Abstract
WRKYs, a large family of transcription factors, are involved in plant response to biotic and abiotic stresses, but the role of them in tomato resistance to Oidium neolycopersici is still unclear. In this study, we evaluate the role of WRKYs in powdery mildew-resistant wild tomato (Solanum habrochaites) LA1777 defense against O. neolycopersici strain lz (On-lz) using a combination of omics, classical plant pathology- and cell biology-based approaches. A total of 27 WRKYs, belonging to group I, II, and III, were identified as differentially expressed genes in LA1777 against On-lz. It was found that expression of ShWRKY41 was increased after Pseudomonas syringae pv. tomato (Pst) DC3000, On-lz and Botrytis cinerea B05 inoculation or ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) treatment. GUS staining of ShWRKY41 promoter indicated that the expression of ShWRKY41 could be induced by SA and ethylene. Furthermore, ShWRKY41 gene silencing reduced the resistance to On-lz infection by decreasing the generation of H2O2 and HR in LA1777 seedlings. Overall, our research suggests that ShWRKY41 plays a positive role in defense activation and host resistance to O. neolycopersici in wild tomato (S. habrochaites) LA1777.
Keywords: tomato powdery mildew, Oidium neolycopersici, WRKYs, resistance
1. Introduction
Tomato powdery mildew, mainly caused by the obligate biotrophic fungus Oidium neolycopersici [1], is a fungal disease that commonly occurs in tomato leaves, characterized by chlorosis, white plaque and occasional necrosis [2,3]. Generally, this disease causes up to 50% yield (fruit) losses in heavily infected fields. Lack of resistant cultivars makes tomato powdery mildew become the limiting factors in tomato production [4]. Fortunately, S. habrochaites, a wild tomato germplasm resource and closely related to tomato cultivar S. lycopersicum, has a high resistance to the powdery mildew [5]. After O. neolycopersici infection, defense responses, including increased production of reactive oxygen species (ROS) and hypersensitive response (HR) are activated in resistant wild tomato S. habrochaites or S. hirsutum, but not in the susceptible S. lycopersicum cultivar tomato [5,6,7]. In wild tomato, some alleles have been identified as resistance genes, including Ol-1, ol-2, Ol-3, Ol-4, Ol-5, Ol-6, etc., the mechanisms of which are similar to the MLO gene in barley [5,6,7,8]. And ShARPC3, encoding a subunit protein of the actin-related protein 2/3 complex, is involved in resistance to O. neolycopersici in wild tomato S. habrochaites LA1777 by regulating the induction of hypersensitive cell death and the generation of reactive oxygen [9]. Also, ShORR-1 (S. habrochaites Oidium Resistance Required-1), identified from S. habrochaites G1.1560, enhances host resistance to O. neolycopersici by effecting the extensive hydrogen peroxide accumulation and formation of abnormal haustoria [10], but the role of WRKYs in wild tomato S. habrochaites LA1777-O. neolycopersici interaction is still unclear.
WRKYs are a large family of transcription factors (TF) that seem to have originated in early eukaryotes [11]. As transcription factors, WRKYs are involved in plant responses to biotic and abiotic stresses [12,13,14,15,16] by altering the expression of other genes. SWEET POTATO FACTOR1 (SPF1) is the first WRKY protein, which is identified from sweet potato in 1994 and binds to the SP8a (ACTGTGTA) and SP8b (TACTATT) sequences of target genes [17]. Usually, WRKY proteins contain WRKY domain, which is a DNA-binding region of approximately 60 amino acids in length and comprises the conserved sequence motif WRKY adjacent to a novel zinc-finger motif. To date, lots of WRKY genes have been identified from Arabidopsis [18], tomato [19,20], rice [21], wheat [22], tobacco [23], etc. The WRKYs are divided into three groups, based on the number of WRKY domains and the types of zinc-finger-like motifs [24].
Functional redundancy among WRKY family members is a hindrance to linking specific WRKY TFs to plant defenses [25]. Some WRKYs directly/indirectly interact with pathogen-associated molecular patterns (PAMPs) or effector proteins to activate or suppress PAMP-triggered immunity (PTI) and/or effector-triggered immunity (ETI) [26], which are two tiers in plant defense responses against invading pathogens [27,28]. In Arabidopsis, WRKY18, WRKY40, and WRKY60 redundantly and cooperatively function as negative regulators of PTI to Pseudomonas syringae [29] and HvWRKY2 (Hv, Hordeum vulgare) also functions as a negative regulator of PTI to powdery mildew fungus Golovinomyces orontii by interacting with barley mildew A (MLA) R protein [30]. In rice, OsWRKY62 gene functions as a negative regulator of innate immunity and serves as a critical mediator of both basal and race-specific defense responses [31]. Meanwhile, some WRKYs work as a positive regulator to participate in plant resistance against phytopathogens. In rice (Oryza sativa), OsWRKY13 [32], OsWRKY45 [33], OsWRKY53 [34], and OsWRKY67 [35] defend against invasion of different pathogens by activating or suppressing the expression of diverse downstream targeting genes to finally moderate plant physiological performance, alter plant phytohormone balance, or modify plant transcriptomic network.
In tomato, a total of 83 WRKYs are identified [20], some of which function as positive regulators of plant responses to biotic stresses, e.g., SlWRKY31 [36], SlWRKY33 [37], SlWRKY39 [38]. Lots of WRKY genes have been identified from tomato-pathogens interaction: SlWRKY45 enhancing tomato susceptibility to the root-knot nematode Meloidogyne javanica [39], SlWRKY39 enhancing resistance to P. syringae [38], and SlWRKY33 enhancing resistance to hemi-biotrophic oomycete Phytophthora infestans, etc. However, there is a little knowledge about the function of WRKYs in wild tomato S. habrochaites LA1777 defense against O. neolycopersici. An understanding the role of WRKYs between wild tomato LA1777 and O. neolycopersici will provide a foundation for tomato anti-disease and breeding. Here, we reveal the expression patterns of WRKY genes in wild tomato LA1777, a powdery mildew resistance tomato, under O. neolycopersici strain lz infection. And further studies find that ShWRKY41, induced by ethylene, is positively related to wild tomato LA1777 defense against On-lz.
2. Results
2.1. A Total of 27 WRKYs Are Differentially Expressed in LA1777 under On-lz Infection
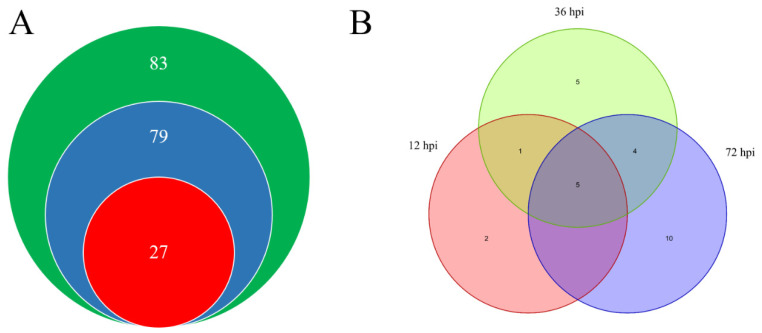
A total of 83 WRKYs were identified in S. lycopersicum in previous research [19,20]. In this study, 69 transcripts were mapped to WRKY genes (Supplementary Table S1). With the criteria of padj (the adjusted p value) < 0.05 and |log2FoldChange| > 1, a total of 27 WRKYs were identified as differentially expressed genes (DEGs) (Figure 1A) in wild tomato S. habrochaites LA1777 under On-lz infection. We found that eight WRKYs were differentially expressed in LA1777 at 12 hpi, and 15 WRKYs were differentially expressed in LA1777 at 36 hpi, and 19 WRKYs were differentially expressed in LA1777 at 72 hpi (Figure 1B). With progress of infection, the number of differentially expressed WRKY genes increased.
Figure 1.
The differentially expressed WRKY genes in LA1777 under On-lz infection. In figure (A), red color represents the differentially expressed WRKY genes, and blue color represents the WRKY transcripts in LA1777 under On-lz infection and green color represents the WRKY genes in S. lycopersicum genome. (B) The distribution of differentially expressed WRKY genes at different time points.
2.2. Categorization and Function Analysis of Differentially Expressed WRKYs in Wild Tomato LA1777 under On-lz Infection
According to a former study [40], the 27 differentially expressed WRKY genes were classified as I, II-a, II-c, II-d, II-e, and III group (Table 1) based on the sequence variation among WRKY proteins.
Table 1.
Categorization of differentially expressed ShWRKYs under On-lz infection in wild tomato LA1777.
