Abstract
The aim of this study was to compare two different tomographs for the evaluation of the role of semiquantitative PET/CT parameters and radiomics features (RF) in the prediction of thyroid incidentalomas (TIs) at 18F-FDG imaging. A total of 221 patients with the presence of TIs were retrospectively included. After volumetric segmentation of each TI, semiquantitative parameters and RF were extracted. All of the features were tested for significant differences between the two PET scanners. The performances of all of the features in predicting the nature of TIs were analyzed by testing three classes of final logistic regression predictive models, one for each tomograph and one with both scanners together. Some RF resulted significantly different between the two scanners. PET/CT semiquantitative parameters were not able to predict the final diagnosis of TIs while GLCM-related RF (in particular GLCM entropy_log2 e GLCM entropy_log10) together with some GLRLM-related and GLZLM-related features presented the best predictive performances. In particular, GLCM entropy_log2, GLCM entropy_log10, GLZLM SZHGE, GLRLM HGRE and GLRLM HGZE resulted the RF with best performances. Our study enabled the selection of some RF able to predict the final nature of TIs discovered at 18F-FDG PET/CT imaging. Classic semiquantitative and volumetric PET/CT parameters did not reveal these abilities. Furthermore, a good overlap in the extraction of RF between the two scanners was underlined.
Keywords: thyroid incidentalomas, radiomics, texture analysis, 18F-FDG, PET/CT, positron emission tomography, thyroid cancer
1. Introduction
Differentiated thyroid cancer (DTC) represents about 1% of all malignant tumors; moreover, it is the most frequent form of endocrine carcinoma and is usually characterized by good prognosis [1,2,3,4]. In recent years, its incidence has been growing due to the increasing use of needle aspiration and thyroid ultrasound [5,6,7].
The role of nuclear medicine in the diagnostic and therapeutic work-up of DTC is pivotal. In fact, nowadays, exams performed with 131I are fundamental for the staging, the restaging, and the therapy of this carcinoma [4,8].
In recent years, we have been continuously experiencing an increase in the use of positron emission tomography/computed tomography (PET/CT) with 18F-fluorodeoxyglucose (18F-FDG) for the evaluation of various pathologies, both neoplastic and inflammatory. In this context, even in the diagnostic work-up of DTC this hybrid imaging modality has a central role, in particular in the evaluation of patients with no evidence of 131I avid disease but a persistence of elevated thyroglobulin levels [4,9,10].
With the increasing use of 18F-FDG PET/CT in the clinical practice, we have also been experiencing an increase in the detection of thyroid incidentalomas (TI) [11,12,13]. TIs are defined as thyroid lesions detected at imaging studies performed for non-thyroid pathologies [14,15].
The precise evaluation of TIs is mandatory, given the non-negligible risk of presence of CTD in a high amount of these findings [16,17,18]. In this context, a lot of authors have tried to clarify the role of 18F-FDG PET/CT for the definition of the precise nature of TIs, in terms of malignancy or benignancy [4]. However, the role of some PET/CT semiquantitative parameters, such as standardized uptake value (SUV), metabolic tumor volume (MTV) and total lesion glycolysis (TLG) has not yet been fully clarified and the results in literature are really heterogeneous [4].
Furthermore, in recent years we have been appreciating an increase in the extraction of specific quantitative features from PET images, called radiomics or texture analysis. In this setting, the use of radiomics for the correct evaluation of every type of incidentalomas is waking increasing interest [19,20]. The case of TIs is not an exception and some works about the use of texture analysis for their correct classification have been produced [21,22,23,24]. However, similarly to semiquantitative parameters, the use of radiomics in this setting has given non-clarifying and initial results.
The aim of this retrospective study is to evaluate the role of semiquantitative PET/CT parameters and radiomics features for the correct classification of TIs discovered at 18F-FDG PET/CT scans. Furthermore, the impact of different PET/CT tomographs on texture analysis and on its ability to predict the final outcome is a fundamental part of this work.
2. Materials and Methods
2.1. Patients Selection
We retrospectively analyzed the 18F-FDG PET/CT scans performed in our center between January 2012 and December 2020 in order to find presence of TIs. All of the patients performed PET/CT exams for staging or restaging purpose of various diseases, but no one had a previous history of DTC. Specifically, 82 patients suffered from lymphoma, 19 from carcinomas of the head and neck, 51 from lung cancer, 6 from fever of unknown origin, 12 from vasculitis, 38 from breast cancer, 3 from esophageal cancer, 2 from ovarian cancer, 5 from colorectal cancer and 2 from endocarditis, while 1 patient performed the examination in order to characterize a formation of the right adrenal gland.
Tis were defined as focal uptakes of 18F-FDG inside the thyroid gland with an uptake higher than the background uptakes. Given the fact that diffuse uptakes on thyroid gland are usually expression of benign conditions, they were excluded from the study [4]. Furthermore, other inclusion criteria were the presence of an ultrasound follow-up of at least 1 year for suspected benignant uptakes and the execution of a cytological evaluation and/or histological examination for suspected malignant uptakes. A total of 237 patients were therefore included in the study and data about the lobe of TIs and ultrasound dimension were collected.
2.2. 18F-FDG PET/CT Acquisition and Interpretation
18F-FDG PET/CT scans were acquired after at least 6 h of fasting and with blood glucose levels below 150 mg/dL. An activity of 3.5–4.5 MBq/kg of 18F-FDG was intravenously administrated to the patients 1 hour before images acquisition. Images were acquired from the base of the skull to the mid-thigh. All of the patients were instructed to void before the PET/CT acquisition and no type of oral or intravenous contrast agents were given for the execution of the scan. Similarly, none of the patients had performed any intestinal preparation.
In our study, we made use of 2 different PET/CT tomographs: the first (scanner 1) was a Discovery 690 PET/CT (General Electric Company-Milwaukee, WI, USA) while the second (scanner 2) was a Discovery STE PET/CT (General Electric Company, Milwaukee, WI, USA). On both of them standard acquisition parameters (CT: 80 mA, 120 Kv without contrast; 2.5–4 min per bed- PET-step, axial width 15 cm) and standard reconstruction parameters were used (256 × 256 matrix and 60 cm field of view).
Furthermore, scanner 1 was characterized by the presence of LYSO (cerium-doped lutetium yttrium oxyorthosilicate) scintillator crystals with a decay time of 45 ns, while scanner 2 had BGO (bismuth germanate) scintillator crystals with a decay time of 300 ns. Scanners were not harmonized with a cross-calibration program and all PET/CT scans were acquired at free-breath, instructing the patients to have regular breathing. For both scanners, a low dose CT at free breathing and without contrast agent was acquired in order to perform attenuation correction and for anatomical correlation. In particular, CT acquisition parameters for scanner 1 were: 120 kV, fixed tube current ≈ 60 mAs (40–100 mAs), 64 slices × 3.75 mm and 3.27 mm interval, pitch 0.984:1, tube rotation 0.5 s. CT acquisition parameters for scanner 2 were: 120 kV, fixed tube current ≈ 73 mAs (40–160 mAs), 4 slices × 3.75 mm and 3.27 mm interval, pitch 1.5:1, tube rotation 0.8 s. Furthermore, on scanner 1 time of flight (TOF) and point spread function (PSF) algorithm were used for the reconstruction of images, with filter cut-off 5 mm, 18 subsets and 3 iterations. Again, on scanner 2, an ordered subset expectation maximization (OSEM) algorithm with filter cut-off 5 mm, 21 subsets and 2 iterations were used.
