ABSTRACT
Objective:
To identify predictive features associated with the course of sarcoidosis at initial evaluation and to develop a predictive score.
Methods:
This was a retrospective study involving pulmonary sarcoidosis patients, classified as having a self-limited or persistent course of disease, comparing data between the outcomes by univariate analysis. Features related to persistent disease were selected by multivariate analysis and a prognostic score was designed.
Results:
The sample comprised 200 patients (mean age = 49 years). The median duration of symptoms to diagnosis was 12 months, and delayed diagnosis (> 12 months) was found in 43% of the cases. The most common radiological stage was II; 37% had reduced FVC. Relevant systemic involvement was detected in 37% of the patients. Treatment for tuberculosis was prescribed in 44 patients prior to sarcoidosis diagnosis. Treatment for sarcoidosis was required in 77% of the sample, and the disease course was persistent in 115 cases. Excluding 40 patients with fibrotic disease, prognostic factors to persistent disease were parenchymal involvement, delayed diagnosis, dyspnea, relevant systemic involvement, and reduced FVC. On the basis of the analysis, a 3-letter scoring system (A, B and C) was developed according to the selected factors. The positive predictive values for persistent course for A (≤ 1 point) and C scores (≥ 4 points) were 12.5% and 81.8%, respectively.
Conclusions:
A score can be derived by selected features at initial evaluation, allowing the prediction of outcomes in a significant number of sarcoidosis patients.
Keywords: Sarcoidosis, Prognosis, Chronic disease, Tuberculosis
RESUMO
Objetivo:
Identificar características preditivas associadas à evolução da sarcoidose na avaliação inicial e desenvolver um escore preditivo.
Métodos:
Estudo retrospectivo com pacientes com sarcoidose pulmonar, classificados como tendo evolução autolimitada ou persistente da doença, comparando dados entre os desfechos por meio de análise univariada. Características relacionadas à doença persistente foram selecionadas por meio de análise multivariada, e foi desenvolvido um escore prognóstico.
Resultados:
A amostra foi composta por 200 pacientes (média de idade = 49 anos). A mediana da duração dos sintomas até o diagnóstico foi de 12 meses, e houve diagnóstico tardio (> 12 meses) em 43% dos casos. O estádio radiológico mais frequente foi o II; 37% apresentavam redução da CVF. Envolvimento sistêmico relevante foi detectado em 37% dos pacientes. Tratamento para tuberculose foi prescrito em 44 pacientes antes do diagnóstico de sarcoidose. Tratamento para sarcoidose foi necessário em 77% da amostra, e a evolução da doença foi persistente em 115 casos. Com a exclusão de 40 pacientes com doença fibrótica, os fatores prognósticos para doença persistente foram comprometimento parenquimatoso, diagnóstico tardio, dispneia, comprometimento sistêmico relevante e redução da CVF. Com base na análise, foi desenvolvido um sistema de pontuação por letras (A, B e C) de acordo com os fatores selecionados. Os valores preditivos positivos para evolução persistente para as pontuações A (≤ 1 ponto) e C (≥ 4 pontos) foram de 12,5% e 81,8%, respectivamente.
Conclusões:
É possível derivar um escore por meio de características selecionadas na avaliação inicial, permitindo a predição de desfechos em um significativo número de pacientes com sarcoidose.
Descritores: Sarcoidose, Prognóstico, Doença crônica, Tuberculose
INTRODUCTION
Sarcoidosis is a multisystem granulomatous disease with highly variable clinical expression, natural history, and prognosis. 1 , 2 It ranges from self-limited, often asymptomatic disease to one with severe loss of function that can lead to death. 1 - 3
A number of studies derived models for predicting a higher risk of death, respiratory failure, or chronic disease. 3 - 14 Some have suggested complex classifications after a long-term follow-up period. 15 - 17 On an individual basis, it may be difficult to establish the prognosis of sarcoidosis, but it would be important to try to identify possible outcomes at diagnosis. Several manifestations are strongly associated with a worse prognosis. These include treatment-resistant pulmonary sarcoidosis phenotypes (e.g., fibrosis and pulmonary hypertension) and multiorgan sarcoidosis. 18 , 19 Other features are associated with chronic or persistent sarcoidosis in some studies, but not in others. 7 , 8 , 10 , 12 , 16 , 17 , 20 These include older age, male gender, smoking, respiratory/constitutional symptoms, race, level of dyspnea, absence of erythema nodosum, abnormal lung function, lung parenchymal involvement in the absence of fibrosis, and systemic involvement. 7 , 8 , 10 , 12 , 16 , 17 , 20
The influence of duration of symptoms to diagnosis in sarcoidosis on outcomes is rarely reported. In a study involving the development of a score by experts, a greater time to diagnosis of sarcoidosis was associated to greater disease severity in the univariate analysis, but not in the multivariate analysis. 16 ) In another study, subacute and chronic course groups (i.e., if estimated disease duration was less or more than 2 years, respectively) had a similar mean duration of symptoms to diagnosis (2 months only). 9 In a North-American study, 21 sarcoidosis was not diagnosed in more than one fourth of the subjects within 6 months of initial symptoms. Those patients had lower FEV1 (but not lower FVC) and more advanced stages of disease. 21 The median duration of symptoms to diagnosis was 12 months in a study in Brazil. 22 In the group of time to diagnosis > 12 months, both, FVC and FEV1 were lower, and tuberculosis was more commonly misdiagnosed.
