Abstract
The number of patients placed on kidney transplant waiting lists is rapidly increasing, resulting in a growing gap between organ demand and the availability of kidneys for transplantation. This organ shortage has forced medical professionals to utilize marginal kidneys from expanded criteria donors (ECD) to broaden the donor pool and shorten wait times for patients with end-stage renal disease. However, recipients of ECD kidney grafts tend to have worse outcomes compared to those receiving organs from standard criteria donors (SCD), specifically increased risks of delayed graft function (DGF) and primary nonfunction incidence. Thus, representative methods for graft-quality assessment are strongly needed, especially for ECDs. Currently, graft-quality evaluation is limited to interpreting the donor’s recent laboratory tests, clinical risk scores, the visual evaluation of the organ, and, in some cases, a biopsy and perfusion parameters. The last few years have seen the emergence of many new technologies designed to examine organ function, including new imaging techniques, transcriptomics, genomics, proteomics, metabolomics, lipidomics, and new solutions in organ perfusion, which has enabled a deeper understanding of the complex mechanisms associated with ischemia-reperfusion injury (IRI), inflammatory process, and graft rejection. This review summarizes and assesses the strengths and weaknesses of current conventional diagnostic methods and a wide range of new potential strategies (from the last five years) with respect to donor graft-quality assessment, the identification of IRI, perfusion control, and the prediction of DGF.
Keywords: kidney transplantation, graft quality assessment, biomarkers, machine perfusion, IRI, DGF
1. Introduction
Kidney transplantation (KTx) is a life-saving treatment for patients with end-stage renal dysfunction that is characterized by higher survival rates and greater quality of patient life compared to dialysis treatment [1]. Unfortunately, the number of patients placed on kidney transplant waiting lists is rapidly increasing, resulting in a growing gap between organ demand and the availability of kidneys for transplantation. Standard criteria donors (SCD) are preferred for kidney transplants because organs from these individuals typically result in more favourable outcomes compared to other donor types [2]. However, the shortage of available kidneys has forced medical professionals to utilize marginal kidneys from expanded criteria donors (ECD) to broaden the donor pool and shorten wait times for patients with end-stage renal disease. Nonetheless, it is well known that donor organ quality affects long-term outcomes for renal transplant recipients, and ECD kidney grafts have been shown to have worse outcomes compared to SCD grafts, including an increased risk of delayed graft function (DGF) and primary nonfunction incidence (PNF) [2,3]. Thus, representative methods of assessing graft-quality are urgently needed, especially for ECDs. Currently, the surgeon decides whether to accept or decline a kidney based on their interpretation of the donor’s recent laboratory tests and a visual evaluation of the organ, with a biopsy being employed in some cases for direct tissue analysis [4,5]. Notably, the rapid emergence of techniques such as imaging, omics, and organ perfusion has provided surgeons with a wide range of new potential tools and biomarkers that could be used to evaluate graft quality.
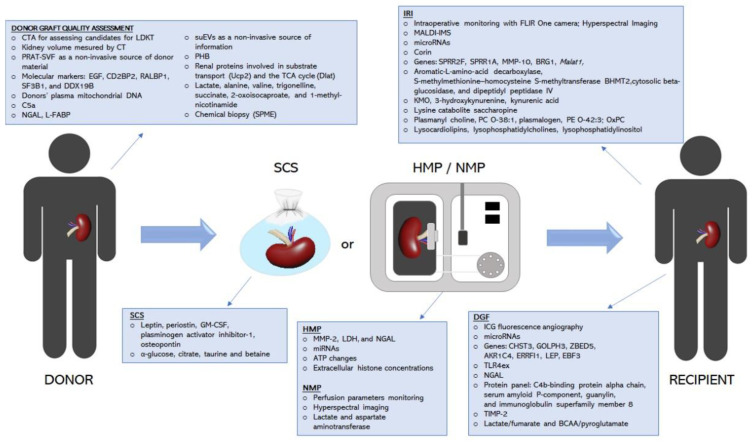
In this paper, we review and evaluate the limits and advantages of current conventional diagnostic methods and a range of new potential tools (from the last five years) with respect to donor graft-quality assessment, the identification of ischemia-reperfusion injury (IRI), perfusion control, and the prediction of DGF (Figure 1).
Figure 1.
Emerging techniques and biomarkers in graft quality assessment, the identification of ischemia-reperfusion injury, perfusion control, and the prediction of DGF.
2. Current Conventional Diagnostic Methods
2.1. Visual Assessment
A visual evaluation of the kidney by the transplant team is a critical step in determining whether it will be accepted for transplantation or rejected. Macroscopic examination is useful for identifying kidney tumors, anatomical changes, damage, fibrosis, and scars that indicate the quality of the graft. However, this method is subjective and depends on the transplant team’s level of experience [4]. Recent findings showed that surgeons were able to reliably predict the occurrence of postperfusion syndrome through visual assessments of liver graft quality, thus emphasizing the importance of visual appraisals by the surgical team [6]. However, no prior studies have evaluated intra-observer variability and the predictive value of visual kidney assessment. Thus, there is a need for new standardized diagnostic solutions for graft-quality assessment.
2.2. Clinical Risk Scores
Clinical information and laboratory results for a potential donor are crucial for an initial assessment of organ quality. Consequently, several scoring systems have been created to comprehensively analyse the risk of long-term graft failure or DGF [7,8,9,10]. At present, the Kidney Donor Risk Index (KDRI) and the Kidney Donor Profile Index (KDPI) are recognized as the most effective systems for scoring kidney graft quality. The KDRI was created by Rao et al., to quantify the risk of graft failure from deceased donors (DDs) based on donor and transplant variables, such as age, serum creatinine (CR), diabetes, HCV status, and cause of death [10]. The KDPI is a percentile measure based on the KDRI that was designed to assess how long a kidney from a DD is expected to function relative to all kidneys recovered in the U.S. during the previous year. The KDPI score is calculated based on ten variably weighted donor parameters that relevantly affect organ quality, with an emphasis on nephron mass. Lower KDPI scores are linked with longer estimated organ function, while higher KDPI scores are associated with a shorter estimated organ lifespan [11,12]. The KDRI and KDPI are regarded as reliable predictors of graft outcomes, and they are expected to increase the prevalence of marginal kidney grafting and reduce the unnecessary discard rate [11,13]. However, these indexes are not intended to be used as the only metric for determining donor suitability; rather, they should be utilized as a part of a comprehensive assessment along with other factors, including pre-implant biopsy histopathology and hypothermic mechine perfusion (HMP) parameters [11,14]. Because age is the most influential factor in calculating the KDRI and KDPI scores, it is unclear whether the scores for these indexes can be applied to elderly and pediatric DDs. Recent studies suggest that the KDPI does not precisely predict pediatric kidney graft survival, while the KDRI has been found to be more reliable for elderly DDs. Overall, more research is needed to assess how reliably KDPI and KDRI scores predict postoperative renal function for grafts using kidneys from pediatric and elderly donors [13,15].
2.3. Biopsy
Pretransplant biopsy is currently one of the most widely used diagnostic methods and is recognized as the gold standard for confirming allograft injury. However, the frequency with which biopsies are performed varies between medical facilities and countries. In the United States, up to 85% of higher-risk kidneys are biopsied, whereas pretransplant biopsies are rarely conducted in European medical facilities. Histological evaluation is usually applied selectively, predominantly in ECD and donor after cardiac death (DCD) kidneys, and can help surgeons decide whether a kidney should be selected for transplantation or rejected [4,5,16].
In contrast to most laboratory data, histopathological assessments of biopsies do not yield a single value; rather, they produce comprehensive diagnoses that consider all available information. Although glomerulosclerosis, vascular disease, and interstitial fibrosis are the most frequently reported kidney parameters associated with worse graft outcomes [4,16], there is no consensus on the relative importance of each factor and which threshold values should be used to define the acceptable limit values. A further difficulty is the low reproducibility of kidney biopsy evaluations between on-call pathologists and renal pathologists described in many prior studies. The clear need to improve reproducibility and to objectivize the procedure and reporting of results prompted the development of several new composite histopathological scoring systems, including the Remuzzi score, the Maryland Aggregate Pathology Index, Banff criteria, and the Chronic Allograft Damage Index. Nevertheless, even with all these scoring systems, there are still doubts relating to the sampling, processing, and evaluation of biopsies [4,5,16].
In daily practice, it may be necessary to obtain quick results. In such circumstances, frozen section (FS) evaluation is often used for decision making. Producing paraffin sections (PS) is time consuming, which can cause histological evaluations to require up to 3 h to complete, even with the use of high-speed processing methods [5,17,18]. However, reports of reproducibility and prognostic value are based on paraffin-embedded tissue [18]. Recent studies have shown discrepancies in the results obtained with the use of FS and PS, but these variances had no significant impact on the outcomes for the transplanted organs [18]. Observed changes could be subtler in frozen sections than in paraffin sections, which may be a limitation, particularly in the hands of inexperienced pathologists [17,19]. On the other hand, it is also critical to consider logistics when choosing an optimal biopsy technique. For instance, FS is able to provide a diagnosis in less than 30 min, whereas PS requires at least 3 h. In selecting the proper technique, it is important to strike a balance between the benefits and risks associated with increased cold ischemia [4,18].
A lack of uniformity with respect to procedural standards has resulted in the use of a variety of biopsy techniques. The majority of medical facilities seem to prefer wedge biopsy (WB) over needle biopsy (NB) because NB carries a greater risk of injuring larger blood vessels, potentially resulting in uncontrolled bleeding after reperfusion. However, most recent reports comparing WB and NB have found that NB provides a much better evaluation of vascular lesions and has a higher overall correlation with the state of the whole kidney [5,16,17].
Ultimately, the most crucial factor is how the histopathological results correlate with long-term graft survival. Many studies have attempted to address the predictive value of renal biopsy with respect to graft outcomes, but the results of these studies have been predominantly inconclusive [20,21,22,23]. For instance, Traynor et al., conducted a retrospective study that examined kidney transplants over a 10-year period to determine whether pretransplant histology is able to predict graft outcomes at 5 years, and whether donor histology adds incremental data to the current clinical parameters. While the results of these reports suggest that that histological assessment adds little additional prognostic information aside from clinical parameters [20], Yap et al., found that the histological evaluation of ECD kidneys was associated with improved long-term graft survival. Their results suggest that pretransplant biopsy assessment can enable ECD kidneys to be used as a safe and viable option during persistent shortages of kidney donors [21]. The divergence between recent studies highlights the need for a prospective controlled trial to evaluate the predictive value of pretransplant biopsies. Until a standardized and comprehensive evaluation protocol has been developed, biopsy findings remain only one component of a donor organ assessment and should not be taken as the sole determinant in deciding whether to discard or transplant donor kidneys [19,24,25].
2.4. Perfusion Control
Static cold storage (SCS) and HMP are the main techniques of kidney graft preservation [26]. HMP has become a frequently and widely used procedure in kidney transplantation over the past few years [26,27,28]. Indeed, several reports have shown that the HMP reconditioning effect results in better postoperative outcomes with respect to reducing DGF and better long-term graft survival after transplantation [29,30,31]. An important benefit of HMP is that it enables the monitoring of perfusion parameters that could predict post-transplant organ viability. In particular, flow rate and renal resistance (RR) have been among the most frequently used perfusion parameters in predicting post-transplant function [27,32,33,34]. Previous studies have produced findings suggesting that real-time RR detection provides good predictive value. As Bissolati et al., showed, the RR trend during HMP can be used to predict post-transplantation outcomes, especially in relation to kidneys procured from ECD [28]. Patel et al., conducted a retrospective study that included 190 kidneys in order to evaluate the prognostic utility of HMP in DD transplantation. Their findings showed that resistances at two hours and beyond predicted DGF, while initial resistance to machine perfusion predicted one-year graft survival post-transplantation [35]. On the other hand, some studies found no association between hemodynamic parameters during HMP and the development of DGF [27]. Thus, due to these inconclusive results, the perfusion parameters cannot be regarded as stand-alone criteria. However, the undoubted advantage of perfusion parameters is that they are easy to obtain in a non-invasive manner. As such, Jochmans et al., and Zheng et al., have suggested that HMP parameters should be included as part of a comprehensive graft assessment [14,32]. DGF has a complex pathogenesis and cannot be predicted with precision using the HMP parameters as a stand-alone assessment tool. However, RR represents an additional source of information that can help clinicians in their decision-making process. Attaining more accurate predictions of graft outcomes will require integrating the perfusion parameters into multifactorial graft quality scoring systems. A combination of the donor’s clinical data, kidney pre-implant histopathology, and HMP parameters may provide a more effective prediction of DGF than any of the measures alone [14,32].
2.5. Microbiological Analysis of Preservation Fluid
Organ transplant recipients are prone to infectious complications, and despite many advances, post-operative infections remain associated with significant morbidity and mortality [36,37,38]. Early post-transplant infections among kidney transplant recipients may be transmitted via the donor, or the donated organ may be contaminated during the transplantation procedure [36,38]. Moreover, pathogens can be transmitted via preservation solution, which is required to maintain kidney viability, but due to its biochemical characteristics, it can also keep microorganisms alive and serve as an infection vector [36,38,39]. For that reason, some transplant centres collect preservation fluid for microbiological analysis in addition to standard screening for donor infections. However, there are no widely accepted recommendations for managing positive preservation fluid cultures [36,38]. Moreover, it remains unanswered whether intra-operative preservation fluid routine screening should be performed because the clinical impact of this practice is still not well established. Some studies have evaluated the risk factors associated with culture-positive preservation fluid and determined the benefit of routine screening of preservation solutions for the management of kidney transplant recipients [36,37,38,40]. Corbel et al., demonstrated that 24% of DD preservation fluid cultures were positive, and these contaminations were mainly a consequence of procurement procedures [37]. Reticker et al. [36] and Oriol et al. [38] showed that the prevalence of culture-positive preservation fluid was up to 60%; however, the vast majority of microbial growth was consistent with skin flora or low-virulence pathogens. In addition, Oriol et al., indicated that pre-emptive antibiotic therapy for recipients with high-risk culture-positive preservation fluid might improve the outcomes and help to avoid preservation-fluid-related infections [38]. Moreover, Stern et al., reported that fungal contamination of preservation fluid was infrequent, although yeast contamination of preservation solutions was associated with high mortality [40]. In parallel, Reticker et al., suggested that antibiotic therapy for recipients with preservation solutions contaminated by low virulence pathogens may not be necessary, reducing antibiotic overuse [36]. In conclusion, routine screening of preservation solutions could improve graft outcomes and pre-emptive antibiotic therapy and be helpful to avoid preservation-fluid-related infections. However, future studies are needed to establish guidelines for preservation fluid microbiological analysis and handling culture-positive preservation fluid.
3. Emerging Techniques
3.1. Imaging
Diagnostic imaging methods are mainly used to evaluate kidneys from living donors (LD) prior to acceptance for transplantation, as well as for assessing post-renal transplant complications. In the case of living donor surgeries, non-invasive preoperative evaluation of the quality of the graft organ is especially critical, which allows surgeons to assess certain vital features, such as size, the presence/absence of focal cystic or solid lesions, and the condition of vascular structures, to establish whether it is appropriate for transplantation. While most of these features can be visualized via Doppler ultrasound, computed tomography angiography (CTA) is usually necessary for a more accurate assessment of the vascular anatomy [41,42,43]. However, given the critical role of careful evaluation and suitable preparation when dealing with living donor transplantation, it will be imperative to continue to conduct new research aimed at improving transplantation outcomes.
