Abstract
Several COVID-19 vaccines have been developed and approved for use around the world from December 2020, to combat the pandemic caused by the novel SARS-CoV-2 virus. Several ophthalmic manifestations of the COVID-19 vaccines have been reported by ophthalmologists. This review was undertaken to recognize, encourage active reporting and determine the pathogenesis and time of appearance for better awareness and understanding of the ophthalmic manifestations of COVID-19 vaccines. A literature search was performed for publications on the ophthalmic manifestations of COVID-19 vaccines between January 1, 2021 and November 7, 2021. 23 case reports, 17 letters to editors, 3 ophthalmic images, 4 brief communications, 4 retrospective cohort studies and 2 case control studies were included. Posterior segment, including the uvea, choroid and retinal vasculature, was most commonly affected and the reported clinical features developed at a median of four days from the time of vaccination. The possible mechanisms include molecular mimicry of the vaccine components with host ocular tissues, antigen-specific cell and antibody-mediated hypersensitivity reactions to viral antigens and adjuvants present in the vaccines. The causal relationship of the ocular signs and symptoms and COVID-19 vaccines has not been established and requires long-term and large multicentre data. Most of the reported manifestations are mild, transient and adequately treated when diagnosed and managed early. The benefits of COVID-19 vaccination outweighs the reported rare adverse events and should not be a deterrent to vaccination.
Keywords: Corneal graft rejection, COVID-19 vaccine, COVID-19, inactivated vaccine, mRNA vaccine, ophthalmic manifestations, SARS-CoV-2, vascular occlusion, vector based vaccine
The year 2020 was unlike any other, a year of uncertainty, distancing, and loss. Man has always found his way through obstacles and challenges; David must come to fell Goliath. The best minds around the world worked at an unprecedented pace to develop vaccines against the novel SARS-CoV-2 virus. By December 2020, vaccines against COVID-19 were authorized for emergency use through expedited clinical trials. Like any change, any revolution, it too faced trials and tribulations, hype and hysteria, media and political might. As we enter the last quarter of 2021, it may be safe to say that the vaccines have “lived and let live.” A large population has been vaccinated, 7,027,377,238 vaccine doses, to be exact, as of November 7, 2021, have been administered, lockdowns are being lifted and gradually life seems to be returning to normal, albeit a new one.[1]
The main vaccines approved for use around the world are the nucleoside-modified RNA vaccine (BNT162b2) by Pfizer-BioNTech, the recombinant adenoviral vector encoding SARS-CoV-2 spike (S) glycoprotein (ChAdOx1 nCoV-19 Coronavirus vaccine Recombinant COVISHIELD by Serum Institute of India based on the Oxford AstraZeneca Chimpanzee Adenovirus Vectored Vaccine (AZD1222)), and the inactivated SARS-CoV-2 vaccine (BBIBP-CorV, Sinopharm). Additionally, there are other vaccines including Sputnik V, Russia, mRNA-1273 Moderna, Janssen, Johnson and Johnson COVAXIN by Bharat Biotech, India, and CoronaVac from China. The vaccines have been found to be 94%–95% effective.[2,3,4] The common side effects of these vaccines are mild to moderate pain, swelling, and erythema at the injection site, chills, fatigue, malaise, headache, and fever. Diarrhea, nausea, vomiting, and dermatitis are less frequent side effects documented. These usually start within 24––48 hours of the inoculation and last for 1–2 days. Table 1 shows the vaccines, their mechanism of action, and doses recommended.[2,3,4,5]
Table 1.
COVID-19 vaccines approved for use around the world
| Vaccine | Manufacturer | Type | Details | Dosage | Safety data from clinical trials: serious adverse events |
|---|---|---|---|---|---|
| BNT162b2 | Pfizer, Inc. and BioNTech (COMIRNATY) | mRNA | Lipid nanoparticle formulated nucleoside- modified mRNA vaccine encoding prefusion stabilized, membrane-anchored full length SARS-CoV-2 spike (S) glycoprotein | 12 years or older 2 doses 21 days apart Additional dose- recommended for moderate to severely immunocompromised people- 4 weeks after the 2nd dose Booster- Some groups of people are recommended to get a booster shot at least 6 months after getting their second shot | Myocarditis or pericarditis: rare, 12.6/100000 adolescents and young adults |
| mRNA-1273 | ModernaTX, Inc | mRNA | Nucleoside-modified mRNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 | 18 years or older 2 doses, 28 days apart Additional dose- recommended for moderate to severely immunocompromised people- 4 weeks after the 2nd dose | Myocarditis or pericarditis: rare, 12.6/100000 adolescents and young adults |
| ChAdOx1 nCoV-19 Corona Virus Vaccine AZD1222 | Serum Institute of India- Covishield AstraZeneca- Vaxzevria | Viral vector- based | Recombinant vaccine from the genetically modified human embryonic kidney (HEK) 293 cells with replication- deficient chimpanzee adenovirus vector encoding SARS-CoV-2 Spike (S) glycoprotein. Induces binding and neutralizing antibodies and interferon-gamma enzyme-linked immunospot responses. | 2 doses 12-16 weeks apart | Cerebral venous sinus thrombosis, thrombosis with thrombocytopenia |
| JNJ-78436735 Ad26COV2.S | Janssen Pharmaceuticals Companies of Johnson and Johnson | Viral vector-based | Recombinant, replication-incompetent Ad26 vector, encoding a stabilized variant of the SARS-CoV-2 DNA encoding Spike (S) protein | 18 years or older 1 shot Additional doses- not recommended Booster- At least 2 months after receiving your vaccine. You can get any of the COVID-19 vaccines authorized in the United States for your booster shot | Thrombosis with thrombocytopenia syndrome: 7/100000 vaccinated women 18-49 years Guillain-Barré syndrome |
| Gam- COVID-Vac | Sputnik V Gamaleya National Research Centre | Viral vector-based | Heterologous recombinant adenovirus approach with adenovirus (Ad26) and adenovirus 5 (Ad5) as vectors for expression of SARS-CoV-2 spike protein | 18 years or older Two doses, 21 days apart | |
| BBV152 | COVAXIN Bharat Biotech | Inactivated coronavirus Vaccine | The whole virion inactivated Imidazoquinoline class molecule (TLR 7/8 agonist) adsorbed to alum | 18 years or older 2 doses, 28 days apart | |
| BBIBP-CorV | Sinopharm | Inactivated coronavirus vaccine | An inactivated vaccine with aluminum-based adjuvant | 2 doses 3-4 weeks apart | Inflammatory demyelination syndrome, acute disseminated encephalomyelitis |
| CoronaVac | Sinovac Biotech Ltd Beijing | Inactivated coronavirus vaccine | The whole virion inactivated vaccine. Aluminum hydroxide adjuvant | 18 years or older 2 doses, 2-4 weeks apart Additional dose- recommended for moderate to severely immunocompromised people - 4 weeks after the 2nd dose | Booster - not recommended yet |
Still, in infancy, there is a paucity of data on the ocular manifestations folllowing COVID-19 vaccines. At the beginning of this year, we reviewed the ophthalmic manifestations of the SARS-CoV-2 virus.[6] In this article, we decided to review the ophthalmic manifestations of the vaccines against COVID-19.
Methods
A literature search was performed on PubMed with search words, COVID-19 vaccine, ophthalmology, eye, adverse effects, SARS-CoV-2, ocular manifestations, graft rejection, retina, vitreous, orbit, facial palsy, uveitis, nerve palsies, choroiditis, retinal vascular occlusion, mRNA vaccine, adenovirus vector vaccine, and inactivated vaccine. The articles included in this review are in English language, published between January 2021 and November 7, 2021. It is not an exhaustive review, but the authors have tried to include all the important reports with 23 case reports, 17 letters to editors, 3 image-based reports, 4 brief clinical communications, 4 retrospective cohort, and 2 casecontrol studies.
Eyelid, Ocular Surface and Anterior Segment Manifestations of COVID-19 Vaccine
The eyelid, ocular surface, and cornea are easily observable by patients and, therefore, present as soon as the symptoms develop. Eighteen cases of ocular adnexal and anterior segment manifestations have been reported [Table 2].[7,8,9,10,11,12,13,14,15,16] The mean age of the patients was 57.5 ± 15.6 (median 62.5) years, and there were 9 females and 6 males (3 gender unspecified). The Pfizer-BioNTech mRNA vaccine was the most common vaccine, used in 13 patients, two had received the viral vector-based Covishield, and three had received the inactivated Sinopharm vaccine. The majority of the symptoms were after the first dose,[11] whereas four patients presented after the second dose. The median interval from vaccination to development of symptoms was 7 (mean 9.8 ± 8) days.
Table 2.
Review of literature of eyelid, ocular surface, and corneal manifestations of COVID-19 vaccines
| Author | Type | Location | Sample | Age | Sex | Systemic/ocular illness | Vaccine | Dose | Duration between vaccine and symptoms (days) | Systemic adverse reaction |
|---|---|---|---|---|---|---|---|---|---|---|
| Austria et al.[7] | Letter to editor | New York | 3 | Mean 39.3 | F | - | BNT162b2 mRNA | - | 1-2 | - |
| F | - | BNT162b2 mRNAl | - | 1-2 | - | |||||
| F | - | BNT162b2 mRNA | - | 1-2 | - | |||||
| Mazzatenta et al.[8] | Letter to editor | Italy | 3 | 44 | F | - | BNT162b2 mRNA | 2 | 21-25 | - |
| 63 | M | - | BNT162b2 mRNA | 2 | 21 | - | ||||
| 67 | F | - | BNT162b2 mRNA | 1 | 10 | - | ||||
| Rallis et al.[9] | Brief communication | UK | 1 | 68 | F | OU lamellar DSAEK for Fuchs’ corneal endothelial dystrophy and a OS re-do PK for failed DSAEK in October 2020. On topical prednisolone OS and dexamethasone OD | BNT162b2 mRNA | 1 | 4 | moderate- chills, myalgia, tiredness |
| Abousy et al.[10] | Case report | USA | 1 | 73 | F | Fuchs’- OU DSEK- 8 years | BNT162b2 mRNA | 2 | 4 | - |
| Phylactou et al.[11] | Case report | UK | 2 | 66 | F | Fuchs’- OD DMEK 14 days before vaccination. Patient on dexamethasone. HIV+: undetectable viral load, CD4>600 on antiviral therapy | BNT162b2 mRNA | 1 | 7 | - |
| 83 | F | Fuchs’- OU DMEK- 3 and 6 years ago (OD DSEK®DMEK). | BNT162b2 mRNA | 2 | 21 | - | ||||
| Wasser et al.[12] | Case report | Jerusalem | 2 | 73 | M | PK- keratoconus and regraft for late endothelial failure. On dexamethasone 0.1% once daily | BNT162b2 mRNA | 1 | 13 | - |
| 56 | M | OU PK- keratoconus. Repeat PK in OD due to late endothelial failure. | BNT162b2 mRNA | 1 | 14 | - | ||||
| Ravichandran et al.[13] | Photo essay | India | 1 | 62 | M | PK- corneal scar OD, 2 years ago. Eye aphakic and amblyopic. On topical corticosteroids | ChAdOx1 nCoV-19 viral vector based | 1 | 21 | - |
| Crnej et al.[14] | Letter to editor | Lebanon | 1 | 71 | M | DMEK OD for endothelial decompensation following cataract surgery. HTN, smoking, CAD | BNT162b2 mRNA | 1 | 7 | - |
| Parmar et al.[15] | Case report | India | 1 | 35 | M | Therapeutic PK 3 yaers ago. Re-PK for graft failure 6 months ago; on topical corticosteroids | ChAdOx1 nCoV-19 viral vector based | 1 | 2 | - |
| Pichi et al.[16] | Case series | Abu Dhabi | 3 (7) | Mean 41.4 | - | BB1Bp-CorV inactivated | 1 | Mean 5.2 | ||
| - | RA on sulfasalazine | BB1Bp-CorV inactivated | 1 | |||||||
| - | BB1Bp-CorV inactivated | 1 | ||||||||
|
| ||||||||||
| Author | Laterality | Clinical features | Diagnosis | Management | Outcome | |||||
|
| ||||||||||
| Austria et al.[7] | Unilateral | UL>LL erythema and edema | Transient eyelid edema | Observation | Resolved 1-2 days | |||||
| unilateral | UL>LL erythema and edema | Transient eyelid edema | Antihistamines | Resolved 1-2 days | ||||||
| UL>LL erythema and edema | Transient eyelid edema | Oral corticosteroids | Resolved 1-2 days | |||||||
| Mazzatenta et al.[8] | OU | purpuric lesions on OU UL. | Transient purpuric lesions on eyelid | Observation | Spontaneously resolved 10 days | |||||
| OU | purpuric lesions on OU UL | Transient purpuric lesions on eyelid | Observation | Spontaneously resolved 15 days | ||||||
| OU | ecchymotic lesions- moderately itchy | Transient purpuric lesions on eyelid | Observation | Spontaneously resolved 12 days | ||||||
| Rallis et al.[9] | OS | Pain, redness. Diffuse punctate corneal staining, graft edema, Descemets folds, KPs, AC acivity | Acute corneal endothelial graft rejection | Hourly topical dexamethasone 0.1% and a week of oral acyclovir 400 mg 5x/day | Resolved, 3 weeks | |||||
| Abousy et al.[10] | OU | Va OD 20/200, Os 20/40, ocular pain, and photophobia, corneal edema, AC cells. Increased CCT | Acute corneal endothelial graft rejection | Prednisolone acetate 1% every 1 to 2 hours with Muro ointment at bedtime. Then tapered to qid | Improved. | |||||
| Phylactou et al.[11] | OD | Acute onset blurred vision, redness, photophobia, OD 6/36, diffuse corneal edema, KPs, AC 1+cells | Acute corneal endothelial graft rejection | Topical corticosteroids- 1 hourly | Improved by day 7. Irreversible endothelial loss | |||||
| OU | OD 6/24, OS 6/12 photophobia, redness, circumcorneal congestion, KPs, AC cells | Acute endothelial graft rejection | Topical corticosteroids- 1 hourly | Improved by day 7 | ||||||
| Wasser et al.[12] | OS | 20/200, ciliary injection, corneal edema, descemet’s folds, KPs | Graft rejection | Topical hourly corticosteroids, oral prednisone 60 mg per day | Resolved 1 week | |||||
| OD | Diffuse corneal edema, KPs, AC | Graft rejection | Topical hourly corticosteroids, oral prednisone 60 mg per day | Resolved 4 weeks | ||||||
| Ravichandran et al.[13] | OD | Congestion, diminution of vision, advancing Khodadoust rejection line, graft edema, AC reaction | Acute corneal Graft rejection | Appropriate | - | |||||
| Crnej et al.[14] | OD | Sudden painless vision loss, 20/125, diffuse corneal edema, increasing CCT | Acute corneal endothelial graft rejection | Topical dexamethasone 2 hourly, oral valacyclovir 1000 mg TID | Resolved 1 week | |||||
| Parmar et al.[15] | OS | Acute vision loss, epithelial and stromal edema, KPs | Acute corneal endothelial graft rejection | Hourly prednisolone, atropine TID, IVMP 1000 mg for 3 days- oral prednisolone. Considering immunologist opinion for NSAID before second dose of vaccine. | Resolved 3 weeks | |||||
| Pichi et al.[16] | Episcleritis | Topical corticosteroids | Resolved | |||||||
| OU | Pain, redness, diffuse scleral hyperaemia, positive phenylephrine test | Anterior scleritis | Topical corticosteroids | Resolved, 7 days | ||||||
| Anterior scleritis | Topical corticosteroids | Resolved | ||||||||
AC: Anterior chamber, CAD: Coronary artery disease, CCT: Central corneal thickness, DMEK: Descemet membrane endothelial keratoplasty, DSAEK: Descemet stripping automated endothelial keratoplasty, DSEK: Descemet stripping endothelial keratoplasty, F: Female, HIV: Human immunodeficiency virus, HTN: Hypertension, IVMP: Intravenous methylprednisolone, KP: Keratic precipitates, LL: Lower eyelid, M: Male, NSAID: Non-steroidal anti-inflammatory drug, OD: Right eye, OS: Left eye, OU: Both eyes, PK: Penetrating keratoplasty, RA: Rheumatoid arthritis, TID: Three times a day, UL: Upper eyelid
Eyelid
The eyelid involvement was transient in all cases limited to unilateral erythematous edema and bilateral purpuric rash. All cases were after the Pfizer-BioNTech mRNA vaccine. The three cases of acute onset eyelid edema reported by Austria et al.[7] resolved with observation, antihistamine, and oral corticosteroids, respectively, within 1–2 days. The purpuric rash observed in three patients developed at a median of 18 days and resolved spontaneously within 10–15 days. The coagulation profile was normal in all three.[8] [Fig. 1] Eyelid edema can be a part of an anaphylactic reaction, but none of the patients reported any systemic adverse effects. Periorbital swelling is seen in ocular vaccinia after the smallpox vaccine, and ocular respiratory syndrome is reported with the influenza vaccine.[8] The mechanisms proposed after COVID-19 vaccination include complement-induced reaction and molecular mimicry with an autoimmune response.
