Keywords: ischemia-reperfusion injury, oxygen generators, skeletal muscle function, tourniquet
Abstract
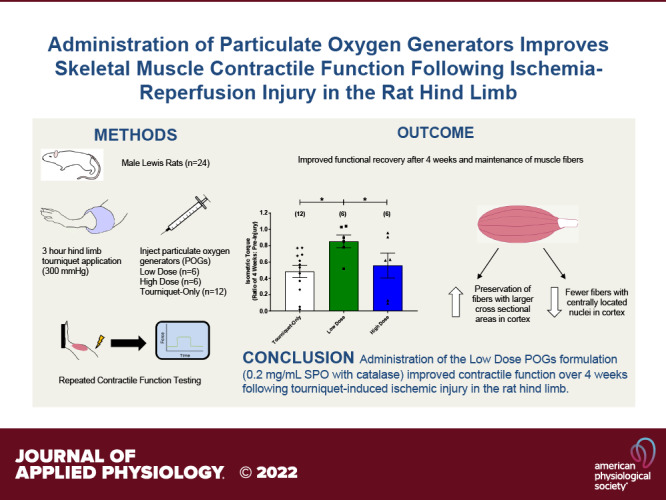
Extended tourniquet application, often associated with battlefield extremity trauma, can lead to severe ischemia-reperfusion (I/R) injury in skeletal muscle. Particulate oxygen generators (POGs) can be directly injected into tissue to supply oxygen to attenuate the effects of I/R injury in muscle. The goal of this study was to investigate the efficacy of a sodium percarbonate (SPO)-based POG formulation in reducing ischemic damage in a rat hindlimb during tourniquet application. Male Lewis rats were anesthetized and underwent tourniquet application for 3 h at a pressure of 300 mmHg. Shortly after tourniquet inflation, animals received intramuscular injections of either 0.2 mg/mL SPO with catalase (n = 6) or 2.0 mg/mL SPO with catalase (n = 6) directly into the tibialis anterior (TA) muscle. An additional Tourniquet-Only group (n = 12) received no intervention. Functional recovery was monitored by in vivo contractile testing of the hindlimb at 1, 2, and 4 wk after injury. By the 4 wk time point, the Low-Dose POG group continued to show improved functional recovery (85% of baseline) compared with the Tourniquet-Only (48%) and High-Dose POG (56%) groups. In short, the low-dose POG formulation appeared, at least in part, to mitigate the impact of ischemic tissue injury, thus improving contractile function after tourniquet application. Functional improvement correlated with maintenance of larger muscle fiber cross-sectional area and the presence of fewer fibers containing centrally located nuclei. As such, POGs represent a potentially attractive therapeutic solution for addressing I/R injuries associated with extremity trauma.
NEW & NOTEWORTHY Skeletal muscle contraction was evaluated in the same animals at multiple time points up to 4 wk after injury, following administration of particulate oxygen generators (POGs) in a clinically relevant rat hindlimb model of tourniquet-induced ischemia. The observed POG-mediated improvement of muscle function over time confirms and extends previous studies to further document the potential clinical applications of POGs. Of particular significance in austere environments, this technology can be applied in the absence of an intact circulation.
INTRODUCTION
Proper tourniquet use during extremity hemorrhage has proven to save lives and has decreased mortality rates in recent years during military conflict (1, 2). Furthermore, recent studies have shown that combat tourniquet use did not impact amputation rates for lower extremity injuries with vascular damage (3, 4). During Operations Iraqi Freedom and Enduring Freedom (OIF and OEF), rapid evacuation to definitive surgical care limited tourniquet application time to 70 min (5), limiting tourniquet-associated complications. However, the current state of global conflicts takes place under remote conditions, with delayed access to definitive care, making tourniquet-associated complications a greater concern. Extended tourniquet application induces ischemic injury followed by reperfusion injury after tourniquet removal, both of which may result in tissue damage (6–9). The neuromuscular damage becomes more severe as the length of the ischemic period increases, beginning 2–3 h after tourniquet application and continuing to progress to a point of irrecoverable damage after ∼6 h (9–16).
In this regard, ischemic preconditioning, hypothermic treatment of the limb, and hyperbaric oxygen therapy have all been investigated for improving muscle damage following ischemia-reperfusion (I/R) injury [reviewed in Gillani et al. (8)]. However, these interventions often require advanced knowledge of injury, special equipment, and/or frequent monitoring, none of which is generally practical in emergency situations. More pragmatic therapeutics have proven effective and span an array of management options (8, 17–25), although treatments that are designed for use at the onset of ischemia are limited, and many of these treatments are intended to be administered systemically as opposed to into the muscle during the time of ischemia. As such, there is a pressing need for an intervention(s) that could be easily implemented intramuscularly in the field, without interfering with tourniquet application, to attenuate muscle injury.
One viable solution in this challenging environment is to implement particulate oxygen generators (POGs), which are compounds that decompose to form oxygen (26, 27). Common POG formulations may incorporate sodium percarbonate (SPO), calcium peroxide (CPO), or liquid hydrogen peroxide (H2O2), and these POG formulations have been investigated for use in a wide array of tissue engineering applications, as discussed in recent reviews (28–30). POGs provide an advantage over conventional methods in that they are shelf stable, are easy to implement/transport, and can be used at the time of injury, in the absence of an intact vasculature, to focus on injury mitigation—in austere environments and without any requisite pretreatment. However, POG solutions also release reactive oxygen species (ROS), thus generating free radicals in vivo (31–33) and thereby enabling free radical damage to muscle cells (6–9, 34, 35). To offset ROS damage, the antioxidant enzyme catalase is frequently incorporated in POG formulations to facilitate the rapid conversion of hydrogen peroxide into water and oxygen (27–30).
Although preclinical studies have demonstrated the utility of POGs to prolong oxygen generation and cell viability in hypoxic environments, there is still a dearth of information regarding long-term effects on skeletal muscle structure and function. For example, SPO with catalase has demonstrated improved maintenance of muscle contractile function in vitro as well as in a 24-h model of partial ischemia in vivo (27), although these studies did not assess the durability and magnitude of functional recovery over time. Since POGs can be directly injected into ischemic tissue, this approach provides an ideal solution for injuries where there is no functional vasculature (i.e., no blood flow), such as during tourniquet application.
Thus, the goal of the present study was to evaluate the ability of intramuscular SPO injections, given at the time of ischemia, to mitigate the ensuing muscle tissue damage to the anterior compartment of the rat hindlimb. Previous work has demonstrated that SPO with catalase decomposes quickly and is effective in an acute ischemic injury (27), making this POG composition an attractive candidate for long-term studies. To this end, we established a reproducible model of tourniquet-induced ischemic damage to skeletal muscle and evaluated the impact of SPO injection on peroneal nerve-mediated isometric torque responses of the anterior crural muscles at 1–4 wk after ischemia. The model allowed us to compare the functional and morphological characteristics of postischemic recovery of both SPO-injected and untreated animals during a 4-wk recovery period. These initial observations, on a biologically relevant animal model, may have important implications for limb salvage after extremity trauma.
MATERIALS AND METHODS
Animal Care
Animal procedures were approved by the US Army Medical Research and Materiel Command Animal Care and Use Review Office and the Institutional Animal Care and Use Committee at the University of Virginia. Twenty-four adult male Lewis rats (Charles River Laboratories), weighing 357.3 ± 7.3 g at the time of injury, were evaluated over the course of this study. Food and water were provided ad libitum, and all animals were housed in an accredited vivarium (Association for Assessment and Accreditation of Laboratory Animal Care International).
Injury Creation and Particulate Oxygen Generator Administration
Tourniquet-induced ischemic injury was created in the rat hindlimb as previously described (36, 37). Briefly, the left hindlimb of anesthetized rats (1.0–2.0% isoflurane) was shaved and prepared with aseptic technique. Animals were then placed on their side, with the experimental leg facing upward, and the foot was elevated for ∼5 min to exsanguinate the leg. A pneumatic digit cuff (model UDC1.6, D.E. Hokanson, Inc.) was placed high up on the experimental limb, to create a secure fit on the upper thigh. The cuff was inflated to a pressure of 300 mmHg with an aneroid sphygmomanometer (model DS400, D.E. Hokanson, Inc.), which, based on previous studies from our group, was sufficient to stop distal blood flow in a rat tourniquet model (37–40). After inflation, the cuff hose was clamped, using a hemostat to maintain the tourniquet pressure for 3 h. At the time of the experiment, two POG formulations were created by directly dissolving hand-ground sodium percarbonate (SPO, Sigma Aldrich) and bovine liver catalase (lyophilized powder, 2,000–5,000 U/mg, Sigma Aldrich) into saline at different concentrations. For this experiment, the two formulations used consisted of 2.0 mg/mL SPO + 1 U/µL catalase and 0.2 mg/mL SPO + 0.1 U/µL catalase, using an average of 3,500 U/mg catalase; note that the rationale for selecting these doses was based on previous studies with a ligation-induced ischemic injury model in the tibialis anterior (TA) muscle (27). Approximately 5 min after cuff inflation, POG treatment groups received four intramuscular POG injections (10 µL each) equidistant along the length of the experimental TA, with ∼0.5 cm between each injection, again as previously described (27). The treatment groups consisted of a 0.2 mg/mL SPO group (Low Dose) (n = 6) and a 2.0 mg/mL SPO group (High Dose) (n = 6). The Tourniquet-Only group (n = 12) did not receive any intervention or sham injections over the course of tourniquet application. After 3 h, the cuff was removed, and animals were returned to their cages after becoming alert. Buprenorphine was administered subcutaneously on the day of injury creation and after 48 h (0.05 mg/kg).
