ABSTRACT
Human pegivirus (HPgV-1), previously known as GB virus C (GBV-C) or hepatitis G virus (HGV), is a single-stranded positive RNA virus belonging to the genus Pegivirus of the Flaviviridae family. It is transmitted by percutaneous injuries (PIs), contaminated blood and/or blood products, sexual contact, and vertical mother-to-child transmission. It is widely prevalent in general population, especially in high-risk groups. HPgV-1 viremia is typically cleared within the first 1–2 years of infection in most healthy individuals, but may persist for longer periods of time in immunocompromised individuals and/or those co-infected by other viruses. A large body of evidences indicate that HPgV-1 persistent infection has a beneficial clinical effect on many infectious diseases, such as acquired immunodeficiency syndrome (AIDS) and hepatitis C. The beneficial effects seem to be related to a significant reduction of immune activation, and/or the inhabitation of co-infected viruses (e.g. HIV-1). HPgV-1 has a broad cellular tropism for lymphoid and myeloid cells, and preferentially replicates in bone marrow and spleen without cytopathic effect, implying a therapeutic potential. The paper aims to summarize the natural history, prevalence and distribution characteristics, and pathogenesis of HPgV-1, and discuss its association with other human viral diseases, and potential use in therapy as a biovaccine or viral vector.
KEYWORDS: Human pegivirus, prevalence, pathogenesis, human immunodeficiency virus type-1, hepatitis C virus
Introduction
Human pegivirus (HPgV-1) is a spherical enveloped virus of about 50 nm in diameter [1]. It belongs to the genus Pegivirus of the family Flaviviridae and has a 9.4 kb positive-sense single-strand RNA genome that is organized similar to hepatitis C virus (HCV) [2,3]. HPgV included type 1 (HPgV-1) and type 2 (HPgV-2). HPgV-1 can cause persistent infection, but is not associated with hepatitis and other obvious clinical symptoms or diseases in healthy people [2]. In particular, a large number of studies have shown that HPgV-1 persistent infection slows the disease progression caused by human immunodeficiency virus type 1 (HIV-1) and/or other viruses and improves the survival of patients, suggesting that HPgV-1 infection plays a beneficial role when co-infected with other viruses [4–7,]. Currently, the natural history, pathogenic mechanisms, and potential impact of HPgV-1 on human health remain to be seen. In this paper, we summarize the history, prevalence and pathogenesis of HPgV-1, and discuss its relationship with other viral diseases, and the possibility of HPgV-1 as therapeutic tools or viral vectors.
Discovery of pegivirus
HPgV-1 was formerly known as GB virus type C (GBV-C) or hepatitis G virus (HGV). The abbreviation “GB” came from a surgeon with acute hepatitis. In 1967, serum from the surgeon was experimentally inoculated into tamarins, and resulted in hepatitis in tamarins [3,6]. Therefore, the presence of a new unknown virus that causes hepatitis was predicted. Until 1995, two new RNA viruses were identified from tamarins that received inoculation of GB passage and developed hepatitis. Because the two viruses belong to the family Flaviviridae, and are different from the previously identified hepatitis A-E viruses, they were named as GB virus A (GBV-A) and GB virus B (GBV-B) [7]. In the same year, another novel RNA virus was identified in the serum of non-AE hepatitis patients from West Africa. The virus had 53% to 59% similarity to GBV-A and GBV-B nucleic acid sequences, respectively, and approximately 47% homology to HCV sequences. Based on sequence homology and phylogenetic analysis, the virus was classified as a new member of the Flaviviridae family and named as GBV-C [5,8]. In 1996, HGV was identified from a patient with chronic hepatitis [9]. Because HGV is closely genetically related to GBV-C, rather than GBV-A and GBV-B, GBV-C and HGV represent different isolates of the same virus species. GBV-C and HGV initially were believed to be associated with non-AE hepatitis in human [5–13]. In 2010, GBV-D was identified and described from free-ranging bats [10]. It shares about 50% identity to GBV-A and GBV-C at the amino acid level, and represents a distinct species within the family Flaviviridae [10].
Among GB viruses, only GBV-B was found to cause hepatitis, and was assigned to the genus Hepacivirus (Figure 1) [11,12]. GBV-C and other two viruses (GBV-A and GBV-D) were later found not to be associated with hepatitis. In 2011, Stapleton et al. assigned GBV-C, GBV-A, and GBV-D to the fourth genus of the family Flaviviridae according to their phylogenetic relationships, genome organization, and pathogenic features (Figure 1) [3]. The new genus was named as Pegivirus (pe, persistent; g, GB, or G). Mammals are the main hosts of pegiviruses, including primates [13–18], horses [19–22], bats, and rodents [10,23–25]. Recent studies showed that pegiviruses can also infect non-mammals, such as geese [26,27], illustrating a wide range of hosts. Because GBV-C/HGV infects human beings, it was renamed as human pegivirus type 1 (HPgV-1). The second human pegivirus (HPgV-2, also known as HHpgV-1) was firstly identified from blood transfusion recipients in the US in 2015 [28,29], and later detected in other countries (e.g. China [30,31], Vietnam [32], Cameroon [33]) (Figure 1). Figure 1 shows the phylogenetic relationship of pegiviruses.
Figure 1.
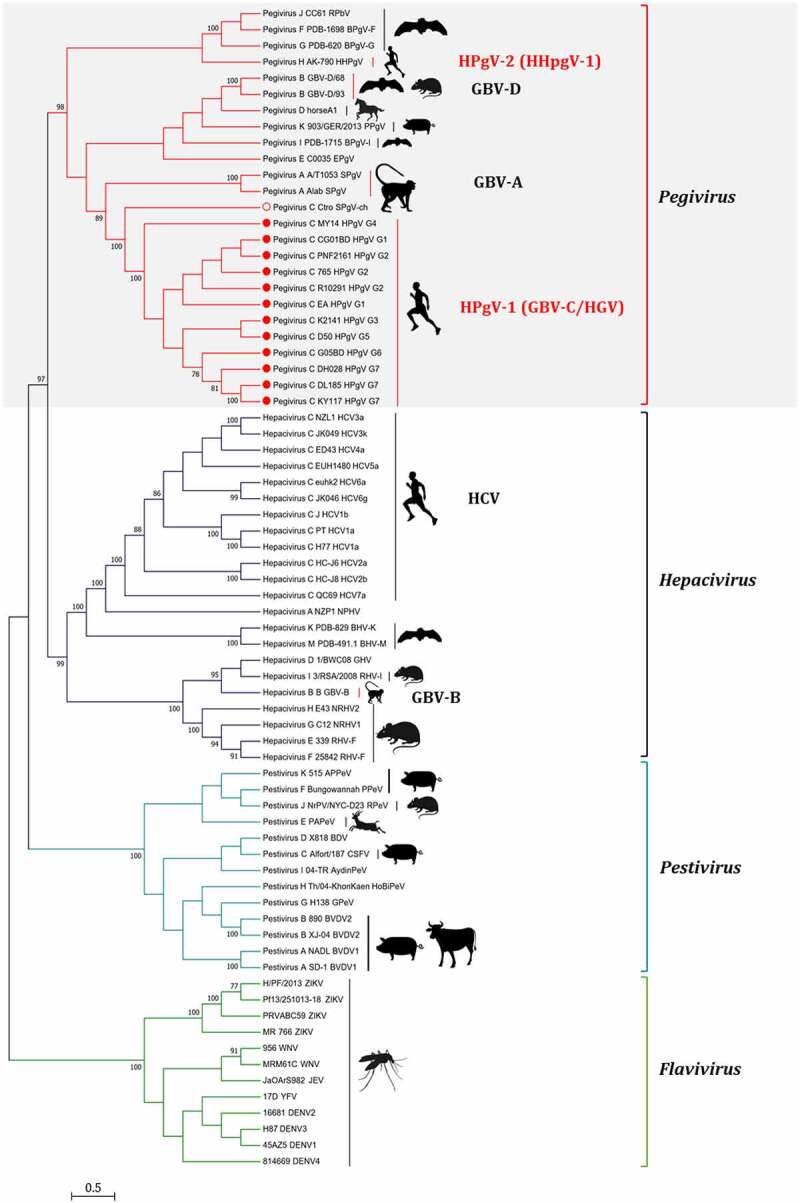
Phylogenetic relationship of pegivirus. The phylogenetic tree was constructed based on RdRp gene sequences of selected Flaviviridae members using the maximum-likelihood (ML) method (MEGA 7.0.26). Four genera are classified in the Flaviviridae family. The main hosts of these viruses are also shown in the figure. The red branches highlight the members of the genus Pegivirus.
Genome organization and protein products of HPgV-1
Like other members of the family Flaviviridae, HPgV-1 genome encodes an open reading frame (ORF) that is translated into a single pre-polyprotein consisting of approximately 3000 amino acid residues (Figure 2) [3,8]. The coding region is flanked by long 5’ and 3’ untranslated regions (UTRs). The 5’-UTR contains an internal ribosome entry site (IRES), which recruits ribosomes to guide viral mRNA translation [3,34]. The pre-polyprotein is further cleaved into two structural proteins (envelope proteins E1 and E2) and six non-structural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) by cellular and viral proteases (Figure 2).
Figure 2.
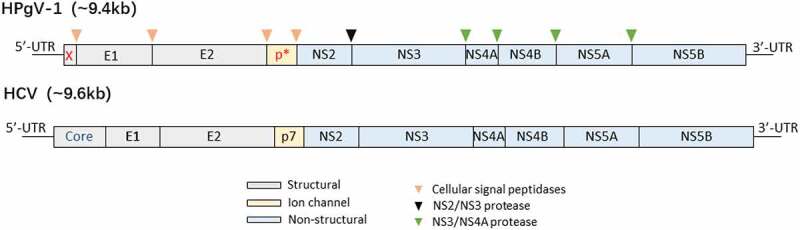
Genome organization of HPgV-1 and HCV. The genome encodes a single pre-polyprotein that is cleaved into mature viral proteins after co-translation and post-translation. Compared to HCV, HPgV-1 genome encodes two additional predicted proteins (protein X at upstream of E1, and protein p* between E2 and NS2), but does not encode a core protein that is an RNA-binding protein and forms the virion nucleocapsid.
Because of sharing similar genome organization and homologous genes to HCV [3], HPgV-1 is believed to have similar life cycle to HCV, including i. viral attachment and entry; ii. endocytosis; iii. fusion and uncoating; iv. translation and polyprotein processing; v. RNA replication; vi. virion assembly; vii. virion release [35]. Its proteins are also predicted to have similar functions with their counterparts in HCV [36–39]. Compared with HCV, the encoding region for a core protein is not identified for HPgV-1 (Figure 2) [3,40]. However, a basic protein is predicted at upstream of the signalase site before E1 in HPgV-1 genome [40]. This protein may participate in RNA packaging during virion assembly. Another additional protein (p*) is an about 6 kDa protein analogous to the HCV p7. Structural proteins E1 and E2 are envelope glycoproteins [36]. They are released from pre-polyprotein via enzymatic hydrolysis by a host signal peptidase [3,36]. By forming heterodimers on the surface of viral particles, they participate in viral assembly and are responsible for virus entry. E2 glycoprotein is responsible for the binding of the virus to cell receptors, which induces membrane fusion and promotes the entry of HPgV-1 into host cells [36,41]. E2 glycoprotein possesses immunogenicity and induces humoral immune response [2,42,43]. Furthermore, E2 glycoprotein interacts with co-infected viruses (e.g. HIV-1) and host proteins, and further participates in the regulation of host immune activation [44,45]. It alters IL-2-signaling pathways by reducing TCR-induced IL-2 production to inhibit the T-lymphocyte activation, and inhibits the IL-12 signaling pathway to reduce the proliferation of NK cell [46,47].
NS2, NS3, and NS4A are responsible for the cleavage of non-structural proteins [41,48,49]. The cleavage of NS2/NS3 is mediated by NS2 protease, and the cleavage of other NS proteins is mediated by NS3 protease with NS4A as a cofactor [49,50]. NS4B is a highly hydrophobic protein that may be involved in the formation of membranous structures supporting RNA replication. NS5A is known as a cytoplasmic phosphorylated protein that may participate in and regulate RNA replication [48]. NS5B is a RNA-dependent RNA polymerase that is responsible for genome replication of HPgV-1 [8,49]. HPgV-1 proteins and their functions are summarized in Table 1.
Table 1.
