Abstract
The use of phytochemicals is gaining interest for the treatment of metabolic syndromes over the synthetic formulation of drugs. Senna is evolving as one of the important plants which have been vastly studied for its beneficial effects. Various parts of Senna species including the root, stem, leaves, and flower are found rich in numerous phytochemicals. In vitro, in vivo, and clinical experiments established that extracts from Senna plants have diverse beneficial effects by acting as a strong antioxidant and antimicrobial agent. In this review, Senna genus is comprehensively discussed in terms of its botanical characteristics, traditional use, geographic presence, and phytochemical profile. The bioactive compound richness contributes to the biological activity of Senna plant extracts. The review emphasizes on the in vivo and in vitro antioxidant and anti-infectious properties of the Senna plant. Preclinical studies confirmed the beneficial effects of the Senna plant extracts and its bioactive components in regard to the health-promoting activities. The safety, side effects, and therapeutic limitations of the Senna plant are also discussed in this review. Additional research is necessary to utilize the phenolic compounds towards its use as an alternative to pharmacological treatments and even as an ingredient in functional foods.
1. Introduction
Senna—a genus belonging to family Fabaceae, subfamily Caesalpinioideae, tribe Cassieae ser. Aphyllae—has roughly 350 species of tree shrubs and subshrubs [1, 2]. It was set apart from Cassia s. l. with the identification of three definite genera, viz., Senna, Cassia L. (s.s), and Chamaecrista Moench [3, 4]. This genus can be found in wide-ranging habitats, in distinct climatic conditions, latitudes, and continents such as America, Africa, and Oceania and to a minor extent in Asia and Pacific islands [5]. Senna plants colonized forests (both humid and dry), deserts (both cold and dry), and rock outcrops [6]. Some ornamental species are widely used for landscape gardening due to the attractive yellow inflorescences and the high adaptability in terms of soil and environmental conditions [7]. Recently, some species from desert climates were proposed to prevent or block desertification in arid zones. The use of Cassia species is reported in the ancient Ayurvedic literature as a laxative, antimalarial, relaxant, and anti-inflammatory [8]. To date, the genus is also commonly recognized for its biologically active compounds and medicinal properties [9, 10].
The cosmopolitan presence of the Senna genus and its medicinal properties lead to its various traditional medicinal uses and health-promoting effects. These beneficial effects of the Senna genus are contributed by the diverse group of phytoconstituents present in its leaves, stem, and seeds. By phytochemical research, more than 350 compounds were extracted from Senna, together with forty secondary metabolites extracted from Senna spectabilis (DC.) H.S.Irwin & Barneby. These phytochemicals majorly included classes of pentacyclic triterpenes and piperidine alkaloids displaying health-promoting properties [11]. Many of the parts such as leaves, pods, roots, and fruits of the natural plants have beneficial pharmacological properties against diseases. The studied pharmacological activities of Senna plants include anti-infectious, antioxidant, anticryptococcus, antitumor, antimutagenic, antiplasmodial, anti-inflammatory, anticancer, antidiabetic, wound healing, and antihelmintic activities [12, 13]. Some studies have shown the antidiabetic activity of Senna plants due to the content of phenols and flavonoids [14]. The antidiabetic effects have as mechanisms the decrease of the expression levels of different adipokines and the reduction of glucose absorption [15].
Its anti-infectious and antioxidant properties are established using various experiments, i.e., in vitro or in vivo.
The current review is focused on the traditional medicinal uses, phytoconstituents, antioxidant and anti-infectious properties, clinical trials, and toxicological data of Senna species.
2. Review Methodology
Information on the antioxidant and anti-infective pharmacological studies of Senna species has been collected from various scientific databases such as PubMed, ScienceDirect, and Google Scholar. The selected studies were analyzed for the phytochemical, antioxidant and anti-infective, toxicological aspects of Senna plants. The next MeSH keywords have been used for searching: “Senna Plant/growth & development,” “Senna Plant/metabolism,” “Senna Plant/chemistry,” “Senna Extract,” “Cassia/chemistry,” “Plant Extracts,” “Plant Extracts/chemistry,” “Oxidative Stress,” “Reactive Oxygen Species,” “Antioxidants,” “Antioxidants/chemistry,” “Malondialdehyde,” “Anti-Infective Agents/pharmacology “Antioxidants/pharmacology,” “Anti-Bacterial Agents,” “Anti-HIV Agents,” “Reverse Transcriptase Inhibitors,” “Antifungal “Agents/pharmacology,” “Antiprotozoal Agents/pharmacology,” “Senna Plant/toxicity,” “Animals,” and “Humans.” The scientific names of the Senna species were validated using the Plant List database and the chemical formulas with ChemSpider [16, 17].
3. Botanical Description and Distribution
Among the plants of the genus Senna, there is a semishrubby or shrubby habit, reaching 4-9 meters in height. Senna plants will tolerate moistly and very poorly draining soils in which it grows naturally. Giving a unique description of general botanical characteristics is tedious given the numerous species included in this genus. Senna has paripinnate compound leaves, with leaflets facing opposite, and globose, cylindrical, or clavate glands on rachis, petiole, or stalk [18]. The flowers are generally yellow and appear in dense racemes. It has large, lateral, terminal inflorescences with branched leafy panicles and can be up to 15–30 cm long. The flowers have fragrance and are made of 5 bristly bracts that usually are oval, 4–5 mm long, and caduceus and pedicles (2–3 mm). The sepals/calyx are unequal, oval to circular, coloured yellow-orange, and 5–7 mm long in size. The flower has 5 (uneven) golden-yellow-coloured petals and an ellipsoidal or spoon-like structure and is 2–3.5 cm in length. Anthers are opening by apical pores and a slit. It has sterile stamens that are 7 large and 3 small, while the pistil is curvy, slender, and hairless. The ovary is smooth and recurved with an inconspicuous style and stigma. The fruits of Senna are green in colour that turns black or dark with ripening, and their shape is cylindrical or column-like long pods. These pods are hard, end in a short, none splitting [7]. The size of the seeds is nearly 5 mm in diameter as they are brown coloured with flattened shapes.
The flowers of genus Senna present an interesting structural specialization that includes outstanding androecial diversity and several floral asymmetry patterns [7]. Classification of Senna flower traits becomes even more complicated due to its extraordinary level of specialization of the buzz-pollination. Ten stamens are present in heterantherous flowers of Senna, out of which 3 adaxial stamens are staminodial and the rest are fertile. These are further divided into two sets, viz., one set of four middle stamens from which the bees buzz and extract pollen, while another set includes 2-3 abaxial stamens, and the pollen from here is deposited on bees through the buzzing and is carried to the stigma of another flower [19]. Senna genus has 3-colporate pollen grains, ranging from size small to medium, and is euripalynic, radiosymmetric, and isopolar; however, the shape is oblates-spheroidic to prolate, nearly circular, and copli is long, subtriangular to triangular. Floral asymmetry is also due to the corolla and androecium. Extrafloral nectaries represent an “archaic feature” of numerous Senna species [5]. This appears in ca. 76% of the American species, several Australian species, scarcely in African, and none in Southeast Asian species. These glands secreting nectar can draw insects like ants that eat the nectar thus protecting the plant from the herbivores [20]. The fruits of Senna are long, enlarged, and tubular/cylindric, with the pods having 25-32 cm size, and the colour is black that has brown seeds equipped with pleurogram [11].
Senna can be propagated by seeds that remain viable for several years [21]. Most of the species of Senna require the scarification of the seeds to favour germination. The plant has numerous lateral roots and a robust primary root that contribute to the colonization of different substrates. Among the several species of Senna the series Aphyllae (Benth.) H.S.Irwin & Barneby is a taxonomically complex group of xeromorphic shrubs and subshrubs of the caesalpinioid legume Senna Mill., from arid, semiarid, and xerophilous areas of southern South America. Among all the Senna species, these seven are morphologically distinct. Fully grown mature plants are without leaves, and stems are junciform, green, and photosynthetic, while roots are woody and deep. These xerophytic attributes assist their survival in harsh conditions [22].
The monophyletic nature of Senna was revealed by phylogenetic investigations making it occupy the place next to Cassia sensu stricto and Chamaecrista [6], and all of these together form the subtribe Cassiinae are morphologically identified based on traits of their androecium, floral architecture, corolla, bracteoles, and fruits [23]. To date, taxonomy is not simply based on floral and vegetative characters, but on several other information, such as anatomy, cytology, serological, and molecular biology, that is useful for determining relationships and affinities among the Senna genus. DNA sequencing of various chloroplast gene sections of Senna plants (matK, rpL16, rpS16) depicted that majority of them are polyphyletic [5]. The chromosome counts exist only for about 20% of Senna species, with a prevalence of 2n = 28. There are also records of 2n = 22, 24, and 26 [24, 25] and records of polyploidy, such as 2n = 42, 56, and 112 in Senna rugosa (G.Don) H.S.Irwin & Barneby [26]; 2n = 56 in Senna aversiflora (Herbert) H.S.Irwin & Barneby; and 2n = 52 and 104 in Senna gardneri (Benth.) H.S.Irwin & Barneby [27]. Recently, Cordeiro and Felix [23] demonstrated that the karyotypic differences noted in Senna, either interspecific or intraspecific, are making this genus among the most representative taxa of the Fabaceae in several world territories [22].
Plants of Senna genus are present in all the tropical regions and grow well on wasteland, river banks, damp/moist uncultivated fields, or similar areas in the low-lying coastal region; they also grow at places with altitudes up to 1000-1400 meters [28] (Figure 1).
Figure 1.
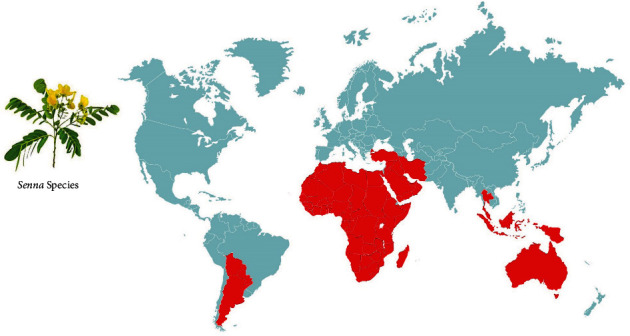
Geographical distribution of Senna species. All the regions where Senna plants are most common are highlighted in red
Senna's evolutionary history is also linked to the arid lands that this genus currently populates, such as deserts and xerophilous regions of South America in southern Bolivia, southeastern Paraguay, and central and northwestern Argentina [22]. Several types of research conducted in plants of genus Senna, growing in diverse climatic conditions, revealed a variation in phenotype between individuals within species that could arise from phenotypic plasticity.
Geographical separation and/or morphological variation among individuals of Senna causes the formation of species and subspecies in a different habitat, thanks to the adaptive strategies. America has the majority of Senna species (74%), followed by Australia with 13 percent of species and Africa and/or Madagascar having 10 percent, while only a few species are obtained from Near East, South-East Asia, and on the Pacific Islands [29]. Soladoye et al. [30] reported about 19 species in the West African floristic region with the whole 19 species in Nigeria and at least 8 species in South-Western Nigeria, with a high variety in habits, ranging from trees (approaching 34 m in height) to prostrate annual herbs. There are about 18 species of Senna in southern Africa, of which the majority is naturalized, but only Senna italica subsp. arachoides (Burch.) Lock and Senna petersiana (Bolle) Lock are native [31].
In Thailand, Larsen [32] studied Senna and stated that there are three native species, namely, Senna timoriensis (DC.) H.S.Irwin & Barneby, Senna siamea (Lam.) H.S.Irwin & Barneby, and Senna garrettiana (Craib) H.S.Irwin & Barneby, and fourteen exotic species, namely, Senna alata (L.) Roxb. (syn. Cassia alata L.), Senna singueana (Delile) Lock (syn. Cassia singueana Delile), Senna alexandrina Mill. (syn. Cassia angustifolia M.Vahl), Senna bicapsularis (L.) Roxb., Senna hirsuta (L.) H.S.Irwin & Barneby, Senna fruticosa (Mill.) H.S.Irwin & Barneby, Senna occidentalis (L.) Link, Senna pallida (Vahl) H.S.Irwin & Barneby, Senna surattensis (Burm.f.) H.S.Irwin & Barneby, Senna septemtrionalis (Viv.) H.S.Irwin & Barneby, Senna sophera (L.) Roxb., S. spectabilis, Senna sulfurea (Collad.) H.S.Irwin & Barneby, and Senna tora (L.) Roxb (syn. Cassia tora L.) [33].
4. Ethnobotanical Uses
Senna genus is widely used in southern countries in different spheres of life such as building, decoration, rituals, nutrition, poisons, and medicine. Some plants of Senna genus are used as building wood and as a shade plant and landscape ornamental [33, 34]. S. alata bark decoction has been applied by the west and east Africans while tribal mark incision and tattoo was making on to the cuts [12].
In Uganda Senna obtusifolia (L.) H.S.Irwin & Barneby is used as a good luck charm before travelling [35]. Shoots and leaves of S. garrettiana and S. siamea are cooked in a dish called kaeng khi lek (a kind of curry) which is found in two forms—with and without coconut milk [33].
Other species consumed as boiled vegetables along with chili sauce include S. timoriensis for its tender leaves and flowers and S. sophera for its tender fruits and shoots [33]. The crude pounded bark of S. alata is used as fish poison [36]. And the most popular usage of Senna genus is as a traditional medicine used as a remedy for a vast range of diseases in various countries and cultures (Table 1).
Table 1.
Traditional and folk medical usage of Senna species.
| Senna species | Country/culture | Part of plant | Internal usage | External usage | Ref |
|---|---|---|---|---|---|
| Senna alata (L.) Roxb. | Bangladesh | Leaves | Helminthiasis | Ringworm, eczema | [37, 38] |
| Benin Republic | Whole plant | Diabetes | — | [12] | |
| Bolivia | Root, leaves | Malaria, salmonella, fever, cold | Bath | [39] | |
| Brazil | Root, whole plant, flower, leaves | Flu, cough, malaria | Ringworms, scabies, blotch, eczema, tinea infections | [12, 40] | |
| Cameroon | Stem, bark, leaves | Gastroenteritis, hepatitis | Ringworm, dermal infections | [12] | |
| China | Stem, bark, leaves seed, root, leaves, flower, whole plant | Intestinal parasitosis, helminthiasis, diabetes, uterus disorder, asthma, constipation, fungal infections, poor eyesight diabetes | — | [12] | |
| Cuba | Whole plant | Diabetes | — | [41] | |
| Egypt | Leaves | Constipation | — | [12] | |
| Ghana | Whole plant | Diabetes | — | [12] | |
| Guatemala | Whole plant flower, leaves | Flu, malaria | Ringworms, tinea infections scabies, eczema, blotch | [12] | |
| Guinea | Whole plant flower, leaves | Flu, malaria | Ringworms, scabies, blotch, eczema, tine infections | [12] | |
| India | Stem, bark, leaves, seed, root leaves, flower the whole plant, leaves | Diabetes, hemorrhoids, inguinal hernia, intestinal parasitosis, syphilis, uterus disorder, helminthiasis constipation, fungal infection diabetes | Skin diseases, ringworm | [12, 42] | |
| Nigeria | Stem, leaves, root whole plant | Constipation, diarrhoea, respiratory tract infection, body and abdominal pain, stress, convulsion, diabetes | Wound, skin diseases, burns, toothache, dermal infections | [12] | |
| Philippines | Stem, bark, leaves seed, root leaves, flower leaves | Hemorrhoids, inguinal hernia, syphilis, intestinal parasitosis, diabetes, uterus disorder, helminthiasis, constipation, fungal infections | Skin diseases, wound | [12, 43] | |
| Sierra Leone | Leaves | Abortion pain, facilitate delivery | — | [12] | |
| Thailand | Leaves | Constipation, flatulence, inflammation | Abscesses, wounds, ringworm, itching | [33, 44] | |
| Togo | Whole plant | Diabetes | — | [12] | |
|
| |||||
| Senna alexandrina Mill. | Cyprus | Fruit | Constipation | — | [45] |
| Djibouti | Leaves | Constipation, injuries | Skin diseases | [46] | |
| Egypt | Leaves | Constipation | — | [47] | |
| Pakistan | Leaves, pod | Constipation, rheumatism, backache, asthma, anaemia typhoid fever, jaundice, pneumonia, leprosy | Wound, pimples | [48] | |
| Qatar | Leaves | Constipation, stomach cramps | — | [49] | |
| Sudan | Leaves, fruits | Constipation, git-disorders | — | [50] | |
| Thailand | Leaf pod | Constipation stomach pain | — | [33] | |
| UAE | Leaves | Constipation, stomach cramps | — | [49] | |
|
| |||||
| Senna auriculata (L.) Roxb. | India | Flower leaves | Diabetes | — | [51] |
|
| |||||
| Senna didymobotrya (Fresen.) H.S.Irwin & Barneby | South Africa | Leaves | Blood coagulation | — | [52] |
|
| |||||
| Senna fruticosa (Mill.) H.S.Irwin & Barneby | Panama | Stem, leaves | — | Body ache | [53] |
|
| |||||
| Senna garrettiana (Craib) H.S.Irwin & Barneby | Thailand | Heartwood | Constipation, cough, emmenagogue | — | [33] |
|
| |||||
| Senna hirsuta (L.) H.S.Irwin & Barneby | Thailand | Debarked stem | Fever, muscle spasm, poisoning, drunkenness | — | [33, 44] |
|
| |||||
| Senna italica Mill. | Bahrain | Leaves, seed | Constipation, stomach cramps | — | [49] |
| Djibouti | Leaves | Constipation | — | [46] | |
| Egypt | Leaves | Constipation, bacterial infection, tumors | — | [47] | |
| Iran | Leaves | Constipation, obesity, hemorrhoids | — | [54] | |
| Pakistan | Leaves | Backache joints pain, headache, migraine | — | [55] | |
| Qatar | Leaves, seed | Constipation, stomach cramps | — | [49] | |
| Saudi Arabia | Leaves, seed | Constipation, stomach cramps | — | [49] | |
| UAE | Leaves, seed | Constipation, stomach cramps | — | [49] | |
|
| |||||
| Senna multiglandulosa (Jacq.) H.S.Irwin & Barneby | Peru | Not specified | — | Wound disinfectant agent | [56] |
|
| |||||
| Senna occidentalis (L.) Link | Bolivia | Root, seed | Dysentery | Bath, ringworm | [39] |
| Cuba | Not specified | Liver pain, rheumatism, arthrosis, catarrh, muscular pain, hemorrhoids, pneumonia, venereal diseases, impotence | — | [41] | |
| Guatemala | Leaves, aerial part | Fever, measles, chickenpox | — | [57] | |
| India | Leaves, root seed | Respiratory diseases, cough, constipation, malaria, diabetes, indigestion, urinary disorder | Skin problems, skin disorders, pimples | [42, 58, 59] | |
| Tanzania | Root | Spasms, malaria, helminthiasis | — | [60] | |
| Thailand | Leaves, fruit | Diarrhoea | — | [44] | |
| Uganda | Leaves | Malaria | — | [35] | |
|
| |||||
| Senna petersiana (Bolle) Lock | Eastern Africa | Not specified | Flatulence | — | [61] |
| Tropical Africa | Not specified | Constipation, gonorrhoea | — | [61] | |
| South Africa | Seed | Venereal diseases, infertility constipation, gonorrhoea | — | [61, 62] | |
|
| |||||
| Senna siamea (Lam.) H.S.Irwin & Barneby | Thailand | Leaves, flower | Constipation, insomnia hypertension | — | [33, 44] |
|
| |||||
| Senna singueana (Delile) Lock | Sudan | Root | Constipation | — | [63] |
| Tanzania | Root | Diabetes | — | [64] | |
|
| |||||
| Senna sophera (L.) Roxb. | Bangladesh | Leaves root | Dyspepsia, asthma, bronchitis, hiccup, gonorrhoea dyspepsia | — | [37, 38, 65] |
| India | Bark | Respiratory disorders | — | [42] | |
|
| |||||
| Senna timoriensis (DC.) H.S.Irwin & Barneby | Thailand | Heartwood | Stimulate menstruation | — | [33] |
|
| |||||
| Senna tora (L.) Roxb. | China | Not specified | Stomach disorders, liver diseases, poor eyesight, weakness, diuretic | — | [66] |
| Thailand | Seed leaves | Constipation, urethral stones, diuretic, constipation, insomnia | — | [33, 44] | |
| India | Seed leaves | Rheumatic swelling and pain, skin diseases | [42, 67] | ||
|
| |||||
| Senna uniflora (Mill.) H.S.Irwin & Barneby | Cuba | Not specified | Bleeding, rheumatism, arthrosis | — | [41] |
5. Phytoconstituents
Ahmed and Shohael [68] reported the presence of anthraquinones named aloe-emodin, chrysophanol, emodin, and rhein from the S. alata leaves. Bradley Morris et al. [3] studied the variation in the concentration of sennosides A and B from pods and leaves of S. alata, S. alexandrina, Senna covesii (A.Gray) H.S.Irwin & Barneby, Senna angulata (Vogel) H.S.Irwin & Barneby, S. hirsuta, S. occidentalis, and Senna uniflora (Mill.) H.S.Irwin & Barneby [3]. Essien et al. [69] isolated oils from hydrodistillation of S. alata, S. hirsuta, and S. occidentalis. The following compounds are reported after analyzing samples using GC-MS (gas chromatography-mass spectrometry) analysis, viz., ar-turmerone, β-caryophyllene, (E)-phytol, and 6,10,14-trimethyl-2-pentadecanone. (E)-Phytol and pentadecanal were the main components of S. hirsuta while S. occidentalis had (E)-phytol, hexadecanoic acid, and 6,10,14-trimethyl-2-pentadecanone. Epifano et al. [70] isolated madagascin (3-isopentenyloxyemodin) and 3-geranyloxyemodine from dried fruits and leave samples of S. alexandrina.
Ahmed et al. [71] isolated the flavonoids quercimeritrin, scutellarein, and rutin from the leaves. Arrieta-Baez et al. [72] reported the isolation of alizarin and purpurin from S. alexandrina.
New compounds of pyridine alkaloids (12′-hydroxy-8′-multijuguinol, 12′-hydroxy-7′-multijuguinol, methyl multijuguinate, 7′-multijuguinol, and 8′-multijuguinol) were isolated using leaves of Senna multijuga (Rich.) H.S.Irwin & Barneby by Francisco et al. [73]. Similarly, Serrano et al. [74] in leaves identified compounds like isolated 7′-multijuguinone and 12′-hydroxy-7′-multijuguinone. Vargas Rechia et al. [75] extracted from seed (aqueous) extract compounds, viz., galactomannan and O-acetyl-glucuronoarabinoxylan. Abegaz et al. [76] separated anthraquinones, emodin, floribundone-1, torosanin-9′, 10′-quinone, anhydrophlegmacin, and 9-(physcion-7′-yl)-5,10-dihydroxy-2-methoxy-7-methyl-1,4-anthraquinone from Senna multiglandulosa (Jacq.) H.S.Irwin & Barneby.
Alemayehu and Abegaz [77] reported the presence of physcion, torosachrysone, floribundone-1, anhydrophlegmacin, and 9-(physcion-7′-yl)-5,10-dihydroxy-2-methyl-7-methoxy-1,4-anthraquinone (isosengulone) from the seeds of S. multiglandulosa.
Essien et al. [78] identified the following volatile oils from the fruits of S. hirsuta and S. occidentalis by GC-MS analysis. Compounds identified in S. hirsuta are as follows: α-pinene, germacrene, camphene, selinene, β-pinene, valencene, viridiflorene, 2-tridecanone, p-cymene, α-muurolene, limonene, 1,8-cineole, (Z,Z)-α-earnesene, γ-terpinene, β-bisabolene, trans-γ-cadinene, δ-cadinene, methyl chavicol, (E)-α-bisabolene, isothymol methyl ether, occidentalol, methyl thymol, caryophyllene oxide, bornyl acetate, cedrol, 1,10-di-epicubenol, α-copaene, 1-epi-cubenol, cyperene, τ-cadinol, β-caryophyllene, α-cadinol, 2,5-dimethoxy-pcymene, valerianol, α-humulene, cyperotundone, pentadecanal, benzyl benzoate, and γ-muurolene. Compounds identified in S. occidentalis are as follows: α-pinene, selinene, β-pinene, valencene, myrcene, α-selinene, α-phellandrene, viridiflorene, δ-3-carene, p-cymene, limonene, β-himachalene, β-bisabolene, terpinolene, 1,8-cineole, linalool, 7-epi-α-selinene, α-terpineol, δ-cadinene, methyl chavicol, caryophyllene oxide, bornyl acetate, myrtenyl acetate, humulene epoxide II, α-terpinyl acetate, α-copaene, 1-epi-cubenol, daucene, γ-eudesmol, cyperene, τ-cadinol, β-caryophyllene, valerianol, trans-α-bergamotene, (Z)-6,7-dihydrofarnesol, α-humulene, α-patchoulene, alloaromadendrene, γ-himachalene, and γ-muurolene.
Maia et al. [79] from methanolic extracts of S. gardneri and Senna georgica H.S.Irwin & Barneby separated compounds, viz., vanillic acid, 3,4-dihydroxybenzoic acid, syringic acid, dihydromyricetin, rutin glucoside, quercetin diglucoside, rutin pentoside, kaempferol rhamnodiglucoside, quercetin glucoarabinoside, kaempferol diglucoside, ellagic acid, rutin, oxyresveratrol, methoxy oxyresveratrol, quercetin glucoside, rubrofusarin tetraglucoside, quercitrin, kaempferol rhamnoglucoside, rubrofusarin triglucoside, rubrofusarin gentobioside, myricetin, quercetin, rubrofusarin glucoside, and emodin.
Monteiro et al. [80] reported the preliminary investigation on the qualitative phytochemicals present in Senna cana (Nees & Mart.) H.S.Irwin & Barn and Senna pendula (Willd.) H.S.Irwin & Barneby and reported the presence of saponins, anthraquinones, triterpenoids, steroids, flavonols, flavones, tannins, and xanthones.
Barba et al. [81] extracted different compounds from the leaves of Senna corymbosa (Lam.) H.S.Irwin & Barneby and roots of Senna lindheimeriana (Scheele) H.S.Irwin & Barneby. They were chrysophanol, methoxyhydroquinone, emodin, 5,7′-biphyscion (floribundone-l), physcion, p-hydroxybenzaldehyde, hydroquinone monomethyl ether, 3-hydroxy-4-methoxyphenol, β-sitosterol, stigmasterol, and linoleic acid in S. corymbose; while S. lindheimeriana had chrysophanol, xanthorin, chrysophanol 8-methyl ether, emodin, questin, physcion, 1-hydroxy-3-methyl-2,6,7,8-tetramethoxy-9,10-anthraquinone, 3,4,3′5′-tetrahydroxystilbene (piceatannol), 4,2′,4′-trihydroxychalcone (isoliquiritigenin), 2,4,5-trimethoxyphenol, betulinic acid, and stigmasterol.