In Vivo Functional Assessment
Contractile function of the injured TA muscle was measured at 1, 2, and 4 wk after injury with protocols that have been described previously (41–45). Briefly, the animals were anesthetized and placed in a supine position. The left foot was taped to a foot plate connected to a force transducer (model 305C-LR-FP, Aurora Scientific), and two electrodes were inserted into the skin, surrounding the common peroneal nerve. An electrical stimulator (model 701C, Aurora Scientific) was used to stimulate the nerve in constant-current mode, and the stimulation voltage and electrode placement were optimized with 1-Hz twitch contractions. Once optimized, the muscle was subjected to a standardized stimulation protocol using frequencies ranging from 10 to 150 Hz, as the stimulus-response curve plateaus at 150 Hz, and the maximal isometric torque response was calculated from the measured force values. Several twitch contractions are used to confirm injury immediately after tourniquet application, but the full functional testing protocol is not evaluated before the 1 wk time point to allow animals to recover from the procedure, as previous experience has yielded no functional responses immediately after injury using twitch contractions.
Histology
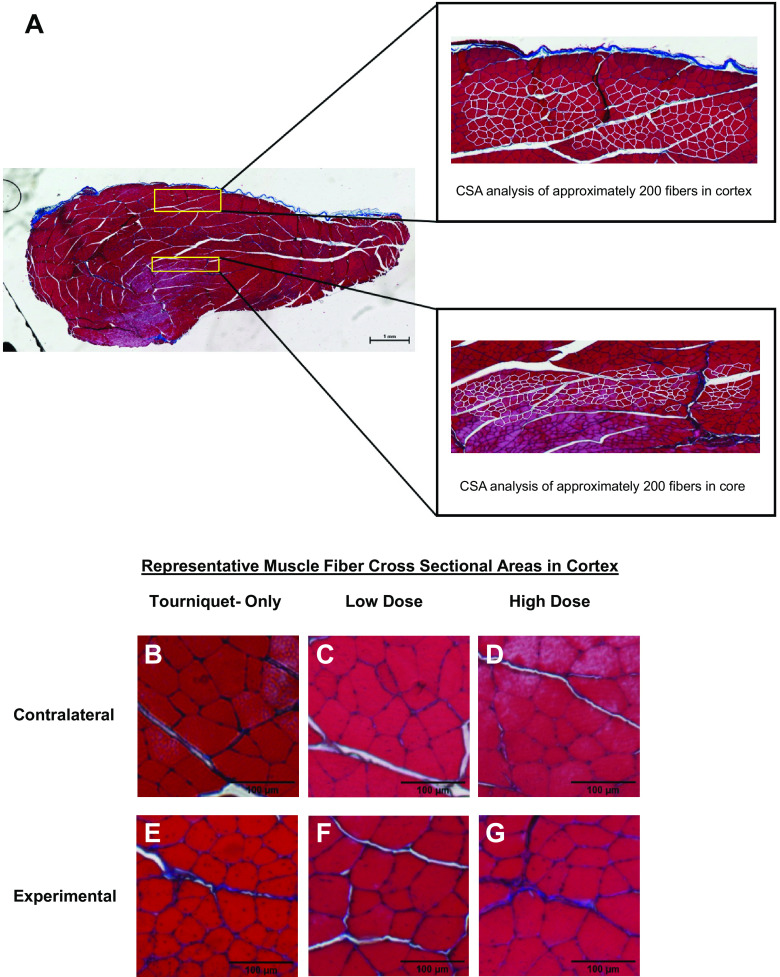
Histological studies were conducted on n = 4 animals per group. After 5 wk post-tourniquet application, the animals were euthanized by CO2 inhalation, and the experimental and contralateral TA muscles were explanted. Harvested muscles were fixed in 10% formalin at 4°C and embedded in paraffin wax. Processing and embedding of samples were conducted by the Research Histology Core at the University of Virginia School of Medicine. Serial cross sections measuring 5 µm thick were taken from the muscle belly for both the experimental and contralateral TA muscles. Whole muscle sections were stained with a standard Masson’s trichrome (MTS) protocol, and the whole section was imaged at ×4 magnification via tile scan (Nikon Eclipse E600 upright microscope and NIS-Elements software, Nikon Instruments Inc.). Muscle fiber cross-sectional areas (CSAs) were manually measured with ImageJ software (National Institutes of Health, Bethesda, MD), as previously described (41). In short, ∼200 muscle fibers were measured in both the cortex and the core of the TA muscle, with one experimental muscle and one contralateral muscle section analyzed per animal (n = 4 animals/group; see Fig. 2A). CSA analysis was conducted in both the cortex and the core of the TA muscle to evaluate the effects of the injury and subsequent POG administration on oxygen diffusion. Fiber analyses were within areas that were >1,000 µm from the lateral edges of the section. Cortex measurements were within 800 µm from the muscle surface. Core measurements were between an area >1,000 µm from the muscle surface and >500 µm from the bottom of the muscle. Whole muscle sections were also stained with hematoxylin and eosin (H&E) and imaged in the cortex (×20 objective, Leica Thunder Imager with Leica Application Suite X software, Leica Microsystems GmbH). Approximately 200 muscle fibers were manually analyzed for centrally located nuclei (CLNs) in the cortex of the experimental muscle (n = 4 animals/group), in the approximate location where the CSA analysis was also conducted. The percentage of fibers containing CLNs was calculated for each muscle section.
Figure 2.
A: a representative cross section from an experimental muscle in the Low-Dose particulate oxygen generator (POG) group that has been stained with Masson’s trichrome is shown to demonstrate the manual fiber analysis as described in materials and methods. B–G: representative examples of Masson’s trichrome images of muscle fibers in the cortex taken from within the ×4 cross sections are shown for the contralateral controls (B–D) as well as for the experimental muscles (E–G) for the Tourniquet-Only (B and E), Low-Dose (C and F), and High-Dose (D and G) groups.
Statistics
Statistical analyses were conducted with GraphPad Prism for Windows (GraphPad Software, La Jolla, CA; version 8). Data are presented as means ± standard error of the mean (SE) unless otherwise noted. Histological and functional data were analyzed by one-way or two-way analyses of variance (ANOVAs) as specified in the figure legends, unless otherwise noted, followed by post hoc comparison testing using Fisher’s least significant difference (LSD) method and α = 0.05. All repeated-measures two-way ANOVA data were first analyzed in SPSS Statistics for Windows (IBM Corp., Armonk, NY; version 25), using Mauchly’s test to determine the validity of the assumption of sphericity. When necessary, the Greenhouse–Geisser correction was applied. Simple linear regression analyses were performed as part of the CSA and CLN data analysis, and the line of best fit is reported as well as the coefficient of determination (R2), the P value, and the Pearson correlation coefficient (r).
RESULTS
In Vivo Functional Assessment
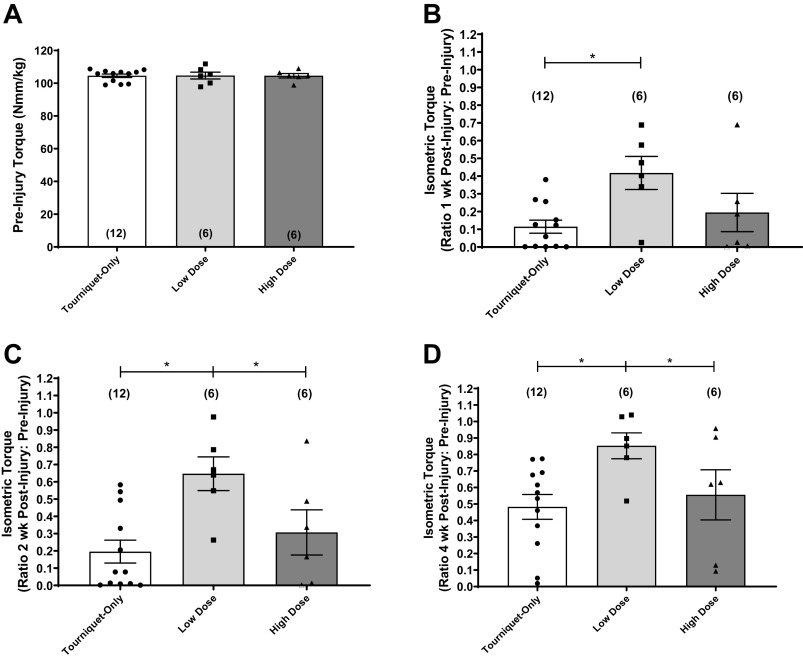
No differences were detected in animal body mass between treatment groups at any point during the time course of the experiment (Table 1). Since the animal masses were not static over the course of the study, torque values were normalized to animal body mass at each time point, to account for changes in torque values that were a result of mass gain over time. The maximal isometric torque generated by each animal during nerve stimulation was measured before injury, and there were no differences in mean preinjury (baseline) maximal isometric torque values between experimental groups (Fig. 1A). All pre- and postinjury animal masses and corresponding torque values are summarized in Table 1.