Figure 1.
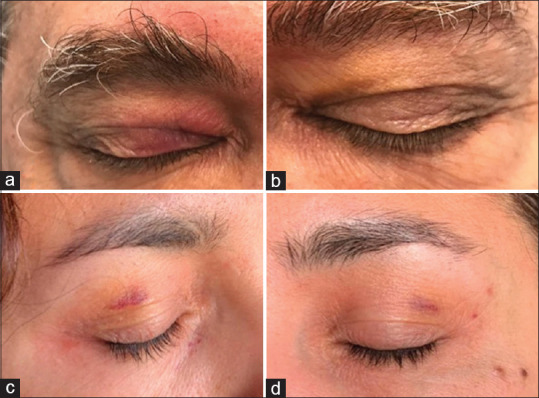
Purpuric lesions on the upper eyelids in patient 2 (a and b) and patient 1 (c and d). (Reproduced with permission from Mazzatenta C, Piccolo V, Pace G, Romano I, Argenziano G, Bassi A. Purpuric lesions on the eyelids developed after BNT162b2 mRNA COVID-19 vaccine: another piece of SARS-CoV-2 skin puzzle?. Journal of the European Academy of Dermatology and Venereology. 2021 May 28.)
Cornea
Corneal graft rejection was the most common anterior segment manifestation of the COVID-19 vaccine. Seven of the nine cases were following the Pfizer-BioNTech mRNA vaccine at a median duration of 7 (mean 10.3 ± 7.2) days. The remaining two received the viral vector-based Covishield vaccine. Four patients had undergone penetrating keratoplasty, whereas the rest had undergone lamellar keratoplasty. Five of eight patients were on topical corticosteroids at the time of vaccination. In the case reported by Phylactou et al., the patient also had a retroviral infection and was on treatment for the same.[11] Three patients had bilateral corneal transplants and of these, one patient developed rejection in only one eye. Five eyes had undergone repeat transplants following prior failures. Only one patient developed systemic symptoms, moderate chills, and myalgia following the vaccination.[9] The symptoms and signs were typical of endothelial graft rejection with acute onset of diminution of vision, redness, photophobia with or without pain, graft edema, Descemet’s folds, and anterior chamber (AC) reaction. [Figs. 2 and 3] Treatment was with frequent (1–2 hourly) administration of topical corticosteroids with or without oral steroids. Acyclovir/Valacyclovir was started in two patients to counter a possible viral infection.[9,14] All the patients recovered within 1–4 weeks.
Figure 2.
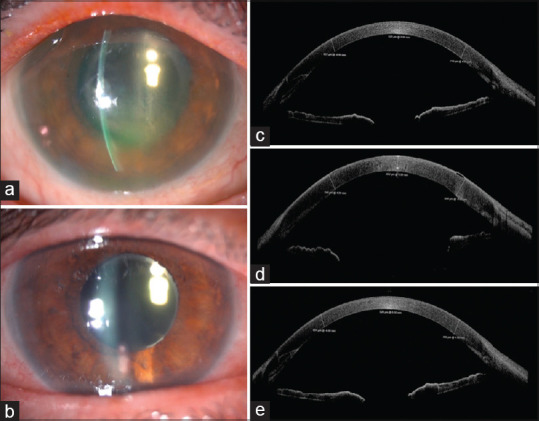
Early acute endothelial rejection post-DMEK following vaccination. Slit-lamp image at presentation on day 7 postvaccination with rejection and corneal edema (a), and on day 14 postvaccination and intensive treatment with topical dexamethasone showing improved stromal transparency (b). Anterior segment OCT on day 7 post-DMEK, indicating full graft attachment and CCT of 525 μm (c), on day 21 post-DMEK (day 7 postvaccination) at presentation with rejection and CCT of 652 μm corresponding to observed stromal edema and inflammation (d), and on day 28 post-DMEK (day 14 post-vaccination), following increased frequency of topical steroids and CCT of 526 μm (e). (Reproduced with permission from Phylactou M, Li JP, Larkin DF. Characteristics of endothelial corneal transplant rejection following immunization with SARS-CoV-2 messenger RNA vaccine. British Journal of Ophthalmology. 2021 Jul 1;105(7):893-6.)
Figure 3.
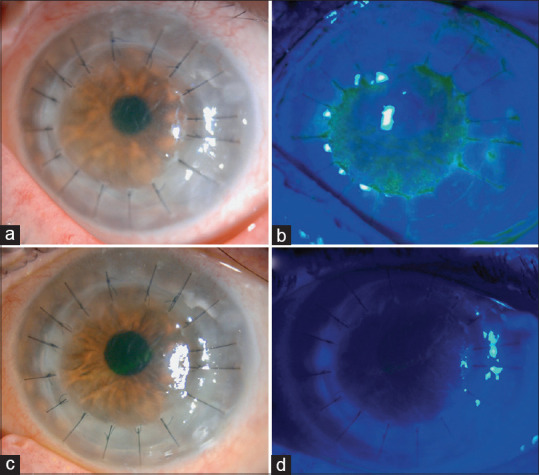
A case of acute corneal endothelial graft rejection after COVID-19 vaccine. A, B Slit-lamp photography demonstrating conjunctival hyperemia, corneal graft haze, diffuse corneal epithelial, and stromal edema (within the graft), Descemet’s folds, scattered keratic precipitates (KPs), and 1+ cells in the anterior chamber. An unusual distribution of fluorescein staining with coarse punctate epitheliopathy over the corneal graft was observed. The central corneal thickness (CCT) was 730 μm. C, D At 3-week post-treatment, the corneal graft rejection was successfully treated with considerable improvement in the graft transparency, reduction in epithelial and stromal edema, and resolution of epitheliopathy and anterior chamber inflammation. The best-corrected visual acuity improved to 6/12, with a CCT of 609 μm (Reproduced with permission from Rallis KI, Ting DS, Said DG, Dua HS. Corneal graft rejection following COVID-19 vaccine. Eye. 2021 Aug 23:1-2.)
The cornea is an immunoprivileged organ, and corneal transplantation has the highest rate of success. Lamellar keratoplasty has ensured further protection of the graft. Graft rejection can follow the breakdown of the blood-ocular barrier following intraocular inflammation or recurrent viral infection.[11] Corneal graft rejection after immunization has been reported with influenza, hepatitis B, tetanus, and yellow fever vaccines.[10,11] Bilateral graft failure in two of three patients with transplants in both eyes, and the temporal sequence of events points towards the role of the COVID-19 vaccine. The suggested mechanisms of graft failure are the vaccine-induced immune enhancement and cross-reactivity of virus antigen-specific T-cells with HLA antigen-disparate corneal allograft endothelial cells, increased vascular permeability after the vaccination, and presence of mRNA in the aqueous following a prior COVID-19 infection.[14] The COVID-19 vaccine induces a strong antibody response with CD4 + Th1 cells, which are also mediators of corneal graft rejection.[10] Systemic reactogenicity would be more likely after the second dose of vaccination than the first one, but seven of the nine patients developed rejection after the first dose itself. Also, none of the patients were reported to have had prior COVID-19 infection, but one cannot rule out mild, asymptomatic infection. Nevertheless, the possibility of graft rejection following immunization necessitates considering deferral of elective corneal transplants by 3–6 months after the final dose of COVID-19 vaccine and initiating corneal transplant patients on topical corticosteroids before the first dose of vaccine and continuing for a month after the second dose.[10] Patients with repeat grafts and immunocompromised state are at a higher risk of rejection and should be forewarned. While all patients recovered, it must be borne in mind that endothelial cell loss following graft rejection is irreplaceable.
Ocular Surface and Sclera
Pichi et al. reported a case series of seven patients with a mean age of 41.4 years who developed ocular complaints following the first dose of the inactivated Sinopharm vaccine.[16] Four eyes of three patients developed episcleritis and scleritis at a mean of 5.2 days. One patient had a history of rheumatoid arthritis, was on sulfasalazine and presented with scleritis. Tapering doses of topical corticosteroid were prescribed, and the inflammation resolved in all the cases. Scleritis and episcleritis have been reported after the administration of live attenuated vaccines as well as post-COVID-19 infection.[6,17]
Posterior Segment Manifestations of COVID-19 Vaccine
The retina and the uvea are the most commonly involved ocular structures following COVID-19 vaccination with 50 cases reported in the literature, 21 being contributed by a retrospective study from Israel [Table 3].[16,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] The mean age was 43.6 ± 17.2 (median 40) years with 23 males and 23 females (unspecified in 4). The symptoms developed at a median interval of 4 (mean 7.4 ± 8.5 ) days from the time of vaccination. None of the patients had a prior history of COVID-19 although one patient with panuveitis tested positive for COVID-19 post-vaccination.[27] Thirty patients had received the Pfizer-BNT mRNA vaccine, the adenoviral vector-based vaccines by AstraZeneca and Serum Institute of India were given to 11 patients, whereas the vector-based vaccine by Johnson and Johnson had been used in one patient. One patient had received the Sputnik V vaccine, one had received Moderna mRNA vaccine, and six had received inactivated vaccine. The symptoms developed after the first dose in 25 patients and after the second dose in 21 patients. Two patients developed adverse effects after both the first and the second dose. Systemic side effects of the vaccine were seen in 12 patients, the majority being mild cases of headache, myalgia, and fever. Valenzuela reported a case of acute macular neuroretinopathy in a patient who developed cervical and supraclavicular lymphadenopathy and difficulty in swallowing, which required treatment with prednisolone and diphenhydramine.[37]
Table 3.
