Abstract
The recent move to require sex as a biological variable (SABV), which includes gender, into the reporting of research published by the American Journal of Physiology-Heart and Circulatory Physiology follows a growing, and much-needed, trend by journals. Understandably, there is concern over how to do this without adding considerable work, especially if one’s primary research focus is not on elucidating sex/gender differences. The purpose of this article is to provide additional guidance and examples on how to incorporate SABV into the conduct and reporting of basic and clinical research. Using examples from our research, which includes both studies focused and not focused on sex/gender differences, we offer suggestions for how to incorporate SABV into basic and clinical research studies.
Keywords: cardiovascular, gender, heart failure, sex, study design
In 2021, the Editors of the American Journal of Physiology-Heart and Circulatory Physiology (AJP-Heart Circ) reinforced their commitment to integrate sex as a biological variable (SABV), which includes gender, into the experimental design and reporting of studies published by the journal (1). Although the National Institutes of Health and other funding organizations have required SABV in grant applications since 2016 (e.g., https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html), journals generally have not enforced the same expectation in published manuscripts. In basic science, researchers have been slow to fully incorporate male and female animals in experiments and report sex-specific analyses. In clinical science, although more studies are inclusive of women, studies of cardiovascular disease (CVD) still remain woefully underrepresentative of women (2), and publications often do not explore or account for sex/gender differences in analyses. From grants to publications, SABV should be incorporated into all aspects of basic and clinical research to better understand normal and abnormal cardiovascular function (Fig. 1). The purpose of this article is to provide additional guidance and examples on how to incorporate SABV into the conduct and reporting of basic and clinical research. Our hope is that by sharing examples, we can demystify this issue and demonstrate that incorporating SABV can be accomplished without considerable additional work and/or doubling the size of experimental groups and samples.
Figure 1.
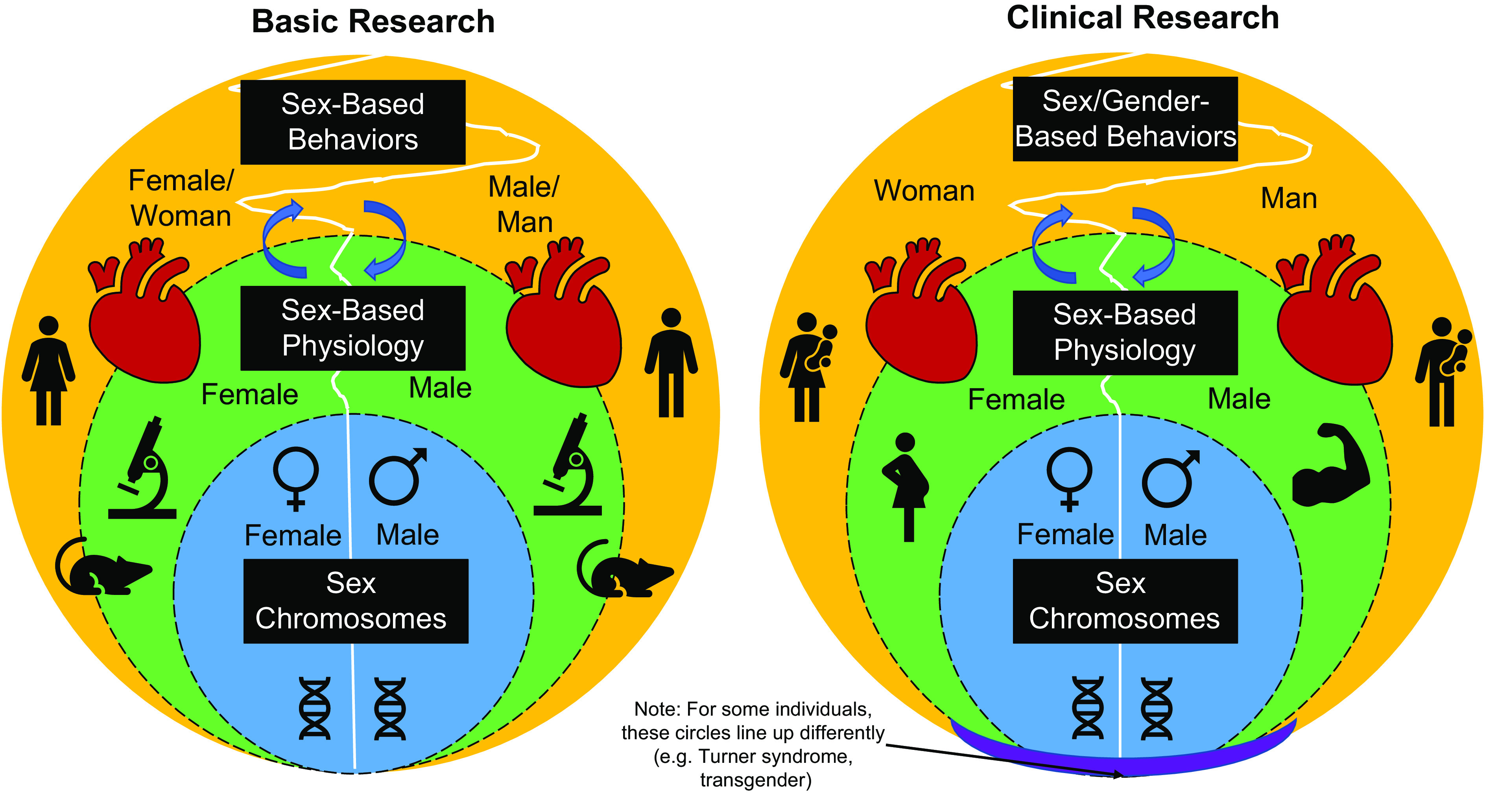
Considerations for incorporating sex as a biological variable into basic and clinical cardiovascular research. There are differences in sex chromosomes, which dictate sex-based differences in physiology. In turn, sex-based differences in physiology can dictate sex/gender-based behaviors. Notably, this relationship is bidirectional such that sex/gender-based behaviors can influence sex-based physiology (noted with arrows). Collectively, sex chromosome, physiology, and behaviors all impact cardiovascular health with some differences and similarities between women/female and men/male. Although the terminology is still being debated, the important thing is to make sure that both sides are considered when conducting and reporting research, especially in augmenting our understanding of the left sides of these diagrams.
BASIC RESEARCH
