Abstract
A hospital cafeteria-associated outbreak of gastroenteritis due to Salmonella enterica serotype Infantis was retrospectively evaluated using modified repetitive element PCR (rep-PCR) fingerprinting with the ERIC2 and BOXA1R primers and computer-assisted gel analysis and dendrogram construction. Rep-PCR yielded objective between-cycler, same-strain similarity values of from 92% (composite fingerprints) to 96% (ERIC2 fingerprints). The 70 Salmonella isolates (which included 19 serotype Infantis isolates from the hospital outbreak, 10 other serotype Infantis isolates, and 41 isolates representing 14 other serotypes) were resolved well to the serotype level with each of the three fingerprint types (ERIC2, BOXA1R, and composite). Rep-PCR typing uncovered several historical serotyping errors and provided presumptive serotype assignments for other isolates with incomplete or undetermined serotypes. Analysis of replicate fingerprints for each isolate, as generated on two different thermal cyclers, indicated that most of the seeming subserotype discrimination noted in single-cycler dendrograms actually represented assay variability, since it was not reproducible in combined-cycler dendrograms. Rep-PCR typing, which would have been able to identify the presence of the hospital-associated serotype Infantis outbreak after the second outbreak isolate, could be used as a simple surrogate for serotyping by clinical microbiology laboratories that are equipped for diagnostic PCR.
Salmonella enterica is a major endemic and epidemic cause of gastrointestinal and extraintestinal infections worldwide (12). In industrialized nations, subspecific typing of Salmonella isolates is fundamental to the detection and investigation of point-source disease outbreaks, which can involve food-borne, water-borne, zoonotic, and person-to-person transmission of the pathogen (9, 12, 28, 30, 31). The traditional subspecific typing method for Salmonella, serotyping (13), requires specialized skills and reagents, and hence is largely restricted to central reference laboratories to which Salmonella isolates must be sent from the primary clinical microbiology laboratories. Similar limitations, plus a possible need for expensive equipment, also apply to the various advanced typing methods that have been used to subtype Salmonella isolates below the serotype level, including phage typing, ribotyping, plasmid profiling, conventional restriction endonuclease analysis, and macrorestriction analysis by pulsed-field gel electrophoresis (PFGE) (1, 9, 14, 23–25, 27, 38). A more universally available subspecific and subserotype typing method for Salmonella, if affordable and reliable, could facilitate the evaluation of Salmonella isolates.
Amplification fingerprinting, which is fairly simple to perform and has been used to subtype diverse bacterial species (33), has been explored to a limited extent as a molecular typing method for Salmonella, with mixed results (4, 7, 15, 19–22, 34). Although some investigators have reported discrimination of Salmonella at the subserotype level (4, 15, 19, 20, 22, 24), others have found resolution limited to the serotype level, with some serotypes not differentiated (21, 34), or have found no serotype specificity at all (7). Reproducibility, although not rigorously assessed, has been noted in several studies to be somewhat or highly problematical (6, 7, 22, 34).
Repetitive-element PCR (rep-PCR) is an amplification fingerprinting method that uses primers specific for defined repeat elements of uncertain function, e.g., BOX elements and enterobacterial repeat intergenic consensus (ERIC) sequences, that are broadly conserved among diverse gram-positive and gram-negative bacteria and are dispersed in multiple copies about the genome (35, 36). We recently showed that rep-PCR exhibits a high degree of day-to-day and cycler-to-cycler reproducibility with both Salmonella enterica and Escherichia coli when performed using annealing temperatures up to 23°C higher than are customarily used with this technique (16, 17). For Salmonella, 12 isolates representing 11 different serotypes were well resolved according to serotype, evidence suggesting that with modified amplification conditions rep-PCR might provide a molecular surrogate for conventional Salmonella serotyping (16).
We undertook the present study to further define the potential utility of rep-PCR as a subspecific typing method for Salmonella. Specifically, we sought to retrospectively determine whether rep-PCR would have been able to identify a serologically confirmed outbreak of gastrointestinal infections due to serotype Infantis that occurred at the Minneapolis VA Medical Center (MVAMC) from a point source within the hospital cafeteria shortly before the inception of the present study. In the process, we objectively assessed the reproducibility of rep-PCR typing for Salmonella and the ability of rep-PCR to resolve Salmonella isolates according to serotype.
MATERIALS AND METHODS
Description of hospital outbreak.
In November 1996, the MVAMC Epidemiology Section learned that four cases of gastrointestinal salmonellosis, confirmed by the Minnesota Department of Health (MDH) as involving serotype Infantis, had occurred among MVAMC outpatients. All four patients had had MVAMC clinic appointments on the same day.
The Epidemiology Section initiated an investigation. A random sample of clinic patients seen on the day in question (onset date) were contacted by telephone and surveyed. A convenience sample of employees who had taken sick leave in the 5 days after the onset date were contacted. All VA Canteen Service (VCS) food-handling employees were interviewed and cultured. In addition, employees and volunteers who were at the MVAMC on the onset date were notified that if they had symptoms of diarrhea, nausea, vomiting, or fever since that date, they should contact the Epidemiology Section. All who had illness consistent with the case definition were asked to provide a stool specimen.
Overall, 331 interviews were conducted and eight additional cases were identified. No specific food item was identified, but foods served from the VCS steam table appeared to be involved. The MVAMC Nutrition and Food Service conducted an internal inspection, and a multidisciplinary task force was convened to review institutional food hygiene. No problems were identified.
Although the outbreak appeared to be limited, in January 1997, the MVAMC Epidemiology Service learned of two new cases of salmonellosis (later typed by the MDH as serotype Infantis) that had occurred in persons who had eaten at the VCS in December 1996. The first was an employee who had eaten a turkey dinner, whereas the second was a visitor who recalled having eaten a turkey dinner 4 days after the first patient. The turkey from these dinners was served from the steam table.
The investigation was reopened,and the MDH was recontacted. All VCS foodhandlers were again interviewed and cultured. All employees and others associated with the Medical Center were requested to report their symptoms to the Epidemiology Section. Additional interviews were conducted with 54 VCS and MVAMC employees and persons with gastrointestinal illness who had concerns about possible exposure. Twelve further cases were identified.