Table 1.
Summary of animal mass and isometric torque values over time
| Tourniquet Only | Low Dose | High Dose | |
|---|---|---|---|
| Sample size | 12 | 6 | 6 |
| Baseline | |||
| Body mass, g | 332.7 ± 13.5 | 328.0 ± 5.0 | 344.4 ± 8.2 |
| Maximal isometric torque, Nmm/kg | 104.6 ± 1.1 | 104.7 ± 2.1 | 104.6 ± 1.4 |
| Tourniquet application | |||
| Body mass, g | 344.3 ± 13.2 | 371.5 ± 6.4 | 369.1 ± 6.6 |
| 1 wk postinjury | |||
| Body mass, g | 333.1 ± 11.0 | 351.8 ± 5.2 | 351.7 ± 5.2 |
| Maximal isometric torque, Nmm/kg | 12.2 ± 4.0 | 43.9 ± 9.7* | 19.8 ± 10.7 |
| Ratio of maximal isometric torque to baseline, Nmm/kg:Nmm/kg | 0.11 ± 0.04 | 0.42 ± 0.09* | 0.19 ± 0.11 |
| 2 wk postinjury | |||
| Body mass, g | 348.7 ± 9.2 | 367.5 ± 6.0 | 366.2 ± 4.7 |
| Maximal isometric torque, Nmm/kg | 20.4 ± 6.9 | 68.2 ± 10.6*† | 31.4 ± 13.0 |
| Ratio of maximal isometric torque to baseline, Nmm/kg:Nmm/kg | 0.20 ± 0.07 | 0.65 ± 0.10*† | 0.31 ± 0.13 |
| 4 wk postinjury | |||
| Body mass, g | 390.3 ± 9.1 | 395.1 ± 6.0 | 388.5 ± 5.2 |
| Maximal isometric torque, Nmm/kg | 50.2 ± 7.8 | 89.7 ± 9.3*† | 57.5 ± 15.5 |
| Ratio of maximal isometric torque to baseline, Nmm/kg:Nmm/kg | 0.48 ± 0.07 | 0.85 ± 0.08*† | 0.56 ± 0.15 |
Data presented as means ± SE. *Significantly different (P < 0.05) compared with Tourniquet-Only group for the time point, †significantly different (P < 0.05) compared to High-Dose group for the time point; body mass and postinjury torque data analyzed using Fisher’s least significant difference (LSD) pairwise comparisons following 2-way repeated-measures ANOVA for matched samples, baseline torque data analyzed with ordinary 1-way ANOVA.
Figure 1.
A: mean maximal isometric torque values normalized to body mass (Nmm/kg) were calculated prior to ischemic injury. No difference was found between experimental groups by ordinary 1-way ANOVA. B–D: mean maximal isometric torque values at 1 wk (B), 2 wk (C), and 4 wk (D) after injury are expressed as a fraction of the baseline measurements made on the same animals for the Tourniquet-Only (n = 12 animals), Low-Dose (n = 6 animals), and High-Dose (n = 6 animals) treatment groups, respectively. Experimental groups different at *P < 0.05; Fisher’s least significant difference (LSD) pairwise comparisons following 2-way repeated-measures ANOVA for matched samples. All data are presented as means ± SE. Number of animals per group shown in parentheses.
Ischemic injury was created by tourniquet application for 3 h at 300 mmHg. Contractile function was evaluated 1, 2, and 4 wk after tourniquet application, and these data are graphically depicted in Fig. 1, B–D. More specifically, the isometric torque values for each experimental group are shown as the ratio of the maximum torque response (normalized to body mass) to the same animal’s preinjury isometric torque (mean ± SE). The Low-Dose group generated greater maximal isometric torque values compared with the Tourniquet-Only group at all time points (P < 0.05). Contractile function for the Low-Dose group was 42% of baseline after 1 wk postinjury (Fig. 1B), and by 4 wk postinjury, the Low-Dose group generated 85% of baseline contractile function, which was greater than both the Tourniquet-Only group (48%; P = 0.0037) and the High-Dose group (56%; P = 0.0400 (Fig. 1D). Table 1 provides a summary of the mean ± SE of all maximal isometric torque values for each pre- and postinjury time point, with identical statistical conclusions.
Histological Analysis
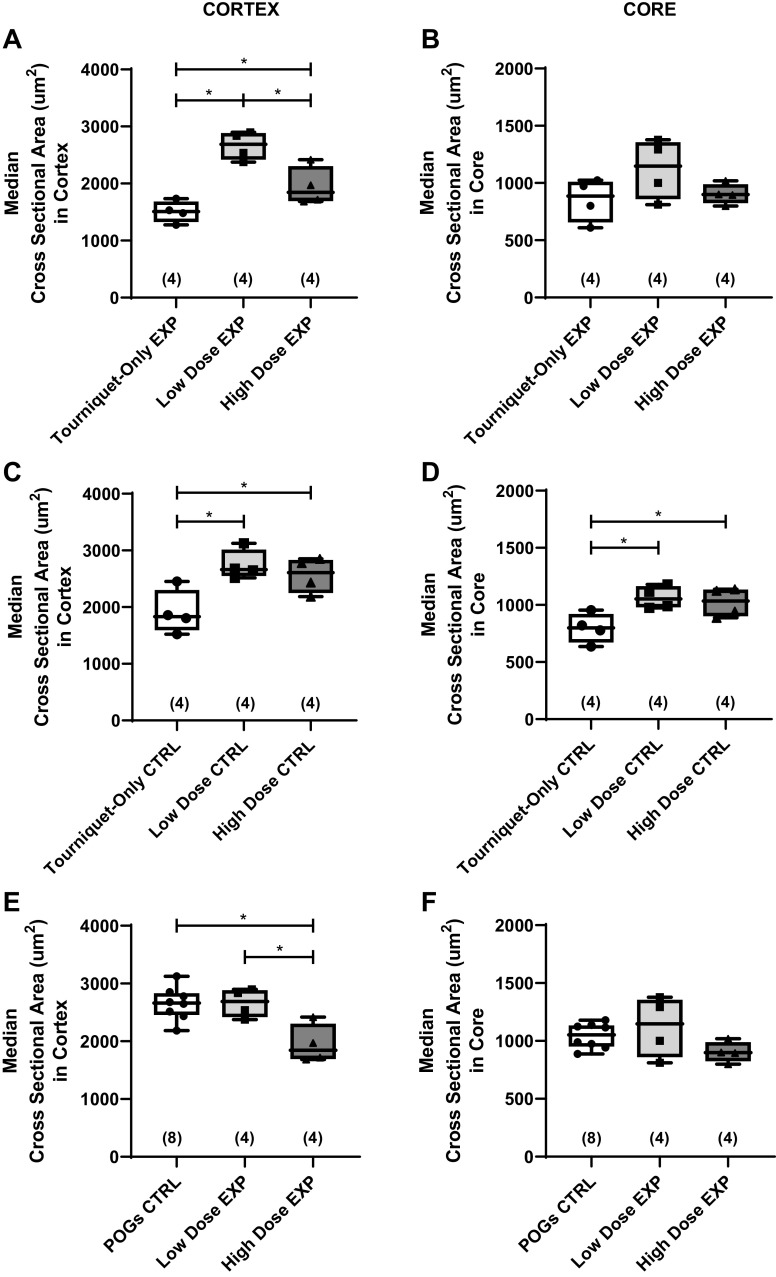
We conducted detailed histological studies to provide insight into the impact of tourniquet application and treatment on muscle fiber CSA. Representative images after Masson’s trichrome staining are shown in Fig. 2. The fiber CSA was measured for ∼200 fibers in the cortex and 200 fibers in the core of each measured muscle section as outlined above (Fig. 3). Fiber CSA analyses were conducted on a subset of animals in each of the experimental groups (n = 4 animals/group). For each animal, both the experimental (EXP) and the contralateral control (CTRL) muscle were analyzed. The median CSA values in the EXP muscles were compared to determine the effects of POGs in the injured leg. In the cortex, the median fiber CSA value for the Low-Dose EXP group (2,687 μm2) was higher than both the Tourniquet-Only EXP (1,507 μm2, P = 0.0002) and High-Dose EXP (1,844 μm2, P = 0.0042) groups (Fig. 3A). Additionally, the High-Dose EXP median CSA was greater than the Tourniquet-Only EXP group (P = 0.0432; Fig. 3A). In the core, there were no differences between the Tourniquet-Only (886.3 μm2), Low-Dose (1,147 μm2), and High-Dose (898.4 μm2) EXP groups (Fig. 3B). Further comparisons between the POG EXP groups and CTRL groups were made to evaluate differences between the injured and uninjured limbs. Once again, the median CSA value for the CTRL muscle was determined for n = 4 animals in each group, in both the cortex and the core. As shown in Fig. 3C, there were noted differences in the CTRL median fiber CSA values between the Tourniquet-Only group and both POG groups. Specifically, in the cortex, there was a difference between the median CSA values of the Tourniquet-Only CTRLs (1,832 µm2) compared with both the Low-Dose (2,663 µm2, P = 0.0056) and High-Dose (2,607 µm2, P = 0.0196) CTRLs (Fig. 3C). Similarly in the core, there was a difference between the median CSA values of the Tourniquet-Only CTRLs (798.8 µm2) compared with both the Low-Dose (1,053 µm2, P = 0.0116) and High-Dose (1,034 µm2, P = 0.0258) CTRLs (Fig. 3D). Subsequently, the Tourniquet-Only CTRL CSA values were excluded and the CSA values for the POG-treated groups were compared to the pooled POG contralateral control (POGs CTRL, n = 8). In the cortex, the median CSA value of Low-Dose EXP muscles did not differ from the POGs CTRL muscles (2,687 µm2 and 2,663 µm2, respectively; Fig. 3E). However, the median CSA value for the High-Dose EXP muscles (1,844 µm2) displayed a difference compared with both the POGs CTRL (P = 0.0016) and the Low-Dose EXP (P = 0.0040) groups (Fig. 3E). In the core, no differences were noted between the POGs CTRL (1,053 µm2), Low-Dose EXP (1,147 µm2), and High-Dose EXP (898.4 µm2) groups (Fig. 3F).