For basic research, our comments are based on years of carrying out studies related to autonomic control of the heart using both male and female rodents. After an early study using only male rats, we switched to using male and female animals in our studies for several reasons (beyond colleagues down the hall constantly asking us about including females). First, we were investigating something we expected would be similar by sex. Second, the notion of using only half the transgenic mice seemed needlessly expensive and wasteful. We were aware of potential sex differences in parameters relevant to our study, so our approach was to use age-matched male and female animals and to track everything carefully by sex, as well as genotype and treatment group so that we could identify any parameters that seemed to differ by sex. We did not control for gonadal hormone levels in either sex. We reasoned that if a sex-based difference was apparent, then we could investigate the underlying mechanism later.
Despite fears that sex-based differences would require use of additional animals, physiological parameters were generally indistinguishable between male and female rodents in our studies. For example, basic cardiovascular parameters were similar between male and female animals even when they differed significantly by genotype (3, 4). Pathologies like spontaneous arrhythmias were likewise similar in male and female mice of the same genotype and exhibited the same circadian variation (3). That does not preclude the possibility that male and female mice use distinct mechanisms to achieve homeostasis, but generally we observed similar changes in both sexes in cardiac and autonomic parameters after myocardial infarction. Group sizes of n = 6–8 animals have been sufficient for most experiments, evenly divided between males and females. On the rare occasion where it appeared that male and female mice might be segregating, additional mice were analyzed. However, we recognize that analyzing additional mice is not always feasible, especially for early stage investigators. We appreciate the clear statement from the editors of AJP-Heart Circ that if sex differences are identified, authors will not be expected to elucidate the underlying mechanism for manuscript acceptance. An important caveat is that we generated myocardial infarction using the same method in both sexes, which is distinct from clinical circumstances where the development and symptoms of cardiac ischemia may differ between males and females (5).
In contrast to the consistency of physiological measures, molecular parameters may exhibit significant sex-based differences, particularly with analyses like RNA-Seq where the presence of X and/or Y chromosomes generates sex-based differences. RNA-Seq in stellate ganglia revealed additional genes beyond the X or Y chromosomes that were differentially expressed between male and female mice (6), and differential expression was confirmed by qPCR analysis. The genes associated with noradrenergic transmission, neuronal structure, and ion channels were similar in males and females, so the connection of differential gene expression to physiology was often lacking. For example, the Tcfl5 gene, which encodes the transcription factor Cha (associated with Chagas disease), was significantly more abundant in male stellates compared with female stellates (6). However, the levels of Tcfl5 mRNA in the superior cervical ganglia were orders of magnitude lower compared with the stellate ganglia, and exhibited no sex-based differences in expression. This finding suggests that differential expression of Tcfl5 in stellates might be a false positive that has a limited biological impact even though it was consistent across multiple animals. Highly sensitive genomics methods can readily identify sex-based differences in gene expression, but it is important to distinguish which connect to sex-based physiological differences.
