Abstract
Despite the COVID 19 pandemic and mostly virtual congresses, innovation in the treatment of breast cancer patients continues at an unabated pace. This review summarises the current developments. Initial overall survival data for CDK4/6 inhibitor treatment in combination with an aromatase inhibitor as the first advanced line of therapy in treatment-naive postmenopausal patients have been published. Similarly, a trial comparing trastuzumab-deruxtecan versus trastuzumab-emtansine revealed a clear benefit regarding progression-free survival. Understanding of biomarkers making checkpoint inhibitor therapy particularly effective is increasing, and new compounds such as oral selective estrogen receptor destabilisers (SERDs) are entering clinical development and completing the first phase III trials.
Key words: BRCA (breast cancer associated gene), genetics, Her-2/neu (human epidermal growth factor receptor), hormonal receptor, breast cancer, breast
Introduction
Almost 50 years ago, tamoxifen was one of the first targeted drugs to be approved for the treatment of patients with breast cancer 1 . Similarly, trastuzumab, a monoclonal antibody targeting HER2 was approved almost 25 years ago 2 . These targeted medications have profoundly improved the prognosis in breast cancer patients and changed the therapeutic landscape of breast cancer forever. Despite the initial success, it was obvious that a large percentage of patients would become resistant to these regimens. That is why new therapeutic options have been developed over the past decades, based on the specific knowledge of these resistance mechanisms. Assessment of CDK4/6 inhibitors is coming to an end in the sense that overall survival data are now also available for first-line therapy in pre- and postmenopausal patients. Moreover, convincing data are available on the new antibody drug conjugate (ADC) trastuzumab-deruxtecan. After the initial enthusiasm for immunotherapies, there is also increasing evidence on those situations when these treatments are more, or less, effective. The latest developments based on newly published, clinically significant trials, recent publications in international journals and international congresses such as ASCO 2021 and ESMO 2021 are presented below.
Long-term Data on Treatment with CDK4/6 Inhibitors in HR-positive, HER2-negative Breast Cancer Patients
Long-term data on overall survival have now been published from some of the initial large-scale trials on CDK4/6 inhibitors 3 , 4 , 5 , 6 . While these data were collected through supplemental analyses in the PALOMA-3, MONALEESA-3 and MONALEESA-7 trials, the data presented by the MONALEESA-2 trial were the first on overall survival. Median follow-up times ranged from 54 months in MONALEESA-7 to 80 months in MONALEESA-2 ( Table 1 ). The primary analysis of overall survival demonstrated benefits in overall survival with hazard ratios ranging from 0.71 to 0.81. Long-term follow-up analysis, when the vast majority of patients were no longer on therapy, revealed that the hazard ratios remained similar over time ( Table 1 ).
Table 1 Summary of current trials with a CDK4/6 inhibitor in advanced treatment settings.
| Trial | Combined partner | Focused on | Enrolment from to (n) | PFS 95%-CI |
OS 95%-CI |
median FU primary OS analysis |
OS
§
95% CI |
median FU longest OS analysis § | References |
|---|---|---|---|---|---|---|---|---|---|
| * Prior chemotherapy allowed in advanced treatment setting. ** The analysis of the longest OS available is also the primary analysis. § If the long-term follow-up analyses are not the primary analyses, they must be considered exploratory. NA = not applicable (not published yet) | |||||||||
| MONALEESA-2 | Ribociclib Letrozol |
Pt. w/o endocrine resistance (first-line) | 02/2014 – 03/2015 (n = 668) | 0.56 (0.43 – 0.72) | 0.76 (0.63 – 0.93) | 80 | 0.76 **(0.63 – 0.93) | 80** | 6 , 43 , 44 |
| MONARCH 3 | Abemacliclib Aromatase inhibitor |
Pt. w/o endocrine resistance (first-line) | 11/2014 – 11/2015 (n = 493) | 0.54 (0.41 – 0.72) | Yet unknown | NA | NA | NA | 45 |
| PALOMA-2 | Palbociclib Letrozol |
Pt. w/o endocrine resistance (first-line) | 02/2013 – 07/2014 (n = 666) | 0.58 (0.46 – 0.72) | Yet unknown | NA | NA | NA | 46 |
| MONALEESA-7 | Ribociclib Premenopausal endocrine therapy |
Pt. w/o endocrine resistance (first-line)* | 12/2014 – 08/2016 (n = 672) | 0.55 (0.44 – 0.69) | 0.71 (0.54 – 0.95) | 34.6 | 0.76 (0.61 – 0.96) | 53.5 | 47 , 48 |
| MONALEESA-3 | Ribociclib Fulvestrant |
Pt. with and w/o endocrine resistance | 06/2015 – 06/2016 (n = 726) | 0.593 (0.48 – 0.73) | 0.72 (0.57 – 0.92) | 39.4 | 0.73 (0.59 – 0.90) | 56.3 | 49 , 50 |
| MONARCH 2 | Abemaciclib Fulvestrant |
Pt. with endocrine resistance | 08/2014 – 12/2015 (n = 669) | 0.553 (0.45 – 0.68) | 0.757 (0.61 – 0.95) | 47.7 | 0.757 ** (0.61 – 0.95) | 47.7** | 51 , 52 |
| PALOMA-3 | Palbociclib Fulvestrant |
Pt. with endocrine resistance | 10/2013 – 08/2014 (n = 521) | 0.46 (0.36 – 0.59) | 0.81 (0.64 – 1.03) | 44.8 | 0.81 (0.65 – 0.99) | 73.3 | 43 , 44 |
| DAWNA-1 | Dalpiciclib | Pt. with endocrine resistance | unknown (n = 361) | 0.45 (0.32 – 0.64) | NA | NA | NA | NA | |
The recent publication of the primary overall survival analysis of the MONALEESA-2 trial 3 was important in interpreting the treatment situation, as this trial only enrolled patients with first-line treatment and did not include patients with evident endocrine resistance. Thus, this patient population corresponds to most patients also treated in clinical practice. The MONALEESA-2 trial enrolled patients who were de novo metastatic or had a disease-free interval of more than 12 months following primary treatment. At the time of the overall survival analysis, these 668 patients had a median follow-up of 80 months and 400 deaths were recorded, 181 of which occurred in the ribociclib arm and 219 in the monotherapy arm at 1 : 1 randomisation. Thus, the benefit favouring the ribociclib arm was 24% with a hazard ratio of 0.74 (95% CI: 0.63 – 0.93) 3 . This difference was statistically significant. The therapeutic benefit was detectable across almost all subgroups, but in the analysis of de novo metastatic patients vs. patients after relapse a trend was noted, as the positive effect favouring ribociclib was mainly seen in the group of de novo patients 3 .
Although there had already been data on first-line treatment from the other trials, this was the first study to collect these data for postmenopausal patients without specific resistance criteria when combined with an aromatase inhibitor. Thus, combined treatment with CDK4/6 inhibitors and endocrine therapy was confirmed as the standard first-line treatment.
The data from the PALOMA-2 and MONARCH 3 trials have not yet been published, but the current (as of December 2021) minimum follow-up times (PALOMA-2 trial: 88 months; MONARCH 3: 72 months) should indicate that these publications are imminent ( Table 1 ).
Apart from the large randomised phase III trials, another trial has now been presented, which had been conducted in China with the CDK4/6 inhibitor dalpiciclib developed for the Chinese market. Patients after progression on endocrine therapy could be randomised to fulvestrant monotherapy versus fulvestrant in combination with dalpiciclib. With a median follow-up of 10.7 months, the centrally calculated hazard ratio for progression-free survival was 0.45 (95% CI: 0.32 – 0.64) ( Table 1 ).