Figure 3.
A and B: the median fiber cross-sectional area (CSA, µm2) for the experimental (EXP) tibialis anterior (TA) muscle was determined for n = 4 animals/group in the cortex (A) and the core (B). C and D: the median fiber CSA was also determined for the contralateral (CTRL) TA muscle (n = 4 animals/group) in the cortex (C) and core (D). Because the Tourniquet-Only CTRL CSA values were different from the particulate oxygen generator (POG) CTRL values, a pooled POG contralateral control group (POGs CTRL) was used for further comparison. E and F: the median fiber CSAs for the EXP TA muscle for the Low-Dose group (n = 4 animals) and High-Dose group (n = 4 animals) were compared with the POGs CTRL group (n = 8 animals) in both the cortex (E) and core (F). For A–F, Different at *P < 0.05; ordinary 1-way ANOVA followed by Fisher’s least significant difference (LSD) multiple comparison test. The median values for each muscle were calculated from approximately n = 200 fibers measured in the cortex and n = 200 fibers measured in the core. Data presented as medians, with the top and bottom of the boxplots representing the 25th and 75th percentiles and whiskers representing minimum and maximum values. Note that median values were used as the whole CSA distribution is nonnormal for each muscle. However, when the individual median values are combined for each group, the data are normal, allowing the use of parametric analyses in A–F. Number of animals analyzed per group shown in parentheses.
Fiber size distributions were further analyzed for the EXP groups in the cortex (Fig. 4A) and the core (Fig. 4B), with a 600-μm2 bin width. In the cortex (Fig. 4A), the Low-Dose EXP group demonstrated a lower percentage of smaller (<1,800 μm2) fibers and a higher percentage of larger fibers (2,400–4,200 μm2) compared with the Tourniquet-Only EXP group. Comparatively, the Tourniquet-Only EXP group demonstrated a leftward shift in frequency distribution (toward smaller fiber sizes), with the distribution of the Low-Dose EXP group shifted rightward (toward larger fiber sizes) and the High-Dose EXP distribution lying between the other EXP groups. In the core (Fig. 4B), there were fewer overall differences between the Tourniquet-Only EXP group and POG EXP groups, although the Tourniquet-Only EXP group demonstrates a higher percentage of fibers <600 μm2 compared with the POG EXP groups. The fiber size distributions were also compared for the two POG EXP groups and the combined/pooled POGs CTRL in both the cortex and the core, again with a 600-μm2 bin width (Fig. 4, C and D). Once more, the Low-Dose EXP group demonstrated an overall rightward shift in the fiber size frequency distribution (toward larger fiber size ranges) compared with the High-Dose EXP group, while displaying similarity to the POGs CTRL fiber distribution in the cortex (Fig. 4C). However, the High-Dose EXP group displayed a difference from the POGs CTRL group in the 600–4,200 µm2 range, as well as a higher percentage of fibers in the 600–2,400 µm2 range compared with the Low-Dose EXP group and a lower percentage of larger fibers (3,000–4,200 µm2) compared with the Low-Dose EXP group. In the core, the Low-Dose EXP group again shows no differences compared with the POGs CTRL group (Fig. 4D), whereas the High-Dose EXP group shows differences compared with the other two groups, the most notable being a higher percentage of smaller (600–1,200 µm2) fibers compared with the POGs CTRL and Low-Dose EXP groups. Of note, as also shown in Fig. 4, E and F, regression analysis performed on data derived from animals in all three treatment groups documented a positive correlation between median fiber CSA and maximal isometric torque, in both the cortex (r = 0.8029, P = 0.0017) and the core (r = 0.5824, P = 0.0469), although the correlation was higher in the former.
Figure 4.
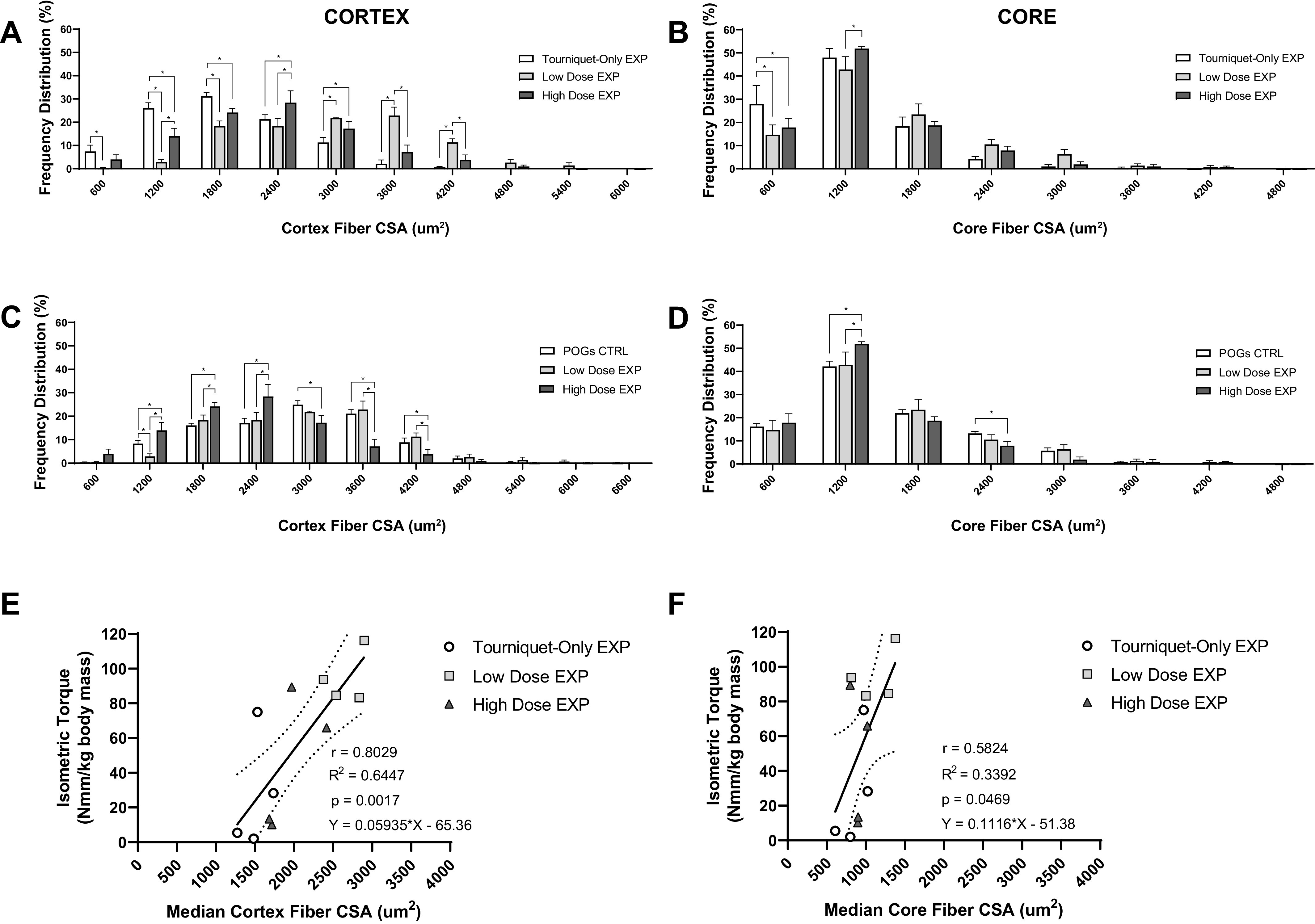
A and B: bar graphs show the muscle fiber cross-sectional area (CSA, µm2) frequency distribution (%) of the Tourniquet-Only (n = 4 animals), Low-Dose (n = 4 animals), and High-Dose (n = 4 animals) groups in both the cortex (A) and the core (B). C and D: additionally, the muscle fiber CSA frequency distributions of the Low-Dose (n = 4 animals) and High Dose (n = 4 animals) groups were compared with the pooled particulate oxygen generator (POG) contralateral muscles (POGs CTRL, n = 8 animals), in both the cortex (C) and the core (D). Note that the tick marks on the x-axis occur in intervals of 600, and each tick on the x-axis represents the upper limit of the corresponding bin (e.g., 600 is the bin from 0 to 600, and 1,200 is the bin from 601 to 1,200, etc.). For each animal, the frequency distributions consist of approximately n = 200 fibers measured in the cortex and n = 200 fibers measured in the core. For all bins, groups are different at *P < 0.05, ordinary 2-way ANOVA followed by Fisher’s least significant difference (LSD) multiple comparison test. Frequency distribution data are presented as means ± SE. EXP, experimental. E and F: regression analyses of individual maximal isometric torque normalized to body mass (Nmm/kg) at 4 wk after injury vs. the median experimental muscle fiber CSA (µm2) for individual animals (n = 4 animals/experimental group) are shown in the muscle cortex (E) and core (F). Median CSA values for each individual animal are used as the unbinned CSA data are nonnormal. Slopes deviate from 0 for analyses in both the cortex (P = 0.0017) and the core (P = 0.0469). Pearson correlation coefficient (r), R2, P value, and line equation are shown for each plot. Straight line fits of the data with 95% confidence bands are shown.
