Abstract
Aim This update of the interdisciplinary S3 guideline on the Diagnosis, Therapy and Follow-up of Cervical Cancer (AWMF Registry No. 032/033OL) was published in March 2021. This updated guideline was funded by German Cancer Aid (Deutsche Krebshilfe) as part of the German Guideline Program in Oncology. The guideline was coordinated by the German Society of Gynecology and Obstetrics ( Deutsche Gesellschaft für Gynäkologie und Geburtshilfe , DGGG) and the Working Group on Gynecological Oncology ( Arbeitsgemeinschaft Gynäkologische Onkologie , AGO) of the German Cancer Society ( Deutsche Krebsgesellschaft , DKG).
Method The process of updating the S3 guideline dating from 2014 was based on an appraisal of the available evidence using the criteria of evidence-based medicine, adaptations of existing evidence-based national and international guidelines or – if evidence was lacking – on a consensus of the specialists involved in compiling the update. After an initial review of the current literature was carried out according to a prescribed algorithm, several areas were identified which, in contrast to the predecessor version from September 2014, required new recommendations or statements which took account of more recently published literature and the appraisal of the new evidence.
Recommendations The short version of this guideline consists of recommendations and statements on the epidemiology, screening, diagnostic workup and therapy of patients with cervical cancer. The most important new aspects included in this updated guideline include the newly published FIGO classification of 2018, the radical open surgery approach for cervical cancers up to FIGO stage IB1, and use of the sentinel lymph node technique for tumors ≤ 2 cm. Other changes include the use of PET-CT, new options in radiotherapy (e.g., intensity-modulated radiotherapy, image-guided adaptive brachytherapy), and drug therapies to treat recurrence or metastasis.
Key words: guideline, cervical cancer, cervical intraepithelial neoplasia, therapy, follow-up
I Guideline Information
Publishing body
The German Guideline Program in Oncology of the Association of Scientific Medical Societies in Germany ( Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e. V. , AWMF), the German Cancer Society ( Deutsche Krebsgesellschaft e. V. , DKG) and German Cancer Aid ( Deutsche Krebshilfe , DKH).
Guidelines program of the DGGG, OEGGG and SGGG
For more information, please refer to the end of this guideline.
Guideline funding
This guideline was funded by German Cancer Aid ( Deutsche Krebshilfe , DKH) as part of the German Guideline Program in Oncology.
Citation format
Diagnosis, Therapy and Follow-up of Cervical Cancer. Guideline of the DGGG, DKG and DKH (S3-Level, AWMF Registry No. 032/033OL, May 2021) – Part 1 with Recommendations on Epidemiology, Screening, Diagnostics and Therapy. Geburtsh Frauenheilk 2022; 82: 139 – 180
Guideline documents
The complete long version, a version for patients and a slide version of this guideline, all of them in German, together with a list of the conflicts of interest of all of the authors are available on the homepage of the AWMF: https://www.awmf.org/leitlinien/detail/ll/032-033OL.html
The German-language version of the guideline is also available via the App of the German Guideline Program in Oncology: https://www.leitlinienprogramm-onkologie.de/app/
Guideline authors
The organizations listed in Tables 1 and 2 and their representatives were involved in the compilation of this guideline and are the authors of the guideline. The guideline was compiled with the direct involvement of a patient representative with voting rights. Physicians from the Oncology Competence Center of the National Association of Statutory Health Insurance Funds in Germany (GKV-Spitzenverband) and the Medical Advisory Service of the German Health Insurance Funds (MDK-Gemeinschaft) were involved in the preparation of this guideline in an advisory capacity on various socio-medical aspects. They did not participate in the voting on individual recommendations and are not responsible for the contents of this guideline.
Tab. 1 Lead and/or coordinating guideline author.
| Author | AWMF professional society |
|---|---|
| Prof. Dr. Matthias W. Beckmann | German Society of Gynecology and Obstetrics ( Deutsche Gesellschaft für Gynäkologie und Geburtshilfe e. V. , DGGG); Working Group on Gynecological Oncology ( Arbeitsgemeinschaft Gynäkologische Onkologie e. V. , AGO) |
| Prof. Dr. Tanja Fehm | German Society of Gynecology and Obstetrics (DGGG) |
Tab. 2 Contributing guideline authors.
| Author Mandate holder |
DGGG working group (AG)/AWMF/non-AWMF professional society/organization/association |
|---|---|
| Prof. Dr. Jan Menke | Imaging in Oncology Working Group ( Arbeitsgemeinschaft Bildgebung in der Onkologie , ABO) |
| Prof. Dr. Olaf Ortmann | Working Group of German Tumor Centers ( Arbeitsgemeinschaft Deutscher Tumorzentren , ADT) |
| PD Dr. Carmen Stromberger Proxy: Prof. Dr. Karin Oechsle |
Working Group for Palliative Medicine ( Arbeitsgemeinschaft für Palliativmedizin , APM) |
| Dipl.-Psych. Beate Hornemann Proxy: Dr. Friederike Mumm |
Working Group for Psychooncology ( Arbeitsgemeinschaft für Psychoonkologie , PSO) |
| Prof. Dr. Peter Mallmann (senior coordinator) Prof. Dr. Tanja Fehm (mandate holder) |
Working Group on Gynecological Oncology (AGO) |
| Prof. Dr. Christoph Grimm (mandate holder) Dr. Alina Sturdza (deputy) |
Working Group on Gynecological Oncology of the Austrian Society of Gynecology and Obstetrics ( Arbeitsgemeinschaft Gynäkologische Onkologie der Österreichischen Gesellschaft für Gynäkologie und Geburtshilfe , AGO der OEGGG) |
| PD Dr. Edward Wight (mandate holder) Dr. Kristina Loessl (deputy) |
Working Group on Gynecological Oncology of the Swiss Society of Gynecology and Obstetrics ( Arbeitsgemeinschaft Gynäkologische Onkologie der Schweizer Gesellschaft für Gynäkologie und Geburtshilfe , AGO der SGGG) |
| Prof. Dr. Michael Golatta (until 03/20) | Working Group on Gynecological Radiology ( Arbeitsgemeinschaft für gynäkologische Radiologie , AGR) |
| Dr. Volker Hagen | Working Group on Internal Oncology ( Arbeitsgemeinschaft Internistische Onkologie , AIO) |
| Dr. Timm Dauelsberg (mandate holder) Prof. Dr. Ingo Diel (deputy) |
Working Group on Oncological Rehabilitation and Social Medicine ( Arbeitsgemeinschaft Onkologische Rehabilitation und Sozialmedizin , AGORS) |
| Prof. Dr. Ingo Diel | Working Group on Supportive Measures in Oncology ( Arbeitsgemeinschaft Supportive Maßnahmen in der Onkologie , AGSMO) |
| Prof. Dr. Karsten Münstedt | Working Group on Prevention and Integrative Oncology ( Arbeitsgemeinschaft Prävention und integrative Onkologie , PRIO) |
| Prof. Dr. Eberhard Merz | Working Group on Ultrasound Diagnostics in Gynecology and Obstetrics, ( Arbeitsgemeinschaft für Ultraschalldiagnostik in Gynäkologie und Geburtshilfe , ARGUS) |
| Prof. Dr. Dirk Vordermark (mandate holder) Prof. Dr. Katja Lindel (deputy) |
Working Group on Radiological Oncology ( Arbeitsgemeinschaft Radiologische Onkologie , ARO) |
| Prof. Dr. Christian Wittekind | Working Group on Tumor Classification in Oncology ( Arbeitsgemeinschaft Tumorklassifikation in der Onkologie , ATO) |
| PD Dr. Volkmar Küppers (mandate holder) Prof. Dr. Ralph Lellé (deputy) |
Working Group on Cervical Pathology and Colposcopy ( Arbeitsgemeinschaft Zervixpathologie und Kolposkopie , AG-CPC) |
| Prof. Dr. med. Klaus Joachim Neis (until August 31, 2019) Prof. Dr. Henrik Griesser (from September 1, 2019) |
Professional Association of German Physicians Working in Cytology ( Arbeitsgemeinschaft zytologisch tätiger Ärzte in Deutschland , AZÄD) |
| Birgit Pöschel | Federal Association of German Pathologists ( Bundesverband Deutscher Pathologen e. V. , BDP) |
| Dr. Manfred Steiner (mandate holder) Dipl.-Med. Ulrich Freitag (deputy) |
Professional Association of Gynecologists in Germany ( Berufsverband der Frauenärzte , BVF) |
| Tobias Gilster | Professional Association of Gynecological Oncologists in Private Practice in Germany ( Berufsverband Niedergelassener Gynäkologischer Onkologen in Deutschland , BNGO) |
| PD Dr. Alexander Schmittel | Professional Association of Hematologists in Private Practice ( Berufsverband der niedergelassenen Hämatologen , BNHO) |
| Prof. Dr. Michael Friedrich | Federal Working Group of Leading Doctors in Gynecology and Obstetrics ( Bundesarbeitsgemeinschaft Leitender Ärztinnen und Ärzte in der Frauenheilkunde und Geburtshilfe , BLFG) |
| Heidemarie Haase (mandate holder) Marion Gebhardt (deputy) |
Federal Association of Womenʼs Self-help After Cancer ( Bundesverband Frauenselbsthilfe nach Krebs , FSH) |
| Prof. Dr. Ludwig Kiesel | German Society of Endocrinology ( Deutsche Gesellschaft für Endokrinologie , DGE) |
| Prof. Dr. Matthias W. Beckmann (guideline coordinator) Prof. Dr. Christian Dannecker (mandate holder) |
German Society of Gynecology and Obstetrics (DGGG) |
| Prof. Dr. Michael Reinhardt (mandate holder) Prof. Dr. Michael Kreißl (deputy) |
German Society for Nuclear Medicine ( Deutsche Gesellschaft für Nuklearmedizin , DGN) |
| Dr. Marianne Kloke | German Society for Palliative Medicine ( Deutsche Gesellschaft für Palliativmedizin , DGP) |
| Prof. Dr. Lars-Christian Horn | German Society for Pathology ( Deutsche Gesellschaft für Pathologie , DGP) |
| Prof. Dr. Regina Wiedemann | German Society for Nursing Science ( Deutsche Gesellschaft für Pflegewissenschaft , DGP) |
| Prof. Dr. Simone Marnitz-Schulze | German Society for Radiooncology ( Deutsche Gesellschaft für Radioonkologie , DEGRO) |
| Prof. Dr. Eberhardt Merz | German Society for Ultrasound in Medicine ( Deutsche Gesellschaft für Ultraschall in der Medizin e. V. , DEGUM) |
| Prof. Dr. Anne Letsch | German Society for Hematology and Oncology ( Deutsche Gesellschaft für Hämatologie und Onkologie , DGHO) |
| Dr. Isabella Zraik | German Society for Urology ( Deutsche Gesellschaft für Urologie , DGU) |
| Dr. Bernhard Mangold (mandate holder) Dr. Jochen Möckel (deputy) |
German Society for Cytology ( Deutsche Gesellschaft für Zytologie , DGZ) |
| PD Dr. Céline Alt | German X-Ray Society ( Deutsche Röntgengesellschaft , DRG) |
| Prof. Dr. Pauline Wimberger | European Society for Gynaecological Oncology (ESGO) |
| Prof. Dr. Peter Hillemanns | Complementary Guideline on Screening, Certification Commission for Gynecological Cancer Centers (Zertifizierungskommission gynäkologischer Krebszentren) |
| Kerstin Paradies | Conference on Oncology Nursing and Pediatric Nursing ( Konferenz onkologischer Kranken- und Kinderkrankenpflege , KOK) |
| Prof. Dr. Alexander Mustea | North-Eastern German Society for Gynecological Oncology ( Nord-Ostdeutsche Gesellschaft für Gynäkologische Onkologie , NOGGO) |
| Prof. Dr. Dominik Denschlag | Study Group of the Gynecological Oncology Working Group ( Studiengruppe der Arbeitsgemeinschaft Gynäkologische Onkologie , AGO) |
| Ulla Henscher (mandate holder) Reina Tholen (deputy) |
Central Association of Physiotherapists ( Zentralverband der Physiotherapeuten/Krankengymnasten, ZVK) |
The Office of the German Guideline Program in Oncology and the AWMF provided the methodological supervision ( Table 3 ). The guideline authors were supported by the project team and the Guidelines Office ( Table 4 ).