The objectives of present study were to identify predictive features at initial evaluation associated with the clinical course of sarcoidosis and to develop a simple predictive prognostic score of disease outcome in routine practice. In particular, the role of delayed diagnosis and of treatment for misdiagnosed tuberculosis was analyzed.
METHODS
Patients with a diagnosis of pulmonary sarcoidosis were retrospectively evaluated at three specialized centers (two tertiary hospitals and a private clinic) in interstitial lung diseases (ILDs) in the city of São Paulo, Brazil, between January of 1990 and December of 2015.
Diagnosis of sarcoidosis was based on a joint statement of the American Thoracic Society, the European Respiratory Society, and the World Association of Sarcoidosis and Other Granulomatous Disorders. 1 Patients with typical clinical features, patients unable to undergo biopsy procedures, and those without definite histological findings were diagnosed by a multidisciplinary team involving pulmonology and radiology experts in ILDs. Patients with complete initial evaluation within 3 months of initial treatment were included. Data were recorded using a standardized assessment form and included the following variables: age, sex, diagnostic procedure, symptoms, pulmonary function and chest radiological test results, systemic staging, treatment, and outcome. A previous diagnosis of tuberculosis was carefully reviewed. No bacteriological diagnosis was confirmed in the cohort. Rare cases of patients with presumed or confirmed sarcoidosis but having a definitive diagnosis of tuberculosis that were seen during the study period were excluded. Exclusion criteria included absence of thoracic involvement (stage 0) or missing data at diagnosis or regarding disease outcome.
Dyspnea was classified in accordance with Mahler and Wells’ magnitude of task 23 and was considered relevant if present in moderate/light activities or at rest. Thoracic images were classified in accordance with the Scadding staging system, 24 based on CT findings. FVC was classified as abnormal using reference values for the Brazilian population. 25 Organ involvement was considered in the presence of definite or probable involvement using criteria from two reference studies. 1 , 26 Relevant systemic involvement was defined by the presence of extrathoracic sarcoidosis, excluding acute manifestations (facial palsy, peripheral adenopathy, orbital/salivary gland involvement, erythema nodosum, arthritis, or calcium metabolism disorders).
Physicians specialized in ILDs individually decided on the therapy. The records included type of drugs and time to initiation and duration of treatment, considered when performed for at least 3 consecutive months.
The disease course was classified as follows: self-limited-spontaneous involution within 2 years after diagnosis or stability at least for 1 year after a course of therapy; and persistent-treatment resistance, relapse after stopping treatment, maintenance treatment deemed necessary, or evidence of stable chronic disease 2 years after diagnosis. 27 In order to classify the disease course, a 1-year-interval from the latest outcome criteria was awaited. Some patients experienced relapse or treatment resistance during the last evaluation, and there was not enough time to determine whether the disease was chronically stable or whether it required permanent treatment.
Statistical analysis
The results were expressed as absolute and relative frequencies, mean ± standard deviation, or median and lower and upper quartiles (Q1 and Q3). The normal distribution of the variables was tested using the Kolmogorov-Smirnov test after visual inspection of the curves.
Time from first symptom to diagnosis was considered zero in asymptomatic patients (incidental diagnosis). Clinical, radiological, and functional data were compared between patients with self-limited and persistent courses by using Fisher’s exact or Pearson’s chi-square tests for categorical variables and by using Student’s t-test and Mann-Whitney U test for continuous variables with normal and non-normal distribution, respectively.
Variables initially selected by univariate analysis with a p-value < 0.10 were entered in a multivariate analysis using the Wald method (forward stepwise technique) to calculate ORs and 95% CIs for independent features related to persistent course in the non-fibrotic sarcoidosis group.
After logistic regression, relative weights were assigned to significant OR values, and a prognosis score was developed, assigning points for each variable identified. Internal consistency of the final model was tested using the bootstrapping method (1,000 samples).
The analyses were performed with the IBM SPSS Statistics software package, version 21.0 (IBM Corporation, Armonk, NY, USA). The study was approved by the Research Ethics Committees of Hospital São Paulo/Universidade Federal de São Paulo (CAAE no. 55830016.0.1001.5505) and Hospital do Servidor Público Estadual de São Paulo (CAAE no. 55830016.0.2001.5463).