Sarier et al., conducted a retrospective study wherein they compared pretransplant CTA images to intraoperative findings to evaluate renal artery variations in a large sample of LD. They found that laparoscopic donor nephrectomy enabled the detection of the same number of renal arteries as CTA in 97.9% of the analysed kidneys, but less than CTA in the remaining 2.1%. Notably, a greater number of renal arteries were not detected in any of the studied kidneys via nephrectomy compared to CTA. These results indicate that CTA is more accurate than intraoperative findings, and is an effective method for evaluating candidate donors for living donor kidney transplantation (LDKT), as well as for identifying renovascular variations [42].
Al-Adra et al., employed computed tomography (CT) scans to assess the influence of donor kidney volume on recipient estimated glomerular filtration rate (eGFR) in a large cohort of patients undergoing LDKT. The resultant statistical models showed a significant correlation between donor kidney volume and recipient eGFR at 1, 3, and 6 months (p < 0.001). These findings indicate that donor kidney volume is a strong independent predictor of recipient eGFR in LDKT and may therefore be a valuable addition to predictive models of eGFR after transplantation. Further research could examine whether addition of donor kidney volume in matching algorithms can improve recipient outcomes [43].
Although the ability to monitor graft status intraoperatively is limited at present, several novel solutions have been proposed over the past few years to evaluate graft quality during transplantation and predict DGF.
In 2019, Fernandez et al., proposed a novel approach that utilized infrared imaging to monitor the reperfusion phase during kidney transplantation in real-time. To this end, they used a long-wave infrared camera (FLIR One) with a visual resolution of 1440 × 1080 pixels and a thermal resolution of 160 × 120 to study the grafts in 10 pediatric patients undergoing kidney transplantation. During the study, images were acquired at several key time points. The authors observed a correlation between changes in intraoperative graft temperature and decreases in postoperative creatinine levels in all of the analysed subjects. Given these results, Fernandez et al., concluded that infrared thermal imaging could be a promising option for non-invasive graft perfusion monitoring. However, additional work is required to confirm Fernandez et al.’s results because they were somewhat limited due to the relatively small number of patients included and the short follow-up period [44].
In another study, Sucher et al., employed Hyperspectral Imaging (HSI) as a noncontact, non-invasive, and non-ionizing method of acquiring quantitative information relating to kidney viability and performance during transplantation. Specifically, they used HSI to study seventeen consecutive deceased donor kidney transplants prior to transplantation, while stored on ice, and again at 15 and 45 min after reperfusion. After computation time of less than 8 s, the analysis software was able to provide an RGB image and 4 false color images representing the physiological parameters of the recorded tissue area, namely, tissue oxygenation, perfusion, organ hemoglobin, and tissue water index. The obtained results revealed that allograft oxygenation and microperfusion were significantly lower in patients with DGF. Future applications might also utilize HSI during donor surgery to assess kidney quality prior to cold perfusion and procurement. However, HSI can only be used intraoperatively and requires a direct view of the kidney because the maximum penetration depth for microcirculation measurements is currently 4–6 millimetres, making transcutaneous applications impossible. Thus, this technique’s main limitations are its inability to provide continuous or intermittent transcutaneous follow-up measurements, as well as its small sample size. Thus, further studies are required to confirmed these results [45].
In the recent article, Gerken et al., documented a prospective diagnostic study that they had conducted in two German transplantation centres wherein allograft microperfusion was assessed intraoperatively via near-infrared fluorescence angiography with indocyanine green (ICG). While previous studies have shown that ICG fluorescence angiography can be applied safely during kidney transplantation, none have provided a quantitative assessment of the use of fluorescence video. To fill this gap, Gerken et al., evaluated the benefits of coupling quantitative intraoperative fluorescence angiography with ICG to predict post-operative graft function and the occurrence of DGF. Their findings indicated that the impairment of intraoperative microperfusion in the allograft cortex is a risk factor for the occurrence of DGF, and that ICG Ingress is an independent predictor of DGF. Further studies are warranted to analyse the effect of applying early therapeutic approaches to prevent DGF in kidney transplant recipients, thus improving long-term graft success [46].
The use of imaging techniques to diagnose post-renal transplant complications has been discussed extensively in recent reviews [47,48,49]; therefore, the present work will only examine a few of the most recent studies in this field. Promising results have been reported with respect to combining positron emission tomography (PET) with CT or magnetic resonance imaging (MRI) using the glucose analogue radiotracer, 2-deoxy-2-fluoro-D-glucose (FDG), to detect acute kidney allograft rejection, for diagnostic applications, for the functional assessment of grafts, and for therapeutic monitoring [50,51]. In another study, the utility of arterial spin labeling (ASL) magnetic resonance imaging was evaluated for its ability to identify kidney allografts with underlying pathologies. ASL uses endogenous water as a tracer, and it has previously been used in applications relating to the brain. Moreover, there have been reports demonstrating that ASL can be used to categorize stages of chronic kidney disease [52]. Wang et al., demonstrated that ASL might be a non-invasive tool for differentiating kidneys with subclinical pathology from those with stable graft function. However, more research should be performed to verify these findings [53].
3.2. Omics
The last few years has seen the emergence of many new technologies that examine organ function on a molecular level, which has enabled the discovery of numerous potential biomarkers of renal injury. High-throughput omics technologies allow researchers to obtain a large amount of data about specific types of molecules, providing a holistic picture that captures the complex and dynamic interactions within a biological system. These innovative methods, including transcriptomics, genomics, proteomics, metabolomics, and lipidomics, provide a deeper understanding of the complex mechanisms associated with IRI, inflammatory processes, and graft rejection [5,54]. This section surveys some promising methods and techniques that could be successfully translated to clinical settings in the foreseeable future (Table 1).
Table 1.
Emerging trends in donor graft quality assessment techniques.
| Application | Category | Model | Type of Sample | Main Conclusions | Author |
|---|---|---|---|---|---|
| Evaluation of gene expression profile of kidney submitted to ischemic injury | Donor graft quality | Pig | Tissue |
|
Giraud et al. [55] |
| Investigation of the features of perirenal adipose tissue as an indicator of the detrimental impact of the ECD microenvironment on a renal transplant | Donor graft quality | Human | Perirenal adipose tissue |
|
Boissier et al. [56] |
| Evaluation of donor category influence on borderline changes in kidney allografts by molecular fingerprints | Donor graft quality | Human | Tissue |
|
Hruba et al. [57] |
| Exploration of the association between plasma mtDNA levels and post-transplant renal allograft function | Donor graft quality | Human | Plasma |
|
Han et al. [58] |
| Searching for urinary miRs that can be a biomarker for AKI | IRI |
|
|
|
Chen et al. [59] |
| Determination of the role of miR-17- 92 in IRI-induced AKI | IRI | Mouse | Tissue |
|
Song et al. [61] |
| Investigation of the expression of renal miRNAs following renal IRI | IRI | Rat | Tissue |
|
Wang et al. [62] |
| Identification of candidate genes involved in renal IRI | IRI | Mouse | Tissue |
|
Su et al. [64] |
| Examination of a link between activation of IL-33 transcription by BRG1 in endothelial cells and renal IRI | IRI | Mouse | Tissue |
|
Liu et al. [65] |
| Screening for differentially expressed genes in renal IR-injured mice using a high-throughput assay | IRI; DGF |
|
|
|
Hu et al. [67] |
| Unbiased urinary microRNA profiling to identify DGF predictors after kidney transplantation. | DGF | Human | Urine |
|
Khalid et al. [68] |
| High-throughput sequencing to expression profiling of exosomal miRNAs obtained from the peripheral blood of patients with DGF | DGF | Human | Plasma |
|
Wang et al. [69] |
| Examination of miR-146a-5p expression in kidney transplant recipients with DGF | DGF | Human | Tissue; Whole blood |
|
Milhoransa et al. [71] |
| Evaluation of PBMC TLR4 expression of renal graft recipients with DGF | DGF | Human | Tissue; Whole blood |
|
Zmonarski et al. [72] |
| Profiling of molecular changes associated with decreased resilience and impaired function of human renal allografts | DGF | Human | Tissue |
|
McGuinness et al. [3] |
| Searching for urinary biomarkers that predict reduced graft function after DD kidney transplantation | RGF | Human | Urine |
|
Koo et al. [74] |
| Evaluation of associations between DD urine injury biomarkers and kidney transplant outcomes | DGF | Human | Urine |
|
Reese et al. [75] |
| Assessment of C3a and C5a in urine samples as biomarkers for post-transplant outcomes | DGF | Human | Urine |
|
Schröppel et al. [76] |
| Assessment of urinary and perfusate concentrations of MCP-1 from kidneys on HMP as an organ function indicator | AKI; DGF | Human | Urine; Perfusate |
|
Mansour et al. [77] |
| Evaluation of the proteome of suEVs and its changes throughout LD transplantation | Donor graft quality | Human | Urine; Tissue |
|
Braun et al. [80] |
| Proteomic study of differentially expressed proteins in BD rabbits kidneys | Donor graft quality | Rabbit | Tissue; Serum |
|
Li et al. [81] |
| Investigation of the influence of BD on systemic and specifically hepatic and renal metabolism in a rodent BD model | Donor graft quality | Rat | Plasma; Urine; Tissue |
|
Van Erp et al. [82] |
| Unbiased integrative proteo-metabolomic study in combination with mitochondrial function analysis of kidneys exposed to IRI to investigate its effects at the molecular level | IRI | Rat | Tissue |
|
Huang et al. [83] |
| Integrative proteome analysis of potential and predominantly renal injury biomarkers considering changes occurring in the tissue and echo in serum and urine protein profiles | IRI | Pig | Serum; Urine; Tissue |
|
Malagrino et al. [84] |
| Evaluation of the changes in the proteome of kidney subjected to ischemia during machine cold perfusion with doxycycline | IRI | Rat | Tissue; Perfusate |
|
Moser et al. [85] |
| Proteomics analysis determinating the molecular differences between NMP human kidneys with URC and UR | IRI | Human | Tissue |
|
Weissenbacher et al. [86] |
| TUPA to identify protein biomarkers of delayed recovery following KTx | DGF | Human | Urine |
|
Williams et al. [87] |
| Assessment of the diagnostic and prognostic role of NGAL in DGF and chronic allograft nephropathy | DGF | Human | Serum; Urine |
|
Lacquaniti et al. [88] |
| Investigation of changes of urinary TIMP-2 and IGFBP7 in the first days after KTx and their diagnostic utility for predicting DGF outcomes | DGF | Human | Urine |
|
Bank et al. [89] |
| Investigation of organ-specific metabolic profiles of the liver and kidney during BD and afterwards during NMP of the kidney | Donor graft quality | Rat | Tissue; Plasma; Urine |
|
van Erp et al. [90] |
| Investigation of the acute and prolonged metabolic consequences associated with IRI, and elucidation whether the early injury mediated metabolic reprogramming can predict the outcome of the injury | IRI | Rat | Tissue; Plasma |
|
Nielsen et al. [91] |
| NMR identification of metabolic alterations to the kidney following IRI | IRI | Mouse | Urine; Serum; Tissue |
|
Chihanga et al. [92] |
| Monitoring of the effect of oxidative stress and ischemia on the condition of kidneys using SPME-LC-HRMS platform |
Organ ischemia | Rabbit | Tissue |
|
Stryjak et al. [93] |
| Assessment of the role of kynurenine 3-monooxygenase as an essential regulator of renal IRI | IRI | Mouse | Plasma; Urine; Tissue |
|
Zheng et al. [95] |
| Unbiased tissue metabolomic profiling of IRI and ACR in murine models to identify novel biomarkers and to provide a better understanding of the pathophysiology | IRI; ACR | Mouse | Tissue |
|
Beier et al. [96] |
| Detection of early lipid changes in AKI using SWATH lipidomics coupled with MALDI tissue imaging | IRI | Mouse | Tissue |
|
Rao et al. [97] |
| Determination of the individual OxPC molecules generated during renal IRI | IRI | Rat | Tissue |
|
Solati et al. [98] |
| Rapid identification of IRI in renal tissue by Mass-Spectrometry Imaging | IRI | Pig | Tissue |
|
Van Smaalen et al. [99] |
| Evaluation of the involvement of the hypoxanthine-XO axis in the IRI that occurs during kidney transplantation | IRI | Human | Plasma; Tissue |
|
Wijermars et al. [100] |
| Prediction of prolonged duration of DGF in DCD kidney transplant recipients by urinary metabolites profiling | DGF | Human | Urine |
|
Kostidis et al. [79] |
| Explorative metabolic assessment based on an integrated, time-resolved strategy involving sequential evaluation of AV differences over reperfused grafts and parallel profiling of graft biopsies | DGF | Human | Tissue; Plasma |
|
Lindeman et al. [101] |
| Analysis of the proteins and peptides that are passed from the kidneys to the preservation fluid during organ preservation | Perfusion control | Human | Preservation fluid |
|
Coskun et al. [106] |
| Searching for proteins accumulating in preservation solutions during SCS as biomarkers to predict posttransplantation graft function | Perfusion control | Human | Preservation fluid |
|
van Balkom et al. [107] |
| Analysis of perfusates during SCS to obtain the metabolite profiles of DGF and IGF allografts | Perfusion control | Human | Preservation fluid |
|
Wang et al. [108] |
| Proteomic study of perfusate from HMP of transplant kidneys | Perfusion control | Human | Perfusate |
|
Moser et al. [115] |
| Proteomic perfusate analysis of DBD kidneys preserved using HMP to identify the differences between proteomic profiles of kidneys with a good and suboptimal outcome | Perfusion control | Human | Perfusate |
|
van Leeuwen et al. [2] |
| Evaluation of miRNAs in kidney machine perfusion fluid as novel biomarkers for graft function | Perfusion control | Human | Perfusate |
|
Gómez-Dos-Santos et al. [116] |
| Influence of method of kidney storage on oxidative stress and post-transplant kidney function parameters | Perfusion control | Human | Perfusate; Whole blood |
|
Tejchman et al. [117] |
| Ex vivo evaluation of kidney graft viability during perfusion using 31P MRI spectroscopy | Perfusion control | Pig | n.a. |
|
Longchamp et al. [118] |
| Assessment of an association between the presence of extracellular histones in machine perfusates and deceased donor kidney viability | Perfusion control | Human | Perfusate |
|
van Smaalen et al. [119] |
| Organ quality assessment during NMP | Perfusion control | Pig | Perfusate; Whole blood; Urine |
|
Kaths et al. [120] |
| Hyperpolarized MRI and spectroscopy using pyruvate and other 13C-labeled molecules as a novel tool for monitoring the state of ex vivo perfused kidneys | Perfusion control | Pig | n.a. |
|
Mariager et al. [123] |
| Examination of the relationship between urinary biomarkers and NMP parameters in a series of human kidneys | Perfusion control | Human | Urine; Serum |
|
Hosgood et al. [124] |
↑—increase of expression; ↓—decrease of expression; n.a—not applicable.
3.2.1. Transcriptomics/Genomics
Several studies have examined how graft quality and donor category impact graft and patient survival. Giraud et al., proposed an open-ended approach based on microarray technology to understand IRI occurring in DCD kidneys in a preclinical porcine model that had been subjected to warm ischemia (WI) followed by cold ischemia. Giraud et al.’s findings indicated that hundreds of cortex and corticomedullary junction genes were significantly regulated after WI or after WI followed by cold storage compared to healthy kidneys. In addition, they also analysed the kinetics of the most differentially expressed genes. They hypothesized that these genes played a key role in IRI and could be divided into eight categories: mitochondria and redox state regulation; inflammation and apoptosis; and protein folding and proteasome; cell cycle, cellular differentiation and proliferation; nucleus genes and transcriptional regulation; transporters; metabolism regulation; mitogen-activated protein kinase and GTPase (guanosine triphosphate, GTP) activity [55].