Because of an apparent association with the VCS steam table, the steam table was closed, and environmental cultures were obtained from it. During the culturing, a laminated plastic cutting board was noted to have worn edges and visible seams, which were specifically cultured. The board had just been washed in a unit that had been inspected and found to have intact operating systems and adequate water temperatures. One of the cutting board cultures grew S. enterica serotype Infantis. After replacement of all cutting boards and further review of cleaning, sanitation, and monitoring, the steam table was reopened. There have been no further episodes of salmonellosis due to serotype Infantis at the MVAMC.
Strains.
The study population comprised 70 clinical isolates of S. enterica. Of these, 19 isolates were serotype Infantis, from the MVAMC outbreak of 1996 to 1997, all of which had been isolated in the MVAMC clinical microbiology laboratory by standard methods (18). Another 10 Infantis isolates, which were provided by the MDH, had been isolated at other medical centers. For comparison with the Infantis isolates were 41 non-Infantis isolates of S. enterica. These represented all available non-Infantis S. enterica isolates from the MVAMC from 1973 to the inception of the study (1997). According to historical serotyping results, the serotypes represented among the non-Infantis isolates, in descending order of frequency (number of isolates; year of isolation), included Heidelberg (11; 1985 to 1992), Typhimurium (seven; 1983 to 1994), Enteritidis (six; 1973 to 1994), Ohio (two; 1982 and 1984), “Salmonella sp.” (two; serogroup D and serogroup B; both 1973); Newport (two; 1991 and 1993), and one isolate each of London (1982), Hadar (1985), Havana (1987), “Mbandaka” (1990) (previously shown to actually be serotype Tennessee [16]), Saintpaul (1990), Tennessee (1990), Glostrup (1991), Agona (1991), “serotype undetermined (rough variant)” (1991), Java (1992), and “subgroup I” (1996). Within the molecular laboratory, isolates were labeled only with code numbers, which effectively blinded the operator to their serotype identity.
rep-PCR fingerprinting.
Template DNA was purified from bacteria representing a single colony of each isolate after overnight growth in shaking broth by using a commercial kit, as described (16, 17). Fingerprints were generated for each isolate from the same template DNA preparation on two different thermal cyclers using (separately) both the ERIC2 and BOXA1R primers (36). Amplification was done using Ready to Go PCR beads (Pharmacia; Piscataway, N.J.). Cycling parameters, which were as previously described, included an initial 10-cycle, 5°C “touchdown” routine beginning at an annealing temperature of 75°C, leading to a final 25-cycle plateau phase during which the annealing temperature was 70°C (16). PCR products were visualized by agarose gel electrophoresis and ethidium bromide staining.
Image analysis and dendrogram construction.
Gel images were captured and analyzed by using a computerized video image analysis system (Gel Doc and Molecular Analyst Fingerprinting Bio-Rad, Hercules, Calif.), as described (16, 17). Molecular weight standards were included in each gel to allow normalization of gel images for valid between-gel comparisons of fingerprints. If for a particular isolate one or more of the initial replicate fingerprints appeared by inspection to be unsuitable for analysis (as occurred for three isolates), repeat PCR was done using either the same template DNA preparation or a new DNA preparation from the same isolate, at the operator's discretion.
Once suitable fingerprints were available for all isolates, these were analyzed quantitatively as analog densitometric scans of gel tracks, which were digitally compared with each other in a pairwise fashion by using Pearson's correlation coefficient (16). This analysis was done strictly by computer, without subjective operator input, which precluded any contribution of operator bias to the results. ERIC2 and BOXA1R fingerprints were analyzed separately and were also digitally combined in a head-to-toe fashion to create a “virtual” composite fingerprint for each isolate, which was then analyzed in the same manner as the individual single-primer fingerprints (16). For each isolate, paired fingerprints of the same type (ERIC2, BOXA1R, or composite) from the two cyclers were then compared with each other to quantitatively assess cycler-to-cycler reproducibility. If the same-strain similarity for any pair of fingerprints was <85% (as occurred for nine isolates), a new round of fingerprints were generated on one of the cyclers using the original template DNA preparation. After substitution of the replacement fingerprints, pairwise comparisons between all fingerprints of a particular type for the 70 isolates were used to construct similarity matrices. One matrix was constructed for each fingerprint type for fingerprints from cycler A alone (n = 70), one matrix per fingerprint type for fingerprints from cycler B alone (n = 70), and one matrix per fingerprint type for fingerprints from both cyclers (n = 140). Dendrograms were then inferred from the resulting (nine) similarity matrices according to the unweighted pair group method with averaging (UPGMA) (16, 29).
In the combined-cycler dendrograms (not shown), an isolate was considered to be resolved according to serotype if its replicate fingerprints from cyclers A and B were placed together either as nearest neighbors to one another or as part of a cluster that contained only representatives of the same serotype. An isolate was considered to be fully resolved if its replicate fingerprints from cyclers A and B were placed together as nearest neighbors, without interposition of fingerprints from another isolate, regardless of serotype. A serotype was considered to be resolved if the minimal cluster that included all representatives of the serotype contained only representatives of that serotype. In the single-cycler dendrograms, similar definitions (but based on a single representative of each isolate) were used for resolution of isolates according to serotype and for resolution of serotypes. Since there was only one fingerprint of each type for each isolate per cycler, in single-cycler dendrograms resolution of individual isolates could not be assessed.
Initial and confirmatory serotyping.
Historical and current serotypes were identified by conventional methods (11).
PFGE.
XbaI macrorestriction analysis of genomic DNA was done for selected isolates by PFGE according to a standardized protocol (8). Newly determined profiles were designated as indistinguishable from, similar to, or unrelated to previously encountered profiles of diverse S. enterica isolates as maintained in a computerized database by the MDH based on the extent of correspondence with respect to individual bands (32). PFGE profiles were compared by using the Jaccard coefficient and Molecular Analyst Fingerprinting software (Bio-Rad). DNA patterns were evaluated with a 1% molecular weight tolerance, and matches were confirmed visually.
Statistical analysis.
Paired comparisons of similarity scores between different rep-PCR fingerprint types and between the initial and the revised PCR fingerprints for a particular fingerprint type were evaluated using a two-tailed paired t test.
RESULTS
Reproducibility of rep-PCR fingerprints.