RESULTS
A total of 200 sarcoidosis patients with complete initial evaluation and follow-up were included in the study. Table 1 summarizes the baseline features and the comparative data between subjects with non-fibrotic and fibrotic sarcoidosis. Most of the subjects were female (63%), and the mean age at diagnosis was 49.4 years. The median duration of symptoms to diagnosis was 12 months. Cough and dyspnea were the most common pulmonary symptoms. Half of the sample manifested systemic symptoms (fever and/or weight loss).
Table 1. General features in the cohort of patients with sarcoidosis (N = 200) and comparison between the non-fibrotic and fibrotic disease groups.a .
| Features | Whole sample | Group | p | |
|---|---|---|---|---|
| Non-fibrotic disease | Fibrotic disease | |||
| (N = 200) | (n = 160) | (n = 40) | ||
| Gender, female | 126 (63) | 101 (63) | 25 (62) | 1.00 |
| Age, years | 49.4 ± 12.2 | 48.3 ± 11.5 | 53.6 ± 14.1 | 0.02 |
| Granulomas on biopsy | 176 (88) | 147 (92) | 29 (72) | < 0.01 |
| Duration of symptoms to diagnosis, months | 12 [4, 25] | 10 [4, 24] | 24 [8, 68] | 0.01 |
| Follow-up, months | 80 [42, 123] | 88 [40, 132] | 77 [44, 96] | 0.22 |
| Smoking (current or former smoker) | 68 (34) | 48 (30) | 20 (50) | 0.02 |
| Dyspnea | 78 (39) | 56 (35) | 22 (55) | 0.02 |
| Cough | 111 (56) | 84 (52) | 27 (60) | 0.09 |
| Wheezing | 59 (30) | 43 (27) | 16 (40) | 0.10 |
| Weight loss | 83 (42) | 69 (43) | 14 (35) | 0.35 |
| Fever | 40 (20) | 30 (19) | 10 (25) | 0.38 |
| Relevant systemic involvementb | 76 (38) | 60 (38) | 16 (40) | 0.77 |
| Treatment for presumed tuberculosis | 44 (22) | 33 (21) | 11 (28) | 0.35 |
| Radiological staging, n | ||||
| I | 38 | |||
| II | 97 | |||
| III | 25 | |||
| IV | 40 | |||
| FVC, % predicted | 84.9 ± 18.8 | 88.5 ± 16.8 | 70.7 ± 19.9 | < 0.01 |
| Reduced FVC | 74 (37) | 47 (29) | 27 (68) | < 0.01 |
| FEV1 /FVC ratio | 0.79 ± 0.09 | 0.78 ± 0.09 | 0.78 ± 0.10 | 0.56 |
| Pharmacological treatment | 153 (76) | 113 (71) | 40 (100) | < 0.01 |
Values expressed as n (%), mean ± SD, or median [Q1, Q3]. bExcluding facial palsy, peripheral adenopathy, orbital and salivary glands involvement, erythema nodosum, arthritis, and calcium metabolism disorder.
Delayed diagnosis was characterized when diagnosis was confirmed more than 12 months after the onset of symptoms (in 43% of the cases). Delayed diagnosis was more common in females than in males (75% vs. 54%; p < 0.01), in patients over than 50 years of age (64% vs. 38%; p < 0.01), and in those presenting with wheezing (38% vs. 23%; p = 0.02) in comparison with non-delayed diagnosis (≤ 12 months).
The most common radiological staging was II, followed by stages I and IV, the latter ones with similar proportions. Mean FVC was below the lower limit of normal in 37% of the cases.
Treatment for presumed tuberculosis was performed prior to the diagnosis of sarcoidosis in 44 patients (22%). Patients treated for presumed tuberculosis, in comparison with those who were not, were associated with weight loss (61% vs. 35%; p < 0.001) and parenchymal lung involvement (95% vs. 77%; p = 0.01). In this group, a longer duration of disease prior to sarcoidosis treatment was observed: median [Q1-Q3] = 17.0 [9.0-62.5] months vs. 9.0 [2.8-18.0] months; p = 0.024.
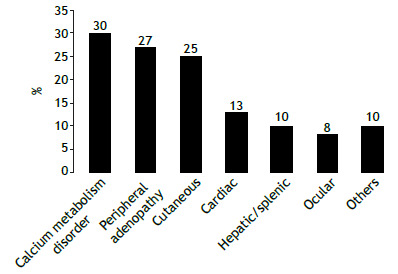
Systemic involvement is shown in Figure 1. Relevant systemic involvement (excluding acute manifestations) was present in 74 cases (37%).
Figure 1. Proportion of systemic manifestations in the cohort (N = 200).
During the course of the disease, 77% of the cases needed treatment, mainly corticosteroids (in 74%), followed by methotrexate and/or leflunomide (in 39%). Only 4% of the patients demanded TNF inhibitors. Two or more drugs were prescribed to 51% of the patients.