Boissier et al., performed a comparative study of cellular components, transcriptomics, and the vasculogenic profiles obtained from 22 optimal donors and 31 deceased ECDs. They hypothesized that as an easily accessible source of donor-derived material, perirenal adipose tissue (PRAT) can be used to assess the quantitative and functional features that characterize donor cells. In addition, adipose tissue can be enzymatically processed to obtain stromal vascular fraction (SVF), which is a heterogeneous cellular mixture free of adipocytes. In their study, Bossier et al., performed a transcriptomic analysis in order to differentiate the PRAT-SVF molecular transcript in ECD and other donors. The upregulated genes demonstrated a strong association with the inflammatory response, cytokine secretion, and circulatory system development, while the downregulated genes were associated with regulating metabolic processes and circulatory system development. Importantly, Bossier et al.’s findings provide new evidence that PRAT-SVF serves as a non-invasive source of donor material that can be highly valuable in the assessment of inflammatory features affecting the quality and function of the graft [56].
The midterm outcomes of kidney transplant recipients with early borderline changes between ECD, SCD, and LD were compared in a retrospective observational study. In the ECD group, microarray analysis showed a higher expression of 244 transcripts than the SCD group, and 437 more than the LD group. Compared to both the SCD and LD groups, gene annotation analysis of transcripts with elevated expression in ECD group revealed enhancement in the inflammatory response, the response to wounding, the defence response, and the ECM-receptor interaction pathway. ECD-related transcripts were likely increased by already occurred vascular changes compared to SCD group, and, similarly in SCD group, by longer ischemia compared with LD group. Therefore, chronic vascular changes and cold ischemia time enhance inflammation and thus contribute to poor outcomes for these grafts [57].
Another novel organ-evaluation tool was proposed in a retrospective open-cohort study that examined donors’ plasma mitochondrial DNA (mtDNA), which can be easily and non-invasively assayed in the pre-transplant period, and may be a promising predictive biomarker for allograft function [58]. The mtDNA levels in the plasma of DCD were determined via real-time polymerase chain reaction (RT-PCR) and then statistically analysed in relation to the recipient’s mtDNA levels and DGF. The linear prediction model, which included plasma mtDNA, donor serum creatinine, and warm ischemia time (WIT), showed high predictive value for reduced graft function. Moreover, the findings indicated that plasma mtDNA might be a novel non-invasive predictor of DGF and allograft function at six months after transplantation, in addition to correlating to allograft survival. Furthermore, mtDNA may serve as a surrogate predictive marker for PNF [58].
The vast majority of studies aiming to identify novel biomarkers involved in IRI have used murine or rat models. A growing body of evidence indicates that the aberrant expression of microRNAs (miRNA/miR) is closely associated with IRI pathogenesis [59,60,61,62,63,64]. MiRNAs are small, noncoding RNAs that mediate mRNA cleavage, translational repression, or mRNA destabilization [59]. For instance, Chen et al.’s findings suggest that miR-16 may serve as a potential biomarker of IRI-induced acute kidney injury (AKI) [59], while Zhu et al., found that miR-142-5p and miR-181a might be responsible for modulating renal IRI development [63]. On the other hand, some studies have pointed that miR-17-92, miR-139-5p, and miR-27a may play a protective role in IRI [61,62,64]. For example, Song et al., suggest that the overexpression of miR-17-92 could partly reverse the side-effects of IRI on the proximal tubules in vivo [61]. Furthermore, Wang et al., have reported that the overexpression of miR-27a results in the downregulation of toll-like receptor 4 (TLR4), which in turn inhibits inflammation, cell adhesion, and cell death in IRI [62].
Other murine-model-based studies have explored new candidate genes associated with renal IRI. In one such study, Su et al., found that IRI caused the upregulation of SPRR2F, SPRR1A, MMP-10, and long noncoding RNA (lncRNA) Malat1 in kidney tissues. These genes are involved in keratinocyte differentiation, regeneration, and the repair of kidney tissues; extracellular matrix degradation and remodeling; inflammation; and cell proliferation in renal IRI [64]. In a separate study, Liu et al., investigated the role of BRG1 in IRI-induced AKI with a focus on its role in regulating IL-33 expression in endothelial cells. Their findings revealed that endothelial BRG1 deficiency reduces renal inflammation following ischemia-reperfusion in mice with a simultaneous reduction in IL-33 levels [65].
Comparisons of IRI in murine-based models and clinical studies have yielded valuable results [66,67]. For instance, Cippà et al., employed RNA-sequencing-mediated transcriptional profiling and machine learning computational approaches to analyse the molecular responses associated with IRI, which emphasized early markers of kidney disease progression and outlined transcriptional programs involved in the transition to chronic injury [66]. Other studies have demonstrated that Corin is downregulated in renal IRI and may be associated with DGF after kidney transplantation. Researchers have also screened differentially expressed genes in a murine model of IRI, with findings identifying Corin as one of the most relevant downregulated genes among 2218 differentially expressed genes. Moreover, 11 recipients with complications due to DGF and 16 without DGF were recruited for an ELISA to determine their plasma Corin concentrations. The findings of this study showed downregulation of plasma Corin concentrations in transplant recipients with DGF complications, indicating that Corin could be a potential biomarker of DGF [67]. DGF may result from early ischemic injury and potentially contribute to poor long-term survival following kidney transplantation [68,69]. For this reason, much research has been devoted to devising reliable methods for predicting the extent of IRI, and hence, DGF.
Hence, as with the IRI, miRNA was evaluated as a biomarker of DGF. In one study, Khalid et al., quantified microRNAs in urine samples from kidney transplant patients to determine whether this approach can be used to predict who will develop DGF following kidney transplantation. To this end, they used unbiased profiling to identify microRNAs that are predictive of DGF following kidney transplantation (i.e., miR-9, -10a, -21, -29a, -221, and -429), and afterward confirmed their findings by measuring specific microRNAs via RT-PCR. The biomarker panel was then assessed using an independent cohort at a separate transplant centre, with urine samples being collected at varying times during the first week after transplantation. When considered individually, all miRs in the panel showed a trend towards an increase or relevant increase in patients with DGF [68].
Wang et al., used high-throughput sequencing to investigate the miRNA expression profiling of exosomes in the peripheral blood of kidney recipients with and without DGF, and explain the regulation of miRNAs in the DGF pathogenesis [69]. Exosomes are cell-derived membrane vesicles present in numerous bodily fluids that play a crucial role in processes such as the regulation of cellular activity, intercellular communication, and waste management [69,70]. Wang et al., identified 52 known and 5 conserved exosomal miRNAs specifically expressed in transplant recipients with DGF. Additionally, their findings showed that transplant recipients with DGF also exhibited the upregulation of three co-expressed miRNAs: hsa-miR-33a-5p R-1, hsa-miR-98-5p, and hsa-miR-151a-5p. Moreover, hsa-miR-151a-5p was positively correlated with the kidney recipients’ serum CR, blood urea nitrogen (BUN), and uric acid (UA) levels in the first week post-transplantation [69].
MicroRNA expression in kidney transplant recipients with DGF has also been assessed in another recently published study [71]. In this work, the researchers employed RT-PCR to analyse the expression of miRNA-146-5p in peripheral blood and renal tissue obtained from kidney transplant recipients who had undergone a surveillance graft biopsy during the DGF period. In the renal tissue, the expression of miR-146a-5p was significantly increased among the DGF patients compared to the stable and acute rejection (AR) patients. Similarly, microRNA 146a-5p had heightened expression in the peripheral blood samples from the DGF group compared to those of the acute rejection and stable groups; however, these differences were not statistically significant (p = 0.083) [71].
Overall, all these reports indicate that miRNAs are emerging as essential biomarkers in the molecular diagnosis of DGF. The above-discussed findings identify biomarkers that could contribute to the development of tools for predicting DGF and, as such, represent an important area of focus for future research.
Zmonarski et al., applied PCR to nonstimulated peripheral blood mononuclear cells (PBMCs) to examine the averaged mRNA toll-like receptor 4 expression (TLR4ex). The sample for this study consisted of 143 kidney transplant patients, 46 of whom had a history of DGF, and a control group of 38 healthy volunteers. The patients with a history of DGF were divided into two subgroups based on the median TLRex: low-TLR4 expression and high-TLR expression. Zmonarski et al.’s findings showed that patients with DGF had a much lower TLR4ex and worse parameters of kidney function. In addition, while a comparison of the DGF patients with low and high TLR4ex revealed no initial differences in kidney transplant function, differences were observed in the post-follow-up period. Furthermore, regression analysis showed that TLR4ex was related to recipient age, tacrolimus concentration, and uremic milieu. Consequently, the authors concluded that the low TLR4 expression in patients with DGF may be associated with poor graft-capacity prognosis, and that analysis of changes in TLR4ex may be valuable for assessing immunosuppression efficacy [72].
Another study aiming to identify potential biomarkers of DGF and AKI was recently conducted by Bi et al. [73]. In this study, the authors obtained two mRNA expression profiles from the National Center of Biotechnology Information Gene Expression Omnibus repository, including 20 DGF and 68 immediate graft function (IGF) samples. Differentially expressed genes (DEGs) in the DGF and IGF groups were identified, and pathway analysis of these DEGs was conducted using the Gene Ontology and Kyoto Encyclopedia of Genes and Genomes. Next, a protein–protein interaction analysis extracted hub genes. The essential genes were then searched in the literature and cross-validated based on the training dataset. In total, 330 DEGs were identified in the DGF and IGF samples, including 179 upregulated and 151 downregulated genes. Of these, OLIG3, EBF3, and ETV1 were transcription factor genes, while LEP, EIF4A3, WDR3, MC4R, PPP2CB, DDX21, and GPT served as hub genes in the PPI network. In addition, the findings suggested that EBF3 may be associated with the development of AKI following renal transplantation because it was significantly upregulated in the validation dataset (GSE139061), which is consistent with the initial gene differential expression analysis. Moreover, the authors found that LEP had a good diagnostic value for AKI (AUC = 0.740). Overall, these findings provided more profound insights into the diagnosis of AKI following kidney transplantation [73].
Elsewhere, McGuinness et al., combined epigenetic and transcriptomic data sets to determine a molecular signature for loss of resilience and impaired graft function. Notably, at a translational level, this study also provided a platform for developing a universal IRI signature and the ability to link it to post-transplant outcomes. Furthermore, McGuinness et al.’s findings relate DNA methylation status to reperfusion injury and DGF outcome. In this study, 24 paired pre- and post-perfusion renal biopsies defined as either meeting the extreme DGF phenotype or exhibiting IGF were selected for analysis. The findings of this analysis showed that the molecular signature contained 42 specific transcripts, related through IFNγ signaling, which, in allografts displaying clinically impaired function (DGF), exhibited a major change in transcriptional amplitude and increased expression of noncoding RNAs and pseudogenes, which is consistent with increased allostatic load. This phenomenon was attended by an increase in DNA methylation within the promoter and intragenic regions of the DGF panel in pre-perfusion allografts with IGF. Overall, McGuinness et al.’s findings suggest that kidneys exhibiting DGF suffer from an impaired ability to restore physiological homeostasis in response to stress that is commensurate to their biological age and associated allostatic load. This outcome is reflected in changes in the epigenome and transcriptome, as well as in the dysregulation of RNA metabolism [3].
3.2.2. Proteomics
Proteomics approaches have also been used to identify donor biomarkers that may predict graft dysfunction in order to alleviate organ shortages and address the lack of representative methods for assessing graft quality. To date, several studies have focused on identifying novel proteomic biomarkers of graft quality in donor urine [74,75,76,77]. Koo et al.’s study aimed to investigate the viability of using the levels of neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and L-type fatty acid binding protein (L-FABP) in donor urine samples to predict reduced graft function (RGF). In addition, Koo et al., also created a prediction model of early graft dysfunction based on these donor biomarkers. This model, which includes donor urinary NGAL, L-FABP, and serum CR, has been shown to provide better predictive value for RGF than donor serum CR alone. Based on this model, a nomogram for a scoring method to predict RGF was created to help guide the allocation of DD and maximize organ utilization [74]. On the other hand, another large prospective study has shown that donor injury biomarkers such as microalbumin, NGAL, KIM-1, IL-18, and L-FABP have limited utility in predicting outcomes among kidney transplant recipients [75]. This study evaluated the associations between injury biomarkers in the urine of DD and donor AKI, recipient DGF, and recipient six-month eGFR. Each of the tested biomarkers was strongly associated with donor AKI in the adjusted analyses. However, although the levels of all five donor biomarkers were higher in recipients with DGF than in those without DGF, the fully adjusted analyses revealed an association between higher donor urinary NGAL concentrations and a modest increase in the relative risk of recipient DGF. Moreover, the results of this study indicated that donor urinary biomarkers add minimal value in predicting recipient allograft function at six months post-transplantation [75]. In both studies, the tested biomarkers were strongly associated with donor AKI, while NGAL concentration was associated with DGF. A potential explanation for the different conclusions of these studies may be that Koo et al., used RGF as an outcome in their study, while Reese et al., used DGF due to different donor characteristics. Furthermore, it is worth emphasizing that, while these proteins are upregulated and secreted in urine in response to tubular injury, they were reported to have low specificity for tubular epithelial cell injury and were observed to increase in patients with urinary tract infections and sepsis [78,79].
In another study, the potential utility of C3a and C5a in DD urine samples as biomarkers for early post-transplant outcomes was investigated [76]. The results of this large, prospective, observational cohort study indicated a three-fold increase in C5a concentrations in urine samples from donors with stage 2 and 3 AKI compared to donors without AKI. In addition, donor C5a was positively correlated with the occurrence of DGF in recipients. In adjusted analyses, C5a remained independently correlated with recipient DGF only for donors without AKI. Moreover, the authors observed a tendency to indicate better 12-month organ functioning from donors with the lowest urinary C5a [76].
Monocyte chemoattractant protein-1 (MCP-1) has also been proposed as a potential biomarker of donor kidney quality. For example, Mansour et al., evaluated the association between graft outcomes and levels of MCP-1 in urine from DD at the time of organ procurement. In particular, they measured MCP-1 concentration to determine its correlation to donor AKI, recipient DGF, six-month estimated eGFR, and graft failure. Unfortunately, Mansour et al.’s results suggested that urinary MCP-1 has minimal clinical utility. Although median urinary MCP-1 concentrations were elevated in donors with AKI compared to those without AKI, higher MCP-1 levels were independently associated with a higher six-month eGFR in those without DGF. However, MCP-1 was not independently associated with DGF, and no independent associations between MCP-1 and graft failure were observed over a median follow-up of ~two years [77].
Recently, Braun et al., demonstrated the potential of using small urinary extracellular vesicles (suEVs) as a non-invasive source of data regarding early molecular processes in transplant biology. Their unbiased proteomic analysis revealed temporal patterns in the signature of suEV proteins, as well as cellular processes involved in both early response and longer-term graft adaptation. In addition, a subsequent correlative analysis identified potential prognostic markers of future graft function, such as phosphoenol pyruvate carboxykinase (PCK2). However, while Braun et al.’s study showed the potential of suEVs as biomarkers, the small number of patients in their sample did not allow for a conclusive statement on the predictive value of suEV PCK2. Therefore, the potential use of this biomarker will depend on larger trials in the future [80].