Centrally located nuclei (CLNs) within the muscle fibers were evaluated as another index of ischemic tissue damage and repair following 3 h of tourniquet application. Representative examples of fibers containing CLNs are provided in Fig. 5, A–C. The percentage of CLN-containing fibers in the cortex was calculated from ∼200 fibers per animal (n = 4 animals/group). The Low-Dose group had fewer myofibers containing CLNs (3.3%) compared with both the Tourniquet-Only (54%) and High-Dose (52%) groups (Fig. 5D), although only the former comparison yielded statistical significance (P = 0.0488 and P = 0.0549, respectively). Regression analysis of the data showed a negative correlation between the percentage of fibers with CLNs and maximal isometric torque generation (r = −0.9598, P < 0.0001; Fig. 5E) as well as median myofiber CSA in the cortex (r = −0.6772, P = 0.0156; Fig. 5F).
Figure 5.
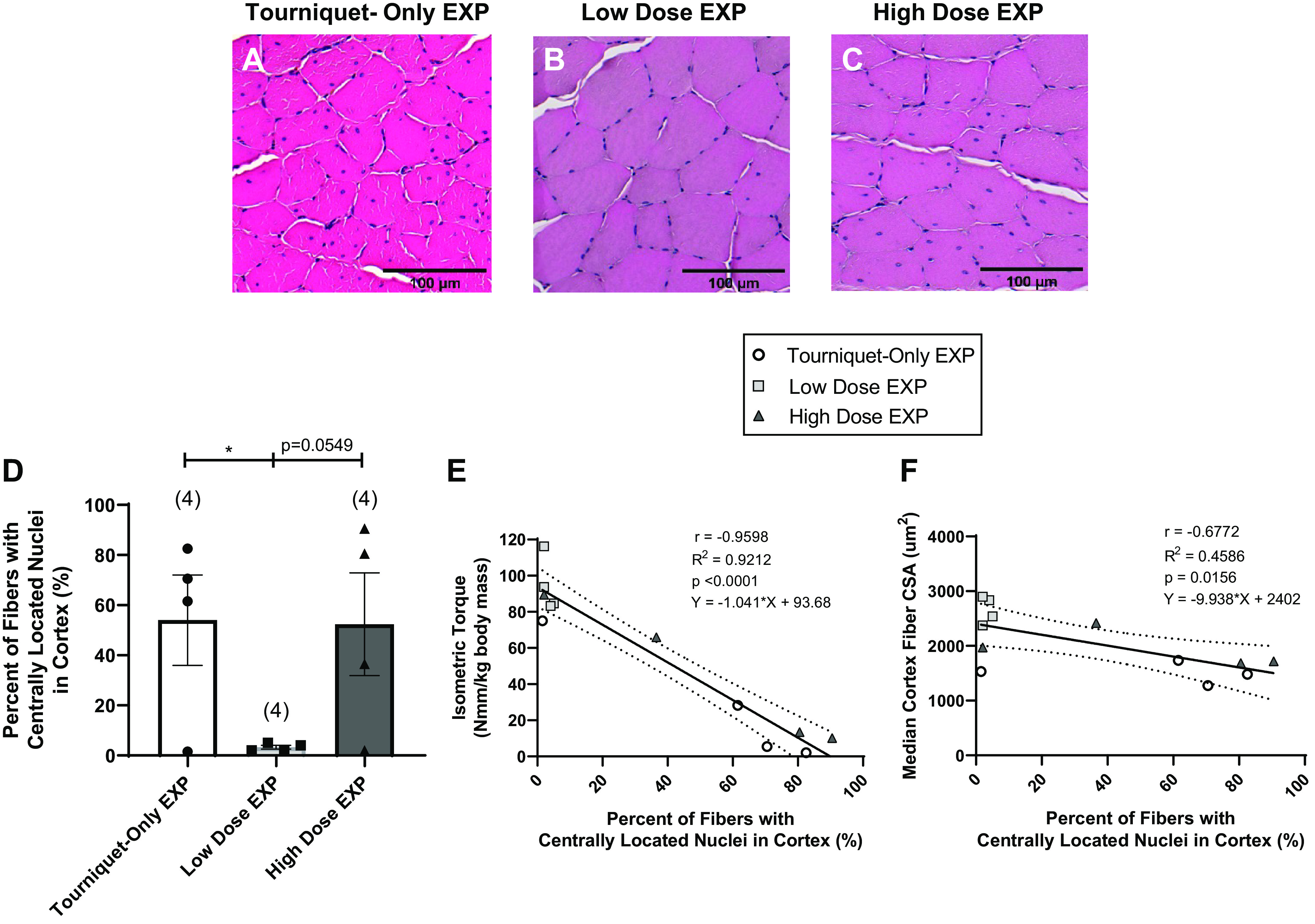
A–C: representative hematoxylin and eosin (H&E) images show the centrally located nuclei within fibers for the Tourniquet-Only (A), Low-Dose (B), and High-Dose (C) groups. EXP, experimental. D: the percentage of fibers containing centrally located nuclei was determined for n = 4 animals/group by manually analyzing ∼200 fibers in the cortex of each muscle, and the percentage of fibers identified with centrally located nuclei was calculated. Different at *P < 0.05; ordinary 1-way ANOVA with post hoc pairwise comparisons using Fisher’s least significant difference (LSD). Data are presented as means ± SE. There is a difference between the Tourniquet-Only group and the Low-Dose group (P = 0.0488). E and F: Straight line fit of the data showed an inverse correlation between appearance of centrally located nuclei and both isometric torque and fiber cross-sectional area (CSA) in the cortex. More specifically, a lower percentage of centrally located nuclei at the time of explant corresponded to higher isometric torque at 4 wk postinjury (E) as well as higher median cortex fiber CSAs (F), with slopes deviating from 0 (P < 0.0001 and P = 0.0156, respectively). Pearson correlation coefficient (r), R2, P value, and line equation are shown for each plot; 95% confidence bands are shown by the dotted lines for linear regression plots. Number of animals analyzed per group shown in parentheses.
DISCUSSION
The goal of the present study was to evaluate the potential utility of intramuscular injection of POGs to mitigate skeletal muscle tissue injury in a clinically/battlefield-relevant tourniquet-induced ischemic injury model. In short, our initial observations support the supposition that the delivery of POGs into the rat TA muscle can maintain greater contractile function over the course of a 4-wk time period following 3 h of tourniquet-induced ischemia. More specifically, the absence of treatment (i.e., Tourniquet-Only group) produced pronounced and substantial tissue (I/R) damage. Of note, the sustained contractile function deficits reported here are in alignment with various models of rodent hindlimb tourniquet application (11, 21, 23, 36, 37, 39, 46–51). Somewhat surprisingly, the Low-Dose POG group demonstrated greater contractile function compared with the Tourniquet-Only group at all time points (Fig. 1, Table 1), consistent with a reduction in the magnitude of initial injury.
With respect to mechanism of action, presumably the provision of oxygen shortly after tourniquet application may have reduced the magnitude of ischemic injury (52). This, in turn, could explain the rapid return of function seen in the Low-Dose POG group, as early as 1 wk compared with the Tourniquet-Only group. Consistent with these findings, the Low-Dose group also displayed an improved maintenance/preservation of larger muscle fiber CSA (Fig. 3 and Fig. 4). That is, the median CSA values for the Low-Dose EXP muscles were higher than both the Tourniquet-Only EXP and High-Dose EXP groups in the cortex (Fig. 3A). Furthermore, the Low-Dose EXP group did not differ from the pooled POGs CTRL muscles in the cortex (Fig. 3E) or the core (Fig. 3F). Additionally, the Low-Dose EXP group displayed a greater percentage of larger fibers (3,000–4,200 µm2) in the cortex compared with the High-Dose EXP and Tourniquet-Only EXP groups (Fig. 4, A and C), as well as a lower percentage of smaller (<1,800 µm2) fibers compared with the untreated Tourniquet-Only EXP group (Fig. 4A). Previous reports have shown that the TA is primarily made up of type II fibers, particularly in the cortex, which contains few if any type I oxidative fibers and consists of higher percentages of type IIb glycolytic fibers compared with the core (53–55). In general, type IIb fibers are larger than type I fibers in the TA (54, 55), and since fast-twitch glycolytic fibers have been shown to sustain greater damage after I/R injury (37, 56, 57), it corresponds to intuition that the provision of POGs to attenuate the damaging effects of I/R injury could improve the preservation/maintenance of the larger type IIb fibers, resulting in the maintenance of larger fibers in the cortex.