PET images were visually and semiquantitatively analyzed by a nuclear physician with at least 10 years of experience, measuring parameters of TIs: the maximum standardized uptake value corrected for body weight (SUVmax), mean SUV corrected for body weight (SUVmean), maximum standardized uptake value lean body mass (SUVlbm), maximum standardized uptake value body surface area (SUVbsa), MTV and TLG. SUV-related parameters were measured on a Xeleris 3.1 GE workstation. MTV was calculated by drawing a volume of interest (VOI) on TIs on 18F-FDG PET/CT images corrected for attenuation, using a SUV-based automated contouring program (Advantage Workstation 4.6, GE HealthCare) with an isocounter threshold method based on 41% of the SUVmax, as previously recommended by the European Association of Nuclear Medicine (EANM) because of its high inter-observer reproducibility [25]. TLG values were calculated as the product of the MTV of the VOI for its SUVmean.
2.3. Radiomics Features Extraction
Image features were extracted from PET images by using LIFEx 2.20 software (LIFEx, by the French Alternative Energies and Atomic Energy Commission (CEA), Gif-sur-Yvette, France) (http://www.lifexsoft.org, accessed on 10 September 2021) [26] with the same procedure previously described for SUV-related parameters extraction, with similar VOI and after a new segmentation process.
The extraction of radiomics features (RF) was performed without spatial resampling, with an intensity discretization of 64 grey levels and with a distance from neighbors of 1 voxel for the extraction of GLCM parameters.
A total of 42 RF were generated (Table 1), divided in first-order statistics (histogram-related and shape-related) and second-order statistics: grey level co-occurrence matrix (GLCM) related, grey-level run length matrix (GLRLM) related, neighborhood grey level different matrix (NGLDM) related and grey-level zone length matrix (GLZLM) related.
Table 1.
List of semiquantitative parameters and of radiomics features considered in the study.
| Semiquantitave Parameters |
|---|
| SUV-related |
| SUVmax |
| SUVmean |
| SUVlbm |
| SUVbsa |
| Volumetric parameters |
| MTV |
| TLG |
| Radiomics features |
| First order features |
| Histogram related |
| Histo skewness |
| Histo kurtosis |
| Histo excess kurtosis |
| Histo entropy_log10 |
| Histo entropy_log2 |
| Histo energy |
| Shape related |
| Shape volume_mL |
| Shape volume_vx |
| Shape sphericity |
| Shape compacity |
| Second order features |
| Grey level co-occurrence matrix (GLCM) related |
| GLCM homogeneity |
| GLCM energy |
| GLCM contrast |
| GLCM correlation |
| GLCM entropy_log10 |
| GLCM entropy_log2 |
| GLCM dissimilarity |
| Grey-level run length matrix (GLRLM) related |
| GLRLM SRE |
| GLRLM LRE |
| GLRLM LGRE |
| GLRLM HGRE |
| GLRLM SRLGE |
| GLRLM SRHGE |
| GLRLM LRLGE |
| GLRLM LRHGE |
| GLRLM GLNU |
| GLRLM RLNU |
| GLRLM RP |
| Neighborhood grey level different matrix (NGLDM) related |
| NGLDM coarseness |
| NGLDM contrast |
| NGLDM busyness |
| Grey-level zone length matrix (GLZLM) related |
| GLZLM SZE |
| GLZLM LZE |
| GLZLM LGZE |
| GLZLM HGZE |
| GLZLM SZLGE |
| GLZLM SZHGE |
| GLZLM LZLGE |
| GLZLM LZHGE |
| GLZLM GLNU |
| GLZLM ZLNU |
| GLZLM ZP |
SUVmax: standardized uptake value body weight max; SUVmean: standardized uptake value body weight mean; SUVlbm: standardized uptake value lean body mass, SUVbsa: standardized uptake value body surface area; MTV: metabolic tumor volume; TLG: total lesion glicolysis; SRE: short-run emphasis; LRE: long-run emphasis; LGRE: Low Gray-level Run Emphasis; HGRE: High Gray-level Run Emphasis; SRLGE: Short-Run Low Gray-level Em-phasis; SRHGE: Short-Run High Gray-level Emphasis; LRLGE: Long-Run Low Gray-level Emphasis; LRHGE: Long-Run High Gray-level Emphasis; GLNU: Gray-Level Non-Uniformity; RLNU: Run Length Non-Uniformity; RP: Run Percentage; SZE: Short-zone emphasis; LZE: Long-zone emphasis; LGZE: Low Gray-level Zone Emphasis; HGZE: High Gray-level Zone Emphasis; SZLGE: Short-Zone Low Gray-level Emphasis; SZHGE: Short-Zone High Gray-level Em-phasis; LZLGE: Long-Zone Low Gray-level Emphasis; LZHGE: Long-Zone High Gray-level Emphasis; ZLNU: Zone Length Non-Uniformity.Extraction of RF by LIFEx is only possible for VOI of at least 64 voxels, therefore 16 patients were excluded from the study because the volume of the TIs uptake was below this limit. As a consequence, the final number of patients included in the study was 221.
2.4. Statistical Analysis
Statistical analysis was performed using MedCalc Software version 18.1 (8400, Ostend, Belgium) and R (http://www.R-project.org/) software version 4.1.1 (Statistics Department of the University of Auckland, Auckland, New Zealand). In the descriptive analysis, the categorical variables were represented as simple and relative frequencies, while the numeric variables with mean, standard deviation, and range values. For both scanners, the kernel density estimation built on the RF values were qualitatively compared and the presence of significant differences were evaluated with the Mann–Whitney test.
The general statistical analysis line of the study was structured of various steps. First of all, a univariate analysis (with a logistic regressor, in a 10-cross-fold validation) was performed for the group of patients evaluated on scanner 1, 1 for the group of patients of scanner 2 and 1 for the entire group of patients (scanner 1 and scanner 2 considered together). This first analysis had the purpose to evaluate the influence of the two scanners on the ability of RF to correlate with the final clinical outcome.
Furthermore, a bivariate analysis was performed with the purpose of developing 3 predictive models (1 for scanner 1, 1 for scanner 2 and 1 for both scanners considered together), by analyzing all of the possible couples of variables (the cartesian product of semiquantitative parameters, RF and the major clinical features such as age, gender and ultrasound dimension of the Tis). This bivariate analysis was performed with a bivariate logistic regression model was applied in order to classify them on the basis of the area under the curve (AUC) under the receiving operator curve (ROC) after a 10-cross fold validation training/testing test. This bivariate model had the purpose to clearly explore all the space of RF presented in the study. Similarly, for each couple of variables, the accuracy was extrapolated and to obtain a more complete statistic, the p-value were also extracted.