Studies focusing on the use of kidney tissue as a sample matrix to evaluate donor organ quality have also been performed. Using a rabbit model of brain death (BD), Li et al., employed two-dimensional gel electrophoresis and Matrix Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF-MS)-based comparative proteomic analysis to profile the differentially-expressed proteins between BD and renal tissue collected from a control group. The authors were able to acquire five downregulated proteins and five upregulated proteins, which were then classified according to their function, including their association with proliferation and differentiation, signal transduction, protein modification, electron transport chain, and oxidation-reduction. Moreover, immunohistochemical analysis indicated that the expression of prohibitin (PHB) gradually elevated in a time-dependent manner. These data showed alterations in the levels of certain proteins in the organs from the BD group, even in the case of non-obvious functional and morphological changes. Given their results, Li et al., suggested that PHB may be an innovative biomarker for the primary assessment of the quality of kidneys from BD donors [81].
Conversely, van Erp et al., used a multi-omics approach and a rat model to investigate organ-specific responses in the kidneys and liver during BD. The application of proteomics analysis enabled them to quantify 50 proteins involved in oxidative phosphorylation, tricarboxylic acid (TCA) cycle, fatty acid oxidation (FAO), substrate transport, and several antioxidant enzymes in isolated hepatic and renal mitochondria. The most relevant changes were observed in the reduced peptide levels in the kidneys, which were related to complex I (Ndufs1), the TCA cycle (Aco2, Fh, and Suclg2), FAO (Hadhb), and the connection between FAO and the electron transport chain (Etfdh). The expression of two renal proteins, which were associated with substrate transport (Ucp2) and the TCA cycle (Dlat), was significantly increased in samples from the BD group compared to the sham-operated group. Interestingly, van Erp et al.’s findings showed that BD pathophysiology affects systemic metabolic processes, alongside organ-explicit metabolic changes, manifest in the kidneys by metabolic shutdown and suffering from oxidative stress, and a shift to anaerobic energy production, while kidney perfusion decreases. Ultimately, van Erp et al., concluded that an organ-specific strategy focusing on metabolic changes and graft perfusion should be part of novel procedures for assessing graft quality in organs from brain-dead donor, and may be the key to improving transplantation outcomes [82].
The vast majority of studies focusing on IRI have used animal models. In one proteo-metabolomics study using rat models, coagulation, complement pathways, and fatty acid (FA) signaling were observed following the elevation of proteins belonging to acute phase response due to IRI. Moreover, after 4 h of reperfusion, analysis of metabolic changes showed an increase in glycolysis, lipids, and FAs, while mitochondrial function and adenosine triphosphate (ATP) production were impaired after 24 h [83]. The authors of another study that used a porcine model of IRI found that integrative proteome analysis can provide a panel of potential—and predominantly renal—biomarkers at many levels, as changes occurring in the tissue are reflected in serum and urine protein profiles. This conclusion was based on the use of urine, serum, and renal cortex samples. In the renal cortex proteome, the authors observed an elevation in the synthesis of proteins in the ischemic kidney (vs. the contralateral kidney), which was highlighted by transcription factors and epithelial adherens junction proteins. Intersecting the set of proteins up- or downregulated in the ischemic tissue with both serum and urine proteomes, authors identified six proteins in the serum that may provide a set of targets of kidney injury. In addition, four urinary proteins with predominantly renal gene expression were also identified: aromatic-L-amino-acid decarboxylase (AADC), S-methylmethionine–homocysteine S-methyltransferase BHMT2 (BHMT2), cytosolic beta-glucosidase (GBA3), and dipeptidyl peptidase IV (DPPIV) [84]. Recent research by Moser et al., has examined kidney preservation injury and the nephroprotective activity of doxycycline (Doxy). In this work, rat kidneys were cold perfused with and without Doxy for 22 h, followed by the extraction of proteins from the renal tissue. Subsequent analysis showed a significant difference in eight enzymes involved in cellular and mitochondrial metabolism. Interestingly, the levels of N(G),N(G)-dimethylarginine dimethylaminohydrolase and phosphoglycerate kinase 1 decreased during cold perfusion on its own but increased during cold perfusion with Doxy [85]. The influence of perfusion type on graft quality has also been evaluated by Weissenbacher et al., who applied proteomics analysis to determine the differences between normothermically perfused (normothermic machine perfusion, NMP) human kidneys with urine recirculation (URC) and urine replacement (UR). Their findings revealed that damage-associated patterns in the kidney tissue decreased after 6 h of NMP with URC, suggesting decreased inflammation. Furthermore, they also observed that vasoconstriction in the kidneys was also attenuated with URC, as indicated by a reduction in angiotensinogen levels. The kidneys became metabolically active during NMP, which could be improved and prolonged by applying URC. The application of URC also enhanced mitochondrial succinate dehydrogenase enzyme levels and carbonic anhydrase, which contributed to pH stabilization. Key enzymes involved in glucose metabolism increased after 12 and 24 h of NMP with URC, including mitochondrial malate dehydrogenase and glutamic-oxaloacetic transaminase, predominantly in DCD tissue. The authors concluded that NMP with URC can prolong organ preservation and revitalize metabolism to possibly better mitigate IRI in discarded kidneys [86].
Ischemic injury may result in DGF, which is associated with a more complicated post-operative course, including a higher risk of AR [87]. Therefore, the early evaluation of kidney function following transplantation is essential for predicting graft outcomes [88]. Several studies have applied proteomic analysis to recipient urine samples in an attempt to identify protein biomarkers of DGF [87,88,89]. For instance, Lacquaniti et al., evaluated the usefulness of NGAL levels both for the early detection of DGF and as a long-term predictor of graft outcome. Their findings revealed that serum and urine samples from DGF patients contained high levels of NGAL beginning the first day after transplantation. Moreover, in patients who had received a kidney from a living related donor with excellent allograft function, NGAL concentrations lowered quickly during the first 24 h post-transplant period, reflecting a more pronounced reversible short-term injury. Importantly, NGAL levels in urine provided a better diagnostic profile than serum NGAL. Hence, urinary biomarkers on day 1 post-transplant may not only be useful in predicting who will need dialysis within one week, but they may also allow clinicians to discriminate between more subtle allograft recovery patterns [88]. However, as mentioned above, NGAL is characterized by low specificity; hence, its clinical application is limited due to inconclusive results [78,79]. Williams et al., used a Targeted Urine Proteome Assay (TUPA) to identify biomarkers of DGF following kidney transplantation. After employing data quality consideration and rigorous statistical analysis, they identified a panel of the top 4 protein biomarkers, including the C4b-binding protein alpha chain, serum amyloid P-component, guanylin, and immunoglobulin superfamily member 8, which had an AUC of 0.891, a specificity of 82.6%, and a sensitivity of 77.4% [87]. Similarly, urinary tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor binding protein-7 (IGFBP7) have been evaluated as biomarkers for DGF [89]. The findings of these studies indicated that TIMP-2 was able to adequately identify patients with DGF and prolonged DGF (AUC 0.89 and 0.77, respectively), whereas IGFBP7 was not. Moreover, correcting TIMP-2 for urine osmolality improved predictability (AUC 0.91 for DGF, AUC 0.80 for prolonged DGF), and 24-h urinary CR excretion and TIMP-2/mOsm were found to be significant predictors of DGF, with an AUC of 0.90. Hence, the obtained results indicated that TIMP-2 might be a promising, non-invasive indicator for predicting the occurrence and duration of DGF in individual patients [89].
3.2.3. Metabolomics and Lipidomics
In the absence of good quantitative biomarkers correlating to pre-transplantation organ quality, van Erp et al., examined metabolic alterations during BD using hyperpolarized magnetic resonance (MR) spectroscopy and ex vivo graft glucose metabolism during normothermic isolated perfused kidney (IPK) machine perfusion [90]. To this end, they employed hyperpolarized 13C-labeled pyruvate MR spectroscopy to quantify pyruvate metabolism in the kidneys and liver at three time points during BD in a rat model. Following BD, glucose oxidation was measured using tritium-labeled glucose (D-6-3H-glucose) during IPK reperfusion. In addition, enriched 13C-pyruvate was injected repetitively to evaluate the metabolic profile at T = 0, T = 2, and T = 4 h via the relative conversion of pyruvate into lactate, alanine, and bicarbonate. The rats showed significantly higher lactate levels immediately following the induction of BD, with alanine production decreasing in the kidneys 4 h post-BD. However, it should be emphasized that this study’s results did not assess whether these metabolic alterations can be associated with graft quality, or if they are suitable predictors of transplant outcome [90].
Another study using a rodent model of IRI examined the potential of using Hyperpolarized 13C-labeled pyruvate to evaluate the metabolic profile directly in the kidneys [91]. The in vivo responses observed at 24 h and 7 d following ischemic injury demonstrated a similar trend towards a general decrease in the overall metabolism in the ischemic kidney and a compensatory increase in anaerobic metabolism, which is evidenced by elevated lactate production, compared to aerobic metabolism. In addition, a correlation was found between the intra-renal metabolic profile 24 h after reperfusion and 7 d after injury induction, as well as a correlation with the plasma CR. As a result, the authors suggest that using hyperpolarized 13C-labeled pyruvate to identify the balance between anaerobic and aerobic metabolism has great future potential as a prognostic biomarker [91].
Increased lactate levels due to IRI were also observed in another study [92]. However, analysis of urine samples via nuclear magnetic resonance (NMR) spectroscopy showed higher levels of valine and alanine and decreased levels of metabolites such as trigonelline, succinate, 2-oxoisocaproate, and 1-methyl-nicotinamide following IRI, which was likely due to altered kidney function or metabolism [92].
A novel and minimally invasive metabolomic and lipidomic diagnostic protocol based on solid-phase microextraction (SPME) has been proposed to address the lack of representative methods of assessing graft quality [93,94]. The small size of the SPME probe allows the performance of chemical biopsy, which enables metabolites to be extracted directly from the kidney without any tissue collection. Furthermore, SPME’s minimally invasive nature permits multiple analyses over time. For instance, ischemia-induced alterations in the metabolic profile of the kidneys and oxidative stress as a function of cold storage were observed in one study that used an animal model, with the most pronounced alterations being observed in the levels of essential amino acids and purine nucleosides [93]. However, more work is required to discriminate a set of characteristic compounds that could serve as biomarkers of graft quality and indicators of possible development of organ dysfunction.
In response to reports that the pharmacological inhibition of kynurenine 3-monooxygenase (KMO), and, separately, the transcriptional blockage of the Kmo gene, reduces 3-hydroxykynurenine formation and protects against secondary AKI, Zheng et al., investigated whether mice lacking functional KMO (Kmonull mice) are protected from AKI experimentally induced by the direct induction of renal IRI [95]. KMO plays a crucial role in kynurenine metabolism. Kynurenine metabolites are generated by tryptophan catabolism and are involved in the regulation of various biological processes, including host-microbiome signaling, immune cell response, and neuronal excitability. The kynurenine pathway diverges into two distinct branches, which are regulated by kynurenine aminotransferases (KATs) and KMO, respectively. KMO is the only route of 3-hydroxykynurenine production that is known to be injurious to cells and tissue. Kynurenine may also be metabolized into kynurenic acid by KATs and to anthranilic acid by kynureninase [95]. Following the experimental induction of AKI via renal IRI, Zheng et al., observed that the Kmonull mice had kept renal function, decreased renal tubular cell injury, and fewer infiltrating neutrophils than the wild-type control mice. Given these results, they suggested that KMO is a critical regulator of renal IRI. Moreover, higher levels of kynurenine and kynurenic acid were observed in the Kmonull IRI mice compared to the Kmonull sham-operated mice. This result may indicate that these metabolites help to protect against AKI after renal IRI, particularly because kynurenic acid has been demonstrated to have protective properties in other inflammatory situations due to its activity at glutamate receptors [95].
A 12.5-fold increase in the lysine catabolite saccharopine in IRI kidneys was observed in a recent study examining the differences between renal allograft acute cellular rejection (ACR) and IRI. The findings of this work indicated that the accumulation of saccharopine causes mitochondrial toxicity and may contribute to IRI pathophysiology. Moreover, similar to other reports, increased levels of itaconate and kynurenine were also observed in ACR kidneys. However, the detected changes in metabolites seemed to be unique for IRI and ACR, respectively, indicating that these two conditions have distinct tissue metabolomic signatures [96].
Several reports have also demonstrated that IRI can alter the lipidome. For example, Rao et al., evaluated lipid changes in an IRI mouse model using sequential window acquisition of all theoretical spectra-mass spectrometry (SWATH-MS) lipidomics. Their findings indicated that four lipids increased significantly at 6 h after IRI: plasmanyl choline, phosphatidylcholine (PC) O-38:1 (O-18:0, 20:1), plasmalogen, and phosphatidylethanolamine (PE) O-42:3 (O-20:1, 22:2). As anticipated, statistically significant changes were observed in many more lipids at 24 h after IRI. Interestingly, elevated levels of PC O-38:1 persisted at 24 h post-IRI, while renal levels of PE O-42:3 decreased alongside all ether PEs detected by SWATH-MS at this later time point. Overall, the authors found that coupling SWATH-MS lipidomics with MALDI-IMS (Imaging Mass Spectrometry, IMS) for lipid localization provided a better understanding of the role played by lipids in the pathobiology of acute kidney injury [97].
Researchers have also tested whether oxidized phosphatidylcholine (OxPC) molecules are generated following renal IRI. Solati et al., identified fifty-five distinct OxPC molecules in rat kidneys following IRI, including various fragmented (aldehyde and carboxylic-acid-containing species) and nonfragmented products. Among these, 1-stearoyl-2-linoleoyl-phosphatidylcholine (SLPC-OH) and 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine (PAzPC) were the most abundant after 6 h and 24 h IRI, respectively. The total number of fragmented aldehyde OxPC molecules was significantly elevated in the 6 h and 24 h IRI groups compared to the sham-operated group, while an increase in the level of fragmented carboxylic acid was observed in the 24 h group compared to the sham and 6 h groups. In addition, fragmented OxPC levels were found to be significantly correlated with CR levels [98].
In their recent paper, van Smaalen et al., introduced and employed an interesting new approach based on IMS to rapidly and accurately evaluate acute ischemia in kidney tissue from a porcine model. First, ischemic tissue damage was systematically evaluated by two pathologists; this was followed by the application of MALDI-IMS to study the spatial distributions and compositions of lipids in the same tissues. Whereas the histopathological analysis revealed no significant difference between the tested groups, the MALDI-IMS analysis provided detailed discrimination of severe and mild ischemia based on the differential expression of characteristic lipid-degradation products throughout the tissue. In particular, elevated levels of lysolipids, including lysocardiolipins, lysophosphatidylcholines, and lysophosphatidylinositol, were present after severe ischemia. This data shows IMS’s potential for use in differentiating and identifying early ischemic injury molecular patterns, and as a future tool that can be deployed in kidney assessment [99].
