Abstract
Purpose:
Intraocular infection in patients with COVID-19 could be different in the presence of treatment with systemic corticosteroid and immunosuppressive agents. We describe the epidemiology and microbiological profile of intraocular infection in COVID-19 patients after their release from the hospital.
Methods:
We analyzed the clinical and microbiological data of laboratory-confirmed COVID-19 patients from April 2020 to January 2021 presenting with features of endogenous endophthalmitis within 12 weeks of their discharge from the hospital in two neighboring states in South India. The data included demography, systemic comorbidities, COVID-19 treatment details, time interval to visual symptoms, the microbiology of systemic and ocular findings, ophthalmic management, and outcomes.
Results:
The mean age of 24 patients (33 eyes) was 53.6 ± 13.5 (range: 5–72) years; 17 (70.83%) patients were male. Twenty-two (91.6%) patients had systemic comorbidities, and the median period of hospitalization for COVID-19 treatment was 14.5 ± 0.7 (range: 7–63) days. Infection was bilateral in nine patients. COVID-19 treatment included broad-spectrum systemic antibiotics (all), antiviral drugs (22, 91.66% of patients), systemic corticosteroid (21, 87.5% of patients), supplemental oxygen (18, 75% of patients), low molecular weight heparin (17, 70.8% of patients), admission in intensive care units (16, 66.6% of patients), and interleukin-6 inhibitor (tocilizumab) (14, 58.3% of patients). Five (20.8%) patients died of COVID-19-related complications during treatment for endophthalmitis; one eye progressed to pan ophthalmitis and orbital cellulitis; eight eyes regained vision >20/400. Fourteen of 19 (73.7%) vitreous biopsies were microbiologically positive (culture, PCR, and microscopy), and the majority (11 patients, 78.5%) were fungi.
Conclusion:
Intraocular infection in COVID-19 patients is predominantly caused by fungi. We suggest a routine eye examination be included as a standard of care of COVID-19.
Keywords: COVID-19, endogenous endophthalmitis, intraocular infection
Coronavirus disease 2019 (COVID-19) has affected almost all countries in the world.[1] The infection typically starts with pulmonary involvement and acute respiratory failure, sometimes progressing to a fatal multiorgan system affection and death in older individuals with existing comorbidities.[2,3] An enveloped RNA beta coronavirus, SARS-Cov-2 causes COVID-19. Bacterial coinfection in hospitalized SARS-Cov-2-infected patients is reported up to 7%, and it is increased up to 14% in people who need intensive care unit (ICU) admission.[4] The fungal coinfection in hospitalized SARS-Cov-2-infected patients is also not uncommon.[4,5,6] The reported ophthalmic manifestations include conjunctivitis, keratoconjunctivitis, episcleritis, central retinal vein and artery occlusion, acute retinal necrosis, optic neuritis, neuroretinitis, ptosis, sixth cranial nerve palsy, dacryoadenitis, and orbital cellulitis.[7,8,9,10,11,12,13,14,15] There are very few reports of intraocular infection, such as endophthalmitis, in patients hospitalized and treated for COVID-19.[16,17,18]
In this communication, we report a series of patients who presented to us over 9 months period at the peak of the pandemic in two adjoining southern states of India.
Methods
We analyzed the patients reporting to our out-patient service after being treated for COVID-19 in designated hospitals and discharged after such treatment. We collected the data from the electronic medical record of consecutive patients with a clinical diagnosis of endogenous endophthalmitis/panophthalmitis from April 2020 to January 2021. All of them were laboratory (reverse transcriptase-real time polymerase chain reaction, RT-PCR)-confirmed SARS-CoV-2 infection and had reported within 12 weeks of discharge from the hospital treated for viral infection. Appropriate consent and institutional review board (IRB) approval were obtained (LEC-BHR-P-09-20-512), and all patients were treated as per the declaration of tenets of Helsinki. The collected data included age, gender, associated co-morbidities, time to onset of COVID-19 symptoms, time to onset of ocular symptoms, history of admission to hospital/ICU, systemic medications including administration of intravenous fluid, blood chemistry including the inflammatory markers, the occurrence of sepsis, ventilator use, culture report of blood/urine/tissue biopsy, and oxygen therapy. Patients were classified into mild, moderate, and severe COVID-19 as per the oxygen requirement.[19] In brief, it was mild COVID-19 (uncomplicated upper respiratory tract infection without evidence of breathlessness or hypoxia), moderate COVID-19 (pneumonia with dyspnoea, hypoxia, fever and cough, respiratory rate >24/min, and blood oxygen saturation between 90% and 94% on room air), severe COVID-19 (respiratory rate >30/min and blood oxygen saturation <90% on room air, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock).
Each patient received a comprehensive eye examination. This included presenting visual acuity (PVA), slit-lamp, and fundus examination (indirect ophthalmoscopy). Essential ophthalmic investigations included ocular ultrasonogram and fundus photography when possible. Endophthalmitis was suspected clinically based on the cluster of symptoms (pain, redness, and reduced vision) and signs (hypopyon, exudates in the anterior chamber, and vitreous opacities). These patients were managed as per the endophthalmitis treatment protocol of the institute, which essentially included a vitrectomy and intravitreal antibiotic injections, microbiology of vitreous sample, repeat vitreous surgery, and/or repeat culture-susceptibility adjusted intravitreal antibiotic/antifungal agents.[20,21]
Undiluted vitreous (0.5–1.0 mL) was collected from eyes at the time of vitrectomy and sent for a detailed microbiological study. Grams staining and calcofluor white (CFW) mount were done for the undiluted vitreous for direct microscopy. The sample was inoculated onto solid (5% sheep blood agar, chocolate agar, Sabouraud dextrose agar, potato dextrose agar) and liquid (brain heart infusion, thioglycolate broth, anaerobic bacteria broth) media to detect any growth of bacteria/fungi. All media were incubated aerobically at 37°C except Sabouraud dextrose agar and potato dextrose agar, which were incubated at 27°C for 2 weeks. Chocolate agar was incubated in 5% CO2 at 37°C. Species identification (bacteria and yeast) was done whenever possible using the Vitek 2 compact system (bioMérieux, France). PCR for eubacteria (16S rDNA), panfungus (ITS), and herpes virus type I and II (Glycoprotein D gene) detection was performed where possible. A small volume of vitreous sample was set aside for RT-PCR under the appropriate cold chain for SARS-CoV-2 detection wherever possible.
We documented the PVA and best-corrected visual acuity (BCVA), ocular findings, and response to treatment in the subsequent examinations. Systemic antibiotic/antifungal agents and topical antibiotics/steroids were considered as and when appropriate to manage the eye conditions. The vitreous biopsy and vitrectomy, along with single or multiple antibiotics/antifungals, were performed when the systemic condition of the patient allowed; the remaining patients were treated with systemic antibiotics/antifungals only with/without intravitreal antibiotics. Vitrectomy and silicone oil tamponade (1000 centistoke) was considered for eyes with necrotic retina and half-dose intravitreal antibiotics/antifungals. Evisceration was considered whenever the globe was not salvageable. Persistent exudates in the vitreous cavity after primary vitrectomy needed vitreous lavage with intravitreal antibiotic/antifungal agents.