HPgV-1 proteins and their functions
| Protein | Function |
|---|---|
| E1 | Envelope glycoproteins |
| E2 | Envelope glycoproteins, receptor binding |
| p7-like | Similar in size to HCV p7 |
| NS2 | Component of the NS2-3 protease, mediating cleavage at the NS2/NS3 junction |
| NS3 | Protease, mediating the cleavage of NS proteins, C-terminal NTPase and helicase |
| NS4A | Cofactor for NS3-mediated cleavages of NS proteins |
| NS4B | Membrane alteration inducer |
| NS5A | Multifunctional phosphoprotein |
| NS5B | RNA-dependent RNA polymerase, genomic RNA replication |
Note: The functions of some HPgV-1 proteins are predicted according to their counterparts in HCV.
Prevalence and distribution
HPgV-1 has a high global prevalence. About one-sixth of the global population was estimated to be sero-positive for HPgV-1, and approximately 750 million people had viremia [2,3,51,52]. The at-risk population had substantially higher prevalence of HPgV-1 than the general population, and the prevalence of HPgV-1 varied considerably in different countries/regions of the world (Figure 3). In the general population and healthy blood donors, HPgV-1 prevalence ranged from 0.8% to 44.6%, while in the at-risk population, the prevalence rate ranged from 1.8% to 75.3% [53–96]. HPgV-1 has a higher prevalence in the developing world than in the developed world (Figure 3). For example, HPgV-1 prevalence in healthy blood donors was 0.8–46.6% in the developing world (e.g. Asia, Africa, and South America), while the rate was 1.1–6% in the developed world (e.g. North America, Europe, and Australia). Similar trend was also observed in the at-risk population with high HPgV prevalence (1.8–75.3%) in the developing world, but relatively low prevalence (9–48.6%) in the developed world. Geographic difference in HPgV-1 prevalence was believed to be associated with the socio-economic situation of a country/region, which reflects the income and welfare levels of local people, and affects their medical and health conditions [51]. People with lower income and welfare levels appeared to have a higher risk of HPgV-1 infection than those with higher income and welfare levels since the former more likely participate in illegal or paid blood donation and reuse unsterilized needles and/or contaminated instruments.
Figure 3.
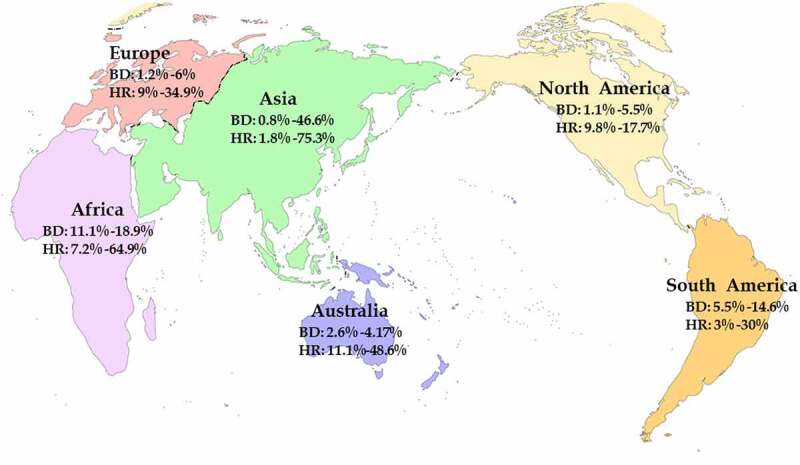
Global prevalence and distribution of HPgV-1. BD: blood donors; HR: high-risk population mainly including IDUs, CSWs, and MSM.
On the other hand, HPgV-1 prevalence appears to have obvious genotypic and geographical characteristics. HPgV-1 genotypes 1 and 2 are mainly distributed in Africa [54,55,78]; genotype 2 is more prevalent in Europe [79,80]; genotype 3 is prevalent in Asian countries and South America [81–84]; genotype 4 and 5 remains dominant in Philippines and other countries in Southeast Asia [85,86]; genotype 6 is circulating in Indonesia [87]. Genotype 7 was recently found in Yunnan Province of China, and some other Asian countries, such as Qatar [88,89]. The difference in the distribution of HPgV-1 genotypes might be associated with origin, evolution, and transmission of these genotypes.
Transmission and at-risk population of HPgV-1
Like HIV-1, HBV, and HCV, HPgV-1 is a blood-borne virus [2,90]. It is efficiently transmitted by percutaneous injuries (PIs) and blood transfusion, which explains why high proportion of HPgV-1 infection was found among healthy blood donors. Because of high-frequency exposure behavior, intravenous drug users (IDUs) are the major high-risk group for HPgV-1 infection, and have very high positive rate for this virus (Figure 3). Furthermore, people who received acupuncture were found to have significantly higher prevalence of HPgV-1 (16.5%) than those who never received acupuncture (9.4%) [91], implying that acupuncture increases the risk of HPgV-1 infection.
Apart from occupational exposure to PIs, and contaminated blood and/or blood components, HPgV-1 can also be transmitted by sexual contact (including heterosexual and homosexual contacts) and vertical mother-to-child transmission [87,89–94]. Commercial sex workers (CSWs) and men who have sex with men (MSM) are also the major high-risk groups for HPgV-1 infection. Because of sharing the same transmission routes with HIV-1, HCV, and HBV, high proportion (3.2–47.9%) of HPgV-1 co-infection was often reported in the individuals who are positive for the above-mentioned viruses [95–104].
Blood donors
HPgV-1 prevalence in blood donors varied largely in different countries/regions (0.8–46.6%) (Figure 3). The vast majority of the studies reported HPgV-1 prevalence less than 5% in blood donors, while few studies showed higher HPgV-1 prevalence (>10%) in some countries/regions (e.g. India [105,106], China [107–122], Kuwait [108]). The global prevalence of HPgV-1 was estimated to be 3.1% in blood donors [51]. The pooled prevalence of HPgV-1 was 1.7% in North America, 9.1% in South America, 2.3% in Europe, and 2.4% in Asia [51]. Based on 67,348 blood donors, HPgV-1 prevalence was estimated to be 3.3% in China [110]. Currently, HPgV-1 is not included in the routine blood donor screening test. The prevalence of HPgV-1 in general population and blood donors highlight the risk of post-transfusion infection even though HPgV-1 infection was largely believed to be benign. Concerns are being raised on whether screening for HPgV-1 should be included in the routine blood donor assay.
IDUs
IDUs are the most important high-risk group for HPgV-1 infection, and have prevalence of 11.6–89.2% in different studies. HPgV-1 viremia was more common among IDUs compared to healthy volunteers [79]. Based on 3779 IDUs from different studies, the pooled prevalence of HPgV-1 was estimated to be 33.6% [79,89,92,101,111–138]. Furthermore, HPgV-1 prevalence among IDUs appeared to be higher in developed world than those in developing world. For example, few studies showed that HPgV-1 prevalence among IDUs reached 89.2% in North America [117], 41.9% in Australia [113,121], while in Africa and South America, the pooled prevalence was 20.8% and 25.8%, respectively. In Asia and Europe, the pooled prevalence was 32% and 34%, respectively.
Because of frequent needle sharing behavior, co-infection of HPgV-1 with HIV-1, HBV, and/or HCV was very common among IDUs. The co-infection rate ranged from 11.6% to 85.8% [101,111,114,121,122,129,131,133,137–139]. In particular, the prevalence of triple infection with HPgV-1, HIV-1, and HCV was often higher than that of dual infection by HPgV-1 and HIV-1 or HCV [111,132,133].
CSWs and MSM
Sexual transmission routes of HPgV-1 include heterosexual and homosexual transmission. CSWs and MSM are the most predominant high-risk groups for heterosexual and homosexual infection of HPgV-1, respectively. HPgV-1 prevalence varied from 0% to 35.5% among CSWs and from 12.5% to 36.2% among MSM [52,89,92,120,121,126,128,131–133,135,136,138,140–143]. The pooled prevalence of HPgV-1 was 17.3% and 19.7% in CSWs and MSM, respectively, suggesting similar transmission risk of HPgV-1 among the two high-risk groups. Importantly, the worst-hit continent of HPgV-1 prevalence was CSWs in Asia (17.9%) and MSM in Australia (36.2%), respectively.
Pathogenesis
The pathogenicity of HPgV-1 remains controversial. A large number of epidemiological and clinical studies did not support an association of HPgV-1 infection with any known clinical diseases (reviewed in [4,144,145]). Although the virus was detected in the saliva and serum of healthy people and replicates in vivo at high titer, neither obvious clinical symptoms were observed, nor significant immune activation in any cell types was detected [146,147]. Approximately 80% of healthy people or immune competent individuals spontaneously clear viraemia within 2 years of HPgV-1 infection [2,3,117,148]. However, in immunocompromised individuals and/or individuals with other infectious diseases, HPgV-1 viraemia can persist for up to decades [2]. The maintenance of persistent infection may be ascribed to the ability of HPgV-1 to avoid immune recognition and T cells immune activation [36,149]. HPgV-1 E2 glycoprotein is believed to contain T cell receptor-inhibitory motifs, and contributes to viral persistence by reducing T cells immune activation [149]. On the other hand, HPgV-1 does not induce broad antibody responses. The specific antibody response appears to be restricted to E2 [2,3,42,117]. Anti-E2 antibody is associated with the clearance of HPgV-1 viraemia, and can prevent HPgV-1 reinfection [2,43,150].
HPgV-1 is frequently co-infected with other blood-borne viruses, such as HIV-1 and HCV. HPgV-1 persistent infection inhibits abnormal and excessive immune activation in patient co-infected with HIV-1, HCV, or EBOV, and often shows beneficial clinical effects in these patients [4,144,145]. In particular, HPgV-1 infection slows disease progression and prolong survival time of HIV-1 infected individuals by directly inhibiting HIV-1 infection and replication, and/or reducing immune activation of T lymphocytes [151–158].
Although most HPgV-1 infections are self-limited, few immunocompromised individuals with HPgV-1 infection developed lymphoma [159–164]. In 2018, Fama et al. reported that HPgV-1 infection was closely associated with the overall risk of lymphoma [165]. The association was observed for almost every major lymphoma subtype except chronic lymphomatous leukemia (CLL)/small lymphocytic lymphoma (SLL) and Hodgkin’s lymphoma (HL). A recent meta-analysis supported the positive association of HPgV-1 persistent infection with lymphoma risk [166]. HPgV-1 is a lymphotropic virus that causes persistent infection in both T and B lymphocytes [2,167]. Persistent HPgV-1 infection may induce DNA mutations and potentially malignant transformation in lymphocytes, which promote the development of lymphoma [165,166]. The possible causal relationship between HPgV-1 viremia and lymphoma risk suggests that HPgV-1 may be a risk marker and a potential therapeutic target for lymphoma.
Furthermore, Balcom et al. reported two cases of HPgV-1 related fatal brain leukocyte encephalitis, in which lymphocytic infiltration and gliosis were detected in the brain tissue, suggesting neurotropism of HPgV-1 [168]. The neural cell tropism of HPgV-1 was supported by another recent study that showed that HPgV-1 infects specific nerve cells in the human brain, such as astrocytes and microglia [169]. By inhibiting antiviral signaling pathways, HPgV-1 can establish persistent infection and promote the development of neurological diseases [169].
Given the potential association of HPgV-1 infection with the lymphoma risk [165,166], development of anti-HPgV-1 small molecule drugs might be beneficial for the treatment and prophylaxis of lymphoma. However, it is very time- and resource-consuming for the development of efficient antiviral small molecule drugs, which needs a suitable in vitro culture system for virus growth. Currently, there was no suitable culture system of HPgV-1 production (reviewed in [2]). Therefore, repurposing of existing anti-drugs may be an alternative strategy. One exciting development in antiviral researches is the development of direct-acting antivirals (DAAs) against HCV [170–173]. DAAs target the NS3/4A protease, the NS5A protein, and the NS5B polymerase of HCV, and cure HCV infection in over 90% of patients [172]. Despite the fact that HPgV-1 shares homogenous genes and has close epidemiological association with HCV, DAAs seem not to inhibit HPgV [174]. In view of the fact that HPgV-1 infection is benign in healthy individuals, whether it is necessary to develop anti-HPgV-1 therapeutic drugs deserves to be cautiously assessed.