In reproducibility analyses based on the initial fingerprint data set, mean same-strain similarity values were >90% for each fingerprint type, with ERIC2 fingerprints exhibiting significantly greater overall reproducibility than BOX or composite fingerprints (Table 1). For nine isolates, one or more paired fingerprint comparisons (all of which involved BOX and/or composite fingerprints) exhibited a similarity score of <85%, with several even <80% (Table 1). Substitution of new fingerprints from one cycler for these nine isolates (revised data set) left no isolates with a similarity value of <80% for any fingerprint type and yielded slightly (ERIC and composite) or significantly (BOX) improved overall mean similarity values (Table 1).
TABLE 1.
Reproducibility of duplicate rep-PCR fingerprints for 70 isolates of S. enterica before (initial dataset) and after (revised dataset) replacement of discrepant fingerprints
| Fingerprint type | Initial dataset
|
Revised dataset
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean % similarity (SD) | No. of isolates with indicated % similaritya
|
Mean % similarity (SD) | No. of isolates with indicated % similaritya
|
|||||||
| >90% | 85–90% | 80–85% | <80% | >90% | 85–90% | 80–85% | <80% | |||
| ERIC2 | 96.2 (2.2)bc | 99 | 1 | 0 | 0 | 96.4 (2.1)cd | 99 | 1 | 0 | 0 |
| BOXAIR | 92.5 (5.3)be | 82 | 12 | 3 | 3 | 93.2 (3.8)de | 85 | 14 | 1 | 0 |
| Composite | 91.3 (5.0)bf | 79 | 17 | 2 | 2 | 92.0 (3.4)df | 81 | 16 | 3 | 0 |
Based on Pearson's correlation coefficient analysis of paired fingerprints for the same isolate as generated on different cyclers.
With the initial dataset, for ERIC2 versus either BOXAIR or composite fingerprints, P < 0.001; for BOXAIR versus composite fingerprints, P = 0.07.
With ERIC2 fingerprints, for the initial versus the revised dataset, P > 0.10.
With the revised dataset, for ERIC2 versus either BOXAIR or composite fingerprints, P < 0.001; for BOXAIR versus composite fingerprints, P = 0.004.
With BOXAIR fingerprints, for the initial versus the revised dataset, P = 0.019.
With composite fingerprints, for the initial versus the revised dataset, P > 0.10.
Dendrogram analysis of rep-PCR fingerprints.
Using the revised fingerprint data set for the 70 isolates (420 fingerprints total), three dendrograms were constructed for each fingerprint type (ERIC2, BOXA1R, and composite), including two single-cycler dendrograms, i.e., one each for cycler A (Fig. 1, 2, and 3) and cycler B (not shown), and one for both cyclers combined (not shown). In each of these dendrograms, the same fundamental groups of isolates were placed together in clusters that largely corresponded with traditional serotypes (e.g., Fig. 1 to 3). For a given fingerprint type, the three different dendrograms all exhibited a consistent conformation irrespective of cycler (not shown). In contrast, for a particular cycler (or combination) the three different fingerprint types yielded dendrograms with quite different conformations, as illustrated for cycler A in Fig. 1 to 3. The conformational differences between the dendrograms based on different fingerprint types were attributable both to different inferred similarity relationships between the various (conserved) broad strain clusters and to differences in the internal structure of the clusters themselves (Fig. 1 to 3).
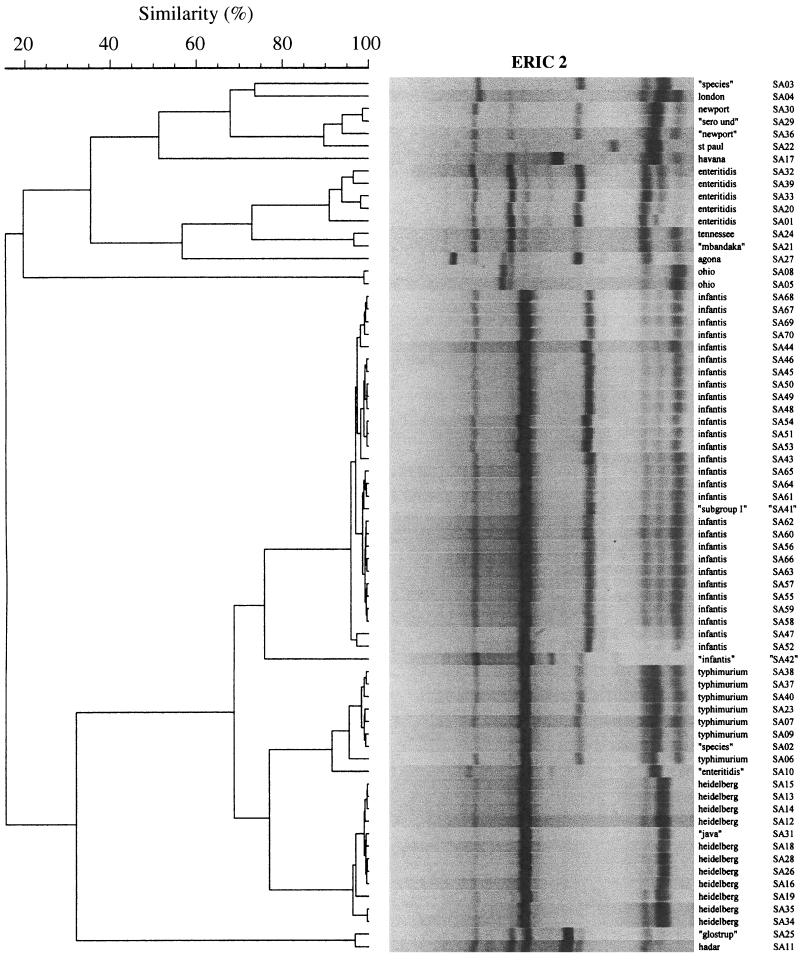
FIG. 1.
Single-cycler dendrogram (cycler A) of ERIC2 fingerprints for 70 isolates of S. enterica. Cluster analysis (by UPGMA) was based on Pearson's correlation coefficient similarity values for pairwise comparisons between fingerprints, which were analyzed as analog densitometric scans without delineation of discrete bands. Serotype designations shown in quotation marks are for isolates that were subsequently reclassified based on the results of confirmatory serotyping or confirmatory rep-PCR typing. Gel strips as shown are computer reconstructions and hence underestimate the clarity of the actual gel images. See reference 16 for examples of primary rep-PCR fingerprints.
