Abstract
Short sleep duration and poor sleep quality are associated with cardiovascular risk, and sympathetic nervous system (SNS) dysfunction appears to be a key contributor. The present review will characterize sympathetic function across several sleep disorders and insufficiencies in humans, including sleep deprivation, insomnia, narcolepsy, and obstructive sleep apnea (OSA). We will focus on direct assessments of sympathetic activation, e.g., plasma norepinephrine and muscle sympathetic nerve activity, but include heart rate variability (HRV) when direct assessments are lacking. The review also highlights sex as a key biological variable. Experimental models of total sleep deprivation and sleep restriction are converging to support several epidemiological studies reporting an association between short sleep duration and hypertension, especially in women. A systemic increase of SNS activity via plasma norepinephrine is present with insomnia and has also been confirmed with direct, regionally specific evidence from microneurographic studies. Narcolepsy is characterized by autonomic dysfunction via both HRV and microneurographic studies but with opposing conclusions regarding SNS activation. Robust sympathoexcitation is well documented in OSA and is related to baroreflex and chemoreflex dysfunction. Treatment of OSA with continuous positive airway pressure results in sympathoinhibition. In summary, sleep disorders and insufficiencies are often characterized by sympathoexcitation and/or sympathetic/baroreflex dysfunction, with several studies suggesting women may be at heightened risk.
Keywords: microneurography, muscle sympathetic nerve activity, norepinephrine, obstructive sleep apnea, sex differences, short sleep
INTRODUCTION
Sleep is increasingly acknowledged as a key pillar of human health and quality of life. Most adults require 7 to 9 h of sleep according to the American Academy of Sleep Medicine (1), yet the trend for self-imposed sleep restriction has evolved through the 20th century to become an epidemic in the 21st century (2), with 35% of adults sleeping an average of less than 7 h per night (3). Moreover, another significant portion of the United States population (i.e., 10–30%) suffers from diagnosed sleep disorders such as insomnia, narcolepsy, and sleep apnea (4, 5).
Short sleep duration is often the result of self-imposed restriction or clinical diagnosis of insomnia, which is characterized as consistent difficulties with sleep initiation and/or maintenance. In stark contrast, narcolepsy is characterized as dysfunctional control of sleep and failure to maintain wakefulness. Finally, sleep apnea consists of regular apneic events from airway collapse or complete cessation of respiratory effort during sleep and results in oxygen desaturations and fragmented sleep that are deleterious to overall health (6). Whether it be chronic sleep restriction or sleep apnea, there is growing evidence that sleep disorders and insufficiencies are accompanied by heightened sympathetic activity and/or dysfunction, known contributors to increased cardiovascular disease (CVD) risk (7–11).
Until recently, sleep has been an often-ignored contributor to sympathetic regulation, and this review will highlight sympathetic regulation within sleep disorders and insufficiencies. Sympathetic assessment methodologies discussed will include indirect assessments [i.e., heart rate variability, (HRV)], global sympathetic activity [i.e., plasma norepinephrine (NOR)], and regional, direct assessment of sympathetic activity to muscle vasculature (i.e., microneurography). Indirect HRV estimates of sympathetic activity are often overinterpreted and can vary across experimental conditions and offer opposite conclusions in comparison to other direct techniques (i.e., microneurography) (12). Plasma NOR is correlated with other direct assessments of sympathetic activity (13) and offers methodological advantages such as temporal pattern quantification of global sympathetic traffic but lacks regional specificity. Microneurography remains the only technique in humans to directly assess postganglionic muscle sympathetic nerve activity (MSNA) to the peripheral vasculature, and while measurements are known to be stable and highly repeatable within individuals, the technique can only be done under highly controlled conditions with specialized trained investigators (14).
The American Journal of Physiology-Heart and Circulatory Physiology has published a number of recent studies examining the complex relations between sleep and cardiovascular health (15–22). The purpose of the present review is to highlight sympathetic and cardiovascular dysfunction associated with experimental sleep deprivation/restriction, insomnia, narcolepsy, and obstructive sleep apnea (OSA), with a particular focus on the important role of biological sex (i.e., male vs. female). We will focus on sympathetic assessments of MSNA and plasma/urinary NOR but also include HRV and/or blood pressure when direct assessments are lacking.
SLEEP DEPRIVATION
Muscle Sympathetic Nerve Activity
Epidemiological studies have reported a significant association between habitual sleep restriction and hypertension (7, 9), and increased sympathetic outflow has been suggested as a key contributing mechanism. Total sleep deprivation (TSD) experimental models (most often 24-h sleep deprivation) have been used to interrogate the relationships between sleep, blood pressure, and MSNA. Early studies composed of primarily male participants reported an acute increase of blood pressure during TSD (23, 24) but did not observe increased MSNA as hypothesized. Instead, these early studies documented a baroreflex-mediated reduction in MSNA after TSD (23, 24).
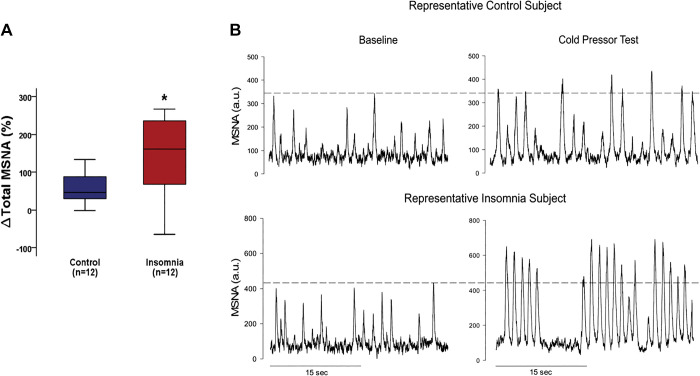
More recently, epidemiological literature on the relationship between short sleep and hypertension indicates sex disparities, with women at elevated risk for hypertension compared with their male counterparts (8), including across the adult life span (10). Studies from our laboratory using the 24-h experimental sleep deprivation paradigm were the first to intentionally recruit both men and women and reported that while TSD elicited similar increases in blood pressure, the MSNA responses differed between sexes. Specifically, we observed that TSD elicited a greater sympathetic profile response and baroreflex dysfunction in young women compared with young men (25) and sympathoexcitation in older postmenopausal women when compared with age-matched men (26) (Fig. 1). These findings demonstrate that men and women have different mechanisms contributing to the TSD-mediated rise in blood pressure and suggest that the sympathetic nervous system serves as a more predominant contributor in women when compared with men.
Figure 1.
Muscle sympathetic nerve activity (MSNA) after a night of normal sleep (NS) and after 24-h total sleep deprivation (TSD). The TSD-mediated hypertensive response was accompanied by significant sympathoinhibition in young males and sympathetic predominance in young females. In contrast, significant sympathoexcitation was evident in older women, but not older men. Modified with permission from Carter et al. (25, 26). *P < 0.05, from corresponding NS condition.
While there remains a lack of information on sleep restriction (i.e., 4–6 h of sleep over multiple, consecutive days) and MSNA, one study has attempted to examine selective sleep stage deprivation for one night. Sayk and colleagues (27) used loud acoustic stimulation timed with polysomnography to deprive slow wave sleep (SWS) but did not observe changes in next-day ambulatory blood pressure or MSNA. The impact of multiple nights of selective SWS on MSNA has not yet been conducted. Moreover, the impact of rapid eye movement (REM)-specific sleep deprivation (or restriction) on MSNA remains equivocal. Finally, while Sayk and colleagues (27) included both sexes (6 women and 5 men), the sample size was insufficient to probe for sex differences. Given the reported epidemiological (10) and experimental (25, 26) sex differences for sleep insufficiencies and neural cardiovascular control, future work on selective SWS- and/or REM-deprivation models would benefit from robust samples of men and women to ensure sex as a biological variable (28).
Plasma and Urinary Norepinephrine
Despite the reported reductions of MSNA after TSD by Kato et al. (23) and Ogawa et al. (24), both studies with predominantly male participants reported no change in plasma NOR between conditions (i.e., normal sleep vs. TSD). Unlike the MSNA studies conducted to date, there have been a number of studies examining the impact of sleep restriction on plasma and urinary NOR. These more ecologically valid models of sleep restriction have reported a sympathetic predominance in cross-sectional and crossover study designs (29–34), but this remains inconclusive (35–38). Sleep restriction study designs vary in the number of nights studied (i.e., 1–13 days), amount of sleep opportunity (i.e., 2–5.5 h), and distribution of men and women. There is evidence that either prolonged sleep restriction (31) or extremely short sleep opportunity (34) is associated with increased plasma or urinary NOR. Covassin et al. (31) reported elevated plasma NOR following a 9-day, 4-h sleep restriction model. In their 2-h sleep restriction model, Faraut et al. (34) observed a significant increase in afternoon urinary NOR. However, this relationship was abolished when morning and afternoon nap opportunities were provided following sleep restriction. To date, the majority of sleep restriction models which have examined NOR were either majority or all male (30, 32–34, 37, 38). Covassin et al. (31) was the first study with similar male and female representation to report elevated plasma NOR after sleep restriction (i.e., 4 h), but the elevation was not different between sexes. In contrast, Nedeltcheva et al. (35, 36) equal sex distributed study did not document an elevation in plasma NOR following 13-day sleep restriction with a longer 5.5-h sleep opportunity. The elevation in plasma NOR reported in Covassin et al. (31) was accompanied by an increased 24-h systolic blood pressure only in women. Yang et al. (39) employed a more complex model composed of four sleep restriction blocks (3-day, 4-h sleep opportunity followed by a 1-day, 8-h sleep opportunity) with equal sex distribution and noted no differences of plasma NOR between sleep restriction and normal sleep but rather a gradual decrease in NOR in both conditions.