Review of literature of posterior segment manifestations of COVID-19 vaccines
| Author | Type | Location | Sample | Age | Sex | Systemic/ocular illness | Vaccine | Dose |
|---|---|---|---|---|---|---|---|---|
| Saraceno et al.[18] | Letter to editor | Brazil | 1 | 62 | F | - | ChAdOx1 nCoV-19 AZD1222 viral vector based | - |
| Koong et al.[19] | Letter to editor | Singapore | 1 | 54 | M | DM, hyperlipidemia | BNT162b2 mRNA | 1 |
| Papasavvas et al.[20] | Case report | Switzerland | 1 | 43 | F | VKH treated with corticosteroids, infliximab. In remission for 6 years | BNT162b2 mRNA | 2 |
| ElSheikh et al.[21] | Letter to editor | USA | 1 | 18 | F | JIA, ANA positive, no prior history of uveitis | BB1Bp-CorV inactivated | 2 |
| Jain et al.[22] | Letter to editor | India | 1 | 27 | M | JIA with HLAB27 on adalimumab for 3 years, discontinued. | ChAdOx1 nCoV-19 viral vector based | 1 |
| Renisi et al.[23] | Case report | Italy | 1 | 23 | M | Recurrent panic attacks on benzodiazepines | BNT162b2 mRNA | (1), 2 |
| Rabinovitch et al.[24] | Retrospective study | Israel | 21 | BNT162b2 mRNA | ||||
| 43 | F | OU anterior uveitis | 1 | |||||
| 34 | M | Ankylosing spondylitis, OU anterior uveitis | 1 | |||||
| 34 | F | Mild psoriasis, Os anterior uveitis | 1 | |||||
| 78 | M | - | 2 | |||||
| 53 | M | Crohn’s | 1 | |||||
| 64 | M | - | 1 | |||||
| 68 | M | - | 1 | |||||
| 61 | F | OU anterior uveitis | 1 | |||||
| 59 | M | - | 2 | |||||
| 72 | M | Ankylosing spondylitis | 2 | |||||
| 51 | M | Ankylosing spondylitis | 2 | |||||
| 42 | F | OU anterior uveitis | 2 | |||||
| 74 | M | - | 2 | |||||
| 39 | M | - | 2 | |||||
| 64 | F | Herpes zoster ophthalmicus, OD keratouveitis | 2 | |||||
| 50 | F | OU anterior uveitis | 2 | |||||
| 23 | F | - | 2 | |||||
| 65 | F | - | 2 | |||||
| 36 | M | OS anterior uveitis | 2 | |||||
| 41 | M | - | 1 | |||||
| 28 | F | - | 2 | |||||
| Ishay et al.[25] | Case series | Israel | 1 (8) | 28 | M | Behcet’s disease, on colchicine | BNT162b2 mRNA | 1 |
| Mudie et al.[26] | Case report | USA | 1 | 43 | F | BNT162b2 mRNA | 2 | |
| Goyal et al.[27] | Letter to editor | India | 1 | 34 | M | - | ChAdOx1 nCoV-19 viral vector based | 2 |
| Pan et al.[28] | Case report | China | 1 | 50 | F | - | Inactivated vaccine | 1 |
| Fowler et al.[29] | Case report | USA | 1 | 33 | M | - | BNT162b2 mRNA | 1 |
| Pichi et al.[16] | Case series | Abu Dhabi | 1 (7) | BB1BP-CorV inactivated | 1 | |||
| Bialasiewicz et al.[30] | Letter to editor | Qatar | 1 | 50 | M | Atopic dermatitis on topical treatment | BNT162b2 mRNA | 2 |
| Endo et al.[31] | Case report | Colombia | 1 | 52 | M | - | BNT162b2 mRNA | 1 |
| Goyal et al.[32] | Letter to editor | India | 1 | 28 | M | - | Gam-COVID-Vac viral vector based | 2 |
| Mambretti et al.[33] | Lettle to editor | Italy, Austria | 2 | 22 | F | OCP | ChAdOx1 nCoV-19 AZD1222 viral vector based | 1 |
| 28 | F | OCP | ChAdOx1 nCoV-19 AZD1222 viral vector based | 1 | ||||
| Patel et al.[34] | Case report | USA | 1 | 26 | F | OCP | JNJ-78436735 viral vector based | 1 |
| Vinzamuri et al.[35] | Case report | India | 1 | 35 | M | - | ChAdOx1 nCoV-19 viral vector based | (1), 2 |
| Book et al.[36] | Images | Germany | 1 | 21 | F | OCP | ChAdOx1 nCoV-19 AZD1222 viral vector based | 1 |
| Valenzuela[37] | Case report | USA | 1 | 20 | F | Vaginal ring with ethinyl estradiol | BNT162b2 mRNA | 2 |
| Drüke et al.[38] | Case report | Germany | 1 | 23 | F | JIA associated iritis, OCP | ChAdOx1 nCoV-19 AZD1222 viral vector based | - |
| Bøhler et al.[39] | Brief communication | Norway | 1 | 27 | F | OCP | ChAdOx1 nCoV-19 AZD1222 viral vector based | 1 |
| Pichi et al.[16] | Case series | Abu Dhabi | 3 (7)* | OU CSCR with chronic serous PED in OS | BB1BP-CorV inactivated | 1 | ||
| BB1BP-CorV inactivated | 1 | |||||||
| BB1BP-CorV inactivated | 1 | |||||||
| Michel et al.[40] | Case report | 1 | 21 | F | OCP | ChAdOx1 nCoV-19 AZD1222 viral vector based | 1 | |
| Mishra et al.[41] | Case report | India | 1 | 71 | M | Chickenpox 25 years ago, DM, HTN | ChAdOx1 nCoV-19 viral vector based | 1 |
| Maleki et al.[42] | Case report | USA | 1 | 33 | F | Preeclampsia, unexplained miscarriage | mRNA-1273 mRNA | 2 |
|
| ||||||||
| Author | Duration between vaccine and symptoms (days) | Systemic adverse reaction | Laterality | Clinical features | Diagnosis | Management | Outcome | |
|
| ||||||||
| Saraceno et al.[18] | 4 | Headache, tinnitus | OU | Acute vision loss, AC and vitreous cells, serous RD | VKH | Oral prednisolone 1.5 mg/kg/day | Resolved, 3 weeks | |
| Koong et al.[19] | 1 | OU | Bilateral serous RD, disc staining in late stages of FA | VKH | IVMP, oral corticosteroids | Improved, on follow up | ||
| Papasavvas et al.[20] | 42 | - | OU | AC inflammation, mutton fat KPs, subretinal fluid, increasing choroidal thickness on EDI OCT | Reactivation of VKH | Oral corticosteroids, infliximab | Resolved | |
| ElSheikh et al.[21] | 5 | - | OU | Anterior uveitis | JIA associated uveitis | Topical corticosteroids and cycloplegics | Resolved, 6 weeks | |
| Jain et al.[22] | 2 | - | OS | Pain, redness, AC cells 2+, non granulomatous KPs | Uveitis | Topical corticosteroids, cycloplegics | - | |
| Renisi et al.[23] | 14 | - | OS | Pain, photophobia, post synechiae, AC cells, KPs | Acute anterior uveitis | Topical corticosteroids, cycloplegics | Resolved 6 weeks | |
| Rabinovitch et al.[24] | ||||||||
| 2 | Fatigue | OD | Redness, pain, blurred vision, C+3, F+1, fibrin | Anterior uveitis | PF q1hwa, Dex-oint nocte, Cyclo x3/d | Complete resolution | ||
| 4 | OD | Redness, pain C+1, nongranulomatous KPs | Anterior uveitis | Dex-SP q3hwa, Tropi x1/d | Complete resolution | |||
| 1 | Pain, fatigue | OS | Redness, pain, photophobia C+2, nongranulomatous KPs | Anterior uveitis | Dex-SP q2hwa, Dex-oint nocte, Tropi x3/d | Complete resolution | ||
| 3 | OS | Redness, pain, blurred vision C+2, F+2, posterior synechiae | Anterior uveitis | PF q2hwa, Dex-oint nocte, Cyclo x3/d, | Complete resolution | |||
| 13 | Pain, headache | OS | Pain C+0.5 | Anterior uveitis | Dex-SP x4/d, Tropi x1/d | Complete resolution | ||
| 14 | - | OS | Pain, redness, photophobia, C+0.5 | Anterior uveitis | Dex-SP x4/d, Tropi x1/d | Complete resolution | ||
| 5 | Nausea | OD | Redness, pain, C+1 | Anterior uveitis | PF q3hwa, Cyclo x1/d | Complete resolution | ||
| 12 | - | OD | Pain, photophobia, C+2 | Anterior uveitis | PF q2hwa, Dex-oint nocte Cyclo x3/d, | Complete resolution | ||
| 8 | Fatigue | OS | Pain, photophobia, blurred vision C+2 | Anterior uveitis | PF q2hwa, Cyclo x3/d, Dex-oint nocte | Complete resolution | ||
| 16 | - | OD | Redness C+1 | Anterior uveitis | Dex-SP q3hwa, Tropi x1/d | Complete resolution | ||
| 2 | - | OS | Redness, pain C+2 | Anterior uveitis | Dex-SP q2hwa, Dex-oint nocte, Tropi x3/d | Complete resolution | ||
| 20 | - | OU | Pain, blurred vision C+2 | Anterior uveitis | Dex-SP q2hwa, Tropi x3/d, Dex-oint nocte, | Complete resolution | ||
| 7 | - | OS | Pain C+1, F+2 | Anterior uveitis | PF q3hwa, Cyclo x3/d | Complete resolution | ||
| 5 | - | OS | Blurred vision, visual field defect, photopsia Outer retinal changes | MEWDS | No treatment | Significant improvemnt | ||
| 6 | - | OD | photophobia C+1 | Anterior uveitis | po Valacy 1g x3/d, Dex-SP q3hwa, Tropi x1/day | Complete resolution | ||
| 2 | Pain | OS | Pain C+1 | Anterior uveitis | Dex-SP q3hwa, Tropi x1/d | Complete resolution | ||
| 2 | Pain, fatigue | OU | Redness, blurred vision, photophobia C+1, F+1 | Anterior uveitis | PF q3hwa, Tropi x2/d | Complete resolution | ||
| 3 | Pain | OD | Redness, pain, photophobia, blurred vision C+2, F+2 | Anterior uveitis | PF q2hwa, Dex-oint nocte, Cyclo x3/d | Complete resolution | ||
| 1 | Pain, flu like, fatigue | OS | Redness, photophobia, blurred vision, C+3, F+3, granulomatous KPs | Anterior uveitis | PF q1hwa, Dex-oint nocte, Cyclo x3/d | Complete resolution | ||
| 2 | Pain | OD | Redness, photophobia, blurred vision, C+2, F+2 | Anterior uveitis | PF q2hwa, Dex-oint nocte, Cyclo x3/d | Complete resolution | ||
| 30 | Fever | OS | Blurred vision, visual field defect, photopsia, outer retinal changes | MEWDS | No treatment | Significant improvement | ||
| Ishay et al.[25] | 10 | OS | Pain, redness, photophobia, leukocytosis, elevated ESR, CRP | Panuveitis | IVMP, topical prednisolone, oral corticosteroids, azathioprine | Improvement | ||
| Mudie et al.[26] | 3 | Asymptomatic COVID-19 after vaccination | OU | AC and vitreous cells, OCT- choroidal thickening, FA- peripheral vascular leakage | Panuveitis | Oral and topical corticosteroids | Recurrence after 3 weeks, on extended tapering of corticosteroids | |
| Goyal et al.[27] | 4 | Headache, ocular pain, myalgia, injection site pain | OU | 6/36, large serous RD, nilateral yelloweye oval lesions in choroid from macula to mid periphery | Bilateral multifocal choroiditis | Oral corticosteroids | Improved, 2 weeks | |
| Pan et al.[28] | 5 | OU | Bilateral posterior uveitis, FA | Bilateral choroiditis | Periocular, oral corticosteroids | Improved, 5 weeks | ||
| Fowler et al.[29] | 3 | Fatigue, soreness at injection site | OD | Metamorphopsia, serous detachemnt of neurosensory retina | CSCR | Spironolactone 50 mg/day | Resolved, 3 months | |
| Pichi et al.[16] | OU | SRF, hypertrophy of photorecetor layer | SRF, forme fruste CSCR | |||||
| Bialasiewicz et al.[30] | 15 mins | - | OS | Retrobulbar pain, redness, diminution of vision | Hemorrhagic CRVO | Low dose acetylsalicylic acid, monthly aflibercept | Resolved CME, on follow up | |
| Endo et al.[31] | 15 | - | OS | Sudden blurring of vision, retinal venous dilatation, tortuosity, dot hemrrhage, exudates | Non ischemic CRVO | Intravitreal dexamethasone, bevacizumab, oral apixaban | Visual acuity improved | |
| Goyal et al.[32] | 11 | - | OD | Visual deterioration, CME | Superior HRVO | Oral prednisolone 40 mg, apixaban 2.5 mg | Improving, 10 days, on follow up | |
| Mambretti et al.[33] | 2 | Fever 24 hrs | OD | Acute paracentral scotoma, barely visible parafoveal lesions | AMN | - | - | |
| 2 | Fever 24 hrs | OD | Acute paracentral scotoma. | AMN | - | - | ||
| Patel et al.[34] | 2 | - | OU | Paracentral scotoma. OCT parafoveal hyperreflective bands in outer retina | AMN | |||
| Vinzamuri et al.[35] | 28 | - | OU | 1st dose- mild blurring of vision. 2nd dose- burry vision, AS Ps normal, reduced brightness sensitivity | AMN, PAMM | Observation | Better 3 weeks | |
| Book et al.[36] | 3 | - | OU | Paracentral scotoma, circumscribed paracentral dark areas | AMN | Observation | ||
| Valenzuela[37] | 2 | Myalgia, headache and bilateral anterior cervical and supraclavicular lymphadenopathy, difficulty in swallowing- treated with oral prednisolone and diphenhydramine | OU | Photopsia, paracentral scotomata on VF, OCT | AMN | obs | Resolved 14 days | |
| Drüke et al.[38] | 1 | Headache, cervical pain | OU | Paracentral scotoma, subtle brownish rimmed parafoveal lesion, IR, disruption of EZ and IZ | AMN | 40 mg prednisolone x 1 week | Improved, 15 weeks | |
| Bøhler et al.[39] | 2 | Flu like symptoms | OS | Paracentral scotoma, tear drop shaped lesion nasal to fovea, perimetry, SS OCT | AMN | - | - | |
| Pichi et al.[16] | OS | Acute vision loss, SDOCT findings | AMN | Observation | Resolved, 2 months | |||
| AMN | ||||||||
| Persistent tachycardia, SBP 210 mmHg | OS | Blurry vision, headache, inferior scotoma, OCT and OCT-A characteristic | PAMM | |||||
| Michel et al.[40] | 2 | Fever and chills | OS | Central scotomas, 20/20, IR well demarcated dark lesion near fovea, SDOCT findings; high ESR, CRP | AMN | Observation | Improvement, on follow up | |
| Mishra et al.[41] | 3 | Fever, myalgia | OD | FC1m, panuveitis, vitritis, disc hyperemia, large areas of yellow white retinal opacification | Reactivation of VZV ARN | valacyclovir 1 g TID + topical corticosteroids, cyclplegic, gancyclovir- ntravitreal, oral corticosteroids | Resolved | |
| Maleki et al.[42] | 10 | OU | OCT- disruption of outer retinal layers, nasal visual field defect, multifocal ERG defect. High ESR, CRP | AZOOR | OS intravitreal dexamethasone implant | |||
*Case series with 7 patients, 3 developed AMN/PAMM. AC: anterior chamber, AMN: acute macular neuroretinopathy, ANA: anti-nuclear antibody, AZOOR: acute zonal occult outer retinopathy, C: cells, CME: cystoid macular edema, CRP: C-reactive protein, CRVO: central retinal vein occlusion, CSCR: central serous chorioretinopathy, Cyclo, cyclopentolate 1%; Dex-oint, ointment containing dexamethasone 1mg, neomycin sulphate 3500 I.U. Polymyxin B sulphate 6000 I.U; Dex-Sp, dexamethasone sodium phosphate 0.1%, DM: diabetes mellitus, EDI: enhanced depth imaging, ERH: electroretinography, ESR: erythrocyte sedimentation rate, F: female, F+: flare, FA: fluorescein angiography, FC: finger counting, IVMP: intravenous methylprednisolone, JIA: juvenile idiopathic arthritis, HRVO: hemiretinal vein occlusion, HTN: hypertension, KP: keratic precipitates, M: male, MEWDS: multiple evanescent white dot syndrome, OCP: oral contraceptive pill, OCT: optical coherence tomography, OD: right eye, OS: left eye, OU: both eyes, PAMM: paracental acute middle maculopathy, PED: pigment epithelial detachment, PF, prednisolone acetate 1%; po, peroral; q-hwa, every – hours while awake, RD: retinal detachment, SBP: systolic blood pressure, SD: spectral domain, SRF: subretinal fluid, SS: swept source, Tropi, tropicamide 0.5%; Valacy, Valacyclovir hydrochloride VF: visual field, VKH: Vogt-Koyanagi-Harada disease, VZV: varicella zoster virus
Uvea
Vogt-Koyanagi-Harada Syndrome (VKH)
The three cases of VKH following COVID-19 vaccination were characterized by the typical acute onset of bilateral painless loss of vision, anterior chamber granulomatous inflammation, and exudative retinal detachment [Fig. 4]. Papasavvas reported a case of a 43-year-old female who had a past history of VKH, on infliximab and without inflammation for 6 years. There was a reactivation 42 days after the second dose of the mRNA vaccine.[20] Two of the three cases were following the mRNA Pfizer-BioNTech vaccine. All the cases were treated with systemic corticosteroids and resolved.
