Dear Editor,
Recent study by Dimeglio et al., demonstrated that the infection with Omicron variant generate higher immune response in affected individuals than the Delta variant.1 The emergence of SARS-CoV-2 Variant of Concern (VOC) Omicron has caused a major spike in new COVID-19 infections worldwide.2 Although, the Delta variant still found be the most prevalent VOC across the globe, the Omicron has displaced Delta in Southern Africa and rapidly becoming dominant in the United Kingdom and United States of America.2 Since January 2022, India has also seen the sudden surge in COVID-19 cases with the Omicron and Delta variant. Omicron has shown higher transmissibility and immune escape as compared to the other VOCs including Alpha, Beta, and Delta leading to many reported breakthrough and re-infections across the globe. However, the severity of the disease is lesser as observed with other VOCs.3 Further research are crucial to evaluate the immune evasion potential of the Omicron in the individuals with natural infection and/or vaccination. In this study, we have analyzed the IgG and neutralizing antibodies (NAbs) against B.1, Alpha, Beta, Delta and Omicron variants with the sera of individuals infected with the Omicron variant (BA.1).
The study individuals (n = 39) were mainly the foreign returnees (n = 28) [UAE, South/ West/ East Africa, Middle east, USA and UK] and their high-risk contacts (n = 11) confirmed as Omicron by next generation sequencing. The sera samples of all the individuals were collected at the post onset date of 10.5 ± 6.3 days, where oro/naso-phyrangeal swabs of eight individuals were positive for SARS-CoV-2 (Ct range: 16–29) and rest of the samples were negative. Further, the participants were grouped into three categories, breakthrough infections after two dose of vaccines [ChAdOx1 nCoV-19 (n = 25); 147 ± 60 days post vaccination and BNT162b2 mRNA (n = 8) 113 ± 57 days post vaccination] and unvaccinated individuals (n = 6). We have analyzed the IgG antibody with S1-RBD, N protein and whole inactivated antigen ELISA and NAb responses using plaque reduction neutralization test (PRNT50) as described earlier.4, 5, 6
The mean age of the ChAdOx1 nCoV-19 vaccinated individuals was 39 years [11F/14 M] with thirtheen of them being asymptomatic while twelve had mild fever, sore-throat cold and cough lasting for 1–3 days. Of these, two cases were of reinfection with documented past infection in July 2020. The individuals vaccinated with two doses of BNT162b2 had mean age of 33 years [7F/1 M] and only one case reported to have mild fever and sore-throat while rest seven were asymptomatic. Unvaccinated group (n = 6) included all female individuals [mean age 16 years, peadiatric (n = 5) and adult (n = 1)]. Of these, four were asymptomatic while two peadiatric cases were mildly symptomatic.
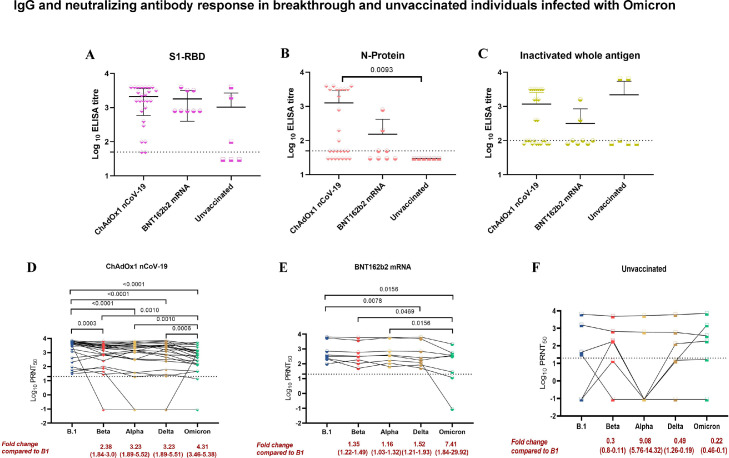
The geometric mean titres (GMTs) of the S1-RBD IgG antibodies in the sera of the ChAdOx1 nCoV-19 [1179] and BNT162b2 mRNA [1383] breakthrough individuals showed no significant difference. However, 2.5 to 3.6 times reduction in the GMTs was observed with N protein and inactivated whole antigen IgG ELISA in the sera of BNT162b2 mRNA breakthrough individuals compared to the ChAdOx1 nCoV-19. In the unvaccinated group, varied response in the GMTs of IgG antibodies were observed with S1-RBD (166.9), N protein (30) and inactivated whole antigen (357.8) [Fig. 1 A–C].
Fig. 1.
IgG antibody response in breakthrough and unvaccinated individuals was assessed using ELISA (A) S1-RBD (B) N protein (C) Inactivated whole antigen. The IgG antibody titers of the different groups were compared using the two-tailed Kruskal Wallis test and the p-value less than 0.05 were considered to be statistically significant. The neutralizing antibody response among (D) ChAdOx1 nCoV-19 breakthrough (E) BNT162b2 mRNA breakthrough and (F) unvaccinated individuals against B.1, Alpha, Beta, Delta and Omicron were determined using the plaque reduction neutralization test (PRNT50). The titers amongst the groups were compared with B.1 using the Wilcoxon matched-pairs signed-rank test to assess the statistical significance.