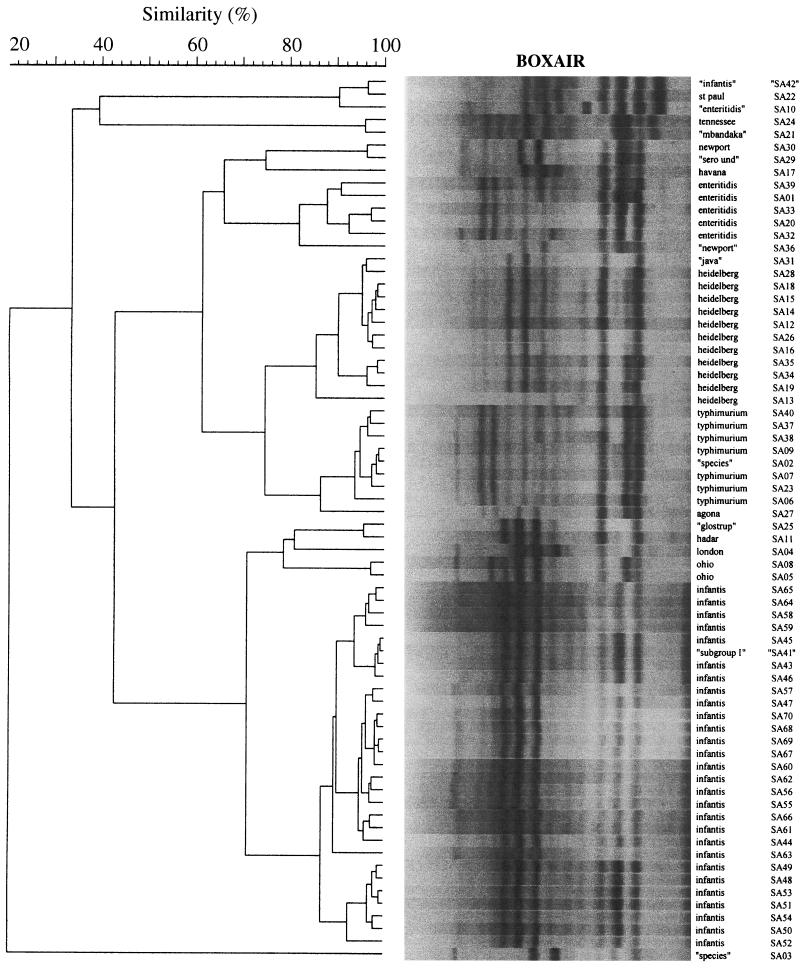
FIG. 2.
Single-cycler dendrogram (cycler A) of BOXA1R fingerprints for 70 isolates of S. enterica. Methods and labels are as described in the legend to Fig. 1. Gel strips as shown are computer reconstructions and hence underestimate the clarity of the actual gel images. See reference 16 for examples of primary rep-PCR fingerprints.
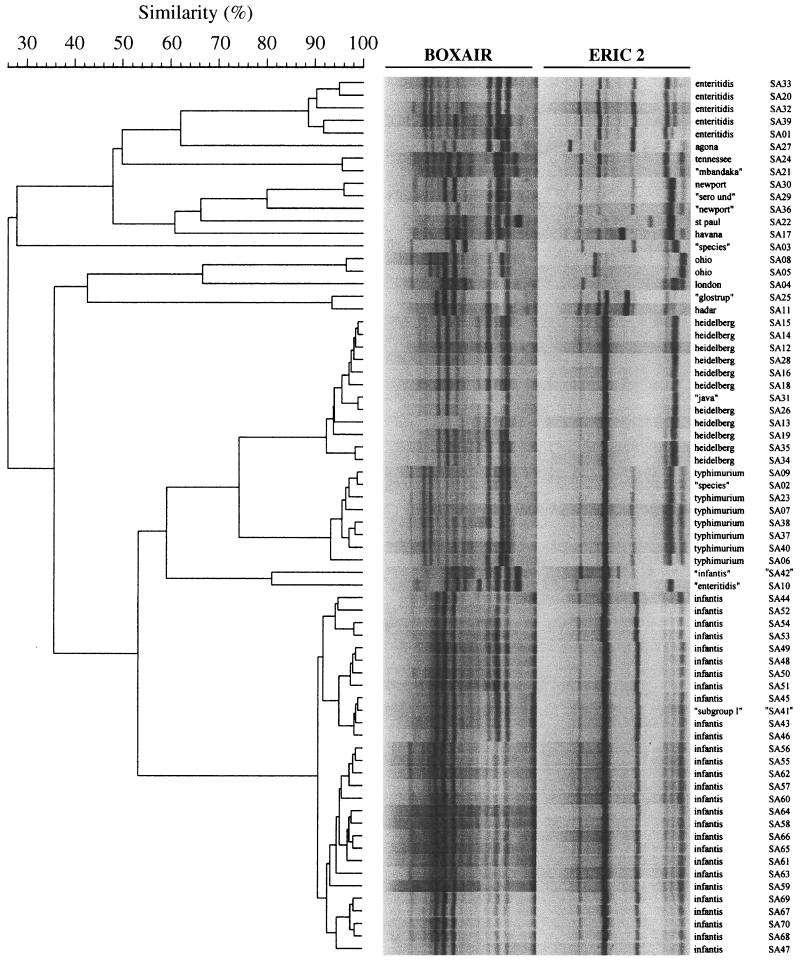
FIG. 3.
Single-cycler dendrogram (cycler A) of composite (ERIC2 plus BOXA1R) fingerprints for 70 isolates of S. enterica. The individual primer fingerprints as shown in Fig. 1 and 2 were combined head-to-toe to create the virtual composite fingerprints shown here, which were analyzed in the same manner as the individual fingerprints. Methods and labels are as described in the legend to Fig. 1. Gel strips as shown are computer reconstructions and hence underestimate the clarity of the actual gel images. See reference 16 for examples of primary rep-PCR fingerprints.
Correspondence of PCR with serotype.
In each of the combined cycler dendrograms (i.e., based on ERIC2, BOXA1R, or composite fingerprints), with notable exceptions (see below) each isolate's replicate fingerprints from the two cyclers were placed together either as nearest neighbors or within a cluster that contained only representatives of the isolate's serotype and included all representatives of that serotype (not shown). This demonstrated the reproducibility of the correspondence between rep-PCR types and serotypes, as suggested by single-cycler dendrograms (Fig. 1 to 3).