Lastly, a selection of the models with the best bivariate logistic regression was performed for scanner 1, scanner 2 and for both scanners considered together. In this setting an AUC higher than 0.8 was arbitrarily considered optimal to predict the final diagnosis of TIs, while an AUC between 0.6 and 0.8 was considered acceptable. Similarly, a p-value < 0.05 was arbitrarily considered as statistically significant.
3. Results
3.1. Patients Characteristics
A total of 221 patients were included in the study (Table 2), with a mean age of 66 years (range 16–88). The majority of the patients were female (n = 149, 67%) while 72 (33%) were male. No significant difference in terms of sex between the 2 groups of malignant TIs and benignant TIs was underlined (p value = 0.07).
Table 2.
Characteristics of the 221 patients included in the study.
| Characteristic | N. (%) |
|---|---|
| Age, mean ± SD (range) | 66 ± 14 (16–88) |
| Sex | |
| Male | 72 (33%) |
| Female | 149 (67%) |
| Thyroid Lobe | |
| Right | 123 (56%) |
| Left | 87 (39%) |
| Isthmus | 11 (5%) |
| Ultrasound diameter (mm), mean ± SD (range) | 17 ± 12 (5–75) |
| Final Diagnosis | |
| Benign | 150 (68%) |
| Malign | 71 (32%) |
| Cytology (N. = 118) | |
| TIR2 | 35 (30%) |
| TIR3a | 24 (20%) |
| TIR3b | 30 (25%) |
| TIR4 | 13 (11%) |
| TIR5 | 16 (14%) |
| Histology (N. = 71) | |
| Anaplastic carcinoma | 3 (4%) |
| Follicular carcinoma | 7 (10%) |
| Papillary carcinoma | 61 (86%) |
| PET/CT Scanner | |
| Scanner 1 (Discovery 690) | 128 (58%) |
| Scanner 2 (Discovery STE) | 93 (42%) |
| Semiquantitative PET/CT parameters | |
| SUVmax, mean ± SD (range) | 7.9 ± 8 (1.3–56.7) |
| SUVmean, mean ± SD (range) | 4.3 ± 4 (1.0–37.1) |
| SUVlbm, mean ± SD (range) | 5.8 ± 6 (1.0–41.3) |
| SUVbsa, mean ± SD (range) | 2.0 ± 2 (0.4–12.6) |
| MTV, mean ± SD (range) | 9.2 ± 18 (0.4–198.0) |
| TLG, mean ± SD (range) | 35.0 ± 75 (1.9–722.4) |
N.: number, SD: standard deviation, mm: millimeters, SUVmax: standardized uptake value body weight max, SUVmean: standardized uptake value body mean, SUVlbm: standardized uptake value lean body mass, SUVbsa: standardized uptake value body surface area, MTV: metabolic tumor volume, TLG: total lesion glicolysis.
TIs were most frequent findings on the right thyroid lobe with 123 (56%) subjects, while in 87 (39%) the incidental uptake were discovered on the left lobe and only in 11 (5%) cases they were underlined at the isthmus. Again, the site of TIs was not significantly correlated with the final diagnosis (p value = 0.79).
The mean diameter of the TIs, evaluated on subsequent ultrasound evaluation, was of 17 mm (range 5–75).
Overall, the final diagnosis of TIs was malignant for 71 (32%) patients and benignant in 150 (68%) patients. In this setting, for the correct evaluation of their final diagnosis, 97 (44%) subjects were evaluated only with ultrasound exams, with a mean follow-up of 24 months (range 12–168). Five (2%) patients performed a 99mTc thyroid scintigraphy, that revealed the presence of an hyperfunctioning adenoma.
For 118 (53%) patients, a cytological examination for the correct diagnosis of incidental 18F-FDG uptakes was performed, classifying the results according to the Italian Thyroid Cytology Classification System [27]. In particular, in 16 (14%) cases the result of the cytological examination was TIR5, in 13 (11%) it was TIR4, in 54 (45%) it was TIR3 while in 35 (30%) it was TIR2. Furthermore, of the 54 patients with a TIR3 classification, 24 (44%) had a TIR3a result while TIR3b was the final cytological result for 30 (56%) patients. A histological diagnosis of the TIs was performed in 71 (32%) cases and all of them revealed the presence of malignancy. In particular, in 3 (4%) cases the presence of anaplastic carcinoma was revealed, in 7 (10%) the presence of follicular carcinoma was underlined and in 61 (86%) there was a final diagnosis of papillary carcinoma. An evaluation of the predictive abilities of semiquantitative PET/CT parameters and of RF to predict the final cytological or histologic diagnosis was not performed because of the low sample of subjects beneath all the subgroups mentioned before.
A total of 128 (58%) scans were performed on the Discovery 690 tomograph (scanner 1), while 93 (42%) of them were acquired on the Discovery STE tomograph (scanner 2). The mean value of the SUVmax of the TIs was 7.9, it was 4.3 for SUVmean, 5.8 for SUVlbm, 2.0 for SUVbsa, 9.2 for MTV and 35.0 for TLG. (Figure 1).
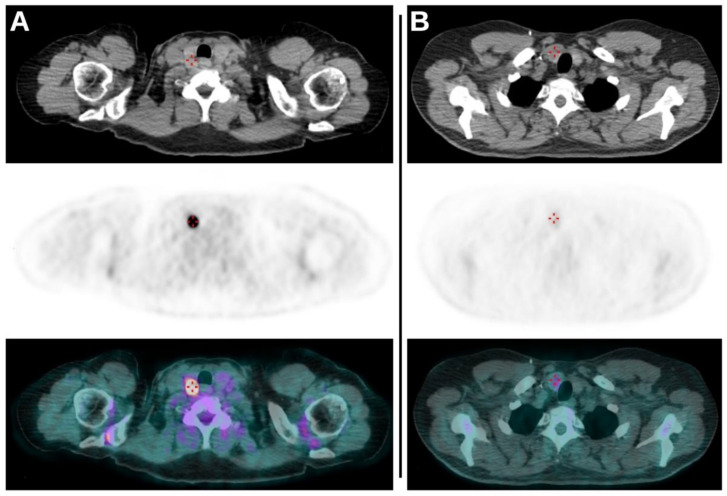
Figure 1.
(A): Axial CT, axial PET and axial fused PET/CT images demonstrating the presence of TI revealed as intense focal uptake of 18F-FDG on the right lobe of thyroid. The lesion had a SUVmax of 44.47, an MTV of 0.7 and a TLG of 18.1 and subsequent cytological exam revealed no malignancy (TIR2). (B): Axial CT, axial PET and axial fused PET/CT images of another scan demonstrating again the presence of TI as a faint uptake on the right lobe of thyroid. The values of SUVmax, MTV and TLG of the lesion were 2.64, 6.9 and 10.3, respectively. Cytological evaluation (TIR5) and subsequent total thyroidectomy revealed the presence of papillary carcinoma.
