in Human Umbilical Vein Endothelial Cells (HUVECs) Artif. Cells Nanomed. Biotechnol. 2019;47:2618–2623. doi: 10.1080/21691401.2019.1629949. [DOI] [PubMed] [Google Scholar]
- 111.Lou C., Deng A., Zheng H., Sun G., Zhao H., Li A., Liu Q., Li Y., Lv Z. Pinitol Suppresses TNF-α-Induced Chondrocyte Senescence. Cytokine. 2020;130:155047. doi: 10.1016/j.cyto.2020.155047. [DOI] [PubMed] [Google Scholar]
- 112.Cruciani S., Garroni G., Ginesu G.C., Fadda A., Ventura C., Maioli M. Unravelling Cellular Mechanisms of Stem Cell Senescence: An Aid from Natural Bioactive Molecules. Biology. 2020;9:57. doi: 10.3390/biology9030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daniele S., Da Pozzo E., Iofrida C., Martini C. Human Neural Stem Cell Aging Is Counteracted by α-Glycerylphosphorylethanolamine. ACS Chem. Neurosci. 2016;7:952–963. doi: 10.1021/acschemneuro.6b00078. [DOI] [PubMed] [Google Scholar]
- 114.Xie J., Li B., Zhang P., Wang L., Lu H., Song X. Osteogenic Protein-1 Attenuates the Inflammatory Cytokine-Induced NP Cell Senescence through Regulating the ROS/NF-ΚB Pathway. Biomed. Pharmacother. 2018;99:431–437. doi: 10.1016/j.biopha.2018.01.053. [DOI] [PubMed] [Google Scholar]
- 115.Kandhaya-Pillai R., Miro-Mur F., Alijotas-Reig J., Tchkonia T., Kirkland J.L., Schwartz S. TNFα-Senescence Initiates a STAT-Dependent Positive Feedback Loop, Leading to a Sustained Interferon Signature, DNA Damage, and Cytokine Secretion. Aging. 2017;9:2411–2435. doi: 10.18632/aging.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hubackova S., Kucerova A., Michlits G., Kyjacova L., Reinis M., Korolov O., Bartek J., Hodny Z. IFNγ Induces Oxidative Stress, DNA Damage and Tumor Cell Senescence via TGFβ/SMAD Signaling-Dependent Induction of Nox4 and Suppression of ANT2. Oncogene. 2016;35:1236–1249. doi: 10.1038/onc.2015.162. [DOI] [PubMed] [Google Scholar]
- 117.Arai Y., Martin-Ruiz C.M., Takayama M., Abe Y., Takebayashi T., Koyasu S., Suematsu M., Hirose N., von Zglinicki T. Inflammation, But Not Telomere Length, Predicts Successful Ageing at Extreme Old Age: A Longitudinal Study of Semi-Supercentenarians. EBioMedicine. 2015;2:1549–1558. doi: 10.1016/j.ebiom.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Avery P., Barzilai N., Benetos A., Bilianou H., Capri M., Caruso C., Franceschi C., Katsiki N., Mikhailidis D., Panotopoulos G., et al. Editorial: Ageing, Longevity, Exceptional Longevity and Related Genetic and Non Genetics Markers: Panel Statement. CVP. 2014;12:659–661. doi: 10.2174/1570161111666131219101226. [DOI] [PubMed] [Google Scholar]
- 119.Berghella A.M., Contasta I., Marulli G., D’Innocenzo C., Garofalo F., Gizzi F., Bartolomucci M., Laglia G., Valeri M., Gizzi M., et al. Ageing Gender-Specific “Biomarkers of Homeostasis”, to Protect Ourselves against the Diseases of the Old Age. Immun. Ageing. 2014;11:3. doi: 10.1186/1742-4933-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu Y., Shen J., Ran Z. Emerging Views of Mitophagy in Immunity and Autoimmune Diseases. Autophagy. 2020;16:3–17. doi: 10.1080/15548627.2019.1603547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Finsterer J. Inherited Mitochondrial Disorders. In: Scatena R., Bottoni P., Giardina B., editors. Advances in Mitochondrial Medicine. Volume 942. Springer; Dordrecht, The Netherlands: 2012. pp. 187–213. Advances in Experimental Medicine and Biology. [DOI] [PubMed] [Google Scholar]
- 122.Pek N.M.Q., Phua Q.H., Ho B.X., Pang J.K.S., Hor J.-H., An O., Yang H.H., Yu Y., Fan Y., Ng S.-Y., et al. Mitochondrial 3243A > G Mutation Confers Pro-Atherogenic and pro-Inflammatory Properties in MELAS IPS Derived Endothelial Cells. Cell Death Dis. 2019;10:802. doi: 10.1038/s41419-019-2036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schlesinger M., Bendas G. Vascular Cell Adhesion Molecule-1 (VCAM-1)-An Increasing Insight into Its Role in Tumorigenicity and Metastasis: VCAM-1 in Tumorigenicity. Int. J. Cancer. 2015;136:2504–2514. doi: 10.1002/ijc.28927. [DOI] [PubMed] [Google Scholar]