Figure 4.
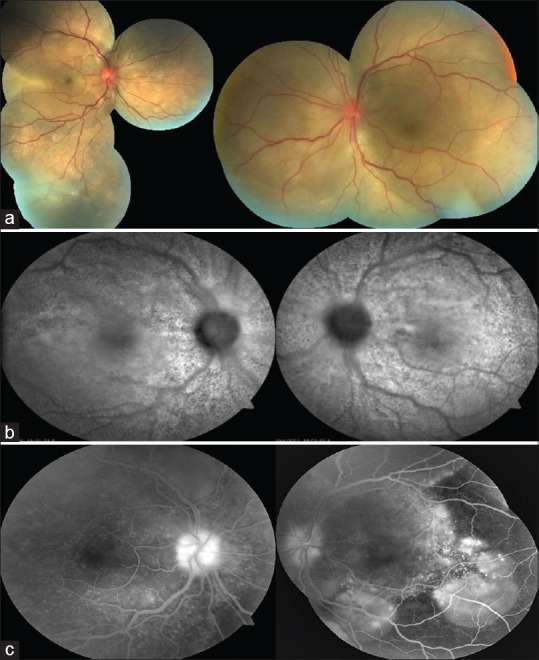
(a) Fundoscopy, (b) autofluorescence, and (c) fluorescein angiography of both eyes showing serous retinal detachment, optic disc hyperemia, and choroidal inflammation in a patient with Vogt-Koyanagi-Harada syndrome after COVID-19 vaccination. (Reproduced with permission from Saraceno JJ, Souza GM, dos Santos Finamor LP, Nascimento HM, Belfort R. Vogt-Koyanagi-Harada Syndrome following COVID-19 and ChAdOx1 nCoV-19 (AZD1222) vaccine. International Journal of Retina and Vitreous. 2021 Dec;7(1):1-7.)
VKH is a T-cell–mediated multisystem autoimmune disease against melanocytes with ocular, neurological, audiovestibular, and dermatological manifestations. Viral infections have been known to trigger VKH with cytomegalovirus (CMV), influenza A, hepatitis C being implicated.[18] Antigenic cross-reactivity, HLA-DR4 status of patient (with influenza A), and interferon have been the mechanisms suggested in relation to viral infections causing VKH. Bacillus Calmette-Guerin (BCG) vaccine, influenza, hepatitis B, and yellow fever vaccinations have all been reported to cause cases of VKH, the onset ranging from 3 days to 4 weeks.[18] VKH after COVID-19 vaccination may be a result of T- and B-cell–induced hypersensitivity reaction, an inflammatory reaction to adjuvants in a vaccine, or sensitization to melanocytic antigens by viral antigens. mRNA-based vaccines do not have any viral antigens or adjuvants but induce humoral immunity, T helper (CD4 + cells) and cytotoxic T cell response which could potentially trigger the inflammatory response.[19]
Uveitis
Renisi et al. reported one of the earliest cases of anterior uveitis following both the first and second doses of the mRNA vaccine. The first episode was within a few hours of the first dose of the vaccine, whereas the second episode was delayed by 14 days from the date of vaccination. Systemic work-up for autoimmune diseases and serology for infections were negative, and it responded to topical corticosteroids and cycloplegics.[23] The second case reported by ElSheikh et al., triggered bilateral anterior uveitis in a patient with juvenile idiopathic arthritis (JIA) without any prior history of uveitis. This was after the second dose of the inactivated Sinopharm vaccine.[21] Jain et al. reported another case of JIA-associated uveitis in a 27-year-old male with HLAB27, 2 days after the Covishield vaccine. [22] A multicentric, retrospective study in Israel by Rabinovitch et al.[reported 19 cases of anterior uveitis following vaccination by the BNT162b2 mRNA vaccine.[24] All the cases were classified as possible or probable consistent with the other articles where a definitive causal relationship with the vaccine could not be established. Two cases of panuveitis have also been reported.[25,26] One of the patients had Behcet’s disease and required azathioprine in addition to topical and oral corticosteroids for control of inflammation.[25] Thus, all three types of vaccines have been associated with uveitis.
Vaccine-induced uveitis is more common in females and is often seen when two or more vaccines are administered simultaneously. It has been seen most frequently after hepatitis B (40.5%) and human papillomavirus (15.6%) vaccines.[28] More than 200 cases of uveitis after the mRNA or vector-borne vaccines have been reported as per the US Food and Drug Administration Vaccine Adverse Event Reporting System.[28] In the review of published literature, a definite predilection for females was not observed. Only three patients had any known pre-existing autoimmune disease. In addition to the concept of molecular mimicry between the vaccine peptide fragments and uveal self-peptides, delayed hypersensitivity and immune complex deposition as triggers for the inflammation have also been suggested.[21] The role of vaccine-induced type 1 interferon secretion driving autoimmune phenotypes in the background of autoimmunity is another concept that may explain the high frequency of anterior uveitis after mRNA vaccines. The uveitis, however, is mild and easily controlled with topical corticosteroids and cycloplegics.
Multiple evanescent white dot syndrome
Two cases of multiple evanescent white dot syndrome (MEWDS) were reported by Rabinovitch et al.24 Both presented shortly after the second dose and improved without any treatment.
Choroid
Choroiditis
In multifocal choroiditis, the pathological process lies in the retinal pigment epithelium (RPE). Two cases of bilateral choroiditis presenting 4–5 days after vaccination have been published. The case from India was in a healthy male who developed symptoms after the second dose of the viralvector-based vaccine, Covishield. The patient had systemic symptoms of headache, ocular pain, and pain at the injection site for 2 days after both doses of the vaccine.[27] The second case from China was following the first dose of the inactivated vaccine.[28] Both patients responded to corticosteroids.
Central serous chorioretinopathy (CSCR)
In January 2021, Fowler et al. reported the only case of central serous chorioretinopathy in a 33-year-old healthy male after 3 days of the first dose of the mRNA vaccine.[29] The OCT and fluorescein angiography (FA) showed the typical features of CSCR. [Fig. 5] The patient was initiated on spironolactone, and it resolved in 3 months. In the absence of any other risk factor for CSCR, (space)like the use of corticosteroids or type A personality, the authors attributed it to the vaccine.[29] A second case of forme fruste CSCR has been reported by Pichi et al.after the Sinopharm inactivated COVID-19 vaccine.[16] Rare cases of CSCR after influenza, yellow fever, anthrax, and small pox vaccines have been described. mRNA-induced endogenous glucocorticoid release and increased permeability of endothelial cells and leaky choriocapillaris on exposure to extracellular naked RNA are the possible pathological mechanisms for the development of CSCR after COVID-19 vaccination.[29]
Figure 5.
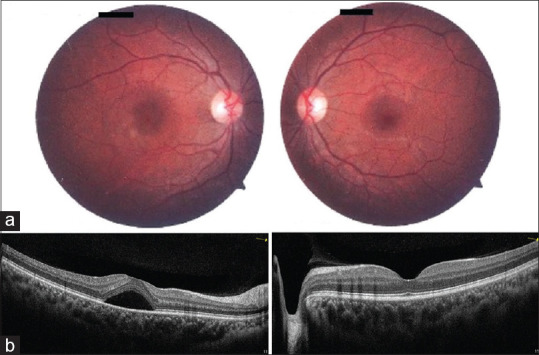
Clinical evaluation of a patient with unilateral central serous retinopathy. The right eye (left column) and left eye (right column) are shown. Fundus photography of the posterior pole (a) of the right eye shows an inferotemporal parafoveal depigmented lesion. The left eye fundus was normal. Optical coherence tomography (b) of the right eye shows a serous detachment of the neurosensory retina in the central macula. (Reproduced with permission from Fowler N, Martinez NR, Pallares BV, Maldonado RS. Acute-onset central serous retinopathy after immunization with COVID-19 mRNA vaccine. American Journal of Ophthalmology Case Reports. 2021 Sep 1;23:101136.)
Retinal vascular occlusions
Central and hemi- retinal vein occlusion
Bialasiewicz et al. and Endo et al. reported a case of central retinal vein occlusion (CRVO) each from Qatar and Colombia, respectively.[30,31] [Fig. 6] Systemic vascular events have been reported following the adenoviral vector–based vaccines, but these are rare with mRNA vaccines with isolated cases being reported of deep vein thrombosis and thrombocytopenia.[43,44] In the eye, both were following the Pfizer-BNT mRNA vaccine. One patient, with atopic dermatitis developed acute onset of pain, redness, and reduction of vision within a few minutes of administration of the second dose of the vaccine. Fundus examination showed a hemorrhagic CRVO and was treated with monthly intravitreal aflibercept injections and low dose acetylsalicylic acid as an antithrombotic measure. The retinal findings improved, and the patient is still under active treatment.[30] The second case was of a healthy 52-year-old male who developed nonischemic CRVO after 15 days of the first dose of the vaccine. He was treated with intravitreal corticosteroid injection initially, and intravitreal bevacizumab was added later along with oral apixaban.[31] This issue of Indian Journal of Ophthalmology (IJO) has a case of hemiretinal vein occlusion (HRVO)reported in a young healthy male following the second dose of Sputnik V vaccine.[32] The patient’s blood work-up, inflammatory markers, and coagulation profile were unremarkable as was the history for any systemic diseases increasing the risk of vascular occlusive events. The patient had strongly positive antibodies IgG to the vaccine and may represent an unusually enhanced immune and possible inflammatory response to the vaccine.[32] The development of retinal vascular occlusions in patients with no other systemic risk factors suggests a possible association of the vaccine to the event.
Figure 6.
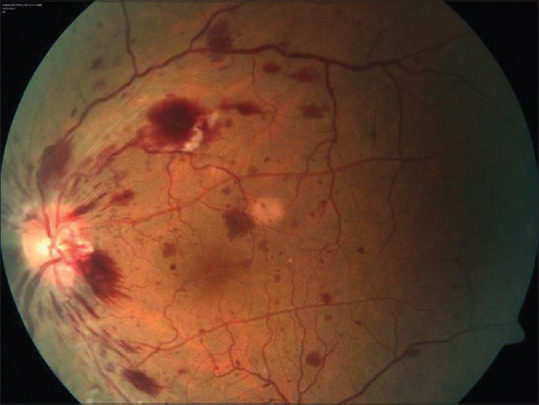
Color fundus photograph of the left eye of a patient with central retinal vein occlusion following COVID-19 vaccination showing dot-blot and flame-shaped hemorrhages, dilated tortuous veins, and blurred margins in the disc, especially in the temporal quadrant (Reproduced with permission from Endo B, Bahamon S, Martínez-Pulgarín DF. Central retinal vein occlusion after mRNA SARS-CoV-2 vaccination: A case report. Indian Journal of Ophthalmology. 2021 Oct 1;69 (10):2865-6.)