The analysis of features associated with the proposed outcomes was performed after excluding 40 patients in stage IV, since this stage is related to irreversible pulmonary lesions. In comparison with non-fibrotic sarcoidosis patients, those in stage IV (n = 40) were older, more often had a delayed diagnosis, more frequently had dyspnea, had lower FVC (in % of the predicted value), and more frequently had a smoking habit (p < 0.05 for all). All fibrotic patients needed treatment (82% of those with two or more drugs), and compared with those without fibrosis, also had a higher death rate (28% vs. 9%).
The final sample of patients with non-fibrotic pulmonary sarcoidosis was divided into self-limited (n = 85) and persistent (n = 75) course subgroups according to disease outcome (Figure 2). Prognostic features of the two subgroups were compared (Table 2). The univariate analysis associated the persistent course subgroup with parenchymal lung involvement (stages II and III), delayed diagnosis, dyspnea, relevant systemic involvement in two or more organs (but not in only one organ), reduced FVC, and treatment for presumed tuberculosis. Erythema nodosum was observed in 16 patients (10% of the cases), mainly in women (88%), but its presence was not associated with a better outcome (10 in the self-limited disease subgroup vs. 6 in the persistent disease subgroup). Smoking history was similar in the two subgroups. Other significant associations were as follows: 42/122 (34%) of the patients with parenchymal involvement had reduced FVC, compared with 5/38 (13%) of those without parenchymal involvement (p = 0,01); and 24/56 (43%) of the patients with dyspnea had reduced FVC, compared with 23/104 (22%) of those without dyspnea (p = 0,01). There was no significant association between parenchymal involvement and dyspnea (p = 0.29). Although there were significant associations among these variables, they were all selected for the final multivariate analysis.
Figure 2. Outcomes in the non-fibrotic pulmonary sarcoidosis group (n = 160). Patients with spontaneous involution or stability after first treatment were classified as having self-limited disease, the remaining being classified as having persistent disease.
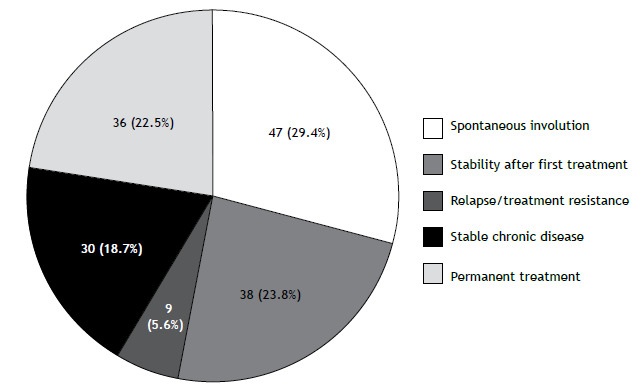
Table 2. General features of 160 patients with non-fibrotic pulmonary sarcoidosis classified as having a self-limited or persistent course.a .
| Feature | Group | p | |
|---|---|---|---|
| Self-limited course | Persistent course | ||
| (n = 85) | (n = 75) | ||
| Female/male | 55 (65)/30 (35) | 46 (61)/29 (39) | 0.74 |
| Age, years | 48.2 ± 12.8 | 48.5 ± 9.9 | 0.64 |
| Time to diagnosis: early/delayed | 60 (71)/25 (29) | 38 (51)/37 (49) | 0.01 |
| Follow-up, months | 76 [41, 114] | 98 [40, 163] | 0.20 |
| Smoking (current or former smoker) | 23 (27) | 25 (33) | 0.75 |
| Dyspnea | 23 (27) | 33 (44) | 0.03 |
| Weight loss | 37 (44) | 32 (43) | 1.00 |
| Fever | 15 (18) | 15 (20) | 0.84 |
| Erythema nodosum | 10 (12) | 6 (8) | 0.43 |
| Tuberculosis treatment | 10 (12) | 23 (31) | < 0.01 |
| Radiological staging | |||
| I | 27 (32) | 11 (15) | 0.04 |
| II | 46 (54) | 51 (68) | |
| III | 12 (14) | 13 (17) | |
| Reduced FVC | 17 (20) | 30 (40) | < 0.01 |
| FEV1/FVC ratio | 0.79 ± 0.09 | 0.79 ± 0.10 | 0.72 |
| Two or more relevant extrathoracic involvements | 4 (5) | 13 (17) | 0.01 |
| Pharmacological treatment | 38 (45) | 75 (100) | < 0.01 |
Values expressed as n (%), mean ± SD, or median [Q1, Q3].
In the final logistic regression model, two patients were excluded because they were considered discrepant by the selected statistical model. The results of the multivariate analysis (OR and 95% CI) are shown in Table 3. Treatment for tuberculosis did not reach statistical significance. However, more patients treated for tuberculosis developed pulmonary fibrosis during follow-up. Evolution imaging was available in 154/158 of the non-fibrotic sarcoidosis patients; 15/32 (47%) and 31/122 (25%) of those who had and had not received tuberculosis treatment, respectively, developed fibrosis (p = 0.018).