| Gene_Description | Group | log2FoldChange | padj | |
|---|---|---|---|---|
| 12 hpi | ShWRKY44 | I | 4.99 | 0.000017 |
| ShWRKY1 | I | 2.58 | 0.000000 | |
| ShWRKY40 | II-a | 2.57 | 0.000442 | |
| ShWRKY51 | II-c | −1.83 | 0.000173 | |
| ShWRKY IId-1 | II-d | 1.46 | 0.010183 | |
| ShWRKY22-like | II-e | 1.90 | 0.021111 | |
| ShWRKY29 | II-e | 1.64 | 0.000160 | |
| ShWRKY41 | III | 2.37 | 0.000000 | |
| 36 hpi | ShWRKY44 | I | 5.73 | 0.007329 |
| ShWRKY33B | I | 2.71 | 0.000026 | |
| ShWRKY1 | I | 1.66 | 0.003228 | |
| ShWRKY3 | I | −1.33 | 0.047367 | |
| ShWRKY45 | II-a | 4.45 | 0.011781 | |
| ShWRKY40 | II-a | 2.76 | 0.000469 | |
| ShWRKY9 | II-b | 5.18 | 0.029976 | |
| ShWRKY30 | II-c | 2.72 | 0.009789 | |
| ShWRKY30-like | II-c | 1.48 | 0.029717 | |
| ShWRKY IId-1 | II-d | 1.51 | 0.013714 | |
| ShWRKY22-like | II-e | 2.06 | 0.004722 | |
| ShWRKY26 | II-e | 1.75 | 0.029760 | |
| ShWRKY53 | III | 2.49 | 0.000276 | |
| ShWRKY53 | III | 2.26 | 0.000432 | |
| ShWRKY41 | III | 2.10 | 0.000002 | |
| 72 hpi | ShWRKY33B | I | 3.13 | 0.000000 |
| ShWRKY1 | I | 3.04 | 0.000000 | |
| ShWRKY31 | I | 1.76 | 0.000000 | |
| ShWRKY14 | I | −2.69 | 0.038904 | |
| ShWRKY40 | II-a | 4.54 | 0.000000 | |
| ShWRKY48 | II-c | 2.28 | 0.000019 | |
| ShWRKY23 | II-c | 1.68 | 0.001020 | |
| ShWRKY56 | II-c | 5.45 | 0.009433 | |
| ShWRKY75 | II-c | −1.57 | 0.048565 | |
| ShWRKY IId-1 | II-d | 2.28 | 0.000000 | |
| ShWRKY7 | II-d | 1.44 | 0.000000 | |
| ShWRKYIId-4 | II-d | 1.20 | 0.003540 | |
| ShWRKY21 | II-d | −1.14 | 0.025213 | |
| ShWRKY22-like | II-e | 3.26 | 0.000000 | |
| ShWRKY26 | II-e | 3.12 | 0.000000 | |
| ShWRKY70 | II-e | 1.55 | 0.000000 | |
| ShWRKY41 | III | 3.06 | 0.000000 | |
| ShWRKY53 | III | 3.00 | 0.000000 | |
| ShWRKY53 | III | 2.11 | 0.000000 |
Note: padj is the adjusted p value using the Benjamini and Hochberg’s approach.
At 12 hpi, eight WRKYs were identified as DEGs, and the most were up-regulated except ShWRKY51. ShWRKY44 and ShWRKY1 that formed group I, and the others (ShWRKY40, ShWRKY51, ShWRKY IId-1, ShWRKY22-like, ShWRKY29, and ShWRKY41) belonged to groups II-a, II -c, II -d, II –e, II –e, and III, respectively. At 36 hpi, the number of WRKY DEGs was increased to 15. Like to 12 hpi, only ShWRKY3, belonging to group I, was a down-regulated DEG, and the others were up-regulated DEGs. At 72 hpi, the number of WRKY DEGs was up to 19. Meanwhile, we found three down-regulated DEGs: ShWRKY14 (group I), ShWRKY75 (group II -c), and ShWRKY21 (group II -d).
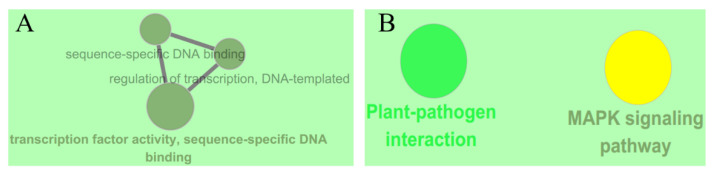
To know the function of the differentially expressed WRKYs, all of those WRKYs were analyzed using ClueGO in Cytoscope. As Figure 2A shown, like other WRKYs, the molecular function of wild tomato ShWRKYs was transcription factor activity, sequence-specific DNA binding. And KEGG analysis (Figure 2B) demonstrated that differentially expressed WRKYs were significantly enriched to MAPK signal and plant-pathogen interaction pathway.
Figure 2.
The function analysis of differentially expressed WRKYs in wild tomato S. habrochaites LA1777 under On-lz infection. (A) The result of GO enrichment on molecular function term, and (B) The result of KEGG enrichment. The GO and KEGG enrichment were both analyzed in Cytoscope using ClueGO app.
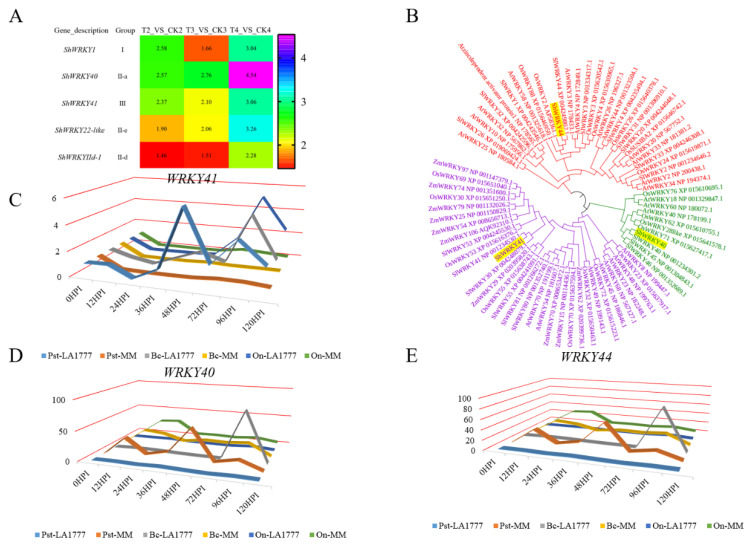
Among those DEGs, five WRKYs (ShWRKY1, ShWRKY40, ShWRKY41, ShWRKY22-like, and ShWRKY IId-1) were identified as simultaneous genes at three time points, and all of them were up-regulated (Figure 3A), and ShWRKY44 had highest expression level at 12 and 36 hpi (4.99 and 5.73, respectively), in wild tomato LA1777 under On-lz infection. Group I ShWRKY44, group II ShWRKY40, and group III ShWRKY41 were selected as a representative of each of three groups for a further analysis. The result of phylogenetic analysis (Figure 3B), based on the amino acid sequence, showed that ShWRKY40, ShWRKY41, and ShWRKY44 were classified to different cluster. ShWRKY40, SlWRKY40/45, OsWRKY71, AtWRKY40, etc. formed one cluster, and ShWRKY41, SlWRKY41, OsWRKY53, etc. formed one cluster, and ShWRKY44, SlWRKY44, OsWRKY88, etc. formed one cluster. The result of categorization and phylogenetic analysis indicated that ShWRKY40, ShWRKY41, and ShWRKY44 belonged to different WRKY groups.
Figure 3.
The screening and identification of WRKY41. (A) The co-expressed WRKY genes among the RNA-seq analysis. (B) The result of phylogenetic analysis of WRKY40, WRKY41 and WRKY44 using NJ method with JTT model. (C–E) The result of WRKY40, WRKY41 and WRKY44 relative expression levels in wild tomato LA1777 or cultivar tomato MM under Pst DC3000, On-lz and B. cinerea infection using qRT-PCR.
The expression levels of ShWRKY40, ShWRKY41, and ShWRKY44 under biotrophic pathogens (On-lz and Pst DC3000) and nectrophic pathogen B. cinerea B05 were analyzed. The results (Figure 3C–E) showed that ShWRKY41, different from ShWRKY40 and ShWRKY44, had higher expression levels in wild tomato LA1777, but lower expression levels in cultivar tomato MM, under On-lz, Pst DC3000, and B. cinerea B05 infections.
Based on the above result, we found that ShWRKY41, a member of group III, had high expression levels in wild tomato LA1777 under biotrophic and nectrophic pathogens. So, the molecular function of ShWRKY41 was further studied.
2.3. The Molecular Function Analysis of ShWRKY41
The amino acid sequence of ShWRKY41 was used for STRING analysis based on the Solanum lycopersicum database. We found that ShWRKY41 putatively interacted with pathogen-related protein, PR1 protein and MLO-like protein 6 (Figure S1A), which indicated ShWRKY41 was involved in disease resistance in wild tomato LA1777. While, the accumulation of mRNAs, coding the putative interacted proteins, was analyzed using RNA-seq data. Our data showed that they were mainly down-regulated genes (Figure S1C), but the expression of them were not found to be statistically significant at the level padj < 0.05 except PR1 at 72 hpi (marked with T4). The result of Pfam analysis (Figure S1B) showed that ShWRKY41 contained one WRKY domain (137–197 aa).
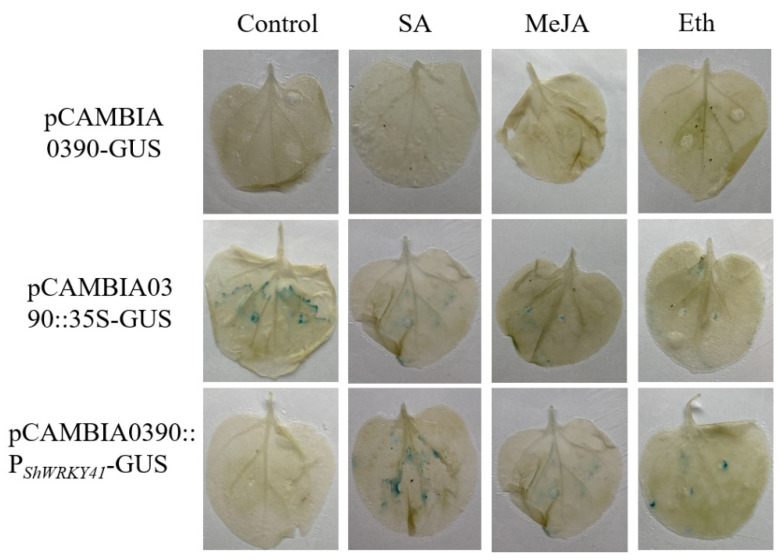
To know more about the expression activity of ShWRKY41, a promoter analysis was analyzed by PlantCARE and Softberry. The cis-acting elements, involved in the SA, MeJA, or Eth responsiveness, were not found in the promoter of ShWRKY41. GUS assay was employed to reveal promoter responsiveness, and as shown in Figure 4, after SA or Eth treatment, GUS activity was highly induced in pCAMBIA0390:: PShWRKY41-GUS, which indicated that the promoter fusions were responsive to SA, MeJA, and ethylene precursor ACC treatment.
Figure 4.
The GUS analysis of wild tomato ShWRKY41 promoter. Histochemical GUS assay was performed in the transient expression N. benthamiana, which were cultivated in a 22 °C chamber with 16 h light/8 h dark cycle for 2 days after the treatment with 100 mM MeJA, 10 mM SA, 0.5 mM ACC (1-aminocyclopropanecarboxylic acid, the ethylene precursor) (Sigma, Shanghai, China), and water (Control), respectively.