Tab. 3 Methodological supervision.
| Name | City |
|---|---|
| Dr. Markus Follmann MPH M. Sc. (Office of the German Guideline Program in Oncology – German Cancer Society) | Berlin |
| Dipl.-Soz. Wiss. Thomas Langer (Office of the German Guideline Program in Oncology – German Cancer Society) | Berlin |
| Dr. Monika Nothacker MPH (Deputy Head – AWMF Institute for Medical Knowledge Management) | Berlin |
| PD Dr. Simone Wesselmann, MBA (German Cancer Society – certification, quality indicators) | Berlin |
| Biologist Gregor Wenzel | Berlin |
Tab. 4 Guidelines Office and project team.
| Name | City |
|---|---|
| Dr. Martin C. Koch (Guidelines Office) | Erlangen |
| Dr. Frederik A. Stübs (Guidelines Office) | Erlangen |
| Dr. Anna K. Dietl (project team) | Erlangen |
| Anna Sevnina (project team) | Erlangen |
| Dr. Franziska Mergel (project team) | Erlangen |
| PD Dr. Laura Lotz (project team) | Erlangen |
| PD Dr. Carolin C. Hack (project team) | Erlangen |
| Dr. Anne Ehret (project team) | Düsseldorf |
| Dr. Daniel Gantert (project team) | Düsseldorf |
| Dr. Franca Martignoni (project team) | Düsseldorf |
Abbreviations
- AIS
adenocarcinoma in situ
- CAM
complementary and alternative medicine
- CHT
chemotherapy
- CI
confidence interval
- CIN
cervical intraepithelial neoplasia
- CT
computed tomography
- EC
expert consensus
- FIGO
Fédération Internationale de Gynécologie et dʼObstétrique (International Federation of Gynecology and Obstetrics)
- GKV
statutory health insurance in Germany (Gesetzliche Krankenversicherung)
- GoR
grade of recommendation
- HE
hysterectomy
- HPV
human papillomavirus
- ICG
indocyanine green
- IECC
International Endocervical Adenocarcinoma Classification
- LoE
level of evidence
- MDK
Medical Advisory Service of the German Association of Health Insurance Funds (Medizinischer Dienst der Krankenkassen)
- MRI
magnetic resonance imaging
- NECC
neuroendocrine cervical carcinoma
- Pap
cervical cytology by Pap smear
- PET
positron emission tomography
- R(CH)T
simultaneous radio(chemo)therapy
- SMILE
stratified mucin-producing intraepithelial lesion
- SNB
sentinel lymph node biopsy
- STIKO
German Standing Committee on Vaccinations at the Robert Koch Institute
- TNM
tumor–nodes–metastasis
- UICC
Union internationale contre le cancer
II Guideline Application
Purpose and objectives
The rationale for this guideline was problems relating to security of care as well as the fact that mortality and morbidity rates have not decreased much in the last 15 years, and that the current therapies administered to patients with cervical cancer can vary greatly. The aim of this updated guideline remains the same as that of the previous version of 2014. This guideline on Diagnosis, Therapy and Follow-up of Cervical Cancer is an evidence- and consensus-based instrument for the care of patients with cervical cancer. It provides patients with scientific, up-to-date, economically viable procedures for diagnosis, therapy, follow-up and rehabilitation which are appropriate for the various stages of disease. The current version of the guideline aims to provide a basis for clinical decision-making on the appropriate treatment. The guideline also incorporates the concept of shared decision-making.
Targeted areas of patient care
The area covered by the guideline ranges from diagnosis to therapy and the follow-up of patients with cervical cancer and includes patients with microinvasive lesions/high-grade precursor lesions (but excludes patients with early precursor lesions/preinvasive lesions). The guideline has an intersectoral scope. It covers both outpatient and in-patient care as well as rehabilitation.
Target user groups
This S3 guideline addresses all patients with cervical cancer (including microinvasive lesions/high-grade precursor lesions but excluding early precursor lesions/preinvasive lesions) and their families.
Intended audience
The recommendations of the guideline are for all physicians and professional groups involved in the outpatient and/or in-patient care and rehabilitation of patients with cervical cancer.
The guideline is also intended for
medical and scientific specialist societies and professional associations,
special interest groups representing women (womenʼs health organizations, patient organizations and self-help organizations),
quality assurance institutions and federal and state-level projects (e.g., AQUA, ADT, IQWiG, GEKID, gesundheitsziele.de, IQTIG),
health policy institutions and decision-making bodies at federal and state levels,
certification institutions (e.g., DKG)
funding bodies
Adoption and period of validity
The validity of this guideline was confirmed by the executive boards/heads of the participating medical professional societies, working groups, organizations and associations as well as by the boards of the DGGG, SGGG and OEGGG and the DGGG/OEGGG/SGGG guidelines commission and was thus approved in its entirety. This guideline is valid until October 2025. Because of the contents of this guideline, this period of validity is only an estimate. If changes are urgently required, the guideline can be updated earlier; if the information in the guideline still represents the current state of knowledge, then the guidelineʼs period of validity can be extended.
III Methodology
Basic principles
The method used to prepare this guideline was determined by the class to which this guideline was assigned. The AWMF Guidance Manual (version 1.0) has set out the respective rules and requirements for different classes of guidelines. Guidelines are differentiated into lowest (S1), intermediate (S2), and highest (S3) class. The lowest class is defined as consisting of a set of recommendations for action compiled by a non-representative group of experts. In 2004, the S2 class was divided into two subclasses: a systematic evidence-based subclass (S2e) and a structural consensus-based subclass (S2k). The highest S3 class combines both approaches. This guideline has been classified as: S3.
The methodological approach used when compiling this guideline is described in the guideline report. The guideline report is freely available on the homepage of the German Guideline Program in Oncology ( https://www.leitlinienprogramm-onkologie.de/leitlinien/zervixkarzinom/ ) and the homepage of the AWMF ( http://www.awmf.org/ ).
Grading of evidence based on SIGN
To classify the risk of bias in identified studies, this guideline used the system of the Scottish Intercollegiate Guidelines Network (SIGN) shown in Table 5 (cf. https://www.sign.ac.uk/media/1050/sign50_2019.pdf ).
Tab. 5 Classification of levels of evidence according to SIGN.
| Level | Description |
|---|---|
| 1++ | High-quality meta-analyses, systematic review of RCTs, or RCTs with a very low risk of bias. |
| 1+ | Well-conducted meta-analyses, systematic reviews, or RCTs with a low risk of bias. |
| 1− | Meta-analyses, systematic reviews, or RCTs with a high risk of bias. |
| 2++ | High-quality systematic reviews of case-control or cohort studies, or High-quality case-control or cohort studies with a very low risk of confounding, bias or “chance” and a high probability that the relationship is causal. |
| 2+ | Well-conducted case-control studies or cohort studies with a low risk of confounding, bias or “chance” and a moderate probability that the relationship is causal. |
| 2− | Case-control studies or cohort studies with a high risk of confounding, bias or “chance” and a significant risk that the relationship is not causal. |
| 3 | Non-analytical studies, e.g., case reports, case series |
| 4 | Expert opinion |
Grading of recommendations
In this guideline, the level of evidence (using the SIGN classification) in the underlying studies and, when making recommendations, the strength of the recommendation (grade of recommendation) is given for all evidence-based statements and recommendations. As regards the strength of recommendations, the guideline differentiates between three grades of recommendation ( Table 6 ), and the level of recommendation is reflected in the wording used in the respective recommendation:
Tab. 6 Grade of recommendation.
| Symbol | Description of level of obligation to comply with the recommendation | Term |
|---|---|---|
| A | Strong recommendation | must/must not |
| B | Simple recommendation | should/should not |
| 0 | Open recommendation, not binding | may/may not |
In principle, the grade of recommendation is based on the strength of the available evidence. For example, if there is a high level of evidence (provided by high-quality meta-analyses/systematic reviews of RCTs or several methodologically high-quality RCTs), then a strong recommendation is given (grade of recommendation: A, “must”).
But the following criteria are also taken into account and can result in the level of recommendation being upgraded or downgraded:
Consistency of study results
Example: The effect estimates for study results diverge, showing no consistent tendency.
Clinical relevance of endpoints and effect sizes
Example: Although studies with results which point in a specific direction are available, the importance of the selected endpoints and/or effect sizes are not considered relevant.
Benefit-to-risk ratio
Example: Although the intervention has a proven benefit, it is also associated with a relevant harm which mitigates against giving an unqualified recommendation.
Ethical obligations
Example: Downgrading: For ethical reasons, an intervention with a proven benefit cannot be offered without restrictions. Upgrading: Strong recommendation based on case-control studies, because an RCT cannot be carried out for ethical reasons.
Patient preferences
Example: An intervention with a proven benefit is not strongly recommended as it is rejected by patients who consider it to be onerous or not feasible.
Applicability, practicability of care
Example: An intervention with proven positive effects cannot be recommended because it is not available in regional healthcare systems for structural reasons.
Statements
Statements are expositions or explanations of specific facts, circumstances or problems without directly calling to action. They are adopted using the same approach used for recommendations and following a formal consensus procedure, and they may be based either on study results or on expert opinions.
Achieving consensus and level of consensus
At structured NIH-type (S2k/S3 level) consensus conferences, authorized participants attending the conference voted on draft statements and recommendations. Conferences are structured as follows: a recommendation is presented; participants can ask about the contents of the recommendation; amendments can be proposed; all proposed amendments are voted on. If a consensus (> 75% of votes) cannot be reached, there is another round of discussions, followed by another vote. At the end of the session, the strength of the consensus is determined based on the number of persons who participated in the session ( Table 7 ).