Table 3. Independent predictive factors for persistent course in the non-fibrotic pulmonary sarcoidosis group at initial evaluation (n = 158 patients).a .
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Reduced FVC | 2.35 | 1.05-5.15 | 0.038 |
| Delayed diagnosis | 2.37 | 1.50-4.93 | 0.020 |
| Dyspnea | 2.55 | 1.18-5.49 | 0.017 |
| Parenchymal lung involvement | 3.94 | 1.54-10.1 | 0.004 |
| Two or more relevant extrathoracic involvements | 5.78 | 1.66-20.2 | 0.006 |
Ajusted for treatment for presumed tuberculosis. Two discrepant patients were excluded from the analysis.
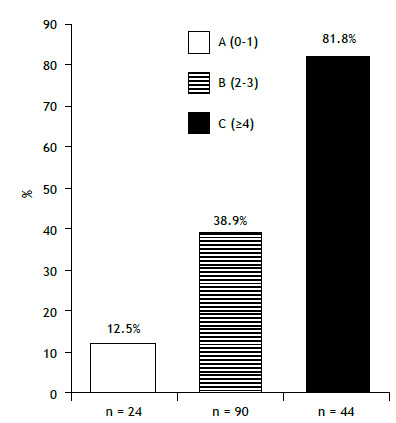
By using the relative ORs of significant variables, a point score system was developed: reduced FVC, delayed diagnosis, and dyspnea-1 point each-parenchymal lung involvement and two or more relevant extrathoracic involvements-2 points each. By grouping the most relevant sum of points to distinct outcomes, three cutoff points and a staging system were developed: 0-1 point = stage A (24 patients); 2-3 points = stage B (90 patients); and ≥ 4 points = stage C (44 patients). Persistent course occurred in 12.5% (21/24) of the patients in stage A, in 38.9% (35/90) of those in stage B, and in 81.8% (36/44) of those in stage C (x2 = 39.94; p < 0.001; Figure 3).
Figure 3. Positive predictive value in the non-fibrotic pulmonary sarcoidosis group (n = 158) for persistent course according to stages A (0-1 point), B (2-3 points) and C (≥ 4 points). p < 0.01.
According to the proposed score, adding 40 cases of fibrotic disease (persistent course), the model would make a correct prediction in 97 of 200 cases (48%).
Internal validation of the staging system (bootstrapping resampling method) was performed, based in 1,000 samples, and statistically significant variables remained in the multivariate analysis (p < 0.05), except again for tuberculosis treatment (p = 0.16).
DISCUSSION
Predicting the course of sarcoidosis at initial evaluation is a hard task in clinical practice. 28 , 29 ) In the current study, we were able to identify prognostic features at presentation that were associated with self-limited or persistent course in patients with non-fibrotic pulmonary sarcoidosis in a significant, albeit limited number of cases. These included delayed diagnosis (> 12 months), presence of dyspnea, parenchymal lung involvement (stages II and III), reduced FVC, and relevant extrathoracic disease in two or more organs. Treatment for presumed tuberculosis in sarcoidosis patients was associated with delayed diagnosis and more persistent disease.
Patients with no thoracic involvement (stage 0) were excluded from the present study. In comparison with a large prospective North-American study, the present study included a smaller number of stage I cases and a larger number of stage IV cases, probably due to selection bias. 30
Several factors have been associated with the outcome of sarcoidosis in different studies. 4 - 14 In the present study, the course of sarcoidosis was classified as self-limited or persistent. 27 At presentation, all patients with pulmonary fibrosis (stage IV) had persistent disease and demanded treatment. These patients were older, more often had a delayed diagnosis, more often reported having significant dyspnea, more frequently were smokers or former smokers, and had lower FVC%. Weight loss was more common in those treated for misdiagnosed tuberculosis, but the presence of fever was not significantly different.
Smoking seems to have a protective effect on sarcoidosis development, but this is not a universal finding. In the present study, those subjects in radiological stage IV were most commonly smokers or former smokers, suggesting a higher risk for the fibrotic phenotype in smokers, similar to that observed in hypersensitivity pneumonitis. 31
No influence of gender on prognosis was found. Race was not evaluated, due to the high interbreeding in the Brazilian population.