Putative discrepancies between PCR and serotype.
Each dendrogram contained 9 or 10 examples of what appeared to represent PCR-serotype discrepancies, as indicated in Fig. 1 to 3 by quotation marks around the putative serotype designations of the corresponding isolates. Several clusters contained isolates putatively of two different defined serotypes. Examples included the placement of “Glostrup” (SA25) with Hadar (SA11), of “Mbandaka” (SA21) with Tennessee (SA24), and of “Java” (SA 31) with Heidelberg (many isolates). Other clusters contained an isolate of ambiguous serotype together with one or more isolates of a defined serotype. Examples included the placement of the “subgroup I” isolate (SA41) with the Infantis group (many isolates), of the “serotype undetermined” isolate (SA29) with Newport (SA30), and of the “Salmonella sp.” isolate (SA02) with the Typhimurium group (many isolates). Finally, several isolates were separated from other isolates with the same putative serotype designation. Examples included putative Infantis isolate SA42 versus all other Infantis isolates (multiple isolates; BOX and composite dendrograms only); putative Enteritidis isolate SA10 versus all other Enteritidis isolates (multiple isolates); “Salmonella sp.” isolates SA03 versus SA02; and putative “Newport” isolate SA36 versus Newport isolate SA30 (Fig. 1 to 3).
Confirmatory serotyping.
To further evaluate these possible PCR-serotype discrepancies, confirmatory serotyping was done for 12 isolates (Table 2). The new serotypes, which differed to various degrees from the historical serotypes for seven of the isolates, resolved the seeming PCR-serotype discrepancies in favor of rep-PCR for four isolates, SA10, SA21, SA25, and SA31 (Table 2). For two other isolates (Hadar SA11 and Tennessee SA24), although repeat serotyping confirmed the historical results for the isolates themselves, these isolates' respective PCR look-alikes (“Glostrup” SA25 and “Mbandaka” SA21, respectively) received new serotype designations that now corresponded with those of Hadar SA11 and Tennessee SA24 (Table 2).
TABLE 2.
Results of confirmatory serotyping and rep-PCR typing for selected S. enterica isolates
| Isolate | Historical identification or serotype | Confirmatory serotype | Inferred serotype (matching isolate)
|
Comment | |
|---|---|---|---|---|---|
| Initial PCR | Confirmatory PCR | ||||
| SA02 | Salmonella sp. (group B) | Typhimurium | Typhimurium (many) | NAa | Enhanced discrimination with PCR |
| SA03 | Salmonella sp. (group D) | Probable Typhib | Unique (none) | NA | Correctly identified as unique by PCR |
| SA10 | Enteritidis | Montivideo | Unique (none) | NA | Historical serotype incorrect |
| SA11 | Hadar | Hadar | Undefinedc (SA25) | NA | Pseudo-discrepancy (due to SA25) |
| SA25 | Glostrup | Hadar | Undefinedc (SA11) | NA | Historical serotype incorrect |
| SA21 | Mbandakad | Tennesseed | Undefinedc (SA24) | NA | Historical serotype incorrect |
| SA24 | Tennesseed | Tennesseed | Undefinede (SA21) | NA | Pseudo-discrepancy (due to SA21) |
| SA29 | Undetermined (rough) | Undetermined (rough) | Newport (SA30) | NA | Enhanced discrimination with PCRf |
| SA36 | Newport | Newport/bardo | Unique (none) | NA | Correctly identified as unique by PCR |
| SA31 | Java | Heidelberg | Heidelberg (many) | NA | Historical serotype incorrect |
| SA41 | Subgroup I | Subgroup I | Infantis (many) | Unique (none) | Initial PCR result incorrectf |
| SA42 | Infantis | Infantis | Unique (none) | Infantis (many) | Initial PCR result incorrectf |
NA, not applicable (confirmatory PCR not done).
Consistent with Typhi except for absence of Vi antigen.
Two potential serotypes, Hadar (SA11) versus Glostrup (SA25).
As previously reported (16).
Two potential serotypes, Mbandaka (SA211) versus Tennessee (SA24).
Serotype as inferred from final rep-PCR was consistent with epidemiological data and PFGE result.
After confirmatory serotyping, only three ambiguities and/or discrepancies remained between PCR and serotype. The “serotype undetermined (rough)” isolate SA29, which by rep-PCR was indistinguishable from Newport isolate SA30, was serologically confirmed as serotype undetermined (rough). This isolate was subsequently discovered to have been recovered within the same month from the same patient as Newport isolate SA30 and to be indistinguishable from SA30 by PFGE (not shown) and hence probably represented a rough derivative of SA30. Thus, PCR appeared to have identified a serologically inapparent clonal relationship between these isolates.
The putative serogroup I isolate SA41, which by rep-PCR initially appeared to be indistinguishable from most of the Infantis isolates, was serologically confirmed as serogroup I. Conversely, putative Infantis isolate SA42, which according to initial ERIC fingerprints constituted an outgroup within the larger Infantis cluster (Fig. 1), and according to both initial BOX and composite fingerprints was distant from the larger Infantis cluster (Fig. 2 and 3), was serologically confirmed as Infantis (Table 2). PFGE analysis yielded results consistent with the serotyping, i.e., SA41 (putatively Infantis) exhibited a PFGE profiles unrelated to any Infantis profile previously encountered by the MDH (not shown), whereas SA42 (putatively subgroup I) exhibited PFGE pattern SIN2, the predominant PFGE pattern of the MVAMC Infantis outbreak (see below). Confirmatory PCR fingerprinting using newly extracted DNA from these two isolates yielded results that were precisely the reverse of the initial PCR fingerprinting results but consistent with the serotyping and PFGE results (Table 2), evidence that these two isolates or their typing results had been reversed during the initial PCR evaluation.
Subserotype resolving power of rep-PCR.
With this preliminary evidence of the ability of rep-PCR to resolve S. enterica isolates to the serotype level as well as or better than conventional serotyping, we next analyzed the subserotype discriminating power of rep-PCR. In each of the six single-cycler dendrograms (cycler A, Fig. 1 to 3; cycler B, data not shown), each isolate appeared to be resolved apart from all other isolates, including those within the same cluster and from the same serotype (e.g., all serotype Infantis isolates) (Fig. 3). Moreover, subclusters of isolates were seemingly resolved within several of the larger serotype-specific clusters, suggestive of subclones within certain serotypes (Fig. 1, Heidelberg SA35 and SA25; Fig. 2, Typhimurium SA40, SA37, and SA38; and Fig. 3, Enteritidis SA33 and SA20, as well as six or more subclusters of serotype Infantis isolates, e.g., SA44, SA52, SA54, and SA53).