While the majority of sleep deprivation or restriction studies have focused on plasma NOR, Grimaldi and colleagues (40) reported 24-h urinary NOR after 8 consecutive days of 5-h sleep restriction. Interestingly, this study included a second arm that replicated the 8-day, 5-h sleep restriction paradigm, but also introduced circadian misalignment by delaying the sleep opportunity by roughly 8 h on half of the nights. While sleep restriction alone did not appear to impact urinary NOR, the sleep restriction combined with circadian misalignment significantly increased 24-h urinary NOR. Moreover, the increase of urinary NOR during sleep restriction with circadian misalignment was most severe during sleep and early morning hours, which are times of heightened risk for adverse cardiovascular events (40). Similar to several studies highlighted throughout this review, this work is limited by a study population of predominantly men, thus not allowing for investigation into potential mediating role of sex.
Taken together, while the proof-of-concept model of TSD has demonstrated acute sympathoexcitation in women via MSNA, more ecologically valid sleep restriction models provide marginal evidence of changes in sympathetic activity but lack rigor into potential role of sex differences with plasma NOR. Furthermore, the literature is completely void of direct sympathetic measurement following a sleep restriction paradigm. Importantly, the majority of studies examining sleep deprivation conditions are applied to otherwise healthy, younger adults who may effectively buffer the acute stressor of short/poor sleep in regard to sympathetic activation. We suggest three steps to improving rigor and understanding in this important field of research. First is a call for more hybrid studies that examine sleep deprivation/restriction with other factors in statistically robust samples sizes of men and women. For example, when sleep restriction was combined with circadian misalignment, increases in urinary NOR were observed (40), yet we have no insights on the potential mediating role of sex in that relationship. In combination with other sleep complexities such as circadian misalignment, sleep fragmentation, and other sleep disruptions, it might result in sympathoexcitation. Second, short sleep is related to increased incidence of CVD across the life span in women (10). Future work must improve representation of midlife and older adults, particularly women, because of lack of representation and higher overall stress levels during the midlife periods (41). Lastly, sleep extension offers another lens to examine the relation between short sleep and sympathetic activation to determine whether behavioral sleep extension interventions lower sympathetic activity via indirect or direct methods and if this differs between men and women. In summary, future studies related to sleep deprivation/restriction, sympathetic activity, and sex differences are warranted.
INSOMNIA
Insomnia is a common sleep disorder characterized by problems with sleep initiation or sleep maintenance and is associated with a 45% greater risk of cardiovascular disease (42). Given the importance of the sympathetic nervous system on cardiovascular health, studies have attempted to characterize various indices of sympathetic activity in individuals with chronic insomnia. To date, the majority of studies examining sympathetic function within the insomnia population have examined either plasma or urinary NOR, and results have been inconsistent. Seelig et al. (43) and Grimaldi et al. (44) observed lower rates of urinary and plasma NOR compared with controls, respectively, and both study samples were predominantly women. Floam et al. (45) examined a younger cohort of insomniacs and reported no differences in urinary NOR compared with controls. In contrast, there is evidence to suggest elevated sympathetic tone via increased plasma NOR in participants with insomnia (46–49). Moreover, plasma and/or urinary NOR levels were associated with detriments in sleep quality (47), with a negative correlation between urinary NOR and sleep onset latency and a positive correlation between urinary NOR and total wake time. A case study of fatal familial insomnia observed an increase of plasma NOR with disease progression and a lack of sensitivity (i.e., poor pressor response) to NOR infusion (48). Roehrs and Roth (49) assessed urinary NOR levels in participants who underwent a multiple sleep latency test (MSLT), and participants with insomnia who took 15 min or longer to fall asleep had higher daytime urinary NOR.
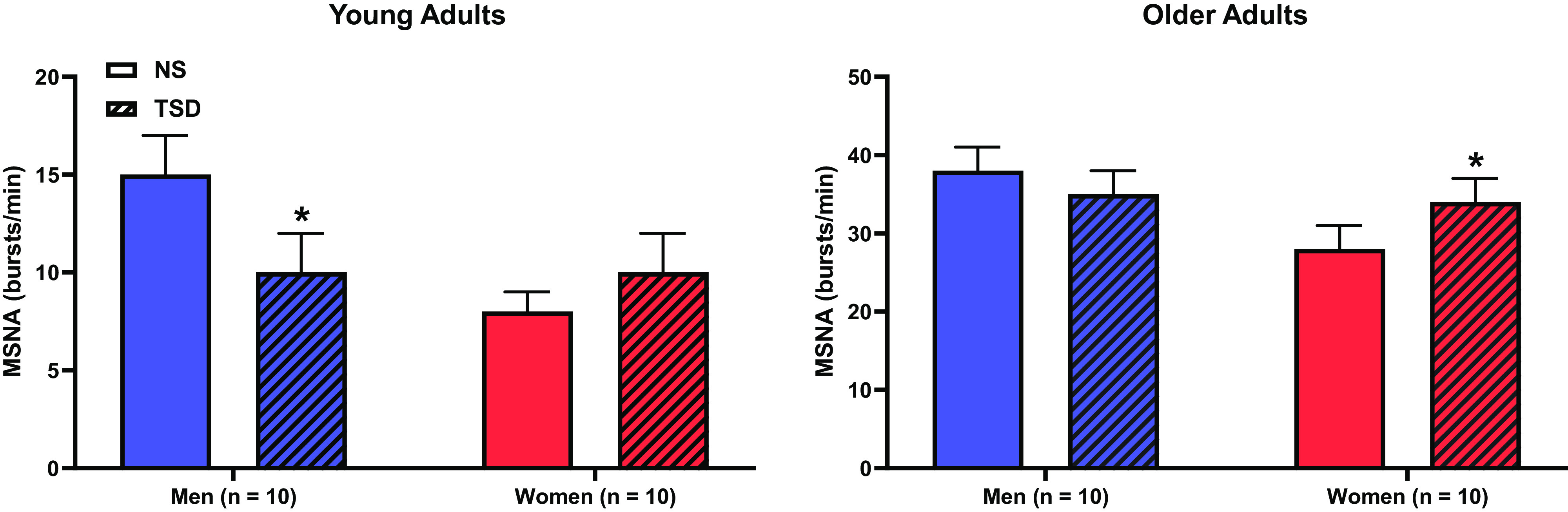
At present, one study has examined direct sympathetic recordings (i.e., MSNA) in participants with chronic insomnia. Carter et al. (50) reported MSNA was not different between insomniacs and controls at baseline. However, participants with chronic insomnia demonstrated blunted sympathetic baroreflex sensitivity and augmented blood pressure and total MSNA responses to the cold pressor test (Fig. 2). These recent findings support the hyperarousal theory of insomnia (51, 52). A sex-specific comparison of sympathetic function within participants with insomnia is lacking, which is alarming given an apparently greater risk of insomnia in women compared with men (53). Further work in this area is warranted as women increase MSNA at a much faster rate with age (54) and have a heightened risk of CVD with sleep disturbances (10).
Figure 2.
Muscle sympathetic nerve activity (MSNA) at baseline and a during sympathoexcitatory maneuver [cold pressor test (CPT)] in control normal sleepers and chronic insomniacs. Change in total MSNA during CPT was augmented in a group of insomniacs compared with controls. A: total MSNA responsiveness to CPT. B: representative recordings from control and insomnia subjects; a.u., arbitrary units. *P < 0.05 from control participants. Reprinted with permission from Carter et al. (50).
Hyperarousal Theory of Insomnia
The pathogenesis of an insomnia diagnosis is often idiopathic or is comorbid with psychological disorders like anxiety and depression (55, 56). However, the physiological arousal theory of insomnia has gained momentum (52), where participants with insomnia present with differences in basal heart rate (57, 58), intrusions of beta electroencephalographic frequencies into normal sleep (59), and a sympathetic dominance via plasma NOR (46, 47) and total MSNA reactivity (50). However, it remains unclear if these neural cardiovascular characteristics of insomnia are a cause of disease onset or the effect of disease progression. A plausible explanation for the mentioned pathophysiology associated with insomnia, and perhaps a contributor to insomnia hyperarousal, is hyperactivity of the orexin system within the brain. Orexin (also known as hypocretin) is a major regulator of the sleep/wake cycle and functions to maintain wake and suppress REM sleep. Neurons within the lateral hypothalamic areas, which release orexin target neurons to release NOR, serotonin, and dopamine. This action is thought to facilitate wakefulness. There is evidence from animal models that overexpression of orexin is related to longer periods of wakefulness and fewer sleep/rest periods (60) in zebrafish, as well as poor nonrapid eye movement (NREM) and REM sleep in mice (61). Relevant to the current review, orexin system activity has also been associated with cardiovascular and sympathetic control in both healthy and hypertensive animal models (62). Increased orexin in human cerebrospinal fluid has not been confirmed in humans with insomnia; however, there is evidence to suggest this relationship might exist given the significant association between increased plasma orexin and insomnia severity (63). Future work examining orexin system hyperactivity and sympathetic regulation in insomnia appear warranted. Moreover, direct sympathetic function has only been measured during morning wakefulness (50), and it may be relevant to examine MSNA immediately before sleep to determine if there is evening hyperarousal not detected during morning daytime measures in participants with chronic insomnia. Finally, Lanfranchi et al. (64) and Lyu et al. (65) have reported a blunted blood pressure dipping profile in participants with chronic insomnia, where evening sympathoexcitation is a suspected mechanism in addition to disturbed sleep interfering with normal blood pressure dipping.