Zavala-Sánchez et al. [82] analyzed the GC-MS result from the Senna crotalarioides (Kunth) H.S.Irwin & Barneby leaf (chloroform) extracts and reported the following compounds. 1-ocyacosanol, 1-triacontanol, palmitic acid, beta-sitosterol, neophytadiene, 1-hexacosanol, and stigmasterol.
Alemayehu et al. [83] from the pods of Senna didymobotrya (Fresen.) H.S.Irwin & Barneby isolated compounds, namely, knipholone, emodin, chrysophanol, 10-hydroxy-10-(physcion-7′-yl)-chrysophanol anthrone, physcion, and 5,10-dihydroxy-2-methyl-9-(physcion-7′-yl)-1,4-anthraquinone.
Ochieng et al. [84] reported that the root extracts (ethyl acetate) resulted in nataloemodin-8-methyl ether, obtusifolin, 1,6-di-O-methylemodin, chrysophanol, physcion, physcion-10,10′-bianthrone, chrysophanol-10,10′-bianthrone, and stigmasterol. Rao et al. [85] extracted compounds, namely, kaempferol 3-O-α-L-rhamnopyranosyl (1→2)-α-L-rhamnopyranoside, kaempferol 3-O-rutinoside, and rutin from the flowers of S. hirsuta.
Silva et al. [86] identified the following compounds from S. gardneri, Senna macranthera (Collad.) H.S.Irwin & Barneby, Senna splendida (Vogel) H.S.Irwin & Barneby, and Senna trachypus (Benth.) H.S.Irwin & Barneby through GC-MS. S. gardneri containing succinic acid, glyceric acid, β-caryophyllene, malic acid, pyroglutamic acid, 3-hydroxy-3-methylglutaric acid, 3,4-dihydroxy benzoic acid, citric acid, neophytadiene, gluconic acid, hexadecanoic acid, linolenic acid methyl ester, phytol, quercetin, α-linolenic acid, linoleic acid, stearic acid, α-tocopherol, eicosanoic acid, squalene, tetracosanoic acid, β-sitosterol, stigmasterol, 1-triacontanol. S. macranthera contains succinic acid, β-caryophyllene, malic acid, pyroglutamic acid, eicosanoic acid, hexadecanoic acid, docosanoic acid, α-linolenic acid, phytol, linoleic acid, stearic acid, chrysin, squalene, trans-catechin, β-tocopherol, α-tocopherol, quercetin, stigmasterol, β-sitosterol, β-amyrin, 1-triacontanol, and α-amyrin.
S. splendida contains succinic acid, glyceric acid, pentanedioic acid, pyroglutamic acid, 3-hydroxy-3-methylglutaric acid, stearic acid, galactonic acid, gluconic acid, hexadecanoic acid, linoleic acid, α-tocopherol, linolenic acid methyl ester, phytol, α-linolenic acid, docosanoic acid, squalene, tetracosanoic acid, stigmasterol, β-sitosterol, quercetin, β-amyrin, 1-triacontanol, α-amyrin. S. trachypus contains succinic acid, linoleic acid, hexadecanoic acid, neophytadiene, linolenic acid ethyl ester, α-linolenic acid, galactonic acid, gluconic acid, eicosanoic acid, phytol, stearic acid, stigmasterol, β-sitosterol, docosanoic acid, squalene, tetracosanoic acid, α-tocopherol, quercetin, β-amyrin, 1-triacontanol, and triacontanoic acid. Gololo et al. [87] identified the phytol (3,7,11,15-tetramethyl-2-hexadecen-1-ol); 1,2-benzenedicarboxylic acid, mono (2-ethylheptyl) ester; n-tetracontane; 13-docosenamide; squalene (2,6,10,14,18,22-hexamethyltetracosane),1-heptacosanol; α-tocopherol-β-D-mannoside; 1,2-epoxynonadecane; stigmasterol; γ-sitosterol and lupeol from hexane extract of Senna italica Mill. leaves through GC-MS analysis.
Khalaf et al. [88] used aerial parts and isolated physcion, emodin, 2-methoxy-emodin-6-O-D-glucopyranoside, quercetin 3-O-L-rhamnopyranosyl-(16)-D-glucopyranoside (rutin), 1-hydroxy-2-acetyl-3-methyl-6-hydroxy-8-methoxynaphthalene (tinnevellin), and 1,6,8-trihydroxy-3-methoxy-9,10-dioxo-9,10-dihydroanthracene. Similarly, Madkour et al. [89] identified n-hexadecanoic acid, (Z,Z,Z)9,12,15-octadecadienoic acid, vitamin E, from hexane extract and 3-methyl-4-oxopentanoic acid, (E)-stilbene, and 2,6-di-tert-butylphenol from methylene chloride extract by GC-MS analysis. Mokgotho et al. [90] extracted 3,4′,5-trihydroxystilbene (resveratrol) from aqueous extracts of the roots.
Alemayehu et al. [91] isolated 1,8,1′,8′-tetrahydroxy-6′-methoxy-3,3′-dimethyl-(10,10′-bianthracen)-9,9′-dione (or chrysophanol-physcion), 1,8,1′,8′-tetrahydroxy-7′methoxy-3,3′-dimethyl-(10,10′-bianthracen)-9,9′-dione (or chrysophanol- isophyscion-10,10′-bianthrone) and 1,8,1′,8′-tetrahydroxy-7,7′-dimethoxy-3,3′-dimethyl-(10,10′-bianthracen)-9,9′-dione (or isophyscion-10,10′-bianthrone) from the leaves and root bark of Senna longiracemosa (Vatke) Lock. Branco et al. [92] communicated the presence of rubrofusarin (5,6-dihydroxy-8-methoxy-2-methylbenzo[g]cromen-4-one, 1) in S. macranthera. Klika et al. [93] confirmed the (2R,3S,4S,2″R,3″S)-guibourtinidol-(4α→8)-catechin (procyanidin) in root isolates.
Pires et al. [94] isolated mannose and galactose from the endosperm of S. macranthera seeds. Messana et al. [95] isolated 10-demethylflavasperone-10-sulphate, 10-demethylflavasperone, 10-demethylflavasperone-10-O-β-D-apiofuranosyl-(1→6)-O-β-D-glucopyranoside, and cassiapyrone-10-sulphate (7-methyl-10-demethylflavasperone-10-suophate); quinquangulin-6-O-β-D-apiofuranosyl-(l→6)-O-β-D-glucopyranoside, rubrofusarin-6-O-β-D-glucopyranoside, quinquangulin-6-O-β-D-glucopyranoside and chrysophanol dimethyl ether, chrysophanol, physcion, cis-3,3′,5,5′-tetrahydroxy-4-methoxystilbene, trans-3,3′,5,5′-tetrahydroxy-4-methoxystilbene, and cassiaside B from the root methanolic extracts [96]. de Macedo et al. [97] reported the presence of bianthrone glycoside, namely, martianine 1 (10,10′-il-chrysophanol-10-oxi-10,10′-bi-glucosyl) from the stalks of Senna martiana (Benth.) H.S.Irwin & Barneby.
Graham et al. [98] isolated quinquangulin and rubrofusarin from the stem and fruit extract (methanolic) of Senna obliqua (G.Don) H.S.Irwin & Barneby.
Pang et al. [99] communicated extractions from seeds of S. obtusifolia and those included obtusifolin-2-O-β-D-(6′-O-α, β-unsaturated butyryl)-glucopyranoside (1) and epi-9-dehydroxyeurotinone-β-D-glucopyranoside. Saidu et al. [100] described the existence of cardenolides, flavonoids, saponins, alkaloids and anthraquinones in the leaves of S. occidentalis.
Javaid et al. [101] extracted 1,3-benzenedicarboxylic acid, bis(2-ethylhexyl) ester, 9,10-dimethyltricyclo [4.2.1.1(2,5)]decane-9,10-diol, 2(2-hydroxy-2-propyl)-5-methyl-cyclohexanol, 1,2-benzenedicarboxylic acid mono(2-ethylhexyl) ester, 7-hydroxy-3,7-dimethyl-octanal, and 5,6,6-trimethyl5-(3-oxobut-1-enyl)-1-oxaspiro[2.5]octan-4-one from the aerial parts. Kim et al. [102] isolated N-methylmorpholine from the seeds.
Kumar et al. [103] identified rutin, quercetin, kaempferol, catechin, ferulic acid, gallic acid, caffeic acid, and coumaric acid using LC-MS (liquid chromatography-mass spectrometry).
Li et al. [104] isolated cycloccidentalic acids A and B, cycloccidentalisides I-V, quercetin, luteolin, eriodictyol, robtein, chrysoeriol, 3-methylquercetin, 7,4′-dihydroxy-3′-methoxyflavone, 7,3′,4′-trihydroxyflavone, 3-methoxy-7,3′,4′-trihydroxyflavone, chrysoeriol 5-methyl ether, 2′,3,4′,4-tetrahydroxychalcone, ajugasterone C, 20-hydroxyecdysone 2-acetate, 20-hydroxyecdysone 3-acetate, calonysterone, and poststerone. S. F. Li and S. L. Li [105] isolated cycloccidentalic acid C and cycloccidentaliside VI.
Ogunwande et al. [106] identified the (E)-geranyl acetone, hexahydrofarnesylacetone, and (E)-phytol acetate through GC-MS. Qin et al. [107] extracted nor-sesquiterpene, 3-isopropyl-1,6-dimethoxy-5-methyl-naphthalen-7-ol, and 2,7-dihydroxy-4-isopropyl-6-methyl-naphthalene-1-carbaldehyde. Singh et al. [108] reported the isolation of emodin, rhamnetin 3-neohesperidoside, chrysophanol, physcion, cassiollin, quercetin, 5,7,2′,4′-tetrahydroxyfavanol, β-sitosterol, and chrysophanol.
Tshikalange et al. [109] extracted luteolin from the seeds of S. petersiana. Gamal-Eldeen et al. [110] isolated 7-acetonyl-5-hydroxy-2-methylchromone (petersinone 1), 7-(propan-2′-ol-1′-yl)-5-hydroxy-2-methylchromone (petersinone 2), 5-methyl-3-(propan-2′-on-1′-yl) benzoic acid (petersinone 3), 5-(methoxymethyl)-3-(propan-2′-ol-1′-yl) benzoic acid (petersinone 4), glyceryl-1-tetracosanoate, and sistosterol-3-β-D-glycoside from the leaves. Coetzee et al. [111] extracted cassiaflavan-(4α→8)-epicatechin, cassiaflavan-(4α→8)-epigallocatechin, cassiaflavan-(4β→8)-epicatechin, cassiaflavan-(4β→8)-epigallocatechin, cassiaflavan-(4β→8)-gallocatechin, ent-cassiaflavan-(4β→8)-epicatechin, and cassiaflavan-(4α→6)-epicatechin from the bark. Ajiboye et al. [112] isolated β-elemene, phytol, caryophyllene oxide chrysophanol, 3-oxo-methyl ester, α-humulene, β-caryophyllene, rhein, emodin, and α-copaene from the leaves of Senna podocarpa (Guill. & Perr.) Lock.
Malmir et al. [113] isolated rhein, emodin, chrysophanol, physcion, and sennosides A and B from the hydroethanol extracts of leaves and roots. Genta-Jouve et al. [114] isolated schoepfins A and D from Senna quinquangulata, while Ogura et al. [115] isolated quinquangulin.
Mena-Rejón et al. [116] isolated 8,9-dihydroxy-3-methoxy-2,2,6-trimethyl-(2H)-anthracen-1-one (racemochrysone) from Senna racemosa (Mill.) H.S.Irwin & Barneby bark extracts (hexane extract). Sansores-Peraza et al. [117] isolated cassine and inositol methyl ether from the leaves. Dos Santos et al. [118] extracted compounds from the wood of Senna reticulata (Willd.) H.S.Irwin & Barneby, and they include chrysophanol, emodin, physcion, aloe-emodin, 1,3,8-trihydroxyanthraquinone, 3-methoxy-1,6,8-trihydroxyanthraquinone, chrysophanol-10,10′-bianthrone, stigmasterol, α and β-amyrin, β-sitosterol, and kaempferol. Barbosa et al. [119] isolated chrysophanol, physcion, quinquangulin, and rubrofusarin from the roots of S. rugosa.
Alemayehu et al. [120] isolated chrysophanol, physcion, emodin, floribundone-1,5,7′-physcion-fallacinol, 5,7′-physcion-physcion-10′-C-α-arabinopyranoside from the stem bark of S. septemtrionalis. Similarly from the pods, Alemayehu et al. [121] isolated bianthraquinone, 5,7′-physcion-fallacinol (1,1′,8,8′,-tetrahydroxy-6,6′-dimethoxy-3-methyl-3′-hydroxymethylene-5,7′-bianthracene-9,9′,10,10′-tetraone) chrysophanol, physcion, torosachrysone, emodin, floribundone-1, and torosanin-9′,10′-quinone. Ingkaninan et al. [122] isolated luteolin, cassia chromone (5-acetonyl-7-hydroxy-2-methylchromone), 4-(trans)-acetyl, 3,6,8-trihydroxy-3-methyldihydronaphthalenone, 5-acetonyl-7-hydroxy-2-hydroxymethyl-chromone, and 4-(cis)-acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone from the leaves of S. siamea.
The leaves are also reported to contain barakol [123], cassiarins A and B [124], and chrobisiamone A [125].
The floral parts of Senna plants species are reported to have cassiarins C-E, 10,11-dihydroanhydrobarakol [126], and cassibiphenols A and B [127]. The compounds such as 1,1′,3,8,8′-pentahydroxy-3′,6-dimethyl [2,2′-bianthracene]-9,9′,10,10′-tetrone, 7-chloro-1,1′,6,8,8′-pentahydroxy-3,3′-dimethyl [2,2′-bianthracene]-9,9′,10,10′-tetrone, emodin, cassiamin A, chrysophanol, friedelin, physcion, and cycloart-25-en-3β,24-diol were isolated from the root [128, 129].
The stems of Senna plant species are identified with physcion, chrysophanol, betulinic acid, lupeol, and emodin [130, 131]. In other studies, Lü et al. [132–134] reported the extraction of chrysophanol, 1-[(β-D-glucopyranosyl-(1→6)-O-β-D-glucopyranosyl)oxy]-8-hydroxyl-3-methy-9,10-anthraquinone, chrysophanol-1-O-beta-D-glucopyranoside [132], sucrose, β-sitosterol, n-octacosanol, 2-methyl-5-2′-hydroxypropyl)-7-hydroxy-chromone-2′-O-β-D-glucopyranoside, piceatannol [133], and 1,8,10-trihydroxyl-1-O-β-D-glucopyranosyl-3-methyl-10-C (S)-β-D-glucopyranosyl-anthrone-9 [134] from stem. Hu et al. [135] isolated siamchromones A-G, 7-hydroxy-2-methyl-5-(2-oxopropyl)-4H-chromen-4-one, O-methylalloptaeroxylin, perforatic acid, uncinoside A, peucenin-7-methyl ether, 8-methyleugenitol, urachromone A, 11-hydroxy-sec-O-glucosylhamaudol, sec-O-glucosylhamaudol, barakol, 4-cis-acety1-3,6,8-trihydroxy-3-methyldihydronaphthalenone, and 2-methyl-5-(2′-hydroxypropy1)-7-hydroxychromone-2′-O-D-glucopyranoside from the stem. In an independent work, Ledwani and Singh [136] reported the isolation of 1,8-dihydroxy-3-methyl anthraquinone and cassiamin from stem. Li et al. [137] isolate 6-hydroxy-7-methoxy-3-(4-methoxyphenyl)-2H-chromen-2-one, 7-hydroxy-6-methoxy-3-(4-methoxyphenyl)-2H-chromen-2-one, piceatanno1, 2,2′,3,3′-tetrahydroxyldiphenylethylene, candenatenin E, kaempferol, quercetin, and nonin A from the stems.
Thengyai et al. [138] isolated lupeol, β-amyrin, α-amyrin, betulin, betulinic acid, and scopoletin from the stem bark.
Baez et al. [139] isolated rutin, quercetin, 5,7-dimethoxyrutin, aglycon 5,7-dimethoxyquercetin, D-3-O-methyl-chiro-inositol, and piceatannol from roots of Senna skinneri (Benth.) H.S.Irwin & Barneby. Also, Baez et al. [140] isolated 5,7-di-O-methylrutin and 5,7-di-O-methylquercetin from S. skinneri and quercetin and rutin from Senna wislizeni (A.Gray) H.S.Irwin & Barneby. Alemayehu et al. [141] separated different compounds from the seeds of S. sophera, and these included presengulone [9-(6′methoxy-3′-methyl-3′,8′,9′-trihydroxy-1′-oxo-1′,2′,3′,4′-tetrahydro-anthracene-7′yl)-5,10-dihydroxy-2-methoxy-7-methyl-1,4-anthraquinone], physcion bianthrone, xanthorin, floribundone-1, isosengulone, sengulone, and anhydrophlegmacin-9,10-quinones A2 and B2. Kharat et al. [142] extracted hexahydroxydiphenic acid and kaempferol from methanolic extract of leaves.
Malhotra and Misra [143] isolated 1,3,6,8-tetrahydroxy 2-methyl 7-vinyl anthraquinone (sopheranin), 3-sitosterol, chrysophanol, physcion, and emodin from the roots and flowers. Mondal et al. [144] isolated 2-(3,4-dihydroxy-phenyl)-3,5-dihydroxy-7-methoxy-chromen-4-one.
Mushtaq et al. [145] isolated palmitic acid, palmitoleic acid, oleic acid, phytol, neophytadiene, and solasodine from S. sophera and S. tora. S. spectabilis is one of plant widely studied and reported. Selegato et al. [11] have reviewed the chemical aspects of S. spectabilis. Silva et al. [146] isolated caffeine, lupeol, α-amyrin, β-amyrin, cycloeucalenol, friedelin, ursolic, oleanolic, and betulinic acids, sitosterol, and stigmasterol and their respective glucosides from the leaves. Lim et al. [147] isolated (+)-spectaline and iso-6-spectaline from the leaves.
For this plant, flowers are recognized by (-)-cassine, (-)-cassine, (-)-spectaline, and iso-6-spectaline [148–150]. Sriphong et al. [151] isolated 3(R)-benzoyloxy-2(R)-methyl-6(R)-(11′-oxododecyl)-piperidine, 5-hydroxy-2-methyl-6-(11′-oxododecyl)-pyridine, 5-hydroxy-2-methyl-6-(11′-oxododecyl)-pyridine N-oxide, and (-)-cassine from the flowers. Viegas Junior et al. [152] isolated (-)-7-hydroxycassine), (-)-cassine, (-)-spectaline, (-)-3-O-acetylspectaline, (-)-7-hydroxyspectaline and (-)-iso-6-spectaline, β-sitosterol, luteolin, 3-methoxyluteolin, betulinic acid, and trans-cinnamic acid from the green fruits and flowers, whereas few other researchers reported piperidine alkaloid (-)-3-O-acetylspectaline, (-)-3-O-acetyl-spectalin, (-)-spectaline cassine, (–)-3-O-acetylcassine, iso-6-cassine, (–)-3-O-acetylspectaline, (-)-cassine, and (-)-spectaline [153–157].
Maia et al. [79] isolated quercetin diglucoside from the leaves, methoxy oxyresveratrol from the roots, quercetin-3-O-rhamnoside-4′-O-glucoside from the flowers (2.885 g/kg), while the bark of S. splendida had quercetin rhamnoside. Valencia et al. [158] isolated 5-(3-formyl-4-hydroxyfenoxy)-2-hydroxybenzaldehyde from stems and leaves of Senna stipulacea (Aiton) H.S.Irwin & Barneby.
El-Sawi and Sleem [159] isolated quercetin 3-O-glucoside 7-O-rahmnoside, quercetin, and rutin from the leaves of S. surattensis. Anu and Madhusudana [160] isolated kleinioxanthrone-1 and 2 from the aerial sections of S. tora [161] while roots had kleinioxanthrone-3 and 4. el-Halawany et al. [162] isolated torachrysone 8-O-[β-D-glucopyranosyl (1→3)-O-β-D-glucopyranosyl (1→6)-O-β-D-glucopyranoside], toralactone 9-O-[β-D-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→6)-O-β-D-glucopyranoside], aurantio-obtusin 6-O-b-D-glucoside, torachrysone 8-O-b-D-gentiobioside, toralactone 9-O-b-D-gentiobioside, 6-hydroxymusizin 8-O-b-D-glucoside, torachrysone tetraglucoside, rubrofusarin triglucoside, and chrysophanol triglucoside from ethanolic extract of the seed. In another work, Fathalla et al. [163] identified chrysophanol, chrysarobin, 10-hydroxy-5-methoxy-2-methyl-1,4-anthracenedione, rubrofusarin, parietin, griseoxanthone-B, isotorachrysone, and cumbiasin B from the seeds through GC-MS. Lee et al. [164] isolated rubrofusarin-6-O-β-D-gentiobioside, cassiaside, and toralactone-9-O-β-D-gentiobioside from the seeds.
Hatano et al. [165] isolated rubrofusarin-6-O-β-gentiobioside, cassiaside, cassiaside C, chrysophanol-1-O-β-tetraglucoside, torosachrysone-8-O-β-gentiobioside, cassiaside C2, rubrofusarin triglucoside, torachrysone tetraglucoside, demethylflavasperone gentiobioside, nor-rubrofusarin gentiobioside, torachrysone gentiobioside, and torachrysone apioglucoside from the seeds. Lee et al. [166, 167] extracted emodin, 7-methoxy-obtusifolin, chrysoobtusin, obtusin, aurantio-obtusin, chrysophanol, obtusifolin, physcion, cassiaside, rubrofusarin-6-O-gentiobiosideol, obtusifolin-2-glucoside, cassitoroside, toralactone-9-O-gentiobioside, chryso-obtusin-2-O-glucoside, physcion-8-O-gentiobioside, glucoaurantio-obtusin, and alaternin 2-O-β-D-glucopyranoside from the seeds. In an independent study, Park and Kim [168] isolated chryso-obtusin-6-glucoside, norrubrofusarin-6-glucoside, and obtusifolin-2-glucoside, using seeds. Cherng et al. [169] extracted aloe-emodin, emodin, chrysophanol, and rhein. Hyun et al. [170] extracted emodin, alaternin, gluco-aurantioobtusin, gluco-obtusifolin, cassiaside, cassitoroside, chrysophanol triglucoside, toralactone gentiobioside, questin, and 2-hydroxyemodin 1-methylether from the methanol extract. Jimenez-Coello et al. [171] isolated (8-hydroxymethylen)-trieicosanyl acetate from the Senna villosa (Mill.) H.S.Irwin & Barneby. Guzmán et al. [172] isolated (8-hydroxymethylen)-trieicosanyl acetate from the leaf extract (chloroform extract).
The chemical structures of some representative phytochemical compounds with therapeutic potencies in Senna plants are represented in Figure 2.
Figure 2.
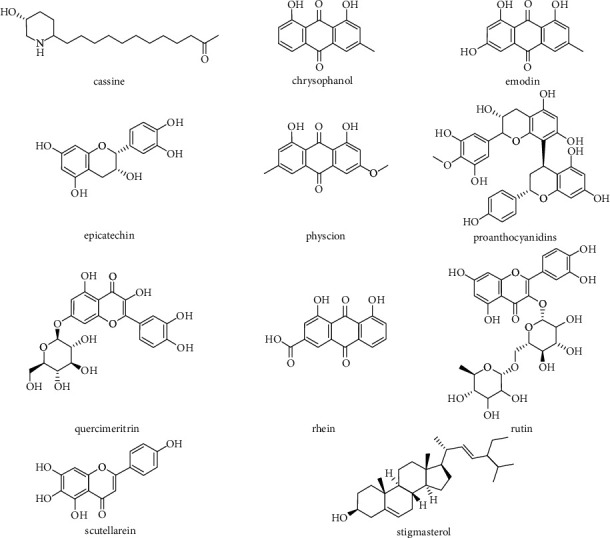
Chemical structures of mostly identified phytochemical compounds in Senna plants.
6. Antioxidant Activity of Senna Plants
Antioxidants are chemical compounds which are naturally present in food and also in human body [173–175]. These substances play a vital role for preventing cell damage caused by oxidative destruction as a result of free radical generation [176–178].
According to the literature, there are different pathways to acting as antioxidant agents [179, 180]:
Inhibiting the spread of free radicals or peroxide radicals by exchange of one or more protons
Reducing or blocking free radical formations with help of “metal chelating agents”
Reduction in reactive oxygen species (ROS) formation
Decreasing cellular ROS creation by hindering the oxidant enzymes
Influencing the complete antioxidant mechanism in the body by synergies of different antioxidant-rich ingredients
ROS are considered causative for various detrimental effects and persistent diseases like cancer, cardiovascular diseases (CVD), neurodegenerative dysfunction, like Alzheimer's, Parkinson's, and Huntington's diseases, sepsis, and diabetes [181–183].
The antioxidant activity of Senna genus was correlated with phenolic and flavonoid content which includes chemical compounds such as catechins, proanthocyanidins, scutellarein, rutin, quercimeritrin, kaempferol glycosides, rhein, chrysophanol, aloe-emodin, and physcion [184–186].
Neutralization of free radicals by the contained polyphenols justifies the antioxidant activities of the genus Senna. These polyphenols also quench singlet, and triplet oxygen, or decompose peroxides [187]. The antioxidant capacity and total polyphenol content of genus Senna were investigated by conducting both in vitro and in vivo experiments (Figure 3).
Figure 3.
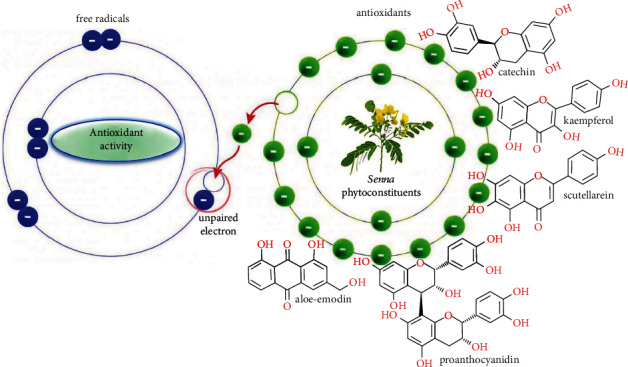
Antioxidant activity of bioactive compounds of Senna plants. The antioxidant bioactive molecules contained in Senna species neutralize free radicals by releasing electrons.