HPgV-1 co-infection and human diseases
HPgV-1 has a high co-infection rate with other human viruses such as HIV-1 and HCV. HPgV-1 persistent infection can lead to significant improvements in clinical parameters and outcomes in patients co-infected with other viruses (Table 2), showing a beneficial effect on other viral diseases to some extent [4,144]. The beneficial outcome of HPgV-1 persistent infection was mainly associated with the inhibition or reduction of abnormal and excessive immune activation, especially the immune activation of T lymphocytes [4,144,145].
Table 2.
HPgV-1 prevalence in persons co-infected with HIV-1 or HCV or EBOV and clinical outcomes
| HPgV-1 co-infected cohorts | HPgV-1 prevalence | Clinical outcomes |
|---|---|---|
| HIV-1 infected | 5−47.9% | Higher survival, CD4 cell counts, and CD4+/CD8+ ratio Lower HIV-1 viral loads, and T-cell activation Slower progression to AIDS Decreased levels of cytokines and chemokines Down-regulation of CCR5 and CXCR4 expression Improved response to HAART Superior quality of life |
| HIV-1/HCV co-infecteda | 11.8−37.2% | Reduction in cirrhosis, hepatic fibrosis and inflammation Down-regulation of LCK and DOK2 expression Lower ALT and AST levels |
| EBOV infected | 26.5% b | Higher survival |
a: HPgV-1 infection does not show significant beneficial effect for HCV-mono-infected individuals. Furthermore, the data and clinical findings are mostly based on patients with HIV-1/HCV/HPgV-1 triple co-infection.
b: Data from one report.
Co-infection with HIV-1
Approximately 5–47.9% HIV-1 infected individuals were co-infected with HPgV-1 [55,89,95–100,151,153–156,175–185]. People who co-infected with HIV-1/HPgV-1 generally had relatively slower disease progression of AIDS and prolonged survival [89,153,155,156,181]. CD4 + T cell count and HIV-1 viral load are two crucial predictors of HIV/AIDS disease progression, and are used to determine the initiation and to evaluate the efficacy of highly active antiretroviral therapy (HAART) [186,187]. A large number of studies revealed that HPgV-1 viral load is significantly positively correlated to CD4 + T cell number, but negatively correlated to HIV-1 viral load [97,98,151,153,154,175,176,178–182,188–191] (Table 2). These findings indicate that HPgV-1 persistent infection is associated with a beneficial effect on HIV/AIDS.
There are diverse mechanisms involving in the beneficial effect of HPgV-1 co-infection on HIV-1 disease progression. First, HPgV-1 infection reduces surface expression of chemokine receptors CCR5 and CXCR4, both of which serve as co-receptors for HIV-1 entry into host cells (including CD4 + T cells, macrophages, DC cells) [183,192–195]. On the other hand, HPgV-1 E2 glycoprotein and NS5A protein can up-regulate the productions of the CCR5 ligands (e.g. RANTES, MIP-1α, and MIP-1β) and the CXCR4 ligand SDF-1, respectively [97,190,193,196]. Down-regulation of HIV-1 co-receptors and increased release of their ligands inhibit HIV-1 entry and reduce viral cell-to-cell transmission. Second, HPgV-1 E2 glycoprotein can reduce the production of mature capsid protein P24 and matrix protein P17 by inhibiting the processing of HIV-1 Gap precursor (P55), and thereby inhibit HIV-1 assembly [144,145,197,198]. Third, persistent immune activation and decreased Th1/Th2 cytokine ratio in HIV-1 infection are associated with rapid progression to AIDS. HPgV-1 co-infection reduces HIV-1-mediated activation of T, B, and/or NK cells, and contributes to the maintenance of balance between T-helper 1 (Th1) cytokines and Th2 cytokines, which delay the development of AIDS [199–202]. For example, HPgV-1 E2 glycoprotein inhibits T cell activation by reducing TCR-induced IL-2 production and altering IL-2 signaling pathways [152,156,202–205]. Furthermore, HPgV-1 infection is associated with an increase of CD4 and CD8 double-negative T cells (CD4-CD8-CD3+), which also contribute to reduction of immune activation and maintenance of immune homeostasis, further improving the survival of HIV-1 infected individuals [144,184,206]. Fourth, HPgV-1 E2 glycoprotein can induce antibodies to neutralize and precipitate diverse HIV-1 isolates possibly by cross-reaction with a cellular antigen on HIV-1 particles [45]. Fifth, HPgV-1 co-infection was reported to control HIV-1 replication by activating the endogenous interferon system, and to reduce Fas-mediated apoptosis of CD4 + T cells by down-regulating Fas expression [207]. Furthermore, HPgV-1 co-infection appeared to improve the response to HAART in HIV-infected individuals and the duration of HAART did not reduce HPgV-1 viremia [178,208].
Co-infection with HCV
HPgV-1 infection was closely related to HCV infection because of sharing the same transmission routes. About 11.8–37.2% HCV-infected individuals were co-infected with HPgV-1 [101–103,209–212]. Recent studies showed that HCV-infected individuals were also found to have a high proportion of HPgV-2 infection [30–32,174,213,214]. HPgV-1 infection was found to be associated with a significant reduction in the severity of HCV-related liver disease in HCV/HIV-1-co-infected patients, showing a beneficial influence [101,103,210]. In HCV/HIV-1-co-infected patients, HPgV-1 persistent infection remarkably decreases AST and ALT levels by down-regulating some crucial genes from intra-hepatic T-cell signal transduction, and then significantly improves chronic hepatitis C-related liver injury and reduces the incidence of hepatopathy (Table 2) [101,210]. These genes include LCK, DOK2, interleukin 2 receptor gamma (IL2R-γ), and cyclin D3 (CCND3), and are closely associated with T-cell receptor complex (TCR) [210]. However, a similar beneficial influence of HPgV-1 infection was not observed in HCV mono-infected patients [103]. The possible reason for this difference is that HPgV-1 is also a lymphotropic virus that may interact with HIV-1 by infecting the same cells, but not with HCV because of different cell targets.
Co-infection with Ebola virus
Ebola virus (EBOV) is an aggressive virus that causes highly lethal Ebola hemorrhagic fever (EHF) on humans and non-human primates. In Sierra Leone, Liberia and Guinea, the worst-hit areas by Ebola epidemic, about 11.1–18.9% of healthy individuals were infected by HPgV-1 (Figure 3). In a retrospective study that analyzed previous deep-sequencing data, 13 (26.5%) of 49 EBOV-infected individuals were found to be co-infected with HPgV-1 [215]. The survival rate of HPgV-1 co-infected Ebola patients was 53.8%, significantly higher than that (22.2%) of HPgV-1 negative Ebola patients, suggesting that HPgV-1 co-infection may attenuate the pathogenicity of EBOV [215]. The beneficial effect of HPgV-1 co-infection on Ebola patient might be also associated with reduced proinflammatory cytokines production and excessive T-cell activation.
Virus isolation and animal models
Cell tropism and host range
Because HPgV-1 was first identified from patients with acute or chronic non-A-E hepatitis, it was initially considered as a hepatotropic virus [3,6,5,8,9,37]. However, subsequent evidences did not support an association of HPgV-1 infection with either acute and/or chronic hepatitis. In particular, HPgV-1 RNA was found to be more frequently detected in circulating lymphocytes, but not or in a very low level in liver biopsies of infected people [2,216–218]. Furthermore, HPgV-1 RNA level remained relatively stable in patients with pre-transplantation HPgV-1 infection after liver transplantation, while HCV RNA level increased steady in patients with chronic hepatitis C after liver transplantation [219]. These evidences suggest that HPgV-1 is lymphotropic, rather than hepatotropic.
HPgV-1 RNA was detected in multiple lineages of peripheral blood mononuclear cells (PBMCs, including T lymphocytes, B lymphocytes, NK cells, and monocytes), indicating a wide tropism [167,220–222]. However, HPgV-1 negative-strand RNA, the marker of viral RNA replication, was preferentially detected in bone marrow and spleen, but less in PBMCs, suggesting that progenitor haematopoietic stem cell (HSC) may also be the primary target of HPgV-1 infection (reviewed in [2]). The presence of HPgV-1 in PBMCs indicates that the virus persists and replicates during and following subsequent lymphocyte maturation [2,218,220,223,224].
Old world primates are believed to be the natural hosts of HPgV-1 [18,225]. Apart from humans, HPgV-1 can also infect chimpanzees and macaques [225,226]. Whether other primates and/or animals are also susceptible to HPgV-1 infection remains to be determined.
In vitro culture of HPgV-1
Establishment of an in vitro cell culture system is crucial for studying the biological characteristics and molecular mechanisms of HPgV-1, as well as developing strategies for prophylactic and therapeutic interventions. Development of an efficient cell culture system depends on permissive cells (primary cells or cell lines) supporting infection and production of infectious virion, and a virus or its infectious clone capable of replicating and assembling virion in permissive cells.
As a lymphotropic virus, HPgV-1 extensively exists in multiple lineages of PBMCs [2,167], and PBMCs from HPgV-1 infected people were demonstrated to transfer the virus to primary PBMCs of healthy individuals in vitro [220,221]. Serum from HPgV-1-infected individuals was also demonstrated to establish infection in PBMCs in vitro [167]. Using primary PBMCs, in vitro HPgV-1 culture systems have been previously established, and the culture could be maintained up to 35 days [220,221]. However, HPgV-1 replication appears to be very limited in PBMCs. The virus can be poorly produced in vitro PBMCs culture system, and average less than 10 HPgV-1 genomic copies can be detected among per 100 PBMCs [167,223]. These imply that only a very small proportion of PBMCs support HPgV-1 replication, or there are some potential cellular restriction factors to inhibit HPgV-1 replication in PBMCs [227]. On the other hand, because cellular receptors for HPgV-1 infection remain unknown, the permissive cell lines supporting HPgV-1 infection and replication need to be determined [2].
In recent years, reverse genetics systems have been developed and provide powerful tools to recover some uncultivated viruses [228]. Using the systems, infectious clones of some emerging viruses, including HCV [229,230], Zika virus, and dengue virus from the Flaviviridae family [231–234], and the newly emerging SARS-CoV-2 [235–237], have been constructed. Although currently the infectious clone of HPgV-1 was not available, two full-length cDNA clones of HPgV-1 were previously constructed and their in vitro full-length RNA transcripts were proved to be infectious in primary CD4 + T cells [238] and in macaques (Macaca mulatta) [239]. In view of sharing similar genome organization and homogenous genes to HCV, the success in the development of efficient cell culture systems for HCV and other Flaviviridae viruses [229,230] and availability of increasing number of complete HPgV-1 genome sequences provide avenues for the development of HPgV-1 infectious clones using reverse genetics tools in the future.
Animal models
The lack of appropriate animal models for HPgV-1 infection limits the understanding of its pathogenesis. Non-human primates (NHPs) are considered to be the ideal animal models for viral diseases since they are closely genetically related to humans than other animals. As the most widely used animal models for viral diseases such as HIV/AIDS, macaques and chimpanzees are considered as primary animal models of HPgV-1 infection since they might be susceptible to HPgV-1 infection [18,225,226,239–241]. For ethical and financial reasons, macaques are preferred to be used for the NHP model of HPgV-1. However, macaques often failed to be experimentally infected with HPgV-1 [3].
Fortunately, some simian pegiviruses (SPgV) that are closely genetially related to HPgV-1 were recently identified and characterized from some old world monkey species (e.g. red colobus monkeys, red-tailed guenons, and olive baboon) [18]. Using a SPgV strain isolated from yellow baboons in Mikumi National Park, Tanzania, a macaque model of HPgV-1 infection was recently established [225]. The SPgV-infected macaques showed similar clinical characteristics (e.g. persistent infection, high-titer viremia, and lack of obvious pathogenic symptoms) to HPgV-1 infected humans. In this model, bone marrow and spleen were further confirmed to be the predominant tissues for HPgV-1 replication and production [225].
On the other hand, development of NHP models for human infectious diseases was largely limited by extremely high cost, difficulty to reach sufficient sample size, as well as raising ethical concerns of experimentation on NHPs. As the most widely used small animal models, humanized mice might represent a rapid, convenient, and promising direction for the development of animal models for HPgV-1 infection and other human viral diseases due to their rapid reproductive capacity, clear genetic background, and well-defined immune systems [242].