Insomnia and Alcohol Self-Medication
A frequent and unfortunate circumstance that accompanies insomnia within the adult population is substance abuse (i.e., alcohol) as an attempt at self-medication. In a group of participants with insomnia, Roehrs and Roth (66) demonstrated an acute beneficial effect of a high dose of alcohol (i.e., 0.6 g/kg) with improved metrics of sleep quality compared with a low alcohol dose (i.e., 0.3 g/kg) and placebo dose (i.e., 0.0 g/kg). However, the acute beneficial effect of alcohol consumption was abolished following repeated consumption of a high dose of alcohol for five consecutive nights. This experimental evidence supports the alcohol feed-forward allostatic framework posited by Koob and Colrain (67), where alcohol consumption before sleep can approach that of binge drinking (i.e., consumption of 4 to 5 alcoholic beverages within 2 h) as an alcohol tolerance develops and reward centers in the brain become active. While sleep latency is often decreased with evening alcohol consumption, this can be a subjective and flawed indicator of improved sleep quality. More importantly, evening alcohol consumption consistently increases overall wakefulness and decreases REM sleep, especially during the second half of the night. With proper intervention, abstinence from drinking or withdrawal phase can also impair sleep quality and lead to preoccupation/anticipation or craving stage. Within this vicious cycle, a comorbid alcohol use disorder (AUD) has the potential to develop, where the interactions of AUD, insomnia, sympathetic tone, and cardiovascular disease have yet to be fully elucidated. Recent evidence from our laboratory on binge alcohol consumption has demonstrated sympathetic dysfunction in social binge drinkers during sympathoexcitatory maneuvers (i.e., Valsalva’s maneuver) (68) and impaired vagal activation during prominent NREM and REM sleep (69), but evidence from clinical populations of AUD and chronic insomnia is lacking.
In summary, sympathetic methodologies within the insomnia literature offer conflicting results. However, two prominent explanations may explain this discrepancy. First, plasma NOR is sampled only under resting conditions to ensure the sample is not artificially inflated because of acute stress. As such, plasma NOR and resting MSNA are in concordance and perhaps only is elevated at rest with severe insomnia. Second, nested within the cognitive hyperarousal theory, and in alignment with Roehrs and Roth (49), sympathetic elevation may occur when sleep is initiated, in part contributing to poor nocturnal blood pressure dipping in insomnia (64). This finding has yet to be confirmed with direct MSNA measurement. Furthermore, prominent samples of women report lower resting plasma NOR in insomnia (43, 44) but increased plasma NOR when sex distribution is improved (47, 49). This suggests that differing sympathetic outflow patterns may exist between sexes (e.g., baseline versus stressor-based sympathoexcitation comparisons), again highlighting the need for sympathetic neural sex differences research within the insomnia population.
NARCOLEPSY
HRV
There is increasing evidence to support autonomic dysfunction in narcolepsy (and with cataplexy) (70). Narcolepsy is a debilitating sleep disorder characterized by a reduction in orexin system activity and production within the lateral hypothalamic areas (71, 72). With less orexin availability, reduced activity of target neurons facilitate narcolepsy symptoms (i.e., excessive sleepiness), sudden onset of REM sleep periods during narcoleptic episodes, or even REM like characteristics persisting into wakefulness (73). Prolonged autonomic dysfunction is thought to be a contributor to CVD risk and is a common comorbidity within narcoleptics. However, there is limited and conflicting evidence regarding sympathetic regulation via direct (i.e., MSNA) and indirect assessment (i.e., HRV). Wake sympathetic activity in narcolepsy derived from frequency domain analysis of HRV suggests increased sympathetic activity and reduced parasympathetic activity, although all study samples were completely (74, 75) or majority male (76). Fronczek et al. (74) reported that narcoleptics had higher HRV and blood pressure variability (BPV), whereas low-frequency (LF) and high-frequency (HF) and total HRV power were increased. However, the conclusion of reduced sympathetic tone taken from these studies must be interpreted with caution. The LF component of HRV is often used as a surrogate for sympathetic activity at the heart, but numerous methodological concerns are acknowledged in the literature (12, 77). Studies by Grimaldi et al. (76) and Silvani et al. (75) concluded increased sympathetic activity and decreased parasympathetic activity, respectively. Grimaldi et al. (76) noted higher baseline heart rate (HR) and higher normalized LF component of HRV. Silvani et al. (75) were attentive to limitations to sympathetic assumptions associated with HRV analyses and reported reductions in time-domain HRV associated with more reliable parasympathetic modulation of HR. HRV studies can serve an important clinical purpose when used as a risk stratification of disease severity, but findings should not be used to probe between sympathetic and parasympathetic neve activation, especially in studies with limited sample sizes.
MSNA
Microneurography studies in the narcoleptic population seem to directly counter the majority of HRV studies. Donadio et al. (78, 79) reported significant reduction of BP and HR in narcoleptics when compared with control sample, and this was accompanied by lower MSNA (78) or a trend for lower MSNA (79). When narcoleptics were exposed to cold noxious stimuli (e.g., cold pressor task), no difference was observed between MSNA, BP, or HR compared with controls. During a mental stressor, narcoleptics exhibited blunted MSNA, BP, and HR reactivity compared with controls.
Common pharmacological treatments for narcolepsy include stimulants to limit excessive daytime sleepiness and antidepression medication to aid in cataplexy management. Sodium oxybate (XYREM) is an effective medication at reducing narcolepsy symptomology, but a side effect can be high blood pressure. In a small 6-mo case study, two patients with narcolepsy were prescribed XYREM and developed hypertension by the end of the treatment, which was accompanied by sympathoexcitation measured via MSNA. This finding should be confirmed in a larger sample but highlights a significant side effect of XYREM treatment in a population who already appear to have autonomic instability (80).
In summary, narcolepsy is a great example where methodologies can dramatically impact interpretation and conclusions. Sympathetic dysfunction appears evident with lower MSNA in patients with narcolepsy (78, 79), but this conflicts the HRV interpretations (76). This divergence follows other conditions such as exercise where an increase in MSNA may be accompanied by an opposing change in LF HRV (12). This is an important methodological consideration that highlights the lack of reliability of LF HRV in comparison to direct, reliable, and reproducible MSNA recordings. We posit HRV can be a powerful tool for disease stratification (81) but should be limited in forming conclusions of cardiac sympathetic activity in disease populations unless confirmed by more reliable measures of sympathetic activity like plasma NOR or MSNA. Furthermore, it is worth noting that sample characteristics including baseline HR appear higher in Grimaldi et al. (76), but lower in Donadio et al. (78). Finally, it is important to note that Donadio et al. (78) sample included a roughly equal male to female population, where decreased sympathetic activity was concluded, but opposite conclusions are drawn in HRV studies of all men (76). Further work to confirm sex differences in narcolepsy appear warranted.
SLEEP APNEA
Sleep apnea, and principally obstructive sleep apnea (OSA), is a common sleep disorder characterized by repetitive airflow limitation and/or breathing cessation during normal sleep. OSA is common in men, obese individuals, and metabolic syndrome (82, 83) but is also prevalent in otherwise healthy adults. Untreated OSA is associated with the pathogenesis of stroke, heart failure, myocardial ischemia and acts as a precursor to other cardiovascular diseases, including hypertension. Sympathetic dysregulation and sympathoexcitation are well-documented mechanisms for OSA and associated cardiovascular risk (84). However, different aspects of OSA pathology (i.e., repetitive apneas, minimal oxygen desaturation, arousals, etc.) may have differential influence on autonomic control within the brain (i.e., MRI studies) and peripheral MSNA (e.g., single- or multiunit recordings).
Repetitive Apneas
Severity of OSA can be classified by the apnea-hypopnea index (AHI) as mild (i.e., 5–15 events/h), moderate (15–30 events/h), or severe (≥30 events/h). With increasing OSA severity, sympathoexcitation is evident (85). Both plasma and urinary NOR are shown to be increased in all male samples with untreated OSA (86, 87). These findings are complemented by numerous microneurography studies have confirmed the elevation of MSNA in OSA (11, 85, 86, 88–91). The repetitive nature of apneas, especially in severe OSA, place added stress on the vasculature. During a normal apnea, there is threefold activation of the sympathetic nervous system via the baroreflex, central chemoreflex, and peripheral chemoreflex. The arterial baroreflex is active from the transient reduction of BP from decreased venous return as the respiratory pump function is halted (91, 92), which becomes blunted in severe OSA. The peripheral chemoreflex detects hypoxia, and the central chemoreflex is activated via hypercapnia and decreased pH (93–95), which trigger increases of sympathetic activity. In untreated OSA, chemoreflex sensitivity is altered to either a tonic active state or hypersensitivity to hypoxic stimuli. Narkiewicz et al. (93) documented an elevated daytime MSNA in patients with OSA and suggested this may result from tonic chemoreflex activation through the experimental deactivation of chemoreflex via hyperoxia. Furthermore, Narkiewicz et al. (94) reported more robust activation of the chemoreflex via increased ventilatory, pressor, and HR responses to hypoxic breathing, and marked sympathoexcitation to voluntary apnea. This “sympathetic storm” leads to an exaggerated pressor response at apnea termination when venous return is increased. Taken together, the hypersensitivity/tonic activation of the chemoreflex, blunted baroreflex function, and vascular stress highlight the comorbid nature of OSA and cardiovascular disease.