Commonly used in vitro techniques for determining the antioxidant activities of extracts are DPPH (2,2-diphenyl-1-picrylhydrazyl radical) and FRAP (ferric reducing antioxidant power) assay. The literature study indicates that various species under Senna genus were investigated using different methodologies, and they are indicated in Table 2. According to the study of Silva et al. [188] with four species of Senna from northeast Brazil, some of the phenolic compounds such as anthraquinones and flavonoids which are detected in the phytochemical screening especially in root extracts more than other parts can act as radical scavengers by donating hydrogen. They also mentioned that root extract of S. trachypus had a higher radical scavenging activity level than two standards (butylated hydroxyanisole (BHA) and quercetin) used in the assays.
Table 2.
Summary of several in vitro studies about the antioxidant activity and total phenolic content of genus Senna.
| Senna genus | Part of plant—solvent—procedure (if any) | Method | Result | References | |||||
|---|---|---|---|---|---|---|---|---|---|
|
Senna gardneri (Benth.) H.S.Irwin & Barneby Senna macranthera (Collad.) H.S.Irwin & Barneby Senna splendida (Vogel) H.S.Irwin & Barneby Senna trachypus (Benth.) H.S.Irwin & Barneby |
Root, leaves—ethanol | DPPH, ABTS—Folin-Ciocalteu | DPPH (IC50 mg/mL) | ABTS (TEAC) | TPC (mg GAE/100 g) | [188] | |||
| Root = Sg | 0,396 | 57,13 | 214,25 | ||||||
| Sm | 0,534 | 53,72 | 122,09 | ||||||
| Ss | 0,502 | 36,02 | 146,60 | ||||||
| St | 0,253 | 64,47 | 1277,34 | ||||||
| Leaves = Sg | 0,089 | 47,91 | 338,76 | ||||||
| Sm | 0,424 | 26,72 | 207,71 | ||||||
| Ss | 0,286 | 29,63 | 148,24 | ||||||
| St | 0,401 | 30,71 | 322,09 | ||||||
| Senna velutina (Vogel) H.S.Irwin & Barneby | Leaves—ethanol | DPPH | [185] | ||||||
| Senna reticulata (Willd.) H.S.Irwin & Barneby | Aerial parts—Methyl tert-butyl ether (MTBE)/methanol (90 : 10) | DPPH, ORAC-Folin-Ciocalteu | DPPH (𝜇g/mL) | ORAC (mmol TE/mg extract) | TPC (mg GAE/g) | [193] | |||
| 72.90 | 2.68 | 79.3 | |||||||
| Senna alata (L.) Roxb. | Roots—acetone, ethanol, water | DPPH, ABTS (IC50) | DPPH (𝜇g/mL) | ABTS (mmol TE/mg extract) | TPC (mg GAE/g) | [189] | |||
| Acetone | 82.42 | 64,93 | 21,42 | ||||||
| Ethanol | 45,18 | 39,14 | 78,21 | ||||||
| Water | 61,15 | 48,3 | 46,3 | ||||||
| Senna bicapsularis (L.) Roxb. | Flowers—ethanol, water | DPPH, FRAP—Folin-Ciocalteu | % DPPH inhibition | FRAP (𝜇moles Fe(II)/100 g). | TPC (mg GAE/100 g) | [194] | |||
| Ethanol | 99,51 | 2403.15 | 26223.78 | ||||||
| Water | 96,51 | 1966.30 | 9468.18 | ||||||
| Senna italica mill. | Aerial parts—ethyl acetate, n-butanol | ABTS | % Inhibition | [88] | |||||
| Ethyl acetate | 82,9 | ||||||||
| n-Butanol | 85,7 | ||||||||
| Senna siamea (Lam.) H.S.Irwin & Barneby | Leaves—ethanol (49%)—ultrasound-assisted (UA) | DPPH, FRAP—Folin-Ciocalteu | %DPPH inhibition. | FRAP (mM FeSO4/g) | TPC (mg GAE/g) | [190] | |||
| Ethanol | 80,49 | 8,08 | 455,42 | ||||||
| UA | 91,83 | 11,41 | 575,23 | ||||||
| Senna alexandrina Mill. | Flowers, leaves—ethanol (70%)—microwave, Soxhlet, marination, reflux and sonication | DPPH—HPLC-ESI-MS/MS | %Inhibition (IC50) = microwave | Soxhlet | Marination | Reflux | Sonication | [191] | |
| Flowers | 3,1 | 3,4 | 3,5 | 6,5 | 5,9 | ||||
| Leaves | 3,6 | 4,2 | 5,6 | 6,2 | 7,4 | ||||
| Senna alata (L.) Roxb. | Leaves—ethanol | Chemiluminescence measurement | [192] | ||||||
| Senna alata (L.) Roxb. | Root, stem, seed, leaves and flower—methanol/water (80%) | FRAP-Folin-Ciocalteu | FRAP (g/100 g) | TPC (g/100 g) | [195] | ||||
| Root | 0,255 | 1,69 | |||||||
| Stem | 0,457 | 2,27 | |||||||
| Seed | 0,345 | 2,59 | |||||||
| Leave | 0,560 | 2,33 | |||||||
| Flower | 0,565 | 1,36 | |||||||
Campos et al. [185] examined the chemical makeup of Senna velutina (Vogel) H.S.Irwin & Barneby leaf extracts (ethanol) and antioxidant activities with the DPPH method. In this study, IC50 (minimum sample concentration needed for scavenging 50 percent free radicals) values of the extract of S. velutina leaf extract; ascorbic acid and butylated hydroxytoluene (BHT) were found (6.3 μg/mL, 2.6 μg/mL, and 21.3 μg/mL, respectively). This indicates that the antioxidant activity of S. velutina leaves is higher with a 3.5-fold than BHT but lower than ascorbic acid according to these results.
Ita and Ndukwe [189] studied the antioxidant activity of S. alata roots in different in vitro models. They used three different solvents such as acetone, ethanol, and water for extraction and measured its ferric reducing power, DPPH, ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical-scavenging abilities, and metal chelating activity to determine antioxidant properties of roots. Researchers stated that ethanol extract had high amounts of total phenolics and flavonoids with values of 78.21 mg gallic acid equivalent (GAE)/g and 39.29 mg quercetin equivalent (QE)/g and exhibited the best antioxidant capacity in terms of DPPH and ABTS protocols. Besides, the aqueous extract showed more potential in metal chelating and reducing power. Khalaf et al. [88] analyzed the phenolic compounds, antioxidant, antimicrobial, and anticancer activities of S. italica aerial parts extracted using ethyl acetate and n-butanol. The researchers isolated and identified six compounds from this plant as they did bioguided fractionation. The names of these compounds are as follows: quercetin 3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (rutin), physcion, emodin, 1-hydroxy-2-acetyl-3-methyl-6-hydroxy-8-methoxynaphthalene (tinnevellin), 2-methoxy-emodin-6-O-β-D-glucopyranoside, and 1,6,8-trihydroxy-3-methoxy-9,10-dioxo-9,10-dihydroanthracene. Antioxidant activity was measured with ABTS method, and the ethyl acetate and n-butanol extracts showed 82.9% and 85.7% inhibition against ABTS radical, respectively, in comparison with ascorbic acid (89.2% inhibition). According to the literature, anthraquinone compounds which are already in this plant are given to their antioxidant potentials. Therefore, the researchers said that these anthraquinone-rich extracts (ethyl acetate and n-butanol) might be the reasons behind the high antiradical capacity. At last, it is noted that the aerial parts of S. italica may possess antioxidant activity and can serve as natural sources of antimicrobial and anticancer factors.
Phaiphan and Baharin [190] focused on determining effects of various extraction methods on some bioactive properties of S. siamea leaf. Researchers focused the study on comparing the solvent extraction with that of ultrasound-assisted extraction with regard to total phenolic content and antioxidant and antibacterial activity. In solvent extraction and ultrasound-assisted extraction (UAE), ethanol/water mixture (49%) and ethanol/water mixture (40%) were used, respectively, under the optimized conditions which were predetermined. The study showed that extracts from the ultrasound-assisted extraction had higher yield, total phenolic content (TPC), and antioxidant activities than those acquired from the solvent extraction. Furthermore, UAE extracts had greater antibacterial activity compared to solvent extracts. This can be attributed to the fact that the cavitational effect caused by ultrasound resulted in a more porous cell wall causing more release of phenolic bioactive in the solvent. It is evident from the literature that higher concentrations of bioactive have a direct correlation with antioxidant activity and antimicrobial activity. Similarly, Laghari et al. [191] investigated the comparison between 5 different extraction methods (microwave, Soxhlet, marination, reflux, and sonication) during the extraction of flavonoids to evaluate the antioxidative properties of S. alexandrina. As a result of this study, a greater quantity of flavonoids was obtained with microwave extraction in the aqueous ethanol (70%) fractions of S. alexandrina flowers and leaves.
In some of the studies, the antioxidant activity of some plants is compared with each other. In a study, five different medicinal plants (S. alata, Eleusine indica (L.) Gaertn., Eremomastax speciosa (Hochst.) Cufod., Carica papaya L., and Polyscias fulva (Hiern) Harms) collected from Cameroon were examined according to their scavenger activities against superoxide anion and hydrogen peroxide [192]. The results show that S. alata plant extracts at less than 12.5 μg had the best scavenger activity with a 67% reduction in luminol-amplified chemiluminescence signal.
Navarro et al. [193] obtained and characterized (UPLC-DAD-EST-TQ-MS) phenolic extracts from Petiveria alliaceae L., Phyllanthus niruri L., and S. reticulata. Researchers also evaluated the antioxidant potential via conducting DPPH and ORAC (oxygen radical absorbance capacity) assay, and TPC was measured by the Folin-Ciocalteu method. Correlation analysis was carried out as well. It was reported that P. niruri has the highest phenolic content with 328.8 GAE/g, followed by S. reticulata with 79.3 GAE/g. In addition, P. niruri exhibited the best DPPH and ORAC values among these three plants. About the phenolic acid's characterization, for S. reticulata, the main compound was ferulic acid (52.6%) followed by 4-hydroxybenzoic acid, caffeic acid, vanillic acid, p-coumaric acid, and protocatechuic acid. S. reticulata had IC50 of 72.9 μg/mL for DPPH and 2.68 mmol Trolox equivalents (TE)/g for ORAC. It was concluded that as TPC and UPLC increased ORAC values increased indicating a strong correlation.
Mak et al. [194] investigated the antioxidant capacity and antibacterial properties of ethanolic and distilled water extracts of hibiscus (Hibiscus rosa-sinensis L.) and S. bicapsularis flower. DPPH radical scavenging activity and FRAP were used as antioxidant assay while total phenolic content was analyzed by using the Folin-Ciocalteu method. DPPH inhibition values were 99.51 ± 0.2 for ethanol extracts and 96.51 ± 0.3 for aqueous extracts. The FRAP values found in the study were like 2403.15 ± 307.3 μmol Fe (II)/100 g for ethanol extract and 1966.30 ± 12.7 for aqueous extract. Total phenolics were also determined in the study, and results are as follows: 26223.78 ± 450.3 mg GAE/100 g for ethanol extract and 9468.18 ± 91.9 mg GAE/100 g for aqueous extract. Researchers stated that these results were significantly different from each other and the other hibiscus flower extracts. Similarly, too many studies in literature, Cassia flower extracts (ethanolic) exhibited the highest TPC, total flavonoid, and flavonol content, which in turn had the highest DPPH radical scavenging activity. In addition to that, they suggested that all hibiscus and cassia flowers—because of their significant antioxidant activities—can be used as a natural preservative in formulations of new and creative functional products or nutraceuticals.
Channa et al. [195] studied medicinal properties, biochemical parameters, and antibacterial activity of S. alata's various sections such as roots, stem, seed, leaves, and flower. To analyze the antioxidant capacity, the FRAP method was chosen and 80% methanol-water was used as a solvent. Researchers noted that the seeds were found rich in phenolic compounds compared to other parts. The seeds of S. alata contained a sufficient amount of total flavonoid whereas the leaves of the plant were quite rich in tannins. However, flowers were found the strongest antioxidative content. As a result, researchers suggested that these extracts have important potential for health benefits, so the plant needs to be isolated and test in detail.
Madubunyi and Ode [196] investigated the antioxidant potential of the S. singueana leaves with an in vivo malondialdehyde test. Malondialdehyde is an oxidative stress marker which is the end product of lipid peroxidation in the cells. In this study, all doses (0.25, 0.50, and 1.00 g/kg feed) of S. singueana extract significantly decreased malondialdehyde (MDA) level in the blood samples of test rats in comparison to the control group up to day 56. Similar to that study, treating rats using the methanolic extract of S. singueana root extracts was able to decrease malondialdehyde levels, the same as aspartate aminotransferase, alanine aminotransferase, and bilirubin level which are the indices of liver damage and lipid peroxidation, in all tissues especially in the liver and kidney [197].
7. Anti-infectious Activity of Senna Plants
7.1. Antibacterial and Antifungal
The most studied genus Senna for its anti-infectious activity was found to be S. alata. Different parts of S. alata are used as a vermicide, astringent, purgative, and expectorant and for treating skin diseases such as eczema, pruritus, itching, ulcers, scabies, and especially ringworm [198, 199]. Other species having antimicrobial activity are S. spectabilis, S. alexandrina, S. occidentalis, S. podocarpa, S. tora, S. racemosa, and S. siamea. The bioactive substances that provide bioactivity to genus Senna are steroids, flavonoids, anthraquinones, anthrones, and miscellaneous other compounds. They are located in the leaves, stems, roots, flowers, bark, seeds, and fruits.
Especially antibacterial and antifungal activities of Senna extracts are obtained from the extraction of leaves mostly. In the studies generally, minimum inhibitory concentration (MIC) is calculated which is described as the smallest concentration of sample necessary to prevent microbial growth. The MIC value of 100–200 μg/mL is generally acceptable for plant materials [200]. Although the extracts of the parts of the genus Senna could not reach such MIC values, when the bioactive compounds are isolated from the extracts, MIC values decrease, and the antimicrobial properties increase [201]. Some of these bioactive components include stigmasterol, beta-sitosterol, kaempferol, luteolin, santal, alatonal, aloe-emodin, alquinone, chrysophanol, emodin, physcion, rhein, alarone, benzoquinone, coumarin, ellagitannin, naphthalene, phenolic acid, purine, xanthone, and cassine [202]. Anti-infectious effects of genus Senna are presented in Table 3.
Table 3.
Anti-infectious activity of genus Senna.
| Effect | Microorganism | Antimicrobial assay | Senna genus | Plant part-solvent | Result-solvent | References |
|---|---|---|---|---|---|---|
| Antiprotozoal | Haemonchus contortus | Effective dose determination for ED50 | Senna occidentalis | Crude plant-aqueous extract | 0.13 mg/mL | [221] |
| Haemonchus contortus | Effective dose determination for ED50 | Senna occidentals | Crude plant-hydroalcoholic extract | 0.17 mg/mL | [221] | |
| Schistosoma mansoni | Effective dose determination for ED50 | Senna spectabilis | Flower-ethanol extract | 495.4 μg/mL | [201] | |
|
| ||||||
| Antibacterial | Bacillus cereus | Diameter of the inhibition zone | Senna alexandrina | Leaves-methanol | 11.0 mm | [211] |
| Bacillus cereus | Diameter of the inhibition zone | Senna alexandrina | Leaves-infusion | 10.0 mm | [211] | |
| Bacillus cereus | Diameter of the inhibition zone | Senna alexandrina | Leaves-decoction | ND | [211] | |
| Bacillus cereus | Diameter of the inhibition zone | Senna alexandrina | Leaves-hydrosol | ND | [211] | |
| Bacillus cereus | Agar disk diffusion method, zone of inhibition | Senna bicapsularis | Flower-ethanol extract | 7 mm | [194] | |
| Bacillus cereus | Agar disk diffusion method, zone of inhibition | Senna bicapsularis | Flower-distilled water | 8 mm | [194] | |
| Bacillus cereus | Paper disk diffusion method, MIC | Senna siamea | Leaf ethanol/water mixture extract | 300 mg/mL | [190] | |
| Bacillus subtilis | Disc agar technique, inhibition zone | Senna italica | Aerial part-n-butanol extract | 9.3 mm | [88] | |
| Bacillus subtilis | Disc agar technique, inhibition zone | Senna italica | Aerial part-ethyl acetate extract | 14 mm | [88] | |
| Candida albicans | Disc agar technique, inhibition zone | Senna italica | Aerial part-n-butanol extract | 12 mm | [88] | |
| Candida albicans | Disc agar technique, inhibition zone | Senna italica | Aerial part-ethyl acetate extract | 6 mm | [88] | |
| Enterobacter aerogenes | Disc agar technique, inhibition zone | Senna italica | Aerial part-n-butanol extract | 12.4 mm | [88] | |
| Enterobacter aerogenes | Disc agar technique, inhibition zone | Senna italica | Aerial part-ethyl acetate extract | 9 mm | [88] | |
| Erwinia spp. | Disc agar technique, inhibition zone | Senna italica | Aerial part-n-butanol extract | 10 mm | [88] | |
| Erwinia spp. | Disc agar technique, inhibition zone | Senna italica | Aerial part-ethyl acetate extract | 8 mm | [88] | |
| Erwinia chrysanthemi | Agar well diffusion | Senna spectabilis | Leaf-dichloromethane Leaf-methanol |
12.00 ± 1.70 mm 13.00 ± 2.10 mm |
[223] | |
| Erwinia chrysanthemi | Agar well diffusion | Senna spectabilis | Flower-dichloromethane Flower-methanol |
9.70 ± 0.60 mm 10.00 ± 2.50 mm |
[223] | |
| Erwinia chrysanthemi | Agar well diffusion | Senna spectabilis | Stem-dichloromethane Stem-methanol |
9.30 ± 1.20 mm 16.00 ± 1.20 mm |
[223] | |
| Escherichia coli | Agar well diffusion | Senna alata | Leaf-ethanol | 17.2 ± 0.3 mm | [203] | |
| Escherichia coli | Agar well diffusion | Senna alata | Leaf-water | 10.2 ± 0.2 mm | [203] | |
| Escherichia coli | Inhibition zone (filter paper disc diffusion method) | Senna occidentalis | Whole plant ethanol extract | 7-8 mm | [212] | |
| Escherichia coli | Disc agar technique, inhibition zone | Senna italica | Aerial part-n-butanol extract | 19 mm | [88] | |
| Escherichia coli | Disc agar technique, inhibition zone | Senna italica | Aerial part-ethyl acetate extract | 16 mm | [88] | |
| Escherichia coli | The cup plate agar diffusion method | Senna alata | Leaf hot water/leaf-methanol/leaf-acetone | 3 mm/4 mm/3 mm | [205] | |
| Escherichia coli | The cup plate agar diffusion method | Senna alata | Root hot water/root-methanol/root-acetone | 4 mm/4 mm/3 mm | [205] | |
| Escherichia coli | Minimum inhibitory concentration | Senna alata | Leaf-methanol/root-methanol | 8 mg/mL/6 mg/mL | [205] | |
| Escherichia coli | Minimum microbicidal concentration | Senna alata | Leaf-methanol/root-methanol | 8 mg/mL/6 mg/mL | [205] | |
| Klebsiella aerogenes | Inhibition zone (filter paper disc diffusion method) | Senna occidentalis | Whole plant ethanol extract | ND | [212] | |
| Klebsiella pneumoniae | Agar disk diffusion method, zone of inhibition | Senna bicapsularis | Flower-ethanol extract | 7 mm | [194] | |
| Klebsiella pneumoniae | Agar disk diffusion method, zone of inhibition | Senna bicapsularis | Flower-distilled water | 9 mm | [194] | |
| Listeria monocytogenes | Agar disk diffusion method, zone of inhibition | Senna bicapsularis | Flower-ethanol extract | ND | [194] | |
| Listeria monocytogenes | Agar disk diffusion method, zone of inhibition | Senna bicapsularis | Flower-distilled water | ND | [194] | |
| Neisseria gonorrhoeae | Minimum inhibitory concentration | Senna podocarpa | Root hydroethanol extract | 100 to 400 mg/L | [113] | |
| Propionibacterium acnes | Disc diffusion assay, minimum inhibitory concentration | Senna alata | Crude plant extract | 0.625 mg/mL | [224] | |
| Propionibacterium acnes | Disc diffusion assay, minimum inhibitory concentration | Senna occidentalis | Crude plant extract | 2.5 mg/mL | [224] | |
| Propionibacterium acnes | Disc diffusion assay, minimum inhibitory concentration | Senna siamea | Crude plant extract | 1.25 mg/mL | [224] | |
| Proteus mirabilis | The cup plate agar diffusion method | Senna alata | Leaf hot water/leaf-methanol/leaf-acetone | 2 mm/3 mm/2 mm | [205] | |
| Proteus mirabilis | The cup plate agar diffusion method | Senna alata | Root hot water/root-methanol/root-acetone | 3 mm/3 mm/2 mm | [205] | |
| Proteus mirabilis | Minimum inhibitory concentration | Senna alata | Leaf-methanol/root-methanol | 10 mg/mL/8 mg/mL | [205] | |
| Proteus mirabilis | Minimum microbicidal concentration | Senna alata | Leaf-methanol/root-methanol | 10 mg/mL/6 mg/mL | [205] | |
| Proteus vulgaris | Inhibition zone (filter paper disc diffusion method) | Senna occidentalis | Whole plant ethanol extract | 7-10 mm | [212] | |
| Pseudomonas aeruginosa | The cup plate agar diffusion method | Senna alata | Leaf hot water/leaf-methanol/leaf-acetone | 3 mm/3 mm/3 mm | [205] | |
| Pseudomonas aeruginosa | The cup plate agar diffusion method | Senna alata | Root hot water/root-methanol/root-acetone | 3 mm/3 mm/3 mm | [205] | |
| Pseudomonas aeruginosa | Minimum inhibitory concentration | Senna alata | Leaf-methanol/root-methanol | 10 mg/mL/8 mg/mL | [205] | |
| Pseudomonas aeruginosa | Minimum microbicidal concentration | Senna alata | Leaf-methanol/root-methanol | 10 mg/mL/8 mg/mL | [205] | |
| Pseudomonas aeruginosa | Paper disk diffusion method, MIC | Senna siamea | Leaf ethanol/water mixture extract | 300 mg/mL | [190] | |
| Pseudomonas aeruginosa | Diameter of the inhibition zone | Senna alexandrina | Leaves-methanol | 9.0 mm | [211] | |
| Salmonella typhimurium | Agar well diffusion | Senna alata | Leaf-ethanol | 12.1 ± 0.1 mm | [203] | |
| Salmonella typhimurium | Agar well diffusion | Senna alata | Leaf-water | 10.1 ± 0.1 mm | [203] | |
| Salmonella typhimurium | The cup plate agar diffusion method | Senna alata | Leaf hot water/leaf-methanol/leaf-acetone | 3 mm/4 mm/4 mm | [205] | |
| Salmonella typhimurium | The cup plate agar diffusion method | Senna alata | Root hot water/root-methanol/root-acetone | 3 mm/4 mm/4 mm | [205] | |
| Salmonella typhimurium | Minimum inhibitory concentration | Senna alata | Leaf-methanol/root-methanol | 8 mg/mL/6 mg/mL | [205] | |
| Salmonella typhimurium | Minimum microbicidal concentration | Senna alata | Leaf-methanol/root-methanol | 6 mg/mL/8 mg/mL | [205] | |
| Salmonella typhimurium | Paper disk diffusion method, MIC | Senna siamea | Leaf ethanol/water mixture extract | 300 mg/mL | [190] | |
| Shigella spp. | Disc agar technique, inhibition zone | Senna italica | Aerial part-n-butanol extract | 7.8 mm | [88] | |
| Shigella spp. | Disc agar technique, inhibition zone | Senna italica | Aerial part-ethyl acetate extract | 8.6 mm | [88] | |
| Shigella flexneri | The cup plate agar diffusion method | Senna alata | Leaf hot water/leaf-methanol/leaf-acetone | 4 mm/4 mm/4 mm | [205] | |
| Shigella flexneri | The cup plate agar diffusion method | Senna alata | Root hot water/root-methanol/root-acetone | 3 mm/4 mm/3 mm | [205] | |
| Shigella flexneri | Minimum inhibitory concentration | Senna alata | Leaf-methanol/root-methanol | 8 mg/mL/5 mg/mL | [205] | |
| Shigella flexneri | Minimum microbicidal concentration | Senna alata | Leaf-methanol/root-methanol | 6 mg/mL/5 mg/mL | [205] | |
| Staphylococcus aureus | Inhibition zone (filter paper disc diffusion method) | Senna occidentalis | Whole plant ethanol extract | 8-9 mm | [212] | |
| Staphylococcus aureus | Agar disk diffusion method, zone of inhibition | Senna bicapsularis | Flower-ethanol extract | ND | [194] | |
| Staphylococcus aureus | Agar disk diffusion method, zone of inhibition | Senna bicapsularis | Flower-distilled water | 7 mm | [194] | |
| Staphylococcus aureus | Agar well diffusion | Senna alata | Leaf-ethanol | 20.1 ± 0.1 mm | [203] | |
| Staphylococcus aureus | Agar well diffusion | Senna alata | Leaf-water | 18.2 ± 0.3 mm | [203] | |
| Staphylococcus aureus | Disc agar technique, inhibition zone | Senna italica | Aerial part-n-butanol extract | 11 mm | [88] | |
| Staphylococcus aureus | Disc agar technique, inhibition zone | Senna italica | Aerial part-ethyl acetate extract | 6 mm | [88] | |
| Staphylococcus aureus | The cup plate agar diffusion method | Senna alata | Leaf hot water/leaf-methanol/leaf-acetone | 5 mm/5 mm/5 mm | [205] | |
| Staphylococcus aureus | The cup plate agar diffusion method | Senna alata | Root hot water/root-methanol/root-acetone | 4 mm/4 mm/4 mm | [205] | |
| Staphylococcus aureus | Minimum inhibitory concentration | Senna alata | Leaf-methanol/root-methanol | 6 mg/mL/5 mg/mL | [205] | |
| Staphylococcus aureus | Minimum microbicidal concentration | Senna alata | Leaf-methanol/root-methanol | 6 mg/mL/5 mg/mL | [205] | |
| Staphylococcus epidermidis | Disc diffusion assay, minimum inhibitory concentration | Senna alata | Crude plant extract | 2.5 mg/mL | [224] | |
| Staphylococcus epidermidis | Disc diffusion assay, minimum inhibitory concentration | Senna occidentalis | Crude plant extract | >5 mg/mL | [224] | |