Continued Development of Antihormonal Therapy
Patient outcomes after CDK4/6 inhibitor therapy
With the establishment of CDK4/6 inhibitors as standard first-line therapy and the first evidence of benefit in early-stage patients 7 , the question of meaningful treatment options following CDK4/6 inhibitor therapy is becoming increasingly important. Research is being vigorously pursued into molecular markers that can predict the efficacy of CDK4/6 inhibitor-based therapy. In addition, research is being conducted on the mechanism of progression under – or at the end of – CDK4/6 inhibitor-based therapy and how to harness it for subsequent treatments.
A number of biomarker analyses have already been carried out as part of the prospective randomised trials. In the PALOMA-3 study, for example, mutation analyses and amplification analyses of circulating tumour DNA (ctDNA) were correlated with progression-free survival. Amplifications in FGFR1 and a TP53 mutation appeared to be predictive for treatment with fulvestrant and palbociclib, while TP53 and ESR1 mutations seemed to play a role in treatment with fulvestrant alone 8 . Pooled ctDNA analyses from the MONALEESA trials identified several genes as possible predictors of better or worse ribociclib activity (FRS2, MDM2, PRKCA, ERBB2, AKT1 E17K, BRCA1/2, CHD4, ATM and CDKN2A/2B/2C) 9 . In the PADA-1 trial, patients treated with palbociclib and fulvestrant were shown to have a worse prognosis if an ESR1 mutation was detected in the ctDNA or if the mutation load of ESR1 mutations was not reduced 10 . These data and the known information on the efficacy of new anti-endocrine agents have led to study designs making use of the knowledge of molecular mechanisms of progression, such as the SERENA-6 trial (see below).
First phase III trial with oral SERDs (selective estrogen receptor degraders) in patients with advanced breast cancer positive
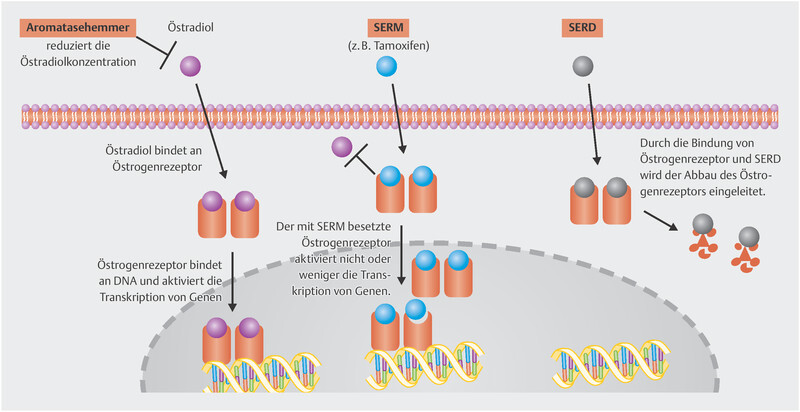
Fulvestrant was the first SERD approved for treatment of metastatic breast cancer. Together with aromatase inhibitors and tamoxifen as SERM, these three substances constitute the foundation of anti-endocrine therapy in breast cancer patients. The mode of action of these substances is summarised in Fig. 1 .
Fig. 1.
Mode of action of oestrogen, selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs).
Establishing the SERD fulvestrant clinically has been difficult. For a long time after approval (initially in 2004), the introduction of this drug was accompanied by difficulties in defining the correct dosage, and the EMA approval as first-line treatment in advanced stages was only granted in 2017 11 . The only adjuvant trial with fulvestrant was terminated prematurely 12 . Partly responsible for this long development phase was a rather unfavourable pharmacokinetic profile, which requires intramuscular drug injection and, even with this mode of administration, it takes months for the plasma levels to stabilise 13 . This is the reason why the known dose of 500 mg is needed to reach adequate plasma levels even in the initial treatment period. This illustrates that the development of oral SERDs with more stable bioavailability could improve therapy. Table 2 gives an overview of the SERDs under development. A press release recently announced that the EMBER trial of the oral SERD elacestrant met the primary study objective. Patients were included after treatment with a CDK4/6 inhibitor in combination with either an aromatase inhibitor or fulvestrant. Patients were then randomised to monotherapy with elacestrant or standard endocrine therapy (either fulvestrant or an aromatase inhibitor). The trial demonstrated that elacestrant significantly prolonged PFS 14 . The trial enrolled patients with and without somatic ESR1 mutation, and the oral SERD had a benefit in both patients with and without the mutation.
Table 2 Current selective estrogen receptor degraders (SERDs).
| SERD Substance Code (Name) | Name of study programme | References |
|---|---|---|
| * New class of SERD (Proteolysis Targeting Chimera, PROTAC) ** New class of SERD (Selective Estrogen Receptor Covalent Antagonist; SERCA) | ||
| LSZ102 | unknown | 53 |
| G1T48 (rintodestrant) | PRESERVE | 54 , 55 |
| RAD1901 (elacestrant) | EMERALD | 14 , 56 |
| GDC-9545 (giredestrant) | …ERA (coopERA, lidERA, perseveERA) | 57 , 58 , 59 |
| SAR439859 (amcenestrant) | AMEERA | 60 , 61 , 62 |
| AZD9833 (camizestrant) | SERENA | 63 |
| LY3484356 (imlunestrant) | EMBER | 64 , 65 , 66 |
| Zn-c5 | unknown | 67 |
| D-0502 | unknown | 68 |
| ARV-471* | unknown | 15 |
| H3B-5942** | unknown | 69 |
PROTAC – New class of substances made useful as SERD
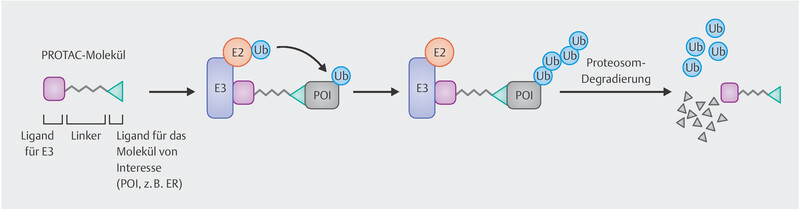
In addition to the SERDs known to date, there are other substances with this effect belonging to a new class of drugs called PROTACs (Proteolysis Targeting Chimeras), which are hetero-bifunctional molecules with a ligand for a protein of interest (in this case the oestrogen receptor) on one side and another ligand on the other side acting as a substrate for the E3 ubiquitin ligase complex. This binds the protein to be degraded to the ubiquitin-proteasome system triggering the degradation ( Fig. 2 ). ARV-471 is a PROTAC targeted against the oestrogen receptor 15 . In a phase I trial, objective response was achieved in 4 out of 14 patients with advanced breast cancer and massive prior treatment. None of the patients experienced primary progression 15 .
Fig. 2.
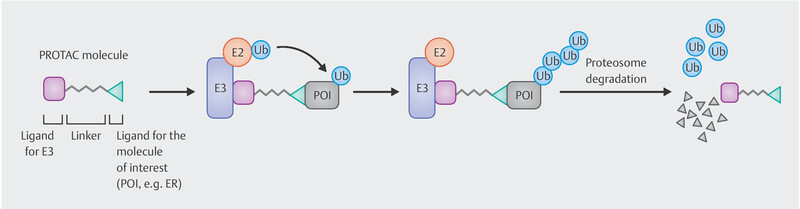
Mode of action of PROTACS such as ARV-471 degrading the oestrogen receptor.