Spontaneous remission has been reported to occur in 90% of the patients in stage I, in 40-70% of those in stage II, and in 10-20% of those in stage III. 32 In the present study, patients in stage I more often presented with self-limited disease, but those in stages II and III had similar frequencies of self-limited and persistent courses and, consequently, were merged. The same was observed in the classic study by Neville et al. 2
Parenchymal involvement, dyspnea, and reduced FVC remained significant predictors of disease outcome in the multivariate analysis. There is a dissociation among the level of dyspnea, pulmonary function results, and chest imaging in sarcoidosis. 33 , 34 We found that even stage I cases can have reduced FVC and relevant dyspnea, similarly to what has been reported in other studies. 8 , 32 Parenchymal lung disease can be present when HRCT is performed, even in the absence of radiographic abnormalities, demonstrating the higher sensitivity of CT. 35 We found that FVC below the lower limit of normal was a predictor of a persistent course of the disease. In one study, FVC < 80% of the predicted value was also a predictor of chronic disease. 36 ) The FEV1/FVC ratio was similar between the two groups. Few studies have evaluated the role of dyspnea for predicting the course of sarcoidosis. In a study to determine features at initial presentation that are associated with continued treatment (2 years after onset), Baughman et al. 8 found that the level of dyspnea and predicted VC were significant features.
Dyspnea is multifactorial and can be the result of several aspects in sarcoidosis, not only due to pulmonary abnormalities. Conditions such as pulmonary hypertension, cardiac disease, anemia, musculoskeletal/neurological involvement, joint involvement, parasarcoidosis syndromes (chronic fatigue, anxiety, and depression), and hyperventilation can result in dyspnea. 37
The diagnosis of sarcoidosis from the onset of symptoms is often delayed for several reasons, including a great number of health care visits prior to diagnosis. 21 , 22 , 38 The presentation of sarcoidosis is too diverse, the disease is uncommon, and very few physicians have training in and experience with the disease.
The influence of the delay in diagnosis on the outcomes has been rarely reported. In the present sample, half of the cases had the diagnosis delayed for more than 1 year, and most of these cases were associated with chronic disease. Delayed diagnosis was more common in females, older patients (> 50 years of age), those with wheezing, and those misdiagnosed with tuberculosis.
Persistent sarcoidosis was more common when relevant multiorgan, extrapulmonary involvement was present, a well-known association. 7 , 10 , 19 , 29 , 39
In developing countries, due to the high incidence of tuberculosis, many patients who are diagnosed with sarcoidosis will have received treatment for tuberculosis. In our study, patients presenting with weight loss had more frequently been treated for tuberculosis. To the best of our knowledge, the impact of such decision on the final outcome of sarcoidosis has not been studied.
Although Mycobacterium tuberculosis does not seem to be the etiologic trigger for sarcoidosis, there is increasing evidence for mycobacteria to be a cause of at least some cases of sarcoidosis. In the present study, we found that a great number of patients treated for tuberculosis had a chronic course and developed imaging-confirmed fibrosis that was not present at initial evaluation. This suggests that treatment for presumed tuberculosis can worsen the course of the disease. A study postulated that the antigen(s) from this mycobacterium could be released during the death of the organism, with a complex of host and mycobacterial proteins in response to the infection leading to sarcoidosis. 40 That study also suggested that the failure to clear these antigen/protein complexes could lead to chronic disease in some patients. 40 There seems to be a bidirectional association between sarcoidosis and tuberculosis. Some patients with sarcoidosis have confirmed tuberculosis, before or after sarcoidosis. However, such cases were excluded from the present cohort.
Several studies have developed complex classifications for assessing the course of sarcoidosis after a long period of follow-up. Drug categories used in treatment were included in those classifications. 15 - 17
A study carried out in Turkey evaluated the course of 275 patients with sarcoidosis separated according to disease course (subacute vs. chronic groups) and developed a model to predict the course using simple clinical and demographic variables. 9 Logistic regression indicated that erythema nodosum, arthralgia and/or arthritis, and stage I disease were more frequent in the subacute group, whereas respiratory/constitutional symptoms and stage II-III disease were more frequently seen in the chronic disease group. In our study, erythema nodosum was uncommon and did not have predictive values. Constitutional symptoms were not a predictor of outcome.
The major strengths of present study are the long-term follow-up period of the patients and the simplicity of the developed model. However, several limitations are present in our study. Patients were retrospectively evaluated and treated in referral centers, so they might not be representative of sarcoidosis at large. The derived model managed to estimate the prognosis in only half of the cases and must be reviewed using a validation cohort. Better markers of prognosis, including biomarkers, are necessary in sarcoidosis. DLCO measurements and investigation for pulmonary hypertension were performed in a limited number of cases and were not included in final analysis. Specific parenchymal findings on CT, which might be helpful in predicting the prognosis, were not evaluated. Finally, this study did not include a protocol for therapy.
In conclusion, a predictive score for sarcoidosis outcome can be derived by multiple variables at initial evaluation in order to predict the clinical course of the disease in a significant, albeit limited number of cases. The results should be confirmed in future studies involving a validation cohort.
ACKNOWLEDGMENTS
The corresponding author would like to thank the institutions and the participants involved the study, especially the Universidade Federal de São Paulo for receiving her since the beginning of my medical residency program.
Footnotes
Financial support: None.