Because ischemia and reperfusion are inevitable consequences of kidney transplantation, and because DGF is a manifestation of IRI, Wijermars et al., used kidney transplantation as a clinical model of IRI to evaluate the role of the hypoxanthine-xanthine oxidase (XO) axis in human IRI. The sample group for this study consisted of patients undergoing renal allograft transplantation (n = 40), who were classified into three groups based on the duration of ischemia: short, intermediate, and prolonged. The results of the analysis confirmed the progressive accumulation of hypoxanthine during ischemia. However, differences in arteriovenous concentrations of UA and an in situ enzymography of XO did not indicate relevant XO activity in IRI kidney grafts. Moreover, renal malondialdehyde and isoprostane levels and allantoin formation were assessed during the reperfusion period to determine whether a putative association exists between hypoxanthine accumulation and renal oxidative stress. The absence of the release of these markers indicated the lack of an association between ischemic hypoxanthine accumulation and post-reperfusion oxidative stress. Based on these results, the authors suggest that the hypoxanthine-xanthine oxidase axis is not involved in the initial phase of clinical IRI [100]. In their clinical study, Kostidis et al., employed NMR spectroscopy to analyse the urinary metabolome of DCD transplant recipients at multiple time points in an attempt to identify markers that predict the prolonged duration of functional DGF [79]. To this end, urine samples were collected at 10, 42, 180, and 360 days post-transplantation. Their analysis revealed that samples collected on day 10 had a different profile than samples obtained at the other time points. At day 10, D-glucose, 2-aminobutyrate, valine, p-hydroxyhippurate, fumarate, 2-ethylacrylate, leucine, and lactate were significantly elevated in patients with DGF compared to those without DGF, while asparagine, DMG, 3-hydroxyisobutyrate, 3-hydroxyisovalerate, 2-hydroxy-isobutyrate, and histidine were significantly reduced in the DGF group. Urine samples from patients with prolonged DGF (≥21 days) showed increased levels of lactate and lower levels of pyroglutamate compared to participants with limited DGF (<21 days). Moreover, the ratios of all metabolites were analysed via logistic regression analysis in an attempt to further distinguish prolonged DGF from limited DGF. The results of this analysis showed that the combination of lactate/fumarate and branched chain amino acids (BCAA)/pyroglutamate provided the best outcome, predicting prolonged DGF with an AUC of 0.85. Given these results, the authors concluded that it is possible to identify kidney transplant recipients with DGF based on their altered urinary metabolome, and that it may also be possible to use these two ratios to predict prolonged DGF [79].
In another study, Lindeman et al., examined possible metabolic origins of clinical IRI by integrating data from 18 pre- and post-reperfusion tissue biopsies with 36 sequential arteriovenous blood samplings from grafts in three groups of subjects, including LD and DD grafts with and without DGF. The integration of metabolomics data enabled Lindeman et al., to determine a discriminatory profile that can be used to identify future DGF. This profile was characterized by impaired recovery of the high-energy phosphate-buffer, phosphocreatine, in DGF grafts post-reperfusion, as well as by persistent post-reperfusion ATP/GTP catabolism and significant ongoing tissue damage. The impaired recovery of high-energy phosphate occurred despite the activation of glycolysis, fatty acid oxidation, glutaminolysis, autophagia and was found to be related to a defect at the level of the oxoglutarate dehydrogenase complex in the Krebs cycle. Hence, Lindeman et al.’s findings suggest that DGF is preceded by a post-reperfusion metabolic collapse, leading to an inability to sustain the organ’s energy requirements. Thus, efforts aimed at preventing DGF should aim to preserve or restore metabolic competence [101].
3.3. New Solutions in Perfusion Control
Organ-preservation technologies have been garnering significant interest for graft quality assessment, advanced organ monitoring, and treating transplanted kidneys during machine perfusion. As mentioned above, SCS and HMP are two of the more common methods of hypothermic preservation applied in clinical settings at present. In SCS, the kidney is submerged in a cold preservation fluid and placed on ice in an icebox; in HMP, a device pumps cold preservation fluid through the renal vasculature, which has been revealed to improve post-transplant outcomes [102]. NMP is another dynamic preservation strategy that involves the circulation of a perfusion solution through the kidney. The NMP conditions are designed to nearly replicate physiological conditions, which makes a real-life assessment of the graft possible prior to transplantation [103,104]. NMP has been recently translated into clinical practice, but this application is still at an experimental stage. However, early clinical results are promising [103,105]. Because preservation/perfusion solutions serve as a non-invasive source for the analysis of biomarkers, numerous studies have employed it for the purposes of graft quality assessment. In this section of this paper, we summarize the latest findings and studies that have used preservation/perfusion fluid and perfusion control in kidney transplantation (Table 1).
Coskun et al., used proteomic techniques to analyse the protein profiles of preservation fluid used in SCS kidneys. Their findings revealed significant correlations between protein levels and donor age (23 proteins), cold ischemia time (5 proteins), recipients’ serum BUN (12 proteins), and CR levels (7 proteins). The identified proteins belonged to groups related to the structural constituent of the cytoskeleton, serine-type endopeptidase inhibitor activity, peptidase inhibitor activity, cellular component organization or biogenesis, and cellular component morphogenesis, among others [106]. In another proteomic study of preservation fluid, five potential biomarkers (leptin, periostin, granulocyte-macrophage colony-stimulating factor (GM-CSF), plasminogen activator inhibitor-1, and osteopontin) were identified in a discovery panel for differentiating kidneys with immediate function from those with DGF. Further analysis yielded a prediction model based on leptin and GM-CSF. Receiver-operating characteristic analysis revealed an AUC of 0.87, and the addition of recipient BMI significantly increased the model’s predictive power, resulting in an AUC of 0.89 [107]. The metabolomic study compared the level of metabolites in perfusate samples collected prior to transplantation, during static cold storage, and between the allografts exhibiting DGF and IGF, while an integrated NMR-based analysis revealed a significant elevation in α-glucose and citrate levels, and significant decreases in taurine and betaine levels in the perfusate of DGF allografts [108].
In the last few years, several studies have documented the benefits of HMP over SCS, including improved short-term outcomes and reduced risk of DGF [109,110,111]. However, reports suggesting that HMP improves long-term graft function are inconclusive [102,111]. Some research groups have compared HMP with SCS to evaluate HMP’s potential to improve kidney-graft outcomes [109,112] and to better understand the long-term benefits associated with its use [111,113]. At the same time, other groups have investigated how the use of oxygenated HMP impacts post-transplant outcomes, and how it can be used to further optimize kidney preservation, thereby expanding the number of organs available for transplant [102,114]. Furthermore, perfusion solution has been used in the search for useful biomarkers of graft quality and potential therapeutic targets. The analysis of perfusates from donor after brain death (DBD), DCD, and LD kidneys showed that DCD kidneys contained the highest levels of matrix metalloproteinase-2 (MMP-2), lactate dehydrogenase (LDH), and NGAL, followed by DBD and LD kidneys, respectively, suggesting a greater amount of injury in the DCD kidneys. Moreover, the DCD kidney perfusate contained significantly higher levels of protein compared to the DBD and LD perfusates, with quantitative analysis of the protein spots revealing significant differences between the groups in relation to seven spots: peroxiredoxin-2, FABP, A1AT, heavy chain of immunoglobulin, serum albumin, fragment of collagen 1, and protein deglycase (DJ-1) [115]. In another proteomic study, perfusate analysis of DBD kidneys preserved via HMP was performed to identify differences between the proteomic profiles of kidneys with good (GO) and suboptimal outcomes (SO) one-year post-transplantation. Analysis of samples collected 15 min after the start of HMP (T1) and before the termination of HMP (T2) indicated that the 100 most abundant proteins demonstrated discrimination between grafts, with a GO and SO at T1. Increased proteins were involved in classical complement cascades at both T1 and T2, while a decreased abundance of lipid metabolism at T1 and cytoskeletal proteins at T2 in GO (vs. SO) was also observed. Perfusate analysis at T1 revealed a predictive value of 91% for ATP-citrate synthase and fatty acid-binding protein 5, and analysis at T2 showed a predictive value of 86% for immunoglobulin heavy variable 2–26 and desmoplakin. In summary, HMP perfusate profiles for DBD kidneys can distinguish between outcomes one-year post-transplantation, providing a potential non-invasive method of assessing donor organ quality [2].
MicroRNAs in kidney machine perfusion solutions have also been considered as new biomarkers for graft function. For instance, Gómez dos Santos et al., conducted a prospective cohort study to investigate graft dysfunction in kidney transplantation from ECD. To this end, they employed a mean expression value approach, which confirmed the significance of a subset of the miRNAs previously identified with the development of delayed graft function, namely, miR-486-5p, miR-144-3p, miR-142-5p, and miR-144-5p. These results confirmed that perfusion fluid can be a valuable pre-transplantation source of organ-viability biomarkers [116].
In another study, Tejchman et al., assessed oxidative stress markers from the hypothermic preservation of transplanted kidneys. In particular, they sought to analyse the activity of enzymes and levels of non-enzymatic compounds involved in antioxidant defense mechanisms. These compounds, which included glutathione (GSH), glutathione peroxidase (GPX), catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), glutathione transferase (GST), thiobarbituric acid reactive substances (TBARS), malondialdehyde (MDA), were measured in preservation solutions before the transplantation of human kidneys grafted from DBD. The study group was divided into two groups based on the method of kidney storage, with Group 1 consisting of HMP kidneys (n = 26) and Group 2 consisting of SCS kidneys (n = 40). There were aggregations of significant correlations between kidney function parameters after KTx and oxidative stress markers, namely: diuresis and CAT; Na+ and CAT; K+ and GPX; and urea and GR. Moreover, there were aggregations of correlations between recipient blood count and oxidative stress markers, including CAT and monocyte count; SOD and white blood cell count; and SOD and monocyte count. However, there was an issue of unequivocal interpretation because none of the observed aggregations constituted conditions that supported the authors’ hypothesis that kidney function after KTx can be predicted based on oxidative stress markers measured during preservation. Moreover, it would be hard to conclude that the blood count alterations observed in the repeated measurements after KTx were unrelated to factors other than oxidative stress or acidosis. As the authors suggest, many other factors may modify blood count, including operative stress, bleeding, immunosuppression, and microaggregation [117].
Longchamp et al., presented an interesting and non-invasive method of assessing graft quality during perfusion based on the use of 31P pMRI spectroscopy to detect high-energy phosphate metabolites, such as ATP. Thus, pMRI can be used to predict the energy state of a kidney and its viability before transplantation. In addition, Longchamp et al., also performed gadolinium perfusion sequences, which allowed them to observe the internal distribution of the flow between the cortex and the medulla. pMRI showed that warm ischemia caused a reduction in ATP levels, but not its precursor, adenosine monophosphate (AMP). Moreover, they found that ATP levels and cortical and medullary gadolinium elimination were inversely correlated with the severity of kidney histological injury. Thus, the measured parameters may be considered as biomarkers of kidney injury after warm ischemia, and Longchamp et al.’s method provides an innovative non-invasive approach to assessing kidney viability prior to transplantation [118].
Other researchers have examined whether a correlation exists between the level of extracellular histones in machine perfusates and the viability of DD kidneys. Extracellular histone levels were significantly elevated in the perfusates of kidneys with post-transplant graft dysfunction, and they were considered an independent risk factor for DGF and one-year graft failure, but not for PNF. One-year graft survival was 12% higher in the low-histone-concentration group (p = 0.008) compared to the higher-histone-concentration group. Hence, the quantitation of extracellular histones might contribute to the evaluation of post-transplant graft function and survival [119].
NMP is an emerging approach for donor organ preservation and functional improvements in kidney transplantation. However, methods for evaluating organs via NMP have yet to be developed, and the development of novel graft quality assessment solutions has only recently come into focus.
Kaths et al., used a porcine model to investigate whether NMP is suitable for graft quality assessment prior to transplantation. They found that intra-renal resistance was lowest in the HBD group and highest in the severely injured DCD group (60 min of warm ischemia), and that the initiation of NMP was correlated with post-operative renal function. Markers of acid-base homeostasis (pH, HCO3–, base excess) correlated with post-transplantation renal function. Furthermore, concentrations of lactate and aspartate aminotransferase were lowest in perfusate from non-injured grafts (vs. DCD kidneys) and were correlated with post-transplantation kidney function. Kaths et al., found that perfusion characteristics and clinically available perfusate biomarkers during NMP were correlated with post-transplantation kidney graft injury and function. However, further research is needed to identify perfusion parameter thresholds for DGF and PNF [120].
HSI combined with NMP was introduced as a novel approach for monitoring physiological kidney parameters. The experimental results of an HSI-based oxygen-saturation calculation indicated that HSI is useful for monitoring oxygen saturation distribution and identifying areas with a reduced oxygen supply prior to transplantation. Moreover, camera-based measurements are easy to integrate with a perfusion setup and allow the fast and non-invasive measurement of tissue characteristics [121]. Subsequent research has explored how to improve algorithms for determining kidney oxygen saturation [122]. Unfortunately, the application of HSI is limited by the propagating light’s low penetration depth, which makes it impossible to detect deeper tissue injuries. However, based on the fact that most metabolic activity occurs in the kidney cortex, the combined use of HSI and NMP offers a promising and easy-to-use method for assessing the status of the organ and for chemical imaging [121,122].
Hyperpolarized MRI and spectroscopy (MRS) using pyruvate and other 13C-labeled molecules offers a novel approach to monitoring the state of ex vivo perfused kidneys. In one study, the state of a porcine kidney was quantified using acquired anatomical, functional, and metabolic data. The findings showed an apparent reduction in pyruvate turnover during renal metabolism compared with the typical in vivo levels observed in pigs, while perfusion and blood gas parameters were found to be in the normal ex vivo range. Mariager et al.’s findings demonstrate the applicability of these techniques for monitoring ex vivo graft metabolism and function in a large animal model that resembles human renal physiology [123].
In another study, researchers sought to investigate the link between the urinary biomarkers, endothelin-1 (ET-1), NGAL, and KIM-1, and NMP parameters in order to improve kidney assessment prior to transplantation. Fifty-six kidneys from DD were used in this work, with each kidney being subjected to 1 h of NMP, followed by assessment based on macroscopic examination, renal blood flow, and urine output. The levels of ET-1 and NGAL measured in the urine samples after 1 h of NMP were significantly associated with perfusion parameters during NMP. These biomarkers and NMP perfusion parameters were also significantly associated with terminal graft function in the donor. However, KIM-1 was not correlated with the perfusion parameters or the donor’s renal function. Larger studies are required to determine the usefulness of using these biomarkers with NMP to predict transplant outcomes. Despite this limitation, this study undoubtedly demonstrates that measuring urinary biomarkers during NMP provides additional information about graft quality [124].
4. Conclusions
New diagnostic solutions for accurately assessing renal graft quality are needed to improve the process for selecting suitable donors, more efficiently managing complications, and prolonging graft survival. Rapid advances in imaging, omics technology, and perfusion methods have led to the development of a wide range of new tools and biomarkers that could be applied to evaluate graft quality. Unfortunately, most of the methods mentioned in the review are based on animal models or require sophisticated technology with a long turn-around time to obtain the results, which significantly limits their potential for clinical use in the form of rapid commercial tests at present. However, non-invasive solutions, including imaging and the measurement of biomarkers in urine, blood, and perfusion fluid, appear to be promising with respect to their ability to be translated to a clinical setting. These studies include mtDNA and miRNAs determination based on commercially available kits for the isolation of genetic material in combination with the RT-PCR technique widely used in laboratory practice. A similar clinical potential is demonstrated by the determination of biomarkers such as NGAL, KIM-1, L-FABP and C5a in urine by ELISA, also routinely used in diagnostics. Nevertheless, the translation of biomarkers from the discovery stage to clinical practice is still challenging due to the complex and multifactorial type of injuries, the absence of standard guidelines for method validation, and adequate prospective and retrospective cohort studies. Larger, multi-centre validation studies are needed before new solutions can be widely implemented in clinics. Moreover, it will be imperative for future research to explore new technologies and integrate molecular measurements from large data sets reported in different experiments.