| Staphylococcus epidermidis | Disc diffusion assay, minimum inhibitory concentration | Senna siamea | Crude plant extract | >5 mg/mL | [224] | |
| Streptococcus pyogenes | The cup plate agar diffusion method | Senna alata | Leaf hot water/leaf-methanol/leaf-acetone | 6 mm/6 mm/5 mm | [205] | |
| Streptococcus pyogenes | The cup plate agar diffusion method | Senna alata | Root hot water/root-methanol/root-acetone | 5 mm/6 mm/5 mm | [205] | |
| Streptococcus pyogenes | Minimum inhibitory concentration | Senna alata | Leaf-methanol/root-methanol | 6 mg/mL/3 mg/mL | [205] | |
| Streptococcus pyogenes | Minimum microbicidal concentration | Senna alata | Leaf-methanol/root-methanol | 6 mg/mL/3 mg/mL | [205] | |
| Xanthomonas axonopodis | Agar well diffusion | Senna spectabilis | Leaf-dichloromethane leaf-methanol | 9.70 ± 0.60 mm 110.0 ± 0.60 mm |
[223] | |
| Xanthomonas axonopodis | Agar well diffusion | Senna spectabilis | Flower-dichloromethane flower-methanol | 11.00 ± 1.20 mm 14.00 ± 3.50 mm |
[223] | |
| Xanthomonas axonopodis | Agar well diffusion | Senna spectabilis | Stem-dichloromethane stem-methanol | 12.00 ± 2.60 mm 25.00 ± 50.0 mm |
[223] | |
|
| ||||||
| Antifungal | Aspergillus flavus | Inhibition zone (filter paper disc diffusion method) | Senna occidentalis | Whole plant ethanol extr80t | 12-30 mm | [212] |
| Aspergillus flavus | Agar well diffusion | Senna alata | Leaf-ethanol | 22.1 ± 0.1 mm | [203] | |
| Aspergillus flavus | Agar well diffusion | Senna alata | Leaf-water | 20.1 ± 0.1 mm | [203] | |
| Aspergillus flavus | The cup plate agar diffusion method | Senna alata | Leaf hot water/leaf-methanol/leaf-acetone | 2 mm/3 mm/2 mm | [205] | |
| Aspergillus flavus | The cup plate agar diffusion method | Senna alata | Root hot water/root-methanol/root-acetone | 2 mm/3 mm/2 mm | [205] | |
| Aspergillus flavus | Minimum inhibitory concentration | Senna alata | Leaf-methanol/root-methanol | 50 mg/mL/50 mg/mL | [205] | |
| Aspergillus flavus | Minimum microbicidal concentration | Senna alata | Leaf-methanol/root-methanol | 50 mg/mL/50 mg/mL | [205] | |
| Aspergillus niger | Inhibition zone (filter paper disc diffusion method) | Senna occidentalis | Whole plant ethanol extract | 14-22 mm | [212] | |
| Aspergillus niger | Agar well diffusion | Senna alata | Leaf-ethanol | 25.2 ± 0.3 mm | [203] | |
| Aspergillus niger | Agar well diffusion | Senna alata | Leaf-water | 27.2 ± 0.2 mm | [203] | |
| Aspergillus niger | Cup-plate method, mean zone of inhibition | Senna alata | Ethanolic leaf extract | 17.6-25.8 mm | [207] | |
| Aspergillus niger | Cup-plate method, mean zone of inhibition | Senna alata | Aqueous leaf extracts | 10.5-33.8 mm | [207] | |
| Aspergillus niger | The cup plate agar diffusion method | Senna alata | Leaf hot water/leaf-methanol/leaf-acetone | 2 mm/3 mm/2 mm | [205] | |
| Aspergillus niger | The cup plate agar diffusion method | Senna alata | Root hot water/root-methanol/root-acetone | 2 mm/3 mm/3 mm | [205] | |
| Aspergillus niger | Minimum inhibitory concentration | Senna alata | Leaf-methanol/root-methanol | 50 mg/mL/50 mg/mL | [205] | |
| Aspergillus niger | Minimum microbicidal concentration | Senna alata | Leaf-methanol/root-methanol | 50 mg/mL/50 mg/mL | [205] | |
| Candida albicans | Agar well diffusion | Senna alata | Leaf-ethanol | 18.2 ± 0.2 mm | [203] | |
| Candida albicans | Agar well diffusion | Senna alata | Leaf-water | 14.1 ± 0.1 mm | [203] | |
| Candida albicans | Cup-plate method, mean zone of inhibition | Senna alata | Ethanolic leaf extract | 19.8-36 mm | [207] | |
| Candida albicans | Cup-plate method, mean zone of inhibition | Senna alata | Aqueous leaf extracts | 20.2-30.0 mm | [207] | |
| Candida albicans | Agar cup method, clearing zone | Senna alata | Leaf-chloroform extract | ND | [222] | |
| Candida albicans | Agar cup method, clearing zone | Senna alata | Leaf-ethyl acetate extract | 15-20 mm | [222] | |
| Candida albicans | The cup plate agar diffusion method | Senna alata | Leaf hot water/leaf-methanol/leaf-acetone | 2 mm/4 mm/3 mm | [205] | |
| Candida albicans | The cup plate agar diffusion method | Senna alata | Root hot water/root-methanol/root-acetone | 3 mm/4 mm/4 mm | [205] | |
| Candida albicans | Minimum inhibitory concentration | Senna alata | Leaf-methanol/root-methanol | 35 mg/mL/25 mg/mL | [205] | |
| Candida albicans | Minimum microbicidal concentration | Senna alata | Leaf-methanol/root-methanol | 25 mg/mL/25 mg/mL | [205] | |
| Candida albicans | Agar cup method, clearing zone | Senna alata | Leaf-hexane extract | 12 mm | [222] | |
| Colletotrichum gloeosporioides | Percent inhibition at 1,000 ppm | Senna spectabilis | Leaf-dichloromethane Leaf-methanol |
0.00 ± 0.00 1.85 ± 1.15 |
[223] | |
| Colletotrichum gloeosporioides | Percent inhibition at 1,000 ppm | Senna spectabilis | Flower-dichloromethane Flower-methanol |
17.78 ± 1.73 2.59 ± 0.58 |
[223] | |
| Colletotrichum gloeosporioides | Percent inhibition at 1,000 ppm | Senna spectabilis | Stem-dichloromethane Stem-methanol |
1.48 ± 1.15 15.93 ± 0.58 |
[223] | |
| Curvularia lunata | Inhibition zone (filter paper disc diffusion method) | Senna occidentalis | Whole plant ethanol extract | 16-26 mm | [212] | |
| Cryptococcus neoformans | The cup plate agar diffusion method | Senna alata | Leaf hot water/leaf-methanol/leaf-acetone | 3 mm/4 mm/3 mm | [205] | |
| Cryptococcus neoformans | The cup plate agar diffusion method | Senna alata | Root hot water/root-methanol/root-acetone | 3 mm/4 mm/4 mm | [205] | |
| Cryptococcus neoformans | Minimum inhibitory concentration | Senna alata | Leaf-methanol/root-methanol | 13 mg/mL/6 mg/mL | [205] | |
| Cryptococcus neoformans | Minimum microbicidal concentration | Senna alata | Leaf-methanol/root-methanol | 13 mg/mL/6 mg/mL | [205] | |
| Epidermophyton floccosum | Agar diffusion and broth dilution method, minimum inhibitory concentration | Senna alata | Leaf-crude ethanol extract | 3.75 mm | [208] | |
| Epidermophyton floccosum | Agar diffusion method | Senna alata | Ethanolic steam bark 5.00 mg/mL & 10 mg/mL | 15.50 mm/20.05 mm | [206] | |
| Epidermophyton floccosum | Minimum inhibitory concentration | Senna alata | Steam bark-ethanol | 5 mg/mL | [206] | |
| Epidermophyton floccosum | Minimum fungicidal concentration | Senna alata | Steam bark-ethanol | 10 mg/mL | [206] | |
| F. moniliforme | Inhibition zone (filter paper disc diffusion method) | Senna occidentalis | Whole plant ethanol extract | 12-36 mm | [212] | |
| Fusarium oxysporum | Percent inhibition at 1,000 ppm | Senna spectabilis | Leaf-dichloromethane Leaf-methanol |
4.81 ± 1.115 7.04 ± 0.58 |
[223] | |
| Fusarium oxysporum | Percent inhibition at 1,000 ppm | Senna spectabilis | Flower-dichloromethane Flower-methanol |
17.78 ± 1.73 19.26 ± 2.31 |
[223] | |
| Fusarium oxysporum | Percent inhibition at 1,000 ppm | Senna spectabilis | Stem-dichloromethane Stem-methanol |
5.19 ± 0.58 44.44 ± 0.00 |
[223] | |
| Helminthosporium oryzae | Minimum inhibitory concentration | Senna alata | Aqueous flower extracts | 15 mg/mL | [198] | |
| Microsporum audouinii | Minimum inhibitory concentration | Senna alata | Aqueous flower extracts | 15 mg/mL | [198] | |
| Microsporum canis | Cup-plate method, mean zone of inhibition | Senna alata | Ethanolic leaf extract | 14.4-30 mm | [207] | |
| Microsporum canis | Cup-plate method, mean zone of inhibition | Senna alata | Aqueous leaf extracts | 17.20-32.0 mm | [207] | |
| Microsporum canslaslomyces | Agar diffusion method | Senna alata | Ethanolic steam bark 5.00 mg/mL & 10 mg/mL | 12 mm/13.5 mm | [206] | |
| Microsporum canslaslomyces | Minimum inhibitory concentration | Senna alata | Steam bark-ethanol | 5 mg/mL | [206] | |
| Microsporum canslaslomyces | Minimum fungicidal concentration | Senna alata | Steam bark-ethanol | 5 mg/mL | [206] | |
| Microsporum gypseum | Agar diffusion and broth dilution method, minimum inhibitory concentration | Senna alata | Leaf-crude ethanol extract | 10.42 mm | [208] | |
| Microsporum gypseum | Hyphal growth inhibition concentration (IC50) | Senna tora | Leaf-methanol | 1.8 mg/mL | [209] | |
| Microsporum gypseum | Hyphal growth inhibition concentration (IC50) | Senna alata | Leaf-methanol | 0.8 mg/mL | [209] | |
| Microsporum gypseum | Agar diffusion and broth dilution method, minimum inhibitory concentration | Senna alata | Leaf-crude ethanol extract | 10.42 mm | [208] | |
| Penicillium notatum | Cup-plate method, mean zone of inhibition | Senna alata | Ethanolic leaf extract | 19.4-30 mm | [207] | |
| Penicillium notatum | Cup-plate method, mean zone of inhibition | Senna alata | Aqueous leaf extracts | 15.20-22.0 mm | [207] | |
| Penicillium marneffei | Hyphal growth inhibition concentration (IC50) | Senna tora | Leaf-methanol | 1.8 mg/mL | [209] | |
| Penicillium marneffei | Hyphal growth inhibition concentration (IC50) | Senna alata | Leaf-methanol | 6.6 mg/mL | [209] | |
| Phytophthora parasitica | Percent inhibition at 1,000 ppm | Senna spectabilis | Leaf-dichloromethane Leaf-methanol |
−28.57 ± 0.00 −24.29 ± 2.65 |
[223] | |
| Phytophthora parasitica | Percent inhibition at 1,000 ppm | Senna spectabilis | Flower-dichloromethane Flower-methanol |
−27.14 ± 1.99 −1.90 ± 2.31 |
[223] | |
| Phytophthora parasitica | Percent inhibition at 1,000 ppm | Senna spectabilis | Stem-dichloromethane stem-methanol | −17.62 ± 2.08 44.76 ± 1.15 |
[223] | |
| Rhizoctonia solani | Percent inhibition at 1,000 ppm | Senna spectabilis | Leaf-dichloromethane Leaf-methanol |
0.00 ± 0.00 27.41 ± 0.58 |
[223] | |
| Rhizoctonia solani | Percent inhibition at 1,000 ppm | Senna spectabilis | Flower-dichloromethane Flower-methanol |
47.04 ± 2.52 22.22 ± 4.58 |
[223] | |
| Rhizoctonia solani | Percent inhibition at 1,000 ppm | Senna spectabilis | Stem-dichloromethane Stem-methanol |
37.78 ± 1.00 29.63 ± 0.00 |
[223] | |
| Trichophyton mentagrophyte | Cup-plate method, mean zone of inhibition | Senna alata | Aqueous leaf extracts | 20.20-35.0 mm | [207] | |
| Trichophyton mentagrophyte | Agar diffusion and broth dilution method, minimum inhibitory concentration | Senna alata | Leaf-crude ethanol extract | 19.64 mm | [208] | |
| Trichophyton mentagrophyte | Cup-plate method, mean zone of inhibition | Senna alata | Ethanolic leaf extract | 16.4-30 mm | [207] | |
| Trichophyton mentagrophytes | Agar cup method, clearing zone | Senna alata | Leaf-hexane extract | 14-18 mm | [222] | |
| Trichophyton mentagrophytes | Agar cup method, clearing zone | Senna alata | Leaf-chloroform extract | 22-26 mm | [222] | |
| Trichophyton mentagrophytes | Agar cup method, clearing zone | Senna alata | Leaf-ethyl acetate extract | 16-18 mm | [222] | |
| Trichophyton mentagrophytes | Agar diffusion method | Senna alata | Ethanolic steam bark 5.00 mg/mL & 10 mg/mL | 17 mm/19 mm | [206] | |
| Trichophyton mentagrophytes | Minimum inhibitory concentration | Senna alata | Steam bark-ethanol | 5 mg/mL | [206] | |
| Trichophyton mentagrophytes | Minimum fungicidal concentration | Senna alata | Steam bark-ethanol | 5 mg/mL | [206] | |
| Trichophyton rubrum | Hyphal growth inhibition concentration (IC50) | Senna tora | Leaf-methanol | 1.2 mg/mL | [209] | |
| Trichophyton rubrum | Hyphal growth inhibition concentration (IC50) | Senna alata | Leaf-methanol | 0.5 mg/mL | [209] | |
| Trichophyton rubrum | Agar diffusion and broth dilution method, minimum inhibitory concentration | Senna alata | Leaf-crude ethanol extract | 18.75 mm | [208] | |
| Trichophyton verrucosum | Agar diffusion method | Senna alata | Ethanolic steam bark 5.00 mg/mL & 10 mg/mL | 15 mm/21 mm | [206] | |
| Trichophyton verrucosum | Minimum inhibitory concentration | Senna alata | Steam bark-ethanol | 5 mg/mL | [206] | |
| Trichophyton verrucosum | Minimum fungicidal concentration | Senna alata | Steam bark-ethanol | 5 mg/mL | [206] | |
|
| ||||||
| Antiviral activity | Herpes simplex | Plaque-inhibition method, reduction factor was measured | Senna occidentalis | Whole plant-ethanolic extract | 1 0 μg/mL | [212] |
| HIV-1 | HIV-1 RT inhibitory assay, % inhibition ratio | Senna alata | Aerial part-ethanolic extract | 35.86 | [216] | |
| HIV-1 | HIV-1 RT inhibitory assay, % inhibition ratio | Senna alata | Aerial part-water extracts | 37 | [216] | |
| Coxsackie | Plaque-inhibition method, reduction factor was measured | Senna occidentalis | Whole plant-ethanolic extract | 1 μg/mL | [212] | |
| Measles | Plaque-inhibition method, reduction factor was measured | Senna occidentalis | Whole plant-ethanolic extract | 1 μg/mL | [212] | |
| Poliomyelitis | Plaque-inhibition method, reduction factor was measured | Senna occidentalis | Whole plant-ethanolic extract | 1 μg/mL | [212] | |
| Semliki forest | Plaque-inhibition method, reduction factor was measured | Senna occidentalis | Whole plant-ethanolic extract | 1 μg/mL | [212] | |
| Vesicular stomatitis | Plaque-inhibition method, reduction factor was measured | Senna occidentalis | Whole plant-ethanolic extract | 1 μg/mL | [212] | |
|
| ||||||
| Herbicidal activity | Brassica chinensis | Percent inhibition germination at 10,000 ppm | Senna spectabilis | Leaf-dichloromethane Leaf-methanol |
12.66 ± 2.89 25.28 ± 7.77 |
[223] |
| Brassica chinensis | Percent inhibition germination at 10,000 ppm | Senna spectabilis | Flower-dichloromethane Flower-methanol |
71.38 ± 3.06 8.03 ± 0.58 |
[223] | |
| Brassica chinensis | Percent inhibition germination at 10,000 ppm | Senna spectabilis | Stem-dichloromethane Stem-methanol |
6.90 ± 1.00 1.14 ± 1.53 |
[223] | |
| Brassica chinensis | Percent inhibition hypocotyl at 10,000 ppm | Senna spectabilis | Leaf-dichloromethane Leaf-methanol |
68.09 ± 4.00 97.33 ± 1.31 |
[223] | |
| Brassica chinensis | Percent inhibition hypocotyl at 10,000 ppm | Senna spectabilis | Flower-dichloromethane Flower-methanol |
99.67 ± 0.58 91.40 ± 1.31 |
[223] | |
| Brassica chinensis | Percent inhibition hypocotyl at 10,000 ppm | Senna spectabilis | Stem-dichloromethane Stem-methanol |
−42.94 ± 5.18 34.75 ± 2.88 |
[223] | |
| Brassica chinensis | Percent inhibition radical 10,000 ppm | Senna spectabilis | Leaf-dichloromethane Leaf-methanol |
84.48 ± 2.63 100.00 ± 0.00 |
[223] | |
| Brassica chinensis | Percent inhibition radical 10,000 ppm | Senna spectabilis | Flower-dichloromethane Flower-methanol |
100.00 ± 0.00 100.00 ± 0.00 |
[223] | |
| Brassica chinensis | Percent inhibition radical 10,000 ppm | Senna spectabilis | Stem-dichloromethane Stem-methanol |
−46.94 ± 7.82 99.94 ± 1.74 |
[223] | |
| Chloris barbata | Percent inhibition germination at 10,000 ppm | Senna spectabilis | Leaf-dichloromethane Leaf-methanol |
72.71 ± 0.00 100.00 ± 0.00 |
[223] | |
| Chloris barbata | Percent inhibition germination at 10,000 ppm | Senna spectabilis | Flower-dichloromethane Flower-methanol |
100.00 ± 0.00 95.50 ± 0.58 |
[223] | |
| Chloris barbata | Percent inhibition germination at 10,000 ppm | Senna spectabilis | Stem-dichloromethane Stem-methanol |
4.50 ± 1.00 95.50 ± 0.58 |
[223] | |
| Chloris barbata | Percent inhibition shoot at 10,000 ppm | Senna spectabilis | Leaf-dichloromethane Leaf-methanol |
85.31 ± 7.45 100.00 ± 0.00 |
[223] | |
| Chloris barbata | Percent inhibition shoot at 10,000 ppm | Senna spectabilis | Flower-dichloromethane Flower-methanol |
100.00 ± 0.00 98.82 ± 2.68 |
[223] | |
| Chloris barbata | Percent inhibition shoot at 10,000 ppm | Senna spectabilis | Stem-dichloromethane Stem-methanol |
38.82 ± 3.19 96.93 ± 4.59 |
[223] | |
| Chloris barbata | Percent inhibition root at 10,000 ppm | Senna spectabilis | Leaf-dichloromethane Leaf-methanol |
88.18 ± 8.75 100.00 ± 0.00 |
[223] | |
| Chloris barbata | Percent inhibition root at 10,000 ppm | Senna spectabilis | Flower-dichloromethane Flower-methanol |
100.00 ± 0.00 98.72 ± 1.79 |
[223] | |
| Chloris barbata | Percent inhibition root at 10,000 ppm | Senna spectabilis | Stem-dichloromethane Stem-methanol |
25.56 ± 2.55 96.49 ± 3.53 |
[223] | |
↓: inhibition; HIV: human immunodeficiency virus; RT: reverse transcriptase.
The antifungal and antibacterial activity of the genus Senna varies depending on the species of the plant, the species of the microorganism, and the factors that affect the yield of the extraction process, such as the extraction method, the solvent used, the portion of the plant, and the secondary metabolite.
Ogunjobi and Abiala [203] investigated in vitro antimicrobial effect of different solvent extracts of S. alata leaves by using the agar well diffusion method. Except for Aspergillus niger inhibition, ethanol extract of S. alata showed a more inhibition zone than water extract. The best antimicrobial properties of S. alata ethanolic extract were shown in A. niger with a 25.2 mm zone of inhibition, and the least was Salmonella typhimurium with a 12.1 mm inhibition zone. Escherichia coli and Candida albicans had similar inhibition zone with 17.2 mm and 18.2 mm. In addition, ethanol extract of S. alata demonstrated effective antimicrobial activity of Staphylococcus aureus and Aspergillus flavus with 20.1 mm and 22.1 mm zone of inhibition. S. alata water extract observed the best effective antimicrobial characteristics against A. niger and A. flavus with 27.2 mm and 20.1. The other zone of inhibition was followed by S. aureus with 18.2 mm and C. albicans with 14.1 mm. The least effective antimicrobial activity was of aqueous extracts of S. alata against E. coli and S. typhimurium having inhibition zones of 10.2 mm and 10.1 mm, respectively.
Makinde et al. [204] research was about the methanol-water extract of S. alata leaves, and extract was assessed for antimicrobial activity by using a disc diffusion method (in vitro assay). The results indicated that S. alata leaves are more effective against fungi. S. alata phenolics and terpenoids, alkaloid salt, alkaloid base, and aqueous extract showed antimicrobial activity against Microsporum canis, Blastomyces dermatitidis, Trichophyton mentagrophytes, C. albicans, and A. flavus with 10–30 mm zone of inhibition. Phenolics and terpenoids, alkaloid salt, and alkaloid base extract of S. alata leaves had provided 5 mm of inhibition of S. aureus, Corynebacterium parvum, Nocardia asteroides, and Clostridium septicum; however, the aqueous extract had not shown antimicrobial activity of these bacteria. Phenolic and terpenoids and aqueous extract of S. alata leaf had 5–10 mm inhibition zone of Dermatophilus congolensis. Alkaloid salt and alkaloid base S. alata extract's inhibition zone of D. congolensis was 10–20 mm and 20–30 mm. Besides, S. alata antimicrobial activity was not observed against Proteus vulgaris and Bacillus pumilus.
Ehiowemwenguan et al. [205] examined the S. alata leaves and roots antimicrobial effect by using the cup plate agar diffusion method. Except for Streptococcus pyogenes, all inhibition zone is less than 5 mm; moreover, hot water extract, methanol extract, and acetone extract S. alata root and leaves did not differentiate among their inhibition zone. S. pyogenes had the highest zone inhibition at 5–6 mm both root and leaf extract independent of solvent type. S. alata root and leaves exhibited antimicrobial and antifungal reaction against E. coli, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhi, Shigella flexneri, S. aureus, A. flavus, A. niger, C. albicans, and Cryptococcus neoformans. S. alata root extract's MIC level was changed (5–8 mg/mL) for bacteria species except for S. pyogenes (3 mg/mL); however, fungi needed more concentration, approximately 25-50 mg/mL for inhibition except for C. neoformans (6 mg/mL). The results of MIC level of leaves for bacteria were similar to root extract; yet, MIC range was between 6 and 10 mg/mL for bacteria. The leaf extract MIC was 35-50 mg/mL except C. neoformans (13 mg/mL). The minimum microbial concentration of S. alata leaf extract for bacteria was determined between 6 and 10 mg/mL, and fungi had more minimum microbial concentration at 25–50 mg/mL except C. neoformans (13 mg/mL). The minimum microbial concentration of root extract results was similar to leaf extract except for S. pyogenes (3 mg/mL) and C. neoformans (6 mg/mL).
Channa et al. [195] also detected antibacterial activity in root, stem, seeds, leaves, and flower extracts (methanol, ethanol, and water) of S. alata. In this study, a good diffusion method was used, and the results were between 8 and 34 mm. The least inhibition zone, 8 mm, was observed against S. aureus and Klebsiella pneumoniae by root-methanol, root-ethanol, leave-ethanol, stem-ethanol, and stem-water extraction. The maximum inhibition zone was observed against E. coli by leaves-methanol extraction. Furthermore, the results showed that flowers and leaves of S. alata possess antibacterial activity as compared to commercial drugs such as ciprofloxacin, penicillin, ampicillin, tetracycline, and gentamicin.
Sule et al. [206] experimented to determine in vitro antifungal activities of S. alata crude stem bark extract by using the agar diffusion method. Zones of inhibition were observed at 5 mg/mL and 10 mg/mL ethanol solvent of S. alata crude steam bark except for T. mentagrophytes. T. mentagrophytes has the highest inhibition zone with 17 mm at 5 mg/mL concentration. The inhibition zone followed the order as Epidermophyton floccosum with 15.5 mm, Trichophyton verrucosum with 15.0 mm, and Microsporum canslaslomyces with 12.0 mm at 5 mg/mL concentration. T. verrucosum and E. floccosum showed the best inhibition of zone with 21.0 mm and 20.5 mm at 10 mg/mL concentration. M. canslaslomyces had again the least zone of inhibition with 13.50 mm at a concentration of 10 mg/mL. However, a concentration of 10 mg/mL was effective against T. mentagrophytes with 19 mm inhibition of the zone. In addition, T. mentagrophytes was the only fungi that affected the inhibition at 2.5 mg/mL concentration with 10 mm zone. The MIC was evaluated at 5 mg/mL for all fungi. Minimum 5 mg/mL fungicidal concentration was appropriate for inhibition of fungi, except E. floccosum. The minimum fungicidal concentration of E. floccosum was determined at 10.0 mg/mL.
Abubacker et al. [198] conducted a study for in vitro antifungal properties of S. alata aqueous flower extracts, using three different fungal groups including fungi that produce aflatoxin (A. flavus and Aspergillus parasiticus), plant pathogenic fungi (Fusarium oxysporum and Helminthosporium oryzae), and human pathogenic fungi (C. albicans and Microsporum audouinii). The results highlighted the strong antifungal activity of S. alata. While 15 mg/mL of flower extract concentration provides 100% inhibition of all the fungus, 10 mg/mL was enough in inhibiting A. flavus. The MIC values of the flower extract of S. alata ranged from 5.75 to 8.0 mg/mL.
In a different investigation, Timothy et al. [207] assessed leaf extracts of S. alata (aqueous and ethanol) against five pathogenic fungi which are C. albicans, M. canis, T. mentagrophyte, Penicillium notatum, and A. niger. According to the calculated zones of inhibition, there was no inhibition for water extract of leaves whereas ethanol extracts exhibited inhibition for all tested microorganisms. Furthermore, MIC of ethanol extracts for all tested fungi was lower than the water extract indicating that ethanol extract includes more bioactive compounds than the water extract. The reason ethanol is being more effective than the water was told to be because of the presence of anthraquinone which is not found in the water extraction. Intense antifungal activities of S. alata were depicted from the study outcomes.
Wuthi-udomlert et al. [208] remarked on the importance of anthraquinone derivatives in the in vitro evaluation. Anthraquinone glycosides including emodin, rhein, and chrysophanol found in S. alata are the source of laxative effects. In the study, extraction of leaves is obtained in five different ways using anthraquinone aglycone, anthraquinone glycoside, anthraquinone aglycone from glycosidic fraction, crude ethanol, and anthraquinone aglycone from crude ethanol extract. Extraction yields were monitored by thin-layer chromatography, and the highest yield is obtained from crude ethanol extraction which was 34.94% w/w. As a result of the in vitro antifungal activity against Trichophyton rubrum, T. mentagrophytes, E. floccosum, and Microsporum gypseum by diffusion and broth dilution methods, anthraquinone aglycone from glycosidic fraction presented greater activity among five different extracts.