Tab. 7 Strength of consensus based on the extent of consensus.
| Symbols | Level of consensus | Extent of agreement in percent |
|---|---|---|
| +++ | Strong consensus | > 95% of participants agree |
| ++ | Consensus | > 75 – 95% of participants agree |
| + | Majority agreement | > 50 – 75% of participants agree |
| – | No consensus | < 51% of participants agree |
Expert consensus
When the guideline authors decide to make statements/recommendations based on the expert consensus of the guideline authors, such statements/recommendations are identified by the phrase “expert consensus” (EC). No symbols are used to grade such recommendations; the strength of the expert consensus is indicated by the wording used (must/should/may) in accordance with the grading shown in Table 6 .
IV Guideline
1 Epidemiology
Data from the Robert Koch Institute and the GEKID from 2019 show that in 2016, 4380 women developed cervical cancer and 1562 women died from cervical cancer in Germany. Compared to the figures for 2002, the incidence of cervical cancer (6500 vs. 4380) has declined significantly although the number of deaths (1700 vs. 1562) from cervical cancer has decreased only slightly. It is expected that the general administration of HPV vaccinations to girls between the ages of 9 and 14 years in 2007 will reduce future incidence and mortality rates even more. Since June 2018, the STIKO has also recommended that boys between the ages of 9 to 14 years be vaccinated. The herd protection obtained by vaccinating a sufficient number of people should further reduce the incidence and mortality of cervical cancer. In 2016, the relative 5-year survival rate for cervical cancer was 67% and the 10-year survival rate was 63% 1 , 2 . The age distribution showed a peak between the ages of 40 and 59 years. The mean age at first diagnosis of cervical cancer is currently 55 years and has dropped by 15 years in the last 25 years 1 . Further information on regional differences, histological subtypes and risk factors are available in the long (English-language) version of the guideline.
2 Prevention and Screening
Because complementary S3 guidelines are already available, this guideline did not develop its own recommendations on prevention and screening. Interested parties should refer to the relevant S3 guidelines “Vaccination for the Prevention of HPV-associated Neoplasia (AWMF Registry No. 082/002)” and “Prevention of Cervical Cancer (AWMF Registry No. 015/027OL)” 3 , 4 .
3 Patient Information
3.1 Informing patients and substance of the information
3.1.1 Informing the patient of the diagnosis
3.1.2 Informing the patient about treatment options
3.1.2.1 Information provided to patients with metastatic or recurrent cervical cancer
4 Diagnosis
The new FIGO classification was introduced in 2018. This new classification now also incorporates findings obtained with radiographic imaging or biopsies to determine the stage of disease (s. Table 19 on p. 261 of the long version of the S3 guideline). In addition, paraaortic lymph nodes are now classified as pN1 and no longer as pM1. Previously, the FIGO classification of cervical cancer consisted of purely clinical staging based on bimanual examination of the patient by a gynecologist. Because the (new) FIGO classification is not congruent with that of the (old) TNM, the old FIGO version is still used in this updated version of the guideline. There are currently no data from clinical studies using the new classification.
4.1 Diagnostic workup as the basis for therapeutic decisions
4.1.1 Images agreed upon by the guideline authors on the diagnostic workup and staging as the basis for therapeutic decisions
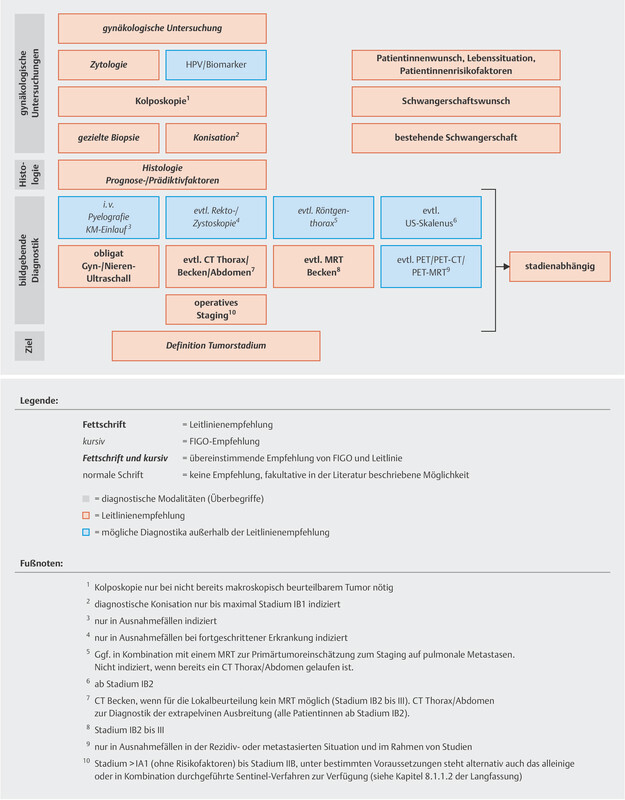
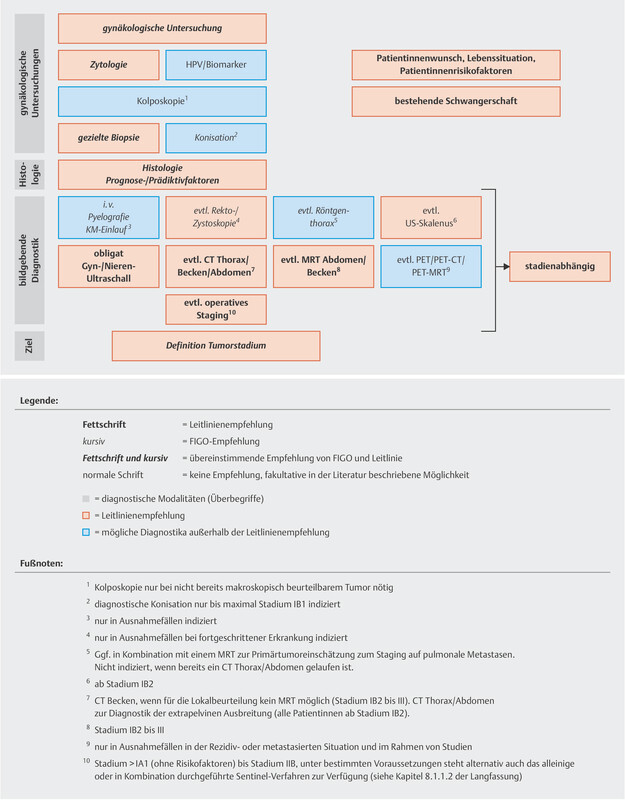
The guideline authors agreed on two graphs outlining the diagnostic workup and staging of lesions, as this affects the therapeutic decisions regarding lesions classified as FIGO ≤ IIB or > IIB ( Figs. 1 and 2 ).
Abb. 1.
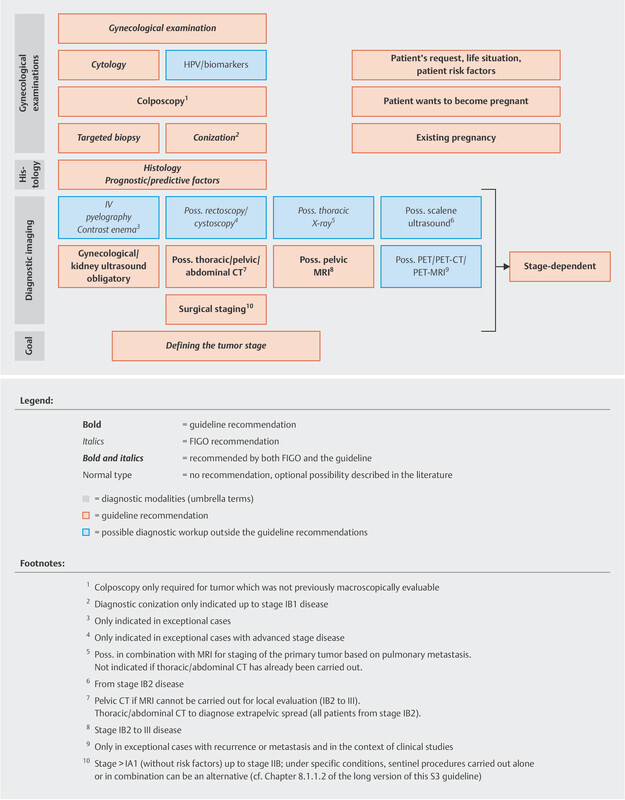
Diagnostic workup and staging as the basis for therapeutic decision for lesions which are ≤ FIGO stage IIB (reviewed in 2021). [rerif]
Abb. 2.
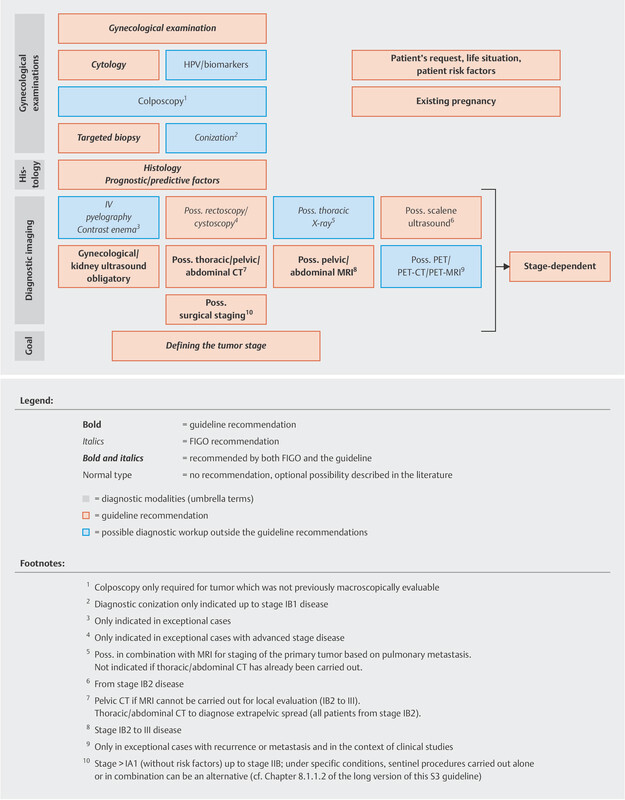
Diagnostic workup and staging as the basis for therapeutic decision for lesions which are > FIGO stage IIB (reviewed in 2021). [rerif]
4.1.2 Recommendations for the diagnostic workup
5 Pathology
5.1 Classification of invasive cervical cancers
5.1.1 Tumor typing
The majority of invasive cervical cancers are squamous cell carcinomas (~ 80%) and adenocarcinomas (~ 5 – 20%) 15 . Other tumor entities are rare. Neuroendocrine (large or small cell) carcinoma and non-HPV-associated adenocarcinoma with the exception of clear cell adenocarcinoma are tumor types which have a particularly unfavorable prognosis.
5.1.2 Staging of cervical cancer
Postoperative staging is based on the TNM classification 16 . Lesions are differentiated into micro- and macroinvasive cancers.