Results
This analysis included 24 consecutive subjects examined between April 2020 and January 2021 in two adjoining states in South India, Andhra Pradesh (AP) and Telangana state (TS) [Fig. 1].
Figure 1.
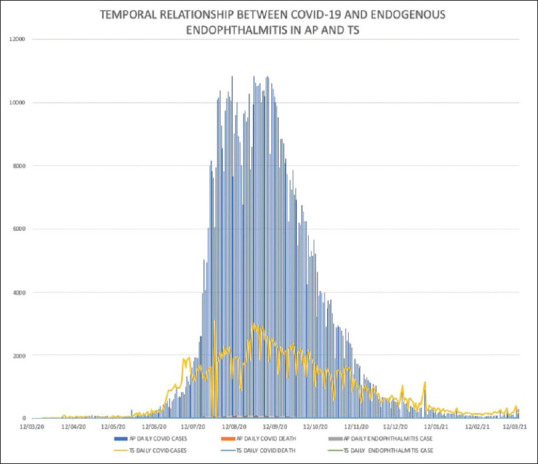
Temporal relationship between daily COVID-19 and endophthalmitis cases (onset of COVID-19 symptoms and eye symptoms of same patients) in Andhra Pradesh (AP) and Telangana states (TS)
Cases of endogenous endophthalmitis (EE) coincided with the peak of COVID-19 in both states. Severe, moderate, and mild cases of COVID-19 were present in 14 (58.3%), seven (29.2%), and two subjects, respectively, and one subject was asymptomatic. All patients were RT-PCR SARS-CoV-2 infection positive (nasopharyngeal swab) and were hospitalized for COVID-19 treatment within 12 weeks of presentation. The mean interval of COVID-19 systemic symptoms to ophthalmic symptoms was 14.9 ± 8.9 (range: 6–72) days in 23 symptomatic patients. The mean age of the patients was 53.6 ± 13.5 (range: 5–72) years, and 17 (70.8%) patients were male. Over 90% (22, 91.6%) patients had multiple pre-COVID-19 systemic comorbidities, and 16 (66.6%) patients were admitted to the intensive care unit (ICU) [Table 1].
Table 1.
Clinical characteristics of 24 COVID-19 patients with endogenous endophthalmitis
| # | Age/Gender | Comorbidities | Clinical Classification of COVID-19 /Hospitalization/Oxygen use/ICU admission Ventilator | Steroid/Broad-spectrum antibiotics/Anticoagulants/Tocilizumab/Antiviral | Time to eye symptom and COVID-19 symptoms in days | Laterality |
|---|---|---|---|---|---|---|
| 1 | 53/F | DM, CKD | Severe COVID-19, ARDS, Sepsis, Hospitalisation, O2, ICU | Broad-spectrum antibiotics | 33 | BE |
| 2 | 58/M | DM | Moderate COVID-19, Hospitalization, ICU | IVMP, Oral steroid, Broad-spectrum antibiotic, Enoxaparin, Tocilizumab, Remdesivir | 37 | RE |
| 3 | 72/M | DM | Moderate COVID-19, Hospitalization, ICU | IVMP, Broad-spectrum antibiotic, Flavipiravir | 32 | RE |
| 4 | 55/M | DM, CKD, EPN (Stenting and PCN) | Severe COVID-19, Hospitalisation, ICU, O2 | IVMP, Broad-spectrum antibiotics, Remdesivir, Favipiravir | 11 | RE |
| 5 | 49/M | HTN, DM*, EPN (Stenting and PCN) | Severe COVID-19, ARDS, Sepsis, Hospitalisation, ICU, Dialysis, O2 | IVMP, Broad-spectrum antibiotic, Remdesivir, Favipiravir, Enoxaparin | 10 | BE |
| 6 | 49/F | DM | Mild COVID-19, Hospitalisation | Oral steroid, Broad-spectrum antibiotic, Favipiravir | 17 | LE |
| 7 | 53/F | DM, Pots spine (TB reactivation)∫ | Severe COVID-19, ARDS, Sepsis, Septic shock, Hospitalisation, ICU, O2, Ventilator | IVMP, Enoxaparin, Broad-spectrum antibiotics, Remdesivir | 17 | BE |
| 8 | 56/F | DM, CKD, HTN, EPN (JJ stenting) | Severe COVID-19, Hospitalisation, ICU, O2, Sepsis | Oral steroid, Broad-spectrum antibiotics, Favipiravir | 37 | LE |
| 9 | 65/M | DM, CKD, EPN, JJ stenting with PCN | Severe COVID-19, Hospitalisation, ICU, O2, Sepsis | IVMP, Enoxaparin, Broad-spectrum antibiotics, Tocilizumab, Remdesivir | 17 | BE |
| 10 | 54/F | DM, HTN | Mild COVID-19, Hospitalisation | Oral steroid, Broad-spectrum antibiotics, Favipiravir | 12 | RE |
| 11 | 40/M | DM | Severe COVID-19, Hospitalisation, ICU, O2 | IVMP, Oral steroid, Enoxaparin, Broad-spectrum antibiotics, tocilizumab, Remdesivir | 10 | BE |
| 12 | 35/M | Sinusitis, Mastoiditis, Meningitis | Severe COVID-19, ARDS, Sepsis, Septic shock, Hospitalisation, ICU, O2, Sepsis | IVMP, Enoxaparin, Broad-spectrum antibiotics, Remdesivir | 16 | BE |
| 13 | 65/M | DM | Severe COVID-19, ARDS, Sepsis, Septic shock, Hospitalisation, ICU, O2, Ventilator, Sepsis | IVMP, Enoxaparin, Broad-spectrum antibiotics, Tocilizumab, Remdesivir | 6 | RE |
| 14 | 59/M | DM, HTN | Severe COVID-19, ARDS, Sepsis, Hospitalisation, ICU, O2 | IVMP, Oral steroid, Enoxaparin, Broad-spectrum antibiotics, Tocilizumab, Remdesivir | 10 | RE |
| 15 | 63/F | DM, HTN, Hypothyroidism | Severe COVID-19, ARDS, Sepsis, Septic shock, Hospitalisation, ICU, O2, Sepsis | IVMP, Oral steroid, Enoxaparin, Broad-spectrum antibiotics, Tocilizumab, Remdesivir | 40 | BE |
| 16 | 55/M | DM* | Moderate COVID-19, Hospitalisation, O2 | IVMP, Oral steroid, Broad spectrum ant78ibiotics, Tocilizumab, Remdesivir | 40 | RE |
| 17 | 67/M | DM, HTN, EPN | Moderate COVID-19, Hospitalisation, O2, Sepsis | Oral steroid, Ecosprine, Broad spectrum antibiotics, Remdesivir, Favipiravir | 60 | LE |
| 18 | 55/M | DM | Moderate COVID-19, Hospitalisation, O2 | IVMP, Oral steroid, Enoxaparin, Broad-spectrum antibiotics, Tocilizumab, Remdesivir | 54 | RE |
| 19 | 53/M | HTN, CKD | Severe COVID-19, Hospitalisation, ICU, O2 | IVMP, Enoxaparin, Ecosprin, Broad-spectrum antibiotics, Tocilizumab, Remdesivir | 47 | LE |
| 20 | 5/M | Nil | Asymptomatic, Hospitalisation | Oral steroid, broad-spectrum antibiotics, | 70 | BE |
| 21 | 49/M | DM*, CKD, DKA, Anaemia | Severe COVID-19, Hospitalisation, ICU, O2, Sepsis | IVMP, Oral steroid, Enoxaparin, Broad-spectrum antibiotics, Tocilizumab, Remdesivir, Favipiravir | 30 | BE |
| 22 | 57/M | DM | Moderate COID, Hospitalisation, O2 | Oral steroid (DEXA), Enoxaparin, Broad-spectrum antibiotics, Remdesivir, | 48 | LE |
| 23 | 71/F | DM* | Moderate COVID-19, Hospitalisation, O2 | IVMP, Enoxaparin, Broad-spectrum antibiotics, Tocilizumab, Remdesivir, | 10 | BE |
| 24 | 50/M | DM*, GBS | Severe COVID-19, Hospitalisation, ICU, O2 | Oral (Dexa), Enoxaparin, Broad spectrum antibiotics, Tocilizumab, Remdesivir, Favipiravir | 72 | RE |
|
| ||||||
| # | Management of endophthalmitis | Result of microbiology/histopathology investigations on vitreous fluid/eviscerated content (microscopy/culture/PCR/histopathology) | Systemic investigations (culture/tissue biopsy) | FU (days) | Outcome | |
|
| ||||||
| 1 | Oral ciprofloxacin, Single intraocular broad-spectrum antibiotic injection in the emergency room. | Not done | BC: -Ve UC:-Ve | 2 | Deceased | |
