Abstract
We present the case of a 23-year-old young man with left-eye abducens nerve palsy following the COVID-19 vaccination. Given the temporal relationship between vaccination and the onset of symptoms, the lack of systemic history, and unremarkable magnetic resonance imaging, the patient’s abducens nerve palsy was related to his vaccination. The ophthalmologist should be aware of this neurotropic sequela of COVID-19 vaccination in young adults.
Keywords: Abducens nerve palsy, COVID-19, vaccination
Sixth nerve palsy in the young adult population is rare and presents with acute esotropia and limited abduction on the affected side, commonly due to neoplasm, trauma, elevated intracranial pressure, inflammation, and infections.[1,2]
According to reports of the US Vaccine Adverse Event Reporting System, the most common motor palsy following vaccinations in the pediatric age group are third, fourth, and abducens nerve.[3,4,5]
In the pediatric age group, the onset of nerve palsies secondary to immunization ranges from days to months, resolves by 6 months, and has a predilection to affect the left eye.[5,6]
We present the case of a young man with unilateral abducens nerve palsy post Covishield vaccination.
Case Report
A healthy 23 year male presented to the clinic for sudden-onset diplopia along with severe headache since 1 week. He denied any history of trauma or any recent illness. He had received his first dose of COVID-19 (Covishield, Serum Institute of India) vaccination 1 week prior. BCVAs were 20/20 OD, OS. Cover test revealed a 40 PD left esotropia with limited abduction of the left eye [Fig. 1a]. Dilated fundus examination was unremarkable. The patient had neuroimaging with MRI and MRA, which revealed no abnormality. Reverse transcription-polymerase chain reaction (RT-PCR) test was negative. All blood investigations, including complete blood count, fasting blood sugar, and antinuclear antibodies (ANA), were normal.
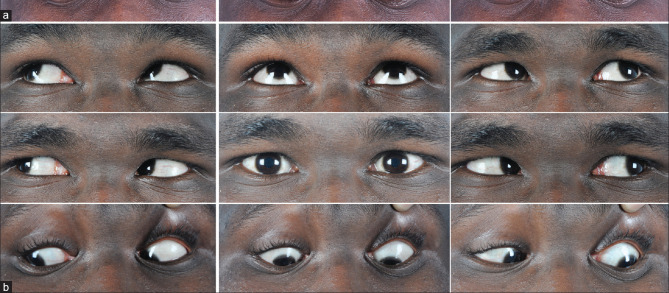
Figure 1.
(a) Cardinal 9 gazes showing LE esotropia of 40 PD with mild limitation of abduction. (b) Nine cardinal gaze showing minimal esotropia in LE after 6 weeks of vaccination and no diplopia in primary gaze
He had history of two similar episodes in the past. During both episodes, his MRI and blood investigations were unremarkable. He had the first episode of LE sixth nerve palsy following fever 5 years back for which LE Botox injection to medial rectus was given with complete resolution after 2 months. He had a similar episode in LE after 2 years of the initial episode following chickenpox with complete resolution in 3 months.
In his current visit, he had LE sixth nerve palsy following the COVID-19 vaccination. Subsequent follow-up examinations showed abduction improved to near normal [Fig. 1b].
Discussion
Cranial nerve palsies have been reported after multiple vaccines. The most common vaccines include the hepatitis A vaccine and the measles, mumps, and rubella vaccine; diphtheria and tetanus toxoids and acellular pertussis vaccine; type b Hemophilus influenza vaccine; and pneumococcal conjugate vaccine.[1,2,3,4,5] Predisposition of live or inactivated vaccines for particular cranial nerves palsies has not been established. Williams et al.[4] assessed the adverse events following influenza H1N1 vaccination. They included a single case of a 15-year-old male who had a history of head trauma in the distant past and now came with VI nerve palsy 13 days after the H1N1 vaccine and trivalent seasonal influenza vaccine. Most of the studies and case reports concluded that cranial neuropathies, as well as the abducens palsy, had a “possible” causal relationship with vaccination according to the modified World Health Organization criteria.[1]
Numerous cases of cranial nerve palsies post SARS-COV-19 have also been reported.[7,8,9,10] Falcone et al. and Faucher et al. reported cases of isolated abducens nerve palsies following a febrile period after COVID-19 symptom onset.[7,8] Out of various proposed mechanisms of neurological insults by the SARS-COV-19 virus, the most accepted is by attaching its S1 spike protein to the angiotensin-converting enzyme receptors, which have been detected in various organ systems, including the neurologic tracts. The abducens nerve palsy may represent part of the neurologic spectrum of COVID-19. Besides abducens nerve palsy, COVID-19 infection can also have other impairments of ocular motility. SARS-COV-19 virus is a neurotropic virus and can indirectly affect motility secondary to venous/arterial thrombosis.[9,10]
Reyes-Capo et al.[9] reported acute abducens palsy in a healthy 59-year-old woman 2 days after receiving the Pfizer-BioNTech COVID-19 vaccine. They emphasized recognition of the potential complication of the COVID-19 vaccine as neurologic sequelae similar to those that as have been reported with the COVID-19 virus itself as well as with other vaccines.