Abbreviations
| ACR | acute cellular rejection |
| AKI | acute kidney injury |
| AMP | adenosine monophosphate |
| AR | acute rejection |
| ASL | arterial spin labelling |
| ATP | adenosine triphosphate |
| AUC | area under the curve |
| BCAA | branched chain amino acids |
| BD | brain death |
| BMI | body mass index |
| BUN | blood urea nitrogen |
| CAT | catalase |
| CR | creatinine |
| CTA | computed tomography |
| CTA | computed tomography angiography |
| DBD | donor after brain death |
| DCD | donor after cardiac death |
| DD | deceased donors |
| DEG | Differentially expressed genes |
| DGF | delayed graft function |
| DJ-1 | protein deglycase |
| Doxy | doxycycline |
| ECD | expanded criteria donors |
| eGFR | estimated glomerular filtration rate |
| ET-1 | endothelin-1 |
| FA | fatty acid |
| FABP | fatty acid-binding protein |
| FAO | fatty acid oxidation |
| FC | fold change |
| FS | frozen section |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GO | good outcome |
| GPX | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | glutathione |
| GST | glutathione transferase |
| GTP | guanosine triphosphate |
| HMP | Hypothermic machine perfusion |
| HSI | Hyperspectral Imaging |
| ICG | indocyanine green |
| IGF | immediate graft function |
| IGFBP7 | insulin-like growth factor binding protein-7 |
| IL-18 | Interleukin-18 |
| IPK | normothermic isolated perfused kidney |
| IRI | ischemia-reperfusion injury |
| KATs | kynurenine aminotransferases |
| KDPI | Kidney Donor Profile Index |
| KDRI | Kidney Donor Risk Index |
| KIM-1 | kidney injury molecule-1 |
| KMO | kynurenine 3-monooxygenase |
| KTx | Kidney transplantation |
| LD | living donor |
| LDH | lactate dehydrogenase |
| LDKT | living donor kidney transplantation |
| L-FABP | L-type fatty acid binding protein |
| lncRNA | long noncoding RNA |
| MALDI-IMS | Matrix Assisted Laser Desorption/Ionization-Imaging Mass Spectrometry |
| MALDI-TOF-MS | Matrix Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDA | malondialdehyde |
| miRNA/miR | microRNA |
| MMP-2 | matrix metalloproteinase-2 |
| MR | magnetic resonance |
| MRI | magnetic resonance imaging |
| MRS | magnetic resonance spectroscopy |
| MS | mass spectrometry |
| MSI | mass spectrometry imaging |
| mtDNA | mitochondrial DNA |
| NB | needle biopsy |
| NGAL | neutrophil gelatinase-associated lipocalin |
| NMP | normothermic machine perfusion |
| NMR | nuclear magnetic resonance |
| OxPC | oxidized phosphatidylcholine |
| PBMC | peripheral blood mononuclear cells |
| PC | phosphatidylcholine |
| PCK2 | phosphoenol pyruvate carboxykinase |
| PE | phosphatidylethanolamine |
| PET | positron emission tomography |
| PHB | prohibitin |
| pMRI | 31P magnetic resonance imaging |
| PNF | primary nonfunction |
| PRAT | perirenal adipose tissue |
| PS | paraffin sections |
| RGB | Red Green Blue (colour model) |
| RGF | reduced graft function |
| RR | renal resistance |
| RT-PCR | real-time polymerase chain reaction |
| SCD | standard criteria donors |
| SCS | Static cold storage |
| SO | suboptimal outcome |
| SOD | superoxide dismutase |
| SPME | solid-phase microextraction |
| suEVs | small urinary extracellular vesicles |
| SVF | stromal vascular fraction |
| SWATH-MS | sequential window acquisition of all theoretical spectra-mass spectrometry |
| TBARS | thiobarbituric acid reactive substances |
| TCA | tricarboxylic acid |
| TIMP-2 | tissue inhibitor of metalloproteinases-2 |
| TLR4 | Toll-like receptor 4 |
| TUPA | Targeted Urine Proteome Assay |
| UA | uric acid |
| UR | urine replacement |
| URC | urine recirculation |
| WB | wedge biopsy |
| WI | warm ischemia |
| WIT | warm ischemia time |
| XO | hypoxanthine-xanthine oxidase |
Author Contributions
Writing—original draft preparation, N.W.; designing writing—review and editing, N.W., K.Ł., B.B.; funding acquisition, B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by National Science Centre, grant Opus number 2017/27/B/NZ5/01013.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Swanson K.J., Aziz F., Garg N., Mohamed M., Mandelbrot D., Djamali A., Parajuli S. Role of novel biomarkers in kidney transplantation. World J. Transplant. 2020;10:230–255. doi: 10.5500/wjt.v10.i9.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Leeuwen L.L., Spraakman N.A., Brat A., Huang H., Thorne A.M., Bonham S., van Balkom B.W.M., Ploeg R.J., Kessler B.M., Leuvenink H.G.D. Proteomic analysis of machine perfusion solution from brain dead donor kidneys reveals that elevated complement, cytoskeleton and lipid metabolism proteins are associated with 1-year outcome. Transpl. Int. 2021;34:1618–1629. doi: 10.1111/tri.13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGuinness D., Mohammed S., Monaghan L., Wilson P.A., Kingsmore D.B., Shapter O., Stevenson K.S., Coley S.M., Devey L., Kirkpatrick R.B., et al. A molecular signature for delayed graft function. Aging Cell. 2018;17:e12825. doi: 10.1111/acel.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dare A.J., Pettigrew G.J., Saeb-parsy K. Preoperative Assessment of the Deceased-Donor Kidney: From Macroscopic Appearance to Molecular Biomarkers. Transplantation. 2014;97:797–807. doi: 10.1097/01.TP.0000441361.34103.53. [DOI] [PubMed] [Google Scholar]
- 5.Moeckli B., Sun P., Lazeyras F., Morel P., Moll S., Pascual M., Bühler L.H. Evaluation of donor kidneys prior to transplantation: An update of current and emerging methods. Transpl. Int. 2019;32:459–469. doi: 10.1111/tri.13430. [DOI] [PubMed] [Google Scholar]
- 6.Kork F., Rimek A., Andert A., Becker N.J., Heidenhain C., Neumann U.P., Kroy D., Roehl A.B., Rossaint R., Hein M. Visual quality assessment of the liver graft by the transplanting surgeon predicts postreperfusion syndrome after liver transplantation: A retrospective cohort study. BMC Anesthesiol. 2018;18:29. doi: 10.1186/s12871-018-0493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyberg S.L., Matas A.J., Kremers W.K., Thostenson J.D., Larson T.S., Prieto M., Ishitani M.B., Sterioff S., Stegall M.D. Improved scoring system to assess adult donors for cadaver renal transplantation. Am. J. Transplant. 2003;3:715–721. doi: 10.1034/j.1600-6143.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 8.Schold J.D., Kaplan B., Baliga R.S., Meier-Kriesche H.-U. The broad spectrum of quality in deceased donor kidneys. Am. J. Transplant. 2005;5:757–765. doi: 10.1111/j.1600-6143.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 9.Watson C.J.E., Johnson R.J., Birch R., Collett D., Bradley J.A. A simplified donor risk index for predicting outcome after deceased donor kidney transplantation. Transplantation. 2012;93:314–318. doi: 10.1097/TP.0b013e31823f14d4. [DOI] [PubMed] [Google Scholar]
- 10.Rao P.S., Schaubel D.E., Guidinger M.K., Andreoni K.A., Wolfe R.A., Merion R.M., Port F.K., Sung R.S. A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation. 2009;88:231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services Organ Procurement and Transplantation Network: KDPI Calculator. [(accessed on 9 November 2021)]; Available online: https://optn.transplant.hrsa.gov/resources/guidance/kidney-donor-profile-index-kdpi-guide-for-clinicians.
- 12.Dahmen M., Becker F., Pavenstädt H., Suwelack B., Schütte-Nütgen K., Reuter S. Validation of the Kidney Donor Profile Index (KDPI) to assess a deceased donor’s kidneys’ outcome in a European cohort. Sci. Rep. 2019;9:11234. doi: 10.1038/s41598-019-47772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jun H., Yoon H.E., Lee K.W., Lee D.R., Yang J., Ahn C., Han S.Y. Kidney Donor Risk Index Score Is More Reliable Than Kidney Donor Profile Index in Kidney Transplantation From Elderly Deceased Donors. Transplant. Proc. 2020;52:1744–1748. doi: 10.1016/j.transproceed.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Zheng J., Hu X., Ding X., Li Y., Ding C., Tian P., Xiang H., Feng X., Pan X., Yan H., et al. Comprehensive assessment of deceased donor kidneys with clinic al characteristics, pre-implant biopsy histopathology and hypothermic mechanical perfusion parameters is highly predictive of delayed graft function. Ren. Fail. 2020;42:369–376. doi: 10.1080/0886022X.2020.1752716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker W.F., Thistlethwaite J.R., Jr., Ross L.F. Kidney Donor Profile Index (KDPI) Does Not Accurately Predict the Graft Survival of Pediatric Deceased Donor Kidneys. Transplantation. 2016;100:2471–2478. doi: 10.1097/TP.0000000000001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopfer H., Kemény E. Assessment of donor biopsies. Curr. Opin. Organ. Transplant. 2013;18:306–312. doi: 10.1097/MOT.0b013e3283607a6e. [DOI] [PubMed] [Google Scholar]
- 17.Naesens M. Zero-time renal transplant biopsies: A comprehensive review. Transplantation. 2016;100:1425–1439. doi: 10.1097/TP.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 18.Sagasta A., Sánchez-Escuredo A., Oppenheimer F., Paredes D., Musquera M., Campistol J.M., Solé M. Pre-implantation analysis of kidney biopsies from expanded criteria donors: Testing the accuracy of frozen section technique and the adequacy of their assessment by on-call pathologists. Transpl. Int. 2016;29:234–240. doi: 10.1111/tri.12709. [DOI] [PubMed] [Google Scholar]
- 19.Cooper M., Formica R., Friedewald J., Hirose R., O’Connor K., Mohan S., Schold J., Axelrod D., Pastan S. Report of National Kidney Foundation Consensus Conference to Decrease Kidney Discards. Clin. Transplant. 2019;33:e13419. doi: 10.1111/ctr.13419. [DOI] [PubMed] [Google Scholar]
- 20.Traynor C., Saeed A., O’Ceallaigh E., Elbadri A., O’Kelly P., de Freitas D.G., Dorman A.M., Conlon P.J., O’Seaghdha C.M. Pre-transplant histology does not improve prediction of 5-year kidney allograft outcomes above and beyond clinical parameters. Ren. Fail. 2017;39:671–677. doi: 10.1080/0886022X.2017.1363778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yap Y.T., Ho Q.Y., Kee T., Ng C.Y., Chionh C.Y. Impact of pre-transplant biopsy on 5-year outcomes of expanded criteria donor kidney transplantation. Nephrology. 2021;26:70–77. doi: 10.1111/nep.13788. [DOI] [PubMed] [Google Scholar]
- 22.De Vusser K., Lerut E., Kuypers D., Vanrenterghem Y., Jochmans I., Monbaliu D., Pirenne J., Naesens M. The predictive value of kidney allograft baseline biopsies for long-term graft survival. J. Am. Soc. Nephrol. 2013;24:1913–1923. doi: 10.1681/ASN.2012111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips B.L., Kassimatis T., Atalar K., Wilkinson H., Kessaris N., Simmonds N., Hilton R., Horsfield C., Callaghan C.J. Chronic histological changes in deceased donor kidneys at implantation do not predict graft survival: A single-centre retrospective analysis. Transpl. Int. 2019;32:523–534. doi: 10.1111/tri.13398. [DOI] [PubMed] [Google Scholar]
- 24.Liapis H., Gaut J.P., Klein C., Bagnasco S., Kraus E., Farris III A.B., Honsova E., Perkowska-Ptasinska A., David D., Goldberg J., et al. Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies. Am. J. Transplant. 2017;17:140–150. doi: 10.1111/ajt.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall I.E., Parikh C.R., Schröppel B., Weng F.L., Jia Y., Thiessen-Philbrook H., Reese P.P., Doshi M.D. Procurement biopsy findings versus kidney donor risk index for predicting renal allograft survival. Transplant. Direct. 2018;4:e373. doi: 10.1097/TXD.0000000000000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng P., Ding Z., He Y., Zhang J., Wang X., Yang Z. Hypothermic Machine Perfusion Versus Static Cold Storage in Deceased Donor Kidney Transplantation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Artif. Organs. 2019;43:478–489. doi: 10.1111/aor.13364. [DOI] [PubMed] [Google Scholar]
- 27.Peris A., Fulceri G.E., Lazzeri C., Bonizzoli M., Li Marzi V., Serni S., Cirami L., Migliaccio M.L. Delayed graft function and perfusion parameters of kidneys from uncontrolled donors after circulatory death. Perfusion. 2021;36:299–304. doi: 10.1177/0267659120938928. [DOI] [PubMed] [Google Scholar]
- 28.Bissolati M., Gazzetta P.G., Caldara R., Guarneri G., Adamenko O., Giannone F., Mazza M., Maggi G., Tomanin D., Rosati R., et al. Renal Resistance Trend During Hypothermic Machine Perfusion Is More Predictive of Postoperative Outcome Than Biopsy Score: Preliminary Experience in 35 Consecutive Kidney Transplantations. Artif. Organs. 2018;42:714–722. doi: 10.1111/aor.13117. [DOI] [PubMed] [Google Scholar]
- 29.Moers C., Smits J.M., Maathuis M.-H.J., Treckmann J., van Gelder F., Napieralski B.P., van Kasterop-Kutz M., van der Heide J.J.H., Squifflet J.-P., van Heurn E., et al. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2009;360:7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 30.Lindell S.L., Muir H., Brassil J., Mangino M.J. Hypothermic Machine Perfusion Preservation of the DCD Kidney: Machine Effects. J. Transplant. 2013;2013:802618. doi: 10.1155/2013/802618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Deken J., Kocabayoglu P., Moers C. Hypothermic machine perfusion in kidney transplantation. Curr. Opin. Organ. Transplant. 2016;21:294–300. doi: 10.1097/MOT.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 32.Jochmans I., Moers C., Smits J.M., Leuvenink H.G.D., Treckmann J., Paul A., Rahmel A., Squifflet J.P., van Heurn E., Monbaliu D., et al. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. Am. J. Transplant. 2011;11:2214–2220. doi: 10.1111/j.1600-6143.2011.03685.x. [DOI] [PubMed] [Google Scholar]
- 33.Mozes M.F., Skolek R.B., Korf B.C. Use of perfusion parameters in predicting outcomes of machine-preserved kidneys. Transplant. Proc. 2005;37:350–351. doi: 10.1016/j.transproceed.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 34.Bunegin L., Tolstykh G.P., Gelineau J.F., Cosimi A.B., Anderson L.M. Oxygen consumption during oxygenated hypothermic perfusion as a measure of donor organ viability. ASAIO J. 2013;59:427–432. doi: 10.1097/MAT.0b013e318292e865. [DOI] [PubMed] [Google Scholar]
- 35.Patel S.K., Pankewycz O.G., Nader N.D., Zachariah M., Kohli R., Laftavi M.R. Prognostic utility of hypothermic machine perfusion in deceased donor renal transplantation. Transplant. Proc. 2012;44:2207–2212. doi: 10.1016/j.transproceed.2012.07.129. [DOI] [PubMed] [Google Scholar]