Potential use of HPgV-1 in therapy
As a non-pathogenic virus, a large number of epidemiological and clinical studies demonstrated the protective effect of HPgV-1 persistent infection on HIV-1 infection, which was well supported by diverse molecular mechanisms, involving in the inhabitation of HIV-1 entry and replication and the suppression of immune activation [2,36,144]. Similar beneficial effect of HPgV-1 infection was also observed in Ebola patients and HIV-1/HCV co-infected patients [101,210,215]. These imply a high potential of HPgV-1 as a therapeutic bio-vaccine to be used in people living with HIV/AIDS in resource-limited regions where HAART is not common [243]. Furthermore, HPgV-1 was demonstrated to preferentially infect and replicate in HSC without cytopathic effect, implying another potential therapeutic application of HPgV-1 as viral vectors [218,224,225].
Therapeutic potential of HPgV-1 as a biovaccine was recently validated in a macaque model, in which the monkeys were sequentially infected by SPgV and simian immunodeficiency virus (SIV) [244]. In the model, the protective effect of SPgV was found to preferentially occur during the chronic phase of SIV infection [244]. In 2019, Greenhalgh and colleagues evaluated the feasibility of HPgV-1 as a biovaccine for HIV/AIDS [245]. Based on the epidemiological data of AIDS among MSM, they constructed a mathematical model to evaluate the potential impact of HPgV-1 biovaccination on AIDS-associated morbidity and mortality. They revealed that HPgV-1 biovaccination can effectively reduce the incidence of HIV/AIDS, AIDS-associated death and improve disability-adjusted life years (DALYs) of HIV-1 patients. Furthermore, the detrimental impact from HPgV-1 evolution was found to be very small under relatively high biovaccination rates (>12.5% annually) [245]. In fact, HPgV-1 is relatively evolutionarily conservative [246–248]. The SPgV-infected macaque model also supported an extremely low propensity of pegivirus to accumulate sequence variation [225]. In this model, about 1.5 variants were identified per 100 infection-days, and no consensus-level variants were detected, implying a very low risk of the HPgV-1 biovaccine strain evolving to pathogenic variants [225]. On the other hand, HPgV-1 co-infection seemed not to alter the evolution of HIV-1 [245]. Despite the significant progress in this field, it is still a long way for HPgV-1 to be used as a biovaccine to treat human infectious diseases. A major challenge is lack of an efficient in vitro culture system for HPgV-1 growth, which requires to first elucidate the cellular receptor of the virus and then develop appropriate (permissive) cell lines [2]. Furthermore, clinical trials to evaluate the effectiveness of HPgV-1 biovaccination in HIV-1-infected people are mandatory before regulatory approval and clinical application.
Conclusion and future perspective
HPgV-1 is more like a non-pathogenic virus in the Flaviviridae family in spite of recent observation of a positive association of HPgV-1 viremia with increased risk of lymphoma in immunocompromised individuals. It is commonly prevalent in general population with extremely high proportions in high-risk groups such as IDUs and MSM. Persistent HPgV-1 infection slows the disease progression and improves the survival of individuals co-infected with HIV-1 and other pathogens by diverse molecular mechanisms, showing significant beneficial clinical effects. As a non-cytopathic lymphotropic virus that infects and preferentially replicates in bone marrow and spleen, HPgV-1 shows high therapeutic potential for infectious diseases as a biovaccine or a safe viral vector.
Although the promising results in HPgV-1 related epidemiological, experimental, and clinical studies, several underlying questions remain to be addressed. First, lack of an efficient in vitro culture system is a major barrier that limits the molecular biological mechanism researches of HPgV-1, including but not limited to the identification of cellular receptor of the virus, life cycle, persistence and clearance mechanism, viral interactions with the host immune system (interacting with immune cells and regulating immune activation), and with co-infected viruses (e.g. HIV-1, HCV, EBOV) or other pathogens (e.g. malaria) [249], as well as the development of suitable animal models and therapeutic biovaccine and/or vectors. Second, the association of HPgV-1 infection with the development of lymphoma, and even neurological diseases also need to be cautiously and systematically assessed. Furthermore, currently, COVID-19 is still the greatest threat to global public health. Whether there is a difference in the susceptibility to SARS-CoV-2 infection and the disease severity of COVID-19 between HPgV-1 infected and uninfected people deserves to be investigated.
Acknowledgments
We thank our colleague Yingying Ma for providing technical support on phylogenetic tree construction.
Funding Statement
This work was supported in part by the Grant from the National Natural Science Foundation of China (32170147).
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- CCND3
Cyclin D3
- CLL
Chronic lymphomatous leukemia
- COVID-19
Corona virus disease 2019
- CSWs
Commercial sex workers
- DAAs
Direct-acting antivirals
- DALYs
Disability-adjusted life years
- EBOV
Ebola virus
- EHF
Ebola hemorrhagic fever
- GBV-A or -B or -C or -D
GB virus A or B or C or D
- HAART
Highly active antiretroviral therapy
- HCV
Hepatitis C virus
- HGV
Hepatitis G virus
- HIV-1
Human immunodeficiency virus type 1
- HL
Hodgkin<apos;>s lymphoma
- HPgV-1
Human pegivirus type 1
- HPgV-2
Human pegivirus type 2 or Human hepegivirus type 1
- HSC
Haematopoietic stem cell
- IDUs
Intravenous drug users
- IL2R-γ
Interleukin 2 receptor gamma
- IRES
Internal ribosome entry site
- MSM
Men who have sex with men
- NHPs
Non-human primates
- ORF
Open reading frame
- PBMCs
Peripheral blood mononuclear cells
- PIs
Percutaneous injuries
- RdRp
RNA-dependent RNA polymerase
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SIV
Simian immunodeficiency virus
- SLL
Small lymphocytic lymphoma
- SPgV
Simian pegiviruses
- TCR
T-cell receptor complex
- Th1(or 2)
T-helper 1 (or 2) cytokines
- UTRs
Untranslated regions
- DC
Dendritic cell
- NK cell
Natural killer cell
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- [1].Marano G, Franchini M, Farina B, et al. The human pegivirus: a new name for an “ancient” virus. can transfusion medicine come up with something new? Acta Virol. 2017;61:401–412. [DOI] [PubMed] [Google Scholar]
- [2].Chivero ET, Stapleton JT.. Tropism of human pegivirus (formerly known as GB virus C/hepatitis G virus) and host immunomodulation: insights into a highly successful viral infection. J Gen Virol. 2015;96:1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stapleton JT, Foung S, Muerhoff AS, et al. The GB viruses: a review and proposed classification of GBV-A, GBV-C (HGV), and GBV-D in genus pegivirus within the family Flaviviridae. J Gen Virol. 2011;92:233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schwarze-Zander C, Blackard JT, Rockstroh JK. Role of GB virus C in modulating HIV disease. Expert Rev Anti Infect Ther. 2012;10:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Giret MTM, Kallas EG. GBV-C: state of the art and future prospects. Curr HIV/AIDS Rep. 2012;9:26–33. [DOI] [PubMed] [Google Scholar]
- [6].Bhattarai N, Stapleton JT. GB virus C: the good boy virus? Trends Microbiol. 2012;20:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mohr EL, Stapleton JT. GB virus type C interactions with HIV: the role of envelope glycoproteins. J Viral Hepat. 2009;16:757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Deinhardt F, Holmes AW, Capps RB, et al. Studies on the transmission of human viral hepatitis to marmoset monkeys. I. transmission of disease, serial passages, and description of liver lesions. J Exp Med. 1967;125:673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Simons JN, Pilot-Matias TJ, Leary TP, et al. Identification of two flavivirus-like genomes in the GB hepatitis agent. Proc Natl Acad Sci U S A. 1995;92:3401–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Simons JN, Leary TP, Dawson GJ, et al. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med. 1995;1:564–569. [DOI] [PubMed] [Google Scholar]
- [11].Leary TP, Muerhoff AS, Simons JN, et al. Sequence and genomic organization of GBV-C: a novel member of the flaviviridae associated with human non-A-E hepatitis. J Med Virol. 1996;48:60–67. [DOI] [PubMed] [Google Scholar]
- [12].Linnen J, Wages J, Zhang-Keck ZY, et al. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505–508. [DOI] [PubMed] [Google Scholar]
- [13].Epstein JH, Quan P-L, Briese T, et al. Identification of GBV-D, a novel GB-like flavivirus from old world frugivorous bats (pteropus giganteus) in Bangladesh. PLoS Pathog. 2010;6:e1000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bukh J, Apgar CL, Yanagi M. Toward a surrogate model for hepatitis C virus: an infectious molecular clone of the GB virus-B hepatitis agent. Virology. 1999;262:470–478. [DOI] [PubMed] [Google Scholar]
- [15].Martin A, Bodola F, Sangar DV, et al. Chronic hepatitis associated with GB virus B persistence in a tamarin after intrahepatic inoculation of synthetic viral RNA. Proc Natl Acad Sci U S A. 2003;100:9962–9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Adams NJ, Prescott LE, Jarvis LM, et al. Detection in chimpanzees of a novel flavivirus related to GB virus-C/hepatitis G virus. J Gen Virol. 1998;79((Pt 8)):1871–1877. [DOI] [PubMed] [Google Scholar]
- [17].Birkenmeyer LG, Desai SM, Muerhoff AS, et al. Isolation of a GB virus-related genome from a chimpanzee. J Med Virol. 1998;56:44–51. [DOI] [PubMed] [Google Scholar]
- [18].Bukh J, Apgar CL. Five new or recently discovered (GBV-A) virus species are indigenous to new world monkeys and may constitute a separate genus of the Flaviviridae. Virology. 1997;229:429–436. [DOI] [PubMed] [Google Scholar]
- [19].Charrel RN, De Micco P, de Lamballerie X. Phylogenetic analysis of GB viruses A and C: evidence for cospeciation between virus isolates and their primate hosts. J Gen Virol. 1999;80((Pt 9)):2329–2335. [DOI] [PubMed] [Google Scholar]
- [20].Mohr EL, Murthy KK, McLinden JH, et al. The natural history of non-human GB virus C in captive chimpanzees. J Gen Virol. 2011. 92 :91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sibley SD, Lauck M, Bailey AL, et al. Discovery and characterization of distinct simian pegiviruses in three wild African old world monkey species. PLoS One. 2014;9(6):e98569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kapoor A, Simmonds P, Cullen JM, et al. Identification of a pegivirus (GB virus-like virus) that infects horses. J Virol. 2013;87:7185–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].de Souza AJS, Malheiros AP, de Sousa ERP, et al. First report of equine pegivirus in South America, Brazil. Acta Trop. 2015;152:56–59. [DOI] [PubMed] [Google Scholar]
- [24].Tang W, Zhu N, Wang H, et al. Identification and genetic characterization of equine pegivirus in China. J Gen Virol. 2018;99:768–776. [DOI] [PubMed] [Google Scholar]