Analyzing PET/CT acquisition depending on the tomograph used for their execution, in 92 (72%) scans performed on scanner 1 the incidental uptake resulted of benign nature while in 36 (28%) cases the final diagnosis was malignancy (1 anaplastic carcinoma, 4 follicular carcinomas and 31 papillary carcinomas). Regarding scanner 2, in 58 (62%) scans the final diagnosis of incidental uptake was benignancy while in 35 (38%) cases the presence of malignancy was underlined (2 anaplastic carcinomas, 3 follicular carcinomas and 30 papillary carcinomas). No significant difference in terms of final diagnosis was reported between the 2 scanners (p value = 0.1).
3.2. Comparison between the Two Scanners
The major clinical and epidemiological characteristics of the patients (age, sex, ultrasound dimension and final diagnosis of the TIs) were not significantly different between the two scanners.
Regarding semiquantitative parameters of PET/CT, only the values of SUVmax resulted significantly different between the 2 scanners (p value = 0.046), while the remaining parameters were not. In particular, the SUVmax values resulted higher on scanner 1 compared to scanner 2.
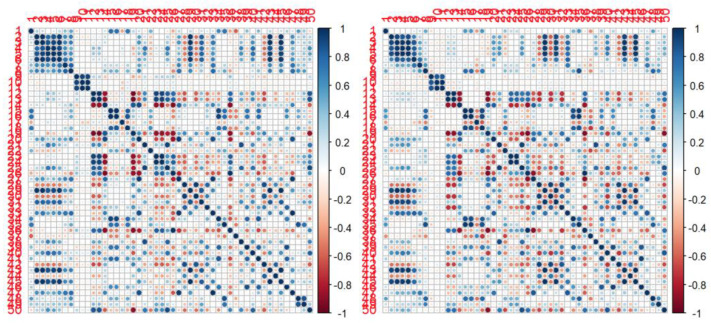
Focusing on RF, only 9 of 42 resulted in significant differences between the 2 scanners. In particular, RF with apparent correlation on the type of scanner used for the acquisition were Histo entropy_log10, Histo entropy_log2, Histo Energy, GLRLM LGRE, GLRLM SRLGE, NGLDM busyness, GLZLM SZE, GLZLM SZHGE and GLZLM ZLNU (Table 3). However, cross-correlation maps of RF between the two scanners were quite similar (Figure 2).
Table 3.
Comparison of clinical parameters, semiquantitative PET/CT parameters and radiomics features between the two scanners.
| Parameters | p-Value |
|---|---|
| Clinical | |
| Age | 0.787 |
| Sex | 0.522 |
| Diameters at ultrasound | 0.446 |
| Semiquantitative PET/CT parameters | |
| SUVmax | 0.046 |
| SUVmean | 0.118 |
| SUVlbm | 0.119 |
| SUVbsa | 0.076 |
| MTV | 0.595 |
| TLG | 0.869 |
| Radiomics features | |
| Histo skewness | 0.193 |
| Histo kurtosis | 0.924 |
| Histo excess kurtosis | 0.924 |
| Histo entropy_log10 | 0.023 |
| Histo entropy_log2 | 0.024 |
| Histo energy | 0.017 |
| Shape volume_mL | 0.211 |
| Shape volume_vx | 0.560 |
| Shape sphericity | 0.088 |
| Shape compacity | 0.518 |
| GLCM homogeneity | 0.104 |
| GLCM energy | 0.638 |
| GLCM contrast | 0.132 |
| GLCM correlation | 0.889 |
| GLCM entropy_log10 | 0.319 |
| GLCM entropy_log2 | 0.315 |
| GLCM dissimilarity | 0.145 |
| GLRLM SRE | 0.123 |
| GLRLM LRE | 0.113 |
| GLRLM LGRE | 0.026 |
| GLRLM HGRE | 0.069 |
| GLRLM SRLGE | 0.036 |
| GLRLM SRHGE | 0.069 |
| GLRLM LRLGE | 0.098 |
| GLRLM LRHGE | 0.135 |
| GLRLM GLNU | 0.260 |
| GLRLM RLNU | 0.962 |
| GLRLM RP | 0.126 |
| NGLDM coarseness | 0.471 |
| NGLDM contrast | 0.476 |
| NGLDM busyness | 0.006 |
| GLZLM SZE | 0.017 |
| GLZLM LZE | 0.168 |
| GLZLM LGZE | 0.053 |
| GLZLM HGZE | 0.086 |
| GLZLM SZLGE | 0.069 |
| GLZLM SZHGE | 0.041 |
| GLZLM LZLGE | 0.102 |
| GLZLM LZHGE | 0.561 |
| GLZLM GLNU | 0.366 |
| GLZLM ZLNU | 0.026 |
| GLZLM ZP | 0.093 |
Figure 2.
Correlation maps for first and second order RF between the two scanners. Scanner 1 (Discovery 690) is presented on the left, while scanner 2 (Discovery STE) is presented on the right. Blue means high positive correlation; red means high negative correlation; white means no correlation.
3.3. Predictive Accuracy
At univariate analysis (Table 4), for scanner 1 (Discovery 690) all PET/CT semiquantitative parameters and RF obtained an AUC value between 0.6 and 0.8. Regarding scanner 2 (Discovery STE), again all of the semiquantitative PET/CT parameters and RF reached a value of AUC between 0.6 and 0.8; in general, these values were lower than the those reported for scanner 1. Furthermore, the evaluation of p-value allowed the selection of some parameters with the best performances for the prediction of the final diagnosis of Tis, for both scanner 1 and scanner 2.
Table 4.
Univariate analysis for semiquantitative PET/CT parameters and for radiomics features for the single scanner and for both scanners considered together. Only values with AUC > 0.6 and p-value < 0.05 are reported.
| Mean AUC | Mean p-Value | |||||
|---|---|---|---|---|---|---|
| Parameters | Scanner 1 | Scanner 2 | Scanner 1 + 2 | Scanner 1 | Scanner 2 | Scanner 1 + 2 |
| SUVmax | 0.762 | 0.679 | 0.748 | <0.01 | 0.02 | <0.01 |
| SUVmean | 0.724 | 0.675 | 0.748 | <0.01 | <0.01 | <0.01 |
| SUVlbm | 0.757 | 0.685 | 0.748 | <0.01 | 0.01 | <0.01 |
| SUVbsa | 0.756 | 0.689 | 0.742 | <0.01 | 0.01 | <0.01 |
| Histo entropy_log10 | 0.709 | 0.674 | 0.724 | <0.01 | <0.01 | <0.01 |
| Histo entropy_log2 | 0.705 | 0.674 | 0.724 | <0.01 | <0.01 | <0.01 |
| GLCM entropy_log10 | 0.713 | 0.664 | 0.702 | 0.02 | 0.03 | <0.01 |
| GLCM entropy_log2 | 0.712 | 0.664 | 0.703 | 0.02 | 0.03 | <0.01 |
| GLCM dissimilarity | 0.719 | 0.682 | 0.727 | 0.01 | <0.01 | <0.01 |
| GLRLM HGRE | 0.731 | 0.693 | 0.741 | 0.03 | 0.03 | <0.01 |
| GLRLM SRHGE | 0.739 | 0.682 | 0.744 | 0.02 | 0.02 | <0.01 |
| GLRLM LRLGE | 0.707 | 0.653 | 0.715 | 0.01 | 0.01 | <0.01 |
| GLZLM SZE | 0.734 | 0.671 | 0.693 | <0.01 | <0.01 | 0.01 |
| GLZLM HGZE | 0.740 | 0.668 | 0.740 | 0.02 | 0.03 | <0.01 |
| GLZLM SZHGE | 0.758 | 0.693 | 0.733 | 0.02 | 0.03 | <0.01 |
| GLZLM ZP | 0.692 | 0.669 | 0.699 | <0.01 | 0.01 | <0.01 |
| Variables with good performances only at Scanner 1 + 2 analysis | ||||||
| GLCM contrast | 0.733 | 0.01 | ||||
| GLZLM ZLNU | 0.729 | 0.04 | ||||
| GLRLM LRLGE | 0.715 | <0.01 | ||||
| GLZLM LGZE | 0.706 | <0.01 | ||||
| GLRLM LGRE | 0.703 | <0.01 | ||||
| GLCM homogeneity | 0.702 | <0.01 | ||||
| GLRLM SRLGE | 0.687 | <0.01 | ||||
| NGLDM busyness | 0.684 | 0.01 | ||||
| GLRLM RP | 0.660 | 0.04 | ||||
| GLZLM SZLGE | 0.651 | <0.01 | ||||
AUC: area under the curve.