- 36.Reticker A., Lichvar A., Walsh M., Gross A.E., Patel S. The Significance and Impact of Screening Preservation Fluid Cultures in Renal Transplant Recipients. Prog. Transplant. 2021;31:40–46. doi: 10.1177/1526924820978608. [DOI] [PubMed] [Google Scholar]
- 37.Corbel A., Ladrière M., Le Berre N., Durin L., Rousseau H., Frimat L., Thilly N., Pulcini C. Microbiological epidemiology of preservation fluids in transplanted kidney: A nationwide retrospective observational study. Clin. Microbiol. Infect. 2020;26:475–484. doi: 10.1016/j.cmi.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Oriol I., Sabe N., Càmara J., Berbel D., Ballesteros M.A., Escudero R., Lopez-Medrano F., Linares L., Len O., Silva J.T., et al. The impact of culturing the organ preservation fluid on solid organ transplantation: A prospective multicenter cohort study. Open Forum Infect. Dis. 2019;6:1–7. doi: 10.1093/ofid/ofz180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu X., Wang R., Peng W., Huang H., Liu G., Yang Q., Zhou J., Zhang X., Lv J.H., Lei W., et al. Incidence, distribution and clinical relevance of microbial contamination of preservation solution in deceased kidney transplant recipients: A retrospective cohort study from China. Clin. Microbiol. Infect. 2019;25:595–600. doi: 10.1016/j.cmi.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 40.Stern S., Bezinover D., Rath P.M., Paul A., Saner F.H. Candida contamination in kidney and liver organ preservation solution: Does it matter? J. Clin. Med. 2021;10:2022. doi: 10.3390/jcm10092022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjekavica I., Novosel L., Rupčić M., Smiljanić R., Muršić M., Duspara V., Lušić M., Perkov D., Hrabak-Paar M., Zidanić M., et al. Radiological imaging in renal transplantation. Acta Clin. Croat. 2018;57:694–712. doi: 10.20471/acc.2018.57.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarier M., Callioglu M., Yuksel Y., Duman E., Emek M., Usta S.S. Evaluation of the Renal Arteries of 2144 Living Kidney Donors Using Computed Tomography Angiography and Comparison with Intraoperative Findings. Urol. Int. 2020;104:637–640. doi: 10.1159/000507796. [DOI] [PubMed] [Google Scholar]
- 43.Al-Adra D.P., Lambadaris M., Barbas A., Li Y., Selzner M., Singh S.K., Famure O., Kim S.J., Ghanekar A. Donor kidney volume measured by computed tomography is a strong predictor of recipient eGFR in living donor kidney transplantation. World J. Urol. 2019;37:1965–1972. doi: 10.1007/s00345-018-2595-x. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez N., Lorenzo A., Chua M., Koyle M.A., Farhat W., Matava C. Real-time kidney graft perfusion monitoring using infrared imaging during pediatric kidney transplantation. J. Pediatr. Urol. 2019;15:222.e1–222.e7. doi: 10.1016/j.jpurol.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 45.Sucher R., Wagner T., Köhler H., Sucher E., Guice H., Recknagel S., Lederer A., Hau H.M., Rademacher S., Schneeberger S., et al. Hyperspectral Imaging (HSI) of Human Kidney Allografts. Ann. Surg. 2020 doi: 10.1097/SLA.0000000000004429. [DOI] [PubMed] [Google Scholar]
- 46.Gerken A.L.H., Nowak K., Meyer A., Weiss C., Krüger B., Nawroth N., Karampinis I., Heller K., Apel H., Reissfelder C., et al. Quantitative Assessment of Intraoperative Laser Fluorescence Angiography with Indocyanine Green Predicts Early Graft Function after Kidney Transplantation. Ann. Surg. 2020 doi: 10.1097/SLA.0000000000004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y.M., Ni Q.Q., Wang Z.J., Chen M.L., Zhang L.J. Multiparametric functional magnetic resonance imaging for evaluating renal allograft injury. Korean J. Radiol. 2019;20:894–908. doi: 10.3348/kjr.2018.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schutter R., Lantinga V.A., Borra R.J.H., Moers C. MRI for diagnosis of post-renal transplant complications: Current state-of-the-art and future perspectives. Magn. Reson. Mater. Physics Biol. Med. 2020;33:49–61. doi: 10.1007/s10334-019-00813-8. [DOI] [PubMed] [Google Scholar]
- 49.Jehn U., Schuette-Nuetgen K., Kentrup D., Hoerr V., Reuter S. Renal allograft rejection: Noninvasive ultrasound- and mri-based diagnostics. Contrast Media Mol. Imaging. 2019;2019:3568067. doi: 10.1155/2019/3568067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pajenda S., Rasul S., Hacker M., Wagner L., Geist B.K. Dynamic 2-deoxy-2[18F] fluoro-D-glucose PET/MRI in human renal allotransplant patients undergoing acute kidney injury. Sci. Rep. 2020;10:8270. doi: 10.1038/s41598-020-65267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jadoul A., Lovinfosse P., Bouquegneau A., Weekers L., Pottel H., Hustinx R., Jouret F. Observer variability in the assessment of renal 18F-FDG uptake in kidney transplant recipients. Sci. Rep. 2020;10:4617. doi: 10.1038/s41598-020-61032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai Y., Li Z., Zuo P., Pfeuffer J., Li Y., Liu F., Liu R. Diagnostic value of renal perfusion in patients with chronic kidney disease using 3D arterial spin labeling. J. Magn. Reson. Imaging. 2017;46:589–594. doi: 10.1002/jmri.25601. [DOI] [PubMed] [Google Scholar]
- 53.Wang W., Yu Y., Li X., Chen J., Zhang Y., Zhang L., Wen J. Early detection of subclinical pathology in patients with stable kidney graft function by arterial spin labeling. Eur. Radiol. 2021;31:2687–2695. doi: 10.1007/s00330-020-07369-5. [DOI] [PubMed] [Google Scholar]
- 54.Bontha S.V., Maluf D.G., Mueller T.F., Mas V.R. Systems Biology in Kidney Transplantation: The Application of Multi-Omics to a Complex Model. Am. J. Transplant. 2017;17:11–21. doi: 10.1111/ajt.13881. [DOI] [PubMed] [Google Scholar]
- 55.Giraud S., Steichen C., Allain G., Couturier P., Labourdette D., Lamarre S., Ameteau V., Tillet S., Hannaert P., Thuillier R., et al. Dynamic transcriptomic analysis of Ischemic Injury in a Porcine Pre-Clinical Model mimicking Donors Deceased after Circulatory Death. Sci. Rep. 2018;8:5986. doi: 10.1038/s41598-018-24282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boissier R., François P., Tellier B.G., Meunier M., Lyonnet L., Simoncini S., Magalon J., Legris T., Arnaud L., Giraudo L., et al. Perirenal Adipose Tissue Displays an Age-Dependent Inflammatory Signature Associated With Early Graft Dysfunction of Marginal Kidney Transplants. Front. Immunol. 2020;11:445. doi: 10.3389/fimmu.2020.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hruba P., Krejcik Z., Dostalova Merkerova M., Klema J., Stranecky V., Slatinska J., Maluskova J., Honsova E., Viklicky O. Molecular Fingerprints of Borderline Changes in Kidney Allografts Are Influenced by Donor Category. Front. Immunol. 2020;11:423. doi: 10.3389/fimmu.2020.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han F., Wan S., Sun Q., Chen N., Li H., Zheng L., Zhang N., Huang Z., Hong L., Sun Q. Donor Plasma Mitochondrial DNA Is Correlated with Posttransplant Renal Allograft Function. Transplantation. 2019;103:2347–2358. doi: 10.1097/TP.0000000000002598. [DOI] [PubMed] [Google Scholar]
- 59.Chen H.-H., Lan Y.-F., Li H.-F., Cheng C.-F., Lai P.-F., Li W.-H., Lin H. Urinary miR-16 transactivated by C/EBPβ reduces kidney function after ischemia/reperfusion-induced injury. Sci. Rep. 2016;6:27945. doi: 10.1038/srep27945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W., Shu L. Upregulation of miR-21 by Ghrelin Ameliorates Ischemia/Reperfusion-Induced Acute Kidney Injury by Inhibiting Inflammation and Cell Apoptosis. DNA Cell Biol. 2016;35:417–425. doi: 10.1089/dna.2016.3231. [DOI] [PubMed] [Google Scholar]
- 61.Song T., Chen M., Rao Z., Qiu Y., Liu J., Jiang Y., Huang Z., Wang X., Lin T. miR-17-92 ameliorates renal ischemia reperfusion injury. Kaohsiung J. Med. Sci. 2018;34:263–273. doi: 10.1016/j.kjms.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y., Wang D., Jin Z. miR-27a suppresses TLR4-induced renal ischemia-reperfusion injury. Mol. Med. Rep. 2019;20:967–976. doi: 10.3892/mmr.2019.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu K., Zheng T., Chen X., Wang H. Bioinformatic analyses of renal ischaemia-reperfusion injury models: Identification of key genes involved in the development of kidney disease. Kidney Blood Press. Res. 2018;43:1898–1907. doi: 10.1159/000496001. [DOI] [PubMed] [Google Scholar]
- 64.Su M., Hu X., Lin J., Zhang L., Sun W., Zhang J., Tian Y., Qiu W. Identification of Candidate Genes Involved in Renal Ischemia/Reperfusion Injury. DNA Cell Biol. 2019;38:256–262. doi: 10.1089/dna.2018.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu L., Mao L., Wu X., Wu T., Liu W., Yang Y., Zhang T., Xu Y. BRG1 regulates endothelial-derived IL-33 to promote ischemia-reperfusion induced renal injury and fibrosis in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:2551–2561. doi: 10.1016/j.bbadis.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 66.Cippà P.E., Sun B., Liu J., Chen L., Naesens M., McMahon A.P. Transcriptional trajectories of human kidney injury progression. JCI insight. 2018;3:e123151. doi: 10.1172/jci.insight.123151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu X., Su M., Lin J., Zhang L., Sun W., Zhang J., Tian Y., Qiu W. Corin is downregulated in renal ischemia/reperfusion injury and is associated with delayed graft function after kidney transplantation. Dis. Markers. 2019;2019:9429323. doi: 10.1155/2019/9429323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khalid U., Newbury L.J., Simpson K., Jenkins R.H., Bowen T., Bates L., Sheerin N.S., Chavez R., Fraser D.J. A urinary microRNA panel that is an early predictive biomarker of delayed graft function following kidney transplantation. Sci. Rep. 2019;9:3584. doi: 10.1038/s41598-019-38642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J., Li X., Wu X., Wang Z., Zhang C., Cao G., Yan T. Expression Profiling of Exosomal miRNAs Derived from the Peripheral Blood of Kidney Recipients with DGF Using High-Throughput Sequencing. Biomed. Res. Int. 2019;2019:1759697. doi: 10.1155/2019/1759697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mirzakhani M., Mohammadnia-Afrouzi M., Shahbazi M., Mirhosseini S.A., Hosseini H.M., Amani J. The exosome as a novel predictive/diagnostic biomarker of rejection in the field of transplantation. Clin. Immunol. 2019;203:134–141. doi: 10.1016/j.clim.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 71.Milhoransa P., Montanari C.C., Montenegro R., Manfro R.C. Micro RNA 146a-5p expression in Kidney transplant recipients with delayed graft function. J. Bras. Nefrol. 2019;41:242–251. doi: 10.1590/2175-8239-jbn-2018-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zmonarski S., Madziarska K., Banasik M., Mazanowska O., Magott-Procelewska M., Hap K., Krajewska M. Expression of PBMC TLR4 in Renal Graft Recipients Who Experienced Delayed Graft Function Reflects Dynamic Balance Between Blood and Tissue Compartments and Helps Select a Problematic Patient. Transplant. Proc. 2018;50:1744–1749. doi: 10.1016/j.transproceed.2018.02.134. [DOI] [PubMed] [Google Scholar]
- 73.Bi H., Zhang M., Wang J., Long G. The mRNA landscape profiling reveals potential biomarkers associated with acute kidney injury AKI after kidney transplantation. PeerJ. 2020;8:e10441. doi: 10.7717/peerj.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koo T.Y., Jeong J.C., Lee Y., Ko K.-P., Lee K.-B., Lee S., Park S.J., Park J.B., Han M., Lim H.J., et al. Pre-transplant evaluation of donor urinary biomarkers can predict reduced graft function after deceased donor kidney transplantation. Medicine. 2016;95:e3076. doi: 10.1097/MD.0000000000003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reese P.P., Hall I.E., Weng F.L., Schröppel B., Doshi M.D., Hasz R.D., Thiessen-Philbrook H., Ficek J., Rao V., Murray P., et al. Associations between deceased-donor urine injury biomarkers and kidney transplant outcomes. J. Am. Soc. Nephrol. 2016;27:1534–1543. doi: 10.1681/ASN.2015040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schröppel B., Heeger P., Thiessen-Philbrook H., Hall I.E., Doshi M.D., Weng F.L., Reese P.P., Parikh C.R. Donor Urinary C5a Levels Independently Correlate with Posttransplant Delayed Graft Function. Transplantation. 2019;103:e29–e35. doi: 10.1097/TP.0000000000002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mansour S.G., Puthumana J., Reese P.P., Hall I.E., Doshi M.D., Weng F.L., Schröppel B., Thiessen-Philbrook H., Bimali M., Parikh C.R. Associations Between Deceased-Donor Urine MCP-1 and Kidney Transplant Outcomes. Kidney Int. Rep. 2017;2:749–758. doi: 10.1016/j.ekir.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mezzolla V., Pontrelli P., Fiorentino M., Stasi A., Franzin R., Rascio F., Grandaliano G., Stallone G., Infante B., Gesualdo L., et al. Emerging biomarkers of delayed graft function in kidney transplantation. Transplant. Rev. 2021;35:100629. doi: 10.1016/j.trre.2021.100629. [DOI] [PubMed] [Google Scholar]
- 79.Kostidis S., Bank J.R., Soonawala D., Nevedomskaya E., van Kooten C., Mayboroda O.A., de Fijter J.W. Urinary metabolites predict prolonged duration of delayed graft function in DCD kidney transplant recipients. Am. J. Transplant. 2019;19:110–122. doi: 10.1111/ajt.14941. [DOI] [PubMed] [Google Scholar]