Sleep Disturbances in OSA
In addition to baroreflex and chemoreflex contributions to sympathoexcitation, there is emerging evidence to suggest other phenomena like oxygen desaturation and/or frequency of arousals may impact sympathetic regulation in OSA. Taylor et al. (96) reported that oxygen desaturation index (ODI) did not appear to directly influence MSNA burst incidence, but rather central changes in OSA. Taylor et al. (6) determined that the best predictor of daytime resting MSNA in OSA was arousal frequency rather than apneic event frequency or severity of OSA diagnosis. This highlights a potential combined impact of hypoxia and fragment sleep and may help explain the variance in daytime MSNA in participants with OSA. However, Taylor et al (6) did not parse out whether arousals in differing stages of sleep influenced the relation between arousal frequency and daytime MSNA. In severe OSA, the majority of apneas occur in light non-REM (NREM) sleep and can impair the transition into SWS and subsequent REM sleep (97). In addition, REM OSA may be most detrimental when sympathoexcitation is evident, and the combined effects of transient arousal and hypoxia may explain a higher variance of daytime MSNA (11).
Multiunit versus Single-Unit MSNA
Multiunit MSNA studies have demonstrated a marked increase of MSNA in OSA, but limited data exist regarding single-unit MSNA. Single-unit MSNA measurements offer further characterization of neural sympathetic activity rate of neural firing, probability of firing, and multiple within burst firing. Elam et al. (98) was the first to record single-unit MSNA in OSA, with the goal to compare to the single-unit MSNA profile of another disease associated with sympathoexcitation (i.e., chronic heart failure). Increased wake integrated multiunit MSNA can result from various single-unit firing strategies including an increased within-burst firing pattern, increased firing probability of already active single-unit vasoconstrictor fibers, as well as recruitment of additional neurons (99). A notably different single-unit MSNA profile was seen in OSA when compared with heart failure (98), where the increase in multiunit MSNA can be explained more so by an increased firing frequency of single neurons (100). In a large sample of OSA, both multiunit MSNA and single-unit MSNA were associated with increased OSA severity (i.e., increased AHI), but this association was strongest in single-unit MSNA. To complement initial work by Elam et al. (98), multiple firing spikes ratios were highest in the most severe cases of OSA (101). To date, there has not been a connection between other OSA detriments (i.e., impaired baroreflex function and overactive/tonic chemoreflex activation) and single-unit MSNA. Interestingly, Incognito et al. (102) have observed that the arterial baroreflex appears to demonstrate lesser control over the incidence of multiple within burst firings, indicating other mechanisms may be partially responsible. In response to acute inspiratory capacity apneas, the incidence of multiple within burst single-unit firings has been shown to increase (103). These findings are supported by recent research by Ott et al. (104), who observed an increased likelihood of a given action potential cluster firing multiple times within an integrated burst during an end expiratory apnea both under normoxic and hypoxic conditions. Lastly, under chronic hypoxemic and hypercapnic conditions (i.e., individuals with chronic obstructive pulmonary disorder), single-unit nerve recruitment strategies closely mimicked those observed in individuals with OSA (105). Evidence of elevated probability of multiple within-burst single-unit spikes appears to be conserved across numerous respiratory disorders (99) pointing to a key role of chemoreflex mechanisms and potentially central sympathetic drive on the underlying sympathetic recruitment strategies observed in individuals with OSA.
Central and Peripheral Changes in OSA
Lundblad et al. (106) and Fatouleh et al. (107) were the first to explore simultaneous changes within the brainstem and cortical areas in relation to resting with peripheral sympathetic activity in OSA, respectively. Importantly, these studies assessed brain function and peripheral sympathetic outflow at rest, reducing the confounding and complex impact of laboratory stressors on central and peripheral activity. Regions with increased grey matter volume in the brainstem included the medullary raphe nuclei, rostral ventrolateral medulla (RVLM), and the right and left dorsolateral pons. These structural differences were paired with reduced MSNA associated functional (f)MRI signal intensity changes in the medullary raphe nuclei, left RVLM, dorsolateral pons, and periaqueductal gray in individuals with OSA. This is interesting, as it would be expected that individuals with OSA, who exhibit elevated MSNA, would have elevated activity within the RVLM, although this was not the case. However, this finding may also indicate reduced active inhibition of the RVLM in individuals with OSA from other brain areas such as the caudal ventrolateral medulla (CVLM), as fMRI signal intensity may reflect synaptic input, as opposed to output of a given brain region (108), as alluded to by the authors (106). Furthermore, MSNA was inversely related to gray matter volume within the medullary raphe, RVLM, and dorsolateral pons, indicating that reduced gray matter volume in the specified brain areas may be related to peripheral sympathetic outflow in OSA (106).
Affected cortical areas included, but were not limited to, dorsolateral and medial prefrontal cortixes, precuneus, and the anterior cingulate cortex (ACC), where increased MSNA was associated with decrease signal intensity, suggesting a complex interaction between cortical areas, brainstem function, and peripheral sympathetic outflow (107). Interestingly, these findings were observed in the absence of structural changes within these brain regions when using a corrected statistical analysis, indicating altered functional neural activity within these areas is primarily responsible for the observed changes in peripheral sympathetic activity. The Floras laboratory took a step further to examine the relationship between intermittent hypoxemia, a common characteristic found in OSA, and changes in cortical thickness in various brain areas in OSA. Taylor et al. (96) observed reduced cortical thickness within the left dorsal posterior insula and increased thickness within the left midcingulate cortex. Furthermore, increased grey matter volume was observed within the bilateral thalamic regions in individuals with OSA. Similar to Lundblad et al. (106), changes in specific regions of the brain were associated with peripheral sympathetic activity. A significant relationship developed between MSNA and the volume and thickness of the left mid cingulate and left posterior thalamic regions. However, it is important to note that brain imaging and MSNA testing were performed on different days, not simultaneously. While intermittent hypoxemia was not explicitly related to MSNA in this cohort of participants with OSA, the ODI was inversely related to cortical thickness within bilateral regions of dpIC and the left posterior cingulate, indicating that the severity of hypoxemia may mediate the relation between cortical thickness and MSNA (96, 106). Furthermore, application of stressors like a Mueller Maneuver (i.e., inspiratory force against closed glottis to simulate obstructive apnea) and end-expiratory breath hold (i.e., lack of respiratory effort to simulate central apnea) to examine wake MSNA elicited similar activation within autonomic regions of the brain via blood-oxygen level-dependent functional magnetic resonance imaging (fMRI) in individuals with and without clinical OSA. However, there was a trend for greater sympathoexcitation during end-expiratory breath hold in OSA compared with control (88). In summary, the link between central brain regions affected from intermittent hypoxemia and peripheral sympathoexcitation in OSA remains equivocal. Of the studies reviewed, a variety of areas within the brainstem and cortical structural and functional brain areas are affected and appear to be related to changes in MSNA outflow. However, a comprehensive study is necessary to 1) determine the combined impact of brainstem and cortical alterations that occur with intermittent hypoxemia in OSA on MSNA, and 2) determine the impact of ODI as a mediator between affected brain areas and autonomic balance in OSA.
CPAP Treatment
Continuous positive airway pressure (CPAP) remains the gold-standard strategy to keep the upper airway from collapsing during normal sleep in patients with severe OSA. Somers et al. (11) (Fig. 3) and Hedner et al. (109) were first to demonstrate that CPAP elicited acute and chronic reductions of sympathetic activity via MSNA and plasma NOR, respectively. The reduction in sympathetic activity has been confirmed in otherwise healthy individuals, as well as those with comorbid cardiovascular disease (11, 110–114). This reduction in sympathetic activity was accompanied by improved nocturnal blood pressure, where lower vascular stress, limited baroreflex and chemoreflex activation, and reflex restoration is thought to contribute to the reduction in wake MSNA.
Figure 3.
Mean blood pressure (MBP) and sympathetic nerve activity (SNA) changes from wake to sleep transition in untreated and treated obstructive sleep apnea. Acute continuous positive airway pressure (CPAP) blunted MBP rise during sleep and reduced SNA during sleep. Reprinted and modified with permission from Somers et al. (11). BP, blood pressure; OSA, obstructive sleep apnea. *P < 0.05 from corresponding CPAP condition.
Recently, tandem MSNA and fMRI imaging examined if the improvements in MSNA with CPAP treatment were related to changes in OSA affected brain areas. Lundblad et al. (113) reported that 6 mo of CPAP significantly reduced MSNA, and restored affected brainstem areas of medullary raphe, RVLM, dorsolateral pons, and ventrolateral midbrain to comparable signal intensity as controls. Importantly, these changes in signal intensity were correlated with reduced MSNA within the medullary raphe and RVLM. Contrary to the original hypothesis, increased activity of the RVLM in treated OSA initially appeared controversial because the RVLM serves as the primary neural output for peripheral sympathetic outflow. However, increased RVLM activity may be reflective of restored baroreflex function and active inhibition of RLVM. Specifically, the baroreflex afferents from the carotid and aortic arteries relay to the nucleus tractus solitarius (NTS), where the NTS innervates the CVLM to inhibit RVLM activity to reduce sympathetic outflow. However, CVLM activity was similar pre- and post-CPAP treatment highlighting the need to further understand changes in RLVM activity in OSA. Fatouleh et al. (107) reported reductions in MSNA related signal intensity in cortical and subcortical regions of the brain such as the precuneus, right and left insula, right medial prefrontal cortex, ACC, right parahippocampus, and right and left retrosplenial cortex following CPAP treatment. Before CPAP, correlations between MSNA and signal intensity where present in all affected brain regions except the left insula and right parahippocampus in OSA compared with controls. Following CPAP, these relationships were largely similar to controls, highlighting the contribution of higher cortical brain areas to overactive sympathetic activity in OSA.