The observed differences between EXP groups in the cortex of the muscle (Fig. 3A and Fig. 4A) were further investigated by measuring the percentage of centrally located nuclei (CLNs) in the cortex of the experimental muscles (Fig. 5). The lower percentage of CLNs in the cortex of the Low-Dose group (Fig. 5D) compared with the Tourniquet-Only group is another indication of the functional benefits of the lower POG dose, consistent with a greater degree of I/R damage in the Tourniquet-Only group, indicating that the CLN fibers are likely undergoing repair/regeneration (37, 58). A higher percentage of CLNs in the cortex is correlated with smaller fiber median CSA (Fig. 5F) and therefore is also correlated with lower torque generation (Fig. 5E).
Although both doses of POGs were coadministered with catalase to offset the free radical damage resulting from SPO decomposition, there seems to be an upper limit to the effect of catalase in this setting. In fact, oxygen-generating peroxides prepared with catalase have been shown to yield poor cell viability results as the concentration of the peroxide was increased (27, 59). Furthermore, Ibrahim and Schlegel (60) observed that when sufficiently high flow rates of H2O2 were introduced into a fermentor with catalase, H2O2 would continue to accrue regardless of whether the starting catalase concentrations were increased. Additionally, higher SPO concentrations will generate more sodium carbonate by-product during decomposition, which in turn could increase the alkalinity of the local environment and subsequently reduce the efficacy of the catalase enzyme (61, 62). Therefore, it seems plausible that an increased SPO concentration, even with the addition of catalase, could lead to lipid peroxidation and subsequent cell membrane damage that could mitigate any positive effects caused by local POG injection. This could explain why the animals treated with the higher dose of POGs in our study, despite nominally receiving a larger supply of oxygen, demonstrated less contractile function over the time course of this investigation, again as characterized by the smaller median fiber CSAs as well as an elevated percentage of CLN in the cortex, and thus were characterized by lower maximal isometric torque (Nmm/kg) generation at the 4 wk time point (Fig. 1D and Table 1).
In this regard, there have been numerous investigations into treatments that address ischemic injury in skeletal muscle [reviewed in Gillani et al. (8)], which include ischemic postconditioning (63–65) regimens as well as controlled hypothermia of the limb (13, 17, 46, 47) and other therapeutics (such as drug, growth factor, and cell therapies) (17–25). Several of these studies involving the restoration of muscle contractile function in the rat hindlimb after extended (2–4 h) ischemia are highlighted in Table 2. In these studies, intervention yielded higher contractile function compared with untreated controls, although the overall percent recovery varied among treatments. Other reports also reported evidence of effective conservation of contractile function for treated groups (46) and correlations between fiber size and force/torque generation (21, 47), indicating that the outcomes of POGs, when applied at the onset of ischemia, are in line with alternative I/R treatment options. Furthermore, POGs are shelf stable, can be transported and implemented without special equipment, and can be injected at the time of injury or tourniquet application, providing a flexibility of use that may fill in gaps within current treatment paradigms.
Table 2.
Summary of functional assessment studies
| Muscle | Injury Model | Ischemic Time, h | Treatment | Functional Assessment (time after injury) | Reference |
|---|---|---|---|---|---|
| Rat gastrocnemius | Tourniquet (350 mmHg) | 2 | Hypothermia | 1 day, 7 days, 14 days, 28 days, 42 days | Fish et al. (46) |
| Rat gastrocnemius | Tourniquet (350 mmHg) | 4 | Hypothermia | 6 wk | Awerbuck et al. (47) |
| Rat EDL | Clamping | 3 | Drug therapies | 3 h, 24 h, 7 days | Barker et al. (24) |
| Rat EDL | Clamping | 3 | Postconditioning | 3 h, 5 days | Park et al. (63) |
| Rat TA | Tourniquet (250 mmHg) | 3 | Cell therapy | 2 wk | Chen et al. (23) |
| Rat gastrocnemius | Tourniquet (250 mmHg) | 2 | Growth factor | 2 wk | Hammers et al. (21) |
EDL, extensor digitorum longus; TA, tibialis anterior.
One limitation of the present study is the lack of metabolic measurements during and immediately after injury. Because of the importance of functional response monitoring during this longitudinal study, tissue samples were not explanted immediately after injury or during early time points, in favor of conducting repeated in vivo contractile measurements over multiple time points in the same animal. Given the results of the present study, future work would logically include evaluation of early time points, that is, within 24 h of injury, as well as over the course of the first week after injury. These data are required to observe and more precisely delineate the mechanisms of action and early effects of POGs in fiber preservation, such as comparisons of glycogen levels in the muscle shortly after injury. Additionally, although we did not evaluate the effects of catalase in the absence of POGs, it is worth noting that previous studies have shown that antioxidants by themselves can improve muscle contraction during hypoxia in various rat diaphragm (66) and sternohyoid (67) muscle models. Thus, forthcoming work should also include a catalase-only group to evaluate the impact that catalase per se has on ROS mediation and the resultant functional outcomes, independent of the presence of POGs. Finally, Ward et al. (27) have previously shown that there is an apparent upper limit to the efficacy of catalase when used in combination with POGs in this system. Thus, although this initial feasibility study was focused on evaluation of the longitudinal effects of POGs in I/R injury, in the future more rigorous evaluation of dose-response curves for the POG formulations, as well as their impact on ROS measurements and markers of ROS damage (such as elevated markers for lipid and DNA damage), at early postinjury time points would be very illuminating.
The linear regression analyses demonstrated higher R2 values when comparing torque to both the median CSA values in the cortex (Fig. 4E) as well as the percentage of CLN fibers in the cortex (Fig. 5E), with lower R2 values relating torque to median core CSA values (Fig. 4F). This may indicate that changes to the muscle cortex have a greater effect on the torque response following injury or that there are other factors affecting the response in the core, such as differences in fiber type/size makeup in response to POGs, or location-specific responses to ROS damage, which could also be further explored in future work. Additionally, 4 wk after injury was chosen for the final time point of this study as it represents a suitable time period to monitor the rapid recovery required for wounded personnel to be able to return to the field. However, comparable rodent models of ischemia have reported continued functional improvements beyond 4 wk (46, 48). Given the continued improvement of contractile torque generation from week to week for all experimental groups in the present study, future investigations may consider additional time points, as well as studies of gait and ambulation at all time points. Contractile function of the contralateral limb should also be assessed in the future, to further elucidate the differences seen between Tourniquet-Only and POG-treated contralateral limbs in the histological studies (Fig. 3, C and D). Nonetheless, these studies still demonstrate an unequivocal value/benefit of POG administration to attenuating the initial insult, and this should be of benefit to treatment of traumatic extremity injuries in both civilians and soldiers, as it would increase return to activity (RTA)/return to duty (RTD).
In summary, the present study confirms and extends our prior work both in vivo and in vitro (27), which has shown that a sodium percarbonate-based POG formulation can be successfully injected intramuscularly for better preservation of skeletal muscle contractile function after tourniquet-induced I/R injury, as would commonly result from extremity trauma to civilians and military personnel. The observed improvement of muscle contractile function over that of the Tourniquet-Only animals shows a positive correlation with maintenance of muscle fiber structure and integrity on a microscopic level. Such therapy has compelling applications not only for emergency medicine but for orthopedic surgery and tissue engineering/regenerative medicine applications as well. Future work will further explore this established rat model to evaluate different POG formulations and rigorously investigate biochemical markers, such as ROS, at early time points to aid in optimization of POG formulation and dose. Further studies may also build on the existing injury model to simulate battlefield injury conditions, such as bone, muscle, or vascular injury in conjunction with tourniquet application, as well as extended tourniquet times beyond 3 h. In addition, the results of the present study will inform future work using a larger and more clinically/battlefield-relevant animal (e.g., porcine) model to further validate the use of POGs. Finally, the results of this study may have important applications for improved limb salvage after extremity trauma in austere environments, as the biomaterial itself is amenable to use when/where many other therapeutics (e.g., biologics) would not be practical.
GRANTS
This work was supported by the US Army Medical Research Acquisition Activity under Contracts No. W81XWH-14-2-0003 (AFIRM II; ER-12, G. J. Christ UVA Site PI, Sean Murphy, WFIRM, PI).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E.D., J.D.R., T.J.W., and G.J.C. conceived and designed research; S.E.D., J.D.R., K.E.H., and A.H. performed experiments; S.E.D., J.D.R., K.E.H., A.H., and G.J.C. analyzed data; S.E.D., J.C.W., T.J.W., and G.J.C. interpreted results of experiments; S.E.D. and G.J.C. prepared figures; S.E.D. and G.J.C. drafted manuscript; S.E.D., J.D.R., K.E.H., A.H., J.C.W., T.J.W., and G.J.C. edited and revised manuscript; S.E.D., J.D.R., K.E.H., A.H., J.C.W., T.J.W., and G.J.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Juliana Passipieri for guidance on functional testing technique, Dr. Ellen Mintz and Dr. Bruna Farjun for guidance in creating histological analysis methodology, and Dr. Sean Murphy for helpful comments and suggestions during the course of study.