| 2 | Intravenous caspofungin Vitreous biopsy, Vitrectomy, Vitreous lavage, Silicone oil injection, 12 times intraocular amphotericin-B | Candida tropicalis | BC:-Ve UC:-Ve | 134 | Systemically doing well, resolved eye infection, 20/800 vision, profound visual impairment | |
| 3 | Intravenous Caspofungin | Not done | BC:-Ve UC:-Ve | 30 | Systemically doing well, resolved eye infection, 20/250 vision, severe visual impairment | |
| 4 | Oral ciprofloxacin Vitreous biopsy, Vitrectomy, Vitreous lavage, Silicone oil injection, 4 times intraocular antibiotics (vancomycin and ceftazidime) | Gram-positive cocci | Streptococcus pneumonia in BC and UC | 164 | Systemically doing well, resolved eye infection, HM vision, near-total blindness | |
| 5 | Intravenous caspofungin Vitreous biopsy, Vitrectomy, Vitreous lavage, Silicone oil injection, 9 times intraocular amphotericin-B | Candida cifferi | Candida sp in BC and UC | 134 | Systemically doing well, resolved eye infection light perception vision, both eyes, extensive scarring of the retina near-total blindness | |
| 6 | Oral ciprofloxacinVitreous biopsy, Vitrectomy, 2 times intraocular antibiotic injections | -Ve | BC: -Ve UC:-Ve | 120 | Systemically doing well, 20/40 vision mild visual impairment | |
| 7 | Systemic antitubercular medication, Intravenous caspofungin | -Ve | Candida sp. In BC and UC | 30 | Deceased | |
| 8 | Oral ciprofloxacin Vitreous biopsy, Vitrectomy, Vitreous lavage, Silicone oil injection, 4 times intraocular vancomycin and ceftazidime injection | Gram-positive cocci | Streptococcus pneumonia in BC and UC | 90 | Systemically doing well, resolved eye infection HM vision, near-total blindness | |
| 9 | Oral ketoconazole Vitreous biopsy, Vitrectomy, 2 times intraocular amphotericin-B injection | Aspergillus flavus | BC:-Ve UC:-Ve Aspergillus sp in kidney biopsy | 90 | Systemically doing well, resolved eye infection light perception vision, both eyes Near-total blindness | |
| 10 | Oral ketoconazole | Not done | Not done | 7 | Systemically doing well, noncompliance lost vision, no light perception Phthisis bulbi, Total blindness | |
| 11 | Oral ciprofloxacin Vitreous biopsy, Vitrectomy, and single intraocular antibiotic (vancomycin and ceftazidime) | -Ve | Escherichia coli in BC and UC | 90 | Systemically doing well resolved eye infection, 20/25 vision mild visual impairment | |
| 12 | Oral and intravenous voriconazole | Not done | BC:-ve UC:-ve Aspergillus sp. in paranasal sinus biopsy culture | 7 | Deceased | |
| 13 | Intravenous amphotericin-B, Oral and intravenous posaconazole, Evisceration | Mucormycete in histopathology | BC:-ve UC:-ve Mucor sp. in paranasal sinus biopsy culture | 60 | Deceased | |
| 14 | Intravenous voriconazole, Vitreous biopsy, Vitrectomy, Silicone oil injection, 2 times intraocular amphotericin and voriconazole | Fusarium equiseti | BC:-Ve UC:-Ve | 90 | Systemically doing well, resolved eye infection HM vision, profound visual impairment | |
| 15 | Intravenous caspofungin | Not done | Candida sp. in BC and UC | 7 | Deceased | |
| 16 | Intravenous voriconazole Vitreous biopsy, Vitrectomy, 3 times intraocular amphotericin and voriconazole injection | -Ve | BC: Candida sp. UC:-Ve | 60 | Systemically doing well lost eye, phthisis bulbi, no light perception, Total blindness | |
| 17 | Oral fluconazole, Vitreous biopsy, Vitrectomy, 6 times intraocular amphotericin-B injection | Candida sp. | BC-Ve UC: Candida sp. | 80 | Systemically doing well, 20/200 vision with foveal scarring, severe visual impairment | |
| 18 | Oral fluconazole Vitreous biopsy, Vitrectomy, 6 times intravitreal amphotericin injection | Candida tropicalis | Not done | 132 | Systemically doing well resolved eye infection 20/25 vision, mild visual impairment | |
| 19 | Intravenous voriconazole Vitreous biopsy, Vitrectomy, 6 times intravitreal amphotericin-B and voriconazole injection | Aspergillus fumigatus | BC:-Ve UC:-Ve | 90 | Systemically doing well, resolved eye infection hand motion vision near-total blindness | |
| 20 | Oral valaciclovir Vitreous biopsy, vitrectomy | HSV-1 DNA | BC; -Ve UC:-Ve | 127 | Systemically doing well, resolved eye infection 20/200 vision, severe visual impairment | |
| 21 | Oral fluconazole Vitreous biopsy, Vitrectomy 3 times intraocular amphotericin and voriconazole | Candida tropicalis | BC:-Ve UC:-Ve | 90 | Systemically doing well, resolved ye infection 20/800 vision, profound visual impairment | |
| 22 | Oral fluconazole, Oral acyclovir Vitreous biopsy, Vitrectomy, Vitreous lavage, 3 times intraocular amphotericin B and voriconazole, 4 times intravitreal ganciclovir | -Ve | BC:-Ve UC:-Ve | 140 | Systemically doing well, resolved eye infection, counting finger close to face vision, scarred retina, profound visual impairment | |
| 23 | Intravenous caspofungin, Vitreous biopsy, Vitrectomy, 3 times intraocular amphotericin b and voriconazole | Fungal DNA | Not done | 90 | Systemically doing well, resolved eye infection 20/40 vision in the right eye, 20/100 vision in the left eye moderate visual impairment | |
| 24 | Oral fluconazole Vitreous biopsy, vitrectomy, 5 times intraocular amphotericin | Candida tropicalis | BC:-Ve UC: Escherichia Coli | 30 | Systemically doing well resolved eye infection, 20/25 vision, mild visual impairment | |
ARDS - acute respiratory distress syndrome; BC - blood culture; CKD - chronic kidney disease; COVID-19 - coronavirus disease; dexa - dexamethasone; DM - diabetes mellitus; EPN, emphysematous pyelonephritis; FU - follow-up; GBS - Guillain-Barre syndrome; HSV-1 - herpes simplex virus-1; HTN - hypertension; ICU - intensive care unit; IVMP - intravenous methyl prednisolone; OS - ocular sample; OSC - ocular sample culture; PCN - percutaneous nephrostomy; PCR - polymerase chain reaction; TB - tuberculosis; UC - urine culture; -Ve-negative. *New-onset diabetes mellitus. ∫During COVID-19 management, she developed reactivation of tuberculosis in the spine
Table 1. Clinical characteristics of 24 COVID-19 patients with endogenous endophthalmitis.