Acute macular neuroretinopathy (AMN) and paracentral acute middle maculopathy (PAMM)
AMN typically affects young women with changes observed in the optical coherence tomography (OCT)and subtle reddish wedge-shaped lesion directed towards the fovea on fundus evaluation with an ischemic origin in the deep capillary plexus or choriocapillaris. PAMMshows hyperreflective band-like lesions in the outer plexiform layer (OPL)/inner nuclear layer (INL) junction on OCT.[35] Thirteen cases of AMN following COVID-19 vaccine have been published to date. There was a definite female predilection (8 of 9 patients), and all of them gave a history of using contraceptives – oral or vaginal ring with ethinyl estradiol.[33,34,36,37,39] Oral contraceptives (OCPs) are a known risk factor for AMN and the vaccine-related inflammation may aggravate a pre-existing thrombophilic state in patients on OCPs.[34] The only male patient with the reported complication had simultaneous PAMM and had ocular manifestations after both doses of the vaccine. This case was also unique in that the ocular symptoms developed almost 4 weeks after the second dose of the vaccine.[35] The remaining five presented with the mild blurring of vision, paracentral scotomas, photopsia, and floaters within 3 days of the vaccination. Drüke et al.[38] reported a case of AMN in a 23-year-old female with JIA-associated iritis treated with methotrexate, sulfasalazine, and topical corticosteroids. This was the only patient who was treated with oral corticosteroid for the AMN, all others were observed with spontaneous resolution of the OCT findings. Eight patients received the viralvector-based vaccine (Janssen, Covishield, AstraZeneca). In the retrospective case series from Abu Dhabi by Pichi et al.,there are reports of three more patients who developed AMNand PAMM[after the first dose of the inactivated Sinopharm vaccine.[16] Viral febrile illness (influenza, dengue, CMV, SARS-CoV-2), vasoconstrictors, hypovolemic shock, anemia, thrombocytopenia, and hyperviscosity are other risk factors for AMN. The development of AMN in a male patient, patients with JIA, response to immunosuppressants, and delayed manifestation indicate an inflammatory, vasculitic mechanism at play rather than a direct result of vaccination. The case following the second dose of mRNA vaccination reported by Valenzuela et al. suggests the role of immune-mediated development of AMN and PAMM.[37] AMN has also been reported following influenza vaccination.[45,46] Five patients had bilateral disease and normal anterior segment findings. The fundus is often normal, and the lesion is best appreciated in infrared reflectance. It is possible that many cases of AMN and PAMM are not diagnosed due to the lack of obvious ocular findings. Awareness of the disease and its association with vaccination, and a high degree of suspicion are essential for the diagnosis.
Retina
Acute retinal necrosis (ARN)
Mishra et al.reported the only case of ARN following the viral vector-based vaccine, Covishield, with reactivation of varicella-zoster infection in an immunocompetent male with a history of chickenpox 25 years ago. It was preceded by fever and myalgia after the vaccination.[41] ARN has been reported after the influenza vaccine and SARS-CoV-2 infection. A case series of seven adults developing varicella zoster virus (VZV) reactivation after COVID-19 vaccination has been published and involves a T-cell– mediated immune mechanism.[47] ARN may resolve spontaneously in immunocompetent patients. In the case of ARN reported, the anti-SARS-CoV-2 spike protein antibodies following vaccination were inadequate, and he was treated with valacyclovir, topical corticosteroids, cycloplegic, intravitreal ganciclovir, and oral corticosteroids. Firstly, it could be immunosenescence, and reduced thymic production of T-cells, which after vaccination caused a relative paucity of T-cell–mediated immunity at the site of VZV infection and its intraocular spread via long ciliary nerves. Secondly, an exhaustion of T-cells following vaccination could lead to a reactivation of VZV, leading to acute retinal necrosis. The third possibility could be a cross-reaction between COVID-19 anti-spike antibodies with varicella zoster spike glycoprotein amino acids amounting to anergy of T-cell–mediated immunity and subsequent reactivation of VZV.[41]
Acute zonal occult outer retinopathy (AZOOR)
A single case of bilateral AZOOR was documented by Maleki et al.in a healthy woman 10 days after vaccination with the Moderna mRNA-1273 vaccine.[42] There was a nasal visual field defect in the left eye, and multifocal electroretinography (ERG) showed defective areas ininferotemporal macula in the right eye and temporal macula in the left eye. OCT showed characteristic disruption of the outer retinal layers. The patient was treated with an intravitreal dexamethasone implant in the left eye. Elevated erythrocyte sedimentation rate (ESR) indicated the inflammatory nature of the pathology. T helper cells and cross-reacting antibodies against the outer retinal layers and retinal pigment epithelial cells were most likely involved in the pathogenesis.
Several posterior segment ophthalmic features of the COVID-19 infection have been reported and are associated with the procoagulant/prothrombotic state created by the cytokines released in response to the virus. The immune dysregulation induced by the virus can also lead to many of the ocular diseases described. The association of the vaccine with the rare retinal and uveal manifestations may be coincidental given the large scale of vaccination drive. However, the adenoviral vector vaccines have been associated with potentially life-threatening cerebral venous sinus thrombosis.[48] The pathogenesis is similar to the autoimmune heparin-mediated thrombocytopenia involving the activation of the coagulation cascade by the platelet-activating autoantibodies against platelet factor 4. The adverse event, vaccine-induced immune thrombotic thrombocytopenia (VITT) has been noted most frequently in young women within the first 3 weeks. The reported rate is close to five cases per million vaccinated adults (April 2021) for the AstraZeneca vaccine that contains chimpanzee replication-incompetent adenovirus without modification to mitigate the shedding of the expressed S protein. The S protein in the circulation can induce a proinflammatory/procoagulant response.[49] The rate is 0.87 cases per million for the Johnson and Johnson vaccine (April 2021) with human replication-incompetent adenovirus. The complication has not been reported with the mRNA-based vaccines.
Vaccine-related uveitis is not uncommon, both mild, transient anterior uveitis and more severe posterior and panuveitis have been reported. Stimulation of cytokine production, modification of surface antigens, and induction of new antigens with molecular mimicry to host tissue antigens, polyclonal B-cell activation, epitope spreading, bystander activation, and reaction to adjuvants are all the suggested mechanisms.[27]
It was difficult to identify any specific trend other than the female gender and OCPs being used by the majority of the cases who developed AMN and association of the Pfizer mRNA vaccine with mild anterior uveitis. There may be multiple mechanisms at play simultaneously. Patients with a history of autoimmune diseases or JIA should be educated about the symptoms of floaters, scotoma, decreased contrast sensitivity, photopsia, and blurring of vision after vaccination and advised to seek medical attention at the earliest.
Neuro-ophthalmic Manifestations of COVID-19 Vaccine
The COVID-19 vaccines have been observed to produce several neuro-ophthalmic manifestations. While most are transient, self-resolving, and mild, there are some that leave a residual effect. At present, there are 22 cases with neuro-ophthalmic adverse effects [Table 4].[39,50,51,52,53,54,55,56,57,58,59] These aside, Shemer et al.have reported 21 cases of patients with acute facial palsy following COVID-19 vaccination in a retrospective case-control study.[60] These cases have not been included in the Table 4 as all the details of the individual cases were not available. The mean age of the patients was 58.3 ± 20.6 (median 61.5) years with 11 males and 11 females. Thirteen patients had received the Pfizer mRNA vaccine, 4 had received Covishield, 3 inactivated CoronaVac from Sinovac, 1 Moderna mRNA vaccine, and 1 Covaxin. The events were equally distributed between the first (9) and second (8) doses, 1 patient developed facial palsy after both the first and second doses, and the dose status was not specified in 4 cases. The median interval of development of symptoms from the vaccination was 4 (mean 7.8 ± 8.1 ) days.
Table 4.
Review of literature of neuro-ophthalmic manifestations of COVID-19 vaccines
| Author | Type | Location | Sample | Age | Sex | Systemic/ocular illness | Vaccine | Dose | Duration between vaccine and symptoms (days) |
|---|---|---|---|---|---|---|---|---|---|
| Maleki et al.[42] | Case report | USA | 1 | 79 | F | Osteoporosis, osteoarthritis | BNT162b2 mRNA | 2 | 2 |
| Leber et al.[50] | Letter to editor | Brazil | 1 | 32 | F | Subacute thyroiditis | CoronaVac inactivated | 2 | 12hours |
| Santovito et al.[51] | Letter to editor | USA | 1 | Middle aged | M | BNT162b2 mRNA | 2 | 3 | |
| Jumroendararasame et al.[52] | Case report | Thailand | 1 | 42 | M | Dyslipidemia | CoronaVac inactivated | 2 | 1 hour |
| Pawar et al.[53] | Letter to editor | India | 4 | 28 | F | ChAdOx1 nCoV-19 viral vector based | 1 | 21 | |
| 24 | F | 1 | 21 | ||||||
| 44 | M | Polio in childhood | 28 | ||||||
| Young adult | M | Chickenpox, recurrent 6th nerve palsy | 6 | ||||||
| Reyes-Capo et al.[54] | Case report | USA | 1 | 59 | F | - | BNT162b2 mRNA | - | 2 |
| Pappaterra et al.[55] | Clinical correspondence | Puerto Rico | 1 | 81 | M | HTN, hypercholesterolemia, uncontrolled DM | mRNA-1273 mRNA | 1 | 4 |
| Shemer et al.[56] | Case report | Israel | 9 | 86 | F | BNT162b2 mRNA | 1 | 14 | |
| 78 | F | 2 | 5 | ||||||
| 79 | M | 1 and 2 | 4,2 | ||||||
| 69 | F | 1 | 3 | ||||||
| 73 | F | 1 | 12 | ||||||
| 77 | M | 2 | 1 | ||||||
| 64 | M | 1 | 7 | ||||||
| 51 | M | 2 | 9 | ||||||
| 35 | M | 1 | 4 | ||||||
| Ish et al.[57] | Letter to editor | India | 1 | 50 | M | Covaxin | 2 | 21 | |
| Yu et al.[58] | Case report | China | 1 | 36 | F | Sinovac inactivated | 1 | 2 | |
| Rodríguez-Martín et al.[59] | Letter to editor | Spain | 1 | 78 | F | Childhood poliomyelitis, HTN | BNT162b2 | - | 3 |
|
| |||||||||
| Author | Systemic adverse reaction | Laterality | Clinical features | Diagnosis | Management | Outcome | |||
|
| |||||||||
| Maleki et al.[42] | OU | OU ON pallor. Temporal artery biopsy, high ESR, CRP | AAION | IVMP, oral corticosteroids | On follow up | ||||
| Leber et al.[50] | OU | Decreased visual acuity, pain with ocular movements, headache. MRI normal. Subacute thyroiditis | Optic neuritis | IVMP, oral corticosteroids | Improved | ||||
| Santovito et al.[51] | Fever chills. unilateral oppressive headache in parietal to frontal lobe | Sudden onset darkening of VF and subjective reduction of visual acuity. Associated with confusion, asthenia and nausea | Acute transient reduction of visual acuity | - | Spontaneously resolvedon | ||||
| Jumroendararasame et al.[52] | - | OU | Blurred vision starting centrally, 20/20, left congruous hemianopia respecting vertical midline. BP 150/90 | Transient VF defect | IV fluids, oral aspirin | Spontaneously resolved | |||
| Pawar et al.[53] | OS | Sudden decrease in vision, blurred disc margins, MRI normal | Optic neuritis | IVMP | Resolved | ||||
| OU | Diplopia, restricted elevation, MRI normal | OU vertical gaze palsy | Systemic corticosteroids | Resolved | |||||
| OS | Diplopia | 6th nerve palsy | OS botox to MR | Miimal residual | |||||
| OS | Headache, esotropia | 6th nerve palsy | Resolved | ||||||
| Reyes-Capo et al.[54] | fever | OD | Acute binocular dipopia, right esotropia, abduction deficit. ESR, CRP mildly elevated | Abducens nerve palsy | Observation | persistent | |||
| Pappaterra et al.[55] | - | OS | Binocular diplopia, ptosis, limited adduction, infraduction, no RAPD. Elevate CRP, normal ESR | Partial oculomotor nerve palsy | Observation | Spontaneously Resolution | |||
| Shemer et al.[56] | OS | Facial asymmetry, lagophthalmos, corneal punctate erosions | Facial palsy | oral glucocorticoids, artificial tears, temporary closeure of eyelids at night | |||||
| OS | Tinnitus, periauricular rash, bilateral SNHL, complete left sided facial palsy | Facial palsy | admitted, antimicrobial treatment, conventional treatment | ||||||
| OD | Facial asymmetry | Facial palsy | oral corticosteroids | ||||||
| OD | Facial asymmetry | Facial palsy | oral corticosteroids, AT | ||||||
| OS | Facial asymmetry | Facial palsy | oral corticosteroids, AT | ||||||
| OS | Facial asymmetry | Facial palsy | oral corticosteroids, AT | ||||||
| OS | Facial asymmetry | Facial palsy | oral corticosteroids, AT | ||||||
| OD | Facial asymmetry | Facial palsy | oral corticosteroids, AT | ||||||
| OS | Facial asymmetry | Facial palsy | oral corticosteroids, AT | ||||||
| Ish et al.[57] | OD | Lagophthalmos, LL temporal ectropion, right sided LMN facial palsy | Facial palsy | topical antibiotics and AT for eye, taping of the eye. Oral prednisolone 1mg/kg for 2 weeks | Improved, day 10, on follow up | ||||
| Yu et al.[58] | OD | Bilateral keratoconjunctivitis, right sided facial weakness | Facial palsy | Prednisolone x 1 week, AT, fluoromethalone eye drops, acupuncture | Resolved, 54 days | ||||
| Rodríguez-Martín et al.[59] | instability, malaise, nausea, severe pain in ext aud canal | OD | Right sided facial palsy, left horizontal nystagmus, gait instability, bilateral snhl, vesicles and crusted lesions on concha | Ramsay Hunt syndrome | Persistent instability and SNHL. Facial palsy improved 2 weeks | ||||
AAION: arteritic anterior ischemic optic neuropathy, AT: artificial tears, BP: blood pressure, CRP: C reactive protein, DM: diabetes mellitus, ESR: erythrocyte sedimentation rate, F: female, HTN: hypertension, IV: intravenous, IVMP: intravenous methylprednisolone, LL: lower eyelid, LMN: lower motor neuron, M: male, MR: medial rectus, MRI: magnetic resonance imaging, OD: right eye, ON: optic nerve, OS: left eye, OU: both eyes, RAPD: relative afferent pupillary defect, SNHL: sensory neural hearing loss, VF: visual fields
Optic nerve
Arteritic anterior ischemic optic neuropathy (AAION)
In the only reported case of AAION, a 79-year-old presented with bilateral loss of vision and optic disc pallor on examination 2 days after the second dose of the Pfizer-mRNA vaccine. The diagnosis was confirmed with elevated ESR and CRP and temporal artery biopsy. The authors attributed it to be an immune-mediated inflammation involving cross-reacting antibodies produced against the SARS-CoV-2 spike protein and helper T cells cross-reacting with proteins and antigens of large arteries. The patient was treated with high-dose intravenous methylprednisolone for 3 days followed by oral corticosteroids.[42]
Optic neuritis
Acute optic neuritis is an idiopathic disease presenting with painless vision loss and is usually unilateral and inflammatory. Leber et al.reported a case of bilateral optic neuritis with symptoms developing within 12 hours of vaccination with the second dose of inactivated CoronaVac vaccine.[50] The patient’s infectious and inflammatory workup was unremarkable barring the detection of reactive MOG-IgG and elevated thyroid-stimulating hormone, antithyroglobulin, and antithyroid peroxidase.[50] The optic neuritis in this patient may be the initial manifestation of a demyelinating disorder associated with MOG-IgG, and the patient needs to be followed up for evaluation. She also had subacute thyroiditis which responded to the corticosteroid therapy. The second case of optic neuritis was in a healthy 28-year-old female after the first dose of vector-based Covishield vaccine.[53] Immune-mediated optic neuritis has been documented with BCG, hepatitis B, and tetanus vaccines.