REFERENCES
- 1.Kragh JF Jr, Littrel ML, Jones JA, Walters TJ, Baer DG, Wade CE, Holcomb JB. Battle casualty survival with emergency tourniquet use to stop limb bleeding. J Emerg Med 41: 590–597, 2011. doi: 10.1016/j.jemermed.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Howard JT, Kotwal RS, Stern CA, Janak JC, Mazuchowski EL, Butler FK, Stockinger ZT, Holcomb BR, Bono RC, Smith DJ. Use of combat casualty care data to assess the US military trauma system during the Afghanistan and Iraq conflicts, 2001–2017. JAMA Surg 154: 600–608, 2019. doi: 10.1001/jamasurg.2019.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kauvar DS, Miller D, Walters TJ. Tourniquet use is not associated with limb loss following military lower extremity arterial trauma. J Trauma Acute Care Surg 85: 495–499, 2018. [Erratum in J Trauma Acute Care Surg 85: 826, 2018]. doi: 10.1097/TA.0000000000002016. [DOI] [PubMed] [Google Scholar]
- 4.Kauvar DS, Thomas SB, Schechtman DW, Walters TJ. Predictors and timing of amputations in military lower extremity trauma with arterial injury. J Trauma Acute Care Surg 87: S172–S177, 2019. doi: 10.1097/TA.0000000000002185. [DOI] [PubMed] [Google Scholar]
- 5.Beekley AC, Sebesta JA, Blackbourne LH, Herbert GS, Kauvar DS, Baer DG, Walters TJ, Mullenix PS, Holcomb JB; 31st Combat Support Hospital Research Group. Prehospital tourniquet use in Operation Iraqi Freedom: effect on hemorrhage control and outcomes. J Trauma 64: S28–S37, 2008. doi: 10.1097/TA.0b013e318160937e. [DOI] [PubMed] [Google Scholar]
- 6.Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, Li CY, Li CJ. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem 46: 1650–1667, 2018. doi: 10.1159/000489241. [DOI] [PubMed] [Google Scholar]
- 7.Paradis S, Charles AL, Meyer A, Lejay A, Scholey JW, Chakfé N, Zoll J, Geny B. Chronology of mitochondrial and cellular events during skeletal muscle ischemia-reperfusion. Am J Physiol Cell Physiol 310: C968–C982, 2016. doi: 10.1152/ajpcell.00356.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillani S, Cao J, Suzuki T, Hak DJ. The effect of ischemia reperfusion injury on skeletal muscle. Injury 43: 670–675, 2012. doi: 10.1016/j.injury.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 10: 620–630, 2002. doi: 10.1016/S0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 10.Doyle GS, Taillac PP. Tourniquets: a review of current use with proposals for expanded prehospital use. Prehosp Emerg Care 12: 241–256, 2008. doi: 10.1080/10903120801907570. [DOI] [PubMed] [Google Scholar]
- 11.Fish JS, McKee NH, Pynn BR, Kuzon WM Jr, Plyley MJ. Isometric contractile function recovery following tourniquet ischemia. J Surg Res 47: 365–370, 1989. doi: 10.1016/0022-4804(89)90149-2. [DOI] [PubMed] [Google Scholar]
- 12.Petrasek PF, Homer-Vanniasinkam S, Walker PM. Determinants of ischemic injury to skeletal muscle. J Vasc Surg 19: 623–631, 1994. doi: 10.1016/S0741-5214(94)70035-4. [DOI] [PubMed] [Google Scholar]
- 13.Bolognesi MP, Chen LE, Seaber AV, Urbaniak JR. Protective effect of hypothermia on contractile force in skeletal muscle. J Orthop Res 14: 390–395, 1996. doi: 10.1002/jor.1100140308. [DOI] [PubMed] [Google Scholar]
- 14.Labbe R, Lindsay T, Walker PM. The extent and distribution of skeletal muscle necrosis after graded periods of complete ischemia. J Vasc Surg 6: 152–157, 1987. doi: 10.1067/mva.1987.avs0060152. [DOI] [PubMed] [Google Scholar]
- 15.Wakai A, Winter DC, Street JT, Redmond PH. Pneumatic tourniquets in extremity surgery. J Am Acad Orthop Surg 9: 345–351, 2001. doi: 10.5435/00124635-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Lee C, Porter KM, Hodgetts TJ. Tourniquet use in the civilian prehospital setting. Emerg Med J 24: 584–587, 2007. doi: 10.1136/emj.2007.046359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozkan H, Ekinci S, Uysal B, Akyildiz F, Turkkan S, Ersen O, Koca K, Seven MM. Evaluation and comparison of the effect of hypothermia and ozone on ischemia-reperfusion injury of skeletal muscle in rats. J Surg Res 196: 313–319, 2015. doi: 10.1016/j.jss.2015.01.053. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder CA Jr, Lee HT, Shah PM, Babu SC, Thompson CI, Belloni FL. Preconditioning with ischemia or adenosine protects skeletal muscle from ischemic tissue reperfusion injury. J Surg Res 63: 29–34, 1996. [Erratum in J Surg Res 64: 216, 1996]. doi: 10.1006/jsre.1996.0217. [DOI] [PubMed] [Google Scholar]
- 19.Bolcal C, Yildirim V, Doganci S, Sargin M, Aydin A, Eken A, Ozal E, Kuralay E, Demirkilic U, Tatar H. Protective effects of antioxidant medications on limb ischemia reperfusion injury. J Surg Res 139: 274–279, 2007. doi: 10.1016/j.jss.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 20.Gürke L, Mattei A, Chaloupka K, Marx A, Sutter PM, Stierli P, Harder F, Heberer M. Mechanisms of ischemic preconditioning in skeletal muscle. J Surg Res 94: 18–27, 2000. doi: 10.1006/jsre.2000.5987. [DOI] [PubMed] [Google Scholar]
- 21.Hammers DW, Sarathy A, Pham CB, Drinnan CT, Farrar RP, Suggs LJ. Controlled release of IGF-I from a biodegradable matrix improves functional recovery of skeletal muscle from ischemia/reperfusion. Biotechnol Bioeng 109: 1051–1059, 2012. doi: 10.1002/bit.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan RA, Cikrit DF, Dalsing MC. Improved recovery of limb function with ATP/MgCl2 in an ischemic canine hind limb. Am J Surg 166: 103–107, 1993. doi: 10.1016/S0002-9610(05)81038-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen XK, Rathbone CR, Walters TJ. Treatment of tourniquet-induced ischemia reperfusion injury with muscle progenitor cells. J Surg Res 170: e65–e73, 2011. doi: 10.1016/j.jss.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 24.Barker JU, Qi WN, Cai Y, Urbaniak JR, Chen LE. Addition of nitric oxide donor S-nitroso-N-acetylcysteine to selective iNOS inhibitor 1400W further improves contractile function in reperfused skeletal muscle. Microsurgery 25: 338–345, 2005. doi: 10.1002/micr.20122. [DOI] [PubMed] [Google Scholar]
- 25.Erkanli K, Kayalar N, Erkanli G, Ercan F, Sener G, Kirali K. Melatonin protects against ischemia/reperfusion injury in skeletal muscle. J Pineal Res 39: 238–242, 2005. doi: 10.1111/j.1600-079X.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 26.Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Oxygen producing biomaterials for tissue regeneration. Biomaterials 28: 4628–4634, 2007. doi: 10.1016/j.biomaterials.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Ward CL, Corona BT, Yoo JJ, Harrison BS, Christ GJ. Oxygen generating biomaterials preserve skeletal muscle homeostasis under hypoxic and ischemic conditions. PLoS One 8: e72485, 2013. doi: 10.1371/journal.pone.0072485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suvarnapathaki S, Wu X, Lantigua D, Nguyen MA, Camci-Unal G. Breathing life into engineered tissues using oxygen-releasing biomaterials. NPG Asia Mater 11: 65, 2019. doi: 10.1038/s41427-019-0166-2. [DOI] [Google Scholar]
- 29.Ashammakhi N, Darabi MA, Kehr NS, Erdem A, Hu SK, Dokmeci MR, Nasr AS, Khademhosseini A. Advances in controlled oxygen generating biomaterials for tissue engineering and regenerative therapy. Biomacromolecules 21: 56–72, 2020. doi: 10.1021/acs.biomac.9b00546. [DOI] [PubMed] [Google Scholar]
- 30.Farris AL, Rindone AN, Grayson WL. Oxygen delivering biomaterials for tissue engineering. J Mater Chem B 4: 3422–3432, 2016. doi: 10.1039/C5TB02635K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett 82-83: 969–974, 1995. doi: 10.1016/0378-4274(95)03532-X. [DOI] [PubMed] [Google Scholar]
- 32.Halliwell B, Gutteridge JM. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med 8: 89–193, 1985. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- 33.Powers SK, Ji LL, Kavazis AN, Jackson MJ. Reactive oxygen species: impact on skeletal muscle. Compr Physiol 1: 941–969, 2011. doi: 10.1002/cphy.c100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girotti AW. Mechanisms of lipid peroxidation. J Free Radic Biol Med 1: 87–95, 1985. doi: 10.1016/0748-5514(85)90011-X. [DOI] [PubMed] [Google Scholar]