Therapeutic sequences and their rationale
The importance of ESR1 mutations as one of the resistance mechanisms against antihormonal treatment or combination therapy with CDK4/6 inhibitors has been postulated for some time 8 , 10 . The SERENA-6 trial 16 is one example of studies making use of this knowledge. Existing and de novo ESR1 mutations in ctDNA are measured before and during treatment with a CDK4/6 inhibitor plus an aromatase inhibitor. These patients are then randomised to continue CDK4/6 inhibitor therapy with the aromatase inhibitor or a SERD as new combined partner 16 .
A number of therapeutic options have been and are being investigated in the post-CDK4/6 inhibitor setting. Although data on the efficacy of alpelisib in patients with PIK3CA mutations have already been collected with the SOLAR-1 trial 17 , few patients received a CDK4/6 inhibitor prior to therapy with alpelisib and fulvestrant. This is why EPIK-B5, a prospective randomised trial still enrolling patients, is studying this question in patients after treatment with CDK4/6 inhibitors 18 .
One trial that has already been conducted in this treatment setting did not achieve its study objective. The VERONICA trial was offered to patients with two or fewer lines of treatment and after CDK4/6 inhibitor therapy. Patients received either fulvestrant monotherapy or a combination of fulvestrant and venetoclax. Venetoclax is a Bcl-2 inhibitor already approved in patients with various haematological neoplasms. The trial did not reveal any difference in PFS between the randomisation arms (HR: 0.94; 95% CI: 0.61 – 1.45). In terms of overall survival, there was even a signal favouring monotherapy (HR: 2.56; 95% CI: 1.11 – 5.89).
It should be noted that CDK4/6 inhibitors will probably remain the standard of care in first-line treatment for a long time 19 . With this context in mind, it will be extremely important to understand the mechanisms of progression. Although the large CDK4/6 inhibitor trials have collected biomaterials, these may not be large enough to apply modern analytical techniques. One trial that may be of interest in this context is the HARMONIA, which compares ribociclib versus palbociclib in the group of PAM50 HER2 enriched patients. An extensive translational research programme is also being undertaken in this trial 20 .
Still Significant Progress in the Treatment of HER2-positive Breast Cancer Patients
Trastuzumab-deruxtecan (T-Dxd) versus T-DM1
With trastuzumab, the trastuzumab biosimilars, lapatinib, pertuzumab, T-DM1, neratinib, tucatinib and T-DXd, a wide range of drugs are available for the treatment of patients with HER2-positive breast cancer. Most of them improved the prognosis significantly, so that patients with HER2-positive breast cancer now belong to the group of patients with better prognosis compared to other molecular subtypes 21 , 22 . Nevertheless, the introduction of new substances has always led to new advances. The latest compound to demonstrate clear benefits in a randomised trial was the receptor tyrosine kinase inhibitor tucatinib, which improved progression-free survival and overall survival in a population largely with pertuzumab and T-DM1 as prior treatment 23 . Data on T-DXd from a prospective randomised trial have also now been published. The study population had to have undergone prior treatment in the advanced therapeutic setting. Thus, almost all patients had received trastuzumab and about 61% also pertuzumab before the trial. The question tested was which of the antibody drug conjugates (ADC), T-DM1 or T-DXd, would result in better progression-free survival and overall survival. The question could be answered clearly: The hazard ratio for PFS was 0.28 (95% CI: 0.22 – 0.37; p = 7.8 E-22) in favour of T-DXd. While the median progression-free survival under T-DM1 was 6.8 months, it had not yet been reached in the T-DXd group at the time of this analysis 24 . The trial thus not only established T-DXd as a new treatment standard in the corresponding therapeutic setting in which T-DM1 had previously been administered, but also demonstrated that there was a real medical need for T-DM1 in the sequence following pertuzumab. In the EMILIA study, the median PFS with T-DM1 was 9.6 months, but it must be remembered that these patients did not receive prior treatment with pertuzumab. Corresponding data from real-world registries are similar to the DESTINY-Breast03 trial, in which the median PFS was 7.7 months in second-line therapy after prior treatment with pertuzumab and 3.4 months in third-line therapy 25 . Hence, in this therapeutic setting, T-DXd significantly improved the treatment of HER2-positive breast cancer. Although the median PFS for T-DXd had not yet been reached, the 12-month PFS rate gives a clear indication. It was 34.1% with T-DM1 and 75.8% with T-DXd. However, it should be noted that the initial phase of the trial during therapy with T-DXd saw a number of deaths resulting from pneumonitis/interstitial lung disease (ILD) 26 . Although there were significantly more ILD cases as a side effect compared with T-DM1 (10.5% vs. 1.9%, a total of 27 cases under T-DXd) in the DESTINY-Breast03 trial, none of these side effects resulted in death 24 . Presumably, this is the consequence of stringent side-effect management, which requires that in respiratory symptoms onset, therapy is stopped immediately, diagnostic workup by high-resolution CT is performed, and corticosteroid therapy is initiated 27 .
Antibody-drug conjugates on the rise
ADC technology has fostered the clinical development of a number of new drugs, of which trial results are now slowly being published. One such study is the TULIP trial, which uses the ADC SYD985 and also trastuzumab-duocarmycin 28 . Duocarmycin is a DNA alkylane first isolated from streptomyces bacteria in the 1970s 29 . The TULIP trial enrolled 437 patients with advanced HER2-positive breast cancer who had completed at least two anti-HER2 regimens in the advanced treatment setting or already received T-DM1. Randomisation was 2 : 1 for treatment with SYD985 every three weeks versus treatment of physicianʼs choice (lapatinib + capecitabine, trastuzumab + capecitabine, trastuzumab + vinorelbine, trastuzumab + eribulin). More than 85% of patients had received prior treatment with T-DM1 and about 60% also with pertuzumab 28 .
Comparison of both randomisation arms found better progression-free survival with trastuzumab-duocarmycin (SYD985). The hazard ratio was 0.64 (95% CI: 0.49 – 0.84; p = 0.002) 28 . Overall survival revealed improvement without statistical significance (HR: 0.83; 95% CI: 0.62 – 1.09; p = 0.153) 28 .
Interestingly enough, this treatment causes side effects that have not been the focus of breast cancer therapeutics so far. Conjunctivitis and keratitis were seen in about 38% of patients 28 . As with T-DXd, 7.6% of patients treated with SYD985 also developed pneumonitis.
The treatment options in patients with HER2-positive breast cancer will definitely undergo significant changes in the next few years. Tucatinib and T-DXd are two new, effective substances currently being tested in extensive trial programmes. The near future will show whether these drugs from the advanced therapeutic setting will also be included in the treatment of patients with early-stage disease. Enrolment in corresponding trials has already started.
Endocrine therapy instead of chemotherapy combined with trastuzumab
In the sysucc-002 trial, patients with hormone receptor-positive, HER2-positive metastatic breast cancer were randomised undergoing first-line treatment were randomised between endocrine therapy plus trastuzumab and chemotherapy plus trastuzumab 30 . Almost two thirds of the 392 patients enrolled in the trial had visceral metastasis, about one quarter were diagnosed with de novo metastasis, and only about one quarter of the patients had previously received HER2-targeted therapy.