However, inspection of the combined-cycler dendrograms (not shown) revealed that this seemingly high degree of resolution at the subserotype or even the individual isolate level was probably an artifact of assay variability. Although each individual fingerprint was still resolved apart from all other fingerprints, duplicate fingerprints from a given isolate usually were not placed as nearest neighbors to one another, but instead were intermingled with fingerprints from other isolates within the same serotype. Moreover, all but one of the apparent subclusters within a larger serotype-specific cluster were found to contain only one of the duplicate fingerprints of the constituent isolates, evidence that these actually represented pseudo-subclusters (not shown). This was true specifically for the serotype Infantis isolates, which despite an appearance of subserotype diversity in the single cycler dendrograms (e.g., Fig. 3: SA44, SA52, SA54, and SA53 as one of six discrete subclusters within the larger Infantis cluster) were found in the combined-cycler dendrograms to be essentially homogeneous (not shown). Historical PFGE data, which were available for 17 of the 18 serotype Infantis isolates from the MVAMC hospital outbreak, showed 15 of these isolates to exhibit pattern SIN2, whereas two exhibited closely related (i.e., a two-band difference) pattern SIN6 (not shown).
Nonetheless, in each of the combined-cycler dendrograms, not only all of the isolates representing a single-isolate serotype, but also three (ERIC and composite fingerprints) or five (BOX fingerprints) of the isolates representing a multiple-isolate serotype were fully resolved, i.e., had both replicate fingerprints as nearest neighbors to one another, without interposition of a fingerprint from a different isolate. This suggested true reproducible subserotype resolution of certain isolates. In addition, the replicate composite fingerprints of Typhimurium isolates SA37 and SA38 were resolved as a two-strain subcluster within the larger Typhimurium cluster, evidence suggesting that these isolates might represent a subclone within serotype Typhimurium (not shown).
DISCUSSION
In the present study we assessed the ability of rep-PCR fingerprinting to discriminate among 70 clinical isolates of S. enterica, including 19 isolates of serotype Infantis from a hospital cafeteria-associated outbreak. We found that rep-PCR reproducibly resolved isolates to the serotype level whether BOX, ERIC2, or composite fingerprints were used, that it superseded serotyping for certain strains that had ambiguous, incomplete, or incorrect serotypes, and that in some instances it differentiated between isolates at the subserotype level.
The observed correspondence of rep-PCR with serotypes is consistent with the known largely clonal distribution of surface antigens within Salmonella spp, (2) and the ability of amplification fingerprinting to assess clonal relationships among members of the family Enterobacteriaceae (10, 17, 37). Exceptions to the “clone-serotype” rule within Salmonella are provided by serotypes Derby, Enteritidis, Newport, and Infantis, each of which comprises isolates from distantly related evolutionary lineages (2, 25). This phenomenon presumably results from horizontal transfer of surface antigen determinants between distant lineages (2). The genetic differences we detected by rep-PCR between Newport and Newport/bardo isolates SA30 and SA36 may correspond with the documented evolutionary diversity within serotype Newport (2). That we detected minimal diversity within serotypes Enteritidis and Infantis suggests that within each of these serotypes the isolates we examined represented only one of the several possible separate clonal groups, consistent with the known geographic and host species-specific segregation of clones within these serotypes (2, 14, 25).
rep-PCR typing outperformed serotyping for several isolates which had incomplete expression of surface antigens or for which previous serotyping results were otherwise incomplete or inaccurate. For some of these isolates, confirmatory serotyping sufficed to resolve the seeming discrepancy between rep-PCR and historical serotypes. For one rough isolate, however, repeat serotyping only confirmed the ambiguous historical serotype. In this instance, PFGE analysis and epidemiological correlation suggested that the rep-PCR-based serotype assignment was correct. On the other hand, in two instances seeming conflicts between rep-PCR and serotyping were found to be due to errors in the initial PCR typing. It appeared that these two strains (which were adjacent to one another in the collection and were processed sequentially) or their typing results were inadvertently reversed during the initial PCR analysis. This and some of the historical serotype inaccuracies illustrate how human error (26), in addition to intrinsic technical limitations of the laboratory methods per se (6, 16, 17), can produce spurious typing results.
By using an objective quantitative measure of reproducibility, i.e., Pearson's correlation coefficient for pairwise comparisons of analog densitometric scans of gel lanes, we found modified rep-PCR to yield highly reproducible results, even when fingerprints were generated on different days using different cyclers. Reproducibility was further improved by selective replacement of discrepant fingerprints following an initial screening of duplicate fingerprints for each isolate. We used two independent cyclers to stringently test reproducibility. Consequently, our reproducibility values probably underestimate what could be achieved with the use of a single cycler. Based on our previous analysis of standard versus modified cycling conditions (16, 17), it is probable that the modified cycling conditions used in the present study contributed substantially to the observed high degree of cycler-to-cycler and day-to-day reproducibility. Nonetheless, for routine laboratory use we would recommend duplicate amplifications for all isolates. Although this would increase workload, it would provide an important internal quality control, allowing detection of the occasional anomalous amplification. In addition, for longitudinal comparisons it might be desirable to include at least one reference strain, to confirm assay stability over time.
The serotype-specific nature of rep-PCR fingerprints could allow individual hospital laboratories to perform their own Salmonella “serotyping” by rep-PCR, thus avoiding the delays associated with referral of isolates to an outside laboratory and possibly hastening the recognition of outbreaks. Rep-PCR also could provide reference laboratories with a simple molecular means to confirm serotyping results or to obtain serotype-level discrimination for isolates with ambiguous serotypes, such as rough strains or those with incomplete antigen expression. A sophisticated digital gel analysis system such as was used in the present study, although useful for constructing databases and for quantitative assessments of similarity and reproducibility (as needed for the present study), would not be essential for routine clinical laboratory use because visual inspection of fingerprints reveals approximately the same similarity relationships as are detected quantitatively by the computer (e.g., Fig. 1 to 3) and probably would suffice for evaluation of temporally clustered isolates from a single laboratory. In retrospect, it seems probable that had rep-PCR fingerprinting been performed on a real-time basis for the MVAMC clinical isolates, it could have identified the MVAMC Infantis outbreak following recovery of the putative second isolate (SA43).