Most patients (n = 15; 62.5%) had anemia, neutrophilia, lymphopenia, thrombocytopenia, and raised inflammatory blood markers (C-reactive protein, lactate dehydrogenase, serum ferritin, D-dimer, and IL-6 level) [Table 2]. One patient was asymptomatic who did not receive blood investigation, and eight patients did not possess a detailed laboratory result.
Table 2.
Systemic health parameters and treatment during COVID-19 hospitalization
| Parameter | Results | ||
|---|---|---|---|
| Laboratory tests at presentation for eye care (n=15) | Blood count. Median (range) | Hb | 10.5 gm% (7.8-12.8) |
| Platelet | 17,000/cu mm (18,000-36,00) | ||
| WBC | 8,700 (1,760-12,050) | ||
| Neutrophil (In DC) | 85 (80-92) | ||
| Lymphocyte (In DC) | 12 (4-8) | ||
| Inflammatory marker. Median (range | CRP | 70 (2.14-125) | |
| IL 6 | 33 pg/ml (0.5-61) | ||
| Enzymes. Median (range) | LDH | 415 U/ml (220-770) | |
| D-Dimer | 3 ug/ml (0.13-20) | ||
| Serum ferritin | 739 ng/ml (18-2001) | ||
| Treatment (n=24) | Antibiotic | Azithromycin | All patients |
| Doxycycline | All patients | ||
| Meropenem/Imipenem | 33.3 (n=8) | ||
| Tocilizumab | 58.3 (n=14) | ||
| Corticosteroid 87.5% (n=21) | IVMP | n=9 | |
| IVMP + Oral steroid | n=6 | ||
| Oral steroid | n=6 | ||
| Low molecular weight heparin | 70.8% (n=17) | ||
| Supportive therapy | Vit C, B-complex, Zinc | All patients | |
CRP - C-reactive protein; DC - differential count; Hb - hemoglobin; IL - interleukin; IVMP - intravenous methylprednisolone; LDH - lactate dehydrogenase
Systemic medications during hospitalization for COVID-19 treatment are listed in Table 2. The mean duration of corticosteroid treatment was 22.1 (range: 5–62) days. Regular microbiological assays such as blood culture, urine culture, samples from the central line, bronco alveolar lavage fluid, and sputum culture were not done in all patients.
In this cohort, we diagnosed endophthalmitis in 33 eyes (including one panophthalmitis) of 24 patients, and both eyes were involved in 9 (37.5%) patients. The ocular features included exudates in the vitreous body and retina (9/33, 27.3%) to complete vitreous abscess (23/33, 69.7%), the involvement of ocular coats, periocular tissue, and orbit (1/33). Figs. 2-5 document description of four representative patients.
Figure 2.
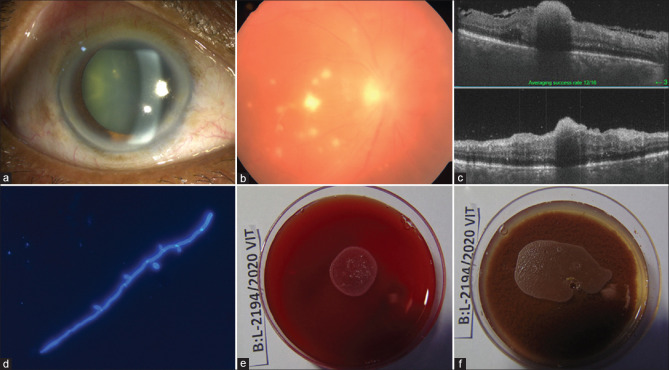
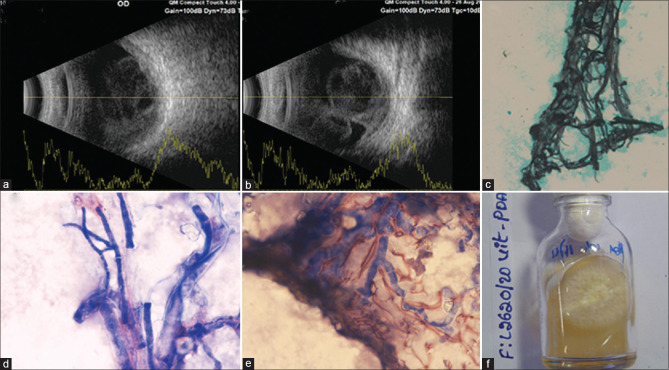
Case 2, Table 1: A 58-year-old man presented with pain, redness, and reduction of vision in the right eye (BCVA 20/320). The eye looked normal externally except for moderate conjunctival and ciliary congestion (a); the retina showed few preretinal exudates (b); optical coherence tomography (OCT) showed involvement of superficial retina (c). He received vitreous biopsy, vitrectomy, and intraocular antibiotics (ceftazidime and vancomycin). Vitreous microscopy showed thin fungal filament with budding cells (d) suggestive of yeast in direct microscopy [calcofluor white stain (CFW), ×400]. The culture was positive [blood agar (BA): e, chocolate agar (CA): f] for Candida tropicalis. The treatment included 5 times vitreo-retina surgery, including silicone oil injection, and 12 times intraocular amphotericin-B injection. No septic foci could be identified systemically; his blood and urine culture reports were negative. At the last follow-up (134 days), the eye was quiet, the retina was attached, and the corrected visual acuity right eye was 20/800
Figure 5.