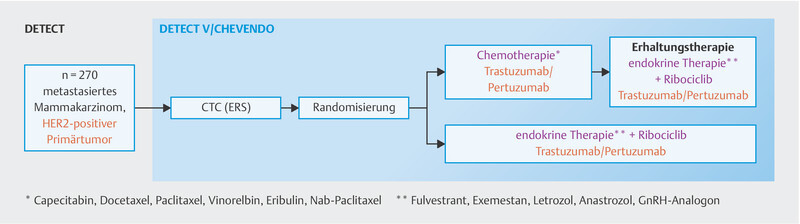
Analysis of progression-free survival revealed no significant difference between both arms (HR: 0.88, 95% CI: 0.71 – 1.09; log-rank: 0.25). Only patients with a disease-free period of less than 24 months experienced a non-significant benefit from chemotherapy (HR: 1.39, 95% CI: 0.97 – 1.98). There was no significant difference in overall survival. This study is the first phase III trial to directly compare chemotherapy with endocrine therapy in the context of HER2-targeted therapy in triple-positive metastatic breast cancer. Weaknesses of this study include the fact that neither a dual blockade with trastuzumab and pertuzumab was employed, which is the global standard in therapy, nor was a CDK4/6 inhibitor included. The DETECT-V trial ( http://www.detect-studien.de , Fig. 3 ), which is actively enrolling patients in Germany, takes this much more modern approach and patients can still be enrolled in it.
Fig. 3.
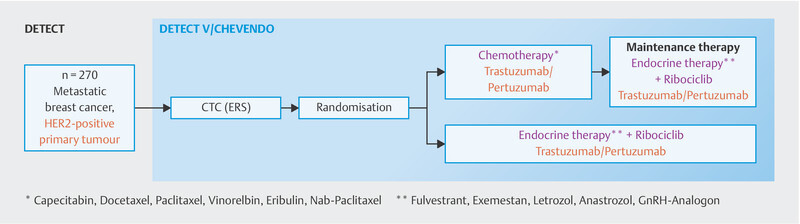
DETECT-V study design.
Immunotherapies – Much Remains to Be Learned
Checkpoint inhibitors and biomarkers
In some indications, PD-L1-positive cells must be identified. The indication for atezolizumab in advanced first-line treatment is linked to the presence of PD-L1-positive immune cells covering at least 1% of the tumour area. The indication for pembrolizumab is linked to a share of PD-L1-expressing immune and tumour cells (combined positive score, CPS) of at least 10. In neoadjuvant settings, PD-L1 expression is not predictive of pembrolizumab efficacy 31 . Although in the neoadjuvant KEYNOTE-522 trial the pCR rates increased with increasing PD-L1 expression, this was the case in both the arm with and the arm without pembrolizumab. Chemotherapy combined with a PD-1/PD-L1 therapeutic agent could also have an impact on efficacy, as the combination of atezolizumab and nab-paclitaxel in IMpassion130 resulted in a better prognosis 32 , while in IMpassion131 the combination of atezolizumab and conventional soluble paclitaxel did not improve prognosis 33 . Similarly, tumour-infiltrating lymphocytes have been linked to both efficacy and prognosis in breast cancer patients 34 , 35 . Immune-related markers of gene expression have previously been associated with response to chemotherapy 36 , 37 .
Data from a comprehensive translational analysis of the IMpassion130 trial have now been presented in light of this context 38 . The tumours of the patients enrolled in this trial were classified according to the following immunophenotypes 39 :
Immune desert: Despite the presence of immune cells., these tumours do not have T-cells that could attack the malignancy. So there is no immune response.
Immune-excluded phenotype: In these tumours, while there is indeed an increased number of immune cells, these are not localised in the parenchyma, but only in the stroma surrounding the tumour.
Immune-inflamed phenotype: In these tumours, the numerous immune cells in the parenchyma appear to be in direct contact with the tumour cells.
Analysis of the IMpassion130 trial in relation to this classification revealed that in PD-L1 positivity, the hazard ratio for overall survival in the immune-inflamed phenotype showed the greatest effect favouring atezolizumab (HR: 0.61; 95% CI: 0.42 – 0.88) 38 .
A classification dividing triple-negative tumours into subtypes based on their gene expression was also tested 40 .
BLIA: strong expression of genes of the immune system
BLIS: high proliferation and glycolysis
LAR: strong expression for the oestrogen and androgen pathway and strong expression for lipid metabolism genes.
MES: strong expression for angiogenesis, myogenesis, oestrogen, and androgen signalling genes, TGF-beta, fibroblasts, and endothelial cells.
It was shown that the BLIA phenotype in particular predisposed to a response to atezolizumab therapy. The hazard ratio for overall survival was 0.54 (95% CI: 0.36 – 0.80).
Despite the success of immune checkpoint inhibitors and their use in standard treatment options, much remains to be learned about the pattern of efficacy of these therapies. Especially with the relevant side effect profile, everything should be tried to better assess the risk-benefit profile of this treatment. Identifying subgroups with particularly high and particularly low levels of efficacy could help.
Pembrolizumab as newly approved treatment option
In the first-line treatment patients with advanced TNBC and a CPS score of 10 or more, data from the KEYNOTE-355 trial already showed that median progression-free survival improved from 5.6 months with chemotherapy to 9.7 months with chemotherapy + pembrolizumab (HR = 0.65; 95% CI: 0.49 – 0.86) 41 . These data have now been supplemented by further analysis of overall survival 42 . Another planned analysis called for a p-value of 0.0113. Indeed, median overall survival was prolonged from 16.1 months to 23.0 months (HR = 0.73; 95% CI: 0.55 – 0.95; p = 0.0093). Thus, a significant improvement in overall survival has also been demonstrated. In the United States, pembrolizumab was available in May 2021 and in Europe in October 2021.
Outlook
The MONALEESA-2 trial was the first to publish overall survival data in first-line treatment combined with an aromatase inhibitor in postmenopausal patients. Data from the MONARCH-3 and PALOMA-2 trials are still pending. Since the last patients were enrolled in July 2014 (PALOMA-2) and November 2015 (MONARCH 3) respectively, publication is expected soon. Only then can the entire study data be comprehensively assessed. The therapeutic benefit of T-DXd over T-DM1 is a significant step forward for the treatment of patients with advanced HER2-positive breast cancer. However, other trials are active – also with another very effective anti-HER2 drug (tucatinib) – studying the benefit in first-line treatment versus pertuzumab, and also trials in the (neo-)adjuvant setting. It may become complex in this context how new therapeutic sequences will establish themselves.
The path towards treatment based on molecular markers is already well underway with new trials such as SERENA-6. Additional trials related to the PI3K pathway and homologous recombination are underway to explore whether these approaches will result in better personalised therapy.
Correction.
Update Breast Cancer 2021 Part 5 – Advanced Breast Cancer Diana Lüftner, Florian Schütz, Elmar Stickeler et al. Geburtsh Frauenheilk 2022; 82: 215–225. doi:10.1055/a-1724-9569
In the above-mentioned article, the institute details were mixed up for two authors. Correct is:
Authors Rachel Würstlein 23 , Andreas D. Hartkopf 24
Affiliations23 Breast Center, Department of Gynecology and Obstetrics and CCC Munich LMU, LMU University Hospital, Munich, Germany 24 Department of Obstetrics and Gynecology, University of Tübingen, Tübingen, Germany
Acknowledgements
This paper evolved in part as a result of company funding from onkowissen.de, Hexal, Pfizer, Lilly, MSD, Gilead, and Novartis. No company had any part in the preparation and recommendations of this manuscript. Sole responsibility for the content of the manuscript rests with the authors.