Based on our years of experience and the broader literature, we have the following recommendations for incorporating SABV more into basic research studies (Table 1). First, know the literature, especially from laboratories focused on elucidating sex differences, which will help with key study design elements such as sample size and potential confounding variables. From sex differences in cerebrovascular endothelial function in mice (7) to sleep deprivation and sympathetic responsiveness in humans (8), take advantage of previous work and build upon it. Second, track and report your results by sex, stratifying or combining the data where appropriate (e.g., stratifying for survival in myocardial infarction models but possibly combining for echocardiographic analyses). Third, consider recording data on variables that may influence sex differences (e.g., estrous cycles, pre-/postmenopause). Finally, consult a statistician or bioinformatician who can help dissect any relevant sex differences with your available data and perform a post hoc power analysis to determine adequate sample sizes for future studies focused on sex-based differences.
Table 1.
Quick guide to incorporating sex (and gender) as a biological variable
| Basic Research | Clinical Research | |
|---|---|---|
| Literature |
|
|
| Sample |
|
|
| Enrollment (for human studies) |
|
|
| Data collection |
|
|
| Data analysis |
|
|
| Reporting |
|
|
CLINICAL RESEARCH
For clinical research, we base examples on our previous work involving adult women and men with heart failure (HF) (9–11). One of our first considerations was to decide on terminology. It is recommended to use the term sex (i.e., biological differences including chromosomes, sex organs, and endogenous hormonal profiles) when focusing on biological parameters and to use the term gender (i.e., socially constructed and enacted roles and behaviors) when focusing on social constructs (12). In practice, however, this is difficult with human subjects because sex and gender are intricately connected (Fig. 1), and depending on the research question, human studies may examine both biological differences and social constructs simultaneously. As pointed out by Robinson et al. (13), while there is ongoing discourse regarding which terminology to use with human participants (sex vs. gender or female vs. women), the important thing is to acknowledge that sex/gender differences need to be considered across all research. For our studies, we ask participants to self-report gender (options: male, female, not listed above or more than one applies, or prefer not to state) and compare with the medical record because we focus on the combined aspects of biological and social parameters. Although sex and gender most frequently overlap, if they do not overlap (<1% in our studies), then we go by self-reported gender.
When designing our studies, there has never been a valid scientific reason to not include women and men; thus, with considerable added effort we have strived to enrich our sample with an adequate sex/gender balance to make comparisons and account for SABV in analyses. Many cardiovascular diseases affect women and men equally, although there are some notable differences (e.g., Takotsubo cardiomyopathy affects women preferentially, whereas myocarditis affects men preferentially), which should be incorporated into the study design. On the whole though, CVD historically and unfortunately was considered a disease of men, and it has taken decades to unravel how CVD affects women (and we are still not there yet).
Moreover, the lack of sex/gender balance in human studies has created a vicious cycle in which our lack of understanding among women perpetuates significant disparities in both knowledge and care. For example, implantation of left ventricular assist devices (LVAD) is abysmally imbalanced (only 1 in 5 LVADs are implanted among women). The lack of women in this population prohibits our understanding of relevant sex/gender similarities and differences, which limits the evidence base and our ability to offer LVADs to more women. Recently, we examined sex/gender differences in echocardiographic parameters, biomarkers, and symptoms from pre- to postimplantation of an LVAD (10). Although sex/gender imbalance was reflected in our secondary analysis, we provided a starting point for future research. For our studies, because HF affects both women and men (14), and there are important sex differences in etiology, pathophysiology, and outcomes (e.g., women are more likely to have HF with preserved ejection than men) (15), we aim to enroll approximately equal numbers of women and men.