- 124.Lin D.-S., Huang Y.-W., Ho C.-S., Hung P.-L., Hsu M.-H., Wang T.-J., Wu T.-Y., Lee T.-H., Huang Z.-D., Chang P.-C., et al. Oxidative Insults and Mitochondrial DNA Mutation Promote Enhanced Autophagy and Mitophagy Compromising Cell Viability in Pluripotent Cell Model of Mitochondrial Disease. Cells. 2019;8:65. doi: 10.3390/cells8010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett Syndrome Is Caused by Mutations in X-Linked MECP2, Encoding Methyl-CpG-Binding Protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 126.Cicaloni V., Pecorelli A., Tinti L., Rossi M., Benedusi M., Cervellati C., Spiga O., Santucci A., Hayek J., Salvini L., et al. Proteomic Profiling Reveals Mitochondrial Alterations in Rett Syndrome. Free Radic. Biol. Med. 2020;155:37–48. doi: 10.1016/j.freeradbiomed.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 127.Scherbakov D., Duscha S., Juskeviciene R., Restelli L., Frank S., Laczko E., Boettger E.C. Mitochondrial Misreading in Skeletal Muscle Accelerates Metabolic Aging and Confers Lipid Accumulation and Increased Inflammation. RNA. 2020;27:265–272. doi: 10.1261/rna.077347.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jia Z., Zhang Y., Li Q., Ye Z., Liu Y., Fu C., Cang X., Wang M., Guan M.-X. A Coronary Artery Disease-Associated TRNAThr Mutation Altered Mitochondrial Function, Apoptosis and Angiogenesis. Nucleic Acids Res. 2019;47:2056–2074. doi: 10.1093/nar/gky1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ji K., Wang W., Lin Y., Xu X., Liu F., Wang D., Zhao Y., Yan C. Mitochondrial Encephalopathy Due to a Novel Pathogenic Mitochondrial TRNA Gln m.4349C>T Variant. Ann. Clin. Transl. Neurol. 2020;7:980–991. doi: 10.1002/acn3.51069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shen X., Han G., Li S., Song Y., Shen H., Zhai Y., Wang Y., Zhang F., Dong N., Li T., et al. Association between the T6459C Point Mutation of the Mitochondrial MT-CO1 Gene and Susceptibility to Sepsis among Chinese Han People. J. Cell. Mol. Med. 2018;22:5257–5264. doi: 10.1111/jcmm.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miquel J., Economos A.C., Fleming J., Johnson J.E. Mitochondrial Role in Cell Aging. Exp. Gerontol. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 132.Fuente M., Miquel J. An Update of the Oxidation-Inflammation Theory of Aging: The Involvement of the Immune System in Oxi-Inflamm-Aging. CPD. 2009;15:3003–3026. doi: 10.2174/138161209789058110. [DOI] [PubMed] [Google Scholar]
- 133.Franceschi C., Ostan R., Santoro A. Nutrition and Inflammation: Are Centenarians Similar to Individuals on Calorie-Restricted Diets? Annu. Rev. Nutr. 2018;38:329–356. doi: 10.1146/annurev-nutr-082117-051637. [DOI] [PubMed] [Google Scholar]
- 134.Komatsu T., Park S., Hayashi H., Mori R., Yamaza H., Shimokawa I. Mechanisms of Calorie Restriction: A Review of Genes Required for the Life-Extending and Tumor-Inhibiting Effects of Calorie Restriction. Nutrients. 2019;11:3068. doi: 10.3390/nu11123068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gabandé-Rodríguez E., Gómez de las Heras M.M., Mittelbrunn M. Control of Inflammation by Calorie Restriction Mimetics: On the Crossroad of Autophagy and Mitochondria. Cells. 2019;9:82. doi: 10.3390/cells9010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Golonka R.M., Xiao X., Abokor A.A., Joe B., Vijay-Kumar M. Altered Nutrient Status Reprograms Host Inflammation and Metabolic Health via Gut Microbiota. J. Nutr. Biochem. 2020;80:108360. doi: 10.1016/j.jnutbio.2020.108360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dias I.R., de Santos C.S., e Magalhães C.O.D., de Oliveira L.R.S., Peixoto M.F.D., De Sousa R.A.L., Cassilhas R.C. Does Calorie Restriction Improve Cognition? IBRO Rep. 2020;9:37–45. doi: 10.1016/j.ibror.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim S.