Danksagung
Diese Arbeit entstand teilweise in Folge von Förderungen der Firmen onkowissen.de, Hexal, Pfizer, Lilly, MSD, Gilead und Novartis. Keine der Firmen hatte einen Anteil an der Erstellung und den Empfehlungen dieses Manuskriptes. Für den Inhalt des Manuskriptes sind alleine die Autoren verantwortlich.
Footnotes
Conflict of Interest/Interessenkonflikt B. A. received honoria and travel grants from AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo and Pfizer. E. B. received honoraria from Novartis, Hexal, BMS, Lilly, Pfizer, Roche, MSD, BBraun and onkowissen.de for consulting, clinical research management or medical education activities. S. B. has no conflict of interest. N. D. has received honoraria from MSD, Roche, AstraZeneca, Teva, Pfizer, Novartis, Seagen,Gilead, MCI Healthcare. P. A. F. reports personal fees from Novartis, grants from Biontech, personal fees from Pfizer, personal fees from Daiichi Sankyo, personal fees from AstraZeneca, personal fees from Eisai, personal fees from Merck Sharp & Dohme, grants from Cepheid, personal fees from Lilly, personal fees from Pierre Fabre, personal fees from SeaGen, personal fees from Roche, personal fees from Hexal, personal fees from Agendia, personal fees from Gilead. T. N. F. has participated on advisory boards for Amgen, Daiichi Sankyo, Novartis, Pfizer, and Roche and has received honoraria for lectures from Amgen, Celgene, Daiichi Sankyo, Roche, Novartis and Pfizer. A. D. H. received speaker and consultancy honoraria from AstraZeneca, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo, Hexal and Pfizer. W. J. has received research grants and/or honoraria from Sanofi-Aventis, Daiichi Sankyo, Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, GSK, Eisai, Cellgene and Johnson & Johnson. C. K.-L. has received honoraria from Roche, AstraZeneca, Celgene, Novartis, Pfizer, Lilly, Hexal, Amgen, Eisai, and SonoScape, honoraria for consultancy from Phaon Scientific, Novartis, Pfizer, and Celgene, research funding from Roche, Novartis, and Pfizer, and travel grants from Novartis and Roche. H.-C. K. has received honoraria from Pfizer, Novartis, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, Teva, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lily, SurgVision, Onkowissen and MSD, travel support from Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo, Tesaro and owns stock of Theraclion SA and Phaon Scientific GmbH. D. L. received honoraria from Amgen, AstraZeneca, Eli Lilly, GSK, Loreal, MSD, Novartis, Pfizer, Teva. M. P. L. has participated on advisory boards for AstraZeneca, Lilly, MSD, Novartis, Pfizer, Eisai, Gilead, Exact Sciences, Pierre Fabre, Grünenthal, Daiichi Sankyo, PharmaMar and Roche and has received honoraria for lectures from MSD, Lilly, Roche, Novartis, Pfizer, Exact Sciences, Daiichi Sankyo, Grünenthal, Gilead, AstraZeneca, and Eisai. He is editorial board member of medactuell from medac. V. M. received speaker honoraria from Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Pfizer, MSD, Novartis, Roche, Teva, Seattle Genetics and consultancy honoraria from Genomic Health, Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Tesaro, Seattle Genetics and Nektar. Institutional research support from Novartis, Roche, Seattle Genetics, Genentech. Travel grants: Roche, Pfizer, Daiichi Sankyo. E. S. received honoraria from Roche, Celgene, AstraZeneca, Novartis, Pfizer, Tesaro, Aurikamed GmbH, MCI Deutschland GmbH, bsh medical communications GmbH, Onkowissen TV. A. S. received research grants from Celgene, Roche, honoraria from Amgen, AstraZeneca, Aurikamed, Bayer, Celgene, Clinsol, Connectmedica, Gilead, GSK, I-MED, Lilly, MCI Deutschland, Metaplan, MSD, Nanostring, Novartis, Onkowissen.de, Promedicis, Pfizer, Pierre Fabre, Roche, Seagen, Streamedup, Teva, Tesaro, Thieme and travel support from Celgene, Pfizer, Roche. F. S. participated on advisory boards for Novartis, Lilly, Amgen and Roche and received honoraria for lectures from Roche, AstraZeneca, MSD, Novartis and Pfizer. H. T. received honoraria from Novartis, Roche, Celgene, Teva, Pfizer, Astra Zeneca and travel support from Roche, Celgene and Pfizer. C. T. received honoraria for advisory boards and lectures from Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Gilead, Lilly, MSD, Mylan, Nanostring, Novartis, Pfizer, Pierre Fabre, Puma, Roche, Seagen, Vifor. M. T. has participated on advisory boards for AstraZeneca, Clovis, Daiichi Sankyo, Eisai, Gilead Science, GSK, Lilly, MSD, Novartis, Organon, Pfizer, Exact Sciences, Pierre-Fabre, Seagen and Roche and has received honoraria for lectures from Clovis, Daiichi Sankyo, Eisai, GSK, Lilly, MSD, Roche, Novartis, Organon, Pfizer, Seagen, Exact Sciences, Viatris, and AstraZeneca and has received trial funding by Exact Sciences and Endomag. Manuscript support was done by Amgen, Celgene, ClearCut, pfm medical, Roche, Servier. M. U. all honoraria went to the institution/employer: Abbvie, Amgen, Astra Zeneca, Celgene, Daichi Sankyo, Eisai, Lilly, MSD Merck, Mundipharma, Myriad Genetics, Pfizer, PUMA Biotechnology, Roche, Sanofi Aventis, Novartis, Pierre Fabre. M. W. has participated on advisory boards for AstraZeneca, Lilly, MSD, Novartis, Pfizer and Roche. A. W. participated on advisory boards for Novartis, Lilly, Amgen, Pfizer, Roche, Tesaro, Eisai and received honoraria for lectures from Novartis, Pfizer, Aurikamed, Roche, Celgene. R. W. has received personal fees/travel support from Agendia, Amgen, Aristo, AstraZeneca, Boeringer Ingelheim, Carl Zeiss, Celgene, Clinsol, Daiichi Sankyo, Eisai, Exact Sciences, Genomic Health, GlaxoSmithKline, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Nanostring, Novartis, Odonate, Paxman, Palleos, Pfizer, Pierre