Acute vision loss
Two separate cases of acute, unspecified, transient episodes of visual loss have been reported in middle-aged, relatively healthy males. The case reported by Santovito et al.was following the second dose of the Pfizer mRNA vaccine.[51] The patient had a prior history of COVID-19 infection. He developed fever with chills after the vaccination. The case description was subjective as no formal evaluation was done, and the patient presented after the spontaneous resolution the same day. There was sudden onset darkening of the visual field and visual distortion associated with nausea, light confusion, and asthenia. The second case was interesting because it was the self-observed report by an ophthalmologist who described the sudden onset of bilateral blurring of vision within an hour of the second dose of the CoronaVac vaccine. Computerized automated perimetry showed left congruous hemianopia respecting the vertical midline. The eye examination, OCT, neurological evaluation, blood investigations including lipid and coagulation profile, magnetic resonance imaging (MRI)were unremarkable. Only the blood pressure of the patient was elevated from the baseline to 150/90 mmHg. There was a spontaneous resolution, though the patient was admitted for observation, intravenous fluids, and oral aspirin. The author gives a logical and acceptable explanation – acute arterial vasospasm in the postchiasmatic visual pathway, most likely the right occipital lobe with macular sparing, with reperfusion to produce complete recovery.[52]
Cranial nerve palsies
Abducens nerve palsy
Reyes-Capo et al.reported one case of acute abducens nerve palsy in a 59-year-old female, 2 days after vaccination with Pfizer mRNA vaccine. She developed sudden onset diplopia and right esotropia. Although the ESR and CRP were mildly elevated, her MRI was normal.[54] Two cases have been reported from India after the vector-based Covishield vaccine.[53] Abducens nerve palsy has been described following immunization with diphtheria-pertussis-tetanus (DPT), measles-mumps-rubella (MMR), hepatitis B, and influenza vaccine and can occur any time between 2 days and 3 weeks with spontaneous, slow resolution. An immune-mediated vasculitis, thrombosis, or demyelination have been proposed as possible mechanisms for the vaccine-associated nerve palsy.
Oculomotor nerve palsy
Pappaterra et al. reported a case of partial, pupil sparing oculomotor nerve palsy in an 81-year-old male with hypertension, diabetes, and hypercholesterolemia, 4 days after the first dose of Moderna mRNA-1273 vaccine.[55] An MRI and MR angiography were done to rule out ischemic or aneurysmal compression. The patient gave a history of COVID-19 infection in the past, and the authors suggest reactivation of the COVID-19–associated immune phenomena with molecular mimicry. In an older individual with known risk factors, a microvascular ischemic event causing pupil sparing 3rd nerve palsy is highly plausible. But the quick recovery and temporal association led the authors to consider the role of the vaccine.[55]
Facial nerve palsy: Bell’s palsy and Ramsay-Hunt syndrome
Cases of facial palsy have been included in this review because of the ocular implications due to lagophthalmos [Fig. 7]. Shemer et al.reported the largest series (9 cases) of facial nerve palsy after vaccination with Pfizer mRNA vaccine in Israel.[56] Bell’s palsy is an acute, idiopathic, unilateral facial palsy often related to a preceding viral infection. The clinical features were typical, and cases of Bell’s palsy responded well to prompt initiation of systemic corticosteroids. The ocular surface must be protected with liberal use of lubricants and night-time taping. Yu et al.reported the first case of Bell’s palsy, following an inactivated vaccine in a patient who had a history of Bell’s palsy in the past.[58] COVID-19 vaccine trials have documented cases of facial palsy, four with Pfizer-BioNTech and three with the Moderna mRNA vaccine compared to a single case in the placebo arm of the trial.[61,62] Ozonoff et al.reviewed the vaccines trials and concluded that the observed incidence of Bell’s palsy following mRNA vaccines was 3.5–7 times higher than the expected incidence in the general population (15-30/100000/year).[63] The risk is also higher after the second dose. In a retrospective case-control study, Shemer et al.found no association between vaccination with BNT162b2 mRNA vaccine and development of new-onset facial palsy after adjustment for pre-existing immune or inflammatory diseases, diabetes, and previous episode of peripheral nerve palsy.[60] Like the other ophthalmic manifestations, Bell’s palsy is not unique to the COVID-19 vaccine and has been described post influenza, hepatitis B, polio, DPT, and MMR vaccines. Conflicting and limited available data make definitive conclusions about a causal relation difficult. The autoimmune process with host cell mimicry, bystander activation of dormant autoreactive T-cells, or induction of innate immunity and interferon production by the combined effect of mRNA and lipids, could be responsible for the resultant facial nerve palsy.
Figure 7.
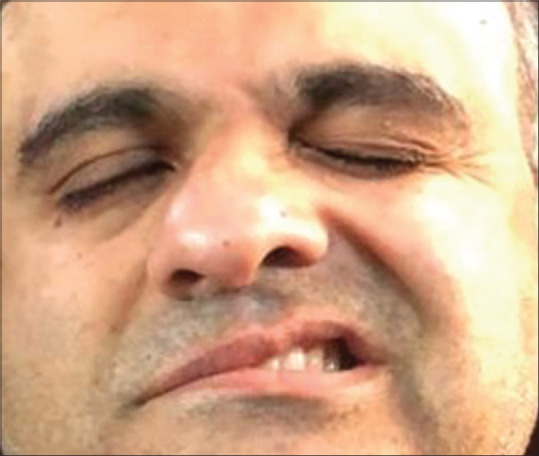
lL of facial symmetry, incomplete right eye closure, loss of nasolabial fold, and drooping of the angle of the mouth of the right side suggestive of right-sided Bell’s palsy (Reproduced with permission from Ish S, Ish P. Facial nerve palsy after COVID19 vaccination – A rare association or a coincidence. Indian J Ophthalmol 2021;69:2550-2.)
Ramsay-Hunt syndrome is caused by reactivation of latent VZV in the geniculate ganglion and is characterized by a vesicular rash on the concha of the ear and external auditory canal and peripheral facial nerve palsy. Influenza, and hepatitis B vaccines have been associated with Ramsay-Hunt syndrome, and the COVID-19 vaccines can be added to the list of vaccines implicated.[59]
In addition to the cases elaborated in the table, Thunstedt el al reported a case of raised intracranial pressure in a healthy 23-year old male a week after receiving the AstraZeneca ChAdOx 1 vaccine. Despite bilateral papilloedema, the patient had normal vision. Headache is a common side effect after the COVID-19 vaccines. However, persistent and pulasatile headache demands complete evaluation to rule out cerebral venous thrombosis, meningitis and drug related raised intracranial tension with CSF analysis and neuroimaging. Patient was successfully treated with acetazolamide and torsemide.[64]
Orbital Manifestations of COVID-19 Vaccine
Orbital complications are uncommon, and there are four published reports to date. [Table 5][65,66,67,68] The mean age affected was 44.6 ± 11.3 (median 47.5) years with 3 females. There was one case each following the two mRNA vaccines from Pfizer and Moderna. Both cases of superior ophthalmic vein thrombosis (SOVT) were following vaccination with ChAdOx1 nCoV-19 vaccine.[67,68] Information about the vaccine dose was available for three patients, two of them were following the first dose and one after the second dose. The onset of symptoms was at a median of 6 (mean 6.3 ± 3 ) days from the time of vaccination.
Table 5.
Review of literature of orbital manifestations of COVID-19 vaccines
| Author | Type | Location | Sample | Age | Sex | Systemic/ocular illness | Vaccine | Dose | Duration between vaccine and symptoms (days) | Systemic adverse reaction |
|---|---|---|---|---|---|---|---|---|---|---|
| Rubinstein[65] | Case report | USA | 1 | 50 | F | Graves’ disease without ophthalmopathy since 11 years treated with radioactive iodine, hypertension, anxiety, hypothyroidism treated with levothyroxine | mRNA | 2 | 3 | |
| Chuang et al.[66] | Case report | USA | 1 | 45 | M | - | BNT162b2 mRNA | - | 5 | |
| Bayas et al.[67] | Clinical picture | Germany | 1 | 55 | F | - | ChAdOx1 nCoV-19 viral vector based | 1 | 7 | fever |
| Panovska-Stavridis[68] | Letter to editor | Republic of North Macedonia | 1 | 29 | F | - | ChAdOx1 nCoV-19 viral vector based | 1 | 10 | fever |
|
| ||||||||||
| Author | Laterality | Clinical features | Diagnosis | Management | Outcome | |||||
|
| ||||||||||
| Rubinstein[65] | OU | Irritation, tearing, orbital pain, abduction limitation, proptosis, normal thyroid functions, normal inflammatory markers. CT- OS>OD enlarged IR, MR without tendon involvement or sinus disease. | Thyroid eye disease | IV teprotumumab | Improvement in congestion and proptosis | |||||
| Chuang et al.[66] | OS | Severe left sided headache, progressive ptosis, decreased vision, RAPD, complete ophthalmoplegia. Inflammation and infection markers normal. Elevated CRP. CT and MRI | Tolosa Hunt syndrome | Initially antibiotics- discontinued after results of blood and CSF. IV methylprednisolone 1 g x 3 days- oral | Pain decreased. Improvement in cranial nerve deficit over 2 months- partial recover, on follow up | |||||
| Bayas et al.[67] | OU | Conjunctival congestion, retro-orbital pain and diplopia. MRI- SOVT. Thrmobocytopenia, antiplatelet IgG Ab positive | SOVT | Heparin, IV dexamethasone | Ischemic stroke | |||||
| Panovska- Stavridis[68] | Severe headache, left proptosis, blurred vision. Thrombocytopenia, high D-dimer. MRI SOVT, PF4 Ab | SOVT | IVIG 1 g/kg x 2 days, Rivaroxaban, oral prednisolone | Improved, on follow up | ||||||
Ab: antibody, CRP: C reactive protein, CSF: cerebrospinal fluid, CT: computed tomography, F: female, IR: inferior rectus, IV: intravenous, IVIG: intravenous immunoglobulin, M: male, MR: medial rectus, MRI: magnetic resonance imaging, OS: left eye, OU: both eyes, PF: platelet factor, RAPD: relative afferent pupillary defect, SOVT: superior ophthalmic vein thrombosis
Thyroid eye disease
Rubinstein et al.[reported a case of thyroid eye disease in a patient with a history of Graves’ disease for 11 years but without any ophthalmic involvement.[65] The onset of eyelid edema, proptosis, ptosis, and diplopia were within 3 days of the second dose of vaccine. Thyroid ophthalmopathy was confirmed on clinical examination (CAS 5), computed tomography (CT) scan, and elevated serum thyroid-stimulating immunoglobulin. The patient responded to teprotumumab.
Tolosa-Hunt syndrome
Chuang et al.reported a case of Tolosa-Hunt syndrome following vaccination.[66] [Fig. 8] A granulomatous inflammatory process involving the cavernous sinus remains a diagnosis of exclusion and responds to corticosteroids. Spontaneous remission has been reported.
Figure 8.
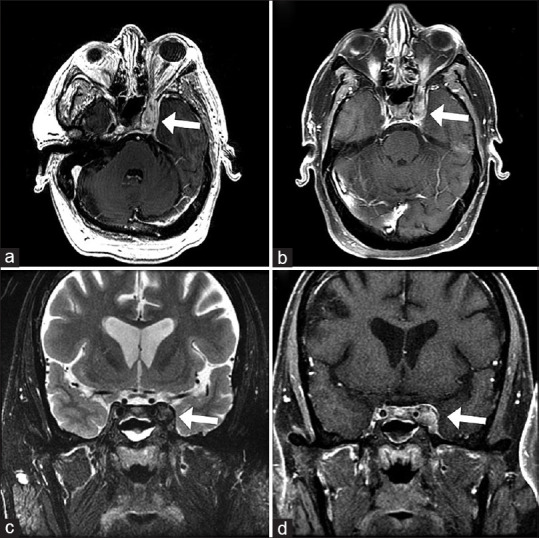
MRI of an inflammatory left cavernous sinus process consistent with Tolosa-Hunt syndrome T2 axial FLAIR (a) and FSE coronal (c) images showing bulky perineural tissue extending into the left cavernous sinus. The perineural tissue has heterogeneous postcontrast enhancement and slightly decreased enhancement centrally consistent with a component of thrombosis on postcontrast T1 axial (b) and coronal (d) images (Reproduced with permission from Chuang TY, Burda K, Teklemariam E, Athar K. Tolosa-Hunt Syndrome Presenting After COVID-19 Vaccination. Cureus. 2021 Jul; 13 (7).)