5.2 Tissue preparation
5.2.1 Diagnostic biopsies
5.2.2 Conization
5.2.3 Trachelectomy
5.2.4 Specimens after radical hysterectomy
5.2.5 Lymphadenectomy specimens
5.2.6 Sentinel lymph nodes
5.3 Morphological prognostic factors
Established prognostic factors for cervical cancer are tumor stage and evidence of pelvic or paraaortic lymph node metastasis 17 – 23 . For more information on resection margins, tumor size, tumor-type histology, HPV status, IECC classification, venous infiltration, perineural sheath infiltration, depth of infiltration, micro- and macrometastasis, immunohistochemical ultrastaging, molecular markers, TCGA classification, mutation load and PD-L1 expression, see Chapter 7.3 of the long version of the guideline.
6 Basic Principles of Therapy
When this guideline was being updated, the new FIGO classification was already available to the guideline group. The underlying data is based on the previous classification. Therefore, all tumor stages refer to the old FIGO classification.
6.1 Primary therapy
Primary therapy consists of either surgery or radiochemotherapy. Surgical therapy is recommended for patients with FIGO stage IIA disease or lower. Primary radio(chemo)therapy is predominantly used to treat later stages of disease with more extensive infiltration (from stage IIB), disease with lymph node involvement or inoperable disease. Radio(chemo)therapy is also recommended when several preoperatively determined risk factors are present (i.e., lymphangitis [L1], R1, G3 [its importance is dubious and only in combination with two additional risk factors], neuroendocrine carcinoma, tumor > 4 cm [stage], or histology positive for lymph node metastasis). The choice of treatment for stage IV disease should be made on an individual basis. In general, when deciding on the appropriate therapy, no differences are made between the different histological tumor entities (e.g., adenocarcinoma or squamous cell carcinoma). Although the diagnosis of stage IB and II disease is different prior to therapy, surgery and simultaneous radio(chemo)therapy have equivalent long-term outcomes despite the differences in patterns of recurrence and the side-effects profiles of the therapies.
6.1.1 Surgery – hysterectomy and lymphadenectomy
6.1.1.1 Uterine surgery
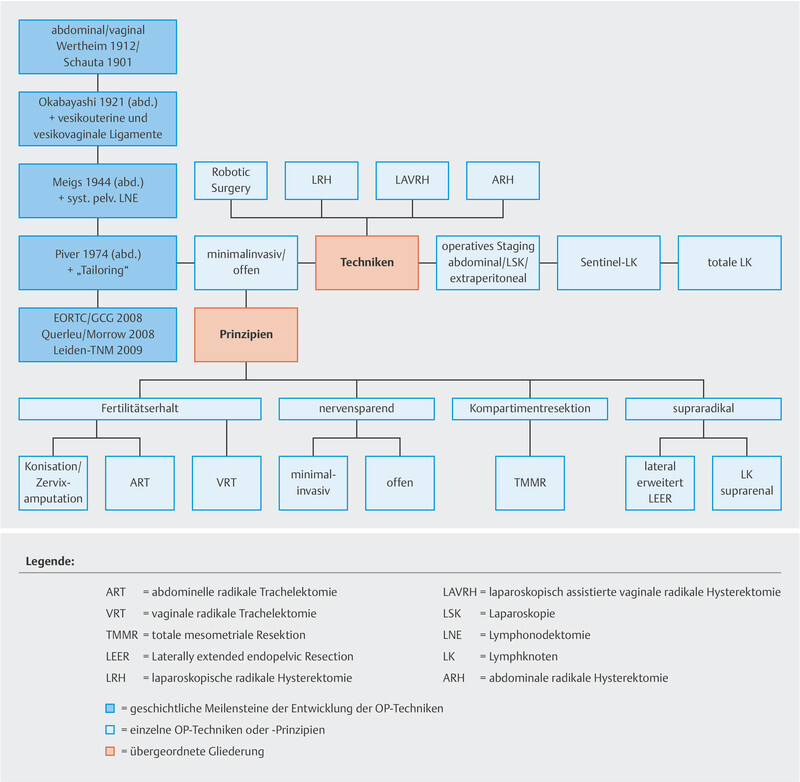
Different surgical techniques and principles can be used for cervical and uterine surgery (conization, simple or radical trachelectomy, simple or radical hysterectomy) ( Fig. 3 ). The Piver-Rutledge classification dating from 1974 is the standard classification to rank radical hysterectomies 24 .
Abb. 3.
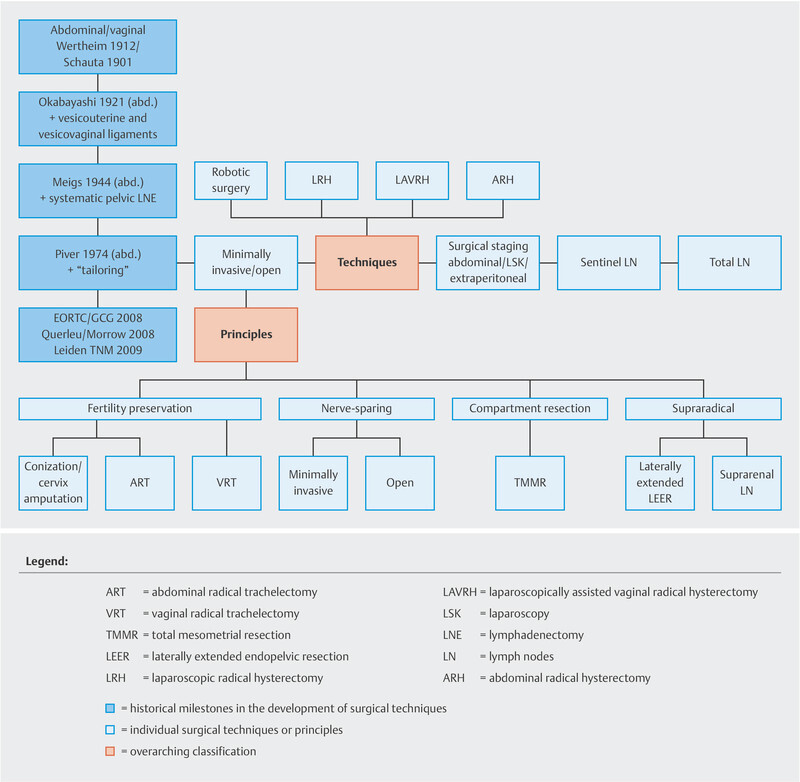
Surgical therapy techniques and principles (reviewed 2021). [rerif]
6.1.1.2 Lymphadenectomy or resection of sentinel lymph nodes to identify tumor stages
In this updated guideline, the use of sentinel lymph nodes as a surgical staging concept for tumors ≤ 2 cm and for pT1a1 and L1 disease was based on new data. It has also been shown that patent blue and radioactive marking are equivalent to intraoperative marking with indocyanine green (ICG).
6.1.1.3 Drainage placement after lymphadenectomy
6.1.2 Radio(chemo)therapy
Radio(chemo)therapy may be administered as neoadjuvant, primary or adjuvant therapy. Standard radio(chemo)therapy is carried out using cisplatin as a radiosensitizer. It is important to differentiate between percutaneous radiotherapy and brachytherapy. Which areas (pelvic/paraaortic) are selected for irradiation depends on histological verification of lymph node involvement and not on any imaging-based presumption of affected lymph nodes. Standard radio(chemo)therapy for stage IIb disease and above as well as lower stages of disease with histologically verified risk factors consists of primary, initially percutaneous, irradiation of the primary tumor and pelvic lymph nodes combined with cisplatin-based chemotherapy followed by brachytherapy.
6.2 Neoadjuvant drug treatment
6.3 Adjuvant therapy
6.3.1 Adjuvant therapy after primary surgery
6.3.2 Adjuvant therapy after primary radiochemotherapy
After primary radio(chemo)therapy has been carried out, the option to perform secondary hysterectomy or expanded chemotherapy may be discussed in specific situations (see Statement).
6.4 Therapy of locally limited cervical cancer ≤ FIGO stage IIA
6.5 Stage-dependent therapy
6.5.1 Standard therapy for invasive cervical cancer
6.5.1.1 FIGO stage IA (synonyms: early stromal invasion, microcarcinoma or microinvasive carcinoma)
6.5.1.2 FIGO stage IB1 and IIA1
6.5.1.3 FIGO stage IB2, IIA2 and IIB
6.5.1.4 FIGO stage III
6.5.1.5 FIGO stage IV
7 Surgical Treatment
Based on new evidence from the LACC trial, the recommendation for the surgical approach to be used for radical hysterectomy in patients with cervical cancer was changed 31 . A clear preference was given to open procedures as opposed to laparoscopic approaches. The relevant recommendation was revised. The guideline authors rated prophylactic salpingectomy carried out in combination with planned hysterectomy as positive. The importance of secondary hysterectomy after radiochemotherapy is still not clear.
7.1 Surgical approach
7.2 Approach after primary radio(chemo)therapy
8 Radiotherapy
After some revision, the recommendations for primary radiochemotherapy including external radiotherapy, simultaneous cisplatin-based chemotherapy and brachytherapy were confirmed. The grading of recommendations on the use of intensity-modulated radiotherapy techniques and for MRI-guided treatment planning for brachytherapy in the context of primary radiochemotherapy was increased as new data is now available which shows the clinical benefits of these techniques.
8.1 Radiochemotherapy
8.1.1 Radiotherapy techniques (percutaneous radiotherapy)
8.1.2 Brachytherapy technique in primary combined radiochemotherapy
8.1.3 Indications for primary radiotherapy or radio(chemo)therapy
8.1.4 Adjuvant radio(chemo)therapy
8.1.5 Adjuvant chemotherapy after completing radio(chemo)therapy
8.1.6 Neoadjuvant radio(chemo)therapy
8.1.7 Ovarian preservation and fertility
8.1.8 Anemia during radiochemotherapy
8.1.9 Hyperthermia to treat cervical cancer

Footnotes
Conflict of Interest/Interessenkonflikt The conflicts of interest of all the authors are listed in the long German-language version of the guideline report./Die Interessenkonflikte der Autoren sind im Leitlinienreport aufgelistet.