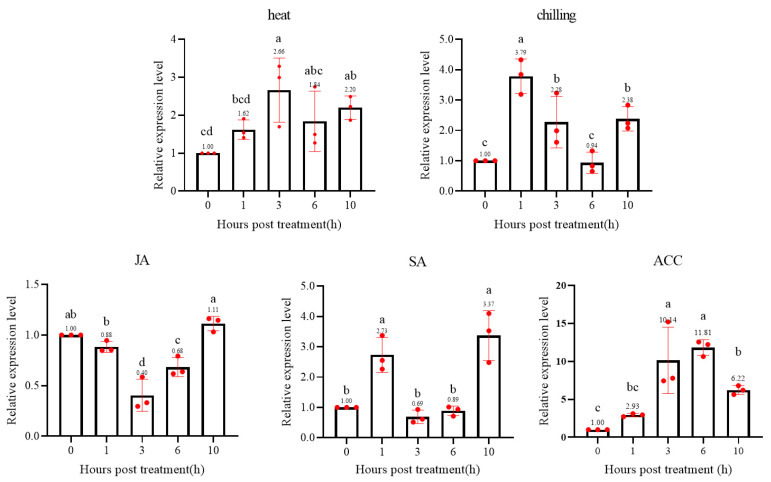
Then, the expression pattern was analyzed using qRT-PCR. As the Figure 5 shown, the expression levels of wild tomato ShWRKY41 was increased after heat (40 °C), chilling (8 °C), SA, and ACC treatments, but decreased after JA treatment (Figure 6). The expression patterns of wild tomato ShWRKY41 after heat and ACC treatments were similar, both of which were continuously increased. Meanwhile, the expression patterns of ShWRKY41 after chilling and SA treatments were similar, both of which had a high expression level at 1 and 10 hpi. While, the expression of wild tomato ShWRKY41 was reduced after JA treatment, down to 0.40 at 3 hpi, and increased back up to 1.11 at 10 hpi.
Figure 5.
The expression of ShWRKY41 in wild tomato S. habrochaites LA1777 under abiotic stresses. ‘heat’ means a 40 °C high temperature stress, and ‘chilling’ represents a 8 °C low temperature stress. ‘JA’, ‘SA’, and ‘ACC’ mean plant hormones stresses. The different lowercase letters (e.g., a, b, c, etc.) represent the significance at p = 0.05 level, and if there are one or more same letters between different groups (e.g., ab, bc, abc, etc.), it means there is a difference between those groups but not significant.
Figure 6.
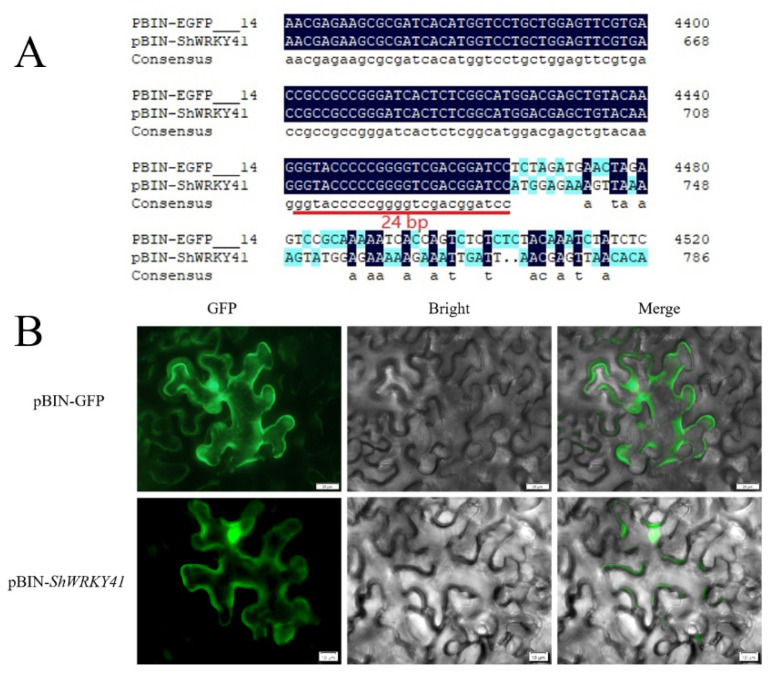
ShWRKY41 located in plasma membrane and nucleus. (A) The sequence analysis of pBIN-ShWRKY41 vector by Sanger sequencing. The 24 bp gap means that the GFP coding sequence and CDS of ShWRKY41 did not exist frameshift mutation. (B) Images were collected by confocal microscopy at 24 h post pBIN-ShWRKY41 inoculation. Green fluorescent protein (GFP) signal was visualized by confocal microscopy.
By in silico analysis, it was predicted that wild tomato ShWRKY41 was primarily located in the plant plasma membrane and nucleus. To validate this prediction, a 1010 bp fragment of ShWRKY41 was cloned into the binary expression vector pBIN-EGFP. After validation using Sanger sequencing (Figure 6A), the recombinant vector was transformed into A. tumefaciens strain GV3101 and transiently expressed in N. benthamiana. As shown in Figure 6B, at 24 h post-co-infiltration, pBIN-ShWRKY41 expressed in plasma membrane and nucleus in tobacco cell, which was similar with the control infiltration (i.e., pBIN-EGFP). This result confirmed the prediction of localization pattern.
2.4. ShWRKY41 Silencing Results in Host Susceptibility to On-lz in Wild Tomato LA1777 Seedlings
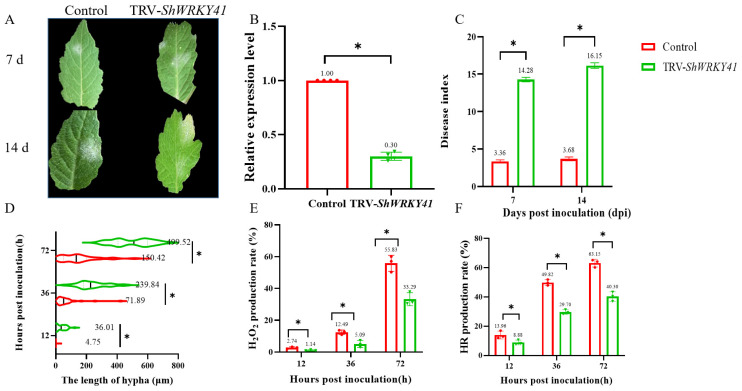
To evaluate the role of ShWRKY41 during the interaction between wild tomato S. habrochaites LA1777 seedlings and On-lz, tobacco rattle virus-based virus-induced gene silencing (TRV-VIGS) was used to silence ShWRKY41 in wild tomato LA1777 seedlings. TRV1, TRV2, TRV2-SlPDS (phytoene desaturase), and TRV2-ShWRKY41 plasmids were transformed into A. tumefaciens strain GV3101, respectively, to silence the target genes in wild tomato LA1777 seedlings. To evaluate the efficiency of the TRV-based approach, TRV2-SlPDS was monitored for the induction of photobleaching. Five weeks after inoculation, a photobleaching phenotype was observed, indicating the success of technical efficiency of TRV-VIGS-mediated gene silencing. The result of qRT-PCR showed that the expression of ShWRKY41 was 0.30 in the TRV2-ShWRKY41 silenced seedlings, which indicated a high degree of silencing efficiency (Figure 7B). In parallel to the analysis of phenotypes, all TRV-VIGS seedlings were inoculated with On-lz and the infection phenotypes were recorded. Compared with control plants, plants carrying TRV2-ShWRKY41 appeared obvious powdery mildew disease lesions (Figure 7A). The disease index values of ShWRKY41 silenced seedlings (7 dpi: 14.28 and 14 dpi: 16.15) were significantly higher than control seedlings (7 dpi: 3.36 and 14 dpi: 3.68) (Figure 7C).
Figure 7.
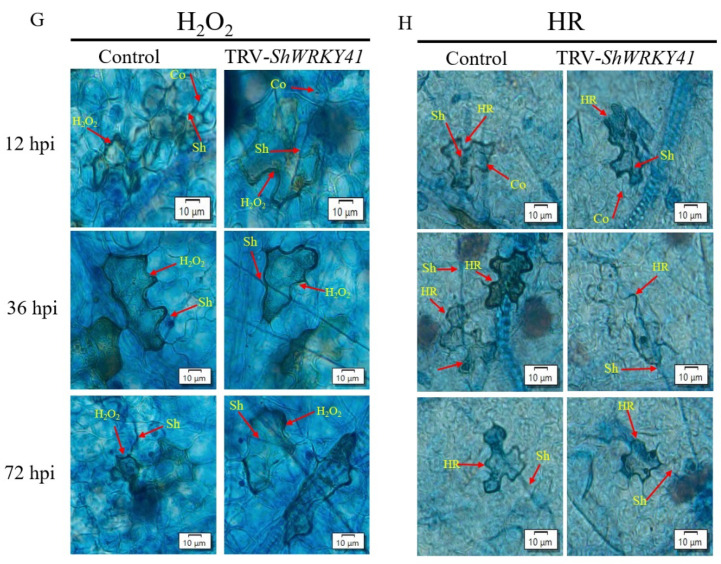
The silence of ShWRKY41 reduces the resistance of wild tomato LA1777 against On-lz. In the figure, the red legend represents the data from tomato LA1777 seedlings and the green legend represents the data from ShWRKY41-silenced LA1777 seedlings. (A) Phenotype of TRV (control) or TRV-ShWRKY41-silenced seedings under On-lz infection at 7&14-day-post-inoculation. (B) The efficiency of ShWRKY41 silencing in LA1777 seedlings. (C) The quantification of disease TRV-ShWRKY41-silenced or control LA1777 seedlings at 7- and 14-day-post-inoculation with On-lz. (D) The statistics of O. neolycopersici hypha on ShWRKY41-silenced or control LA1777 seedlings. (E) The H2O2 production rate in ShWRKY41-silenced or control LA1777 seedlings under On-lz infection. (F) The HR induction rate in ShWRKY41 silenced or control LA1777 seedlings under On-lz infection. The asterisk indicates statistically significant differences at level α = 0.05 with Student’s t-test between control and TRV-ShWRKY41-silenced wild tomato LA1777 seedlings. (G,H) The microscopic detection of H2O2 and HR accumulations at interaction sites of O. neolycopersici with control and silenced ShWRKY41, respectively. Co, conidium; Sh, secondary hyphae. Bar, 10 mm.