Phongpaichit et al.'s [209] experiment was about antifungal activities of S. alata and S. tora. Except Penicillium marneffei, 10 mg/mL methanolic extract obtained from leaves of S. alata and S. tora were enough in inhibiting all the M. gypseum, and T. rubrum. 10 mg/mL S. alata leaves inhibited only 77% of P. marneffei; still, S. tora extract was sufficient to inhibit all P. marneffei. In addition, IC50 result of T. rubrum followed the order as S. alata at 0.5 mg/mL and S. tora at 1.2 mg/mL. S. alata with 0.8 mg/mL IC50 value was the best inhibition for M. gypseum; also S. tora have an IC50 value at 1.8 mg/mL. The IC50 values of P. marneffei of S. alata and S. tora were 6.6 mg/mL and 1.8 mg/mL, respectively.
Malmir et al. [113] drew attention to the bioactive substance called “rhein” isolated from S. podocarpa root hydroethanol extract. In their study, S. podocarpa root extracts were evaluated for in vitro anti-Neisseria gonorrhoeae activity. Gonorrhoea is a widespread sexually transmitted infectious disease induced by N. gonorrhoeae bacterium infection. N. gonorrhoeae infects the mucous membranes of the reproductive organs that include the fallopian tubes, uterus, and cervix in women, while in men and boys it infects the urethra. N. gonorrhea can also harm the mucous membranes of the mouth, throat, and eyes [210]. S. podocarpa root demonstrated anti-N. gonorrhoeae activity against all strains. MIC ranged from 100 to 400 mg/L. The most active fractions having 50–100 mg/L MIC values, had rhein, emodin, chrysophanol, and physician as their key compounds as detected by LC-UV/DAD cochromatography with reference standards. Among all the isolates, rhein (MIC: 3.13 mg/L against all test strains) was the most effective. In addition to rhein, Sansores-Peraza et al. [117] highlighted the antibacterial and antifungal activity of cassine, isolated from S. racemosa, with MIC of 2.5 mg/mL against S. aureus and Bacillus subtilis and 5.0 mg/mL for C. albicans.
Albayrak et al. [211] indicated that infusion of S. alexandrina leaves is the only herb that has antibacterial effect against Bacillus cereus among infusions of eight plants in Turkey which are Foeniculum vulgare Mill. (fennel), Pimpinella anisum L. (anise), Laurus nobilis L. (laurel), Tilia × europaea L. (linden tea), Urtica dioica L. (nettle), Petroselinum crispum (Mill.) Fuss (parsley), and Anethum graveolens L. (dill). In the study, they extracted S. alexandrina leaves by four methods which are methanol extraction, infusion, decoction, and hydrosol. The in vitro antimicrobial activities of S. alexandrina leaves were evaluated, and the results showed that infusion of Senna leaves has antibacterial effect against B. cereus and methanol extracts of Senna have antibacterial activity against B. cereus and P. aeruginosa.
As a result of in vitro antibacterial analysis conducted by Jain et al. [212], although Klebsiella aerogenes exhibited resistance to all extracts, ethanol extracts of flowers and pods of S. occidentalis provide inhibition of growth of E. coli and P. vulgaris. In addition, a descending sort among bioactive compounds according to the antibacterial activities against test bacteria which are E. coli, K. aerogenes, P. vulgaris, and S. aureus was reported as anthraquinones>sennosides>flavonoids. Antifungal activity of ethanol extracts of S. occidentalis was found to be higher than the antibacterial activity. Among the metabolite-rich fractions, the maximum inhibition was shown by sennosides against A. flavus, followed by anthraquinones and flavonoids against Curvularia lunata.
7.2. Antiviral
Antiviral activity of genus Senna is generally found quite low; however, the extraction yield and the isolation of bioactive compounds provide an increase in the antiviral activity.
Jain et al. [212] investigated the antimicrobial, antitumor, and antiviral activity of ethanol extracts of S. occidentalis. They conducted in vitro analysis for antiviral and in vivo analysis for antitumor activity. The antiviral activity against Herpes simplex was quite inadequate; the reduction factor of titre was found 10 μg/mL. In addition, S. occidentalis did not exhibit any antitumor activity or cytotoxicity.
Ogbole et al. [213] highlighted the antiviral agents that S. siamea includes which are lupenone, lupeol, betulinic acid, chrysophanol, physicon, and β-sitosterol glucoside. Among tested anthraquinones and triterpenoids, lupeol was the most effective constituent against poliovirus having 0.014 μg/mL of IC50 value. Antipoliovirus, antitobacco mosaic virus, and anti-HIV-1 effects were observed in the extract of S. siamea stem bark [135, 214].
Another genus which is analyzed for the antiviral activity is S. alata. Shaheen et al. [215] determined the antiviral activity of methanol, chloroform, ethyl acetate, n-butanol, and aqueous extracts of S. alata by in vitro and in vivo experiments. The results justified the antiviral activity of Senna; all extracts exhibited antiviral effects against cardiac coxsackievirus B3. As a result of in vitro analysis, the therapeutic index varied between 0.2 and 12. In vivo, virus titer values were between 0 log10 and 2.5 log10. Both in vitro and in vivo analyses exhibited that the most effective extracts against cardiac coxsackievirus B3 were aqueous extracts. Woradulayapinij et al. [216] investigated in vitro HIV-1 reverse transcriptase inhibitory activity of ethanol and water extracts of aerial part of S. alata. Even though the results were quite close to each other, water extract depicted higher activity than the ethanol extract; inhibition ratios were 37 and 35.86% for water and ethanol extracts, respectively.
7.3. Antiprotozoal
Numerous studies reported antiprotozoal activities of genus Senna. de Castro et al. [201] conducted a study about the schistosomicidal activity of S. spectabilis flower extracts. Schistosoma is an intestinal parasite that causes a chronic disease called Schistosomiasis. The disease has been reported in 78 countries; especially, 90% of the cases have been reported in Africa where access to safe drinking water is a challenge. According to the WHO [217], at least 229 million people needed the treatment of Schistosoma in 2018. de Castro et al. [201] extracted and isolated (-)-cassine and (-)-spectaline substances from S. spectabilis flowers. In vitro activity of extracts, their fractions, and the mixture of (-)-cassine and (-)-spectaline against S. mansoni worms were analyzed. Obtained data indicated that the mixture of (-)-cassine and (-)-spectaline exhibited a multitarget mechanism against the excretory activity, tegument lesions, and neuromotor activity. It also showed a toxic effect on the larval period of cercariae. Therefore, S. spectabilis flower extracts (-)-cassine and (-)-spectaline have a great potential for their schistosomicidal activity. Furthermore, de Albuquerque Melo et al. [218] mentioned about leishmanicidal activity of S. spectabilis and the two major alkaloidal metabolites (−)-cassine/(−)-spectaline. Caamal-Fuentes et al. [219] studied antiprotozoal properties of S. racemosa against Giardia intestinalis and observed that methanolic extracts of S. racemosa bark in both in vitro and in vivo experiments had activity against G. intestinalis [219, 220]. Eguale et al. [221] mentioned the in vitro anthelmintic activity of S. occidentalis, and the extract concentration required to inhibit 50% (ED50) of the eggs of Haemonchus contortus was found to be 0.13 mg/mL and 0.17 mg/mL for aqueous and hydroalcoholic extracts, respectively.
A scheme with anti-infectious properties of Senna plants is summarized in Figure 4.
Figure 4.
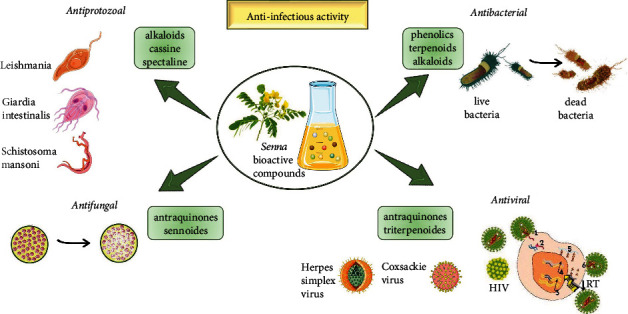
Anti-infectious properties of the most representative bioactive compounds of Senna plants. Botanical molecules such us alkaloids, sennoides, anthraquinones, phenolics, terpenoids, alkaloids, and triterpenoids have anti-infectious activity against bacteria, fungus, protozoa, and viruses (HIV, Coxsackie, and Herpes simplex).
7.4. Other Biological Properties
Villaseñor et al. [222] also conducted research on S. alata leaf extracts with hexane, chloroform, and ethyl acetate to investigate antimutagenic, antifungal, analgesic, anti-inflammatory, and hypoglycemic activities. Chloroform extract exhibited a reduction in the mutagenic activity of tetracycline by 65.8% at a dosage of 2 mg/20 g mouse as a result of the in vivo analysis. Against fungi, T. mentagrophytes chloroform extract was the most effective. The hexane extract was having the highest analgesic property which provides a decrease of 59.9% at a dosage of 5 mg/20 g mouse among other extracts. The analgesic activity of hexane extract was similar to the activity of mefenamic acid which is a widely known analgesic. For the anti-inflammatory activity, all three extracts are observed hourly, for three hours. At the end of three hours, hexane and ethyl acetate extracts demonstrated 65.5% and 68.2% inhibition, respectively, at a dosage of 5 mg/20 g mouse. Ethyl acetate extract also showed hypoglycemic activity more effectively than the other extracts by providing a 56.7% reduction in blood glucose level.
8. Clinical Studies
Health-promoting effects of Senna and its other species have been evaluated by a large number of researchers around the world while clinical trials have been conducted in limited cases (Table 4). Therefore, in this section, we are presenting quantified data on Senna and its clinical trials (previous and latest).
Table 4.
Summary of some clinical trials conducted on Senna spp.
| Samples | Type of study/findings/results | Country | Ref |
|---|---|---|---|
| Senna alata (L.) Roxb. | Randomized controlled trial Trial registration: TCTR0180828004 Evaluating the use and safety of S. alata on bowel function recovery among women with gynecologic cancer 90 women candidates diagnosed with gynecologic cancer were randomly assigned to postoperative consumption (45 with S. alata tea and 45 with warm water) Usage of S. alata significantly reduced the time of first passage of flatus (mean difference: -8.5 h; 95% confidence interval: -3.7, -13.4 h) and time of first defecation (mean difference: -19.8 h; 95% confidence interval: -11.2, -28.5 h) compared with the controls The use of S. alata showed a positive impact during the postoperative care of gynecologic cancer patients |
Thailand | [231] |
|
| |||
| Senna | Randomized controlled and crossover study Assessing the efficacy and safety of Senna versus polyethylene glycol in treating constipation in children The proportional formula was used to calculate the sample size and 28 patients were obtained Effectiveness of laxative therapy was evaluated by mean of a three-variable construct (a) Daily bowel movement (b) Faecal soiling (c) S clean abdominal X-ray The study was completed before the time because an interim analysis showed effective results of Senna (p = 0.026) The maximum daily dose of Senna and polyethylene glycol was recorded as 38.7 mg and 17 g Senna therapy showed promising results against constipation in children with anorectal malformation |
Mexico | [232] |
|
| |||
| Senna | Comparative study Evaluating of Senna and other oral bowel medicines for treating constipation in pediatric oncology patients getting opioids The results of 5-year investigation demonstrated that 41.8% (n = 245) had blood cancer, 50.3% (n = 295) had solid cancer, and 7.9% (n = 46) had brain cancer out of 586 matched samples (age: 0-20 years, ave. age: 11.5 years) Initializing Senna therapy, over another oral bowel medication, reduced the subsequent risk of surrogate markers of problematic constipation. Adjusted effect of Senna on enema (hazard ratio, 0.31; 95% confidence interval, 0.11-0.91), abdominal radiographic imaging (hazard ratio, 0.74; 95% confidence interval, 0.55-0.98), and escalation of oral bowel medicine (hazard ratio, 0.78; 95% confidence interval, 0.59-1.03) were recorded |
Philadelphia | [233] |
|
| |||
| Senna | Control single-blinded randomized study Assessing the efficacy and safety of gum chewing added to high dose Senna before colonoscopy promotes bowel cleaning 129 candidates participated and were further divided into two groups (a) n = 65 patients treated with Senna solution (150 mL) and sennoside tablet (80 mg) daily for 3 days before the colonoscopy (b) n = 64 patients were additionally advised to chew sugarless gum half an hour (three times) daily for 3 days The results demonstrated that gum chewing enhanced colonoscopy bowel preparation quality and is considered a physiologically sound, safe, and impassive part of the colonoscopy bowel preparation. The gum chewing group showed better cleaning compared to other groups |
Turkey | [234] |
|
| |||
| Senna | Placebo-controlled, double-blinded, randomized study Evaluating the use of Senna with docusate for constipation after pelvic surgery 96 candidates completed a baseline seven-day bowel diary pre- and postsurgery. After pelvic surgery, candidates were divided into two groups: (a) n = 45 in the placebo group and (b) n = 48 in Senna (8.6 mg) with docusate (50 mg) group. The findings demonstrated that the use of Senna with docusate decreases the time to first bowel movement in those undergoing pelvic surgery than placebo (3.00 vs. 4.05 days; p = 0.001). |
Philadelphia | [235] |
|
| |||
| Senna | Case study Case of a 31-year-old female patient who, after prolonged ingestion of Senna extract, developed severe weight loss, cyclic oedema, and dyspepsia, accompanied by an asymptomatic increase in markers of liver and muscle damage, dyslipidemia, electromyographic alterations, and mitochondrial myopathy in the muscle biopsy This clinical case is of particular significance, given that Senna is widely used for its pharmacological properties, with failure to consider its potentially toxic effects |
Portugal | [236] |
|
| |||
| Senna | Single-blinded randomized study The effectiveness of Senna tables and sodium phosphate solution for bowel preparation before colonoscopy was examined for its efficiency A total of 134 candidates were treated with Senna tablets (180 mg) and sodium phosphate solution (95 mL) on the day before colonoscopy The results demonstrated that the mean cleanliness scores in the four segments of the colon (rectum, sigmoid segments, descending colon, and transverse colon) except the cecum were higher in the sodium phosphate group than in the Senna group (7.9 vs. 8.3, 8.0 vs. 8.5, 7.9 vs. 8.5, 7.9 vs. 8.2, and 7.2 vs. 6.9, respectively) The taste of Senna was more effective compared to sodium phosphate solutions |
Thailand | [237] |
|
| |||
| Senna tora (L.) Roxb. | Experimental study Supplementation of S. tora fibre on the serum lipid profile of diabetic Korean patients was evaluated. S. tora fibre supplement of a combination of soluble fibre extracted from S. tora (2 g), alpha-tocopherol (200 mg), ascorbic acid (500 mg), and maltodextrin (300 mg) was prepared in a pack and given to a total of 15 candidates 2 packs per day up to 2 months The results demonstrated that S. tora fibre products were safe for consumption and additionally provided the necessary amount of dietary fibre for helping in the maintenance of lipid status in diabetic (type II) patients |
Korea | [238] |
|
| |||
| Senna | Controlled randomized single-blinded study Evaluating efficiency and acceptability of high dose Senna tablets and its comparison with standard polyethylene glycol in adult patients 192 patients participated and were treated into two groups: (a) n = 91 in polyethylene glycol group and (b) n = 101 in Senna group The Senna tablet group showed acceptable results for colon cleansing and tolerance compared to the polyethylene glycol group (p < 0.001) |
— | [239] |
|
| |||
| Senna | Controlled study Highly purified Senna extract was evaluated against cell proliferation, crypt length in the entire colon and gene expression (p53 and bcl-2). 171 patients (84 with sennoside-containing syrup and 87 without sennoside-containing syrup) were included 15 patients with Senna and 17 without Senna from 32 randomized patients were used for biopsies Proliferation activity in four areas of colon and gene expression (p53 and bcl-2) was evaluated by using 5-bromo-2′-deoxyuridine labelling, immunohistochemistry, and immunohistochemical The results demonstrated that crypts were shorter in the Senna group than without Senna group in the transverse and sigmoid colon. In the entire colon, the labelling index was higher in the Senna group than without the Senna group. In addition, bcl-2 expression was higher in both groups when crypts were shorter and proliferation was enhanced while no difference was recorded in p53 expression |
Netherlands | [240] |
|
| |||
|
Senna and MaZiRenWan (MZRW) (1st phase) |
A double-blinded, double-dummy, randomized, and controlled trial Trial registration: NCT01695850 The protocol evaluated the effectiveness of MaZiRenWan (MZRW) with laxative Senna for functional constipation 291 candidates were recruited, and after a 2-week run-in period, the suitable candidates were randomly grouped into the three viz (a) Chinese medicine arm (MZRW and western medicine placebo) (b) Western medicine arm (Senna and Chinese medicine placebo) (c) Placebo arm (Chinese medicine placebo and western medicine placebo) The results of the eight-week treatment showed the increased responder rate for a complete spontaneous bowel movement (CSBM≧1/week) in the course of the treatment while the eight-week follow-up period showed changes of colonic transit, individual and global symptom assessments, and adverse effects |
China | [241] |
|
| |||
|
Senna and MaZiRenWan (MZRW) (2nd phase) |
A double-blind, double-dummy, randomized, and controlled trial Trial registration: NCT01695850 Evaluating the efficacy and safety of Chinese herbal medicine MZRW and its comparison with the stimulant laxative Senna and placebo against functional constipation Primary and secondary outcomes demonstrated that the MZRW showed well-accepted effects in increasing complete spontaneous bowel movement per week compared to the Senna group (68.0% vs. 57.7%, with p = 0.14) during the treatment. After the eight-week-follow-up period, 47.4% of patients had a complete response to MZRW, 20.6% had a complete response to Senna, and 17.5% had a complete response to placebo (p < 0.005 for MZRW vs. placebo) |
China | [242] |
Mcnicol [225] performed a clinical experiment to evaluate the activity of tablets prepared using Senna (standardized preparation) on human bowel function and its possible side effects. The experiment was carried out in two phases: (a) first is the administration of the drug to 52 ward patients; (b) the drug was administered to 126 volunteer medical students. The Senna tablets were prepared in two different batches. The results demonstrated that the mean values for “speed of action” of Senna preparation (3 tablets) were recorded as 9.7 hours with ward patients and 12.15 hours among student volunteers, respectively. The frequency of griping, looseness of stool, and multiple bowel movements in ward patients have been recorded in dose-dependent patterns (increased with rising dosage). In addition, results confirmed that there is no significant difference between male and female responses. Thamlikitkul et al. [226] performed a randomized controlled experiment to evaluate the efficacy of S. alata against constipation. A total of 80 candidates participated in this study, and the differences observed between both groups (placebo & mist. Alba; and placebo & S. alata) were statistically highly significant (p < 0.001).
Kinnunen et al. [227] evaluated the safety and efficacy profile of laxatives containing Senna in treating constipation patients using lactulose as standard medication. The present study was carried out in a total of 30 patients (mainly bed-ridden due to degenerative diseases, age: 65-94 years). One week run-in without laxatives was followed by 5 weeks (a) of a daily dose of 14.8 mg (20 mL) laxative plus Senna or 20.1 mg (30 mL) lactulose and (b) crossed medicines (5-week period). The results indicated that bulk laxative plus Senna (14.8 mg dose) when given daily resulted in significantly (p < 0.005) more frequent bowel habits (4.5 vs. 2.2-19/week) compared to that of lactulose (daily dose of 14.8 mg). In other words, bulk laxative plus Senna produced efficiently treated constipation patients.
Damodaran and Venkataraman [228] from India reported the therapeutic effectiveness of S. alata leaves against Pityriasis versicolor in humans. The study was completed among 200 candidates (age: 16-60 years) of Tamil Nadu (Indian State) within 10 years. Different concentrations of plant extract (80%, 90%, and 100%) were used at affected areas (trunk, neck, hands, and face) of the body. The results indicated that S. alata leaf extract could be employed as a herbal remedy having no side effects, for curing P. versicolor.
Ramesh et al. [229] carried out a controlled comparative study of Misrakasneham (Ayurvedic formulation) and laxative Senna tablets (purified Senna extract) against opioid-induced constipation. Misrakasneham (a combination of 21 different types of herbs, castor oil, purified butter, and milk) is a centuries-old Ayurvedic medicine. The present study was conducted in 50 patients with advanced cancer aged 15 years and categorized into two groups (25 each). The first group received Misrakasneham while the second group received Senna tablets in three steps during the 14-day study. The results demonstrated that 85% of the Misrakasneham group and 69% of the laxative Senna group had satisfactory bowel movements with no statistical difference (p > 0.2). In addition, Misrakasneham data showed interesting results in terms of efficacy and was recommended as a possible candidate for opioid-induced constipation.
van Gorkom et al. [230] reported the effects of sennosides on histology of colonic mucosa and bowel preparation. In this experiment, a total of 171 candidates participated who were further split into two groups: (a) n = 84 candidates treated with 1 mL/kg of a syrup containing 2 mg/mL sennosides A and B and 3-5 L of a lavage solution and (b) n = 87 candidates treated with 3-5 L of lavage solution. The results demonstrated that both groups showed no difference in tolerance or quality of bowel preparation. In addition, group a (10/19) also showed a rapid increase of mononuclear infiltrate in the lamina propria compared to group b (2/21), respectively (p = 0.0005).
9. Safety and Side Effects
In traditional medicine, the leaves of Senna traditionally are used as laxatives in the form of pellets prepared with dried figs and plums. The anthraquinone laxatives like Senna are extremely useful drugs, but appropriate usage is highly important, although most of the reported side effects are mild and transit.
Senna is generally safe and well tolerated but can cause adverse events when it is used in high doses and for a long period (Figure 5). Most of the adverse effects are mild and transient. The liver injury, including hepatotoxicity, has been reported in several case studies when Senna has been used prolonged, and the symptoms were mild-to-moderate in severity and solved rapidly with discontinuation [243–245]. In all cases, the correlation between side effects was explained by abuse of Senna in laxative purpose.
Figure 5.

Summarized scheme with side effects and clinical therapeutic limitations of Senna plant.
Derivatives of sennosides present in the leaves and pods may affect increasing irritability on the intestinal mucosa, which could cause abdominal pain and spasm in a sensitive person. It can also lead to diarrhoea, intensification of menstrual bleeding, and dark urine. It is recommended to take herbal tea or capsules/tablets/syrup of Senna in the evening before sleep, as effects start 6 to 12 hours later. Also, drugs that contain Senna are available in the form of rectal suppositories.
The prolonged use of Senna causing the spasm is the sign that it is necessary to stop future taken. In rare cases, vomiting and nausea may occur. Chronic use of Senna and other laxative herbs leads to increased potassium excretion, resulting in spasms, muscle weakness, and heart failure. However, in very well-explained patient conditions, these types of herbal drugs should be avoided. However, the full safety profile of these herbals is controversial like the opponent attitude of FDA and EMA regarding their consumption in some vulnerable groups of people.
10. Therapeutic Perspectives and Clinical Gaps
Traditional and modern medicines, in case of decreasing the intestine motility, take into consideration two classes of curative substances: drugs that increase the volume of the gut contents and facilitate mass flow [246, 247]. Among herbal substances, here, we have substances rich in sugars (dried plums and figs) and herbals with mucus, such as flax seeds. Another approach is medicines that contain substances that have a mild irritant effect on the intestinal mucosa to promote intestinal motility [248, 249]. Among these remedies are the species from the genus Senna.
Genus Senna is well recognized as the most used laxative herbal treatment, also available without a prescription. Even before we knew its composition, Senna was used for centuries in phytotherapy for the same purpose. The main type of Senna genus used in medicine is S. alexandrina, known in commerce as Alexandria Senna, and Tinnevelly Senna [250]. Senna plants are widely used herbal medicine in the treatment of functional constipation. As the beneficial parts of the plant in phytotherapy, both the mature pods and the dried leaves are used. They contain natural chemical compounds, called anthraquinone, which are glycoside derivatives of anthracene, and the major compounds are sennosides A and B, which are available in the market [251]. The sennosides A and B have been broken down by the bacterial flora in the colon and result in the production of the main active metabolites rhein and rheinanthrone [252]. The working of anthraquinones includes the hindrance of NaCl absorption in the colon and the stimulation of Cl secretion, by inhibiting the (Na+, K+)-ATPase [253].
Additionally, S. alexandrina is used in case of bowel irritable colon, as a pretreatment before diagnostic tests like colonoscopy [254] and as a supplement for weight reduction [255]. While the treatment with active compounds from genus Senna is widely used in different laxative drugs taken orally in liquid or solid dosage forms, in the form of instant tea and herbal tea, however, there are controversies in their usage.
European Medical Agency (EMA) reference the use of Senna [256] only in cases of periodical constipation, while long term is not recommended due to acute dehydration which is followed by loss of electrolytes. Also, EMA do not recommend Senna in case of pregnancy, breast feeding, dehydration, different forms of intestinal obstructions, ulcers, and ulcerative colitis, inflammatory bowel disease including Crohn's disease, pain and spasm in stomach, unknown etiology, and rectal bleeding.
EMA does not recommend using the Senna as a laxative treatment in children under 12, but off-label use has been reported (Figure 5). On the other hand, the USA Food and Drug Administration (FDA) prescribes 17.2 mg (7.5 to 30 mg) per day for people 12 years and older and 8.5 mg for children under 12 and allowing the usage of botanical laxatives containing Senna in children under 12. Based on a recently published review on Senna side effects as a long-term therapy in children by Vilanova-Sanchez et al. [257], Senna can be a safely employed option in treating functional constipation in children. However, more evidences are needed to confirm this conclusion and to change the attitude of EMA, who recently revised the herbal monography of Senna still stated that Senna is not recommended for children under 12 years [256].
Although some researches of Senna have found that it is effective in a short-term usage of constipation treatment in pregnancy [258–260] and does not have the teratogenic potential [261], intake of Senna during the pregnancy is allowed only in some countries like the USA.
Senna is still contraindicated by EMA recommendation because of experimental data that indicated possibly a genotoxic risk of several anthranoids, e.g., emodin and aloe-emodin [262]. While the use of Senna in breastfeeding women is not recommended, there is evidence that anthraquinone drugs in lactating mothers do not carry a risk of producing a laxative effect in the infant [263–265]. However, there are available data from other studies in which laxative effect on the bowels was observed in infants [258]. Despite controversial findings, still, the official recommendation is to avoid the use of it.
Senna should not be used for a longer period, no longer than 1-2 weeks, nor with medicines that lead to loss of potassium (diuretics, cardiotonic drugs, and corticosteroids). The caution should be exercised when used with antiarrhythmic and cardiotonic drugs and medicinal products inducing QT-prolongation, as it may potentiate their effect. All of these effects are correlated with hypokalemia [266, 267]. It has been found that usage of sennosides and digoxin in combination is linked with a modestly increased risk of digoxin toxicity in heart failure patients [268].