- 80.Braun F., Rinschen M., Buchner D., Bohl K., Plagmann I., Bachurski D., Späth M.R., Antczak P., Göbel H., Klein C., et al. The proteomic landscape of small urinary extracellular vesicles during kidney transplantation. J. Extracell. Vesicles. 2020;10:e12026. doi: 10.1002/jev2.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L., Li N., He C., Huang W., Fan X., Zhong Z., Wang Y., Ye Q. Proteomic analysis of differentially expressed proteins in kidneys of brain dead rabbits. Mol. Med. Rep. 2017;16:215–223. doi: 10.3892/mmr.2017.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Erp A.C., Rebolledo R.A., Hoeksma D., Jespersen N.R., Ottens P.J., Nørregaard R., Pedersen M., Laustsen C., Burgerhof J.G.M., Wolters J.C., et al. Organ-specific responses during brain death: Increased aerobic metabolism in the liver and anaerobic metabolism with decreased perfusion in the kidneys. Sci. Rep. 2018;8:4405. doi: 10.1038/s41598-018-22689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang H., Van Dullemen L.F.A., Akhtar M.Z., Faro M.-L.L., Yu Z., Valli A., Dona A., Thézénas M.-L., Charles P.D., Fischer R., et al. Proteo-metabolomics reveals compensation between ischemic and non-injured contralateral kidneys after reperfusion. Sci. Rep. 2018;8:8539. doi: 10.1038/s41598-018-26804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malagrino P.A., Venturini G., Yogi P.S., Dariolli R., Padilha K., Kiers B., Gois T.C., Cardozo K.H.M., Carvalho V.M., Salgueiro J.S., et al. Proteome analysis of acute kidney injury—Discovery of new predominantly renal candidates for biomarker of kidney disease. J. Proteomics. 2017;151:66–73. doi: 10.1016/j.jprot.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 85.Moser M.A.J., Sawicka K., Sawicka J., Franczak A., Cohen A., Bil-Lula I., Sawicki G. Protection of the transplant kidney during cold perfusion with doxycycline: Proteomic analysis in a rat model. Proteome Sci. 2020;18:3. doi: 10.1186/s12953-020-00159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weissenbacher A., Huang H., Surik T., Faro M.L.L., Ploeg R.J., Coussios C.C., Friend P.J., Kessler B.M. Urine recirculation prolongs normothermic kidney perfusion via more optimal metabolic homeostasis—a proteomics study. Am. J. Transplant. 2020;21:1740–1753. doi: 10.1111/ajt.16334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams K.R., Colangelo C.M., Hou L., Chung L., Belcher J.M., Abbott T., Hall I.E., Zhao H., Cantley L.G., Parikh C.R. Use of a Targeted Urine Proteome Assay (TUPA) to identify protein biomarkers of delayed recovery after kidney transplant. Proteomics Clin. Appl. 2017;11:1600132. doi: 10.1002/prca.201600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lacquaniti A., Caccamo C., Salis P., Chirico V., Buemi A., Cernaro V., Noto A., Pettinato G., Santoro D., Bertani T., et al. Delayed graft function and chronic allograft nephropathy: Diagnostic and prognostic role of neutrophil gelatinase-associated lipocalin. Biomarkers. 2016;21:371–378. doi: 10.3109/1354750X.2016.1141991. [DOI] [PubMed] [Google Scholar]
- 89.Bank J.R., Ruhaak R., Soonawala D., Mayboroda O., Romijn F.P., Van Kooten C., Cobbaert C.M., De Fijter J.W. Urinary TIMP-2 Predicts the Presence and Duration of Delayed Graft Function in Donation after Circulatory Death Kidney Transplant Recipients. Transplantation. 2019;103:1014–1023. doi: 10.1097/TP.0000000000002472. [DOI] [PubMed] [Google Scholar]
- 90.Van Erp A.C., Qi H., Jespersen N.R., Hjortbak M.V., Ottens P.J., Wiersema-Buist J., Nørregaard R., Pedersen M., Laustsen C., Leuvenink H.G.D., et al. Organ-specific metabolic profiles of the liver and kidney during brain death and afterwards during normothermic machine perfusion of the kidney. Am. J. Transplant. 2020;20:2425–2436. doi: 10.1111/ajt.15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nielsen P.M., Qi H., Bertelsen L.B., Laustsen C. Metabolic reprogramming associated with progression of renal ischemia reperfusion injury assessed with hyperpolarized [1-13C]pyruvate. Sci. Rep. 2020;10:8915. doi: 10.1038/s41598-020-65816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chihanga T., Ma Q., Nicholson J.D., Ruby H.N., Edelmann R.E., Devarajan P., Kennedy M.A. NMR spectroscopy and electron microscopy identification of metabolic and ultrastructural changes to the kidney following ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2018;314:F154–F166. doi: 10.1152/ajprenal.00363.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stryjak I., Warmuzińska N., Bogusiewicz J., Łuczykowski K., Bojko B. Monitoring of the influence of long-term oxidative stress and ischemia on the condition of kidneys using solid-phase microextraction chemical biopsy coupled with liquid chromatography–high-resolution mass spectrometry. J. Sep. Sci. 2020;43:1867–1878. doi: 10.1002/jssc.202000032. [DOI] [PubMed] [Google Scholar]
- 94.Stryjak I., Warmuzińska N., Łuczykowski K., Hamar M., Urbanellis P., Wojtal E., Masztalerz M., Selzner M., Włodarczyk Z., Bojko B. Using a chemical biopsy for graft quality assessment. J. Vis. Exp. 2020:e60946. doi: 10.3791/60946. [DOI] [PubMed] [Google Scholar]
- 95.Zheng X., Zhang A., Binnie M., McGuire K., Webster S.P., Hughes J., Howie S.E.M., Mole D.J. Kynurenine 3-monooxygenase is a critical regulator of renal ischemia–reperfusion injury. Exp. Mol. Med. 2019;51:1–14. doi: 10.1038/s12276-019-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beier U.H., Hartung E.A., Concors S., Hernandez P.T., Wang Z., Perry C., Baur J.A., Denburg M.R., Hancock W.W., Gade T.P., et al. Tissue metabolic profiling shows that saccharopine accumulates during renal ischemic-reperfusion injury, while kynurenine and itaconate accumulate in renal allograft rejection. Metabolomics. 2021;16:65. doi: 10.1007/s11306-020-01682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rao S., Walters K.B., Wilson L., Chen B., Bolisetty S., Graves D., Barnes S., Agarwal A., Kabarowski J.H. Early lipid changes in acute kidney injury using SWATH lipidomics coupled with MALDI tissue imaging. Am. J. Physiol. Ren. Physiol. 2016;310:F1136–F1147. doi: 10.1152/ajprenal.00100.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Solati Z., Edel A.L., Shang Y., Karmin O., Ravandi A. Oxidized phosphatidylcholines are produced in renal ischemia reperfusion injury. PLoS ONE. 2018;13:e0195172. doi: 10.1371/journal.pone.0195172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Smaalen T.C., Ellis S.R., Mascini N.E., Siegel T.P., Cillero-Pastor B., Hillen L.M., van Heurn L.W.E., Peutz-Kootstra C.J., Heeren R.M.A. Rapid Identification of Ischemic Injury in Renal Tissue by Mass-Spectrometry Imaging. Anal. Chem. 2019;91:3575–3581. doi: 10.1021/acs.analchem.8b05521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wijermars L.G.M., Bakker J.A., de Vries D.K., van Noorden C.J.F., Bierau J., Kostidis S., Mayboroda O.A., Tsikas D., Schaapherder A.F., Lindeman J.H.N. The hypoxanthine-xanthine oxidase axis is not involved in the initial phase of clinical transplantation-related ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2017;312:F457–F464. doi: 10.1152/ajprenal.00214.2016. [DOI] [PubMed] [Google Scholar]
- 101.Lindeman J.H., Wijermars L.G., Kostidis S., Mayboroda O.A., Harms A.C., Hankemeier T., Bierau J., Gupta K.B.S.S., Giera M., Reinders M.E., et al. Results of an explorative clinical evaluation suggest immediate and persistent post-reperfusion metabolic paralysis drives kidney ischemia reperfusion injury. Kidney Int. 2020;98:1476–1488. doi: 10.1016/j.kint.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 102.Jochmans I., Brat A., Davies L., Hofker H.S., van de Leemkolk F.E.M., Leuvenink H.G.D., Knight S.R., Pirenne J., Ploeg R.J. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet. 2020;396:1653–1662. doi: 10.1016/S0140-6736(20)32411-9. [DOI] [PubMed] [Google Scholar]
- 103.Hosgood S.A., Hoff M., Nicholson M.L. Treatment of transplant kidneys during machine perfusion. Transpl. Int. 2021;34:224–232. doi: 10.1111/tri.13751. [DOI] [PubMed] [Google Scholar]
- 104.Resch T., Cardini B., Oberhuber R., Weissenbacher A., Dumfarth J., Krapf C., Boesmueller C., Oefner D., Grimm M., Schneeberger S. Transplanting Marginal Organs in the Era of Modern Machine Perfusion and Advanced Organ Monitoring. Front. Immunol. 2020;11:631. doi: 10.3389/fimmu.2020.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hamar M., Selzner M. Ex-vivo machine perfusion for kidney preservation. Curr. Opin. Organ. Transplant. 2018;23:369–374. doi: 10.1097/MOT.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 106.Coskun A., Baykal A.T., Kazan D., Akgoz M., Senal M.O., Berber I., Titiz I., Bilsel G., Kilercik H., Karaosmanoglu K., et al. Proteomic analysis of kidney preservation solutions prior to renal transplantation. PLoS ONE. 2016;11:e0168755. doi: 10.1371/journal.pone.0168755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van Balkom B.W.M., Gremmels H., Ooms L.S.S., Toorop R.J., Dor F.J.M.F., de Jong O.G., Michielsen L.A., de Borst G.J., De Jager W., Abrahams A.C., et al. Proteins in preservation fluid as predictors of delayed graft function in kidneys from donors after circulatory death. Clin. J. Am. Soc. Nephrol. 2017;12:817–824. doi: 10.2215/CJN.10701016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Z., Yang H., Zhao C., Wei J., Wang J., Han Z., Tao J., Xu Z., Ju X., Tan R., et al. Proton nuclear magnetic resonance (1H-NMR)-based metabolomic evaluation of human renal allografts from donations after circulatory death. Med. Sci. Monit. 2017;23:5472–5479. doi: 10.12659/MSM.905168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nath J., Smith T.B., Patel K., Ebbs S.R., Hollis A., Tennant D.A., Ludwig C., Ready A.R. Metabolic differences between cold stored and machine perfused porcine kidneys: A 1H NMR based study. Cryobiology. 2017;74:115–120. doi: 10.1016/j.cryobiol.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 110.Adani G.L., Pravisani R., Crestale S., Baccarani U., Scott C.A., D’Alì L., Demaglio G., Tulissi P., Vallone C., Isola M., et al. Effects of delayed hypothermic machine perfusion on kidney grafts with a preliminary period of static cold storage and a total cold ischemia time of over 24 hours. Ann. Transplant. 2020;25:e918997. doi: 10.12659/AOT.918997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Foucher Y., Fournier M.-C., Legendre C., Morelon E., Buron F., Girerd S., Ladrière M., Mourad G., Garrigue V., Glotz D., et al. Comparison of machine perfusion versus cold storage in kidney transplant recipients from expanded criteria donors: A cohort-based study. Nephrol. Dial. Transplant. 2020;35:1051–1059. doi: 10.1093/ndt/gfz175. [DOI] [PubMed] [Google Scholar]
- 112.Tejchman K., Sierocka A., Kotowski M., Zair L., Pilichowska E., Ostrowski M., Sieńko J. Acid-Base Balance Disorders During Kidney Preservation in Cold Ischemia. Transplant. Proc. 2020;52:2036–2042. doi: 10.1016/j.transproceed.2020.01.099. [DOI] [PubMed] [Google Scholar]
- 113.He N., Li J.-H., Jia J.-J., Xu K.-D., Zhou Y.-F., Jiang L., Lu H.-H., Yin S.-Y., Xie H.-Y., Zhou L., et al. Hypothermic Machine Perfusion’s Protection on Porcine Kidney Graft Uncovers Greater Akt-Erk Phosphorylation. Transplant. Proc. 2017;49:1923–1929. doi: 10.1016/j.transproceed.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 114.Patel K., Smith T.B., Neil D.A.H., Thakker A., Tsuchiya Y., Higgs E.B., Hodges N.J., Ready A.R., Nath J., Ludwig C. The Effects of Oxygenation on Ex Vivo Kidneys Undergoing Hypothermic Machine Perfusion. Transplantation. 2019;103:314–322. doi: 10.1097/TP.0000000000002542. [DOI] [PubMed] [Google Scholar]
- 115.Moser M.A.J., Sawicka K., Arcand S., O’Brien P., Luke P., Beck G., Sawicka J., Cohen A., Sawicki G. Proteomic analysis of perfusate from machine cold perfusion of transplant kidneys: Insights into protection from injury. Ann. Transplant. 2017;22:730–739. doi: 10.12659/AOT.905347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gómez-Dos-Santos V., Ramos-Muñoz E., García-Bermejo M.L., Ruiz-Hernández M., Rodríguez-Serrano E.M., Saiz-González A., Martínez-Perez A., Burgos-Revilla F.J. MicroRNAs in Kidney Machine Perfusion Fluid as Novel Biomarkers for Graft Function. Normalization Methods for miRNAs Profile Analysis. Transplant. Proc. 2019;51:307–310. doi: 10.1016/j.transproceed.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 117.Tejchman K., Sierocka A., Kotfis K., Kotowski M., Dolegowska B., Ostrowski M., Sienko J. Assessment of oxidative stress markers in hypothermic preservation of transplanted kidneys. Antioxidants. 2021;10:1263. doi: 10.3390/antiox10081263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Longchamp A., Klauser A., Songeon J., Agius T., Nastasi A., Ruttiman R., Moll S., Meier R.P.H., Buhler L., Corpataux J.-M., et al. Ex Vivo Analysis of Kidney Graft Viability Using 31P Magnetic Resonance Imaging Spectroscopy. Transplantation. 2020;104:1825–1831. doi: 10.1097/TP.0000000000003323. [DOI] [PubMed] [Google Scholar]
- 119.Van Smaalen T.C., Beurskens D.M.H., Hoogland E.R.P., Winkens B., Christiaans M.H.L., Reutelingsperger C.P., van Heurn L.W.E., Nicolaes G.A.F. Presence of Cytotoxic Extracellular Histones in Machine Perfusate of Donation after Circulatory Death Kidneys. Transplantation. 2017;101:e93–e101. doi: 10.1097/TP.0000000000001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaths J.M., Echeverri J., Chun Y.M., Cen J.Y., Goldaracena N., Linares I., Dingwell L.S., Yip P.M., John R., Bagli D., et al. Continuous Normothermic Ex Vivo Kidney Perfusion Improves Graft Function in Donation after Circulatory Death Pig Kidney Transplantation. Transplantation. 2017;101:754–763. doi: 10.1097/TP.0000000000001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tetschke F., Markgraf W., Gransow M., Koch S., Thiele C., Kulcke A., Malberg H. Hyperspectral imaging for monitoring oxygen saturation levels during normothermic kidney perfusion. J. Sensors Sens. Syst. 2016;5:313–318. doi: 10.5194/jsss-5-313-2016. [DOI] [Google Scholar]
- 122.Markgraf W., Feistel P., Thiele C., Malberg H. Algorithms for mapping kidney tissue oxygenation during normothermic machine perfusion using hyperspectral imaging. Biomed. Tech. 2018;63:557–566. doi: 10.1515/bmt-2017-0216. [DOI] [PubMed] [Google Scholar]
- 123.Mariager C.Ø., Hansen E.S.S., Bech S.K., Munk A., Kjærgaard U., Lyhne M.D., Søberg K., Nielsen P.F., Ringgaard S., Laustsen C. Graft assessment of the ex vivo perfused porcine kidney using hyperpolarized [1-13C]pyruvate. Magn. Reson. Med. 2020;84:2645–2655. doi: 10.1002/mrm.28363. [DOI] [PubMed] [Google Scholar]
- 124.Hosgood S.A., Nicholson M.L. An assessment of urinary biomarkers in a series of declined human kidneys measured during ex vivo normothermic kidney perfusion. Transplantation. 2017;101:2120–2125. doi: 10.1097/TP.0000000000001504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.