Superior ophthalmic vein thrombosis
Patients with SOVT present with headache, proptosis, ophthalmoplegia, diplopia, and diminished vision and filling defect on CEMRI with a dilated SOV confirms the diagnosis. Two cases of superior ophthalmic vein thrombosis after the viral vector-based vaccine ChAdOx1 nCov-19 vaccine have been published. In the report by Panovska-Stavridis et al., the patient had thrombocytopenia, high D-dimer levels, and platelet factor 4 antibodies.[68] She was treated with intravenous immunoglobulin (IVIG) and tapering doses of oral corticosteroids. The second patient was an older female, fifty-five-year-old with features suggestive of secondary immune thrombocytopenia. She was treated with heparin but developed ischemic stroke.[67]
As discussed in the previous section, cases of CSVT after the adenoviral vector vaccines have been described and are amongst the most serious adverse effects. Thrombotic thrombocytopenia is an immune-mediated post-vaccination complication. A multicentric cohort study by Perry et al found that patients with VITT related CSVT were more likely to have received the ChAdOx1 vaccine, younger, without systemic risk factors, had more extensive venous thrombosis, intracranial hemorrhage and more concurrent extracranial and arterial thrombosis with worse outcome as compared to patients without VITT associated CSVT. The outcome improved with use of IVIG and non-heparin anticoagulants.[69] Ophthalmologists should be aware of the occurrence of such thrombotic events. Frequently, they may be the ones to diagnose the condition which may pre-empt fatal cerebral ischemia.
Patients with underlying thyroid disease should undergo a baseline ophthalmic examination before vaccination and be informed about the possibility of thyroid eye disease flaring.
Discussion
Ocular manifestations of the COVID-19 vaccine are not uncommon but relatively mild and transient except for cases of retinal and ophthalmic vascular occlusions and graft rejections. In a cross-sectional study based on an online survey questionnaire, Kadali et al. found that of the 803 healthcare workers who received the BNT162b2 mRNA vaccine, very few had non-specific ocular symptoms of blurred vision (4/803, 0.5%), eye pain (7/803, 0.87%), and flashes (2/803, 0.25%).[70] A similar study on 432 healthcare workers who received the mRNA-1273 vaccine showed that frequency of ophthalmic side effects was rare (eye pain: 15/432, 3.47%), to extremely rare (blurring of vision: 4/432, 0.93%; flashing lights: 3/432, 0.69%).[71] The posterior segment, the retinal vasculature, and the uvea are most frequently affected. Comparison by age shows that patients with posterior segment manifestation were relatively younger (mean age 43.6 years) as compared to patients with neuro-ophthalmic lesions (mean age 58.3 years) and corneal graft rejections (mean 65 years). The median time for the development of ocular signs and symptoms was 7 days for eyelid, ocular surface, and anterior segment, 6 days for orbit and 4 days for the posterior segment and neuro-ophthalmological cases. These details are important to follow up high-risk patients in the ophthalmic clinic. [Fig. 9] Patients with corneal grafts should be followed within a week, whereas those with JIA or prior history of uveitis need to be called in earlier.
Figure 9.
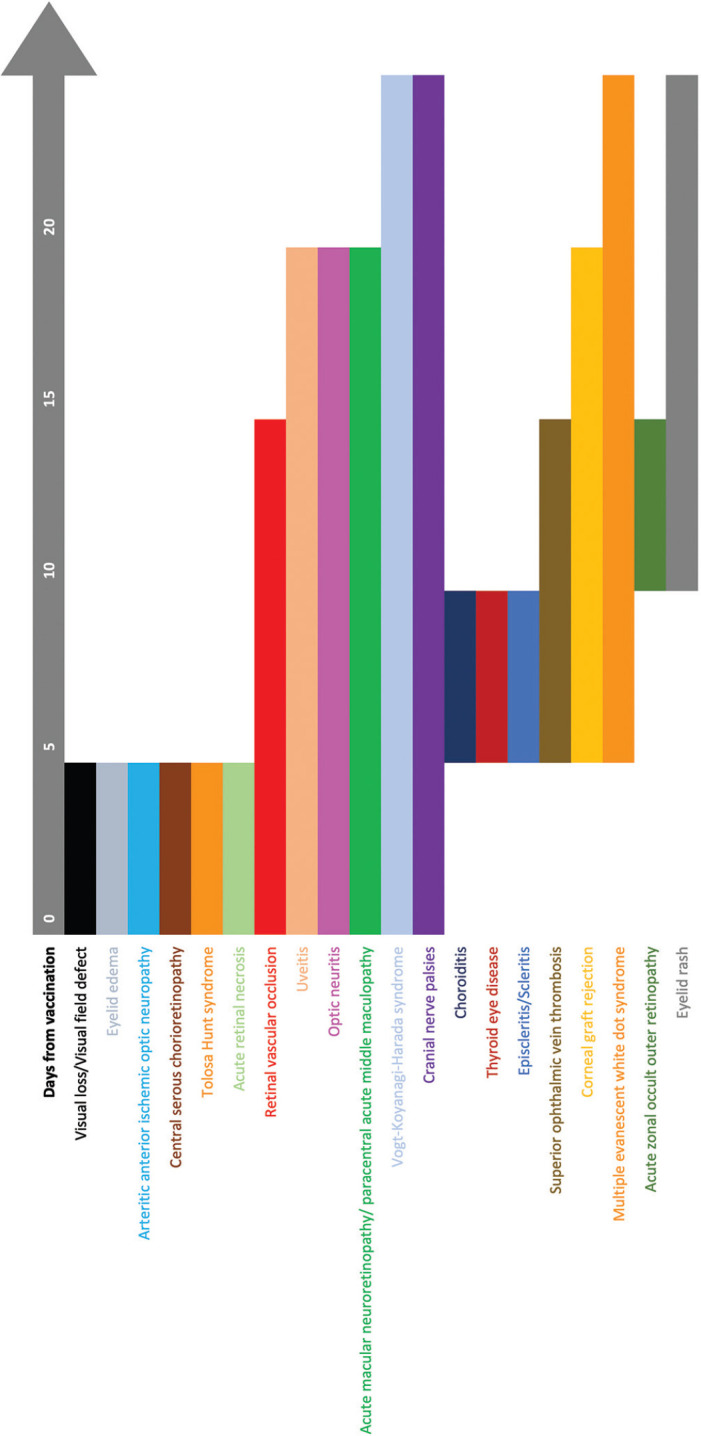
Timeline showing the onset of signs and symptoms from the time of COVID-19 vaccination (Day 0)
The vaccines have been found to be safe before receiving approval from the appropriate authority. Adverse effects following vaccination are infrequent, benign, transient, and treatable. Serious adverse effects including anaphylaxis and thrombocytopenia are rare. The ocular manifestations have been attributed to molecular mimicry (similarities in the structure of SARS-CoV-2, human or chimpanzee adenoviral components), antigen-specific cell, and antibody-mediated hypersensitivity reactions to spike antigen, or other viral antigens and adjuvants present in the vaccines that enhance immunogenic activity. The mechanisms are similar to those seen with various other vaccines that have been used for several years including influenza, MMR, hepatitis A and B, yellow-fever, BCG, DPT, and HPV. Almost all the manifestations have been managed conservatively or with steroidal and nonsteroidal immunosuppressants.
That being said, it is important to report all manifestations to improve the knowledge base of physicians and patients. For ophthalmologists, this awareness is necessary to be able to counsel patients for vaccination, informing them of the possible symptoms for early presentation, diagnosis, and initiation of treatment. Patients with corneal grafts can be started on frequent topical corticosteroids a week before vaccination, and this can be tapered slowly after the second dose. Those who require non-urgent corneal transplantation may be scheduled after 4–6 months of the completion of all doses of vaccination. Patients with a history of inflammatory eye diseases should be warned of possible reactivation or recurrence and educated about early symptoms. Knowledge about the adverse effects is also important to encourage patients for vaccination and counter false information.
It is encouraging to note that all the authors have reported with great maturity, highlighting rightly that the causal relationship of all the manifestations to the COVID-19 vaccine are only possibilities and not established with certainty because of the lack of large-scale real-world studies. They have also vehemently supported the need for every individual to get vaccinated as the benefits far outweigh the risks. Responsible reporting is the need of the hour.
Future Directions and Conclusion
As further information is acquired and shared, the long-term implications of the vaccines will become more clear. Patients who developed the signs after the first dose need to be followed up after the second dose to document if they had a similar reaction or not. This will throw further light on the true association of the vaccines with the ocular events as well as the risk factors for the same. It may also be prudent to classify the associations as possible, probable, definite, or unrelated as more cases come to light. Corneal graft rejection after the first dose raises the question of the timing of the second dose. The permanent or residual effects of the manifestations are also to be determined.
From the review of cases, it is evident that a maladaptive immune system at work in response to the vaccine may reactivate some autoimmune diseases in the patients like uveitis, thyroid ophthalmopathy, and thrombocytopenic thrombosis. New-onset uveitis may be the first sign of an underlying autoimmune disease. This raises the question of the need for complete systemic evaluation of a patient with uveitis following vaccination. COVID-19 vaccines do not have any live virus and therefore, individuals on immunomodulators can have the vaccine safely, but the efficacy in them may be questionable.[4] The currently available studies have not demonstrated an increased risk of reactivation following vaccination with COVID-19 including in patients with multiple sclerosis.[72]
New vaccines are on the horizon, booster doses are definite in the near future, and children are soon to be vaccinated, creating a platform for a plethora of new and exciting information. As of October 29, 2021, there are 128 vaccines in clinical development and 194 vaccines in the preclinical development stage.[3] Many of the adverse events are treated with immunosuppressants, and one is curious to see if the vaccine efficacy is affected by drugs used for the reactions like corticosteroids, and teprotumumab. With travel also returning to the forefront, the mixing of vaccines may also bring about more information.
COVID-19 vaccines are a boon that needs to be studied, improved, and harnessed to their full potential for safety as well as returning to as normal a life as possible. As ophthalmologists, we need to be aware of the emerging manifestations and complications pertaining to the eye following the vaccination with different vaccines, to be better prepared to diagnose, manage, and give correct information to patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1. [Last accessed on 2021 Oct 26]. Available from: https://covid19.who.int .
- 2. [Last accessed on 2021 Oct 26]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html .
- 3. [Last accessed on 2021 Oct 26]. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines .
- 4. [Last accessed on 2021 Oct 26]. Available from: https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html#about-the-vaccine .