Particular attention, based on the animal studies, should be exerted in the patients with kidney and liver disorders during chronic use of Senna-based products [269]. Additionally, studies performed on rats showed that long-term administration of extracts of Senna does not promote gastrointestinal, liver, kidney, or adrenal tumors in the rats [270–272].
11. Concluding Remarks
This review showed that various parts of the Senna plant such as roots, stem, leaves, and seeds are traditionally used to treat many ailments and its extract has antioxidant, antimicrobial, and important health-promoting activities. These biological activities are attributed to the many phytochemicals contained in the genus Senna. Epicatechin, proanthocyanidins, scutellarein, rutin, and sennoides are just a few bioactive compounds of the genus Senna that are responsible for their bioactivity. Numerous studies in vitro and in vivo have been performed to establish the anti-infective and antioxidant properties of Senna extracts. Studies on the consumption of Senna over a period have shown that Senna is safe, but chronic use has adverse and limiting effects in medical practice. Among them, the laxative disease is a condition related to the massive use of Senna-based laxatives with an increased loss of potassium ions and the possibility of interaction with other drugs prescribed for heart disease. Based on the analysis of the studies selected in the study, this review opens new therapeutic perspectives of the Senna plant for antioxidant and especially anti-infective effects in the digestive tract.
Contributor Information
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Monica Butnariu, Email: monicabutnariu@yahoo.com.
Manoj Kumar, Email: manojkumarpuniya114@gmail.com.
Daniela Calina, Email: calinadaniela@gmail.com.
William C. Cho, Email: chocs@ha.org.hk.
Data Availability
The data supporting this review are from previously reported studies and datasets, which have been cited. The processed data are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Azani N., Babineau M., Bailey C. D., et al. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny: the legume phylogeny working group (LPWG) Taxon . 2017;66(1):44–77. doi: 10.12705/661.3. [DOI] [Google Scholar]
- 2.Robbiati F. O., Anton A., Marazzi B., Vásquez-Cruz M., Fortunato R. H. The evolutionary history of Senna ser. Aphyllae (Leguminosae–Caesalpinioideae), an endemic clade of southern South America. Plant Systematics and Evolution . 2017;303(10):1351–1366. doi: 10.1007/s00606-017-1450-7. [DOI] [Google Scholar]
- 3.Bradley Morris J., Tonnis B. D., Wang M. L. Variability for Sennoside a and B concentrations in eight Senna species. Industrial Crops and Products . 2019;139, article 111489 doi: 10.1016/j.indcrop.2019.111489. [DOI] [Google Scholar]
- 4.Irwin H. S., Barneby R. C. The American cassiinaea synoptical revision of leguminosae tribe cassieae subtribe cassiinae in the New World . New York USA: New York Botanical Garden Bronx; 1982. [Google Scholar]
- 5.Marazzi B., Endress P. K., Queiroz L. P. ., Conti E. Phylogenetic relationships within Senna (Leguminosae, Cassiinae) based on three chloroplast DNA regions: patterns in the evolution of floral symmetry and extrafloral nectaries. American Journal of Botany . 2006;93(2):288–303. doi: 10.3732/ajb.93.2.288. [DOI] [PubMed] [Google Scholar]
- 6.Acharya L., Mukherjee A. K., Panda P. C. Separation of the genera in the subtribe Cassiinae (Leguminosae: Caesalpinioidae) using molecular markers. Acta Botânica Brasílica . 2011;25(1):223–233. doi: 10.1590/S0102-33062011000100026. [DOI] [Google Scholar]
- 7.Marazzi B., Sanderson M. J. Large-scale patterns of diversification in the widespread legume genus Senna and the evolutionary role of extrafloral nectaries. Evolution: International Journal of Organic Evolution . 2010;64(12):3570–3592. doi: 10.1111/j.1558-5646.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 8.Rahman M. O., Rahman M. Z., Begum A. Numerical taxonomy of the genus Senna mill. from Bangladesh. Bangladesh Journal of Plant Taxonomy . 2013;20(1):77–83. doi: 10.3329/bjpt.v20i1.15467. [DOI] [Google Scholar]
- 9.Spiller H. A., Winter M. L., Weber J. A., Krenzelok E. P., Anderson D. L., Ryan M. L. Skin breakdown and blisters from Senna-containing laxatives in young children. Annals of Pharmacotherapy . 2003;37(5):636–639. doi: 10.1345/aph.1C439. [DOI] [PubMed] [Google Scholar]
- 10.Nassar M. A., Ramadan H. R., Ibrahim H. M. Morphological characteristics of vegetative and reproductive growth of Senna occidentalis (L.) link (Caesalpiniaceae) Research Journal of Agriculture and Biological Sciences . 2011;7(2):260–270. [Google Scholar]
- 11.Selegato D. M., Monteiro A. F., Vieira N. C., et al. Update: biological and chemical aspects of Senna spectabilis. Journal of the Brazilian Chemical Society . 2017;28(3):415–426. doi: 10.21577/0103-5053.20160322. [DOI] [Google Scholar]
- 12.Oladeji O. S., Adelowo F. E., Oluyori A. P., Bankole D. T. Ethnobotanical description and biological activities of Senna alata. Evidence-based Complementary and Alternative Medicine . 2020;2020 doi: 10.1155/2020/2580259.2580259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farid A., Kamel D., Abdelwahab Montaser S., Mohamed Ahmed M., el Amir M., el Amir A. Synergetic role of Senna and Fennel extracts as antioxidant, anti-inflammatory and anti-mutagenic agents in irradiated human blood lymphocyte cultures. Journal of Radiation Research and Applied Science . 2020;13(1):191–199. doi: 10.1080/16878507.2020.1723948. [DOI] [Google Scholar]
- 14.Nambirajan G., Karunanidhi K., Ganesan A., et al. Evaluation of antidiabetic activity of bud and flower of Avaram Senna (Cassia auriculata L.) in high fat diet and streptozotocin induced diabetic rats. Biomedicine & Pharmacotherapy . 2018;108:1495–1506. doi: 10.1016/j.biopha.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Malematja R. O., Bagla V. P., Njanje I., et al. Potential hypoglycaemic and antiobesity effects of Senna italica leaf acetone extract. Evidence-based Complementary and Alternative Medicine . 2018;2018 doi: 10.1155/2018/5101656.5101656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PlantList T. 2021, http://www.theplantlist.org/
- 17.Heinrich M., Appendino G., Efferth T., et al. Best practice in research - overcoming common challenges in phytopharmacological research. Journal of Ethnopharmacology . 2020;246, article 112230 doi: 10.1016/j.jep.2019.112230. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Pacella L. Morfología polínica de especies del género Senna (Fabaceae) del Sureste del Iberá, Corrientes, Argentina. Argentina. Revista de Biología Tropical . 2014;62(2):769–782. doi: 10.15517/rbt.v62i2.9159. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho D. A., Oliveira P. E. Biologia reprodutiva e polinização de Senna sylvestris (Vell.) H.S. Irwin & Barneby (Leguminosae, Caesalpinioideae) Brazilian Journal of Botany . 2003;26(3):319–328. doi: 10.1590/S0100-84042003000300005. [DOI] [Google Scholar]
- 20.Heil M., McKey D. Protective ant-plant interactions as model systems in ecological and evolutionary research. Annual Review of Ecology, Evolution, and Systematics . 2003;34(1):425–553. doi: 10.1146/annurev.ecolsys.34.011802.132410. [DOI] [Google Scholar]
- 21.Jothy S. L., Torey A., Darah I., et al. Cassia spectabilis (DC) Irwin et barn: a promising traditional herb in health improvement. Molecules . 2012;17(9):10292–10305. doi: 10.3390/molecules170910292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbiati F. O., Amarilla L. D., Anton A. M., Fortunato R. H. Phenotypic variation in arid and semi-arid zones of southern South America: the case of Senna series Aphyllae (Fabaceae, Caesalpinioideae) Botanical Journal of the Linnean Society . 2017;183(3):454–473. doi: 10.1093/botlinnean/bow012. [DOI] [Google Scholar]
- 23.Cordeiro J. M. P., Felix L. P. Intra- and interspecific karyotypic variations of the genus Senna mill. (Fabaceae, Caesalpinioideae) Acta Botânica Brasílica . 2018;32(1):128–134. doi: 10.1590/0102-33062017abb0274. [DOI] [Google Scholar]
- 24.Resende K. F., Davide L. C., Torres G. A. Chromosome number and meiosis in populations of Senna species (Caesalpinioideae – Fabaceae) from Southeast Brazil. Caryologia . 2013;66(1):1–5. doi: 10.1080/00087114.2012.760883. [DOI] [Google Scholar]
- 25.Rice A., Glick L., Abadi S., et al. The chromosome counts database (CCDB)–a community resource of plant chromosome numbers. New Phytologist . 2015;206(1):19–26. doi: 10.1111/nph.13191. [DOI] [PubMed] [Google Scholar]
- 26.Resende K., Prado C., Davide L., Torres G. Polyploidy and apomixis in accessions of Senna rugosa (G.Don) H.S.Irwin & Barneby. Turkish Journal of Biology . 2014;38(4):510–515. doi: 10.3906/biy-1312-66. [DOI] [Google Scholar]
- 27.Matos L. P., Barreto K. L., Conceição A. S., Queiroz L. P., Andrade M. J. Análise citogenética em 16 espécies dos gêneros Senna Mill. e Cassia L.(Leguminosae), com ênfase nas espécies ocorrentes na Bahia. XV Semic-Seminário de Iniciação Científica . Feira de Santana: Universidade Estadual de Feira de Santana; 2011. [Google Scholar]
- 28.Singh S., Singh S. K., Yadav A. A review on Cassia species: pharmacological, traditional and medicinal aspects in various countries. American Journal of Phytomedicine and Clinical Therapeutics . 2013;1(3):291–312. [Google Scholar]
- 29.Abubakar B. Y., Adamu A. K., Ambi A. A., Nuru F. G. Taxonomic delimitation of species of Senna and Cassia in Ahmadu Bello University, Zaria. Trends in Science and Technology Journal . 2018;3(2):448–495. [Google Scholar]
- 30.Soladoye M. O., Onakoya M. A., Chukwuma E. C., Sonibare M. A. Morphometric study of the genus Senna Mill. in South-Western Nigeria. African Journal of Plant Science . 2010;4(3):044–052. [Google Scholar]
- 31.Jaca T., Condy G. Senna didymobotrya. The Flowering Plants of Africa . 2017;65:68–75. [Google Scholar]
- 32.Larsen K. Leguminosae-Caesalpinioideae. Flora of Thailand . The Chutima Press; 1984. [Google Scholar]
- 33.Monkheang P., Sudmoon R., Tanee T., Noikotr K., Bletter N., Chaveerach A. Species diversity, usages, molecular markers and barcode of medicinal Senna species (Fabaceae, Caesalpinioideae) in Thailand. Journal of Medicinal Plant Research . 2011;5(26):6173–6181. [Google Scholar]
- 34.Okullo J. B., Obua J., Kaboggoza J. R., Aluma J. R. Traditional agroforestry systems, tree uses and management in northern Uganda. Uganda Journal of Agricultural Sciences . 2003;8:5–12. [Google Scholar]
- 35.Ssegawa P., Kasenene J. M. Medicinal plant diversity and uses in the Sango bay area, southern Uganda. Journal of Ethnopharmacology . 2007;113(3):521–540. doi: 10.1016/j.jep.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Kalita B. C., Tag H., Gogoi B. J., Hui P. K. Diversity and traditional uses of some poisonous plants of Arunachal Pradesh. International Journal of Advance Research and Innovative Ideas in Education . 2017;3(1):755–763. [Google Scholar]
- 37.Hossain U., Rahman M. O. Ethnobotanical uses and informant consensus factor of medicinal plants in Barisal district, Bangladesh. Bangladesh Journal of Plant Taxonomy . 2018;25(2):241–255. doi: 10.3329/bjpt.v25i2.39530. [DOI] [Google Scholar]
- 38.Rahman A. H., Biswas M. C., Islam A. K., Zaman A. T. Assessment of traditional medicinal plants used by local people of Monirampur Upazilla under Jessore district of Bangladesh. Wudpecker Journal of Medicinal Plants . 2013;2:99–109. [Google Scholar]
- 39.Hajdu Z., Hohmann J. An ethnopharmacological survey of the traditional medicine utilized in the community of Porvenir, Bajo Paragua Indian reservation, Bolivia. Journal of Ethnopharmacology . 2012;139(3):838–857. doi: 10.1016/j.jep.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 40.Castro J. A., Brasileiro B. P., Lyra D. H., de Almeida Pereira D., Chaves J. L. Ethnobotanical study of traditional uses of medicinal plants: the flora of caatinga in the community of Cravolândia-BA, Brazil. Journal of Medicinal Plant Research . 2011;5(10):1905–1917. [Google Scholar]
- 41.Cano J. H., Volpato G. Herbal mixtures in the traditional medicine of eastern Cuba. Journal of Ethnopharmacology . 2004;90(2-3):293–316. doi: 10.1016/j.jep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Kar S., Das D., Das A., Datta B. K. Ethnomedicinal uses of some legumes in Tripura, India. Pleione . 2019;13(2):258–268. doi: 10.26679/Pleione.13.2.2019.258-268. [DOI] [Google Scholar]
- 43.Balinado L., Chan M. An ethnomedicinal study of plants and traditional health care practices in district 7, Cavite, Philippines. 2017 International Conference on Chemical, Agricultural, Biological and Medical Sciences (CABMS-17); 2017; Manila (Philippines). [Google Scholar]
- 44.Sutjaritjai N., Wangpakapattanawong P., Balslev H., Inta A. Traditional uses of Leguminosae among the Karen in Thailand. Plants . 2019;8(12):p. 600. doi: 10.3390/plants8120600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karousou R., Deirmentzoglou S. The herbal market of Cyprus: traditional links and cultural exchanges. Journal of Ethnopharmacology . 2011;133(1):191–203. doi: 10.1016/j.jep.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 46.Hassan-Abdallah A., Merito A., Hassan S., et al. Medicinal plants and their uses by the people in the region of Randa, Djibouti. Journal of Ethnopharmacology . 2013;148(2):701–713. doi: 10.1016/j.jep.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 47.Mahmoud T., Gairola S. Traditional knowledge and use of medicinal plants in the Eastern Desert of Egypt: a case study from Wadi El-Gemal National Park. Journal of Medicinal Plants . 2013;1(6):10–17. [Google Scholar]
- 48.Husain S. Z., Malik R. N., Javaid M., Bibi S. A. Ethonobotanical properties and uses of medicinal plants of Morgah Biodiversity Park, Rawalpindi. Pakistan Journal of Botany . 2008;40(5):1897–1911. [Google Scholar]
- 49.Phondani P. C., Bhatt A., Elsarrag E., Horr Y. A. Ethnobotanical magnitude towards sustainable utilization of wild foliage in Arabian Desert. Journal of Traditional and Complementary Medicine . 2016;6(3):209–218. doi: 10.1016/j.jtcme.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karar M. G. E., Kuhnert N. Herbal drugs from Sudan: traditional uses and phytoconstituents. Pharmacognosy Reviews . 2017;11(22):83–103. doi: 10.4103/phrev.phrev_15_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manoranjotham M., Ramaraj T., Kamaraj M. An edthnobotanical study on traditional uses of medicinal plants in Musiri taluk, Tiruchirappalli District, Tamil Nadu. Journal of Applied and Advanced Research . 2016;1(3):16–24. doi: 10.21839/jaar.2016.v1i3.30. [DOI] [Google Scholar]
- 52.Semenya S., Potgieter M., Tshisikhawe M., Shava S., Maroyi A. Medicinal utilization of exotic plants by Bapedi traditional healers to treat human ailments in Limpopo province, South Africa. Journal of Ethnopharmacology . 2012;144(3):646–655. doi: 10.1016/j.jep.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Gupta M., Solís P. N., Calderón A. I., et al. Medical ethnobotany of the Teribes of Bocas del Toro, Panama. Journal of Ethnopharmacology . 2005;96(3):389–401. doi: 10.1016/j.jep.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 54.Amiri M. S., Joharchi M. R. Ethnobotanical investigation of traditional medicinal plants commercialized in the markets of Mashhad, Iran. Avicenna Journal of Phytomedicine . 2013;3(3):254–271. [PMC free article] [PubMed] [Google Scholar]
- 55.Qureshi R., Bhatti G. R., Memon R. A. Ethnomedicinal uses of herbs from northern part of Nara desert Pakistan. Pakistan Journal of Botany . 2010;42(2):839–851. [Google Scholar]
- 56.Hammond G. B., Fernández I. D., Villegas L. F., Vaisberg A. J. A survey of traditional medicinal plants from the Callejon de Huaylas, Department of Ancash, Peru. Journal of Ethnopharmacology . 1998;61(1):17–30. doi: 10.1016/S0378-8741(98)00009-9. [DOI] [PubMed] [Google Scholar]
- 57.Kufer J., Förther H., Pöll E., Heinrich M. Historical and modern medicinal plant uses—the example of the Ch'orti ‘Maya and Ladinos in eastern Guatemala. Journal of Pharmacy and Pharmacology . 2005;57(9):1127–1152. doi: 10.1211/jpp.57.9.0008. [DOI] [PubMed] [Google Scholar]
- 58.Rani J. Ethanobotanical survey and traditional uses of medicinal plants in Jind district of Haryana India. Plant Archives . 2019;19(1):1241–1247. [Google Scholar]
- 59.Yaseen G., Ahmad M., Shinwari S., et al. Medicinal plant diversity used for livelihood of public health in deserts and arid regions of Sindh-Pakistan. Pakistan Journal of Botany . 2019;51(2):2409–2419. doi: 10.30848/PJB2019-2(31). [DOI] [Google Scholar]
- 60.Moshi M. J., Otieno D. F., Weisheit A. Ethnomedicine of the Kagera region, north western Tanzania. Part 3: plants used in traditional medicine in Kikuku village, Muleba District. Journal of Ethnobiology and Ethnomedicine . 2012;8(1):1–11. doi: 10.1186/1746-4269-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tshikalange T. E. The traditional use of medicinal plants to treat sexually transmitted diseases . University of Pretoria; 2006. [Google Scholar]
- 62.Masevhe N. A., McGaw L. J., Eloff J. N. The traditional use of plants to manage candidiasis and related infections in Venda, South Africa. Journal of Ethnopharmacology . 2015;168:364–372. doi: 10.1016/j.jep.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 63.Sobeh M., Mahmoud M., Hasan R., Cheng H., el-Shazly A., Wink M. Senna singueana: antioxidant, hepatoprotective, antiapoptotic properties and phytochemical profiling of a methanol bark extract. Molecules . 2017;22(9):p. 1502. doi: 10.3390/molecules22091502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keter L. K., Mutiso P. C. Ethnobotanical studies of medicinal plants used by traditional health practitioners in the management of diabetes in lower Eastern Province, Kenya. Journal of Ethnopharmacology . 2012;139(1):74–80. doi: 10.1016/j.jep.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 65.Rahman A., Keya M. A. Traditional medicinal plants used by local people at the village Sabgram under Sadar Upazila of Bogra district Bangladesh. Research in Plant Sciences . 2015;3(2):31–37. [Google Scholar]
- 66.Fu Y., Yang J. C., Cunningham A. B., et al. A billion cups: the diversity, traditional uses, safety issues and potential of Chinese herbal teas. Journal of Ethnopharmacology . 2018;222:217–228. doi: 10.1016/j.jep.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 67.Pattnaik S. S. M., Behera L. Traditional use of herbal medicines against rheumatism by the tribals of Bargarh district in Odisha (India) by sk sen1_ mr pattnaik2 and lm behera3. Life Sciences Leaflets . 2014;51:59–68. [Google Scholar]
- 68.Ahmed S., Shohael A. M. In silico studies of four anthraquinones of Senna alata L. as potential antifungal compounds. Pharmacology . 2019;2:259–268. [Google Scholar]
- 69.Essien E. E., Walker T. M., Ogunwande I. A., Bansal A., Setzer W. N., Ekundayo O. Volatile constituents, antimicrobial and cytotoxicity potentials of Three Senna Species from Nigeria. Journal of Essential Oil-Bearing Plants . 2011;14(6):722–730. doi: 10.1080/0972060X.2011.10643995. [DOI] [Google Scholar]
- 70.Epifano F., Fiorito S., Locatelli M., Taddeo V. A., Genovese S. Screening for novel plant sources of prenyloxyanthraquinones: Senna alexandrina Mill. and Aloe vera (L.) Burm. F. Natural Product Research . 2015;29(2):180–184. doi: 10.1080/14786419.2014.971792. [DOI] [PubMed] [Google Scholar]
- 71.Ahmed S. I., Hayat M. Q., Tahir M., et al. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complementary Medicine and Therapies . 2016;16(1):p. 460. doi: 10.1186/s12906-016-1443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arrieta-Baez D., Roman R., Vazquez-Duhalt R., Jiménez-Estrada M. Peroxidase-mediated transformation of hydroxy-9,10-anthraquinones. Phytochemistry . 2002;60(6):567–572. doi: 10.1016/S0031-9422(02)00173-5. [DOI] [PubMed] [Google Scholar]
- 73.Francisco W., Pivatto M., Danuello A., et al. Pyridine alkaloids from Senna multijuga As acetylcholinesterase inhibitors. Journal of Natural Products . 2012;75(3):408–413. doi: 10.1021/np200814j. [DOI] [PubMed] [Google Scholar]
- 74.Serrano M. A. R., Pivatto M., Francisco W., et al. Acetylcholinesterase inhibitory pyridine alkaloids of the leaves of Senna multijuga. Journal of Natural Products . 2010;73(3):482–484. doi: 10.1021/np900644x. [DOI] [PubMed] [Google Scholar]
- 75.Vargas Rechia C. G., Sierakowski M. R., Ganter J. L. M. S., Reicher F. Polysaccharides from the seeds of Senna multijuga. International Journal of Biological Macromolecules . 1995;17(6):409–412. doi: 10.1016/0141-8130(96)81854-X. [DOI] [PubMed] [Google Scholar]
- 76.Abegaz B. M., Bezabeh M., Alemayehu G., Duddeck H. Anthraquinones from Senna multiglandulosa. Phytochemistry . 1994;35(2):465–468. doi: 10.1016/S0031-9422(00)94783-6. [DOI] [Google Scholar]
- 77.Alemayehu G., Abegaz B. M. Bianthraquinones from the seeds of Senna multiglandulosa. Phytochemistry . 1996;41(3):919–921. doi: 10.1016/0031-9422(95)00645-1. [DOI] [Google Scholar]
- 78.Essien E. E., Thomas P. S., Ascrizzi R., Setzer W. N., Flamini G. Senna occidentalis(L.) link and Senna hirsuta (L.) H. S. Irwin & Barneby: constituents of fruit essential oils and antimicrobial activity. Natural Product Research . 2019;33(11):1637–1640. doi: 10.1080/14786419.2018.1425842. [DOI] [PubMed] [Google Scholar]
- 79.Maia I. R. . O., Trevisan M. T. S., Silva M. G. . V., Breuer A., Owen R. W. Characterization and quantitation of polyphenolic compounds in Senna gardneriand S. Georgicafrom the northeast of Brazil. Natural Product Communications . 2018;13(11, article 1934578X1801301) doi: 10.1177/1934578X1801301125. [DOI] [Google Scholar]
- 80.Monteiro J. A., Ferreira Júnior J. M., Oliveira I. R., et al. Bioactivity and toxicity of Senna cana and Senna pendula extracts. Biochemistry Research International . 2018;2018 doi: 10.1155/2018/8074306.8074306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barba B., Díaz J. G., Herz W. Anthraquinones and other constituents of two Senna species. Phytochemistry . 1992;31(12):4374–4375. doi: 10.1016/0031-9422(92)80483-U. [DOI] [Google Scholar]
- 82.Zavala-Sánchez M. Á., Rodríguez-Chávez J. L., Figueroa-Brito R., et al. Bioactivity of 1-octacosanol from Senna crotalarioides (Fabaceae: Caesalpinioideae) to control Spodoptera frugiperda (Lepidoptera: Noctuidae) Florida Entomologist . 2019;102(4):731–737. doi: 10.1653/024.102.0410. [DOI] [Google Scholar]
- 83.Alemayehu G., Hailu A., Abegaz B. M. Bianthraquinones from Senna didymobotrya. Phytochemistry . 1996;42(5):1423–1425. doi: 10.1016/0031-9422(96)00102-1. [DOI] [Google Scholar]
- 84.Ochieng C. O., Shrivastava A., Chaturvedi U., et al. Effects of Senna didymobotrya root extract and compounds on Tritoninduced hyperlipidaemic rats and differentiation of 3T3-Li preadipocytes. Natural Products Journal . 2013;3(3):212–217. doi: 10.2174/22103155113039990011. [DOI] [Google Scholar]
- 85.Rao K. V., Damu A. G., Jayaprakasam B., Gunasekar D. Flavonol glycosides from Cassiahirsuta. Journal of Natural Products . 1999;62(2):305–306. doi: 10.1021/np980195c. [DOI] [PubMed] [Google Scholar]
- 86.Silva J. G., Silva A. A., Coutinho I. D., Pessoa C. O., Cavalheiro A. J., Silva M. G. Chemical profile and cytotoxic activity of leaf extracts from Senna spp. from northeast of Brazil. Journal of the Brazilian Chemical Society . 2016;27(10):1872–1880. [Google Scholar]
- 87.Gololo S. S., Mapfumari N. S., Sethoga L. S., Olivier M. T., Shai L. J., Mogale M. A. Identification of phytochemical constituents within the n-hexane leaf extract of Senna italica (mill) using gas chromatography-mass spectrometry (GC-MS) analysis. Journal of Pharmaceutical Sciences and Research . 2016;8(10):1141–1143. [Google Scholar]
- 88.Khalaf O. M., Ghareeb M. A., Saad A. M., Madkour H. M. F., el-Ziaty A. K., Abdel-Aziz M. S. Phenolic constituents, antimicrobial, antioxidant, and anticancer activities of ethyl acetate andn-butanol extracts of Senna italica. Acta Chromatographica . 2019;31(2):138–145. doi: 10.1556/1326.2018.00412. [DOI] [Google Scholar]