Fabre, PumaBiotechnology, Riemser, Roche, Sandoz/Hexal, Seattle Genetics, Tesaro Bio, Teva, Veracyte and Viatris./ B. A. erhielt Honorare und Reisekostenzuschüsse von AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo und Pfizer. E. B. erhielt Honorare von Novartis, Hexal, BMS, Lilly, Pfizer, Roche, MSD, BBraun und onkowissen.de für Beratung, klinisches Forschungsmanagement bzw. medizinische Fortbildungsaktivitäten. S. B. gibt keinen Interessenkonflikt an. N. D. erhielt Honorare von MSD, Roche, AstraZeneca, Teva, Pfizer, Novartis, Seagen, Gilead, MCI Healthcare. P. A. F. erhielt persönliche Honorare von Novartis, Zuschüsse von Biontech, persönliche Honorare von Pfizer, persönliche Honorare von Daiichi Sankyo, persönliche Honorare von AstraZeneca, persönliche Honorare von Eisai, persönliche Honorare von Merck Sharp & Dohme, Zuschüsse von Cepheid, persönliche Honorare von Lilly, persönliche Honorare von Pierre Fabre, persönliche Honorare von SeaGen, persönliche Honorare von Roche, persönliche Honorare von Hexal, persönliche Honorare von Agendia, persönliche Honorare von Gilead. T. N. F. war für Amgen, Daiichi Sankyo, Novartis, Pfizer und Roche in Beratungsgremien tätig und erhielt für Vortragstätigkeit Honorare von Amgen, Celgene, Daiichi Sankyo, Roche, Novartis und Pfizer. A. D. H. erhielt für Vortrags- und Beratertätigkeiten Honorare von AstraZeneca, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo, Hexal und Pfizer. W. J. hat Forschungszuschüsse und/oder Honorare von Sanofi-Aventis, Daiichi Sankyo, Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, GSK, Eisai, Cellgene und Johnson & Johnson erhalten. C. K.-L. erhielt Honorare von Roche, AstraZeneca, Celgene, Novartis, Pfizer, Lilly, Hexal, Amgen, Eisai und SonoScape, Honorare für Beratungstätigkeit von Phaon Scientific, Novartis, Pfizer und Celgene, Forschungsgelder von Roche, Novartis und Pfizer sowie Reisekostenzuschüsse von Novartis und Roche. H.- C. K. hat Honorare von Pfizer, Novartis, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, Teva, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lily, SurgVision, Onkowissen und MSD erhalten, Reiseunterstützung von Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo, Tesaro und besitzt Aktien von Theraclion SA und Phaon Scientific GmbH. D. L. erhielt Honorare von Amgen, AstraZeneca, Eli Lilly, GSK, Loreal, MSD, Novartis, Pfizer und Teva. M. P. L. war in Beiräten für AstraZeneca, Lilly, MSD, Novartis, Pfizer, Eisai, Gilead, Exact Sciences, Pierre Fabre, Grünenthal, Daiichi Sankyo, PharmaMar und Roche tätig und erhielt Honorare für Vortragstätigkeit von MSD, Lilly, Roche, Novartis, Pfizer, Exact Sciences, Daiichi Sankyo, Grünenthal, Gilead, AstraZeneca und Eisai. Er ist Redaktionsmitglied bei medactuell von medac. V. M. erhielt Honorare für Vortragstätigkeit von Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Pfizer, MSD, Novartis, Roche, Teva, Seattle Genetics und Honorare für Beratungstätigkeit von Genomic Health, Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Tesaro, Seattle Genetics und Nektar. Institutionelle Forschungsunterstützung von Novartis, Roche, Seattle Genetics, Genentech. Reisekostenzuschüsse: Roche, Pfizer, Daiichi Sankyo. E. S. erhielt Honorare von Roche, Celgene, AstraZeneca, Novartis, Pfizer, Tesaro, Aurikamed GmbH, MCI Deutschland GmbH, bsh medical communications GmbH, Onkowissen TV. A. S. erhielt Forschungszuschüsse von Celgene, Roche, Honorare von Amgen, AstraZeneca, Aurikamed, Bayer, Celgene, Clinsol, Connectmedica, Gilead, GSK, I-MED, Lilly, MCI Deutschland, Metaplan, MSD, Nanostring, Novartis, Onkowissen.de, Promedicis, Pfizer, Pierre Fabre, Roche, Seagen, Streamedup, Teva, Tesaro, Thieme und Reiseunterstützung von Celgene, Pfizer, Roche. F. S. war in Beiräten für Novartis, Lilly, Amgen und Roche tätig und erhielt Honorare für Vortragstätigkeit von Roche, AstraZeneca, MSD, Novartis und Pfizer. H. T. erhielt Honorare von Novartis, Roche, Celgene, Teva, Pfizer, AstraZeneca und Reisekostenunterstützung von Roche, Celgene und Pfizer. C. T. erhielt Honorare für Beratungsgremien und Vorträge von Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Gilead, Lilly, MSD, Mylan, Nanostring, Novartis, Pfizer, Pierre Fabre, Puma, Roche, Seagen, Vifor. M. T . war in Beiräten für AstraZeneca, Clovis, Daiichi Sankyo, Eisai, Gilead Science, GSK, Lilly, MSD, Novartis, Organon, Pfizer, Exact Sciences, Pierre-Fabre, Seagen und Roche tätig und erhielt Honorare für Vorträge von Clovis, Daiichi Sankyo, Eisai, GSK, Lilly, MSD, Roche, Novartis, Organon, Pfizer, Seagen, Exact Sciences, Viatris und AstraZeneca und erhielt Studienfinanzierung von Exact Sciences und Endomag. Das Manuskript wurde von Amgen, Celgene, ClearCut, pfm medical, Roche und Servier unterstützt. M. U. alle Honorare gingen an die Einrichtung/den Arbeitgeber: Abbvie, Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Lilly, MSD Merck, Mundipharma, Myriad Genetics, Pfizer, PUMA Biotechnology, Roche, Sanofi Aventis, Novartis, Pierre Fabre. M. W. hat in Beiräten für AstraZeneca, Lilly, MSD, Novartis, Pfizer und Roche mitgewirkt. A. W. war in Beiräten für Novartis, Lilly, Amgen, Pfizer, Roche, Tesaro, Eisai tätig und erhielt Honorare für Vortragstätigkeit von Novartis, Pfizer, Aurikamed, Roche, Celgene. R. W. hat persönliche Honorare/Reiseunterstützung erhalten von Agendia, Amgen, Aristo, AstraZeneca, Boeringer Ingelheim, Carl Zeiss, Celgene, Clinsol, Daiichi Sankyo, Eisai, Exact Sciences, Genomic Health, GlaxoSmithKline, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Nanostring, Novartis, Odonate, Paxman, Palleos, Pfizer, Pierre Fabre, Puma Biotechnology, Riemser, Roche, Sandoz/Hexal, Seattle Genetics, Tesaro Bio, Teva, Veracyte und Viatris.