The GMTs of neutralizing antibodies of ChAdOx1 nCoV-19 breakthrough individuals showed significant fold-reductions compared to B.1 against Alpha (3.23), Beta (2.38), Delta (3.23) and Omicron (4.31) variants, respectively. Similarly, BNT162b2 mRNA breakthrough individuals demonstrated significant fold-reduction in GMTs of 1.52 and 7.41 for Delta and Omicron, respectively. While, non-significant fold-reduction was observed with Alpha (1.16) and Beta (1.35). In contrary, Alpha variant (9.08) was modestly more resistant to neutralization than Beta (0.3), Delta (0.49) and Omciron (0.22) in the unvaccinated individuals compared to B.1 [Fig. 1D–F]. Our study suggest a 3-fold reduction in the NAb titres in BNT162b2 mRNA breakthrough individuals as compared with ChAdOx1 nCoV-19.
The earlier studies from other groups demonstrated that immune response generated through natural infection or vaccination showed weaker immune response against Omicron.7, 8, 9, 10, 11 Our study demonstrated that the individuals infected with Omicron have significant immune response which could neutralize not only the Omicron but also the other VOCs including most prevalent Delta variant. This suggest that the immune response induced by the Omicron could effectively neutralize the Delta variant making the re-infection with Delta less likely, thereby displacing the Delta as dominant strain.This emphasizes the need for the Omicron specific vaccine strategy.
Declaration of Competing Interest
Authors do not have a conflict of interest among themselves.
Acknowledgments
Funding
Financial support was provided by the Indian Council of Medical Research (ICMR), New Delhi at ICMR-National Institute of Virology, Pune under intramural funding ‘COVID-19′.
Acknowledgement
Authors are thankful to the support provided by Dr. Kiran Bhise, In-charge COVID care center Pune; Dr. Balaji Barure, District Surveillance officer (DSO), Latur; Dr. Bodke, DSO Osmanabad; Dr. Sachin Patil, DSO Satara for the coordination of the sample collection and shipment. The authors would like to gratefully acknowledge the staff of ICMR-NIV, Pune including Mrs. Asha Salunkhe, Mr. Chetan Patil Dr. Rajlaxmi Jain, Mr. Prasad Sarkale, Mr. Hitesh Dighe, Mr. Annasaheb Suryavanshi, Mr. Manjunath Holeppanawar, Mr. Sanjay Thorat, Mrs. Priyanka Waghmare, Mrs. Poonam Bodke, Mrs. Shilpa Ray for extending the excellent technical support. The authors also acknowledge the contribution of Dr. ML Choudhary, Dr. Sumit Bharadwaj and the team of National Influenza center (NIC), ICMR-NIV, Pune.
References
- 1.Dimeglio C., Migueres M., Mansuy J.M., Saivin S., Miedougé M., Chapuy-Regaud S., et al. Antibody titers and breakthrough infections with Omicron SARS-CoV-2. J Infect. 2022 doi: 10.1016/j.jinf.2022.01.044. , S0163-4453–3. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Enhancing response to Omicron SARS-CoV-2 variant: Technical brief and priority actions for Member States. https://www.who.int/docs/default-source/coronaviruse/2022-01-07-global-technical-brief-and-priority-action-on-omicron---corr2.pdf?sfvrsn=918b09d_20. Accessed 18 January 2022.
- 3.Wolter N., Jassat W., Walaza S., Welch R., Moultrie H., Groome M., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ella R., Reddy S., Jogdand H., Sarangi V., Ganneru B., Prasad S., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21(7):950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshpande G.R., Sapkal G.N., Tilekar B.N., Yadav P.D., Gurav Y., Gaikwad S., et al. Neutralizing antibody responses to SARS-CoV-2 in COVID-19 patients. Indian J Med Res. 2020;152(1–2):82. doi: 10.4103/ijmr.IJMR_2382_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadav P.D., Gupta N., Potdar V., Mohandas S., Sahay R.R., Sarkale P., et al. An in vitro and in vivo approach for the isolation of Omicron variant from human clinical specimens. bioRxiv. 2022 doi: 10.1101/2022.01.02.474750. [DOI] [Google Scholar]
- 7.Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2021:1–5. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. medRxiv. 2021 doi: 10.1101/2021.12.14.21267615. [DOI] [Google Scholar]
- 9.Wilhelm A., Widera M., Grikscheit K., Toptan T., Schenk B., Pallas C., et al. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. medRxiv. 2021 doi: 10.1101/2021.12.07.21267432. [DOI] [Google Scholar]
- 10.Roessler A., Riepler L., Bante D., von Laer D., Kimpel J. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N Engl J Med. 2022;386:698–700. doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carreno J.M., Alshammary H., Tcheou J., Singh G., Raskin A., Kawabata H., et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2021:1–8. doi: 10.1038/s41586-022-04399-5. [DOI] [PubMed] [Google Scholar]