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 4.1. reviewed 2021 |
Consensus-based statement Recommendations on the prevention and early detection of cervical carcinoma are presented in the Level 3 guidelines (S3) “Vaccine Prevention of HPV-Associated Neoplasia” (AWMF register no. 082/002) and “Prevention of Cervical Carcinoma” (AWMF register no. 015/027OL). |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 5.1. reviewed 2021 |
Consensus-based recommendation High-quality and pertinent information materials (using print or Internet media) must be produced in accordance with defined quality criteria for health information and made available to patients, to support them in independent decision-making for or against medical measures by providing generally comprehensible risk information (e.g., with details of absolute risk reductions). |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 5.2. reviewed 2021 |
Consensus-based recommendation The patient must be informed that her partner or a relative can be invited to be included in the discussion(s). |
EC | ||
| 5.3. reviewed 2021 |
Consensus-based recommendation During the medical discussion, the patientʼs individual preferences, needs, worries and anxieties must be identified and taken into account. If a patient needs several discussions for the purpose, an offer of further discussions must be available. |
EC | ||
| 5.4. modified 2021 |
Consensus-based recommendation Providing the patient with medical information is primarily a task for the attending physician, but for specific topics it should be provided by other professional groups such as nurses, psycho-oncologists, etc. |
EC | ||
| 5.5. modified 2021 |
Consensus-based recommendation Information must be communicated and provided to the patient as early as possible on the basis of the following basic principles of patient-centered communication allowing participatory decision-making:
|
EC | ||
| 5.6. reviewed 2021 |
Consensus-based recommendation The patient should be offered psychosocial and psycho-oncological support for psychological, sexual, and relationship problems. |
EC | ||
| 5.7. reviewed 2021 |
Consensus-based recommendation The patient must be informed about the option of contacting self-help groups. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 5.8. reviewed 2021 |
Consensus-based recommendation In accordance with the “Law on Improving Patientsʼ Rights,” the patient must be informed about all of the treatment options described in this guideline that are relevant to her and about their prospects of success and possible effects. In particular, effects on her physical appearance, sexual life, urinary and rectal continence, and aspects of female identity (self-image, fertility) should be mentioned. |
EC | ||
| 5.9. reviewed 2021 |
Consensus-based statement Principles, intended treatment goals, duration and implementation of the individual treatment measures Surgical treatment measures:
Late sequelae of the disease and therapy and ways of treating them Complementary therapy:
|
EC | ||
| 5.10. reviewed 2021 |
Consensus-based recommendation The patient must be informed about the patient guideline on diagnosis, treatment, and follow-up for patients with cervical carcinoma. |
EC | ||
| 5.11. reviewed 2021 |
Consensus-based statement Cervical carcinoma is not an emergency case. The patient can and must be given sufficient time for her own decision-making processes. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 5.12. reviewed 2021 |
Consensus-based recommendation The following points can be mentioned as forming the content of a discussion in the palliative situation: Aims of palliative medical therapy (alleviating suffering, treatment of pain – foremost goal: the patientʼs quality of life)
|
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 6.1. modified 2021 |
Consensus-based recommendation Vaginal ultrasonography must be used for clinical imaging to establish the extent of local tumor spread, and renal ultrasonography to exclude urinary transport disturbance. |
EC | ||
| 6.2. modified 2021 |
Evidence-based recommendation Patients with histologically confirmed cervical carcinoma from FIGO stage IB2 to III inclusive should undergo pelvic MRI for assessment of locoregional tumor spread. Patients who are unable to undergo pelvic MRI for technical reasons should have a pelvic CT. |
B | 1+ | 5 – 7 |
| 6.3. modified 2021 |
Consensus-based recommendation Starting from FIGO IB2 to III, patients in whom pelvic MRI cannot be carried out for technical reasons should undergo locoregional imaging of the pelvis for staging purposes during staging CT examinations of the chest, abdomen, and pelvis. |
EC | ||
| 6.4. new 2021 |
Consensus-based recommendation Patients in FIGO stage IVA who are unable to undergo pelvic MRI for technical reasons should receive locoregional imaging staging of the pelvis as part of staging CT of the chest/abdomen/pelvis. |
EC | ||
| 6.5. modified 2021 |
Consensus-based recommendation Patients with histologically confirmed cervical carcinoma FIGO stage IB2 or above should undergo chest/abdominal/pelvic CT for assessment of tumor spread. |
EC | ||
| 6.6. reviewed 2021 |
Consensus-based recommendation If a tumor of the vaginal part of the cervix cannot be clearly assessed macroscopically, a differential colposcopy and targeted biopsy must be carried out. |
EC | ||
| 6.7. reviewed 2021 |
Consensus-based recommendation The histologically confirmed tumor stage should be the basis for interdisciplinary treatment decision-making at the tumor conference. |
EC | ||
| 6.8. modified 2021 |
Evidence-based recommendation PET-CT should not be used for treatment planning in primary cervical carcinoma. |
B | 2+ | 6 , 8 – 11 |
| 6.9. modified 2021 |
Evidence-based recommendation When a local procedure (radiochemotherapy or exenteration) is being considered for treatment of a recurrence, PET-CT should be carried out to exclude lymph-node metastases and distant metastases. |
B | 2+ | 6 , 12 – 14 |
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 7.1. reviewed 2021 |
Consensus-based recommendation Tumor classification must be carried out on the basis of the currently valid edition of the WHO classification. |
EC | ||
| 7.2. reviewed 2021 |
Consensus-based recommendation In cervical carcinomas with neuroendocrine components, the latter must be reported along with the percentage of the total tumor that they represent. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 7.3. modified 2021 |
Consensus-based recommendation Staging must be carried out in accordance with the current edition of the TNM classification. |
EC | ||
| 7.4. reviewed 2021 |
Consensus-based recommendation A diagnosis of microinvasive cervical carcinoma must be based on the definitions given in the current editions of both the WHO and TNM classifications. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 7.5. reviewed 2021 |
Consensus-based recommendation The biopsy sample that has been taken must be processed in step sections. |
EC | ||
| 7.6. modified 2021 |
Consensus-based recommendation The report on the findings should mention the evidence and the grade of CIN, ACIS (and its variants in the form of stratified mucin-producing intraepithelial lesions [SMILE]), virus-associated changes, and possible invasion. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 7.7. reviewed 2021 |
Consensus-based recommendation The pathology report must state the size and characteristics of the excised (conization) specimen. The conization specimen must be completely processed and step sections must be prepared from each paraffin block. |
EC | ||
| 7.8. modified 2021 |
Consensus-based recommendation The histological report must note the type of lesion (CIN, ACIS and its variants in the form of stratified mucin-producing intraepithelial lesions [SMILE]), its location (endocervical, ectocervical), and its extent, as well as the presence of invasive tumor. When there is evidence of invasion, details must also be given of its extent and of lymphatic, vascular and perineural sheath invasion, as well as grading. The status of the resection margins must also be noted. |
EC | ||
| 7.9. new 2021 |
Consensus-based recommendation A multifocal microinvasive carcinoma is defined as evidence of invasive foci that are histologically clearly separate from each other at a minimum distance of 0.2 cm. The size of each invasive tumor focus must be reported separately, with the largest single lesion being relevant for staging. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 7.10. reviewed 2021 |
Consensus-based recommendation Morphological processing must take place in such a way that all therapeutically and prognostically relevant parameters can be assessed. The report must be produced on the basis of the currently valid WHO classification for tumor type and the current TNM classification for staging, as well as the R classification (UICC). |
EC | ||
| 7.11. modified 2021 |
Consensus-based recommendation The trachelectomy report must include the following details:
|
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 7.12. reviewed 2021 |
Consensus-based recommendation Morphological processing must take place in such a way that all therapeutically and prognostically relevant parameters can be assessed. The report must be produced on the basis of the currently valid WHO classification for tumor type and the current TNM classification for staging, as well as the R classification (UICC). |
EC | ||
| 7.13. new 2021 |
Consensus-based recommendation To document intratumoral heterogeneity, macroscopically visible tumors ≤ 2 cm in size should be completely processed and at least one block per centimeter at the greatest tumor extension should be embedded from tumors larger than 2 cm. |
EC | ||
| 7.14. reviewed 2021 |
Consensus-based statement Deep stromal infiltration is defined as invasion by the cervical carcinoma into the outer third of the cervical stroma (> 66%). |
EC | ||
| 7.15. modified 2021 |
Consensus-based recommendation The radical hysterectomy report must include the following details:
|
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 7.16. reviewed 2021 |
Consensus-based recommendation Micrometastases are defined as histological evidence of tumor cells in lymph nodes measuring ≥ 0.2 mm, but no larger than 0.2 cm. |
EC | ||
| 7.17. reviewed 2021 |
Consensus-based recommendation In lymphadenectomy specimens obtained during surgical treatment for cervical carcinoma, all removed lymph nodes must be histologically examined. |
EC | ||
| 7.18. reviewed 2021 |
Consensus-based recommendation Lymph nodes up to approx. 0.3 cm in size should be completely paraffin-embedded, and larger lymph nodes should be halved along the long axis and also completely paraffin-embedded. |
EC | ||
| 7.19. new 2021 |
Consensus-based recommendation Evidence of isolated tumor cells or micrometastases should be mentioned in the histological report and included in the TNM classification. |
EC | ||
| 7.20. reviewed 2021 |
Consensus-based recommendation The report on lymph nodes must include the following details: number of affected lymph nodes relative to the number of lymph nodes removed, correlated with the location of removal (pelvic/para-aortic). |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 7.21. reviewed 2021 |
Consensus-based recommendation Sentinel lymph nodes in cervical carcinoma must be completely paraffin-embedded and examined in step sections. |