It should be noted that the range of serotypes examined was limited compared with the 2,463 that are currently recognized within the genus Salmonella (5), and that nine of the included serotypes were represented by only a single isolate. Confirmation of the serotype specificity of modified rep-PCR using a larger and more diverse population of Salmonella isolates would be desirable.
In contrast to its seeming ability to discriminate at the serotype level, rep-PCR exhibited limited evidence of discrimination at the subserotype level. Although the present study was not designed specifically to assess subserotype discrimination, the available data suggest that for reference laboratories rep-PCR is unlikely to supersede PFGE, the subserotype typing method currently used by the allied public health laboratories of the PulseNet network (3, 9, 28). However, if primers specific for Salmonella could be developed, it might be possible in the future to use PCR to both detect and “serotype” Salmonella spp. from clinical specimens, thereby bypassing the traditional isolation step.
The present study can be compared with prior work in the field. Others have explored rep-PCR, ribosomal DNA PCR, or random amplified polymorphic DNA PCR for typing of Salmonella spp. and, with few exceptions (7), have reported discrimination between isolates at the serotype level (21, 34) or lower (4, 15, 19, 20, 22, 24). However, in these studies, reproducibility, which in some reports was described as good (19, 24), but in others as somewhat (22, 34) or highly (7) problematical, was not formally or quantitatively assessed, nor were fingerprints evaluated in an objective or blinded fashion. To our knowledge, the present study is the first to evaluate PCR fingerprinting of Salmonella with this degree of stringency. Additional reproducibility challenges that deserve evaluation include the analysis of serial subcultures or sequential stool cultures from the same patient, operator-to-operator and run-to-run variability, and reproducibility over time.
The pseudo-discrimination we observed in single-cycler dendrograms when assay variability was not taken into consideration (Fig. 1 to 3) suggests that some of the putative “types,” as noted in previous studies, may have been artifactual. Of note, previous studies that evaluated rep-PCR fingerprinting for Salmonella used conventional cycling conditions (4, 7, 21, 34), which we have found to yield significantly poorer reproducibility and net discriminating power than the elevated annealing temperature cycling conditions that were used in the present study (16, 17). The present study is also unique in being the first to use an amplification typing method to evaluate a hospital-associated outbreak of salmonellosis or one due to serotype Infantis.
In summary, we used modified rep-PCR fingerprinting to retrospectively evaluate a hospital cafeteria-associated outbreak of serotype Infantis. All outbreak isolates but one were identified as being members of a large Infantis cluster, the only exception resulting from a probable clerical error. Diverse other Salmonella isolates, including some with incomplete or inaccurate historical serotypes, were resolved to the serotype level. Minimal subserotype discrimination was obtained. Modified rep-PCR typing was highly reproducible and simple to perform. It may allow serotype-level discrimination of Salmonella spp. in any laboratory equipped for diagnostic PCR.
ACKNOWLEDGMENTS
This material is based on work supported by Office of Research and Development, Medical Research Service, Department of Veterans Affairs, National Institutes of Health grant DK-47504, and National Research Initiative (NRI) Competitive Grants Program/U.S. Department of Agriculture grant 00-35212-9408 (J.R.J.).
The MVAMC Clinical Microbiology Laboratory provided the S. enterica isolates. Confirmatory serotyping was done by Wanda Boyer at the Minnesota Department of Health Microbiology Laboratory. Dave Prentiss (MVAMC) prepared the figures. Ann Emery (MVAMC) helped prepare the manuscript.
REFERENCES
- 1.Arbeit R D. Laboratory procedures for the epidemiologic analysis of microorganisms. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, editors. Manual of clinical microbiology. Washington, D.C.: American Society for Microbiology; 1995. pp. 230–235. [Google Scholar]
- 2.Beltran P, Musser J M, Helmuth R, Farmer J J, I. I I, Frerichs W M, Wachsmuth I K, Ferris K, McWhorter A C, Wells J G, Cravioto A, Selander R K. Toward a population genetic analysis of Salmonella: genetic diversity and relationships among strains of serotypes S. choleraesuis, S. derby, S. dublin, S. enteritidis, S. heidelberg, S. infantis, S. newport, and S. typhimurium. Proc Natl Acad Sci USA. 1988;85:7753–7757. doi: 10.1073/pnas.85.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender J B, Hedberg C W, Besser J M, Boxrud D J, MacDonald K L, Osterholm M T. Surveillance for Escherichia coli O157:H7 infections in Minnesota by molecular subtyping. N Engl J Med. 1997;337:388–394. doi: 10.1056/NEJM199708073370604. [DOI] [PubMed] [Google Scholar]
- 4.Beyer W, Mukendi F M, Kimmig P, Bohm R. Suitability of repetitive-DNA-sequence-based PCR fingerprinting for characterizing epidemic isolates of Salmonella enterica serovar Saintpaul. J Clin Microbiol. 1998;36:1549–1554. doi: 10.1128/jcm.36.6.1549-1554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner F W, Villar R G, Angulo F J, Tauxe R, Swaminathan B. Salmonella nomenclature. J Clin Microbiol. 2000;38:2465–2467. doi: 10.1128/jcm.38.7.2465-2467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burr M D, Pepper I L. Variability in presence-absence scoring of AP PCR fingerprints affects computer matching of bacterial isolates. J Microbiol Methods. 1997;29:63–68. [Google Scholar]
- 7.Burr M K, Josephson K L, Pepper I L. An evaluation of ERIC PCR and AP PCR fingerprinting for discriminating Salmonella serotypes. Lett Appl Microbiol. 1998;27:24–30. doi: 10.1046/j.1472-765x.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Standardized molecular subtyping of Escherichia coli by pulsed-field gel electrophoresis. Training course. Atlanta, Ga: Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 9.Cimons M. Rapid food-borne pathogen ID system is making a difference. ASM News. 2000;66:617–619. [Google Scholar]