- [25].Tomlinson JE, Wolfisberg R, Fahnøe U, et al. Equine pegiviruses cause persistent infection of bone marrow and are not associated with hepatitis. PLoS Pathog. 2020;16:e1008677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kapoor A, Simmonds P, Scheel TKH, et al. Identification of rodent homologs of hepatitis C virus and pegiviruses. mBio. 2013;4:e00216–e00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Quan P-L, Firth C, Conte JM, et al. Bats are a major natural reservoir for hepaciviruses and pegiviruses. Proc Natl Acad Sci U S A. 2013;110:8194–8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Van Nguyen D, Van Nguyen C, Bonsall D, et al. Detection and characterization of homologues of human hepatitis viruses and pegiviruses in rodents and bats in Vietnam. Viruses. 2018;10 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu Z, Wu Y, Zhang W, et al. The first nonmammalian pegivirus demonstrates efficient replication and high lymphotropism. J Virol. 2020. 94 :e01150–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhen W, Wu Y, Zhang W, et al. Emergence of a novel pegivirus species in southwest China showing a high rate of coinfection with parvovirus and circovirus in geese. Poult Sci. 2021;100:101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kapoor A, Kumar A, Simmonds P, et al. Virome analysis of transfusion recipients reveals a novel human virus that shares genomic features with hepaciviruses and pegiviruses. mBio. 2015;6:e01466–e01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Berg MG, Lee D, Coller K, et al. Discovery of a novel human pegivirus in blood associated with hepatitis C virus co-infection. PLoS Pathog. 2015;11:e1005325. DOI: 10.1371/journal.ppat.1005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liang Y, Hu F, Fan H, et al. Difference of intrahost dynamics of the second human pegivirus and hepatitis C virus in HPgV-2/HCV-Coinfected patients. Front Cell Infect Microbiol. 2021;11:728415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang H, Wan Z, Sun Q, et al. Second human pegivirus in hepatitis c virus-infected and hepatitis c virus/HIV-1-Co-infected persons who inject drugs, China. Emerg Infect Dis. 2018;24:908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Anh NT, Hong NTT, Nhu LNT, et al. Detection and characterization of human pegivirus 2, Vietnam. Emerg Infect Dis. 2018;24:2063–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rodgers MA, Holzmayer V, Vallari A, et al. Hepatitis C virus surveillance and identification of human pegivirus 2 in a large Cameroonian cohort. J Viral Hepat. 2019;26:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Simons JN, Desai SM, Schultz DE, et al. Translation initiation in GB viruses A and C: evidence for internal ribosome entry and implications for genome organization. J Virol. 1996;70:6126–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim CW, Chang K-M. Hepatitis C virus: virology and life cycle. Clin Mol Hepatol. 2013;19:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim JP, Fry KE. Molecular characterization of the hepatitis G virus. J Viral Hepat. 1997;4:77–79. [DOI] [PubMed] [Google Scholar]
- [40].Agnello V, Abel G, Elfahal M, et al. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1999;96:12766–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. [DOI] [PubMed] [Google Scholar]
- [42].Xiang J, Klinzman D, McLinden J, et al. Characterization of hepatitis G virus (GB-C virus) particles: evidence for a nucleocapsid and expression of sequences upstream of the E1 protein. J Virol. 1998;72:2738–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dubuisson J. Hepatitis C virus proteins. World J Gastroenterol. 2007;13:2406–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tacke M, Schmolke S, Schlueter V, et al. Humoral immune response to the E2 protein of hepatitis G virus is associated with long-term recovery from infection and reveals a high frequency of hepatitis G virus exposure among healthy blood donors. Hepatology. 1997;26:1626–1633. [DOI] [PubMed] [Google Scholar]
- [45].Tillmann HL, Heringlake S, Trautwein C, et al. Antibodies against the GB virus C envelope 2 protein before liver transplantation protect against GB virus C de novo infection. Hepatology. 1998;28:379–384. [DOI] [PubMed] [Google Scholar]
- [46].Eissmann K, Mueller S, Sticht H, et al. HIV-1 fusion is blocked through binding of GB Virus C E2-derived peptides to the HIV-1 gp41 disulfide loop [corrected]. PLoS One. 2013;8:e54452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mohr EL, Xiang J, McLinden JH, et al. GB virus type C envelope protein E2 elicits antibodies that react with a cellular antigen on HIV-1 particles and neutralize diverse HIV-1 isolates. J Immunol. 2010;185:4496–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chivero ET, Bhattarai N, McLinden JH, et al. Human pegivirus (HPgV; formerly known as GBV-C) inhibits IL-12 dependent natural killer cell function. Virology. 2015;485:116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bhattarai N, McLinden JH, Xiang J, et al. GB virus C envelope protein E2 inhibits TCR-induced IL-2 production and alters IL-2-signaling pathways. J Immunol. 2012;189:2211–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Penin F, Dubuisson J, Rey FA, et al. Structural biology of hepatitis C virus. Hepatology. 2004. 39 :5–19. [DOI] [PubMed] [Google Scholar]
- [51].Neddermann P, Tomei L, Steinkühler C, et al. The nonstructural proteins of the hepatitis C virus: structure and functions. Biol Chem. 1997;378:469–476. [PubMed] [Google Scholar]
- [52].Belyaev AS, Chong S, Novikov A, et al. Hepatitis G virus encodes protease activities which can effect processing of the virus putative nonstructural proteins. J Virol. 1998;72:868–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yang N, Dai R, Zhang X. Global prevalence of human pegivirus-1 in healthy volunteer blood donors: a systematic review and meta-analysis. Vox Sang. 2020;115:107–119. [DOI] [PubMed] [Google Scholar]
- [54].Zimmerman J, Blackard JT. Human pegivirus type 1 infection in Asia-A review of the literature. Rev Med Virol. 2021:e2257. DOI: 10.1002/rmv.2257. [DOI] [PubMed] [Google Scholar]
- [55].El-Zayadi AR, Abe K, Selim O, et al. Prevalence of GBV-C/hepatitis G virus viraemia among blood donors, health care personnel, chronic non-B non-C hepatitis, chronic hepatitis C and hemodialysis patients in Egypt. J Virol Methods. 1999;80:53–58. [DOI] [PubMed] [Google Scholar]
- [56].Ben Dhifallah I, Ayouni K, Chouiha A, et al. Genotype distribution and prevalence of human pegivirus among high-risk populations in Tunisia. Intervirology. 2016;59:170–178. [DOI] [PubMed] [Google Scholar]
- [57].de Pina-araujo IIM, Horta MA, Do Amaral Mello FC, et al. Human pegivirus 1 in Cabo Verde: prevalence and genotypic distribution among HIV-infected individuals. Arch Virol. 2021;166:1345–1353. [DOI] [PubMed] [Google Scholar]
- [58].Sathar MA, Soni PN, Naicker S, et al. GB virus C/hepatitis G virus infection in KwaZulu Natal, South Africa. J Med Virol. 1999;59:38–44. [PubMed] [Google Scholar]
- [59].Casteling A, Song E, Sim J, et al. GB virus C prevalence in blood donors and high risk groups for parenterally transmitted agents from Gauteng, South Africa. J Med Virol. 1998;55:103–108. [DOI] [PubMed] [Google Scholar]
- [60].Shaker EK, Al-Jebouri MM, Al-Mayah QS, et al. Phylogenetic analysis of human pegivirus from anti-hepatitis C virus IgG- positive patients. Infect Genet Evol. 2021;96:105099. [DOI] [PubMed] [Google Scholar]
- [61].Ghanbari R, Ravanshad M, Hosseini SY, et al. Genotyping and infection rate of GBV-C among Iranian HCV- infected patients. Hepat Mon. 2010;10:80–87. [PMC free article] [PubMed] [Google Scholar]
- [62].Abu Odeh RO, Al-Moslih MI, Al-Jokhdar MW, et al. Detection and genotyping of GBV-C virus in the United Arab Emirates. J Med Virol. 2005;76:534–540. [DOI] [PubMed] [Google Scholar]
- [63].Yan J, Chen LL, Luo YH, et al. High frequencies of HGV and TTV infections in blood donors in Hangzhou. World J Gastroenterol. 2001;7:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nakatsuji Y, Shih JW, Tanaka E, et al. Prevalence and disease association of hepatitis G virus infection in Japan. J Viral Hepat. 1996;3:307–316. [DOI] [PubMed] [Google Scholar]
- [65].Brown KE, Wong S, Buu M, et al. High prevalence of GB virus C/hepatitis G virus in healthy persons in Ho Chi Minh City, Vietnam. J Infect Dis. 1997;175:450–453. [DOI] [PubMed] [Google Scholar]
- [66].Nakai K, Win KM, Oo SS, et al. Molecular characteristic-based epidemiology of hepatitis B, C, and E viruses and GB virus C/hepatitis G virus in Myanmar. J Clin Microbiol. 2001;39:1536–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jeon MJ, Shin JH, Suh SP, et al. TT virus and hepatitis G virus infections in Korean blood donors and patients with chronic liver disease. World J Gastroenterol. 2003;9:741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kondo Y, Mizokami M, Nakano T, et al. Prevalence and molecular epidemiology of GB virus C/hepatitis G virus infection in Mongolia. J Med Virol. 1997;52:143–148. [PubMed] [Google Scholar]
- [69].Moaven LD, Hyland CA, Young IF, et al. Prevalence of hepatitis G virus in Queensland blood donors. Med J Aust. 1996;165:369–371. [DOI] [PubMed] [Google Scholar]
- [70].Hitzler WE, Runkel S. Prevalence, persistence and liver enzyme levels of HGV RNA-positive blood donors determined by large-scale screening and transmission by blood components. Clin Lab. 2004;50:25–31. [PubMed] [Google Scholar]
- [71].Mercier B, Barclais A, Botte C, et al. Prevalence of GBV C/HGV RNA and GBV C/HGV antibodies in French volunteer blood donors: results of a collaborative study. Vox Sang. 1999;76:166–169. [DOI] [PubMed] [Google Scholar]
- [72].Blair CS, Davidson F, Lycett C, et al. Prevalence, incidence, and clinical characteristics of hepatitis G virus/GB virus C infection in Scottish blood donors. J Infect Dis. 1998;178:1779–1782. [DOI] [PubMed] [Google Scholar]
- [73].Césaire R, Martial J, Maier H, et al. Infection with GB virus C/hepatitis G virus among blood donors and hemophiliacs in Martinique, a Caribbean island. J Med Virol. 1999;59:160–163. [PubMed] [Google Scholar]
- [74].Alter HJ, Nakatsuji Y, Melpolder J, et al. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997;336:747–754. [DOI] [PubMed] [Google Scholar]
- [75].Giulivi A, Slinger R, Tepper M, et al. Prevalence of GBV-C/hepatitis G virus viremia and anti-E2 in Canadian blood donors. Vox Sang. 2000;79:201–205. [DOI] [PubMed] [Google Scholar]
- [76].Alvarado-Mora MV, Botelho L, Nishiya A, et al. Frequency and genotypic distribution of GB virus C (GBV-C) among Colombian population with hepatitis B (HBV) or hepatitis C (HCV) infection. Virol J. 2011;8:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Silva ADSN, Silva CP, Barata RR, et al. Human pegivirus (HPgV, GBV-C) RNA in volunteer blood donors from a public hemotherapy service in Northern Brazil. Virol J. 2020;17:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Slavov SN, Maraninchi Silveira R, Hespanhol MR, et al. Human pegivirus-1 (HPgV-1) RNA prevalence and genotypes in volunteer blood donors from the Brazilian Amazon. Transfus Clin Biol. 2019;26:234–239. [DOI] [PubMed] [Google Scholar]
- [79].Konomi N, Miyoshi C, La Fuente Zerain C, et al. Epidemiology of hepatitis B, C, E, and G virus infections and molecular analysis of hepatitis G virus isolates in Bolivia. J Clin Microbiol. 1999;37:3291–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].N’Guessan KF, Boyce C, Kwara A, et al. Human pegivirus (HPgV) infection in Ghanaians co-infected with human immunodeficiency virus (HIV) and hepatitis B virus (HBV). Virus Genes. 2018;54:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jõgeda E-L, Huik K, Pauskar M, et al. Prevalence and genotypes of GBV-C and its associations with HIV infection among persons who inject drugs in Eastern Europe. J Med Virol. 2017;89:632–638. [DOI] [PubMed] [Google Scholar]
- [82].Neibecker M, Schwarze-Zander C, Rockstroh JK, et al. Evidence for extensive genotypic diversity and recombination of GB virus C (GBV-C) in Germany. J Med Virol. 2011;83:685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Li Z, Li Y, Liang Y, et al. Prevalence and risk factors of human pegivirus type 1 infection in hematopoietic stem cell transplantation patients. Int J Infect Dis. 2019;85:111–113. [DOI] [PubMed] [Google Scholar]