EC | ||
| 7.22. new 2021 |
Consensus-based recommendation Sentinel lymph nodes in cervical carcinoma should be processed as follows:
|
EC | ||
| 7.23. new 2021 |
Consensus-based recommendation Intraoperative rapid frozen-section examination (when clinically indicated) of sentinel lymph nodes in cervical carcinoma should be performed as follows:
|
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 8.1. reviewed 2021 |
Consensus-based recommendation The aim of treatment for primary cervical carcinoma should be individualized therapy. The choice of treatment should take the following factors into account:
|
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 8.2. reviewed 2021 |
Consensus-based recommendation Treatment must be administered relative to the histological tumor stage, verified using surgical staging or interventional diagnosis. |
EC | ||
| 8.3. modified 2021 |
Consensus-based statement Sentinel lymphadenectomy alone should be used:
|
EC | ||
| 8.4. modified 2021 |
Evidence-based statement If the sentinel lymphadenectomy method alone is being carried out, the following staining methods must be used:
|
A | 2++ | 25 – 27 |
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 8.5. reviewed 2021 |
Evidence-based recommendation Following pelvic lymphadenectomy, placement of a retroperitoneal drain in the surgical area should be avoided, in order to prevent lymphoceles. |
B | 1+ | 28 |
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 8.6. reviewed 2021 |
Evidence-based recommendation Neoadjuvant drug therapy can be carried out in selected patients who are at high-risk. |
0 | 1− | 29 , 30 |
| 8.7. reviewed 2021 |
Consensus-based recommendation after systematic research The significance of tumor-affected lymph nodes after neoadjuvant chemotherapy for further treatment planning is unclear. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 8.8. reviewed 2021 |
Consensus-based recommendation after systematic research Adjuvant therapy following primary surgical therapy should be administered on the basis of the postoperative histological tumor stage as follows: Negative lymph nodes; R0; no risk factors
|
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 8.9. reviewed 2021 |
Consensus-based recommendation after systematic research In stages ≤ FIGO stage IIA, primary surgical therapy should be carried out if adjuvant therapy is not expected (no preoperative risk factors). |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 8.10. modified 2021 |
Consensus-based recommendation In stage IA1 without any risk factors, treatment must be administered as follows: Surgery:
|
EC | ||
| 8.11. new 2021 |
Consensus-based recommendation In stage IA1 with lymphatic infiltration (L1), treatment must be administered as follows: Surgery:
|
EC | ||
| 8.12. modified 2021 |
Consensus-based recommendation In stage IA1 with at least two risk factors, and stage IA2 with up to one risk factor, treatment should be administered as follows: Surgery:
|
EC | ||
| 8.13. modified 2021 |
Consensus-based recommendation In stage IA2 with at least two risk factors, treatment should be administered as follows: Surgery (preserving fertility is not possible) with SNB:
|
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 8.14. modified 2021 |
Consensus-based recommendation In stages IB1 and IIA1, treatment should be administered as follows: Surgery:
|
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 8.15. reviewed 2021 |
Consensus-based recommendation In stages IB2, IIA2, and IIB with a maximum of two risk factors, treatment should be administered as follows: Surgery:
|
EC | ||
| 8.16. modified 2021 |
Consensus-based statement after systematic research Radical hysterectomy before planned radio(chemo)therapy offers no benefits for the patient in relation to disease-free survival or overall survival. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 8.17. reviewed 2021 |
Consensus-based recommendation In stage III , the following treatment should be administered: Surgery:
|
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 8.18. reviewed 2021 |
Consensus-based recommendation In stage IVA, treatment should be administered as follows: Surgery:
|
EC | ||
| 8.19. reviewed 2021 |
Consensus-based recommendation In stage IVB, treatment should be administered as follows: Surgery:
|
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 9.1. reviewed 2021 |
Consensus-based recommendation In postmenopausal patients with macroinvasive carcinoma, bilateral adnexectomy should be carried out during hysterectomy. |
EC | ||
| 9.2. new 2021 |
Evidence-based recommendation Open radical hysterectomy should be offered to patients up to FIGO stage IB1. |
B | 1+ | 31 |
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 9.3. reviewed 2021 |
Consensus-based statement after systematic research The value of secondary hysterectomy after primary radio(chemo)therapy is unclear in relation to the rate of local recurrence, disease-free survival, metastasis-free survival, and overall survival. |
EC | ||
| 9.4. reviewed 2021 |
Consensus-based statement Hysterectomy after primary radio(chemo)therapy in patients with complete remission on clinical and imaging findings is associated with a higher morbidity rate in comparison with primary radio(chemo)therapy alone. |
EC | ||
| 9.5. reviewed 2021 |
Consensus-based statement after systematic research It is unclear whether secondary hysterectomy should be carried out in the form of simple or radical hysterectomy after primary R(CH)T. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 10.1. modified 2021 |
Evidence-based recommendation Intensity-modulated techniques should be used to achieve optimal sparing of the surrounding tissue during primary radiochemotherapy for cervical carcinoma. |
B | 1+ | 32 |
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 10.2. reviewed 2021 |
Guideline-adapted recommendation Brachytherapy should be a component of the curative treatment approach in primary treatment for cervical carcinoma that includes radio(chemo)therapy. |
B | 4 | 6 |
| 10.3. modified 2021 |
Consensus-based recommendation MRI-planned brachytherapy should be used in primary radiochemotherapy for cervical carcinoma, to reduce the rate and severity of gastrointestinal and urogenital toxicities. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 10.7. reviewed 2021 |
Consensus-based recommendation Neoadjuvant radio(chemo)therapy should not be administered outside of research studies. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 10.8. reviewed 2021 |
Consensus-based recommendation Young patients should be offered ovariopexy and high-conformal radiotherapy techniques to preserve ovarian hormone function. |
EC | ||
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 10.9. modified 2021 |
Guideline-adapted recommendation During radiotherapy or radio(chemo)therapy for cervical carcinoma, the patientʼs hemoglobin values should be monitored and corrected via transfusion at values below 10 g/dL (6.2 mmol/L). |
B | 2++ | 6 |
| No. | Recommendations/Statements | GoR | LoE | Sources |
|---|---|---|---|---|
| 10.10. reviewed 2021 |
Evidence-based recommendation Locoregional hyperthermia can be used in combination with percutaneous radiotherapy to treat locoregional recurrence or primary cervical carcinoma ≥ FIGO stage IIB. |
0 | 1− | 39 |
| 10.11. modified 2021 |
Evidence-based statement No advantage in relation to overall survival or disease-free survival has so far been confirmed in randomized trials, with the addition of locoregional hyperthermia to primary radiochemotherapy for cervical carcinoma. |
ST | 1− | 40 |
| 10.12. reviewed 2021 |
Consensus-based recommendation Locoregional hyperthermia must be administered in a quality-assured and standardized fashion, preferably in the framework of scientific studies. |
EC | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 4.1. geprüft 2021 |
Empfehlungen zu Prävention und Früherkennung des Zervixkarzinoms werden in den S3-Leitlinien „Impfprävention HPV-assoziierter Neoplasien (AWMF-Registernummer 082/002)“ und „Prävention des Zervixkarzinoms (AWMF-Registernummer 015/027OL)“ bearbeitet. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 5.1. geprüft 2021 |
Qualifizierte und sachdienliche Informationsmaterialien (Print- oder Internetmedien) sollen nach definierten Qualitätskriterien für Gesundheitsinformationen erstellt und Patientinnen zur Verfügung gestellt werden, um sie durch eine allgemeinverständliche Risikokommunikation (z. B. Angabe von absoluten Risikoreduktionen) in ihrer selbstbestimmten Entscheidung für oder gegen medizinische Maßnahmen zu unterstützen. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 5.2. geprüft 2021 |
Der Patientin soll angeboten werden, den Partner/die Partnerin oder Angehörige in das Gespräch bzw. die Gespräche einzubeziehen. | EK | ||
| 5.3. geprüft 2021 |
Im ärztlichen Gespräch sollen die individuellen Präferenzen, Bedürfnisse, Sorgen und Ängste der Patientin eruiert und berücksichtigt werden. Wenn eine Patientin dafür mehrere Gespräche benötigt, soll das Angebot zu weiteren Gesprächen bestehen. | EK | ||
| 5.4. mod. 2021 |
Die medizinische Aufklärung der Patientin ist primär Aufgabe des behandelnden Arztes, sie sollte jedoch bei spezifischen Themen durch andere Berufsgruppen wie Pflege, Psychoonkologen etc. erbracht werden. | EK | ||
| 5.5. mod. 2021 |
Die Art der Vermittlung von Informationen und der Aufklärung der Patientin soll möglichst frühzeitig nach folgenden Grundprinzipien einer patientinnenzentrierten
Kommunikation, die eine partizipative Entscheidungsfindung ermöglicht, erfolgen:
|
EK | ||
| 5.6. geprüft 2021 |
Der Patientin sollte eine psychosoziale und psychoonkologische Unterstützung bei psychischen, sexuellen oder partnerschaftlichen Problemen angeboten werden. | EK | ||
| 5.7. geprüft 2021 |
Die Patientin soll auf die Möglichkeit, Selbsthilfegruppen zu kontaktieren, hingewiesen werden. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 5.8. geprüft 2021 |
Gemäß dem „Gesetz zur Verbesserung der Rechte von Patientinnen und Patienten“ soll die Patientin über alle in dieser Leitlinie beschriebenen für sie relevanten Therapieoptionen, deren Erfolgsaussichten und deren mögliche Auswirkungen informiert werden. Insbesondere soll auf die Auswirkungen auf ihr körperliches Erscheinungsbild, ihr Sexualleben, ihre Harn- und Stuhlkontrolle (Inkontinenz) und Aspekte des weiblichen Selbstverständnisses (Selbstbild, Fertilität) eingegangen werden. | EK | ||
| 5.9. geprüft 2021 |
Prinzipien, angestrebte Behandlungsziele, Dauer und die Durchführung der einzelnen Therapiemaßnahmen Operative Therapiemaßnahmen:
Spätfolgen der Erkrankung und der Therapie und ihre Behandlungsmöglichkeiten komplementäre Therapie:
|
EK | ||
| 5.10. geprüft 2021 |
Die Patientin soll auf die Patientinnen-Leitlinie zur Diagnostik, Therapie und Nachsorge der Patientin mit Zervixkarzinom hingewiesen werden. | EK | ||
| 5.11. geprüft 2021 |
Die Erkrankung Zervixkarzinom ist kein Notfall! Der Patientin ist für ihren Entscheidungsprozess ausreichend Zeit einzuräumen. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
| 5.12. geprüft 2021 |
Als Inhalte eines Gesprächs in der Palliativsituation können folgende Punkte angesprochen werden: Ziele der palliativmedizinischen Therapie (Linderung von Leiden, Behandlung von Schmerzen – oberstes Ziel: Lebensqualität der Patientin):
|
EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
| 6.1. mod. 2021 |