References/Literatur
- 1.Jordan V C. Third annual William L. McGuire Memorial Lecture. “Studies on the estrogen receptor in breast cancer” – 20 years as a target for the treatment and prevention of cancer. Breast Cancer Res Treat. 1995;36:267–285. doi: 10.1007/BF00713399. [DOI] [PubMed] [Google Scholar]
- 2.Drugs.com Herceptin FDA Approval HistoryAccessed November 14, 2021 at:https://www.drugs.com/history/herceptin.html%23
- 3.Hortobagyi G N, Stemmer S M, Burris H A., III LBA17_PR – Overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib (RIB) Ann Oncol. 2021;32 05:S1283–S1346. [Google Scholar]
- 4.Slamon D J, Neven P, Chia S. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021;32:1015–1024. doi: 10.1016/j.annonc.2021.05.353. [DOI] [PubMed] [Google Scholar]
- 5.Tripathy D, Im S A, Colleoni M. Abstract PD2-04: Updated overall survival (OS) results from the phase III MONALEESA-7 trial of pre- or perimenopausal patients with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib. Cancer Res. 2021;81 (4 Supplement):PD2-04. [Google Scholar]
- 6.Cristofanilli M, Rugo H S, Im S A. Overall survival (OS) with palbociclib (PAL) + fulvestrant (FUL) in women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (ABC): Updated analyses from PALOMA-3. J Clin Oncol. 2021;39(15_suppl):1000. [Google Scholar]
- 7.Harbeck N, Rastogi P, Martin M. Adjuvant Abemaciclib Combined With Endocrine Therapy for High-Risk Early Breast Cancer: Updated Efficacy and Ki-67 Analysis From the monarchE Study. Ann Oncol. 2021;32:1571–1581. doi: 10.1016/j.annonc.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 8.OʼLeary B, Cutts R J, Huang X. Circulating Tumor DNA Markers for Early Progression on Fulvestrant With or Without Palbociclib in ER+ Advanced Breast Cancer. J Natl Cancer Inst. 2021;113:309–317. doi: 10.1093/jnci/djaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andre F, Su F, Solovieff N. Pooled ctDNA analysis of the MONALEESA (ML) phase III advanced breast cancer (ABC) trials. J Clin Oncol. 2020;38(15_suppl):1009. doi: 10.1016/j.annonc.2023.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Bidard F C, Callens C, Dalenc F. Prognostic impact of ESR1 mutations in ER+HER2-MBC patients prior treated with first line AI and palbociclib: An exploratory analysis of the PADA-1 trial. J Clin Oncol. 2020;38(15_suppl):1010. [Google Scholar]
- 11.European Medicines Agency Summary of opinion (post authorisation): Faslodex (fulvestrant)Accessed October 16, 2021 at:https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-faslodex_en-0.pdf
- 12.Ruíz-Borrego M, Guerrero-Zotano A, Bermejo B. Phase III evaluating the addition of fulvestrant (F) to anastrozole (A) as adjuvant therapy in postmenopausal women with hormone receptor-positive HER2-negative (HR+/HER2-) early breast cancer (EBC): results from the GEICAM/2006–10 study. Breast Cancer Res Treat. 2019;177:115–125. doi: 10.1007/s10549-019-05296-8. [DOI] [PubMed] [Google Scholar]
- 13.Robertson J F. Fulvestrant (Faslodex) – how to make a good drug better. Oncologist. 2007;12:774–784. doi: 10.1634/theoncologist.12-7-774. [DOI] [PubMed] [Google Scholar]
- 14.Menari Group Radius Health Menarini Group and Radius Health Announce Positive Phase 3 Topline Results from the EMERALD Trial Evaluating Elacestrant in Breast CancerAccessed October 22, 2021 at:https://ir.radiuspharm.com/news-releases/news-release-details/menarini-group-and-radius-health-announce-positive-phase-3
- 15.Snyder L B, Flanagan J J, Qian Y. Abstract 44: The discovery of ARV-471, an orally bioavailable estrogen receptor degrading PROTAC for the treatment of patients with breast cancer. Cancer Res. 2021;81 (13 Supplement):44. [Google Scholar]
- 16.ClinicalTrials.gov Phase III Study to Assess AZD9833+ CDK4/6 Inhibitor in HR+/HER2-MBC With Detectable ESR1 m Before Progression (SERENA-6) (SERENA-6)Accessed October 24, 2021 at:https://clinicaltrials.gov/ct2/show/NCT04964934
- 17.André F, Ciruelos E, Rubovszky G. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov Study to Assess the Efficacy and Safety of Alpelisib Plus Fulvestrant in Participants With HR-postitive (HR+), HER2-negative, Advanced Breast Cancer After Treatment With a CDK4/6 Inhibitor and an Aromatase Inhibitor. (EPIK-B5)Accessed October 24, 2021 at:https://clinicaltrials.gov/ct2/show/NCT05038735
- 19.Lux M P, Schneeweiss A, Hartkopf A D. Update Breast Cancer 2020 Part 5 – Moving Therapies From Advanced to Early Breast Cancer Patients. Geburtshilfe Frauenheilkd. 2021;81:469–480. doi: 10.1055/a-1397-7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SOLTI Study Group SOLTI launches HARMONIA, an international phase III study to identify the best therapeutic option for selected patients with aggressive HR+/HER2- advanced breast cancerAccessed October 24, 2021 at:https://www.gruposolti.org/ndp-harmonia-2021/?lang=en
- 21.Katzorke N, Rack B K, Haeberle L. Prognostic value of HER2 on breast cancer survival. J Clin Oncol. 2013;31(15_suppl):640. [Google Scholar]
- 22.Taran F A, Fasching P A, Volz B. Abstract P5-21-09: Overall survival of metastatic breast cancer patients – data from the PRAEGNANT breast cancer registry. Cancer Res. 2018;78:P5-21-09. [Google Scholar]
- 23.Murthy R K, Loi S, Okines A. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 24.Cortés J, Kim S, Chung W. LBA1 – Trastuzumab deruxtecan (T-DXd) vs. trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): Results of the randomized phase III DESTINY-Breast03 study. Ann Oncol. 2021;32(suppl_5):S1283–S1346. [Google Scholar]
- 25.Michel L L, Hartkopf A D, Fasching P A. Progression-Free Survival and Overall Survival in Patients with Advanced HER2-Positive Breast Cancer Treated with Trastuzumab Emtansine (T-DM1) after Previous Treatment with Pertuzumab. Cancers (Basel) 2020;12:3021. doi: 10.3390/cancers12103021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modi S, Saura C, Yamashita T. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Medicines Agency Enhertu – Annex I. Summary of Product CharacteristicsAccessed October 24, 2021 at:https://www.ema.europa.eu/en/documents/product-information/enhertu-epar-product-information_en.pdf
- 28.Saura Manich C, OʼShaughnessy J, Aftimos P F. LBA15 – Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physicianʼs choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol. 2021;3 05:S1283–S1346. [Google Scholar]
- 29.Hanka L J, Dietz A, Gerpheide S A. CC-1065 (NSC-298223), a new antitumor antibiotic. Production, in vitro biological activity, microbiological assays and taxonomy of the producing microorganism. J Antibiot (Tokyo) 1978;31:1211–1217. doi: 10.7164/antibiotics.31.1211. [DOI] [PubMed] [Google Scholar]
- 30.Yuan Z, Huang J J, Hua X. Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for metastatic breast cancer with hormone receptor-positive and HER2-positive: The sysucc-002 randomized clinical trial. J Clin Oncol. 2021;39(15_suppl):1003. doi: 10.1158/1078-0432.CCR-21-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rugo H S, Schmid P, Cescon D W. Abstract GS3-01: Additional efficacy endpoints from the phase 3 KEYNOTE-355 study of pembrolizumab plus chemotherapy vs. placebo plus chemotherapy as first-line therapy for locally recurrent inoperable or metastatic triple-negative breast cancer. Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium, San Antonio, TX, USA. December 8–11, 2020. [DOI]
- 32.Schmid P, Rugo H S, Adams S. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 33.Miles D, Gligorov J, André F. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32:994–1004. doi: 10.1016/j.annonc.2021.05.801. [DOI] [PubMed] [Google Scholar]