Although it is a great starting point to build SABV into the study design, the actual process of recruiting women can be difficult. For example, in two studies, we had to close enrollment to men for several months to achieve our target of 50% women (9, 11). Thus, it is important to be prepared to extend enrollment windows to recruit adequate numbers of women and men. Furthermore, it is important to work with the clinical team to recruit; consider expanding your approach to include women and men providers as well as different clinics (e.g., internal medicine) (2). Throughout recruitment, you should monitor the sex/gender balance and, as needed, understand where you are losing women (or men): is it when they visit their clinical providers (e.g., is the imbalance in the setting), or do they decline or withdraw more often? Even with the best intentions, it may not be feasible to achieve adequate sex/gender balance for a myriad of reasons. Some suggestions, in this case, are 1) to consider applying for additional funding to reach underrepresented/underserved women (e.g., administrative supplement such as https://grants.nih.gov/grants/guide/notice-files/NOT-OD-22-031.html), 2) to collect data on reasons for lack of adequate inclusion of a particular sex/gender (in turn, this could be an interesting paper), and 3) to address the imbalance as a limitation and offer suggestions for future research.
There also are key SABV considerations for statistical analyses. As with all studies, the degree to which you integrate SABV depends on your research question. Even if your research question is not focused on elucidating SABV, it is important to explore all outcome data by sex/gender and account for sex/gender as a control variable where appropriate. Approaches can be simple [e.g., using comparative statistics to examine sex/gender differences or performing stratified analyses (9, 10)] or they can be more complex (e.g., using propensity score analysis to account for confounding differences). In addition, you can use an interaction term (also called moderation) to determine if associations between variables are different between women and men. For example, using moderation analysis, we found that the association between the diameter of the left ventricle at end diastole and physical HF symptoms differed between women and men (11). You may be underpowered to examine these relationships given sample-size limitations, but you can generate effect sizes to power larger studies and identify signals to pursue with future research.
Finally, clinical research studies are consistent in reporting the sex/gender breakdown of samples; however, it often stops there. Although an extensive analysis of SABV does not need to be performed, if there are any relevant differences that emerge, they need to be reported. Equally important, any similarities should be noted, and a brief mention is often all that is needed. Moreover, note any limitations in your paper if you had an imbalance of women and men, which should, in turn, be an area of future research.
Our compiled list of recommendations for incorporating SABV into clinical research studies is outlined in Table 1. In addition to the suggestions outlined previously, and similar to basic research studies, we strongly recommend performing a quick literature search to glean important insights from previous work that you can build upon [e.g., see Lala et al. (15) for review in HF]. We also encourage investigators to check out previous reviews on design considerations (16) and relevant resources from organizations such as the Office of Research on Women’s Health (https://orwh.od.nih.gov/).
Conclusions
Although we have made progress with SABV initiatives to elucidate important sex and gender differences, we still need to move the needle. Herein, we demonstrated that this is not a daunting or complicated endeavor. Even though incorporating SABV requires time, planning, and foresight, given how uncharted women’s health is, exploring sex/gender differences may provide a niche opportunity and yield a significant return on investment. In sum, SABV initiatives have encouraged better study designs and inclusion of females/women, but there is little marked progress in outcomes and clinically meaningful knowledge of sex/gender differences. Hence, we applaud the move by AJP-Heart Circ, who joins a small but growing list of journals (17, 18), to require SABV in experimental design, analysis, and reporting unless there is strong scientific justification.
GRANTS
This work was funded by the National Institutes of Health Grants HD043488 (to Q.E.D. and C.S.L.), NR019054 (to Q.E.D.), HL093056 (to B.A.H.), HL146833 (to B.A.H.), and NR013492 (to C.S.L.).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.E.D. and B.A.H. conceived and designed research; Q.E.D. and B.A.H. drafted manuscript; Q.E.D., C.S.L., and B.A.H. edited and revised manuscript; Q.E.D., C.S.L., and B.A.H. approved final version of manuscript.