- [84].Yu ML, Chuang WL, Wang LY, et al. Status and natural course of GB virus C/hepatitis G virus infection among high-risk groups and volunteer blood donors in Taiwan. J Gastroenterol Hepatol. 2000;15:1404–1410. [DOI] [PubMed] [Google Scholar]
- [85].Loureiro CL, Alonso R, Pacheco BA, et al. High prevalence of GB virus C/hepatitis G virus genotype 3 among autochthonous Venezuelan populations. J Med Virol. 2002;68:357–362. [DOI] [PubMed] [Google Scholar]
- [86].Lee CK, Tang JW-T, Chiu L, et al. Epidemiology of GB virus type C among patients infected with HIV in Singapore. J Med Virol. 2014;86:737–744. [DOI] [PubMed] [Google Scholar]
- [87].Handajani R, Soetjipto L, Suryohudoyo MI, et al. Prevalence of GB virus C/hepatitis G virus infection among various populations in Surabaya, Indonesia, and identification of novel groups of sequence variants. J Clin Microbiol. 2000;38:662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Naito H, Abe K. Genotyping system of GBV-C/HGV type 1 to type 4 by the polymerase chain reaction using type-specific primers and geographical distribution of viral genotypes. J Virol Methods. 2001;91:3–9. [DOI] [PubMed] [Google Scholar]
- [89].Anggorowati N, Yano Y, Subronto YW, et al. GB virus C infection in Indonesian HIV-positive patients. Microbiol Immunol. 2013;57:298–308. [DOI] [PubMed] [Google Scholar]
- [90].AbuOdeh RO, Al-Absi E, Ali NH, et al. Detection and phylogenetic analysis of human pegivirus (GBV-C) among blood donors and patients infected with hepatitis B virus (HBV) in Qatar. J Med Virol. 2015;87:2074–2081. [DOI] [PubMed] [Google Scholar]
- [91].Miao Z, Gao L, Song Y, et al. Prevalence and clinical impact of human pegivirus-1 infection in HIV-1-infected individuals in Yunnan, China. Viruses. 2017;9 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Deuffic-Burban S, Delarocque-Astagneau E, Abiteboul D, et al. Blood-borne viruses in health care workers: prevention and management. J Clin Virol. 2011. 52 :4–10. [DOI] [PubMed] [Google Scholar]
- [93].Nordbø SA, Krokstad S, Winge P, et al. Prevalence of GB virus C (also called hepatitis G virus) markers in Norwegian blood donors. J Clin Microbiol. 2000;38:2584–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nerurkar VR, Chua PK, Hoffmann PR, et al. High prevalence of GB virus C/hepatitis G virus infection among homosexual men infected with human immunodeficiency virus type 1: evidence for sexual transmission. J Med Virol. 1998;56:123–127. [PubMed] [Google Scholar]
- [95].Scallan MF, Clutterbuck D, Jarvis LM, et al. Sexual transmission of GB virus C/hepatitis G virus. J Med Virol. 1998;55:203–208. [DOI] [PubMed] [Google Scholar]
- [96].Bourlet T, Guglielminotti C, Evrard M, et al. Prevalence of GBV-C/hepatitis G virus RNA and E2 antibody among subjects infected with human immunodeficiency virus type 1 after parenteral or sexual exposure. J Med Virol. 1999;58:373–377. [PubMed] [Google Scholar]
- [97].Chakraborty R, Rees G, Bourboulia D, et al. Viral coinfections among African children infected with human immunodeficiency virus type 1. Clin Infect Dis. 2003;36:922–924. [DOI] [PubMed] [Google Scholar]
- [98].Compston LI, Li C, Sarkodie F, et al. Prevalence of persistent and latent viruses in untreated patients infected with HIV-1 from Ghana, West Africa. J Med Virol. 2009;81:1860–1868. [DOI] [PubMed] [Google Scholar]
- [99].Rodríguez AK, Garzaro DJ, Loureiro CL, et al. HIV-1 and GBV-C co-infection in Venezuela. J Infect Dev Ctries. 2014;8:863–868. [DOI] [PubMed] [Google Scholar]
- [100].Alcalde R, Nishiya A, Casseb J, et al. Prevalence and distribution of the GBV-C/HGV among HIV-1-infected patients under anti-retroviral therapy. Virus Res. 2010;151:148–152. [DOI] [PubMed] [Google Scholar]
- [101].Bhanich Supapol W, Remis RS, Raboud J, et al. Prevalence and correlates of GB virus C infection in HIV-infected and HIV-uninfected pregnant women in Bangkok, Thailand. J Med Virol. 2011;83:33–44. [DOI] [PubMed] [Google Scholar]
- [102].Keyvani H, Mohammadi A, Haji-Abdolbaghi M. Prevalence of GBV-C RNA in HIV infected individuals in Tehran, Iran. Iran J Public Health. 2010;39:22–27. [PMC free article] [PubMed] [Google Scholar]
- [103].Feng Y, Liu L, Feng Y-M, et al. GB virus C infection in patients with HIV/Hepatitis C virus coinfection: improvement of the liver function in chronic hepatitis C. Hepat Mon. 2014;14:e14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Shahid M, Idrees M, Butt AM, et al. Short article: hepatitis C and G virus coinfection in Punjab, Pakistan: incidence and its correlation analysis with clinical data. Eur J Gastroenterol Hepatol. 2019;31:389–392. [DOI] [PubMed] [Google Scholar]
- [105].Hofer H, Aydin I, Neumueller-Guber S, et al. Prevalence and clinical significance of GB virus type C/hepatitis G virus coinfection in patients with chronic hepatitis C undergoing antiviral therapy. J Viral Hepat. 2011;18:513–517. [DOI] [PubMed] [Google Scholar]
- [106].Alhetheel A, El-Hazmi MM. Hepatitis G virus in Saudi blood donors and chronic hepatitis B and C patients. J Infect Dev Ctries. 2014;8:110–115. [DOI] [PubMed] [Google Scholar]
- [107].Kar P, Bedi P, Berry N, et al. Hepatitis G virus (HGV) infection in voluntary and commercial blood donors in India. Diagn Microbiol Infect Dis. 2000. 38 :7–10. [DOI] [PubMed] [Google Scholar]
- [108].Arankalle VA, Deshmukh TM, Chobe LP, et al. Hepatitis G virus infection in India: prevalence and phylogenetic analysis based on 5’ non-coding region. Indian J Gastroenterol. 2001;20:13–17. [PubMed] [Google Scholar]
- [109].Wang X, Sun D, Zhuang H. Follow-up studies on hepatitis G infection in plasma donors with positive antibody against hepatitis C (in Chinese). Zhonghua Yu Fang Yi Xue Za Zhi. 1997;31:269–271. [PubMed] [Google Scholar]
- [110].Odeh RA, Yasin S, Nasrallah G, et al. Rates of infection and phylogenetic analysis of GB virus-C among Kuwaiti and Jordanian blood donors. Intervirology. 2010;53:402–407. [DOI] [PubMed] [Google Scholar]
- [111].Wang HL, Jin DY. Prevalence and genotype of hepatitis G virus in Chinese professional blood donors and hepatitis patients. J Infect Dis. 1997;175:1229–1233. [DOI] [PubMed] [Google Scholar]
- [112].Wang T, Chen J, Zhang Q, et al. Prevalence of hepatitis G virus infection among 67,348 blood donors in mainland China. BMC Public Health. 2019;19:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Feng Y, Zhao W, Feng Y, et al. A novel genotype of GB virus C: its identification and predominance among injecting drug users in Yunnan, China. PLoS One. 2011;6:e21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wu RR, Mizokami M, Cao K, et al. GB virus C/hepatitis G virus infection in southern China. J Infect Dis. 1997;175:168–171. [DOI] [PubMed] [Google Scholar]
- [115].Hyland CA, Mison L, Solomon N, et al. Exposure to GB virus type C or hepatitis G virus in selected Australian adult and children populations. Transfusion. 1998;38:821–827. [DOI] [PubMed] [Google Scholar]
- [116].Löve A, Stanzeit B, Gudmundsson S, et al. Hepatitis G virus infections in Iceland. J Viral Hepat. 1999;6:255–260. [DOI] [PubMed] [Google Scholar]
- [117].Christensen PB, Fisker N, Mygind LH, et al. GB virus C epidemiology in Denmark: different routes of transmission in children and low- and high-risk adults. J Med Virol. 2003;70:156–162. [DOI] [PubMed] [Google Scholar]
- [118].Jongerius J, Boland G, van der Poel C, et al. GB virus type C viremia and envelope antibodies among population subsets in The Netherlands. Vox Sang. 1999;76:81–84. [DOI] [PubMed] [Google Scholar]
- [119].Gutierrez RA, Dawson GJ, Knigge MF, et al. Seroprevalence of GB virus C and persistence of RNA and antibody. J Med Virol. 1997;53:167–173. [DOI] [PubMed] [Google Scholar]
- [120].Dawson GJ, Schlauder GG, Pilot-Matias TJ, et al. Prevalence studies of GB virus-C infection using reverse transcriptase-polymerase chain reaction. J Med Virol. 1996. 50 :97–103. [DOI] [PubMed] [Google Scholar]
- [121].Oubiña JR, Mathet V, Feld M, et al. Genetic diversity of GBV-C/HGV strains among HIV infected-IVDU and blood donors from Buenos Aires, Argentina. Virus Res. 1999;65:121–129. [DOI] [PubMed] [Google Scholar]
- [122].Tian D-Y, Yang D-F, Xia N-S, et al. The serological prevalence and risk factor analysis of hepatitis G virus infection in Hubei Province of China. World J Gastroenterol. 2000;6:585–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Berzsenyi MD, Bowden DS, Bailey MJ, et al. Male to male sex is associated with a high prevalence of exposure to GB virus C. J Clin Virol. 2005;33:243–246. [DOI] [PubMed] [Google Scholar]
- [124].Asim M, Potukuchi SK, Arora A, et al. Hepatitis-G virus infection in multi-transfused patients and intravenous drug abusers: new Delhi experience. Dig Dis Sci. 2008;53:1383–1389. [DOI] [PubMed] [Google Scholar]
- [125].Katayama Y, Apichartpiyakul C, Handajani R, et al. GB virus C/hepatitis G virus (GBV-C/HGV) infection in Chiang Mai, Thailand, and identification of variants on the basis of 5’-untranslated region sequences. Arch Virol. 1997;142:2433–2445. [DOI] [PubMed] [Google Scholar]
- [126].Shrestha SM, Shrestha S, Tsuda F, et al. Infection with GB virus C and hepatitis C virus in drug addicts, patients on maintenance hemodialysis, or with chronic liver disease in Nepal. J Med Virol. 1997;53:157–161. [DOI] [PubMed] [Google Scholar]
- [127].Ru H, Wen X, Liang W. [Investigation of HGV infection in various populations in Guangxi]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1998;12:336–339. [PubMed] [Google Scholar]
- [128].Hwang SJ, Chu CW, Lu RH, et al. Seroprevalence of GB virus C/hepatitis G virus-RNA and anti-envelope antibody in high-risk populations in Taiwan. J Gastroenterol Hepatol. 2000;15:1171–1175. [DOI] [PubMed] [Google Scholar]
- [129].Li G, Ma -H-H, Lau GKK, et al. Prevalence of hepatitis G virus infection and homology of different viral strains in Southern China. World J Gastroenterol. 2002;8:1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Poovorawan Y, Theamboonlers A, Chongsrisawat V, et al. Prevalence of infection with hepatitis G virus among various groups in Thailand. Ann Trop Med Parasitol. 1998;92:89–95. [DOI] [PubMed] [Google Scholar]
- [131].Lu L, Ng MH, Zhou B, et al. Detection and genotyping of GBV-C/HGV variants in China. Virus Res. 2001;73:131–144. [DOI] [PubMed] [Google Scholar]
- [132].Noguchi S, Sata M, Suzuki H, et al. GB virus C (GBV-C)/hepatitis G virus (HGV) infection among intravenous drug users in Japan. Virus Res. 1997;49:155–162. [DOI] [PubMed] [Google Scholar]
- [133].Suganuma N, Ikeda S, Taketa K, et al. Risk analysis of the exposure to GB virus C/hepatitis G virus among populations of intravenous drug users, commercial sex workers and male outpatients at STD clinic in Chiang Mai, Thailand: a cross-sectional case-control study. Acta Med Okayama. 1998;52:161–167. [DOI] [PubMed] [Google Scholar]
- [134].Taklual W, Tang S, Yue W. Effect of human pegivirus route of transmission on the genetic distribution of the virus: an institution based cross-sectional study. Virol J. 2019;16:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Ramezani A, Mohraz M, Vahabpour R, et al. Frequency of hepatitis G virus infection among HIV positive subjects with parenteral and sexual exposure. J Gastrointestin Liver Dis. 2008;17:269–272. [PubMed] [Google Scholar]
- [136].Zhou B, Ma W, Wang H, et al. Investigation on hepatitis G virus (HGV) infection among different populations in Shenzhen. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1997;11:348–351. [PubMed] [Google Scholar]