Considering the combined analysis of both the scanners together (scanner 1 + 2), in general PET/CT semiquantitative parameters revealed a higher AUC compared to RF, with significant p-value. Interestingly, the combined evaluation of both scanners revealed acceptable values of UAC with significant p-value for some RF, even if the same RF did not reach these values at the analysis for single scanner.
After performing a bivariate analysis, for both the single scanners and for both of the scanners considered together, the best combinations between PET/CT semiquantitative parameters and RF are summarized in Table 5. Similarly to univariate analysis, none of the combinations reached an optimal AUC of 0.8 and the couples of parameters generally obtained higher AUC values on scanner 1 than on scanner 2. Furthermore, for this analysis, the p-values were statistically more significant on scanner 1 than on scanner 2. In this setting, even if a comparison between the couples of variables obtained before is complex given the heterogeneity between the two scanners, in general GLCM-related parameters variously combined resulted the ones with best performances. This is true for both scanner 1 and scanner 2 and these findings are confirmed by the good results at univariate analysis previously described. The GLRLM-related and GLZLM-related RF also revealed good performances in this setting. Interestingly, PET/CT semiquantitative parameters were confirmed as good predictors only for scanner 2 (Figure 3).
Table 5.
Bivariate analysis for clinical, semiquantitative PET/CT parameters and radiomics features for the single scanner and for both scanners considered together. For each analysis, only the couples with best performances are reported.
| Covariate 1 | Covariate 2 | Mean p-Value 1 | Mean p-Value 2 | Mean AUC |
|---|---|---|---|---|
| Scanner 1 | ||||
| GLZLM GLNU | MTV | <0.01 | 0.01 | 0.779 |
| GLRLM RLNU | MTV | 0.02 | 0.03 | 0.776 |
| GLCM energy | GLCM entropy_log2 | 0.04 | <0.01 | 0.771 |
| GLCM energy | GLCM entropy_log10 | 0.04 | <0.01 | 0.771 |
| GLCM entropy_log2 | GLRLM HGRE | 0.01 | 0.03 | 0.763 |
| GLCM entropy_log10 | GLZLM HGZE | 0.02 | 0.02 | 0.762 |
| GLCM entropy_log10 | GLRLM HGRE | 0.01 | 0.03 | 0.761 |
| GLCM entropy_log2 | GLZLM HGZE | 0.02 | 0.02 | 0.760 |
| GLCM entropy_log10 | GLZLM SZHGE | 0.01 | 0.02 | 0.760 |
| GLCM entropy_log2 | GLZLM SZHGE | 0.01 | 0.02 | 0.759 |
| GLRLM RP | GLZLM SZHGE | 0.04 | 0.02 | 0.751 |
| GLRLM HGRE | GLRLM RP | 0.02 | 0.03 | 0.745 |
| MTV | TLG | <0.01 | 0.01 | 0.741 |
| GLRLM SRE | GLZLM HGZE | 0.03 | 0.01 | 0.740 |
| NGLDM coarseness | NGLDM busyness | <0.01 | 0.01 | 0.738 |
| Shape volume_mL | GLRLM GLNU | 0.03 | 0.01 | 0.736 |
| GLRLM GLNU | NGLDM coarseness | 0.03 | <0.01 | 0.734 |
| GLRLM SRE | GLZLM SZHGE | 0.03 | 0.02 | 0.732 |
| GLRLM SRE | GLRLM HGRE | 0.03 | 0.02 | 0.730 |
| GLRLM LRLGE | NGLDM coarseness | <0.01 | 0.04 | 0.730 |
| Shape volume_vx | GLRLM GLNU | 0.02 | 0.02 | 0.723 |
| GLCM entropy_log10 | GLZLM SZHGE | 0.04 | <0.01 | 0.713 |
| Shape compacity | GLZLM GLNU | 0.01 | <0.01 | 0.707 |
| Shape volume_mL | MTV | 0.02 | 0.02 | 0.693 |
| Ultrasound dimension | MTV | 0.01 | 0.02 | 0.691 |
| GLCM correlation | NGLDM coarseness | <0.01 | <0.01 | 0.690 |
| Shape compacity | NGLDM coarseness | 0.03 | 0.01 | 0.680 |
| Ultrasound dimension | GLRLM GLNU | 0.01 | 0.01 | 0.677 |
| Scanner 2 | ||||
| GLRLM SRE | SUVmean | 0.04 | 0.01 | 0.712 |
| GLCM entropy_log10 | SUVbsa | 0.05 | 0.01 | 0.697 |
| GLCM entropy_log2 | SUVbsa | 0.05 | 0.02 | 0.696 |
| GLCM entropy_log2 | GLZLM SZHGE | 0.03 | 0.02 | 0.689 |
| GLCM entropy_log10 | GLZLM SZHGE | 0.03 | 0.02 | 0.689 |
| GLRLM RP | SUVmean | 0.05 | 0.02 | 0.686 |
| GLCM entropy_log2 | GLZLM HGZE | 0.05 | 0.02 | 0.682 |
| GLCM entropy_log10 | GLZLM HGZE | 0.05 | 0.02 | 0.680 |
| GLCM entropy_log10 | GLRLM HGRE | 0.04 | 0.02 | 0.679 |
| GLCM energy | GLRLM LRHGE | 0.01 | 0.02 | 0.679 |
| GLCM entropy_log2 | GLRLM HGRE | 0.04 | 0.02 | 0.679 |
| NGLDM coarseness | GLZLM ZP | 0.03 | <0.01 | 0.677 |
| Histo energy | GLRLM HGRE | 0.04 | 0.03 | 0.676 |
| GLCM homogeneity | NGLDM coarseness | <0.01 | 0.06 | 0.675 |
| GLCM contrast | GLCM entropy_log10 | 0.04 | 0.04 | 0.673 |
| GLCM contrast | GLCM entropy_log2 | 0.04 | 0.04 | 0.673 |
| Histo energy | GLZLM SZHGE | 0.04 | 0.03 | 0.669 |
| GLRLM SRE | GLRLM HGRE | 0.04 | 0.03 | 0.669 |
| GLRLM SRE | NGLDM coarseness | 0.01 | 0.05 | 0.668 |
| GLRLM LRE | SUVmean | 0.04 | 0.01 | 0.668 |
| NGLDM coarseness | NGLDM busyness | 0.02 | 0.02 | 0.666 |
| GLZLM GLNU | MTV | 0.02 | 0.02 | 0.663 |