REFERENCES
- 1.Lindsey ML, LeBlanc AJ, Ripplinger CM, Carter JR, Kirk JA, Hansell Keehan K, Brunt KR, Kleinbongard P, Kassiri Z. Reinforcing rigor and reproducibility expectations for use of sex and gender in cardiovascular research. Am J Physiol Heart Circ Physiol 321: H819–H824, 2021. doi: 10.1152/ajpheart.00418.2021. [DOI] [PubMed] [Google Scholar]
- 2.Cho L, Vest AR, O'Donoghue ML, Ogunniyi MO, Sarma AA, Denby KJ, Lau ES, Poole JE, Lindley KJ, Mehran R; Cardiovascular Disease in Women Committee Leadership Council. Increasing participation of women in cardiovascular trials: JACC Council perspectives. J Am Coll Cardiol 78: 737–751, 2021. doi: 10.1016/j.jacc.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Lorentz CU, Alston EN, Belcik T, Lindner JR, Giraud GD, Habecker BA. Heterogeneous ventricular sympathetic innervation, altered beta-adrenergic receptor expression, and rhythm instability in mice lacking the p75 neurotrophin receptor. Am J Physiol Heart Circ Physiol 298: H1652–H1660, 2010. doi: 10.1152/ajpheart.01128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parrish DC, Gritman K, Van Winkle DM, Woodward WR, Bader M, Habecker BA. Postinfarct sympathetic hyperactivity differentially stimulates expression of tyrosine hydroxylase and norepinephrine transporter. Am J Physiol Heart Circ Physiol 294: H99–H106, 2008. doi: 10.1152/ajpheart.00533.2007. [DOI] [PubMed] [Google Scholar]
- 5.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 133: 916–947, 2016. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 6.Bayles RG, Olivas A, Denfeld Q, Woodward WR, Fei SS, Gao L, Habecker BA. Transcriptomic and neurochemical analysis of the stellate ganglia in mice highlights sex differences. Sci Rep 8: 8963, 2018. [Erratum in Sci Rep 9: 9506, 2019]. doi: 10.1038/s41598-018-27306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuloaga KL, Davis CM, Zhang W, Alkayed NJ. Role of aromatase in sex-specific cerebrovascular endothelial function in mice. Am J Physiol Heart Circ Physiol 306: H929–H937, 2014. doi: 10.1152/ajpheart.00698.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter JR, Fonkoue IT, Greenlund IM, Schwartz CE, Mokhlesi B, Smoot CA. Sympathetic neural responsiveness to sleep deprivation in older adults: sex differences. Am J Physiol Heart Circ Physiol 317: H315–H322, 2019. doi: 10.1152/ajpheart.00232.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denfeld QE, Habecker BA, Camacho SA, Roberts Davis M, Gupta N, Hiatt SO, Medysky ME, Purnell JQ, Winters-Stone K, Lee CS. Characterizing sex differences in physical frailty phenotypes in heart failure. Circ Heart Fail 14: e008076, 2021. doi: 10.1161/CIRCHEARTFAILURE.120.008076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denfeld QE, Faulkner KM, Davis MR, Habecker BA, Chien CV, Gelow JM, Mudd JO, Hiatt SO, Grady KL, Lee CS. Exploring gender differences in trajectories of clinical markers and symptoms after left ventricular assist device implantation. Eur J Cardiovasc Nurs 20: 648–656, 2021. doi: 10.1093/eurjcn/zvab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CS, Hiatt SO, Denfeld QE, Chien CV, Mudd JO, Gelow JM. Gender-specific physical symptom biology in heart failure. J Cardiovasc Nurs 30: 517–521, 2015. doi: 10.1097/JCN.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NIH Office of Research on Women's Health. Sex & Gender. https://orwh.od.nih.gov/sex-gender. [2021 Nov 12]
- 13.Robinson AT, Wenner MM, Bunsawat K, Watso JC, Giersch GEW, Charkoudian N. When it’s time for the sex talk, words matter. Am J Physiol Heart Circ Physiol 322: H66–H70, 2022. doi: 10.1152/ajpheart.00556.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics 2021 Update: a report from the American Heart Association. Circulation 143: e254–e743, 2021. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 15.Lala A, Tayal U, Hamo CE, Youmans Q, Al-Khatib SM, Bozkurt B, Davis MB, Januzzi J, Mentz R, Sauer A, Walsh MN, Yancy C, Gulati M. Sex differences in heart failure. J Card Fail, 2021. doi: 10.1016/j.cardfail.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Rich-Edwards JW, Kaiser UB, Chen GL, Manson JE, Goldstein JM. Sex and gender differences research design for basic, clinical, and population studies: essentials for investigators. Endocr Rev 39: 424–439, 2018. doi: 10.1210/er.2017-00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prager EM. Addressing sex as a biological variable. J Neurosci Res 95: 11, 2017. doi: 10.1002/jnr.23979. [DOI] [PubMed] [Google Scholar]
- 18.Lillemoe KD. Joint Statement by the Surgery Journal Editors Group 2018. Ann Surg 267: 991, 2018. doi: 10.1097/SLA.0000000000002740. [DOI] [PubMed] [Google Scholar]