Despite an evident temporal association between presumed postimmunization abducens nerve palsy and immunization, the pathophysiological mechanism and site of cellular injury remain ambiguous. Hypothetically, abducens nerve injury includes a neurotropic effect of the infectious agent, an immune-mediated reaction or a parainfectious etiology causing demyelination, and sectarian arteritis or microinfarction of the abducens nerve.
Covishield is a recombinant, replication-deficient chimpanzee adenovirus vector encoding the SARS-CoV-2 spike (S) glycoprotein. Following administration, the genetic material of the coronavirus is expressed, which stimulates an immune response similar to post viral infections. The common adverse effects include lethargy, fever, and flu-like symptoms.
With a history of recurrence supporting viral etiology, RT-PCR negative report, a normal neuroimaging, and vaccination 7 days prior, we believe that in our patient, sixth nerve palsy was caused by a viral-like inflammatory reaction to the vaccine, inciting an immune-mediated indirect insult along the abducens nerve.
Conclusion
Acute benign abducens nerve palsy in young adults is a diagnosis of exclusion. The temporal proximity to the immunization is not a definitive cause but an important consideration for the clinician regarding proper management, future booster immunizations, and awareness in cases with recurrent nerve palsies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1. Brazis PW. Isolated palsies of cranial nerves III, IV, and VI. Semin Neurol. 2009;29:14–28. doi: 10.1055/s-0028-1124019. [DOI] [PubMed] [Google Scholar]
- 2. Holmes JM, Mutyala S, Maus TL, Grill R, Hodge DO, Gray DT. Pediatric third, fourth, and sixth nerve palsies: A population-based study. Am J Ophthalmol. 1999;127:388–92. doi: 10.1016/s0002-9394(98)00424-3. [DOI] [PubMed] [Google Scholar]
- 3. Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, et al. Understanding vaccine safety information from the vaccine adverse event reporting system. Pediatr Infect Dis J. 2004;23:287–94. doi: 10.1097/00006454-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 4. Williams SE, Pahud BA, Vellozzi C, Donofrio PD, Dekker CL, Halsey N, et al. Causality assessment of serious neurologic adverse events following 2009 H1N1 vaccination. Vaccine. 2011;29:8302–8. doi: 10.1016/j.vaccine.2011.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woo EJ, Winiecki SK, Ou AC. Motor palsies of cranial nerves (excluding VII) after vaccination: Reports to the US vaccine adverse event reporting system. Hum Vaccin Immunother. 2014;10:301–5. doi: 10.4161/hv.27032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falcone MM, Rong AJ, Salazar H, Redick DW, Falcone S, Cavuoto KM. Acute abducens nerve palsy in a patient with the novel coronavirus disease (COVID19) J AAPOS. 2020;24:216–7. doi: 10.1016/j.jaapos.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faucher A, Rey PA, Aguadisch E, Degos B. Isolated post SARS-CoV-2 diplopia. J Neurol. 2020;267:3128–9. doi: 10.1007/s00415-020-09987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–73. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reyes-Capo DP, Stevens SM, Cavuoto KM. Acute abducens nerve palsy following COVID-19 vaccination. J AAPOS. 2021 doi: 10.1016/j.jaapos.2021.05.003. S1091-8531(21)00109-9. doi: 10.1016/j.jaapos.2021.05.003. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna vaccines. Eur Rev Med Pharmacol Sci. 2021;25:1663–9. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]