Histological observations showed that the growth of On-lz conidia on the ShWRKY41 silenced seedlings was faster than that on the control seedlings. At 12 hpi, the hypha length showed that the average of hypha length was 36.01 μm on the ShWRKY41 silenced seedlings, which was significantly higher than 4.75 μm on the control seedlings. At 36 hpi, the average of hypha length on the ShWRKY41 silenced seedlings was 239.84 μm, while the average of hypha length on the control seedlings was only 71.89 μm. At 72 hpi, the length of On-lz hyphae on the control seedlings was 150.42 μm, significantly lower than 499.52 μm on the ShWRKY41 silenced seedlings (Figure 7D).
To further demonstrate the function of ShWRKY41 in wild tomato LA1777 resistance to On-lz infection, we evaluated the induction of early defense signaling processes, such as the accumulation of H2O2 and the induction of hypersensitive response (HR). In the case of H2O2 response signaling (Figure 7E,G), at 12 hpi, 2.74% of the infection sites from control seedlings produced H2O2, which was higher than that of ShWRKY41-silenced seedlings. At 36 hpi, in control seedlings, the production of H2O2 was observed to be 12.49%, which was significantly higher than that of ShWRKY41-silenced seedlings (p value = 0.05). At 72 hpi, control seedlings produced more H2O2 (55.83%) than ShWRKY41-silenced seedlings (33.29%). A similar trend was observed for the induction of the HR, which revealed that ShWRKY41 silencing reduced the induction of HR (Figure 7F,H). At 12 hpi, the rate of HR induction was 13.96% in control seedlings, which was higher than 8.88% in ShWRKY41-silenced seedlings. At 36 hpi, in ShWRKY41-silenced seedlings, the rate of HR induction was 49.82%, which was significantly higher than 29.70% in ShWRKY41-silenced seedlings. At 72 hpi, control seedlings were induced more HR (63.15%) than ShWRKY41-silenced seedlings (40.30%).
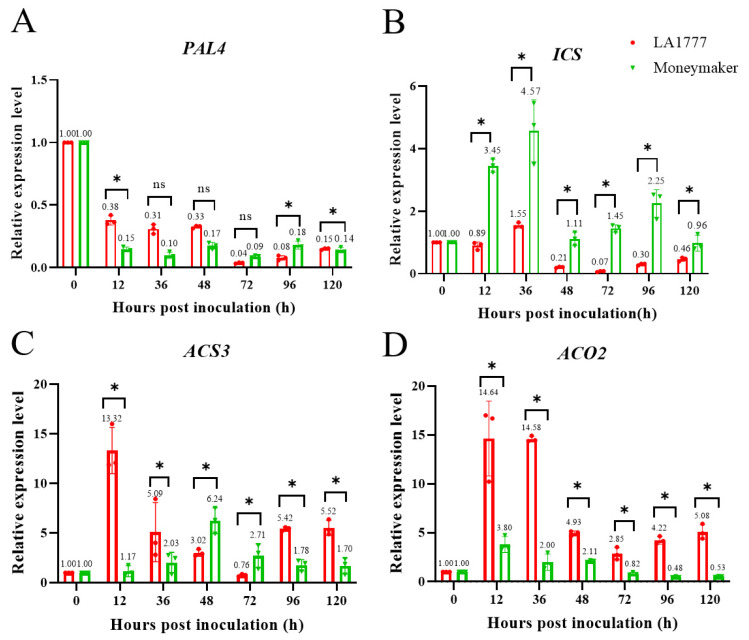
Based on the above result, we found that ShWRKY41, which could be induced by SA and Eth, was involved in wild tomato LA1777 defense against On-lz. To know which plant hormone was involved in LA1777 against On-lz, genes, PAL4 and ICS, which were related to SA biosynthesis, ACS3 and ACO2, which were related to Eth biosynthesis, were analyzed using qRT-PCR. We found that the expression levels of PAL4 and ICS were decreased in wild tomato LA1777 under On-lz infection (Figure 8A,B), while the expression levels of ACS3 and ACO2 were increased in wild tomato LA1777 under On-lz infection (Figure 8C,D). This result indicated that Eth was involved in wild tomato LA1777 defense against On-lz, instead of SA. So, the gene ShWRKY41 was induced by Eth in the process of wild tomato LA1777 against On-lz.
Figure 8.
The expression of PAL4 and ICS are suppressed, but the expression of ACS3 and ACO2 are activated in wild tomato LA1777 under On-lz infection. In the figure, the red legend represents the data from tomato LA1777 seedlings and the green legend represents the data from cultivar tomato MM. (A) The expression level of PAL4. (B) The expression level of ICS. (C) The expression level of ACS3. (D) The expression level of ACO2. The asterisk indicates statistically significant differences and ‘ns’ indicates no statistically significant differences at level α = 0.05 with Student’s t-test between wild tomato LA1777 and cultivar tomato MM seedlings.
3. Discussion
Oidium neolycopersici, a biotrophic fungus, is the main pathogen responsible for tomato powdery mildew [1]. A lack of resistant cultivars makes tomato powdery mildew become the limiting factor in tomato production [4]. Fortunately, previous research has led to the discovery of wild germplasm resources, e.g., Solanum habrochaites, which have resistance to O. neolycopersici infection [5], and our former studies found that ShARP3 and ShNPSN11 are involved in resistance to powdery mildew in wild tomato S. habrochaites LA1777 [14,20]. Nevertheless, what the function of WRKYs in the crosstalk between resistant wild tomato LA1777 and O. neolycopersici is unclear. WRKYs, as a large family of transcription factors, functionally connect and form a transcriptional network, then involves in plant immune responses through the regulation of transcriptional reprogramming [18,25,26]. In this study, we used next generation sequencing (NGS) technology to illuminate the possible role of WRKYs in LA1777 against O. neolycopersici strain lz (On-lz). A total of 27 WRKYs were identified as differentially expressed genes with padj ≤ 0.05 and |fold-change| ≥1. These genes were mainly enriched to the transcription factor activity, sequence-specific DNA binding at GO term of molecular function and enriched to MAPK signal pathway and plant-pathogen interaction in KEGG analysis.
WRKYs are categorized into group I, II, and III based on the number of WRKY domains and the type of zinc-finger-like motif [24]. In this study, the differentially expressed genes were distributed into tomato WRKY group I, II and III (Table 1). Among them, 18 differentially expressed WRKYs belonged to group II, accounting for 66.67%, and six differentially expressed WRKYs belonged to group I, accounting for 22.22%, and there are only three differentially expressed genes in group III, accounting for 11.11%. We also found three down-regulated expressed genes, WRKY21, WRKY51, and WRKY75, belonged to II group, and two down-regulated expressed genes, WRKY3 and WRKY14, belonged to I group.
Some studies have revealed that WRKY75, a member of group II, is involved in response to necrotrophic pathogens infection and abiotic stresses [41,42], and WRKY3, a member of group I, is involved in resistance to Phytophthora infestans infection and salt stress tolerance [43,44], in tomato. In Arabidopsis, the mutation of AtWRKY18, AtWRKY40 and AtWRKY60, members of the WRKY II subfamily, result in increased resistance to P. syringae and increased susceptibility to B. cinerea through inducing the expression of SA–regulated PR1 and JA-regulated PDF1.2 [29]. The result above suggest that members of this subfamily may work as negative regulators in plant defense biotrophic pathogens. While the other 22 WRKYs had high expression levels, five of them (Figure 3A), including WRKY1, WRKY40, WRKY41, WRKY22-like, and WRKYIId-1, always had high expression levels at 12, 36, and 72 hpi in wild tomato LA1777 under On-lz infection conditions. Of them, WRKY1 had been identified as a positive regulator in tomato P. infestans interaction [45,46]. We also found that WRKY44 had highest expression levels at 12 and 36 h after On-lz inoculation.
Next, three highly expressed WRKYs—ShWRKY40, ShWRKY41, and ShWRKY44—belonging to the group II, III, and I, respectively, were selected for further analysis. Based on the amino acid sequence, we found that ShWRKY40 had a high sequence similarity to SlWRKY40/45, rice OsWRKY71, and Arabidopsis AtWRKY40, and ShWRKY41 had a high sequence similarity to SlWRKY41, and OsWRKY53, and ShWRKY44 had a high sequence similarity to SlWRKY44, and OsWRKY88, indicating that ShWRKY40, ShWRKY41, and ShWRKY44 belonged to different WRKY groups (Figure 3B). And we also analyzed the expression levels of ShWRKY40, ShWRKY41, and ShWRKY44 in wild tomato LA1777 and cultivar tomato MM under Pst DC 3000, On-lz, or B. cinerea B05 infection. The result indicated that the transcription of ShWRKY41 was activated by those three pathogens, which revealed that ShWRKY41 may be a positive regulator in wild tomato LA1777 response to different pathogens’ infection. OsWRKY53, having a high sequence similarity to ShWRKY41, has a functional diversity in rice, by activating or suppressing the expression of diverse downstream targeting genes to finally moderate plant physiological performance and interfere plant balance [34,47,48,49,50,51]. Phytohormones play a critical role in plant response to pathogens infection, and the biosynthesis of SA, Eth and JA is triggered after pathogens infection and then these hormones lead to activation of downstream signaling in cells producing them. Based on the previous reports, during powdery mildew resistance, the wild tomato resistance gene Ol-1 and Ol-qtls need ET to generate cell death, and JA deficiency can compromise resistance mediated by ol-2, whereas Ol-4-mediated resistance depends on SA [5,6,7,8]. In this study, we found that expression of ShWRKY41 was induced by plant hormones SA and Eth (Figure 5 and Figure 6) using GUS-promoter assay and qRT-PCR analysis, two major hormones involved in response to different stresses in plant [52].