Zur Festlegung der lokalen Tumorausbreitung soll klinisch bildgebend der vaginale Ultraschall und zum Ausschluss einer Harntransportstörung der Nierenultraschall durchgeführt werden. | EK | ||
| 6.2. mod. 2021 |
Patientinnen mit histologisch gesichertem Zervixkarzinom ab mindestens FIGO-Stadium IB2 und bis einschließlich III sollten eine MRT-Becken zur Beurteilung der lokoregionären Tumorausbreitung erhalten. | B | 1+ | 5 – 7 |
| 6.3. mod. 2021 |
Bei Patientinnen ab mindestens FIGO IB2 bis einschließlich III, bei denen aus technischen Gründen eine MRT-Becken nicht durchgeführt werden kann, sollte das lokoregionäre bildgebende Staging des Beckens im Rahmen der Staging-CT-Thorax/Abdomen/Becken-Untersuchung erfolgen. | EK | ||
| 6.4. neu 2021 |
Bei Patientinnen im Stadium FIGO IVA, bei denen aus technischen Gründen eine MRT-Becken nicht durchgeführt werden kann, sollte das lokoregionäre bildgebende Staging des Beckens im Rahmen der Staging-CT-Thorax/Abdomen/ Becken-Untersuchung erfolgen. | EK | ||
| 6.5. mod. 2021 |
Patientinnen mit histologisch gesichertem Zervixkarzinom ab mindestens FIGO-Stadium IB2 sollten ein CT-Thorax/Abdomen/Becken zur Beurteilung der Tumorausbreitung erhalten. | EK | ||
| 6.6. geprüft 2021 |
Bei makroskopisch nicht sicher beurteilbarem Tumor der Portio soll eine Differenzialkolposkopie und gezielte Biopsie erfolgen. | EK | ||
| 6.7. geprüft 2021 |
Grundlage der interdisziplinären Therapieentscheidung in der Tumorkonferenz sollte das histologisch gesicherte Tumorstadium sein. | EK | ||
| 6.8. mod. 2021 |
Das PET-CT sollte zur Therapieplanung des primären Zervixkarzinoms nicht eingesetzt werden. | B | 2+ | 6 , 8 – 11 |
| 6.9. mod. 2021 |
Bei Erwägung eines lokalen Verfahrens (RCHT, Exenteration) zur Therapie eines Rezidivs sollte zum Ausschluss von Lymphknoten- und Fernmetastasen eine PET-CT durchgeführt werden. | B | 2+ | 6 , 12 – 14 |
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 7.1. geprüft 2021 |
Die Tumortypisierung des Zervixkarzinoms soll nach der aktuell gültigen Auflage der WHO-Klassifikation erfolgen. | EK | ||
| 7.2. geprüft 2021 |
Bei Zervixkarzinomen mit neuroendokriner Komponente soll diese mit Angabe des Prozentsatzes am Gesamttumor ausgewiesen werden. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 7.3. mod. 2021 |
Die Stadieneinteilung des Zervixkarzinoms soll nach der aktuellen Auflage der TNM-Klassifikation erfolgen. | EK | ||
| 7.4. geprüft 2021 |
Der Diagnose eines mikroinvasiven Zervixkarzinoms soll die Definition der jeweils aktuellen Auflage der WHO- und TNM-Klassifikation zugrunde gelegt werden. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 7.5. geprüft 2021 |
Das entnommene Biopsat soll in Stufenschnitten aufgearbeitet werden. | EK | ||
| 7.6. mod. 2021 |
Der Befundbericht sollte zum Nachweis und zum Grad der CIN, eines AIS (und dessen Variante in Form der stratifizierten muzinproduzierenden Läsion [SMILE]) sowie zu virusassoziierten Veränderungen und einer eventuellen Invasion Stellung nehmen. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 7.7. geprüft 2021 |
Der pathologische Befundbericht soll zur Größe und Beschaffenheit des Exzidates (Konisates) Stellung nehmen. Das Konisat soll vollständig aufgearbeitet und von jedem Paraffinblock sollen Stufenschnitte angefertigt werden. | EK | ||
| 7.8. mod. 2021 |
Im histologischen Befundbericht vermerkt sein sollen die Art der Läsion (CIN, AIS und dessen Variante in Form der stratifizierten muzinproduzierenden Läsion [SMILE]), deren Lokalisation (endo-, ektozervikal) und deren Ausdehnung sowie das Vorhandensein eines invasiven Tumors. Beim Nachweis einer Invasion soll zusätzlich die Angabe der Größenausdehnung erfolgen und zur Lymph-, Blutgefäß- sowie Perineuralscheideninvasion sowie zum Grading Stellung bezogen werden. Zum Status der Resektionsränder soll Stellung genommen werden. | EK | ||
| 7.9. neu 2021 |
Ein multifokales mikroinvasives Karzinom ist definiert als der Nachweis voneinander histologisch klar separierter invasiver Foci, die einen minimalen Abstand von 0,2 cm
aufweisen. Jeder invasive Tumorfokus soll separat in seiner Größe angegeben werden, wobei die größte Einzelläsion stagingrelevant ist. |
EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 7.10. geprüft 2021 |
Die morphologische Aufarbeitung soll so erfolgen, dass alle therapeutisch und prognostisch relevanten Parameter erhoben werden können. Der Befunderstellung soll die jeweils gültige WHO-Klassifikation zur Tumortypisierung und die aktuelle TNM-Klassifikation zur Stadieneinteilung sowie die R-Klassifikation (UICC) zugrunde gelegt werden. | EK | ||
| 7.11. mod. 2021 |
Der Befundbericht zur Trachelektomie soll folgende Angaben beinhalten:
|
EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 7.12. geprüft 2021 |
Die morphologische Aufarbeitung soll so erfolgen, dass alle therapeutisch und prognostisch relevanten Parameter erhoben werden können. Der Befunderstellung soll die jeweils gültige WHO-Klassifikation zur Tumortypisierung und die aktuelle TNM-Klassifikation zur Stadieneinteilung sowie die R-Klassifikation (UICC) zugrunde gelegt werden. | EK | ||
| 7.13. neu 2021 |
Zur Dokumentation einer intratumoralen Heterogenität sollen makroskopisch sichtbare Tumoren ≤ 2 cm vollständig aufgearbeitet und von Tumoren ab 2 cm Größe mindestens ein Block pro Zentimeter größter Tumorausdehnung eingebettet werden. | EK | ||
| 7.14. geprüft 2021 |
Eine tiefe Stromainfiltration ist definiert als die Invasion des Zervixkarzinoms bis in das äußere Drittel des zervikalen Stromas (> 66%). | EK | ||
| 7.15. mod. 2021 |
Der Befundbericht zur radikalen Hysterektomie soll folgende Angaben beinhalten:
|
EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 7.16. geprüft 2021 |
Mikrometastasen sind definiert als der histologische Nachweis von Tumorzellen im Lymphknoten von ≥ 0,2 mm, aber nicht größer als 0,2 cm. | EK | ||
| 7.17. geprüft 2021 |
Bei Lymphonodektomiepräparaten im Rahmen der operativen Therapie beim Zervixkarzinom sollen alle entfernten Lymphknoten histologisch untersucht werden. | EK | ||
| 7.18. geprüft 2021 |
Lymphknoten bis ca. 0,3 cm Größe sollten komplett eingebettet und größere Lymphknoten entlang ihrer Längsachse halbiert und ebenfalls komplett eingebettet werden. | EK | ||
| 7.19. neu 2021 |
Der Nachweis von isolierten Tumorzellen bzw. von Mikrometastasen soll im histologischen Befundbericht erwähnt werden und in die TNM-Klassifikation einfließen. | EK | ||
| 7.20. geprüft 2021 |
Der Befundbericht zu den Lymphknoten soll folgende Angaben beinhalten: Angabe der Zahl der befallenen Lymphknoten im Verhältnis zur Zahl der entfernten Lymphknoten in Zuordnung zur Entnahmelokalisation (pelvin/paraaortal) | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 7.21. geprüft 2021 |
Sentinel-Lymphknoten beim Zervixkarzinom sollen vollständig eingebettet und in Stufenschnitten untersucht werden. | EK | ||
| 7.22. neu 2021 |
Sentinel-Lymphknoten beim Zervixkarzinom sollten wie folgt aufgearbeitet werden:
|
EK | ||
| 7.23. neu 2021 |
Die intraoperative Schnellschnittuntersuchung (wenn klinisch indiziert) von Sentinel-Lymphknoten beim Zervixkarzinom soll wie folgt durchgeführt werden:
|
EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 8.1. geprüft 2021 |
Ziel der Therapie des primären Zervixkarzinoms sollte eine individuelle Therapie sein. Die Therapiewahl sollte unter Berücksichtigung folgender Faktoren erfolgen:
|
EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 8.2. geprüft 2021 |
Die Therapie soll in Abhängigkeit des histologischen Tumorstadiums erfolgen, verifiziert mittels operativem Staging oder interventioneller Diagnostik. | EK | ||
| 8.3. mod. 2021 |
Die alleinige Sentinel-Lymphonodektomie sollte eingesetzt werden bei:
|
EK | ||
| 8.4. mod. 2021 |
Wenn die alleinige Sentinel-Lymphonodektomiemethode durchgeführt wird, sollen folgende Färbemethoden durchführt werden:
|
A | 2++ | 25 – 27 |
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 8.5. geprüft 2021 |
Nach pelviner Lymphonodektomie sollte auf die Einlage einer retroperitonealen Drainage in das OP-Gebiet zur Vermeidung von Lymphozysten verzichtet werden. | B | 1+ | 28 |
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 8.6. geprüft 2021 |
Eine medikamentöse neoadjuvante Therapie kann bei ausgewählten Risikopatientinnen durchgeführt werden. | 0 | 1− | 29 , 30 |
| 8.7. geprüft 2021 |
Die Bedeutung tumorbefallener Lymphknoten nach neoadjuvanter Chemotherapie für die weitere Therapieplanung ist unklar. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 8.8. geprüft 2021 |
Die adjuvante Therapie nach primär operativer Therapie sollte anhand des postoperativen histologischen Tumorstadiums folgendermaßen erfolgen: negative Lymphknoten; R0; keine Risikofaktoren
|
EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 8.9. geprüft 2021 |
In den Stadien ≤ FIGO-Stadium IIA sollte bei nicht zu erwartender adjuvanter Therapie (fehlende präoperative Risikofaktoren) die primär operative Therapie erfolgen. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 8.10. mod. 2021 |
Im
Stadium IA1
ohne
Risikofaktor
soll folgendermaßen therapiert werden: Operation:
|
EK | ||
| 8.11. neu 2021 |
Im
Stadium IA1
mit
Lymphgefäßinfiltration (L1)
soll folgendermaßen therapiert werden: Operation:
|
EK | ||
| 8.12. mod. 2021 |
Im
Stadium IA1
mit mindestens 2 Risikofaktoren
und Stadium IA2 mit bis zu 1 Risikofaktor sollte folgendermaßen therapiert werden: Operation:
|
EK | ||
| 8.13. mod. 2021 |
Im
Stadium IA2
mit mindestens 2 Risikofaktoren
sollte folgendermaßen therapiert werden: Operation (kein Fertilitätserhalt möglich) mit SNB:
|
EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 8.14. mod. 2021 |
Im
Stadium IB1 und IIA1
sollte folgendermaßen therapiert werden: Operation:
|
EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 8.15. geprüft 2021 |
Im
Stadium IB2, IIA2 und IIB
mit maximal 2 Risikofaktoren
sollte folgendermaßen therapiert werden: Operation:
|
EK | ||
| 8.16. mod. 2021 |
Die radikale Hysterektomie vor der geplanten Radio(chemo)therapie hat keinen Vorteil für das krankheitsfreie Überleben oder das Gesamtüberleben der Patientin. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 8.17. geprüft 2021 |
Im
Stadium III
sollte folgendermaßen therapiert werden: Operation:
|
EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 8.18. geprüft 2021 |
Im
Stadium IVA
sollte folgendermaßen therapiert werden: Operation:
|
EK | ||
| 8.19. geprüft 2021 |
Im
Stadium IVB
sollte folgendermaßen therapiert werden: Operation:
|
EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 9.1. geprüft 2021 |
In der Postmenopause sollte bei makroinvasivem Karzinom eine beidseitige Adnexektomie im Rahmen einer Hysterektomie durchgeführt werden. | EK | ||
| 9.2. neu 2021 |
Die offene radikale Hysterektomie sollte den Patientinnen bis FIGO IB1 angeboten werden. | B | 1+ | 31 |
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 9.3. geprüft 2021 |
Die Wertigkeit der sekundären Hysterektomie nach primärer Radio(chemo)therapie bezogen auf die Lokalrezidivrate, das krankheitsfreie Überleben, das metastasenfreie Überleben oder das Gesamtüberleben ist unklar. | EK | ||
| 9.4. geprüft 2021 |
Eine Hysterektomie nach primärer Radio(chemo)therapie bei klinischer und bildgebender Komplettremission hat eine höhere Morbidität im Vergleich zur alleinigen primären Radio(chemo)therapie. | EK | ||
| 9.5. geprüft 2021 |