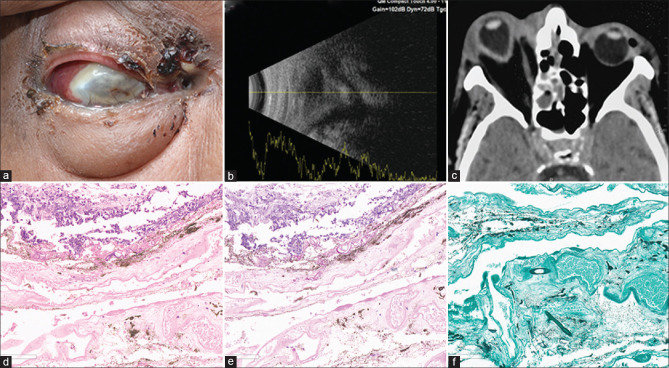
Case 13, Table 1: A 69-year-old man presented with periocular swelling, discharging fistula, and exudates externally (a) with light perception vision in the right eye. USG showed disorganized eyeball (b), the computer tomography (CT) scan revealed protrusion of the right eye with elongated axial length (c). His eviscerated material and tissue from paranasal sinuses were suggestive of mucormycosis. He received intravenous amphotericin-B and posaconazole. Eyeball was not salvageable; evisceration was done. The histopathology of the eviscerated contents showed broad aseptate fungal filaments with right angle branching suggestive of mucormycosis [hematoxylin and eosin (H and E) stain, ×200 (d); periodic acid Schiff stain (PAS), ×200 (e); Gomori methenamine silver stain (GMS), ×200 (f)]. At 60 days, he expired due to COVID-19-related complications
The mean presenting visual acuity was 0.0415 ± 0.1445 (range: 0.0013–0.8). All patients complained of pain, redness, and blurring of vision at presentation. Vitreous biopsy could not be obtained in five patients (eight eyes); three of them were critically ill with fluctuating blood oxygen saturation, unfit for any surgical procedure, and expired due to COVID-19-related complications during the course of systemic treatment; and two patients refused any surgical interventions. Fourteen of 19 vitreous biopsies (73.68%) were microbiologically positive: 11 (78.6%) fungi, 2 bacteria, and 1 virus [Table 1]. The systemic focus of infection was identified in 11 of 21 subjects (tests were not performed in three subjects); five had candidemia, three had bacteremia (two Streptococcus pneumoniae, one Escherichia coli), two Aspergillus spp. (one renal biopsy and one paranasal sinus biopsy), one Mucormycosis (Mucor in paranasal sinuses). RT-PCR for COVID-19 did not detect any virus in the vitreous sample in any of these patients.
At a median follow-up of 90 ± 19.8 (range: 2–164) days, 19 patients recovered, and five patients expired due to COVID-19-related complications [Fig. 6].
Figure 6.
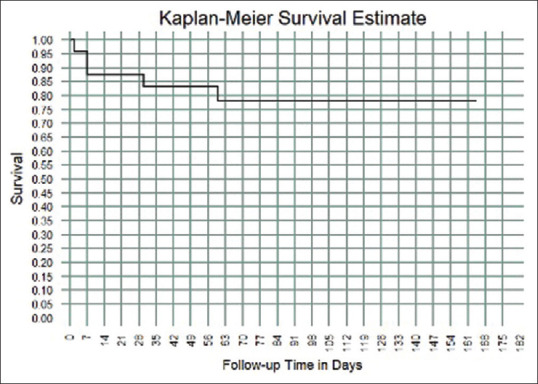
Kaplan–Meier curve in COVID-19 patients treated for endogenous endophthalmitis between April 2020 and January 2021. The endpoint was death in five patients in a median follow-up of 90 ± 19.8 (range: 2–164) days
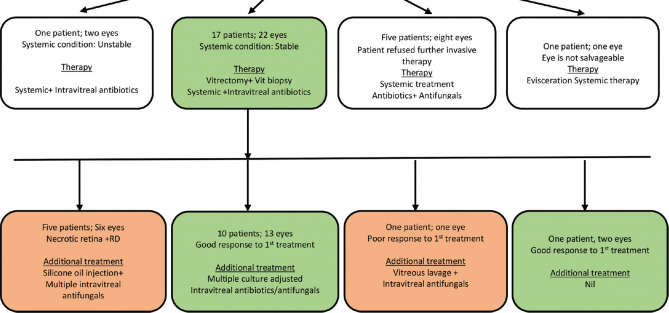
Table 1 lists the treatment for the eye ailment, and the summary is shown in Fig. 7.
Figure 7.
Flow diagram illustrative of eye treatment
The ophthalmic management included primary vitrectomy in 17 patients (22 eyes), repeat vitreous surgery in five patients (six eyes) including silicone oil tamponade in five patients (six eyes), two-times intravitreal antibiotic (one patient. one eye), multiple intravitreal antifungals in 10 patients (13 eyes), systemic antibiotic in five patients, and systemic antifungal in 19 patients. The systemic antibiotic was ciprofloxacin, and antifungals were caspofungin, voriconazole, posaconazole, fluconazole, and ketoconazole. The intravitreal antibiotics were vancomycin and ceftazidime, and intravitreal antifungals were amphotericin-B and voriconazole.
At the last follow-up, all the surviving people (19 of 24 patients; 22 eyes) had recovered from COVID-19-related systemic complications. The visual outcome [Table 1] was as follows: severe vision impairment (BCVA ≤20/400) in 13 (59.1%) eyes of 11 (57.9%) patients; functionally improved vision (BCVA > 20/400) in 9 (40.9%) of 8 (42.1%) patients. Four of five patients who died had bilateral involvement.
Brief descriptions of few cases
Case 2, Table 1: A 58-year-old man presented with pain, redness, and reduction of vision in the right eye (BCVA 20/320). The eye looked normal externally except for moderate conjunctival and ciliary congestion [Fig. 2a]; the retina showed few preretinal exudates [Fig. 2b]; optical coherence tomography (OCT) showed involvement of superficial retina [Fig. 2c]. He received vitreous biopsy, vitrectomy, and intraocular antibiotics (ceftazidime and vancomycin). Vitreous microscopy showed thin fungal filament with budding cells [Fig. 2d] suggestive of yeast in direct microscopy [calcofluor white stain (CFW), ×400]. The culture was positive [blood agar (BA): Fig. 2e chocolate agar (CA): Fig. 2f] for Candida tropicalis. The treatment included 5 times vitreo-retina surgery, including silicone oil injection, and 12 times intraocular amphotericin-B injection. No septic foci could be identified systemically; his blood and urine culture reports were negative. At the last follow-up (134 days), the eye was quiet, the retina was attached, and the corrected visual acuity right eye was 20/800.