Normally, to respond to biotic or abiotic stresses, plant hormones change the transcription level of related genes through transcription factors, e.g., WRKY transcription factors. Ectopic overexpression of Capsicum annum WRKY27 confers resistance to Ralstonia solanacearum in Nicotiana tabacum through modulation of SA-, JA- and ET-mediated signaling pathways [53]. MusaWRKY18, from banana, PMusaWRKY18-β-D-glucuronidase (GUS) assay reveals that the expression of MusaWRKY18 is strongly induced after application of abscisic acid, SA, methyl jasmonate, and ethephon, which indicates that plant hormones SA and Eth can induce the expression of MusaWRKY18 [54]. MiWRKY53, identified from mulberry (Morus indica var. K2) and induced by SA, functions as a positive regulator of plant defense response through SA-mediated mechanisms [55]. In this study, to know which of them, SA and Eth, could induce the expression of ShWRKY41, and was involved in wild tomato LA1777 against On-lz, the expression level of genes, PAL4 and ICS, coding the key enzymes of SA biosynthesis, ACS3 and ACO2, coding the key enzymes of Eth biosynthesis, were analyzed using qRT-PCR. And the result (Figure 8) showed that ACS3 and ACO2 had high expression levels in wild tomato LA1777 under On-lz infection. Oppositely, PAL4 and ICS had low expression levels. So, we inferred that Eth was involved in the resistance of wild tomato LA1777 against On-lz.
Some new studies reveal that Eth is involved in plant defense responses to biotrophic pathogens. During plant-biotrophic pathogen interactions, Eth has been demonstrated to be involved in Arabidopsis resistance to powdery mildew by feedback-attenuated RPW8.1-mediated cell death and disease resistance [56]. In contrast, Eth is needed in Triticum urartu resistance to Blumeria graminis f. sp. tritici (Bgt) infection because the expression of TuACO3, positively related to Eth biosynthesis, is induced by Bgt infection and accompanied by increased Eth content. TuACO3-silenced decreases Eth production and wheat resistance to Bgt, but both processes are enhanced in the overexpressed TuACO3 wheat [57]. Similar to this, Eth biosynthesis-related genes had high expression levels in wild tomato S. habrochaites LA1777 under On-lz infection. Normally, ethylene could induce the generation of ROS by modulating the activity of NADPH oxidase-dependent H2O2 synthesis and a high ROS level could induce HR, in plant tissues [58,59]. Plants, resistance to biotrophic pathogens, e.g., O. neolycopersici, is closely related to the H2O2 accumulation and generation of HR triggered by the fungal haustoria [7]. In the study of tomato powdery mildew resistance, more HR and H2O2 are found in resistant tomatoes, including wild tomato S. habrochaites G1.1560 and S. habrochaites LA1777, compared to the susceptible S. lycopersicum MM tomato cultivar [9,10,60]. If the generation of HR and H2O2 is reduced, the resistance to biotrophic pathogens will be decreased [28,61]. Herein, we found that the silencing of ShWRKY41 decreased the resistance to On-lz in wild tomato S. habrochaites LA1777 seedlings through reducing the production of H2O2 and HR (Figure 7), so the resistance, induced by Eth, was related to the ability of Eth-induced H2O2 generation. In conclusion, herein we reveal that ShWRKY41 was involved in wild tomato S. habrochaites LA1777 defense against On-lz by regulating the generation of H2O2 and HR.
4. Materials and Methods
4.1. Plant Materials and Plant, Pathogen Growth, and Inoculation Experiments
Oidium neolycopersici strain lz (On-lz) was propagated and preserved according to the previously reported method [9,60]. Wild tomato S. habrochaites LA1777, obtained from the Tomato Genetics Resource Center (Department of Plant Sciences, University of California, Davis, CA, USA), and tomato Moneymaker (MM) (S. lycopersicum), were used in this research. Wild tomato S. habrochaites LA1777 is highly resistant to On-lz, while tomato Moneymaker is highly susceptible to On-lz. For germination and growth, tomato seeds were surface sterilized according to the method of the formers [60].
Nicotiana benthamiana seedlings were grown in a growth chamber at 20 °C under a 16 h light/8 h dark cycle with 60% relative humidity and a light intensity of 120-mmol photons m−2 s−1.
Escherichia coli strain DH5α was grown at 37 °C on Luria-Bertani (LB) medium containing antibiotics. Agrobacterium tumefaciens strain GV3101 harboring binary vector constructs was grown on antibiotic-containing LB media at 28 °C.
For pathogen inoculation assays, the conidia of On-lz were sprayed onto 8-day-old plants with a suspension of 105 spores mL−1 according to the method of Zheng et al. [62]. Spore counts were quantified using a hemocytometer. Inoculated tomato seedlings were grown in environmentally controlled growth chambers under the same conditions as described above.
4.2. Sample Collection, Library Preparation and Transcriptome Sequencing
Fresh conidia were inoculated on wild tomato S. habrochaites LA1777 at 5-true-leaf stage by spraying spore suspension as described above. H2O was used as the control treatment. Based on the result of former study, the wild tomato LA1777 leaf tissues samples of 12 hpi, 36 hpi, and 72 hpi, collected from LA1777 under On-lz stress or control conditions, were used to RNA sequencing. Total RNA was extracted from the above samples by using the BioZol reagent (Bioer Technology, Hangzhou, China). RNAs were purified and used to construct sequencing libraries. The libraries were paired-end sequenced on the Illumina HiSeq platform. The library construction and sequencing were carried out at the Novogene Bioinformatics Technology Co., Ltd. (Beijing, China). And the raw data of RNA-seq was available at NCBI with the BioProject ID PRJNA769495.
4.3. Bioinformatics Analysis of WRKYs
Raw reads were processed by filtering out sequencing adapters, short-fragment reads, and other low-quality reads with FastQC v0.11.8 [63], Trimmomatic 0.38 [64] and FastUniq 1.1 [65]. Index of the reference genome S. lycopersicum Solyc2.50 (ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/188/115/GCF_000188115.3_SL2.50/ (accessed on 20 December 2017)) was built and paired-end clean reads were aligned to the reference genome by using Hisat2 v2.0.5 [66]. featureCounts v1.5.0-p3 [67] was used to count the reads numbers mapped to each gene, and then FPKM of each gene was calculated based on the length of the gene and reads count mapped to this gene. DESeq2 R package (1.16.1) [68] was used to identify the differentially expressed genes (DEGs). The resulting p values were adjusted using the Benjamini and Hochberg’s approach, called padj, for controlling the false discovery rate. The genes with padj ≤ 0.05 and |fold-change| ≥1 were identified as DEGs. And then the WRKY genes were obtained with perl scripts.
The predictions of expression-pattern and subcellular location were carried out in ProtParam (https://web.expasy.org/protparam/ (accessed on 21 November 2020)), Uniprot (https://www.uniprot.org/ (accessed on 21 November 2020)), and ProtComp (http://linux1.softberry.com/berry.phtml?topic=protcomppl&group=programs&subgroup=proloc (accessed on 21 November 2020)). The domains of ShWRKY proteins were checked with SMART database (http://smart.embl-heidelberg.de/smart/batch.pl (accessed on 21 November 2020)) [69]. The putative interacted proteins of ShWRKY41 was predicated by STRING with Solanum lycopersicum data [70].
4.4. The Expression Analysis of Target WRKYs
The expression levels of ShWRKY40, ShWRKY41, and ShWRKY44 under biotrophic pathogens On-lz, Pseudomonas syringae pv. tomato (Pst) DC3000 and nectrophic pathogen Botrytis cinerea B05 infections were analyzed using qRT-PCR. On-lz was inoculated with the above method, and Pst DC3000 and B. cinerea B05 were inoculated according to Iwaseer et al. [71] and Lian et al. [72], respectively. All the samples were collected at 0 h, 12 h, 24 h, 36 h, 48 h, 72 h, 96 h, and 120 h post inoculation. Total RNA was extracted according to the above descriptions. Complementary DNA (cDNA) synthesis was performed using a PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer’ s instructions and the generation was diluted 10-fold. DNA primers for qRT-PCR (Supplementary Table S2) were designed using Beacon Designer 7.7 (Premier Biosoft, Palo Alto, CA, USA). PCR reaction components and cycling parameters were same with our former description [60]. mRNA expression values were calculated using the 2–ΔΔCT method [73], using GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE (SlGAPDH) as an internal control.
4.5. The Promoter Analysis of ShWRKY41
Firstly, the promoter of ShWRKY41 (PShWRKY41) (0_to_−2000) was analyzed by PlantCARE and Softberry. Next, the promoter of ShWRKY41 was cloned into the expression vector pCAMBIA0390-GUS using the DNA primers PShWRKY41-F (5′-tggctgcaggtcgacggatccCCCTCAACCTATGTCCGAAATC-3′) and PShWRKY41-R (5′-tcttagaattcccggggatccGGCTATACCCTTCACCCTCTG-3′), and the recombinant vector was transformed into Agrobacterium strain GV3101 for transient expression [74]. Agrobacterium-mediated transient assay was performed on the 4-week-old N. benthamiana leaves, according to the former method. In brief, the N. benthamiana for transient expression were cultivated in a 22 °C chamber with 16 h light/8 h dark cycle for 2 days before the treatment with 100 mM MeJA, 10 mM SA, 0.5 mM ACC (Sigma, Shanghai, China) (1-aminocyclopropanecarboxylic acid, the ethylene precursor and after application, ACC is immediately converted to ethylene by ACC oxidases (ACOs) in plant cells [75]), and water (Control), respectively. All treatments were three replicates, each of which contained three seedlings. After 48 h treatments, the tobacco leaves were collected for detection of GUS activity. Histochemical GUS assay was performed according to the procedure of Jefferson [76].