Ob die sekundäre Hysterektomie nach primärer R(CH)T als einfache oder radikale Hysterektomie durchgeführt werden soll, ist unklar. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 10.1. mod. 2021 |
Intensitätsmodulierte Techniken sollten zur optimalen Schonung des umliegenden Gewebes in der primären Radiochemotherapie des Zervixkarzinoms zum Einsatz kommen. | B | 1+ | 32 |
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 10.2. geprüft 2021 |
Die Brachytherapie sollte Bestandteil des kurativen Therapiekonzeptes in der Primärtherapie des Zervixkarzinoms, die eine Radio(chemo)therapie beinhaltet, sein. | B | 4 | 6 |
| 10.3. mod. 2021 |
In der primären Radiochemotherapie des Zervixkarzinoms sollte die MRT-geplante Brachytherapie eingesetzt werden, um die Rate und den Schweregrad gastrointestinaler und urogenitaler Toxizitäten zu reduzieren. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 10.7. geprüft 2021 |
Die neoadjuvante Radio(chemo)therapie sollte außerhalb von Studien nicht angewandt werden. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 10.8. geprüft 2021 |
Zum Erhalt der hormonellen Funktion des Ovars sollte der jungen Patientin die Ovariopexie und hochkonformale Strahlentherapietechniken angeboten werden. | EK | ||
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 10.9. mod. 2021 |
Während einer Radiotherapie oder Radio(chemo)therapie des Zervixkarzinoms sollte der Hämoglobinwert überwacht werden und bei Werten unterhalb von 10 g/dl (6,2 mmol/l) mittels Transfusion korrigiert werden. | B | 2++ | 6 |
| Nr. | Empfehlungen/Statements | EG | LoE | Quellen |
|---|---|---|---|---|
| 10.10. geprüft 2021 |
Die lokoregionäre Hyperthermie kann bei der Therapie des lokoregionären Rezidivs oder des primären Zervixkarzinoms ≥ FIGO-Stadium IIB in Kombination mit der perkutanen Radiotherapie eingesetzt werden. | 0 | 1− | 39 |
| 10.11. mod. 2021 |
Ein Vorteil im Gesamtüberleben oder krankheitsfreien Überleben durch Hinzunahme der lokoregionären Hyperthermie zur primären Radiochemotherapie des Zervixkarzinoms konnte in randomisierten Studien bisher nicht nachgewiesen werden. | ST | 1− | 40 |
| 10.12. geprüft 2021 |
Die lokoregionäre Hyperthermie soll qualitätsgesichert und standardisiert erfolgen, möglichst im Rahmen von wissenschaftlichen Studien. | EK | ||
References/Literatur
- 1.RKI Krebs in Deutschland für 2015/2016 2019. Online (Stand: 28.09.2021):https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2019/krebs_in_deutschland_2019.pdf;jsessionid=E24C98E8A5A6CD3A1D73BB6A09D30A39.2_cid363?__blob=publicationFile
- 2.Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (GEKID) 2014Online (Stand: 11.08.2019):https://atlas.gekid.de/CurrentVersion/atlas.html
- 3.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF) Prävention des Zervixkarzinoms, Langversion 1.1, 2020, AWMF Registernummer: 015/027OLOnline (Stand: 28.09.2021):http://www.leitlinienprogramm-onkologie.de/leitlinien/zervixkarzinom-praevention/
- 4.Evidenz- und konsensbasierte Leitlinie Impfprävention HPV-assoziierter Neoplasien – Langfassung – AWMF-Register Nr.: 082-002, 2020Online (Stand: 28.09.2021):https://www.awmf.org/uploads/tx_szleitlinien/082-002l_S3_Impfpraevention-HPV-assoziierter-Neoplasien_2020-07_01.pdf
- 5.Bipat S, Glas A S, van der Velden J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: a systematic review. Gynecol Oncol. 2003;91:59–66. doi: 10.1016/s0090-8258(03)00409-8. [DOI] [PubMed] [Google Scholar]
- 6.SIGN SIGN: Management of Cervical Cancer 2008. Online (Stand: 28.09.2021):https://www.sign.ac.uk/our-guidelines/management-of-cervical-cancer/
- 7.Thomeer M G, Gerestein C, Spronk S. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: systematic review and meta-analysis. Eur Radiol. 2013;23:2005–2018. doi: 10.1007/s00330-013-2783-4. [DOI] [PubMed] [Google Scholar]
- 8.Choi H J, Ju W, Myung S K. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: meta-analysis. Cancer Sci. 2010;101:1471–1479. doi: 10.1111/j.1349-7006.2010.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouy S, Morice P, Narducci F. Prospective multicenter study evaluating the survival of patients with locally advanced cervical cancer undergoing laparoscopic para-aortic lymphadenectomy before chemoradiotherapy in the era of positron emission tomography imaging. J Clin Oncol. 2013;31:3026–3033. doi: 10.1200/JCO.2012.47.3520. [DOI] [PubMed] [Google Scholar]
- 10.Tsai C S, Lai C H, Chang T C. A prospective randomized trial to study the impact of pretreatment FDG-PET for cervical cancer patients with MRI-detected positive pelvic but negative para-aortic lymphadenopathy. Int J Radiat Oncol Biol Phys. 2010;76:477–484. doi: 10.1016/j.ijrobp.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Kang S, Kim S K, Chung D C. Diagnostic value of (18)F-FDG PET for evaluation of paraaortic nodal metastasis in patients with cervical carcinoma: a metaanalysis. J Nucl Med. 2010;51:360–367. doi: 10.2967/jnumed.109.066217. [DOI] [PubMed] [Google Scholar]
- 12.Meads C, Auguste P, Davenport C. Positron emission tomography/computerised tomography imaging in detecting and managing recurrent cervical cancer: systematic review of evidence, elicitation of subjective probabilities and economic modelling. Health Technol Assess. 2013;17:1–323. doi: 10.3310/hta17120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding X P, Feng L, Ma L. Diagnosis of recurrent uterine cervical cancer: PET versus PET/CT: a systematic review and meta-analysis. Arch Gynecol Obstet. 2014;290:741–747. doi: 10.1007/s00404-014-3263-z. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Y, Wei J, Zhang Y. Positron emission tomography alone, positron emission tomography-computed tomography and computed tomography in diagnosing recurrent cervical carcinoma: a systematic review and meta-analysis. Arch Med Sci. 2014;10:222–231. doi: 10.5114/aoms.2014.42572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Womens Health (Larchmt) 2012;21:1031–1037. doi: 10.1089/jwh.2011.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wittekind C, Meyer H. Weinheim: Viley-VCH Verlag; 2010. TNM-Klassifikation maligner Tumoren. [Google Scholar]
- 17.Obrzut B, Semczuk A, Naróg M. Prognostic Parameters for Patients with Cervical Cancer FIGO Stages IA2-IIB: A Long-Term Follow-Up. Oncology. 2017;93:106–114. doi: 10.1159/000471766. [DOI] [PubMed] [Google Scholar]
- 18.Suprasert P, Srisomboon J, Charoenkwan K. Twelve years experience with radical hysterectomy and pelvic lymphadenectomy in early stage cervical cancer. J Obstet Gynaecol. 2010;30:294–298. doi: 10.3109/01443610903585192. [DOI] [PubMed] [Google Scholar]
- 19.Kasamatsu T, Onda T, Sawada M. Radical hysterectomy for FIGO stage I-IIB adenocarcinoma of the uterine cervix. Br J Cancer. 2009;100:1400–1405. doi: 10.1038/sj.bjc.6605048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y J, Kim D Y, Lee S W. A postoperative scoring system for distant recurrence in node-positive cervical cancer patients after radical hysterectomy and pelvic lymph node dissection with para-aortic lymph node sampling or dissection. Gynecol Oncol. 2017;144:536–540. doi: 10.1016/j.ygyno.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Cohen P A, Jhingran A, Oaknin A. Cervical cancer. Lancet. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- 22.Lapuz C, Kondalsamy-Chennakesavan S, Bernshaw D. Stage IB cervix cancer with nodal involvement treated with primary surgery or primary radiotherapy: Patterns of failure and outcomes in a contemporary population. J Med Imaging Radiat Oncol. 2016;60:274–282. doi: 10.1111/1754-9485.12411. [DOI] [PubMed] [Google Scholar]
- 23.Horn L C, Hentschel B, Fischer U. Detection of micrometastases in pelvic lymph nodes in patients with carcinoma of the cervix uteri using step sectioning: Frequency, topographic distribution and prognostic impact. Gynecol Oncol. 2008;111:276–281. doi: 10.1016/j.ygyno.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Piver M S, Rutledge F, Smith J P. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol. 1974;44:265–272. [PubMed] [Google Scholar]
- 25.van de Lande J, Torrenga B, Raijmakers P GHM. Sentinel lymph node detection in early stage uterine cervix carcinoma: a systematic review. Gynecol Oncol. 2007;106:604–613. doi: 10.1016/j.ygyno.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Ruscito I, Gasparri M L, Braicu E I. Sentinel Node Mapping in Cervical and Endometrial Cancer: Indocyanine Green Versus Other Conventional Dyes – A Meta-Analysis. Ann Surg Oncol. 2016;23:3749–3756. doi: 10.1245/s10434-016-5236-x. [DOI] [PubMed] [Google Scholar]
- 27.Frumovitz M, Plante M, Lee P S. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): a randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018;19:1394–1403. doi: 10.1016/S1470-2045(18)30448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charoenkwan K, Kietpeerakool C. Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy for the prevention of lymphocyst formation in patients with gynaecological malignancies. Cochrane Database Syst Rev. 2010;(01):CD007387. doi: 10.1002/14651858.CD007387.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Kim H S, Sardi J E, Katsumata N. Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: an international collaborative meta-analysis. Eur J Surg Oncol. 2013;39:115–124. doi: 10.1016/j.ejso.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Rydzewska L, Tierney J, Vale C L. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst Rev. 2012;(12):CD007406. doi: 10.1002/14651858.CD007406.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez P T, Frumovitz M, Pareja R. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Chen K, Lu Z. Intensity-modulated radiation therapy for definitive treatment of cervical cancer: a meta-analysis. Radiat Oncol. 2018;13:177. doi: 10.1186/s13014-018-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green J, Kirwan J, Tierney J. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005;(03):CD002225. doi: 10.1002/14651858.CD002225.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang N, Guan Q L, Wang K. Radiochemotherapy versus radiotherapy in locally advanced cervical cancer: a meta-analysis. Arch Gynecol Obstet. 2011;283:103–108. doi: 10.1007/s00404-010-1385-5. [DOI] [PubMed] [Google Scholar]
- 35.Rosa D D, Medeiros L RF, Edelweiss M I. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev. 2012;(06):CD005342. doi: 10.1002/14651858.CD005342.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers L, Siu S SN, Luesley D. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst Rev. 2012;(05):CD007583. doi: 10.1002/14651858.CD007583.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC) . Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database Syst Rev. 2010;(01):CD008285. doi: 10.1002/14651858.CD008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dueñas-González A, Zarbá J J, Patel F. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29:1678–1685. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]
- 39.Lutgens L, van der Zee J, Pijls-Johannesma M. Combined use of hyperthermia and radiation therapy for treating locally advanced cervical carcinoma. Cochrane Database Syst Rev. 2010;(01):CD006377. doi: 10.1002/14651858.CD006377.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harima Y, Ohguri T, Imada H. A multicentre randomised clinical trial of chemoradiotherapy plus hyperthermia versus chemoradiotherapy alone in patients with locally advanced cervical cancer. Int J Hyperthermia. 2016;32:801–808. doi: 10.1080/02656736.2016.1213430. [DOI] [PubMed] [Google Scholar]