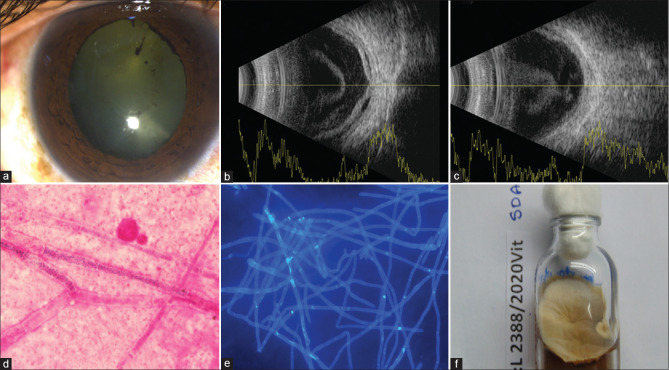
Case 14, Table 1: A 59-year-old man presented with mild conjunctival congestion [Fig. 3a] and hand motion vision in the right eye. Fundus detail was not visible. Ultrasonogram (USG) of the right eye showed echodense vitreous cavity [Fig. 3b], exudative retina detachment (RD), [Fig. 3c], and choroidal thickening (CT); [Fig. 3c]. The vitreous microscopy sample showed septate fungal filaments in direct microscopy [Gram stain, ×1000 [Fig. 3d]; Calcofluor white, ×400 [Fig. 3e]]. Fusarium equiseti grew on all media, including Sabouraud dextrose agar (SDA), [Fig. 3f]. Treatment included two vitreous procedures (vitrectomy vitreous lavage with silicone oil injection) and 2 times intravitreal amphotericin-B/voriconazole injections. His blood and urine cultures were negative for any organism. At the last follow-up visit (90 days), the eye was quiet; there was extensive scarring of the retina with hand motion vision in the right eye.
Figure 3.
Case 14, Table 1: A 59-year-old man presented with mild conjunctival congestion (a) and hand motion vision in the right eye. Fundus detail was not visible. Ultrasonogram (USG) of the right eye showed echodense vitreous cavity (b), exudative retina detachment (RD), (b), and choroidal thickening (CT); (c). The vitreous microscopy sample showed septate fungal filaments in direct microscopy [Gram stain, ×1000 (d); Calcofluor white, ×400 (e)]. Fusarium equiseti grew on all media, including Sabouraud dextrose agar (SDA), (f). Treatment included two vitreous procedures (vitrectomy vitreous lavage with silicone oil injection) and 2 times intravitreal amphotericin-B/voriconazole injections. His blood and urine cultures were negative for any organism. At the last follow-up visit (90 days), the eye was quiet; there was extensive scarring of the retina with hand motion vision in the right eye
Case 9, Table 1: A 65-year-old man presented with bilateral endogenous endophthalmitis with light perception vision in both eyes. The ultrasound of the eye showed echodense vitreous cavity [Fig. 4a and b]. Vitreous microscopy showed septate fungal filaments in various vital stains [Gomori methenamine silver (GMS), ×400 [Fig. 4c]; Giemsa stain, ×1000 [Fig. 4d and e]] and grew Aspergillus flavus [Fig. 4f]) on SDA. His renal biopsy had also grown Aspergillus spp. He received vitrectomy and intravitreal amphotericin-B (2 times). At 90 days, his eyes were quiet, but the vision did not improve beyond light perception in either eye.
Figure 4.
Case 9, Table 1: A 65-year-old man presented with bilateral endogenous endophthalmitis with light perception vision in both eyes. The ultrasound of the eye showed echodense vitreous cavity (a and b). Vitreous microscopy showed septate fungal filaments in various vital stains [Gomori methenamine silver (GMS), ×400 (c); Giemsa stain, ×1000 (d and e)] and grew Aspergillus flavus (f)) on SDA. His renal biopsy had also grown Aspergillus spp. He received vitrectomy and intravitreal amphotericin-B (two times). At 90 days, his eyes were quiet; but the vision did not improve beyond light perception in either eye
Case 13, Table 1: A 69-year-old man presented with periocular swelling, discharging fistula, and exudates externally [Fig. 5a] with light perception vision in the right eye. USG showed disorganized eyeball [Fig. 5b], computer tomography (CT) scan revealed protrusion of the right eye with elongated axial length [Fig. 5c]. His eviscerated material and tissue from paranasal sinuses were suggestive of mucormycosis. He received intravenous amphotericin-B and posaconazole. Eyeball was not salvageable; evisceration was done. The histopathology of the eviscerated contents showed broad aseptate fungal filaments with right-angle branching suggestive of mucormycosis [hematoxylin and eosin (H and E) stain, ×200 [Fig. 5d]; periodic acid Schiff stain (PAS), ×200 [Fig. 6e]; Gomori methenamine silver stain (GMS), ×200 [Fig. 6f]]. At 60 days, he expired due to COVID-19-related complications.
Discussion
Endogenous endophthalmitis results from the hematogenous spread of septic embolus from the bloodstream.[22] Untreated or inadequately treated, endophthalmitis initially confined to the vitreous cavity spreads to the ocular coats, resulting in panophthalmitis and orbital cellulitis.[23] Endogenous endophthalmitis has been reported in patients with systemic comorbidities such as diabetes mellitus, hepato-biliary disease, prolonged hospitalization, ICU admission, intravenous medication, indwelling urinary catheter, and use of corticosteroid/immunosuppressive agents.[24] In our cohort, all patients were hospitalized and had received intravenous medications; 91.6% (n = 22) patients had systemic illness (diabetes mellitus, chronic kidney disease, and hypertension); 66.6% (n = 16) patients were treated in the ICU, and 8.3% (n = 2) needed a ventilator. In our cohort, prolonged administration of three classes of drugs might have predisposed to endogenous endophthalmitis. These drugs are systemic corticosteroids, IL-6 inhibitors (tocilizumab), and broad-spectrum antibiotics.
Corticosteroid is known to cause immunosuppression and increases the risk of bacterial/fungal infection.[25] The RECOVERY trial recommended dexamethasone 6 mg daily for up to 10 days in hospitalized COVID-19 patients who require oxygen supplementation.[26] The other corticosteroids used in COVID-19 are methylprednisolone, prednisolone, and hydrocortisone.[27] In our cohort, the majority (21/24, 87.5%) of the patients were treated with corticosteroid, 71.42% (15/24) with intravenous methylprednisolone, and the mean duration of such treatment was 22.12 days.
Broad-spectrum antibiotics kill the bacteria and commensals that keep the yeast at bay and allow yeast multiplication.[28] Their use has been associated with systemic fungal infection.[29] In our cohort, all patients were treated with systemic antibiotics (Invariably azithromycin/doxycycline, or meropenem/Imipenem) during hospital admission for COVID-19, and the median treatment duration was 21 ± 4.9 (range: 5–35) days.
IL-6 inhibitors impair the function of neutrophils, macrophages, and T cells and increase the risk of fungal infection.[30] Tocilizumab is an IL-6 receptor monoclonal blocking agent used for rheumatoid arthritis for several years.[31] In COVID-19 patients, tocilizumab is administered in patients with severe pneumonia with cytokine storm, increased demand for oxygen, raised inflammatory markers, and worsened CT chest.[32] Bacterial infection associated with tocilizumab has been reported earlier.[31] Recently, candidemia in hospitalized COVID-19 patients has been reported after tocilizumab use,[33] An experimental study has shown severe impairment of macrophage, neutrophil, helper T-cell functioning leading to candidemia in IL-6 deficient mice.[30] In our cohort, 58.3% (14/24) had received tocilizumab.
In this cohort, laboratory confirmation of infection was obtained in 79.2% (n = 19) patients. This included 52.6% (n = 10 of 19) positive vitreous culture, 57.1% (n = 12 of 21) positive nonocular samples (blood/urine/sinus/ear discharge) and 23.5% (n = 4 of 17) positive ocular and non-ocular samples. All culture-positive ocular samples grew fungus, and Candida spp. was the most common. Systemic fungal infection in hospitalized COVID-19 patients is not new.[4,6,34] Like the systemic infection, the spectrum of fungal infection in our patients was wide: Candida, Aspergillus, Fusarium, and Mucor.