4.6. Expression Analysis of ShWRKY41 in Wild Tomato LA1777 under Abiotic Stresses
For the evaluation of ShWRKY41 mRNA accumulation under abiotic stresses, 2-week-old wild tomato LA1777 seedlings were used. The samples were collected at 0, 1, 3, 6, and 10 h post treatment, including heat (40 °C), chilling (8 °C), 1 mM SA (salicylic acid), 100 μM MeJA (methyl jasmonate), and 0.5 mM ACC (1-aminocyclopropanecarboxylic acid, the ethylene precursor). Total RNA, cDNA, and qRT-PCR analysis were same with the above descriptions. DNA primers for qRT-PCR (Supplementary Table S2) were designed using Beacon Designer 7.7.
4.7. TRV Vectors Construction and Plant Transformation
Virus-induced gene silencing (VIGS) vectors were constructed using tobacco rattle virus (TRV1 and TRV2). The region of target gene for genome-wide off-target gene silencing was selected using SGN VIGS Tool [77]. A 257 bp fragment of ShWRKY41, containing a BamHI restriction enzyme site, was amplified from the wild tomato LA1777 cDNA and cloned into the expression vector pTRV2 via forward primer (5′- agaaggcctccatggggatccATCCCTAAAGCAACTTCACTTG-3′) and reverse primer (5′- cgtgagctcggtaccggatccATTCCTCAGGAGAAGCTAATGG-3′) according to the method of Senthil-Kumar et al. [78]. After verification using Sanger sequence, the recombinant plasmid was extracted using the E.Z.N.A. Plasmid DNA Mini Kit (Omega Bio-tek, Inc, Doraville, GA) and transformed into A. tumefaciens strain GV3101 using the heat shock method [79]. A. tumefaciens srtain GV3101 carrying pTRV2 or pTRV2 derivatives, were cultured and infiltrated as previously described [9,60]. Following inoculation, seedlings were transferred to an environmentally controlled growth chamber (25 °C, 16-h light/8-h dark photoperiod). A. tumefaciens strain GV3101 carrying pTRV2-PDS, constructed before, was used in this study. Photobleaching symptoms in the PDS-silenced seedlings were observed at approximately 35 days after inoculation.
4.8. Subcellular Localization Analysis
The full-length cDNA of ShWRKY41 was cloned into the binary vector pBIN-EGFP (harboring GFP label) via BamH I restriction enzyme digestion followed by ligation of gene-specific DNA primers (Supplementary Table S2). The resultant expression plasmid was transformed into A. tumefaciens strain GV3101. A. tumefaciens harboring pBIN-ShWRKY41 was cultured and centrifuged according to the former report [9,60]. Leaves of N. benthamiana were inoculated with strains containing recombinant pBIN-ShWRKY41 or the empty vector pBIN-EGFP. GFP fluorescence was detected using a FV1000 confocal microscope (Olympus GmbH, Hamburg, Germany) equipped with a 488 nm filter. The experiment was repeated three times.
4.9. Fungal Biomass Analysis and Quantification of Disease Severity
For each experiment, two subsets of plants were maintained from each treatment (i.e., TRV2, TRV2-SlPDS, or TRV2-ShWRKY41). After 35 days inoculation, samples were collected from TRV2, TRV2-SlPDS, or TRV2-ShWRKY41 silenced seedlings. Total RNA, synthesis of cDNA was performed according to the above descriptions. Silencing efficiency was evaluated by qRT-PCR using gene-specific primers (Supplementary Table S2). In parallel to sample for mRNA analysis, samples were collected at 3-time points (12, 36, and 72 hpi) for histological observation. Disease severity was assessed by the former description with 0–9 disease rating scale [80]: 0 = no disease symptoms; 1 = 0–5% of leaves having disease symptoms; 3 = leaves with infection lesions comprising up to 6–10% of the total leaf surface; 5 = leaves with infection lesions up to 11–20% of the total leaf surface; 7 = leaves with infection lesions up to 21–40% of the total leaf surface; and 9 = leaves with infection lesions up to 41–100% of the total leaf surface.
Disease severity indices were calculated using the following equation: Disease index (DI) = [Σ (number of diseased plant leaves at a given disease severity × the disease severity)/(total plant leaves analyzed × 9)] × 100. An average DI was calculated at three independent time points for each infected plant.
To quantify the accumulation of H2O2 (H2O2 production rate = H2O2 numbers per 100 penetration site) and the induction of HR cell death (HR production rate = HR numbers per 100 penetration site) during On-lz infection, the 3,3-diaminobenzidine (DAB; AMERCO, Solon, OH, USA) and trypan blue staining methods were used, respectively [61,81]. In brief, samples collected from 5-time points (12, 36, and 72 hpi) were cut into 2–3 cm2 segments without the edge and main vein, and then stained as previous description [61,81]. In addition, the samples, staining with DAB, were re-staining with 5% Coomassie brilliant blue G-250 for infection structures observation. At least 30 penetration sites on each of four-leaf samples were observed at each time point. Statistical significance was assessed by a Student’s t-test (α = 0.05.) using the SPSS software 20.0 (IBM, New York, NY, USA).
4.10. The expression Analysis of Plant Hormone Biosynthesis Related Genes
To know which hormone involved in wild tomato S. habrochaites LA1777 against On-lz, based on the result of ShWRKY41 expression analysis and GUS-promoter assay, PLA4, ICS, ACS3, and ACO2 were chosen to monitor the hormone biosynthesis process. PLA4 and ICS, coding protein and conferring 10% and 90% SA biosynthesis in plant [82,83], are the key genes in SA biosynthesis. And the products of ACS3 and ACO2 are the key enzymes of Eth biosynthesis [84,85,86]. The samples were collected from wild tomato LA1777 and cultivar tomato MM after On-lz inoculation 0, 12, 36, 48, 72, 96, and 120 h. And total RNA, cDNA and qRT-PCR were carried out according to the above description.
4.11. Data Collection and Analysis
All experiments were performed in triplicate and at least 30 penetration sites were scored by microscopy at each time point. Statistical analyses were carried out using the IBM SPSS statistics software package (version 20.0). Comparisons between control samples and each treatment were evaluated using a Student’s t-test at a significance level of α = 0.05.
Acknowledgments
The authors would like to acknowledge Tomato Genetics Resource Center for offering the seeds of wild tomato S. habrochaites LA1777. We thank Novogene Bioinformatics Technology Co., Ltd. for offering transcriptome sequencing service.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms23031267/s1.
Author Contributions
Q.L., X.H., B.Z. and Q.M. performed the experiments and analyzed data. Y.W. and Q.M. guided the operation of the experiment. X.H. assisted with writing. Q.L. and Q.M. were major contributors in writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key R&D Project of Shaanxi Province (grant no. 2019ZDLNY03-07).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the reported results can be found in the supplementary materials file.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kiss L. Identification of two powdery mildew fungi, Oidium neolycopersici sp. nov. and an Oidium subgenus Reticuloidium, infecting tomato in different parts of the world. Mycol. Res. 2001;105:684–697. doi: 10.1017/S0953756201004105. [DOI] [Google Scholar]
- 2.Whipps J.M., Budge S.P., Fenlon J.S. Characteristics and host range of tomato powdery mildew. Plant Pathol. 1998;47:36–48. doi: 10.1046/j.1365-3059.1998.00207.x. [DOI] [Google Scholar]
- 3.Jones H., Whipps J.M., Gurr S.J. The tomato powdery mildew fungus Oidium neolycopersici. Mol. Plant Pathol. 2001;2:303–309. doi: 10.1046/j.1464-6722.2001.00084.x. [DOI] [PubMed] [Google Scholar]
- 4.Jankovics T., Bai Y., Kovács G.M., Bardin M., Nicot P.C., Toyoda H., Matsuda Y., Niks R.E., Kiss L. Oidium neolycopersici: Intraspecific variability inferred from amplified fragment length polymorphism analysis and relationship with closely related powdery mildew fungi infecting various plant species. Phytopathology. 2008;98:529–540. doi: 10.1094/PHYTO-98-5-0529. [DOI] [PubMed] [Google Scholar]
- 5.Lindhout P., Beek H., Pet G. Wild Lycopersicon Species as Sources for Resistance to Powdery Mildew (Oidium lycopersicum): Mapping of the Resistance Gene OL-1 on Chromosome 6 of L. hirsutum. International Society for Horticultural Science (ISHS); Leuven, Belgium: 1994. pp. 387–394. [Google Scholar]
- 6.Bai Y., Huang C.C., van der Hulst R., Meijer-Dekens F., Bonnema G., Lindhout P. QTLs for tomato powdery mildew resistance (Oidium lycopersici) in Lycopersicon parviflorum G1.1601 co-localize with two qualitative powdery mildew resistance genes. Mol. Plant-Microbe Interact. 2003;16:169–176. doi: 10.1094/MPMI.2003.16.2.169. [DOI] [PubMed] [Google Scholar]
- 7.Bai Y., van der Hulst R., Bonnema G., Marcel T.C., Meijer-Dekens F., Niks R.E., Lindhout P. Tomato defense to Oidium neolycopersici: Dominant Ol genes confer isolate-dependent resistance via a different mechanism than recessive ol-2. Mol. Plant-Microbe Interact. 2005;18:354–362. doi: 10.1094/MPMI-18-0354. [DOI] [PubMed] [Google Scholar]
- 8.Huang C.C., Cui Y.Y., Weng C.R., Zabel P., Lindhout P. Development of diagnostic PCR markers closely linked to the tomato powdery mildew resistance gene Ol-1 on chromosome 6 of tomato. Theor. Appl. Genet. 2000;101:918–924. doi: 10.1007/s001220051562. [DOI] [Google Scholar]