-E., Mori R., Shimokawa I. Does Calorie Restriction Modulate Inflammaging via FoxO Transcription Factors? Nutrients. 2020;12:1959. doi: 10.3390/nu12071959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cătană C.-S., Atanasov A.G., Berindan-Neagoe I. Natural Products with Anti-Aging Potential: Affected Targets and Molecular Mechanisms. Biotechnol. Adv. 2018;36:1649–1656. doi: 10.1016/j.biotechadv.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 140.Kim D.H., Bang E., Jung H.J., Noh S.G., Yu B.P., Choi Y.J., Chung H.Y. Anti-Aging Effects of Calorie Restriction (CR) and CR Mimetics Based on the Senoinflammation Concept. Nutrients. 2020;12:422. doi: 10.3390/nu12020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hrelia P., Sita G., Ziche M., Ristori E., Marino A., Cordaro M., Molteni R., Spero V., Malaguti M., Morroni F., et al. Common Protective Strategies in Neurodegenerative Disease: Focusing on Risk Factors to Target the Cellular Redox System. Oxidative Med. Cell. Longev. 2020;2020:8363245. doi: 10.1155/2020/8363245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moro-García M.A., Mayo J.C., Sainz R.M., Alonso-Arias R. Influence of Inflammation in the Process of T Lymphocyte Differentiation: Proliferative, Metabolic, and Oxidative Changes. Front. Immunol. 2018;9:339. doi: 10.3389/fimmu.2018.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Naismith E., Pangrazzi L. The Impact of Oxidative Stress, Inflammation, and Senescence on the Maintenance of Immunological Memory in the Bone Marrow in Old Age. Biosci. Rep. 2019;39:BSR20190371. doi: 10.1042/BSR20190371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mechta M., Ingerslev L.R., Fabre O., Picard M., Barrès R. Evidence Suggesting Absence of Mitochondrial DNA Methylation. Front. Genet. 2017;8:166. doi: 10.3389/fgene.2017.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mankan A.K., Schmidt T., Chauhan D., Goldeck M., Höning K., Gaidt M., Kubarenko A.V., Andreeva L., Hopfner K.-P., Hornung V. Cytosolic RNA:DNA Hybrids Activate the CGAS-STING Axis. EMBO J. 2014;33:2937–2946. doi: 10.15252/embj.201488726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Collins L.V., Hajizadeh S., Holme E., Jonsson I.-M., Tarkowski A. Endogenously Oxidized Mitochondrial DNA Induces in Vivo and in Vitro Inflammatory Responses. J. Leukoc. Biol. 2004;75:995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- 147.Moya G.E., Rivera P.D., Dittenhafer-Reed K.E. Evidence for the Role of Mitochondrial DNA Release in the Inflammatory Response in Neurological Disorders. Int. J. Mol. Sci. 2021;22:7030. doi: 10.3390/ijms22137030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.De Gaetano A., Solodka K., Zanini G., Selleri V., Mattioli A.V., Nasi M., Pinti M. Molecular Mechanisms of MtDNA-Mediated Inflammation. Cells. 2021;10:2898. doi: 10.3390/cells10112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Burns A., Iliffe S. Alzheimer’s Disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- 150.Butterfield D.A., Boyd-Kimball D. Redox Proteomics and Amyloid Β-peptide: Insights into Alzheimer Disease. J. Neurochem. 2019;151:459–487. doi: 10.1111/jnc.14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hopperton K.E., Mohammad D., Trépanier M.O., Giuliano V., Bazinet R.P. Markers of Microglia in Post-Mortem Brain Samples from Patients with Alzheimer’s Disease: A Systematic Review. Mol. Psychiatry. 2018;23:177–198. doi: 10.1038/mp.2017.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Almanan M., Raynor J., Ogunsulire I., Malyshkina A., Mukherjee S., Hummel S.A., Ingram J.T., Saini A., Xie M.M., Alenghat T., et al. IL-10–Producing Tfh Cells Accumulate with Age and Link Inflammation with Age-Related Immune Suppression. Sci. Adv. 2020;6:eabb0806. doi: 10.1126/sciadv.abb0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Amin J., Boche D., Clough Z., Teeling J., Williams A., Gao Y., Chudley L., Lau L., Smith F., Harris S., et al. Peripheral Immunophenotype in Dementia with Lewy Bodies and Alzheimer’s Disease: An Observational Clinical Study. J. Neurol. Neurosurg. Psychiatry. 