Alternative therapies for OSA treatment are limited, and it remains largely unclear if any of the alternative treatments to CPAP provide similar improvements in sympathetic and cardiovascular metrics. For example, in a study assessing the impacts of 2-wk treatment with CPAP and supplemental oxygen, CPAP significantly increased NOR clearance rates, while oxygen supplementation had no impact on NOR kinetics (115). During an acute exercise intervention, Ueno-Pardi et al. (116) examined the metaboreflex during isometric handgrip and reported decreased metaboreflex control of MSNA in OSA. In a follow-up 6-wk exercise intervention study, Guerra et al. (117) observed improved metabreflex control of MSNA and a reduction in baseline MSNA compared with a nonexercise-trained OSA control. However, inspiratory muscle training can be associated with similar improvements in OSA to CPAP. According to Ramos-Barrera et al. (118), a 6-wk high-intensity inspiratory muscle-training intervention produced a reduction in both nighttime BP and next day morning MSNA in older participants with obesity and with diagnosed moderate-to-severe OSA. Importantly, these effects were observed with training for only 5 min each day during the intervention and highlights an alternative, and perhaps a more achievable lifestyle intervention, to improve OSA symptom severity versus CPAP or dietary and exercise interventions for weight loss, which often lack adherence.
In summary, OSA disease prevalence if far more common in midlife men compared with women (119), but this may be a function of OSA classification. Scoring apneic events based upon 3% oxygen desaturation with an arousal criteria (vs. 4% criteria) can result a higher proportion of severe diagnosis in women, who present similar disease symptomology as men and are associated with an increased odds ratio for the development of CVD (i.e., higher odds ratio than traditional 4% criteria) (120). In addition, menopausal transition in women is paralleled by an increase in incident OSA (121). Prior mechanisms of baroreflex and chemoreflex impairment that contribute to sympathoexcitation and cardiovascular disease in OSA were identified in predominantly male participants with a lack of attention to potential sex differences (11, 82, 92, 94). While cardioprotection to experimental hypoxia may be present in younger females (122), it can be reasonably inferred that this effect would not be present in older women given the combine effect of sleep disturbance and repeated hypoxia. In addition, brain injury in OSA may be more severe in women (123) and potentially contribute to downstream sympathetic dysfunction (106, 107). Future work regarding OSA and cardiovascular disease mechanisms between men and women is needed.
CONCLUSIONS
Sleep serves a foundational role in cardiovascular health. Figure 4 outlines established and proposed mechanisms for sympathetic dysfunction associated with various sleep disorders and insufficiencies. TSD paradigms are converging on the link between short sleep duration and hypertension via sympathetic nervous system activation in women but not men. Urinary NOR has been shown to be increased with experimental sleep restriction, but only when associated with circadian misalignment: a finding of relevance to shift workers (40). Chronic insomnia has been associated with increased sympathetic activation via plasma NOR and sympathoexcitation from microneurography studies, providing mechanistic evidence for the hyperarousal theory of insomnia. Narcolepsy is characterized by autonomic dysfunction from loss of function of the orexin system and decreased MSNA. Finally, increased sympathetic activation in OSA is linked to decreased baroreflex function and/or tonic activity of the chemoreflex from recurring apneic events, but proper autonomic function can be restored with CPAP treatment. The preponderance of studies highlighted have not adequately accounted for sex as a biological variable, oversampling of either men (i.e., sleep deprivation, narcolepsy, sleep apnea) or women (i.e., insomnia). There is accumulating epidemiological evidence to suggest women are more susceptible to sleep disorder-mediated CVD (8). Indeed, in studies that have included equal representation of men and women (31, 78), or specifically focused their aims on sex differences (25, 26, 39), there is growing evidence that the complex relations between sleep disorders/insufficiencies and sympathetic activity are indeed impacted by sex. Future studies aimed to determine cardiovascular and/or sympathetic neural changes across sleep disorders/insufficiencies should ensure a priori power to explore sex differences, even if responses are similar between sexes. Furthermore, when HRV and plasma/urinary NOR are used in various sleep paradigms, reliability of such measures should be critically evaluated or confirmed against direct measures of sympathetic activity via microneurography.
Figure 4.
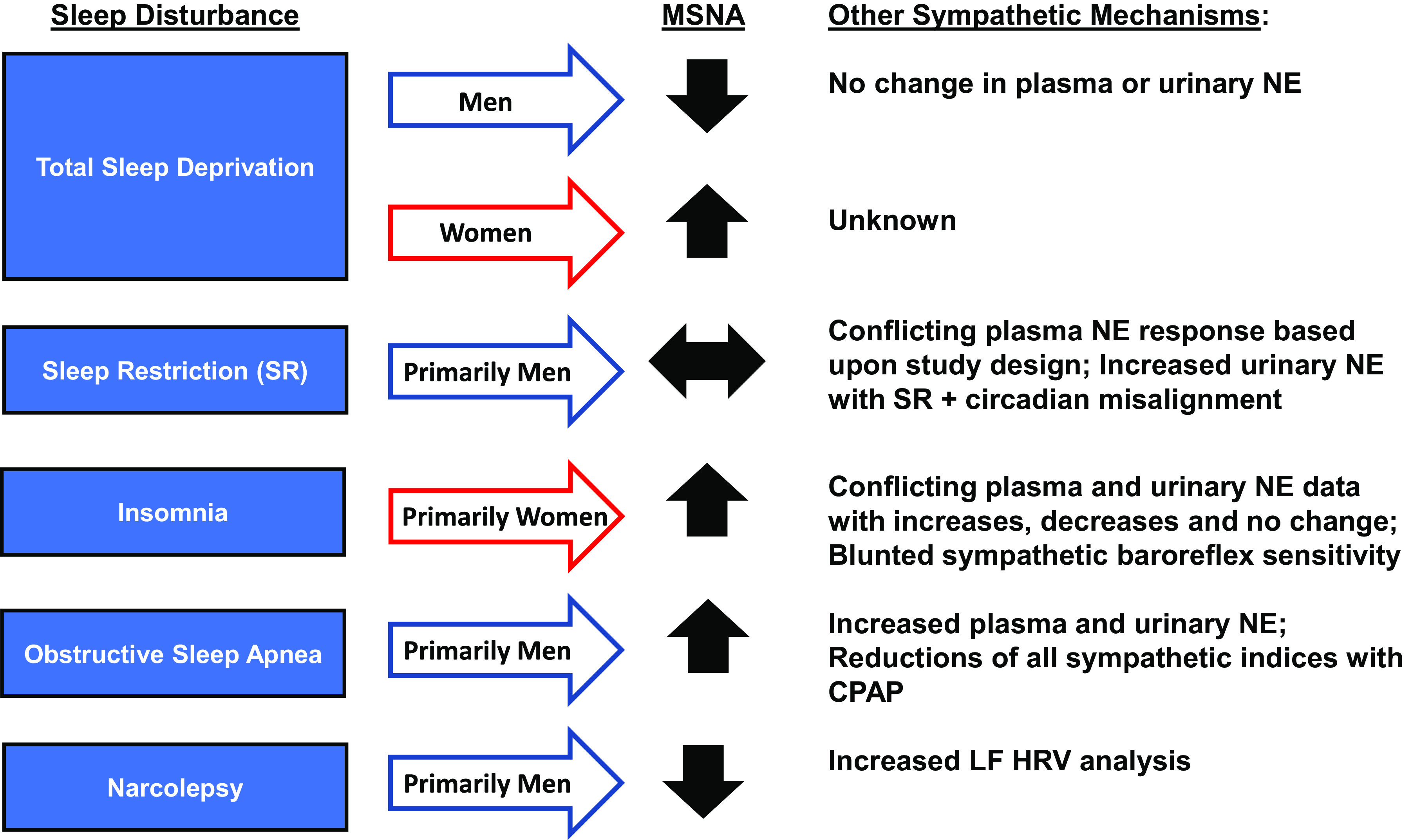
Sleep disturbances of total sleep deprivation (TSD), insomnia, and obstructive sleep apnea with associated sympathetic response and defined mechanism. TSD paradigms have interrogated the sympathetic divergence between men and women, but a preponderance of sex-specific evidence is lacking in clinical populations of insomnia (prominently women) and obstructive sleep apnea (prominently men). CPAP, continuous positive airway pressure; LF, low frequency: HRV, heart rate variability; NE, norepinephrine; MSNA, muscle sympathetic nerve activity; SR, sleep restriction.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA-024892 (to J.R.C.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M.G. and J.R.C. conceived and designed research; I.M.G. and J.R.C. prepared figures; I.M.G. and J.R.C. drafted manuscript; I.M.G. and J.R.C. edited and revised manuscript; I.M.G. and J.R.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jeremy Bigalke for critical review of the manuscript.
