Dear Editor,
While ocular arterial and venous thromboembolic events have been frequently reported following COVID-19 infection,[1] there are fewer reports of these occurring after vaccination.[2] We report a 28-year-old healthy male with visual deterioration in his right eye 11 days after receiving the second dose of the Gam-COVID-Vac/Sputnik V (Gamaleya Institute).
At presentation, the best-corrected visual acuity (BCVA) in right eye was 6/9, N8. There were no anterior or posterior segment signs of inflammation. The intraocular pressures and pupillary responses were normal. Fundus examination revealed superior hemi-retinal vein occlusion with severe cystoid macular edema while the left eye was unremarkable [Fig. 1a-d]. The patient underwent a comprehensive examination by our internist and his vital parameters were normal. Complete hemogram, blood sugars, serum lipid profile, serum thyroid profile, serum homocysteine, anti-nuclear antibody, anti-neutrophil cytoplasmic antibodies (C and P), serum calcium, serum angiotensin-converting enzyme (ACE), Treponema pallidum hemagglutination assay, serum HIV test, chest X-ray, d-Dimer, coagulation profile, and COVID-19 antibodies were unremarkable. Serum IgG COVID-19 antibodies were strongly positive (Spike-S1 neutralizing antibodies by chemiluminescent microparticle immunoassay method) at 2946.4 arbitrary units (AU) per milliliter (positive threshold: 50 AU/mL; as a reference COVID-19 infection is associated with serum antibody levels of 112.40 AU/ml at 3 weeks after infection[3]). Oral prednisolone 40 mg daily was prescribed in tapering doses and the anticoagulant drug apixaban 2.5 mg twice daily was advised for six weeks. Three days later, the VA improved to 6/6, N6 and the macula showed decreased edema [Fig. 2a and b]. A week later, significant resolution of macular edema with trace intraretinal and subretinal fluid was noted [Fig. 2c and d]. He was advised to follow up after 2 weeks.
Figure 1.
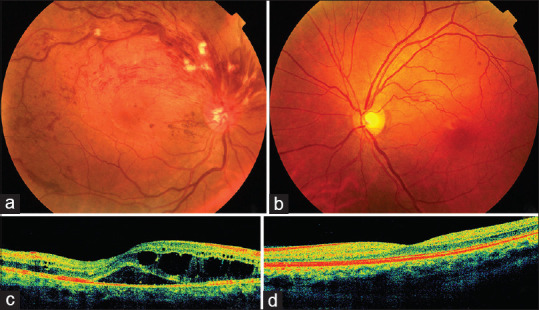
(a) Fundus photograph of the right eye at presentation showing superior hemi-retinal vein occlusion with dilated tortuous retinal veins, extensive retinal hemorrhages, and occasional exudates in superior retinal quadrants; there is clinically obvious macular edema. (b) Normal fundus of the left eye. (c) Spectral-domain optical coherence tomography (SD-OCT) of the right eye at presentation showing severe macular edema with significant intraretinal and subretinal fluid. (d) Corresponding SD-OCT of the left eye shows normal macula
Figure 2.
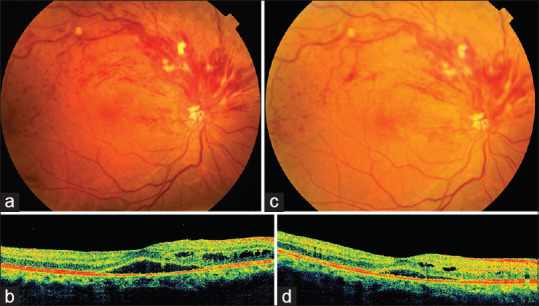
(a) Fundus photograph of the right eye 3 days after therapy showing some resolution of the venous dilation, tortuosity, and decreased retinal hemorrhages and macular edema. (b) Corresponding SD-OCT showing decreased intra- and subretinal fluid. (c) Ten days later, there is a further reduction in superior venous dilation, tortuosity, retinal hemorrhages, and macular edema. (d) Corresponding SD-OCT showing near-complete resolution of intra- and subretinal fluid
The association of vein occlusion with vaccination is well established due to the temporal sequence (within 5–30 days of immunization), and the strong positive COVID-19 antibodies titers indicate an unusually strong immune (and possibly inflammatory) response to the vaccine.
Systemic arterial and venous thromboembolic events have been extensively reported following other adenoviral vector-based vaccines such as ChAdOx1 CoV-19 (AstraZeneca, University of Oxford, and Covishield, Serum Institute of India)[4] and Ad26.COV2.S vaccine (Janssen, Johnson and Johnson).[5] Central retinal vein occlusion has been reported to occur after the second dose of the m-RNA SARS-CoV-2 vaccine (Pfizer-BioNTech, USA).[2] However, so far, neither ocular nor systemic thromboembolic events have been reported following the SPUTNIK V vaccine and we believe this to be the first such report of ocular venous occlusion. The exact pathophysiology is uncertain especially as there were no preexisting risk factors in our case.
In conclusion, we reemphasize that vaccination is the most promising approach for containing the COVID-19 pandemic. There is broad consensus among regulatory agencies and expert panels that the benefits of vaccination greatly outweigh the potential risks of rare vaccine side effects. While retinal arterial and vein occlusions are probably extremely rare, based on the paucity of reports, in view of the long-lasting impact on the vision, patients should be gently cautioned to report immediately in case of any visual side effects.
Financial support and sponsorship
Funding was provided by Hyderabad Eye Research Foundation, Hyderabad, India for Dr. Somasheila I. Murthy. The funders had no role in the preparation, review, or approval of the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1. Venkatesh R, Reddy NG, Agrawal S, Pereira A. COVID-19-associated central retinal vein occlusion treated with oral aspirin. BMJ Case Rep. 2021;14:e242987. doi: 10.1136/bcr-2021-242987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bialasiewicz AA, Farah-Diab MS, Mebarki HT. Central retinal vein occlusion occurring immediately after 2nd dose of mRNA SARS-CoV-2 vaccine. Int Ophthalmol. 2021:1–4. doi: 10.1007/s10792-021-01971-2. doi: 10.1007/s10792-021-01971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xiao AT, Gao C, Zhang S. Profile of specific antibodies to SARS-CoV-2: The first report. J Infect. 2020;81:147–78. doi: 10.1016/j.jinf.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pottegård A, Lund LC, Karlstad Ø, Dahl J, Andersen M, Hallas J, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;5:373. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC Health Alert Network. Cases of cerebral venous sinus thrombosis with thrombocytopenia after receipt of the Johnson and Johnson COVID-19 vaccine. [Last accessed on 2021 April 17]. Available from: https://emergency.cdc.gov/han/2021/han00442.asp .