Study carried out at Hospital São Paulo, Hospital do Servidor Público Estadual de São Paulo, and Clínica Dr. Carlos Alberto de Castro Pereira, São Paulo (SP) Brasil.
REFERENCES
- 1.Statement on sarcoidosis Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis an analysis of 818 patients. Q J Med. 1983;52(208):525–533. [PubMed] [Google Scholar]
- 3.Walsh SL, Wells AU, Sverzellati N, Keir GJ, Calandriello L, Antoniou KM. An integrated clinicoradiological staging system for pulmonary sarcoidosis a case-cohort study. Lancet Respir Med. 2014;2(2):123–130. doi: 10.1016/S2213-2600(13)70276-5. [DOI] [PubMed] [Google Scholar]
- 4.Baughman RP, Winget DB, Bowen EH, Lower EE. Predicting respiratory failure in sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14(2):154–158. [PubMed] [Google Scholar]
- 5.Vestbo J, Viskum K. Respiratory symptoms at presentation and long-term vital prognosis in patients with pulmonary sarcoidosis. Sarcoidosis. 1994;11(2):123–125. [PubMed] [Google Scholar]
- 6.Viskum K, Vestbo J. Vital prognosis in intrathoracic sarcoidosis with special reference to pulmonary function and radiological stage. Eur Respir J. 1993;6(3):349–353. [PubMed] [Google Scholar]
- 7.Baughman RP, Lower EE. Features of sarcoidosis associated with chronic disease. Sarcoidosis Vasc Diffuse Lung Dis. 2015;31(4):275–281. [PubMed] [Google Scholar]
- 8.Baughman RP, Judson MA, Teirstein A, Yeager H, Rossman M, Knatterud GL. Presenting characteristics as predictors of duration of treatment in sarcoidosis. QJM. 2006;99(5):307–315. doi: 10.1093/qjmed/hcl038. [DOI] [PubMed] [Google Scholar]
- 9.Demirkok SS, Basaranoglu M, Akinci ED, Karayel T. Analysis of 275 patients with sarcoidosis over a 38 year period; a single-institution experience. Respir Med. 2007;101(6):1147–1154. doi: 10.1016/j.rmed.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Doubková M, Pospíšil Z, Skřičková J, Doubek M. Prognostic markers of sarcoidosis an analysis of patients from everyday pneumological practice. Clin Respir J. 2015;9(4):443–449. doi: 10.1111/crj.12160. [DOI] [PubMed] [Google Scholar]
- 11.Ungprasert P, Crowson CS, Carmona EM, Matteson EL. Outcome of pulmonary sarcoidosis a population-based study 1976-2013. Sarcoidosis Vasc Diffuse Lung Dis. 2018;35(2):123–128. doi: 10.36141/svdld.v35i2.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva AL, Melo N, Caetano Mota P, Lima B, Pereira JM, Cunha R. Pulmonary Sarcoidosis Prognostic Factors at Diagnosis in Patients from North of Portugal. Reumatol Clin (Engl Ed) 2020;16(6):468–472. doi: 10.1016/j.reuma.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Judson MA, Baughman RP, Thompson BW, Teirstein AS, Terrin ML, Rossman MD. Two year prognosis of sarcoidosis the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20(3):204–211. [PubMed] [Google Scholar]
- 14.Mañá J, Salazar A, Manresa F. Clinical factors predicting persistence of activity in sarcoidosis a multivariate analysis of 193 cases. Respiration. 1994;61(4):219–225. doi: 10.1159/000196341. [DOI] [PubMed] [Google Scholar]
- 15.Baughman RP, Nagai S, Balter M, Costabel U, Drent M, du Bois R. Defining the clinical outcome status (COS) in sarcoidosis results of WASOG Task Force. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(1):56–64. [PubMed] [Google Scholar]
- 16.Wasfi YS, Rose CS, Murphy JR, Silveira LJ, Grutters JC, Inoue Y. A new tool to assess sarcoidosis severity. Chest. 2006;129(5):1234–1245. doi: 10.1378/chest.129.5.1234. [DOI] [PubMed] [Google Scholar]
- 17.Prasse A, Katic C, Germann M, Buchwald A, Zissel G, Müller-Quernheim J. Phenotyping sarcoidosis from a pulmonary perspective. Am J Respir Crit Care Med. 2008;177(3):330–336. doi: 10.1164/rccm.200705-742OC. [DOI] [PubMed] [Google Scholar]
- 18.Judson MA. Strategies for identifying pulmonary sarcoidosis patients at risk for severe or chronic disease. Expert Rev Respir Med. 2017;11(2):111–118. doi: 10.1080/17476348.2017.1281745. [DOI] [PubMed] [Google Scholar]