REFERENCES
- 1.Consensus Conference Panel; Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. J Clin Sleep Med 11: 931–952, 2015.doi: 10.5664/jcsm.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hublin C, Haasio L, Kaprio J. Changes in self-reported sleep duration with age–a 36-year longitudinal study of Finnish adults. BMC Public Health 20: 1373, 2020. doi: 10.1186/s12889-020-09376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults—United States, 2014. MMWR Morb Mortal Wkly Rep 65: 137–141, 2016. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- 4.Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Family Med Prim Care 5: 780–784, 2016. doi: 10.4103/2249-4863.201153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 108: 246–249, 2009. [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor KS, Murai H, Millar PJ, Haruki N, Kimmerly DS, Morris BL, Tomlinson G, Bradley TD, Floras JS. Arousal from sleep and sympathetic excitation during wakefulness. Hypertension 68: 1467–1474, 2016. doi: 10.1161/HYPERTENSIONAHA.116.08212 [DOI] [PubMed] [Google Scholar]
- 7.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension 47: 833–839, 2006. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 8.Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension 50: 693–700, 2007. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep 29: 1009–1014, 2006. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 10.Grandner M, Mullington JM, Hashmi SD, Redeker NS, Watson NF, Morgenthaler TI. Sleep duration and hypertension: analysis of >700,000 adults by age and sex. J Clin Sleep Med 14: 1031–1039, 2018. doi: 10.5664/jcsm.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34: 623–648, 1997. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 13.Wallin BG, Sundlöf G, Eriksson BM, Dominiak P, Grobecker H, Lindblad LE. Plasma noradrenaline correlates to sympathetic muscle nerve activity in normotensive man. Acta Physiol Scand 111: 69–73, 1981. doi: 10.1111/j.1748-1716.1981.tb06706.x. [DOI] [PubMed] [Google Scholar]
- 14.Fonkoue IT, Carter JR. Sympathetic neural reactivity to mental stress in humans: test-retest reproducibility. Am J Physiol Regul Integr Comp Physiol 309: R1380–R1386, 2015. doi: 10.1152/ajpregu.00344.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plouffe AA, Vranish JR. What keeps us up at night? The cardiovascular impact of nighttime arousals from sleep. Am J Physiol Heart Circ Physiol 321: H613–H614, 2021. doi: 10.1152/ajpheart.00462.2021. [DOI] [PubMed] [Google Scholar]
- 16.Bigalke JA, Greenlund IM, Nicevski JR, Smoot CA, Oosterhoff B, John-Henderson NA, Carter JR. Blunted heart rate recovery to spontaneous nocturnal arousals in short-sleeping adults. Am J Physiol Heart Circ Physiol 321: H558–H566, 2021. doi: 10.1152/ajpheart.00329.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stockelman KA, Bain AR, Dow CA, Diehl KJ, Greiner JJ, Stauffer BL, DeSouza CA. Regular aerobic exercise counteracts endothelial vasomotor dysfunction associated with insufficient sleep. Am J Physiol Heart Circ Physiol 320: H1080–H1088, 2021. doi: 10.1152/ajpheart.00615.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holwerda SW, Carter JR, Yang H, Wang J, Pierce GL, Fadel PJ. CORP: Standardizing methodology for assessing spontaneous baroreflex control of muscle sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol 320: H762–H771, 2021. doi: 10.1152/ajpheart.00704.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrera A, Morales-Loredo H, Garcia JM, Fregoso G, Pace CE, Mendiola PJ, Naik JS, Gonzalez Bosc LV, Kanagy NL. Simulated sleep apnea alters hydrogen sulfide regulation of blood flow and pressure. Am J Physiol Heart Circ Physiol 320: H511–H519, 2021. doi: 10.1152/ajpheart.00672.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherubini JM, Cheng JL, Williams JS, MacDonald MJ. Sleep deprivation and endothelial function: reconciling seminal evidence with recent perspectives. Am J Physiol Heart Circ Physiol 320: H29–H35, 2021. doi: 10.1152/ajpheart.00607.2020. [DOI] [PubMed] [Google Scholar]
- 21.Limberg JK, Smith JA, Soares RN, Harper JL, Houghton KN, Jacob DW, Mozer MT, Grunewald ZI, Johnson BD, Curry TB, Baynard T, Manrique-Acevedo C, Padilla J. Sympathetically mediated increases in cardiac output, not restraint of peripheral vasodilation, contribute to blood pressure maintenance during hyperinsulinemia. Am J Physiol Heart Circ Physiol 319: H162–H170, 2020. doi: 10.1152/ajpheart.00250.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyavanapalli J. Novel approaches to restore parasympathetic activity to the heart in cardiorespiratory diseases. Am J Physiol Heart Circ Physiol 319: H1153–H1161, 2020. doi: 10.1152/ajpheart.00398.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension 35: 1173–1175, 2000. doi: 10.1161/01.hyp.35.5.1173. doi:. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa Y, Kanbayashi T, Saito Y, Takahashi Y, Kitajima T, Takahashi K, Hishikawa Y, Shimizu T. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep 26: 986–989, 2003. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- 25.Carter JR, Durocher JJ, Larson RA, DellaValla JP, Yang H. Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am J Physiol Heart Circ Physiol 302: H1991–H1997, 2012. doi: 10.1152/ajpheart.01132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter JR, Fonkoue IT, Greenlund IM, Schwartz CE, Mokhlesi B, Smoot CA. Sympathetic neural responsiveness to sleep deprivation in older adults: sex differences. Am J Physiol Heart Circ Physiol 317: H315–H322, 2019. doi: 10.1152/ajpheart.00232.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayk F, Teckentrup C, Becker C, Heutling D, Wellhöner P, Lehnert H, Dodt C. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 298: R191–R197, 2010. doi: 10.1152/ajpregu.00368.2009. [DOI] [PubMed] [Google Scholar]
- 28.Lindsey ML, LeBlanc AJ, Ripplinger CM, Carter JR, Kirk JA, Hansell Keehan K, Brunt KR, Kleinbongard P, Kassiri Z. Reinforcing rigor and reproducibility expectations for use of sex and gender in cardiovascular research. Am J Physiol Heart Circ Physiol 321: H819–H824, 2021. doi: 10.1152/ajpheart.00418.2021. [DOI] [PubMed] [Google Scholar]
- 29.Broussard JL, Chapotot F, Abraham V, Day A, Delebecque F, Whitmore HR, Brunt KR, Kleinbongard P, Kassiri Z. Sleep restriction increases free fatty acids in healthy men. Diabetologia 58: 791–798, 2015. doi: 10.1007/s00125-015-3500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 59: 2126–2133, 2010. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Covassin N, Bukartyk J, Singh P, Calvin AD, St Louis EK, Somers VK. Effects of experimental sleep restriction on ambulatory and sleep blood pressure in healthy young adults: a randomized crossover study. Hypertension 78: 859–870, 2021. doi: 10.1161/HYPERTENSIONAHA.121.17622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dettoni JL, Consolim-Colombo FM, Drager LF, Rubira MC, Cavasin de Souza SB, Irigoyen MC, Mostarda C, Borile S, Krieger EM, Moreno H Jr, Lorenzi-Filho G. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J Appl Physiol (1985) 113: 232–236, 2012. doi: 10.1152/japplphysiol.01604.2011. [DOI] [PubMed] [Google Scholar]
- 33.Takase B, Akima T, Satomura K, Ohsuzu F, Mastui T, Ishihara M, Kurita A. Effects of chronic sleep deprivation on autonomic activity by examining heart rate variability, plasma catecholamine, and intracellular magnesium levels. Biomed Pharmacother 58, Suppl 1: S35–39, 2004. doi: 10.1016/S0753-3322(04)80007-6. [DOI] [PubMed] [Google Scholar]
- 34.Faraut B, Nakib S, Drogou C, Elbaz M, Sauvet F, De Bandt JP, Léger D. Napping reverses the salivary interleukin-6 and urinary norepinephrine changes induced by sleep restriction. J Clin Endocrinol Metab 100: E416–E426, 2015. doi: 10.1210/jc.2014-2566. [DOI] [PubMed] [Google Scholar]
- 35.Nedeltcheva AV, Imperial JG, Penev PD. Effects of sleep restriction on glucose control and insulin secretion during diet-induced weight loss. Obesity (Silver Spring) 20: 1379–1386, 2012. doi: 10.1038/oby.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 94: 3242–3250, 2009. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid SM, Jauch-Chara K, Hallschmid M, Schultes B. Mild sleep restriction acutely reduces plasma glucagon levels in healthy men. J Clin Endocrinol Metab 94: 5169–5173, 2009. doi: 10.1210/jc.2009-0969. doi: 10.1210/jc.2009-0969. [DOI] [PubMed] [Google Scholar]
- 38.Klingenberg L, Chaput JP, Holmbäck U, Visby T, Jennum P, Nikolic M, Astrup A, Sjödin A. Acute sleep restriction reduces insulin sensitivity in adolescent boys. Sleep 36: 1085–1090, 2013. doi: 10.5665/sleep.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang H, Baltzis D, Bhatt V, Haack M, Meier-Ewert HK, Gautam S, Veves A, Mullington JM. Macro- and microvascular reactivity during repetitive exposure to shortened sleep: sex differences. Sleep 44: zsaa257, 2021. doi: 10.1093/sleep/zsaa257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grimaldi D, Carter JR, Van Cauter E, Leproult R. Adverse impact of sleep restriction and circadian misalignment on autonomic function in healthy young adults. Hypertension 68: 243–250, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindfors P, Lundberg O, Lundberg U. Allostatic load and clinical risk as related to sense of coherence in middle-aged women. Psychosom Med 68: 801–807, 2006. doi: 10.1097/01.psy.0000232267.56605.22. [DOI] [PubMed] [Google Scholar]