| GLCM energy | GLZLM SZHGE | 0.06 | <0.01 | 0.660 |
| GLCM energy | GLRLM HGRE | 0.06 | <0.01 | 0.659 |
| GLRLM RP | NGLDM coarseness | 0.01 | 0.05 | 0.657 |
| GLRLM RLNU | MTV | 0.01 | 0.01 | 0.650 |
| NGLDM coarseness | MTV | 0.04 | 0.03 | 0.627 |
| Scanner 1 + 2 | ||||
| GLCM entropy_log2 | GLZLM SZHGE | <0.01 | <0.01 | 0.769 |
| GLCM entropy_log10 | GLRLM HGRE | <0.01 | <0.01 | 0.769 |
| GLCM entropy_log10 | GLZLM SZHGE | <0.01 | <0.01 | 0.769 |
| GLCM entropy_log10 | GLZLM HGZE | <0.01 | <0.01 | 0.769 |
| GLCM entropy_log2 | GLRLM HGRE | <0.01 | <0.01 | 0.768 |
| GLCM entropy_log2 | GLZLM HGZE | <0.01 | <0.01 | 0.768 |
| GLRLM SRE | SUVmean | <0.01 | <0.01 | 0.763 |
| GLRLM GLNU | NGLDM Coarseness | <0.01 | <0.01 | 0.756 |
| GLCM homogeneity | GLRLM HGRE | <0.01 | <0.01 | 0.749 |
| GLCM homogeneity | GLZLM HGZE | <0.01 | <0.01 | 0.749 |
| Histo energy | GLRLM HGRE | <0.01 | <0.01 | 0.749 |
| Histo energyUniformity | GLZLM SZHGE | <0.01 | <0.01 | 0.748 |
| GLCM homogeneity | GLZLM SZHGE | <0.01 | <0.01 | 0.748 |
| NGLDM coarseness | NGLDM busyness | <0.01 | <0.01 | 0.746 |
| GLRLM SRE | GLRLM HGRE | <0.01 | <0.01 | 0.742 |
| GLRLM RP | GLZLM HGZE | <0.01 | <0.01 | 0.742 |
| GLRLM SRE | GLZLM HGZE | <0.01 | <0.01 | 0.742 |
| GLRLM HGRE | GLRLM RP | <0.01 | <0.01 | 0.742 |
| NGLDM coarseness | GLZLM ZP | <0.01 | <0.01 | 0.741 |
| GLZLM GLNU | MTV | <0.01 | <0.01 | 0.738 |
| GLRLM SRE | GLZLM SZHGE | <0.01 | <0.01 | 0.738 |
| GLRLM RP | GLZLM SZHGE | <0.01 | <0.01 | 0.737 |
| GLRLM LRE | GLRLM LRHGE | <0.01 | <0.01 | 0.737 |
| GLRLM RLNU | MTV | <0.01 | <0.01 | 0.730 |
| Histo energy | GLCM energy | <0.01 | <0.01 | 0.717 |
| Shape compacity | NGLDM coarseness | <0.01 | <0.01 | 0.681 |
| GLCM correlation | NGLDM coarseness | <0.01 | <0.01 | 0.654 |
| Shape compacity | GLZLM GLNU | <0.01 | <0.01 | 0.640 |
AUC: area under the curve.
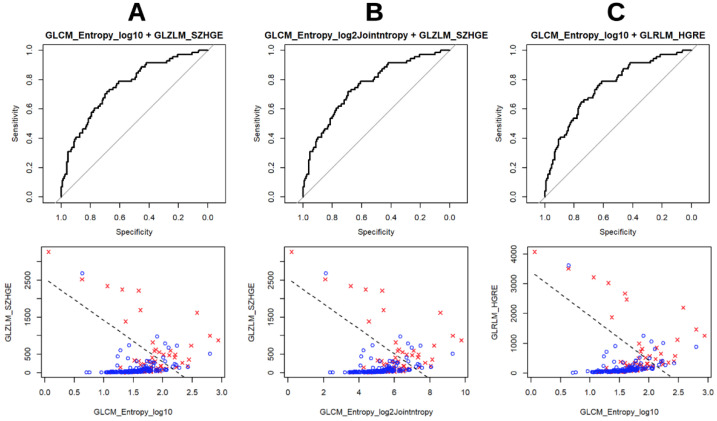
Figure 3.
Visual representations of the three combinations ((A) GLCM Entropy_log10+GLZLM_SZHGE, (B) GLCM Entropy_log2+GLZLM:SZHGE; (C) GLCM Entropy_lo10+GLRLM_HGRE) with best performances at bivariate analysis for both scanners considered together.
4. Discussion
The aim of this study was to verify the predictive abilities of semiquantitative PET/CT parameters and of RF to discriminate between benignant and malignant nature of TIs revealed at 18F-FDG imaging.
On the basis of the resulting evidence we identified some remarkable points concerning the effect of different PET scanners on RF extraction and the predictive features and associated models.
In our experimental setting, we had to deal with images coming from different PET/CT tomographs and this fact required a preliminary investigation of the effect of different technologies in producing images and subsequent image features. The results showed that the scanner technology concretely affects some RF, as previously underlined in literature, and in clinical day practice the use of different tomographs in the same department is frequent [20,28,29,30,31,32,33,34]. In particular, the acquisition of the same phantoms on different tomographs with different scintillators and algorithm used for the reconstruction (number of iterations, number of subsets or on the presence of partial volume correction) demonstrated this evidence.
These findings suggest two relevant points: the former indicates that different scanners can potentially have different preferred features in terms of correlations with a clinical outcome; the second point suggests that we must critically consider radiomics models coming from centers adopting different technologies. In other words, on one hand a unique radiomic best model trained on many scanners is probably suboptimal for each of them and on the other hand, any radiomic model coming from different centers should be internally validated before considering its use in the daily practice. In particular, in the literature only one study which evaluated the predictive role of RF in TIs [23] used different scanners for the extraction of RF: this means that the reproducibility of the results (which is one of the biggest challenges in radiomics) still remain uninvestigated in this field. Furthermore, in our evaluation, only a small amount of RF demonstrated to be significantly different between the two scanners, together with SUVmax, but nevertheless the cross-correlation maps resulted quite similar, adding value to our results. In this setting, of the parameters that after bivariate analysis demonstrated the best performances, GLZLM SZHGE was the only one significantly different between the two scanners.