- 34.Denkert C, von Minckwitz G, Darb-Esfahani S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 35.Würfel F, Erber R, Huebner H. TILGen: A Program to Investigate Immune Targets in Breast Cancer Patients – First Results on the Influence of Tumor-Infiltrating Lymphocytes. Breast Care (Basel) 2018;13:8–14. doi: 10.1159/000486949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fasching P A, Denkert C, Benz S. Abstract PD5-08: Tumor immune-cell activity assessed by RNAseq is an independent predictor of therapy response and prognosis after neoadjuvant chemotherapy in HER2 negative breast cancer patients – An analysis of the GeparSepto trial. Proceedings of the 2019 San Antonio Breast Cancer Symposium, San Antonio, TX, USA. December 10–14, 2019. [DOI]
- 37.Sinn B V, Loibl S, Hanusch C A. Immune-related Gene Expression Predicts Response to Neoadjuvant Chemotherapy but not Additional Benefit from PD-L1 Inhibition in Women with Early Triple-negative Breast Cancer. Clin Cancer Res. 2021;27:2584–2591. doi: 10.1158/1078-0432.CCR-20-3113. [DOI] [PubMed] [Google Scholar]
- 38.Emens L A, Goldstein L D, Schmid P. The tumor microenvironment (TME) and atezolizumab + nab-paclitaxel (A+nP) activity in metastatic triple-negative breast cancer (mTNBC): IMpassion130. J Clin Oncol. 2021;39(15_suppl):1006. [Google Scholar]
- 39.Chen D S, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 40.Burstein M D, Tsimelzon A, Poage G M. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–1698. doi: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortes J, Cescon D W, Rugo H S. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 42.Rugo H S, Cortés J, Cescon D W. LBA16 – KEYNOTE‑355: Final results from a randomized, double-blind phase III study of first-line pembrolizumab + chemotherapy vs. placebo + chemotherapy for metastatic TNBC. Ann Oncol. 2021;32(suppl_5):S1283–S1346. [Google Scholar]
- 43.Turner N C, Ro J, André F. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 44.Turner N C, Slamon D J, Ro J. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 45.Goetz M P, Toi M, Campone M. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 46.Finn R S, Martin M, Rugo H S. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 47.Im S A, Lu Y S, Bardia A. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N Engl J Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 48.Tripathy D, Im S A, Colleoni M. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19:904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 49.Slamon D J, Neven P, Chia S. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2020;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 50.Slamon D J, Neven P, Chia S. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 51.Sledge G W, jr., Toi M, Neven P. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020;6:116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sledge G W, jr., Toi M, Neven P. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 53.Jhaveri K, Juric D, Yap Y S. A Phase I Study of LSZ102, an Oral Selective Estrogen Receptor Degrader, with or without Ribociclib or Alpelisib, in Patients with Estrogen Receptor–Positive Breast Cancer. Clin Cancer Res. 2021;27:5760–5770. doi: 10.1158/1078-0432.CCR-21-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maglakelidze M, Bulat I, Ryspayeva D. Rintodestrant (G1 T48), an oral selective estrogen receptor degrader, in combination with palbociclib for ER+/HER2– advanced breast cancer: Phase 1 results. J Clin Oncol. 2021;39(15_suppl):1063. [Google Scholar]
- 55.Aftimos P, Neven P, Pegram M. Abstract PS12-04: Rintodestrant (G1 T48), an oral selective estrogen receptor degrader in ER+/HER2- locally advanced or metastatic breast cancer: Updated phase 1 results and dose selection. Cancer Res. 2021;81 (4 Supplement):PS12-04. [Google Scholar]
- 56.Bardia A, Kaklamani V, Wilks S. Phase I Study of Elacestrant (RAD1901), a Novel Selective Estrogen Receptor Degrader, in ER-Positive, HER2-Negative Advanced Breast Cancer. J Clin Oncol. 2021;39:1360–1370. doi: 10.1200/JCO.20.02272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurvitz S A, Park Y H, Bardia A. LBA14 – Neoadjuvant giredestrant (GDC-9545) + palbociclib (palbo) vs. anastrozole (A) + palbo in post-menopausal women with oestrogen receptor-positive, HER2-negative, untreated early breast cancer (ER+/HER2– eBC): Interim analysis of the randomised, open-label, phase II coopERA BC study. Ann Oncol. 2021;32(suppl_5):S1283–S1346. doi: 10.1016/j.annonc.2021.08.2086. [DOI] [Google Scholar]
- 58.Liang J, Zbieg J R, Blake R A. GDC-9545 (Giredestrant): A Potent and Orally Bioavailable Selective Estrogen Receptor Antagonist and Degrader with an Exceptional Preclinical Profile for ER+ Breast Cancer. J Med Chem. 2021;64:11841–11856. doi: 10.1021/acs.jmedchem.1c00847. [DOI] [PubMed] [Google Scholar]
- 59.Metcalfe C, Ingalla E, Blake R A. Abstract P5-04–07: GDC-9545: A novel ER antagonist and clinical candidate that combines desirable mechanistic and pre-clinical DMPK attributes. Cancer Res. 2019;79 (4 Supplement):P5-04-07. [Google Scholar]
- 60.Chandarlapaty S, Linden H M, Neven P. 264P – AMEERA-1: Subgroup analyses of phase I/II study of amcenestrant (SAR439859), an oral selective estrogen receptor (ER) degrader (SERD), with palbociclib in postmenopausal women with ER+/human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (aBC) Ann Oncol. 2021;32(suppl_5):S457–S515. [Google Scholar]
- 61.Chandarlapaty S, Linden H M, Neven P. AMEERA-1: Phase 1/2 study of amcenestrant (SAR439859), an oral selective estrogen receptor (ER) degrader (SERD), with palbociclib (palbo) in postmenopausal women with ER+/ human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (mBC) J Clin Oncol. 2021;39(15):1058–1058. doi: 10.1200/JCO.2021.39.15_suppl.1058. [DOI] [Google Scholar]
- 62.Shomali M, Sun F, Besret L.Abstract 739: Preclinical and clinical activity of SAR439859, Amcenestrant, a next generation SERD Cancer Res 202181(Suppl.)739. 10.1158/1538-7445.AM2021-739 [DOI] [Google Scholar]
- 63.Scott J S, Moss T A, Stokes S. Abstract 5674: Discovery of AZD9833, an oral small molecule selective degrader of the estrogen receptor (SERD) Cancer Res. 2020;80 (16 Supplement):5674. [Google Scholar]
- 64.Bhagwat S V, Zhao B, Shen W. Abstract 1236: Preclinical characterization of LY3484356, a novel, potent and orally bioavailable selective estrogen receptor degrader (SERD) Cancer Res. 2021;81 (13 Supplement):1236. [Google Scholar]
- 65.Lim E, Beeram M, Prawira A. Abstract OT-09-03: EMBER: A phase 1a/b trial of LY3484356, a novel, oral selective estrogen-receptor degrader (SERD), in advanced ER+ breast cancer and endometroid endometrial cancer. Cancer Res. 2021;81 (4 Supplement):OT-09-03. [Google Scholar]
- 66.Jhaveri K L, Lim E, Hamilton E P. A first-in-human phase 1a/b trial of LY3484356, an oral selective estrogen receptor (ER) degrader (SERD) in ER+ advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): Results from the EMBER study. J Clin Oncol. 2021;39(15_suppl):1050. [Google Scholar]
- 67.Samatar A A, Li J, Hegde S. Abstract 4373: Discovery of ZN-c5, a novel potent and oral selective estrogen receptor degrader. Cancer Res. 2020;80 (16 Supplement):4373. [Google Scholar]
- 68.Osborne C, Richards D A, Wilks S T. Abstract PS11–26: A phase 1 study of D-0502, an orally bioavailable SERD, for advanced or metastatic HR-positive and HER2-negative breast cancer. Cancer Res. 2021;81 (4 Supplement):PS11–26. [Google Scholar]
- 69.Hamilton E P, Wang J S, Pluard T. Abstract PD8-06: Phase I/II trial of H3B-6545, a novel selective estrogen receptor covalent antagonist (SERCA), in estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer. Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium, San Antonio, TX, USA. December 8–11, 2020. [DOI]