- [137].Schwarze-Zander C, Blackard JT, Zheng H, et al. GB virus C (GBV-C) infection in hepatitis C virus (HCV)/HIV-coinfected patients receiving HCV treatment: importance of the GBV-C genotype. J Infect Dis. 2006;194:410–419. [DOI] [PubMed] [Google Scholar]
- [138].Ramia S, Mokhbat J, Sibai A, et al. Exposure rates to hepatitis C and G virus infections among HIV-infected patients: evidence of efficient transmission of HGV by the sexual route. Int J STD AIDS. 2004;15:463–466. [DOI] [PubMed] [Google Scholar]
- [139].Baklan Z, Gorisek JR, Poljak M, et al. Prevalence of HIV, hepatitis B, C and G virus infections among injecting drug users on methadone maintenance treatment in Maribor. Wien Klin Wochenschr. 2004;116(Suppl 2):5–7. [PubMed] [Google Scholar]
- [140].Ibáñez A, Giménez-Barcons M, Tajahuerce A, et al. Prevalence and genotypes of GB virus C/hepatitis G virus (GBV-C/HGV) and hepatitis C virus among patients infected with human immunodeficiency virus: evidence of GBV-C/HGV sexual transmission. J Med Virol. 1998;55:293–299. [DOI] [PubMed] [Google Scholar]
- [141].Kojima M, Kanazawa K, Hakamada T, et al. Infection with hepatitis GB virus C in intravenous drug abusers with type C chronic liver diseases. Nihon Rinsho. 1997;55:549–553. [PubMed] [Google Scholar]
- [142].Nübling CM, Bialleck H, Fürsch AJ, et al. Frequencies of GB virus C/hepatitis G virus genomes and of specific antibodies in German risk and non-risk populations. J Med Virol. 1997;53:218–224. [PubMed] [Google Scholar]
- [143].Kotaki T, Khairunisa SQ, Sukartiningrum SD, et al. High prevalence of HIV-1 CRF01_AE viruses among female commercial sex workers residing in Surabaya, Indonesia. PLoS One. 2013;8:e82645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Hattori J, Ibe S, Nagai H, et al. Prevalence of infection and genotypes of GBV-C/HGV among homosexual men. Microbiol Immunol. 2003;47:759–763. [DOI] [PubMed] [Google Scholar]
- [145].Liu Z, Li L, Chen Z, et al. Prevalence of GB virus type C viraemia in MSM with or without HIV-1 infection in Beijing, China. Epidemiol Infect. 2012;140:2199–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Seemayer CA, Viazov S, Philipp T, et al. Detection of GBV-C/HGV RNA in saliva and serum, but not in urine of infected patients. Infection. 1998;26:39–41. [DOI] [PubMed] [Google Scholar]
- [147].Stapleton JT. GB virus type C/Hepatitis G virus. Semin Liver Dis. 2003;23:137–148. [DOI] [PubMed] [Google Scholar]
- [148].Tanaka E, Kiyosawa K, Shimoda K, et al. Evolution of hepatitis G virus infection and antibody response to envelope protein in patients with transfusion-associated non-A, non-B hepatitis. J Viral Hepat. 1998;5:153–159. [DOI] [PubMed] [Google Scholar]
- [149].Stapleton JT, Xiang J, McLinden JH, et al. A novel T cell evasion mechanism in persistent RNA virus infection. Trans Am Clin Climatol Assoc. 2014. 125 :14–24. [PMC free article] [PubMed] [Google Scholar]
- [150].Elkayam O, Hassoba HM, Ferrell LD, et al. GB virus C (GBV-C/HGV) and E2 antibodies in children preliver and postliver transplant. Pediatr Res. 1999;45:795–798. [DOI] [PubMed] [Google Scholar]
- [151].Heringlake S, Ockenga J, Tillmann HL, et al. GB virus C/hepatitis G virus infection: a favorable prognostic factor in human immunodeficiency virus-infected patients? J Infect Dis. 1998;177:1723–1726. [DOI] [PubMed] [Google Scholar]
- [152].Nunnari G, Nigro L, Palermo F, et al. Slower progression of HIV-1 infection in persons with GB virus C co-infection correlates with an intact T-helper 1 cytokine profile. Ann Intern Med. 2003;139:26–30. [DOI] [PubMed] [Google Scholar]
- [153].Tillmann HL, Heiken H, Knapik-Botor A, et al. Infection with GB virus C and reduced mortality among HIV-infected patients. N Engl J Med. 2001;345:715–724. [DOI] [PubMed] [Google Scholar]
- [154].Toyoda H, Fukuda Y, Hayakawa T, et al. Effect of GB virus C/hepatitis G virus coinfection on the course of HIV infection in hemophilia patients in Japan. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:209–213. [DOI] [PubMed] [Google Scholar]
- [155].Williams CF, Klinzman D, Yamashita TE, et al. Persistent GB virus C infection and survival in HIV-infected men. N Engl J Med. 2004;350:981–990. [DOI] [PubMed] [Google Scholar]
- [156].Xiang J, Wünschmann S, Diekema DJ, et al. Effect of coinfection with GB virus C on survival among patients with HIV infection. N Engl J Med. 2001;345:707–714. [DOI] [PubMed] [Google Scholar]
- [157].Zhang W, Chaloner K, Tillmann HL, et al. Effect of early and late GB virus C viraemia on survival of HIV-infected individuals: a meta-analysis. HIV Med. 2006;7:173–180. [DOI] [PubMed] [Google Scholar]
- [158].Vahidnia F, Petersen M, Stapleton JT, et al. Acquisition of GB virus type C and lower mortality in patients with advanced HIV disease. Clin Infect Dis. 2012;55(7):1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chang CM, Stapleton JT, Klinzman D, et al. GBV-C infection and risk of NHL among U.S. adults. Cancer Res. 2014;74:5553–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Civardi G, Tanzi E, Ferrari B, et al. High prevalence of anti-HGV/E2 antibodies in HCV-positive patients with non Hodgkin’s lymphoma. Haematologica. 1998;83:957–958. [PubMed] [Google Scholar]
- [161].De Renzo A, Persico E, de Marino F, et al. High prevalence of hepatitis G virus infection in Hodgkin’s disease and B-cell lymphoproliferative disorders: absence of correlation with hepatitis C virus infection. Haematologica 2002. . 87:714–718. [PubMed] [Google Scholar]
- [162].Ellenrieder V, Weidenbach H, Frickhofen N, et al. HCV and HGV in B-cell non-Hodgkin’s lymphoma. J Hepatol. 1998;28:34–39. [DOI] [PubMed] [Google Scholar]
- [163].Nakamura S, Takagi T, Matsuda T. Hepatitis G virus RNA in patients with B-cell non-Hodgkin’s lymphoma. Br J Haematol. 1997;98:1051–1052. [PubMed] [Google Scholar]
- [164].Krajden M, Yu A, Braybrook H, et al. GBV-C/hepatitis G virus infection and non-Hodgkin lymphoma: a case control study. Int J Cancer. 2010;126(12):2885–2892. [DOI] [PubMed] [Google Scholar]
- [165].Fama A, Xiang J, Link BK, et al. Human pegivirus infection and lymphoma risk and prognosis: a North American study. Br J Haematol. 2018;182:644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Fama A, Larson MC, Link BK, et al. Human pegivirus infection and lymphoma risk: a systematic review and meta-analysis. Clin Infect Dis. 2020;71:1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Chivero ET, Bhattarai N, Rydze RT, et al. Human pegivirus RNA is found in multiple blood mononuclear cells in vivo and serum-derived viral RNA-containing particles are infectious in vitro. J Gen Virol. 2014;95:1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Balcom EF, Doan MAL, Branton WG, et al. Human pegivirus-1 associated leukoencephalitis: clinical and molecular features. Ann Neurol. 2018;84:781–787. [DOI] [PubMed] [Google Scholar]
- [169].Doan MAL, Roczkowsky A, Smith M, et al. Infection of glia by human pegivirus suppresses peroxisomal and antiviral signaling pathways. J Virol. 2021;95:e0107421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Cheinquer H, Sette H, Wolff FH, et al. Treatment of chronic HCV infection with the new Direct Acting Antivirals (DAA): first report of a real world experience in Southern Brazil. Ann Hepatol. 2017;16:727–733. [DOI] [PubMed] [Google Scholar]
- [171].Feeney ER, Chung RT. Antiviral treatment of hepatitis C. Bmj. 2014;348:g3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Pawlotsky J-M. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176–1192. [DOI] [PubMed] [Google Scholar]
- [173].Sievert W, Razavi H, Estes C, et al. Enhanced antiviral treatment efficacy and uptake in preventing the rising burden of hepatitis C-related liver disease and costs in Australia. J Gastroenterol Hepatol. 2014;29(Suppl 1):1–9. [DOI] [PubMed] [Google Scholar]
- [174].Wan Z, Liu J, Hu F, et al. Evidence that the second human pegivirus (HPgV-2) is primarily a lymphotropic virus and can replicate independent of HCV replication. Emerg Microbes Infect. 2020;9(1):485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].N’Guessan KF, Anderson M, Phinius B, et al. The impact of human pegivirus on CD4 cell count in HIV-positive persons in Botswana. Open Forum Infect Dis. 2017;4(4):ofx222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Yirrell DL, Wright E, Shafer LA, et al. Association between active GB virus-C (hepatitis G) infection and HIV-1 disease in Uganda. Int J STD AIDS. 2007;18(4):244–249. [DOI] [PubMed] [Google Scholar]
- [177].Weintrob AC, Hamilton JD, Hahn C, et al. Active or prior GB virus C infection does not protect against vertical transmission of HIV in coinfected women from Tanzania. Clin Infect Dis. 2004;38:e46–e48. [DOI] [PubMed] [Google Scholar]
- [178].Mosam A, Sathar MA, Dawood H, et al. Effect of GB virus C co-infection on response to generic HAART in African patients with HIV-1 clade C infection. AIDS. 2007;21:1377–1379. [DOI] [PubMed] [Google Scholar]
- [179].Horemheb-Rubio G, Ramos-Cervantes P, Arroyo-Figueroa H, et al. High HPgV replication is associated with improved surrogate markers of HIV progression. PLoS One. 2017;12:e0184494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].de Miranda BKB, de Sá KSG, Da Silva ANR, et al. GBV-C/HIV-1 coinfection is associated with low HIV-1 viral load and high CD4 T lymphocyte count. Arch Virol. 2017;162:3431–3438. [DOI] [PubMed] [Google Scholar]
- [181].Sahni H, Kirkwood K, Kyriakides TC, et al. GBV-C viremia and clinical events in advanced HIV infection. J Med Virol. 2014;86(3):426–432. [DOI] [PubMed] [Google Scholar]
- [182].Li C, Collini P, Danso K, et al. GB virus C and HIV-1 RNA load in single virus and co-infected West African individuals. AIDS. 2006;20(3):379–386. [DOI] [PubMed] [Google Scholar]
- [183].Schwarze-Zander C, Neibecker M, Othman S, et al. GB virus C coinfection in advanced HIV type-1 disease is associated with low CCR5 and CXCR4 surface expression on CD4+ T-cells. Antivir Ther. 2010;15(5):745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Maidana-Giret MT, Silva TM, Sauer MM, et al. GB virus type C infection modulates T-cell activation independently of HIV-1 viral load. AIDS. 2009;23:2277–2287. [DOI] [PubMed] [Google Scholar]
- [185].Björkman P, Flamholc L, Nauclér A, et al. GB virus C during the natural course of HIV-1 infection: viremia at diagnosis does not predict mortality. AIDS. 2004;18(6):877–886. [DOI] [PubMed] [Google Scholar]
- [186].Gill CJ, Griffith JL, Jacobson D, et al. Relationship of HIV viral loads, CD4 counts, and HAART use to health-related quality of life. J Acquir Immune Defic Syndr. 2002;30:485–492. [DOI] [PubMed] [Google Scholar]
- [187].van Leth F, Andrews S, Grinsztejn B, et al. The effect of baseline CD4 cell count and HIV-1 viral load on the efficacy and safety of nevirapine or efavirenz-based first-line HAART. AIDS. 2005;19(5):463–471. [DOI] [PubMed] [Google Scholar]
- [188].Liu K, Li Y, Xu R, et al. HIV-1 infection alters the viral composition of plasma in men who have sex with men. mSphere. 2021;6 e00081–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Xiang J, Sathar MA, McLinden JH, et al. South African GB virus C isolates: interactions between genotypes 1 and 5 isolates and HIV. J Infect Dis. 2005;192:2147–2151. [DOI] [PubMed] [Google Scholar]
- [190].Lanteri MC, Vahidnia F, Tan S, et al. Downregulation of cytokines and chemokines by GB virus C after transmission via blood transfusion in HIV-positive blood recipients. J Infect Dis. 2015;211:1585–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Tenckhoff S, Kaiser T, Bredeek F, et al. Role of GB virus C in HIV-1-infected and hepatitis C virus-infected hemophiliac children and adolescents. J Acquir Immune Defic Syndr. 2012;61:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Xiang J, George SL, Wünschmann S, et al. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1alpha, MIP-1beta, and SDF-1. Lancet. 2004;363:2040–2046. [DOI] [PubMed] [Google Scholar]