- 9.Sun G., Feng C., Guo J., Zhang A., Xu Y., Wang Y., Day B., Ma Q. The tomato Arp2/3 complex is required for resistance to the powdery mildew fungus Oidium neolycopersici. Plant Cell Environ. 2019;42:2664–2680. doi: 10.1111/pce.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Xu K., Pei D., Yu D., Zhang J., Li X., Chen G., Yang H., Zhou W., Li C. ShORR-1, a novel tomato gene, confers enhanced host resistance to Oidium neolycopersici. Front. Plant Sci. 2019;10:1400. doi: 10.3389/fpls.2019.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulker B., Somssich I.E. WRKY transcription factors: From DNA binding towards biological function. Curr. Opin. Plant Biol. 2004;7:491–498. doi: 10.1016/j.pbi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Poll A.A., Lee J., Sanderson R.A., Byrne E., Gatehouse J.A., Sadanandom A., Gatehouse A.M.R., Edwards M.G. Septoria leaf blotch and reduced nitrogen availability alter WRKY transcription factor expression in a codependent manner. Int. J. Mol. Sci. 2020;21:4165. doi: 10.3390/ijms21114165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosado D., Ackermann A., Spassibojko O., Rossi M., Pedmale U.V. WRKY transcription factors and ethylene signaling modify root growth during the shade avoidance response. Plant Physiol. 2021;2021:kiab493. doi: 10.1093/plphys/kiab493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y., Zheng W., Li J., Liu P., Zhong K., Jin P., Xu M., Yang J., Chen J. NbWRKY40 positively regulates the response of Nicotiana benthamiana to Tomato Mosaic Virus via salicylic acid signaling. Front. Plant Sci. 2020;11:603518. doi: 10.3389/fpls.2020.603518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahammed G.J., Li X., Mao Q., Wan H., Zhou G., Cheng Y. The SlWRKY81 transcription factor inhibits stomatal closure by attenuating nitric oxide accumulation in the guard cells of tomato under drought. Physiol. Plant. 2021;172:885–895. doi: 10.1111/ppl.13243. [DOI] [PubMed] [Google Scholar]
- 16.Shu P., Zhang S., Li Y., Wang X., Yao L., Sheng J., Shen L. Over-expression of SlWRKY46 in tomato plants increases susceptibility to Botrytis cinerea by modulating ROS homeostasis and SA and JA signaling pathways. Plant Physiol. Biochem. 2021;166:1–9. doi: 10.1016/j.plaphy.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Ishiguro S., Nakamura K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol. Gen. Genet. 1994;244:563–571. doi: 10.1007/BF00282746. [DOI] [PubMed] [Google Scholar]
- 18.Eulgem T., Rushton P.J., Robatzek S., Somssich I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/S1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y., Li M.-Y., Wu P., Xu Z.-S., Que F., Wang F., Xiong A.-S. Members of WRKY Group III transcription factors are important in TYLCV defense signaling pathway in tomato (Solanum lycopersicum) BMC Genom. 2016;17:788. doi: 10.1186/s12864-016-3123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karkute S.G., Gujjar R.S., Rai A., Akhtar M., Singh M., Singh B. Genome wide expression analysis of WRKY genes in tomato (Solanum lycopersicum) under drought stress. Plant Gene. 2018;13:8–17. doi: 10.1016/j.plgene.2017.11.002. [DOI] [Google Scholar]
- 21.Abdullah-Zawawi M.R., Ahmad-Nizammuddin N.F., Govender N., Harun S., Mohd-Assaad N., Mohamed-Hussein Z.A. Comparative genome-wide analysis of WRKY, MADS-box and MYB transcription factor families in Arabidopsis and rice. Sci. Rep. 2021;11:19678. doi: 10.1038/s41598-021-99206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye H., Qiao L., Guo H., Guo L., Ren F., Bai J., Wang Y. Genome-wide identification of wheat WRKY gene family reveals that TaWRKY75-A is referred to drought and salt resistances. Front. Plant Sci. 2021;12:663118. doi: 10.3389/fpls.2021.663118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos R.N., Martin G.B., Pombo M.A., Rosli H.G. WRKY22 and WRKY25 transcription factors are positive regulators of defense responses in Nicotiana benthamiana. Plant Mol. Biol. 2021;105:65–82. doi: 10.1007/s11103-020-01069-w. [DOI] [PubMed] [Google Scholar]
- 24.Rinerson C.I., Rabara R.C., Tripathi P., Shen Q.J., Rushton P.J. The evolution of WRKY transcription factors. BMC Plant Biol. 2015;15:66. doi: 10.1186/s12870-015-0456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eulgem T., Somssich I.E. Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 2007;10:366–371. doi: 10.1016/j.pbi.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Bakshi M., Oelmüller R. WRKY transcription factors: Jack of many trades in plants. Plant Signal. Behav. 2014;9:e27700. doi: 10.4161/psb.27700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J.M., Zhang Y. Plant immunity: Danger perception and signaling. Cell. 2020;181:978–989. doi: 10.1016/j.cell.2020.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Xu X., Chen C., Fan B., Chen Z. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell. 2006;18:1310–1326. doi: 10.1105/tpc.105.037523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen Q.H., Saijo Y., Mauch S., Biskup C., Bieri S., Keller B., Seki H., Ulker B., Somssich I.E., Schulze-Lefert P. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science. 2007;315:1098–1103. doi: 10.1126/science.1136372. [DOI] [PubMed] [Google Scholar]
- 31.Peng Y., Bartley L.E., Chen X., Dardick C., Chern M., Ruan R., Canlas P.E., Ronald P.C. OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol. Plant. 2008;1:446–458. doi: 10.1093/mp/ssn024. [DOI] [PubMed] [Google Scholar]
- 32.Qiu D., Xiao J., Ding X., Xiong M., Cai M., Cao Y., Li X., Xu C., Wang S. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant-Microbe Interact. 2007;20:492–499. doi: 10.1094/MPMI-20-5-0492. [DOI] [PubMed] [Google Scholar]
- 33.Shimono M., Sugano S., Nakayama A., Jiang C.J., Ono K., Toki S., Takatsuji H. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell. 2007;19:2064–2076. doi: 10.1105/tpc.106.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chujo T., Takai R., Akimoto-Tomiyama C., Ando S., Minami E., Nagamura Y., Kaku H., Shibuya N., Yasuda M., Nakashita H., et al. Involvement of the elicitor-induced gene OsWRKY53 in the expression of defense-related genes in rice. Biochim. Biophys. Acta. 2007;1769:497–505. doi: 10.1016/j.bbaexp.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q., Li X., Yan S., Yu T., Yang J., Dong J., Zhang S., Zhao J., Yang T., Mao X., et al. OsWRKY67 positively regulates blast and bacteria blight resistance by direct activation of PR genes in rice. BMC Plant Biol. 2018;18:257. doi: 10.1186/s12870-018-1479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B., Hong Y.B., Zhang Y.F., Li X.H., Huang L., Zhang H.J., Li D.Y., Song F.M. Tomato WRKY transcriptional factor SlDRW1 is required for disease resistance against Botrytis cinerea and tolerance to oxidative stress. Plant Sci. Int. J. Exp. Plant Biol. 2014;227:145–156. doi: 10.1016/j.plantsci.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J., Wang J., Zheng Z., Fan B., Yu J.Q., Chen Z. Characterization of the promoter and extended C-terminal domain of Arabidopsis WRKY33 and functional analysis of tomato WRKY33 homologues in plant stress responses. J. Exp. Bot. 2015;66:4567–4583. doi: 10.1093/jxb/erv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang S., Gao Y., Liu J., Peng X., Niu X., Fei Z., Cao S., Liu Y. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genom. 2012;287:495–513. doi: 10.1007/s00438-012-0696-6. [DOI] [PubMed] [Google Scholar]
- 39.Chinnapandi B., Bucki P., Braun Miyara S. SlWRKY45, nematode-responsive tomato WRKY gene, enhances susceptibility to the root knot nematode M. javanica infection. Plant Signal. Behav. 2017;12:e1356530. doi: 10.1080/15592324.2017.1356530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akbudak M.A., Filiz E. Genome-wide investigation of proline transporter (ProT) gene family in tomato: Bioinformatics and expression analyses in response to drought stress. Plant Physiol. Biochem. 2020;157:13–22. doi: 10.1016/j.plaphy.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Finiti I., Leyva M.d.l.O., Vicedo B., Gómez-Pastor R., López-Cruz J., García-Agustín P., Real M.D., González-Bosch C. Hexanoic acid protects tomato plants against Botrytis cinerea by priming defence responses and reducing oxidative stress. Mol. Plant Pathol. 2014;15:550–562. doi: 10.1111/mpp.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.López-Galiano M.J., González-Hernández A.I., Crespo-Salvador O., Rausell C., Real M.D., Escamilla M., Camañes G., García-Agustín P., González-Bosch C., García-Robles I. Epigenetic regulation of the expression of WRKY75 transcription factor in response to biotic and abiotic stresses in Solanaceae plants. Plant Cell Rep. 2018;37:167–176. doi: 10.1007/s00299-017-2219-8. [DOI] [PubMed] [Google Scholar]
- 43.Hichri I., Muhovski Y., Žižková E., Dobrev P.I., Gharbi E., Franco-Zorrilla J.M., Lopez-Vidriero I., Solano R., Clippe A., Errachid A., et al. The Solanum lycopersicum WRKY3 Transcription Factor SlWRKY3 is involved in salt stress tolerance in tomato. Front. Plant Sci. 2017;8:1343. doi: 10.3389/fpls.2017.01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui J., Xu P., Meng J., Li J., Jiang N., Luan Y. Transcriptome signatures of tomato leaf induced by Phytophthora infestans and functional identification of transcription factor SpWRKY3. Theor. Appl. Genet. 2018;131:787–800. doi: 10.1007/s00122-017-3035-9. [DOI] [PubMed] [Google Scholar]
- 45.Li J.-B., Luan Y.-S., Liu Z. SpWRKY1 mediates resistance to Phytophthora infestans and tolerance to salt and drought stress by modulating reactive oxygen species homeostasis and expression of defense-related genes in tomato. Plant Cell Tissue Organ Cult. (PCTOC) 2015;123:67–81. doi: 10.1007/s11240-015-0815-2. [DOI] [Google Scholar]
- 46.Cui J., Jiang N., Meng J., Yang G., Liu W., Zhou X., Ma N., Hou X., Luan Y. LncRNA33732-respiratory burst oxidase module associated with WRKY1 in tomato-Phytophthora infestans interactions. Plant J. Cell Mol. Biol. 2019;97:933–946. doi: 10.1111/tpj.14173. [DOI] [PubMed] [Google Scholar]