- 89.Madkour H. M., Ghareeb M. A., Abdel-Aziz M. S., et al. Gas chromatography-mass spectrometry analysis, antimicrobial, anticancer and antioxidant activities of n-hexane and methylene chloride extracts of Senna italica. Journal of Applied Pharmaceutical Science . 2017;7(6):023–032. [Google Scholar]
- 90.Mokgotho M. P., Gololo S. S., Masoko P., et al. Isolation and chemical structural characterisation of a compound with antioxidant activity from the roots of Senna italica. Evidence-based Complementary and Alternative Medicine . 2013;2013 doi: 10.1155/2013/519174.519174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alemayehu G., Abegaz B., Snatzke G., Duddeck H. Bianthrones from Senna longiracemosa. Phytochemistry . 1993;32(5):1273–1277. doi: 10.1016/S0031-9422(00)95104-5. [DOI] [Google Scholar]
- 92.Branco A., Pinto A. C., Braz-Filho R., Silva E. F., Grynberg N. F., Echevarria A. Rubrofusarina, um policetídeo natural inibidor da topoisomerase II-α humana. Brazilian Journal of Pharmacognosy . 2008;18:703–708. doi: 10.1590/S0102-695X2008000500012. [DOI] [Google Scholar]
- 93.Klika K. D., Ricarte I., Trevisan M. T. S., de Vasconcelos Silva M. G., Owen R. W. (2R∗,3S∗,4S∗,2″R∗,3″S∗)-Guibourtinidol-(4α->8)-catechin, a biflavanoid procyanidin of the proguibourtinidin group from Senna macranthera: its relative stereochemistry and conformation. Tetrahedron: Asymmetry . 2015;26(5-6):247–250. doi: 10.1016/j.tetasy.2015.01.013. [DOI] [Google Scholar]
- 94.Pires L., Gorin P. A. J., Reicher F., Sierakowski M. R. An active heparinoid obtained by sulphation of a galactomannan extracted from the endosperm of Senna macranthera seeds. Carbohydrate Polymers . 2001;46(2):165–169. doi: 10.1016/S0144-8617(00)00298-8. [DOI] [Google Scholar]
- 95.Messana I., Ferrari F., Cavalcanti M. S., Gacs Baitz E. New naphthopyrone derivatives from Cassia pudibunda. Heterocycles . 1990;31(10):p. 1847. doi: 10.3987/COM-90-5519. [DOI] [Google Scholar]
- 96.Messana I., Ferrari F., Cavalcanti M. S. B., Morace G. An anthraquinone and three naphthopyrone derivatives from Cassia pudibunda. Phytochemistry . 1991;30(2):708–710. doi: 10.1016/0031-9422(91)83762-A. [DOI] [PubMed] [Google Scholar]
- 97.de Macedo E. M. S., Wiggers H. J., Silva M. G. V., Braz-Filho R., Andricopulo A. D., Montanari C. A. A new bianthron glycoside as inhibitor of Trypanosoma cruzi glyceraldehyde 3-phosphate dehydrogenase activity. Journal of the Brazilian Chemical Society . 2009;20(5):947–953. doi: 10.1590/S0103-50532009000500021. [DOI] [Google Scholar]
- 98.Graham J. G., Zhang H., Pendland S. L., et al. Antimycobacterial naphthopyrones from Senna obliqua. Journal of Natural Products . 2004;67(2):225–227. doi: 10.1021/np030348i. [DOI] [PubMed] [Google Scholar]
- 99.Pang X., Wang L. M., Zhang Y. C., et al. New anthraquinone and eurotinone analogue from the seeds of Senna obtusifolia and their inhibitory effects on human organic anion transporters 1 and 3. Natural Product Research . 2019;33(23):3409–3416. doi: 10.1080/14786419.2018.1480621. [DOI] [PubMed] [Google Scholar]
- 100.Saidu A. N., Aina E. O., Mann A., Leje U. I. The effect of aqueous extract of Senna occidentalis leaves on rats infected with salmonella typhi. Australian Journal of Basic and Applied Sciences . 2011;5(12):1863–1867. [Google Scholar]
- 101.Javaid A., Qudsia H., Shoaib A. Bioassays guided fractionation of Senna occidentalis for identification of natural antifungal constituents against Macrophomina phaseolina. Planta Daninha . 2017;35 doi: 10.1590/s0100-83582017350100002. [DOI] [Google Scholar]
- 102.Kim H. L., Camp B. J., Grigsby R. D. Isolation of N-methylmorpholine from the seeds of Cassia occidentalis L. (coffee Senna) Journal of Agricultural and Food Chemistry . 1971;19(1):198–199. doi: 10.1021/jf60173a026. [DOI] [PubMed] [Google Scholar]
- 103.Kumar N., Singh G., Singh S., Singh A. Standardization and simultaneous quantification of flavonoids and phenolic contents in Cassia occidentalis using liquid chromatography triple quadrupole mass spectrometry (LC-MS/MS) Research Journal of Chemistry and Environment . 2017;21(1):1–8. [Google Scholar]
- 104.Li S. F., di Y. T., Luo R. H., et al. Cycloartane triterpenoids from Cassia occidentalis. Planta Medica . 2012;78(8):821–827. doi: 10.1055/s-0031-1298376. [DOI] [PubMed] [Google Scholar]
- 105.Li S. F., Li S. L. Cycloartane triterpenoid and its glucoside isolated from Cassia occidentalis. Chinese Journal of Natural Medicines . 2017;15(12):950–954. doi: 10.1016/S1875-5364(18)30012-8. [DOI] [PubMed] [Google Scholar]
- 106.Ogunwande I. A., Avoseh N. O., Flamini G., et al. Essential oils from the leaves of six medicinal plants of Nigeria. Natural Product Communications . 2013;8(2):243–248. doi: 10.1177/1934578X1300800229. [DOI] [PubMed] [Google Scholar]
- 107.Qin R. X., Zuo Q., Huang X. H., et al. A new nor-sesquiterpene from Cassia occidentalis and its bioactivity. China Journal of Chinese Materia Medica . 2016;41(23):4389–4392. doi: 10.4268/cjcmm20162316. [DOI] [PubMed] [Google Scholar]
- 108.Singh H., Chahal P., Mishra A., Mishra A. K. An up-to-date review on chemistry and biological activities of Senna occidentalis (L.) link family: Leguminosae. Advances in Traditional Medicine . 2020;20(3):263–278. doi: 10.1007/s13596-019-00391-z. [DOI] [Google Scholar]
- 109.Tshikalange T. E., Meyer J. J. M., Hussein A. A. Antimicrobial activity, toxicity and the isolation of a bioactive compound from plants used to treat sexually transmitted diseases. Journal of Ethnopharmacology . 2005;96(3):515–519. doi: 10.1016/j.jep.2004.09.057. [DOI] [PubMed] [Google Scholar]
- 110.Gamal-Eldeen A. M., Djemgou P. C., Tchuendem M., Ngadjui B. T., Tane P., Toshifumi H. Anti-cancer and immunostimulatory activity of chromones and other constituents from Cassia petersiana. Zeitschrift fur Naturforschung - Section C Journal of Biosciences . 2007;62(5-6):331–338. doi: 10.1515/znc-2007-5-622. [DOI] [PubMed] [Google Scholar]
- 111.Coetzee J., Mciteka L., Malan E., Ferreira D. Structure and synthesis of the first procassinidin dimers based on epicatechin, and gallo- and epigallo-catechin. Phytochemistry . 2000;53(7):795–804. doi: 10.1016/S0031-9422(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 112.Ajiboye B. O., Ojo O. A., Fatoba B., et al. In vitro antioxidant and enzyme inhibitory properties of the n-butanol fraction of Senna podocarpa (Guill. and Perr.) leaf. Journal of Basic and Clinical Physiology and Pharmacology . 2020;31(1) doi: 10.1515/jbcpp-2019-0123. [DOI] [PubMed] [Google Scholar]
- 113.Malmir M., Ferreira E., Serrano R., Gomes E. T., Caniça M., Silva O. In vitro anti- Neisseria gonorrhoeae activity of Senna podocarpa root extracts. Industrial Crops and Products . 2015;76:467–471. doi: 10.1016/j.indcrop.2015.07.022. [DOI] [Google Scholar]
- 114.Genta-Jouve G., Weinberg L., Cocandeau V., Maestro Y., Thomas O. P., Holderith S. Revising the absolute configurations of coatlines via density functional theory calculations of electronic circular dichroism spectra. Chirality . 2013;25(3):180–184. doi: 10.1002/chir.22129. [DOI] [PubMed] [Google Scholar]
- 115.Ogura M., Cordell G. A., Farnsworth N. R. Quinquangulin, a new naphthopyrone from Cassia quinquangulata (Leguminosae) Lloydia . 1977;40(4):347–351. [PubMed] [Google Scholar]
- 116.Mena-Rejóna G. J., Pérez-Rivas K., Sansorez-Peraza P., Rios T., Quijano L. Racemochrysone, a dihydroanthracenone from Senna racemosa. Zeitschrift fur Naturforschung - Section C Journal of Biosciences . 2002;57(9-10):777–779. doi: 10.1515/znc-2002-9-1003. [DOI] [PubMed] [Google Scholar]
- 117.Sansores-Peraza P., Rosado-Vallado M., Brito-Loeza W., Mena-Rejón G. J., Quijano L. Cassine, an antimicrobial alkaloid from Senna racemosa. Fitoterapia . 2000;71(6):690–692. doi: 10.1016/S0367-326X(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 118.Dos Santos R. N., Silva M. G. D. V., Braz Filho R. Constituintes químicos do caule de Senna reticulata Willd. (Leguminoseae): chemical constituents isolated from the wood of Senna reticulata Willd. Quimica Nova . 2008;31(8):1979–1981. doi: 10.1590/S0100-40422008000800011. [DOI] [Google Scholar]
- 119.Barbosa F. G., de Oliveira M. . C. F., Braz-Filho R., Silveira E. R. Anthraquinones and naphthopyrones from Senna rugosa. Biochemical Systematics and Ecology . 2004;32(3):363–365. doi: 10.1016/j.bse.2003.07.005. [DOI] [Google Scholar]
- 120.Alemayehu G., Adane L., Abegaz B. M. A new bianthracene C-arabinopyranoside from Senna septemtrionalis. Natural Product Communications . 2010;5(5):747–750. doi: 10.1177/1934578X1000500514. [DOI] [PubMed] [Google Scholar]
- 121.Alemayehu G., Woldeyesus B., Abegaz B. M. (+)-Floribundone 3 from the pods of Senna septemtrionalis. Bulletin of the Chemical Society of Ethiopia . 1997;11(1):25–29. doi: 10.4314/bcse.v11i1.21010. [DOI] [Google Scholar]
- 122.Ingkaninan K., Ijzerman A. P., Verpoorte R. Luteolin, a compound with adenosine A1Receptor-binding activity, and chromone and dihydronaphthalenone constituents from Senna siamea. Journal of Natural Products . 2000;63(3):315–317. doi: 10.1021/np9904152. [DOI] [PubMed] [Google Scholar]
- 123.Sakunpak A. P., Suksaeree J. I., Monton C. H., Pathompak P. A. Development and quantitative determination of barakol in Senna siamea leaf extract by TLC-image analysis method. International Journal of Pharmacy and Pharmaceutical Sciences . 2014;6(3):267–270. [Google Scholar]
- 124.Morita H., Oshimi S., Hirasawa Y., et al. Cassiarins a and B, novel antiplasmodial alkaloids from Cassia siamea. Organic Letters . 2007;9(18):3691–3693. doi: 10.1021/ol701623n. [DOI] [PubMed] [Google Scholar]
- 125.Oshimi S., Tomizawa Y., Hirasawa Y., et al. Chrobisiamone a, a new bischromone from Cassia siamea and a biomimetic transformation of 5-acetonyl-7-hydroxy-2-methylchromone into cassiarin a. Bioorganic and Medicinal Chemistry Letters . 2008;18(13):3761–3763. doi: 10.1016/j.bmcl.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 126.Oshimi S., Deguchi J., Hirasawa Y., et al. Cassiarins C-E, antiplasmodial alkaloids from the flowers of Cassia siamea. Journal of Natural Products . 2009;72(10):1899–1901. doi: 10.1021/np9004213. [DOI] [PubMed] [Google Scholar]
- 127.Deguchi J., Sasaki T., Hirasawa Y., et al. Two novel tetracycles, cassibiphenols a and B from the flowers of Cassia siamea. Tetrahedron Letters . 2014;55(7):1362–1365. doi: 10.1016/j.tetlet.2014.01.023. [DOI] [Google Scholar]
- 128.Koyama J., Morita I., Tagahara K., Aqil M. Bianthraquinones from Cassia siamea. Phytochemistry . 2001;56(8):849–851. doi: 10.1016/S0031-9422(01)00025-5. [DOI] [PubMed] [Google Scholar]
- 129.Kumar D., Karmase A., Jagtap S., Shekhar R., Bhutani K. K. Pancreatic lipase inhibitory activity of cassiamin a, a bianthraquinone from Cassia siamea. Natural Product Communications . 2013;8(2):195–198. doi: 10.1177/1934578X1300800216. [DOI] [PubMed] [Google Scholar]
- 130.Ogbole O. O., Akinleye T. E., Faleye T. O. C., Adeniji A. J. Enterovirus inhibiting activities of two Lupane triterpenoids and anthraquinones from senna siamea stem bark against three serotypes of echovirus. Acta Pharmaceutica Sciencia . 2019;57(3):105–115. doi: 10.23893/1307-2080.APS.05720. [DOI] [Google Scholar]
- 131.Ajaiyeoba E. O., Ashidi J. S., Okpako L. C., Houghton P. J., Wright C. W. Antiplasmodial compounds from Cassia siamea stem bark extract. Phytotherapy Research . 2008;22(2):254–255. doi: 10.1002/ptr.2254. [DOI] [PubMed] [Google Scholar]
- 132.Lü T. S., Yi Y. H., Mao S. L., et al. Studies on the anthraquinones of Cassia siamea. Yao xue xue bao = Acta Pharmaceutica Sinica . 2001;36(7):547–548. [PubMed] [Google Scholar]
- 133.Lu T. S., Yi Y. H., Mao S. L., et al. A new chromone glycoside from Cassia siamea Lam. Yaoxue Xuebao . 2003;38(2):113–115. [PubMed] [Google Scholar]
- 134.Lu T. S., Yi Y. H., Zhang Z. G., Zhang Z. Q., Hua N. A new 10-hydroxyl anthrone glycoside from Cassia siamea Lam. Chinese Chemical Letters . 2002;13(8):731–733. [Google Scholar]
- 135.Hu Q. F., Zhou B., Gao X. M., et al. s. Journal of Natural Products . 2012;75(11):1909–1914. doi: 10.1021/np300395m. [DOI] [PubMed] [Google Scholar]
- 136.Ledwani L., Singh M. Isolation and characterization of anthraquinones from the stem bark of Cassia species. Journal of the Indian Chemical Society . 2006;83(4):383–385. [Google Scholar]
- 137.Li Y. K., Zhou B., Wu X. X., et al. Phenolic compounds from Cassia siamea and their anti-tobacco mosaic virus activity. Chemistry of Natural Compounds . 2015;51(1):50–53. doi: 10.1007/s10600-015-1201-3. [DOI] [Google Scholar]
- 138.Thengyai S., Thiantongin P., Sontimuang C., Ovatlarnporn C., Puttarak P. α -glucosidase and α -amylase inhibitory activities of medicinal plants in Thai antidiabetic recipes and bioactive compounds from Vitex glabrata R. Br. Stem bark. Journal of Herbal Medicine . 2020;19:p. 100302. doi: 10.1016/j.hermed.2019.100302. [DOI] [Google Scholar]
- 139.Baez D. A., Zepeda Vallejo L. G., Jimenez-estrada M. Phytochemical studies on Senna skinneri and Senna wislizeni. Natural Product Letters . 1999;13(3):223–228. doi: 10.1080/10575639908048789. [DOI] [Google Scholar]
- 140.Baez D. A., Vallejo G. Z., Sánchez P. C., Valle M. B., Chilpa R. R., Estrada M. J. Use of the SOS-chromotest spot assay as a screening system for detecting genotoxic compounds in crude plant extracts. ATLA, Alternatives to Laboratory Animals . 2002;30(1):87–92. [PubMed] [Google Scholar]
- 141.Alemayehu G., Abegaz B., Kraus W. A 1,4-anthraquinone-dihydroanthracenone dimer from Senna sophera. Phytochemistry . 1998;48(4):699–702. doi: 10.1016/S0031-9422(98)00031-4. [DOI] [Google Scholar]
- 142.Kharat A. R., Kharat K., Jadhav M., Makhija S. J. Antihyperglycemic, antihyperlipidemic and antioxidative evaluation of compounds from Senna sophera (L.) Roxb in streptozotocin-induced diabetic rats. Natural Product Research . 2019;33(4):602–605. doi: 10.1080/14786419.2017.1399389. [DOI] [PubMed] [Google Scholar]
- 143.Malhotra S., Misra K. A new anthraquinone fromCassia sopheraHeartwood. Planta Medica . 1982;46(12):247–249. doi: 10.1055/s-2007-971225. [DOI] [PubMed] [Google Scholar]
- 144.Mondal A., Rajalingam D., Kumar Maity T. Anti-inflammatory effect of O -methylated flavonol 2-(3,4-dihydroxy- phenyl)-3,5-dihydroxy-7-methoxy-chromen-4-one obtained from Cassia sophera Linn in rats. Journal of Ethnopharmacology . 2013;147(2):525–529. doi: 10.1016/j.jep.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 145.Mushtaq W., Ain Q., Siddiqui M. B., Alharby H., Hakeem K. R. Allelochemicals change macromolecular content of some selected weeds. South African Journal of Botany . 2020;130:177–184. doi: 10.1016/j.sajb.2019.12.026. [DOI] [Google Scholar]
- 146.Silva F. . O., Oliveira Í. R. ., Silva M. G. . V., Braz-Filho R. Constituintes químicos das folhas de Senna spectabilis (DC) Irwin & Barneby var. excelsa (Schrad.) Irwin & Barneby. Quimica Nova . 2010;33(9):1874–1876. doi: 10.1590/S0100-40422010000900010. [DOI] [Google Scholar]
- 147.Lim K. T., Amanah A., Chear N. J. Y., Zahari Z., Zainuddin Z., Adenan M. I. Inhibitory effects of (+)-spectaline and iso-6-spectaline from Senna spectabilis on the growth and ultrastructure of human-infective species Trypanosoma brucei rhodesiense bloodstream form. Experimental Parasitology . 2018;184:57–66. doi: 10.1016/j.exppara.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 148.da Silva K. A. B. S., Manjavachi M. N., Paszcuk A. F., et al. Plant derived alkaloid (−)-cassine induces anti-inflammatory and anti- hyperalgesics effects in both acute and chronic inflammatory and neuropathic pain models. Neuropharmacology . 2012;62(2):967–977. doi: 10.1016/j.neuropharm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 149.Pivatto M., Baccini L. R., Sharma A., et al. Antimalarial activity of piperidine alkaloids from Senna spectabilis and semisynthetic derivatives. Journal of the Brazilian Chemical Society . 2014;25(10):1900–1906. [Google Scholar]
- 150.Silva F. O., Silva M. G. V., Cerqueira G. S., et al. Central nervous system effects of Iso-6-spectaline isolated from Senna spectabilis var. excelsa (schrad) in mice. Journal of Young Pharmacists . 2011;3(3):232–236. doi: 10.4103/0975-1483.83772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sriphong L., Sotanaphun U., Limsirichaikul S., Wetwitayaklung P., Chaichantipyuth C., Pummangura S. Cytotoxic alkaloids from the flowers of Senna spectabilis. Planta Medica . 2003;69(11):1054–1056. doi: 10.1055/s-2003-45156. [DOI] [PubMed] [Google Scholar]
- 152.Viegas Junior C., Pivatto M., Rezende A. D., Hamerski L., Silva D. H., Bolzani V. D. 7-Hydroxycassine: a new 2,6-dialkylpiperidin-3-ol alkaloid and other constituents isolated from flowers and fruits of Senna spectabilis (Fabaceae) Journal of the Brazilian Chemical Society . 2013;24(2):230–235. [Google Scholar]
- 153.Valli M., Betti A. H., Danuello A., et al. Pyridinic analog of the natural product (−)-spectaline as potential adjuvant for the treatment of central nervous system disorders. Bioorganic and Medicinal Chemistry Letters . 2015;25(10):2247–2250. doi: 10.1016/j.bmcl.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 154.Viegas C., Jr., Bolzani V. S., Pimentel L. S. B., et al. New selective acetylcholinesterase inhibitors designed from natural piperidine alkaloids. Bioorganic and Medicinal Chemistry . 2005;13(13):4184–4190. doi: 10.1016/j.bmc.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 155.Silva F. . O., Silva M. G. . V., Feng D., de Freitas R. M. Evaluation of central nervous system effects of iso-6-cassine isolated from Senna spectabilis var. excelsa (Schrad) in mice. Fitoterapia . 2011;82(2):255–259. doi: 10.1016/j.fitote.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 156.Lacerda R. B., Freitas T. R., Martins M. M., et al. Isolation, leishmanicidal evaluation and molecular docking simulations of piperidine alkaloids from Senna spectabilis. Bioorganic and Medicinal Chemistry . 2018;26(22):5816–5823. doi: 10.1016/j.bmc.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 157.Pereira R. M., Ferreira-Silva G. Á., Pivatto M., et al. Alkaloids derived from flowers of Senna spectabilis, (−)-cassine and (−)-spectaline, have antiproliferative activity on HepG2 cells for inducing cell cycle arrest in G1/S transition through ERK inactivation and downregulation of cyclin D1 expression. Toxicology In Vitro . 2016;31:86–92. doi: 10.1016/j.tiv.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 158.Valencia E., Valenzuela E., Barros E., et al. Estudio fitoquimico y actividad antialimentaria de Senna stipulaceae. Boletin de la Sociedad Chilena de Quimica . 2000;45(2):297–301. doi: 10.4067/S0366-16442000000200017. [DOI] [Google Scholar]
- 159.El-Sawi S. A., Sleem A. A. Flavonoids and hepatoprotective activity of leaves of Senna Surattensis (Burm.f.) in CCl4 induced hepatotoxicity in rats. Australian Journal of Basic and Applied Sciences . 2010;4(6):1326–1334. [Google Scholar]
- 160.Anu S. J., Madhusudana Rao J. Oxanthrone esters from the roots of Cassia kleinii. Phytochemistry . 2002;59(4):425–427. doi: 10.1016/S0031-9422(01)00439-3. [DOI] [PubMed] [Google Scholar]
- 161.Anu S. J., Rao J. M. Oxanthrone esters from the aerial parts of Cassia kleinii. Phytochemistry . 2001;57(4):583–585. doi: 10.1016/S0031-9422(01)00114-5. [DOI] [PubMed] [Google Scholar]
- 162.el-Halawany A. M., Chung M. H., Nakamura N., Ma C. M., Nishihara T., Hattori M. Estrogenic and anti-estrogenic activities of Cassia tora phenolic constituents. Chemical and Pharmaceutical Bulletin . 2007;55(10):1476–1482. doi: 10.1248/cpb.55.1476. [DOI] [PubMed] [Google Scholar]
- 163.Fathalla N., Bishr M., Nasser Singab A., Salama O. GC-MS and LC-MS identification of the phenolic compounds present in the ethyl acetate fraction obtained fromSenna tora,L. Roxb. Seeds. Natural Product Letters . 2019;33(19):2878–2881. doi: 10.1080/14786419.2018.1508138. [DOI] [PubMed] [Google Scholar]
- 164.Lee G. Y., Jang D. S., Lee Y. M., Kim J. M., Kim J. S. Naphthopyrone glucosides from the seeds of Cassia tora with inhibitory activity on advanced glycation end products (AGEs) formation. Archives of Pharmacal Research . 2006;29(7):587–590. doi: 10.1007/BF02969270. [DOI] [PubMed] [Google Scholar]
- 165.Hatano T., Uebayashi H., Ito H., Shiota S., Tsuchiya T., Yoshida T. Phenolic constituents of cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chemical and Pharmaceutical Bulletin . 1999;47(8):1121–1127. doi: 10.1248/cpb.47.1121. [DOI] [PubMed] [Google Scholar]
- 166.Lee G. Y., Kim J. H., Choi S. K., Kim Y. H. Constituents of the seeds of Cassia tora with inhibitory activity on soluble expoxide hydrolease. Bioorganic and Medicinal Chemistry Letters . 2015;25(22):5097–5101. doi: 10.1016/j.bmcl.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 167.Lee H. J., Choi J. S., Jung J. H., Kang S. S. Alaternin glucoside isomer from Cassia tora. Phytochemistry . 1998;49(5):1403–1404. doi: 10.1016/S0031-9422(98)00151-4. [DOI] [Google Scholar]
- 168.Park Y. B., Kim S. B. Isolation and identification of antitumor promoters from the seeds of Cassia tora. Journal of Microbiology and Biotechnology . 2011;21(10):1043–1048. doi: 10.4014/jmb.1103.03040. [DOI] [PubMed] [Google Scholar]
- 169.Cherng J. M., Chiang W., Wang J. H., et al. Anthraquinones of edible wild vegetable Cassia tora stimulate proliferation of human CD4+ T lymphocytes and secretion of interferon- gamma or interleukin 10. Food Chemistry . 2008;107(4):1576–1580. doi: 10.1016/j.foodchem.2007.10.005. [DOI] [Google Scholar]
- 170.Hyun S. K., Lee H., Kang S. S., Chung H. Y., Choi J. S. Inhibitory activities of Cassia tora and its anthraquinone constituents on angiotensin - converting enzyme. Phytotherapy Research . 2009;23(2):178–184. doi: 10.1002/ptr.2579. [DOI] [PubMed] [Google Scholar]
- 171.Jimenez-Coello M., Acosta-Viana K. Y., Guzman-Marin E., Perez Gonzalez C., Salud Perez Gutierrez M. Anti-trypanosomal activity of (8-hydroxymethylen)-trieicosanyl acetate against infective forms of Trypanosoma cruzi. Pharmaceutical Biology . 2010;48(6):666–671. doi: 10.3109/13880200903241853. [DOI] [PubMed] [Google Scholar]
- 172.Guzmán E., Pérez C., Zavala M. A., Acosta-Viana K. Y., Pérez S. Antiprotozoal activity of (8-hydroxymethylen)-trieicosanyl acetate isolated from Senna villosa. Phytomedicine . 2008;15(10):892–895. doi: 10.1016/j.phymed.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 173.Salehi B., Shivaprasad Shetty M., Anil Kumar N. V., et al. Veronica plants-drifting from farm to traditional healing, food application, and phytopharmacology. Molecules . 2019;24(13):p. 2454. doi: 10.3390/molecules24132454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sharifi-Rad M., Anil Kumar N. V., Zucca P., et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Frontiers in Physiology . 2020;11:p. 21. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mititelu R. R., Pădureanu R., Băcănoiu M., et al. Inflammatory and oxidative stress markers—Mirror tools in rheumatoid arthritis. Biomedicine . 2020;8(5):p. 125. doi: 10.3390/biomedicines8050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Salehi B., Sharifi-Rad J., Cappellini F., et al. The therapeutic potential of anthocyanins: current approaches based on their molecular mechanism of action. Frontiers in Pharmacology . 2020;11:p. 20. doi: 10.3389/fphar.2020.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tsoukalas D., Fragkiadaki P., Docea A. O., et al. Association of nutraceutical supplements with longer telomere length. International Journal of Molecular Medicine . 2019;44(1):218–226. doi: 10.3892/ijmm.2019.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Salehi B., Sharifi-Rad, Capanoglu, et al. Cucurbita plants: from farm to industry. Applied Sciences-Basel . 2019;9(16):p. 3387. doi: 10.3390/app9163387. [DOI] [Google Scholar]