- 5. [Last accessed on 2021 Nov 07]. Available from: https://covid19.trackvaccines.org/agency/who/
- 6. Sen M, Honavar SG, Sharma N, Sachdev MS. COVID-19 and eye:A review of ophthalmic manifestations of COVID-19. Indian J Ophthalmol. 2021;69:488–509. doi: 10.4103/ijo.IJO_297_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Austria QM, Lelli GJ, Segal KL, Godfrey KJ. Transient eyelid edema following COVID-19 vaccination. Ophthalmic Plast Reconstr Surg. 2021;37:501–2. doi: 10.1097/IOP.0000000000002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mazzatenta C, Piccolo V, Pace G, Romano I, Argenziano G, Bassi A. Purpuric lesions on the eyelids developed after BNT162b2 mRNA COVID-19 vaccine:Another piece of SARS-CoV-2 skin puzzle? J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17340. doi:10.1111/jdv. 17340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rallis KI, Ting DS, Said DG, Dua HS. Corneal graft rejection following COVID-19 vaccine. Eye. 2021 doi: 10.1038/s41433-021-01671-2. doi:10.1038/s41433-021-01671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abousy M, Bohm K, Prescott C, Bonsack JM, Rowhani-Farid A, Eghrari AO. Bilateral EK rejection after COVID-19 vaccine. Eye Contact Lens. 2021;47:625–8. doi: 10.1097/ICL.0000000000000840. [DOI] [PubMed] [Google Scholar]
- 11. Phylactou M, Li JP, Larkin DF. Characteristics of endothelial corneal transplant rejection following immunisation with SARS-CoV-2 messenger RNA vaccine. Br J Ophthalmol. 2021;105:893–6. doi: 10.1136/bjophthalmol-2021-319338. [DOI] [PubMed] [Google Scholar]
- 12. Wasser LM, Roditi E, Zadok D, Berkowitz L, Weill Y. Keratoplasty rejection after the BNT162b2 messenger RNA vaccine. Cornea. 2021;40:1070–2. doi: 10.1097/ICO.0000000000002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ravichandran S, Natarajan R. Corneal graft rejection after COVID-19 vaccination. Indian J Ophthalmol. 2021;69:1953–4. doi: 10.4103/ijo.IJO_1028_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crnej A, Khoueir Z, Cherfan G, Saad A. Acute corneal endothelial graft rejection following COVID-19 vaccination. J Fr Ophtalmol. 2021;44:e445–7. doi: 10.1016/j.jfo.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parmar DP, Garde PV, Shah SM, Bhole PK. Acute graft rejection in a high-risk corneal transplant following COVID -19 vaccination: A case report. Indian J Ophthalmol. 2021;69:3757–8. doi: 10.4103/ijo.IJO_2515_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pichi F, Aljneibi S, Neri P, Hay S, Dackiw C, Ghazi NG. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139:1131–5. doi: 10.1001/jamaophthalmol.2021.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moorthy RS, Moorthy MS, Cunningham ET., Jr Drug-induced uveitis. Curr Opin Ophthalmol. 2018;29:588–603. doi: 10.1097/ICU.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 18. Saraceno JJ, Souza GM, dos Santos Finamor LP, Nascimento HM, Belfort R. Vogt-Koyanagi-Harada syndrome following COVID-19 and ChAdOx1 nCoV-19 (AZD1222) vaccine. Int J Retina Vitreous. 2021;7:49. doi: 10.1186/s40942-021-00319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koong LR, Chee WK, Toh ZH, Ng XL, Agrawal R, Ho SL. Vogt-Koyanagi-Harada disease associated with COVID-19 mRNA vaccine. Ocul Immunol Inflamm. 2021:1–4. doi: 10.1080/09273948.2021.1974492. doi:10.1080/09273948.2021.1974492. [DOI] [PubMed] [Google Scholar]
- 20. Papasavvas I, Herbort CP. Reactivation of Vogt-Koyanagi-Harada disease under control for more than 6 years, following anti-SARS-CoV-2 vaccination. J Ophthalmic Inflamm Infect. 2021;11:21. doi: 10.1186/s12348-021-00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ElSheikh RH, Haseeb A, Eleiwa TK, Elhusseiny AM. Acute uveitis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021:1–3. doi: 10.1080/09273948.2021.1962917. doi:10.1080/09273948.2021.1962917. [DOI] [PubMed] [Google Scholar]
- 22. Jain A, Kalamkar C. COVID-19 vaccine-associated reactivation of uveitis. Indian J Ophthalmol. 2021;69:2899–900. doi: 10.4103/ijo.IJO_1435_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Renisi G, Lombardi A, Stanzione M, Invernizzi A, Bandera A, Gori A. Anterior uveitis onset after bnt162b2 vaccination:Is this just a coincidence? Int J Infect Dis. 2021;110:95–7. doi: 10.1016/j.ijid.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 24. Rabinovitch T, Ben-Arie-Weintrob Y, Hareuveni-Blum T, Shaer B, Vishnevskia-Dai V, Shulman S, et al. Uveitis following the BNT162b2 mRNA vaccination against SARS-CoV-2 infection:A possible association. Retina (Philadelphia, Pa.) 2021 doi: 10.1097/IAE.0000000000003277. doi:10.1097/IAE.0000000000003277. [DOI] [PubMed] [Google Scholar]
- 25. Ishay Y, Kenig A, Tsemach-Toren T, Amer R, Rubin L, Hershkovitz Y, et al. Autoimmune phenomena following SARS-CoV-2 vaccination. Int Immunopharmacol. 2021;99:107970. doi: 10.1016/j.intimp.2021.107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mudie LI, Zick JD, Dacey MS, Palestine AG. Panuveitis following vaccination for COVID-19. Ocul Immunol Inflamm. 2021;29:741–2. doi: 10.1080/09273948.2021.1949478. [DOI] [PubMed] [Google Scholar]
- 27. Goyal M, Murthy SI, Annum S. Bilateral multifocal choroiditis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29:753–7. doi: 10.1080/09273948.2021.1957123. [DOI] [PubMed] [Google Scholar]
- 28. Pan L, Zhang Y, Cui Y, Wu X. Bilateral uveitis after inoculation with COVID-19 vaccine:A case report. Int J Infect Dis. 2021;113:116–8. doi: 10.1016/j.ijid.2021.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fowler N, Martinez NR, Pallares BV, Maldonado RS. Acute-onset central serous retinopathy after immunization with COVID-19 mRNA vaccine. Am J Ophthalmol Case Rep. 2021;23:101136. doi: 10.1016/j.ajoc.2021.101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bialasiewicz AA, Farah-Diab MS, Mebarki HT. Central retinal vein occlusion occurring immediately after 2nd dose of mRNA SARS-CoV-2 vaccine. Int Ophthalmol. 2021;41:3889–92. doi: 10.1007/s10792-021-01971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Endo B, Bahamon S, Martínez-Pulgarín DF. Central retinal vein occlusion after mRNA SARS-CoV-2 vaccination:A case report. Indian J Ophthalmol. 2021;69:2865–6. doi: 10.4103/ijo.IJO_1477_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goyal M, Murthy SI, Srinivas Y. Unilateral retinal vein occlusion in a young, healthy male following Sputnik V vaccination. Indian J Ophthalmol. 2021;69:3793–4. doi: 10.4103/ijo.IJO_2412_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mambretti M, Huemer J, Torregrossa G, Ullrich M, Findl O, Casalino G. Acute macular neuroretinopathy following coronavirus disease 2019 vaccination. Ocul Immunol Inflamm. 2021;29:730–3. doi: 10.1080/09273948.2021.1946567. [DOI] [PubMed] [Google Scholar]
- 34. Patel SN, Yonekawa Y. Acute macular neuroretinopathy following SARS-CoV-2 vaccination. Retin Cases Brief Rep. 2021 doi: 10.1097/ICB.0000000000001195. doi:10.1097/ICB.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 35. Vinzamuri S, Pradeep TG, Kotian R. Bilateral paracentral acute middle maculopathy and acute macular neuroretinopathy following COVID-19 vaccination. Indian J Ophthalmol. 2021;69:2862–4. doi: 10.4103/ijo.IJO_1333_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Book BA, Schmidt B, Foerster AM. Bilateral acute macular neuroretinopathy after vaccination against SARS-CoV-2. JAMA Ophthalmol. 2021;139:e212471. doi: 10.1001/jamaophthalmol.2021.2471. [DOI] [PubMed] [Google Scholar]
- 37. Valenzuela DA, Groth S, Taubenslag KJ, Gangaputra S. Acute macular neuroretinopathy following Pfizer-BioNTech COVID-19 vaccination. Am J Ophthalmol Case Rep. 2021;24:101200. doi: 10.1016/j.ajoc.2021.101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drüke D, Pleyer U, Hoerauf H, Feltgen N, Bemme S. Acute macular neuroretinopathy (AMN) following COVID-19 vaccination. Am J Ophthalmol Case Rep. 2021;24:101207. doi: 10.1016/j.ajoc.2021.101207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bøhler AD, Strøm ME, Sandvig KU, Moe MC, Jørstad ØK. Acute macular neuroretinopathy following COVID-19 vaccination [published online ahead of print, 2021 Jun 22] Eye (Lond) 2021;1-2 doi:10.1038/s41433-021-01610-1. [Google Scholar]
- 40. Michel T, Stolowy N, Gascon P, Dupessey F, Comet A, Attia R, et al. Acute macular neuroretinopathy after COVID-19 Vaccine. 2021 doi: 10.1016/j.jfo.2022.01.022. doi:10.21203/rs. 3.rs-632137/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mishra SB, Mahendradas P, Kawali A, Sanjay S, Shetty R. Reactivation of varicella zoster infection presenting as acute retinal necrosis post COVID 19 vaccination in an Asian Indian male. Eur J Ophthalmol. 2021 doi: 10.1177/11206721211046485. 11206721211046485. doi:10.1177/11206721211046485. [DOI] [PubMed] [Google Scholar]
- 42. Maleki A, Look-Why S, Manhapra A, Foster CS. COVID-19 recombinant mRNA vaccines and serious ocular inflammatory side effects:Real or coincidence? J Ophthalmic Vis Res. 2021;16:490–501. doi: 10.18502/jovr.v16i3.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carli G, Nichele I, Ruggeri M, Barra S, Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. 2021;16:803–4. doi: 10.1007/s11739-021-02685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee E-J, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–7. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shah P, Zaveri JS, Haddock LJ. Acute macular neuroretinopathy following the administration of an influenza vaccination. Ophthalmic Surg Lasers Imaging Retina. 2018;49:e165–8. doi: 10.3928/23258160-20181002-23. [DOI] [PubMed] [Google Scholar]
- 46. Liu JC, Nesper PL, Fawzi AA, Gill MK. Acute macular neuroretinopathy associated with influenza vaccination with decreased flow at the deep capillary plexus on oct angiography. Am J Ophthalmol Case Rep. 2018;10:96–100. doi: 10.1016/j.ajoc.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Psichogiou M, Samarkos M, Mikos N, Hatzakis A. Reactivation of varicella zoster virus after vaccination for SARS-CoV-2. Vaccines (Basel) 2021;9:572. doi: 10.3390/vaccines9060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–6. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jampol LM, Tauscher R, Schwarz HP. COVID-19, COVID-19 vaccinations, and subsequent abnormalities in the retina:Causation or coincidence? JAMA Ophthalmol. 2021;139:1135–6. doi: 10.1001/jamaophthalmol.2021.3483. [DOI] [PubMed] [Google Scholar]
- 50. Leber HM, Sant'Ana L, Konichi da Silva NR, Raio MC, Mazzeo TJMM, Endo CM, et al. Acute thyroiditis and bilateral optic neuritis following SARS-CoV-2 vaccination with CoronaVac:A case report. Ocul Immunol Inflamm. 2021:1–7. doi: 10.1080/09273948.2021.1961815. doi:10.1080/09273948.2021.1961815. [DOI] [PubMed] [Google Scholar]
- 51. Santovito LS, Pinna G. Acute reduction of visual acuity and visual field after Pfizer-BioNTech COVID-19 vaccine 2nd dose:A case report. Inflamm Res. 2021;70:931–3. doi: 10.1007/s00011-021-01476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jumroendararasame C, Panyakorn S, Othong R, Jumroendararasame A, Srimanan W, Tipparut K. Transient visual field loss after COVID-19 vaccination:Experienced by ophthalmologist, case report. Am J Ophthalmol Case Rep. 2021;24:101212. doi: 10.1016/j.ajoc.2021.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pawar N, Maheshwari D, Ravindran M, Padmavathy S. Ophthalmic complications of COVID-19 vaccination. Indian J Ophthalmol. 2021;69:2900–2. doi: 10.4103/ijo.IJO_2122_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reyes-Capo DP, Stevens SM, Cavuoto KM. Acute abducens nerve palsy following COVID-19 vaccination. J AAPOS. 2021;S1091-8531(21):00109–9. doi: 10.1016/j.jaapos.2021.05.003. doi:10.1016/j.jaapos.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pappaterra MC, Rivera EJ, Oliver AL. Transient Oculomotor Palsy Following the Administration of the Messenger RNA-1273 Vaccine for SARS-CoV-2 Diplopia Following the COVID-19 Vaccine. J Neuroophthalmol. 2021 doi: 10.1097/WNO.0000000000001369. Aug 4. doi:10.1097/WNO.0000000000001369. Epub ahead of print. PMID:34369471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shemer A, Pras E, Hecht I. Peripheral facial nerve palsy following BNT162b2 (COVID-19) vaccination. Isr Med Assoc J. 2021;23:143–4. [PubMed] [Google Scholar]
- 57. Ish S, Ish P. Facial nerve palsy after COVID19 vaccination –A rare association or a coincidence. Indian J Ophthalmol. 2021;69:2550–2. doi: 10.4103/ijo.IJO_1658_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yu BY, Cen LS, Chen T, Yang TH. Bell's palsy after inactivated COVID-19 vaccination in a patient with history of recurrent Bell's palsy:A case report. World J Clin Cases. 2021;9:8274–9. doi: 10.12998/wjcc.v9.i27.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodríguez-Martín M, Corriols-Noval P, López-Simón E, Morales-Angulo C. Ramsay Hunt syndrome following mRNA SARS-COV-2 vaccine. Enferm Infecc Microbiol Clin (Engl Ed) 2021 doi: 10.1016/j.eimce.2021.06.003. doi:10.1016/j.eimce.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shemer A, Pras E, Einan-Lifshitz A, Dubinsky-Pertzov B, Hecht I. Association of COVID-19 vaccination and facial nerve palsy:A case-control study. JAMA Otolaryngology Head Neck Surg. 2021;147:739–43. doi: 10.1001/jamaoto.2021.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaccines and Related Biological Products Advisory Committee Meeting-FDA Briefing Document. [Last accessed on 2021 Jun 26]. Available from: https://www.fda.gov/media/144245/download .
- 62.Vaccines and Related Biological Products Advisory Committee Meeting. [Last accessed on 2021 Jun 26]. Downloaded from: https://www.fda.gov/media/144434/download .
- 63. Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21:450–2. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thunstedt DC, Straube A, Schöberl F. Isolated intracranial hypertension following COVID-19 vaccination:A case report. Cephalalgia Reports. 2021;4 Sep 9. 25158163211044797. [Google Scholar]
- 65. Rubinstein TJ. Thyroid eye disease following COVID-19 vaccine in a patient with a history Graves' disease:A case report. Ophthalmic Plast Reconstr Surg. 2021;37:e221–3. doi: 10.1097/IOP.0000000000002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chuang TY, Burda K, Teklemariam E, Athar K. Tolosa-Hunt syndrome presenting after COVID-19 vaccination. Cureus. 2021;13:e16791. doi: 10.7759/cureus.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet. 2021;397:e11. doi: 10.1016/S0140-6736(21)00872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Panovska-Stavridis I, Pivkova-Veljanovska A, Trajkova S, Lazarevska M, Grozdanova A, Filipche V. A Rare Case of Superior Ophthalmic Vein Thrombosis and Thrombocytopenia Following ChAdOx1 nCoV-19 Vaccine Against SARS-CoV-2. Mediterr J Hematol Infect Dis. 2021;13(1):e2021048. doi: 10.4084/MJHID.2021.048. Published 2021 Mar 1. doi:10.4084/MJHID.2021.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Perry RJ, Tamborska A, Singh B, Craven B, Marigold R, Arthur-Farraj P, Yeo JM, Zhang L, Hassan-Smith G, Jones M, Hutchcroft C. Cerebral venous thrombosis after vaccination against COVID-19 in the UK:a multicentre cohort study. The Lancet. 2021;398(10306):1147–56. doi: 10.1016/S0140-6736(21)01608-1. Sep 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kadali RA, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID-19 vaccine:A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–81. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kadali RAK, Janagama R, Peruru S, Gajula V, Madathala RR, Chennaiahgari N, et al. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine:A randomized, cross sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. 2021;93:4420–9. doi: 10.1002/jmv.26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ng XL, Betzler BK, Testi I, Ho SL, Tien M, Ngo WK, et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021:1–9. doi: 10.1080/09273948.2021.1976221. doi:10.1080/09273948.2021.1976221. [DOI] [PMC free article] [PubMed] [Google Scholar]