In our group, there was fewer bacterial endophthalmitis: only three patients (two Streptococcus pneumoniae and one Escherichia coli in blood culture). It is probable that bacterial endophthalmitis, if any, did not manifest due to the systemic antibiotics used in these patients for COVID-19 treatment. All three antibiotics—azithromycin, doxycycline, meropenem/Imipenem—are known to cross the blood–retinal barrier.[35,36] However, the use of these drugs and prolonged systemic steroid use could have resulted in endogenous fungal endophthalmitis. Viral coinfection in hospitalized COVID-19 patients is 3%, including respiratory syncytial virus (RSV) and Influenza.[4] In our cohort, one patient was positive for HSV-1 in viral PCR analysis of the ocular sample. The negative RT-PCR of the vitreous samples for SARS-CoV-2 precluded intraocular inflammation directly caused by the virus.
We compared the current endogenous endophthalmitis data in COVID-19 treated patients with other recently published series from other parts of the world[37,38,39,40] [Table 3]. In the current cohort, the mean patient age (53.66 years vs. 23.41 years), the identification of the source of infection (100% vs. 23.7%), presence of systemic symptoms (95.89% vs. 23.7%), positive blood culture (29.16% vs. 0.57%), and positive urine culture (29.16% vs. 6.35%) were higher than our earlier report of endogenous endophthalmitis without COVID-19 infection;[37] this trend was similar to reports from other parts of the world.[38,40] In the current series, there was higher fungal infection (58.33% vs. 15%) and lesser gram-negative infection (4.1% vs. 7%).[37]
Table 3.
Comparison of features of endogenous endophthalmitis in patients with COVID-19 (current study) or without COVID-19 (published literature)
| Criteria | Current study | Comparing with Dave et al. 2020 (India/South Asia)[37] | Comparing with Muda R et al. 2018 (Malayasia/South-East Asia)[38] | Comparing with Ratra et al. 2015 (India/South Asia)[39] | Comparing with Binder et al. .l 2003 (USA/North America)[40] | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| n | P (95% CI) | n | P (95% CI) | n | P (95% CI) | n | P (95% CI) | ||
| Sample size | 24 | 173 | 143 | 61 | 34 | ||||
| Age | 53.66 (+-13.53) | 25.41+-20.46 | <0.0001 (36.74-19.75) | 52.6+-15.1 | P=0.7473 | 34.6+-14.9 | P<0.0001 (26.025-12.09) | 63.3 | - |
| Gender (Male) | 17 (70.83) | 96 (55.55) | P=0.1571 | 59 (49.2) | P=0.0503 | 36 (62.1) | P=0.4515 | 19 (55.5) | P=0.2406 |
| Identificationof primary source of infection | 24 (100) | 56 (23.7) | P<0.0001 (52.02-74.15) | 90 (75) | P=0.0059 | 31 (53.4) | P<0.0001 (28.35-58.94) | 33 (97) | P=0.3961 |
| Systemic symptoms | 23 (95.89) | 41 (23.7) | P<0.0001 (54.73-78.83) | 84 (70) | P=0.0078 | 22 (37.9) | P < -0001 (37.61-69.60) | 23 (67.64) | P=0.0095 |
| Blood culture | 7 (29.16) | 1 (0.57) | P<0.0001 (14.10-48.59) | 50 (42) | P=0.2365 | 2 (5.88) | P=0.0036 | 9 (33.33) | P=0.7388 |
| Urine culture | 7 (29.16) | 11 (6.35) | P=0.0003 | 19 (41.3) | P=0.2618 | 4 (11.6) | P=0.0513 | 7 (25.9) | P=0.7854 |
| Vitreous culture | 10/19 (52.63) | 161 (93.06) | P<0.0001 (21.62-59.56) | 27 (22.3) | P=0.0019 | 16 (47.05) | 0.6451 | 24 (70.58) | P=0.1665 |
| Gram negative infection | 1 (4.1) | 64 (37) | P=0.0014 | 66 (80.8) | P<0.0001 (59.1-83.25) | 20 (58.82) | P<0.0001 (34.37-66.67) | 4 (11.76) | P=0.3094 |
| Fungal infection | 14/24 (58.33) | 24 (15) | P<0.0001 (22.90-61.12) | 16 (19.5) | P=0.0001 | 5 (14.7) | P=0.0001 | 14 (41.17) | P=0.2016 |
Bilgic et al.[16] reported three consecutive cases of endogenous endophthalmitis, all bacterial origin in the COVID-19 recovery stage. The better visual outcome after vitreous biopsy, vitrectomy, and intraocular antibiotic in their series could be related to bacterial endogenous endophthalmitis. In our series, the majority belonged to fungal endogenous endophthalmitis, which could have led to poor anatomical and functional outcomes. We did not see any SARS-Cov-2 virus in the vitreous samples in eight patients where vitreous biopsy material was subjected to RT-PCR for SARS-Cov-2 whereas Bilgic et al. had found one vitreous biopsy sample positive for the same virus.[16]
Limitations of this study
The tertiary care referral nature of our practice could have skewed some of the clinical presentations. The lack of the denominator of total hospitalized COVID-19 patients and their systemic comorbidities and interventions limits the conclusions drawn in our study.
Conclusion
Endophthalmitis is a rare but not uncommon occurrence in patients even after hospitalized care for COVID-19. This is associated with high mortality and blindness. Ocular infection is correlated with associated comorbidities, hospitalization, ICU admission, systemic therapy with a broad-spectrum antibiotic, corticosteroid, IL-6 inhibitor, raised inflammatory markers, and indwelling catheter. Candida spp. are the most common infecting organism. The treating physician should keep these facts in mind while treating patients with COVID-19. We recommend the inclusion of a routine eye examination (external eye and fundus) and estimation of vision as a part of the standard of care for hospitalized patients with COVID-19 who develop eye symptoms like blurring of vision and or redness.
Financial support and sponsorship
Hyderabad Eye Research Foundation, Hyderabad, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Dr Jay Kumar Chhablani, MD; University of Pittsburg, Pennsylvania.
References
- 1.Coronavirus Disease (COVID-19) - events as they happen. [Last accessed on 2020 Oct 21]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen .
- 2. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: A systematic review and meta-analysis. J Infect. 2020;81:266–75. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al-Hatmi AMS, Mohsin J, Al-Huraizi A, Khamis F. COVID-19 associated invasive candidiasis. J Infect. 2021;82:e45–6. doi: 10.1016/j.jinf.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verweij PE, Gangneux J-P, Bassetti M, Brüggemann RJM, Cornely OA, Koehler P, et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e53–5. doi: 10.1016/S2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Acharya S, Diamond M, Anwar S, Glaser A, Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020;21:e00867. doi: 10.1016/j.idcr.2020.e00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R. Retinal findings in patients with COVID-19. Lancet. 2020;395:1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28:391–5. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheth JU, Narayanan R, Goyal J, Goyal V. Retinal vein occlusion in COVID-19: A novel entity. Indian J Ophthalmol. 2020;68:2291–3. doi: 10.4103/ijo.IJO_2380_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–8. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cyr DG, Vicidomini CM, Siu NY, Elmann SE. Severe bilateral vision loss in 2 patients with coronavirus disease 2019. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2020;40:403–5. doi: 10.1097/WNO.0000000000001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mangana CM, Kargacin AB, Barraquer RI. Episcleritis as an ocular manifestation in a patient with COVID-19. Acta Ophthalmol (Copenh) 2020;98:e1056–7. doi: 10.1111/aos.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sindhuja K, Lomi N, Asif M, Tandon R. Clinical profile and prevalence of conjunctivitis in mild COVID-19 patients in a tertiary care COVID-19 hospital: A retrospective cross-sectional study. Indian J Ophthalmol. 2020;68:1546–50. doi: 10.4103/ijo.IJO_1319_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sen M, Honavar SG, Sharma N, Sachdev MS. COVID-19 and eye: A review of ophthalmic manifestations of COVID-19. Indian J Ophthalmol. 2021;69:488–509. doi: 10.4103/ijo.IJO_297_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bilgic A, Sudhalkar A, Gonzalez-Cortes JH, March de Ribot F, Yogi R, Kodjikian L, et al. Endogenous endophthalmitis in the setting of Covid-19 infection: A case series. Retina. 2021;41:1709–14. doi: 10.1097/IAE.0000000000003168. [DOI] [PubMed] [Google Scholar]
- 17. Shah KK, Venkatramani D, Majumder PD. A case series of presumed fungal endogenous endophthalmitis in post COVID-19 patients. Indian J Ophthalmol. 2021;69:1322–5. doi: 10.4103/ijo.IJO_3755_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goyal M, Murthy SI, Annum S. Retinal manifestations in patients following COVID-19 infection: A consecutive case series. Indian J Ophthalmol. 2021;69:1275–82. doi: 10.4103/ijo.IJO_403_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Available from: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf .
- 20. Das T, Hussain A, Naduvilath T, Sharma S, Jalali S, Majji AB. Case control analyses of acute endophthalmitis after cataract surgery in south india associated with technique, patient care, and socioeconomic status. J Ophthalmol 2012. 2012 doi: 10.1155/2012/298459. e298459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol Chic Ill 1960. 1995;113:1479–96. [PubMed] [Google Scholar]
- 22. Relhan N, Forster RK, Flynn HW. Endophthalmitis: Then and now. Am J Ophthalmol. 2018;187:xx–xxvii. doi: 10.1016/j.ajo.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pappuru RR, Dave VP, Pathengay A, Gangakhedkar S, Sharma S, Narayanan R, et al. Endophthalmitis progressing to panophthalmitis: Clinical features, demographic profile, and factors predicting outcome. Semin Ophthalmol. 2018;33:671–4. doi: 10.1080/08820538.2017.1416411. [DOI] [PubMed] [Google Scholar]
- 24. Cunningham ET, Flynn HW, Relhan N, Zierhut M. Endogenous endophthalmitis. Ocul Immunol Inflamm. 2018;26:491–5. doi: 10.1080/09273948.2018.1466561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954–63. doi: 10.1093/clinids/11.6.954. [DOI] [PubMed] [Google Scholar]
- 26. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corticosteroids. COVID-19 Treat. Guidel. [Last accessed on 2020 Dec 10]. Available from: https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticesteroids/
- 28. Gutierrez D, Weinstock A, Antharam VC, Gu H, Jasbi P, Shi X, et al. Antibiotic-induced gut metabolome and microbiome alterations increase the susceptibility to Candida albicans colonization in the gastrointestinal tract. FEMS Microbiol Ecol. 2020;96:fiz187. doi: 10.1093/femsec/fiz187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krcméry V, Matejicka F, Pichnová E, Jurga L, Sulcova M, Kunová A, et al. Documented fungal infections after prophylaxis or therapy with wide spectrum antibiotics: Relationship between certain fungal pathogens and particular antimicrobials? J Chemother Florence Italy. 1999;11:385–90. doi: 10.1179/joc.1999.11.5.385. [DOI] [PubMed] [Google Scholar]
- 30. Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, et al. Impaired neutrophil response and CD4+T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J Exp Med. 1996;183:1345–55. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pawar A, Desai RJ, Solomon DH, Santiago Ortiz AJ, Gale S, Bao M, et al. Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: A multidatabase cohort study. Ann Rheum Dis. 2019;78:456–64. doi: 10.1136/annrheumdis-2018-214367. [DOI] [PubMed] [Google Scholar]
- 32. Keske Ş, Tekin S, Sait B, İrkören P, Kapmaz M, Çimen C, et al. Appropriate use of tocilizumab in COVID-19 infection. Int J Infect Dis. 2020;99:338–43. doi: 10.1016/j.ijid.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Antinori S, Bonazzetti C, Gubertini G, Capetti A, Pagani C, Morena V, et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: An increased risk for candidemia? Autoimmun Rev. 2020;19:102564. doi: 10.1016/j.autrev.2020.102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pemán J, Ruiz-Gaitán A, García-Vidal C, Salavert M, Ramírez P, Puchades F, et al. Fungal co-infection in COVID-19 patients: Should we be concerned? Rev Iberoam Micol. 2020;37:41–6. doi: 10.1016/j.riam.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hosoya K, Tomi M, Tachikawa M. Strategies for therapy of retinal diseases using systemic drug delivery: Relevance of transporters at the blood-retinal barrier. Expert Opin Drug Deliv. 2011;8:1571–87. doi: 10.1517/17425247.2011.628983. [DOI] [PubMed] [Google Scholar]
- 36. Varela-Fernández R, Díaz-Tomé V, Luaces-Rodríguez A, Conde-Penedo A, García-Otero X, Luzardo-Álvarez A, et al. Drug delivery to the posterior segment of the eye: Biopharmaceutic and pharmacokinetic considerations. Pharmaceutics. 2020;12:269. doi: 10.3390/pharmaceutics12030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dave VP, Pathengay A, Panchal B, Jindal A, Datta A, Sharma S, et al. Clinical presentations, microbiology and management outcomes of culture-proven endogenous endophthalmitis in India. Indian J Ophthalmol. 2020;68:834–9. doi: 10.4103/ijo.IJO_1091_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muda R, Vayavari V, Subbiah D, Ishak H, Adnan A, Mohamed SO. Endogenous endophthalmitis: A 9-year retrospective study at a tertiary referral hospital in Malaysia. J Ophthalmic Inflamm Infect. 2018;8:14. doi: 10.1186/s12348-018-0158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ratra D, Saurabh K, Das D, Nachiappan K, Nagpal A, Rishi E, et al. Endogenous endophthalmitis: A 10-year retrospective study at a tertiary hospital in South India. Asia-Pac J Ophthalmol Phila Pa. 2015;4:286–92. doi: 10.1097/APO.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 40. Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis: An 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore) 2003;82:97–105. doi: 10.1097/00005792-200303000-00004. [DOI] [PubMed] [Google Scholar]