- [193].Nattermann J, Nischalke H-D, Kupfer B, et al. Regulation of CC chemokine receptor 5 in hepatitis G virus infection. AIDS. 2003;17:1457–1462. [DOI] [PubMed] [Google Scholar]
- [194].Lederman MM, Penn-Nicholson A, Cho M, et al. Biology of CCR5 and its role in HIV infection and treatment. Jama. 2006;296:815–826. [DOI] [PubMed] [Google Scholar]
- [195].Lalle E, Sacchi A, Abbate I, et al. Activation of interferon response genes and of plasmacytoid dendritic cells in HIV-1 positive subjects with GB virus C co-infection. Int J Immunopathol Pharmacol. 2008;21:161–171. [DOI] [PubMed] [Google Scholar]
- [196].Moore JP, Kitchen SG, Pugach P, et al. The CCR5 and CXCR4 coreceptors–central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20:111–126. [DOI] [PubMed] [Google Scholar]
- [197].Herrera E, Gomara MJ, Mazzini S, et al. Synthetic peptides of hepatitis G virus (GBV-C/HGV) in the selection of putative peptide inhibitors of the HIV-1 fusion peptide. J Phys Chem B. 2009;113:7383–7391. [DOI] [PubMed] [Google Scholar]
- [198].Koedel Y, Eissmann K, Wend H, et al. Peptides derived from a distinct region of GB virus C glycoprotein E2 mediate strain-specific HIV-1 entry inhibition. J Virol. 2011;85:7037–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Spellberg B, Edwards JE. Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001. 32 :76–102. [DOI] [PubMed] [Google Scholar]
- [200].Johann-Liang R, Cervia J, Noel GJ. Endogenous interleukin-2 serum levels in children infected with human immunodeficiency virus. Clin Infect Dis. 1997;25:1233–1236. [DOI] [PubMed] [Google Scholar]
- [201].Clerici M, Balotta C, Salvaggio A, et al. Human immunodeficiency virus (HIV) phenotype and interleukin-2/ interleukin-10 ratio are associated markers of protection and progression in HIV infection. Blood. 1996;88:574–579. [PubMed] [Google Scholar]
- [202].Barcellini W, Rizzardi GP, Borghi MO, et al. TH1 and TH2 cytokine production by peripheral blood mononuclear cells from HIV-infected patients. AIDS. 1994;8:757–762. [DOI] [PubMed] [Google Scholar]
- [203].Nel AE. T-cell activation through the antigen receptor. Part 1: signaling components, signaling pathways, and signal integration at the T-cell antigen receptor synapse. J Allergy Clin Immunol. 2002;109:758–770. [DOI] [PubMed] [Google Scholar]
- [204].Paredes R, López Benaldo de Quirós JC, Fernández-Cruz E, et al. The potential role of interleukin-2 in patients with HIV infection. AIDS Rev. 2002;4:36–40. [PubMed] [Google Scholar]
- [205].Bhattarai N, McLinden JH, Xiang J, et al. GB virus C particles inhibit T cell activation via envelope E2 protein-mediated inhibition of TCR signaling. J Immunol. 2013;190:6351–6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. [DOI] [PubMed] [Google Scholar]
- [207].Moenkemeyer M, Schmidt RE, Wedemeyer H, et al. GBV-C coinfection is negatively correlated to fas expression and fas-mediated apoptosis in HIV-1 infected patients. J Med Virol. 2008;80:1933–1940. [DOI] [PubMed] [Google Scholar]
- [208].Björkman P, Flamholc L, Molnegren V, et al. Enhanced and resumed GB virus C replication in HIV-1-infected individuals receiving HAART. AIDS. 2007;21:1641–1643. [DOI] [PubMed] [Google Scholar]
- [209].Berzsenyi MD, Bowden DS, Kelly HA, et al. Reduction in hepatitis C-related liver disease associated with GB virus C in human immunodeficiency virus coinfection. Gastroenterology. 2007;133:1821–1830. [DOI] [PubMed] [Google Scholar]
- [210].Berzsenyi MD, Woollard DJ, McLean CA, et al. Down-regulation of intra-hepatic T-cell signaling associated with GB virus C in a HCV/HIV co-infected group with reduced liver disease. J Hepatol. 2011;55(3):536–544. [DOI] [PubMed] [Google Scholar]
- [211].Pawlotsky JM, Roudot-Thoraval F, Muerhoff AS, et al. GB virus C (GBV-C) infection in patients with chronic hepatitis C. influence on liver disease and on hepatitis virus behaviour: effect of interferon alfa therapy. J Med Virol. 1998;54:26–37. [DOI] [PubMed] [Google Scholar]
- [212].Goeser T, Seipp S, Wahl R, et al. Clinical presentation of GB-C virus infection in drug abusers with chronic hepatitis C. J Hepatol. 1997;26:498–502. [DOI] [PubMed] [Google Scholar]
- [213].Li T, Tang S, Su Y, et al. High prevalence and viremia of human pegivirus 2 in the HIV-infected population in honghe prefecture, Yunnan province. Arch Virol. 2020;165:619–626. [DOI] [PubMed] [Google Scholar]
- [214].Sridhar S, Yip CCY, Chew NFS, et al. Epidemiological and clinical characteristics of human hepegivirus 1 infection in patients with hepatitis C. Open Forum Infect Dis. 2019;6:ofz329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Lauck M, Bailey AL, Andersen KG, et al. GB virus C coinfections in West African Ebola patients. J Virol. 2015;89:2425–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Fan X, Xu Y, Solomon H, et al. Is hepatitis G/GB virus-C virus hepatotropic? Detection of hepatitis G/GB virus-C viral RNA in liver and serum. J Med Virol. 1999;58:160–164. [PubMed] [Google Scholar]
- [217].Pessoa MG, Terrault NA, Detmer J, et al. Quantitation of hepatitis G and C viruses in the liver: evidence that hepatitis G virus is not hepatotropic. Hepatology. 1998;27:877–880. [DOI] [PubMed] [Google Scholar]
- [218].Tucker TJ, Smuts HE, Eedes C, et al. Evidence that the GBV-C/hepatitis G virus is primarily a lymphotropic virus. J Med Virol. 2000;61:52–58. [PubMed] [Google Scholar]
- [219].Berg T, Müller AR, Platz KP, et al. Dynamics of GB virus C viremia early after orthotopic liver transplantation indicates extrahepatic tissues as the predominant site of GB virus C replication. Hepatology. 1999;29(1):245–249. [DOI] [PubMed] [Google Scholar]
- [220].George SL, Varmaz D, Stapleton JT. GB virus C replicates in primary T and B lymphocytes. J Infect Dis. 2006;193:451–454. [DOI] [PubMed] [Google Scholar]
- [221].Kisiel E, Cortez KC, Pawełczyk A, et al. Hepatitis G virus/GBV-C in serum, peripheral blood mononuclear cells and bone marrow in patients with hematological malignancies. Infect Genet Evol. 2013;19:195–199. [DOI] [PubMed] [Google Scholar]
- [222].Mellor J, Haydon G, Blair C, et al. Low level or absent in vivo replication of hepatitis C virus and hepatitis G virus/GB virus C in peripheral blood mononuclear cells. J Gen Virol. 1998;79((Pt 4)):705–714. [DOI] [PubMed] [Google Scholar]
- [223].George SL, Xiang J, Stapleton JT. Clinical isolates of GB virus type C vary in their ability to persist and replicate in peripheral blood mononuclear cell cultures. Virology. 2003;316:191–201. [DOI] [PubMed] [Google Scholar]
- [224].Radkowski M, Kubicka J, Kisiel E, et al. Detection of active hepatitis C virus and hepatitis G virus/GB virus C replication in bone marrow in human subjects. Blood. 2000;95:3986–3989. [PubMed] [Google Scholar]
- [225].Bailey AL, Lauck M, Mohns M, et al. Durable sequence stability and bone marrow tropism in a macaque model of human pegivirus infection. Sci Transl Med. 2015;7:305ra144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Bukh J, Kim JP, Govindarajan S, et al. Experimental infection of chimpanzees with hepatitis G virus and genetic analysis of the virus. J Infect Dis. 1998;177:855–862. [DOI] [PubMed] [Google Scholar]
- [227].Wolf D, Goff SP. Host restriction factors blocking retroviral replication. Annu Rev Genet. 2008;42(1):143–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Neumann G, Kawaoka Y. Reverse genetics systems for the generation of segmented negative-sense RNA viruses entirely from cloned cDNA. Curr Top Microbiol Immunol. 2004;283:43–60. [DOI] [PubMed] [Google Scholar]
- [229].Zhong J, Gastaminza P, Cheng G, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A. 2005;102:9294–9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1974. [DOI] [PubMed] [Google Scholar]
- [231].Shan C, Xie X, Muruato AE, et al. An infectious cdna clone of zika virus to study viral virulence, mosquito transmission, and antiviral inhibitors. Cell Host Microbe. 2016;19(6):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Tsetsarkin KA, Kenney H, Chen R, et al. A full-length infectious cDNA clone of zika virus from the 2015 epidemic in Brazil as a genetic platform for studies of virus-host interactions and vaccine development. mBio. 2016;7 e01114–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Puri B, Polo S, Hayes CG, et al. Construction of a full length infectious clone for dengue-1 virus Western Pacific,74 strain. Virus Genes. 2000;20:57–63. [DOI] [PubMed] [Google Scholar]
- [234].Kinney RM, Butrapet S, Chang GJ, et al. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology. 1997;230:300–308. [DOI] [PubMed] [Google Scholar]
- [235].Xie X, Muruato A, Lokugamage KG, et al. An Infectious cDNA Clone of SARS-CoV-2. Cell Host Microbe. 2020. 27 :841–848.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Thi Nhu Thao T, Labroussaa F, Ebert N, et al. Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform. Nature. 2020;582:561–565. [DOI] [PubMed] [Google Scholar]
- [237].Hou YJ, Okuda K, Edwards CE, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory Tract. Cell. 2020. 182 :429–446.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Xiang J, Wünschmann S, Schmidt W, et al. Full-length GB virus C (hepatitis G virus) RNA transcripts are infectious in primary CD4-positive T cells. J Virol. 2000;74:9125–9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Ren H, Zhu F-L, Cao -M-M, et al. Hepatitis G virus genomic RNA is pathogenic to Macaca mulatta. World J Gastroenterol. 2005;11:970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Cheng Y, Zhang W, Li J, et al. Serological and histological findings in infection and transmission of GBV-C/HGV to macaques. J Med Virol. 2000;60:28–33. [DOI] [PubMed] [Google Scholar]
- [241].Evans DT, Silvestri G. Nonhuman primate models in AIDS research. Curr Opin HIV AIDS. 2013;8:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. [DOI] [PubMed] [Google Scholar]
- [243].Bagasra O, Bagasra AU, Sheraz M, et al. Potential utility of GB virus type C as a preventive vaccine for HIV-1. Expert Rev Vaccines. 2012;11:335–347. [DOI] [PubMed] [Google Scholar]
- [244].Bailey AL, Buechler CR, Matson DR, et al. Pegivirus avoids immune recognition but does not attenuate acute-phase disease in a macaque model of HIV infection. PLoS Pathog. 2017;13:e1006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Greenhalgh S, Schmidt R, Day T. Fighting the public health burden of AIDS with the human pegivirus. Am J Epidemiol. 2019;188:1586–1594. [DOI] [PubMed] [Google Scholar]
- [246].Thézé J, Lowes S, Parker J, et al. Evolutionary and phylogenetic analysis of the hepaciviruses and pegiviruses. Genome Biol Evol. 2015;7:2996–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Ghai RR, Sibley SD, Lauck M, et al. Deep sequencing identifies two genotypes and high viral genetic diversity of human pegivirus (GB virus C) in rural Ugandan patients. J Gen Virol. 2013;94:2670–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Nakao H, Okamoto H, Fukuda M, et al. Mutation rate of GB virus C/hepatitis G virus over the entire genome and in subgenomic regions. Virology. 1997;233:43–50. [DOI] [PubMed] [Google Scholar]
- [249].Tumbo A-M, Schindler T, Dangy J-P, et al. Role of human pegivirus infections in whole plasmodium falciparum sporozoite vaccination and controlled human malaria infection in African volunteers. Virol J. 2021;18:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