2020;91:1219–1226. doi: 10.1136/jnnp-2020-323603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pradeepkiran J.A., Reddy P.H. Defective Mitophagy in Alzheimer’s Disease. Ageing Res. Rev. 2020;64:101191. doi: 10.1016/j.arr.2020.101191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He Z., Yang Y., Xing Z., Zuo Z., Wang R., Gu H., Qi F., Yao Z. Intraperitoneal Injection of IFN-γ Restores Microglial Autophagy, Promotes Amyloid-β Clearance and Improves Cognition in APP/PS1 Mice. Cell Death Dis. 2020;11:440. doi: 10.1038/s41419-020-2644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Magalhães T.N.C., Weiler M., Teixeira C.V.L., Hayata T., Moraes A.S., Boldrini V.O., dos Santos L.M., de Campos B.M., de Rezende T.J.R., Joaquim H.P.G., et al. Systemic Inflammation and Multimodal Biomarkers in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease. Mol. Neurobiol. 2018;55:5689–5697. doi: 10.1007/s12035-017-0795-9. [DOI] [PubMed] [Google Scholar]
- 157.Wharton W., Kollhoff A.L., Gangishetti U., Verble D.D., Upadhya S., Zetterberg H., Kumar V., Watts K.D., Kippels A.J., Gearing M., et al. Interleukin 9 Alterations Linked to Alzheimer Disease in African Americans. Ann. Neurol. 2019;86:407–418. doi: 10.1002/ana.25543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cisbani G., Koppel A., Knezevic D., Suridjan I., Mizrahi R., Bazinet R.P. Peripheral Cytokine and Fatty Acid Associations with Neuroinflammation in AD and AMCI Patients: An Exploratory Study. Brain Behav. Immun. 2020;87:679–688. doi: 10.1016/j.bbi.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 159.Porro C., Cianciulli A., Panaro M.A. The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules. 2020;10:1017. doi: 10.3390/biom10071017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liang C.-S., Su K.-P., Tsai C.-L., Lee J.-T., Chu C.-S., Yeh T.-C., Su M.-W., Lin G.-Y., Lin Y.-K., Chu H.-T., et al. The Role of Interleukin-33 in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. Alzheimer’s Res. Ther. 2020;12:86. doi: 10.1186/s13195-020-00652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fang E.F., Hou Y., Palikaras K., Adriaanse B.A., Kerr J.S., Yang B., Lautrup S., Hasan-Olive M.M., Caponio D., Dan X., et al. Mitophagy Inhibits Amyloid-β and Tau Pathology and Reverses Cognitive Deficits in Models of Alzheimer’s Disease. Nat. Neurosci. 2019;22:401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Manczak M., Kandimalla R., Yin X., Reddy P.H. Hippocampal Mutant APP and Amyloid Beta-Induced Cognitive Decline, Dendritic Spine Loss, Defective Autophagy, Mitophagy and Mitochondrial Abnormalities in a Mouse Model of Alzheimer’s Disease. Hum. Mol. Genet. 2018;27:1332–1342. doi: 10.1093/hmg/ddy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leal N.S., Dentoni G., Schreiner B., Naia L., Piras A., Graff C., Cattaneo A., Meli G., Hamasaki M., Nilsson P., et al. Amyloid β-Peptide Increases Mitochondria-Endoplasmic Reticulum Contact Altering Mitochondrial Function and Autophagosome Formation in Alzheimer’s Disease-Related Models. Cells. 2020;9:2552. doi: 10.3390/cells9122552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reddy P.H., Yin X., Manczak M., Kumar S., Pradeepkiran J.A., Vijayan M., Reddy A.P. Mutant APP and Amyloid Beta-Induced Defective Autophagy, Mitophagy, Mitochondrial Structural and Functional Changes and Synaptic Damage in Hippocampal Neurons from Alzheimer’s Disease. Hum. Mol. Genet. 2018;27:2502–2516. doi: 10.1093/hmg/ddy154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Parkinson’s Disease Information Page, National Institute of Neurological Disorders and Stroke. Web Resourse. [(accessed on 2 December 2021)]; Available online: https://www.ninds.nih.gov/Disorders/All-Disorders/Parkinsons-Disease-Information-Page.