- 47.Van Eck L., Davidson R.M., Wu S., Zhao B.Y., Botha A.M., Leach J.E., Lapitan N.L. The transcriptional network of WRKY53 in cereals links oxidative responses to biotic and abiotic stress inputs. Funct. Integr. Genom. 2014;14:351–362. doi: 10.1007/s10142-014-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoo S.J., Kim S.H., Kim M.J., Ryu C.M., Kim Y.C., Cho B.H., Yang K.Y. Involvement of the OsMKK4-OsMPK1 cascade and its downstream transcription factor OsWRKY53 in the wounding response in Rice. Plant Pathol. J. 2014;30:168–177. doi: 10.5423/PPJ.OA.10.2013.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chujo T., Miyamoto K., Ogawa S., Masuda Y., Shimizu T., Kishi-Kaboshi M., Takahashi A., Nishizawa Y., Minami E., Nojiri H., et al. Overexpression of phosphomimic mutated OsWRKY53 leads to enhanced blast resistance in rice. PLoS ONE. 2014;9:e98737. doi: 10.1371/journal.pone.0098737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu L., Ye M., Li R., Zhang T., Zhou G., Wang Q., Lu J., Lou Y. The rice transcription factor WRKY53 suppresses herbivore-induced defenses by acting as a negative feedback modulator of mitogen-activated protein kinase activity. Plant Physiol. 2015;169:2907–2921. doi: 10.1104/pp.15.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian X., Li X., Zhou W., Ren Y., Wang Z., Liu Z., Tang J., Tong H., Fang J., Bu Q. Transcription factor OsWRKY53 positively regulates brassinosteroid signaling and plant architecture. Plant Physiol. 2017;175:1337–1349. doi: 10.1104/pp.17.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L., Song Y., Li S., Zhang L., Zou C., Yu D. The role of WRKY transcription factors in plant abiotic stresses. Biochim. Biophys. Acta. 2012;1819:120–128. doi: 10.1016/j.bbagrm.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Dang F., Wang Y., She J., Lei Y., Liu Z., Eulgem T., Lai Y., Lin J., Yu L., Lei D., et al. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection. Physiol. Plant. 2014;150:397–411. doi: 10.1111/ppl.12093. [DOI] [PubMed] [Google Scholar]
- 54.Tak H., Negi S., Ganapathi T.R. The 5′-upstream region of WRKY18 transcription factor from banana is a stress-inducible promoter with strong expression in guard cells. Physiol. Plant. 2021;173:1335–1350. doi: 10.1111/ppl.13326. [DOI] [PubMed] [Google Scholar]
- 55.Negi N., Khurana P. A salicylic acid inducible mulberry WRKY transcription factor, MiWRKY53 is involved in plant defence response. Plant Cell Rep. 2021;40:2151–2171. doi: 10.1007/s00299-021-02710-8. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Z.X., Feng Q., Liu P.Q., He X.R., Zhao J.H., Xu Y.J., Zhang L.L., Huang Y.Y., Zhao J.Q., Fan J., et al. RPW8.1 enhances the ethylene-signaling pathway to feedback-attenuate its mediated cell death and disease resistance in Arabidopsis. New Phytol. 2021;229:516–531. doi: 10.1111/nph.16857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng H., Dong L., Han X., Jin H., Yin C., Han Y., Li B., Qin H., Zhang J., Shen Q., et al. The TuMYB46L-TuACO3 module regulates ethylene biosynthesis in einkorn wheat defense to powdery mildew. New Phytol. 2020;225:2526–2541. doi: 10.1111/nph.16305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ge X.-M., Cai H.-L., Lei X., Zhou X., Yue M., He J.-M. Heterotrimeric G protein mediates ethylene-induced stomatal closure via hydrogen peroxide synthesis in Arabidopsis. Plant J. 2015;82:138–150. doi: 10.1111/tpj.12799. [DOI] [PubMed] [Google Scholar]
- 59.Pan R., Han H., Medison M.B., Abou-Elwafa S.F., Liu Y., Yang X., Zhang W. Aerenchyma formation in the root of leaf-vegetable sweet potato: Programmed cell death initiated by ethylene-mediated H2O2 accumulation. Physiol. Plant. 2021;173:2361–2375. doi: 10.1111/ppl.13587. [DOI] [PubMed] [Google Scholar]
- 60.Lian Q., Meng Y., Zhao X., Xu Y., Wang Y., Day B., Ma Q. ShNPSN11, a vesicle-transport-related gene, confers disease resistance in tomato to Oidium neolycopersici. Biochem. J. 2020;477:3851–3866. doi: 10.1042/BCJ20190776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J., Wang Y., Liu X., Xu Y., Ma Q. Microtubule polymerization functions in hypersensitive response and accumulation of h2o2 in wheat induced by the stripe rust. Biomed. Res. Int. 2016;2016:7830768. doi: 10.1155/2016/7830768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng Z., Appiano M., Pavan S., Bracuto V., Ricciardi L., Visser R.G.F., Wolters A.M.A., Bai Y. Genome-wide study of the tomato SlMLO gene family and its functional characterization in response to the powdery mildew fungus Oidium neolycopersici. Front. Plant. Sci. 2016;7:380. doi: 10.3389/fpls.2016.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wingett S.W., Andrews S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research. 2018;7:1338. doi: 10.12688/f1000research.15931.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu H., Luo X., Qian J., Pang X., Song J., Qian G., Chen J., Chen S. FastUniq: A fast de novo duplicates removal tool for paired short reads. PLoS ONE. 2012;7:e52249. doi: 10.1371/journal.pone.0052249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim D., Langmead B., Salzberg S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao Y., Smyth G.K., Shi W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 68.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Letunic I., Khedkar S., Bork P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021;49:D458–D460. doi: 10.1093/nar/gkaa937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szklarczyk D., Gable A.L., Nastou K.C., Lyon D., Kirsch R., Pyysalo S., Doncheva N.T., Legeay M., Fang T., Bork P., et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49:D605–D612. doi: 10.1093/nar/gkaa1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iwase A., Kondo Y., Laohavisit A., Takebayashi A., Ikeuchi M., Matsuoka K., Asahina M., Mitsuda N., Shirasu K., Fukuda H., et al. WIND transcription factors orchestrate wound-induced callus formation, vascular reconnection and defense response in Arabidopsis. New Phytol. 2021;232:734–752. doi: 10.1111/nph.17594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lian Q., Zhang J., Gan L., Ma Q., Zong Z., Wang Y. The biocontrol efficacy of Streptomyces pratensis LMM15 on Botrytis cinerea in tomato. Biomed. Res. Int. 2017;2017:9486794. doi: 10.1155/2017/9486794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Prot. 2008;6:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 74.Wang L., Xie X., Yao W., Wang J., Ma F., Wang C., Yang Y., Tong W., Zhang J., Xu Y., et al. RING-H2-type E3 gene VpRH2 from Vitis pseudoreticulata improves resistance to powdery mildew by interacting with VpGRP2A. J. Exp. Bot. 2017;68:1669–1687. doi: 10.1093/jxb/erx033. [DOI] [PubMed] [Google Scholar]
- 75.Wakeel A., Ali I., Wu M., Liu B., Gan Y. Dichromate-induced ethylene biosynthesis, perception, and signaling regulate the variance in root growth inhibition among Shaheen basmati and basmati-385 rice varieties. Environ. Sci. Pollut. Res. Int. 2021;28:38016–38025. doi: 10.1007/s11356-021-13477-6. [DOI] [PubMed] [Google Scholar]
- 76.Jefferson R.A. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Report. 1987;5:387–405. doi: 10.1007/BF02667740. [DOI] [Google Scholar]
- 77.Fernandez-Pozo N., Rosli H.G., Martin G.B., Mueller L.A. The SGN VIGS tool: User-friendly software to design virus-induced gene silencing (VIGS) constructs for functional genomics. Mol. Plant. 2015;8:486–488. doi: 10.1016/j.molp.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 78.Senthil-Kumar M., Hema R., Anand A., Kang L., Udayakumar M., Mysore K. A systematic study to determine the extent of gene silencing in Nicotiana benthamiana and other Solanaceae species when heterologous gene sequences are used for virus-induced gene silencing. New Phytol. 2007;176:782–791. doi: 10.1111/j.1469-8137.2007.02225.x. [DOI] [PubMed] [Google Scholar]
- 79.Weigel D., Glazebrook J. Transformation of Agrobacterium using the freeze-thaw method. CSH Protoc. 2006;2006 doi: 10.1101/pdb.prot4666. [DOI] [PubMed] [Google Scholar]
- 80.Correll J.C., Gordon T.R., Elliot V.J. Powdery mildew of tomato: The effect of planting date and triadimefon on disease onset, progress, incidence, and severity. Phytopathology. 1988;78:512–519. doi: 10.1094/Phyto-78-512. [DOI] [Google Scholar]
- 81.Wang J., Hai Z., Yan H., Feng C., Yang W., Ma Q. Evaluation of actin cytoskeleton in non-host resistance of pepper to Puccinia striiformis f. sp. tritici stress. Phyiol. Mol. Plant Pathol. 2015;92:112–118. doi: 10.1016/j.pmpp.2015.09.003. [DOI] [Google Scholar]
- 82.Rekhter D., Lüdke D., Ding Y., Feussner K., Zienkiewicz K., Lipka V., Wiermer M., Zhang Y., Feussner I. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science. 2019;365:498–502. doi: 10.1126/science.aaw1720. [DOI] [PubMed] [Google Scholar]
- 83.Torrens-Spence M.P., Bobokalonova A., Carballo V., Glinkerman C.M., Pluskal T., Shen A., Weng J.K. PBS3 and EPS1 complete salicylic acid biosynthesis from isochorismate in Arabidopsis. Mol. Plant. 2019;12:1577–1586. doi: 10.1016/j.molp.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 84.Adams D.O., Yang S.F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA. 1979;76:170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang C. Q&A: How do plants respond to ethylene and what is its importance? BMC Biol. 2016;14:7. doi: 10.1186/s12915-016-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frankowski K., Kesy J., Kopcewicz J. Regulation of ethylene biosynthesis in plants. Postepy Biochem. 2007;53:66–73. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the reported results can be found in the supplementary materials file.