- 10.Desjardins P, Picard B, Kaltenbock B, Elion J, Denamur E. Sex in Escherichia coli does not disrupt the clonal structure of the population: evidence from random amplified polymorphic DNA and restriction-fragment-length polymorphism. J Mol Evol. 1995;41:440–448. doi: 10.1007/BF00160315. [DOI] [PubMed] [Google Scholar]
- 11.Ewing W H. Identification of Enterobacteriaciae. 4th ed. New York, N.Y: Elsevier; 1986. [Google Scholar]
- 12.Goldberg M B, Rubin R H. The spectrum of Salmonella infection. Infect Dis Clin North Am. 1988;2:571–598. [PubMed] [Google Scholar]
- 13.Gray L D. Escherichia, Salmonella, Shigella, and Yersinia. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: ASM Press; 1995. pp. 450–456. [Google Scholar]
- 14.Helmuth R, Schroeter A. Molecular typing methods for S. enteritidis. Int J Food Microbiol. 1994;21:69–77. doi: 10.1016/0168-1605(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 15.Hilton A C, Banks J G, Penn C W. Optimization of RAPD for fingerprinting of Salmonella. Lett Appl Microbiol. 1997;24:243–248. doi: 10.1046/j.1472-765x.1997.00385.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson J R, Clabots C. Improved repetitive element-polymerase chain reaction fingerprinting of Salmonella with the use of extremely elevated annealing temperatures. Clin Diagn Lab Immunol. 2000;7:258–264. doi: 10.1128/cdli.7.2.258-264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson J R, O'Bryan T T. Improved repetitive element-polymerase chain reaction fingerprinting for resolving pathogenic and nonpathogenic phylogenetic groups within Escherichia coli. Clin Diagn Lab Immunol. 2000;7:265–273. doi: 10.1128/cdli.7.2.265-273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lennette E H, Balows A, Hausler W J, Shadomy H J. Manual of clinical microbiology. 4th ed. Washington, D.C.: American Society for Microbiology; 1985. [Google Scholar]
- 19.Lin A W, Usera M A, Barrett T J, Goldsby R A. Application of random amplified polymorphic DNA analysis to differentiate strains of Salmonella enteritidis. J Clin Microbiol. 1996;34:870–876. doi: 10.1128/jcm.34.4.870-876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling J M, Koo I C, Kam K M, Cheng A F. Antimicrobial susceptibilities and molecular epidemiology of Salmonella enterica serotype Enteritidis strains isolated in Hong Kong from 1986 to 1996. J Clin Microbiol. 1998;36:1693–1699. doi: 10.1128/jcm.36.6.1693-1699.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Molina N, Laconcha I, Rementeria A, Audicana A, Perales I, Garaizar J. Typing of Salmonella enteritidis of different phage types of PCR fingerprinting. J Appl Microbiol. 1998;84:877–882. doi: 10.1046/j.1365-2672.1998.00425.x. [DOI] [PubMed] [Google Scholar]
- 22.Martin M C, Gonzalez-Hevia M A, Moro I, Mendoza M C. Genetic typing methods applied to the differentiation of clonal lines among Salmonella enterica serogroup G strains causing human salmonellosis. FEMS Immunol Med Microbiol. 1997;19:215–221. doi: 10.1111/j.1574-695X.1997.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 23.Murase T, Okitsu T, Suzuki R, Morozumi H, Matsushima A, Nakamura A, Yamai S. Evaluation of DNA fingerprinting by PFGE as an epidemiologic tool for Salmonella infections. Microbiol Immunol. 1995;39:673–676. doi: 10.1111/j.1348-0421.1995.tb03255.x. [DOI] [PubMed] [Google Scholar]
- 24.Nastasi A, Mammina C. Epidemiological evaluation by PCR ribotyping of sporadic and outbreak-associated strains of Salmonella enterica serotype Typhimurium. Res Microbiol. 1995;146:99–106. doi: 10.1016/0923-2508(96)80274-9. [DOI] [PubMed] [Google Scholar]
- 25.Pelkonen S, Romppanen E L, Siitonen A P J. Differentiation of Salmonella enterica serovar Infantis isolates from human and animal sources by fingerprinting IS200 and 16S rrn loci. J Clin Microbiol. 1994;32:2128–2133. doi: 10.1128/jcm.32.9.2128-2133.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reason J T. Human error. Cambridge, England: Cambridge University Press; 1990. [Google Scholar]
- 27.Shlaes D M, Currie-McCumber C A. Plasmid analysis in molecular epidemiology: a summary and future directions. Rev Infect Dis. 1986;8:738–746. doi: 10.1093/clinids/8.5.738. [DOI] [PubMed] [Google Scholar]
- 28.Slutsker L, Altekruse S F, Swerdlow S F. Foodborne diseases: emerging pathogens and trends. Infect Dis Clin North Am. 1998;12:199–216. doi: 10.1016/s0891-5520(05)70418-9. [DOI] [PubMed] [Google Scholar]
- 29.Sokal R R, Sneath P H A. Principles of numerical taxonomy. W. H. San Francisco, Calif: Freeman; 1963. [Google Scholar]
- 30.Tauxe R V. Emerging food-borne diseases: an evolving public health challenge. Emerg Infect Dis. 1997;3:425–434. doi: 10.3201/eid0304.970403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauxe R V. Salmonella: a postmodern pathogen. J Food Protect. 1991;54:563–568. doi: 10.4315/0362-028X-54.7.563. [DOI] [PubMed] [Google Scholar]
- 32.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Muray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Belkum A, Meis J. Polymerase chain reaction-mediated genotyping in microbial epidemiology. Clin Infect Dis. 1994;18:1017–1018. doi: 10.1093/clinids/18.6.1017. [DOI] [PubMed] [Google Scholar]
- 34.van Lith L A, Aarts H J. Polymerase chain reaction identification of Salmonella serotypes. Lett Appl Microbiol. 1994;19:273–276. doi: 10.1111/j.1472-765x.1994.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 35.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Versalovic J, Schneid M, de Bruijn F J, Lupski J R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol. 1994;5:25–40. [Google Scholar]
- 37.Wang G, Whittam T S, Berg C M, Berg D E. RAPD (arbitrary primer) PCR is more sensitive than multilocus enzyme electrophoresis for distinguishing related bacterial strains. Nucleic Acids Res. 1993;21:5930–5933. doi: 10.1093/nar/21.25.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson M, Nordholm G E. DNA fingerprinting analysis of standard, intermediate, and variant antigenic types of Salmonella enterica subspecies enterica serovar Gallinarum biovar pullorum. Avian Dis. 1995;39:594–598. [PubMed] [Google Scholar]