- 42.Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol 21: 57–64, 2014. doi: 10.1177/2047487312460020. [DOI] [PubMed] [Google Scholar]
- 43.Seelig E, Keller U, Klarhöfer M, Scheffler K, Brand S, Holsboer-Trachsler E, Hatzinger M, Bilz S. Neuroendocrine regulation and metabolism of glucose and lipids in primary chronic insomnia: a prospective case-control study. PLoS One 8: e61780, 2013. doi: 10.1371/journal.pone.0061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grimaldi D, Reid KJ, Papalambros NA, Braun RI, Malkani RG, Abbott SM, Ong JC, Zee PC. Autonomic dysregulation and sleep homeostasis in insomnia. Sleep 44: zsaa274, 2021. doi: 10.1093/sleep/zsaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Floam S, Simpson N, Nemeth E, Scott-Sutherland J, Gautam S, Haack M. Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. J Sleep Res 24: 296–304, 2015. doi: 10.1111/jsr.12259. [DOI] [PubMed] [Google Scholar]
- 46.Irwin M, Clark C, Kennedy B, Gillin JC, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun 17: 365–372, 2003. doi: 10.1016/S0889-1591(03)00031-X. [DOI] [PubMed] [Google Scholar]
- 47.Vgontzas AN, Tsigos C, Bixler EO, Stratakis CA, Zachman K, Kales A, Vela-Bueno A, Chrousos GP. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosomat Res 45: 21–31, 1998. doi: 10.1016/S0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 48.Cortelli P, Parchi P, Contin M, Pierangeli G, Avoni P, Tinuper P, Montagna P, Baruzzi A, Gambetti PL, Lugaresi E. Cardiovascular dysautonomia in fatal familial insomnia. Clin Auton Res 1: 15–21, 1991. doi: 10.1007/BF01826053. [DOI] [PubMed] [Google Scholar]
- 49.Roehrs TA, Roth T. Hyperarousal in insomnia and hypnotic dose escalation. Sleep Med 23: 16–20, 2016. doi: 10.1016/j.sleep.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carter JR, Grimaldi D, Fonkoue IT, Medalie L, Mokhlesi B, Van Cauter E. Assessment of sympathetic neural activity in chronic insomnia: evidence for elevated cardiovascular risk. Sleep 41: zsy048, 2018. doi: 10.1093/sleep/zsy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev 14: 9–15, 2010. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Bonnet M, Arand D. Hyperarousal and insomnia. Sleep Med Rev 1: 97–108, 1997. doi: 10.1016/S1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 53.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep 29: 85–93, 2006. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 54.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 55.Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res 40: 700–708, 2006. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Freeman D, Stahl D, McManus S, Meltzer H, Brugha T, Wiles N, Bebbington P. Insomnia, worry, anxiety and depression as predictors of the occurrence and persistence of paranoid thinking. Soc Psychiatry Psychiatr Epidemiol 47: 1195–1203, 2012. doi: 10.1007/s00127-011-0433-1. [DOI] [PubMed] [Google Scholar]
- 57.Haynes SN, Adams A, Franzen M. The effects of presleep stress on sleep-onset insomnia. J Abnorm Psychol 90: 601–606, 1981. doi: 10.1037/0021-843X.90.6.601. [DOI] [PubMed] [Google Scholar]
- 58.Monroe LJ. Psychological and physiological differences between good and poor sleepers. J Abnorm Psychol 72: 255–264, 1967. doi: 10.1037/h0024563. [DOI] [PubMed] [Google Scholar]
- 59.Freedman RR. EEG power spectra in sleep-onset insomnia. Electroencephalogr Clin Neurophysiol 63: 408–413, 1986. doi: 10.1016/0013-4694(86)90122-7. [DOI] [PubMed] [Google Scholar]
- 60.Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci 26: 13400–13410, 2006. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willie JT, Takahira H, Shibahara M, Hara J, Nomiyama M, Yanagisawa M, Sakurai T. Ectopic overexpression of orexin alters sleep/wakefulness states and muscle tone regulation during REM sleep in mice. J Mol Neurosci 43: 155–161, 2011. doi: 10.1007/s12031-010-9437-7. [DOI] [PubMed] [Google Scholar]
- 62.Li A, Nattie E. Orexin, cardio-respiratory function, and hypertension. Front Neurosci 8: 22, 2014. doi: 10.3389/fnins.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang S, Huang W, Lu S, Lu L, Li G, Chen X, Liu X, Lv X, Zhao Z, Duan R, Du Y, Tang J. Increased plasma orexin-A levels in patients with insomnia disorder are not associated with prepro-orexin or orexin receptor gene polymorphisms. Peptides 88: 55–61, 2017. doi: 10.1016/j.peptides.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 64.Lanfranchi PA, Pennestri MH, Fradette L, Dumont M, Morin CM, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep 32: 760–766, 2009. doi: 10.1093/sleep/32.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyu B, Hagen EW, Ravelo LA, Peppard PE. Blood pressure dipping and sleep quality in Wisconsin Sleep Cohort. J Hypertens 38: 448–455, 2020. doi: 10.1097/HJH.0000000000002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roehrs T, Roth T. Insomnia as a path to alcoholism: tolerance development and dose escalation. Sleep 41: zsy091, 2018. doi: 10.1093/sleep/zsy091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koob GF, Colrain IM. Alcohol use disorder and sleep disturbances: a feed-forward allostatic framework. Neuropsychopharmacology 45: 141–165, 2020. doi: 10.1038/s41386-019-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greenlund IM, Cunningham HA, Tikkanen AL, Bigalke JA, Smoot CA, Durocher JJ, Carter JR. Morning sympathetic activity after evening binge alcohol consumption. Am J Physiol Heart Circ Physiol 320: H305–H315, 2021. doi: 10.1152/ajpheart.00743.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greenlund IM, Bigalke JA, Tikkanen AL, Durocher JJ, Smoot CA, Carter JR. Evening binge alcohol disrupts cardiovagal tone and baroreflex function during polysomnographic sleep. Sleep 44: zsab130, 2021. doi: 10.1093/sleep/zsab130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jennum P, Ibsen R, Knudsen S, Kjellberg J. Comorbidity and mortality of narcolepsy: a controlled retro- and prospective national study. Sleep 36: 835–840, 2013. doi: 10.5665/sleep.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron 27: 469–474, 2000. doi: 10.1016/S0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep 32: 993–998, 2009. doi: 10.1093/sleep/32.8.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thannickal TC. A decade of hypocretin/orexin: accomplishments in sleep medicine. Sleep Med Rev 13: 5–8, 2009. doi: 10.1016/j.smrv.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Fronczek R, Overeem S, Reijntjes R, Lammers GJ, van Dijk JG, Pijl H. Increased heart rate variability but normal resting metabolic rate in hypocretin/orexin-deficient human narcolepsy. J Clin Sleep Med 04: 248–254, 2008. doi: 10.5664/jcsm.27188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silvani A, Grimaldi D, Barletta G, Bastianini S, Vandi S, Pierangeli G, Plazzi G, Cortelli P. Cardiovascular variability as a function of sleep–wake behaviour in narcolepsy with cataplexy. J Sleep Res 22: 178–184, 2013. doi: 10.1111/jsr.12007. [DOI] [PubMed] [Google Scholar]
- 76.Grimaldi D, Pierangeli G, Barletta G, Terlizzi R, Plazzi G, Cevoli S, Franceschini C, Montagna P, Cortelli P. Spectral analysis of heart rate variability reveals an enhanced sympathetic activity in narcolepsy with cataplexy. Clin Neurophysiol 121: 1142–1147, 2010. doi: 10.1016/j.clinph.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 77.Lord S, Senior R, Das M, Whittam A, Murray A, McComb J. Low-frequency heart rate variability: reproducibility in cardiac transplant recipients and normal subjects. Clin Sci (Lond) 100: 43–46, 2001. doi: 10.1042/CS20000111. [DOI] [PubMed] [Google Scholar]
- 78.Donadio V, Liguori R, Vandi S, Pizza F, Dauvilliers Y, Leta V, Giannoccaro MP, Baruzzi A, Plazzi G. Lower wake resting sympathetic and cardiovascular activities in narcolepsy with cataplexy. Neurology 83: 1080–1086, 2014. doi: 10.1212/WNL.0000000000000793. [DOI] [PubMed] [Google Scholar]
- 79.Donadio V, Plazzi G, Pizza F, Liguori R. Abnormal sympathetic and cardiovascular reactivity during mental stress in patients with narcolepsy and cataplexy. Clin Neurophysiol 127: e143, 2016. doi: 10.1016/j.clinph.2015.09.054. [DOI] [Google Scholar]
- 80.Giannoccaro MP, Donadio V, Plazzi G, Pizza F, Vandi S, Leta V, Liguori R. Sympathetic and cardiovascular changes induced by sodium oxybate treatment in patients with narcolepsy and cataplexy. Clin Neurophysiol 124: e218, 2013. doi: 10.1016/j.clinph.2013.06.159. [DOI] [Google Scholar]
- 81.Carter JR, Mokhlesi B, Thomas RJ. Obstructive sleep apnea phenotypes and cardiovascular risk: is there a role for heart rate variability in risk stratification? Sleep 44: zsab037, 2021. doi: 10.1093/sleep/zsab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Narkiewicz K, Van De Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 98: 772–776, 1998. doi: 10.1161/01.CIR.98.8.772. [DOI] [PubMed] [Google Scholar]
- 83.Toschi-Dias E, Trombetta IC, Silva VJ, Maki-Nunes C, Cepeda FX, Alves MJ, Carvalho GL, Drager LF, Lorenzi-Filho G, Negrão CE, Rondon MU. Diet associated with exercise improves baroreflex control of sympathetic nerve activity in metabolic syndrome and sleep apnea patients. Sleep Breath 23: 143–151, 2019. doi: 10.1007/s11325-018-1675-x. [DOI] [PubMed] [Google Scholar]