- 179.Padureanu R., Albu C. V., Mititelu R. R., et al. Oxidative stress and inflammation interdependence in multiple sclerosis. Journal of Clinical Medicine . 2019;8(11):p. 1815. doi: 10.3390/jcm8111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Calina D., Buga A. M., Mitroi M., et al. The treatment of cognitive, behavioural and motor impairments from brain injury and neurodegenerative diseases through cannabinoid system modulation-evidence from in vivo studies. Journal of Clinical Medicine . 2020;9(8):p. 2395. doi: 10.3390/jcm9082395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Salehi B., Quispe C., Chamkhi I., et al. Pharmacological properties of chalcones: a review of preclinical including molecular mechanisms and clinical evidence. Frontiers in Pharmacology . 2021;11:592654–592654. doi: 10.3389/fphar.2020.592654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sharifi-Rad J., Kamiloglu S., Yeskaliyeva B., et al. Pharmacological activities of psoralidin: a comprehensive review of the molecular mechanisms of action. Frontiers in Pharmacology . 2020;11:p. 11. doi: 10.3389/fphar.2020.571459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Salehi B., Prakash Mishra A., Nigam M., et al. Ficus plants: state of the art from a phytochemical, pharmacological, and toxicological perspective. Phytotherapy Research . 2021;35(3):1187–1217. doi: 10.1002/ptr.6884. [DOI] [PubMed] [Google Scholar]
- 184.Thabit S., Handoussa H., Roxo M., el Sayed N. S., Cestari de Azevedo B., Wink M. Evaluation of antioxidant and neuroprotective activities of Cassia fistula (L.) using theCaenorhabditis elegansmodel. PeerJ . 2018;6, article e5159 doi: 10.7717/peerj.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Campos J. F., de Castro D. T. H., Damião M. J., et al. The chemical profile of Senna velutina leaves and their antioxidant and cytotoxic effects. Oxidative Medicine and Cellular Longevity . 2016;2016:12. doi: 10.1155/2016/8405957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ahmed S. I., Hayat M. Q., Tahir M., et al. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complementary and Alternative Medicine . 2016;16(1):1–9. doi: 10.1186/s12906-016-1443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guarize L., da Costa J. C., Dutra L. B., Mendes R. F., Lima I. V. A., Scio E. Anti-inflammatory, laxative and intestinal motility effects of Senna macranthera leaves. Natural Product Research . 2012;26(4):331–343. doi: 10.1080/14786411003754264. [DOI] [PubMed] [Google Scholar]
- 188.Silva G. A., Monteiro J. A., Ferreira E. B., et al. Total phenolic content, antioxidant and anticancer activities of four species of Senna mill. from Northeast Brazil. International Journal of Pharmacy and Pharmaceutical Sciences . 2014;6:199–202. [Google Scholar]
- 189.Ita B., Ndukwe G. Antioxidant activity of Senna alata root extracts. Journal of Natural Products and Resources . 2017;3(1):94–96. [Google Scholar]
- 190.Phaiphan A., Baharin B. Ultrasound-assisted extraction produce better antibacterial and antioxidant activities of Senna siamea (Lam.) leaf extracts than solvent extraction. Malaysian Journal of Microbiology . 2019;15:34–43. [Google Scholar]
- 191.Laghari A. Q., Memon S., Nelofar A., Laghari A. H. Extraction, identification and Antioxidative properties of the flavonoid-rich fractions from leaves and flowers of Cassia angustifolia. American Journal of Analytical Chemistry . 2011;2(8):871–878. doi: 10.4236/ajac.2011.28100. [DOI] [Google Scholar]
- 192.Sagnia B., Fedeli D., Casetti R., Montesano C., Falcioni G., Colizzi V. Antioxidant and anti-inflammatory activities of extracts from Cassia alata, Eleusine indica, Eremomastax speciosa, Carica papaya and Polyscias fulva medicinal plants collected in Cameroon. PLoS One . 2014;9(8, article e103999) doi: 10.1371/journal.pone.0103999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Navarro M., Moreira I., Arnaez E., et al. Proanthocyanidin characterization, antioxidant and cytotoxic activities of three plants commonly used in traditional medicine in Costa Rica: Petiveria alliaceae L., Phyllanthus niruri L. and Senna reticulata Willd. Plants . 2017;6(4):p. 50. doi: 10.3390/plants6040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mak Y. W., Chuah L. O., Ahmad R., Bhat R. Antioxidant and antibacterial activities of hibiscus (Hibiscus rosa- sinensis L.) and Cassia (Senna bicapsularis L.) flower extracts. Journal of King Saud University-Science . 2013;25(4):275–282. doi: 10.1016/j.jksus.2012.12.003. [DOI] [Google Scholar]
- 195.Channa U., Shah A. M., Bhatti S., Memon A. A., Ghanghro A. B., Memon M. N. Phytochemical analysis and antibacterial properties of Cassia Senna alata. Rawal Medical Journal . 2020;45(1):223–226. [Google Scholar]
- 196.Madubunyi I. I., Ode O. J. In vitro and in vivo antioxidant potential of the methanolic extract of Cassia singueana Delile (Fabaceae) lock leaves. Comparative Clinical Pathology . 2012;21(6):1565–1569. doi: 10.1007/s00580-011-1328-y. [DOI] [Google Scholar]
- 197.Ottu O., Atawodi S., Onyike E. Antioxidant, hepatoprotective and hypolipidemic effects of methanolic root extract of Cassia singueana in rats following acute and chronic carbon tetrachloride intoxication. Asian Pacific Journal of Tropical Medicine . 2013;6(8):609–615. doi: 10.1016/S1995-7645(13)60105-4. [DOI] [PubMed] [Google Scholar]
- 198.Abubacker M. N., Ramanathan R., Kumar T. S. In vitro antifungal activity of Cassia alata Linn. Flower extract. Natural Product Radiance . 2008;7(1):6–9. [Google Scholar]
- 199.Mohideen S., Sasikala E., Aruhaj P. Pharmacognosy of Cassia alata Linn–leaves. Ancient Science of Life . 2005;24(4):192–198. [PMC free article] [PubMed] [Google Scholar]
- 200.Borges-Argáez R., Canche-Chay C. I., Peña-Rodríguez L. M., Said-Fernández S., Molina-Salinas G. M. Antimicrobial activity of Diospyros anisandra. Fitoterapia . 2007;78(5):370–372. doi: 10.1016/j.fitote.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 201.de Castro A. T., Castro A. P., Silva M. S., et al. In vitro evaluation of the schistosomicidal effect of the extracts, fractions and major 3-hydroxy-2,6-dialkyl-substituted piperidine alkaloids from the flowers of Senna spectabilis (Fabaceae) Bioorganic & Medicinal Chemistry Letters . 2016;26(17):4197–4204. doi: 10.1016/j.bmcl.2016.07.058. [DOI] [PubMed] [Google Scholar]
- 202.Hennebelle T., Weniger B., Joseph H., Sahpaz S., Bailleul F. Senna alata. Fitoterapia . 2009;80(7):385–393. doi: 10.1016/j.fitote.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 203.Ogunjobi A., Abiala M. Antimicrobial activity of Senna alata and Phyllanthus amarus. Global journal of pharmacology . 2013;7(2):198–202. [Google Scholar]
- 204.Makinde A. A., Igoli J. O., Ta’Ama L., Shaibu S. J., Garba A. Antimicrobial activity of Cassia alata. African Journal of Biotechnology . 2007;6(13) [Google Scholar]
- 205.Ehiowemwenguan G., Inetianbor J., Yakubu J. Antimicrobial qualities of Senna alata. IOSR Journal of Pharmacy and Biological Sciences . 2014;9(2):47–52. doi: 10.9790/3008-09244752. [DOI] [Google Scholar]
- 206.Sule W. F., Okonko I. O., Omo-Ogun S., et al. Phytochemical properties and in-vitro antifungal activity of Senna alata Linn. Crude stem bark extract. Journal of Medicinal Plant Research . 2011;5(2):176–183. [Google Scholar]
- 207.Timothy S. Y., Wazis C. H., Adati R. G., Maspalma I. D. Antifungal activity of aqueous and ethanolic leaf extracts of Cassia alata Linn. Journal of Applied Pharmaceutical Science . 2012;2(7):p. 182. doi: 10.7324/JAPS.2012.2728. [DOI] [Google Scholar]
- 208.Wuthi-udomlert M., Kupittayanant P., Gritsanapan W. In vitro evaluation of antifungal activity of anthraquinone derivatives of Senna alata. Journal of Health Research . 2010;24(3):117–122. [Google Scholar]
- 209.Phongpaichit S., Pujenjob N., Rukachaisirikul V., Ongsakul M. Antifungal activity from leaf extracts of Cassia alata L., Cassia fistula L. and Cassia tora L. Songklanakarin Journal of Science and Technology . 2004;26(5):741–748. [Google Scholar]
- 210.Sharifi-Rad J., Quispe C., Rahavian A., et al. Bioactive compounds as potential agents for sexually transmitted diseases management: a review to explore molecular mechanisms of action. Frontiers in Pharmacology . 2021;12(1886) doi: 10.3389/fphar.2021.674682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Albayrak S., Aksoy A., Sagdic O., Albayrak S. Antioxidant and antimicrobial activities of different extracts of some medicinal herbs consumed as tea and spices in Turkey. Journal of Food Biochemistry . 2012;36(5):547–554. doi: 10.1111/j.1745-4514.2011.00568.x. [DOI] [Google Scholar]
- 212.Jain S., Sharma R. A., Jain R., Mittal C. Antimicrobial screening of Cassia occidentalis l.in vivo andin vitro. Phytotherapy Research . 1998;12(3):200–204. doi: 10.1002/(SICI)1099-1573(199805)12:3<200::AID-PTR226>3.0.CO;2-B. [DOI] [Google Scholar]
- 213.Ogbole O. O., Adeniji J. A., Ajaiyeoba E. O. Anthraquinones and triterpenoids from Senna siamea (Fabaceae) Lam inhibit poliovirus activity. African Journal of Microbiology Research . 2014;8(31):2955–2963. doi: 10.5897/AJMR2014.6911. [DOI] [Google Scholar]
- 214.Ogbole O. O., Adeniji J. A., Ajaiyeoba E. O., Adu D. F. Anti-polio virus activity of medicinal plants selected from the Nigerian ethno-medicine. Academic Journals . 2013;12(24):3878–3883. [Google Scholar]
- 215.Shaheen M., Borsanyiova M., Mostafa S., Bopegamage S., El-Esnawy N. Antiviral activity of Cassia alata extracts against cardiac coxsackievirus B3 infections in vitro and in vivo. Tropical Journal of Pharmaceutical Research . 2009;8(2):117–125. [Google Scholar]
- 216.Woradulayapinij W., Soonthornchareonnon N., Wiwat C. In vitro HIV type 1 reverse transcriptase inhibitory activities of Thai medicinal plants and Canna indica L. rhizomes. Journal of Ethnopharmacology . 2005;101(1-3):84–89. doi: 10.1016/j.jep.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 217.WHO. Schistosomiasis . Wolrd Health Organization; 2020. [Google Scholar]
- 218.de Albuquerque Melo G. M., Silva M. C. R., Guimarães T. P., et al. Leishmanicidal activity of the crude extract, fractions and major piperidine alkaloids from the flowers of Senna spectabilis. Phytomedicine . 2014;21(3):277–281. doi: 10.1016/j.phymed.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 219.Caamal-Fuentes E. E., Graniel-Sabido M., Mena-Rejón G. J., Moo-Puc R. E. Anti-giardia activity and acute toxicity of a methanol extract of Senna racemosa bark. Journal of Ethnopharmacology . 2016;193:604–606. doi: 10.1016/j.jep.2016.09.055. [DOI] [PubMed] [Google Scholar]
- 220.Moo-Puc R. E., Mena-Rejon G. J., Quijano L., Cedillo-Rivera R. Antiprotozoal activity of Senna racemosa. Journal of Ethnopharmacology . 2007;112(2):415–416. doi: 10.1016/j.jep.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 221.Eguale T., Tadesse D., Giday M. In Vitro anthelmintic activity of crude extracts of five medicinal plants against egg-hatching and larval development of Haemonchus contortus. Journal of Ethnopharmacology . 2011;137(1):108–113. doi: 10.1016/j.jep.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 222.Villaseñor I. M., Canlas A. P., Pascua M. P., Sabando M. N., Soliven L. A. Bioactivity studies on Cassia alata Linn. Leaf extracts. Phytotherapy Research . 2002;16(S1):93–96. doi: 10.1002/ptr.768. [DOI] [PubMed] [Google Scholar]
- 223.Chomnawang M. T., Surassmo S., Nukoolkarn V. S., Gritsanapan W. Antimicrobial and weed inhibitory activities of Senna spectabilis extracts against plant pathogens. International Journal of Agricultural Technology . 2018;14(7 Special Issue):1445–1454. [Google Scholar]
- 224.Chomnawang M. T., et al. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. Journal of Ethnopharmacology . 2005;101(1-3):330–333. doi: 10.1016/j.jep.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 225.Mcnicol G. P. The effect of a standardised senna preparation on the human bowel. Journal of Pharmacy and Pharmacology . 2011;10(1):499–506. doi: 10.1111/j.2042-7158.1958.tb10334.x. [DOI] [PubMed] [Google Scholar]
- 226.Thamlikitkul V. I., Bunyapraphatsara N., Dechatiwongse T., et al. Randomized controlled trial of Cassia alata Linn. For constipation. Journal of the Medical Association of Thailand . 1990;73(4):217–222. [PubMed] [Google Scholar]
- 227.Kinnunen O., Winblad W., Koistinen P., Salokannel I. Safety and efficacy of a bulk laxative containing Senna versus lactulose in the treatment of chronic constipation in geriatric patients. Pharmacology . 1993;47(1):253–255. doi: 10.1159/000139866. [DOI] [PubMed] [Google Scholar]
- 228.Damodaran S., Venkataraman S. A study on the therapeutic efficacy of Cassia alata, Linn. Leaf extract against Pityriasis versicolor. Journal of Ethnopharmacology . 1994;42(1):19–23. doi: 10.1016/0378-8741(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 229.Ramesh P., Kumar K. S., Rajagopal M. R., Balachandran P., Warrier P. K. Managing morphine-induced constipation: a controlled comparison of an Ayurvedic formulation and Senna. Journal of Pain and Symptom Management . 1998;16(4):240–244. doi: 10.1016/S0885-3924(98)00080-3. [DOI] [PubMed] [Google Scholar]
- 230.van Gorkom B. A., Karrenbeld A., Limburg A. J., Kleibeuker J. H. The effect of sennosides on colonic mucosal histology and bowel preparation. Zeitschrift für Gastroenterologie . 1998;36(1):13–18. [PubMed] [Google Scholar]
- 231.Phutsisen J., Kietpeerakool C., Jampathong N., et al. Effects of Cassia alata Linn on bowel function recovery following surgery for gynecological cancer: a randomized controlled trial. Complementary Therapies in Medicine . 2019;47, article 102222 doi: 10.1016/j.ctim.2019.102222. [DOI] [PubMed] [Google Scholar]
- 232.Santos-Jasso K. A., Arredondo-García J. L., Maza-Vallejos J., Lezama-del Valle P. Effectiveness of senna vs. polyethylene glycol as laxative therapy in children with constipation related to anorectal malformation. Journal of Pediatric Surgery . 2017;52(1):84–88. doi: 10.1016/j.jpedsurg.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 233.Feudtner C., Freedman J., Kang T., Womer J. W., Dai D., Faerber J. Comparative effectiveness of Senna to prevent problematic constipation in pediatric oncology patients receiving opioids: a multicenter study of clinically detailed administrative data. Journal of Pain and Symptom Management . 2014;48(2):272–280. doi: 10.1016/j.jpainsymman.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 234.Ergül B., Filik L., Koçak E., Doğan Z., Sarıkaya M. Efficacy and safety of gum chewing in adjunct to high-dose Senna for bowel cleansing before colonoscopy: a single-blind randomized controlled trial. Saudi Journal of Gastroenterology . 2014;20(6):356–359. doi: 10.4103/1319-3767.145325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Patel M., Schimpf M. O., O'Sullivan D. M., LaSala C. A. The use of Senna with docusate for postoperative constipation after pelvic reconstructive surgery: a randomized, double-blind, placebo-controlled trial. American Journal of Obstetrics and Gynecology . 2010;202(5):479.e1–479.e5. doi: 10.1016/j.ajog.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 236.Raposo J., Velho P. Prolonged ingestion of senna: weight loss, cyclic edema, dyspepsia and hepatoneuromyopathy. Medicină Internă . 2010;17(2):81–84. [Google Scholar]
- 237.Kositchaiwat S., Suwanthanmma W., Suvikapakornkul R., Tiewthanom V., Rerkpatanakit P., Tinkornrusmee C. Comparative study of two bowel preparaton regimens for colonoscopy: Senna tabletsvssodium phosphate solution. World Journal of Gastroenterology . 2006;12(34):5536–5539. doi: 10.3748/wjg.v12.i34.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cho S.-H., Kim T. H., Lee N. H., Son H. S., Cho I. J., Ha T. Y. Effects of Cassia tora fiber supplement on serum lipids in Korean diabetic patients. Journal of Medicinal Food . 2005;8(3):311–318. doi: 10.1089/jmf.2005.8.311. [DOI] [PubMed] [Google Scholar]
- 239.Radaelli F., Meucci G., Terruzzi V., et al. Efficacy and acceptability of high dose Senna compared with standard polyethylene glycol solution for colon cleansing prior to colonoscopy: preliminary results of a randomized, investigator-blind trial. Gastrointestinal Endoscopy . 2005;61(5):p. AB119. doi: 10.1016/S0016-5107(05)00730-3. [DOI] [Google Scholar]
- 240.van Gorkom B. A., Karrenbeld A., van der Sluis T., Koudstaal J., de Vries E. G. E., Kleibeuker J. H. Influence of a highly purified senna extract on colonic epithelium. Digestion . 2000;61(2):113–120. doi: 10.1159/000007743. [DOI] [PubMed] [Google Scholar]
- 241.Zhong L. L., Cheng C. W., Chan Y., et al. Chinese herbal medicine (Ma Zi Ren wan) for functional constipation: study protocol for a prospective, double-blinded, double-dummy, randomized controlled trial. Trials . 2013;14(1):p. 366. doi: 10.1186/1745-6215-14-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhong L. L., Cheng C. W., Kun W., et al. Efficacy of MaZiRenWan, a Chinese herbal medicine, in patients with functional constipation in a randomized controlled trial. Clinical Gastroenterology and Hepatology . 2019;17(7):1303–1310.e18. doi: 10.1016/j.cgh.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 243.Beuers U., Spengler U., Pape G. R. Hepatitis after chronic abuse of senna. Lancet . 1991;337(8737):372–373. doi: 10.1016/0140-6736(91)91012-J. [DOI] [PubMed] [Google Scholar]
- 244.Vanderperren B., Rizzo M., Angenot L., Haufroid V., Jadoul M., Hantson P. Acute liver failure with renal impairment related to the abuse of Senna anthraquinone glycosides. Annals of Pharmacotherapy . 2005;39(7-8):1353–1357. doi: 10.1345/aph.1E670. [DOI] [PubMed] [Google Scholar]
- 245.Soyuncu S., Cete Y., Nokay A. E. Portal vein thrombosis related to Cassia angustifolia. Clinical Toxicology . 2008;46(8):774–777. doi: 10.1080/15563650701682097. [DOI] [PubMed] [Google Scholar]
- 246.Sharifi-Rad J., Rodrigues C. F., Stojanović-Radić Z., et al. Probiotics: versatile bioactive components in promoting human health. Medicina . 2020;56(9):p. 433. doi: 10.3390/medicina56090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Islam M. T., Quispe C., Martorell M., et al. Dietary supplements, vitamins and minerals as potential interventions against viruses: perspectives for COVID-19. International Journal for Vitamin and Nutrition Research . 2021:1–18. doi: 10.1024/0300-9831/a000694. [DOI] [PubMed] [Google Scholar]
- 248.Mitrut P., Docea A. O., Kamal A. M., et al. Rodrigo L., editor. Colorectal cancer and inflammatory bowel disease. Colorectal Cancer - from Pathogenesis to Treatment . 2016. pp. 185–199.
- 249.Zlatian O. M., Comănescu M. V., Roşu A. F., et al. Histochemical and immunohistochemical evidence of tumor heterogeneity in colorectal cancer. Romanian Journal of Morphology and Embryology . 2015;56(1):175–181. [PubMed] [Google Scholar]
- 250.Mascolo N., Capasso R., Capasso F. Senna. A safe and effective drug. Phytotherapy Research . 1998;12(S1):S143–S145. doi: 10.1002/(SICI)1099-1573(1998)12:1+<S143::AID-PTR277>3.0.CO;2-G. [DOI] [Google Scholar]
- 251.Cirillo C., Capasso R. Constipation and botanical medicines: an overview. Phytotherapy Research . 2015;29(10):1488–1493. doi: 10.1002/ptr.5410. [DOI] [PubMed] [Google Scholar]
- 252.Wang X., Yin J. Complementary and alternative therapies for chronic constipation. Evidence-based Complementary and Alternative Medicine . 2015;2015 doi: 10.1155/2015/396396.396396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Capasso F., Gaginella T. S. Laxative. A Practical Guide . Milan: Springer-Verlag Italia; 1997. [Google Scholar]
- 254.Labenz J., Hopmann G., Leverkus F., Börsch G. Bowel cleansing prior to colonoscopy. A prospective, randomized, blind comparative study. Medizinische Klinik . 1990;85(10):581–585. [PubMed] [Google Scholar]
- 255.Park S. B., Kim Y. S. Simultaneous separation of three isomeric sennosides from senna leaf (Cassia acutifolia) using counter-current chromatography. Journal of Separation Science . 2015;38(20):3502–3507. doi: 10.1002/jssc.201500672. [DOI] [PubMed] [Google Scholar]
- 256. https://www.cbsnews.com/news/norway-covid-19-vaccine-elderly-deaths-no-link/
- 257.Vilanova-Sanchez A., Gasior A. C., Toocheck N., et al. Are Senna based laxatives safe when used as long term treatment for constipation in children? Journal of Pediatric Surgery . 2018;53(4):722–727. doi: 10.1016/j.jpedsurg.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 258.Greenhalf J. O., Leonard H. S. Laxatives in the treatment of constipation in pregnant and breast-feeding mothers. Practitioner . 1973;210(256):259–263. [PubMed] [Google Scholar]
- 259.Prather C. M. Pregnancy-related constipation. Current Gastroenterology Reports . 2004;6(5):402–404. doi: 10.1007/s11894-004-0057-7. [DOI] [PubMed] [Google Scholar]
- 260.Gattuso J. M., Kamm M. A. Adverse effects of drugs used in the management of constipation and diarrhoea. Drug Safety . 1994;10(1):47–65. doi: 10.2165/00002018-199410010-00004. [DOI] [PubMed] [Google Scholar]
- 261.Ács N., Bánhidy F., Puhó E. H., Czeizel A. E. No association between severe constipation with related drug treatment in pregnant women and congenital abnormalities in their offspring: a population-based case-control study. Congenital Anomalies . 2010;50(1):15–20. doi: 10.1111/j.1741-4520.2009.00252.x. [DOI] [PubMed] [Google Scholar]
- 262.Müller S. O., Eckert I., Lutz W. K., Stopper H. Genotoxicity of the laxative drug components emodin, aloe-emodin and danthron in mammalian cells: topoisomerase II mediated? Mutation Research . 1996;371(3-4):165–173. doi: 10.1016/S0165-1218(96)90105-6. [DOI] [PubMed] [Google Scholar]
- 263.Baldwin W. F. Clinical study of Senna administration to nursing mothers: assessment of effects on infant bowel habits. Canadian Medical Association Journal . 1963;89(11):566–568. [PMC free article] [PubMed] [Google Scholar]
- 264.Faber P., Strenge-Hesse A. Relevance of rhein excretion into breast milk. Pharmacology . 1988;36(1):212–220. doi: 10.1159/000138442. [DOI] [PubMed] [Google Scholar]
- 265.Capasso F., Gaginella T. S., Grandolini G., Izzo A. A. Phytotherapy: A Quick Reference to Herbal Medicine . Heidelberg, Germany: Springer-Verlag; 2003. [Google Scholar]
- 266.Ritsema G. H., Eilers G. Potassium supplements prevent serious hypokalaemia in colon cleansing. Clinical Radiology . 1994;49(12):874–876. doi: 10.1016/S0009-9260(05)82879-X. [DOI] [PubMed] [Google Scholar]
- 267.Waldhäusl V. H. Laxative-induced hypokalemic myopathy. A case history. Wiener Klinische Wochenschrift . 1980;92(3):101–103. [PubMed] [Google Scholar]
- 268.Wang M. T., Li I. H., Lee W. J., Huang T. Y., Leu H. B., Chan A. L. F. Exposure to sennoside-digoxin interaction and risk of digoxin toxicity: a population-based nested case-control study. European Journal of Heart Failure . 2011;13(11):1238–1243. doi: 10.1093/eurjhf/hfr091. [DOI] [PubMed] [Google Scholar]
- 269.Cao Y., He Y., Wei C., et al. Aquaporins alteration profiles revealed different actions of Senna, Sennosides, and Sennoside a in diarrhea-rats. International Journal of Molecular Sciences . 2018;19(10):p. 3210. doi: 10.3390/ijms19103210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mascolo N., Mereto E., Borrelli F., et al. Does senna extract promote growth of aberrant crypt foci and malignant tumors in rat colon? Digestive Diseases and Sciences . 1999;44(11):2226–2230. doi: 10.1023/A:1026696402212. [DOI] [PubMed] [Google Scholar]
- 271.Lyden-Sokolowski A., Nilsson A., Sjöberg P. Two-year carcinogenicity study with sennosides in the rat: emphasis on gastro-intestinal alterations. Pharmacology . 1993;47(1):209–215. doi: 10.1159/000139860. [DOI] [PubMed] [Google Scholar]
- 272.Mereto E., Ghia M., Brambilla G. Evaluation of the potential carcinogenic activity of Senna and cascara glycosides for the rat colon. Cancer Letters . 1996;101(1):79–83. doi: 10.1016/0304-3835(96)04129-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this review are from previously reported studies and datasets, which have been cited. The processed data are available from the corresponding authors upon request.