- 166.Sveinbjornsdottir S. The Clinical Symptoms of Parkinson’s Disease. J. Neurochem. 2016;139:318–324. doi: 10.1111/jnc.13691. [DOI] [PubMed] [Google Scholar]
- 167.Shulman J.M., De Jager P.L., Feany M.B. Parkinson’s Disease: Genetics and Pathogenesis. Annu. Rev. Pathol. Mech. Dis. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 168.Clark E.H., Vázquez de la Torre A., Hoshikawa T., Briston T. Targeting Mitophagy in Parkinson’s Disease. J. Biol. Chem. 2021;296:100209. doi: 10.1074/jbc.REV120.014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lin T.-K., Lin K.-J., Lin K.-L., Liou C.-W., Chen S.-D., Chuang Y.-C., Wang P.-W., Chuang J.-H., Wang T.-J. When Friendship Turns Sour: Effective Communication Between Mitochondria and Intracellular Organelles in Parkinson’s Disease. Front. Cell Dev. Biol. 2020;8:607392. doi: 10.3389/fcell.2020.607392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Malpartida A.B., Williamson M., Narendra D.P., Wade-Martins R., Ryan B.J. Mitochondrial Dysfunction and Mitophagy in Parkinson’s Disease: From Mechanism to Therapy. Trends Biochem. Sci. 2020;46:329–343. doi: 10.1016/j.tibs.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 171.Borsche M., König I.R., Delcambre S., Petrucci S., Balck A., Brüggemann N., Zimprich A., Wasner K., Pereira S.L., Avenali M., et al. Mitochondrial Damage-Associated Inflammation Highlights Biomarkers in PRKN/PINK1 Parkinsonism. Brain. 2020;143:3041–3051. doi: 10.1093/brain/awaa246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhi L., Qin Q., Muqeem T., Seifert E.L., Liu W., Zheng S., Li C., Zhang H. Loss of PINK1 Causes Age-Dependent Decrease of Dopamine Release and Mitochondrial Dysfunction. Neurobiol. Aging. 2019;75:1–10. doi: 10.1016/j.neurobiolaging.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu Y., Lear T.B., Verma M., Wang K.Z.Q., Otero P.A., McKelvey A.C., Dunn S.R., Steer E., Bateman N.W., Wu C., et al. Chemical Inhibition of FBXO7 Reduces Inflammation and Confers Neuroprotection by Stabilizing the Mitochondrial Kinase PINK1. JCI Insight. 2020;5:e131834. doi: 10.1172/jci.insight.131834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Igarashi R., Yamashita S., Yamashita T., Inoue K., Fukuda T., Fukuchi T., Kanki T. Gemcitabine Induces Parkin-Independent Mitophagy through Mitochondrial-Resident E3 Ligase MUL1-Mediated Stabilization of PINK1. Sci. Rep. 2020;10:1465. doi: 10.1038/s41598-020-58315-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ingram T.L., Shephard F., Sarmad S., Ortori C.A., Barrett D.A., Chakrabarti L. Sex Specific Inflammatory Profiles of Cerebellar Mitochondria Are Attenuated in Parkinson’s Disease. Aging. 2020;12:17713–17737. doi: 10.18632/aging.103937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xia S., Zhang X., Zheng S., Khanabdali R., Kalionis B., Wu J., Wan W., Tai X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016;2016:8426874. doi: 10.1155/2016/8426874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.