- 35.Magder S. Reactive oxygen species: toxic molecules or spark of life? Crit Care 10: 208, 2006. doi: 10.1186/cc3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walters TJ, Garg K, Corona BT. Activity attenuates skeletal muscle fiber damage after ischemia and reperfusion. Muscle Nerve 52: 640–648, 2015. doi: 10.1002/mus.24581. [DOI] [PubMed] [Google Scholar]
- 37.Walters TJ, Kragh JF, Baer DG. Influence of fiber-type composition on recovery from tourniquet-induced skeletal muscle ischemia-reperfusion injury. Appl Physiol Nutr Metab 33: 272–281, 2008. doi: 10.1139/H07-180. [DOI] [PubMed] [Google Scholar]
- 38.Kim JG, Lee J, Roe J, Tromberg BJ, Brenner M, Walters TJ. Hemodynamic changes in rat leg muscles during tourniquet-induced ischemia-reperfusion injury observed by near-infrared spectroscopy. Physiol Meas 30: 529–540, 2009. doi: 10.1088/0967-3334/30/7/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walters TJ, Kragh JF, Kauvar DS, Baer DG. The combined influence of hemorrhage and tourniquet application on the recovery of muscle function in rats. J Orthop Trauma 22: 47–51, 2008. doi: 10.1097/BOT.0b013e31815b3591. [DOI] [PubMed] [Google Scholar]
- 40.Kauvar DS, Baer DG, Walters TJ. Influence of systemic hypotension on skeletal muscle ischemia-reperfusion injury after 4-hour tourniquet application. J Surg Educ 64: 273–277, 2007. doi: 10.1016/j.jsurg.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Mintz EL, Passipieri JA, Franklin IR, Toscano VM, Afferton EC, Sharma PR, Christ GJ. Long-term evaluation of functional outcomes following rat volumetric muscle loss injury and repair. Tissue Eng Part A 26: 140–156, 2020. doi: 10.1089/ten.tea.2019.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Passipieri JA, Baker HB, Siriwardane M, Ellenburg MD, Vadhavkar M, Saul JM, Tomblyn S, Burnett L, Christ GJ. Keratin hydrogel enhances in vivo skeletal muscle function in a rat model of volumetric muscle loss. Tissue Eng Part A 23: 556–571, 2017. doi: 10.1089/ten.tea.2016.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mintz EL, Passipieri JA, Lovell DY, Christ GJ. Applications of in vivo functional testing of the rat tibialis anterior for evaluating tissue engineered skeletal muscle repair. J Vis Exp 116: 54487, 2016. doi: 10.3791/54487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng Part A 20: 705–715, 2014. doi: 10.1089/ten.TEA.2012.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Criswell TL, Corona BT, Ward CL, Miller M, Patel M, Wang Z, Christ GJ, Soker S. Compression-induced muscle injury in rats that mimics compartment syndrome in humans. Am J Pathol 180: 787–797, 2012. doi: 10.1016/j.ajpath.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Fish JS, McKee NH, Kuzon WM Jr, Plyley MJ. The effect of hypothermia on changes in isometric contractile function in skeletal muscle after tourniquet ischemia. J Hand Surg Am 18: 210–217, 1993. doi: 10.1016/0363-5023(93)90348-7. [DOI] [PubMed] [Google Scholar]
- 47.Awerbuck D, Luong V, Plyley MJ, McKee NH. skeletal muscle form and function after 4 hr ischemia-hypothermia. J Surg Res 57: 480–486, 1994. doi: 10.1006/jsre.1994.1173. [DOI] [PubMed] [Google Scholar]
- 48.Vignaud A, Hourde C, Medja F, Agbulut O, Butler-Browne G, Ferry A. Impaired skeletal muscle repair after ischemia-reperfusion injury in mice. J Biomed Biotechnol 2010: 724914, 2010. doi: 10.1155/2010/724914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corona BT, Wenke JC, Walters TJ, Rathbone CR. Intramuscular transplantation and survival of freshly isolated bone marrow cells following skeletal muscle ischemia-reperfusion injury. J Trauma Acute Care Surg 75: S142–S149, 2013. doi: 10.1097/TA.0b013e31829ac1fa. [DOI] [PubMed] [Google Scholar]
- 50.Oyster N, Witt M, Gharaibeh B, Poddar M, Schneppendahl J, Huard J. Characterization of a compartment syndrome-like injury model. Muscle Nerve 51: 750–758, 2015. doi: 10.1002/mus.24461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aurora A, Roe JL, Umoh NA, Dubick M, Wenke JC, Walters TJ. Fresh whole blood resuscitation does not exacerbate skeletal muscle edema and long-term functional deficit after ischemic injury and hemorrhagic shock. J Trauma Acute Care Surg 84: 786–794, 2018. doi: 10.1097/TA.0000000000001806. [DOI] [PubMed] [Google Scholar]
- 52.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298: 229–317, 2012. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deveci D, Marshall JM, Egginton S. Relationship between capillary angiogenesis, fiber type, and fiber size in chronic systemic hypoxia. Am J Physiol Heart Circ Physiol 281: H241–H252, 2001. doi: 10.1152/ajpheart.2001.281.1.H241. [DOI] [PubMed] [Google Scholar]
- 54.Egginton S, Fairney J, Bratcher J. Differential effects of cold exposure on muscle fibre composition and capillary supply in hibernator and non-hibernator rodents. Exp Physiol 86: 629–639, 2001. doi: 10.1113/eph8602260. [DOI] [PubMed] [Google Scholar]
- 55.Pullen AH. The distribution and relative sizes of three histochemical fibre types in the rat tibialis anterior muscle. J Anat 123: 1–19, 1977. [PMC free article] [PubMed] [Google Scholar]
- 56.Chan RK, Austen WG Jr, Ibrahim S, Ding GY, Verna N, Hechtman HB, Moore FD Jr.. Reperfusion injury to skeletal muscle affects primarily type II muscle fibers. J Surg Res 122: 54–60, 2004. doi: 10.1016/j.jss.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Charles AL, Guilbert AS, Guillot M, Talha S, Lejay A, Meyer A, Kindo M, Wolff V, Bouitbir J, Zoll J, Geny B. Muscles susceptibility to ischemia-reperfusion injuries depends on fiber type specific antioxidant level. Front Physiol 8: 52, 2017. doi: 10.3389/fphys.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cadot B, Gache V, Gomes ER. Moving and positioning the nucleus in skeletal muscle—one step at a time. Nucleus 6: 373–381, 2015. doi: 10.1080/19491034.2015.1090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park S, Park KM. Hyperbaric oxygen-generating hydrogels. Biomaterials 182: 234–244, 2018. doi: 10.1016/j.biomaterials.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 60.Ibrahim M, Schlegel HG. Efficiency of bovine liver catalase as a catalyst to cleave H2O2 added continually to buffer solutions. Biotechnol Bioeng 22: 1895–1906, 1980. doi: 10.1002/bit.260220909. [DOI] [PubMed] [Google Scholar]
- 61.Chance B. Effect of pH upon the reaction kinetics of the enzyme-substrate compounds of catalase. J Biol Chem 194: 471–481, 1952. doi: 10.1016/S0021-9258(18)55799-9. [DOI] [PubMed] [Google Scholar]
- 62.Trawczyńska I. New method of determining kinetic parameters for decomposition of hydrogen peroxide by catalase. Catalysts 10: 323, 2020. doi: 10.3390/catal10030323. [DOI] [Google Scholar]
- 63.Park JW, Kang JW, Jeon WJ, Na HS. Postconditioning protects skeletal muscle from ischemia-reperfusion injury. Microsurgery 30: 223–229, 2010. doi: 10.1002/micr.20756. [DOI] [PubMed] [Google Scholar]
- 64.Lintz JA, Dalio MB, Joviliano EE, Piccinato CE. Ischemic pre and postconditioning in skeletal muscle injury produced by ischemia and reperfusion in rats. Acta Cir Bras 28: 441–446, 2013. doi: 10.1590/S0102-86502013000600007. [DOI] [PubMed] [Google Scholar]
- 65.Mase VJ Jr, Roe JL, Christy RJ, Dubick MA, Walters TJ. Postischemic conditioning does not reduce muscle injury after tourniquet-induced ischemia-reperfusion injury in rats. Am J Emerg Med 34: 2065–2069, 2016. doi: 10.1016/j.ajem.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 66.Mohanraj P, Merola AJ, Wright VP, Clanton TL. Antioxidants protect rat diaphragmatic muscle function under hypoxic conditions. J Appl Physiol (1985) 84: 1960–1966, 1998. doi: 10.1152/jappl.1998.84.6.1960. [DOI] [PubMed] [Google Scholar]
- 67.Skelly JR, Bradford A, Jones JF, O’Halloran KD. Superoxide scavengers improve rat pharyngeal dilator muscle performance. Am J Respir Cell Mol Biol 42: 725–731, 2010. doi: 10.1165/rcmb.2009-0160OC. [DOI] [PubMed] [Google Scholar]