- 19.Korsten P, Strohmayer K, Baughman RP, Sweiss NJ. Refractory pulmonary sarcoidosis - proposal of a definition and recommendations for the diagnostic and therapeutic approach. Clin Pulm Med. 2016;23(2):67–75. doi: 10.1097/CPM.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mihailovic-Vucinic V, Zugic V, Videnovic-Ivanov J. New observations on pulmonary function changes in sarcoidosis. Curr Opin Pulm Med. 2003;9(5):436–441. doi: 10.1097/00063198-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Judson MA, Thompson BW, Rabin DL, Steimel J, Knattereud GL, Lackland DT. The diagnostic pathway to sarcoidosis. Chest. 2003;123(2):406–412. doi: 10.1378/chest.123.2.406. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues MM, Coletta EN, Ferreira RG, Pereira CA. Delayed diagnosis of sarcoidosis is common in Brazil. J Bras Pneumol. 2013;39(5):539–546. doi: 10.1590/S1806-37132013000500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 24.SCADDING JG. Prognosis of intrathoracic sarcoidosis in England A review of 136 cases after five years' observation. Br Med J. 1961;2(5261):1165–1172. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira CA, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. 2007;33(4):397–406. doi: 10.1590/S1806-37132007000400008. [DOI] [PubMed] [Google Scholar]
- 26.Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H., Jr Defining organ involvement in sarcoidosis the ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(1):75–86. [PubMed] [Google Scholar]
- 27.Pereira CA, Dornfeld MC, Baughman R, Judson MA. Clinical phenotypes in sarcoidosis. Curr Opin Pulm Med. 2014;20(5):496–502. doi: 10.1097/MCP.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 28.Nagai S, Handa T, Ito Y, Ohta K, Tamaya M, Izumi T. Outcome of sarcoidosis. Clin Chest Med. 2008;29(3):565–56x. doi: 10.1016/j.ccm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Kobak S. Catch the rainbow Prognostic factor of sarcoidosis. Lung India. 2020;37(5):425–432. doi: 10.4103/lungindia.lungindia_380_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA. Clinical characteristics of patients in a case control study of sarcoidosis. Pt 1Am J Respir Crit Care Med. 2001;164(10):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsuka Y, Munakata M, Tanimura K, Ukita H, Kusaka H, Masaki Y. Smoking promotes insidious and chronic farmer's lung disease, and deteriorates the clinical outcome. Intern Med. 1995;34(10):966–971. doi: 10.2169/internalmedicine.34.966. [DOI] [PubMed] [Google Scholar]
- 32.Lynch 3rd JP, Ma YL, Koss MN, White ES. Pulmonary sarcoidosis. Semin Respir Crit Care Med. 2007;28(1):53–74. doi: 10.1055/s-2007-970333. [DOI] [PubMed] [Google Scholar]
- 33.Baydur A, Alsalek M, Louie SG, Sharma OP. Respiratory muscle strength, lung function, and dyspnea in patients with sarcoidosis. Chest. 2001;120(1):102–108. doi: 10.1378/chest.120.1.102. [DOI] [PubMed] [Google Scholar]
- 34.Baughman RP, Lower EE. Six-minute walk test in managing and monitoring sarcoidosis patients. Curr Opin Pulm Med. 2007;13(5):439–444. doi: 10.1097/MCP.0b013e328273bc2b. [DOI] [PubMed] [Google Scholar]
- 35.Akten HS, Kilic H, Celik B, Erbas G, Isikdogan Z, Turktas H. Diagnostic Yield of Transbronchial Biopsy in Comparison to High Resolution Computerized Tomography in Sarcoidosis Cases. Asian Pac J Cancer Prev. 2018;19(4):1029–1033. doi: 10.22034/APJCP.2018.19.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mañá J, Salazar A, Pujol R, Manresa F. Are the pulmonary function tests and the markers of activity helpful to establish the prognosis of sarcoidosis? Respiration. 1996;63(5):298–303. doi: 10.1159/000196564. [DOI] [PubMed] [Google Scholar]
- 37.de Boer S, Kolbe J, Wilsher ML. The relationships among dyspnoea, health-related quality of life and psychological factors in sarcoidosis. Respirology. 2014;19(7):1019–1024. doi: 10.1111/resp.12359. [DOI] [PubMed] [Google Scholar]
- 38.Gerke AK, Tang F, Pendergast J, Cavanaugh JE, Polgreen PM. The high frequency of healthcare use in patients one year prior to a sarcoidosis diagnosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(3):256–261. [PMC free article] [PubMed] [Google Scholar]
- 39.Takada K, Ina Y, Noda M, Sato T, Yamamoto M, Morishita M. The clinical course and prognosis of patients with severe, moderate or mild sarcoidosis. J Clin Epidemiol. 1993;46(4):359–366. doi: 10.1016/0895-4356(93)90150-Y. [DOI] [PubMed] [Google Scholar]
- 40.Moller DR. Potential etiologic agents in sarcoidosis. Proc Am Thorac Soc. 2007;4(5):465–468. doi: 10.1513/pats.200608-155MS. [DOI] [PMC free article] [PubMed] [Google Scholar]