- 84.Narkiewicz K, Somers V. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 177: 385–390, 2003. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 85.Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation 98: 1071–1077, 1998. doi: 10.1161/01.CIR.98.11.1071. [DOI] [PubMed] [Google Scholar]
- 86.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 103: 1763–1768, 1993. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 87.Elmasry A, Lindberg E, Hedner J, Janson C, Boman G. Obstructive sleep apnoea and urine catecholamines in hypertensive males: a population-based study. Eur Respir J 19: 511–517, 2002. doi: 10.1183/09031936.02.00106402. [DOI] [PubMed] [Google Scholar]
- 88.Taylor KS, Keir DA, Haruki N, Kimmerly DS, Millar PJ, Murai H, Floras JS. Comparison of cortical autonomic network-linked sympathetic excitation by mueller maneuvers and breath-holds in subjects with and without obstructive sleep apnea. Front Physiol 12: 678630, 2021. doi: 10.3389/fphys.2021.678630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goya TT, Silva RF, Guerra RS, Lima MF, Barbosa ER, Cunha PJ, Lobo DM, Buchpiguel CA, Busatto-Filho G, Negrão CE, Lorenzi-Filho G, Ueno-Pardi LM. Increased muscle sympathetic nerve activity and impaired executive performance capacity in obstructive sleep apnea. Sleep 39: 25–33, 2016. doi: 10.5665/sleep.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl 6: S529–531, 1988. doi: 10.1097/00004872-198812040-00166. [DOI] [PubMed] [Google Scholar]
- 91.Carlson JT, Hedner JA, Sellgren J, Elam M, Wallin BG. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med 154: 1490–1496, 1996. doi: 10.1164/ajrccm.154.5.8912770. [DOI] [PubMed] [Google Scholar]
- 92.Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension 32: 1039–1043, 1998. doi: 10.1161/01.HYP.32.6.1039. [DOI] [PubMed] [Google Scholar]
- 93.Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation 97: 943–945, 1998. doi: 10.1161/01.CIR.97.10.943. [DOI] [PubMed] [Google Scholar]
- 94.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99: 1183–1189, 1999. doi: 10.1161/01.CIR.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 95.Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest 131: 1406–1413, 2007. doi: 10.1378/chest.06-2580. [DOI] [PubMed] [Google Scholar]
- 96.Taylor KS, Millar PJ, Murai H, Haruki N, Kimmerly DS, Bradley TD, Floras JS. Cortical autonomic network gray matter and sympathetic nerve activity in obstructive sleep apnea. Sleep 41: zsx208, 2018. doi: 10.1093/sleep/zsx208. [DOI] [PubMed] [Google Scholar]
- 97.Penzel T, Kantelhardt JW, Lo CC, Voigt K, Vogelmeier C. Dynamics of heart rate and sleep stages in normals and patients with sleep apnea. Neuropsychopharmacology 28, Suppl 1: S48–53, 2003. doi: 10.1038/sj.npp.1300146. [DOI] [PubMed] [Google Scholar]
- 98.Elam M, McKenzie D, Macefield V. Mechanisms of sympathoexcitation: single-unit analysis of muscle vasoconstrictor neurons in awake OSAS subjects. J Appl Physiol (1985) 93: 297–303, 2002. doi: 10.1152/japplphysiol.00899.2001. [DOI] [PubMed] [Google Scholar]
- 99.Macefield VG. Firing patterns of muscle vasoconstrictor neurons in respiratory disease. Front Physiol 3: 153, 2012. doi: 10.3389/fphys.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Macefield VG, Rundqvist B, Sverrisdottir YB, Wallin BG, Elam M. Firing properties of single muscle vasoconstrictor neurons in the sympathoexcitation associated with congestive heart failure. Circulation 100: 1708–1713, 1999. doi: 10.1161/01.CIR.100.16.1708. [DOI] [PubMed] [Google Scholar]
- 101.Hamaoka T, Murai H, Kaneko S, Usui S, Okabe Y, Tokuhisa H, Kato T, Furusho H, Sugiyama Y, Nakatsumi Y, Takata S, Takamura M. Single-unit muscle sympathetic nerve activity reflects sleep apnea severity, especially in severe obstructive sleep apnea patients. Front Physiol 7: 66, 2016. doi: 10.3389/fphys.2016.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Incognito AV, Samora M, Shepherd AD, Cartafina RA, Guimarães GM, Daher M, Millar PJ, Vianna LC. Arterial baroreflex regulation of muscle sympathetic single-unit activity in men: influence of resting blood pressure. Am J Physiol Heart Circ Physiol 318: H937–H946, 2020. doi: 10.1152/ajpheart.00700.2019. [DOI] [PubMed] [Google Scholar]
- 103.Macefield VG, Wallin BG. Firing properties of single vasoconstrictor neurones in human subjects with high levels of muscle sympathetic activity. J Physiol 516: 293–301, 1999. doi: 10.1111/j.1469-7793.1999.293aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ott EP, Baker SE, Holbein WW, Shoemaker JK, Limberg JK. Effect of varying chemoreflex stress on sympathetic neural recruitment strategies during apnea. J Neurophysiol 122: 1386–1396, 2019. doi: 10.1152/jn.00319.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ashley C, Burton D, Sverrisdottir YB, Sander M, McKenzie DK, Macefield VG. Firing probability and mean firing rates of human muscle vasoconstrictor neurones are elevated during chronic asphyxia. J Physiol 588: 701–712, 2010. doi: 10.1113/jphysiol.2009.185348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lundblad LC, Fatouleh RH, Hammam E, McKenzie DK, Macefield VG, Henderson LA. Brainstem changes associated with increased muscle sympathetic drive in obstructive sleep apnoea. Neuroimage 103: 258–266, 2014. doi: 10.1016/j.neuroimage.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 107.Fatouleh RH, Hammam E, Lundblad LC, Macey PM, McKenzie DK, Henderson LA, Macefield VG. Functional and structural changes in the brain associated with the increase in muscle sympathetic nerve activity in obstructive sleep apnoea. Neuroimage Clin 6: 275–283, 2014. doi: 10.1016/j.nicl.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 109.Hedner J, Darpo B, Ejnell H, Carlson J, Caidahl K. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur Respir J 8: 222–229, 1995. doi: 10.1183/09031936.95.08020222. [DOI] [PubMed] [Google Scholar]
- 110.Waradekar NV, Sinoway LI, Zwillich CW, Leuenberger UA. Influence of treatment on muscle sympathetic nerve activity in sleep apnea. Am J Respir Crit Care Med 153: 1333–1338, 1996. doi: 10.1164/ajrccm.153.4.8616563. [DOI] [PubMed] [Google Scholar]
- 111.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 100: 2332–2335, 1999. doi: 10.1161/01.CIR.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 112.Donadio V, Liguori R, Vetrugno R, Contin M, Elam M, Wallin BG, Karlsson T, Bugiardini E, Baruzzi A, Montagna P. Daytime sympathetic hyperactivity in OSAS is related to excessive daytime sleepiness. J Sleep Res 16: 327–332, 2007. doi: 10.1111/j.1365-2869.2007.00602.x. [DOI] [PubMed] [Google Scholar]
- 113.Lundblad LC, Fatouleh RH, McKenzie DK, Macefield VG, Henderson LA. Brain stem activity changes associated with restored sympathetic drive following CPAP treatment in OSA subjects: a longitudinal investigation. J Neurophysiol 114: 893–901, 2015. doi: 10.1152/jn.00092.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henderson LA, Fatouleh RH, Lundblad LC, McKenzie DK, Macefield VG. Effects of 12 months continuous positive airway pressure on sympathetic activity related brainstem function and structure in obstructive sleep apnea. Front Neurosci 10: 90, 2016. doi: 10.3389/fnins.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol (1985) 100: 343–348, 2006. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- 116.Ueno-Pardi LM, Guerra RS, Goya TT, Silva RF, Gara EM, Lima MF, Nobre TS, Alves MJ, Trombetta IC, Lorenzi-Filho G. Muscle metaboreflex control of sympathetic activity in obstructive sleep apnea. Med Sci Sports Exerc 49: 1424–1431, 2017. doi: 10.1249/MSS.0000000000001242. [DOI] [PubMed] [Google Scholar]
- 117.Guerra RS, Goya TT, Silva RF, Lima MF, Barbosa ER, Alves M, Rodrigues AG, Lorenzi-Filho G, Negrão CE, Ueno-Pardi LM. Exercise training increases metaboreflex control in patients with obstructive sleep apnea. Med Sci Sports Exerc 51: 426–435, 2019. doi: 10.1249/MSS.0000000000001805. [DOI] [PubMed] [Google Scholar]
- 118.Ramos-Barrera GE, DeLucia CM, Bailey EF. Inspiratory muscle strength training lowers blood pressure and sympathetic activity in older adults with OSA: a randomized controlled pilot trial. J Appl Physiol (1985) 129: 449–458, 2020. doi: 10.1152/japplphysiol.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 20: 705–706, 1997. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 120.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nunez N, Selma-Ferrer MJ, Punjabi NM, Farre R. Impact of different hypopnea definitions on obstructive sleep apnea severity and cardiovascular mortality risk in women and elderly individuals. Sleep Med 27-28: 54–58, 2016. doi: 10.1016/j.sleep.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 121.Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep 23: 165–170, 2000. [PubMed] [Google Scholar]
- 122.Jacob DW, Ott EP, Baker SE, Scruggs ZM, Ivie CL, Harper JL, Manrique-Acevedo CM, Limberg JK. Sex differences in integrated neurocardiovascular control of blood pressure following acute intermittent hypercapnic hypoxia. Am J Physiol Regul Integr Comp Physiol 319: R626–R636, 2020. doi: 10.1152/ajpregu.00191.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Macey PM, Kumar R, Yan-Go FL, Woo MA, Harper RM. Sex differences in white matter alterations accompanying obstructive sleep apnea. Sleep 35: 1603–1613, 2012. doi: 10.5665/sleep.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]