Regarding the predictive role of RF for the correct evaluation of Tis, at univariate and bivariate analysis a good percentage of the aforementioned parameters revealed an acceptable AUC between 0.6 and 0.8. However, none of them demonstrated an AUC above 0.8. Similarly, these AUC were coupled with a significant p-value in a high percentage of the cases. It is worth underlining the fact that at bivariate analysis performed for both the scanner considered together, the AUC values and the p-values were the best in the whole study. This fact underlines a good predictive ability of some RF such as GLCM-related (in particular GLCM entropy_log2 e GLCM entropy_log10), GLRLM-related and GLZLM-related.
Only a small amount of works that investigate the predictive role of radiomics in the evaluation of TIs at 18F-FDG PET/CT are available in literature [21,22,23,24].
Even if not clearly characterized by the presence of a proper texture analysis, the first study to evaluate the distributive heterogeneity of 18F-FDG in TIs was produced by Kim et al. [24]. In this work, the authors revealed that this heterogeneity was a promising parameter which was able to predict the final nature of these TIs.
Subsequently, Sollini et al. [23] were the first to evaluate the predictive abilities of texture analysis in this setting. Data of this study underlined the fact that SUVstd (the standard deviation of the distribution of SUV inside the considered VOI), SUVmax, MTV, TLG, Histo skewness, Histo kurtosis and GLCM correlation were the only parameters that were able to predict the final diagnosis of TIs, with a general positive predictive value of 54% and a general negative predictive value of 85%.
A similar analysis was also performed by Aksu et al. [22], who underlined how the semiquantitative PET/CT parameters and some shape-related, GLCM-related, GLRLM-related and GLZLM-related RF obtained AUC values superior to 0.7. These findings were partially confirmed in our study, where the same parameters confirmed these good results, with the exception of semiquantitative parameters and shape-related RF. Furthermore, the authors of the study developed a machine-learning algorithm using GLRLM RLNU e SUVmax with a good general AUC value (0.731).
Lastly, Ceriani et al. [21] demonstrated the ability to predict the final nature of TIs of some PET/CT semiquantitative parameters (SUVmax, SUVmean, SUVpeak, MTV e TLG) and some RF. In this case, the authors performed texture analysis with a different software from LIFEx and so RF resulted partly different in comparison to the ones used in our study. In general, some shape-related and GLCM-related features demonstrated good performances and multivariate analysis confirmed TLG, SUVmax and Shape sphericity as able to predict the final nature of Tis.
It is interesting to underline that PET/CT semiquantitative parameters resulted good predictors in all of the studies, while in our work only SUVmean obtained a certain predictive role at bivariate analysis. In this setting, we reported that AUC of semiquantitative parameters were quite similar to AUC of RF only at monovariate analysis. Given the fact that the bivariate predictive model did not confirm this evidence, we can assume that these parameters do not perform well when trying to build models with multiple variables as in our case. Furthermore, as previously described, data in literature about the role of these parameters for the assessment of TIs are really heterogeneous and our findings confirm these insights. Moreover, RF describe quality and parameters of images that cannot be visually assessed and this is why we focused our attention on the evaluation of these features, allowing us to better understand the role of 18F-FDG PET/CT in the prediction of Tis nature.
Our study surely presents some limitations. First of all, this is a retrospective study with the use of tomography that are not the actual state. Furthermore, the relatively low sample of patients included in the work, even if higher than similar studies, appears sub-optimal to clearly evaluate the predictive abilities of texture analysis. Furthermore, RF extrapolation with a single software appears another limit of our analysis. Lastly, the aforementioned problem of the reproducibility of radiomics analysis in terms of multicentric evaluation is still an open issue and, in this setting, further research in this field are mandatory.
5. Conclusions
In conclusion, our study enabled the selection of some RF that are able to predict with a certain good accuracy, the final nature of TIs discovered at 18F-FDG PET/CT imaging. Classic semiquantitative and volumetric PET/CT parameters did not reveal this ability. Furthermore, a good overlap in the extraction of RF between the two scanners was underlined.
Author Contributions
Conceptualization, F.D., R.G. (Roberto Gattaand) and D.A.; methodology, R.G. (Raffaele Giubbini) and N.P.; formal analysis, F.D. and R.G. (Roberto Gattaand); writing—original draft preparation, F.D., D.A. and R.G. (Raffaele Giubbini); writing—review and editing, N.P. and F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the retrospective design of the study according to the local laws.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are not public, but are present in our institution.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DeGroot L.J., Kaplan E.L., McCormick M., Straus F.H. Natural history, treatment, and course of papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 1990;71:414–424. doi: 10.1210/jcem-71-2-414. [DOI] [PubMed] [Google Scholar]
- 2.Schlumberger M.J. Papillary and follicular thyroid carcinoma. N. Engl. J. Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 3.Stokkel M.P., Duchateau C.S., Dragoiescu C. The value of FDG-PET in the follow-up of differentiated thyroid cancer: A review of the literature. Q. J. Nucl. Med. Mol. Imaging. 2006;50:78–87. [PubMed] [Google Scholar]
- 4.Bertagna F., Treglia G., Piccardo A., Giubbini R. Diagnostic and clinical significance of F-18-FDG-PET/CT thyroid incidentalomas. J. Clin. Endocrinol. Metab. 2012;97:3866–3875. doi: 10.1210/jc.2012-2390. [DOI] [PubMed] [Google Scholar]
- 5.Filetti S., Durante C., Hartl D., Leboulleux S., Locati L.D., Newbold K., Papotti M.G., Berruti A., ESMO Guidelines Committee Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019;30:1856–1883. doi: 10.1093/annonc/mdz400. [DOI] [PubMed] [Google Scholar]
- 6.Vaccarella S., Franceschi S., Bray F., Wild C.P., Plummer M., Dal Maso L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N. Engl. J. Med. 2016;375:614–617. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 7.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luster M., Clarke S.E., Dietlein M., Lassmann M., Lind P., Oyen W.J., Tennvall J., Bombardieri E. European Association of Nuclear Medicine (EANM). Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:1941–1959. doi: 10.1007/s00259-008-0883-1. [DOI] [PubMed] [Google Scholar]
- 9.Bertagna F., Bosio G., Biasiotto G., Rodella C., Puta E., Gabanelli S., Lucchini S., Merli G., Savelli G., Giubbini R., et al. F-18 FDG-PET/CT evaluation of patients with differentiated thyroid cancer with negative I-131 total body scan and high thyroglobulin level. Clin. Nucl. Med. 2009;34:756–761. doi: 10.1097/RLU.0b013e3181b7d95c. [DOI] [PubMed] [Google Scholar]
- 10.Dong M.J., Liu Z.F., Zhao K., Ruan L.X., Wang G.L., Yang S.Y., Sun F., Luo X.G. Value of 18F-FDG-PET/PET-CT in differentiated thyroid carcinoma with radioiodine-negative whole-body scan: A meta-analysis. Nucl. Med. Commun. 2009;30:639–650. doi: 10.1097/MNM.0b013e32832dcfa7. [DOI] [PubMed] [Google Scholar]
- 11.Elzein S., Ahmed A., Lorenz E., Balasubramanian S.P. Thyroid incidentalomas on PET imaging—Evaluation of management and clinical outcomes. Surgeon. 2015;13:116–120. doi: 10.1016/j.surge.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Bomanji J.B., Costa D.C., Ell P.J. Clinical role of positron emission tomography in oncology. Lancet Oncol. 2001;2:157–164. doi: 10.1016/S1470-2045(00)00257-6. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y.K., Ding H.J., Chen K.T., Chen Y.L., Liao A.C., Shen Y.Y., Su C.T., Kao C.H. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for cancer screening in healthy subjects. Anticancer Res. 2005;25:1421–1426. [PubMed] [Google Scholar]
- 14.Shi H., Yuan Z., Yuan Z., Yang C., Zhang J., Shou Y., Zhang W., Ping Z., Gao X., Liu S. Diagnostic Value of Volume-Based Fluorine-18-Fluorodeoxyglucose PET/CT Parameters for Characterizing Thyroid Incidentaloma. Korean J. Radiol. 2018;19:342–351. doi: 10.3348/kjr.2018.19.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burguera B., Gharib H. Thyroid incidentalomas. Prevalence, diagnosis, significance, and management. Endocrinol. Metab. Clin. N. Am. 2000;29:187–203. doi: 10.1016/S0889-8529(05)70123-7. [DOI] [PubMed] [Google Scholar]
- 16.Are C., Hsu J.F., Schoder H., Shah J.P., Larson S.M., Shaha A.R. FDG-PET detected thyroid incidentalomas: Need for further investigation? Ann. Surg. Oncol. 2007;14:239–247. doi: 10.1245/s10434-006-9181-y. [DOI] [PubMed] [Google Scholar]
- 17.Kim T.Y., Kim W.B., Ryu J.S., Gong G., Hong S.J., Shong Y.K. 18F-fluorodeoxyglucose uptake in thyroid from positron emission tomogram (PET) for evaluation in cancer patients: High prevalence of malignancy in thyroid PET incidentaloma. Laryngoscope. 2005;115:1074–1078. doi: 10.1097/01.MLG.0000163098.01398.79. [DOI] [PubMed] [Google Scholar]
- 18.Kim B.H., Na M.A., Kim I.J., Kim S.J., Kim Y.K. Risk stratification and prediction of cancer of focal thyroid fluorodeoxyglucose uptake during cancer evaluation. Ann. Nucl. Med. 2010;24:721–728. doi: 10.1007/s12149-010-0414-6. [DOI] [PubMed] [Google Scholar]
- 19.Wilson R., Devaraj A. Radiomics of pulmonary nodules and lung cancer. Transl. Lung Cancer Res. 2017;6:86–91. doi: 10.21037/tlcr.2017.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albano D., Gatta R., Marini M., Rodella C., Camoni L., Dondi F., Giubbini R., Bertagna F. Role of 18F-FDG PET/CT Radiomics Features in the Differential Diagnosis of Solitary Pulmonary Nodules: Diagnostic Accuracy and Comparison between Two Different PET/CT Scanners. J. Clin. Med. 2021;10:5064. doi: 10.3390/jcm10215064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceriani L., Milan L., Virili C., Cascione L., Paone G., Trimboli P., Giovanella L. Radiomics Analysis of [18F]-Fluorodeoxyglucose-Avid Thyroid Incidentalomas Improves Risk Stratification and Selection for Clinical Assessment. Thyroid. 2021;31:88–95. doi: 10.1089/thy.2020.0224. [DOI] [PubMed] [Google Scholar]
- 22.Aksu A., Şen N.P.K., Acar E., Kaya G.Ç. Evaluating Focal 18F-FDG Uptake in Thyroid Gland with Radiomics. Nucl. Med. Mol. Imaging. 2020;54:241–248. doi: 10.1007/s13139-020-00659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sollini M., Cozzi L., Pepe G., Antunovic L., Lania A., Di Tommaso L., Magnoni P., Erba P.A., Kirienko M. [18F]FDG-PET/CT texture analysis in thyroid incidentalomas: Preliminary results. Eur. J. Hybrid Imaging. 2017;1:3. doi: 10.1186/s41824-017-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S.J., Chang S. Predictive value of intratumoral heterogeneity of F-18 FDG uptake for characterization of thyroid nodules according to Bethesda categories of fine needle aspiration biopsy results. Endocrine. 2015;50:681–688. doi: 10.1007/s12020-015-0620-z. [DOI] [PubMed] [Google Scholar]
- 25.Boellaard R., Delgado-Bolton R., Oyen W.J., Giammarile F., Tatsch K., Eschner W., Verzijlbergen F.J., Barrington S.F., Pike L.C., Weber W.A., et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging. 2014;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nioche C., Orlhac F., Boughdad S., Reuzè S., Goya-Outi J., Robert C., Pellot-Barakt C., Soussan M., Frouin F., Buvat I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018;78:4786–4789. doi: 10.1158/0008-5472.CAN-18-0125. [DOI] [PubMed] [Google Scholar]
- 27.Nardi F., Basolo F., Crescenzi A., Fadda G., Frasoldati A., Orlandi F., Palombini L., Papini E., Zini M., Pontecorvi A., et al. Italian consensus for the classification and reporting of thyroid cytology. J. Endocrinol. Investig. 2014;37:593–599. doi: 10.1007/s40618-014-0062-0. [DOI] [PubMed] [Google Scholar]
- 28.Reynes-Llompart G., Sabatè-Llobera A., Linares-Tello E., Martì-Climent J., Gamez-Cenzano C. Image quality evaluation in a modern PET system: Impact of new reconstructions methods and a radiomics approach. Sci. Rep. 2019;9:10640. doi: 10.1038/s41598-019-46937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha S., Choi H., Paeng J.C., Cheon G.J. Radiomics in Oncological PET/CT: A Methodological Overview. Nucl. Med. Mol. Imaging. 2019;53:14–29. doi: 10.1007/s13139-019-00571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lodge M.A. Repeatability of SUV in Oncologic 18F-FDG PET. J. Nucl. Med. 2017;58:523–532. doi: 10.2967/jnumed.116.186353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook G.J.R., Azad G., Owczarczyk K., Siddique M., Goh V. Challenges and Promises of PET Radiomics. Int. J. Radiat. Oncol. Biol. Phys. 2018;102:1083–1089. doi: 10.1016/j.ijrobp.2017.12.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zwanenburg A. Radiomics in nuclear medicine: Robustness, reproducibility, standardization, and how to avoid data analysis traps and replication crisis. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:2638–2655. doi: 10.1007/s00259-019-04391-8. [DOI] [PubMed] [Google Scholar]
- 33.Lovinfosse P., Visvikis D., Hustinx R., Hatt M. FDG PET radiomics: A review of the methodological aspects. Clin. Transl. Imaging. 2018;6:379–391. doi: 10.1007/s40336-018-0292-9. [DOI] [Google Scholar]
- 34.Pfaehler E., van Sluis J., Merema B.B.J., van Ooijen P., Berendsen R.C.M., van Velden F.H.P., Boellaard R. Experimental Multicenter and Multivendor Evaluation of the Performance of PET Radiomic Features Using 3-Dimensionally Printed Phantom Inserts. J. Nucl. Med. 2020;61:469–476. doi: 10.2967/jnumed.119.229724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not public, but are present in our institution.